Significance
Here, we have utilized nucleoside-modified messenger RNA-lipid nanoparticle (mRNA-LNP) vaccines to deliver a combination of influenza virus antigens targeting different conserved proteins to induce strong immune responses with substantial breadth. We believe this report represents a major advancement in influenza virus vaccine research as it describes a unique vaccination approach that induces broadly protective immune responses against multiple influenza A group 2 hemagglutinin viruses in mice after administration of a single dose. The inclusion of multiple conserved antigens considerably broadens the breadth and levels of protection against a panel of heterologous (including avian) influenza virus strains. Taken together, our data demonstrate that the mRNA-LNP quadrivalent group 2 influenza virus vaccine is a promising vaccine candidate that targets diverse influenza virus strains.
Keywords: influenza virus, mRNA vaccine, nucleoside modification, lipid nanoparticle, T cells
Abstract
Combined vaccine formulations targeting not only hemagglutinin but also other influenza virus antigens could form the basis for a universal influenza virus vaccine that has the potential to elicit long-lasting, broadly cross-reactive immune responses. Lipid nanoparticle (LNP)-encapsulated messenger RNA (mRNA) vaccines can be utilized to efficiently target multiple antigens with a single vaccine. Here, we assessed the immunogenicity and protective efficacy of nucleoside-modified mRNA-LNP vaccines that contain four influenza A group 2 virus antigens (hemagglutinin stalk, neuraminidase, matrix protein 2, and nucleoprotein) in mice. We found that all vaccine components induced antigen-specific cellular and humoral immune responses after administration of a single dose. While the monovalent formulations were not exclusively protective, the combined quadrivalent formulation protected mice from all challenge viruses, including a relevant H1N1 influenza virus group 1 strain, with minimal weight loss. Importantly, the combined vaccine protected from morbidity at a dose of 125 ng per antigen after a single vaccination in mice. With these findings, we confidently conclude that the nucleoside-modified mRNA-LNP platform can be used to elicit protection against a large panel of influenza viruses.
Infections with seasonal influenza viruses result in significant global morbidity and mortality annually (1). In addition, influenza pandemics occurring at irregular and unpredictable intervals and can result in devastating mortality. Current seasonal influenza virus vaccines induce narrow, strain-specific immune responses and have variable effectiveness depending on how well vaccine antigens match those of circulating strains (2). These vaccines rely on targeting the immuno-dominant surface glycoprotein of the influenza virus, hemagglutinin (HA). However, the HA is subject to rapid antigenic drift due to polymerase error rate and immune selection pressure (3). Accordingly, seasonal influenza virus vaccines are reformulated annually (4–6). As such, the investigations of new viral targets for influenza virus vaccines that are broadly protective and do not require frequent changes to match antigenically drifted viruses is warranted.
The nucleoside-modified messenger RNA-lipid nanoparticle (mRNA-LNP) platform provides the ideal vaccine platform for investigating the protective capacity of new infectious disease vaccine targets (7). The flexibility of the platform allows the encapsulation of several mRNA-encoded antigens into a single formulation (8–12). Additionally, the production of in vitro transcribed synthetic mRNA vaccines is simple, scalable, and, most importantly, it is sequence-independent (the same manufacturing procedure can be applied to any mRNA sequence). In addition, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleoside-modified mRNA-LNP vaccines have demonstrated safety and high efficacy in humans, suggesting that this platform can be utilized to develop safe and broadly protective influenza virus vaccines (13–15).
Our earlier work showed that targeting the A/Brisbane/59/2007 H1N1 influenza virus HA using a mini HA construct, and neuraminidase (NA), nucleoprotein (NP) and matrix 2 (M2) protein from A/Michigan/45/2015 H1N1 influenza virus with a quadrivalent nucleoside-modified mRNA-LNP formulation, provided mice with broad protective immunity (16). The HA stalk contains conserved epitopes that when targeted by antibodies can provide broad protection. Repeated targeting of the HA stalk proved to be a successful vaccination strategy (17–21). NA is a conserved glycoprotein that was shown to contribute to protection from influenza virus infection (22, 23). Despite its potential to reduce morbidity and viral shedding (24, 25), it is a largely ignored vaccine target. While NA-specific immunity is mainly restricted to a specific subtype, cross-protection within that subtype has been observed. For example, mice vaccinated with the NA of the A/Puerto Rico/8/1934 (H1N1) laboratory strain were protected from challenge with both a 2009-pandemic H1N1 isolate as well as an H5N1 virus (26). In addition to the influenza virus surface glycoproteins, NP is a highly conserved antigen that will likely provide cell-based cross-protection against a large number of influenza virus strains (19, 27). The M2 is a highly conserved viral antigen in both human and avian influenza A viruses (28) and immune responses against it have been shown to correlate well with protection in preclinical and clinical settings (29, 30). Indeed, the ectodomain of M2 (M2e) is a very attractive vaccine antigen as it is only 23 amino acids long, and it is almost invariant in all human epidemic strains regardless of subtype. As demonstrated in early studies, constructs containing several M2e sequences in tandem induced high titers of M2e-specific antibodies and improved protection from viral challenge (31). Here, we again utilized the nucleoside-modified mRNA-LNP vaccine platform to effectively target the HA stalk (32), NA, NP and M2 to protect against challenge with group 2 hemagglutinin expressing influenza viruses. We found that the delivery of a quadrivalent vaccine that targets a combination of influenza virus antigens provides broad protection in mice, even after administration of a single, low dose.
Results
Selection of Group 2 Influenza Virus Vaccine Antigens and Confirmation of Antigen Expression.
The overall goal of these vaccination studies was to define influenza virus antigens that protect against group 2 HA and group 2 NA expressing influenza viruses in mice. Before undertaking vaccination experiments, we first confirmed protein production from mRNA vaccine antigens in cell transfection experiments. HEK293T cells were transfected with mRNAs encoding a group 2 stabilized headless HA (H3ss-TM), A/Singapore/INFIMH-16-0019/2016 NA, NP, M2, or firefly luciferase (Luc) and protein production was assessed by flow cytometry or Western blotting. Binding of CR9114, an anti-HA antibody (33), and 1G01, an anti-NA antibody (34), were observed for both the HA and NA proteins, respectively (SI Appendix, Fig. S1 A and B). Protein expression from NP and M2-encoding mRNAs was confirmed by Western blotting (SI Appendix, Fig. S1 C and D).
Vaccination with Group 2 Influenza Nucleoside-Modified mRNA-LNPs Results in Robust Humoral Immune Responses.
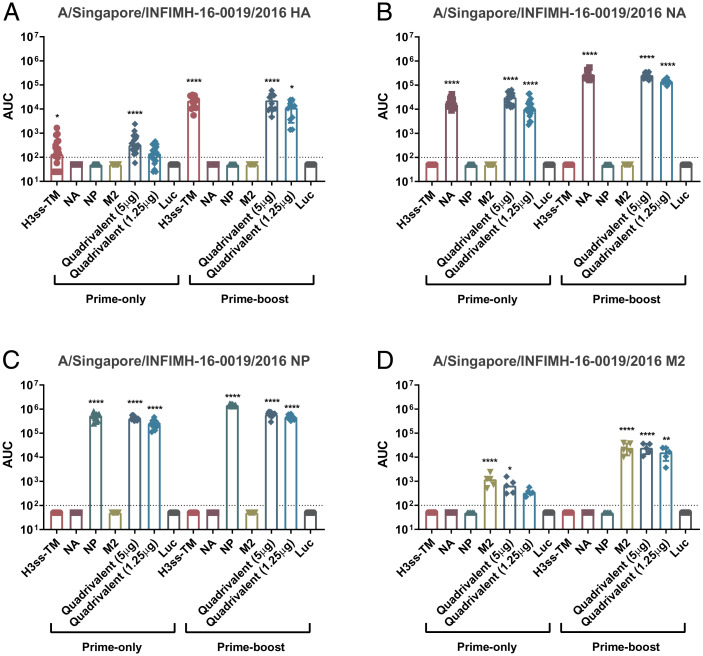
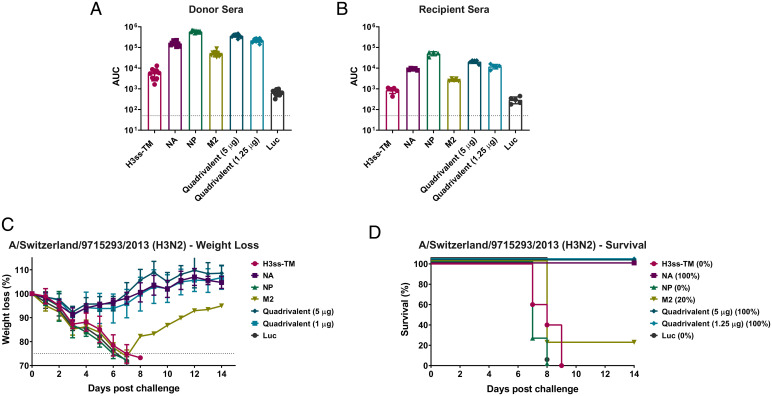
We next determined titers and functionality of serum antibodies elicited 28 d after a prime-only or a prime-boost vaccination regimen (SI Appendix, Fig. S2). Mice were vaccinated with mRNA-LNPs encoding different conserved virus antigens (as monovalent or quadrivalent formulations) or a control formulation encoding firefly luciferase. Enzyme-linked immunosorbent assays (ELISAs) indicated that vaccination with both the monovalent and quadrivalent vaccine formulations resulted in potent antigen-specific antibodies to similar titers (Fig. 1). As anticipated, we observed increased antibody titers in the prime-boost sera for all antigens (Fig. 1).
Fig. 1.
Assessment of binding to vaccine antigens in the prime-only and prime-boost sera using ELISA. Mice were vaccinated once or twice (4 wk apart) ID with 5 μg of monovalent mRNA-LNP or with 5 μg/antigen or 1.25 μg/antigen of the quadrivalent mRNA-LNP formulation. Negative control animals received 5 μg of Luc mRNA-LNP. Sera were collected on day 28 post single- or double-shot vaccination and binding of antibodies to A/Singapore/INFIMH-16-0019/2016 (H3N2) HA (A) NA (B), NP (C) and M2 (D) was assessed using ELISA. Each symbol represents one animal, sera from 5 to 20 animals were assessed. AUCs with a cutoff value of the average background plus three SDs are shown. Bars represent the geometric mean for each group and error bars depict SD. Statistical significance was calculated using one-way ANOVA and groups were compared to the Luc control for their respective vaccination regimen (prime-only/prime-boost vaccination). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
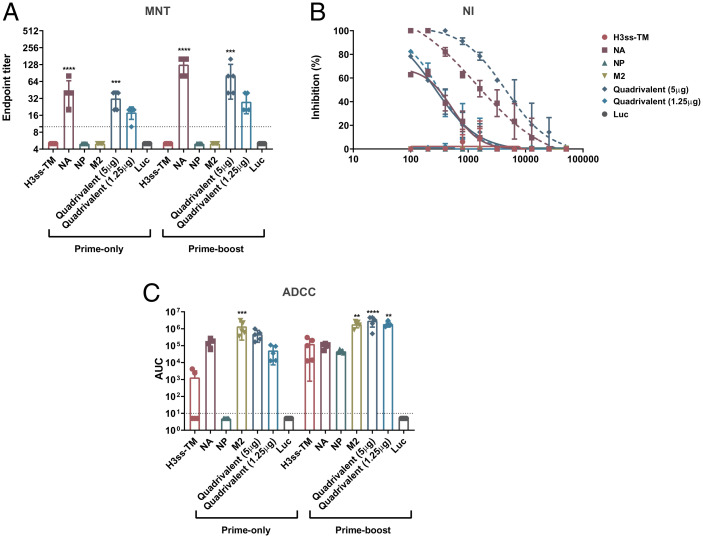
To further characterize nucleoside-modified mRNA-LNP vaccine-elicited antibodies, a multicycle neutralization assay was performed using the A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186 reassortant) influenza virus. Only mice vaccinated with NA-encoding mRNA-LNP formulations displayed neutralizing antibody responses in both the prime-only and prime-boost sera (Fig. 2A), with a dose-dependent response observed between the 5 µg/antigen and 1.25 µg/antigen quadrivalent formulations. This result was replicated in the NA inhibition (NI) assay performed against A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186 reassortant) influenza virus (Fig. 2B), although no NI was observed in mice administered the quadrivalent formulation containing 1.25 µg of NA after prime-only. To assess Fc-mediated effector functions elicited by serum antibodies, a murine antibody-dependent cellular cytotoxicity (ADCC) reporter assay was conducted using A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186). Vaccination with mRNA-LNP formulations in a single-dose regimen containing the H3ss-TM, NA and M2 resulted in robust ADCC reporter activity (Fig. 2C). All vaccination groups had robust ADCC reporter activity in the prime-boost vaccination studies.
Fig. 2.
Determination of functional antibody responses in the prime-only and prime-boost sera. Mice were vaccinated once or twice (4 wk apart) ID with 5 μg of monovalent mRNA-LNP or with 5 μg/antigen or 1.25 μg/antigen of the quadrivalent mRNA-LNP formulation. Negative control animals received 5 μg of Luc mRNA-LNP. Functional characterization of antibodies in the sera of vaccinated mice was assessed using MNT (A), NI (B) and ADCC reporter (C) assay against the A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) virus. MNT data are presented as endpoint titers of five randomly selected animals. ADCC data are presented as AUCs with a cutoff value of the average background plus five SDs. For MNT and ADCC data, bars represent the geometric mean for each group and error bars depict SD. Statistical significance was calculated using one-way ANOVA and groups were compared to the Luc control for their respective vaccination regimen (single/double vaccination). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. NI data are presented as inhibition calculated against a virus-only control. Each data point represents inhibition of sera from five randomly selected animals and error bars are representative of SD. Complete lines represent data obtained from prime-only sera, whereas dashed lines represent data obtained from prime-boost sera.
Vaccination with Group 2 Influenza mRNA-LNPs Induces Antigen-Specific Cellular Immune Responses.
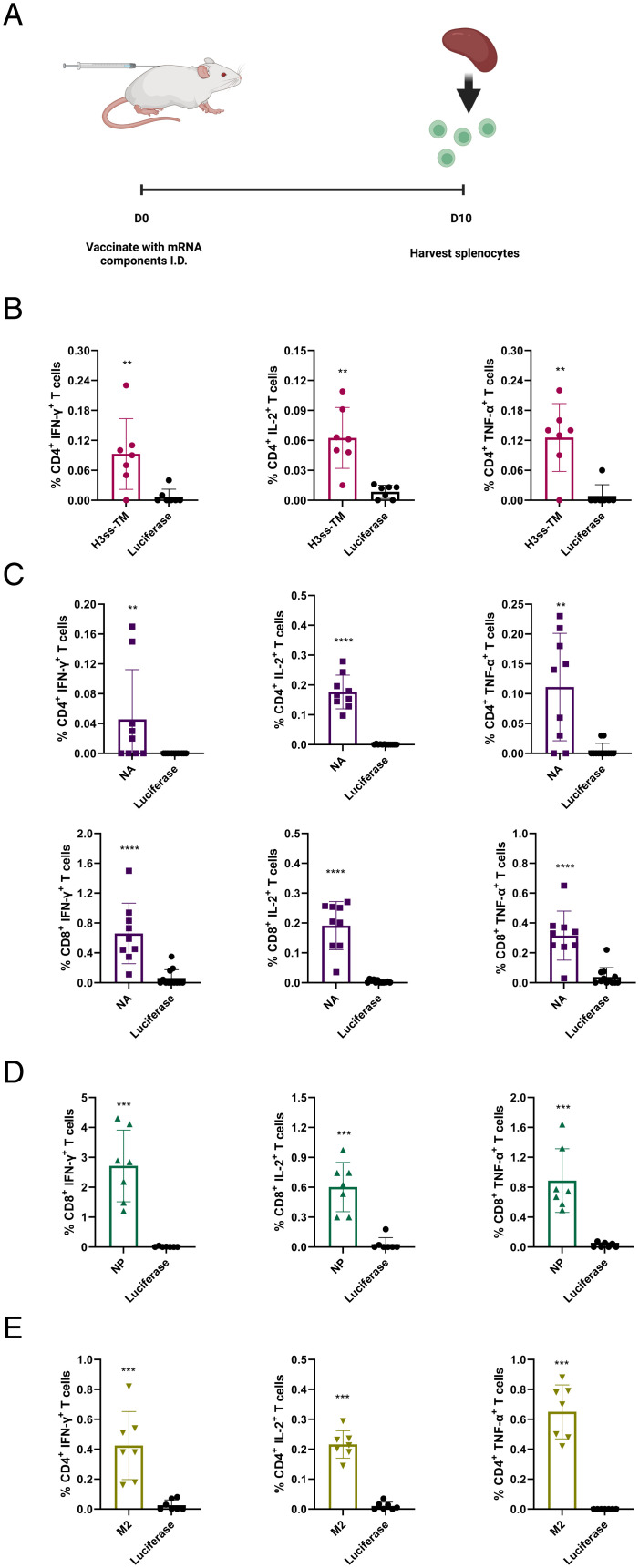
We and others previously demonstrated that nucleoside-modified mRNA-LNP vaccines induce robust cellular immune responses against various pathogens (8, 11, 35, 36). We immunized mice with a single I.D. dose of 5 µg of monovalent headless HA, NA, NP, M2, or control luciferase mRNA-LNPs and determined splenic antigen-specific CD4+ and CD8+ T cell responses 10 d later (Fig. 3A and SI Appendix, Fig. S3). Overall, all four monovalent vaccines elicited antigen-specific cellular responses after a single immunization. Strain-matched peptides or peptide pools were not available for us to determine HA- and NA-specific cellular immune responses, therefore, we used peptide pools specific for related influenza A virus subtypes. We could measure antigen-specific immune responses against HA and NA, but they were not as robust as against NP and M2, likely due to the utilization of nonstrain-matched peptide pools (Fig. 3 B and C). NP-specific CD8+ T cell responses and M2-specific CD4+ T cell responses were determined with immunodominant major histocompatibility complex (MHC) class I-restricted and MHC class II-restricted peptides, respectively, and we detected robust immune responses against both NP and M2 (Fig. 3 D and E).
Fig. 3.
Cellular immune responses induced by influenza group 2 mRNA-LNP vaccines. Mice were vaccinated ID with a single dose of 5 μg of H3ss-TM (HA), NA, NP, M2, or control luciferase mRNA-LNPs. Splenocytes collected from immunized animals 10 d after immunization were stimulated with NP or M2-specific immunodominant MHC class I-restricted and MHC class II-restricted peptides, respectively, or NA or HA overlapping peptide pools, and cytokine production by CD4+ and CD8+ T cells was assessed by flow cytometry. (A) Schematic illustration of the experiment. (B) Percentages of cytokine producing HA-specific CD4+ T cells. (C) Percentages of cytokine producing NA-specific CD4+ (Upper panels) and CD8+ (Lower panels) T cells. (D) Percentages of cytokine producing NP-specific CD8+ T cells. (E) Percentages of cytokine producing M2-specific CD4+ T cells. Each symbol represents one animal and error is shown as SEM (n = 6 to 9 mice per group). Statistical analysis: two-tailed unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To gain further insights into the quality of NP-specific CD8+ T cell responses, an in vivo killing assay was performed (SI Appendix, Fig. S4). Our data demonstrate that NP-specific CD8+ T cells in mice vaccinated with a single dose of NP mRNA-LNPs produced a very robust cytotoxic effect on cells loaded with NP peptides and transferred to immunized mice.
Mice Vaccinated With the Group 2 Quadrivalent Nucleoside-Modified mRNA-LNPs Are Protected from a Broad Panel of Influenza A Viruses.
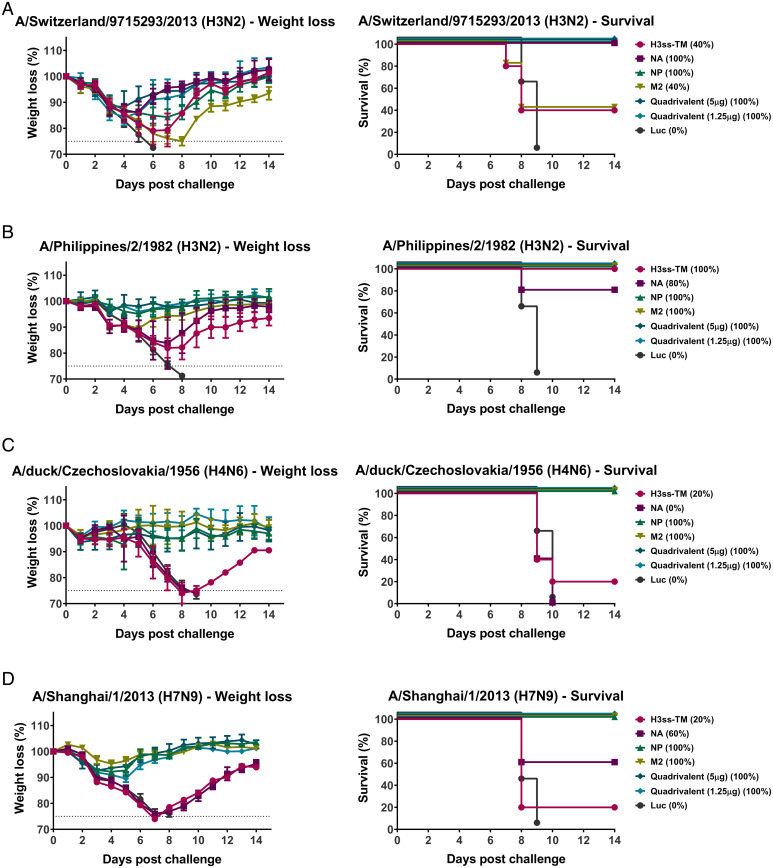
To assess if a prime-only approach can provide protection from group 2 influenza viruses, challenge with a number of group 2 HA expressing influenza viruses was performed. Mice were challenged with 5x the 50% murine lethal dose (mLD50) of A/Switzerland/9715293/2013 (H3N2), A/Philippines/2/1982 (H3N2, X-79, A/PR/8/1934 reassortant), A/duck/Czechoslovakia/1956 (H4N6, A/PR/8/1934 reassortant) or A/Shanghai/1/2013 (H7N9, A/PR/8/1934 reassortant) viruses and weight loss and survival were monitored over a 14 d time course (Fig. 4). The similarity of each vaccine antigen to each of the influenza virus challenge strains was determined using ClustalOmega (SI Appendix, Table S1).
Fig. 4.
A prime-only vaccination with the quadrivalent mRNA-LNP formulation protects mice from challenge with influenza virus. Mice were ID vaccinated once with monovalent or quadrivalent mRNA-LNP as described in Fig. 1. Four weeks later, animals were IN challenged with 5mLD50 of group 2 influenza viruses and morbidity and mortality were assessed. Morbidity and mortality in mice challenged with A/Switzerland/9715293/2013 (H3N2) (A), A/Philippines/2/1982 (H3N2, A/PR/8/1934 reassortant) (B), A/duck/Czechoslovakia/1956 (H4N6, A/PR/8/1934 reassortant) (C) or A/Shanghai/1/2013 (H7N9, A/PR/8/1934 reassortant) (D) influenza viruses. Survival is also represented in parentheses. Data are shown as mean and error bars represent SD (n = 5 per group).
We found that mice vaccinated with NA, NP, or the quadrivalent formulations were protected from A/Switzerland/9715293/2013 (H3N2) virus challenge, despite observable weight loss (Fig. 4A). Mice vaccinated with NP, M2, or the quadrivalent formulations displayed minimal weight loss and were protected from challenge with A/Philippines/2/1982 (H3N2) (Fig. 4B), A/duck/Czechoslovakia/1956 (H4N6, A/PR/8/1934 reassortant) (Fig. 4C) or A/Shanghai/1/2013 (H7N9, A/PR/8/1934 reassortant) (Fig. 4D) influenza viruses.
As vaccination with monovalent formulations did not prevent morbidity upon challenge with the A/Switzerland/9715293/2013 (H3N2) virus, we also assessed protection against this strain in prime-boost vaccinated mice. Similar to the prime-only vaccination challenge studies, we observed morbidity in animals immunized with H3ss-TM, NP, and M2 monovalent formulations, although mice were protected from challenge (SI Appendix, Fig. S5). Mice vaccinated with the NA monovalent formulation and the quadrivalent formulation containing 1.25 µg showed limited weight loss and no weight loss was observed in mice receiving the quadrivalent formulation containing 5 µg of each antigen.
Next, we assessed protection against the phylogenetically distant A/canine/Illinois/41915/2015 (H3N2) strain in a single immunization experiment with the monovalent and quadrivalent mRNA-LNP formulations. We observed weight loss after challenge in all vaccination groups, but only the luciferase controls succumbed to infection. Mice vaccinated with the low or high dose quadrivalent formulation displayed limited (5% to 10%) weight loss and almost completely recovered by the end of the observation period (SI Appendix, Fig. S6).
To further test the breadth of protective immune responses induced by the group 2 mRNA-LNP vaccines, animals were immunized with a single dose of the monovalent or quadrivalent mRNA-LNP formulations and challenged with a group 1 H1N1 virus, A/Netherlands/602/2009, 4 weeks later. The monovalent vaccines induced partial protection from mortality, except the NP vaccine that proved to be highly protective and induced protection without significant morbidity (SI Appendix, Fig. S7). Similarly, the quadrivalent vaccines (both the low and high doses) provided protection from death with a low level of morbidity. Collectively, our extensive challenge experiments demonstrate that a single immunization with the group 2 quadrivalent mRNA-LNP vaccine elicited broad protection from influenza A viruses.
Antibodies That Target the NA Provide Protection against Influenza Virus Challenge in Passive Transfer Experiments.
To further assess the protective capacity of antibodies induced following mRNA-LNP vaccination, a serum transfer study was performed. Animals were immunized with 5 µg of mRNA-LNP vaccines (monovalent and quadrivalent formulations) in a prime-boost vaccination regimen, 28 d apart. Four weeks post boost, mice were terminally bled and sera were collected. Sera from immunized animals showed reactivity against A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) influenza virus-infected cells (Fig. 5A). Serum samples from immunized animals were then pooled and transferred into naive mice. Two hours post transfer, sera from recipient mice were collected and subsequently tested using the same assay (Fig. 5B). The post transfer sera reacted similarly to the pretransfer sera, although, as expected, at a lower potency. Animals were then challenged with 5mL50 of A/Switzerland/9715293/2013 (H3N2) virus and morbidity and mortality were monitored for 14 days post infection. Animals that received serum from mice vaccinated with the quadrivalent formulations and the NA monovalent formulation were protected from challenge (Fig. 5 C and D), indicating that antibody responses that inhibit virus replication are required to protect mice from challenge in a passive transfer experiment.
Fig. 5.
Humoral protection from group 2 influenza virus challenge is afforded by antibodies that target the NA. Mice were vaccinated twice (4-wk intervals) ID with 5 µg of monovalent or quadrivalent mRNA-LNPs. Sera were collected from vaccinated animals 4 wk after the boost and the sera were assessed for antibody reactivity toward A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) influenza virus-infected cells by cell-based ELISA (A). Sera from vaccinated mice were then transferred into naïve mice. Two hours after the transfer, sera from passively transferred naïve mice were assessed for antibody reactivity toward A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) influenza virus-infected cells by ELISA (B). Each symbol represents one animal, sera from 5 to 10 animals were assessed. AUCs with a cutoff value of the average background plus three SDs are shown. Bars represent the geometric mean for each group and error bars depict SD. Naïve mice were then I.N. challenged with 5mLD50 of A/Switzerland/9715293/2013 (H3N2) and weight loss (C) and survival (D) were monitored for 14 d. Survival is also represented in parentheses in panel D. Data are shown as mean and error bars represent SD (n = 4 or 5 per group). One mouse passively transferred NP sera was excluded due to a failed transfer.
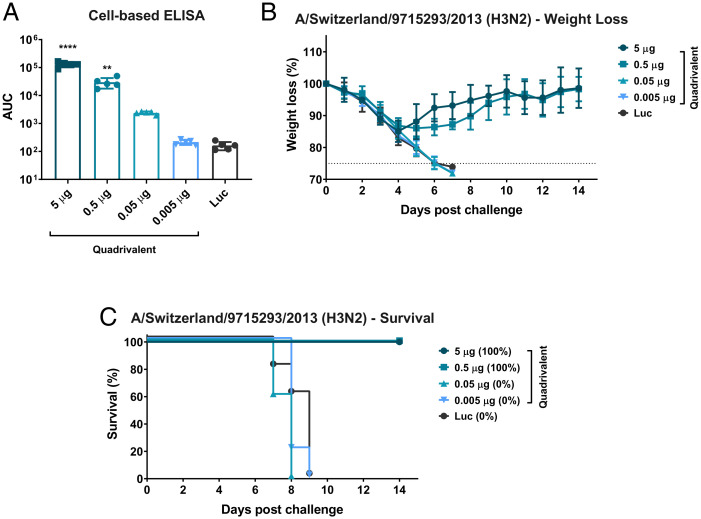
A Single Immunization with a Low Dose of Quadrivalent mRNA-LNP Vaccine Induces Protective Immunity.
After observing improved protection afforded by the quadrivalent formulation when compared to the monovalent formulations (Fig. 4), we determined the minimal dose at which the quadrivalent vaccine was protective. Animals were administered the quadrivalent formulation at 10-fold decreasing doses or a Luc control. Twenty-eight days after vaccination, mice were bled and antibodies in the sera were detected in an ELISA against cells infected with A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) influenza virus (Fig. 6A). Antibody responses were detected in animals vaccinated with 5 µg, 0.5 µg, and 0.05 µg of the quadrivalent vaccine, albeit at lower levels in the 0.05 µg group. Minimal or no humoral immune responses were measured in mice vaccinated with 0.005 µg of the quadrivalent formulation. Mice were then challenged with 5mLD50 of A/Switzerland/9715293/2013 (H3N2) influenza virus and morbidity and mortality were monitored for 14 d post challenge (Fig. 6 B and C). Mice vaccinated with 5 µg or 0.5 µg doses were protected from challenge. Despite the observed antibody responses in mice vaccinated with 0.05 µg of the quadrivalent formulation, these mice succumbed to infection, as did mice vaccinated with 0.005 µg quadrivalent formulation and the Luc control (Fig. 6C).
Fig. 6.
A single immunization with the quadrivalent mRNA-LNP formulation protects mice in the nanogram range. Mice were ID vaccinated with 5, 0.5, 0.05, or 0.005 µg of the quadrivalent mRNA-LNP formulation. Twenty-eight days later sera were collected and antibody response toward A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) influenza virus-infected cells was assessed by cell-based ELISA (A). Each symbol represents one animal, sera from five animals were assessed. AUCs with a cutoff value of the average background plus three SDs are shown. Bars represent the geometric mean for each group and error bars depict SD. Statistical significance was calculated using a one-way ANOVA and groups were compared to the Luc control for their respective vaccination regimen (prime-only/prime-boost vaccination). **P < 0.01, ****P < 0.0001. Mice were then I.N. infected with 5mL50 A/Switzerland/9715293/2013 (H3N2) influenza virus and morbidity (B) and mortality (C) were monitored for 14 d post challenge. Survival is also represented in parentheses in panel C. Data are shown as mean and error bars represent SD (n = 5 per group).
Discussion
The development of a single-dose multivalent mRNA-LNP based vaccine that targets diverse influenza virus strains may be achievable by targeting multiple conserved viral antigens. Using emerging vaccine platforms, such as nucleoside-modified mRNA-LNP, provides the ability to readily target multiple antigens and induce potent antibody responses against each antigen. We previously used mRNA-encoded influenza virus immunogens (A/Brisbane/59/2007 H1 mini HA construct, a membrane-bound A/Michigan/45/2015 N1 NA, A/Michigan/45/2015 NP and A/Michigan/45/2015 M2) to elicit broad protection against group 1 HA influenza challenge viruses (16). In a follow-up study, we also demonstrated that introduction of modifications into the mRNA-encoded influenza virus antigens could improve the safety and immunogenicity of nucleoside-modified mRNA-LNP vaccines (37). In this work, we aimed to identify influenza virus antigens that protect against challenge with group 2 HA and NA expressing influenza viruses. We generated monovalent mRNA-LNP vaccines encoding a stabilized group 2 HA stalk construct (H3ss-TM) (32) or full-length NA, NP or M2 from the A/Singapore/INFIMH-16-0019/2016 (H3N2) influenza virus. Additionally, we generated a quadrivalent vaccine containing all four antigen-encoding mRNAs in a single formulation. We observed strong antibody responses against the vaccine antigens in mice vaccinated with both the monovalent and quadrivalent formulations. We could also measure antigen-specific cellular immune responses against each vaccine component after a single immunization. Of note, animals immunized with the monovalent vaccines were not protected from challenge with group 2 HA and NA expressing influenza viruses to the same extent as mice vaccinated with the quadrivalent formulation.
Our work establishes that simultaneous targeting of multiple conserved viral epitopes with combined mRNA-LNP vaccines results in improved protection from challenge when compared to monovalent formulations. For example, when vaccinating with the NA monovalent formulation in the prime-only regimen, all mice were protected with minimal weight loss from challenge with A/Switzerland/9715293/2013 (H3N2) virus but variable levels of protection were observed upon challenge with the other group 2 influenza viruses. Mice receiving the M2 monovalent vaccine were protected from challenge with A/Philippines/2/1982 (H3N2, A/PR/8/1934 reassortant), A/duck/Czechoslovakia/1956 (H4N6, A/PR/8/1934 reassortant) and A/Shanghai/1/2013 (H7N9, A/PR/8/1934 reassortant) viruses but were not protected against challenge with A/Switzerland/9715293/2013 (H3N2) influenza virus. The three viruses that M2 monovalent-vaccinated mice were protected from contain the same M2 segment. Mice vaccinated with the quadrivalent formulation containing either 5 µg or 1.25 µg of each of the vaccine antigens were protected from challenge with all group 2 influenza viruses, despite differences in antibody levels detected in assessments of functional antibody responses. Indeed, mice vaccinated with 1.25 µg of each antigen in the quadrivalent formulation had lower antibody responses in microneutralization (MNT), NI, and ADCC reporter assays in the prime-only and prime-boost sera when compared to mice receiving 5 µg quadrivalent vaccine, although this did not appear to affect protection. The only obvious differences were in the prime-boost protection studies, where mice vaccinated with the 5 µg quadrivalent formulation showed no weight loss but mice receiving the 1.25 µg quadrivalent formulation showed an average loss of 10% body weight. Additionally, the quadrivalent formulations (both the low and high doses) demonstrated protection from a group 1 H1N1 virus, A/Netherlands/602/2009.
Most interesting are the undetectable and reduced NI antibody responses in prime-only and prime-boost sera, respectively, of mice receiving the 1.25 µg of the quadrivalent formulation when compared to the NA monovalent formulation and 5 µg quadrivalent formulation. This result suggests that higher doses of the NA may be required to elicit strong responses that target the NA active site. We also observed that NI antibody responses following a prime-only vaccination regimen with the NA monovalent formulation and 5 µg quadrivalent formulation failed to fully inhibit virus activity. In 2014, a double mutation in the NA of influenza A H3N2 viruses was first detected and by 2016/2017 almost all circulating H3N2 viruses in humans carried these mutations that introduced a new glycosylation site at amino acid positions 245-247 (38). These mutations have since become fixed in circulating H3N2 viruses and the newly introduced glycan might contribute to shielding of certain epitopes that are targeted during vaccination. The virus used in the NI assay, A/Singapore/INFIMH-16-0019/2016 (H3N2), contains this glycosylation and hence antibody responses may be reduced. Testing of additional N2 viruses in the NI assay may yield different results.
A prime-only vaccination regimen that targets the H3ss-TM and M2 resulted in limited antibody responses. Improved antibody responses were observed after two immunizations, suggesting that a prime-boost vaccination regimen may be necessary to elicit potent HA stalk- and M2-specific immune responses. Indeed, prime-boost and sequential vaccination regimens have been investigated for targeting the HA stalk (2, 39–41).
As mentioned above, mice receiving the M2 monovalent vaccine were protected from challenge with A/Philippines/2/1982 (H3N2, A/PR/8/1934 reassortant), A/duck/Czechoslovakia/1956 (H4N6, A/PR/8/1934 reassortant) and A/Shanghai/1/2013 (H7N9, A/PR/8/1934 reassortant) viruses but were not protected against challenge with A/Switzerland/9715293/2013 (H3N2) influenza virus. For the group 2 influenza challenge viruses used in these studies, it should be noted that A/Philippines/2/1982 (H3N2), A/duck/Czechoslovakia/1956 (H4N6) and A/Shanghai/1/2013 (H7N9) are A/Puerto Rico/8/1934 (H1N1) reassortant strains that contain the HA and NA of the respective viruses. The use of these reassortant strains increases safety (42–46), however, the protection afforded by the NP and M2 formulations should be carefully considered as the NP and M2 within these three challenge viruses are exactly the same. In addition, challenges with the mouse-adapted A/Switzerland/9715293/2013 (H3N2) influenza virus were performed in DBA/2J mice. DBA/2J mice have a CD94 deficiency, and hence dysregulated NK cells (47), which may be why increased lethality is observed in this animal model.
Many vaccine platforms have been assessed for their potential as broadly protective influenza vaccines with increased effectiveness compared to the standard of care QIV. Broadly reactive antibody responses toward the group 2 influenza virus HA have been achieved with chimeric HAs, which contain avian head domains but conserved stalk domains, and mini-HA antigens (32, 48, 49). Use of recombinantly produced N2 NAs has also been shown to induce broadly protective antibody responses in mice (26). Targeting influenza virus internal proteins (NP and M1) using viral vector vaccines has been shown to induce protective immune responses against group 2 HA expressing influenza viruses (19, 27). These viral vector vaccines have also been shown to stimulate broadly reactive T cell responses when the vaccines contain influenza virus NP and M1 (19, 27, 50, 51). These vaccines have generally focused on the protective effects afforded by targeting one or two influenza virus antigens. In our work, we add to this body of research and demonstrate that a quadrivalent nucleoside-modified mRNA-LNP vaccine formulation induces broad protection against group 2 influenza viruses after administration of a single low dose in mice. In agreement with our previous work (16), this study further highlights the importance of developing combined nucleoside-modified mRNA-LNP vaccine formulations that target multiple influenza virus antigens to provide broad protection. As such, further investigation of a multivalent mRNA-LNP vaccine that protects against challenge with group 1, group 2 and influenza B viruses is warranted.
Materials and Methods
Cells.
Baculovirus rescue was performed in Sf9 cells (CRL-1711, ATCC). Sf9 cells were grown in Trichoplusia ni medium-formulation Hink insect cell medium (TNM-FH, Gemini Bioproducts) containing 10% fetal bovine serum (FBS; Sigma) and penicillin (100 U/mL)-streptomycin (100 μg/mL) solution (Gibco). BTI-TN-5B1-4 (High Five) cells used for protein expression were cultured in serum-free SFX medium (HyClone) containing penicillin (100 U/mL)-streptomycin (100 μg/mL) solution. Expi293F cells (ThermoFisher) used for protein expression were grown in Expi293 Expression Medium (Gibco) in 1 L Erlenmeyer shake flasks (Corning) at 37 °C and 125 RPM in a humidified incubator with 8% CO2. Madin Darby canine kidney (MDCK) and human embryonic kidney (HEK) 293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and penicillin (100 U/mL)-streptomycin (100 μg/mL) solution.
Viruses.
The influenza A virus strains A/Singapore/INFIMH-16-0019/2016 IVR-186 (H3N2), A/Switzerland/9715293/2013 (H3N2) (mouse-adapted), A/Philippines/2/1982 (H3N2, X-79, A/PR/8/1934 reassortant), A/duck/Czechoslovakia/1956 (H4N6, A/PR/8/1934 reassortant) (52), A/Shanghai/1/2013 (H7N9, A/PR/8/1934 reassortant), A/canine/Illinois/41915/2015 (H3N2) and A/Netherlands/602/09 (H1N1) were grown in 10-d-old embryonated chicken eggs (Charles River) for 48 h at 37 °C. After virus growth, the eggs were chilled at 4 °C overnight. The following day the allantoic fluid was harvested from the chilled eggs and cellular debris was removed via centrifugation (4,000 × g for 10 min at 4 °C). Viruses were aliquoted and stored at −80 °C. Virus titers (plaque forming units/mL) were determined via plaque assay.
A/Singapore/INFIMH-16-0019/2016 IVR-186 (H3N2), A/Philippines/2/1982 (H3N2, X-79), A/duck/Czechoslovakia/1956 (H4N6) and A/Shanghai/1/2013 (H7N9) are reassortant viruses and contain the internal genome segments of A/Puerto Rico/8/1934 (H1N1).
Protein Production.
Recombinant HAs and NAs from A/Singapore/INFIMH-16-0019/2016 were expressed in High Five insect cells (53). A/Singapore/INFIMH-16-0019/2016 recombinant NP was expressed in HEK293F cells (16). Recombinant HAs, NAs and NP were purified from cell culture supernatant or cell lysates using Ni2+-nitrilotriacetic acid (Ni-NTA) chromatography (53–55).
mRNA-LNP Production.
mRNA-LNP vaccines were produced as previously described (16, 37). Codon-optimized firefly luciferase, a stabilized transmembrane domain (TM)-containing headless group 2 HA construct (H3ss-TM) based on the HA of the A/Finland/486/2004 (H3N2) influenza virus (32), and A/Singapore/INFIMH-16-0019/2016 (H3N2) NA, NP and M2 were synthesized (Genscript) and were ligated into mRNA production vectors. Vectors were then linearized, and an in vitro transcription reaction (Megascript, Ambion) was performed using T7 RNA polymerase to generate mRNAs with 101 nucleotide-long poly(A) tails. m1Ψ-5′-triphosphate (TriLink) was used in the in vitro transcription reaction instead of UTP. mRNA capping was performed alongside transcription through the addition of a trinucleotide cap1 analog, CleanCap (TriLink), and mRNA was purified with cellulose-based purification, as previously described (56). The size and integrity of mRNAs were analyzed on an agarose gel before storing at −20 °C.
Purified mRNAs were LNP-encapsulated using a self-assembly process where a mixture of an ionizable cationic lipid, phosphatidylcholine, cholesterol, and polyethylene glycol-lipid in ethanol were rapidly combined with an aqueous solution containing mRNA at acidic pH as previously described (57). The ionizable cationic lipid (pKa in the range of 6.0 to 6.5, proprietary to Acuitas Therapeutics) and LNP composition are described in the patent application WO 2017/004143. The average hydrodynamic diameter was ∼80 nm with a polydispersity index of 0.02 to 0.06 as measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd) and an encapsulation efficiency of ∼95% was achieved as determined using a Ribogreen assay.
Cell Transfections.
HEK293T cells were transfected with mRNA using TransIT-mRNA (Mirus Bio) as per the manufacturer’s instructions: mRNA (0.3 µg) was combined with TransIT-mRNA Reagent (0.34 µL) and Boost Reagent (0.22 µL) in 17 µL of serum-free medium, and the complex was added to 6 × 104 cells in 183 µL complete medium.
Western Blot Analysis of mRNA-Transfected Cells.
Cell lysates of Luc, NP or M2 mRNA-transfected and nontransfected HEK293T cells were used. Cells were lysed for 1 h on ice in radioimmunoprecipitation assay buffer (Sigma) 24 h after transfection. Samples were combined with 355 mM 2-mercaptoethanol (Bio-Rad) containing 4× Laemmli buffer (Bio-Rad) and were boiled for 5 min and spun at 18,000 × g for 5 min at room temperature (RT). The samples were separated on a 4% to 15% precast polyacrylamide Criterion TGX gel (Bio-Rad) for 45 min at 200 V. Transfer to polyvinylidene fluoride membrane (Thermo Fisher Scientific) was performed using a semidry apparatus (Bio-Rad) at 10 V for 1 h. The membrane was blocked in 5% milk powder-TBST (25 mM Tris, 150 mM NaCl, pH 7.5 + 0.1% Tween-20) for 1.5 h at RT and then, the membrane was incubated with the anti-NP (mouse monoclonal, 1:2,000, BioXCell, #BE0159), anti-M2 (mouse monoclonal, E10, 1:2,000) (58) and anti-glyceraldehyde 3-phosphate dehydrogenase (rabbit monoclonal, 1:5,000; Cell Signaling Technology, #14C10) primary antibodies O/N at 4 °C. The membrane was washed with 1× TBST three times for 10 min and incubated with the horse radish peroxidase (HRP)-conjugated anti-rabbit (donkey polyclonal, 1:10,000, Thermo Fisher Scientific, #31458) or anti-mouse (donkey polyclonal, 1:10,000, Jackson ImmunoResearch, #715-035-150) secondary antibodies for 1 h at RT. After washing three times for 20 min with 1× TBST at RT, the signal was developed with the HRP Substrate solution (GE Healthcare, Amersham ECL Western Blotting Detection Reagents) and imaged using a ChemiDoc XRS+ machine (BioRad).
Staining and Flow Cytometric Analyses of mRNA-Transfected Cells and Mouse Splenocytes.
Luc, H3ss-TM or NA mRNA transfected HEK293T cells were collected 24 h after transfection and were washed once with 1% fetal FBS (HyClone) in phosphate buffered saline (PBS) (Corning, DPBS 1× [without calcium and magnesium]). Next, cells were incubated with 7.5 μg/mL anti-NA [1G01 (34)] or 7.5 μg/mL anti-HA [CR9114 (33)] antibodies in V-bottom 96-well plates (Greiner) for 30 min at 4 °C. After washing with 1% FBS in PBS, cells were incubated with anti-human AlexaFluor-647 (goat polyclonal, 1:300, Thermo Fisher Scientific, #A21445) secondary antibody for 30 min at 4 °C in dark. The samples were washed and resuspended with 1% FBS in PBS. Flow cytometric data were acquired on a BD LSRII flow cytometer. At least 50,000 events for each sample were recorded and data were analyzed with the FlowJo 10 software.
Spleen single-cell suspensions were made in complete Roswell Park Memorial Institute (RPMI) medium 1640 (ATCC). Then, 3 × 106 cells per sample were stimulated for 6 h at 37 °C and 5% CO2, in the presence of overlapping HA (JPT Peptide Technologies, PM-INFA-HAHK), NA (BEI Resources, NR-19251) peptide pools or specific NP (GenScript, NP147-155, TYQRTRALV) or M2 (BEI Resources, NR-2614, M21-16 MSLLTEVETPIRNEWG & M26-22 EVETPIRNEWGCRCNDS) peptides at 2.5 µg/mL (NA) or 5.0 µg/mL (NP, M2, HA) per peptide. GolgiPlug (5 mg/mL; brefeldin A; BD Biosciences) and GolgiStop (10 mg/mL; monensin; BD Biosciences) were added to each sample after 1 h of stimulation. Unstimulated samples for each animal were included. A phorbol 12-myristate-13-acetate (10 mg/mL; Sigma) and ionomycin (200 ng/mL; Sigma)-stimulated sample was included as a positive control. After stimulation, cells were washed with PBS and stained for 10 min in dark at 25 °C with the LIVE/DEAD fixable aqua dead cell stain kit (Life Technologies). Samples were incubated in the Fc blocker (Purified Rat Anti-Mouse CD16/CD32, BD Biosciences) for 10 min in dark at 4 °C and then surface-stained with the monoclonal antibodies anti-CD4 PerCP/Cy5.5 (clone GK1.5, BioLegend) and anti-CD8 Pacific Blue (clone 53-6.7, BioLegend) for 30 min at 4 °C. After surface staining, cells were washed with FACS buffer, fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) and washed again using the permeabilization buffer (BD Biosciences). Cells were intracellularly stained with anti-CD3 APC-Cy7 (clone, 145-2C11, BD Biosciences), anti-TNF-α PE-Cy7 (clone MP6-XT22, BD Biosciences) anti-IFN-γ AF700 (clone XMG1.2, BD Biosciences), and anti-IL-2 BV711 (clone JES6-5H4, BioLegend) monoclonal antibodies for 30 min at 4 °C. Next, the cells were washed twice with the permeabilization buffer (BD Biosciences) and once with FACS buffer, fixed with 4% paraformaldehyde in PBS, and stored at 4 °C until analysis. Splenocytes were analyzed on a LSR II flow cytometer (BD Biosciences). In all, 300,000 events were collected per specimen. Data were analyzed with the FlowJo 10 program. Data were expressed by subtracting the percentages of the unstimulated stained cells from the percentages of the peptide pool-stimulated stained samples.
mRNA-LNP Vaccination and Virus Challenge.
Female BALB/c or DBA/2J mice (aged 6 to 8 wk) were intradermally (ID) vaccinated with 100 μL of mRNA-LNP vaccines in PBS at two different sites on the back. Four weeks after vaccination, blood was collected from anesthetized mice for serological assessment. Following blood collection mice were challenged intranasally (IN) with 50 µL of influenza virus containing 5× the 50% mouse lethal dose (mLD50) (SI Appendix, Fig. S2A). DBA/2J mice were used for A/Switzerland/9715293/2013 (H3N2) challenge studies due to influenza virus susceptible in this mouse model. BALB/c mice were used for challenge with other influenza viruses. Weight loss was monitored for 14 d following challenge. Any mouse that lost more than 25% of its initial body weight was humanely euthanized. For prime-boost vaccination studies, 4 wk after the prime mice were vaccinated as described above. Mice were IN challenged with 5mLD50 of A/Switzerland/9715293/2013 (H3N2) influenza virus four weeks after the boost (SI Appendix, Fig. S2B).
All animal experiments were performed according to guidelines stated in the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee protocol.
In Vivo Cell Killing Assay.
Single cell splenocyte suspensions from naïve mice were divided into two aliquots and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) at final concentrations of 7 μM (CFSEhigh) or 0.7 μM (CFSElow). CFSEhigh cells were pulsed with 5 µg/mL of the NP peptide (GenScript, NP147-155, TYQRTRALV) in RPMI + 10% FBS medium at 37 °C for 40 min. Equal numbers of pulsed and nonpulsed cells from naïve mice were intravenously injected (a total of 2 × 107 cells/mouse) into mice ID immunized with the NP vaccine 10 d earlier. Splenocyte single cell suspensions from immunized mice were prepared 18 h later and were analyzed for the CFSE signal by flow cytometry using LSR II flow cytometer (BD Biosciences). The numbers of CFSEhigh and CFSElow cells were used to calculate the percentage of peptide-pulsed target cell killing, determined by using the formula: 100 to 100 × [(% CFSEhigh NP-immunized/% CFSElow NP-immunized)/(% CFSEhigh Luciferase-injected/% CFSElow Luciferase-injected)].
Passive Transfer Studies in Mice.
Female BALB/c mice aged 6 to 8 wk were vaccinated in a prime-boost vaccination regimen with mRNA-LNP vaccines 4-wk apart. Four weeks after the boost whole blood was collected and coagulated for 1 h at RT before being placed at 4 °C for 30 min. After chilling, the blood was centrifuged at 12,000 × g for 10 min at 4 °C to separate the sera from the blood components. The sera were then stored at 4 °C until passive transfer studies. Two hundred µL of sera were intraperitoneally transferred into naïve DBA/2J mice 2 h prior to challenge with 5mLD50 of A/Switzerland/9715293/2013 (H3N2). Mice were bled posttransfer, and sera were tested against A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) influenza virus-infected MDCK cells to ensure successful transfer.
ELISA.
Immulon plates (Immulon 4HBX; Thermo Scientific) were coated with 2 μg/mL recombinant protein (50 μL per well) in PBS at 4 °C, O/N. The following day, the coated plates were washed three times with PBS containing 0.1% Tween-20 (PBS-T). The plates were then blocked for 1 h at RT with blocking solution (3% goat serum, 0.5% milk in PBS-T). To measure antibody levels in the sera, prediluted serum was added to the first well at a final concentration of 1:100 in blocking solution and serially diluted 1:3 in blocking solution. The serially diluted sera were incubated for 2 h at RT. After the incubation, the plates were washed three with PBS-T and a goat anti-mouse IgG HRP conjugate (Rockland) in blocking solution was added to the plates. The plates were incubated with the goat anti-mouse IgG HRP conjugate for 1 h at RT. The plates were then washed 4× with PBS-T. One hundred microliters of O-phenylenediamine dihydrochloride (OPD) substrate (SigmaFast OPD; Sigma-Aldrich) was then added to each well for 10 min. The OPD reaction was then quenched by the addition of 50 μL of 3 M hydrochloric acid (HCl) to each well. The optical density at 490 nm (OD490) was measured on a Synergy 4 plate reader (BioTek). A cutoff value of the average of the OD values of blank wells plus 3 SDs was determined for each plate and used for calculating the area under the curve (AUC). The limit of detection of the assay was a titer of 1:100. Samples that did not reach this titer were assigned a value of 1:50.
Cell-based ELISAs utilized 96-well cell culture plates (Corning) seeded with 100 µL/well of MDCK cells at a density of 2 × 105 cells/mL and incubated O/N at 37 °C with 5% CO2. The next day PBS-washed cells were infected with A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) virus at a multiplicity of infection (MOI) of 1. The virus was incubated with the cells for 20 h at 33 °C with 5% CO2. The cells were then fixed with 3.7% paraformaldehyde (Fisher Scientific) and stored at 4 °C for 24 h. After fixation the cells were washed with PBS and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) diluted in PBS. After 15 min at RT the 0.1% Triton X-100 diluted in PBS was removed for the cells and the cells washed twice with PBS. The remainder of the cell-based ELISA was performed as described above.
Microneutralization Assay.
Neutralization of influenza virus was assessed in MDCK cells. Here, MDCK cells were seeded onto 96-well cell culture plates (Corning) at a density of 20,000 cells/well. The cells were then incubated O/N at 37 °C in a humidified incubator, 5% CO2. The next day, receptor destroying enzyme-treated sera were diluted twofold across a 96-well plate in infection medium (1× minimum essential media [MEM]; Gibco), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco), 2 mM L-glutamine (Gibco), 3.2% NaHCO3 (Sigma-Aldrich), 1.2% bovine serum albumin (BSA; MP Biomedicals) supplemented with 1 µg/mL L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich). Sixty microliters of 100× median tissue culture infectious dose (TCID50) of virus in infection medium and 60 µL of serially diluted sera were added to a new 96-well cell culture plate and incubated on a shaker for 1 h at RT. After the incubated 100 µL of the incubated sera-virus mixture was added to PBS-washed MDCK cells. The plates were then incubated for 1 h at 33 °C with 5% CO2. The virus inoculum was then aspirated from the MDCK cells and the MDCK cells were washed with 200 µL PBS. One hundred microliters of the serially diluted sera were added to the respective wells and incubated for 72 h at 33 °C and 5% CO2. After the 72-h incubation, 50 µL of cell culture supernatant was added to V-bottom 96-well plates (Thermo Fisher Scientific) with 50 µL of 0.5% chicken red blood cells and the plates were incubated for 45 min at 4 °C. After the incubation the presence or absence of hemagglutination determined. Data are displayed as the endpoint titer and this value represents the lowest dilution at which no hemagglutination could be detected.
ADCC Reporter Assay.
Serum ADCC activity was assessed using the murine in vitro ADCC bioreporter assays as per the manufacturer’s instructions (Promega). Here, 2 × 105 cells/mL MDCK cells were seeded onto white, flat-bottom, 96-well cell culture plates (Corning) and incubated O/N at 37 °C under 5% CO2. The next day PBS washed cells were infected with A/Singapore/INFIMH-16-0019/2016 (H3N2, IVR-186) virus at an multiplicity of infection (MOI) of 1 and incubated overnight at 37 °C with 5% CO2. The following day, sera from vaccinated mice were serially diluted twofold in RPMI 1640 (Gibco). ADCC bioassay effector cells were diluted to 3 × 106 cells/mL in RPMI 1640 media. Twenty five microliters per well of assay buffer, 25 µL/well of serially diluted sera, and 25 µL/well of diluted ADCC effector cells were then added to the PBS washed infected cells. After a 6-h incubation at 37 °C with 5% CO2 in a humidified incubator and a temperature equilibration for 15 min at RT, 75 µL/well Bio-Glo Luciferase assay reagent was added to each well and the plates were incubated for 10 min in the dark at RT. Luminescence measured using a Synergy H1 microplate reader (BioTek). A cutoff value of the average of the OD values of blank wells plus 5 SDs was defined for each plate and used for calculating the AUC, the readout for this assay. The limit of detection of the assay was a titer of 1:10. Samples that did not reach this titer were assigned a value of 1:5 for graphing purposes.
NA and NA Inhibition Assays.
Flat-bottom Immulon 4BX 96-well plates were coated with 100 μL of 25 μg/mL fetuin (Sigma) in PBS and incubated O/N at 4 °C. Viruses to be tested for NA activity were serially diluted 2-fold in sample diluent (PBS (Gibco) with 0.9 mM CaCl2 and 0.5 mM MgCl2 supplemented with 1% BSA (MP Biomedicals) and 0.5% Tween 20 (Fisher Scientific)). One hundred microliters of the serially diluted virus was then added to the 3× PBS-T washed fetuin-coated plates. The plates were then covered and incubated for 18 h at 37 °C. The following day the plated were washed 3× with PBS-T. One hundred microliters per well of HRP-conjugated peanut agglutinin (PNA) in PBS was then added to the plates and the plates were incubated for 2 h at RT. The PNA incubation was stopped by washing the plates 4× with PBS-T and adding 100 μL of OPD substrate to each well. The OD490 was then measured on a Synergy 4 plate reader (BioTek). The half maximal effective concentration (EC50) was determined using GraphPad Prism.
Prior to undertaking NA inhibition (NI) assays, sera from vaccinated mice were inactivated at 56 °C for 1 h. The sera were then serially twofold diluted in sample diluent at a starting dilution of 1:100 and an equal volume (50 µL) of 2× EC50 virus was added. The sera-virus mixture was then added to PBS-T-washed fetuin-coated plates and incubated for 18 h at 37 °C. The remainder of the assay was performed as described above. Each plate contained a virus control column (virus in sample diluent without sera) and no virus control column (sample diluent without sera). Data were analyzed with GraphPad Prism 8.
Statistical Analyses.
Line graph data are expressed as means. Bar graph data are expressed as individual values and the average presented as the geometric mean. Error is represented by SD or SEM. Significance between antibody responses were analyzed by one-way ANOVA followed by a multiple comparison test. Groups were compared to the Luc control group. Differences were considered statistically significant at P < 0.05. All statistical analyses were performed using GraphPad Prism 8.
Supplementary Material
Acknowledgments
The study was supported by NIH R01-AI146101 (NP and MM) and by the Collaborative Influenza Vaccine Innovation Centers (CIVICs) contract 75N93019C00051 (FK). Cs. B. was a postdoctoral fellow supported by the Biotechnological National Laboratory, Szeged, Hungary. András Sárközy was supported by the Rosztoczy Foundation. The NP and M2 peptides used in the T cell studies were kindly provided by Michael J. Hogan and Laurence C. Eisenlohr (Children’s Hospital of Philadelphia). Figure 3A and Supplemental Figure 4A were created with BioRender.
Footnotes
Competing interests statement: In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that N.P. and Y.K.T. are named on a patent describing the use of nucleoside-modified mRNA in lipid nanoparticles as a vaccine platform. N.P. and F.K. are named on a patent filed on universal influenza vaccines using nucleoside-modified mRNA. F.K. is also named on several patents and patent applications for universal influenza virus vaccine candidates based on other vaccine platforms. We have disclosed those interests fully to the University of Pennsylvania and The Icahn School of Medicine at Mount Sinai, and we have in place an approved plan for managing any potential conflicts arising from licensing of our patents. M.B. and Y.K.T. are employees of Acuitas Therapeutics, a company focused on the development of LNP nucleic acid delivery systems for therapeutic applications. F.K. has consulted for Merck, Curevac, Seqirus and Pfizer and currently consults for Pfizer, Third Rock Ventures and Avimex. M.K. and B.S.G. are named on several patents and patent applications filed by the US Department of Health and Human Services on influenza vaccine candidates including the stabilized HA stem constructs. The remaining authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2206333119/-/DCSupplemental.
Data, Materials, and Software Availability
All data that support the findings in this publication are included in the article and SI Appendix. All study data are included in the article and/or SI Appendix.
References
- 1.Control C. f. D., CDC Seasonal Flu Vaccine Effectiveness Studies (2020). https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm?web=1&wdLOR=c05150D7B-8483-4D38-A562-E59CCC551B64. Accessed 1 December 2021.
- 2.Krammer F., Palese P., Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 14, 167–182 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Heaton N. S., Sachs D., Chen C. J., Hai R., Palese P., Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl. Acad. Sci. U.S.A. 110, 20248–20253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie H., et al. , H3N2 mismatch of 2014-15 northern hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci. Rep. 5, 15279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong J. C., Beyer W. E., Palache A. M., Rimmelzwaan G. F., Osterhaus A. D., Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 61, 94–99 (2000). [PubMed] [Google Scholar]
- 6.CDC, Past Pandemics (2018). https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html. Accessed 1 December 2021.
- 7.Alameh M. G., Weissman D., Pardi N., Messenger RNA-based vaccines against infectious diseases. Curr. Top. Microbiol. Immunol. (2020). Epub Date: 20200417 Date: Apr 17 ISSN: 0070-217X (Print) 0070-217x DOI: 10.1007/82_2020_202 Accession Number: 32300916. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi S., et al. , Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 4, eaaw70803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaTourette P. C. II, et al. , Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mRNA in lipid nanoparticle vaccine. Vaccine 38, 7409–7413 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sajid A., et al. , mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 13, eabj9827 (2021). [DOI] [PubMed] [Google Scholar]
- 11.John S., et al. , Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 36, 1689–1699 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Pardi N., et al. , Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat. Commun. 13, 4677 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden L. R., et al. ; COVE Study Group, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F. P., et al. ; C4591001 Clinical Trial Group, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan M. J., Pardi N., mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 73, 17–39 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Freyn A. W., et al. , A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 28, 1569–1584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nachbagauer R., et al. , A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Nachbagauer R., Krammer F., Albrecht R. A., A live-attenuated prime, inactivated boost vaccination strategy with chimeric hemagglutinin-based universal influenza virus vaccines provides protection in ferrets: A confirmatory study. Vaccines (Basel) 6, 47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon M., et al. , Vaccination with viral vectors expressing chimeric hemagglutinin, NP and M1 antigens protects ferrets against influenza virus challenge. Front. Immunol. 10, 2005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krammer F., Pica N., Hai R., Margine I., Palese P., Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87, 6542–6550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yassine H. M., et al. , Use of hemagglutinin stem probes demonstrate prevalence of broadly reactive group 1 influenza antibodies in human sera. Sci. Rep. 8, 8628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Easterbrook J. D., et al. , Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432, 39–44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohlbold T. J., Krammer F., In the shadow of hemagglutinin: A growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6, 2465–2494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couch R. B., Kasel J. A., Gerin J. L., Schulman J. L., Kilbourne E. D., Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 129, 411–420 (1974). [DOI] [PubMed] [Google Scholar]
- 25.Schulman J. L., Khakpour M., Kilbourne E. D., Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J. Virol. 2, 778–786 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohlbold T. J., et al. , Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 6, e02556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asthagiri Arunkumar G., et al. , Vaccination with viral vectors expressing NP, M1 and chimeric hemagglutinin induces broad protection against influenza virus challenge in mice. Vaccine 37, 5567–5577 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiers W., et al. , M2e-based universal influenza A vaccine. Vaccine 27, 6280–6283 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Schotsaert M., et al. , Long-lasting cross-protection against influenza A by neuraminidase and M2e-based immunization strategies. Sci. Rep. 6, 24402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krammer F., Novel universal influenza virus vaccine approaches. Curr. Opin. Virol. 17, 95–103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Filette M., et al. , Universal influenza A vaccine: Optimization of M2-based constructs. Virology 337, 149–161 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Corbett K. S., et al. , Design of nanoparticulate group 2 influenza virus hemagglutinin stem antigens that activate unmutated ancestor B cell receptors of broadly neutralizing antibody lineages. MBio 10, e02810–e02818 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreyfus C., et al. , Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadlbauer D., et al. , Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 366, 499–504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardi N., et al. , Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215, 1571–1588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laczkó D., et al. , A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53, 724–732.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freyn A. W., et al. , Antigen modifications improve nucleoside-modified mRNA-based influenza virus vaccines in mice. Mol. Ther. Methods Clin. Dev. 22, 84–95 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan H., et al. , The neuraminidase of A(H3N2) influenza viruses circulating since 2016 is antigenically distinct from the A/Hong Kong/4801/2014 vaccine strain. Nat. Microbiol. 4, 2216–2225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachbagauer R., Krammer F., Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 23, 222–228 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margine I., et al. , Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J. Virol. 87, 10435–10446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krammer F., et al. , Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J. Virol. 88, 3432–3442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beare A. S., Schild G. C., Craig J. W., Trials in man with live recombinants made from A/PR/8/34 (H0 N1) and wild H3 N2 influenza viruses. Lancet 2, 729–732 (1975). [DOI] [PubMed] [Google Scholar]
- 43.Belser J. A., et al. , Pathogenicity testing of influenza candidate vaccine viruses in the ferret model. Virology 511, 135–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuoka Y., et al. , Safety evaluation in chickens of candidate human vaccines against potential pandemic strains of influenza. Avian Dis. 47(3, suppl.)926–930 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez A., et al. , Attenuated strains of influenza A viruses do not induce degradation of RNA polymerase II. J. Virol. 83, 11166–11174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell P. J., et al. , The M segment of the 2009 pandemic influenza virus confers increased neuraminidase activity, filamentous morphology, and efficient contact transmissibility to A/Puerto Rico/8/1934-based reassortant viruses. J. Virol. 88, 3802–3814 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frank K., Paust S., Dynamic natural killer cell and T cell responses to influenza infection. Front. Cell. Infect. Microbiol. 10, 425 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margine I., et al. , H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J. Virol. 87, 4728–4737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krammer F., et al. , H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J. Virol. 88, 2340–2343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coughlan L., et al. , Heterologous two-dose vaccination with simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine 29, 146–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berthoud T. K., et al. , Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. 52, 1–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amanat F., Meade P., Strohmeier S., Krammer F., Cross-reactive antibodies binding to H4 hemagglutinin protect against a lethal H4N6 influenza virus challenge in the mouse model. Emerg. Microbes Infect. 8, 155–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margine I., Palese P., Krammer F., Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J. Vis. Exp. 6, e51112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krammer F., et al. , A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7, e43603 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stadlbauer D., et al. , SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 57, e100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baiersdörfer M., et al. , A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maier M. A., et al. , Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez-Sesma A., Schulman J. L., Moran T. M., A bispecific antibody recognizing influenza A virus M2 protein redirects effector cells to inhibit virus replication in vitro. J. Virol. 70, 4800–4804 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings in this publication are included in the article and SI Appendix. All study data are included in the article and/or SI Appendix.