Significance
Relapse to reward seeking progressively increases during abstinence, a behavioral phenomenon termed “incubation of craving.” Mechanistic studies of incubation can lead to novel relapse treatments. However, as previous studies primarily used rat models and targeted region-by-region analyses, a brain-wide functional atlas of incubation of reward seeking is lacking. We established a behavioral procedure for incubation of palatable food seeking in mice and performed whole-brain activity mapping using the neuronal activity marker Fos to identify the brain-wide signature of this incubation. Like rats, mice showed incubation of food seeking during abstinence. Using two complementary activity mapping approaches, we identified a brain-wide pattern of increased neural activation that mirrored incubation of food seeking after 60 but not 1 or 15 abstinence days.
Keywords: addiction, incubation, whole-brain analysis, mice, Fos
Abstract
Studies using rodent models have shown that relapse to drug or food seeking increases progressively during abstinence, a behavioral phenomenon termed “incubation of craving.” Mechanistic studies of incubation of craving have focused on specific neurobiological targets within preselected brain areas. Recent methodological advances in whole-brain immunohistochemistry, clearing, and imaging now allow unbiased brain-wide cellular resolution mapping of regions and circuits engaged during learned behaviors. However, these whole-brain imaging approaches were developed for mouse brains, while incubation of drug craving has primarily been studied in rats, and incubation of food craving has not been demonstrated in mice. Here, we established a mouse model of incubation of palatable food craving and examined food reward seeking after 1, 15, and 60 abstinence days. We then used the neuronal activity marker Fos with intact-brain mapping procedures to identify corresponding patterns of brain-wide activation. Relapse to food seeking was significantly higher after 60 abstinence days than after 1 or 15 days. Using unbiased ClearMap analysis, we identified increased activation of multiple brain regions, particularly corticostriatal structures, following 60 but not 1 or 15 abstinence days. We used orthogonal SMART2 analysis to confirm these findings within corticostriatal and thalamocortical subvolumes and applied expert-guided registration to investigate subdivision and layer-specific activation patterns. Overall, we 1) identified brain-wide activity patterns during incubation of food seeking using complementary analytical approaches and 2) provide a single-cell resolution whole-brain atlas that can be used to identify functional networks and global architecture underlying the incubation of food craving.
Studies using rodent models have shown that nonreinforced drug or food seeking progressively increases during abstinence in the homecage (1–3). This behavioral phenomenon of time-dependent increases in reward-seeking behavior was first identified in rats after cocaine self-administration and was termed “incubation of craving” (4–7). Since then, incubation of craving has been demonstrated for other drugs such as heroin (8), methamphetamine (9), alcohol (10), and nicotine (11), as well as for nondrug rewards such as sucrose (12, 13), standard chow pellets (14), and high-carbohydrate pellets (15). These preclinical behavioral findings inspired clinical studies that showed incubation of cue-induced drug craving and physiological responses in human drug users (16–18). We and others suggested that identifying brain mechanisms of incubation of craving in animal models is relevant to mechanisms of persistent relapse vulnerability to consumption of both palatable unhealthy food during dieting (3, 19) and addictive drugs in humans (1, 2, 20–22).
Immediate early gene (IEG, e.g. Fos, Arc, Zif) expression serves as a proxy for strongly activated neurons, and quantification of Fos-positive (Fos+) cells is routinely used to identify changes in neural activation patterns after exposure to different unconditioned and conditioned stimuli (23–27). Previous activity-mapping studies of incubation of drug and food craving have identified several brain regions (SI Appendix, Table S1) relevant to 1) relapse (increased Fos expression during relapse tests vs. homecage controls), and/or 2) incubation (higher Fos expression during late abstinence test vs. early abstinence test) (1, 3, 5, 28, 29). These studies used targeted one-by-one regional quantification of Fos+ cell counts in thin sectioned tissue samples and focused on changes in specific predetermined brain areas. Thus, it is currently unknown whether brain-wide activity patterns, including multiregional patterns, are altered during incubation of reward seeking during abstinence.
To address this knowledge gap, we leveraged recent developments in brain-wide activity mapping approaches, including whole mouse brain immunofluorescent staining and clearing (30–35), light sheet fluorescence microscopy (LSFM) (36, 37), and open-source analysis tools (38–41) to investigate changes in brain-wide activation patterns during incubation of palatable food seeking in mice.
We trained food-sated CD-1 male mice to self-administer palatable high-carbohydrate food pellets (42, 43) for 7 d and then tested them for relapse to food seeking after 1, 15, or 60 abstinence days. We perfused and extracted their brains 90 min after the relapse tests (or directly from homecage as a baseline activity control), labeled “active” Fos+ nuclei across intact mouse brains using an optimized iDISCO+ Fos immunofluorescent staining protocol (41) and imaged Fos immunofluorescence at single-cell resolution using LSFM. We used the ClearMap pipeline (39, 40) for unbiased mapping of “incubation-associated” neural activation patterns across the entire anterior–posterior (AP) axis of the mouse brain. We also updated the SMART analysis package (41) to conduct targeted analysis of neural activation patterns within LSFM coronal subvolumes and used SMART2 to cross-validate and extend our ClearMap findings within a subset of corticostriatal and thalamocortical brain regions and subdivisions.
Materials and Methods
Subjects.
We used male (n = 60) 4- to 6-mo-old sexually experienced CD-1 mice (Charles River Laboratories) weighing ∼40 g prior to food self-administration training. See SI Appendix for detailed Charles River Laboratories breeding procedures. We excluded 14 mice due to failure to acquire food self-administration. The mice had free access to food and water in the homecage and were maintained on a reverse 12-h:12-h light–dark cycle (light off at 8 AM). We only used male mice because the behavioral study was performed before the implementation of the NIH sex as a biological variable guideline.
Apparatus.
We trained and tested mice in Med Associates operant chambers equipped with a stainless-steel grid floor, a houselight, a retractable active lever, a nonretractable inactive lever, a bright yellow light-emitting diode light conditioned stimulus (CS), a pellet dispenser, and a food receptacle with a photobeam (see SI Appendix for details). Presses on the active lever (only extended during food self-administration sessions or food-seeking tests) resulted in delivery of 20-mg food pellets and a 2-s light CS, while presses on the inactive lever had no programmed consequences.
Behavioral Procedures.
The experimental timeline is shown in Fig. 1A. Details of the food self-administration procedure, abstinence phase, and relapse test are provided in their respective subheadings.
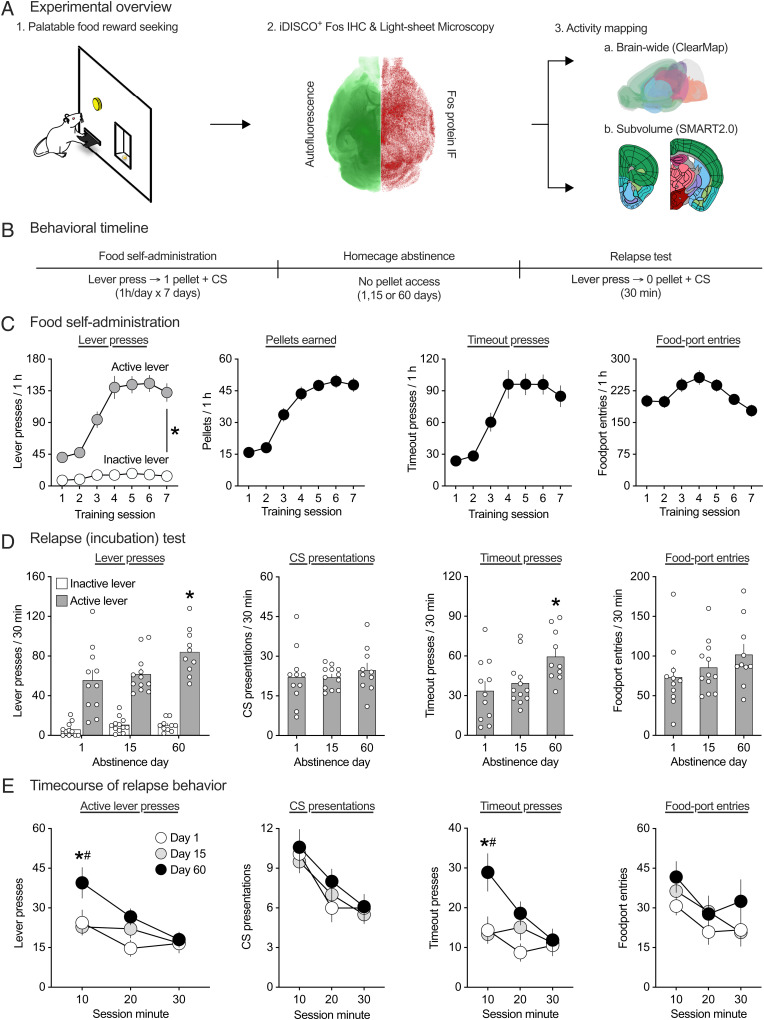
Fig. 1.
Incubation of palatable food seeking in male CD1 mice. (A) Experimental overview. (B) Timeline of food self-administration training, abstinence, and relapse tests. (C) Food self-administration training. Mice learned to self-administer palatable food pellets over seven sessions. Mean (± SEM) number of active and inactive lever presses (Left), food pellets earned (Middle Left), timeout presses (Middle Right), and food-port entries (Right) during each 1-h session. *Significant difference (P < 0.05) between active and inactive lever presses (n = 46). (D) Relapse (incubation) test. Responding on active but not inactive lever progressively increased during abstinence. Mean (± SEM) number of active and inactive lever presses (Left), CS presentations (Middle Left), timeout presses (Middle Right), and food-port entries (Right) during the 30-min relapse test session. *Significant difference (P < 0.05) from day 1. (E) Time course of relapse behavior. Binned 10-min time course of active and inactive lever presses (Left), CS presentations (Middle Left), timeout presses (Middle Right), and food-port entries (Right). *Significant difference (P < 0.05) from day 1. #Significant difference (P < 0.05) from day 15. See Dataset S1 for a detailed listing of all statistical outputs relating to this figure.
Food self-administration.
The food self-administration procedure is based on our previous study (43). Prior to the operant training sessions, we gave all mice one 30-min session of food-magazine training. During this session mice received 15 evenly spaced (every 2 min) deliveries of a 20-mg palatable food pellet (TestDiet, catalog no. 1811142, 12.7% fat, 66.7% carbohydrate, and 20.6% protein) paired with a 2-s discrete cue light (food-paired CS). Next, we trained the mice to lever press for palatable food reward during one 1-h session per day. The start of a session was signaled by the illumination of the house light followed 10-s later by the presentation of the central retractable active lever for 60 min. The houselight remained on for the duration of the session and served as a discriminative stimulus that signaled availability of the palatable food upon lever press.
Throughout the session, responses on the active lever were rewarded under a fixed-ratio-1 (FR1), 20-s timeout reinforcement schedule—active lever presses resulted in illumination of the food-paired CS for 2 s followed by the delivery of a palatable food pellet. Additional active responses during the 20-s timeout had no programmed consequence. Responses on the inactive lever had no programmed consequences throughout the session. We recorded 1) the total number of active lever presses, 2) the total number of inactive lever presses, 3) the total number of head entries into the food port, and 4) the total number of food pellet rewards earned during the entire session. We also calculated timeout responses (number of active lever presses—number of rewards delivered) for each session. Lever-press data for initial training sessions was not recorded for two mice due to technical malfunction so we recorded their values as zero to include them in statistical analysis. We gave mice at least seven training sessions to acquire stable food self-administration behavior before moving to the homecage forced abstinence phase.
We used the above mentioned TestDiet pellets, because both mice and rats prefer this pellet over other nutritional or flavor compositions and show reliable acquisition of food self-administration without any food deprivation (42, 44). Additionally, food-stated CD-1 male mice strongly preferred these food pellets over operant aggression self-administration (43), and food-sated male and female rats strongly prefer these pellets over methamphetamine, heroin, and fentanyl self-administration in rats (45, 46).
Forced abstinence.
We housed mice in individual cages in the animal facility for 1, 15, or 60 d with no access to palatable food pellets. We gave mice ad libitum access to regular chow and water during this phase and handled them once per week.
Relapse tests.
Following abstinence, we tested mice for nonreinforced food seeking during a 30-min relapse test. During the test, responses on the active lever resulted in presentation of the food-paired CS on the same FR1, 20-s timeout reinforcement schedule but were not reinforced with food pellets (extinction conditions). After the test, we returned mice to their homecage for 60 min prior to perfusions and brain tissue collection (n = 11 for day 1, n = 12 for day 15, and n = 10 for day 60). At each incubation timepoint, we also collected brains from food-trained mice directly from the homecage (n = 4 for homecage day 1, n = 6 for homecage day 15, n = 3 for homecage day 60) and collapsed them into a single group (Homecage, n = 13) to serve as baseline controls for whole-brain analysis. We matched mice from the four groups for food-reinforced responding during the training phase. We recorded the total number of 1) active lever presses, 2) inactive lever presses, 3) head entries into the food port, and 4) CS presentations during the entire session. We also calculated timeout responses (number of active lever presses − number of rewards delivered) made during the relapse test sessions.
Whole-Brain Fos Immunohistochemistry.
We perfused mice and collected brains 90 min after the start of the relapse tests (or directly from the homecage) and used a modified version of the iDISCO+ protocol for intact mouse brain Fos immunohistochemistry (41). We processed 37 brains across the four groups following perfusions (n = 11 for Homecage, n = 9 for day 1, n = 9 for day 15, and n = 8 for day 60) based on perfusion quality, level of intactness, and behavioral data. Detailed sample collection, pretreatment, immunolabeling, and clearing steps are provided in SI Appendix.
Whole-Brain Imaging and Analysis.
We imaged stained and cleared intact mouse brains in coronal orientation using LSFM (see SI Appendix for detailed image acquisition parameters). We excluded three brains due to insufficient clearing and staining and two brains due to technical issues during image acquisition. We used the three-dimensional rendering software Arivis Vision 4D (3.0.0) to stitch image tiles, manually corrected coronal alignment where necessary, and exported the images as TIFF files for whole-brain analysis. During sample preprocessing steps, we observed that illumination of the entire coronal plane by the light sheet during the first imaged tile scan led to significant photobleaching of the second imaged tile in all samples. Therefore, we only used the first imaged hemisphere from each sample for analysis and mirrored outputs for all visualizations.
We analyzed 32 brains across the four groups (n = 11 for Homecage, n = 8 for day 1, n = 8 for day 15, and n = 5 for day 60) using the ClearMap analysis pipeline (40) and an updated version of the SMART (41) package as described in SI Appendix. We provide the updated SMART2 package repository (https://github.com/sgoldenlab/SMART2) and include a Docker installation image for rapid and user-friendly installation of SMART2 (https://hub.docker.com/repository/docker/goldenneurolab/wholebrain_smart2).
Statistical Analyses.
We analyzed behavioral and whole-brain Fos data using IBM SPSS Statistics (version 28) and GraphPad Prism (version 9.3). We tested the data for sphericity and homogeneity of variance when appropriate. When the sphericity assumption was not met, we adjusted the degrees of freedom using the Greenhouse–Geisser correction. For the behavioral analysis, alpha (significance) level was set at 0.05, two-tailed. For whole-brain analysis, when screening for significant regions, we corrected for multiple comparisons using Benjamini and Hochberg’s false discovery rate (FDR) correction method (implemented via R p.adjust() function in SPSS) and set alpha (significance) level at 0.1, two-tailed. For between-group comparisons within significant regions, we used Tukey’s honestly significant difference (HSD) post hoc test and set alpha (significance) level at 0.05, two-tailed. Because our analyses yielded multiple main effects and interactions, we report only those that are critical for data interpretation. See SI Appendix, Table S2 for a listing of number of subjects/samples included in each phase of the study and Datasets S1–S4 for raw data (analyzed behavioral outputs, raw counts tables, and z-scored counts tables), statistical results, and summary statistics corresponding to Figs. 1–4.
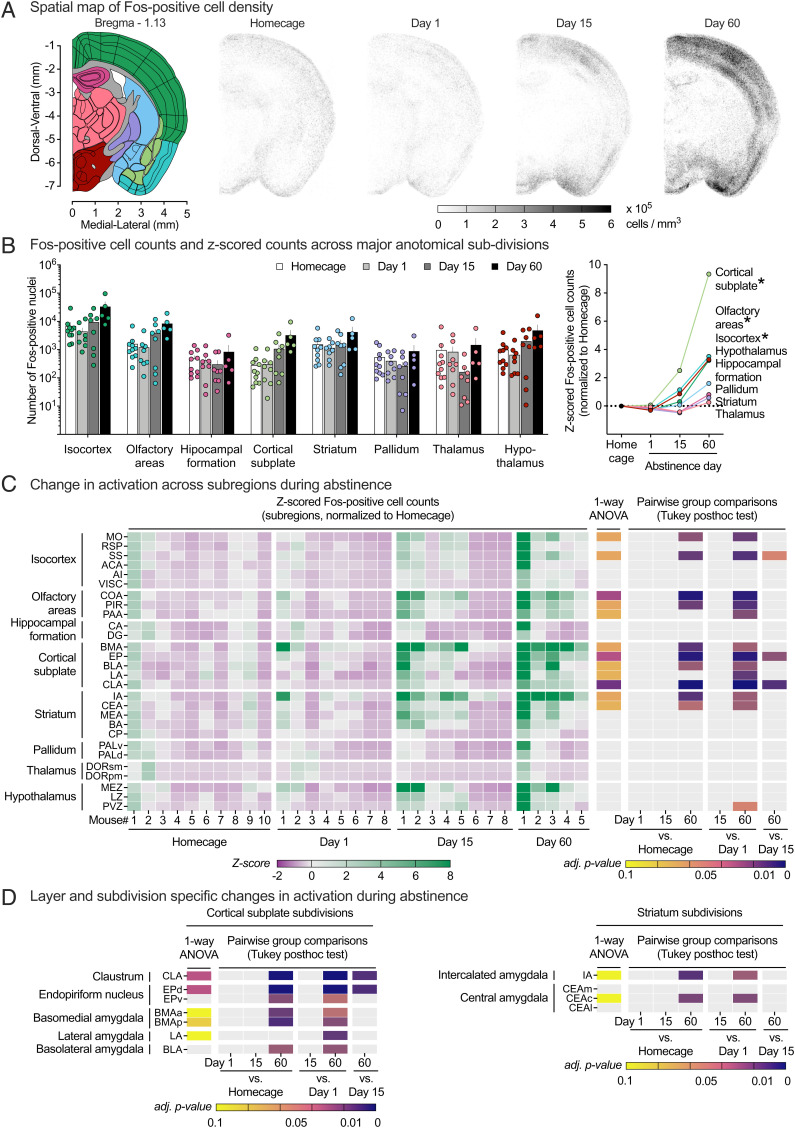
Fig. 4.
Targeted analysis of thalamocortical coronal subvolume (AP −1.08 to AP −1.28 relative to Bregma) using SMART2. (A) Spatial map of Fos+ cell density. Grayscale intensity of individual points (20- × 20- × 200-μm voxel) represents mean cell density in cells per mm3 within AP −1.08 to AP −1.28 coronal subvolume. (B, Left) Fos+ cell counts for eight major anatomical regions within the subvolume. (B, Right) Z-score-normalized counts (normalized to homecage group distribution) for five major anatomical regions within the subvolume. *Significant differences (FDR-adjusted P < 0.1) one-way ANOVAs. (C) Changes in activation across the subvolume. Fos+ cell counts for each region are z-score-normalized to homecage group distribution for statistical analysis. Heat map of individual z-scored Fos+ cell counts (Left), one-way ANOVA FDR-adjusted P values (Middle), and Tukey’s HSD pairwise group comparisons P values (Right) for 28 subregions within the analyzed coronal subvolume. Subregions are organized by eight parent anatomical subdivisions and ranked in descending order of mean day 60 group activation level. (D) Layer- and subdivision-specific changes in activation. Heat map of one-way ANOVA FDR-adjusted P values and Tukey’s HSD pairwise group comparison P values for selected subregion layers or subdivisions within cortical subplate (Left) or striatum (Right). See Dataset S4 for a detailed listing of all statistical results related to this figure.
Behavior.
We analyzed four behavioral measures during food self-administration training: 1) the total number of lever presses on active and inactive lever (denoted as lever presses), 2) the total number of food pellet rewards earned (denoted as rewards during the training phase and as CS presentations during the relapse tests), 3) the total number of timeout lever presses (denoted as timeout presses), and 4) the total number of head entries into the food receptacle (denoted as food-port entries). Following training, we tested separate groups of mice for palatable food reward seeking after 1, 15, or 60 d of abstinence and analyzed nonreinforced responding (lever presses) during the 30-min food-seeking test. We describe the within- and between-subjects factors in the mixed ANOVAs we used to analyze the behavioral data in Results and provide all analyzed behavioral outputs and statistical results in Dataset S1.
ClearMap analysis.
We processed 32 brains using the ClearMap pipeline, of which 3 failed the registration to the reference atlas. We analyzed the remaining 29 brains using ClearMap (n = 9 for Homecage, n = 7 for day 1, n = 8 for day 15, and n = 5 for day 60). We computed Fos+ cell counts within regions at two levels of the atlas hierarchy: 1) 10 major anatomical divisions and 2) 56 subregions across the brain based on hierarchical relationships defined in the Allen Mouse Common Coordinate Framework (47). We selected regions within each level such that there were no parent–child relationships and/or overlapping spatial footprints between them. We transformed the raw Fos+ cell counts to Z-scores prior to the statistical analyses to normalize the data and account for differences in volume across regions of interest. We computed Z-scores for each region of interest relative to the homecage group’s values using the formula z = (x – μ)/σ, where x is the sample Fos+ cell count, μ is the mean Fos+ cell count of the homecage group, and σ is the SD of the homecage group. For each level, we first used two-way mixed ANOVA (GLM procedure in SPSS) with the within-subject factor of region and the between-subjects factor of group (Homecage, day 1, day 15, day 60). Significant main effect and interaction results were followed up with one-way ANOVAs within each region. We used the FDR correction method for multiple comparison correction across all analyzed regions and set alpha (significance) level at 0.1, two-tailed. Within regions that passed this initial screen, we used the Tukey’s HSD test for post hoc comparisons between groups with alpha (significance) level at 0.05, two-tailed. We provide raw counts data, Z-scored data, and statistical results (F values, uncorrected P values, FDR, or Tukey’s adjusted P values, and estimates of effect size) for all analysis pertaining to ClearMap in Dataset S2.
SMART2 analysis.
In addition to the 29 brains used for ClearMap analysis, we used manual registration correction within each subvolume to recover and analyze brains that failed ClearMap registration. We analyzed 32 brains across the four groups (n = 11 for Homecage, n = 8 for day 1, n = 8 for day 15, and n = 5 for day 60) for subvolume 1 and 31 brains across the four groups (n = 10 for Homecage, n = 8 for day 1, n = 8 for day 15, and n = 5 for day 60) for subvolume 2. We extracted Fos+ cell counts within regions of interest at three levels of the atlas hierarchy: 1) major anatomical regions (subvolume 1: five regions; subvolume 2: eight regions), 2) main subregions (subvolume 1: 18 subregions; subvolume 2: 28 regions), and 3) internal subdivisions within the subregions (subvolume 1: 43 subdivisions; subvolume 2: 64 subdivisions). Similar to ClearMap analysis, we selected regions within each level such that there were no parent–child relationships and/or overlapping spatial footprints between them. We transformed the raw Fos+ cell counts to Z-scores prior to statistical testing. For each level, we first used two-way mixed ANOVA (GLM procedure in SPSS) with the within-subject factor of region and the between-subjects factor of group (Homecage, day 1, day 15, day 60). We followed up on significant main effects and interactions with one-way ANOVAs within each region. We used the FDR correction method for multiple comparison correction across all analyzed regions and set alpha (significance) level at 0.1, two-tailed. Within regions that passed this initial screen, we used Tukey’s HSD test for post hoc comparisons between groups with alpha (significance) level at 0.05, two-tailed. We also generated spatial maps of Fos+ cell densities (mean across group) for each subvolume to aid visualization of differences in activation patterns between groups. We provide raw counts data, Z-scored data, and statistical results (F values, uncorrected P values, FDR, or Tukey’s adjusted P values, and estimates of effect size) for all analysis pertaining to SMART2 subvolumes 1 and 2 in Datasets S3 and S4, respectively.
Results
The experimental design and timeline of behavioral training, intact mouse brain activity labeling, and brain-wide activity mapping is shown in Fig. 1 A. See SI Appendix, Table S2 for a listing of number of subjects/samples included in each phase of the study.
Incubation of Palatable Food Seeking in Male CD1 Mice.
We determined whether the time-dependent increases in food seeking during abstinence (incubation of food craving), previously observed in rats (3, 19), generalize to mice. The experimental timeline is shown in Fig. 1 A and B. We trained food-sated CD-1 male mice to lever press for high-carbohydrate food pellets for 7 d. Next, we tested different groups for relapse to food seeking in the presence of contextual (food self-administration chamber) and discrete (light cue paired with food delivery) cues after 1, 15, or 60 d of homecage forced abstinence. Statistical outputs for all analyses pertaining to training and relapse tests are provided in Dataset S1.
Training phase.
The mice showed reliable food self-administration as indicated by increased responding on the food-paired lever during the daily sessions (Fig. 1 C). The repeated-measures ANOVA of rewards, which included the within-subjects factor of training session (sessions 1 to 7), showed a significant effect of this factor (F3.2,144.7 = 67.2, P < 0.001). The repeated measures ANOVA of lever presses, which included the within-subjects factors of training session and lever (inactive, active), showed a significant interaction between the two factors (F3.6,160.3 = 28.9, P < 0.001). The repeated-measures ANOVA of timeout presses, which included the within-subjects factor of training session (sessions 1 to 7), showed a significant effect of this factor (F3.5,158.3 = 24.2, P < 0.001). The repeated-measures ANOVA of food-port entries, which included the within-subjects factor of training session (sessions 1 to 7), showed a significant effect of this factor (F3.0,136.9 = 6.3, P < 0.001).
Relapse (incubation) tests.
Nonreinforced presses on the previously active lever were significantly higher after 60 abstinence days than after 1 or 15 d (Fig. 1 D, Left), indicating “incubation of food craving.” The mixed ANOVA of total lever presses, which included the between-subjects factor of abstinence day (days 1, 15, and 60) and lever (active, inactive), showed a significant interaction between the two factors (F2,30 = 3.4, P = 0.045). This incubation effect was primarily due to higher active lever presses in the day-60 group during the first 10 min of the test session (Fig. 1 E, Left). Additionally, one-way ANOVA of total timeout presses (Fig. 1 D, Middle Right) with between-subjects factor of abstinence day was significant (F2,30 = 4.6, P = 0.017), indicating higher timeout responding in the day-60 group than in day-1 and day-15 groups, specifically during the first 10 min of the test session (Fig. 1 E, Middle Right). There were no significant differences in the total number of CS presentations (F2,30 = 0.37, P = 0.694) or food-port entries (F2,30 = 1.4, P = 0.257) between the three groups. Post hoc group differences (Tukey’s HSD test) are depicted in Fig. 1 D and E (* denotes significant difference vs. day 1; # denotes significant difference vs. day 15).
Unbiased Intact Brain-Wide Activity Mapping of Incubation of Palatable Food Seeking.
We processed brains using the ClearMap pipeline and extracted Fos+ cell counts within regions of interest (ROIs) at two levels of the atlas hierarchy: 1) 10 major anatomical divisions (Fig. 2 A and B, Left ) and 2) 56 subregions of interest (Fig. 2 B, Center and Right) based on hierarchical relationships defined in the Allen Mouse Common Coordinate Framework (47). We selected regions within each level such that there were no parent–child relationships and/or overlapping spatial footprints between them and performed z-score normalization (relative to homecage group mean) for each ROI prior to statistical analyses (Fig. 2 B). Detailed statistical reporting of ClearMap analysis at all levels is provided in Dataset S2.
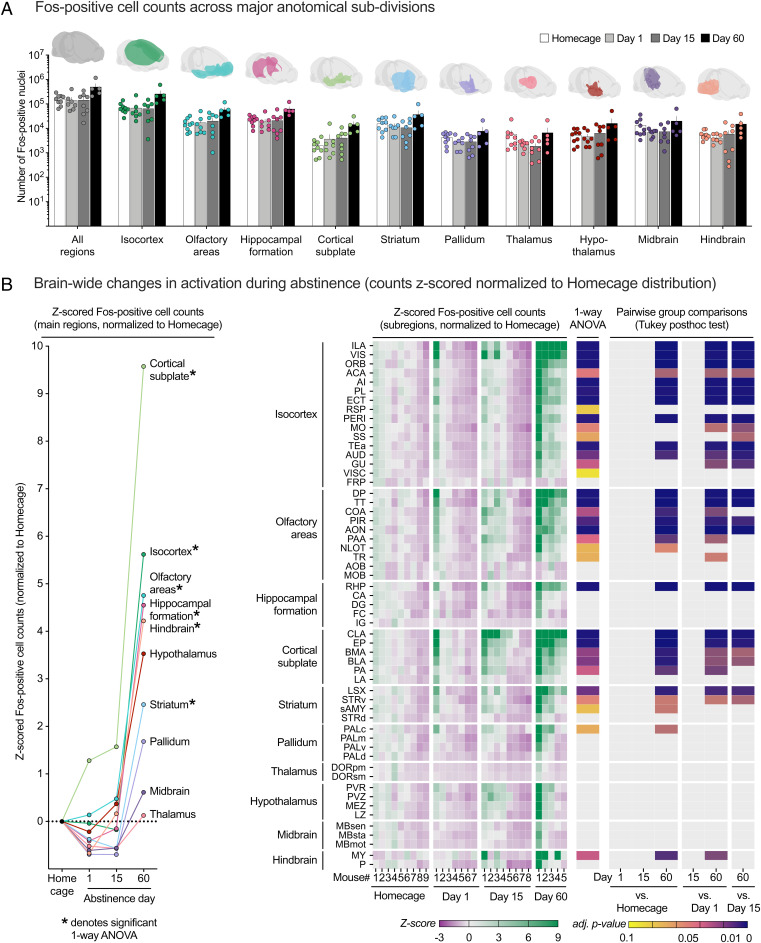
Fig. 2.
Unbiased brain-wide activity mapping of incubated relapse to palatable food seeking using ClearMap. (A) Mean (± SEM) Fos+ cell counts across the whole brain and for 10 major anatomical subdivisions. (B) Brain-wide changes in activation during abstinence. Raw Fos+ cell counts for each region are z-score-normalized to the homecage group distribution for statistical analysis. Mean z-scored cell counts for 10 major anatomical subdivisions showing time-dependent changes in activation pattern in multiple brain regions induced by the relapse tests (Left). *Significant differences (FDR-adjusted P < 0.1) one-way ANOVA. Heat map of individual z-scored Fos+ cell counts, one-way ANOVA FDR-adjusted P values, and Tukey’s HSD pairwise group comparison P values for 56 subregions across the analyzed brain volume (Right). Individual data are sorted by group and ranked in descending order of activation level within each group. Subregions are organized by 10 parent anatomical subdivisions and ranked in descending order of mean day-60 group activation level. See Dataset S2 for a detailed listing of all statistical results associated with this figure.
Main regions.
For Level 1, the mixed ANOVA, using the between-subjects factor of group (homecage, day 1, day 15, day 60) and the within-subjects factor of region, showed significant main effects of group (F3,25 = 4.4, P = 0.013) and region (F1.9,47.7 = 18.2, P < 0.001) and an interaction between the two factors (F5.7,47.7 = 6.1, P < 0.001). Follow-up one-way ANOVAs (between-subjects factor group) were significant (FDR-adjusted P < 0.1) for 6 out of 10 tested regions (denoted by * in Fig. 2 B, Left). These data indicate increased brain-wide activation after the relapse test after 60 abstinence days, but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Subregions.
For Level 2, the mixed ANOVA showed significant main effects of group (F3,25 = 6.0, P = 0.003), and subregion (F1.8,45.3 = 18.7, P < 0.001) and an interaction between the two factors (F5.4,45.3 = 8.0, P < 0.001). Follow up one-way ANOVAs (between-subjects factor group) were significant (FDR-adjusted P < 0.1) for 34 out of 56 tested subregions, reflecting selective increased activation following relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05). One-way ANOVA FDR-adjusted P values for all tested subregions and Tukey’s HSD pairwise post hoc comparison P values for all tested subregions are shown as heat maps in Fig. 2 B, Right.
The most activated areas were isocortex (15 of 16 subregions), cortical subplate (5 of 6 subregions), olfactory area (8 of 10 subregions), and striatum (3 of 4 subregions), collectively designated as “corticostriatal,” with sparse subregional activation in the retrohippocampal region of the hippocampus and medulla of the hindbrain. Increased Fos expression in the day-60 group was not observed in subregions of thalamus, hypothalamus, and midbrain structures.
Targeted Analysis of the Corticostriatal Coronal Subvolume Using SMART2.
We processed a 200-μm-thick coronal subvolume spanning AP +1.55 to AP +1.75 relative to Bregma using the SMART2 pipeline. Spatial maps of groupwise Fos+ cell densities within the subvolume are shown in Fig. 3 A to aid visualization of differences in activation patterns between groups. We extracted Fos+ cell counts within regions of interest (ROIs) at three levels of the atlas hierarchy: 1) 5 major anatomical regions (Fig. 3 B), 2) 18 main subregions (Fig. 3 C), and 3) 43 internal subdivisions within the subregions (Fig. 3 D). We performed z-score normalization (relative to homecage mean) for each ROI prior to statistical analysis. One-way ANOVA FDR-adjusted P values and Tukey’s HSD pairwise post hoc comparison P values for all Level 2 ROIs and a subset of Level 3 ROIs are shown as heat maps in Fig. 3 C and D. Detailed statistical reporting of SMART2 analysis for this subvolume is provided in Dataset S3.
Fig. 3.
Targeted analysis of corticostriatal coronal subvolume (AP +1.55 to AP +1.75 relative to Bregma) using SMART2 (A) Spatial map of Fos+ cell density. Grayscale intensity of individual points (20- × 20- × 200-μm voxel) represents mean cell density in cells per mm3 within AP +1.55 to AP +1.75 coronal subvolume. (B, Left) Fos+ cell counts for five major anatomical regions within the subvolume. (B, Right) Z-score-normalized counts (normalized to homecage group distribution) for five major anatomical regions within the subvolume. *Significant differences (FDR-adjusted P < 0.1) one-way ANOVAs. (C) Changes in activation across the subvolume during abstinence. Fos+ cell counts for each region are z-score-normalized to homecage group distribution for statistical analysis. Heat map of individual z-scored Fos+ cell counts (Left), one-way ANOVA FDR-adjusted P values (Middle), and Tukey’s HSD pairwise group comparisons P values (Right) for 18 subregions within the analyzed coronal subvolume. Subregions are organized by five parent anatomical subdivisions and ranked in descending order of mean day-60 group activation level. (D) Layer- and subdivision-specific changes in activation. Heat map of one-way ANOVA FDR-adjusted P values and Tukey’s HSD pairwise group comparison P values for selected subregion layers or subdivisions within isocortex (Left) or olfactory areas (Right). See Dataset S3 for a detailed listing of all statistical results associated with this figure.
Main regions.
For Level 1, the mixed ANOVA showed significant main effects of region (F1.6,4.7 = 6.5, P = 0.006) and group (F3,28 = 6.9, P = 0.001) and an interaction between the two factors (F4.7,44.0 = 4.7, P = 0.002). Follow-up one-way ANOVAs (FDR-adjusted P < 0.1) were significant for all five tested regions, reflecting increased activation following the relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Subregions.
For Level 2, the mixed ANOVA showed significant main effects of subregion (F1.2,32.7 = 9.5, P = 0.003) and group (F3,28 = 7.2, P = 0.001) and an interaction between the two factors (F3.5,32.7 = 6.3, P = 0.001). Follow-up one-way ANOVAs (FDR-adjusted P < 0.1) were significant for all 18 tested subregions, reflecting increased activation following the relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Subdivisions.
For Level 3, the mixed ANOVA showed significant main effects of subdivision (F1.2,32.2 = 10.3, P = 0.002) and group (F3,28 = 7.1, P = 0.001) and an interaction between the two factors (F3.5,32.2 = 6.5, P = 0.001). Follow-up one-way ANOVAs (FDR-adjusted P < 0.1) were significant for all 43 tested subdivisions, reflecting increased activation following the relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Targeted Analysis of the Thalamocortical Coronal Subvolume Using SMART2.
We processed a 200-μm-thick coronal subvolume spanning AP −1.08 to AP −1.28 relative to Bregma using the SMART2 pipeline. Spatial maps of groupwise Fos+ cell densities within the subvolume are shown in Fig. 4 A to aid visualization of differences in activation patterns between groups. We extracted Fos+ cell counts within ROIs at three levels of the atlas hierarchy: 1) 8 major anatomical regions (Fig. 4 B), 2) 28 main subregions (Fig. 4 C), and 3) 64 internal subdivisions within the subregions (Fig. 4 D). One-way ANOVA FDR-adjusted P values and Tukey’s HSD pairwise post hoc comparison P values for all Level 2 ROIs and a subset of Level 3 ROIs are shown as heat maps in Fig. 4 C and D. Detailed statistical reporting of SMART2 analysis for this subvolume is provided in Dataset S4.
Main regions.
For Level 1, the mixed ANOVA showed significant main effects of region (F1.2,32.3 = 14.0, P < 0.001) and group (F3,27 = 3.2, P = 0.040) and an interaction between the two factors (F3.6,32.3 = 5.5, P = 0.002). Follow-up one-way ANOVAs (FDR-adjusted P < 0.1) were significant for three of eight tested regions, reflecting increased activation following the relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Subregions.
For Level 2, two-way ANOVA showed significant main effects of subregion (F1.1,30.8 = 9.5, P = 0.003) and group (F3,27 = 3.4, P = 0.033) and an interaction between the two factors (F3.4,30.8 = 3.5, P = 0.024). Follow-up one-way ANOVAs (FDR-adjusted P < 0.1) were significant for 12 of 18 tested subregions, reflecting increased activation following relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Subdivisions.
For Level 3, the mixed ANOVA showed significant main effects of region (F1.2,31.2 = 7.8, P = 0.007) and group (F3,27 = 3.1, P = 0.044) and an interaction between the two factors (F3.5,31.2 = 3.0, P = 0.038). Follow-up one-way ANOVAs (FDR-adjusted P < 0.1) were significant for 15 of 64 tested subdivisions, reflecting increased activation following the relapse test after 60 but not 1 or 15 d of abstinence (Tukey’s HSD P < 0.05).
Discussion
We used unbiased intact-brain mapping of Fos expression to investigate brain-wide activation patterns during incubation of palatable food seeking in mice. Relapse to food seeking was higher after 60 abstinence days than after 1 or 15 d, indicating incubation of food seeking. More importantly, unbiased whole-brain analysis of Fos expression using ClearMap showed a strong induction of neural activity across multiple brain regions that mirrored incubation of food seeking after 60, but not 15, days of abstinence. Targeted coronal slice analysis of Fos expression using SMART2 replicated and validated the time-dependent increases in activation patterns within corticostriatal and thalamocortical subvolumes and enabled detailed analysis of subdivision and layer-specific changes during abstinence. Overall, our data indicate that incubation of palatable food craving correlates with widespread activation of many brain regions beyond those previously implicated in incubation of food or drug craving (SI Appendix, Table S1).
Outbred Mice Show Incubation of Palatable Food Seeking Like Outbred Rats.
Food seeking in the male mice increased following 60 but not 1 or 15 d of abstinence from food self-administration training. Previous studies in rats reported incubation of food seeking but with a different time course; robust incubation of sucrose craving was observed in rats after 7, 15, and 30 abstinence days but not 60 d (5). Incubation of food craving has also been reported in rats trained to self-administer standard chow pellets after 30 abstinence days (14) and high-carbohydrate pellets after 21 abstinence days (15). By extending the model to mice we were able to leverage mouse-optimized procedures to investigate brain-wide activation patterns of incubation of food craving. [Note: Consistent with the previous food and drug incubation literature (1, 19), we use the term “incubation of food craving” neither as an explanatory construct nor as a pathological state but as a descriptor of the behavioral phenomenon of time-dependent increases in reward seeking during abstinence.]
Notably, we used outbred CD-1 mice in these experiments rather than more commonly used inbred C57BL/6J. In our experience, outbred and hybrid mice exhibit a more complex spectrum of behavior and acquire learned behavior more robustly than their inbred counterparts(44, 48–50). Additionally, an inbred genetic background limits the generalizability of genotype–phenotype relationships (51) as inbred mice display highly heritable and uniform strain-specific phenotypes in brain volume, scalar diffusion tensor imaging metrics, and quantitative connectomes (52). Similarly, contrary to the assumed relationship, meta-analysis of coefficients of variation do not find evidence of greater trait stability in inbred mice than outbred mice, and hybrid mice show enhanced properties desired for neurobiological research such as reduced anxiety-like behavior, improved learning, and enhanced long-term spatial memory (53). Taken within the context of developing a resource whole-brain atlas for incubation of food craving in mice, outbred populations likely provide a more generalizable and robust platform.
Finally, we used only male CD-1 mice in this study because our behavioral experiments were conducted before the NIH sex as a biological variable mandate. We acknowledge the importance of developing animal models that incorporate both sexes and have done so in recent behavioral and neurobiological studies on incubation of craving and other relapse-related models (21, 54, 55). An important question for future research is whether a similar time course of incubation of food craving (and pattern of brain activation) will be observed in female CD-1 mice.
Brain-Wide Patterns of Increased Neural Activation following Incubation of Food Seeking during Abstinence.
Previous targeted region-by-region analyses in rats have identified several brain regions that are activated during incubation of food and drug craving during abstinence (1, 3, 21, 28, 29, 56). However, an unbiased brain-wide interrogation of regions engaged during “incubated” reward seeking has not previously been performed. We used a modified version of the iDISCO+ procedure to label active (Fos+) neurons in intact mouse brains and employed light-sheet fluorescence microscopy to image these potentially behaviorally relevant neurons across the entire brain volume at single-cell resolution. Our unbiased ClearMap analysis revealed a time-dependent increase in activation of multiple brain regions (e.g., prelimbic, infralimbic, orbitofrontal, and insular cortices, central amygdala and basolateral amygdala, ventral and dorsal striatum) similar to that shown in rats following incubation of food and drug seeking (57–63) (SI Appendix, Table S1). This pattern of time-dependent increase of neural activity was not restricted to these previously identified incubation-related regions. Indeed, over half of the tested regions (6 out of 10 main anatomical divisions and 34 of 56 subregions), including most regions within the isocortex, olfactory areas, cortical subplate, and striatum, showed increased activation after 60 but not 15 abstinence days.
Two recent studies used a similar unbiased approach to investigate brain-wide activation (assessed by Fos) patterns of short-term alcohol abstinence after two-bottle choice and chronic intermittent ethanol vapor exposure (64) and acute withdrawal following experimenter-administered psychostimulants (65). They used hierarchical clustering techniques to demonstrate a strong decrease in modularity after abstinence/withdrawal compared to drug-naïve controls and employed graph theory approaches to identify hub regions that might drive this functional restructuring. It is possible that this observed decrease in modularity might be a result of the recruitment of networks of regions like that seen in our study following food seeking in mice. However, it is important to note that in these studies brains were collected directly from homecage and not after behavioral testing. Thus, the observed changes likely reflect shifts in “resting-state” functional brain architecture and not behaviorally evoked differential network engagement during reward seeking.
Targeted Subvolume Analysis Reveals Variations in Time-Dependent Brain Activation Patterns.
We followed-up on our global volumetric ClearMap findings by analyzing activation patterns within two coronal volumes using an updated version of the SMART pipeline (41). This approach allowed us to use expert-guided registration and Fos-segmentation to 1) isolate ClearMap effects to specific coronal plates along the AP axis, 2) validate our data against previous incubation studies that used selected coronal slices for Fos-mapping, 3) include brains that failed ClearMap due to physical damage during processing, and 4) extend our analysis to subdivisions and layers within these subvolumes for future mechanistic investigation.
In agreement with the ClearMap analyses, SMART2 analysis identified several subregions with increased activation on abstinence day 60 within the isocortex, olfactory areas, cortical subplate, and striatum, several of which have been previously identified after incubation of food (e.g., prelimbic cortex, infralimbic cortex, orbitofrontal cortex, basolateral amygdala, central amygdala nucleus, nucleus accumbens, somatosensory cortex) and drug seeking (e.g., prelimbic cortex, infralimbic cortex, agranular insular area, basolateral amygdala, central amygdalar nucleus, nucleus accumbens) in rat models (57–63, 66–68) (SI Appendix, Table S1).
However, while some identified subregions showed similar increases in activation across both subvolumes (e.g., somatomotor and somatosensory areas, claustrum, piriform area) others were only present in one subvolume (e.g., prelimbic and infralimbic cortex, basolateral and central amygdala) or showed differential engagement along the AP axis (e.g., anterior cingulate and agranular insular cortices). Even within a subvolume and subregion, activation patterns were not uniform but sometimes graded across layers (e.g., layers of piriform area, dorsal peduncular area, infralimbic and prelimbic areas) or isolated to specific subdivisions (e.g., central but not medial or lateral subdivisions of central amygdala), suggesting different degrees of engagement across multiple brain regions and likely circuits after 60 abstinence days.
Putative Mechanisms Underlying the Time-Dependent Increase in Brain-Wide Activation.
One interpretation of the time-dependent brain-wide activation observed on abstinence day 60 is time-dependent increases in food context-induced nonspecific generalized arousal after prolonged abstinence. However, the mice of the day-60 group discriminated the active lever from the inactive lever and were not statistically different from the other groups in either CS presentations or food-port entries. In contrast, the day-60 group showed a significant increase in timeout lever presses following CS presentations. These results suggest that the incubation-related behavior and associated increases in brain-wide activation are potentially driven by time-dependent increases in the incentive value of the previously food-paired CS. This interpretation is consistent with a previous study showing that the conditioned response to CSs previously paired with cocaine, heroin, and sucrose progressively increases during abstinence (69).
The emergence of incubation-associated brain-wide activation on abstinence day 60 may be driven by 1) global time-dependent changes of neuronal sensitivity and/or 2) local time-dependent engagement of critical “hub” region(s) to produce the proposed increase of incentive value for the food-paired CS. Regarding the first idea, repeated neuronal excitation during food self-administration could initially produce a homeostatic decrease in neuronal sensitivity resulting in low CS-induced activation on abstinence day 1 and 15. Gradual recovery of neuronal sensitivity to basal levels following 60 days of low activity in the homecage during abstinence could explain the robust induction of brain-wide activation during the day 60 seeking test (2, 70–73).
Regarding the second idea, one potential “hub” region is the central amygdala, identified in the present study as a region selectively activated during the day-60 relapse test and known to be involved in incubation of drug craving across drug classes (58, 62, 74). Another potential “hub” region is the claustrum, a small nucleus within the cortical subplate that showed increased Fos expression after 60 abstinence days, has reciprocal connections with several reward-related brain regions (75, 76), and has been shown to be involved in a broad range of cognitive functions, including coordinating responses to reward cues (77, 78).
Conclusions
We demonstrated that incubation of palatable food reward seeking is accompanied by an induction of neural engagement in multiple brain regions, many of which extend beyond the traditional brain areas and circuits involved in incubation of food and drug craving. We extended the rat incubation of food-seeking model to male CD1 mice and leveraged mouse-specific unbiased whole-brain staining, clearing, and analysis pipelines to generate a single-cell resolution whole-brain atlas of incubation food seeking during prolonged abstinence. The results of our study suggest that the overarching neural mechanism underlying incubation of reward seeking is more anatomically widespread than suggested by the published literature and likely not localized to a particular brain area or circuit. Whatever neural mechanisms mediate incubation of reward seeking, our findings suggest that these mechanisms affect acute neural responses throughout the brain, either through widespread alterations in all these brain areas or in key brain areas that regulate brain-wide circuitry. Finally, the “incubation atlas” here provides a mineable dataset for better understanding system-level alterations related to incubation of food craving and relapse, a behavioral phenomenon with potential relevance to persistent relapse vulnerability to unhealthy eating habits during dieting and upon exposure to conditioned cues previously paired with palatable foods.
Supplementary Material
Acknowledgments
We thank members of the B.T.H., Y.S., and S.A.G. laboratories for their support and insight during all stages of this study. We also thank Dr. Jennifer M. Bossert and Dr. Ida Fredriksson for assistance with whole-brain immunohistochemical assays and Dr. Carlos A. Mejias-Aponte and Vadim Kashtelyan for assistance with sample processing and light sheet fluorescence microscopy. The research was supported by National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) funds to the laboratories of Y.S. and B.T.H. S.A.G. received funding from NIH Postdoctoral Research Associate Training (PRAT) 1FI2GM117583-01, NIH grants NIDA R00DA045662, NIDA R01DA054317, NIDA 1UG3DA053802, NIDA P30DA048736, and National Alliance for Research on Schizophrenia & Depression Young Investigator Award 27082. R.M. received funding from the NIH Center for Compulsive Behaviors. E.R.S. received funding from the Washington Research Foundation Postdoctoral Fellowship Program. O.R.D. and D.Q.P. were supported by the NIDA IRP Scientific Director’s Fellowship for Diversity in Research.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2209382119/-/DCSupplemental.
Data, Materials, and Software Availability
Images have been deposited in the publicly accessible data repository Figshare (https://figshare.com/articles/media/c-Fos_whole-brain_image_stacks/21207845) (79). All other study data are included in the article (Figs. 1–4) and/or supporting information (SI Appendix, and Supplemental Datasets S1–S4).
References
- 1.Pickens C. L., et al. , Neurobiology of the incubation of drug craving. Trends Neurosci. 34, 411–420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf M. E., Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 17, 351–365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm J. W., Incubation of food craving in rats: A review. J. Exp. Anal. Behav. 113, 37–47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm J. W., Hope B. T., Wise R. A., Shaham Y., Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412, 141–142 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L., Grimm J. W., Hope B. T., Shaham Y., Incubation of cocaine craving after withdrawal: A review of preclinical data. Neuropharmacology 47 (suppl. 1), 214–226 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Neisewander J. L., et al. , Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 20, 798–805 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran-Nguyen L. T. L., et al. , Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19, 48–59 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Shalev U., Morales M., Hope B., Yap J., Shaham Y., Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl.) 156, 98–107 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Shepard J. D., Bossert J. M., Liu S. Y., Shaham Y., The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry 55, 1082–1089 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Bienkowski P., et al. , Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur. Neuropsychopharmacol. 14, 355–360 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Abdolahi A., Acosta G., Breslin F. J., Hemby S. E., Lynch W. J., Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur. J. Neurosci. 31, 733–741 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm J. W., Shaham Y., Hope B. T., Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav. Pharmacol. 13, 379–388 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm J. W., Fyall A. M., Osincup D. P., Incubation of sucrose craving: Effects of reduced training and sucrose pre-loading. Physiol. Behav. 84, 73–79 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darling R. A., Dingess P. M., Schlidt K. C., Smith E. M., Brown T. E., Incubation of food craving is independent of macronutrient composition. Sci. Rep. 6, 30900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krasnova I. N., et al. , Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology 39, 2008–2016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedi G., et al. , Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry 69, 708–711 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvaz M. A., Moeller S. J., Goldstein R. Z., Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73, 1127–1134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G., et al. , Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One 8, e68791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair S. G., Adams-Deutsch T., Epstein D. H., Shaham Y., The neuropharmacology of relapse to food seeking: Methodology, main findings, and comparison with relapse to drug seeking. Prog. Neurobiol. 89, 18–45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y., Taylor J. R., Wolf M. E., Shaham Y., Circuit and synaptic plasticity mechanisms of drug relapse. J. Neurosci. 37, 10867–10876 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredriksson I., et al. , Animal models of drug relapse and craving after voluntary abstinence: A review. Pharmacol. Rev. 73, 1050–1083 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szumlinski K. K., Shin C. B., Kinase interest you in treating incubated cocaine-craving? A hypothetical model for treatment intervention during protracted withdrawal from cocaine. Genes Brain Behav. 17, e12440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bito H., Deisseroth K., Tsien R. W., CREB phosphorylation and dephosphorylation: A Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Cruz F. C., et al. , New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci. 14, 743–754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz F. C., Javier Rubio F., Hope B. T., Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 1628, 157–173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan J. I., Curran T., Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 14, 421–451 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Yap E.-L., Greenberg M. E., Activity-regulated transcription: Bridging the gap between neural activity and behavior. Neuron 100, 330–348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso-Caraballo Y., Guha S. K., Chartoff E. H., The neurobiology of abstinence-induced reward-seeking in males and females. Pharmacol. Biochem. Behav. 200, 173088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Caprioli D., Marchant N. J., Recent updates on incubation of drug craving: A mini-review. Addict. Biol. 20, 872–876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ertürk A., et al. , Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 7, 1983–1995 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Ertürk A., et al. , Three-dimensional imaging of the unsectioned adult spinal cord to assess axon regeneration and glial responses after injury. Nat. Med. 18, 166–171 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Lee E., et al. , ACT-PRESTO: Rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci. Rep. 6, 18631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renier N., et al. , iDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Susaki E. A., et al. , Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Ueda H. R., et al. , Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 21, 61–79 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Power R. M., Huisken J., A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Reynaud E. G., Krzic U., Greger K., Stelzer E. H., Light sheet-based fluorescence microscopy: More dimensions, more photons, and less photodamage. HFSP J. 2, 266–275 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fürth D., et al. , An interactive framework for whole-brain maps at cellular resolution. Nat. Neurosci. 21, 139–149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirst C., et al. , Mapping the fine-scale organization and plasticity of the brain vasculature. Cell 180, 780–795.e25 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Renier N., et al. , Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin M., et al. , SMART: An open-source extension of WholeBrain for intact mouse brain registration and segmentation. eNeuro 9, ENEURO.0482-21.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calu D. J., Chen Y.-W., Kawa A. B., Nair S. G., Shaham Y., The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology 76, 395–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golden S. A., et al. , Compulsive addiction-like aggressive behavior in mice. Biol. Psychiatry 82, 239–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golden S. A., et al. , Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 16, 44–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venniro M., Zhang M., Shaham Y., Caprioli D., Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology 42, 1126–1135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiner D. J., et al. , Role of projections between piriform cortex and orbitofrontal cortex in relapse to fentanyl seeking after palatable food choice-induced voluntary abstinence. J. Neurosci. 40, 2485–2497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q., et al. , The allen mouse brain common coordinate framework: A 3D reference Atlas. Cell 181, 936–953.e20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramsey L. A., Holloman F. M., Hope B. T., Shaham Y., Venniro M., Waving through the window: A model of volitional social interaction in female mice. Biol. Psychiatry 91, 988–997 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golden S. A., Jin M., Shaham Y., Animal models of (or for) aggression reward, addiction, and relapse: Behavior and circuits. J. Neurosci. 39, 3996–4008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golden S. A., et al. , Nucleus accumbens Drd1-expressing neurons control aggression self-administration and aggression seeking in mice. J. Neurosci. 39, 2482–2496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sittig L. J., et al. , Genetic background limits generalizability of genotype-phenotype relationships. Neuron 91, 1253–1259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang N., et al. , Variability and heritability of mouse brain structure: Microscopic MRI atlases and connectomes for diverse strains. Neuroimage 222, 117274 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloin H. E., et al. , Hybrid offspring of C57BL/6J mice exhibit improved properties for neurobehavioral research. eNeuro 9, 10.1523/ENEURO.0221-22.2022 (2022). [DOI] [PMC free article] [PubMed]
- 54.Nicolas C., et al. , Sex differences in opioid and psychostimulant craving and relapse: A critical review. Pharmacol. Rev. 74, 119–140 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madangopal R., et al. , Discriminative stimuli are sufficient for incubation of cocaine craving. eLife 8, e44427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venniro M., et al. , Factors modulating the incubation of drug and non-drug craving and their clinical implications. Neurosci. Biobehav. Rev. 131, 847–864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis I. R., Coldren S. A., Li X., Methamphetamine seeking after prolonged abstinence is associated with activated projections from anterior intralaminar nucleus of thalamus to dorsolateral striatum in female rats. Pharmacol. Biochem. Behav. 200, 173087 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Funk D., et al. , Role of central amygdala neuronal ensembles in incubation of nicotine craving. J. Neurosci. 36, 8612–8623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grimm J. W., et al. , Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Struct. Funct. 221, 2817–2830 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X., et al. , Role of anterior intralaminar nuclei of thalamus projections to dorsomedial striatum in incubation of methamphetamine craving. J. Neurosci. 38, 2270–2282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fanous S., et al. , Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J. Neurosci. 32, 11600–11609 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venniro M., et al. , Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc. Natl. Acad. Sci. U.S.A. 117, 8126–8134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venniro M., et al. , Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520–1529 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimbrough A., et al. , Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proc. Natl. Acad. Sci. U.S.A. 117, 2149–2159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimbrough A., et al. , Characterization of the brain functional architecture of psychostimulant withdrawal using single-cell whole-brain imaging. eNeuro 8, ENEURO.0208-0219.2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altshuler R. D., et al. , Role of orbitofrontal cortex in incubation of oxycodone craving in male rats. Addict. Biol. 26, e12927 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Blackwood C. A., Leary M., Salisbury A., McCoy M. T., Cadet J. L., Escalated oxycodone self-administration causes differential striatal mRNA expression of FGFs and IEGs following abstinence-associated incubation of oxycodone craving. Neuroscience 415, 173–183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi L. M., et al. , Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence. Neuropsychopharmacology 45, 256–265 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Ciano P., Everitt B. J., Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur. J. Neurosci. 19, 1661–1667 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Huang Y. H., Schlüter O. M., Dong Y., Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav. Brain Res. 216, 9–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moulin T. C., Schiöth H. B., Excitability, synaptic balance, and addiction: The homeostatic dynamics of ionotropic glutamatergic receptors in VTA after cocaine exposure. Behav. Brain Funct. 16, 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J., et al. , Cascades of homeostatic dysregulation promote incubation of cocaine craving. J. Neurosci. 38, 4316–4328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf M. E., The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 33, 391–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X., et al. , Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J. Neurosci. 35, 8232–8244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torgerson C. M., Irimia A., Goh S. Y. M., Van Horn J. D., The DTI connectivity of the human claustrum. Hum. Brain Mapp. 36, 827–838 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zingg B., Dong H. W., Tao H. W., Zhang L. I., Input-output organization of the mouse claustrum. J. Comp. Neurol. 526, 2428–2443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith J. B., et al. , A role for the claustrum in salience processing? Front. Neuroanat. 13, 64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Terem A., et al. , Claustral neurons projecting to frontal cortex mediate contextual association of reward. Curr. Biol. 30, 3522–3532.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Madangopal R., et al. , c-Fos whole-brain image stacks. Figshare. https://figshare.com/articles/media/c-Fos_whole-brain_image_stacks/21207845/1. Deposited 30 September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Images have been deposited in the publicly accessible data repository Figshare (https://figshare.com/articles/media/c-Fos_whole-brain_image_stacks/21207845) (79). All other study data are included in the article (Figs. 1–4) and/or supporting information (SI Appendix, and Supplemental Datasets S1–S4).