Abstract
In this review, we discuss the advantages of vegetable sprouts in the development of food products as well as their beneficial effects on a variety of disorders. Sprouts are obtained from different types of plants and seeds and various types of leafy, root, and shoot vegetables. Vegetable sprouts are enriched in bioactive compounds, including polyphenols, antioxidants, and vitamins. Currently, different conventional methods and advanced technologies are used to extract bioactive compounds from vegetable sprouts. Due to some issues in traditional methods, increasingly, the trend is to use recent technologies because the results are better. Applications of phytonutrients extracted from sprouts are finding increased utility for food processing and shelf-life enhancement. Vegetable sprouts are being used in the preparation of different functional food products such as juices, bread, and biscuits. Previous research has shown that vegetable sprouts can help to fight a variety of chronic diseases such as cancer and diabetes. Furthermore, in the future, more research is needed that explores the extraordinary ways in which vegetable sprouts can be incorporated into green-food processing and preservation for the purpose of enhancing shelf-life and the formation of functional meat products and substitutes.
Keywords: sprout, bioactive compound, new extraction, functional product, pharmacological role
1. Introduction
Sprouts are germinated from seeds of crops such as radish, cereals (rice and legumes), soybeans, and trees (Toonasinensis and pepper). Sprouts have been a popular dish in China for over 5000 years and have now spread to other Eastern countries. Sprout consumption has increased in Western cultures due to a shift in lifestyle towards convenience and health [1]. Sprouts have become more popular worldwide because of their nutritional value and health advantages. As compared with adult edible plant portions, sprouts are abundant in health-promoting bioactive chemicals, vitamins, and minerals. Sprouting is a food processing method that boosts the nutritional value of cereals, oilseeds, and vegetable seeds [2], by inducing macronutrient breakdown and increasing the amounts of amino acids, simple sugars, and other nutritional components [3]. Sprouting also helps to reduce anti-nutritional components and to improve sprouts’ digestibility and sensory aspects. Sprouts aid in the synthesis of new beneficial components such as polyphenols and vitamin C. Recently, sprouts have grasped consumers’ consumption interests due to their functional properties and phytonutritional profile. Sprouts have gained popularity as a top healthy food [4]. Due to the presence of biologically active compounds [5], sprouts have an important role in preventing several forms of malignancies. In the literature, sprouts have been shown to have a substantial anti-genotoxic impact against DNA damage [6]. According to Gawlik-Dziki et al. [7], Brassica and vegetable sprouts help to minimize the incidence of lung and colorectal cancer occurrence. According to epidemiological studies, the consumption of broccoli sprout-rich foods has been linked to a lower incidence of many malignancies and chronic degenerative diseases [8]. Cowpea sprouts have been celebrated for reducing cell proliferation and boosting anti-colorectal cancer activity [9]. Isoflavonoids in soybean sprouts defend against cancer and cardiovascular disease [10]. Sprouts include a variety of nutrients that are beneficial to human health and help to avoid a variety of diseases [11]. According to the research, sprouts are an excellent source of a range of phenolic compounds that protect against oxidative reactions. Mung bean sprouts have been reported to lower gastrointestinal issues and heart stroke [12]. Soybean sprouts have been demonstrated to have health-promoting qualities such as lowering cancer and cardiovascular disease risk [13]. Sprouts have been discovered to offer antidiabetic properties. In studies, Brassica oleracea sprouts have been shown to have antidiabetic, hepatoprotective, and antioxidant properties. The extracts had a lowering effect on blood glucose levels in the body and hepatoprotective and antioxidant properties. As a consequence, Brassica sprouts have anti-hyperglycemic activity [14]. Antibacterial activity has also been discovered in sprouts. Broccoli and pea sprouts have antibacterial properties against Helicobacter pylori (bacteria linked to stomach cancer) [15,16]. The purpose of this article is to explore methods for extracting bioactive components from certain vegetable sprouts to make functional meals and their bioavailability against certain diseases.
2. Bioactive Components in Different Vegetables Sprouts
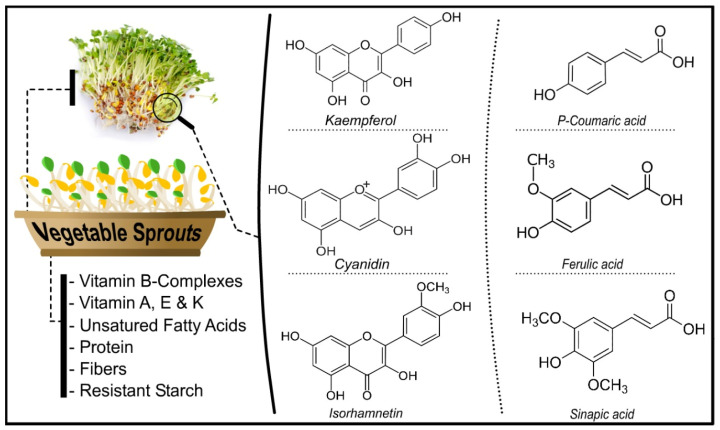
Sprouts are recognized due to their high concentration of bioactive compounds [17]. Bioactive chemicals are present in high concentrations in food and food waste. Several bioactive compounds have been found with a wide range of functional and structural features [18] (Figure 1).
Figure 1.
Different bioactive compounds in vegetables sprouts.
Natural chemical components present in minute amounts in plants are called bioactive chemicals [19]. These substances can interact with one or more live tissue components, resulting in many conceivable outcomes. Bioactive chemicals are naturally occurring essential and nonessential substances present in the food chain that have been shown to impact human health. Antioxidants and polyphenols have been studied for their many biological actions. Due to their unique properties, polyphenols such as protocatechuic acid, vanillic acid, caffeic acid, quercetin, and kaempferol have been shown to protect against oxidative damage [20]. Polyphenols are abundant in sprouts [21]. Polyphenols are reducing agents, hydrogen-donating antioxidants, and singlet oxygen quenchers formed in response to biotic or abiotic stress. Polyphenols’ multifunctionality is due to their dispersion in various tissues and organs of plants at various concentrations. Plants produce phenolic substances that contain at least one aromatic ring with one or more hydroxyl substituents and can be divided into flavonoids and phenolic acids based on their chemical structure. In recent years, phenolic compounds have been extensively studied for their antioxidant, anthelmintic, antiallergenic, anticancer, anti-inflammatory, antiviral, antiulcer, anti-hepatotoxic, antidiarrheal, and antiproliferative properties [3,22]. Micronutrients (vitamins and minerals) are important for tissue maintenance, bone and tooth production, and general health. They help to regulate and coordinate most biological activities and other biochemical and physiological functions by acting as cofactors and coenzymes in diverse enzyme systems. Humans and other creatures require micronutrients at varying levels throughout their lives to coordinate numerous physiological activities and sustain health [23,24]. Vitamins are essential for optimal health and perform vital functions in the human body. Vitamin C, often known as ascorbate, is a micronutrient that humans require. In humans, vitamin C deficiency inhibits the function of several enzymes and can lead to scurvy. Ascorbic acid is a cofactor in a variety of essential enzymatic processes [25], and it is involved in collagen formation. Vitamin E (tocopherols and tocotrienols) protects DNA, low-density lipoproteins, and polyunsaturated fatty acids against oxidative damage. Vitamin E is also involved in hemoglobin production, immune response regulation, and membrane structure stability, and it helps to keep blood coagulation, bone growth, and healing under control. In newborns, vitamin K deficiency can cause hemorrhagic illness and surgical bleeding, muscular hematomas, and intracranial hemorrhages in adults [26].
Minerals are required to perform processes that are necessary for a healthy life. The human body needs calcium for optimal heart and muscle function, bone production, and blood cell creation and function. Copper, molybdenum, selenium, and zinc are key components of various critical enzymes in the human body. In contrast, iron is required for several protein syntheses, including hemoglobin, which helps to avoid anemia. Magnesium is necessary for ATP processing and bone health. Sodium and potassium are electrolytes found throughout the body and are necessary for the coregulation of ATP. Phosphorus is found in bones and cells, and it also plays a role in energy metabolism, DNA and ATP (as phosphate), and a variety of other functions [27]. The extraction of bioactive compounds from different types of vegetable sprouts is shown in Table 1.
Table 1.
Extraction of bioactive compound from vegetables sprouts.
| Vegetable | Type | Extraction/Detection/Methods | Bioactive Compounds | Solvent | References |
|---|---|---|---|---|---|
| Water spinach | Leafy | -- | Carotenoids and phenolic acids | -- | [28] |
| Quinoa | Leafy | Conventional -- |
Phenolics, flavonoids, carotenoids (β-carotene and lycopene), and chlorophylls (a and b) | Ethanol | [29] |
| Brassica | Leafy | -- | Polyphenols and glucosinolates | -- | [30] |
| Kale and broccoli | Leafy | Ultrahigh-performance liquid chromatography high-resolution mass spectrometry | Polyphenols and glucosinolates | Methanol/water | [31] |
| Quinoa | Leafy | Spectrophotometry | Total phenolic compounds and antioxidants | Methanol | [32] |
| Radish, broccoli, leek, and beetroot | Leafy, Root | ABTS, FRAP, and ORAC | Polyphenols, L-ascorbic acid, carotenoids, and chlorophylls | Methanol | [33] |
| Garden cress | Leafy | HPLC, ABTS, and DPPH | Phenolic compound, antioxidant,, and flavonoids | Methanol | [34] |
| Onion | Root | HPLC and FTIR | Flavonoids, quercetin, and glucosides | Ethanol | [35] |
| Broccoli and red radish | Leafy Root |
Conventional and HPLC-DAD |
Glucosinolates and phenolic compounds | Methanol | [36] |
| Turnip | Root | Gas chromatography, mass spectroscopy, and DPPH | Phenolics, glucosinolates, and antioxidant, | Water | [37] |
| Fennel | Root | HPLC | Vitamin C, polyphenols, and antioxidants | Methanol/water | [38] |
| Brassicaceae | Root and leafy | FRAP | Phenolic compound and antioxidants | Methanol | [39] |
| Brussels | Leafy | HPLC and spectroscopic analysis | Chlorophyll, vitamin C, polyphenols, flavonoids, and antioxidants | Methanol | [36] |
| Sweet potato | Root | DPPH, spectrophotometer, LCMS/MS method | Anthocyanin and antioxidant, | Ethanol | [40] |
| Vegetable | Leafy | -- | Glucosinolates, phenolics, and isoflavones | -- | [41] |
| Brassica | Leafy | Conventional | Sulforaphane | -- | [42] |
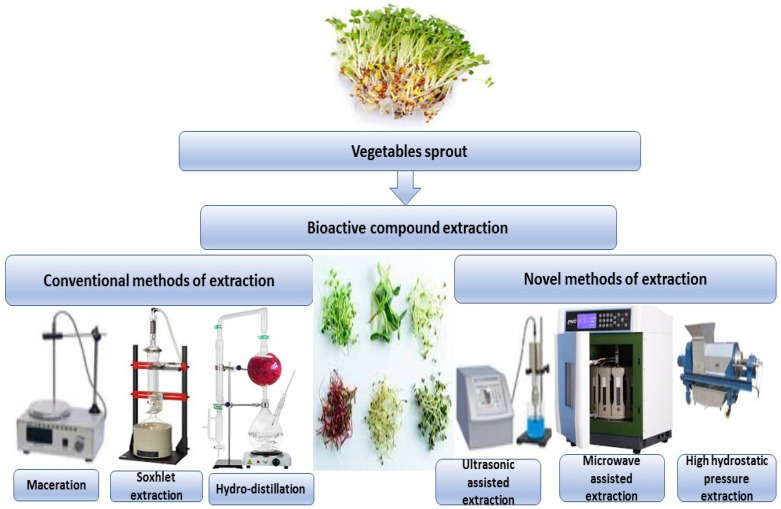
3. Conventional and New Extraction Methods Are Used to Extract the Bioactive Compounds
Several traditional extraction procedures can extract bioactive chemicals from plant sources. The majority of these methods rely on a solvent’s ability to extract and the use of heat and/or mixing. The three most common procedures for extracting bioactive chemicals from plants are Soxhlet extraction, maceration, and hydro distillation [43]. There are both traditional and modern ways of obtaining isoflavones from plants. Maceration, percolation, decoction, infusion, Soxhlet extraction, and hot reflux extraction are all examples of traditional extraction procedures that have been employed to extract bioactive chemicals [44]. As a result, developing quick, safe, and environmentally acceptable technology for analyzing and separating bioactive chemicals is critical. Isoflavones have been separated via “ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), and pressured liquid extraction with green solvents such as ionic water liquids and supercritical carbon dioxide (PLE)”. These methods use organic solvents, take less time, and produce higher yields and quality [45].
In recent years, natural deep eutectic solvents (DESs) have received much attention as good green solvents for extracting bioactive chemicals from natural resources [46]. The current research has looked at the feasibility and effectiveness of extracting isoflavones from chickpea sprouts using various polarities of natural deep eutectic solvents (DESs), by testing 20 different DESs that included hydrogen bond acceptors such as “choline chloride, betaine, and L-proline with different hydrogen bond donors (carboxylic acids, alcohols, sugars, and amine). The researchers looked at the yields of four isoflavones (ononin, sissotrin, formononetin, and biochanin A), total flavonoid concentration, and antioxidant activity to estimate extraction efficiency. Using a Box–Behnken design in conjunction with response surface techniques, “the components that contribute to optimal ultrasound-assisted extraction conditions were then examined.” The extraction yields of isoflavones were significantly affected by DES water content and extraction temperature. Our findings suggest that DESs might be utilized to extract bioactive chemicals from a variety of biomaterials [47]. Sprouts from peanuts yield trans-resveratrol in accelerated solvent extraction [48].
Soxhlet extraction has been used to recover a large number of phytochemicals from Azadirachta indica (Neem) leaf powder, predominantly nonpolar components [49]. Evaluation of Soxhlet extraction for Moringa oliefera leaves resulted in lower yield, as well as phenolic and flavonoid contents [50]. Centella asiatica extraction was optimized using Soxhlet extraction, which produced the best results at 25 °C, a sample-solvent ratio of 1:45, 200 rpm agitation speed, and 1.5 h [51]. After removing lipoidal components from powdered Clitorea ternate flowers with petroleum ether at 60–80 °C, the yield was 2.2 percent w/w [52].
After more ethanol extraction from the marc, alkaloids and saponins were confirmed to be present. However, the anthocyanin, i.e., the main pigment of Clitorea ternate flowers, was not present, indicating that oxidation and degradation had taken place. As compared with other solvents such as petroleum ether, chloroform, and water, the extraction of Psidium guajava L. [53] leaves using ethanolic and hydro alcohol extracts (4:1 v/v) produced the highest extraction yield with the greatest presence of phytoconstituents (alkaloids, saponins, carbohydrates, tannins, and flavonoids) [50]. Nonpolar solvents such as petroleum ether and chloroform revealed no retained active chemicals and very low tannin content in the extracts, respectively. With the exception of the absence of any alkaloids, water was shown to be as effective as ethanol. Polar solvents have been shown to work better for removing bioactive compounds from Psidium guajava [54]. As compared with aqueous extracts of Garnicia atriviridis, methanol extracts (1:10 w/v) had stronger antioxidant activities, while the aqueous extracts had better anti-hyperlipidemic activities [50]. Based on total phenolics, maceration with various solvents at a ratio of 1:10 w/v sample to solvent, for an hour, revealed that 70% acetone was an effective solvent for Portucala oleracea and 70% methanol was an effective solvent for flavonoids in Cosmos caudatus [55]. As compared with Soxhlet extraction and percolation using a comparable solvent, maceration with 70% ethanol and powdered dried materials at 1:40 w/v showed the greatest phenolic and flavonoid concentrations for Moringa oliefera [56]. Using 100% ethanol as the solvent at 75 °C and an irradiation power of 600 W for four cycles, MAE has been evaluated as a new technique to extract triterpene from Centella asiatica and the yield was increased by two times over Soxhlet extraction [50]. Combining MAE with enzyme lysis (such as cellulase) has been shown to increase extraction; the ideal conditions of sample/solvent ratio at 1:36, enzyme pretreatment at 45 °C for 30 min, and irradiation at 650 W for 110 s produced a yield of 27.10 percent. However, Trusheva et al.’s observation that an extra MAE cycle had an impact on the phytochemical degradation was not examined. The best extraction was obtained using MAE with 100 W and 1:12.5 sample/solvent ratios on Dioscorea hispida. The UAE has been shown to be the most productive technique for extracting propolis based on its high yield, lengthy (10–30 min) extraction duration, and excellent selectivity. To shorten extraction times and to prevent exposure to high temperatures, UAE was used to extract thermolabile chemicals such as anthocyanin from floral components. When extracting Withania somnifera using water as the solvent for 15 min, the yield was at its highest, reaching 11.85 percent as compared with ethanol and water-ethanol at various 5, 15, and 20 min extraction times. A higher effectiveness on phenolics was observed when Cratoxylum formosum was extracted using ultrasonic at 45 kHz, 50.33 percent ethanol by volume, at 65 °C for 15 min. Free radical production at irradiation frequencies higher than 20 kHz, however, may need to be taken into account [50].
Sprouts and microgreens are edible seedlings of different vegetables and herbs that have become increasingly popular due to their positive health benefits and are now referred to as functional foods or superfoods. Bioactive components have long been appreciated in broccoli seedlings (Brassica oleracea L. var. Italica). Secondary metabolites have been linked to several positive health outcomes. In in vitro and animal studies, broccoli seedlings have been shown to have health benefits. A current study has summarized previous research on the bioactive components and bioactivities of various broccoli derivatives, as well as the mechanisms of action associated with them [57]. Conventional and new techniques can be used to extract bioactive compounds from different types of vegetable sprouts, as shown in Figure 2.
Figure 2.
Conventional and new techniques can be used to extract bioactive compounds from different types of vegetable sprouts.
4. Food Applications of Vegetable Sprouts
Nowadays, the food industry has been focused on developing healthier products that are more responsive to changing customer demands. Recently, sprouted grains have become a new element in the culinary world. Sprouted grains have a higher nutritional value, lower antinutrient content, a prime source of bioactive compounds, and a sweeter flavor, making them a potential new food component [58]. Sprouted grains were formerly only used in bread, but they may now be found in tortillas, granola, cookies, crackers, muffins, snacks, bars, morning cereals, side dishes, and salads [59]. Sprouted grains may be used in various culinary applications without requiring any formulation adjustments, and they can assist considerably in differentiating products.
After sprouting and drying, a whole grain kernel can be milled into flour or processed into grits, coarse meals, or flakes, among other granulations. Wheat, rye, spelt, barley, brown rice, oat, sorghum, millet, quinoa, buckwheat, and amaranth may all be sprouted and used in several nutritious applications as long as the germ is intact. Bars, cereals, granola, bread, tortillas, frozen dough, candies, snacks, side dishes, soups, and pasta are common components. Gluten-free foods such as sprouted sorghum, millet, quinoa, amaranth, buckwheat, brown rice, and purity protocol oats are naturally gluten-free foods. They can be added to gluten-free diets to boost nutrition. Because of the wide variety of grains available, bakers, food scientists, and chefs have a lot of creative freedom. The functional differences between sprouted grains and their unsprouted counterparts must be recognized and addressed for optimal formulation, processing, and end-product attributes. Due to their high nutritional content, interesting technical possibilities, and sensory qualities, sprouted grains are being exploited as a component in a variety of food product innovations. The quality of sprouts and their specific culinary behavior depends on the germination circumstances. In this article, we look at two applications of sprouted grains: the effect of wheat sprouting time on the production of innovative baking flours and the microbiological risk of homemade rejuvelac, a sprouted wheat-based fermented beverage [60]. Bakers may notice a shortening of the proofing period or an increase in bread absorption, contributing to higher yields.
In tortillas, flour made of sprouted whole wheat can help to soften them, lengthen their shelf-life, and improve their sensory qualities [40]. The potential of sprouted wheat to improve the likability of bread and tortillas might lead to an increase in whole grain consumption, which would be a tremendous step forward in human health, especially because these staples are frequently consumed on a regular basis [52]. Table 2 shows the food applications of vegetable sprouts.
Table 2.
Food applications of vegetables sprouts.
| Sprout Source | Application in Food Products | Improvement | References |
|---|---|---|---|
| Vegetable sprout | -- | Improve nutritional value of different food product | [61] |
| Radish, red cabbage, vegetable green, buckwheat and broccoli seeds | -- | Vegetable sprouts are rich in nutrients | [62] |
| Fresh alfalfa and flax sprouts | Rabbit meat | Modified the fat content, fatty acid, and phytochemical profile of the meat | [63] |
| Wheat seeds | Bread | To examined the profile of phenolic acids and antioxidant properties of wheat bread | [64] |
| Brown rice | Wheat bread | Sensory acceptance and longer shelf-life | [65] |
| Broccoli | Broccoli sprout juice | Broccoli sprouts are naturally enriched in glucoraphanin (GR) | [66] |
| Brussels | Juice | It may reduce the risk of cancer of the alimentary tract | [67] |
5. Bioavailability of Sprout against Different Diseases
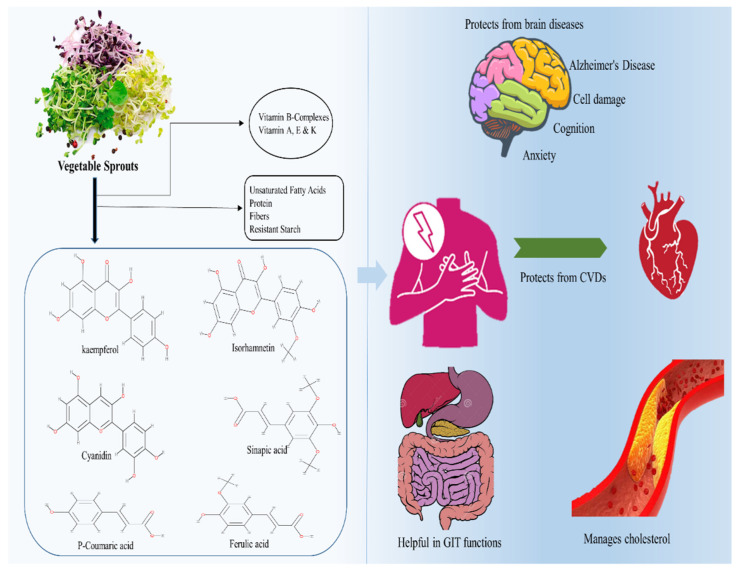
With the intake of plant sprouts, bioavailability has long been regarded as crucial. The bioavailability of phytochemicals in various sprout diets varies substantially depending on several parameters. Interindividual factors, including delivery mode, and even intraindividual biochemical variances and the makeup and function of the gut microbiota are all factors to consider. In one study, to test iso-thiocyanate bioavailability, mice were administered either thermally processed broccoli sprout powders or pure isothiocyanate sulforaphane. The greatest quantities of the isothiocyanate metabolite were discovered in slightly cooked broccoli sprout powdered meals. In vivo, nonheated broccoli sprouts were followed by powdered broccoli sprout meals. They identified erusin and sulforaphane interconversion and observed that erusin was the preferred form in the kidney, liver, and bladder even when just sulforaphane was digested. It is worth mentioning that the bioavailability of sulforaphane from broccoli sprouts varies greatly depending on the delivery method [68]. The study suggested the inhibitory effect of broccoli sprout extracts on the properties of two prostate cancer cell lines characterized by low (AT-2) and high (MAT-LyLu) metastatic potential. These effects may be due to the fact that broccoli sprouts contained flavonoids and phenolic acids [7]. A previous study suggested that fava bean sprouts had higher antioxidant activity because they contained more polyphenols and l-3,4-dihydroxyphenylalanine (l-DOPA) than the bean itself [69]. The study suggested that lentil sprouts contained melatonin that is a multifunctional antioxidant neurohormone. The results showed that germination of lentils increased the content of melatonin. In another study, Sprague Dawley rats were used to investigate the pharmacokinetic profile of melatonin after oral administration of a lentil sprout extract and to evaluate plasma and urine melatonin and related biomarkers and antioxidant capacity. The outcomes showed that lentil sprout intake increased melatonin plasmatic concentration and attenuate plasmatic oxidative stress [70]. Figure 3 shows the bioavailability of sprouts against different disorders.
Figure 3.
Bioavailability of sprouts against different disorders.
5.1. Bioavailability of Sprouts against Brain Issues
Nervous system diseases are a common ailment that will become more common as populations age. Axonopathy, also known as dying-back axonopathy, is a neurological illness in which axons become disconnected from their destinations, resulting in functional impairment. Axons can renew or sprout in response to several neurologic illnesses to re-establish synaptic function and to reconnect with the target before motor neuron death. Compensatory motor axon sprouting and neuromuscular junction reinnervation has been demonstrated in ALS patients, although the disease’s course has typically outpaced these advantages. In ALS and kindred illnesses defined by dying-back axonopathy, potential therapeutics that encourage compensatory sprouting and reinnervation may delay symptom onset and may sustain muscle function for extended periods. Many questions concerning the impact of various disease-causing mutations on axonal outgrowth and regeneration, especially in motor neurons derived from patient-induced pluripotent stem cells, remain unsolved. Researchers must mimic the human neuromuscular circuit using motor neurons created from human-induced pluripotent stem cells to uncover drugs that stimulate axonal regeneration, sprouting, and reinnervation of neuromuscular junctions [71]. Regarding colored flavonoids, anthocyanins, the majority of which are highly acylated, and glycosylated forms of cyanidin are abundant in broccoli, radishes, cabbages, and kale sprouts [72]. Recently, anthocyanins have attracted more attention due to their potential to improve brain function and their role in the prevention and treatment of disorders including diabetes and obesity. One study suggested that two crude juices of broccoli sprouts had a protective effect on SH-SY5Y cells treated with the fragment Aβ25–35 because they contained different amounts of polyphenols and sulforaphane. The sprouts’ juices both protected against Aβ-induced cytotoxicity and apoptotic cell death as evidenced by cell viability, nuclear chromatin condensation, and apoptotic body formation measurements [73]. Another study suggested that cruciferous vegetables were a good source of sulforaphane. The results of this study showed that sulforaphane protected against acute brain injuries and neurodegenerative diseases through activating the Nrf2 signaling pathway [74].
5.2. Compensatory Sprouting as a Potential Therapeutic Strategy for Amyotrophic Lateral Sclerosis
Functional motor recovery can be aided by the sprouting of motor axons and the reinnervation of denervated NMJs. Axonal sprouting allows motor units to increase 5–8 times their initial size. In amyotrophic lateral sclerosis, there is evidence of motor axon sprouting [75]. ALS is more common in certain motor neuron subpopulations that are also less prone to sprouting. In people with amyotrophic lateral sclerosis, compensatory sprouting may be employed to slow the onset of muscle denervation and weakness. The global number of ALS cases is expected to rise by 2040. Any drug that can improve the quality of life for ALS patients is badly needed. Axonal sprouting, which involves the functional reinnervation of NMJs, has the potential to improve life quality [76]. Phenolic acids are present in different vegetable sprouts. The diverse neuroprotective effects of phenolic acids make them interesting candidates for better ALS therapies. Study outcomes have shown that protocatechuic acid administration at 100 mg/kg in SOD1G93A mice prolonged survival, recovered motor functions, and decreased gliosis [4,77]. An in vitro study suggested that antioxidant molecules were capable of rescuing NSC34 motor neuron cells expressing an ALS-associated mutation of superoxide dismutase 1 [78].
5.3. Bioavailability of Sprouts against Gastrointestinal Tract (GIT) Health Problems
Sprouts may make it easier for you to digest your diet. According to a study, sprouted seeds increased the amount of fiber in them, making them more accessible. According to one study, cereals sprouted for five days had up to 133 percent more fiber than non-sprouted grains. Another study found that growing beans until the sprouts were 5 mm long boosted the overall fiber content by 226 percent. Sprouting appears to enhance the amount of insoluble fiber, a type of fiber that aids stool creation and passage through the stomach, reducing constipation risk. Finally, sprouted beans, grains, vegetables, nuts, and seeds have lower antinutrient levels than their non-sprouted counterparts. This makes it easier for the body to absorb nutrients during digestion [79]. Fiber that the human gut cannot digest on its own, but some bacteria can digest, is an essential source of nutrients that your gut microbe need to stay healthy. Fiber helps to stimulate the growth of colonic flora, to increase the weight of the stool, and to enhance the number of bacteria in the gut. The growth of bacteria present in the gut enhances the health of the intestines. However, short-chain fatty acids are produced by anaerobic gut bacteria through saccharolytic fermentation of complex resistant carbohydrates, which escape digestion and absorption in the small intestine [80]. In contrast to micro- and macronutritional contents, dietary polyphenols tend to be recognized as xenobiotic by humans during absorption, and therefore, their biological accessibility is significantly low. Furthermore, polymerization and structural complexity influence digestion in the small intestine [81]. The small intestine usually consumes approximately 5–10% of the absorbed polyphenols. The residual polyphenols (90–95%) might develop up to millimolar proportions in the large intestine linked to bile conjugates spilled into lumen in which they are susceptible to the enzymatic reactions of the gut bacteria species [82]. According to current data, dietary polyphenols that penetrate gut microflora, also including volatile compounds produced, manufacture and generate differences in the microbiota community through their prebiotic properties and functioning as an antiseptic towards infectious intestinal microbiota [83].
Onions have been proven to offer digestive system-protective properties, such as preventing stomach ulcers, regulating gut flora, and alleviating colitis. In rats, raw onion sprouts were shown to suppress histamine-induced stomach acid release and to attenuate ethanol-stimulated gastric ulcers. However, boiling the onion was less effective. In common carp juveniles, dietary supplementation with onion sprout powder has been shown to alter gut microbiota by increasing the number of lactic acid bacteria [84]. In rats, bioactive substances produced from onions, such as quercetin and quercetin monoglycosides, were found to boost the enzymatic activity of the gut microbiota. In colitis mice caused by dextran sodium sulfate, quercetin monoglycosides were shown to affect a variety of gut bacteria. Furthermore, onions and other Allium species have been demonstrated to protect against upper aerodigestive tract and gastrointestinal tract cancers [85]. Peanut sprout ethanolic extract at a purification of 80% (v/v) has been administered to loperamide-induced constipated SD rats, which revealed its laxative effects [86].
5.4. Bioavailability of Sprouts against Cardiovascular Diseases (CVDs)
In hypercholesterolemic Wistar rats, dietary supplementation with onion reversed high-cholesterol diet-induced changes in lipid mediators such as oxylipin and sphingolipid profiles [87]. Using an animal model to study increased blood pressure, researchers investigated the relationship between oxidative stress and a diet rich in broccoli sprouts with a high quantity of glucoraphanin. After 14 weeks, rats were fed broccoli sprouts that were either low in the chemical or rich in glucoraphanin. After the trial, they observed that rats fed a glucoraphanin-rich diet had lower blood pressure and less heart inflammation. According to the researchers, the benefits were attributed to better antioxidant defense systems and a decreased glucoraphanin-induced inflammatory response. Broccoli and broccoli sprouts contain different antioxidants (vitamin E, β-carotene, α-tocopherol, and ascorbic acid) that may aid in the prevention of cardiovascular diseases. In laboratory rats, the chemical glucoraphanin increased heart function, decreased inflammation, and boosted natural antioxidant defenses. When unstable molecules, called free radicals, react with oxygen in the body, they promote inflammation and cell death, raising the risk of heart disease and cancer. Antioxidants are supposed to help reduce oxidative stress in the body, preventing these detrimental consequences. Glucoraphanin is a chemical that boosts the body’s antioxidant defenses by acting as an indirect antioxidant. It is naturally found in broccoli and broccoli sprouts [88].
Onions have been shown in trials to enhance lipid profiles and to prevent platelet aggregation, lowering the risk of heart disease. Onions and their bioactive components have been widely researched for their hypocholesterolemia effects in rats fed high-cholesterol or high-fat diets. Onion sprouts successfully reduced total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels in hyperlipidemic rats [89]. Polyphenol-rich onion extract alleviated hyperlipidemia in Sprague-Dawley rats’ livers by upregulating the low-density lipoprotein receptor (LDLR) and downregulating the 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMGCR). In addition, Lee et al. [90] found that quercetin-rich onion peel extract increased fecal cholesterol, reduced the atherogenic index, cardiac risk factor, and activation of LDLR and cholesterol 7-monooxygenase (CYP7A1) in high-cholesterol diet-fed mice, indicating that onion had a cholesterol-lowering effect via fecal excretion. They proved that fecal excretion of onions lowered cholesterol. When onions were added to a high-cholesterol diet supplied to rats, the bile acid levels in their stools changed. Dietary onion increased antioxidant enzyme activity and enhanced anti-inflammatory response and cardiovascular risk markers in rats fed a high-cholesterol diet [66]. An overview of vegetable sprouts’ bioavailability against different diseases is given in Table 3.
Table 3.
Bioavailability of sprouts against different diseases.
| Vegetable Sprout Types | Study Design | Disease | Recovery | References |
|---|---|---|---|---|
| Cruciferous | Human (male and female) | Cancer | Cruciferous vegetables reduce the risk of cancer by decreasing the damage to DNA | [91] |
| Broccoli | -- | -- | The biological properties of broccoli are antioxidant, anticancer, anticancer, antimicrobial, anti-inflammatory, anti-obesity, and antidiabetic activities | [92] |
| Broccoli | Mice and rats | Alleviate pain | The broccoli sprouts have ability in pain therapy | [36] |
| Red cabbage, broccoli, Galega kale and Penca cabbage | ,-- | -- | Different vegetables sprouts have antioxidant and anti-carcinogenic properties | [93] |
| Broccoli | Human | Cancer | Broccoli may reduce the risk of cancer by managing metabolism | [94] |
| Broccoli | Mice | Prostate tumorigenesis | Broccoli sprouts have significant inhibitory effects on prostate tumorigenesis. | [95] |
| Alfalfa | Mice | Inflammation | The study suggests that alfalfa supplementation can suppress the production of proinflammatory cytokines and alleviate acute inflammatory hazards. | [96] |
| Brussels | -- | Cancer | Brussels sprouts have cancer preventive effects which may be due to a reduction in oxidative DNA damage | [97] |
| Spinach, kale, Brussels sprouts, mustard greens, green bell peppers, cabbage, and collards | Human | Binding of bile acids | The results show equal health-promoting potential of spinach, kale, brussels sprouts, mustard greens, green bell peppers, and collards, as indicated by their bile acid binding on dry matter basis | [98] |
| Brussels | Human | -- | The results show that compounds in cooked and autolysed brussels sprouts can enhance lymphocyte resistance towards H2O2-induced DNA strand breaks in vitro | [99] |
| Radish, broccoli, leek, and beetroot | In vitro | Diabetic, obesity and cholinergic | Different vegetable sprouts can be used daily as superfoods or functional food | [33] |
| Turnip, cauliflower, and mustard | In vitro | Cancer | In vitro antiproliferative study supports that sprouts are a good source of anticancer agents | [100] |
| Broccoli | In vitro | Cancer | In vitro study indicates that broccoli sprouts can reduce prostate cancer | [101] |
5.5. Bioavailability of Sprouts against Oxidative Stress-Related Diseases such as Cancer and Diabetes
In addition, secondary metabolites are abundantly present in sprouts, especially the glucosinolates (GLs), as in the case of the Brassicaceae family [102]. Gulcosinolates consist of an amino acid group and a thiohydroximate-O-sulfonate attached to the glucose unit [103]. Myrosinase acts to hydrolyse these GLs to thiocyanates and isothiocyanates [89] when the pH is between 6.0 and 7.0 [79], and then it yields anti-mutagenic activity, having a limiting effect on oxidative stress and playing a role in chemoprotection, especially in cancers and diabetes [104]. Glucoraphenin and glucobrassicin are the GLs excessively present in sprouted radish, which readily enhance antioxidant activity, and consequently decrease carcinogenesis in the body [105]. Kale sprouts do not have dehydroerucin but have a better GL profile as compared with sprouted radish due to gluconapoleiferin, glucoiberin, gluconasturtin, gluconapin, progoitrin, glucobrassicin, neoglucobrassicin, 4-hydroxyglucobrassicin, and sinigrin, which potentially reduce oxidative stress, and hence, decrease the risk of related diseases, i.e., diabetes, cancer, and heart diseases [94]. Taniguchi et al. [106] used Japanese radish sprouts in normal and streptozotin-induced diabetic mice to show the benefits of cruciferous sprouts on DM. It was shown that radish sprout consumption decreased plasma levels of fructosamine, glucose, and insulin, suggesting that the hypoglycemia brought on by radish sprout consumption may not be related to an increase in insulin synthesis but rather to enhanced sensitivity or an insulin-like action [107]. It depends on the ktype of sorghum, enzyme-inducing, and anti-proliferative capabilities. The most effective inducer of quinone oxidoreductase, a phase II detoxifying enzyme, has been shown to be an extract from black tea (non-tannin) that is abundant in 3-deoxyanthocyanins. Comparatively speaking, white sorghum extract has been shown to be a relatively potent inducer. Despite not inducing quinone oxidoreductase, tannin sorghum extracts have provided the most potent antiproliferative effects on human esophageal and colon cancer cells.
5.6. Bioavailability of Protein against Malnutrition
Sprouting enhances protein content, as evidenced from a study conducted by Devi et al. [95] on cowpea (lobia) by enhancing its bioavailability and digestibility. Sprouting is an interesting phenomenon that influences metabolic enzymes, especially proteinases, which increase the content of protein [96]. Sprouted chickpeas have more protein content than black gram. The sprouting process decreases the protease inhibitors and even enhances lipase activity, yielding increased content of fatty acids, and this also improves the digestibility of starches [97].
6. Sprout Vegetables as an Ingredient or Substitute for Meat Products
Dried sprouted food ingredients have been a trend for healthy/functional foods to live healthier, particularly by incorporating them into bread making flours or traditional beverages and juices [108]. With continued research on the influence of sprouted dietary feed on animals, it has revealed increased phytochemicals, particularly antioxidants, in the animals’ meat, as well as enhanced fatty acid content, particularly when sprouted alfalfa and flax were fed to rabbits [54]. Meat product consumption to achieve protein requirements has increased, and currently, it is difficult to rely on just one livestock source. With advancements in in vitro meat technology, tissue culturing engineers have started to develop lab-grown meat [109].
7. Conclusions
It is concluded that sprouts have been introduced as a new food for some years. Vegetables are basically important plant-based foods. Vegetable sprouts are composed of bioactive compounds, including phenolic compounds, antioxidants, etc. These bioactive compounds are extracted from sprouts by using different conventional methods and new techniques. Plant protein content improvement and enhanced protein bioavailability and digestibility can set up a better opportunity to research and develop plant-based meat protein substitutes. Vegetable sprouts are being used to develop functional foods and they also play an important role in maintaining the stability of food products. Furthermore, pharmaceutically, they aid in the defense of different types of chronic disorders.
8. Future Prospective and Recommendations
Vegetable sprouts’ potential involvement in the prevention and treatment of chronic diseases has to be investigated further. The high nutrient content of sprouts may provide extra lipid-lowering advantages. Sprouts are fiber-rich foods that are likely to provide a feeling of fullness. It is also crucial to remember that functional meals must be consumed often in order to offer their somewhat modest benefits. To check the probable positive effect of vegetable sprout foods on chronic diseases or risk factors related to lifestyle, a comprehensive scientific human study is required. The influence of the whole meal, which represents the synergistic effect between components, must be explored by conducting different studies on extracts and components. It is equally crucial to consider the makeup of the background diet because it might bias results and could create challenges in connecting the effects to the fitting dietary elements. The effect of vegetable sprout-based meals on chronic diseases or risk factors related to lifestyle must reflect the whole diet in order to apply the trial’s findings in practice. Unfortunately, in vegetable sprout research, because metabolic changes and their link that may affect biological activity in the body after consumption have not been taken into consideration, it is difficult to characterize the direct antioxidant impact of vegetable sprouts. It is necessary to determine the safety of ingesting the quantities of vegetable sprout extracts utilized in these studies by dietary consumption of foods containing vegetable sprouts. However, information gained from many types of experimental studies has contributed to a broader understanding of how the vegetable sprout food matrix may be advantageous. Keeping in mind the gap between protein supply and demand, more time is needed to research and develop sprouted vegetable protein-based products, which would be a better approach because this source would provide a better choice of protein accompanied by phytochemicals.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author Contributions
Conceptualization, W.K. and A.A. (Afifa Aziz); methodology, W.K.; software, H.K.; validation, A.A. (Afifa Aziz) and S.N.; formal analysis, W.K., A.A. (Anwar Ali) and S.N.; investigation, C.M.G.L., M.k.N. and A.A. (Anwar Ali); data curation, W.S.A., G.S., A.A.E. and S.N.; writing—original draft preparation, M.k.N., W.S.A., G.S., A.A.E. and W.K.; writing—review and editing, M.k.N., F.M., W.K., C.M.G.L. and H.K. supervision, W.K. and A.A. (Afifa Aziz); project administration, M.k.N.; funding acquisition, A.A.-F., W.S.A., G.S. and A.A.E. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sikin A.M.D., Zoellner C., Rizvi S.S.H. Current Intervention Strategies for the Microbial Safety of Sprouts. J. Food Prot. 2013;76:2099–2123. doi: 10.4315/0362-028X.JFP-12-437. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C., Zhao Z., Yang G., Shi Y., Zhang Y., Shi C., Xia X. Effect of slightly acidic electrolyzed water on natural Enterobacteriaceae reduction and seed germination in the production of alfalfa sprouts. Food Microbiol. 2021;97:103414. doi: 10.1016/j.fm.2020.103414. [DOI] [PubMed] [Google Scholar]
- 3.Liu H.K., Kang Y.F., Zhao X.Y., Liu Y.P., Zhang X.W., Zhang S.J. Effects of elicitation on bioactive compounds and biological activities of sprouts. J. Funct. Foods. 2019;53:136–145. doi: 10.1016/j.jff.2018.12.019. [DOI] [Google Scholar]
- 4.Chen F., Zhang M., Yang C. hui Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020;63:104953. doi: 10.1016/j.ultsonch.2019.104953. [DOI] [PubMed] [Google Scholar]
- 5.Ki H.H., Poudel B., Lee J.H., Lee Y.M., Kim D.K. In vitro and in vivo anti-cancer activity of dichloromethane fraction of Triticum aestivum sprouts. Biomed. Pharmacother. 2017;96:120–128. doi: 10.1016/j.biopha.2017.09.118. [DOI] [PubMed] [Google Scholar]
- 6.The Role of Sprouts in Human Nutrition. A Review. [(accessed on 23 July 2022)]. Available online: https://www.cabdirect.org/cabdirect/abstract/20113263994.
- 7.Gawlik-Dziki U., Jezyna M., Świeca M., Dziki D., Baraniak B., Czyz J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012;49:469–476. doi: 10.1016/j.foodres.2012.08.010. [DOI] [Google Scholar]
- 8.Kensler T.W., Ng D., Carmella S.G., Chen M., Jacobson L.P., Muñoz A., Egner P.A., Chen J.G., Qian G.S., Chen T.Y., et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33:101–107. doi: 10.1093/carcin/bgr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendoza-Sánchez M., Pérez-Ramírez I.F., Wall-Medrano A., Martinez-Gonzalez A.I., Gallegos-Corona M.A., Reynoso-Camacho R. Chemically induced common bean (Phaseolus vulgaris L.) sprouts ameliorate dyslipidemia by lipid intestinal absorption inhibition. J. Funct. Foods. 2019;52:54–62. doi: 10.1016/j.jff.2018.10.032. [DOI] [Google Scholar]
- 10.Nakamura Y., Kaihara A., Yoshii K., Tsumura Y., Ishimitsu S., Tonogai Y. Content and Composition of Isoflavonoids in Mature or Immature Beans and Bean Sprouts Consumed in Japan. J. Health Sci. 2001;47:394–406. doi: 10.1248/jhs.47.394. [DOI] [Google Scholar]
- 11.Teixeira-Guedes C.I., Oppolzer D., Barros A.I., Pereira-Wilson C. Phenolic rich extracts from cowpea sprouts decrease cell proliferation and enhance 5-fluorouracil effect in human colorectal cancer cell lines. J. Funct. Foods. 2019;60:103452. doi: 10.1016/j.jff.2019.103452. [DOI] [Google Scholar]
- 12.Tang D., Dong Y., Ren H., Li L., He C. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata) Chem. Cent. J. 2014;8:1–9. doi: 10.1186/1752-153X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prakash D., Upadhyay G., Singh B.N., Singh H.B. Antioxidant and free radical-scavenging activities of seeds and agri-wastes of some varieties of soybean (Glycine max) Food Chem. 2007;104:783–790. doi: 10.1016/j.foodchem.2006.12.029. [DOI] [Google Scholar]
- 14.Sahai V., Kumar V. Anti-diabetic, hepatoprotective and antioxidant potential of Brassica oleracea sprouts. Biocatal. Agric. Biotechnol. 2020;25:101623. doi: 10.1016/j.bcab.2020.101623. [DOI] [Google Scholar]
- 15.Ho C.Y., Lin Y.T., Labbe R.G., Shetty K. Inhibition of helicobacter pylori by phenolic extracts of sprouted peas (Pisum sativum L.) J. Food Biochem. 2006;30:21–34. doi: 10.1111/j.1745-4514.2005.00032.x. [DOI] [Google Scholar]
- 16.Yanaka A., Fahey J.W., Fukumoto A., Nakayama M., Inoue S., Zhang S., Tauchi M., Suzuki H., Hyodo I., Yamamoto M. Dietary Sulforaphane-Rich Broccoli Sprouts Reduce Colonization and Attenuate Gastritis in Helicobacter pylori–Infected Mice and Humans. Cancer Prev. Res. 2009;2:353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 17.Kim W.I., Choi S.Y., Han I., Cho S.K., Lee Y., Kim S., Kang B., Choi O., Kim J. Inhibition of Salmonella enterica growth by competitive exclusion during early alfalfa sprout development using a seed-dwelling Erwinia persicina strain EUS78. Int. J. Food Microbiol. 2020;312:108374. doi: 10.1016/j.ijfoodmicro.2019.108374. [DOI] [PubMed] [Google Scholar]
- 18.Teodoro A.J. Bioactive compounds of food: Their role in the prevention and treatment of diseases. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3765986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uwineza P.A., Waśkiewicz A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules. 2020;25:3847. doi: 10.3390/molecules25173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Jubete L., Wijngaard H., Arendt E.K., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- 21.Erba D., Angelino D., Marti A., Manini F., Faoro F., Morreale F., Pellegrini N., Casiraghi M.C. Effect of sprouting on nutritional quality of pulses. Int. J. Food Sci. Nutr. 2019;70:30–40. doi: 10.1080/09637486.2018.1478393. [DOI] [PubMed] [Google Scholar]
- 22.Costa D.C., Costa H.S., Albuquerque T.G., Ramos F., Castilho M.C., Sanches-Silva A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015;45:336–354. doi: 10.1016/j.tifs.2015.06.009. [DOI] [Google Scholar]
- 23.Gernand A.D., Schulze K.J., Stewart C.P., West K.P., Christian P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016;12:274–289. doi: 10.1038/nrendo.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker K.L. Nutrient intake, nutritional status, and cognitive function with aging. Ann. N. Y. Acad. Sci. 2016;1367:38–49. doi: 10.1111/nyas.13062. [DOI] [PubMed] [Google Scholar]
- 25.Arrigoni O., De Tullio M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta Gen. Subj. 2002;1569:1–9. doi: 10.1016/S0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 26.Poiroux-Gonord F., Bidel L.P.R., Fanciullino A.L., Gautier H., Lauri-Lopez F., Urban L. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects To Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010;58:12065–12082. doi: 10.1021/jf1037745. [DOI] [PubMed] [Google Scholar]
- 27.Godswill A.G., Somtochukwu I.V., Ikechukwu A.O., Kate E.C. Health Benefits of Micronutrients (Vitamins and Minerals) and their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020;3:1–32. doi: 10.47604/ijf.1024. [DOI] [Google Scholar]
- 28.Matsuo T., Asano T., Mizuno Y., Sato S., Fujino I., Sadzuka Y. Water spinach and okra sprouts inhibit cancer cell proliferation. Vitr. Cell. Dev. Biol. Anim. 2022;58:79–84. doi: 10.1007/s11626-022-00650-5. [DOI] [PubMed] [Google Scholar]
- 29.Le L., Gong X., An Q., Xiang D., Zou L., Peng L., Wu X., Tan M., Nie Z., Wu Q., et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021;357:129752. doi: 10.1016/j.foodchem.2021.129752. [DOI] [PubMed] [Google Scholar]
- 30.Ebert A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants. 2022;11:571. doi: 10.3390/plants11040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., Shi J., Wan J., Pham Q., Zhang Z., Sun J., Yu L., Luo Y., Wang T.T.Y., Chen P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2022;2:101–113. doi: 10.1021/acsfoodscitech.1c00355. [DOI] [Google Scholar]
- 32.Choque-Quispe D., Ligarda-Samanez C.A., Ramos-Pacheco B.S., Leguía-Damiano S., Calla-Florez M., Zamalloa-Puma L.M., Colque-Condeña L. Phenolic Compounds, Antioxidant Capacity, and Protein Content of Three Varieties of Germinated Quinoa (Chenopodium quinoa Willd) Ing. Investig. 2021;41:1–7. doi: 10.15446/ing.investig.v41n2.89831. [DOI] [Google Scholar]
- 33.Wojdyło A., Nowicka P., Tkacz K., Turkiewicz I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules. 2020;25:4648. doi: 10.3390/molecules25204648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Aty A.M., Salama W.H., Fahmy A.S., Mohamed S.A. Impact of germination on antioxidant capacity of garden cress: New calculation for determination of total antioxidant activity. Sci. Hortic. 2019;246:155–160. doi: 10.1016/j.scienta.2018.10.062. [DOI] [Google Scholar]
- 35.Majid I., Hussain S., Nanda V., Jabeen F., Mehmood Abbasi A., Alkahtani J., Soliman Elshikh M., Azhar Khan M., Usmani S., Javed Ansari M. Changes in major flavonols and quercetin glycosides upon sprouting in onion cultivars. J. King Saud Univ. Sci. 2021;33:101222. doi: 10.1016/j.jksus.2020.10.019. [DOI] [Google Scholar]
- 36.Baenas N., Gómez-Jodar I., Moreno D.A., García-Viguera C., Periago P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017;127:60–67. doi: 10.1016/j.postharvbio.2017.01.010. [DOI] [Google Scholar]
- 37.Almuhayawi S.M., Almuhayawi M.S., Al Jaouni S.K., Selim S., Hassan A.H.A. Effect of Laser Light on Growth, Physiology, Accumulation of Phytochemicals, and Biological Activities of Sprouts of Three Brassica Cultivars. J. Agric. Food Chem. 2021;69:6240–6250. doi: 10.1021/acs.jafc.1c01550. [DOI] [PubMed] [Google Scholar]
- 38.Maoloni A., Milanović V., Osimani A., Cardinali F., Garofalo C., Belleggia L., Foligni R., Mannozzi C., Mozzon M., Cirlini M., et al. Exploitation of sea fennel (Crithmum maritimum L.) for manufacturing of novel high-value fermented preserves. Food Bioprod. Process. 2021;127:174–197. doi: 10.1016/j.fbp.2021.03.001. [DOI] [Google Scholar]
- 39.Baenas N., Moreno D.A., García-Viguera C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012;60:11409–11420. doi: 10.1021/jf302863c. [DOI] [PubMed] [Google Scholar]
- 40.Yudiono K., Kurniawati L. Effect of sprouting on anthocyanin, antioxidant activity, color intensity and color attributes in purple sweet potatoes. Food Res. 2018;2:171–176. [Google Scholar]
- 41.Miyahira R.F., Lopes J.D., Antunes A.E. The Use of Sprouts to Improve the Nutritional Value of Food Products: A Brief Review. Plant Foods Hum. Nutr. 2021;76:143–152. doi: 10.1007/s11130-021-00888-6. [DOI] [PubMed] [Google Scholar]
- 42.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 43.Blicharski T., Oniszczuk A. Extraction Methods for the Isolation of Isoflavonoids from Plant Material. Open Chem. 2017;15:34–45. doi: 10.1515/chem-2017-0005. [DOI] [Google Scholar]
- 44.Lang Q., Wai C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta. 2001;53:771–782. doi: 10.1016/S0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]
- 45.Shang X., Dou Y., Zhang Y., Tan J.N., Liu X., Zhang Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019;140:111724. doi: 10.1016/j.indcrop.2019.111724. [DOI] [Google Scholar]
- 46.Koraqi H., Qazimi B., Çesko C., Trajkovska Petkoska A. Environmentally Friendly Extraction of BioactiveCompounds from Rosa canina L. fruits Using DeepEutectic Solvent (DES) as Green Extraction Media. Acta Chim. Slov. 2022;69:3. doi: 10.17344/acsi.2022.7559. [DOI] [PubMed] [Google Scholar]
- 47.Le T.N., Chiu C.H., Hsieh P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants. 2020;9:946. doi: 10.3390/plants9080946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T., Luo L., Kim S., Moon S.K., Moon B.K. Trans-resveratrol extraction from peanut sprouts cultivated using fermented sawdust medium and its antioxidant activity. J. Food Sci. 2020;85:639–646. doi: 10.1111/1750-3841.14981. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes S.R., Barreiros L., Oliveira R.F., Cruz A., Prudêncio C., Oliveira A.I., Pinho C., Santos N., Morgado J. Chemistry, bioactivities, extraction and analysis of azadirachtin: State-of-the-art. Fitoterapia. 2019;134:141–150. doi: 10.1016/j.fitote.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Azwanida N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 2015;4:2167-0412. [Google Scholar]
- 51.Kandar P. Phytochemicals and biopesticides: Development, current challenges and effects on human health and diseases. J. Biomed. Res. 2021;2:3–15. [Google Scholar]
- 52.Das A., Sharangi A.B. Medicinal Plants. Apple Academic Press; New York, NY, USA: 2022. Postharvest Care of Medicinal and Aromatic Plants: A Reservoir of Many Health Benefiting Constituents; pp. 387–407. [Google Scholar]
- 53.Tzanova M., Atanasov V., Yaneva Z., Ivanova D., Dinev T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes. 2020;8:1222. doi: 10.3390/pr8101222. [DOI] [Google Scholar]
- 54.Moura P.M., Prado G.H., Meireles M.A., Pereira C.G. Supercritical fluid extraction from guava (Psidium guajava) leaves: Global yield, composition and kinetic data. J. Supercrit. Fluids. 2012;62:116–122. doi: 10.1016/j.supflu.2011.11.014. [DOI] [Google Scholar]
- 55.Moyo S.M. Effects of Cooking and Drying on the Phenolic Compounds, Antioxidant Activity and Antibacterial Activity of Cleome gynandra (Spider Plant) University of Johannesburg; Johannesburg, South Africa: 2016. [Google Scholar]
- 56.Vongsak B., Sithisarn P., Mangmool S., Thongpraditchote S., Wongkrajang Y., Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013;44:566–571. doi: 10.1016/j.indcrop.2012.09.021. [DOI] [Google Scholar]
- 57.Ding J., Feng H. Controlled germination for enhancing the nutritional value of sprouted grains. Sprouted Grains Nutr. Value Prod. Appl. 2019:91–112. [Google Scholar]
- 58.Finnie S., Brovelli V., Nelson D. Sprouted grains as a food ingredient. Sprouted Grains Nutr. Value Prod. Appl. 2019:113–142. [Google Scholar]
- 59.Omary M.B., Fong C., Rothschild J., Finney P. REVIEW: Effects of Germination on the Nutritional Profile of Gluten-Free Cereals and Pseudocereals: A Review. Cereal Chem. 2012;89:1–14. doi: 10.1094/CCHEM-01-11-0008. [DOI] [Google Scholar]
- 60.Liu T., Hou G.G., Cardin M., Marquart L., Dubat A. Quality attributes of whole-wheat flour tortillas with sprouted whole-wheat flour substitution. LWT. 2017;77:1–7. doi: 10.1016/j.lwt.2016.11.017. [DOI] [Google Scholar]
- 61.Physiological Characteristics and Manufacturing of the Processing Products of Sprout Vegetables-Korean Journal of Food and Cookery Science|Korea Science. [(accessed on 24 July 2022)]. Available online: https://koreascience.kr/article/JAKO201025665646714.page.
- 62.Dal Bosco A., Castellini C., Martino M., Mattioli S., Marconi O., Sileoni V., Ruggeri S., Tei F., Benincasa P. The effect of dietary alfalfa and flax sprouts on rabbit meat antioxidant content, lipid oxidation and fatty acid composition. Meat Sci. 2015;106:31–37. doi: 10.1016/j.meatsci.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Gawlik-Dziki U., Dziki D., Pietrzak W., Nowak R. Phenolic acids prolife and antioxidant properties of bread enriched with sprouted wheat flour. J. Food Biochem. 2017;41:e12386. doi: 10.1111/jfbc.12386. [DOI] [Google Scholar]
- 64.Charoenthaikij P., Jangchud K., Jangchud A., Prinyawiwatkul W., Tungtrakul P. Germination Conditions Affect Selected Quality of Composite Wheat-Germinated Brown Rice Flour and Bread Formulations. J. Food Sci. 2010;75:S312–S318. doi: 10.1111/j.1750-3841.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 65.Bello C., Maldini M., Baima S., Scaccini C., Natella F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018;268:249–256. doi: 10.1016/j.foodchem.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 66.Smith T.K., Lund E.K., Clarke R.G., Bennett R.N., Johnson I.T. Effects of Brussels Sprout Juice on the Cell Cycle and Adhesion of Human Colorectal Carcinoma Cells (HT29) In Vitro. J. Agric. Food Chem. 2005;53:3895–3901. doi: 10.1021/jf048025v. [DOI] [PubMed] [Google Scholar]
- 67.Fahey J.W., Zhang Y., Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall K.L., Farah M. Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders. Neural Regen. Res. 2021;16:1901–1910. doi: 10.4103/1673-5374.308077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okumura K., Hosoya T., Kawarazaki K., Izawa N., Kumazawa S. Antioxidant activity of phenolic compounds from fava bean sprouts. J. Food Sci. 2016;81:C1394–C1398. doi: 10.1111/1750-3841.13330. [DOI] [PubMed] [Google Scholar]
- 70.Rebollo-Hernanz M., Aguilera Y., Herrera T., Cayuelas L.T., Dueñas M., Rodríguez-Rodríguez P., Martín-Cabrejas M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods. 2020;9:330. doi: 10.3390/foods9030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malone A., Hamilton C. The Academy of Nutrition and Dietetics/the American Society for Parenteral and Enteral Nutrition consensus malnutrition characteristics application in practice. Nutr. Clin. Pract. 2013;28:639–650. doi: 10.1177/0884533613508435. [DOI] [PubMed] [Google Scholar]
- 72.Petrov D., Mansfield C., Moussy A., Hermine O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 2017;9:68. doi: 10.3389/fnagi.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masci A., Mattioli R., Costantino P., Baima S., Morelli G., Punzi P., Mosca L. Neuroprotective effect of brassica oleracea sprouts crude juice in a cellular model of alzheimer’s disease. Oxid. Medi. Cellular Long. 2015;2015:781938. doi: 10.1155/2015/781938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Y., Yang T., Mao L., Zhang F. Sulforaphane protects against brain diseases: Roles of cytoprotective enzymes. Austin J. Cereb. Dis. Stroke. 2017;4:1054. doi: 10.26420/austinjcerebrovascdisstroke.2017.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goyal M.R., Suleria H., Harikrishnan R. The Role of Phytoconstitutents in Health Care. Apple Academic Press; New York, NY, USA: 2020. The Role of Herbal Medicines in Female Genital Infections; pp. 191–214. [Google Scholar]
- 76.Zhao X.X., Lin F.J., Li H., Li H.B., Wu D.T., Geng F., Ma W., Wang Y., Miao B.H., Gan R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.) Front. Nutr. 2021;8:463. doi: 10.3389/fnut.2021.669805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koza L.A., Winter A.N., Holsopple J., Baybayon-Grandgeorge A.N., Pena C., Olson J.R., Mazzarino R.C., Patterson D., Linseman D.A. Protocatechuic acid extends survival, improves motor function, diminishes gliosis, and sustains neuromuscular junctions in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Nutrients. 2020;12:1824. doi: 10.3390/nu12061824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barber S.C., Higginbottom A., Mead R.J., Barber S., Shaw P.J. An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Rad. Bio. Med. 2009;46:1127–1138. doi: 10.1016/j.freeradbiomed.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gawlik-Dziki U., Świeca M., Dziki D., Sȩczyk Ł., Złotek U., Rózyło R., Kaszuba K., Ryszawy D., Czyz J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. Biomed. Res. Int. 2014;2014:608053. doi: 10.1155/2014/608053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khalid W., Arshad M.S., Jabeen A., Muhammad Anjum F., Qaisrani T.B., Suleria H.A.R. Fiber-enriched botanicals: A therapeutic tool against certain metabolic ailments. Food Sci. Nut. 2022;10 doi: 10.1002/fsn3.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Appeldoorn M.M., Vincken J.P., Gruppen H., Hollman P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009;139:1469–1473. doi: 10.3945/jn.109.106765. [DOI] [PubMed] [Google Scholar]
- 82.Cardona F., Andres-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Majid I., Dhatt A.S., Sharma S., Nayik G.A., Nanda V. Effect of sprouting on physicochemical, antioxidant and flavonoid profile of onion varieties. Int. J. Food Sci. Technol. 2016;51:317–324. doi: 10.1111/ijfs.12963. [DOI] [Google Scholar]
- 85.Müller L., Meyer M., Bauer R.N., Zhou H., Zhang H., Jones S., Robinette C., Noah T.L., Jaspers I. Effect of Broccoli Sprouts and Live Attenuated Influenza Virus on Peripheral Blood Natural Killer Cells: A Randomized, Double-Blind Study. PLoS ONE. 2016;11:e0147742. doi: 10.1371/journal.pone.0147742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gill C.I.R., Haldar S., Porter S., Matthews S., Sullivan S., Coulter J., McGlynn H., Rowland I. The Effect of Cruciferous and Leguminous Sprouts on Genotoxicity, In Vitro and In Vivo. Cancer Epidemiol. Biomark. Prev. 2004;13:1199–1205. doi: 10.1158/1055-9965.1199.13.7. [DOI] [PubMed] [Google Scholar]
- 87.Vale A.P., Santos J., Brito N.V., Fernandes D., Rosa E., Beatriz M., Oliveira P.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry. 2015;115:252–260. doi: 10.1016/j.phytochem.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Clarke J.D., Hsu A., Riedl K., Bella D., Schwartz S.J., Stevens J.F., Ho E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011;64:456–463. doi: 10.1016/j.phrs.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keum Y.S., Oo Khor T., Lin W., Shen G., Han Kwon K., Barve A., Li W., Kong A.N. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: Implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm. Res. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 90.Lee S.G., Parks J.S., Kang H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017;42:62–71. doi: 10.1016/j.jnutbio.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 91.Higdon J.V., Delage B., Williams D.E., Dashwood R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu C., Poulsen H.E., Loft S. Inhibition of oxidative DNA damage in vitro by extracts of brussels sprouts. Free Radic. Res. 2000;33:187–196. doi: 10.1080/10715760000300741. [DOI] [PubMed] [Google Scholar]
- 93.Kahlon T.S., Chapman M.H., Smith G.E. In vitro binding of bile acids by spinach, kale, brussels sprouts, broccoli, mustard greens, green bell pepper, cabbage and collards. Food Chem. 2007;100:1531–1536. doi: 10.1016/j.foodchem.2005.12.020. [DOI] [Google Scholar]
- 94.Zhu C.Y., Loft S. Effects of Brussels sprouts extracts on hydrogen peroxide-induced DNA strand breaks in human lymphocytes. Food Chem. Toxicol. 2001;39:1191–1197. doi: 10.1016/S0278-6915(01)00061-8. [DOI] [PubMed] [Google Scholar]
- 95.Chaudhary A., Choudhary S., Sharma U., Vig A.P., Arora S. In vitro Evaluation of Brassica sprouts for its Antioxidant and Antiproliferative Potential. Indian J. Pharm. Sci. 2016;78:615–623. doi: 10.4172/pharmaceutical-sciences.1000160. [DOI] [Google Scholar]
- 96.Hong Y.H., Chao W.W., Chen M.L., Lin B.F. Ethyl acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit lipopolysaccharide-induced inflammation in vitro and in vivo. J. Biomed. Sci. 2009;16:1–12. doi: 10.1186/1423-0127-16-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abellán A., Domínguez-Perles R., Moreno D.A., García-Viguera C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients. 2019;11:429. doi: 10.3390/nu11020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barba F.J., Nikmaram N., Roohinejad S., Khelfa A., Zhu Z., Koubaa M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016;3:24. doi: 10.3389/fnut.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagner A.E., Terschluesen A.M., Rimbach G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell. Longev. 2013;2013:964539. doi: 10.1155/2013/964539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y., Szép S., Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. USA. 2009;106:20515. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayes J.D., Kelleher M.O., Eggleston I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008;47((Suppl. S2)):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 102.Li R., Zhu Y. The primary active components, antioxidant properties, and differential metabolite profiles of radish sprouts (Raphanus sativus L.) upon domestic storage: Analysis of nutritional quality. J. Sci. Food Agric. 2018;98:5853–5860. doi: 10.1002/jsfa.9137. [DOI] [PubMed] [Google Scholar]
- 103.Jeon J., Kim J.K., Kim H.R., Kim Y.J., Park Y.J., Kim S.J., Kim C., Park S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018;241:7–13. doi: 10.1016/j.foodchem.2017.08.067. [DOI] [PubMed] [Google Scholar]
- 104.Devi C.B., Kushwaha A., Kumar A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata) J. Food Sci. Technol. 2015;52:6821. doi: 10.1007/s13197-015-1832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gulewicz P., Martínez-Villaluenga C., Frias J., Ciesiołka D., Gulewicz K., Vidal-Valverde C. Effect of germination on the protein fraction composition of different lupin seeds. Food Chem. 2008;107:830–844. doi: 10.1016/j.foodchem.2007.08.087. [DOI] [Google Scholar]
- 106.Taniguchi H., Kobayashi-Hattori K., Tenmyo C., Kamei T., Uda Y., Sugita-Konishi Y., Takita T. Effect of Japanese radish (Raphanus sativus) sprout (Kaiware-daikon) on carbohydrate and lipid metabolisms in normal and streptozotocin-induced diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Natural Product Deri. 2006;20:274–278. doi: 10.1002/ptr.1851. [DOI] [PubMed] [Google Scholar]
- 107.Dipnaik K., Bathere D. Effect of soaking and sprouting on protein content and transaminase activity in pulses. Int. J. Res. Med. Sci. 2017;5:4271–4276. doi: 10.18203/2320-6012.ijrms20174158. [DOI] [Google Scholar]
- 108.Benincasa P., Falcinelli B., Lutts S., Stagnari F., Galieni A. Sprouted Grains: A Comprehensive Review. Nutrients. 2019;11:421. doi: 10.3390/nu11020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhat Z.F., Kumar S., Bhat H.F. In vitro meat: A future animal-free harvest. Crit. Rev. Food Sci. Nutr. 2017;57:782–789. doi: 10.1080/10408398.2014.924899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.