Abstract
Background
Millions of people suffer from Alzheimer's disease (AD) and Parkinson's disease (PD) worldwide. Due to their complex pathology, no effective pharmacological treatment has been found to date, despite extensive research. Developing new, effective therapeutic agents to cure these disease remains a major challenge. Although the cause of AD and PD remains illusive, numerous studies indicates that oxidative stress and neuro-inflammation lead to neurodegeneration in the central nervous system and play vital role in AD and PD morbidity and progression. Flavonoids, which are found widely in nature, exhibit anti-oxidative, anti-inflammatory, anti-mutative, anti-microbial, and neuroprotective properties, so have potential to treat these two kinds of diseases.
Methods
In this review, we focus on the anti-oxidative and neuroprotective action of flavonoids in attenuating Alzheimer's and Parkinson's disease, and how they might be harnessed in the development of new pharmacological agents to treat these two diseases.
Result
Some flavonoid compounds, like hesperidin, naringin, naringenin, tangeretin, nobiletin, silibinin, Epigallocatechin-3-gallate, displayed to be effective in both AD and PD.
Conclusion
Considerable studies have demonstrated the anti-AD and anti-PD effects of flavonoids through various in vitro and in vivo models. However, more rigorous studies are needed to be done for flavonoids to develop into effective drugs and apply them to clinical practice.
Keywords: Alzheimer's disease (AD), Parkinson's disease (PD) pathophysiology, Flavonoids, Anti-oxidation, Oxidative stress
Alzheimer's disease (AD); Parkinson's disease (PD) pathophysiology; Flavonoids; Anti-oxidation; Oxidative stress.
1. Introduction
Alzheimer's and Parkinson's disease, are progressive, chronic and severe conditions characterized by functional loss and neuronal death, and are associated with both motor and cognitive function impairment [1, 2, 3]. The number of patients living with these two diseases increases every year worldwide due to the aging population [4, 5]. AD and PD reduce physical and mental health, and are responsible for an increasingly large burden of disease [6, 7]. Despite considerable effort from many researchers and leading pharmaceutical companies over many years, there are no effective therapies for these two diseases. Developing potent pharmaceutical therapies for AD and PD is a tough challenge due to the complexity of pathological mechanisms and clinical features [8]. Some natural products, like flavonoids, alkaloids, and saponins, have been shown to be effective in preventing and treating neurodegenerative changes, indicating a possible therapeutic role in AD and PD [9, 10, 11, 12, 13]. This article focuses on the potential of flavonoids in Alzheimer's and Parkinson's disease therapy.
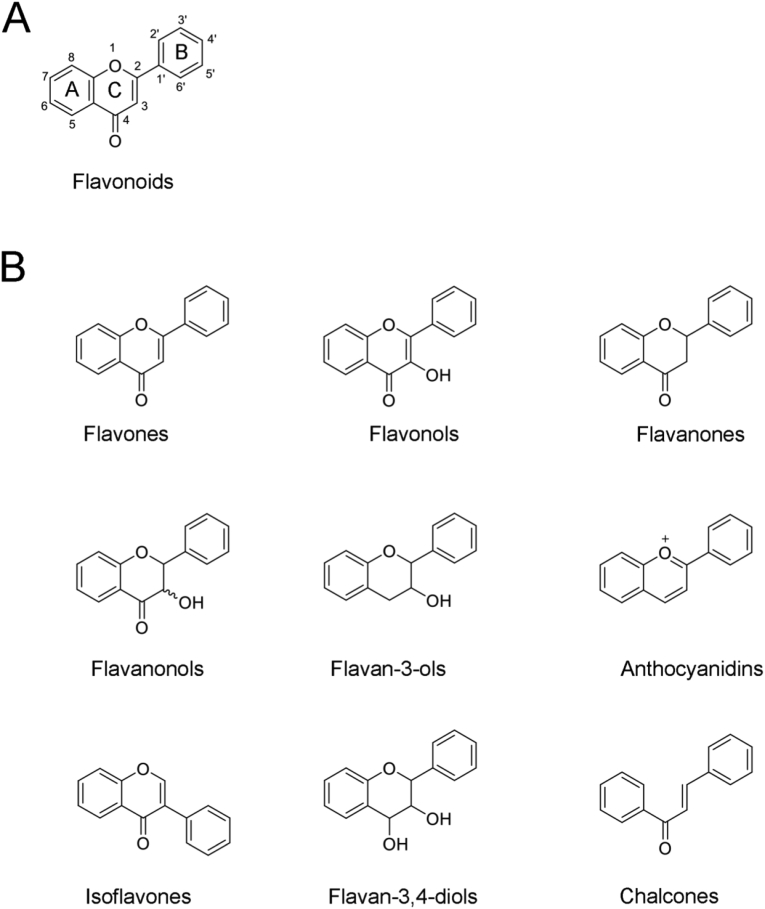
Flavonoids are ubiquitous in nature, and play various biological roles. These phytochemicals are polyphenols with two aromatic rings connected by a three-carbon chain (Figure 1 A). According to their chemical structures, flavonoids are classified into groups such as flavones, flavonols, flavanoes, flavanonols, flavanols (catechins), anthocyanidins (anthocyanins), isoflavones, dihydroflavonols, and chalcones (Figure 1 B). Flavonoids have some unique chemical and pharmacological properties due to their special structures [14, 15]. For example, they exhibit oil and water amphipathy, and have complex or electrostatic interactions with a variety of metal ions, reduce and trap free radicals, and combine with proteins [16, 17, 18, 19]. The strong anti-oxidative and neuroprotective activities of flavonoids are a comprehensive reflection of these properties. Flavonoids react with hyperoxidized free radicals, quench unpaired electrons and/or terminate the chain reactions of free radicals, enabling them to effectively scavenge free radicals. Therefore, flavonoids have been used extensively as powerful antioxidants, and are considered promising candidates for pharmaceutical agents to treat various Alzheimer's and Parkinson's disease [20, 21, 22].
Figure 1.
The structures of flavonoids (A) The basic structure of flavonoids (B) The structures of the main subgroups.
2. Neurodegenerative diseases and oxidative stress
Oxidative stress has both beneficial and deleterious effects on the central nervous system. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play important roles in hippocampal development, synaptic plasticity and axonal form [23, 24, 25]. Moreover, ROS seem to be involved in inflammation [26], signaling transduction [27], immune response [28], apoptosis [29], and are vital in intracellular signaling [30] and growth factor signaling [31, 32]. ROS regulate cytokines [33], tyrosine kinases [34], protein tyrosine phosphatases [35], serine/threonine kinases [36], and nuclear transcription factors [37]. Oxidative stress induces lipid peroxidation and damages nucleic acids and proteins [38]. Polyunsaturated fatty acids, such as arachidonic acid and linoleic acid, are main targets of lipid peroxidation, which induces serious injury to the neuronal membrane [39, 40]. Oxidative stress may damage ribonucleic acid (RNA) and nuclear deoxyribonucleic acid (DNA), especially the mitochondrial DNA [41, 42]. Mitochondrial dysfunction and abnormal energy metabolism are considered the major drivers of neuronal damage [41, 43].
Oxidative stress induces neuronal damage, which is the main pathogeny of AD and PD, and the disease states lead to greater production of ROS and subsequent oxidative injury [44, 45]. Although the molecular mechanisms underlying AD and PD have not been elucidated completely, convincing evidence demonstrates that oxidative stress, which causes numerous biological effects, including abnormal protein secretion and aggregation, mitochondria dysfunction, neuro-inflammation, neuronal death, may be one of the underlying generative mechanisms of these two diseases [46, 47, 48]. Therefore, reduction of oxidative stress is crucial in the treatment of these two diseases, and high-quality antioxidants are potential therapeutic agents.
3. Chemical structure and anti-oxidative activity of flavonoids
Flavonoids can not only remove the active free radicals of chain initiation reaction and metal ions of catalytic action, but directly capture the peroxide free radicals in the chain transfer stage and block the chain reaction [49]. According to the D-value of the heat of formation (△ HOF) and radical scavenging rete examination (including DPPH, ABTS and FRAP test), the anti-oxidative activity of flavonoid compounds is closely related to their structures [50]. The main structural factors affecting the anti-oxidative activity of flavonoids includes the number and location of the hydroxyl, ring-C yoke system, the glycoside or methylation of the hydroxyl, the lipid solubility and charge distribution [51, 52].
3.1. Phenolic hydroxyl and anti-oxidative activity
Flavonoids are compounds containing polyhydroxy groups. The more phenolic hydroxyl groups in the molecule, the more hydrogen atoms are bonded to the active free radicals and the stronger the phenolic hydroxyl groups anti-oxidation [53]. The ring-B is the main active site for anti-oxidation and scavenging of free radicals [54]. The presence of the o-diphenolhydroxy group on the ring-B greatly enhances anti-oxidative activity, because it scavenges superoxide anions (O2-). and is considered to be the structural basis for the anti-oxidative properties of flavonoids [55, 56]. Flavonoids with strong anti-oxidative activity generally have a 3′, 4′-catechol structure, among which the 4 hydroxyl group is particularly important [57]. When the number of phenolic hydroxyl groups in the ring-B increases to a certain amount, the anti-oxidative activity no longer increases with the number of phenolic hydroxyl groups. Besides, the o-dihydroxyl group on the ring-A also has anti-oxidative activity [58, 59]. The 5-and 7-hydroxy groups are beneficial to anti-oxidative activity, and can be complexed with metal ions. Moreover, the 7-hydroxy group can increase the anti-oxidative activity in the presence of 4′hydroxyl [60]. The hydroxyl group on the ring-C also affect the anti-oxidative activity of flavonoids. The absence of the hydroxyl group at the C3 position decreases the anti-oxidative activity of flavonoids [57, 61].
3.2. Ring-C yoke system and anti-oxidative activity
The existence of ring-A and ring-B conjugate systems through the double bond between C2 and C3 and the 4-position carbonyl group promotes the formation of electron delocalization, which helps the flavonoids form relatively stable free radical intermediates after hydrogen supply and improves their anti-oxidative capacity. The anti-oxidative activity decreases if the double bond is hydrogenated [62].
3.3. Other factors
Hydroxyl glycosyl substitution or methylation has an adverse effect on anti-oxidative activity, and the effect is closely related to the substituent position [63, 64]. It is generally believed that 7-oxyglycoside and 6, 8-carboglycoside strongly reduce anti-oxidative activity, while 3-oxyglycoside has little effect [65, 66]. The 4-position carbonyl group also has an effect on the anti-oxidative capacity of flavonoids [65]. The presence of the 4-position carbonyl group prolongs the conjugate system, which is conducive to the formation of more stable free radical intermediates from flavonoids, and therefore is conducive to anti-oxidative activity [62, 67].
4. Flavonoids and Alzheimer's diseases treatment
Alzheimer's disease is the most common neurodegenerative diseases (NDDs), with the gradual increase in the population of the elderly over 65 years old, the number of AD patients is also increasing year by year [5, 68]. Pathologically, it is characterized by atrophy and/or loss of cortical neurons, extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (NFT) [69, 70]. AD is driven by a complex interplay between genetic and environmental factors. More than 20 genes have been proved to be genetic risk factors for AD, among which the APOE gene is considered the most dangerous factor [70, 71]. Abnormal secretion of Aβ, high phosphorylation and distribution of microtubule-associated protein tau (tau), diminished cerebral glucose metabolism, and the downstream pathological consequences (synaptic dysfunction, mitochondrial dysfunction, neuron atrophy and/or loss, macroscopic atrophy of the cerebellum and/or hippocampus) contribute to neurodegeneration [72, 73] As a major public health problem, although the research into the mechanisms of AD has continued for many years, few treatments have been approved.
Oxidative stress contributes to Aβ formation and tau pathology. The oxidation of lipids, proteins, and nucleic acids occurs decades prior to Aβ plaque formation, and seems to precede senile plaques and NFTs. Soluble Aβ at pathological levels reduces the activities of key enzymes (such as cytochrome oxidase, Mn-SOD) and disrupts mitochondrial dynamics [74, 75]. Hyper-phosphorylated tau protein, the major component of NFTs and a hallmark of AD, is involved in the neurodegeneration associated with oxidative stress in AD [76, 77]. Oxidative stress leads to mitochondria dysfunction, induces abnormal Aβ secretion and aggregation, makes tau hyper-phosphorylation, synaptic plasticity deficit and learning and memory impairment. Hence, antioxidants are potential therapeutics due to their ability to reduce Aβ level and tau phosphorylation, eliminate ROS, and protect neurons in AD.
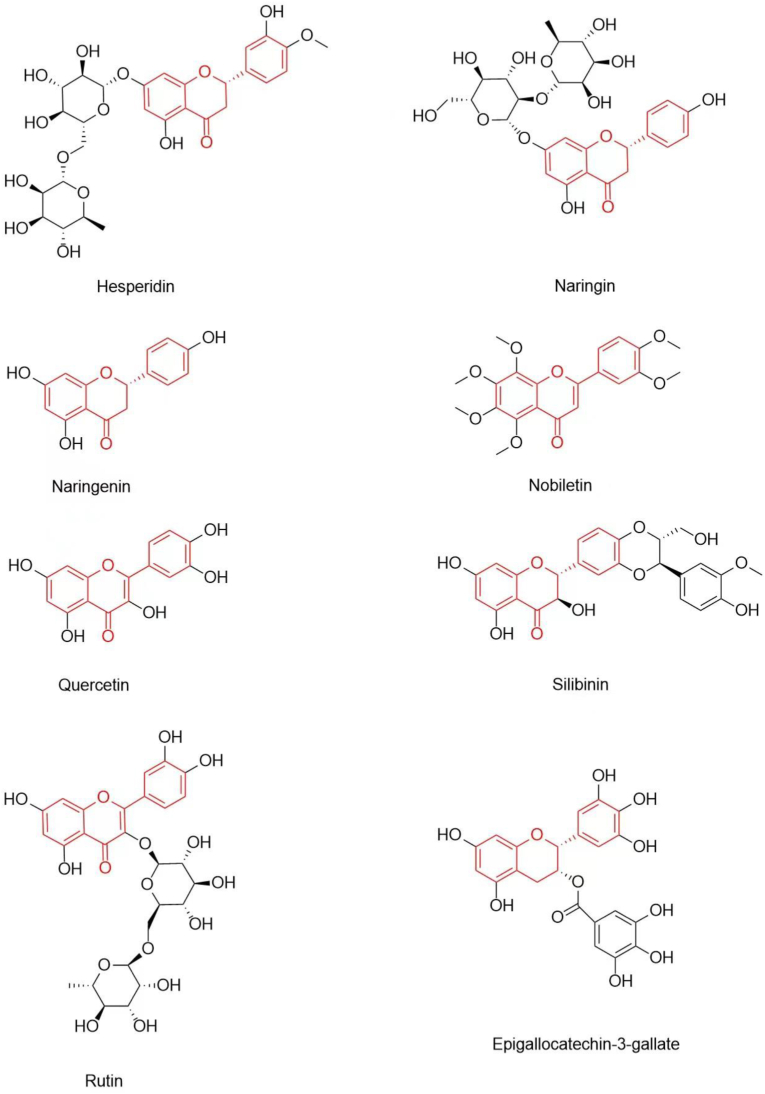
Flavonoids have been described as excellent antioxidants, free radical scavengers, and metal chelators [22, 78, 79]. They have been reported to attenuate cholinergic deficits, reduce neurotoxic Aβ abnormal accumulation, and inhibit tau protein hyper-phosphorylation, which makes them to be promising candidate for AD prevention and treatment [80, 81, 82, 83, 84]. Hesperidin, naringin, naringenin, tangeretin, nobiletin, quercetin, rutin, silibinin, anthocyanins, and epigallocatechin-3-gallate are the most-studied flavonoids exhibiting potential therapeutic activities against AD (Figure 2 and Table 1).
Figure 2.
Chemical structures of flavonoids which exhibited effective activities against AD.
Table 1.
Characteristics of flavonoids exhibiting effective activities against AD in the review.
| Flavonoid | Molecular | Source | Effects | Model and dose |
|---|---|---|---|---|
| Hesperidin | C28H34O15 | unripe citrus fruit, like orange, grapefruit, lemon, etc. | anti-oxidative stress, prevent glutamate excitotoxicity, alleviate mitochondrial dysfunction, reduce Aβ accumulation, attenuate cognitive deficit, etc. | kainic acid (KA)-induced rats model, 10 or 50 mg/kg [138], APP/PS1 mice model, 40 mg/kg for 90 days [87], 100 mg/kg for 10 days [88], APPswe/PS1dE9 transgenic mice, 50 or 100 mg/kg for 16weeks [89]. |
| Naringin | C27H32O14 | citrus fruit, like citrus grandis, citrus paradise, etc. | anti-oxidation, anti-apoptotic, anti-inflammation, neuroprotection, memory improvement, etc. | gp120-induced rats model, 30 mg/kg [95], Swiss albino model mice, UCMS mice, 80 mg/kg for 21 days [93], female Wistar rats, 50 mg/kg [93] |
| Naringenin | C15H12O5 | citrus fruit, like citrus grandis, citrus paradise, etc. | anti-oxidation, anti-nociceptive, anti-inflammation, etc. | KO2-induced mice model, 50,150 mg/kg [139], Aβ-induced rats model [98], lipopolysaccharide (LPS)-induced rats model, 25, 50, or 100 mg/kg/day [99], intracerebroventricular-streptozotocin (ICV-STZ) induced rats model, 50 mg/kg for 2 weeks [100], AlCl3+D-gal induced rats model, 50 mg/kg) for two weeks [101] |
| Nobiletin | C21H22O8 | peel of citrus fruits, like shiikuwasa, oranges, lemons, etc. | anti-oxidation, improve learning and reduce memory impairment, reduce soluble Aβ and Aβ plaque, reverse tau hyperphosphorylation, etc. | OBX-, Aβ-, MK-801, LPS-induced mice model; Tg AD model mice, 30 mg/kg, 100 mg/kg [104, 105, 106, 108, 109, 110] |
| Quercetin | C15H10O7 | onions, apples, broccoli, and red wine, etc. | anti-oxidation, anti-inflammation, anti-apoptotic, etc. | Aβ-induced rats model, 100 mg/kg for 18 day [115], APPswe/PS1dE9 transgenic AD model mice, 20, 40 mg mg/day for 16 weeks [116], triple transgenic AD model (3xTg-AD) mice, 25 mg/kg for 3 months [117], thalassemic patients, 500 mg/day [114] |
| Rutin | C27H30O16 | Ruta graveolens L., Scphora japonica L., etc. | improve memory deficit, scavenge superoxide radicals, increase antioxidant enzymatic activity, etc. | Aβ induced rats model, 100 mg/kg [119], female Wistar rats, 100 mg/kg [93] |
| Silibinin | C25H22O10 | dried fruits and seeds of Silybum marianum (L.) Gaertn | anti-oxidation, anti-inflammation, anti-aging, ameliorates memory deficits, etc. | STZ- induced rats' model, Aβ25-35 induced mice model, 25, 50, and 100 mg/kg [124, 128]; UVB-irradiated mice model, 50 mg/kg [125] |
| Anthocyanins | a kind of polyphenolic flavonoid | fruits, flowers, grains, and vegetables | anti-oxidation, reduce Aβ production, prevent tau phosphorylation, protect neurons damage, improve synapse function, enhance learning and memory, etc. | APP/PS1 mice model, 12 mg/kg [127] LPS-induced mice model, 24 mg/kg [129]; Aβ-induced mice model, 4 mg/kg [131]; ovariectomized rat, 200 mg/kg [134] |
| Epigallocatechin-3-gallate | C37H30O18 | green tea | anti-oxidation, inhibit Aβ fibrillation, restrain tau phosphorylation, improve learning and memory, etc. | morphine-induced rats' model, 5 and 50 mg/kg [137] |
Hesperidin (C28H34O15) (2S)-5-hydroxy-2-(3-hydroxy- 4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl 6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside, is a flavonoid present primarily in unripe citrus fruit, like orange, grapefruit, lemon, and tangerine juice [85]. This natural compound presents potent anti-oxidative activity, exerts neuroprotective effects and could cross the blood–brain barrier properties [86]. Studies have shown that hesperidin could inhibit evoked glutamate release and attenuates KA-induced neuronal death [86]. Hesperidin was demonstrated to promote neuronal differentiation and mediate neuronal survival [86]. Besides, studies have identified that this compound could activate Akt/Nrf2: Akt/Nrf2 signaling (Akt/Nrf2) and inhibit RAGE/NF-κB signaling (RAGE/NF-κB), through which suppresses oxidative stress, shows neuroprotection and attenuates learning and memory deficits in APPswe/PS1De9 (APP/PS1) transgenic AD model mouse [86]. Moreover, hesperidin has been demonstrated to restore non-cognitive nesting ability and social interaction deficit through reducing Aβ deposition and neuro-inflammatory [87]. Furthermore, hesperidin was reported to reduce β-amyloid precursor protein (APP) expression and Aβ accumulation, inhibit inflammatory markers (like NF-κB), attenuate cognitive impairment, and alleviate mitochondrial dysfunction and oxidative stress [88, 89].
Naringin (C27H32O14), 4’, 5, 7-trihydroxyflavanone-7- rhamnoglucoside, is another flavonoid present in citrus grandis, citrus paradise, and other citrus. This phytochemical exhibits strong anti-oxidative, anti-apoptotic and anti-inflammatory activities [90, 91, 92]. Studies have demonstrated that naringin treatment significantly decreased the expression of purinergic receptor P2X7 (P2X7) and a member of nuclear factor-kappaB (P65), reduced the agonist of P2X7 (BzATP) activated current, and could improve glycoprotein 120 (gp120) induced learning and memory dysfunction [98]. Furthermore, it has been identified that narigin could enhance memory in stressed and unstressed mice probably by decreasing AChE activity and by inhibition of inducible NOS [93, 94]. Naringin was reported to have neuroprotective potential with beneficial effects on spatial, emotional and episodic memories [93, 94]. Naringenin (C15H12O5), 4′,5,7-trihydroxyflavanone, is the aglycone of naringin and blood–brain barrier penetrating compound. This natural compound also exhibits excellent anti-oxidative activity probably via activation of the NO-cGMP-PKG-KATP channel signaling involving the induction of Nrf2/HO-1 pathway [95]. Studies have shown that naringenin could rescue Aβ evoked neurotoxicity through maintaining the mitochondrial membrane potential and resisting ROS production [96]. naringenin was identified to reduce Aβ plaque probably by increasing Aβ degradation enzymes [97]. Moreover, studies have demonstrated that naringenin pretreatment could ameliorate Aβ-induced learning and memory impairment through mitigation of lipid peroxidation and apoptosis [98]. In addition, naringenin was reported to alleviate LPS-induced cognitive deficits and neuroinflammation by attenuation of oxidative stress and AChE and modulation of Nrf2/NF-κB/TNFα/COX2/iNOS/TLR4/GFAP [99]. Naringenin was reported to protect against intracerebroventricular-streptozotocin (ICV-STZ) induced cognitive deficits, neuronal injury and oxidative stress [100]. Naringenin was proven to protect AlCl3+D-gal induced AD-like behavioral disturbances and AD-like symptoms [101].
Nobiletin (C21H22O8), 5,6,7,8,3′,4′-hexameth-oxyflavone, is a polymethoxylated flavone found in the peel of citrus fruits such as Citrus depressa (shiikuwasa), Citrus sinensis (oranges), and Citrus limon (lemons). It has been proven to be a retinoic acid-related orphan receptor (ROR) agonist [102]. Nobiletin was identified to modulate cellular antioxidant defense systems and increase the activity of glutathione peroxidase (GPx) [103, 104]. In addition, nobiletin was shown to halt acetylcholinesterase-positive fiber density decrease, reduce soluble Aβ and Aβ plaque levels, and ameliorate the generation of free radicals [105, 106]. Moreover, nobiletin was demonstrated to reverse tau hyperphosphorylation, activate ERK signaling, trigger cAMP transcription, and suppress caspase 3 and Bax expression [107, 108]. This natural flavone attracted great attention when its anti-dementia and neuroprotective properties were discovered [103, 104]. Studies have demonstrated that nobiletin could improve learning and reduce memory impairment in olfactory-bulbectomized (OBX), Aβ, MK-801 (dizocilpine maleate) -induced AD model mice, and could ameliorate these problems in transgenic AD model mice (such as amyloid precursor protein Tg mice, 3XTg-AD mice) [105, 109, 110].
Quercetin (C15H10O7), 3, 3′, 4’, 5, 7-pentahydroxyflavone, is a flavonoid present in onions, apples, broccoli, and red wine. It has been proved to inhibit many enzyme systems, including tyrosine protein kinase, phospholipase A2, phosphodiesterase, mitochondrial ATPase, PI3-kinase, and protein kinase C [111, 112]. This natural phytochemical was proven to reduce the oxidative damage caused by iron overload [113]. Quercetin was reported to reduce iron overload and exhibit anti-oxidative, anti-inflammation, cardio-protective, neuroprotective, and anti-apoptotic properties [114]. Quercetin was identified to decrease MDA, increase SOD and GSH [115]. Studies showed that quercetin could modulate Nrf-2 translocation from cytoplasm to nucleus, protect mitochondrial dysfunction [116]. Besides, Quercetin was identified to inhibit Aβ plaque aggregation and neurofibrillary tangles formation [116, 117]. Studies identified that pretreatment quercetin could improve cognitive memory probably by decreasing Aβ level, antioxidant activity and increasing Nrf2/HO-1 pathway (Nrf2/HO-1) [115]. Quercetin was proven to improve cognitive functioning in the APPswe/PS1dE9 transgenic AD model mouse by reducing plaque mitochondrial dysfunction through the activation of AMPK [116]. Quercetin was identified to reverse histological hallmarks of AD and protect cognitive and emotional function in aged triple transgenic AD model (3xTg-AD) mice [117]. Rutin (C27H30O16), 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl-6-O-(6-deoxy-α-L-mannopyrano-syl)-β-D-glucopyranoside, is a glycone of quercetin with a flavonol structure. This compound was reported to scavenge superoxide radicals, increase antioxidant enzymatic activity, and reduce lipid peroxidation and cytokine production [118]. Moreover, rutin was identified to improve memory deficit induced by Aβ through the MAPK and brain-derived neurotrophic factor (BDNF) pathways [119]. In addition, this compound was proven to alleviate episodic memory deficits and have beneficial effects on spatial, emotional and episodic memories [119].
Silibinin (C25H22O10) (2R, 3R)-3, 5, 7-trihydroxy-2-[(2R, 3R)-3-(4-hydroxy-3-methoxyphenyl)-2- (hydroxymethyl)- 2, 3-dihydro-1, 4-benzodioxin-6-yl]-2,3-dihydro-4H-chromen-4-on, also called silybin and silymarin, is a flavonoid isolated from the dried fruits and seeds of Silybum marianum (L.) Gaertn [120]. it has anti-oxidative, anti-inflammatory, anti-radiation, anti-aging, and delaying skin aging activities [121, 122, 123]. This natural flavonoid has been shown to modulate the pro-inflammatory cytokines IL-1β and IL-4, the antioxidant enzyme glutathione (GSH), malondialdehyde (MDA), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) [124]. It is reportedly involved in the inflammatory process and autophagy [125]. In addition, studies have demonstrated that silybin could suppress Aβ25–35 induced abnormal increase in nitrotyrosine, and inhibit the overexpression of iNOS and TNF-α [126]. Moreover, silybin has been identified to decrease STZ-induced tau hyperphosphorylation (ser404), alleviate Aβ25–35 and streptozocin-induced memory deficits, reverse learning and memory damage [127, 128].
Anthocyanins, a kind of polyphenolic flavonoid commonly present in fruits, flowers, grains, and vegetables, present antioxidant, anti-inflammatory, and anti-apoptotic properties [127, 129]. Studies have demonstrated that anthocyanins could reduce the levels of Aβ, BACE-1, and APP, and elevate the levels of synaptophysin, postsynaptic density protein 95 (PSD95), synaptosome associated protein23 (SNAP23), glutamate receptor 1 phosphorylated at serine 845 (p-GluR1 (Ser845)), and cyclic AMP response element binding protein phosphorylated at serine 133 (p-CREB (Ser133)) [130,131]. In addition, anthocyanins were reported to increase phosphorylated phosphoinositide 3-kinase (p-PI3K), protein kinase B phosphorylated at serine 473 (p-Akt (Ser473)), glycogen synthase kinase 3β phosphorylated at serine 9 (p-GSK3β (Ser9)) levels, and decrease the phosphorylation level of tau at Ser413 and Ser404 [127]. Studies also have demonstrated that anthocyanins could reduce the ratio of ratio of Bax to Bcl-2 (Bax/Bcl2), inhibit Cyt c, caspase-9, cleaved caspase-3, and poly (ADP-ribose) polymerase-1 (PARP-1) [132,133]. Moreover, anthocyanins have been reported to reduce Aβ production, prevent tau phosphorylation, protect neurons damage, improve synapse function, and enhance learning and memory [134].
Epigallocatechin-3-gallate (EGCG, C37H30O18) is a major flavonoid in tea. This compound has been shown to inhibit Al (III)-induced Aβ fibrillation, and remold the preformed, mature, toxic fibrils into low toxic amorphous aggregates [135]. In addition, EGCG was identified to restrain tau phosphorylation, inhibit tau aggregation and recast tau oligomers to an unfolded monomeric state [136]. Furthermore, studies also have shown that EGCG could improve learning and memory impairment [137].
5. Flavonoids and Parkinson's diseases treatment
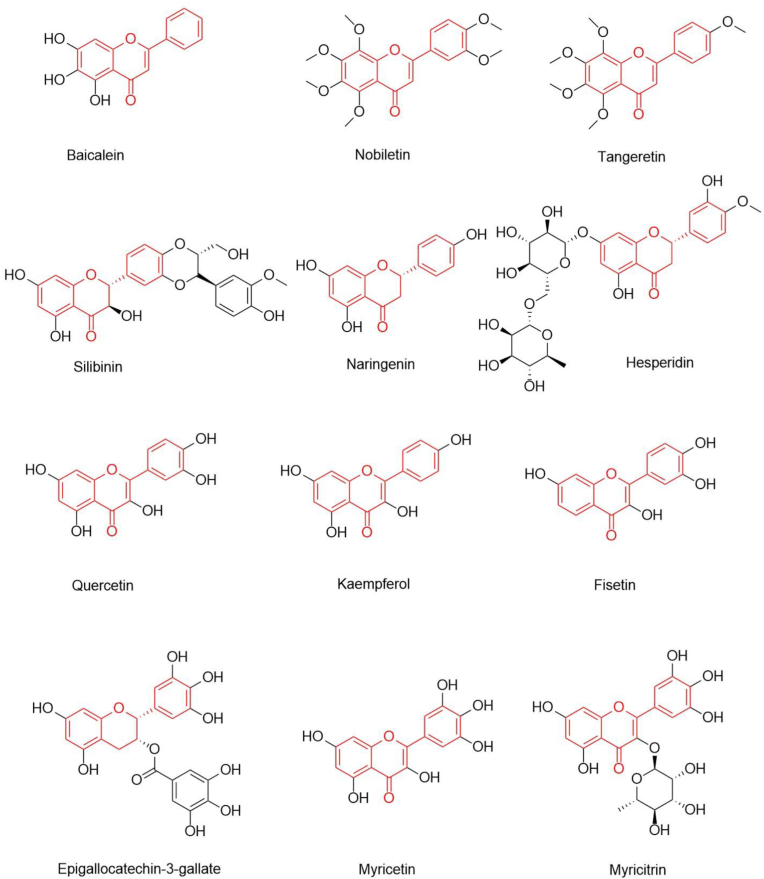
Parkinson's disease, the second most common neurodegenerative disease after AD, is increasing in prevalence worldwide [4, 140]. Chronic and progressive loss of dopaminergic neurons and presence of intracellular Lewy bodies in the substantia nigra (SN) are two major hallmarks of PD. Lesions occurring in the SN induce a decrease in dopamine (DA) content and weakening DA nerve function. This breaks the balance between dopaminergic neurons and cholinergic neurons, resulting in serious movement disturbances, such as tremors, rigidity, and bradykinesia and/or cognitive dysfunction [141, 142]. Although the causes of the lesions of SN and the consequent dopaminergic neurons damage remain unclear, studies have demonstrated that cellular oxidative stress plays a central role in this process. Natural antioxidants are believed to reduce the risk of PD [143, 144], and thus in recent years, the pharmaceutical industry and researchers have turned their attention to flavonoids due to their excellent antioxidative activity and other therapeutic properties [22, 145, 146]. Baicalein, nobiletin, tangeretin, silibinin, naringenin, hesperidin, quercetin, kaempferol, fisetin, epigallocatechin-3-gallate, myricitrin, and myricetin are the most studied flavonoids for their value in PD treatment (Figure 3 and Table 2).
Figure 3.
Chemical structures of flavonoids which have shown efficacy in PD.
Table 2.
Characteristics of flavonoids showing efficacy in PD in the review.
| Flavonoid | Molecular | Source | Effects | Model and dose |
|---|---|---|---|---|
| Baicalein | C15H10O5 | the roots of Scutellaria baicalensis Georgi., etc. | anti-oxidation, prevent α-synuclein accumulation, disaggregate α-synuclein fibrils, prevent dopaminergic neuron loss, attenuate behavioral impairments, etc. | collagenase-induced ICH rats model, 50 mg/kg [153]; AlCl3-induced rats model, 5 mg/kg and 10 mg/kg [154]; MPTP-induced mice model, 10 mg/kg and 50 mg/kg [152] |
| Nobiletin | C21H22O8 | peel of citrus fruits, like shiikuwasa, oranges, lemons, etc. | anti-oxidation, prevent MPP+-induced DA neuron damage, increase TH activity, reduce motor impairment and cognitive deficits, etc. | MPTP-induced rats' model, 10 mg/kg [157] |
| Tangeretin | C20H20O7 | citrus fruit, like grapefruit, tangerines, mandarins, oranges, etc. | anti-oxidation, protect 6-OHDA-/MPTP/P-induced DA declination, dopaminergic neuron damage, reduce behavioral deficits, etc. | 6-OHDA-insuced rats' model, 10 mg/kg [164]; MPTP-induced rats' model, 50, 100 or 200 mg/kg [166] |
| Silibinin | C25H22O10 | dried fruits and seeds of Silybum marianum (L.) Gaertn | anti-oxidation, prevent MPTP-caused TH+ neuronal loss, decrease dopamine depletion, ameliorate motor behavior, etc. | METH induced mice model [168] MPP+ induced model, 10, 50, or 100 mg/kg [169] |
| Naringenin | C15H12O5 | citrus fruit, like, citrus grandis, citrus paradise, etc. | anti-oxidation, protect 6-OHDA-induced TH+ neuronal loss, increase DA and its metabolic levers, etc. | hypercholesterolemic diet (HCD) rats' model, 50 mg/kg [173]; isoflurane induced rats' model, 25,50,100 mg/kg [176] |
| Naringin | C27H32O14 | citrus fruit, like, citrus grandis, citrus paradise, etc. | anti-oxidation, prevent MPP+-induced DA neurotoxicity, activate mTORC1 and anti-inflammation, etc. | KA-induced mice model, 80 mg/kg [177] |
| Hesperidin | C28H34O15 | unripe citrus fruit, like orange, grapefruit, lemon, etc. | anti-oxidation, reduce 6-OHDA-induced memory impairment and depression-like behavior, improve synapse formation and function, enhance memory, etc. | 6-OHDA-induced mice model, 50 mg/kg [179] MTX-induced rats model, 100 mg/kg [180]; adult mice, 10 mg/kg [181] |
| Quercetin | C15H10O7 | onions, apples, broccoli, and red wine, etc. | anti-oxidation, protect 6-OHDA-/MPTP-induced DA depletion, improve behavior impairment, etc. | Cadmium (Cd) induced mice model, 25, 50, 100 mg/kg [182]; CdCl2 induced rats model, 20 mg/kg [183]; 6-OHDA induced rats model, 50 mg/kg [185] |
| Kaempferol | C15H10O6 | kaempferol galanga L, etc. | anti-oxidation, reduce behavior impairment and increase striatal DA and its metabolites, etc. | I/R rats model, 1.75, 3.49, 6.99 mM [192]; ovariectomized (OVX) rats model, 10 mg/kg [193] |
| Fisetin | C15H10O6 | strawberries and lower levels in apples and persimmons, etc. | anti-oxidation, protect MPTP/MPP+-induced dopaminergic toxicity, TH+ loss, and decrease α-synuclein aggregation, etc. | D-gal induced rats' model, 15 mg/kg [195] |
| Epigallocatechin-3-gallate | C37H30O18 | green tea, etc. | anti-oxidation, protect DA depletion and DA neuronal loss, inhibit a-synuclein aggregation and Aβ fibrillogenesis, etc. | sevoflurane-induced mice model, 25, 50, 75 mg/kg [198]; 6-OHDA-induced rats model, 10 mg/kg [200, 201, 205]; MPTP- induced mice model,25,50 mg/kg [204, 206] |
| Myricetin | C15H10O8 | berries, vegetables, tea and wine. | anti-oxidation, reduce MPP+-induced DA-like cell loss, prevent 6-OHDA-/6-hydroxydopamine-induced TH+ and DA neuron degeneration, etc. | 6-OHDA induced rats' model, 0.5 mg/mL [208] |
| Myricitrin | C21H20O12 | Myrica cerifera, Myrica esculenta, Ampelopsis grossedentata, etc. | anti-oxidation, reduce 6-OHDA-/MPP+-induced DA neurotoxicity, dopaminergic neuronal loss, etc. | 6-OHDA induced mice model, 30 mg/kg [212] |
Baicalein (C15H10O5), 5,6,7-trihydroxyflavone, a natural flavonoid extracted from the roots of Scutellaria baicalensis Georgi, possesses neuroprotective, anti-oxidative, anti-inflammatory, and antiapoptotic properties [147]. Studies have shown that this compound could protect against 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenylpyridinium (MPP+), glutamate, Aβ, hydrogen peroxide (H2O2), 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP), and methamphetamine-induced neurotoxicity. α-syn plays a vital role in PD pathomechanism, diagnosis and treatment [148, 149]. Studies have demonstrated that baicalein could prevent α-synuclein accumulation, and disaggregate mature α-synuclein fibrils [150, 151]. Furthermore, this flavonoid has been shown to attenuate behavioral impairments [152], and prevent dopaminergic neuron loss in the SN [153, 154]. In addition, baicalein was reported to restore mitochondrial function, reduce the activation of microglia and astrocytes, and increase the levels of neurotransmitters such as DA, 3,4-dihydroxyphenylacetic acid (DOPAC), and homo vanillic acid (HVA) [155].
Nobiletin, a RORs agonist, can inhibit neuro-inflammation, regulating apoptotic signaling and protect dopaminergic neurons [156, 157, 158]. This natural compound has been reported to prevent MPP+-induced DA neuron damage in the SN [157]. Furthermore, nobiletin has been shown to increase TH activity by stimulating Ca2+/calmodulin-dependent protein kinase Ⅱ (CaMKII)- and PKA-dependent phosphorylation, leading to reduce MPTP-induced motor impairment and cognitive deficits [157, 159, 160].
Tangeretin (C20H20O7), 5,6,7,8-Tetramethoxy-2-(4-methoxyphenyl)-4-benzopyrone, a flavonoid isolated from the peel of citrus fruit such as grapefruit, tangerines, mandarins, and oranges, is a potent inhibitor of Notch-1 [161]. This compound has been proven to inhibit reactive oxygen species production and p47phox phosphorylation, enhancing the expression of heme oxygenase-1 and the DNA binding activity of nuclear factor-erythroid 2-related factor 2 to the antioxidant response element, and thus possess a potent antioxidant effect in LPS-stimulated microglia [162, 163]. Studies demonstrated that tangeretin can reduce 6-OHDA-induced decline in tyrosine hydroxylse-positive (TH+) cells and improve the reduction of striatal DA in a dose-dependent manner [164]. In addition, this natural flavonoid has been reported to upregulate the mRNA levels of UPR-target genes in dopaminergic neurons and astrocytes after chronic MPTP/probenecid (MPTP/P) injections [165]. Tangeretin was also reported to protect against MPTP/P-induced dopaminergic neuron damage, maintain striatonigral integrity, and reduce behavioral deficits [166].
Silibinin exhibits excellent anti-oxidative and anti-inflammatory activities [167]. Treatment with silibinin in aging animals can decrease dopamine depletion, ameliorate motor behavior, reduce MDA content and increase GSH content [168]. Studies have also demonstrated that silibinin could prevent MPTP-caused TH+ neuronal loss in the striatum and SN [169].
Naringenin is a flavonoid present in tomatoes, grapes, grapefruit and other citrus fruits, which has been demonstrated to have anti-nociceptive, anti-inflammatory, and anti-oxidative activities [170, 171]. Studies have shown that naringenin treatment could increase nuclear factor erythroid 2-related factor 2 (Nrf2) protein levels and activate the antioxidant response element pathway in a 6-OHDA-induced PD model [172]. In addition, pre-treatment with naringenin can protect 6-OHDA-induced ROS damage, striatum and SNC TH+ neuronal loss [173, 174]. Moreover, naringenin has been reported to increase DA and its metabolic levers, like DOPAC and HVA [175].
Naringin, naringenin 7-O-rutinoside, has been reported to inhibit oxidative stress and reduce neuronal loss and neuro-inflammation, and therefore has been suggested to have potential as a therapeutic agent against NDDs [176]. Naringin has been shown to prevent MPP+-induced nigrostriatal DA neurotoxicity through anti-inflammatory activity [177]. In addition, naringin was reported to activate mechanistic target of rapamycin complex 1 (mTORC1) and anti-inflammation in DA neurons [176, 178].
Hesperidin has been demonstrated that it’s treatment could effectively reduce the activity of glutathione peroxidase and catalase, and significantly decrease total reactive antioxidant potential, thereby could reduce 6-OHDA-induced memory impairment and depression-like behavior [179, 180]. Furthermore, this nature flavonoid has been proven to induce neuron synapse formation and function between the hippocampus and cortex, and improve memory [181].
Quercetin has been shown to protect against oxidative damage, reduce DA depletion, improve behavior, and maintain the resting membrane potential of neurons in MPTP-induced PD model mice [182, 183]. Studies have demonstrated that quercetin treatment could increase striatal DA, improve neuronal survival, and raise antioxidant enzyme levels in 6-OHDA-induced PD model mice [184, 185, 186]. In addition, quercetin was demonstrated to attenuate the loss of mitochondrial complex-I activity and enhance endogenous antioxidant enzyme activity [187]. Quercetin has been revealed to upregulate the phosphorylation of protein kinase D1, protein kinase B (Akt), cyclic AMP response element binding protein (CREB), and BDNF expression in DA neuronal cells [188]. Rutin, troxerutin (C33H42O19), and isoquercitrin (C21H20O12), the glycosides of quercetin, have been shown to prevent 6-OHDA-induced neurotoxicity [189]. Moreover, troxerutin was reported to improve apomorphine-induced behavior impairment, prevent nigral TH+ neuronal loss, lessen striatal lipid peroxidation, and diminish mitochondrial oxidative stress [190, 191].
Kaempferol (C15H10O6), 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one, is a flavonoid mainly isolated from the rhizome of kaempferol galanga L., which has been shown to possess neuro-protective effect [192]. Studies have demonstrated that this compound could reduce behavior impairment and increase striatal DA and its metabolites [193]. In addition, this flavonoid has been proven to protect against neurotoxicity and preserve the striatal glutamatergic response by autophagy in a PD model [194].
Fisetin (C15H10O6), 3,7,3′, 4’ tetrahydroxyflavone, abundant in strawberries and lower levels in apples and persimmons, is a dietary flavonoid. This natural compound protects brain tissue against oxidative stress, apoptosis and neurodegeneration [195, 196]. Studies have shown that fisetin could protect against MPTP/MPP+-induced dopaminergic toxicity, prevent TH+ loss, and decrease α-synuclein and inflammation [197].
Epigallocatechin-3-gallate has been shown to protect against mitochondrial oxidative stress-induced neuronal apoptosis through CREB/BDNF/TrkB, PI3K/Akt/mTOR, and PI3K/AKT/eNOS signaling [198, 199]. Furthermore, this compound has been identified to protect against 6-OHDA-induced neuronal toxicity by stimulating protein kinase C (PKC) and modulating cell survival/cycle genes [200, 201, 202]. Moreover, studies have demonstrated that EGCG could inhibit a-synuclein aggregation and Aβ fibrillogenesis [203, 204]. EGCG also has been proven to protect striatal DA depletion and DA neuronal loss in MPTP-/6-OHDA-induced PD model mice [205, 206].
Myricetin (C15H10O8), 3, 5, 7, 3′, 4′, 5′-hexahydroxy-flavone, occurs in berries, vegetables, tea and wine. This nature compound was reported to reduce MPP+-induced DA-like cell loss and nuclear condensation by suppressing ROS production and inhibiting mitogen-activated protein kinase 4 (MKK4) and c-Jun N-terminal kinases (JNK) [207]. In addition, studies have demonstrated that myricetin could prevent 6-OHDA- and 6-hydroxydopamine-induced TH+ and DA neuron degeneration [208].
Myricitrin (C21H20O12), 3, 5, 7, 3′, 4′, 5′-hexahydroxyflavone 3-O-rhamnoside, the glycoside of myricetin, is abundant in the root bark of Myrica cerifera, Myrica esculenta, Ampelopsis grossedentata, Nymphaea lotus, and Chrysobalanus icaco. This compound possesses anti-oxidative, anti-inflammatory, and anti-nociceptive, and neuro-protective activities [209]. Studies have identified that, like myricetin, myricitrin could reduce MPP+-induced mitochondrial dysfunction, prevent 6-OHDA-induced mitochondrial oxidation, restore mitochondrial damage, ameliorate apoptosis dysfunction, and protect against DA neurotoxicity through DJ-1 activity [210, 211]. Furthermore, studies have shown that myricitrin could amend 6-hydroxydopamine-induced SN dopaminergic neuronal loss [212].
6. Conclusion
The prevalence of AD and PD is on the rise worldwide, and effective pharmacological treatments are needed urgently. Considerable evidence supports the involvement of oxidative stress in the development and progression of these two NDDs. Oxidative stress induces overproduction of ROS and RNS, along with mitochondrial dysfunction, neuroinflammation and neurodegeneration, and plays a vital role in the pathological processes of these two diseases. Therefore, antioxidants are promising candidates for AD and PD therapeutics.
This review provides an overview of the research on the effects of various plant flavonoids on AD and PD. Interestingly, some flavonoid compounds, like hesperidin, naringin, naringenin, tangeretin, nobiletin, silibinin, Epigallocatechin-3-gallate, displayed to be effective in both AD and PD. Tt is well known that oxidative stress induced damages may be one of the major pathological mechanism in both AD and PD. Oxidative stress leads to abnormal protein (such as Aβ and a-synuclein), neuro-inflammation mitochondrial dysfunction, and neuronal loss (including cholinergic neurons and dopaminergic neurons). Besides, some flavonoid metabolites (especially some specific products of bacterial transformation) attracted researchers and pharmacy's attention, for their stronger bioactivities [213]. Such as anthocyanins phenolic acid metabolites (including 4-Hydroybenzoic acid, protocatechuic acid, gallic acid, vanillic acid, 3-O-methygallic acid, syringic acid) have been demonstrated to anti-oxidant, anti-neuroinflammation, interfere proteins aggregation, and show good neuroprotective activities [214].
Evaluation of studies have demonstrated the anti-AD and anti-PD effect of flavonoids through various in vitro and in vivo models. However, more rigorous studies are necessary to confirm dosage, the possible targets, the absorption and trans-blood brain barrier (BBB) permeability, address safety and other issues. It will be many years before flavonoids are developed into effective pharmaceuticals and applied in clinical settings. Nevertheless, with continued progress in neuroimaging techniques (like PET-CT, DTI, fMRI, NIRS, ECoG) and biochemical biomarkers, the pathological processes of AD and PD will be clarified, and effective strategies of flavonoids for treating AD and PD will also be found.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Hu Wei-yan was supported by National Natural Science Foundation of China [81860254 & 81960666]. The National Natural Science Foundation of China (81860254 & 81960666, W. Y. H.), Yunnan Province Young Academic and Technical Leaders Project (202105AC160078, W. Y. H.) and the joint Program of Yunnan Province and Kunming Medical University (202101AY070001-009, W. Y. H.).
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Yu Hao-fei, Email: yuhaofei@kmmu.edu.cn.
Hu Wei-yan, Email: huweiyan2004@163.com.
References
- 1.Agnihotri A., Aruoma O.I. Alzheimer's disease and Parkinson's disease: a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental chemicals. J. Am. Coll. Nutr. 2020;39(1):16–27. doi: 10.1080/07315724.2019.1683379. [DOI] [PubMed] [Google Scholar]
- 2.Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., et al. Alzheimer's disease. Lancet. 2021;397(10284):1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloem B.R., Okun M.S., Klein C. Parkinson's disease. Lancet. 2021;397(10291):2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 4.Qi S., Yin P., Wang L., Qu M., Kan G.L., Zhang H., et al. Prevalence of Parkinson's disease: a community-based study in China. Mov. Disord. 2021;36(12):2940–2944. doi: 10.1002/mds.28762. [DOI] [PubMed] [Google Scholar]
- 5.Tahami Monfared A.A., Byrnes M.J., White L.A., Zhang Q. Alzheimer's disease: epidemiology and clinical progression. Neurol Ther. 2022;11(2):553–569. doi: 10.1007/s40120-022-00338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y., Tian Y., Han H., Cui J., Ge X., Qin Y., et al. The path linking disease severity and cognitive function with quality of life in Parkinson's disease: the mediating effect of activities of daily living and depression. Health Qual. Life Outcome. 2021;19(1):92. doi: 10.1186/s12955-021-01740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azocar I., Livingston G., Huntley J. The association between impaired awareness and depression, anxiety, and apathy in mild to moderate alzheimer's disease: a systematic review. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.633081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Vicente P., Passarinha L.A., Silvestre S., Gallardo E. Recent developments in new therapeutic agents against alzheimer and Parkinson diseases: in-silico approaches. Molecules. 2021;26(8) doi: 10.3390/molecules26082193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan D., Liu L., Wu Z., Cao M. Combating neurodegenerative diseases with the plant alkaloid berberine: molecular mechanisms and therapeutic potential. Curr. Neuropharmacol. 2019;17(6):563–579. doi: 10.2174/1570159X16666180419141613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohd Sairazi N.S., Sirajudeen K.N.S. Natural products and their bioactive compounds: neuroprotective potentials against neurodegenerative diseases. Evidence-Based complementary and alternative medicine. eCAM. 2020;2020 doi: 10.1155/2020/6565396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher P. The potential of flavonoids for the treatment of neurodegenerative diseases. Int. J. Mol. Sci. 2019;20(12) doi: 10.3390/ijms20123056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong Y.R., Tay K.C., Su Y.X., Wong C.K., Tan W.N., Khaw K.Y. Potential of naturally derived alkaloids as multi-targeted therapeutic agents for neurodegenerative diseases. Molecules. 2021;26(3) doi: 10.3390/molecules26030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalorak P., Sanguanphun T., Limboonreung T., Meemon K. Neurorescue effects of frondoside A and ginsenoside Rg3 in C. elegans model of Parkinson's disease. Molecules. 2021;26(16) doi: 10.3390/molecules26164843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juca M.M., Cysne Filho F.M.S., de Almeida J.C., Mesquita D.D.S., Barriga J.R.M., Dias K.C.F., et al. Flavonoids: biological activities and therapeutic potential. Nat. Prod. Res. 2020;34(5):692–705. doi: 10.1080/14786419.2018.1493588. [DOI] [PubMed] [Google Scholar]
- 15.Wen K., Fang X., Yang J., Yao Y., Nandakumar K.S., Salem M.L., et al. Recent research on flavonoids and their biomedical applications. Curr. Med. Chem. 2021;28(5):1042–1066. doi: 10.2174/0929867327666200713184138. [DOI] [PubMed] [Google Scholar]
- 16.Lebeau J., Furman C., Bernier J.L., Duriez P., Teissier E., Cotelle N. Antioxidant properties of di-tert-butylhydroxylated flavonoids. Free Radic. Biol. Med. 2000;29(9):900–912. doi: 10.1016/s0891-5849(00)00390-7. [DOI] [PubMed] [Google Scholar]
- 17.Luo Z., Murray B.S., Yusoff A., Morgan M.R., Povey M.J., Day A.J. Particle-stabilizing effects of flavonoids at the oil-water interface. J. Agric. Food Chem. 2011;59(6):2636–2645. doi: 10.1021/jf1041855. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Tan N., Wang H., Hu J., Diwu W., Wang X. A systematic analysis of natural alpha-glucosidase inhibitors from flavonoids of Radix scutellariae using ultrafiltration UPLC-TripleTOF-MS/MS and network pharmacology. BMC Compl. Med. Ther. 2020;20(1):72. doi: 10.1186/s12906-020-2871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parcheta M., Swislocka R., Orzechowska S., Akimowicz M., Choinska R., Lewandowski W. Recent developments in effective antioxidants: the structure and antioxidant properties. Materials. 2021;14(8) doi: 10.3390/ma14081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atrahimovich D., Avni D., Khatib S. Flavonoids-macromolecules interactions in human diseases with focus on alzheimer. Atheroscl. Cancer. Antioxidants (Basel) 2021;10(3) doi: 10.3390/antiox10030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahul, Siddique Y.H. Neurodegenerative diseases and flavonoids: special reference to kaempferol. CNS Neurol. Disord. - Drug Targets. 2021;20(4):327–342. doi: 10.2174/1871527320666210129122033. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Arce E., Saldias M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112236. [DOI] [PubMed] [Google Scholar]
- 23.Paloczi J., Varga Z.V., Hasko G., Pacher P. Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxidants Redox Signal. 2018;29(1):75–108. doi: 10.1089/ars.2017.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung A.W.K., Tzvetkov N.T., Georgieva M.G., Ognyanov I.V., Kordos K., Jozwik A., et al. Reactive oxygen species and their impact in neurodegenerative diseases: literature landscape analysis. Antioxidants Redox Signal. 2021;34(5):402–420. doi: 10.1089/ars.2019.7952. [DOI] [PubMed] [Google Scholar]
- 25.Tewari D., Sah A.N., Bawari S., Nabavi S.F., Dehpour A.R., Shirooie S., et al. Role of nitric oxide in neurodegeneration: function, regulation, and inhibition. Curr. Neuropharmacol. 2021;19(2):114–126. doi: 10.2174/1570159X18666200429001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M., Zhang G., Naqvi S., Zhang F., Kang T., Duan Q., et al. Cytotoxicity of Saikosaponin A targets HEKa cell through apoptosis induction by ROS accumulation and inflammation suppression via NF-kappaB pathway. Int. Immunopharm. 2020;86 doi: 10.1016/j.intimp.2020.106751. [DOI] [PubMed] [Google Scholar]
- 27.Bailly C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019;476(20):3019–3032. doi: 10.1042/BCJ20190159. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S., Ghosh S., Mandal A., Ghosh N., Sil P.C. ROS-associated immune response and metabolism: a mechanistic approach with implication of various diseases. Arch. Toxicol. 2020;94(7):2293–2317. doi: 10.1007/s00204-020-02801-7. [DOI] [PubMed] [Google Scholar]
- 29.He R., Jiang Y., Shi Y., Liang J., Zhao L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater. Sci. Engin. C, Mat. Biol. Appl. 2020;117 doi: 10.1016/j.msec.2020.111314. [DOI] [PubMed] [Google Scholar]
- 30.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamed R., Dayati P., Mehr R.N., Kamato D., Seif F., Babaahmadi-Rezaei H., et al. Transforming growth factor-beta1 mediated CHST11 and CHSY1 mRNA expression is ROS dependent in vascular smooth muscle cells. J. Cell Commun. Signal. 2019;13(2):225–233. doi: 10.1007/s12079-018-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palozza P., Parrone N., Simone R., Catalano A. Role of lycopene in the control of ROS-mediated cell growth: implications in cancer prevention. Curr. Med. Chem. 2011;18(12):1846–1860. doi: 10.2174/092986711795496845. [DOI] [PubMed] [Google Scholar]
- 33.Prata C., Maraldi T., Zambonin L., Fiorentini D., Hakim G., Landi L. ROS production and Glut1 activity in two human megakaryocytic cell lines. Biofactors. 2004;20(4):223–233. doi: 10.1002/biof.5520200406. [DOI] [PubMed] [Google Scholar]
- 34.Fang Y., Han S.I., Mitchell C., Gupta S., Studer E., Grant S., et al. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 2004;40(4):961–971. doi: 10.1002/hep.20385. [DOI] [PubMed] [Google Scholar]
- 35.Keilhack H., Muller M., Bohmer S.A., Frank C., Weidner K.M., Birchmeier W., et al. Negative regulation of Ros receptor tyrosine kinase signaling. An epithelial function of the SH2 domain protein tyrosine phosphatase SHP-1. J. Cell Biol. 2001;152(2):325–334. doi: 10.1083/jcb.152.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalpage H.A., Wan J., Morse P.T., Lee I., Huttemann M. Brain-specific serine-47 modification of cytochrome c regulates cytochrome c oxidase activity attenuating ROS production and cell death: implications for ischemia/reperfusion injury and Akt signaling. Cells. 2020;9(8) doi: 10.3390/cells9081843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y., Huynh D.T.N., Myung C.S., Heo K.S. Ginsenoside Rh1 prevents migration and invasion through mitochondrial ROS-mediated inhibition of STAT3/NF-kappaB signaling in MDA-MB-231 cells. Int. J. Mol. Sci. 2021;22(19) doi: 10.3390/ijms221910458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park M.W., Cha H.W., Kim J., Kim J.H., Yang H., Yoon S., et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camins A., Sureda F.X., Gabriel C., Pallas M., Escubedo E., Camarasa J. Modulation of neuronal mitochondrial membrane potential by the NMDA receptor: role of arachidonic acid. Brain Res. 1997;777(1-2):69–74. doi: 10.1016/s0006-8993(97)00947-5. [DOI] [PubMed] [Google Scholar]
- 40.Wood A.H.R., Chappell H.F., Zulyniak M.A. Dietary and supplemental long-chain omega-3 fatty acids as moderators of cognitive impairment and Alzheimer's disease. Eur. J. Nutr. 2022;61(2):589–604. doi: 10.1007/s00394-021-02655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y.H. Genes & genomics; 2020. Trans-cinnamaldehyde Protects C2C12 Myoblasts from DNA Damage, Mitochondrial Dysfunction and Apoptosis Caused by Oxidative Stress through Inhibiting ROS Production. [DOI] [PubMed] [Google Scholar]
- 42.Zhao M., Wang Y., Li L., Liu S., Wang C., Yuan Y., et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics. 2021;11(4):1845–1863. doi: 10.7150/thno.50905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali S.S., Medda N., Dutta S.M., Patra R., Maiti S. Protection against mitochondrial oxidative-stress by flesh-extract of edible freshwater snail bellamya bengalensis prevents arsenic induced DNA and tissue damage. Anti Cancer Agents Med. Chem. 2020;20(10):1266–1273. doi: 10.2174/1871520620666200410081535. [DOI] [PubMed] [Google Scholar]
- 44.Mecocci P., Boccardi V., Cecchetti R., Bastiani P., Scamosci M., Ruggiero C., et al. A long journey into aging, brain aging, and alzheimer's disease following the oxidative stress tracks. J. Alzheim. Dis. : JAD. 2018;62(3):1319–1335. doi: 10.3233/JAD-170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dionisio P.A., Amaral J.D., Rodrigues C.M.P. Oxidative stress and regulated cell death in Parkinson's disease. Ageing Res. Rev. 2021;67 doi: 10.1016/j.arr.2021.101263. [DOI] [PubMed] [Google Scholar]
- 46.Rekatsina M., Paladini A., Piroli A., Zis P., Pergolizzi J.V., Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv. Ther. 2020;37(1):113–139. doi: 10.1007/s12325-019-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng S.T., Wang Z.Z., Yuan Y.H., Sun H.M., Chen N.H., Zhang Y. Update on the association between alpha-synuclein and tau with mitochondrial dysfunction: implications for Parkinson's disease. Eur. J. Neurosci. 2021;53(9):2946–2959. doi: 10.1111/ejn.14699. [DOI] [PubMed] [Google Scholar]
- 48.Samluk L., Ostapczuk P., Dziembowska M. Long-term mitochondrial stress induces early steps of Tau aggregation by increasing reactive oxygen species levels and affecting cellular proteostasis. Mol. Biol. Cell. 2022;33(8):ar67. doi: 10.1091/mbc.E21-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Souza R.F., De Giovani W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep.: communications in free radical research. 2004;9(2):97–104. doi: 10.1179/135100004225003897. [DOI] [PubMed] [Google Scholar]
- 50.Baldim J.L., de Alcantara B.G.V., Domingos O.D.S., Soares M.G., Caldas I.S., Novaes R.D., et al. The correlation between chemical structures and antioxidant, prooxidant, and antitrypanosomatid properties of flavonoids. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/3789856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J.L., Fang X.Y., Wang J.Q., Zhao L.G., Li Y., Tang F., et al. Structures and bioactivities of seven flavonoids from Osmanthus fragrans 'Jinqiu' essential oil extraction residues. Nat. Prod. Res. 2018;32(5):588–591. doi: 10.1080/14786419.2017.1318387. [DOI] [PubMed] [Google Scholar]
- 52.Li C., Dai T., Chen J., Chen M., Liang R., Liu C., et al. Modification of flavonoids: methods and influences on biological activities. Crit. Rev. Food Sci. Nutr. 2022:1–22. doi: 10.1080/10408398.2022.2083572. [DOI] [PubMed] [Google Scholar]
- 53.Chen J., Yang J., Ma L., Li J., Shahzad N., Kim C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020;10(1):2611. doi: 10.1038/s41598-020-59451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J., Yang J., Ma L., Li J., Shahzad N., Kim C.K. Author Correction: structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020;10(1):5666. doi: 10.1038/s41598-020-62493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng Z.F., Strack D., Baumert A., Subramaniam R., Goh N.K., Chia T.F., et al. Antioxidant flavonoids from leaves of Polygonum hydropiper. L. Phytochemistry. 2003;62(2):219–228. doi: 10.1016/s0031-9422(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 57.Gutierrez-Merino C., Lopez-Sanchez C., Lagoa R., Samhan-Arias A.K., Bueno C., Garcia-Martinez V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011;18(8):1195–1212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- 58.Franco J.L., Posser T., Missau F., Pizzolatti M.G., Dos Santos A.R., Souza D.O., et al. Structure-activity relationship of flavonoids derived from medicinal plants in preventing methylmercury-induced mitochondrial dysfunction. Environ. Toxicol. Pharmacol. 2010;30(3):272–278. doi: 10.1016/j.etap.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu J., Huang Y., Xiong M., Luo S., Chen Y., Li Y. The effects of natural flavonoids on lipoxygenase-mediated oxidation of compounds with a benzene ring structure–a new possible mechanism of flavonoid anti-chemical carcinogenesis and other toxicities. Int. J. Toxicol. 2006;25(4):295–301. doi: 10.1080/10915810600746122. [DOI] [PubMed] [Google Scholar]
- 60.Sheahan J., Cheong H., Rechnitz G. The colorless flavonoids of Arabidopsis thaliana (Brassicaceae). I. A model system to study the orthodihydroxy structure. Am. J. Bot. 1998;85(4):467. [PubMed] [Google Scholar]
- 61.Li Y.R., Li G.H., Zhou M.X., Xiang L., Ren D.M., Lou H.X., et al. Discovery of natural flavonoids as activators of Nrf2-mediated defense system: structure-activity relationship and inhibition of intracellular oxidative insults. Bioorg. Med. Chem. 2018;26(18):5140–5150. doi: 10.1016/j.bmc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Guo Y.Q., Tang G.H., Lou L.L., Li W., Zhang B., Liu B., et al. Prenylated flavonoids as potent phosphodiesterase-4 inhibitors from Morus alba: isolation, modification, and structure-activity relationship study. Eur. J. Med. Chem. 2018;144:758–766. doi: 10.1016/j.ejmech.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 63.Li K., Yao F., Xue Q., Fan H., Yang L., Li X., et al. Inhibitory effects against alpha-glucosidase and alpha-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018;12(1):82. doi: 10.1186/s13065-018-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuji P.A., Stephenson K.K., Wade K.L., Liu H., Fahey J.W. Structure-activity analysis of flavonoids: direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer. 2013;65(7):1014–1025. doi: 10.1080/01635581.2013.809127. [DOI] [PubMed] [Google Scholar]
- 65.Choi J.S., Chung H.Y., Kang S.S., Jung M.J., Kim J.W., No J.K., et al. The structure-activity relationship of flavonoids as scavengers of peroxynitrite. Phytother Res.: PT. 2002;16(3):232–235. doi: 10.1002/ptr.828. [DOI] [PubMed] [Google Scholar]
- 66.Ugusman A., Zakaria Z., Hui C.K., Nordin N.A., Mahdy Z.A. Flavonoids of Piper sarmentosum and its cytoprotective effects against oxidative stress. EXCLI J. 2012;11:705–714. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang P., Mak J.C., Man R.Y., Leung S.W. Flavonoids reduces lipopolysaccharide-induced release of inflammatory mediators in human bronchial epithelial cells: structure-activity relationship. Eur. J. Pharmacol. 2019;865 doi: 10.1016/j.ejphar.2019.172731. [DOI] [PubMed] [Google Scholar]
- 68.Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 69.McGrowder D.A., Miller F., Vaz K., Nwokocha C., Wilson-Clarke C., Anderson-Cross M., et al. Cerebrospinal fluid biomarkers of alzheimer's disease: current evidence and future perspectives. Brain Sci. 2021;11(2) doi: 10.3390/brainsci11020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva M.V.F., Loures C.M.G., Alves L.C.V., de Souza L.C., Borges K.B.G., Carvalho M.D.G. Alzheimer's disease: risk factors and potentially protective measures. J. Biomed. Sci. 2019;26(1):33. doi: 10.1186/s12929-019-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park J.E., Kim H.J., Kim Y.E., Jang H., Cho S.H., Kim S.J., et al. Analysis of dementia-related gene variants in APOE epsilon4 noncarrying Korean patients with early-onset Alzheimer's disease. Neurobiol. Aging. 2020;85:155 e5–e8. doi: 10.1016/j.neurobiolaging.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Busche M.A., Hyman B.T. Synergy between amyloid-beta and tau in Alzheimer's disease. Nat. Neurosci. 2020;23(10):1183–1193. doi: 10.1038/s41593-020-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gella A., Durany N. Oxidative stress in Alzheimer disease. Cell Adhes. Migrat. 2009;3(1):88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nunomura A., Hofer T., Moreira P.I., Castellani R.J., Smith M.A., Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 2009;118(1):151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 76.Tramutola A., Lanzillotta C., Perluigi M., Butterfield D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017;133:88–96. doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 77.Akhtar A., Sah S.P. Insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer's disease. Neurochem. Int. 2020;135 doi: 10.1016/j.neuint.2020.104707. [DOI] [PubMed] [Google Scholar]
- 78.Elisha I.L., Dzoyem J.P., McGaw L.J., Botha F.S., Eloff J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Compl. Alternative Med. 2016;16:307. doi: 10.1186/s12906-016-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wasek M., Nartowska J., Wawer I., Tudruj T. Electron spin resonance assessment of the antioxidant potential of medicinal plants. Part I. Contribution of anthocyanosides and flavonoids to the radical scavenging ability of fruit and herbal teas. Acta Pol. Pharm. 2001;58(4):283–288. [PubMed] [Google Scholar]
- 80.Braidy N., Behzad S., Habtemariam S., Ahmed T., Daglia M., Nabavi S.M., et al. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in alzheimer's and Parkinson's disease. CNS Neurol. Disord. - Drug Targets. 2017;16(4):387–397. doi: 10.2174/1871527316666170328113309. [DOI] [PubMed] [Google Scholar]
- 81.Espargaro A., Ginex T., Vadell M.D., Busquets M.A., Estelrich J., Munoz-Torrero D., et al. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-alzheimer drugs. J. Nat. Prod. 2017;80(2):278–289. doi: 10.1021/acs.jnatprod.6b00643. [DOI] [PubMed] [Google Scholar]
- 82.Williams R.J., Spencer J.P. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012;52(1):35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Zhan C., Lao Z., Tang Y., Qiao Q., Wei G. Natural stereoisomeric flavonoids exhibit different disruptive effects and the mechanism of action on Abeta42 protofibril. Chem. Commun. 2021;57(35):4267–4270. doi: 10.1039/d1cc00404b. [DOI] [PubMed] [Google Scholar]
- 84.Shengkai D., Yazhen S. Flavonoids from stems and leaves of Scutellaria baicalensis Georgi regulate the brain tau hyperphosphorylation at multiple sites induced by composited abeta in rats. CNS Neurol. Disord. - Drug Targets. 2022;21(4):367–374. doi: 10.2174/1871527320666210827112609. [DOI] [PubMed] [Google Scholar]
- 85.Pyrzynska K. Hesperidin: a review on extraction methods, stability and biological activities. Nutrients. 2022;14(12) doi: 10.3390/nu14122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res.: PT. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 87.Hong Y., An Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-kappaB signaling. Arch Pharm. Res. (Seoul) 2018;41(6):655–663. doi: 10.1007/s12272-015-0662-z. [DOI] [PubMed] [Google Scholar]
- 88.Li C., Zug C., Qu H., Schluesener H., Zhang Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015;281:32–42. doi: 10.1016/j.bbr.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Wang D., Liu L., Zhu X., Wu W., Wang Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer's disease. Cell. Mol. Neurobiol. 2014;34(8):1209–1221. doi: 10.1007/s10571-014-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen F., Wei G., Xu J., Ma X., Wang Q. Naringin ameliorates the high glucose-induced rat mesangial cell inflammatory reaction by modulating the NLRP3 Inflammasome. BMC Compl. Alternative Med. 2018;18(1):192. doi: 10.1186/s12906-018-2257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hassan R.A., Hozayen W.G., Abo Sree H.T., Al-Muzafar H.M., Amin K.A., Ahmed O.M. Naringin and hesperidin counteract diclofenac-induced hepatotoxicity in male wistar rats via their antioxidant, anti-inflammatory, and antiapoptotic activities. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9990091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y., Liu S. Bioactivity of naringin and related mechanisms. Pharmazie. 2021;76(8):359–363. doi: 10.1691/ph.2021.1504. [DOI] [PubMed] [Google Scholar]
- 93.Maratha S.R., Mahadevan N. Memory enhancing activity of naringin in unstressed and stressed mice: possible cholinergic and nitriergic modulation. Neurochem. Res. 2012;37(10):2206–2212. doi: 10.1007/s11064-012-0844-8. [DOI] [PubMed] [Google Scholar]
- 94.Ramalingayya G.V., Nampoothiri M., Nayak P.G., Kishore A., Shenoy R.R., Mallikarjuna Rao C., et al. Naringin and rutin alleviates episodic memory deficits in two differentially challenged object recognition tasks. Phcog. Mag. 2016;12(Suppl 1):S63–70. doi: 10.4103/0973-1296.176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin S., Chen Q., Wu H., Liu C., Hu J., Zhang D., et al. Effects of naringin on learning and memory dysfunction induced by gp120 in rats. Brain Res. Bull. 2016;124:164–171. doi: 10.1016/j.brainresbull.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 96.Ahsan A.U., Sharma V.L., Wani A., Chopra M. Naringenin upregulates AMPK-mediated autophagy to rescue neuronal cells from beta-amyloid (1-42) evoked neurotoxicity. Mol. Neurobiol. 2020;57(8):3589–3602. doi: 10.1007/s12035-020-01969-4. [DOI] [PubMed] [Google Scholar]
- 97.Yang Z., Kuboyama T., Tohda C. Naringenin promotes microglial M2 polarization and Abeta degradation enzyme expression. Phytother Res.: PT. 2019;33(4):1114–1121. doi: 10.1002/ptr.6305. [DOI] [PubMed] [Google Scholar]
- 98.Ghofrani S., Joghataei M.T., Mohseni S., Baluchnejadmojarad T., Bagheri M., Khamse S., et al. Naringenin improves learning and memory in an Alzheimer's disease rat model: insights into the underlying mechanisms. Eur. J. Pharmacol. 2015;764:195–201. doi: 10.1016/j.ejphar.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Khajevand-Khazaei M.R., Ziaee P., Motevalizadeh S.A., Rohani M., Afshin-Majd S., Baluchnejadmojarad T., et al. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur. J. Pharmacol. 2018;826:114–122. doi: 10.1016/j.ejphar.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Khan M.B., Khan M.M., Khan A., Ahmed M.E., Ishrat T., Tabassum R., et al. Naringenin ameliorates Alzheimer's disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem. Int. 2012;61(7):1081–1093. doi: 10.1016/j.neuint.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 101.Haider S., Liaquat L., Ahmad S., Batool Z., Siddiqui R.A., Tabassum S., et al. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He B., Nohara K., Park N., Park Y.S., Guillory B., Zhao Z., et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metabol. 2016;23(4):610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakajima A., Nemoto K., Ohizumi Y. An evaluation of the genotoxicity and subchronic toxicity of the peel extract of Ponkan cultivar 'Ohta ponkan' (Citrus reticulata Blanco) that is rich in nobiletin and tangeretin with anti-dementia activity. Regul. Toxicol. Pharmacol. : RTP (Regul. Toxicol. Pharmacol.) 2020;114 doi: 10.1016/j.yrtph.2020.104670. [DOI] [PubMed] [Google Scholar]
- 104.Nakajima A., Ohizumi Y., Yamada K. Anti-dementia activity of nobiletin, a citrus flavonoid: a review of animal studies. Clinical Psychopharmacol. Neur.: the official scientific journal of the Korean College of Neuropsychopharmacology. 2014;12(2):75–82. doi: 10.9758/cpn.2014.12.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee H.J., Lee S.K., Lee D.R., Choi B.K., Le B., Yang S.H. Ameliorating effect of Citrus aurantium extracts and nobiletin on betaamyloid (142)induced memory impairment in mice. Mol. Med. Rep. 2019;20(4):3448–3455. doi: 10.3892/mmr.2019.10582. [DOI] [PubMed] [Google Scholar]
- 106.Nakajima A., Yamakuni T., Haraguchi M., Omae N., Song S.Y., Kato C., et al. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007;105(1):122–126. doi: 10.1254/jphs.sc0070155. [DOI] [PubMed] [Google Scholar]
- 107.Ijaz M.U., Tahir A., Samad A., Anwar H. Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol. 2020 doi: 10.1177/0960327120950007. [DOI] [PubMed] [Google Scholar]
- 108.Mao Q., Liang X., Wu Y., Lu Y. Nobiletin protects against myocardial injury and myocardial apoptosis following coronary microembolization via activating PI3K/Akt pathway in rats. N. Schmied. Arch. Pharmacol. 2019;392(9):1121–1130. doi: 10.1007/s00210-019-01661-y. [DOI] [PubMed] [Google Scholar]
- 109.Onozuka H., Nakajima A., Matsuzaki K., Shin R.W., Ogino K., Saigusa D., et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer's disease. J. Pharmacol. Exp. Therapeut. 2008;326(3):739–744. doi: 10.1124/jpet.108.140293. [DOI] [PubMed] [Google Scholar]
- 110.Qi G., Mi Y., Fan R., Li R., Liu Z., Liu X. Nobiletin protects against systemic inflammation-stimulated memory impairment via MAPK and NF-kappaB signaling pathways. J. Agric. Food Chem. 2019;67(18):5122–5134. doi: 10.1021/acs.jafc.9b00133. [DOI] [PubMed] [Google Scholar]
- 111.Singh A., Naidu P.S., Kulkarni S.K. Quercetin potentiates L-Dopa reversal of drug-induced catalepsy in rats: possible COMT/MAO inhibition. Pharmacology. 2003;68(2):81–88. doi: 10.1159/000069533. [DOI] [PubMed] [Google Scholar]
- 112.Wang R.E., Hunt C.R., Chen J., Taylor J.S. Biotinylated quercetin as an intrinsic photoaffinity proteomics probe for the identification of quercetin target proteins. Bioorg. Med. Chem. 2011;19(16):4710–4720. doi: 10.1016/j.bmc.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen X., Li H., Wang Z., Zhou Q., Chen S., Yang B., et al. Quercetin protects the vascular endothelium against iron overload damages via ROS/ADMA/DDAH/eNOS/NO pathway. Eur. J. Pharmacol. 2020;868 doi: 10.1016/j.ejphar.2019.172885. [DOI] [PubMed] [Google Scholar]
- 114.Sajadi Hezaveh Z., Azarkeivan A., Janani L., Hosseini S., Shidfar F. The effect of quercetin on iron overload and inflammation in beta-thalassemia major patients: a double-blind randomized clinical trial. Compl. Ther. Med. 2019;46:24–28. doi: 10.1016/j.ctim.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 115.Li Y., Tian Q., Li Z., Dang M., Lin Y., Hou X. Activation of Nrf2 signaling by sitagliptin and quercetin combination against beta-amyloid induced Alzheimer's disease in rats. Drug Dev. Res. 2019;80(6):837–845. doi: 10.1002/ddr.21567. [DOI] [PubMed] [Google Scholar]
- 116.Wang D.M., Li S.Q., Wu W.L., Zhu X.Y., Wang Y., Yuan H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer's disease. Neurochem. Res. 2014;39(8):1533–1543. doi: 10.1007/s11064-014-1343-x. [DOI] [PubMed] [Google Scholar]
- 117.Sabogal-Guaqueta A.M., Munoz-Manco J.I., Ramirez-Pineda J.R., Lamprea-Rodriguez M., Osorio E., Cardona-Gomez G.P. The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kessler M., Ubeaud G., Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003;55(1):131–142. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- 119.Moghbelinejad S., Nassiri-Asl M., Farivar T.N., Abbasi E., Sheikhi M., Taghiloo M., et al. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 2014;224(1):108–113. doi: 10.1016/j.toxlet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 120.Saad-Allah, K M., Fetouh M I., Elhaak M A. Induction of milk thistle (Silybum marianum L. Gaertn) growth and phytochemicals production by natural stimulants. J. Appl. Res. Medi. Aromat. Plants. 2017;6:101–110. [Google Scholar]
- 121.Kumar R., Deep G., Agarwal R. An overview of ultraviolet B radiation-induced skin cancer chemoprevention by silibinin. Current Pharmacol. Reports. 2015;1(3):206–215. doi: 10.1007/s40495-015-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lim J.O., Shin N.R., Seo Y.S., Nam H.H., Ko J.W., Jung T.Y., et al. Silibinin attenuates silica dioxide nanoparticles-induced inflammation by suppressing TXNIP/MAPKs/AP-1 signaling. Cells. 2020;9(3) doi: 10.3390/cells9030678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raghu R., Karthikeyan S. Zidovudine and isoniazid induced liver toxicity and oxidative stress: evaluation of mitigating properties of silibinin. Environ. Toxicol. Pharmacol. 2016;46:217–226. doi: 10.1016/j.etap.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 124.Song X., Zhou B., Cui L., Lei D., Zhang P., Yao G., et al. Silibinin ameliorates Abeta25-35-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress. Neurochem. Res. 2017;42(4):1073–1083. doi: 10.1007/s11064-016-2141-4. [DOI] [PubMed] [Google Scholar]
- 125.Wang Q., Ye Y., Liu W., Jiang S., Tashiro S., Onodera S., et al. Dual effects of silibinin treatment on autophagy-regulated dermal apoptosis retardation and epidermal apoptosis up-regulation in UVB-induced skin inflammation. J. Asian Nat. Prod. Res. 2012;14(7):688–699. doi: 10.1080/10286020.2012.685725. [DOI] [PubMed] [Google Scholar]
- 126.Tyagi A., Agarwal C., Dwyer-Nield L.D., Singh R.P., Malkinson A.M., Agarwal R. Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol. Carcinog. 2012;51(10):832–842. doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- 127.Ali T., Kim T., Rehman S.U., Khan M.S., Amin F.U., Khan M., et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of alzheimer's disease. Mol. Neurobiol. 2018;55(7):6076–6093. doi: 10.1007/s12035-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 128.Liu P., Cui L., Liu B., Liu W., Hayashi T., Mizuno K., et al. Silibinin ameliorates STZ-induced impairment of memory and learning by up- regulating insulin signaling pathway and attenuating apoptosis. Physiol. Behav. 2020;213 doi: 10.1016/j.physbeh.2019.112689. [DOI] [PubMed] [Google Scholar]
- 129.Khan M.S., Ali T., Kim M.W., Jo M.H., Jo M.G., Badshah H., et al. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016;100:1–10. doi: 10.1016/j.neuint.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 130.Amin F.U., Shah S.A., Badshah H., Khan M., Kim M.O. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Abeta1-42-induced oxidative stress. J. Nanobiotechnol. 2017;15(1):12. doi: 10.1186/s12951-016-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Badshah H., Kim T.H., Kim M.O. Protective effects of anthocyanins against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015;80:51–59. doi: 10.1016/j.neuint.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 132.Anwar S., Fratantonio D., Ferrari D., Saija A., Cimino F., Speciale A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Rep. 2016;14(2):1397–1403. doi: 10.3892/mmr.2016.5397. [DOI] [PubMed] [Google Scholar]
- 133.Wei J., Wu H., Zhang H., Li F., Chen S., Hou B., et al. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018;41(3):1608–1618. doi: 10.3892/ijmm.2018.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Varadinova M.G., Docheva-Drenska D.I., Boyadjieva N.I. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause. 2009;16(2):345–349. doi: 10.1097/gme.0b013e3181847619. [DOI] [PubMed] [Google Scholar]
- 135.Singh N.A., Mandal A.K.A., Khan Z.A. Inhibition of Al(III)-Induced Abeta42 fibrillation and reduction of neurotoxicity by epigallocatechin-3-gallate nanoparticles. J. Biomed. Nanotechnol. 2018;14(6):1147–1158. doi: 10.1166/jbn.2018.2552. [DOI] [PubMed] [Google Scholar]
- 136.Gueroux M., Fleau C., Slozeck M., Laguerre M., Pianet I. Epigallocatechin 3-gallate as an inhibitor of tau phosphorylation and aggregation: a molecular and structural insight. J. Preven. Alzheimer's Disease. 2017;4(4):218–225. doi: 10.14283/jpad.2017.35. [DOI] [PubMed] [Google Scholar]
- 137.Saffar S., Fatemi I., Rahmani M., Hassanshahi J., Sahamsizadeh A., Allahtavakoli M., et al. The effect of epigallocatechin-3-gallate on morphine-induced memory impairments in rat: EGCG effects on morphine neurotoxicity. Hum. Exp. Toxicol. 2020;39(7):994–1002. doi: 10.1177/0960327120909540. [DOI] [PubMed] [Google Scholar]
- 138.Chang C.Y., Lin T.Y., Lu C.W., Huang S.K., Wang Y.C., Chou S.S., et al. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology. 2015;50:157–169. doi: 10.1016/j.neuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 139.Manchope M.F., Calixto-Campos C., Coelho-Silva L., Zarpelon A.C., Pinho-Ribeiro F.A., Georgetti S.R., et al. Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ou Z., Pan J., Tang S., Duan D., Yu D., Nong H., et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson's disease in 204 countries/territories from 1990 to 2019. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.776847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Futami T., Takakusaki K., Kitai S.T. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci. Res. 1995;21(4):331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- 142.Kitai S.T. Afferent control of substantia nigra compacta dopamine neurons: anatomical perspective and role of glutamatergic and cholinergic inputs. Adv. Pharmacol. 1998;42:700–702. doi: 10.1016/s1054-3589(08)60844-3. [DOI] [PubMed] [Google Scholar]
- 143.Costas C., Faro L.R.F. Do naturally occurring antioxidants protect against neurodegeneration of the dopaminergic system? A systematic revision in animal models of Parkinson's disease. Curr. Neuropharmacol. 2022;20(2):432–459. doi: 10.2174/1570159X19666210421092725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Theofanous T., Kourti M. Abrogating oxidative stress as a therapeutic strategy against Parkinson's disease: a mini review of the recent advances on natural therapeutic antioxidant and neuroprotective agents. Med. Chem. 2022;18(7):772–783. doi: 10.2174/1573406418666220304222401. [DOI] [PubMed] [Google Scholar]
- 145.Hritcu L., Ionita R., Postu P.A., Gupta G.K., Turkez H., Lima T.C., et al. Antidepressant flavonoids and their relationship with oxidative stress. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/5762172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kostyuk V.A., Potapovich A.I., Kostyuk T.V., Cherian M.G. Metal complexes of dietary flavonoids: evaluation of radical scavenger properties and protective activity against oxidative stress in vivo. Cell. Mol. Biol. 2007;53(1):62–69. [PubMed] [Google Scholar]
- 147.AlSaad A.M., Mohany M., Almalki M.S., Almutham I., Alahmari A.A., AlSulaiman M., et al. Baicalein neutralizes hypercholesterolemia-induced aggravation of oxidative injury in rats. Int. J. Med. Sci. 2020;17(9):1156–1166. doi: 10.7150/ijms.46108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Prigione A., Piazza F., Brighina L., Begni B., Galbussera A., Difrancesco J.C., et al. Alpha-synuclein nitration and autophagy response are induced in peripheral blood cells from patients with Parkinson disease. Neurosci. Lett. 2010;477(1):6–10. doi: 10.1016/j.neulet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 149.Devine M.J., Gwinn K., Singleton A., Hardy J. Parkinson's disease and alpha-synuclein expression. Mov. Disord. 2011;26(12):2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang M., Porat-Shliom Y., Pei Z., Cheng Y., Xiang L., Sommers K., et al. Baicalein reduces E46K alpha-synuclein aggregation in vitro and protects cells against E46K alpha-synuclein toxicity in cell models of familiar Parkinsonism. J. Neurochem. 2010;114(2):419–429. doi: 10.1111/j.1471-4159.2010.06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu M., Rajamani S., Kaylor J., Han S., Zhou F., Fink A.L. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004;279(26):26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 152.Song Q., Peng S., Zhu X. Baicalein protects against MPP(+)/MPTP-induced neurotoxicity by ameliorating oxidative stress in SH-SY5Y cells and mouse model of Parkinson's disease. Neurotoxicology. 2021;87:188–194. doi: 10.1016/j.neuro.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 153.Wei N., Wei Y., Li B., Pang L. Baicalein promotes neuronal and behavioral recovery after intracerebral hemorrhage via suppressing apoptosis, oxidative stress and neuroinflammation. Neurochem. Res. 2017;42(5):1345–1353. doi: 10.1007/s11064-017-2179-y. [DOI] [PubMed] [Google Scholar]
- 154.Zhou L., Tan S., Shan Y.L., Wang Y.G., Cai W., Huang X.H., et al. Baicalein improves behavioral dysfunction induced by Alzheimer's disease in rats. Neuropsychiatric Dis. Treat. 2016;12:3145–3152. doi: 10.2147/NDT.S117469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y.H., Yu H.T., Pu X.P., Du G.H. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules. 2013;18(12):14726–14738. doi: 10.3390/molecules181214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cui Y., Wu J., Jung S.C., Park D.B., Maeng Y.H., Hong J.Y., et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010;33(11):1814–1821. doi: 10.1248/bpb.33.1814. [DOI] [PubMed] [Google Scholar]
- 157.Jeong K.H., Jeon M.T., Kim H.D., Jung U.J., Jang M.C., Chu J.W., et al. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson's disease. J. Med. Food. 2015;18(4):409–414. doi: 10.1089/jmf.2014.3241. [DOI] [PubMed] [Google Scholar]
- 158.Amarsanaa K., Kim H.J., Ko E.A., Jo J., Jung S.C. Nobiletin exhibits neuroprotective effects against mitochondrial complex I inhibition via regulating apoptotic signaling. Exp Neurobiol. 2021;30(1):73–86. doi: 10.5607/en20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hao Y., Cheung C.S., Yip W.C., Ko W.H. Nobiletin stimulates chloride secretion in human bronchial epithelia via a cAMP/PKA-dependent pathway. Cell. Physiol. Biochem.: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;37(1):306–320. doi: 10.1159/000430355. [DOI] [PubMed] [Google Scholar]
- 160.Kawahata I., Yoshida M., Sun W., Nakajima A., Lai Y., Osaka N., et al. Potent activity of nobiletin-rich Citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: identification of the substances responsible for the pharmacological action. J. Neural. Transm. 2013;120(10):1397–1409. doi: 10.1007/s00702-013-1025-x. [DOI] [PubMed] [Google Scholar]
- 161.Liu L.L., Li F.H., Zhang Y., Zhang X.F., Yang J. Tangeretin has anti-asthmatic effects via regulating PI3K and Notch signaling and modulating Th1/Th2/Th17 cytokine balance in neonatal asthmatic mice. Brazilian J. Med. Biol. Res. = Revista Brasileira de Pesquisas Medicas e Biologicas. 2017;50(8) doi: 10.1590/1414-431X20175991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee Y.Y., Lee E.J., Park J.S., Jang S.E., Kim D.H., Kim H.S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J. Neuroimmune Pharmacol.: the official journal of the Society on NeuroImmune Pharmacology. 2016;11(2):294–305. doi: 10.1007/s11481-016-9657-x. [DOI] [PubMed] [Google Scholar]
- 163.Yang T., Feng C., Wang D., Qu Y., Yang Y., Wang Y., et al. Neuroprotective and Anti-inflammatory Effect of Tangeretin against Cerebral Ischemia-Reperfusion Injury in Rats. Inflammation. 2020 doi: 10.1007/s10753-020-01303-z. [DOI] [PubMed] [Google Scholar]
- 164.Datla K.P., Christidou M., Widmer W.W., Rooprai H.K., Dexter D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson's disease. Neuroreport. 2001;12(17):3871–3875. doi: 10.1097/00001756-200112040-00053. [DOI] [PubMed] [Google Scholar]
- 165.Shu Z., Yang B., Zhao H., Xu B., Jiao W., Wang Q., et al. Tangeretin exerts anti-neuroinflammatory effects via NF-kappaB modulation in lipopolysaccharide-stimulated microglial cells. Int. Immunopharm. 2014;19(2):275–282. doi: 10.1016/j.intimp.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 166.Yang J.S., Wu X.H., Yu H.G., Teng L.S. Tangeretin inhibits neurodegeneration and attenuates inflammatory responses and behavioural deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson's disease dementia in rats. Inflammopharmacology. 2017;25(4):471–484. doi: 10.1007/s10787-017-0348-x. [DOI] [PubMed] [Google Scholar]
- 167.Hackett E.S., Mama K.R., Twedt D.C., Gustafson D.L. Evaluation of antioxidant capacity and inflammatory cytokine gene expression in horses fed silibinin complexed with phospholipid. Am. J. Vet. Res. 2013;74(10):1333–1339. doi: 10.2460/ajvr.74.10.1333. [DOI] [PubMed] [Google Scholar]
- 168.Lu P., Mamiya T., Lu L., Mouri A., Niwa M., Kim H.C., et al. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav. Brain Res. 2010;207(2):387–393. doi: 10.1016/j.bbr.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 169.Jung U.J., Jeon M.T., Choi M.S., Kim S.R. Silibinin attenuates MPP(+)-induced neurotoxicity in the substantia nigra in vivo. J. Med. Food. 2014;17(5):599–605. doi: 10.1089/jmf.2013.2926. [DOI] [PubMed] [Google Scholar]
- 170.Jayaraman J., Namasivayam N. Naringenin modulates circulatory lipid peroxidation, anti-oxidant status and hepatic alcohol metabolizing enzymes in rats with ethanol induced liver injury. Fund. Clin. Pharmacol. 2011;25(6):682–689. doi: 10.1111/j.1472-8206.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 171.Xue N., Wu X., Wu L., Li L., Wang F. Antinociceptive and anti-inflammatory effect of Naringenin in different nociceptive and inflammatory mice models. Life Sci. 2019;217:148–154. doi: 10.1016/j.lfs.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 172.Wang G.Q., Zhang B., He X.M., Li D.D., Shi J.S., Zhang F. Naringenin targets on astroglial Nrf2 to support dopaminergic neurons. Pharmacol. Res. 2019;139:452–459. doi: 10.1016/j.phrs.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 173.Chtourou Y., Kamoun Z., Zarrouk W., Kebieche M., Kallel C., Gdoura R., et al. Naringenin ameliorates renal and platelet purinergic signalling alterations in high-cholesterol fed rats through the suppression of ROS and NF-kappaB signaling pathways. Food Funct. 2016;7(1):183–193. doi: 10.1039/c5fo00871a. [DOI] [PubMed] [Google Scholar]
- 174.Qin W., Ren B., Wang S., Liang S., He B., Shi X., et al. Apigenin and naringenin ameliorate PKCbetaII-associated endothelial dysfunction via regulating ROS/caspase-3 and NO pathway in endothelial cells exposed to high glucose. Vasc. Pharmacol. 2016;85:39–49. doi: 10.1016/j.vph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 175.Liu Y., Xu F., Zhang Z., Song R., Tian Y. Simultaneous determination of naringenin and hesperetin in rats after oral administration of Da-Cheng-Qi decoction by high-performance liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr.: BMC (Biomed. Chromatogr.) 2008;22(7):736–745. doi: 10.1002/bmc.991. [DOI] [PubMed] [Google Scholar]
- 176.Hua F.Z., Ying J., Zhang J., Wang X.F., Hu Y.H., Liang Y.P., et al. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-kappaB-mediated inflammation. Int. J. Mol. Med. 2016;38(4):1271–1280. doi: 10.3892/ijmm.2016.2715. [DOI] [PubMed] [Google Scholar]
- 177.E L, Jh N, MT J Naringin protects the ni- grostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. J. Nutr. Biochem. 2014;25:801–806. doi: 10.1016/j.jnutbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 178.Kim J.H., Park T.S., Yang S.H., Suh J.W., Shim S.M. Microbial bioconversion and processing methods enhance the phenolic acid and flavonoids and the radical scavenging capacity of Smilax China L. leaf. J. Sci. Food Agric. 2016;96(3):878–885. doi: 10.1002/jsfa.7160. [DOI] [PubMed] [Google Scholar]
- 179.Antunes M.S., Goes A.T., Boeira S.P., Prigol M., Jesse C.R. Protective effect of hesperidin in a model of Parkinson's disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30(11-12):1415–1422. doi: 10.1016/j.nut.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 180.Welbat Ju, Naewla S., Pannangrong W., Sirichoat A., Aranarochana A., Wigmore P. Neuroprotective effects of hesperidin against methotrexate-induced changes in neurogenesis and oxidative stress in the adult rat. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114083. [DOI] [PubMed] [Google Scholar]
- 181.Matias I., Diniz L.P., Buosi A., Neves G., Stipursky J., Gomes F.C.A. Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-beta1. Front. Aging Neurosci. 2017;9:184. doi: 10.3389/fnagi.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Halder S., Kar R., Chandra N., Nimesh A., Mehta A.K., Bhattacharya S.K., et al. Alteration in cognitive behaviour, brain antioxidant enzyme activity and their gene expression in F1 generation mice, following Cd exposure during the late gestation period: modulation by quercetin. Metab. Brain Dis. 2018;33(6):1935–1943. doi: 10.1007/s11011-018-0299-y. [DOI] [PubMed] [Google Scholar]
- 183.Ujah G.A., Nna V.U., Agah M.I., Omue L.O., Leku C.B., Osim E.E. Effect of quercetin on cadmium chloride-induced impairments in sexual behaviour and steroidogenesis in male Wistar rats. Andrologia. 2018;50(2) doi: 10.1111/and.12866. [DOI] [PubMed] [Google Scholar]
- 184.Chen P.Y., Vittal R., Nien P.C., Ho K.C. Enhancing dopamine detection using a glassy carbon electrode modified with MWCNTs, quercetin, and Nafion. Biosens. Bioelectron. 2009;24(12):3504–3509. doi: 10.1016/j.bios.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 185.Haleagrahara N., Siew C.J., Ponnusamy K. Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J. Toxicol. Sci. 2013;38(1):25–33. doi: 10.2131/jts.38.25. [DOI] [PubMed] [Google Scholar]
- 186.Naidu P.S., Singh A., Kulkarni S.K. D2-dopamine receptor and alpha2-adrenoreceptor-mediated analgesic response of quercetin. Indian J. Exp. Biol. 2003;41(12):1400–1404. [PubMed] [Google Scholar]
- 187.Li C., Zhang W.J., Frei B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016;9:104–113. doi: 10.1016/j.redox.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zbarsky V., Datla K.P., Parkar S., Rai D.K., Aruoma O.I., Dexter D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic. Res. 2005;39(10):1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 189.Singh S., Kumar P. Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: biochemical and neurochemical evidences. Neurosci. Res. 2018;133:38–47. doi: 10.1016/j.neures.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 190.Geetha R., Sathiya Priya C., Anuradha C.V. Troxerutin abrogates mitochondrial oxidative stress and myocardial apoptosis in mice fed calorie-rich diet. Chem. Biol. Interact. 2017;278:74–83. doi: 10.1016/j.cbi.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 191.Qadiri A., Mirzaei Bavil F., Hamidian G., Zavvari Oskuye Z., Ahmadi M., Oghbaei H., et al. Administration of troxerutin improves testicular function and structure in type-1 diabetic adult rats by reduction of apoptosis. Avic. J. Phyt. 2019;9(4):374–385. [PMC free article] [PubMed] [Google Scholar]
- 192.Wang J., Mao J., Wang R., Li S., Wu B., Yuan Y. Kaempferol protects against cerebral ischemia reperfusion injury through intervening oxidative and inflammatory stress induced apoptosis. Front. Pharmacol. 2020;11:424. doi: 10.3389/fphar.2020.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kouhestani S., Jafari A., Babaei P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018;13(10):1827–1832. doi: 10.4103/1673-5374.238714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Filomeni G., Graziani I., De Zio D., Dini L., Centonze D., Rotilio G., et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson's disease. Neurobiol. Aging. 2012;33(4):767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 195.Singh S., Singh A.K., Garg G., Rizvi S.I. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci. 2018;193:171–179. doi: 10.1016/j.lfs.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 196.Zhang L., Wang H., Zhou Y., Zhu Y., Fei M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem. Int. 2018;118:304–313. doi: 10.1016/j.neuint.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 197.Patel M.Y., Panchal H.V., Ghribi O., Benzeroual K.E. The neuroprotective effect of fisetin in the MPTP model of Parkinson's disease. J. Parkinsons Dis. 2012;2(4):287–302. doi: 10.3233/JPD-012110. [DOI] [PubMed] [Google Scholar]
- 198.Ding M.L., Ma H., Man Y.G., Lv H.Y. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can. J. Physiol. Pharmacol. 2017;95(12):1396–1405. doi: 10.1139/cjpp-2016-0333. [DOI] [PubMed] [Google Scholar]
- 199.Nan W., Zhonghang X., Keyan C., Tongtong L., Wanshu G., Zhongxin X. Epigallocatechin-3-Gallate reduces neuronal apoptosis in rats after middle cerebral artery occlusion injury via PI3K/AKT/eNOS signaling pathway. BioMed Res. Int. 2018 doi: 10.1155/2018/6473580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bitu Pinto N., da Silva Alexandre B., Neves K.R., Silva A.H., Leal L.K., Viana G.S. the 6-OHDA Model of Parkinson's Disease. Evidence-based complementary and alternative medicine: eCAM. 2015. 2015. Neuroprotective properties of the standardized extract from camellia sinensis (green tea) and its main bioactive components, epicatechin and epigallocatechin gallate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leaver K.R., Allbutt H.N., Creber N.J., Kassiou M., Henderson J.M. Oral pre-treatment with epigallocatechin gallate in 6-OHDA lesioned rats produces subtle symptomatic relief but not neuroprotection. Brain Res. Bull. 2009;80(6):397–402. doi: 10.1016/j.brainresbull.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 202.Wang L., Xu S., Xu X., Chan P. (-)-Epigallocatechin-3-Gallate protects SH-SY5Y cells against 6-OHDA-induced cell death through STAT3 activation. J. Alzheim. Dis.: JAD. 2009;17(2):295–304. doi: 10.3233/JAD-2009-1048. [DOI] [PubMed] [Google Scholar]
- 203.Bonanomi M., Natalello A., Visentin C., Pastori V., Penco A., Cornelli G., et al. Epigallocatechin-3-gallate and tetracycline differently affect ataxin-3 fibrillogenesis and reduce toxicity in spinocerebellar ataxia type 3 model. Hum. Mol. Genet. 2014;23(24):6542–6552. doi: 10.1093/hmg/ddu373. [DOI] [PubMed] [Google Scholar]
- 204.Mandel S., Maor G., Youdim M.B. Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (-)-epigallocatechin-3-gallate. J. Mol. Neurosci. : M O. 2004;24(3):401–416. doi: 10.1385/JMN:24:3:401. [DOI] [PubMed] [Google Scholar]
- 205.Guo S.H., Yan J.Q., Yang T.B., Yang X.Q., Bezard E., Zhao B.L. Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson’s disease through inhibition of ROS-NO pathway. Biol. Psychiatr. 2007;62:1353–1362. doi: 10.1016/j.biopsych.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 206.Zhou T., Zhu M., Liang Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson's disease. Mol. Med. Rep. 2018;17(4):4883–4888. doi: 10.3892/mmr.2018.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang K., Ma Z., Wang J., Xie A., Xie J. Myricetin attenuated MPP(+)-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology. 2011;61(1-2):329–335. doi: 10.1016/j.neuropharm.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 208.Ma Z.G., Wang J., Jiang H., Liu T.W., Xie J.X. Myricetin reduces 6-hydroxydopamine-induced dopamine neuron degeneration in rats. Neuroreport. 2007;18(11):1181–1185. doi: 10.1097/WNR.0b013e32821c51fe. [DOI] [PubMed] [Google Scholar]
- 209.Gao J., Chen S., Qiu Z., Fang L., Zhang L., Guo C., et al. Myricitrin ameliorates ethanol-induced steatosis in mouse AML12 liver cells by activating AMPK, and reducing oxidative stress and expression of inflammatory cytokines. Mol. Med. Rep. 2018;17(5):7381–7387. doi: 10.3892/mmr.2018.8740. [DOI] [PubMed] [Google Scholar]
- 210.Cai Z., Zeng W., Tao K., Lu F., Gao G., Yang Q. Myricitrin alleviates MPP(+)-induced mitochondrial dysfunction in a DJ-1-dependent manner in SN4741 cells. Biochem. Biophys. Res. Commun. 2015;458(2):227–233. doi: 10.1016/j.bbrc.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 211.Wang Y.H., Yu H.T., Pu X.P., Du G.H. Myricitrin alleviates methylglyoxal-induced mitochondrial dysfunction and AGEs/RAGE/NF-kappaB pathway activation in SH-SY5Y cells. J. Mol. Neurosci.: M O. 2014;53(4):562–570. doi: 10.1007/s12031-013-0222-2. [DOI] [PubMed] [Google Scholar]
- 212.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Myricitrin ameliorates 6-hydroxydopamine-induced dopaminergic neuronal loss in the substantia nigra of mouse brain. J. Med. Food. 2016;19(4):374–382. doi: 10.1089/jmf.2015.3581. [DOI] [PubMed] [Google Scholar]
- 213.Murota K., Nakamura Y., Uehara M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018;82(4):600–610. doi: 10.1080/09168451.2018.1444467. [DOI] [PubMed] [Google Scholar]
- 214.Winter A.N., Bickford P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants. 2019;8(9) doi: 10.3390/antiox8090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.