Abstract
Transfer RNA-derived fragments (tRFs) are a novel class of non-coding RNA transcripts and play important roles in several physiological/pathological processes. However, the role of tRFs in ocular angiogenesis remains elusive. Herein, we investigate whether the intervention of tRF-1001 expression could suppress pathological ocular angiogenesis. The results show that the levels of tRF-1001 expression were reduced in the retinas of an oxygen-induced retinopathy (OIR) model, choroidal neovascularization model, and endothelial sprouting model in vitro. Increased tRF-1001 expression could suppress ocular angiogenesis and endothelial sprouting in vivo and reduce endothelial migration, specification, and sprouting in vitro. Mechanistically, tRF-1001 regulated endothelial angiogenic effects via tRF-1001/METTL3/RBPJ-MAML1 signaling. The levels of tRF-1001 expression were downregulated in the aqueous humor of age-related macular degeneration (AMD) patients. tRF-1001 upregulation could suppress AMD aqueous humor-induced endothelial sprouting and pathological angiogenesis. Collectively, tRF-1001 acts as an anti-angiogenic factor during ocular angiogenesis. Targeting tRF-1001-mediated signaling is a therapeutic option for ocular neovascular diseases.
Keywords: MT: Non-coding RNAs, transfer RNA-derived fragment, METTL3, oxygen-induced retinopathy, choroidal neovascularization, ocular angiogenesis
Graphical abstract

Targeting endothelial angiogenic biology is a method for anti-angiogenic treatment. This study reveals that tRF-1001 is downregulated in the retinas of oxygen-induced retinopathy (OIR), choroidal neovascularization, and endothelial sprouting model. tRF-1001 acts as an anti-angiogenic factor during ocular angiogenesis. Targeting tRF-1001-mediated signaling is a therapeutic option for ocular neovascular diseases.
Introduction
Angiogenesis is an important process involved in the growth of new capillaries from the pre-existing ones.1 Physiological angiogenesis is required for embryonic development and vascular homeostasis in adulthood. Pathological angiogenesis is also involved in the pathogenesis of several human diseases, such as cancers, metabolic diseases, and immune diseases.2,3 The eye provides a unique window for the non-invasive visualization of retinal vasculature. Ocular angiogenesis is known as the pathological basis of the sight-threatening retinopathies, such as retinopathy of prematurity, diabetic retinopathy, and age-related macular degeneration (AMD).4 Neovascular tissues are often characterized by incompetent and leaky blood vessels, which can cause hemorrhage and retinal detachment. Notably, increased endothelial sprouting is identified as an inducing factor of ocular angiogenesis.5,6
During vascular endothelial sprouting, a key step is the differentiation of endothelial cells into diverse phenotypic states, including tip cells and stalk cells. Tip cells can extend their filopodia and guide the navigation of retinal vessels, whereas stalk cells are mainly involved in vessel elongation. Tip cells and stalk cells have different gene expression profiles, and have morphologic and functional differences.7 Tip-stalk cell specification is the building block of sprouting angiogenesis. Thus, deciphering the mechanism underlying tip-stalk cell specialization contributes to developing novel methods for the prevention and treatment of ocular angiogenesis.
Tip-stalk cell specialization is a complicated processes and is regulated by several signaling pathways, such as Notch-vascular endothelial growth factor receptor (VEGFR) signaling, Slit-Robo signaling, and extracellular matrix (ECM)-binding integrin signaling.8 For example, vascular endothelial growth factor (VEGF) and Notch signaling pathway can regulate angiogenesis by controlling endothelial tip cell and stalk cell specification.9 VEGF treatment leads to the formation of endothelial tip cells. Tip cells expressing Delta-like 4 (Dll4) leads to the activation of Notch signaling and reduces the levels of VEGFR2 and VEGFR3 in the neighboring stalk cells, preventing them from becoming new tip cells.8,10 In addition, cyclic AMP (cAMP)-dependent protein kinase A (PKA) can affect tip cell specialization in a Notch-independent manner.11 However, current understanding of the signaling pathways controlling tip-stalk cell specialization is far from complete.
Recently, epigenetic therapy has opened a new avenue for the treatment of human diseases. The role of non-coding RNAs in human diseases has gradually been recognized, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs).12,13,14 However, few scholars have paid attention to the role of tRNA-derived small RNA fragments (tRFs) in human diseases.15 tRFs are known as the abundant and short non-coding RNAs, which are ubiquitous in all domains of life. They are generated through endonucleolytic cleavage of pre-tRNAs and mature tRNAs, which can regulate gene expression via affecting ribosome biogenesis, translational inhibition, and mRNA stability.16 tRFs play important roles in many biological processes, such as stress tolerance, gene expression, gene silencing, and protein biosynthesis.17 Dysregulation of tRFs have been implicated in diet-induced metabolic disorders and cancer progression.9,18 Despite the emerging interests, the role of tRFs in ocular angiogenesis remains largely unknown.
In this study, we determined the role of a tRNA-derived small RNA fragment, tRF-1001, in pathological ocular angiogenesis. The result shows that tRF-1001 was significantly reduced in pathological angiogenesis. tRF-1001 acted as an anti-angiogenic factor during retinal and choroidal angiogenesis by inhibiting endothelial spouting. Mechanistically, tRF-1001 regulated pathological angiogenesis via tRF-1001/METTL3/RBPJ-MAML1 signaling. Clinically, tRF-1001 expression was downregulated in the clinical samples of AMD patients. tRF-1001 upregulation could ameliorate AMD aqueous humor-induced endothelial sprouting and pathological angiogenesis. This study highlights the potential of tRF-1001 as a therapeutic target for ocular neovascular diseases.
Results
tRF-1001 expression is reduced during ocular angiogenesis
To determine the relationship between tRF-1001 expression and ocular angiogenesis, we examined the expression pattern of tRF-1001 in the retinas of a murine oxygen-induced retinopathy (OIR) model. qRT-PCR assays revealed that, compared with the room air (RA) controls, tRF-1001 expression was significantly reduced in the OIR retinas at the postnatal day (P)17 (Figure 1A). We next examined the expression change of tRF-1001 in the OIR retinas from P7 to P12 and from P12 to P17. The expression of tRF-1001 did not alter from P7 to P12, but the expression of tRF-1001 steadily decreased from P12 to P17 (Figures 1B and 1C). The Bruch’s membrane of C57BL/6 mice were destroyed by laser photocoagulation to induce choroidal neovascularization (CNV). qPCR assays showed that tRF-1001 expression was significantly reduced in the retinal/choroidal complex at 2 weeks after laser photocoagulation (Figure 1D). In addition, human retinal vascular endothelial cells (HRVECs) were stimulated with VEGF to induce endothelial sprouting in vitro. qPCR assays showed that tRF-1001 expression was significantly reduced in HRVECs after VEGF treatment (Figure 1E).
Figure 1.
tRF-1001 expression is reduced during ocular angiogenesis
(A) The neonatal mice with their nursing mothers were maintained in 75% O2 from P7 to P12, and in room air (RA) from P12 to P17. qPCR assays were conducted to detect tRF-1001 expression at P17 (n = 5 mice per group; Mann-Whitney U test; ∗p < 0.05 versus RA-P17). (B and C) The retinas were collected from the OIR model. qPCR assays were conducted to detect tRF-1001 expression at the indicated time points (n = 5 mice per group; Kruskal-Wallis test followed by Bonferroni post hoc test; ∗p < 0.05 versus P7 or P12). (D) qRT-PCRs were used to detect tRF-1001 expression in the retina-choroid complex of C57BL/6J mice at 2 weeks after laser injury (n = 5 mice per group; Mann-Whitney U test; ∗p < 0.05 versus Ctrl). (E) HRVECs were starved for 24 h and then treated with VEGF (25 ng/mL) for 24 h, 48 h, or left untreated (Ctrl). qRT-PCRs were used to detect tRF-1001 expression (n = 4; one-way ANOVA followed by Bonferroni post hoc test; ∗p < 0.05 versus Ctrl).
tRF-1001 regulates ocular angiogenesis and vascular sprouting in vivo
Due to altered tRF-1001 expression in pathological angiogenesis, we speculated that tRF-1001 was a potential regulator of ocular angiogenesis. An OIR model was selected to investigate the role of tRF-1001 in ocular angiogenesis. The results showed that intravitreous injection of tRF-1001 agomir suppressed pathological angiogenesis, reduced the avascular areas, and decreased the numbers of vascular tufts in the OIR retinas at P17 (Figures 2A–2D). Moreover, intravitreous injection of tRF-1001 agomir decreased the number of tip cells and filopodia in the angiogenic areas (Figures 2E–2G). By contrast, intravitreous injection of tRF-1001 antagomir accelerated the angiogenesis process in the OIR model and contributed to vascular sprouting as shown by increased number of vascular tip cells and filopodia (Figures 2A–2G). A CNV model was also selected to examine the role of tRF-1001 in ocular angiogenesis on day 14 after laser injury. Quantification of CNV area revealed that injection of tRF-1001 agomir led to reduced CNV area by >50% compared with the controls (Figure 2H). The CNV lesions were stained with IB-4 and VEGFR2 to label CNV lesions and endothelial tip cells. The result showed that injection of tRF-1001 agomir not only reduced the size of CNV lesions but also decreased the number of tip cells (Figure 2I). By contrast, injection of tRF-1001 antagomir contributed to the development of CNV as shown by aggravated CNV lesions and increased number of tip cells (Figures 2H and 2I).
Figure 2.
tRF-1001 regulates ocular angiogenesis and vascular sprouting in vivo
(A–C) The neonatal mice received an injection of negative control (NC) antagomir, tRF-1001 antagomir, NC agomir, or tRF-1001 agomir, or were left untreated (Ctrl). On P7, they were subjected to hyperoxia (75% O2) for 5 days and then exposed in RA. The retinas were collected on P17 and stained with Isolectin B4 (IB4) to label retinal vessels. Yellow staining indicated the neovascular area; white area indicated the avascular area; low panels show the high magnification of neovascular tufts. Scale bars: upper panel, 1 mm; lower panel, 100 μm (n = 6 mice per group). (E–G) The quantification results of filopodia (yellow dots) and tip cells are shown. Scale bars: upper panel, 100 μm; lower panel, 25 μm (n = 6 mice per group). (H and I) Retina/choroid complexes were collected, flat mounted on day 14, and stained with IB-4 to label retinal/choroidal vessels. The representative images are shown. Green staining indicates CNV lesions; dashed line indicates CNV areas. Scale bar, 200 μm (H; n = 6 mice per group). On day 14 after laser injury, CNV lesions were stained with IB-4 and VEGFR2 to label ocular angiogenesis and tip cells. Scale bar, 100 μm (I; n = 6 mice per group). The significant difference was detected by Kruskal-Wallis test followed by Bonferroni post hoc test; ∗p < 0.05 versus Ctrl; #p < 0.05 between the marked groups.
tRF-1001 regulates vascular endothelial sprouting in vitro and ex vivo
To determine the role of tRF-1001 in endothelial sprouting in vitro, we detected the expression change of the marker genes of tip cells and stalk cells after the intervention of tRF-1001 expression. In HRVECs, transfection of tRF-1001 mimic led to increased levels of tRF-1001 expression. By contrast, transfection of tRF-1001 inhibitor led to decreased levels of tRF-1001 expression (Figure S1). Transfection of tRF-1001 mimic significantly decreased the expression of the marker genes of tip cells (CXCR4, CD34, and VEGFA) and increased the expression of the marker genes of stalk cells (HEY1, HEY2, and DLL4) (Figure 3A). We further conducted spheroid sprouting assays to examine the ability of endothelial sprouting. The results showed that, compared with the control group, transfection of tRF-1001 mimic reduced the sprouting ability of endothelial cells as shown by reduced endothelial sprouting length (Figure 3B). Transwell assays showed that, compared with the control group, transfection of tRF-1001 mimic decreased the number of migrated endothelial cells (Figures 3C and 3D). We further used a choroidal sprouting model to examine the role of tRF-1001 in endothelial sprouting ex vivo. The choroidal tissues were incubated onto the Matrigel to induce endothelial sprouting. tRF-1001 mimic reduced the sprouting areas and decreased the number of vascular sprouting after 7-day culture (Figures 3E and 3F). By contrast, tRF-1001 inhibitor increased the expression of the marker genes of tip cells (CXCR4, CD34, and VEGFA), decreased the expression of the marker genes of stalk cells (HEY1, HEY2, and DLL4), and enhanced the sprouting ability and migration ability of endothelial cells (Figures 3A–3F).
Figure 3.
tRF-1001 regulates vascular endothelial sprouting in vitro and ex vivo
(A) HRVECs were transfected with NC inhibitor, tRF-1001 inhibitor, NC mimic, or tRF-1001 mimic, or were left untreated (Ctrl) for 24 h. qPCR assays were used to detect gene expression change. n = 4; one-way ANOVA followed by the Bonferroni post hoc test; ∗p < 0.05 versus Ctrl group. (B) HRVECs were treated as shown for 24 h. Spheroid sprouting assays were conducted to examine the role of tRF-1001 in endothelial sprouting. Scale bar, 100 μm; n = 4; one-way ANOVA followed by the Bonferroni post hoc test; ∗p < 0.05 versus Ctrl group. (C and D) Transwell assays were used to examine the role of tRF-1001 in endothelial migration ability. Scale bar, 20 μm; n = 4; one-way ANOVA followed by the Bonferroni post hoc test; ∗p < 0.05 versus Ctrl group. (E and F) The mice received an injection of NC antagomir, tRF-1001 antagomir, NC agomir, or tRF-1001 agomir, or were left untreated (Ctrl). On day 14, RPE/choroid complexes were cut into 1 × 1-mm pieces and seeded. The sprouting ability of choroidal explants were observed on day 7 after seeding (n = 6). The representative images and quantification results are shown. Scale bar, 500 μm; ∗p < 0.05 versus Ctrl group.
tRF-1001 regulates endothelial cell function by targeting METTL3
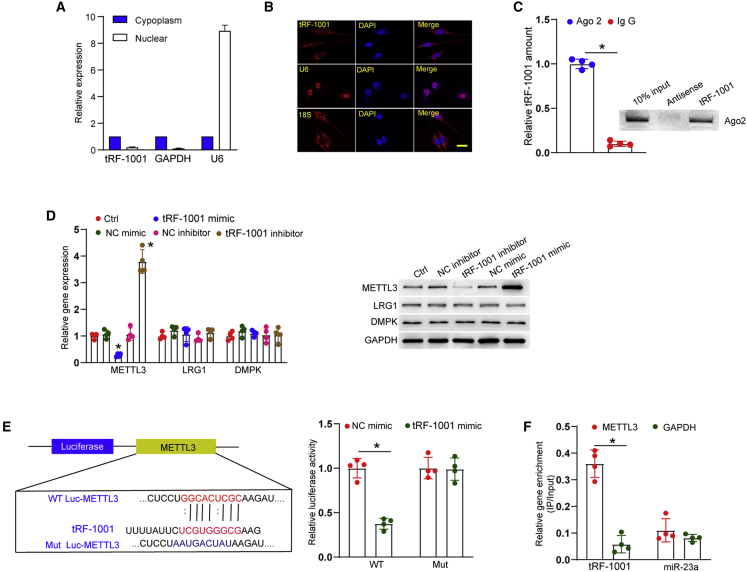
We next explored the molecular mechanism of tRF-1001 in endothelial cells. qRT-PCR analysis of nucleus and cytoplasmic RNAs showed that tRF-1001 was mainly expressed in the cytoplasm of HRVECs (Figure 4A). Fluorescence in situ hybridization (FISH) assays also revealed that tRF-1001 was mainly expressed in the cytoplasm of HRVECs (Figure 4B). Ago2 protein can bind miRNAs to execute miRNA-directed silencing of the target mRNAs.19 RNA immunoprecipitation (RIP) and western blots revealed that tRF-1001 could be immunoprecipitated by anti-Ago2 but not anti-immunoglobulin (Ig) G antibody (Figure 4C).
Figure 4.
tRF-1001 regulates endothelial cell function by targeting METTL3
(A) The amount of nucleus control transcript (U6), cytoplasm control transcript (GAPDH), and tRF-1001 was examined by qRT-PCR assays in the nucleus fractions and cytoplasm fractions of HRVECs (n = 4). (B) FISH assays were conducted to detect the intracellular distribution of tRF-1001. 18S rRNA and U6 were detected as the cytoplasm control and nucleus control. Scale bar, 10 μm. (C) The cellular fractions were isolated from HRVECs and immunoprecipitated using Ago2 or IgG antibody. The amounts of tRF-1001 in the immunoprecipitates were determined by qRT-PCR (n = 4, ∗p < 0.05). Meanwhile, the biotinylated tRF-1001 or its anti-sense RNA was incubated with the total extracts of HRVECs, targeted with streptavidin beads, and washed. The associated proteins were resolved by SDS-PAGE, and western blots were conducted to detect the specific interaction between Ago2 and tRF-1001 (n = 4). (D) The expression of METTL3, LRG1, and DMPK was determined by qRT-PCR assays and western blots in HRVECs after the transfection of tRF-1001 mimic, NC mimic, tRF-1001 inhibitor, or NC inhibitor, or being left untreated (Ctrl) (n = 4, ∗p < 0.05 versus Ctrl group). (E) The luciferase activity of WT-Luc-METTL3 or mutant Luc-METTL3 was determined after the transfection of tRF-1001 mimic or NC mimic in HRVECs (n = 4, ∗p < 0.05 versus tRF-1001 mimics group). (F) The 3ʹ-end biotinylated tRF-1001 or biotinylated miR-23a was transfected into HRVECs. After streptavidin capture, the levels of METTL3 and GAPDH in the input and bound fractions were detected by qRT-PCR assays (n = 4, ∗p < 0.05 versus METTL3 group). All significant differences were detected by Student’s t test or one-way ANOVA followed by the Bonferroni post hoc test.
We speculated that tRF-1001 might play its regulatory role at the post-transcriptional level via an RNAi-like mechanism. The potential target genes of tRF-1001 were predicted by miRDB database (http://www.microrna.org) and Diana Tools (http://diana.imis.athena-innovation.gr/DianaTools/index.php) to obtain the overlapped results to reduce the prediction scope. RNAhybrid tool was used to predict the free binding energy of the interaction between tRF-1001 in METTL3 (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid). METTL3, LRG1, and DMPK were predicted as the potential targets of tRF-1001. qRT-PCR assays and western blots showed that the levels of METTL3 expression were reduced after the transfection of tRF-1001 mimic. By contrast, transfection of tRF-1001 inhibitor led to increased levels of METTL3 expression (Figure 4D). However, the transfection of tRF-1001 inhibitor or mimic did not alter the levels of LRG1 and DMPK expression (Figure 4D). We further used the luciferase assays to verify the direct binding between tRF-1001 and METTL3. Transfection of tRF-1001 mimic reduced the luciferase activity of wild-type Luc-METTL3. However, the luciferase activity of mutant Luc-METTL3 was not altered after the transfection of tRF-1001 mimic (Figure 4E). RNA pull-down experiments were also used to detect the interaction between tRF-1001 and METTL3. Due to the low sequence similarity between miR-23a and tRF-1001, we used biotinylated miR-23a as the negative control in RNA pull-down experiments. The 3ʹ-end biotinylated tRF-1001 or biotinylated miR-23a were transfected into HRVECs. MEETL3 was found to be greatly enriched in the tRF-1001-captured fraction compared with the miR-23a-captured fraction (Figure 4F).
METTL3 usually regulates mRNA activity via N6-methyladenosine (m6A)-mediated modification.20 Since tRF-1001 regulated METTL3 expression, we speculated that tRF-1001 also regulated the process of m6A modification. HRVECs were stimulated with 25 ng/mL of VEGF to induce endothelial sprouting. Colorimetric assays and dot blots revealed that the number of m6A RNA modifications were markedly increased in HRVECs after VEGF treatment (Figures S2A and S2B). Moreover, the amount of m6A RNA modifications were markedly increased in retinal/choroidal tissues at 2 weeks after laser injury (Figures S2C and S2D). Considering that m6A RNA modification is regulated by a series of m6A methyltransferases, we then examined the expression pattern of these methyltransferases in HRVECs. METTL3 expression was markedly increased in HRVECs after VEGF treatment both at mRNA and protein levels, while the expression of METTL14, VIRMA, WTAP, RBM15, and ZC3H13 was not altered after VEGF treatment (Figures S2E and S2F).
We further determined the role of METTL3 in endothelial sprouting, and the expression levels of METTL3 were changed by the transfection of METTL3 small interfering RNA (siRNA) or the gain of function of METTL3. qRT-PCR assays revealed that METTL3 siRNA led to decreased expression of METTL3, while METTL3 overexpression led to increased expression of METTL3 (Figure S2G). METTL3 siRNA could decrease the expression of the marker genes of tip cells (CXCR4, CD34, and VEGFA) and increased the marker genes of stalk cells (HEY1, HEY2, and DLL4). By contrast, METTL3 overexpression had an opposite effect on expression of tip or stalk signature genes (Figure S2H). Spheroid sprouting assays revealed that, compared with the control group, METTL3 silencing suppressed the sprouting ability of endothelial spheroids, whereas METTL3 overexpression enhanced the sprouting ability of endothelial spheroids (Figure S2I).
High-throughput m6A sequencing reveals that RBPJ and MAML1 are the potential targets of METTL3
To search for the potential targets of METTL3 in endothelial sprouting, total RNAs were isolated from METTL3-overexpressed HRVECs and normal HRVECs following VEGF treatment. The above-mentioned RNAs were subjected to m6A-modifed RNA (MeRIP) followed by sequencing. The globe hypermethylation levels in the METTL3-overexpression group were markedly increased compared with the control (Figure 5A). m6A peaks were greatly enriched in the coding sequences (CDSs) (Figure 5B), and 1,392 mRNAs were differentially m6A methylated between the METTL3-overexpresssed group and control group, including 706 m6A hypermethylated mRNAs and 686 m6A hypomethylated mRNAs (Figure 5C; Table S1). Due to the critical role of METTL3 in m6A methylation writing, these m6A peaks with the enhanced abundance were taken as the real m6A peaks.
Figure 5.
High-throughput m6A sequencing reveals that RBPJ and MAML1 are the targets of METTL3
(A) Metagene plots show the abundance of m6A peak and normalized transcript composed of three rescaled non-overlapping segments: 5′ UTR, CDS, and 3′ UTR in control group and METTL3-overexpression group. (B) Pie charts show the distribution of m6A peaks throughout the differentially m6A methylated mRNAs. (C) Volcano plots show the hypermethylated mRNAs and hypomethylated mRNAs between METTL3-overexpression group and control group. (D) KEGG pathway analysis was used to predict the pathways associated with METTL3 overexpression. (E) IGV plots show the MeRIP reads of RBPJ and MAML1. (F) Reduced m6A modifications in RBPJ and MAML1 transcripts after METTL3 silencing as shown by gene-specific m6A-qPCR assays in HRVECs (n = 4, Student’s t test). (G) qRT-PCRs were used to examine the expression of RBPJ and MAML1 mRNAs in HRVECs after METTL3 intervention (n = 4). (H) HRVECs were transfected with pcDNA3.1-YTHDF2 (YTHDF2), pcDNA3.1 vector (Vector), or were left untreated (Ctrl) for 24 h. Western blots were used to examine RBPJ and MAML1 expression (n = 4, ∗p < 0.05 versus Ctrl group, one-way ANOVA, Bonferroni test). (I) RIP assays were conducted to detect the association between YTHDF2 and RBPJ/MAML1. The levels of RBPJ and MAML1 were detected by qRT-PCR assays (n = 4, ∗p < 0.05 versus IgG group, Student’s t test). Meanwhile, western blots were conducted to detect the amount of YTHDF2 in the immunoprecipitates following RBPJ or MAML1-mediated pull-down experiments (n = 4). (J) HRVECs were treated as shown for 24 h. Western blots were conducted to detect the expression of RBPJ and MAML1 (n = 4, ∗p < 0.05 versus Ctrl group, #p < 0.05 between the marked group, one-way ANOVA, Bonferroni test).
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was employed to predict the relevant pathways of m6A hypermethylated mRNAs. Six signaling pathways were predicted to be associated with these mRNAs. Notably, Notch signaling was ranked as the top 1 signaling regulated by METTL3 (Figure 5D). MeRIP sequencing maps showed the hypermethylated sites of RBPJ and MAML1 in the METTL3 overexpression group (Figure 5E). In particular, the m6A abundances of RBPJ and MAML1 were markedly reduced upon METTL3 knockdown, as shown by gene-specific m6A-qPCRs (Figure 5F). We also examined whether METTL3 regulated the expression of RBPJ and MAML1 in HRVECs. METTL3 knockdown led to increased expression of RBPJ and MAML1. Conversely, METTL3 overexpression led to reduced expression of RBPJ and MAML1 in HRVECs (Figure 5G).
We further determined whether the expression of RBPJ and MAML1 was regulated by m6A modification in HRVECs. m6A is usually recognized by m6A-binding proteins to regulate the activity of m6A methylated mRNAs. Overexpression of YTHDF2 but not YTHDC1-3, YTHDF1, YTHDF3, or IGF2 led to reduced expression of RBPJ and MAML1 in HRVECs (Figures 5H and S3). RIP-qPCR assays demonstrated that RBPJ and MAML1 were the potential targets of YTHDF2 (Figure 5I). Overexpression of YTHDF2 inhibited METTL3 silencing-induced upregulation of RBPJ and MAML1 in HRVECs (Figure 5J). Collectively, these results indicate that METTL3 regulates the translation of RBPJ and MAML1 in a YTHDF2-dependent manner.
tRF-1001/METTL3/RBPJ-MAML1 axis regulates endothelial sprouting and ocular angiogenesis
We subsequently investigated the role of the tRF-1001/METTL3/RBPJ-MAML1 axis in endothelial sprouting. In vitro spheroid sprouting assays showed that METTL3 silencing could mimic the effects of tRF-1001 mimic and lead to decreased endothelial spouting. However, the inhibition of RBPJ or MAML1 could partially reverse the effects of METTL3 silencing on endothelial spouting ability (Figures 6A and 6B). METTL3 silencing reduced the expression of marker genes of tip cells (CXCR4, CD34, and VEGFA) and increased the expression of marker genes of stalk cells (HEY1, HEY2, and DLL4), showing similar effects to tRF-1001 mimic. By contrast, the inhibition of RBPJ or MAML1 could partially reverse the effects of METTL3 silencing on the expression of tip/stalk signature genes (Figure 6C).
Figure 6.
tRF-1001/METTL3/RBPJ-MAML1 axis regulates endothelial sprouting and ocular angiogenesis
(A–C) HRVECs were treated as shown for 24 h. Spheroid sprouting assays were used to detect endothelial sprouting ability (A and B). qPCR assays were used to examine the expression of VEGFA, HEY1, HEY2, CXCR4, CD34, and DLL4 (C). For (A)-(C): n = 4; one-way ANOVA followed by the Bonferroni post hoc test. Scale bar, 100 μm. (D–G) The neonatal mice received the injections of the indicated agomir or antagomir, or were left untreated (Ctrl). On P7, the pups with their mothers were exposed to 75% O2 for 5 days and then exposed to room air on P12. The retinas were collected on P17 and stained with IB4 to label retinal vessels. Yellow staining indicates the neovascular areas; white area indicates the avascular areas; low panels indicate the high magnification of neovascular tufts. Scale bars: upper panel, 1 mm; lower panels, 100 μm (n = 6). (H and I) The mice received an injection of the indicated agomir or antagomir. On day 14, RPE/choroid complexes were cut into 1 × 1-mm pieces and seeded. The sprouting ability of choroidal explants was observed on day 7 (H, n = 6). Scale bar, 500 μm. In addition, retinal/choroidal complexes were flat mounted on day 14 after laser injury and stained with IB-4 to label retinal/choroidal vessels (I, n = 6). Green staining indicates the CNV lesions. Scale bar, 100 μm. (D)–(I) Kruskal-Wallis test followed by Bonferroni post hoc test. ∗p < 0.05 versus Ctrl; #p < 0.05 between the marked groups.
We then investigated the role of the tRF-1001/METTL3/RBPJ-MAML1 axis in pathological angiogenesis. As shown in the OIR model, METTL3 silencing led to decreased pathological angiogenesis, reduced avascular area, and reduced number of vascular tufts and tip cells, which could be partially reversed by the inhibition of RBPJ or MAML1 (Figures 6D–6G). The choroidal tissues were incubated onto Matrigel to induce choroidal vascular sprouting. METTL3 silencing could mimic the effects of tRF-1001 mimic and led to reduced area of choroidal sprouting. By contrast, the inhibition of RBPJ or MAML1 could partially reverse the effects of METTL3 silencing on choroidal sprouting ex vivo (Figure 6H). In a CNV model, the quantification of CNV area revealed that METTL3 silencing led to reduced CNV area. Inhibition of RBPJ or MAML1 could partially reverse the inhibitory effects of METTL3 silencing on CNV formation (Figure 6I).
Clinical relevance of tRF-1001-mediated signaling in neovascular ocular diseases
To reveal the clinical relevance of tRF-1001-mediated signaling, we examined the expression levels of tRF-1001 in the patients with exudative AMD and the patients with cataract. qRT-PCRs showed that the levels of tRF-1001 were markedly reduced in the aqueous humor of the patients with exudative AMD (Figure 7A). The levels of METTL3 expression were negatively correlated with the levels of tRF-1001 expression (Figure 7B), whereas the levels of RBPJ and MAML1 expression were positively associated with the levels of tRF-1001 (Figures 7C and 7D). Another cohort of patients, the anti-VEGF treatment cohort, were selected to investigate the effects of anti-VEGF treatment on tRF-1001 expression. Anti-VEGF treatment led to increased tRF-1001 expression in the aqueous humor compared with AMD patients without anti-VEGF treatment (Figure 7E).
Figure 7.
Clinical relevance of tRF-1001-mediated signaling in neovascular ocular diseases
(A) Aqueous humor (AH) was obtained from the patients with exudative AMD (n = 20 eyes) and the patients with cataract (Ctrl, n = 20 eyes). qRT-PCR assays were conducted to detect the expression of tRF-1001. ∗p < 0.05 versus Ctrl group, Mann-Whitney U test. (B–D) AH was obtained from the patients with exudative AMD (n = 10 eyes) and cataract (n = 10 eyes). qRT-PCRs and ELISA assays were used to determine the expression relevance between tRF-1001 and METTL3, RBPJ, or MAML1. (E) AH was obtained from the patients with exudative AMD before anti-VEGF treatment (Ctrl group) and after anti-VEGF treatment. qRT-PCRs were conducted to detect tRF-1001 expression. n = 10 eyes; ∗p < 0.05 versus Ctrl group; Mann-Whitney U test. (F) HRVEC spheroids embedded in the collagen were stimulated with AH from CNV patients without or with NC mimic, tRF-1001 mimic, RBPJ overexpression, or MAML1 overexpression for 24 h. The sprout length was calculated on at least 10 spheroids per experimental group. n = 4; ∗p < 0.05 versus Ctrl group, #p < 0.05 versus AH group. One-way ANOVA followed by Bonferroni post hoc test. (G) Retinal vascular leakage was detected in retinal extracts from the mice injected with PBS (Ctrl), AH from CNV patients, or AH with NC agomir, tRF-1001 agomir, or anti-VEGF after 4-week treatment (G, n = 5 retinas per group, ∗p < 0.05 versus Ctrl group, #p < 0.05 versus AH group, Kruskal-Wallis test followed by Bonferroni post hoc test). Quantifications of retinal neovascular tufts from the mice injected with AH from CNV patients or AH with NC agomir, tRF-1001 agomir, or anti-VEGF were performed after 4-week treatment (H, n = 5 retinas per group, ∗p < 0.05 versus AH group, Kruskal-Wallis test followed by Bonferroni post hoc test).
HRVECs were cultured with the aqueous humor from CNV patients. Spheroid sprouting assay revealed that treatment with CNV aqueous humor led to increased sprouting ability of HRVECs. By contrast, tRF-1001 mimic significantly reduced the sprouting ability of HRVECs. Overexpression of RBPJ and MAML1 could achieve similar effects to tRF-1001 mimic on the sprouting ability of HRVECs (Figure 7F).
The mice received an intravitreal injection of PBS, or aqueous humor from CNV patients without or with tRF-1001 agomir to induce retinal vascular dysfunction. Injection of aqueous humor increased the retinal vasopermeability and enlarged the neovascular tuft area. By contrast, tRF-1001 agomir decreased CNV aqueous humor-induced vasopermeability and reduced the number of neovascular tufts. tRF-1001 agomir could play a similar role to anti-VEGF in the regulation of retinal vasopermeability and neovascular tufts (Figures 7G and 7H).
Discussion
Endothelial sprouting is a critical step during angiogenesis.9,21 In this study, we show that tRF-1001 acts as an anti-angiogenic factor in the progression of ocular angiogenesis. tRF-1001 expression is reduced in the endothelial sprouting model, the retinas of OIR model, and the choroidal neovascularization model. Mechanistically, inhibition of tRF-1001 leads to increased levels of METTL3, which could repress RBPJ and MAML1 expression through YTHDF2-dependent mRNA decay. Collectively, tRF-1001 is a key regulator of endothelial spouting and pathological angiogenesis. Targeting tRF-1001-mediated signaling is a promising method for the treatment of ocular neovascular diseases.
tRFs provide a novel regulatory layer of gene expression.22 Previous studies have shown that some tRFs play their roles via an RNAi-like mechanism or miRNA-mediated mechanism. For example, some tRFs can bind to the 3′ UTR of target mRNAs and repress the repression of target mRNAs.23,24 Some tRFs can affect the translation process by altering the conformation of mRNAs, while some tRFs can affect the stability of the transcripts via displacing the 3′ UTR.25 In this study, RNA pull-down assays, luciferase activity assays, and qRT-PCR assays indicate that tRF-1001 plays its role via an RNAi-like mechanism. The binding between tRF-1001 and METTL3 can lead to reduced expression of METTL3. METTL3 is an RNA methyltransferase that can regulate mRNA biology via N6-methyladenosine (m6A) modification. METTL3 has also be reported to be involved in various biological processes.20,26 This study reveals a novel role of METTL3-mediated m6A modification in endothelial sprouting, which could expand our knowledge about the functions of m6A modification.
We further identify the potential targets of METTL3 during endothelial sprouting. MeRIP followed by sequencing revealed that two members of Notch signaling, RBPJ and MAML1, are the potential targets of METTL3. RBP-J can bind to Notch proteins and act as an activator in Notch signaling pathway.27,28 MAML1 is required for the transcription of mammalian Notch target genes.29 RBPJ and MAML1 are two key mediators of Notch signaling, which is a highly conserved signaling that controls cell-fate determination.30 Previous studies have revealed that Notch signaling is involved in tip-stalk specification and mural cell recruitment.10,31 During endothelial sprouting, reduced tRF-1001 may lead to increased expression of METTL3. Increased METTL3 level could suppress the expression of RBPJ and MAML1, causing the inactivation of Notch signaling. Due to the critical role of Notch in endothelial cell specification during angiogenesis, we can conclude that the tRF-1001/METTL3/RBPJ and MAML signaling axis is critical for endothelial cell specification during angiogenesis.
METLL3-mediated m6A modification plays critical roles in gene expression, which is achieved by m6A reader proteins. A series of YTH domain-containing proteins have been identified as the m6A reader proteins.32,33 Silencing of METTL3 leads to increased levels of RBPJ and MAML1. This process is mainly mediated by YTHDF2. YTHDF2 can bind to the m6A-modified site of RBPJ and MAML1 mRNAs and cause the degradation of RBPJ and MAML1 mRNAs. Increased expression of RBPJ and MAML1 could lead to the activation of Notch signaling and suppress the sprouting of endothelial cells, which in turn causes the inhibition of pathological angiogenesis. Collectively, tRF-1001-mediated METTL3 signaling triggers m6A modification on RBPJ and MAML1 mRNAs and orchestrates YTHDF2 to regulate the expression of RBPJ and MAML1.
In conclusion, we have identified that a tRNA-derived fragment, tRF-1001, plays important roles in endothelial sprouting and pathological angiogenesis via tRF-1001/METTL3/RBPJ-MAML1 signaling. We also provide evidence that tRF-1001 acts as an anti-angiogenic factor and a promising biomarker for ocular neovascular diseases. Upregulation of tRF-1001 by synthetic tRF-1001 agomir is a promising method for the treatment of ocular neovascular diseases.
Materials and methods
Animals
C57BL/6J mice were obtained from Nanjing Qinglongshan Experimental Animal Center (Nanjing, China). All procedures were approved by the authors’ institute and conducted according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision. The animals were maintained in pathogen-free conditions, cultured at 23°C ± 2°C, 50% ± 10% humidity, and 12-h light/12-h dark. They were allowed free access to food and water.
OIR mouse model
Seven-day-old C57BL/6J mice with their mothers were exposed to 75% oxygen for 5 days. On P12, they were exposed to RA (21% oxygen) until P17. In phase I, the hyperoxia between P7 and P12 led to vaso-obliteration and an avascular areas in the retinas. In phase II, the relative hypoxia led to the formation of pathological neovascularization.34
Laser-induced CNV model
C57BL/6J mice (6–8 weeks old, male) were anesthetized by the mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Their pupils were dilated using 0.5% phenylephrine and 0.5% tropicamide eye drops (Alcon, USA). In each eye, laser photocoagulation was conducted at three, six, nine, and twelve o’clock positions surrounding the optic discs using the OcuLight GLx Laser System (Iridex, USA). The parameters were selected as shown below: wavelength, 532 nm; spot size, 50 μm; duration, 70-ms; power, 140 mW. The formation of white bubbles was regarded as the disruption of Bruch’s membrane.35
Cell culture and transfection
HRVECs were cultured in endothelial cell growth medium-2 (cc-3202, Lonza, Switzerland) supplemented with 5% (v/v) fetal bovine serum (FBS; 10099141, Gibco, USA) and 1% (v/v) penicillin streptomycin (Pen Strep; 10378016, Gibco, USA) at 37°C under humidified conditions with 5% CO2. When cell confluence reached about 85%, they were transfected with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocols.
Subcellular fractionation assay
HRVECs were lysed and centrifuged for 5 min to collect the cytoplasmic fractions. The remaining fractions were re-suspended in the nucleus lysis buffer at 4°C and centrifuged for 10 min. RNAs in the cytoplasmic and nuclear fractions were isolated using Trizol reagent (15596018, Invitrogen, USA). The amounts of GAPDH, U6, and tRF-1001 were detected by qRT-PCR assays.
Immunostaining assay
The mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) and sacrificed by cervical dislocation. The ocular globe was fixed with 4% paraformaldehyde for 30 min at room temperature. After washing twice in 1× PBS, the retinas were cut into four quadrants under a light dissection microscope (Olympus, Japan). Then, the flat-mounts were permeabilized with PBS solution containing 0.1% Triton X-100 and 5% BSA (240GR100, Biofroxx, Germany) for 30 min. They were incubated with Isolectin GS-IB4 (1:50; L2895, Sigma-Aldrich, USA) at 4°C for 2 h. The flat-mounts were observed under a fluorescence microscope (Olympus, Japan).
Choroidal flat-mount staining
After laser injury, the eyes were enucleated and fixed in 4% paraformaldehyde for 1 h at 4°C. RPE/choroid tissue was flat mounted and permeabilized with 0.1% Triton X-100 at 37°C for 45 min. The flat-mount tissue was incubated with anti-VEGFR2 (1:200; sc-6251, Santa Cruz Biotechnology, USA) for 12 h at 4°C and then incubated with the Alexa Fluor 594 secondary antibody (1:200; A11005, Invitrogen, USA) for 3 h at room temperature. Finally, the flat-mount tissue was incubated with Isolectin GS-IB4 (1:50; L2895, Sigma-Aldrich, USA) at 4°C for 6 h to label choroidal vessels.
Transwell migration assay
Cell migration was determined using Transwell chambers (8.0-μm pore size; Corning, USA). Briefly, HRVECs were suspended in 200 μL of culture medium and seeded onto the upper chamber. The culture medium with 10% serum was added to the bottom chamber. After incubation, these non-migrated cells were removed with cotton swabs, and the remaining cells were fixed in 4% paraformaldehyde for 30 min and stained with 0.5% crystal violet solution for 15 min. Cells were counted under a microscope (Olympus, Japan).
Tube formation assay
About 50 μL of growth-factor-reduced Matrigel was added to an ice-cold 24-well plate per well (Corning, USA). The plate was shaken gently and placed at 37°C for solidification. HRVECs were seeded onto the Matrigel in a 24-well plate per well. After 8-h culture, the tube-like structure was observed under a bright-field microscope (Olympus, Japan). The quantification of capillary tube formation was conducted by comparing tube length using Image J software.
Luciferase reporter assay
The METTL3 encoding sequence was PCR amplified and inserted into the KpnI and HindIII sites of pGL3-control vector (Promega) to construct the Luc-METTL3 vector. HRVECs were seeded in 96-well plates at a density of 2 × 103 cells/well. After 24-h culture, HRVECs were transfected with Luc-METTL3 WT vector, Luc-METTL3 Mut vector, or empty vector, with negative control mimic or tRF-1001 mimic using Lipofectamine 2000 according to the manufacturer’s instructions. After incubation for 36 h in a cell incubator, the cells were lysed with passive lysis buffer and the luciferase activity of different groups was detected with a microplate reader (Molecular Devices, San Jose, CA, USA). The luciferase activity was measured by the Dual-Luciferase Reporter Assay kit (Promega Corporation).
RIP assay
RIP assays were performed using the EZ-Magna RIP kit (17–701, Millipore, USA). Briefly, HRVECs were lysed in the lysis buffer and incubated with the magnetic beads conjugated with anti-Ago2, anti-IgG antibody (AP-124, Millipore, USA), or anti-YTHDF2 for 6–8 h at 4°C. Finally, the magnetic beads were washed and treated with proteinase K to remove the binding proteins. The remaining RNAs were used for the detection of tRF-1001, RBPJ, and MAML1 expression.
Choroidal sprouting assay ex vivo
The eyes of C57BL/6J mice (4 weeks old, male) were collected and immersed in ice-cold culture medium. The RPE/choroid complex was cut into 1-mm2 pieces and seeded onto the Matrigel (354230, BD Biosciences, USA) in 24-well plates. About 500 μL of medium was added into each well and incubated at 37°C with 5% CO2. Choroid explants were observed under a microscope (Olympus, Japan).36
Aqueous humor collection
The patients who visited the affiliated eye hospital (Nanjing Medical University) from 2019 to 2021 were enrolled. The inclusion criteria were older than 60 years; first diagnosis with exudative AMD; no history of treatments including anti-VEGF treatment, photodynamic treatment, or photocoagulation. The exclusion criteria were other ocular diseases and ocular inflammation. Undiluted aqueous humor (100–200 μL) was collected from 20 exudative AMD patients via anterior chamber paracentesis and 20 control eyes before cataract surgery. Aqueous humor was stored at −80°C until analysis. A second cohort of patients was also included. Undiluted aqueous humor (100–200 μL) was collected from AMD patients via anterior chamber paracentesis before and after anti-VEGF treatment. Informed consent was obtained from all patients. This clinical study was approved by the Institutional Review Board of the authors’ institute and adhered to the tenets of the Declaration of Helsinki.
Statistics
Data analysis was performed using the GraphPad Prism software 8.0. Data normality was analyzed using the D’Agostino-Pearson omnibus normality test. For the normally distributed data, statistical analysis was performed using Student’s t test or one-way analysis of variance (ANOVA). For the non-normally distributed data, statistical analysis was performed by Mann-Whitney U test or Kruskal-Wallis test. p < 0.05 was taken as statistically significant.
Acknowledgments
This study was funded by the grants from the National Natural Science Foundation of China (nos. 82171074 and 81970809 to B.Y., and nos. 81570859 and 82070983 to Q.J.).
Author contributions
B.Y. and Q.J. designed this study. Y.Z., M.-D.Y, Y.M., and Q.-Y.Z. performed the experiments, figure preparation, and manuscript draft. M.-D.Y., Y.M., and Y.Z. developed the methods and data analysis. Y.Z., M.-D.Y., Y.M., and Y.Z. acquired and interpreted the data. M.-D.Y. and B.Y. wrote and revised the paper. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2022.10.016.
Supplemental information
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- 1.Chung A.S., Ferrara N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacca M., Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012;19:622–629. doi: 10.1038/gt.2012.17. [DOI] [PubMed] [Google Scholar]
- 4.Gariano R.F., Gardner T.W. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 5.Das A., McGuire P.G. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Stone J., Maslim J. Mechanisms of retinal angiogenesis. Prog. Retin. Eye Res. 1997;16:157–181. [Google Scholar]
- 7.Eilken H.M., Adams R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Chen W., Xia P., Wang H., Tu J., Liang X., Zhang X., Li L. The endothelial tip-stalk cell selection and shuffling during angiogenesis. J. Cell Commun. Signal. 2019;13:291–301. doi: 10.1007/s12079-019-00511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco R., Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofler N.M., Shawber C.J., Kangsamaksin T., Reed H.O., Galatioto J., Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes Cancer. 2011;2:1106–1116. doi: 10.1177/1947601911423030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nedvetsky P.I., Zhao X., Mathivet T., Aspalter I.M., Stanchi F., Metzger R.J., Mostov K.E., Gerhardt H. cAMP-dependent protein kinase A (PKA) regulates angiogenesis by modulating tip cell behavior in a Notch-independent manner. Development. 2016;143:3582–3590. doi: 10.1242/dev.134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 13.Moran V.A., Perera R.J., Khalil A.M. Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Res. 2012;40:6391–6400. doi: 10.1093/nar/gks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattick J.S. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2:986–991. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su Z., Wilson B., Kumar P., Dutta A. Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu. Rev. Genet. 2020;54:47–69. doi: 10.1146/annurev-genet-022620-101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y.S., Shibata Y., Malhotra A., Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magee R., Rigoutsos I. On the expanding roles of tRNA fragments in modulating cell behavior. Nucleic Acids Res. 2020;48:9433–9448. doi: 10.1093/nar/gkaa657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y., Yu X., Zhu L., Li T., Yan Z., Guo J. Transfer RNA-derived fragments and tRNA halves: biogenesis, biological functions and their roles in diseases. J. Mol. Med. 2018;96:1167–1176. doi: 10.1007/s00109-018-1693-y. [DOI] [PubMed] [Google Scholar]
- 19.Pratt A.J., MacRae I.J. The RNA-induced silencing complex: a versatile gene-silencing machine. J. Biol. Chem. 2009;284:17897–17901. doi: 10.1074/jbc.R900012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maity A., Das B. N6-methyladenosine modification in mRNA: machinery, function and implications for health and diseases. FEBS J. 2016;283:1607–1630. doi: 10.1111/febs.13614. [DOI] [PubMed] [Google Scholar]
- 21.Marcelo K.L., Goldie L.C., Hirschi K.K. Regulation of endothelial cell differentiation and specification. Circ. Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishna S., Raghavan S., DasGupta R., Palakodeti D. tRNA-derived fragments (tRFs): establishing their turf in post-transcriptional gene regulation. Cell. Mol. Life Sci. 2021;78:2607–2619. doi: 10.1007/s00018-020-03720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao E.W., Wang H.L., Cheng W.Y., Liu Q.Q., Chen Y.X., Gao Q.Y. A specific tRNA half, 5’tiRNA-His-GTG, responds to hypoxia via the HIF1α/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021;40:67. doi: 10.1186/s13046-021-01836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong L., Zhang W., Qu B., Zhang F., Wu Z., Shi J., Chen X., Song Y., Wang Z. The tRNA-derived fragment-3017A promotes metastasis by inhibiting NELL2 in human gastric cancer. Front. Oncol. 2020;10:570916. doi: 10.3389/fonc.2020.570916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilusz J.E. Controlling translation via modulation of tRNA levels. Wiley Interdiscip. Rev. RNA. 2015;6:453–470. doi: 10.1002/wrna.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H., Weng H., Chen J. The biogenesis and precise control of RNA m6A methylation. Trends Genet. 2020;36:44–52. doi: 10.1016/j.tig.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borggrefe T., Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell. Mol. Life Sci. 2009;66:1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitagawa M. Notch signalling in the nucleus: roles of Mastermind-like (MAML) transcriptional coactivators. J. Biochem. 2016;159:287–294. doi: 10.1093/jb/mvv123. [DOI] [PubMed] [Google Scholar]
- 29.Shen H., McElhinny A.S., Cao Y., Gao P., Liu J., Bronson R., Griffin J.D., Wu L. The Notch coactivator, MAML1, functions as a novel coactivator for MEF2C-mediated transcription and is required for normal myogenesis. Genes Dev. 2006;20:675–688. doi: 10.1101/gad.1383706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dou G.R., Wang L., Wang Y.S., Han H. Notch signaling in ocular vasculature development and diseases. Mol. Med. 2012;18:47–55. doi: 10.2119/molmed.2011.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mack J.J., Iruela-Arispe M.L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 2018;25:212–218. doi: 10.1097/MOH.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil D.P., Pickering B.F., Jaffrey S.R. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo S., Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc. Natl. Acad. Sci. USA. 2014;111:13834–13839. doi: 10.1073/pnas.1412742111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott A., Fruttiger M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye. 2010;24:416–421. doi: 10.1038/eye.2009.306. [DOI] [PubMed] [Google Scholar]
- 35.Lambert V., Lecomte J., Hansen S., Blacher S., Gonzalez M.L.A., Struman I., Sounni N.E., Rozet E., de, Tullio P., Foidart J.M., et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 36.Tomita Y., Shao Z., Cakir B., Kotoda Y., Fu Z., Smith L.E. An ex vivo choroid sprouting assay of ocular microvascular angiogenesis. JoVE. 2020;162 doi: 10.3791/61677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.