Abstract
Background
Globally, are skincare practices and skin injuries in extremely preterm infants comparable? This study describes skin injuries, variation in skincare practices and investigates any association between them.
Methods
A web-based survey was conducted between February 2019 and August 2021. Quantifying skin injuries and describing skincare practices in extremely preterm infants were the main outcomes. The association between skin injuries and skincare practices was established using binary multivariable logistic regression adjusted for regions.
Results
Responses from 848 neonatal intensive care units, representing all geographic regions and income status groups were received. Diaper dermatitis (331/840, 39%) and medical adhesive-related skin injuries (319/838, 38%) were the most common injuries. Following a local skincare guideline reduced skin injuries [medical adhesive-related injuries: adjusted odds ratios (aOR) = 0.63, 95% confidence interval (CI) = 0.45–0.88; perineal injuries: aOR = 0.66, 95% CI = 0.45–0.96; local skin infections: OR = 0.41, 95% CI = 0.26–0.65; chemical burns: OR = 0.46, 95% CI = 0.26–0.83; thermal burns: OR = 0.51, 95% CI = 0.27–0.96]. Performing skin assessments at least every four hours reduced skin injuries (abrasion: aOR = 0.48, 95% CI = 0.33–0.67; pressure: aOR = 0.51, 95% CI = 0.34–0.78; diaper dermatitis: aOR = 0.71, 95% CI = 0.51–0.99; perineal: aOR = 0.52, 95% CI = 0.36–0.75). Regional and resource settings-based variations in skin injuries and skincare practices were observed.
Conclusions
Skin injuries were common in extremely preterm infants. Consistency in practice and improved surveillance appears to reduce the occurrence of these injuries. Better evidence regarding optimal practices is needed to reduce skin injuries and minimize practice variations.
Keywords: Extremely premature infants, Injuries, Neonatal intensive care unit, Skin care, Wounds
Introduction
The skin plays a vital role in the protection, thermoregulation, and sensory functions of the body [1]. Extremely preterm (EP) infants, born < 28 weeks gestational age (GA), are vulnerable to thermal imbalance, fluid and electrolyte loss, skin injury, and sepsis arising from wound contamination and skin breakdown, all due to developmental immaturity of the skin [2, 3]. Hence, it is imperative that the best evidence-based practices are implemented to promote skin integrity and reduce complications. EP infants may also develop injuries from mechanical causes, and from intensive care practices [4]. The prevalence of skin injuries in term and preterm infants ranges from 9.25% to 43.1% [5].
The influence of geographic region and resource settings on skincare practices, and whether skincare practices for EP infants are comparable across the globe in the delivery of evidence-based medicine are unknown. This international survey was designed to provide a comprehensive description of skin injuries, identify variation in skincare practices, and ascertain any association between these practices and skin injuries in EP infants. We hypothesized that significant variation in practice exists, and that skin injuries occurred frequently with certain practices. The findings of this study have implications for advancing the understanding of skincare practices and therefore improving healthcare delivery and clinical outcomes.
Methods
Neonatal intensive care units (NICUs) providing care to EP infants were identified, either directly from an internet search or through regional professional neonatal or parent organizations. The NICU directors were then contacted by an email and invited to participate.
Research Electronic Data Capture (REDCap, Vanderbilt University, Nashville, TN, USA) was used to create a secure e-questionnaire and capture the responses. The link to access the questionnaire was included in the request-to-participate letter. Most questions were closed ones (either single or multiple-choice), few were open to allow for free text descriptions of other practices or commercial products. This international survey was an extension of a pilot study conducted in Australia and New Zealand [6]. Ethical approval was obtained before commencing the study (LNR/18/WMEAD/288–5770 and REB-20-0213-E). Information regarding the purpose of the study, names of the investigators, informed consenting process, time for completion of the survey, security of data storage, and protection of participants’ privacy was provided in the request-to-participate invitation letter. Participation in the survey was voluntary, and participants consented by clicking “Yes–I agree to participate”. Only one response per NICU was requested. To increase participation in the survey, a reminder was sent twice after the initial invitation. A 5-point unipolar scale was used to record the occurrence of skin injuries. This information was then dichotomized to uncommon (including the responses rare or seldom) and common (often, almost always and always).
Statistics
Data were analyzed using Stata 17 (StataCorp, College Station, TX, USA). Descriptive statistics were used to summarize the responses. Chi-squared test or where appropriate Fisher’s exact test was used to explore region and income status-based differences in skincare practices. The association between skin injuries and skincare practices was first assessed in univariate models. Binary multivariable logistic models adjusted for regions including variables that had a P value of < 0.2 in the univariate models were then created using backward stepwise selection. Results from these models are reported with adjusted odds ratios (aOR) and 95% confidence intervals (CI) (Table 1). A two-tailed P value < 0.05 was considered as significant and no adjustments have been made for multiple comparisons.
Table 1.
Relationship between skin care practices and skin injuries from binary univariate and multivariable logistic regression
| Practices or variables | Yes/no | Occurrence of injuries | Univariate, OR (95% CI) | P | Multivariable, aOR (95% CI) | P | |
|---|---|---|---|---|---|---|---|
| Uncommon (rare/seldom), n (%) | Common (often/almost always/always), n (%) | ||||||
| MARSI | |||||||
| Local skin care guideline available | No | 117 (53) | 104 (47) | 0.58 (0.42–0.80) | 0.001 | 0.63 (0.45–0.88) | 0.008 |
| Yes | 378 (66) | 197 (34) | |||||
| Tapes used for securing tubes to the skin | |||||||
| Transparent film dressing | No | 323 (62) | 199 (38) | 0.99 (0.74–1.32) | 0.960 | NA | NA |
| Yes | 196 (62) | 120 (38) | |||||
| Hydrocolloid base with transparent adhesive tape | No | 340 (61) | 219 (39) | 0.86 (0.64–1.16) | 0.340 | NA | NA |
| Yes | 179 (64) | 100 (36) | |||||
| Silicone tapes | No | 429 (61) | 271 (39) | 0.84 (0.57–1.23) | 0.380 | NA | NA |
| Yes | 90 (65) | 48 (35) | |||||
| Plastic polymer skin barrier film | No | 471 (61) | 295 (39) | 0.79 (0.47–1.33) | 0.380 | NA | NA |
| Yes | 48 (67) | 24 (33) | |||||
| Zinc oxide adhesive | No | 489 (63) | 292 (37) | 1.50 (0.87–2.58) | 0.130 | NA | NA |
| Yes | 30 (53) | 27 (47) | |||||
| Plastic perforated tape | No | 481 (63) | 283 (37) | 1.61 (0.99–2.59) | 0.050 | 1.66 (1–2.75) | 0.04 |
| Yes | 38 (51) | 36 (49) | |||||
| Hydrogel adhesives | No | 470 (62) | 286 (38) | 1.10 (0.69–1.76) | 0.660 | NA | NA |
| Yes | 49 (60) | 33 (40) | |||||
| Other practices | No | 410 (62) | 254 (38) | 0.96 (0.68–1.35) | 0.820 | NA | NA |
| Yes | 109 (63) | 65 (37) | |||||
| Use of barrier film underneath the adhesive for skin protection | No | 218 (60) | 146 (40) | 0.84 (0.62–1.12) | 0.240 | NA | NA |
| Yes | 268 (64) | 151 (36) | |||||
| Use of adhesive removers when removing tapes | No | 141 (54) | 119 (46) | 0.61 (0.45–0.83) | 0.002 | 0.59 (0.42–0.81) | 0.002 |
| Yes | 344 (66) | 179 (34) | |||||
| Type of adhesive remover used when removing tapes | |||||||
| Alcohol/organic-based products | No | 255 (67) | 125 (33) | 1.23 (0.82–1.84) | 0.290 | NA | NA |
| Yes | 89 (62) | 54 (38) | |||||
| Oil-based solvents | No | 215 (66) | 113 (34) | 0.97 (0.66–1.41) | 0.880 | NA | NA |
| Yes | 129 (66) | 66 (34) | |||||
| Silicone-based removers | No | 248 (65) | 131 (35) | 0.94 (0.63–1.42) | 0.790 | NA | NA |
| Yes | 96 (67) | 48 (33) | |||||
| Other removers | No | 297 (67) | 149 (33) | 1.27 (0.77–2.09) | 0.340 | NA | NA |
| Yes | 47 (61) | 30 (39) | |||||
| Additional strategies for MARSI prevention | |||||||
| Remove adhesives slowly using moistened gauze/pad | No | 129 (65) | 68 (35) | 1.22 (0.87–1.70) | 0.240 | NA | NA |
| Yes | 390 (61) | 251 (39) | |||||
| Pull adhesive tapes in a horizontal plane | No | 318 (60) | 210 (40) | 0.82 (0.61–1.09) | 0.180 | 0.76 (0.55–1.05) | 0.090 |
| Yes | 201 (65) | 109 (35) | |||||
| Fold the tape back onto itself while wetting the adhesive-skin interface | No | 300 (64) | 169 (36) | 1.21 (0.91–1.60) | 0.170 | 1.47 (1.07–2.01) | 0.010 |
| Yes | 219 (59) | 150 (41) | |||||
| Other practices | No | 501 (61) | 314 (39) | 0.44 (0.16–1.20) | 0.110 | NA | NA |
| Yes | 18 (78) | 5 (22) | |||||
| Abrasion/friction injuries | |||||||
| Skin assessments at least every 4 h | No | 141 (66) | 72 (34) | 0.48 (0.34–0.68) | < 0.001 | 0.48 (0.33–0.71) | < 0.001 |
| Yes | 441 (80) | 109 (20) | |||||
| Local skin care guideline available | No | 161 (72) | 62 (28) | 0.76 (0.54–1.09) | 0.140 | NA | NA |
| Yes | 442 (77) | 131 (23) | |||||
| Use of alcohol-free skin protectants | No | 291 (76) | 90 (24) | 0.88 (0.60–1.28) | 0.520 | NA | NA |
| Yes | 212 (79) | 58 (21) | |||||
| Strategies for injury prevention | |||||||
| Frequent vigilance | No | 147 (72) | 57 (28) | 0.77 (0.54–1.11) | 0.160 | NA | NA |
| Yes | 487 (77) | 147 (23) | |||||
| Routinely rotating site of monitoring device | No | 86 (67) | 43 (33) | 0.58 (0.39–0.88) | 0.010 | NA | NA |
| Yes | 548 (77) | 161 (23) | |||||
| Routinely changing body position | No | 86 (73) | 32 (27) | 0.84 (0.54–1.31) | 0.440 | 0.58 (0.34–0.98) | 0.040 |
| Yes | 548 (76) | 172 (24) | |||||
| Use of pressure injury prevention devices | No | 360 (75) | 118 (25) | 0.95 (0.69–1.31) | 0.790 | NA | NA |
| Yes | 274 (76) | 86 (24) | |||||
| Special purpose mattress | No | 474 (75) | 160 (25) | 0.81 (0.55–1.18) | 0.280 | NA | NA |
| Yes | 160 (78) | 44 (22) | |||||
| Petroleum based ointments | No | 566 (77) | 173 (23) | 1.49 (0.94–2.35) | 0.080 | 1.73 (1.06–2.82) | 0.020 |
| Yes | 68 (69) | 31 (31) | |||||
| Availability of a skin assessment tool (local tool or none) | No | 91 (64) | 52 (36) | 0.45 (0.22–0.91) | 0.020 | NA | NA |
| Yes | 50 (79) | 13 (21) | |||||
| Frequency of skin assessment differed for infants ≤ 25 wk | No | 389 (76) | 121 (24) | 1.16 (0.83–1.60) | 0.360 | NA | NA |
| Yes | 227 (73) | 82 (27) | |||||
| Head to toe examination at least 6 h | No | 389 (76) | 121 (24) | 0.83 (0.60–1.16) | 0.280 | NA | NA |
| Yes | 261 (77) | 77 (23) | |||||
| Oil-based emollient application | No | 384 (73) | 139 (27) | 0.71 (0.51–1.00) | 0.050 | 0.65 (0.45–0.95) | 0.020 |
| Yes | 250 (79) | 65 (21) | |||||
| Petrolatum-base emollient application | No | 522 (76) | 161 (24) | 1.24 (0.83–1.80) | 0.270 | NA | NA |
| Yes | 112 (72) | 43 (28) | |||||
| Pressure injuries | |||||||
| Skin assessments at least every 4 h | No | 148 (69) | 66 (31) | 0.50 (0.35–0.72) | < 0.001 | 0.51 (0.34–0.78) | 0.002 |
| Yes | 452 (82) | 102 (18) | |||||
| Local skin care guideline available | No | 168 (75) | 57 (25) | 0.71 (0.49–1.03) | 0.070 | 0.71 (0.47–1.09) | 0.120 |
| Yes | 463 (80) | 113 (20) | |||||
| Use of alcohol-free skin protectants | No | 293 (76) | 90 (24) | 0.74 (0.50–1.09) | 0.130 | NA | NA |
| Yes | 222 (81) | 51 (19) | |||||
| Injury prevention strategies | |||||||
| Frequent vigilance | No | 160 (78) | 46 (22) | 0.95 (0.65–1.39) | 0.800 | NA | NA |
| Yes | 500 (78) | 137 (22) | |||||
| Routinely rotating site of monitoring device | No | 102 (79) | 27 (21) | 1.05 (0.66–1.67) | 0.810 | NA | NA |
| Yes | 558 (78) | 156 (22) | |||||
| Routinely changing body position | No | 99 (84) | 19 (16) | 1.52 (0.90–2.56) | 0.110 | NA | NA |
| Yes | 561 (77) | 164 (23) | |||||
| Use of pressure injury prevention devices | No | 380 (79) | 100 (21) | 1.12 (0.81–1.56) | 0.470 | NA | NA |
| Yes | 280 (77) | 83 (23) | |||||
| Special purpose mattress | No | 494 (77) | 144 (23) | 0.80 (0.54–1.19) | 0.280 | NA | NA |
| Yes | 166 (81) | 39 (19) | |||||
| Petrolatum-based ointments | No | 591 (79) | 153 (21) | 1.67 (1.05–2.67) | 0.020 | NA | NA |
| Yes | 69 (70) | 30 (30) | |||||
| Availability of a skin assessment tool (local tool or none) | No | 110 (77) | 33 (23) | 1.23 (0.62–2.42) | 0.540 | NA | NA |
| Yes | 46 (73) | 17 (27) | |||||
| Frequency of skin assessment differed for infants ≤ 25 wk | No | 400 (78) | 121 (22) | 1.04 (0.74–1.46) | 0.810 | NA | NA |
| Yes | 240 (77) | 70 (23) | |||||
| Head to toe examination at least 6 h | No | 363 (77) | 111 (23) | 0.84 (0.60–1.18) | 0.330 | NA | NA |
| Yes | 270 (79) | 70 (21) | |||||
| Petrolatum-based emollient application | No | 550 (80) | 136 (20) | 1.72 (1.17–2.55) | 0.006 | 1.52 (0.94–2.46) | 0.080 |
| Yes | 110 (70) | 47 (30) | |||||
| Perineal injuries | |||||||
| Skin assessments at least every 4 h | No | 142 (66) | 72 (34) | 0.58 (0.41–0.82) | 0.002 | 0.52 (0.36–0.75) | 0.001 |
| Yes | 425 (77) | 126 (23) | |||||
| Local skin care guideline available | No | 159 (71) | 66 (29) | 0.76 (0.54–1.07) | 0.120 | 0.66 (0.45–0.96) | 0.030 |
| Yes | 436 (76) | 138 (24) | |||||
| Strategies for injury prevention | |||||||
| Frequent vigilance | No | 160 (77) | 47 (23) | 1.26 (0.87–1.82) | 0.220 | NA | NA |
| Yes | 462 (73) | 171 (27) | |||||
| Routinely rotating site of monitoring device | No | 97 (76) | 31 (24) | 1.11 (0.71–1.72) | 0.620 | NA | NA |
| Yes | 525 (74) | 187 (26) | |||||
| Routinely changing body position | No | 96 (82) | 21 (18) | 1.71 (1.03–2.82) | 0.030 | 1.94 (0.88–4.25) | 0.09 |
| Yes | 526 (73) | 197 (27) | |||||
| Use of pressure injury prevention devices | No | 359 (75) | 120 (25) | 1.11 (0.81–1.52) | 0.490 | NA | NA |
| Yes | 263 (73) | 98 (27) | |||||
| Special purpose mattress | No | 480 (75) | 157 (25) | 1.31 (0.92–1.86) | 0.120 | 1.33 (0.90–1.97) | 0.150 |
| Yes | 142 (70) | 61 (30) | |||||
| Petrolatum-based ointments | No | 558 (73) | 183 (25) | 1.66 (1.06–2.60) | 0.020 | 1.50 (0.89–2.53) | 0.120 |
| Yes | 64 (65) | 35 (35) | |||||
| Availability of a skin assessment tool (local tool or none) | No | 94 (65) | 50 (35) | 0.87 (0.46–1.64) | 0.670 | NA | NA |
| Yes | 43 (68) | 20 (32) | |||||
| Frequency of skin assessment differed for infants ≤ 25 wk | No | 372 (73) | 137 (27) | 0.89 (0.64–1.24) | 0.510 | NA | NA |
| Yes | 233 (75) | 77 (25) | |||||
| Head to toe examination at least 6 h | No | 347 (73) | 126 (27) | 0.89 (0.64–1.23) | 0.480 | NA | NA |
| Yes | 256 (76) | 83 (24) | |||||
| Oil-base emollient application | No | 382 (73) | 144 (27) | 0.81 (0.59–1.13) | 0.220 | NA | NA |
| Yes | 240 (76) | 74 (24) | |||||
| Petrolatum-base emollient application | No | 523 (76) | 161 (24) | 1.87 (1.29–2.70) | 0.001 | 1.88 (1.21–2.91) | 0.004 |
| Yes | 99 (63) | 57 (37) | |||||
| Diaper dermatitis | |||||||
| Skin assessments at least every 4 h | No | 121 (57) | 93 (43) | 0.77 (0.56–1.06) | 0.110 | 0.71 (0.51–0.99) | 0.040 |
| Yes | 345 (63) | 205 (37) | |||||
| Local skin care guideline available | No | 134 (60) | 91 (40) | 0.90 (0.65–1.23) | 0.530 | NA | NA |
| Yes | 355 (62) | 218 (38) | |||||
| Strategies to injury prevention | |||||||
| Frequent vigilance | No | 128 (62) | 79 (38) | 1.07 (0.77–1.47) | 0.670 | NA | NA |
| Yes | 381 (60) | 252 (40) | |||||
| Routinely rotating site of monitoring device | No | 81 (62) | 49 (38) | 1.08 (0.74–1.60) | 0.660 | NA | NA |
| Yes | 428 (60) | 282 (40) | |||||
| Routinely changing body position | No | 79 (67) | 39 (33) | 1.37 (0.91–2.07) | 0.120 | 1.64 (0.92–2.90) | 0.080 |
| Yes | 430 (60) | 292 (40) | |||||
| Use of pressure injury prevention devices | No | 288 (60) | 191 (40) | 0.95 (0.72–1.26) | 0.740 | NA | NA |
| Yes | 221 (61) | 140 (39) | |||||
| Special purpose mattress | No | 389 (61) | 246 (39) | 1.12 (0.81–1.54) | 0.480 | NA | NA |
| Yes | 120 (59) | 85 (41) | |||||
| Petroleum based ointments | No | 453 (61) | 288 (39) | 1.20 (0.79–1.84) | 0.380 | NA | NA |
| Yes | 56 (57) | 43 (43) | |||||
| Availability of a skin assessment tool (local tool or none) | No | 88 (61) | 56 (39) | 1.38 (0.75–2.51) | 0.290 | NA | NA |
| Yes | 33 (53) | 29 (47) | |||||
| Frequency of skin assessment differed for infants ≤ 25 wk | No | 303 (59) | 208 (41) | 0.92 (0.69–1.24) | 0.620 | NA | NA |
| Yes | 188 (61) | 120 (39) | |||||
| Head to toe examination at least 6 h | No | 290 (61) | 183 (39) | 1.08 (0.81–1.43) | 0.590 | NA | NA |
| Yes | 201 (59) | 137 (41) | |||||
| Oil-based emollient application | No | 305 (58) | 219 (42) | 0.76 (0.57–1.02) | 0.060 | NA | NA |
| Yes | 204 (65) | 112 (35) | |||||
| Petrolatum-based emollient application | No | 429 (63) | 254 (37) | 1.62 (1.14–2.30) | 0.006 | 1.62 (1.12–2.33) | 0.009 |
| Yes | 80 (51) | 77 (49) | |||||
| Complications from emollient use | |||||||
| Increased CONS infection | |||||||
| Prophylactic application | No | 136 (93) | 10 (7) | 0.43 (0.17–1.10) | 0.080 | 0.38 (0.15–0.99) | 0.040 |
| Yes | 279 (97) | 9 (3) | |||||
| Oil-based emollient | No | 178 (94) | 12 (6) | 0.37 (0.14–0.97) | 0.040 | NA | NA |
| Yes | 275 (98) | 7 (2) | |||||
| Petrolatum-based emollient | No | 323 (98) | 8 (2) | 3.41 (1.34–8.69) | 0.010 | 3.66 (1.42–9.46) | 0.007 |
| Yes | 130 (92) | 11 (8) | |||||
| Hyperthermia | |||||||
| Prophylactic application | No | 135 (96) | 5 (4) | 0.87 (0.28–2.64) | 0.800 | NA | NA |
| Yes | 279 (97) | 9 (3) | |||||
| Oil-based emollient | No | 177 (96) | 7 (4) | 1.02 (0.39–2.69) | 0.950 | NA | NA |
| Yes | 271 (96) | 11 (4) | |||||
| Petrolatum-based emollient | No | 320 (98) | 7 (2) | 3.92 (1.48–10.35) | 0.006 | NA | NA |
| Yes | 128 (92) | 11 (8) | |||||
| Tissue burns | |||||||
| Prophylactic application | No | 137 (97) | 4 (3) | 1.36 (0.42–4.34) | 0.600 | NA | NA |
| Yes | 277 (96) | 11 (4) | |||||
| Oil-based emollient | No | 178 (96) | 7 (4) | 0.75 (0.26–2.10) | 0.580 | NA | NA |
| Yes | 271 (97) | 8 (3) | |||||
| Petrolatum-based emollient | No | 318 (98) | 6 (2) | 3.64 (1.27–10.43) | 0.010 | NA | NA |
| Yes | 131 (94) | 9 (6) | |||||
| Interference with adhesive tapes | |||||||
| Prophylactic application | No | 113 (78) | 31 (26) | 1.10 (0.68–1.79) | 0.680 | NA | NA |
| Yes | 221 (77) | 67 (23) | |||||
| Oil-based emollient | No | 143 (77) | 42 (23) | 1.12 (0.73–1.74) | 0.580 | NA | NA |
| Yes | 214 (75) | 71 (25) | |||||
| Petrolatum-based emollient | No | 259 (79) | 70 (21) | 1.62 (1.04–2.52) | 0.030 | NA | NA |
| Yes | 98 (70) | 43 (31) | |||||
| Environmental contamination leading to invasive sepsis | |||||||
| Prophylactic application | No | 133 (96) | 6 (4) | 1.13 (0.42–3.02) | 0.790 | NA | NA |
| Yes | 273 (95) | 14 (3) | |||||
| Oil-based emollient | No | 173 (95) | 9 (5) | 1.00 (0.42–2.37) | 0.990 | NA | NA |
| Yes | 269 (95) | 14 (5) | |||||
| Petrolatum-based emollient | No | 318 (97) | 9 (3) | 4.02 (1.69–9.53) | 0.002 | NA | NA |
| Yes | 123 (90) | 14 (10) | |||||
Responses reported as number (%), percentages rounded to the nearest whole number. Adjusted odds ratio from stepwise backward binary multivariate logistic regression models, adjusted for regions. Uncommon occurrence of skin injuries was arbitrarily used as the reference group (base). Occurrence of injuries uncommon: responses rare and seldom; occurrence of injuries common: responses often, almost always and always. MARSI medical adhesive-related skin injury, CONS coagulase negative staphylococci, OR unadjusted odds ratio, aOR adjusted odds ratio, CI confidence interval, NA effect output not included as P ≥ 0.2 for stepwise regression
Results
Responses from 848 NICUs from six geographic regions (Europe, Asia, North America, Africa, South America, and Oceania) and from low and lower middle-income countries (low and LMIC), upper middle-income countries (UMIC) and high-income countries (HIC) were received. The World Bank assigns each country one of the four groups: low, lower middle, upper middle, and high-income countries based on its economic performance. We used the World Bank report for 2021 to reflect the income status category of the participating unit’s country.
Skin injuries
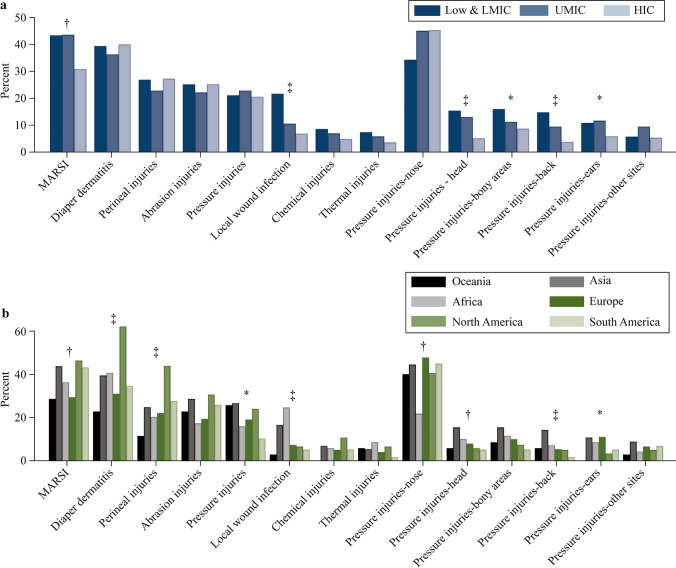
Diaper dermatitis (331/840, 39%) and medical adhesive-related skin injury (MARSI) (319/838, 38%) were the most common injuries, followed by perineal (218/840, 26%), abrasion (204/838, 24%), pressure injuries (183/843, 22%), and local infection (94/840, 11%). Diaper dermatitis differed between geographic regions (Fig. 1). The odds of diaper dermatitis were higher in NICUs from Asia (OR = 1.45, 95% CI = 1.02–2.06; P = 0.03) and North America (OR = 3.77, 95% CI = 2.51–5.89; P < 0.001) compared to European NICUs, and in NICUs applying petrolatum-based emollient (aOR = 1.62, 95% CI = 1.12–2.33; P = 0.009).
Fig. 1.
Occurrence of skin injuries based on income status group (a) and geographic region (b). MARSI medical adhesive-related skin injury, LMIC lower middle-income countries, UMIC upper middle-income countries, HIC high-income countries. *P ≥ 0.01 and < 0.05, †P ≥ 0.001 and < 0.01, ‡P < 0.001
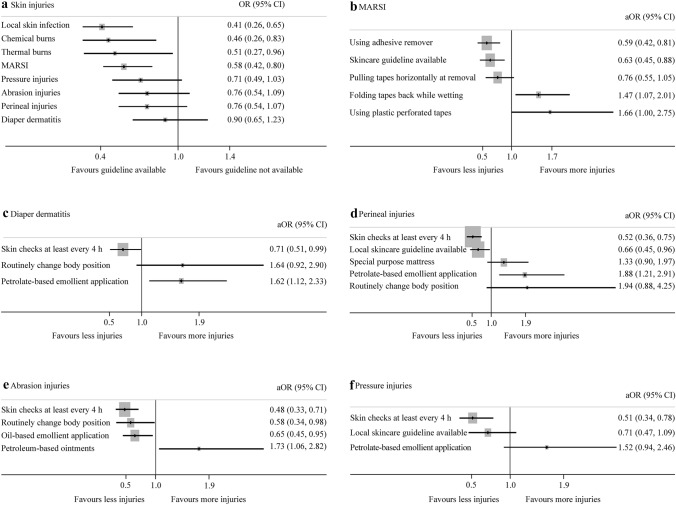
Having a local skincare guideline (aOR = 0.63, 95% CI = 0.45–0.88; P = 0.008) and using adhesive tape removers (aOR = 0.59, 95% CI = 0.42–0.81; P = 0.002) reduced the odds of MARSI. The odds of MARSI were higher in NICUs using plastic perforated tapes (aOR = 1.66, 95% CI = 1.00–2.75; P = 0.04) for securing tubes and folding the adhesive tape backwards and wetting it during its removal (aOR = 1.47, 95% CI = 1.07–2.01; P = 0.01) (Fig. 2). The odds were lower in NICUs from HIC (OR = 0.56, 95% CI = 0.39–0.81; P = 0.002) compared to NICUs from low and LMIC, and UMIC. The odds were higher in NICUs from Asia (OR = 1.91, 95% CI = 1.34–2.71; P < 0.001), North America (OR = 2.08, 95% CI = 1.34–3.23; P = 0.001) and South America (OR = 1.86, 95% CI = 1.04–3.32; P = 0.03) compared to European NICUs.
Fig. 2.
Relationship between practices and the odds of skin injuries. a Availability of skincare guideline and odds of skin injuries (from univariable models); b practices and odds of MARSI (from multivariable model); c practices and odds of diaper dermatitis (from multivariable model); d practices and odds of perineal injuires (from multivariable model); e practices and odds of abrasion injuries (from multivariable model); f practices and odds of pressure injuries (from multivariable model). MARSI medical adhesive-related skin injury, CI confidence interval, OR unadjusted odds ratio from univariable model, aOR adjusted odds ratio from multivariable model
The odds of perineal injuries were higher in NICUs from North America (OR = 2.71, 95% CI = 1.72–4.26; P < 0.001) compared to European NICUs. The odds were lower when skin assessments were performed at least every four hours (aOR = 0.52, 95% CI = 0.36–0.75; P = 0.001) and when a local skincare guideline was available (aOR = 0.66, 95% CI = 0.45–0.96; P = 0.03). The odds of perineal injuries were higher when petrolatum-based emollients were applied (aOR = 1.88, 95% CI = 1.21–2.91; P = 0.004) (Fig. 2).
Abrasion injuries were reported most from North America (37/121, 31%) and least from Africa (12/68, 18%) (Fig. 1). The odds were lower when NICUs performed skin assessments at least every four hours (aOR = 0.48, 95% CI = 0.33–0.71; P < 0.001), applied oil-based emollients (aOR = 0.65, 95% CI = 0.45–0.95; P = 0.02), routinely changed the body position (aOR = 0.58, 95% CI = 0.34–0.98; P = 0.04), routinely rotated the device site (OR = 0.58, 95% CI = 0.39–0.88; P = 0.01) and used a locally developed skin assessment tool (as compared to none; OR = 0.45, 95% CI = 0.22–0.961; P = 0.02) (Fig. 2 and Table 1). The odds were higher when applying petrolatum-based ointments (aOR = 1.73, 95% CI = 1.06–2.82; P = 0.02) and in NICUs from Asia (OR = 1.67, 95% CI = 0.54–2.93; P = 0.01) and North America (OR = 1.8, 95% CI = 1.11–2.92; P = 0.01) compared to European NICUs.
Nasal pressure injuries were most common (363/797, 46%) (Fig. 1). The odds were higher in NICUs from Asia (OR = 1.55, 95% CI = 1.04–2.31; P = 0.03) compared to European NICUs. The odds were lower when NICUs assessed the sites at least every four hours (aOR = 0.51, 95% CI = 0.34–0.78; P = 0.002).
Skincare practices
Local skincare and skin antisepsis guidelines were available for 72% (579/805) and 75% (605/811) NICUs respectively. Availability differed between income status groups and geographic regions (Tables 2 and 3). Skin injuries were lower when a local skincare guideline was available (Fig. 2).
Table 2.
Practices based on income status groups of the respondent units
| Practices | Low and LMIC (175/842, 21%) | UMIC (275/842, 33%) |
HIC (392/842, 47%) |
Level of association, P |
|---|---|---|---|---|
| Local skin care guideline available (n = 799) | 100/167 (60) | 190/260 (73) | 284/372 (76) | < 0.001 |
| Local skin antisepsis guideline available (n = 805) | 99/170 (58) | 195/260 (75) | 306/375 (82) | < 0.001 |
| Skin cleansing solution prior to sterile procedures (n = 842)a | n = 175 | n = 275 | n = 392 | < 0.001 |
| Aqueous chlorhexidine solution | 50 (29) | 93 (34) | 211 (54) | |
| Combination alcohol and antiseptic | 106 (61) | 72 (26) | 107 (27) | |
| Iodine-based solution | 72 (41) | 146 (53) | 93 (24) | |
| Hexachlorophene | 2 (1) | 3 (1) | 8 (2) | |
| Sterile water | 12 (7) | 28 (10) | 50 (13) | |
| Skin cleansing solution prior to clean procedures (n = 828)a | n = 172 | n = 271 | n = 385 | < 0.001 |
| Aqueous chlorhexidine solution | 15 (9) | 44 (16) | 106 (28) | |
| Combination alcohol and antiseptic | 129 (75) | 172 (63) | 180 (47) | |
| Iodine-based solution | 10 (6) | 34 (13) | 9 (2) | |
| Hexachlorophene | 1 (1) | 2 (1) | 3 (1) | |
| Sterile water | 7 (4) | 4 (1) | 16 (4) | |
| Othersb | 10 (6) | 15 (6) | 71 (18) | |
| Differing skin antisepsis for infants ≤ 25 wk GA (n = 833)c | 24/172 (14) | 60/273 (22) | 119/388 (31) | < 0.001 |
| Skin integrity assessment tool (n = 842)a | n = 175 | n = 275 | n = 392 | < 0.001 |
| Braden Q | 6 (3) | 70 (25) | 39 (10) | |
| Neonatal skin risk assessment tool | 66 (38) | 57 (21) | 61 (16) | |
| Neonatal skin condition score | 26 (15) | 55 (20) | 56 (14) | |
| Starkid skin scale | 1 (1) | 5 (2) | 1 (-) | |
| Neonatal skin risk assessment scale | 12 (7) | 65 (24) | 34 (9) | |
| Glamorgan pressure injury risk assessment | 0 (0) | 9 (3) | 12 (3) | |
| Other local toolsd | 4 (2) | 9 (3) | 49 (13) | |
| None | 40 (23) | 32 (12) | 74 (19) | |
| Umbilical cord care practices (n = 842)a | n = 175 | n = 275 | n = 392 | < 0.001 |
| Leave alone | 105 (60) | 162 (59) | 283 (72) | |
| Sterile water | 46 (26) | 33 (12) | 50 (13) | |
| A drying agent | 20 (11) | 57 (21) | 25 (6) | |
| Topical antibiotic agent | 8 (5) | 25 (9) | 11 (3) | |
| Topical antifungal agent | 2 (1) | 5 (2) | 1 (-) | |
| Topical breast milk | 2 (1) | 7 (3) | 0 (0) | |
| Otherse | 16 (9) | 29 (11) | 44 (11) | |
| Routine use of topical emollients (n = 805), of these 41 (5%) were used for specific GA infants | 96/167 (57) | 135/258 (52) | 104/380 (27) | < 0.001 |
| Frequency of emollient use (n = 334) | n = 95 | n = 136 | n = 103 | 0.004 |
| Once daily | 37 (39) | 63 (46) | 38 (37) | |
| Twice daily | 41 (43) | 29 (21) | 32 (31) | |
| More than twice daily | 13 (14) | 31 (23) | 17 (16) | |
| Others | 4 (4) | 13 (10) | 16 (16) | |
| Type of topical emollient useda (n = 842), not just prophylactic | n = 175 | n = 275 | n = 392 | < 0.001 |
| Oil-based | 99 (57) | 123 (45) | 95 (24) | |
| Petrolatum-based | 27 (15) | 70 (25) | 59 (15) | |
| Othersf | 6 (3) | 32 (12) | 52 (13) | |
| Issues (often, almost always and always) from any use of emollientsa | ||||
| Interference with other adhesives (n = 469) | 27/110 (25) | 47/178 (26) | 39/181 (22) | 0.550 |
| Increased incidence of CONS infection (n = 471) | 4/109 (4) | 8/179 (4) | 7/183 (4) | 0.930 |
| Hyperthermia (n = 465) | 4/110 (4) | 11/176 (6) | 3/179 (2) | 0.080 |
| Tissue burns (n = 463) | 6/110 (5) | 5/176 (3) | 4/177 (2) | 0.300 |
| Environmental contamination causing invasive sepsis (n = 463) | 9/109 (8) | 11/176 (6) | 3/178 (2) | 0.020 |
| MARSI prevention | ||||
| Tapes for securing tubes (n = 848)a | n = 175 | n = 275 | n = 392 | 0.001 |
| Transparent film dressing | 70 (40) | 116 (42) | 131 (33) | |
| Hydrocolloid base with transparent film or adhesive tape | 30 (17) | 98 (36) | 154 (39) | |
| Silicone tape | 32 (18) | 39 (14) | 68 (17) | |
| Plastic polymer skin barrier film | 9 (5) | 34 (12) | 29 (7) | |
| Zinc oxide adhesive | 26 (15) | 16 (6) | 16 (4) | |
| Plastic perforated tape | 10 (6) | 26 (10) | 39 (10) | |
| Hydrogel adhesive | 8 (5) | 25 (9) | 49 (13) | |
| Othersg | 27 (15) | 54 (20) | 94 (24) | |
| Use of barrier film underneath the adhesive for skin protection (n = 787) | 69/162 (43) | 132/254 (52) | 220/371 (59) | 0.002 |
| Use of adhesive remover when removing tapes (n = 787) | 56/162 (35) | 170/257 (66) | 298/368 (81) | < 0.001 |
| Type of adhesive remover used (n = 524 as 4 did not identify their country)a | n = 56 | n = 170 | n = 298 | < 0.001 |
| Alcohol/organic-based products | 31 (55) | 58 (34) | 55 (19) | |
| Oil-based solvents | 22 (39) | 80 (47) | 92 (31) | |
| Silicone-based removers | 3 (5) | 45 (27) | 96 (32) | |
| Othersh | 5 (9) | 17 (10) | 56 (19) | |
| Additional strategy for MARSI prevention (n = 842)a | n = 175 | n = 275 | n = 392 | < 0.001 |
| Remove adhesive slowly and carefully using moistened gauze/pad | 121 (69) | 212 (77) | 312 (80) | |
| Pull adhesive tape in a horizontal plane | 55 (31) | 114 (42) | 144 (37) | |
| Fold the tape back onto itself while continuously wetting the adhesive-skin interface | 56 (32) | 138 (50) | 177 (45) | |
| Othersi | 4 (2) | 5 (2) | 14 (4) | |
Six respondents did not identify their country. Responses reported as number (%), percentage rounded to the nearest whole number. LMIC lower middle-income country, UMIC upper middle-income country, HIC high-income country, GA gestational age, MARSI medical adhesive-related skin injury, CONS coagulase-negative Staphylococcal. aMultiple responses allowed; bother solutions were alcohol, chlorine, chlorhexidine/alcohol and benzalkonium, sodium chloride, octenidine and hypochlorite; cother practices such as use of only sterile water, povidone-iodine, weak non-alcoholic solution, octenidine with sterile water and wiping off the cleansing solution with sterile water; dvisual inspection, homegrown local tool, neonatal skin injury and pressure injury risk assessment, Swiss neonatal skin score, Norton pressure sore risk; eother topical cord application practices included application of varying strengths of chlorhexidine or alcohol-based solutions, normal saline, calendula tincture, hydrogen peroxide, iodine-based solutions, octinidine solution, methylated spirits, and use of soap and water; fbaby oil, benzalkonium, ceramide base, cold cream, dimethacone, eucerin, silicone ointment, oil with vitamin E; gadhesive paper or plaster, band aid, brown tape, cotton or cloth tape, polyacrylate tape, silk tape; hcoconut oil, water, soap and water, emollient, saline, octenidine dihydrochloride and 2-phenoxyethanol; ikeep adhesive tapes for 24 hours, olive oil moistened cotton wool, avoid band aids, loosen edges of tape with adhesive remover and carefully peel back dressing until it is removed followed by clean site with saline wipe
Table 3.
Practices based on geographic region of the respondent units
| Practices | Europe (300/848) |
Asia (259/848) |
North America (121/848) | Africa (69/848) |
South America (58/848) | Oceania (35/848) | Level of association, P |
|---|---|---|---|---|---|---|---|
| Local skin care guideline available (n = 799) | 213/288 (74) | 159/240 (66) | 89/115 (77) | 42/67 (63) | 46/55 (84) | 25/34 (74) | 0.020 |
| Local skin antisepsis guideline available (n = 805) | 231/287 (80) | 157/244 (64) | 88/116 (76) | 42/67 (63) | 52/57 (91) | 30/34 (88) | < 0.001 |
| Skin cleansing solution prior to sterile procedures (n = 842)a | n = 300 | n = 259 | n = 121 | n = 69 | n = 58 | n = 35 | < 0.001 |
| Aqueous chlorhexidine solutionb | 109 (36) | 96 (37) | 56 (46) | 16 (23) | 46 (79) | 31 (89) | |
| Combination alcohol and antiseptic | 98 (33) | 93 (36) | 27 (22) | 49 (71) | 13 (22) | 5 (14) | |
| Iodine-based solution | 1000 (33) | 122 (47) | 63 (52) | 24 (35) | 1 (2) | 1 (3) | |
| Hexachlorophene | 3 (1) | 8 (3) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | |
| Sterile water | 37 (12) | 24 (9) | 17 (14) | 6 (9) | 4 (7) | 2 (6) | |
| Skin cleansing solution prior to clean procedures (n = 828)a | n = 295 | n = 254 | n = 120 | n = 67 | n = 57 | n = 35 | < 0.001 |
| Aqueous chlorhexidine solution | 66 (22) | 36 (14) | 24 (20) | 0 (0) | 28 (49) | 11 (31) | |
| Combination alcohol and antiseptic | 166 (66) | 162 (64) | 67 (56) | 53 (79) | 22 (39) | 11 (31) | |
| Iodine-based solution | 13 (4) | 29 (11) | 6 (5) | 4 (6) | 1 (2) | 0 (0) | |
| Hexachlorophene | 1 (-) | 5 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Sterile water | 10 (3) | 5 (2) | 2 (2) | 4 (6) | 1 (2) | 5 (14) | |
| Others | 39 (13) | 17 (7) | 21 (18) | 6 (9) | 5 (9) | 8 (23) | |
| Differing skin antisepsis for infants ≤ 25 wk GA (n = 833) | 78/297 (26) | 57/258 (22) | 40/120 (33) | 4/67 (6) | 12/57 (21) | 12/34 (35) | 0.001 |
| Skin integrity assessment tool (n = 842)a | n = 300 | n = 259 | n = 121 | n = 69 | n = 58 | n = 35 | < 0.001 |
| Braden Q | 48 (16) | 38 (15) | 16 (13) | 1 (1) | 10 (17) | 2 (6) | |
| Neonatal skin risk assessment tool | 53 (18) | 58 (22) | 17 (14) | 34 (49) | 12 (21) | 10 (29) | |
| Neonatal skin condition score | 43 (14) | 49 (19) | 25 (21) | 5 (7) | 5 (9) | 10 (29) | |
| Starkid skin scale | 1 (-) | 5 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | |
| Neonatal skin risk assessment scale | 41 (14) | 44 (17) | 12 (10) | 2 (3) | 7 (12) | 5 (14) | |
| Glamorgan pressure injury risk assessment | 6 (2) | 4 (2) | 3 (2) | 0 (0) | 0 (0) | 8 (23) | |
| Other local tools | 28 (9) | 13 (5) | 14 (12) | 0 (0) | 2 (3) | 5 (14) | |
| None | 54 (18) | 38 (15) | 22 (18) | 15 (22) | 13 (3) | 4 (11) | |
| Umbilical cord care practices (n = 842)a | n = 300 | n = 259 | n = 121 | n = 69 | n = 58 | n = 35 | < 0.001 |
| Leave alone | 221 (74) | 158 (61) | 100 (83) | 13 (19) | 26 (45) | 32 (91) | |
| Sterile water | 41 (14) | 34 (13) | 9 (7) | 34 (49) | 8 (14) | 3 (9) | |
| Drying agent | 26 (9) | 37 (14) | 6 (5) | 13 (19) | 20 (34) | 0 (0) | |
| Topical antibiotic agent | 10 (3) | 25 (10) | 4 (3) | 5 (7) | 0 (0) | 0 (0) | |
| Topical antifungal agent | 1 (-) | 6 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | |
| Topical breast milk | 0 (0) | 6 (2) | 0 (0) | 1 (1) | 2 (3) | 0 (0) | |
| Others | 26 (9) | 32 (12) | 6 (5) | 14 (20) | 9 (16) | 2 (6) | |
| Routine use of topical emollients (n = 805), of these 41 (5%) were used for specific GA infants | 131/290 (45) | 106/242 (44) | 36/120 (30) | 41/65 (63) | 14/54 (26) | 7/34 (20) | < 0.001 |
| Frequency of emollient use (n = 334) | n = 131 | n = 105 | n = 36 | n = 41 | n = 14 | n = 7 | 0.002 |
| Once daily | 53 (40) | 49 (47) | 12 (33) | 12 (29) | 10 (71) | 2 (29) | |
| Twice daily | 36 (27) | 28 (27) | 12 (33) | 24 (59) | 0 (0) | 2 (29) | |
| More than twice daily | 23 (18) | 22 (21) | 7 (19) | 5 (12) | 3 (21) | 1 (14) | |
| Others | 19 (15) | 6 (6) | 5 (14) | 0 (0) | 1 (7) | 2 (29) | |
| Type of topical emollient used (n = 842)a not just prophylactic | n = 300 | n = 259 | n = 121 | n = 69 | n = 58 | n = 35 | < 0.001 |
| Oil-based | 127 (42) | 107 (41) | 21 (17) | 45 (65) | 15 (26) | 2 (6) | |
| Petrolatum-based | 69 (23) | 47 (18) | 24 (20) | 9 (13) | 4 (7) | 3 (9) | |
| Others | 37 (12) | 14 (5) | 21 (17) | 4 (6) | 7 (12) | 7(20) | |
| Issues (often, almost always and always) from any use of emollientsa | |||||||
| Interference with other adhesives (n = 469) | 46/196 (23) | 37/132 (28) | 13/59 (22) | 10/51 (20) | 4/18 (22) | 3/13 (23) | 0.880 |
| Increased incidence of CONS infection (n = 471) | 3/196 (2) | 10/134 (7) | 4/60 (7) | 2/50 (4) | 0/18 (0) | 0/13 (0) | 0.080 |
| Hyperthermia (n = 465) | 8/193 (4) | 5/134 (4) | 2/57 (4) | 3/50 (6) | 0/18 (0) | 0/13 (0) | 0.950 |
| Tissue burns (n = 463) | 2/191 (1) | 10/133 (8) | 1/58 (2) | 2/50 (4) | 0/18 (0) | 0/13 (0) | 0.370 |
| Environmental contamination causing invasive sepsis (n = 463) | 5/192 (3) | 12/132 (9) | 3/58 (5) | 3/50 (6) | 0/18 (0) | 0/13 (0) | 0.150 |
| MARSI prevention | |||||||
| Tapes for securing tubes (n = 842)a | n = 300 | n = 259 | n = 121 | n = 69 | n = 58 | n = 35 | < 0.001 |
| Transparent film dressing | 100 (33) | 117 (45) | 68 (56) | 16 (23) | 15 (26) | 1 (3) | |
| Hydrocolloid base with transparent film or adhesive tape | 91 (30) | 76 (29) | 52 (43) | 11 (16) | 34 (59) | 18 (51) | |
| Silicone tape | 39 (13) | 46 (18) | 22 (18) | 24 (35) | 1 (2) | 7 (20) | |
| Plastic polymer skin barrier film | 33 (11) | 29 (11) | 8 (7) | 2 (3) | 0 (0) | 0 (0) | |
| Zinc oxide adhesive | 170 (6) | 13 (5) | 2 (2) | 21 (30) | 1 (2) | 4 (11) | |
| Plastic perforated tape | 38 (13) | 19 (7) | 13 (11) | 2 (3) | 1 (2) | 2 (6) | |
| Hydrogel adhesive | 32 (11) | 28 (11) | 13 (11) | 1 (2) | 3 (5) | 5 (14) | |
| Other methods | 76 (25) | 42 (16) | 27 (22) | 7 (10) | 16 (28) | 7 (20) | |
| Use of barrier film underneath the adhesive (n = 787) | 143/286 (50) | 132/233 (57) | 70/117 (60) | 22/65 (34) | 34/53 (64) | 20/33 (61) | 0.004 |
| Use of adhesive removers when removing tapes (n = 787) | 236/286 (83) | 141/235 (60) | 80/114 (70) | 12/64 (19) | 31/55 (56) | 24/33 (73) | < 0.001 |
| Type of adhesive remover used (n = 524, country unknown for 4)a | n = 226 | n = 141 | n = 80 | n = 12 | n = 31 | n = 24 | < 0.001 |
| Alcohol/organic-based product | 52 (22) | 59 (42) | 23 (29) | 3 (25) | 4 (13) | 3 (13) | |
| Oil-based solvent | 75 (32) | 64 (45) | 25 (31) | 9 (75) | 17 (55) | 4 (17) | |
| Silicone-based remover | 81 (34) | 19 (14) | 27 (34) | 0 (0) | 6 (19) | 11 (46) | |
| Other agent | 43 (18) | 14 (10) | 9 (11) | 1 (8) | 5 (16) | 6 (25) | |
| Additional strategy for MARSI prevention (n = 842)a | n = 300 | n = 259 | n = 121 | n = 69 | n = 58 | n = 35 | < 0.001 |
| Remove adhesives slowly using moistened gauze | 243 (81) | 201 (78) | 95 (79) | 36 (52) | 39 (67) | 31 (89) | |
| Pull adhesive tapes in a horizontal plane | 109 (36) | 83 (32) | 57 (47) | 31 (45) | 19 (33) | 14 (40) | |
| Fold the tape back onto itself while continuously wetting the adhesive-skin interface | 146 (49) | 109 (42) | 52 (43) | 12 (17) | 32 (55) | 20 (57) | |
| Other methods | 13 (4) | 3 (1) | 6 (5) | 1 (2) | 0 (0) | 0 (0) | |
Responses reported as number (%), percentage rounded to the nearest whole number. GA gestational age, CONS coagulase negative staphylococci, MARSI medical adhesive-related skin injury. aMultiple responses allowed; bthe strength of the chlorhexidine solution varied from 0.01% to 100%
Aqueous chlorhexidine (355/848, 42%), iodine-based solution (314/848, 37%), and a combination of alcohol and antiseptic (286/848, 34%) were the most common skin cleansing agents used prior to sterile procedures. Choice of topical cleansing agent differed based on resource settings (Table 2) and geographic region (Table 3). For skin cleansing prior to clean procedures, 58% (484/834) NICUs used a combination of alcohol and antiseptic solution. NICUs from South America used aqueous chlorhexidine the most (Table 3). A quarter of NICUs followed a differing practice on skin antisepsis for infants ≤ 25 weeks gestation. This practice varied widely across income status groups and regions (Tables 2 and 3). Most NICUs applied nothing to the umbilical cord (553/848, 65%). Sterile water (130/848, 15%) and a drying agent (104/848, 12%) were the most common topical agents used. Umbilical cord practices differed between income status groups and between geographic regions (Tables 2 and 3).
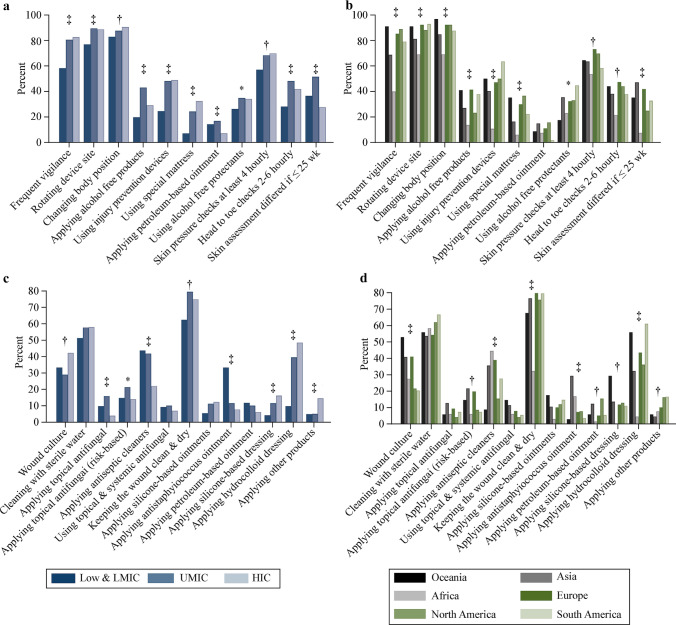
A neonatal skin risk assessment tool (184/848, 22%), neonatal skin condition score (140/848, 17%) and the Braden Q scale (116/848, 14%) were the most common skin integrity assessment tools used. No tool was used in 17% (146/848) of NICUs and 7% (62/848) used a locally developed tool. Income group and region-based use of these tools are shown in Tables 2 and 3. There was no relationship between any skin integrity assessment tool and the occurrence of skin injuries, except for the Braden Q tool for perineal injuries (OR = 0.52, 95% CI = 0.31–0.87; P = 0.01). Most NICUs (556/771, 72%) were assessing the skin at least every four hours, however, only 60% of NICUs from low and LMIC were performing these assessments at least every four hours compared to NICUs from UMIC and HIC (each 75%) (Fig. 3). European and North American NICUs performed this surveillance more than NICUs from other regions. Changing body position (729/848, 86%), rotating the sites of monitoring devices (716/848, 84%) and frequent site surveillance (639/848, 75%) were the three most common practices used to minimize skin injuries. These were more commonly practiced in NICUs from UMIC and HIC (Fig. 3).
Fig. 3.
Skin injury prevention and management strategies. a Responses by income status group; b prevention responses by geographic region; c management responses by income status group; d management responses by geographic region. Other management practices included application of other products such as zinc-based paste, alginate, artificial skin, Leptospermum or medical grade honey and other alternative medicine practices. LMIC lower middle-income countries, UMIC upper middle-income countries, HIC high-income countries, *P ≥ 0.01 and < 0.05, †P ≥ 0.001 and < 0.01, ‡P < 0.001
A transparent (318/848, 38%) or a hydrocolloid-based (283/848, 33%) dressing was mostly used for securing tubes to the skin. These practices were similar between income status groups and geographic regions except for NICUs from Africa (Tables 2 and 3). Just over half of the NICUs were using a barrier film underneath the adhesive for MARSI prevention and 67% (528/792) of NICUs were using adhesive removers when removing tapes. Other MARSI prevention practices included removing adhesives carefully using moistened gauze (649/848, 77%), folding the tape back onto itself while continuously wetting the adhesive-skin interface (373/848, 44%) and pulling off the adhesive tapes horizontally (315/848, 37%).
Keeping a wound clean and dry (589/848, 70%), thorough cleaning with sterile water (449/848, 53%), use of hydrocolloid dressings (298/848, 35%), performing surveillance wound cultures (290/848, 34%) and use of antiseptic cleansers (264/848, 31%) were the most common wound practices. Applying antiseptic cleaners and anti-staphylococcus ointments were more common in NICUs from low and LMIC than in NICUs from UMIC and HIC, and geographic variation for these practices was observed (Fig. 3).
Emollients were used prophylactically by 41% (336/810) NICUs; of these 5% used them for specific gestational ages. They were usually applied either once daily (138/335, 41%) or twice daily (102/335, 30%). Applying an oil-based emollient (318/848, 38%) was more common than applying a petrolatum-based emollient (158/848, 19%). Interference with adhesives was the most common complication (113/470, 24%), the occurrence of other complications was low (< 5%). Emollient use was lower in NICUs from HIC compared to NICUs from other two income groups (Table 2). Its use was lower in NICUs from Oceania, South and North America (Table 3). Petrolatum-based emollient was associated with higher odds of complications [coagulase negative staphylococcus infection (aOR = 3.66, 95% CI = 1.42–9.46; P = 0.007); hyperthermia (OR = 3.92, 95% CI = 1.48–10.35; P = 0.006); tissue burns (OR = 3.64, 95% CI = 1.27–10.43; P = 0.01); interference with adhesives (OR = 1.62, 95% CI = 1.04–2.52; P = 0.03) and environmental contamination (OR = 4.02, 95% CI = 1.69–9.53; P = 0.002)], oil-based emollient which was associated with lower odds of coagulase-negative staphylococcus infection (OR = 0.37, 95% CI = 0.14–0.97; P = 0.04).
Discussion
In this large global survey, skin injuries were common in EP infants. Skin injuries were less when NICUs had a local skincare guideline and performed skin assessments at least every four hours. Geographic region and resource settings-based variation for skin injuries and skincare practices were observed. The reasons for this variation (such as limitations from cost or skills shortage) needs further exploration.
EP infants are at high risk of developing skin injuries [7]. MARSI may occur through various mechanisms [5, 8]. Two common practices included applying a transparent adhesive tape to the skin and applying a hydrocolloid tape in between a transparent tape and the skin. Although hydrogel-based adhesives when removed are gentler on the skin, they were used infrequently by the respondents [9]. While some researchers found certain products or practices reduced MARSI, others reported no effect [9–13]. Evidence is needed regarding which adhesive best secures medical devices and causes the least skin injury. Barrier films protect preterm infants’ skin [14]. At least half of the NICUs were using a barrier film for skin protection. The use of adhesive removers could reduce MARSI when removing tapes, though their efficacy and safety in preterm infants has been questioned [15–17]. MARSI was less in NICUs that followed a local skincare guideline and used an adhesive remover when removing tapes. MARSI can occur with zinc-based adhesives or plastic perforated tapes [9, 18]. Altogether, MARSI was frequent in NICUs from North America, South America and Asia. These NICUs used plastic perforated tapes, which may have contributed to MARSI. Diaper dermatitis is common in term infants [19]. In our survey, diaper dermatitis and perineal injuries occurred frequently in NICUs from North America and in NICUs using petrolatum-based ointment. Perineal injuries (injury of any nature specific to the perineal region) were less in NICUs that assessed skin at least every four hours, had a local skincare guideline or used the Braden Q tool. Although Braden Q tool is widely used for pressure injury risk assessment, its association with lower odds of perineal injuries in our survey could be explained by pressure injury at the perineum [4].
Medical devices can cause pressure injuries [20]. The pressure injury sites reported in this survey are consistent with previous reports [5, 21]. Preventing pressure injury and pressure ulcer is essential, as they affect the patient and the organization [22]. The evidence for pressure injury prevention strategies in EP infants is limited [23–25]. Frequent surveillance, rotating the site of medical devices, routinely changing body position, use of pressure injury prevention devices or special mattresses, alcohol-free products and petrolatum-based ointments are strategies to prevent pressure injuries in newborn infants at high risk of skin injuries [4]. But these practices are often extrapolated from adult and/or pediatric literature [26]. Regular skin assessment, at least every 12 hours, is suggested for the early identification of pressure injuries from medical devices [24, 27]. In this study, diaper dermatitis, pressure, perineal and abrasion injuries were less when skin assessments were performed at least every four hours.
Using topical skin cleansing agents prior to invasive procedures reduces hospital-acquired bloodstream infections [28]. While most NICUs used a topical cleansing agent, the choice of cleansing agent varied, and few used sterile water. In adults, the application of a topical chlorhexidine-based agent is possibly superior to povidone-iodine in reducing catheter-related bloodstream infections [29]. But evidence for its superiority over other agents in EP infants is lacking [30, 31]. Hence, the Centers for Disease Control and Prevention makes no such recommendation for its use in infants < 2 months of age. There are safety concerns regarding systemic absorption of iodine and alcohol-based cleansing solutions and lack of information on long-term neurodevelopment especially as infants born at 22 weeks GA are offered active care [28, 32–35]. Maintaining skin integrity and reducing catheter-related bloodstream infections is vital for their survival. Hence, the question of which cleansing agent is superior in efficacy and safety for EP infants should be addressed.
While daily or more frequent skin assessments are suggested, there is ambiguity regarding its optimal frequency, and its effect on occurrence of skin injury [4]. Most NICUs were performing them at least every four hours and this practice was associated with less skin injuries. It is important to use a valid skin assessment tool to assess skin health objectively. Most skin assessment tools used either did not account for prematurity or were not validated for use in preterm infants [36]. Newer tools for evaluating skin integrity are reported [37, 38]. However, further testing of these tools in EP infants is suggested before making changes in practice [4]. Interestingly, we observed that abrasion injuries were less when NICUs used even a local skin assessment tool compared to none. This highlights the need for use of an objective skin surveillance tool. In this survey, 28% of NICUs did not have a local skincare guideline. Integrating a skincare guideline into practice probably reduces skin injuries by delivering evidence-based care, improving staff education, and reducing variations in practice.
Application of emollients may benefit term infants, but debate continues regarding the benefits for preterm infants [39]. In this survey, oil-based emollient was used most often and a quarter of NICUs reported interference with medical device adherence as the most common complication. Income status-based and region-based variation was observed for application of emollient and complications from its use. Additionally, NICUs applying petroleum-based emollient reported a higher odds of skin injuries. The true reason for this observation needs further exploration. Plausible reasons could include skin barrier disruption by the process of emollient application (e.g., massaging), increased risk of skin colonization and infection from pathogens, and adverse local and systemic effects form absorption of chemicals contained in the emollient [39]. Skin protection from the application of coconut oil has been reported, but concerns have been raised regarding interference with medical device adherence and systemic infection [40–42]. Applying emollients (e.g., sunflower or coconut oil) to preterm infants in LMICs improved weight gain and reduced sepsis [43, 44]. A randomized trial is currently investigating the effect of topical coconut oil application on the development of sepsis in EP infants [45].
Umbilical cord care practices reported by most NICUs aligned with the current international recommendation [46]. Geographic and resource-settings-based variation in using a topical drying agent was observed. Keeping a wound clean and dry, using sterile water for wound cleaning, and applying hydrocolloid dressings were the most consistent practices that aligned with wound management principles [4, 47]. Evidence to support routine application of topical antimicrobial agents for wound healing is lacking. Antiseptic skin cleansers were used by 31% of NICUs. This practice can cause trauma to the healing tissue and delay wound healing [47, 48]. Application of silicone-based or hydrocolloid-based adhesive dressings promotes wound healing and reduces trauma caused by removal of the adhesive [11, 49]. Despite a lack of similar evidence in EP infants, hydrocolloid-based and silicone-based dressings are used. There is emerging evidence of the safety and efficacy of Leptospermum honey in preterm infants, but this needs further exploration in controlled trials [50, 51].
Previous studies have focused on practices within a country [52, 53]. The strength of this study was representative participation from all geographic regions and resource settings, therefore the findings are generalizable to a wider neonatal community. Our study has certain limitations. The questionnaire was prepared only in the English language; this may have excluded participation of NICUs from non-English speaking regions. The COVID-19 pandemic may have affected participation in the survey. Although participating unit’s identifiable information was not recorded, we are confident that duplicate responses from the same unit were not included by checking the demographic data and the survey responses. Finally, an overall survey response rate was not reported, as the total number of NICUs from each participating region was not known.
In conclusion, skin injuries were common in EP infants. Having a local skincare guideline and performing skin assessments at least every four hours were associated with reduced odds of skin injuries. Further evidence on skincare practices in EP infants is needed to formulate region and resource settings-based guidelines, which will reduce variations in practices. Future research may inform strategies on reducing skin injuries and delivering a better quality of health care, leading to improved clinical outcomes.
Acknowledgements
We acknowledge the assistance of many professional neonatal or parent organisation, Dr Sara Sanii, Australia; Dr Euiseok Jung, Asan Medical Center, University of Ulsan College of Medicine, Korea; Dr Simon Lam, The Chinese University of Hong Kong; Dr Shabina Ariff, Aga Khan University, Pakistan who assisted with the distribution of the survey in their region. We acknowledge the assistance of SuperScript Writing and Editing for professional proofreading.
Author contributions
PJ and UM contributed equally to this work. PJ and UM contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing of original draft, reviewing and editing. JB, KW, DG contributed to data curation, project administration, visualization, reviewing and editing. RM and DC contributed to data curation, methodology, visualization, writing of original draft, reviewing and editing. KL, AW, JM, MC, AP contributed to data curation, methodology, visualization, reviewing and editing. KA contributed to formal analysis, visualization, reviewing and editing. JEM, JLO, SV, UV, AK, SG, FCC, WHZ, XJH, and MS contributed to data curation, project administration, visualization, reviewing and editing. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding was received for this study.
Data availability
All data on skincare practices generated or analyzed during this study are included in this published article.
Declarations
Ethical approval
This study protocol was reviewed and approved by The Western Sydney Local Health District’s Human Research Ethics Committee (approval number: LNR/18/WMEAD/288–5770) and by The Mount Sinai Hospital Research Ethics Board (approval number: 20–0213-E). Participation in the survey was voluntary, and participants consented by clicking “Yes–I agree to participate”.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. Wen-Hao Zhou is a member of the Editorial Board for World Journal of Pediatrics. The paper was handled by the other Editor and has undergone a rigorous peer review process. Wen-Hao Zhou was not involved in the journal's review of, or decisions related to, this manuscript. The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Visscher MO, Adam R, Brink S, Odio M. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271–280. doi: 10.1016/j.clindermatol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia J. Fluid and electrolyte management in the very low birth weight neonate. J Perinatol. 2006;26(Suppl 1):S19–21. doi: 10.1038/sj.jp.7211466. [DOI] [PubMed] [Google Scholar]
- 3.Ågren J, Segar JL, Söderstrom F, Bell EF. Fluid management considerations in extremely preterm infants born at 22–24 weeks of gestation. Semin Perinatol. 2022;46:151541. doi: 10.1016/j.semperi.2021.151541. [DOI] [PubMed] [Google Scholar]
- 4.Brandon D, Hill CM, Heimall L, Lund CH, Kuller J, McEwan T, et al. Neonatal skin care: evidence-based clinical practice guideline. 4. Washington, DC: Association of Women’s Health, Obstetric and Neonatal Nurses; 2018. [Google Scholar]
- 5.August DL, New K, Ray RA, Kandasamy Y. Frequency, location and risk factors of neonatal skin injuries from mechanical forces of pressure, friction, shear and stripping: a systematic literature review. J Neonatal Nurs. 2018;24:173–180. doi: 10.1016/j.jnn.2017.08.003. [DOI] [Google Scholar]
- 6.Mishra U, Jani P, Maheshwari R, Shah D, D'Cruz D, Priyadarshi A, et al. Skincare practices in extremely premature infants: a survey of tertiary neonatal intensive care units from Australia and New Zealand. J Paediatr Child Health. 2021;57:1627–1633. doi: 10.1111/jpc.15578. [DOI] [PubMed] [Google Scholar]
- 7.Cousins Y. Wound care considerations in neonates. Nurs Stand. 2014;28:61–70. doi: 10.7748/ns.28.46.61.e8402. [DOI] [PubMed] [Google Scholar]
- 8.Lund C. Medical adhesives in the NICU. Newb Inf NursRev. 2014;14:160–165. doi: 10.1053/j.nainr.2014.10.001. [DOI] [Google Scholar]
- 9.Lund CH, Nonato LB, Kuller JM, Franck LS, Cullander C, Durand DK. Disruption of barrier function in neonatal skin associated with adhesive removal. J Pediatr. 1997;131:367–372. doi: 10.1016/S0022-3476(97)80060-1. [DOI] [PubMed] [Google Scholar]
- 10.Dollison E, Beckstrand J. Adhesive tape vs pectin-based barrier use in preterm infants. Neonatal Netw. 1995;14:35–39. [PubMed] [Google Scholar]
- 11.Morris C, Emsley P, Marland E, Meuleneire F, White R. Use of wound dressings with soft silicone adhesive technology. Paediatr Nurs. 2009;21:38–43. doi: 10.7748/paed2009.04.21.3.38.c7037. [DOI] [PubMed] [Google Scholar]
- 12.Grove GL, Zerweck CR, Ekholm BP, Smith GE, Koski NI. Randomized comparison of a silicone tape and a paper tape for gentleness in healthy children. J Wound Ostomy Cont Nurs. 2014;41:40–48. doi: 10.1097/01.WON.0000436669.79024.b0. [DOI] [PubMed] [Google Scholar]
- 13.Boswell N, Waker CL. Comparing 2 adhesive methods on skin integrity in the high-risk neonate. Adv Neonatal Care. 2016;16:449–454. doi: 10.1097/ANC.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 14.Brandon DH, Coe K, Hudson-Barr D, Oliver T, Landerman LR. Effectiveness of no-sting skin protectant and aquaphor on water loss and skin integrity in premature infants. J Perinatol. 2010;30:414–419. doi: 10.1038/jp.2009.174. [DOI] [PubMed] [Google Scholar]
- 15.Black P. Peristomal skin care: an overview of available products. Br J Nurs. 2007;16(17):1048–1056. doi: 10.12968/bjon.2007.16.17.27249. [DOI] [PubMed] [Google Scholar]
- 16.Lund CH, Tucker JA. Adhesion and newborn skin. In: Hoath SB, Maibach HI, editors. Neonatal skin: structure and function. New York: Marcel Dekker; 2003. pp. 299–324. [Google Scholar]
- 17.Ittmann PI, Bozynski ME. Toxic epidermal necrolysis in a newborn infant after exposure to adhesive remover. J Perinatol. 1993;13:476–477. [PubMed] [Google Scholar]
- 18.Nielsen LF, Blume N, Romme T, Samuelsen P, Everland H, Ifversen P, et al. Skin changes induced by a zinc oxide dressing compared with a hydrocolloid dressing in healthy individuals. Skin Res Technol. 2005;11:140–151. doi: 10.1111/j.1600-0846.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 19.Philipp R, Hughes A, Golding J. Getting to the bottom of nappy rash.ALSPAC survey team Avon longitudinal study of pregnancy and childhood. Br J Gen Pract. 1997;47:493–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Visscher M, Taylor T. Pressure ulcers in the hospitalized neonate: rates and risk factors. Sci Rep. 2014;4:7429. doi: 10.1038/srep07429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newnam KM, McGrath JM, Estes T, Jallo N, Salyer J, Bass WT. An integrative review of skin breakdown in the preterm infant associated with nasal continuous positive airway pressure. J Obstet Gynecol Neonatal Nurs. 2013;42:508–516. doi: 10.1111/1552-6909.12233. [DOI] [PubMed] [Google Scholar]
- 22.Triantafyllou C, Chorianopoulou E, Kourkouni E, Zaoutis TE, Kourlaba G. Prevalence, incidence, length of stay and cost of healthcare-acquired pressure ulcers in pediatric populations: a systematic review and meta-analysis. Int J Nurs Stud. 2021;115:103843. doi: 10.1016/j.ijnurstu.2020.103843. [DOI] [PubMed] [Google Scholar]
- 23.López AA, López EB, Paredes AP, Salgado JG, Dolz MCR, Molina PG. Effectiveness of measures and strategies for the prevention of pressure ulcers in neonates. Gerokomos. 2020;31:193–197. [Google Scholar]
- 24.Visscher M, King A, Nie AM, Schaffer P, Taylor T, Pruitt D, et al. A quality-improvement collaborative project to reduce pressure ulcers in PICUs. Pediatrics. 2013;131:e1950–e1960. doi: 10.1542/peds.2012-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindler CA, Mikhailov TA, Cashin SE, Malin S, Christensen M, Winters JM. Under pressure: preventing pressure ulcers in critically ill infants. J Spec Pediatr Nurs. 2013;18:329–341. doi: 10.1111/jspn.12043. [DOI] [PubMed] [Google Scholar]
- 26.Baharestani MM. An overview of neonatal and pediatric wound care knowledge and considerations. Ostomy Wound Manage. 2007;53:34–6. [PubMed] [Google Scholar]
- 27.Scheans P. Neonatal pressure ulcer prevention. Neonatal Netw. 2015;34:126–132. doi: 10.1891/0730-0832.34.2.126. [DOI] [PubMed] [Google Scholar]
- 28.Sathiyamurthy S, Banerjee J, Godambe SV. Antiseptic use in the neonatal intensive care unit-a dilemma in clinical practice: an evidence based review. World J Clin Pediatr. 2016;5:159–171. doi: 10.5409/wjcp.v5.i2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman AK, Aucott SW, Gilmore MM, Advani S, Clarke W, Milstone AM. Absorption and tolerability of aqueous chlorhexidine gluconate used for skin antisepsis prior to catheter insertion in preterm neonates. J Perinatol. 2013;33:768–771. doi: 10.1038/jp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanzi V, Pitaro R. Skin injuries and chlorhexidine gluconate-based antisepsis in early premature infants: a case report and review of the literature. J Perinat Neonatal Nurs. 2018;32:341–350. doi: 10.1097/JPN.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 32.Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Arch Dis Child Fetal Neonatal Ed. 2014;99:F21–F28. doi: 10.1136/archdischild-2013-303799. [DOI] [PubMed] [Google Scholar]
- 33.Garland JS, Alex CP, Uhing MR, Peterside IE, Rentz A, Harris MC. Pilot trial to compare tolerance of chlorhexidine gluconate to povidone-iodine antisepsis for central venous catheter placement in neonates. J Perinatol. 2009;29:808–813. doi: 10.1038/jp.2009.161. [DOI] [PubMed] [Google Scholar]
- 34.Kieran EA, O’Sullivan A, Miletin J, Twomey AR, Knowles SJ, O’Donnell CPF. 2% chlorhexidine–70% isopropyl alcohol versus 10% povidone–iodine for insertion site cleaning before central line insertion in preterm infants: a randomised trial. Arch Dis Child Fetal Neonatal Ed. 2018;103:F101–F106. doi: 10.1136/archdischild-2016-312193. [DOI] [PubMed] [Google Scholar]
- 35.Rysavy MA, Mehler K, Oberthür A, Ågren J, Kusuda S, McNamara PJ, et al. An immature science: intensive care for infants born at ≤ 23 weeks of gestation. J Pediatr. 2021;233:16–25.e1. doi: 10.1016/j.jpeds.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vance DA, Demel S, Kirksey K, Moynihan M, Hollis K. A Delphi study for the development of an infant skin breakdown risk assessment tool. Adv Neonatal Care. 2015;15:150–157. doi: 10.1097/ANC.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 37.Broom M, Dunk AM, Mohamed ALE. Predicting neonatal skin injury: the first step to reducing skin injuries in neonates. Health Serv Insights. 2019;12:1178632919845630. doi: 10.1177/1178632919845630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.August DL, Edmonds L, Brown DK, Murphy M, Kandasamy Y. Pressure injuries to the skin in a neonatal unit: fact or fiction. J Neonatal Nurs. 2014;20:129–137. doi: 10.1016/j.jnn.2013.08.006. [DOI] [Google Scholar]
- 39.Cleminson J, McGuire W. Topical emollient for preventing infection in preterm infants. Cochrane Database Syst Rev. 2021;5:CD001150. doi: 10.1002/14651858.CD001150.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strunk T, Pupala S, Hibbert J, Doherty D, Patole S. Topical coconut oil in very preterm infants: an open-label randomised controlled trial. Neonatology. 2018;113:146–151. doi: 10.1159/000480538. [DOI] [PubMed] [Google Scholar]
- 41.Kiechl-Kohlendorfer U, Berger C, Inzinger R. The effect of daily treatment with an olive oil/lanolin emollient on skin integrity in preterm infants: a randomized controlled trial. Pediatr Dermatol. 2008;25:174–178. doi: 10.1111/j.1525-1470.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 42.AlKharfy T, Ba-Abbad R, Hadi A, AlFaleh K. Use of topical petroleum jelly for prevention of sepsis in very low-birthweight infants: a prospective, randomised controlled trial. Paediatr Int Child Health. 2014;34:194–197. doi: 10.1179/2046905514Y.0000000117. [DOI] [PubMed] [Google Scholar]
- 43.Salam RA, Das JK, Darmstadt GL, Bhutta ZA. Emollient therapy for preterm newborn infants–evidence from the developing world. BMC Public Health. 2013;13(Suppl 3):S31. doi: 10.1186/1471-2458-13-S3-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nangia S, Paul VK, Deorari AK, Sreenivas V, Agarwal R, Chawla D. Topical oil application and trans-epidermal water loss in preterm very low birth weight infants—a randomized trial. J Trop Pediatr. 2015;61:414–420. doi: 10.1093/tropej/fmv049. [DOI] [PubMed] [Google Scholar]
- 45.Can topical coconut oil reduce the incidence of late-onset sepsis in extremely preterm infants–a pragmatic cluster-randomised controlled trial. 2020. https://www.anzctr.org.au/ACTRN12620001332910.aspx. Accessed 21 Aug 2022.
- 46.Stewart D, Benitz W. Committee on Fetus and Newborn Umbilical cord care in the newborn infant. Pediatrics. 2016;138:e20162149. doi: 10.1542/peds.2016-2149. [DOI] [PubMed] [Google Scholar]
- 47.Rolstad BS, Ovington L, Harris A. Principles of wound management. In: Bryant RA, editor. Acute and chronic wounds: nursing management. St. Louis: Mosby; 2007. pp. 85–112. [Google Scholar]
- 48.Wilson JR, Mills JG, Prather ID, Dimitrijevich SD. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care. 2005;18:373–378. doi: 10.1097/00129334-200509000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Rippon M, White R, Davies P. Skin adhesives and their role in wound dressings. Wounds Uk. 2007;3:76–86. [Google Scholar]
- 50.Amaya R. Safety and efficacy of active Leptospermum honey in neonatal and paediatric wound debridement. J Wound Care. 2015;24:95–103. doi: 10.12968/jowc.2015.24.3.95. [DOI] [PubMed] [Google Scholar]
- 51.Esser M. Leptospermum honey for wound care in an extremely premature infant. Adv Neonat Care. 2017;17:27–32. doi: 10.1097/ANC.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 52.Munson KA, Bare DE, Hoath SB, Visscher MO. A survey of skin care practices for premature low birth weight infants. Neonatal Netw. 1999;18:25–31. doi: 10.1891/0730-0832.18.3.25. [DOI] [PubMed] [Google Scholar]
- 53.Csoma ZR, Doró P, Tálosi G, Machay T, Szabó M. Neonatal skin care in tertiary neonatal intensive care units in Hungary. Orv Hetil. 2014;155:1102–1107. doi: 10.1556/OH.2014.29910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data on skincare practices generated or analyzed during this study are included in this published article.