Abstract
Rhoptry-associated protein 1 (RAP1) of Plasmodium falciparum is a nonpolymorphic merozoite antigen that is considered a potential candidate for a malaria vaccine against asexual blood stages. In this longitudinal study, recombinant RAP1 (rRAP1) proteins with antigenicity similar to that of P. falciparum-derived RAP1 were used to analyze antibody responses to RAP1 over a period of 4 years (1991 to 1995) of 53 individuals naturally exposed to P. falciparum malaria. In any 1 year during the study, between 23 and 39% of individuals who had malaria developed immunoglobulin G (IgG) antibodies detectable with at least one rRAP1 protein. However, the anti-RAP1 antibody responses were detected only during or shortly after clinical malarial infections. RAP1 antibody levels declined rapidly (within 1 to 2 months) following drug treatment of the infections. No anti-RAP1 antibodies were usually detected a few months after the end of malaria transmission, during the dry season, or by the start of the next malaria season. Thus, RAP1 IgG responses were very short-lived. The short duration of RAP1 antibody response may explain the apparent lack of response in a surprisingly high proportion of individuals after clinical malarial infections. For some individuals who experienced more than one malarial infection, a higher anti-RAP1 antibody response to subsequent infections than to earlier infections was observed. This suggested secondary responses to RAP1 and thus the development of immunological memory for RAP1.
Malaria remains a major infectious disease in many parts of the tropics. It is estimated that over 300 to 500 million clinical cases occur each year, with cases in tropical Africa accounting for more than 90% of these figures (52). Infection by Plasmodium falciparum of individuals who have not been exposed to malaria before invariably leads to the disease. The infection can, however, become clinically less severe in individuals living for several years in regions where malaria is endemic. Two situations have been observed, seemingly dependent on distinct epidemiological patterns of, and thus exposure to, malaria (28, 31). First, in populations with a low frequency of the infection due to unstable transmission the development of acquired immunity is often incomplete. In this situation, it is believed, most infections in all age groups are likely to develop into the disease. Second, in populations in which malaria transmission is frequent and stable, acquired immunity does develop, though over a long period of time. In this situation, clinical disease is more frequent in children than in adults, presumably because children have not yet received the repeated exposure believed to be required to achieve the level of immunity shown by the adults in the same community.
There is no clear understanding of the mechanism(s) of this naturally acquired immunity in humans. Nonetheless, it is known that the immunity is partly mediated by antibodies since passive transfers of purified immunoglobulin G (IgG) from immune adult West Africans to P. falciparum-infected patients rapidly reduced the recipients’ parasitemia (8, 32, 41). The identification of parasite antigens that induce protective antibody responses would be an important step toward the understanding of naturally acquired human immunity to malaria. In this context, it is particularly pertinent to understand the development of human responses to several defined merozoite antigens now considered as targets for the development of a blood-stage vaccine because of their ability to induce partial or complete protection against parasite challenge in animal models (21). Importantly, naturally occurring human antibodies to certain merozoite antigens have been correlated with reduced parasite densities or disease by seroepidemiological studies. For example, antibodies detectable with recombinant MSP1 (rMSP1) or P. falciparum MSP1 (10, 51), rMSP2 (1, 49), or rRESA (2) were correlated with lower parasite densities. The results indicate a protective role for antibodies to these antigens, and such field studies on naturally occurring immune responses of humans thus complement animal experiments conducted for the purposes of vaccine development.
Rhoptry-associated protein 1 (RAP1), the subject of this study, is considered an important malaria vaccine antigen. Since the amino acid sequence of RAP1 shows only very limited diversity among P. falciparum isolates (17, 19, 20, 37), antigenic polymorphism should be less of a problem for a RAP1 vaccine than for vaccines based on some other, more polymorphic antigens of the parasite. Immunizations with affinity-purified protein complex containing RAP1 modified the course of parasitemia and protected Saimiri monkeys from a lethal P. falciparum challenge infection (36). In vitro, monoclonal and polyclonal antibodies to P. falciparum RAP1 inhibit merozoite invasion (16, 18, 42, 45), suggesting that antibodies to RAP1 may reduce parasite multiplication. Now it is important to elucidate the natural development of infection-induced human immune responses to RAP1.
Anti-RAP1 antibodies have been detected in people living in different regions where malaria is endemic (13, 19, 22–24, 46); thus, RAP1 is antigenic and a target for human immune responses. It has further been shown that most of the detectable human antibodies are of the IgG1 subclass (13) and are targeted to N-terminal parts of RAP1 (13, 19). Importantly, an association between high levels of IgG antibodies to the N-terminal regions of RAP1 and protection against high densities of P. falciparum parasites in Tanzanian children (25) has suggested a possible role of anti-RAP1 antibodies in human immunity. All these studies were cross-sectional surveys evaluating RAP1 antibodies at one point in time. Since no study has monitored individuals longitudinally, so far it has not been possible to approach even relatively simple questions about the dynamics of anti-RAP1 responses. Thus, it is not known how reliably antibodies to RAP1 are produced in response to a malarial infection, or what may be the duration of anti-RAP1 responses after the infection.
In this study, immunologically well characterized rRAP1 proteins (13) were used to analyze for the first time longitudinal antibody responses to RAP1 in individuals exposed to naturally transmitted P. falciparum infections over a period of several years. We have investigated the frequency of antibody responses to RAP1 following documented clinical infections, the duration of these responses after drug treatment of patients, and the question whether immunological memory to RAP1 is detectable after reinfections.
MATERIALS AND METHODS
Study area.
This longitudinal study was carried out among permanent residents of Daraweesh, a small sorghum and sesame farming village in Gedaref State, 450 km southeast of Khartoum, Sudan. The main vector responsible for transmission is Anopheles arabiense (15, 26), and P. falciparum is the predominant malarial species (3, 39). The climate is characterized by annual rainfalls between July and September, with a very dry season for the rest of the year. Clinical malaria cases occur in residents of Daraweesh from about September to November and peak in October or November. The period when clinical cases are observed, termed malaria season in this study, normally begins in September and ends by January in most years (40, 50). However, the region has been described as having seasonal and unstable malaria transmission (11, 12). The instability of malaria was demonstrated during a severe drought, October 1989 to June 1992, which interrupted malaria transmission in Daraweesh for 2 years, with fewer than 10 cases found in the entire village during the 1990–1991 and 1991–1992 seasons (39).
Study cohort, malaria monitoring, and collection of samples.
The village has a population of approximately 430 inhabitants, most of whom are descendants of a Fulani group, originally from Burkina Faso, who settled in Sudan a century ago. The residents of Daraweesh have participated in a study of acquired immunity to P. falciparum since 1988. This longitudinal study was carried out in a cohort of 53 permanent residents (born 1963 to 1987), from September 1991 to January 1995. The sickle cell hemoglobin trait was absent from all individuals involved in the study (50). The project had an ethical clearance from the Research Board of the Faculty of Medicine, University of Khartoum, and a national clearance granted by the Sudanese Ministry of Health.
All individuals involved in the cohort study were monitored for malaria each year from the beginning of September until the following mid-January as described in detail elsewhere (11, 39, 40). Malaria detection was carried out through a clinic run in the village by the project’s health team at least every second day. A health worker resident in the village was available for consultation daily. Active case detection was performed by health assistants on house visits to the cohort members every 2 weeks. Each individual was questioned about any illness since the previous visit, and oral temperature was measured; if the temperature was higher than 37.5°C or if the individual complained of fever, Giemsa-stained blood film was prepared. Passive case detection involved individuals who reported to the health team with any symptoms suggestive of malaria (constant or intermittent fever, headache, joint pain, vomiting, and diarrhea). The oral temperature of such individuals was measured, and blood film was examined. In this study, clinical malaria was defined as a febrile illness with P. falciparum-positive blood film and a body temperature of 37.5°C, or a positive blood film and reported complaint of fever. During the 1991 malaria season, both active and passive case detection methods were used, but no cases were detected that season. In the subsequent years, only passive case detection was used; thus, all cases reported in this study were detected passively. Chloroquine was administered to patients with malaria, and sulfadoxine-pyrimethamine treatment was used for chloroquine-resistant infections.
Blood samples for immunological analysis and parasitological examination were collected from each cohort member, after an informed consent was obtained, in regular surveys at the beginning (September) and after the end (January) of annual malaria seasons. The present study included samples collected during these surveys of 1991 to 1995, including the period of severe drought (September 1991 to January 1992). Samples were also donated by some cohort members at the time when they presented with a clinical case of P. falciparum malaria (which was then treated) during the 1992 to 1994 seasons. Samples from these patients in subsequent January surveys were taken 1 to 3.5 months (median, 75 days) after the episodes of clinical malaria. In 1994, 12 patients agreed to donate an additional convalescent sample 30 to 40 days after their clinical malaria. Samples from patients during an additional survey in November 1995 were collected 8 to 40 days after clinical malaria. In addition, dry-season samples were collected in June of 1994 and 1995. A total of 499 plasma samples from 53 individuals were analyzed by enzyme-linked immunosorbent assay (ELISA) for anti-RAP1 antibody.
In addition to examination of Giemsa-stained blood films, PCRs were performed as described previously (39) to detect P. falciparum in the blood of the cohort members from September 1993 onward.
rRAP1 antigens.
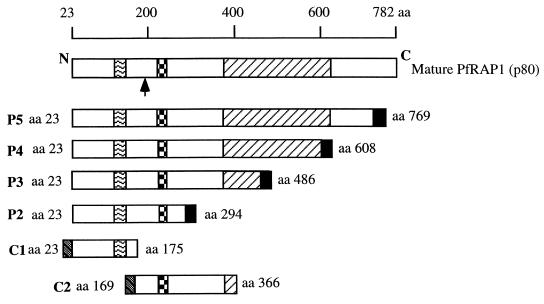
A schematic representation of six rRAP1 proteins derived from P. falciparum RAP1 is shown in Fig. 1. Glutathione S-transferase (GST) and hexahistidine (His6) rRAP1 antigens were expressed in Escherichia coli, purified, and used to evaluate anti-RAP1 antibody responses. The production and detailed immunological characterization of these rRAP1 antigens have been described elsewhere (13). The GST protein alone and coating buffer were used as controls for GST- and His6-RAP1 fusion proteins, respectively.
FIG. 1.
Schematic representation showing mature P.
falciparum RAP1 and rRAP1 proteins. P2 to P5 are proteins fused to
hexahistidine tag (■); C1 and C2 are fused to GST
( ).
).
 , region of repeats related to
the KSSSPS motif (between amino acid 123 and 164).
↑,
cleavage site (between amino acid 190 and 191) of mature RAP1 to yield
p65 fragment (35).
, inhibitory monoclonal antibody
epitope (L202 TPLEELYP210
[16]).
, region of repeats related to
the KSSSPS motif (between amino acid 123 and 164).
↑,
cleavage site (between amino acid 190 and 191) of mature RAP1 to yield
p65 fragment (35).
, inhibitory monoclonal antibody
epitope (L202 TPLEELYP210
[16]).  ,
cysteine-rich region (between amino acid 353 and 616). Amino acid
numbering is as in the work of Ridley et al. (37). This
figure has been modified from the work of Fonjungo et al.
(13).
,
cysteine-rich region (between amino acid 353 and 616). Amino acid
numbering is as in the work of Ridley et al. (37). This
figure has been modified from the work of Fonjungo et al.
(13).
ELISA for detection of IgG antibodies.
All sera were aliquoted and stored at −70°C before use. ELISA procedures were as described elsewhere (13). Briefly, rRAP1 or control antigens were diluted in coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3, pH 9.6) to a concentration of 0.5 μg/ml. Wells of 96-well flat-bottomed microtiter plates (Immunolon 4; Dynatech, Billingshurst, United Kingdom) were coated with 100 μl (50 ng/well) of an antigen at 4°C overnight. Antigen-coated wells were washed three times with 0.05% (vol/vol) Tween 20 in phosphate-buffered saline and blocked with 200 μl of 1% (wt/vol) nonfat dried skimmed milk in phosphate-buffered saline–Tween (blocking buffer) per well for 5 h at room temperature. Human sera were diluted in the blocking buffer for 5 h at room temperature, and 100-μl aliquots of each diluted serum were added to duplicate wells for each antigen or control and reacted at 4°C overnight. The wells were washed as described above and incubated with 100 μl of horseradish peroxidase-conjugated rabbit anti-human IgG (Dako Ltd., High Wycombe, United Kingdom) per well diluted 1:5,000, for 3 h at room temperature. The wells were washed four times, and reactions were developed with 100 μl of substrate buffer (0.024 M citric acid, 0.05 M Na2HPO4, 0.4 mg of o-phenylenediamine [Sigma, Poole, Dorset, United Kingdom] per ml, 0.4 μl of 30% [wt/vol] H2O2 per ml) per well for 10 min. The reactions were stopped with 20 μl of 2 M H2SO4 per well, and the optical density (OD) values were read at 492 nm.
Specific reactivity due to the RAP1 portion of GST-RAP1 proteins was obtained by subtraction of averaged OD due to GST alone from averaged OD for each GST-RAP1 protein. Likewise, for His6 proteins reactivity due to RAP1 was obtained by subtracting averaged OD due to coating buffer from averaged OD for P2, P3, P4, or P5 protein. All sera were tested at a dilution of 1:500. This dilution was chosen after optimization of the assay for specificity with titrations of sera from malaria-exposed and European donors, rRAP1, and antigen controls (GST or coating buffer). Thus diluted, >95% of endemic serum samples give low background with the controls. The assay differentiates well between RAP1 antibody-negative and -positive sera and, within limits, between levels of antibodies in the positive sera.
Control sera.
Negative control sera were collected from 33 healthy European donors to the Scottish Blood Transfusion Service who had not been exposed to malaria. A cutoff ELISA OD value for each recombinant protein was calculated as the mean plus 2 standard deviations of 33 European control serum samples. The cutoff values were 0.219 for C1, 0.142 for C2, 0.176 for P2, 0.233 for P3, 0.252 for P4, and 0.207 for P5.
A positive standard was a pool of sera from adult Gambians immune to malaria. Titration of the standard was performed against each antigen in each experiment to control for interexperimental variation. Titration curves of the positive standard (dilutions of 1:500, 1:1,000, 1:2,000, 1:4,000, and 1:8,000) were also used to determine whether antibody levels increased significantly (fourfold or more) between pretransmission samples and acute malaria and/or posttransmission and/or postinfection samples of each individual. To do this, longitudinal sets of sera (1:500) from each individual were all tested together at one time. Titration curves of the standard were produced at the same time, and OD values obtained for sera of one individual were compared by extrapolation to the standard curve. The working range of the assay, between negative cutoff and OD 2.0, is covered within an 8- to 16-fold change in antibody concentration.
ELISA for IgM antibodies.
Four rRAP1 proteins (C1, C2, P4, and P5) representing different regions of P. falciparum RAP1 were used to screen plasmas for IgM antibodies. The procedure for determining specific anti-RAP1 IgM antibodies was similar to that described above for IgG except that horseradish peroxidase-conjugated rabbit anti-human IgM (Dako Ltd.) diluted 1:2,000 was used for detecting IgM antibodies. A cutoff value for each rRAP1 protein in the IgM assays was calculated as the mean plus 2 standard deviations of 10 normal European serum samples: 0.096 for C1, 0.092 for C2, 0.206 for P4, and 0.077 for P5. Since there was no RAP1-specific IgM antibody standard, the anti-IgM serum was tested in each experiment against a range of concentrations of purified IgM myeloma protein applied as a coating on plates; the serum reliably detected between 1 and 0.03 μg of IgM per well.
RESULTS
Longitudinal IgG antibody responses to RAP1 in the cohort.
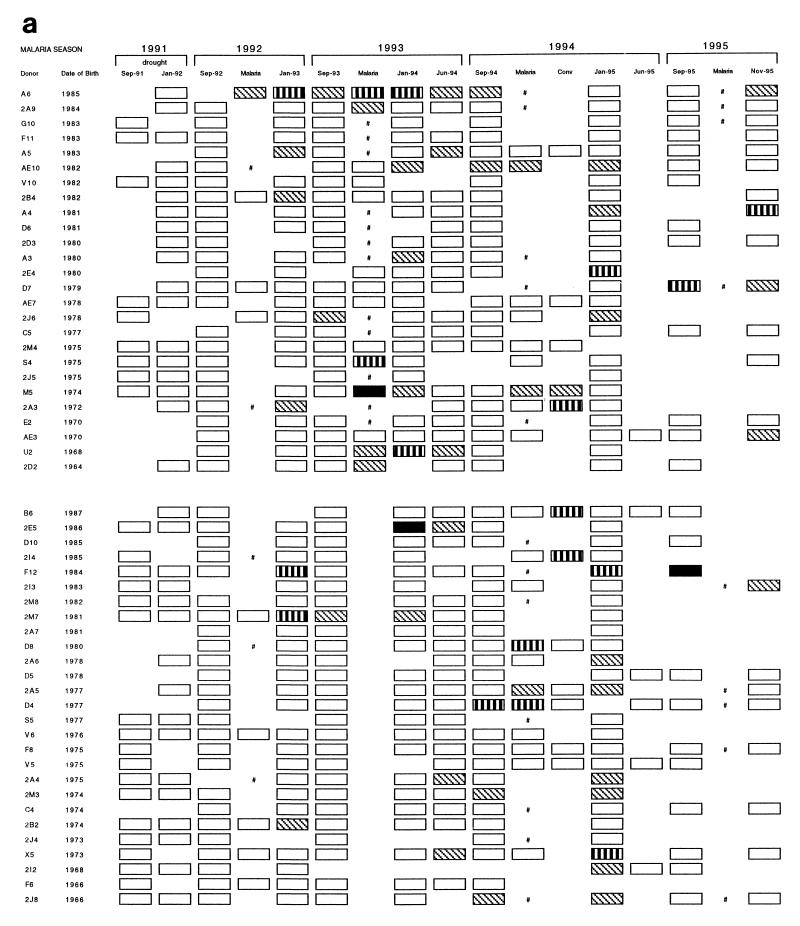
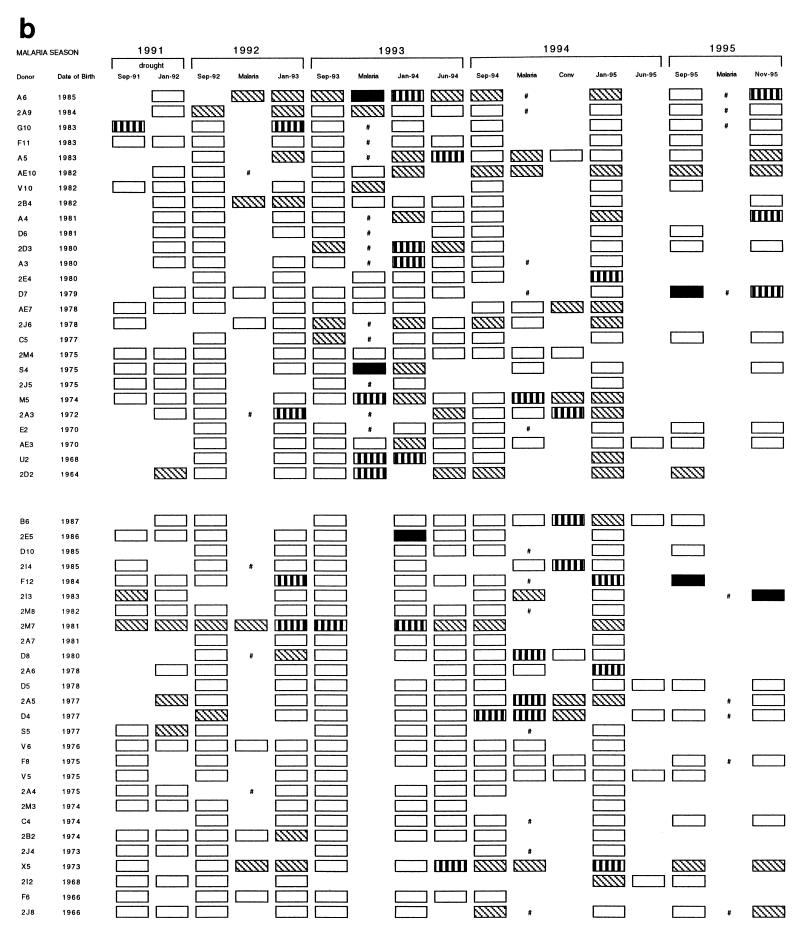
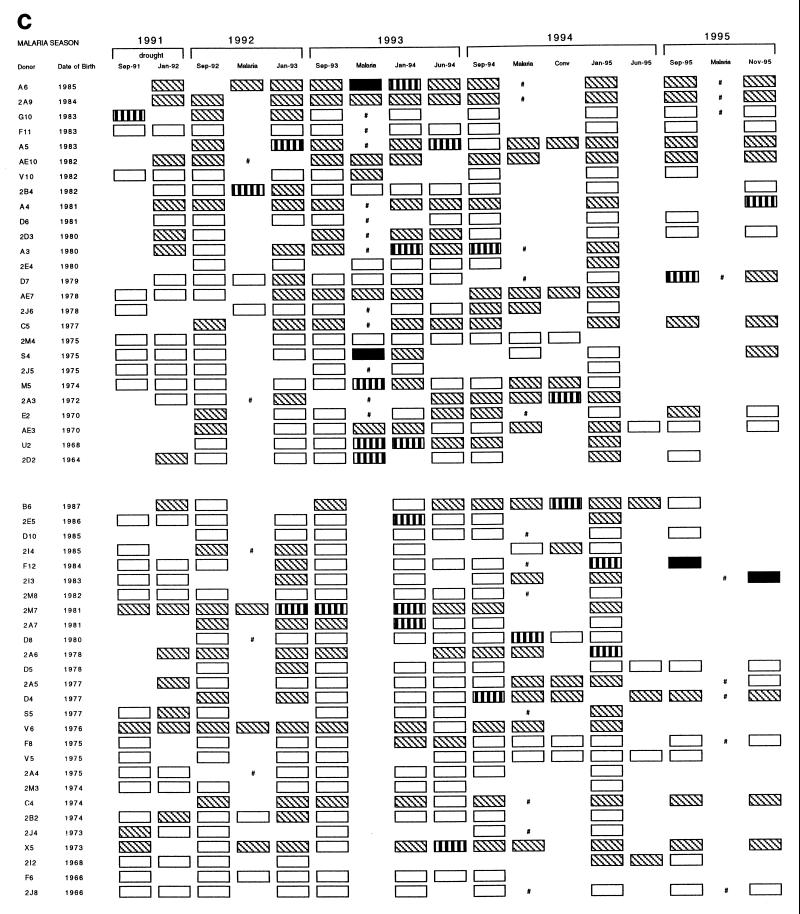
rRAP1 proteins were used to monitor antibody responses to P. falciparum RAP1 in a cohort of 53 individuals from 1991 to 1995. Results obtained with a total of 499 serum samples with three rRAP1 antigens are represented schematically in Fig. 2 (panels a to c correspond to reactivities with antigens C1, P3, and P5, respectively). First, the reactivity of each tested serum was compared to a cutoff level of normal European sera. Second, to estimate whether and, if so, when antibody responses occurred for the individuals studied, changes in the strength of reactivities between pairs of serial serum samples from each individual were assessed by comparisons to titration curves of a positive standard serum against each rRAP1 protein. In an individual, a significant antibody response was defined as a fourfold or higher increase in serum antibody levels from a pretransmission season sample (September) to an acute sample and/or to a posttransmission sample (January).
FIG. 2.
Reactivity profiles of IgG antibodies detectable with rRAP1 proteins C1 (a), P3 (b), and P5 (c) in 499 serum samples donated by 53 individuals between 1991 and 1995. Each serum sample was tested by ELISA at a dilution of 1:500, and reactions were developed with peroxidase-conjugated rabbit anti-human IgG diluted 1:5,000. Cutoff levels (means + 2 standard deviations of 33 European serum samples) and the indicated range of OD values above the cutoffs were used to grade arbitrarily the strength of each reaction. □, below cutoff level; ▧, above cutoff (OD < 0.5); ▥, above cutoff (OD = 0.5 to 1.0); ■, above cutoff (OD > 1.0); #, clinical malaria infection with no available plasma sample; Conv, convalescence sample collected 30 to 40 days after clinical malaria infection.
After 2 years of drought (1989 to 1991), low-positive reactions very close to European negative cutoff values were detected in sera of some individuals between September 1991 and September 1992 (with proteins P3 and P5, illustrated by Fig. 2b and c, respectively). However, none of the 53 individuals had any significant increase of antibody levels (i.e., response) to RAP1 up to September 1992, and this result correlated well with the observed absence of malaria cases during the drought.
During the autumn of 1992, when malaria transmission restarted after the return of rains, 14 individuals in the cohort had a clinical episode of P. falciparum malaria (Fig. 2). Serum samples from 13 individuals, for whom both September 1992 and postinfection (January 1993) samples were available, were analyzed for increases in anti-RAP1 levels following the infections (Table 1). Only 3 to 5 of the 13 individuals had significant antibody responses detectable with different rRAP1 proteins. The number of clinical malaria cases increased in the 1993–1994 and 1994–1995 malaria seasons. Of 26 individuals who became clinically infected in 1993–1994, 23 individuals donated pre- and postinfection samples used for the analysis of response and 30 to 39% had IgG antibody responses detected with the rRAP1 proteins (Table 1). Thirty of 32 individuals who were diagnosed with clinical malaria in 1994–1995 gave samples valid for the analysis, and 30 to 33% had an IgG antibody response detectable with at least one rRAP1 protein.
TABLE 1.
Antibody response to RAP1 in the Daraweesh cohort
| Antigen | Antibody response by
season [no. positive/ no. total (%)] for
individualsa
|
|||||
|---|---|---|---|---|---|---|
| With clinically documented
malarial infection
|
Without clinical malaria
|
|||||
| 1992–1993 | 1993–1994 | 1994–1995 | 1992–1993 | 1993–1994 | 1994–1995 | |
| C1 | 3/13 (23) | 7/23 (30) | 10/30 (33) | 1/39 (3) | 1/27 (4) | 1/21 (5) |
| C2 | 5/13 (38) | 7/23 (30) | 9/30 (30) | 1/39 (3) | 0/27 (0) | 0/21 (0) |
| P2 | 5/13 (38) | 9/23 (39) | 9/30 (30) | 2/39 (5) | 1/27 (4) | 1/21 (5) |
| P3 | 5/13 (28) | 9/23 (39) | 9/30 (30) | 2/39 (5) | 1/27 (4) | 1/21 (5) |
| P4 | 5/13 (38) | 9/23 (39) | 9/30 (30) | 2/39 (5) | 1/27 (4) | 1/21 (5) |
| P5 | 5/13 (38) | 9/23 (39) | 9/30 (30) | 1/39 (3) | 1/27 (4) | 0/21 (0) |
Response was defined as a fourfold or higher increase in antibody level from a level detected in a pretransmission sample (September) to a level in an acute-malaria and/or posttransmission sample (January). Analysis was performed for those individuals with pre- and posttransmission season samples.
Individuals without a recent history of clinical malaria had detectable antibody responses to RAP1 only very rarely (Table 1). In 1992, 2 of 39 such individuals (G10 and F12) had responses detected with some rRAP1 proteins (Fig. 2). Of 27 and 21 individuals without clinical malaria in the 1993–1994 and 1994–1995 seasons, two individuals (2E5 in 1994 and 2E4 in 1995) had an IgG response to RAP1 (Fig. 2).
These results indicate that individuals usually produce detectable IgG antibody responses to RAP1 only during or after a clinical malaria episode. This accords with the absence of detectable antibody responses (and of cases) in the cohort during the drought and up to September 1992.
Different patterns of anti-RAP1 antibody response to clinical malaria in individuals.
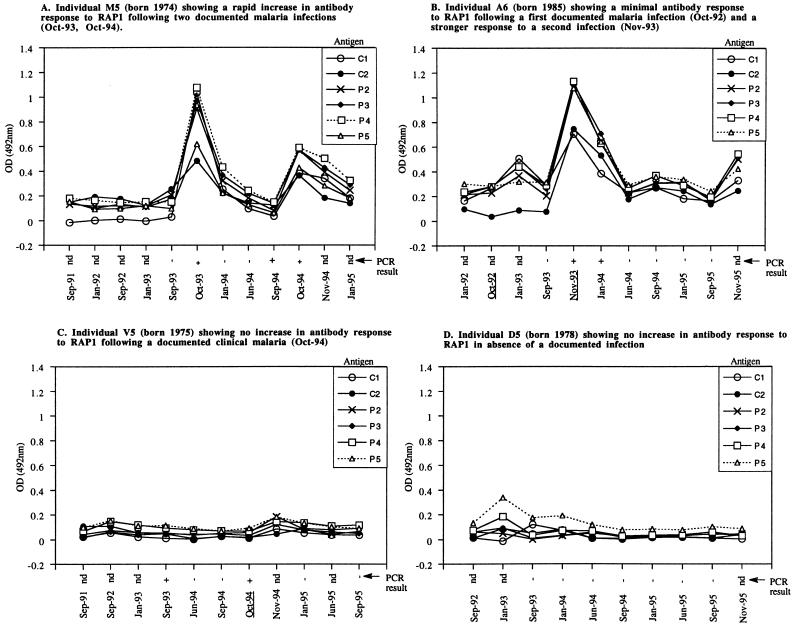
During the study (1991 to 1995), three distinct antibody response patterns were observed among 48 individuals who had at least one episode of documented clinical malaria. The first pattern consisted of a rapid rise in anti-RAP1 antibodies that was detectable already during the clinical infection (for example, see Fig. 3A, Oct-93). Among seven individuals who donated serum samples before and during clinical malaria in 1992–1993, two individuals (X5 and 2B4) had this rapid response detectable with some rRAP1 proteins. Of 14 infected individuals who gave preinfection and acute-malaria samples in the 1993–1994 season, 5 (A6, S4, M5, U2, and 2D2) had the rapid antibody responses. In the 1994–1995 season, 3 individuals (D8, D4, and 2A5) of 18 who gave suitable samples showed the rapid response.
FIG. 3.
Different patterns of IgG antibody responses to RAP1 in natural P. falciparum infections. Each panel illustrates the time course of response(s) of one individual as detected by ELISA with six rRAP1 proteins. The x axes show the month and the year when each test sample was collected. Dates of samples collected during documented episodes of clinical malaria are underlined. PCR results, also along the x axes, indicate the absence (−) or presence (+) of P. falciparum in blood samples of each individual. nd, PCR was not done.
The second pattern (example in Fig. 3B, Oct-92 infection) was where no antibody response was seen at the time of acute infection but a rise did occur later and was detectable during convalescence 30 to 40 days after clinical malaria (1994 and 1995 cases) or at the time of the postseason surveys in January. This was the most commonly observed pattern, and 40% of individuals had significant antibody responses of this slower type to one or more episodes of malaria.
The third pattern was characterized by no detectable IgG antibody response to RAP1 following a documented clinical infection (example in Fig. 3C, Oct-94). A surprisingly high proportion of the cohort were in this category at one time or another over the period of the study (Table 1). More than 60% of individuals appeared to have failed to respond to at least one episode of clinical malaria in the three malaria seasons. In the 1992 season, 8 of 13 individuals with clinical infections had no antibody response detectable by any rRAP1 protein. In 1993–1994 and 1994–1995, 14 of 23 and 20 of 30 infected individuals, respectively, had no detectable antibody response.
We have attempted to find whether there is an immunological memory to RAP1 by monitoring IgG responses of individuals who had two or more clinical infections during the study after the drought. In comparing the first and subsequent infections after the drought, boosting of anti-RAP1 antibody response was observed in some but not all individuals. An example of an apparent boosting is illustrated by Fig. 3B, showing antibody responses of an individual who had a higher response to a second infection (Nov-93) than to a previous infection (Oct-92). Of four individuals with a second infection in 1993–1994, only one (A6) showed a higher antibody response to the second infection. In 1994–1995, 2 (X5 and D8) of 11 individuals with a reinfection had a higher antibody response to their second than to the first infection. However, other individuals appeared to have failed to respond well to two infections (e.g., AE7, 2J6, 2M4, E2, AE3, and V6). Thus, with repeated infections a degree of immunological memory to RAP1 may develop, though this is found relatively infrequently in this cohort.
Patterns of anti-RAP1 antibody response in individuals without malaria.
In individuals without clinical malaria, two patterns of antibody response were observed. No detectable antibody responses in the absence of any documented malarial infection throughout the 1991 to 1995 study were observed in only 3 of the 53 individuals (2A7, D5, and 2I2) (example in Fig. 3D).
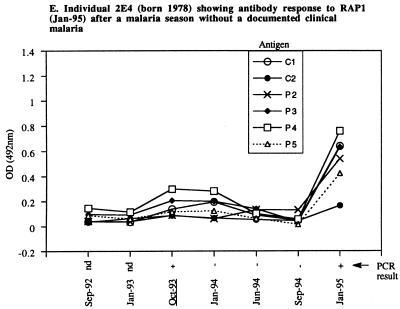
The second, more interesting pattern was seen for a few individuals who had no clinical malaria near the time of a significant antibody response (example in Fig. 3E). This rarest pattern was observed for four individuals (G10 and F12 in 1992–1993, 2E5 in 1993–1994, and 2E4 in 1994–1995). These individuals may have responded while infected asymptomatically. PCR analysis confirmed the presence of P. falciparum in the blood of two individuals who remained well (example in Fig. 3E, positive PCR in Jan-95).
The findings support the overall conclusion that the presence of malarial parasites is required to elicit a specific RAP1 antibody response (and also support the specificity of anti-RAP1 IgG ELISAs).
Duration of IgG antibody response to RAP1.
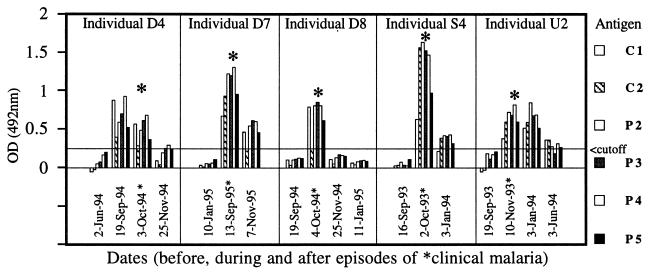
One of the objectives of this longitudinal study was to determine how long anti-RAP1 antibody responses last after an infection. A generally rapid decline of the responses is illustrated by examples shown in Fig. 3 and 4. Following a documented malarial infection at the peak of the transmission season (October or November), antibodies sometimes persisted until a regular January survey (e.g., Fig. 3B, Nov-93 to Jan-94; Fig. 4, individual U2) but usually declined to low or undetectable levels by January (e.g., Fig. 3A, Oct-93 to Jan-94; Fig. 4, individuals D8 and S4). In some cases, responses detected during a malarial infection in September or October waned even by the time of collection of convalescent samples in November (e.g., Fig. 4, individuals D4, D7, and D8). Of 10 individuals who had a significant response to clinical malaria in October, 5 had no detectable antibody by January. In comparison, antibody could still be detected in January in all of seven individuals who responded to an acute infection in November. Collections of serum samples in June of 1994 and 1995 enabled us to monitor the duration of antibody response over the dry season. In the cohort of 53 individuals, no new antibody responses were detected between January and the start of the next malaria season (e.g., Fig. 3A and B, Jan-94 to Sep-94). Instead, antibodies detectable in January declined by June in the majority of individuals from whom both samples were available in 1994 or in 1995 (Fig. 2). Thus, by the start of each of three consecutive malaria seasons (September of 1993, 1994, and 1995), all but a few individuals had very low or not detectable IgG antibodies (Fig. 2). The finding of four individuals with high levels of antibodies in September of 1994 and 1995 could be explained by the fact that three of the four cases were infected (PCR positive), probably due to the earlier onset of malaria transmission in these comparatively wet years (49a).
FIG. 4.
IgG responses to RAP1 are short-lived after recovery from P. falciparum infection. Reactivities of rRAP1 antigens with IgG in sera donated by five different individuals on the indicated dates before, during, and after drug-cured clinical malarial episodes (asterisks) are shown. Note a rapid decline of antibody levels in all cases. A cutoff value indicated by a horizontal line is that of P4; cutoffs for the other antigens were lower (see Materials and Methods).
Together, these results show that the duration of antibody responses to P. falciparum RAP1 is approximately 2 months or less in the absence of reinfection or persistence of the infection.
IgM antibody response to RAP1.
In any one season, 61 to 70% of individuals who experienced clinical malaria had no detectable anti-RAP1 IgG response. We have therefore tested whether such individuals might be producing IgM antibodies to RAP1. To investigate this, 33 IgG-negative postinfection samples were screened for the presence of IgM antibodies with four rRAP1 proteins, C1, C2, P4, and P5, which are representative of different parts of the RAP1 protein. No specific IgM was detected in any of the samples with any of the proteins (data not shown). Thus, RAP1 IgG-negative samples also had no detectable RAP1 IgM antibodies.
DISCUSSION
To study the longitudinal kinetics of antibody responses to RAP1, we have taken advantage of two epidemiological features of P. falciparum malaria in Daraweesh (11, 12, 39, 40, 50). Firstly, Daraweesh has a short and well-defined malaria transmission season, and this allows a reasonably accurate timing of antibody responses to RAP1 relative to the infection. Secondly, the main part of the study was initiated after a 2-year drought period that produced in each individual a low baseline antibody level to which it was possible to compare antibody responses after infections in subsequent transmission seasons. Anti-RAP1 responses of a cohort of 53 permanent residents were monitored over 4 years, and the results led to the following conclusions. (i) Most individuals without a documented malaria episode had anti-RAP1 levels no higher than those of nonexposed European controls. (ii) After the 1989 to 1991 drought, anti-RAP1 antibody responses were observed in the cohort only when transmission and clinical malaria cases reappeared in 1992. Individuals had detectable responses to RAP1 usually only after a documented malarial infection. (iii) Anti-RAP1 antibody response was boostable in at least some individuals, and this indicates that immunological memory to RAP1 may develop after a repeated exposure to the parasite. (iv) However, antibody responses to RAP1 were generally very short-lived.
Several observations strongly indicate that antigenic stimulation by live malarial infection is required for a sustained production of RAP1 IgG antibodies. Firstly, no rise in antibody levels to RAP1 was detected in any cohort member using samples collected until September 1992. This correlated well with the absence of malaria during 2 years of drought that preceded the restart of transmission in September 1992. Also, no infected mosquitoes were reported in the Daraweesh area during the drought period (12). Secondly, antibody responses to RAP1 began to occur only after the first postdrought malaria season (1992–1993), usually only in cases of clinical malaria (Table 1). Thirdly, antibody responses to clinical malaria declined rapidly after drug treatment of the patients, presumably because clearance of the parasites or a decrease in parasite density resulted in a diminished antigenic stimulation of the immune response.
RAP1 antibody response was apparently boostable in some individuals who had a higher IgG antibody response to a second infection than to the first documented clinical infection after the drought. This observation indicates that immunological memory to RAP1 can be retained for some time after an infection. The observation is supported by the report of Stowers and colleagues (46) that two volunteers experimentally challenged with sporozoites of P. falciparum showed a boosting effect of a second infection on RAP1 response. Changes in immune responses of the study cohort probably occurred during the drought while P. falciparum malaria was absent for 2 years. During the 1992 to 1994 transmission seasons, 23 to 39% of individuals with clinical malaria responded to the infection by production of RAP1 antibodies but only nine individuals responded rapidly to their first infection after the drought. This suggested that these individuals had been infected before the drought and that some retained a degree of immunological memory despite the lack of transmission for 2 years. This is somewhat reminiscent of a study in the central highlands of Madagascar which showed that some older people retained immunological memory to P. falciparum 155/RESA or circumsporozoite protein (CSP) following 20 years of absence of malaria transmission (6).
It was quite unexpected to find a high percentage of individuals with clinical malaria who had no detectable IgG antibody response. Between 61 and 70% of individuals apparently failed to respond by production of IgG RAP1 antibodies to the infection at some time. It is unlikely that all these individuals had never been exposed to malaria before the drought, since about 30% of residents of Daraweesh had a confirmed clinical malarial infection before the interruption of malaria transmission (50). One explanation may be that a substantial decline in immunological memory had occurred during the 2 years of the drought in many individuals, who thus produced only transient responses or undetectably low levels of antibodies to their postdrought infections.
Failing to detect RAP1 IgG antibodies after infections in some individuals, we investigated whether these individuals produced IgM antibodies to RAP1. No IgM antibody was detected in 33 IgG-negative serum samples tested. However, since no specific anti-RAP1 IgM positive control serum was available, it is not clear whether our IgM assay was sensitive enough, and the presence of anti-RAP1 IgM responses thus has not been totally excluded.
Several different hypothetical explanations have been offered by others to account for an apparent failure to produce antibodies to a defined antigen after a malarial infection. Antigenic polymorphism is a common feature of malarial parasites (4, 29, 30, 43, 47) that could explain low frequencies of responses to certain antigens. Priming of individuals to epitopes of a highly polymorphic antigen, e.g., block 2 of MSP1, following exposure to one P. falciparum “strain” may not be adequate to produce a response on exposure to different epitopes of another “strain” (5). However, antigenic polymorphism is not likely to account for the apparent lack of response to RAP1, since RAP1 is a largely conserved protein (17, 19, 20, 37) and the same or similar epitopes are likely to be presented on every reinfection with P. falciparum.
An intrinsically poor immunogenicity of a malarial antigen could lead to a lack of or low and undetectable antibody response. For example, a relatively high percentage of children living in an area where malaria is endemic did not have detectable anti-P. falciparum CSP antibodies, while at the same time producing high titers of antibody to P. falciparum asexual blood-stage antigens (48). The RAP1 protein could contact antigen-presenting cells, though probably only transiently due to its intracellular location, if it was released during merozoite invasion. Previous studies have shown RAP1 to be poorly soluble in an aqueous environment due to its chemical structure (7, 37). Thus, it can be envisaged that a reduced exposure, uptake, processing, or presentation of RAP1 by antigen-presenting cells could result from the poor solubility of RAP1 when released in an aqueous physiological environment. However, studies carried out in different areas of endemicity (13, 19, 22–25, 46) indicate that, overall, RAP1 is strongly immunogenic, with up to 86% of individuals having antibodies to RAP1 in some populations. Thus, poor immunogenicity of RAP1 is an unlikely explanation for the failure to detect antibodies to it in many members of our cohort.
Responses to some malarial antigens can be genetically controlled, and this could result in permanently weak responses or nonresponsiveness of some individuals. The hypothesis is supported by studies with inbred mice showing that a failure to produce antibodies is linked to the H-2 genotype in some models (27). Strains of mice with the H-2k haplotype produced antibodies, while H-2b haplotype mice failed to respond or responded weakly to P. falciparum 155/RESA and peptides derived from RESA. Also, when mice were immunized with (NANP)40 repeats of the CSP of P. falciparum, only H-2b mice responded while non-H-2b mice were consistently nonresponsive (9). Immunological nonresponsiveness or low responses to defined malarial antigens such as CSP (14, 34, 44) or P. falciparum 155/RESA, 230-kDa, 48/45-kDa and 25-kDa proteins (34, 38), or the SPf66 vaccine (33) have been reported for individuals from different areas where malaria is endemic. The idea of HLA-dependent restriction of immune responses in humans has been proposed to account for the apparent lack of or low antibody levels to malarial antigens in some individuals (14).
The notion of genetic restriction of immune response to RAP1 has not been investigated here; nonetheless, the results deserve comments regarding this. Some cohort members who failed to respond to RAP1 in one infection had an antibody response upon reinfection or vice versa. Genetic restriction cannot easily explain the apparent lack of RAP1 response to one P. falciparum infection and the presence of the response to another episode of the same infection in the same individual. However, seven individuals did not produce RAP1-IgG antibodies even after a reinfection, though some of these individuals did respond to other malarial antigens such as MSP1 (5) or MSP2 (unpublished data). Thus, we do not entirely exclude a possibility that responsiveness to RAP1 might be genetically restricted.
The most plausible explanation for the lack of detectable antibody after some malarial episodes is the very short duration of anti-RAP1 IgG responses in this cohort. This has been highlighted by the rapid decline of antibody levels during each dry season, the levels becoming undetectable before the start of the next malaria season in the majority of individuals who responded in the previous season (examples in Fig. 3A and B). In many individuals, antibody responses induced by a clinical infection in September or October declined to preinfection levels and were no longer detectable by January. In some cases, responses detected during a malarial episode waned even by the time of collection of convalescent samples 1 to 2 months later (examples in Fig. 4). The short duration of anti-RAP1 antibody response, 2 months or less, can best explain why no antibodies were detected in convalescent or posttransmission samples following many infections in this cohort. Antibody responses to RAP1 in two immunologically naive volunteers experimentally challenged with P. falciparum sporozoites were also found to be short-lived (46).
In conclusion, our observations strongly indicate that the presence of parasites is required first to elicit and then to maintain anti-RAP1 antibody response. So far, sampling of the cohort has not been sufficiently frequent to allow a definitive conclusion on the overall frequency of anti-RAP1 responses that are of short duration in this population. Interestingly, all of the few individuals who were found to be infected asymptomatically had antibody responses to RAP1. The finding of anti-RAP1 responses in such individuals agrees with a report of Elhassan and colleagues (12), who found other antimalarial antibodies in individuals with subpatent infections in this population. If antibodies to RAP1 contribute to acquired immunity, the very short duration of the response could have important implications, as drug treatment of malaria could lead to a rapid loss of immunity in individuals in this area of unstable transmission. These issues should be resolved by our current, more intensive follow-up studies of clinical cases and also of asymptomatic infections.
ACKNOWLEDGMENTS
We are grateful to the people of Daraweesh for continuous cooperation throughout the study. We thank Eleanor Riley for a pool of control sera.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (project 940030), the ENRECA program of the Danish International Development Agency (DANIDA), the Danish Biotechnology Programme, the INCO-DC program of the European Union, and The Wellcome Trust (013163/Z/94/C, 047096/Z/96/Z/077, and 019536/Z/96/C).
REFERENCES
- 1.Al-Yaman F, Genton B, Anders R, Taraika J, Ginny M, Mellor S, Alpers M P. Assessment of the role of the humoral response to Plasmodium falciparumMSP2 compared to RESA and SPf66 in protecting Papua New Guinean children from clinical malaria. Parasite Immunol. 1995;17:493–501. doi: 10.1111/j.1365-3024.1995.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Yaman F, Genton B, Falk M, Anders R F, Lewis D, Hii J, Beck H-P, Alpers M P. Humoral response to Plasmodium falciparumring-infected erythrocyte surface antigen in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg. 1995;52:66–71. doi: 10.4269/ajtmh.1995.52.66. [DOI] [PubMed] [Google Scholar]
- 3.Babiker H, Creasey A M, Fenton B, Bayoumi R A L, Arnot D E, Walliker D. Genetic diversity of Plasmodium falciparumin a village in eastern Sudan. Diversity of enzymes, 2D-PAGE proteins and antigens. Trans R Soc Trop Med Hyg. 1991;85:572–577. doi: 10.1016/0035-9203(91)90347-2. [DOI] [PubMed] [Google Scholar]
- 4.Brown H, Kemp D J, Barzaga N, Brown G V, Anders R F, Coppel R L. Sequence variation in S-antigen genes of Plasmodium falciparum. Mol Biol Med. 1987;4:365–376. [PubMed] [Google Scholar]
- 5.Cavanagh D R, Elhassan I M, Roper C S, Robinson V J, Giha H, Holder A A, Hviid L, Theander T G, Arnot D E, McBride J S. A longitudinal study of type-specific antibody responses to Plasmodium falciparummerozoite surface protein 1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 6.Chougnet C, Deloron P, Lepers J P, Tallet S, Rason M D, Astagneau P, Savel J, Coulanges P. Humoral and cell-mediated immune responses to the Plasmodium falciparumantigens Pf155/RESA and CS protein: seasonal variations in a population recently re-exposed to endemic malaria. Am J Trop Med Hyg. 1990;43:234–242. doi: 10.4269/ajtmh.1990.43.234. [DOI] [PubMed] [Google Scholar]
- 7.Clark J T, Anand R, Akoglu T, McBride J S. Identification and characterisation of proteins associated with the rhoptry organelles of Plasmodium falciparummerozoites. Parasitol Res. 1987;73:425–434. doi: 10.1007/BF00538200. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, McGregor I A, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 9.Del Giudice G, Cooper J A, Merino J, Verdini A S, Pessi A, Togna A R, Engers H D, Corradin G, Lambert P H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoites repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986;137:2952–2955. [PubMed] [Google Scholar]
- 10.Egan A F, Morris J, Barnish G, Allen S, Greenwood B M, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparummalaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 11.Elhassan I M, Hviid L, Arnot D E, Roper C, Theander T G. Is immunity against P. falciparum malaria acquired by individuals living in a seasonal and unstable malaria transmission area? Sudan Med J. 1995;33:101–104. [Google Scholar]
- 12.Elhassan I M, Hviid L, Jakobsen P H, Giha H, Satti G M H, Arnot D E, Jensen J B, Theander T G. High proportion of subclinical Plasmodium falciparuminfections in an area of seasonal and unstable malaria in Sudan. Am J Trop Med Hyg. 1995;53:78–83. [PubMed] [Google Scholar]
- 13.Fonjungo P N, Stüber D, McBride J S. Antigenicity of recombinant proteins derived from rhoptry-associated protein 1 of Plasmodium falciparum. Infect Immun. 1998;66:1037–1044. doi: 10.1128/iai.66.3.1037-1044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good M F, Miller L H, Kumar S, Quakyi I A, Keister D, Adams J H, Moss B, Berzofsky J A, Carter R. Limited immunological recognition of critical malaria vaccine candidate antigens. Science. 1988;242:574–577. doi: 10.1126/science.2902690. [DOI] [PubMed] [Google Scholar]
- 15.Haridi A M. Partial exophily of Anopheles gambiaespecies B in the Khashm Elgirba area in eastern Sudan. Bull W H O. 1972;46:39–46. [PMC free article] [PubMed] [Google Scholar]
- 16.Harnyuttanakorn P, McBride J S, Donachie S, Heidrich H G, Ridley R G. Inhibitory monoclonal antibodies recognise epitopes adjacent to a proteolytic cleavage site on the RAP1 protein of Plasmodium falciparum. Mol Biochem Parasitol. 1992;55:177–186. doi: 10.1016/0166-6851(92)90138-a. [DOI] [PubMed] [Google Scholar]
- 17.Howard R F. The sequence of the p82 rhoptry protein is highly conserved between two Plasmodium falciparumisolates. Mol Biochem Parasitol. 1992;51:327–330. doi: 10.1016/0166-6851(92)90083-v. [DOI] [PubMed] [Google Scholar]
- 18.Howard R F, Jacobson K C, Rickel E, Thurman J. Analysis of inhibitory epitopes in the Plasmodium falciparumrhoptry protein RAP1 including identification of a second inhibitory epitope. Infect Immun. 1998;66:380–386. doi: 10.1128/iai.66.1.380-386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard R F, Jensen J B, Franklin H L. Reactivity profile of human anti-82-kilodalton rhoptry protein antibodies generated during natural infection with Plasmodium falciparum. Infect Immun. 1993;61:2960–2965. doi: 10.1128/iai.61.7.2960-2965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard R F, Peterson C. Limited RAP1 sequence diversity in field isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1996;77:95–98. doi: 10.1016/0166-6851(96)02576-5. [DOI] [PubMed] [Google Scholar]
- 21.Howard R J, Pasloske B L. Target antigens for asexual malaria vaccine development. Parasitol Today. 1993;9:369–372. doi: 10.1016/0169-4758(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson K C, Thurman J, Schmidt C M, Rickel E, De Ferreira J O, Ferreira-Da-Cruz M F, Ribeiro C T D, Howard R F. A study of antibody and T cell recognition of rhoptry-associated protein 1 (RAP1) and RAP2 recombinant proteins and peptides of Plasmodium falciparumin migrants and residents of the state of Rondonia, Brazil. Am J Trop Med Hyg. 1998;59:208–216. doi: 10.4269/ajtmh.1998.59.208. [DOI] [PubMed] [Google Scholar]
- 23.Jakobsen P H, Hviid L, Theander T G, Afare E A, Ridley R G, Heegaard P M H, Stüber D, Dalsgaard K, Nkrumah F K. Specific T-cell recognition of the merozoite proteins rhoptry-associated protein 1 and erythrocyte-binding antigen 1 of Plasmodium falciparum. Infect Immun. 1993;61:268–273. doi: 10.1128/iai.61.1.268-273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsen P H, Kurtzhals J A, Riley E M, Hviid L, Theander T G, Morris-Jones S, Jensen J B, Bayoumi R A L, Ridley R G, Greenwood B M. Antibody responses to rhoptry associated protein-1 (RAP1) of Plasmodium falciparumparasites in humans from areas of different malaria endemicity. Parasite Immunol. 1997;19:387–393. doi: 10.1046/j.1365-3024.1997.d01-234.x. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsen P H, Lemnge M M, Abu-Zeid Y A, Msangeni H A, Salum F M, Mhina J I K, Akida J A, Ruta A S, Ronn A M, Heegaard P M H, Ridley R G, Bygbjerg I C. Immunoglobulin G reactivities to rhoptry-associated protein-1 associated with decreased levels of Plasmodium falciparumparasitaemia in Tanzanian children. Am J Trop Med Hyg. 1996;55:642–646. doi: 10.4269/ajtmh.1996.55.642. [DOI] [PubMed] [Google Scholar]
- 26.Jensen J B, Boland M T, Allan J S, Carlin J M, Waa J A V, Divo A A, Akood M A S. Association between human serum-induced crisis forms in cultured Plasmodium falciparumand clinical immunity to malaria in Sudan. Infect Immun. 1983;41:1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew A M, Langford C J, Pye D, Edwards S, Corcoran L, Anders R F. Class II restriction in mice to the malaria candidate vaccine ring infected erythrocyte surface antigen (RESA) as synthetic peptides or as expressed in recombinant Vaccinia. J Immunol. 1989;142:4012–4016. [PubMed] [Google Scholar]
- 28.MacDonald G. The epidemiology and control of malaria. London, United Kingdom: Oxford University Press; 1957. [Google Scholar]
- 29.McBride J S, Walliker D, Morgan G. Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science. 1982;217:254–256. doi: 10.1126/science.6178159. [DOI] [PubMed] [Google Scholar]
- 30.McBride J S, Welsby P D, Walliker D. Serotyping Plasmodium falciparumfrom acute human infections using monoclonal antibodies. Trans R Soc Trop Med Hyg. 1984;78:32–34. doi: 10.1016/0035-9203(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 31.McGregor I A. The development and maintenance of immunity to malaria in highly endemic areas. Clin Trop Med Commun Dis. 1986;1:29–53. [Google Scholar]
- 32.McGregor I A, Carrington S P, Cohen S. Treatment of East African Plasmodium falciparummalaria with West African human gammaglobulin. Trans R Soc Trop Med Hyg. 1963;57:170–175. [Google Scholar]
- 33.Patarroyo M E, Vinasco J, Amador R, Espejo F, Silva Y, Moreno A, Rojas M, Mora A L, Salcedo M, Valero V, Goldberg A K, Kalil J. Genetic control of the immune response to a synthetic vaccine against Plasmodium falciparum. Parasite Immunol. 1991;13:509–516. doi: 10.1111/j.1365-3024.1991.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 34.Quakyi I A, Otoo L N, Pombo D, Sugars L Y, Menon A, Degroot A S, Johnson A, Alling D, Miller L H, Good M F. Differential non-responsiveness in humans of candidate Plasmodium falciparumvaccine antigens. Am J Trop Med Hyg. 1989;41:125–134. [PubMed] [Google Scholar]
- 35.Ridley R G, Lahm H W, Takacs B, Scaife J G. Genetic and structural relationships between components of a protective rhoptry antigen complex from Plasmodium falciparum. Mol Biochem Parasitol. 1991;47:245–246. doi: 10.1016/0166-6851(91)90184-8. [DOI] [PubMed] [Google Scholar]
- 36.Ridley R G, Takacs B, Etlinger H, Scaife J G. A rhoptry antigen of Plasmodium falciparum is protective in Saimirimonkeys. Parasitology. 1990;101:187–192. doi: 10.1017/s0031182000063228. [DOI] [PubMed] [Google Scholar]
- 37.Ridley R G, Takacs B, Lahm H W, Delves C J, Goman M, Certa U, Matile H, Woollett G R, Scaife J G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990;41:125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- 38.Riley E M, Olerup O, Bennett S, Rowe P, Allen S J, Blackman M J, Troye-Blomberg M, Holder A A, Greenwood B M. MHC and malaria: the relationship between HLA class II alleles and immune responses to Plasmodium falciparum. Int Immunol. 1992;4:1055–1063. doi: 10.1093/intimm/4.9.1055. [DOI] [PubMed] [Google Scholar]
- 39.Roper C, Elhassan I M, Hviid L, Giha H, Richardson W, Babiker H, Sati G M H, Theander T G, Arnot D E. Detection of very low level Plasmodium falciparuminfections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. Am J Trop Med Hyg. 1996;54:325–331. doi: 10.4269/ajtmh.1996.54.325. [DOI] [PubMed] [Google Scholar]
- 40.Roper C, Richardson W, Elhassan I M, Giha H, Hviid L, Sati G M H, Theander T G, Arnot D E. Seasonal changes in the Plasmodium falciparumpopulation in individuals and their relationship to clinical malaria: a longitudinal study in a Sudanese village. Parasitology. 1998;116:501–510. doi: 10.1017/s0031182098002650. [DOI] [PubMed] [Google Scholar]
- 41.Sabchareon A, Outtara D, Attanah P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparummalaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 42.Schofield L, Bushell G R, Cooper J A, Saul A J, Upcroft J A, Kidson C. A rhoptry antigen of Plasmodium falciparumcontains conserved and variable epitopes recognised by inhibitory monoclonal antibodies. Mol Biochem Parasitol. 1986;18:183–195. doi: 10.1016/0166-6851(86)90037-x. [DOI] [PubMed] [Google Scholar]
- 43.Smythe J A, Peterson M G, Coppel R L, Saul A J, Kemp D J, Anders R F. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990;39:227–234. doi: 10.1016/0166-6851(90)90061-p. [DOI] [PubMed] [Google Scholar]
- 44.Stephens H A F, Brown A E, Chandanayingyong D, Webster H K, Sirikong M, Longta P, Vangseratthana R, Gordon D M, Lekmak S, Rungruang E. The presence of the HLA class II allele DPB1*0501 in ethnic Thais correlates with an enhanced vaccine-induced antibody response to a malaria sporozoite antigen. Eur J Immunol. 1995;25:3142–3147. doi: 10.1002/eji.1830251123. [DOI] [PubMed] [Google Scholar]
- 45.Stowers A, Prescott N, Cooper J, Takacs B, Stüber D, Kennedy P, Saul A. Immunogenicity of recombinant Plasmodium falciparumrhoptry associated proteins 1 and 2. Parasite Immunol. 1995;17:631–642. doi: 10.1111/j.1365-3024.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 46.Stowers A, Taylor D, Prescott N, Cheng Q, Cooper J, Saul A. Assessment of the humoral immune response against Plasmodium falciparumrhoptry associated proteins 1 and 2. Infect Immun. 1997;65:2329–2338. doi: 10.1128/iai.65.6.2329-2338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanabe K, Mackay M, Goman M, Scaife J G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 48.Tapchaisri P, Chomcharn W, Poonthong C, Asavanich A, Limsuwan S, Maleevan O, Tharavanij S, Harinasuta T. Anti-sporozoite antibodies induced by natural infection. Am J Trop Med Hyg. 1983;32:1203–1208. doi: 10.4269/ajtmh.1983.32.1203. [DOI] [PubMed] [Google Scholar]
- 49.Taylor R R, Allen S J, Greenwood B M, Riley E M. IgG3 antibodies to Plasmodium falciparummerozoite surface antigen 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;53:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 49a.Theander, T. G. Unpublished data.
- 50.Theander T G, Hviid L, Abu-Zeid Y A, Abdulhadi N H, Saeed B O, Jakobsen P H, Reimert C M, Jepsen S, Bayoumi R A L, Jensen J B. Reduced cellular immune reactivity in health individuals during the malaria transmission season. Immunol Lett. 1990;25:237–242. doi: 10.1016/0165-2478(90)90121-6. [DOI] [PubMed] [Google Scholar]
- 51.Tolle R, Früh K, Doumbo O, Koita O, N’diaye M, Fischer A, Dietz K, Bujard H. A prospective study of the association between the human humoral immune response to Plasmodium falciparumblood stage antigen gp190 and control of malaria infection. Infect Immun. 1993;61:40–47. doi: 10.1128/iai.61.1.40-47.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO Study Group. 1995. WHO vector control for malaria and other mosquito borne diseases. WHO Tech. Rep. Ser. 857. [PubMed]