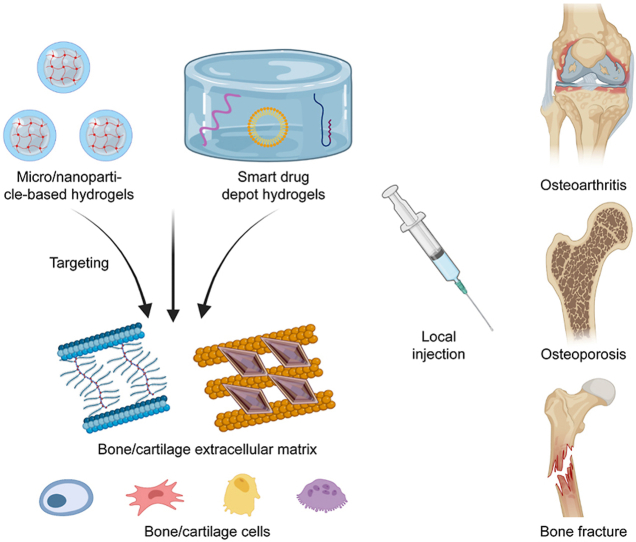
Abstract
The skeletal system is responsible for weight-bearing, organ protection, and movement. Bone diseases caused by trauma, infection, and aging can seriously affect a patient's quality of life. Bone targeted biomaterials are suitable for the treatment of bone diseases. Biomaterials with bone-targeted properties can improve drug utilization and reduce side effects. A large number of bone-targeted micro-nano materials have been developed. However, only a few studies addressed bone-targeted hydrogel. The large size of hydrogel makes it difficult to achieve systematic targeting. However, local targeted hydrogel still has significant prospects. Molecules in bone/cartilage extracellular matrix and bone cells provide binding sites for bone-targeted hydrogel. Drug delivery systems featuring microgels with targeting properties is a key construction strategy for bone-targeted hydrogel. Besides, injectable hydrogel drug depot carrying bone-targeted drugs is another strategy. In this review, we summarize the bone-targeted hydrogel through application environment, construction strategies and disease applications. We hope this article will provide a reference for the development of bone-targeted hydrogels. We also hope this article could increase awareness of bone-targeted materials.
Keywords: Bone, Cartilage, Hydrogel, Target therapy, Bone disease
Graphical abstract
Highlights
-
•
Introducing the microenvironment and target molecules in different parts of long bones.
-
•
Summarizing the construction strategy of micro/nanoparticle hydrogel with bone targeting properties.
-
•
Summarizing the construction strategy of hydrogel based depot carrying bone-targeted drugs.
-
•
Reporting the application and effect of bone targeting hydrogel in common bone diseases.
1. Introduction
The prevalence of automobiles and an aging society have led to a gradual increase in the incidence of orthopedic disorders caused by trauma and aging [[1], [2], [3]]. Comminuted and open fractures caused by high-energy injuries increase the risk of bone defect and osteomyelitis significantly. The increasing elderly population has led to an increase in the number of patients with degenerative orthopedic diseases such as osteoarthritis (OA) and osteoporosis (OP) [4,5]. These orthopedic diseases create a huge social and economic burden. Advances in bioactive materials provide new treatments for orthopedic disorders [6]. Bioactive materials can enhance the stability of fracture internal fixation, promote fracture healing and repair bone defect [7]. Antimicrobial drug-loaded biomaterials can effectively control bone infections and improve the prognosis of osteomyelitis. For the elderly, the development of new biomaterials to repair articular cartilage damage or decrease the bone loss is highly beneficial [8]. The development of bioactive materials provides an important contribution to the advancement of orthopedic diseases treatment.
Biomaterials for orthopedic applications focus on improving osteoconductive and osteoinductive ability. The physical and chemical properties of bone should be considered. Calcium phosphate (CaP) bone cement and polymethyl methacrylate (PMMA) bone cement are widely used for high bone affinity [9]. Materials with high bone affinity and osseointegration, such as materials that target hydroxyapatite (Ha), are used as active coatings for internal fixation devices to enhance internal fixation stability [10]. In recent years, materials with specific bone-targeted properties have been developed. A recent review article by our team provides a comprehensive overview of the current status of research in bone-targeted biomaterials [11]. However, hydrogel, a commonly used material in bone diseases treatment, was not fully discussed in our previous review.
Hydrogels are a class of three-dimensional reticulated macromolecular materials with high hydrophilicity [12]. Bulk hydrogels have been widely reported in bone diseases as scaffolds for bone tissue engineering, carriers for the controlled drugs delivery, and bone repair materials in bone regeneration [13]. Bulk hydrogels have adjustable biophysical and biochemical properties, the ability to program degradation, and flexible modification [14]. The structure of hydrogel is very similar to the ECM of bone and cartilage, which makes hydrogels very suitable for bone repair and regeneration. Hydrogels have developed from simple natural or synthetic hydrogels to smart hydrogels with several properties to achieve response and targeting [15]. However, there are some disadvantages of bulk hydrogels, including large size, low specific surface area, lacking of micropores, and low speed of degradation. Those disadvantages lead to poor cell infiltration and cell viability [16].
Microgels with 10 nm–100 μm diameter were developed to overcome the shortcomings of bulk hydrogels [17]. Microgels can be injected into injury sites or injected through vein. Due to their small size and deformability, microgels can cross some bio-barriers. Due to the controllable micropores and reversible swelling capacity of microgels, microgels serve as mobile drug and cells carriers [18]. Microgels can be functionalized with targeting units on their surfaces [19]. Microgels have several properties, such as injectablility, microporosity, heterogeneity, and modifiable surfaces. Due to those features, microgels are the most suitable materials to manufacture targeted hydrogels. Microgel-based targeting carriers can increase the local drug concentration and enhance the therapeutic effect. However, due to their small size and fast degradation, microgel-based targeting carriers cannot achieve long-term drug delivery. In contrast, smart drug depot hydrogels can achieve long-term drug delivery for a single treatment due to their large block size and large amounts of targeted drugs.
Recently, several publications have reported hydrogels with bone-targeted ability or the ability to release bone-targeted drugs. In this review, we focus on the development of hydrogel microspheres or nano hydrogels with bone-targeted properties and some hydrogel materials that can load bone-targeted drugs. We discuss the application scenarios, construction strategies, and clinical applications of bone-targeted hydrogels. We hope this review could be a reference for the development of bone-targeted hydrogels.
2. Cartilage targeted hydrogel for joint
2.1. Structure and micro-environment of joints
2.1.1. Anatomy and function
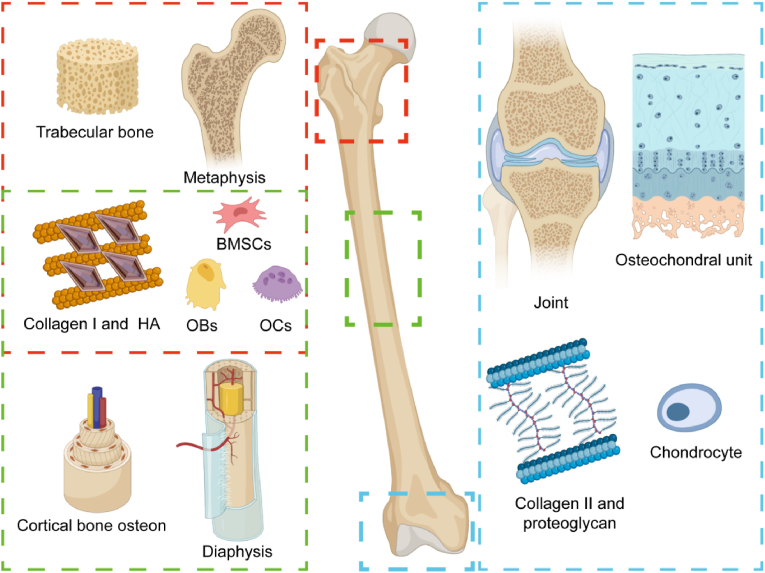
Joints are connections between two bones that are responsible for limb movement and force conduction. Joints consist of soft tissues such as the joint capsule, ligaments, and meniscus, along with hard tissues such as articular cartilage and subchondral bone (Fig. 1). The structural integrity of the joint is the basis for ensuring joint mobility. Articular cartilage and subchondral bone are linked as functional units [20]. The concept of an osteochondral unit considers the cartilage and subchondral bone as a whole [21]. Articular cartilage is primarily responsible for reducing friction and transmitting loads. The subchondral bone is responsible for distributing and transmitting loads from joints [22]. Articular cartilage and subchondral bone exhibit dramatic differences in cellular composition, extracellular matrix structure, and the rate of matrix remodeling. These differences balance the load between articular cartilage and subchondral bone and protect the cartilage in the physiological state [23]. In the pathological state, abnormal changes in either structure can affect the other [24].
Fig. 1.
A schematic figure of the anatomy and micro-structure of long bones The red rectangle indicates the anatomy and microstructure of the metaphysis. The green rectangle indicates the anatomy and microstructure of the diaphysis. The blue rectangle indicates the anatomy and microstructure of the articular osteochondral unit. Metaphysis and diaphysis share similar extracellular matrix and cellular components, including a bone matrix composed of type I collagen and calcium salt crystals. In the metaphysis and diaphysis, bone cells include bone marrow mesenchymal stem cells (BMSCs), osteoblasts (OB), and osteoclasts (OC). Articular cartilage is mainly composed of type II collagen, proteoglycans, and chondrocyte. Created by Biorender.
2.1.2. Micro-structure
Articular cartilage is composed of a large amount of extracellular matrix. The chondrocytes only occupy 5% of the weight of the cartilage [25]. The main component of the extracellular matrix is water, and the rest of the dry weight consists mainly of type II collagen and proteoglycans (Fig. 1). Human articular cartilage is divided into four layers: the superficial, transitional, deep, and calcified cartilage layers. In the superficial layer, the chondrocytes are flattened and the collagen fibers are arranged parallel to the joint surface. In the transitional layer, collagen fibers are gradually arranged in an oblique manner, and multiple chondrocytes are arranged in columns. In the deep layer, the collagen fibers are arranged perpendicular to the articular surface, and the chondrocytes are arranged in clusters. In the calcified cartilage layer, hypertrophic chondrocytes are present. The extracellular matrix appears to be calcified.
There is a demarcation line between the calcified cartilage and the hyaline cartilage that can be revealed by tissue staining. This line is known as the tidemark [26]. The calcified cartilage is embedded with the subchondral bone plate beneath each other, and the junction line appears wavy. The subchondral bone plate is a denser layer of cortical bone which then gradually transitions to subchondral trabeculae perpendicular to the bone plate [27]. As the main components of articular cartilage, type II collagen and proteoglycans, such as hyaluronic acid (HA) and chondroitin sulfate (ChS), are the main targets of bone-targeted materials (Fig. 1). In addition, chondrocytes, as the primary cells in cartilage, are also potential material targets.
The main problem in the application of hydrogel in cartilage is how to cross the tidemark and transport hydrogels to the deep layer of cartilage. Articular cartilage matrix is acidic, mostly negatively charged, with a relatively large gap between collagen [28]. However, it is important to note that the tidemark is a physical barrier. Tidemark could stop larger molecules moving between the hyaline cartilage and calcified cartilage. The tidemark has an average gap of 30 nm and a maximum gap of almost 100 nm. A study reported that the tidemark can allow the passage of molecules up to 150 KDa [29]. These characteristics lead some physical limits for bone-targeted materials. Oversized materials could only be able to stay in the hyaline cartilage.
2.2. Strategies for hydrogels targeted to the articular cartilage
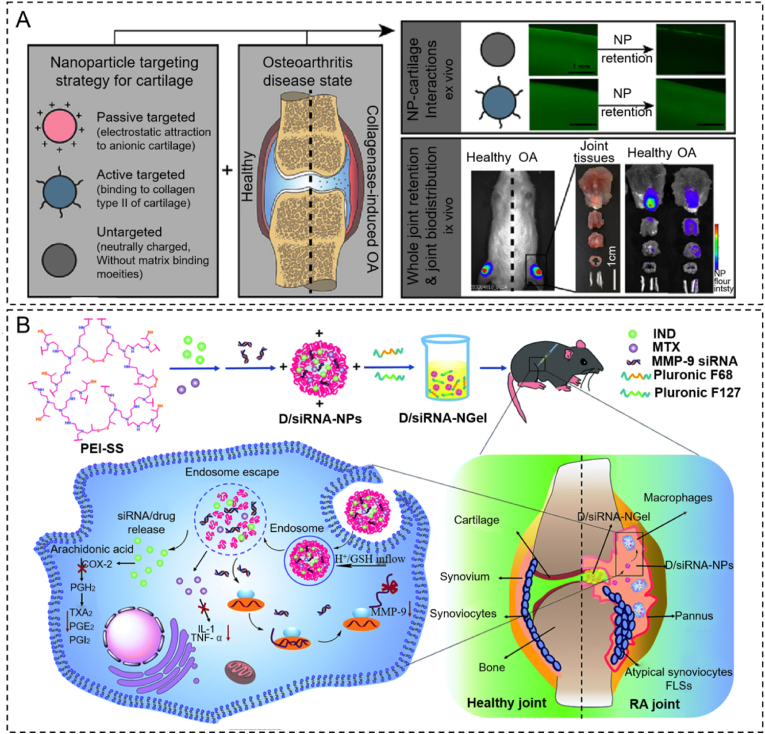
ECM and cells in the articular cartilage are potential targets for targeted materials. Targeted microgel-based hydrogels perform active targeting by special surface moieties that interact with molecules in the cartilage such as collagen II, hyaluronic acid, and ChS. On the other hand, microgel-based hydrogels perform passive targeting via biophysical and biochemical properties such as size and charge (Fig. 2A) [30]. The recommended size of microgel-based hydrogels in cartilage is lower than 30 nm, and positive charged hydrogels could target cartilage ECM. In joint-related diseases such as osteoarthritis, specific molecules emerge during the pathology of the disease, thus providing targets for specific drugs [[31], [32], [33]]. These targeted drugs have already been loaded into large smart drug depot hydrogels (Fig. 2B) [34].
Fig. 2.
Strategies for hydrogels targeted at the articular cartilage. (A) The biodistribution of various targeted (passive or active) hydrogel particles in healthy and osteoarthritic joints. Reproduced with permission [30]. Copyright 2020, Elsevier (B) The scheme of material synthesis and the experimental process of smart drug depot hydrogel developed by our team. Reproduced with permission [34]. Copyright 2020, RSC Publications.
2.2.1. Microgel-based hydrogels targeted to the articular cartilage
Microgel-based hydrogels have been reported in OA [35]. These targeted microgel-based hydrogels are regarded as ideal carriers for drug, liposome, and peptide loading [[36], [37], [38], [39], [40]].
ChS possesses targeting potential because of its easy interaction with collagen II and the receptor CD36 on chondrocytes [41]. Compared to non-functional particles, ChS-decorated delivery carrier systems can target the articular cartilage [35]. Jain et al. reported diacerein-loaded solid lipid nanoparticles modified with ChS hydrogel that could target the cartilage in a rat OA model [40]. Firstly, soy lecithin was dissolved in chloroform and mixed with diacerein dissolved in methanol. This mixture is added to melted stearic acid and stirred quickly to make a primary emulsion. Primary emulsions was added to the hot water phase containing Pluronic F68 and citric acid, and homogenized at high speed for 10 min. Then, the aqueous solution containing ChS was added to the suspension and sonicated for 10 min. Finally, water and organic solvents were evaporated under vacuum to obtain ChS modified lipid nanoparticles. The particle size of these ChS modified lipid nanoparticles was 396 ± 2.7 nm. This study confirmed that the ChS hydrogels could be a cartilage-targeted drug delivery system.
Microgel-based hydrogels with a positive charge such as chitosan and GelMA exhibit the natural ability to target cartilage tissue [42]. Ebada et al. fabricated a micro/nano-hydrogel (average diameter 156.80 ± 0.05 nm) by adding cassic acid (CA) integrated nano-reservoirs (CA-NRs) in positively charged ChS hydrogel [43]. They prepared CA-integrated nanomaterials based on the ionic conjugation between CA and the hydrophobic cationic surface modifier octadecylamine (ODA). Positive-charged CA-NRs (average electric potential 41.83 ± 5.63 mV) combined with ChS improved cartilage-targeting ability and the release of drugs in a long term.
HA is also an important component in cartilage. HA has high affinity for the CD44 receptor, which is expressed on the surface of chondrocytes. Therefore, HA-based microgel containing active molecules could target chondrocytes actively via local injection [44]. To achieve controlled delivery and cartilage cell targeting, HA could also be coated on a polymeric nanoparticle core. Another study reported a HA coated bovine serum albumin nanoparticle (average diameter of 700 nm) that actively interacted with chondrocytes via selective binding to the CD44 receptor [45].
2.2.2. Smart drug depot hydrogels targeted to the articular cartilage
Due to limited drug loading capacity, microgel-based hydrogel systems have an unsatisfactory drug release rate and therapeutic efficiency in long-term treatment. To address those challenges, targeted smart drug depot hydrogels were proposed. This system carries targeted particles or inhibitors in hydrogel to achieve continuous treatment effect [46]. These types of depot hydrogels could release cargoes and degrade gradually [47]. Oliveira reported two novel hydrogel delivery systems based on drugs targeted to rheumatoid arthritis (RA) [48]. By combining ChS with a poly amidoamine encapsulating anti-TNF- antibody, they created a dendrimer nanoparticle. Nanoparticles were loaded into tyramine-gellan gum (Ty-GG) hydrogel and Ty-GG/silk fibroin hydrogel to treat RA via local injection. These hydrogels demonstrated a good drug release rate and reduced RA symptoms via TNFa activation. Hydrogel-based drug depots can release targeted inhibitors such as antimiR-221, circular RNAs3505, ADAMTS-5 inhibitor, and small-interfering RNA targeting MMP 9 to chondrocytes directly and gradually [[49], [50], [51], [52], [53]]. Lolli fabricated a novel targeted depot based on fibrin/hyaluronan hydrogel. This hydrogel contained a microRNA targeting miR-221 [52]. This system promoted chondrogenesis and chondrogenic differentiation from BMSCs and lasted for 14 days.
2.3. Targeted hydrogels for osteoarthritis
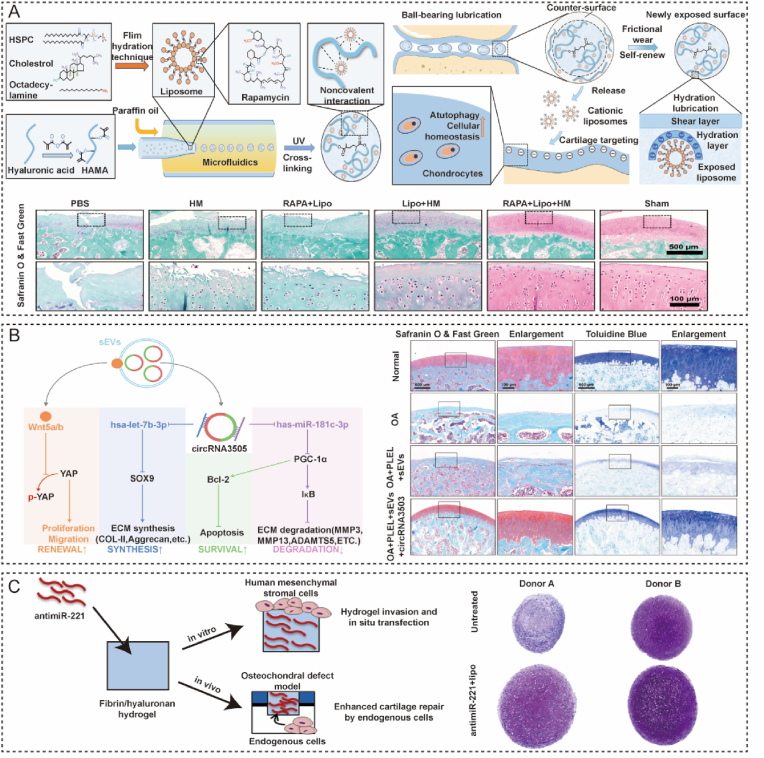
OA is a degenerative disease characterized by cartilage degradation, inflammation, subchondral bone sclerosis, and osteophyte formation. OA has become a common reason for disability in the elderly [22]. OA is a gradual evolutionary process with different characteristics at each stage. Researchers focused on protecting cartilage and slowing the progression of OA in the early stages, when the cartilage had not completely worn away. Healthy joints can move smoothly with little friction due to the hydration lubrication mechanism [54]. However, this function of a joint is impaired in patients with OA. Increasing interchondral lubrication has a significant role in protecting the cartilage. Some bio-materials have been coated with hydration layers to alleviate OA and report good outcomes [55]. However, these biomaterials can be broken by friction and cannot provide long-term lubrication [56,57]. Therefore, biomaterials that target cartilage surfaces with a self-repairing ability are preferred. Lei designed a injectable hydrogel microsphere (HMs) that possessed self-renewable hydration layers and could target cartilage [58]. These hybrid hydrogel microspheres consist of rapamycin (RAPA)-loaded and HA-based hydrogenated soy phosphatidylcholine (HSPC) liposomes. The positive zeta potential of liposomes formed a robust hydration layer on the negatively charged cartilage to provide lubrication. Methacrylated HA (HAMA)-based hydrogel microspheres serve as a liposome reservoir to renew the hydration layers and maintain a stable lubrication environment. RAPA can be sustainably released from liposomes to maintain cellular homeostasis by improving autophagy and reducing ROS regeneration. In vivo experiments showed that the lubricated microgel could alleviate articular damage and delay the progression of OA (Fig. 3A). These targeted microgel used the passive target method to form a long-durative lubricated membrane at the surface of articular cartilage.
Fig. 3.
Application of cartilage-targeted hydrogel in osteoarthritis. (A) Cartilage-targeted hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis [58]. Reproduced with permission Copyright, 2022 American Association for the Advancement of Science. (B) A thermosensitive hydrogel loaded with small extracellular vesicles in combination with circRNA3503 to prevent osteoarthritis [49]. Reproduced with permission Copyright, 2021 Elsevier. (C) Hydrogel-based delivery of antimiR-221 targeted to the chondro-progenitor cells for cartilage regeneration [52]. Reproduced with permission Copyright, 2019 Elsevier.
The degradation of ECM and apoptosis of chondrocytes are key processes in the progression of OA. In the advanced stage of OA, matrix homeostasis is disrupted, leading to cartilage loss and chondrocytes apoptosis. Protection of chondrocytes and correction of the unbalanced ECM metabolism are vital in preventing OA progression. Several treatments have been reported to treat these processes, including stem cell therapy, small molecule therapy, and gene therapy [59,60]. CircRNAs are crucial regulators of chondrocyte apoptosis in OA [61]. Effective delivery of circRNAs could alleviate apoptosis of chondrocytes. An injectable thermosensitive hydrogel combined with small extracellular vesicles (sEVs) loaded with circRNA has been developed to prevent OA progression [49]. sEVs derived from synovium mesenchymal stem cells and encapsulated in a hydrogel composed of poly(D,l-lactide)-b-poly(ethylene glycol)-b-poly(D,l-lactide). CircRNA3503 in sEVs could enhance ECM synthesis, inhibit chondrocyte apoptosis, and relieve ECM degradation by penetrating cartilage to target chondrocyte. The results of histological staining have further demonstrated that this targeted therapeutic strategy may be a potential approach for OA (Fig. 3B).
In the late stages of OA, cartilage defects and subchondral bone exposure are the main pathological features [62]. Chondrocyte catabolism and the expression of MMPs may impede cartilage repair [63]. Furthermore, articular cartilage is an avascular tissue, leading to little regenerative capacity [64]. Osteochondral defects are a significant challenge in the clinic. Recent research has proven that miR-221 in bone marrow mesenchymal cells (BMSCs) may impede cartilage repair in vivo. Chondrogenesis can be effectively induced by silencing miR-221. Lolli utilized a fibrin/hyaluronan (FB/HA) hydrogel to deliver a microRNA targeting miR-221 (antimiR-221) to enhance cartilage regeneration [52]. In this study, antimiR-221 released from FB/HA hydrogel gradually. Results of histology and immunohistochemistry in an osteochondral defect model demonstrated that cartilage regeneration was enhanced by this hydrogel (Fig. 3C).
3. Targeted hydrogel for bone metaphysis
3.1. Structure and micro-environment of the long bone metaphysis
3.1.1. Anatomy and function
The Association for the Study of Internal Fixation defines the metaphysis as a square area at the end of the long bone. The side length of this square is the joint width. The main anatomy of the metaphysis contains the subchondral trabecular bone, the epiphyseal line (the growth plate in immature animals) and the metaphysis trabecular bone (Fig. 1) [65]. The epiphyseal cartilage is an important structure for the growth of human limbs and is a multi-layered structure similar to articular cartilage. Chondrocytes gradually proliferate, undergo hypertrophy, differentiate, and eventually calcify in the deepest layer [66]. In adults, the growth plate is fully calcified to form an epiphyseal line consisting of lamellar bone.
Trabeculae are mainly located in the metaphysis and are arranged in a lamellar or linear pattern with an average of 50–400 μm in thickness [67]. Trabeculae bone is polyporous, and the porosity is 30–40%. The range of distance between bone rods is from 100 to 500 μm. Trabecular osteons are semilunar, and normally approximately 35 μm in thickness [68]. The arrangement of the trabecular bone is distributed along the compressive and tensile stresses. Thus, a complex three-dimensional structure was formed in the trabeculae bone to disperse and transfer load [69]. The blood supply of the trabecular bone at the metaphysis is abundant, and the bone turnover rate is relatively fast [70]. Under physiological conditions, the functions of bone formation and bone resorption in the trabecular bone are coupled. In the pathological state or aging, this precise balance is disrupted and leads to the disruption of bone microstructure. Therefore, the bone-targeted materials in the metaphysis need to regulate the activity of osteoblasts and osteoclasts.
3.1.2. Micro-structure
Bone tissue is predominantly a large amount of bone matrix in addition to a smaller number of osteocytes, osteoblasts, and osteoclasts. Unlike the cartilage matrix, the bone matrix mainly consists of type I collagen and a small amount of proteoglycans and lipids. The bone matrix is characterized by many crystals of inorganic metal salts. Ha is the main form of calcium salt deposition in bone tissue, which appears as fine needle-like crystals on electron microscopy [71]. Type I collagen consists of a triple helix structure consisting of three polypeptide chains that provide attachment points for calcium salt crystals (Fig. 1) [72]. The collagen arrangement in trabecular bone is still somewhat directional. The matrix of trabecular bone is less intact than that of cortical bone. Therefore, the mechanical properties of trabecular bone are weaker than those of cortical bone [73]. It is hard to measure the pH valve of bone in vivo. According to research about acid-base balance in bone, the normal pH valve of bone was estimated at 7.2–7.4 [74]. The bone matrix is composed of Ha and type I collagen, so it could be negatively charged. In cancellous bone, type I collagen and Ha are the main extracellular targets. BMSCs, OB and OC are the main cellular targets that regulate the microenvironment of trabecular bone [75].
Except osteocytes, other bone cells are located in the bone marrow or adhere to the bone surface. The issue lies in applying hydrogel in the metaphysis is accurate delivery rather than penetrating. Targeting the specific cell types directly and realizing multi-targeting are great challenges.
3.2. Strategies for hydrogels targeted to the metaphysis
The metaphysis predominantly consists of trabecular bone and is vulnerable to OP [76]. The porosity of metaphysis trabecular bone is large, and the targets of metapyhsis are on the surface of the bone. Thus, there is no limitation on the diameter of the hydrogels. Besides, the main component of bone matrix is Ha, which is commonly considered an electrical neutral material. Therefore, charge modification is not suitable for metaphysis targeting. Modifying with matrix or cell-targeted molecules on the surface of the hydrogel and embedding bone matrix or cells targeted drugs in the hydrogel are two main strategies for bone-targeted hydrogel.
3.2.1. Microgel-based hydrogels targeted to the metaphysis
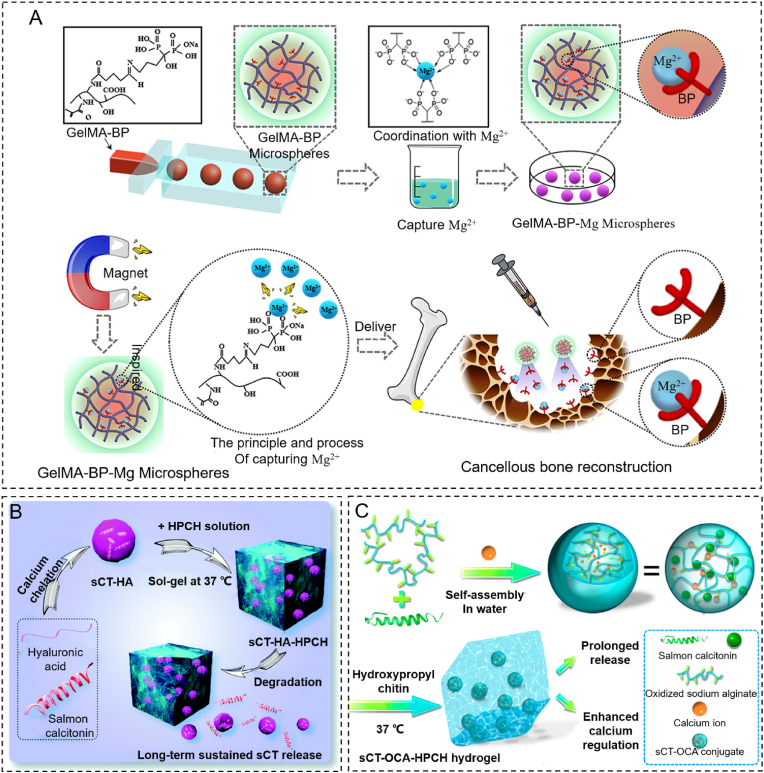
Due to the lack of specific moieties and a low affinity for mineralized tissue, no research showed that non-hybrid hydrogels could target bone tissue. Several bone-targeting drugs, including bisphosphonates and oligopeptides, can be modified on hydrogels [77]. BP has been used to modify the branching chains of natural and synthetic hydrogels in order to achieve active targeting on osteoclasts and inhibit bone resorption [78]. Zhao fabricated a micro scale (265 ± 36 μm) targeted GelMA-BP microparticles hydrogel by microfluidic technology [79]. GelMA-BP was synthesized by grafting BP onto the GelMA branching chains via the Schiff base reaction and aldehyde activation reaction. Mg2+ is an osteogentic metal ion. GelMA-BP microspheres can capture Mg2+ by magnetism to form a GelMA-BP-Mg system and possess a dual targeting function. GelMA-BP-Mg could promote bone formation and inhibit bone resorption simultaneously (Fig. 4A). Farbod developed a biocompatible gelatin nanoparticle (475 ± 66 nm) that displayed a strong affinity with mineralized surfaces by conjugating alendronic acid (ALN) [80]. Gelatin nanoparticles were prepared using a two-step desolvation method. The gelatin nanoparticles were cross-linked with glutaraldehyde (GA). Then, the residual aldehyde group of the GA cross-linking reaction was combined with the amino group on ALN. Interestingly, the affinity of gelatin nanoparticles with calcium phosphate increased after cross-linking with ALN. This nanoparticle hydrogel could stick to the bone surface and inhibit osteoclast activity through ALN.
Fig. 4.
Strategies for hydrogels targeting the metaphysis. (A) The process of microfluidic GelMA-BP microspheres and the construction of GelMA-BP-Mg microspheres that capture Mg2+ for the treatment of osteoporosis via an inspired “magnet” function. Reproduced with permission [79]. Copyright 2021, ACS Publications. (B) The manufacture of sCT-HA hydrogel particles, the sCT-HA-HPCH hydrogel and the sustained release process. Reproduced with permission [84]. Copyright 2020, RSC Publications. (C) Fabrication of sCT-OCA-HPCH hydrogel and its main functions. Reproduced with permission [85]. Copyright 2020, ASC Publications.
3.2.2. Smart drug depot hydrogels targeted to the metaphysis
Due to its unique 3D ECM-like properties, hydrogel is an attractive drug depot for bone-targeted drugs [81]. The targeted drug release from hydrogels is regulated by the intrinsic properties of the hydrogel and drug-network interactions [82]. To improve the biocompatibility and reduce the side effects of ALN. ALN was hybridized into poly (lactide-co-glycolide) (PLGA) by solid-oil-water emulsification. To achieve long-term release, ALN-PLGA nanoparticles were encapsulated in a gellan gum hydrogel [83]. Yu manufactured a nanoparticle by combining sCT and Ha (sCT-Ha) and loading the thermo-sensitive hydrogel based on hydroxypropyl chitin (Fig. 4B) [84]. They also introduced active bone-targeted nanoparticles by combining sCT and oxidized calcium alginate (OCA) via the similar approach. An excellent effect of promoting bone regeneration was reported (Fig. 4C) [85].
Other osteogenetic drugs, such as LLP2A, Apt19S, or bone resorption inhibitors, such as V3 and D-Asp8 peptide, could be embedded in the hydrogel depot [11]. Hydrogels with those targeted molecules may develop into potential materials to treat OP. Gačanin designed a DNA hydrogel system by chemically modifying human serum albumin with DNA [86]. This DNA hydrogel encapsulated the reorganized Rho-inhibiting C3 toxin (C2IN-C3lim-G205C) which selectively targeted osteoclasts to reduce osteoclast formation and resorption activity.
3.3. Targeted hydrogel for osteoporosis and osteoporotic fracture
OP is a systemic, progressive, and metabolic disease that particularly affects the elderly and postmenopausal women [87]. OP is characterized by low bone mass and the disruption of the bone micro-architecture caused by the imbalance between bone formation and bone resorption [88,89]. This degradation of the microstructure of the bone tissue forms a disruption of the bone trabeculae, leading to increased bone fragility and susceptibility to fragility fractures. Several drugs have been approved to suppress bone resorption and improve bone formation in the clinic, such as BP, calcitonin, and parathyroid hormone (PTH). However, some drawbacks and side effects may limit the application of these drugs.
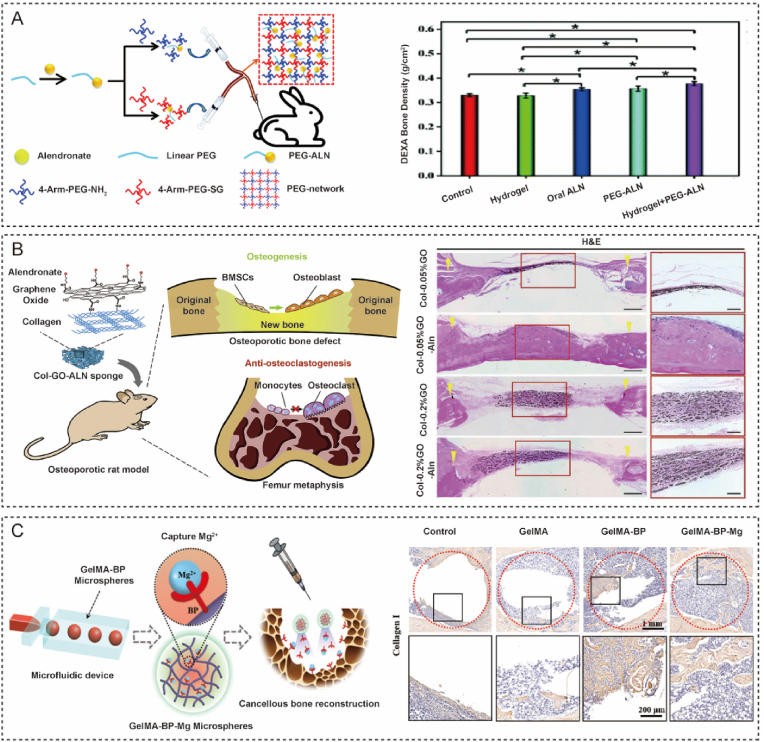
To overcome these difficulties, some researchers have developed bone-targeted hydrogels. Modification of hydrogels with BP creates a way to fabricate bone-targeted hydrogels for OP. BP can inhibit the activity of osteoclasts by binding to osteoclasts via two phosphate groups and prevent bone resorption by covering on bone matrix [11]. A long-term delivery hydrogel system has been developed to release ALN for OP therapy (Fig. 5A) [90]. ALN can be released for 28 days due to the slow degradation of the hydrogel. The anti-osteoporosis effect of this system was evaluated by measuring the bone mineral density (BMD) of a rabbit ovariectomized (OVX) model. The injectable hydrogel loaded with ALN effectively increased BMD in the rabbit OVX model (Fig. 6A). An osteoclast-targeted hydrogel composed of protein and DNA was reported to inhibit osteoclast formation and activity by releasing Rho-inhibiting C3 toxins in a spatiotemporally controlled manner [86]. This hybrid hydrogel was based on hydrogen bonds. It could be degraded by DNase to release cargo. Rho-inhibiting C3 toxins could inhibit osteoclast formation and bone resorption without affecting osteoblast differentiation and mineralization. Hybrid hydrogels targeted to osteoclasts may be a potential method for OP.
Fig. 5.
Application of metaphysis-targeted hydrogels for osteoporosis and osteoporotic fracture. (A) Injectable PEG-based hydrogel loaded with alendronate for osteoporosis therapy [90]. Reproduced with permission. Copyright, 2020 Royal Society of Chemistry. (B) Alendronate-loaded graphene oxide functionalized collagen sponge for osteoporosis therapy [92]. Reproduced with permission. Copyright, 2020 Elsevier. (C) Bone-targeted microfluidic hydrogel microspheres promoted cancellous bone regeneration by capturing magnesium ions [79]. Reproduced with permission. Copyright, 2021 American Chemical Society.
Fig. 6.
Strategies for diaphysis-targeted hydrogels. (A) Schematic of the three-dimensional structure of FMSs, fullerol, and gelatin methacryloyl (GelMA). Reproduced with permission [100]. Copyright 2020, Elsevier. (B) The fabrication of hydrogel Gel/TG/TA-MPs-His6-T4L-BMP2 (Hydrogel/MPs-His6-T4L-BMP2) [110]. Reproduced with permission, Copyright 2021, Elsevier.
OP can cause fragility fractures and impede bone healing [91]. Osteoporotic bone defects are common in fragility fractures due to low bone mass [9]. Due to the acidic, high ROS levels and lack of oxygen microenvironment of osteoporotic bone defects, osteoclast activity is high and hampers bone healing. Therefore, systemic therapy for OP combined therapy for bone regeneration is widely accepted for the treatment of osteoporotic bone defects. Recently, Zeng described a ALN-loaded graphene oxide (GO) modified collagen sponge that could promote osteogenesis and inhibit osteoclasts in osteoporosis [92]. This was a hybrid hydrogel that crosslinked type I collagen with ALN modified GO. GO increased the osteogenic differentiation of BMSCs, the ALN inhibited osteoclasts, and type I collagen served as a scaffold for cell adhesion. This biomaterial showed active anti-osteoclastogenic effects, osteogenesis ability, and enhanced bone formation in osteoporotic rats (Fig. 5B). Consequently, this bone-targeted hydrogel could represent an ideal tool for bone regeneration with OP.
Bone-targeted hydrogel microspheres capturing Mg2+ via metal ion-coordinate bonds have been developed for the treatment of osteoporotic bone defects [79]. Mg2+ plays a vital role in bone formation by promoting osteogenesis and angiogenesis. The microspheres exhibited active capture and slow release of Mg2+. Histological assessments have proved that these composite microspheres promote osteoporotic bone defect repair via Mg2+ capture (Fig. 5C). However, microgel-based hydrogel could only be applied to bone defects without load bearing. Because of the low mechanical properties of ploy microgel-based hydrogel, it can not provide effective support.
4. Bone targeted hydrogel for diaphysis
4.1. Structure and micro-environment of the long bone diaphysis
4.1.1. Anatomy and function
The long bone diaphysis is a tubular structure consisting of a hard peripheral cortical bone and a bone marrow cavity (Fig. 1). Cortical bone has good mechanical properties, especially against axial loads and supports the weight of the body. Cortical bone, on the other hand, has limited lateral strength and is prone to diaphysis fractures during high-energy injuries [93]. The bone marrow cavity contains mainly hematopoietic lineage cells, adipocytes, and BMSCs. These cells maintain bone growth and development, bone metabolism and repair, and the renewal of the hematopoietic system [75,94].
4.1.2. Micro-structure
Compared to trabecular bone, cortical bone is more mineralized and has a regular microstructure. Cortical bone is generally composed of the periosteum, outer circumferential lamellae, osteon, inner circumferential lamellae, and endosteum (Fig. 1). The periosteum is thicker at the attachments of muscles, tendons, and trophoblastic vessels. The periosteum is composed of many collagen fibers and fibroblasts. Both outer circumferential lamellae and inner circumferential lamellae are aligned along the longitudinal axis of the long bones. A large number of osteons are located between the inner and outer circumferential lamellae, also known as the Haversian system [95]. The osteon is cylindrical. The collagen fibers of the osteon are arranged in concentric circles. Haversian systems are approximately 0.5–2.5 mm long and 100–500 μm wide [67]. Osteocytes are located in a trap in the osteon, surrounded by several tiny slits. Osteocytes can extend their pseudopods to connect with the central canal to obtain nutrients and carry away metabolic. Cortical bone porosity is usually less than 5% and the pH valve is closed to 7.1 [96].
Bone defects and fractures are the main orthopedic disorders at the diaphysis. The development of biomaterials needs to target the key cells or structures of the diaphysis to promote fracture repair and bone tissue regeneration [97]. Biomaterials targeting cells in the bone marrow cavity can promote bone regeneration.
4.2. Strategies for hydrogels targeted to the diaphysis
Bone defects caused by fracture and infections in the diaphysis are significant challenges in the clinic. During indirect bone healing, endochondral ossification is the primary process underlying bone repair. BMSCs, hypertrophic chondrocytes, vascular endothelial cells, osteoblasts, and osteoclasts are all involved in this biological process. Targeting bone matrix or important cells in endochondral ossification may be a prospective method.
4.2.1. Microgel-based hydrogels targeted to the diaphysis
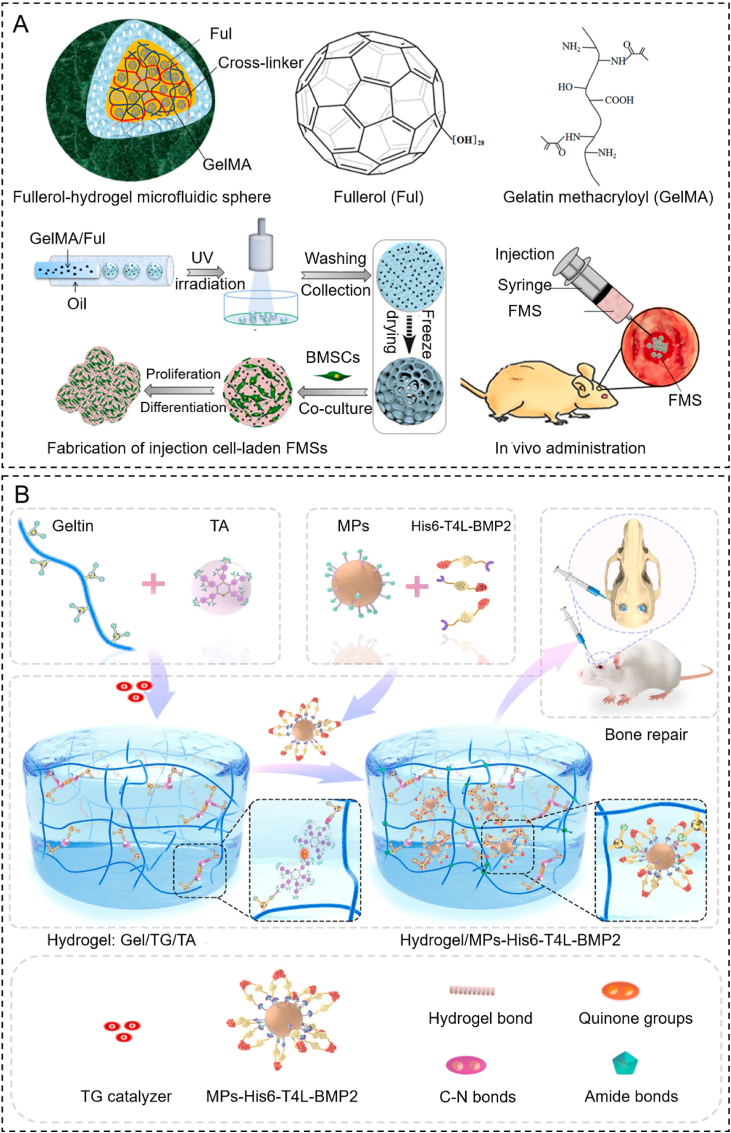
The bone repair process involves local inflammation, vascularization, cartilage matrix formation, and ultimately, bone formation [98]. Microgel, which is made up of loaded particles, cells, drugs, and growth factors, will pave the way for a bone regeneration strategy [[99], [100], [101]]. According to a study from Yang, highly dispersed fullerol nanocrystals were incorporated into hydrogel microspheres to construct fullerol-hydrogel microfluidic spheres (FMSs) by microfluidic technology. Injection of FMSs in situ regulated the redox homeostasis of stem cells and promoted bone healing (Fig. 6A) [100]. Cell-based microgels to achieve in-situ targeted mineralization are being investigated for bone repair [102]. BMSC-entrapped fibrin hydrogel particles have been shown to exhibit enhanced osteogenesis and improved survival of BMSC when compared to BMSCs alone [103]. Moreover, targeted hydrogel treatment strategies are highly suitable for bone repair.
4.2.2. Smart drug depot hydrogels targeted to the diaphysis
Targeted moieties such as cells, genes, peptides, and sEVs loaded into hydrogel-based smart drug depots play a vital role in long-term bone regeneration. Choi manufactured a photo-crosslinked chitosan hydrogel encapsulating BMSCs, and a CaP nanoparticles coated with Gln-OChi (CaP/Gln-OChi) loaded with siRNA targeting noggin [104]. Noggin is a bone morphogenetic protein antagonist. This hydrogel depot induced the osteogenic differentiation of BMSCs through inhibiting noggin. Apart from these active targeted moieties. An amphipathic cell penetrating peptide can be coated on the surface of nanoparticles to improve passive targeting via enhanced BMSC uptake [105]. In another study, Sathy designed an alginate-based hydrogel depot including RALA (an amphipathic cell penetrating peptide) complexed with α-TCP nanoparticles. This system could accelerate mineralization by upregulating osteogenic genes [106].
Recently, several hydrogels have been used to carry targeted RNA to facilitate bone regeneration. Extracellular vesicles (sEVs) encapsulated into alginate hydrogel could improve bone regeneration by releasing osteogenic microRNAs [107,108]. Li constructed an injectable heparin lithium hydrogel (Li-hep-gel) loaded with a tetrahedral DNA nanostructure (TDNs) and miR335-5p. Li-hep-gel could regulate the osteogenic differentiation of BMSCs and promote new bone formation by inhibiting DKK1 [109]. BMP-2 can bind to receptors on BMSCs and induce the osteogenic differentiation of BMSCs. A specific delivery system was designed by Chen [110]. They used polyhistidine-tagged-T4 lysozyme (His6-T4L) and genetically fused the N-terminal of BMP-2 as a protein spacer. The nickel-chelated nitrilotriacetic acid (Ni-NTA) particles could mediate a site-specific covalent between His6-T4L and BMP-2 (MPS-His6-T4L-BMP2). A new gelatin-based hydrogel was constructed by cross-linking transglutaminase (TG) and tannic acid (TA) as a carrier of MPS-His6-T4L-BMP2. This hydrogel provides effective and long-term controlled release of His6-T4L-BMP2, enhancing bone regeneration in severe bone defects (Fig. 6B). In addition, different types of synthetic hydrogel, such as PEG and PAMAM, have been designed for bone tissue engineering; this is due to their variable biophysical and biochemical properties and their good binding affinity to bone [111,112].
4.3. Targeted hydrogels for bone regeneration
Bone defects refer to the extensive destruction of bone structure. These defects are difficult to heal due to the defect size and a lack of bone regeneration capacity. Tumors, trauma, and infection may all lead to bone defects and are difficult to treat [113]. Autografts and allografts are widely used for bone defects in the clinic. However, several limitations restrict the application of bone grafts, including tissue source, donor site bone defect, immune reaction, and infection.
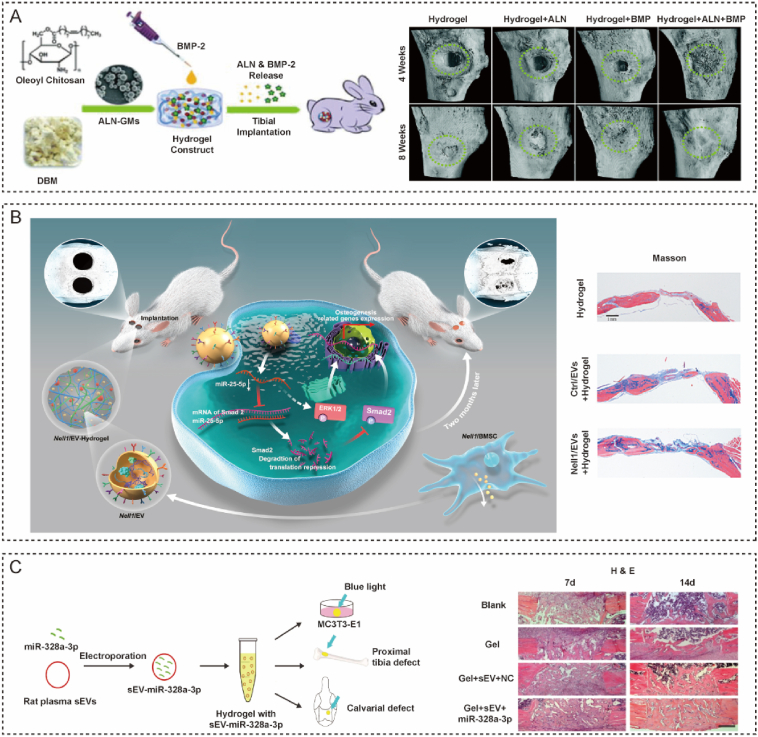
Bone tissue engineering using hydrogels brings new methods for bone regeneration and overcomes the disadvantages of bone grafting. Due to their excellent biocompatibility, flexibility, designability, and multifunctionality, hydrogels have been widely used for bone tissue engineering [114]. The osteogenic differentiation ability of stem cells influences the rate of bone regeneration. To accelerate the repair of bone defects, cytokines and drugs that can promote osteogenic differentiation are used. ALN is widely used to treat bone defects to accelerate bone repair via osteoclast inhibition. ALN-loaded microspheres were incorporated into a chitosan-based hydrogel that could enhance bone regeneration [115]. This pre-gel mixture could self-assemble and embed additional BMP-2. This hydrogel could release ALN and BMP-2 for two weeks and enhance osteogenesis in vitro and in vivo (Fig. 7A).
Fig. 7.
Application of diaphysis-targeted hydrogels for bone defects and fractures. (A) A microsphere-embedded hydrogel for the delivery of alendronate and BMP-2 for bone regeneration [115]. Reproduced with permission Copyright, 2021 Royal Society of Chemistry. (B) A hydrogel-loaded EV system derived from Nell1-modified BMSCs led to improved bone repair [116]. Reproduced with permission Copyright, 2020 Elsevier. (C) A hydrogel carrying traumatic brain injury sEVs targeted osteoprogenitors and enhanced bone formation [108]. Reproduced with permission Copyright, 2021 Nature Publishing Group.
EVs-hydrogel systems are also promising biomaterials with bone-targeted capability for bone tissue engineering. A composite hydrogel consisting of thiol-modified hyaluronan, hydroxyapatite and thiol-modified heparin was loaded with EVs derived from neural EGFL-Like 1 modified mesenchymal stem cells [116]. EVs in this hydrogel depot improved the protein levels of osteogenic markers and enhanced osteogenesis by targeting the Smad2 via SMAD/ERK pathways. As determined by fluorescence images, EVs-hydrogel system could release EVs for a long time after implantation in rat bone defect (Fig. 7B). sEVs released from patients with traumatic brain injury could also target osteoprogenitors to accelerate bone formation by promoting the activity of fibronectin [108]. A methacrylated glycol chitosan hydrogel carrying miR-328a-3p loaded into sEVs could target osteoprogenitors and efficiently increase bone repair both in vitro and in vivo (Fig. 7C).
5. Conclusion and outlook
Two main strategies have been developed to build bone-targeted hydrogels: (1) Construct movable microgel to achieve bone tissue targeted function via cross-linked targeted molecules. (2) Construct hydrogels as drug depots that can encapsulate bone-targeted agents or drugs and release them gradually in situ. To improve therapeutic efficacy and increase utilization, microgel-based hydrogels may be delivered to injury sites or lesions via passive or active targeting [117]. In passive targeting, the size and charge of particles can be controlled to interact with the targeted site. In active targeting, hydrogels can be modified with special molecules or cells on the surface. For hydrogel as a depot for targeted drugs, loaded drugs can be slowly released and bound to key pathological molecules [34].
Those two strategies have advantages and disadvantages. Microgel-based hydrogels has smaller size and high mobility, which make microgel-based hydrogels have several following merits. (1) Due to the micro-nano size and deformability of microgel, microgels can permeate compact tissue like cartilage. (2) Microgels can diffuse and target a larger area due to high flowability. (3) Due to the heterogeneity of microgels, multiple bone-targeted hydrogels can be fabricated. In contrast, the small size of microgel-based hydrogel lead to unsatisfied mechanical properties. Rapid degradation of microgel is an advantage in other system diseases, but not in bone diseases. Besides, microgels have a high cost because of microfluidic technology is expensive. Microgel-base bone-targeted is suitable for targeting diseases which need accurate and multiple drug delivery.
Smart depot hydrogels are a kind of injectable bulk hydrogel with targeted drugs. The main advantages of smart depot hydrogel are low cost, high and adjustable mechanical properties, good biocompatibility, and appropriate degradation speed. The disadvantages of the smart depot are similar to those of bulk hydrogel. Smart depot hydrogels are unmovable and can not diffuse. Smart depot hydrogel could provide mechanical support and long-term release in the bone regeneration process. Therefore, smart depot hydrogels are for bone regeneration of fracture and bone defect.
Natural, synthetic, and hybrid hydrogels are widely used as the basic components of targeted materials [13,118]. Limitations created by the size of the hydrogel material increase the risk of vascular embolism in intravenous injection. Thus, the targeted hydrogels in this review were used in local injections. Compared with systematic injection, injection in situ has several advantages and disadvantages. Most bone diseases, including OA, fractures, and bone defects, only affect one joint or limbs. Local injection to injury sites could improve therapeutic efficiency and decrease toxic effect. Local administration is a common strategy in orthopedics, due to the injury sites of skeletal disorders are superficial. Local injections have a low risk of infection and are simple to perform due to the simple anatomy of bone. We cannot deny the huge advantages of bone-targeted materials through systematic administration in systematic bone diseases. But local injection of bone-targeted materials in several vulnerable sites of osteoporotic fracture may be helpful to decrease fracture risk.
Hydrogel with bone-targeted ability could be useful in creating bone organoids. Organoids refer to self-assembling 3D cultures in vitro which could mimic the structure and function of organs in vivo [119]. Bone organoids are 3D self-renewing and self-organized micro-bone tissues with biomimetic spatial features that are built on bioactive materials and directionally differentiated from stem cells or progenitor cells [120]. Because of the similarity of bone ECM, hydrogel is an appropriate scaffold for bone organoid. Iordachescu first proposed the concept of bone organoid in 2017. They applied a calcium phosphate-containing cellulose hydrogel system to add osteoblasts to self-assemble into bone-like structures, reproducing multiple stages of calcium-salt deposition and bone maturation, and obtaining a multistage structure and osteocyte network similar to natural bone [121,122]. O'connor applied mouse induced pluripotent stem cells (ipscs) in micro spheroidal hydrogel to construct osteochondral organoids [123]. In addition to hydrogel, trabecular particles from animals and de-mineralized bone were found in bone-organoid [120].
The keys to bone organoid construction is to use various engineering means to reduce bone formation and bone resorption processes in vivo and achieve precise cell differentiation regulation on appropriate matrix scaffold materials [120]. There are currently no reports of bone-targeted hydrogel being used to create bone organoids. We still believe that bone-targeted hydrogel could be a cell trap to recruit and tie different stem cells or progenitor cells to the scaffold to form bone organoids. Our team has carried out related experiments to build a hydrogel with a bone-target molecule to create a bone organoid. Applying bone organoids to build highly simulated disease models facilitates material and drug screening and enables high-throughput efficacy evaluation. This is the future development direction of bone bio-materials.
Bone tissue has more extracellular matrix, fewer cellular components, and poorer local perfusion. Therefore, materials with targeted properties are suitable for orthopedic applications. Bone-targeted hydrogels is a novel concept but have not been investigated in detail. The large size of hydrogels creates a major challenge for the development of bone-targeting hydrogels. In contrast, micro-nano scale hydrogels are difficult to prepare. Therefore, fewer bone-targeted hydrogels are available for systemic delivery. Hydrogel microspheres with bone-targeted properties or hydrogels containing bone-targeted drugs are still promising. Hydrogels, as three-dimensional biomaterials, are structurally similar to the extracellular matrix. Research and development of hydrogel microspheres, or the development of new hydrogel drug libraries will be critical in the future.
In this paper, we describe the main application scenarios of bone-targeted hydrogels, beginning with the anatomy and structure of each component of the long bone. The specific microstructure and cellular microenvironment of different parts of long bones provide many targets for bone-targeted hydrogels, including the major components of the extracellular matrix and some cellular marker proteins. Current strategies for the preparation of bone-targeting hydrogels include micron-level hydrogel microspheres with targeting properties and hydrogel drug libraries containing bone-targeting drugs. Although there are few studies on bone-targeted hydrogels, we have also summarized the use of bone-targeted hydrogels in joint-related diseases, long bone metaphysis-related diseases and long bone diaphysis-related diseases. Because the local blood circulation of bone tissue is slightly weaker than in other tissues, the development of bone-targeted materials is of significant importance for orthopedic diseases. We hope that the summary of bone targeted hydrogels in this paper can provide a reference for future research in this field.
Authorship contribution statement
Hao Zhang, Shunli Wu, Weikai Chen contributed equally to this work. Hao Zhang, Shunli Wu, Weikai Chen wrote manuscript and drafted figures of this review. Yan Hu, Zhen Geng revised the manuscript. Jiacan Su guided and revised the manuscript of this review.
The authors declare no conflict of interest in this review
This is no ethics approval and consent to participant involved in this article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by the National Key R&D Program of China (2018YFC2001500); Key Project of The National Natural Science Foundation of China (82230071); National Natural Science Foundation of China (82172098, 32101084).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yan Hu, Email: xjhuyan@shu.edu.cn.
Zhen Geng, Email: nanboshan1987@163.com.
Jiacan Su, Email: drsujiacan@163.com.
References
- 1.Pigeolet M., Jayaram A., Park K.B., Meara J.G. Osteoarthritis in 2020 and beyond. Lancet. 2021;397(10279):1059–1060. doi: 10.1016/S0140-6736(21)00208-7. [DOI] [PubMed] [Google Scholar]
- 2.Compston J.E., McClung M.R., Leslie W.D. Osteoporosis, Lancet. 2019;393(10169):364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 3.Sattui S.E., Saag K.G. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat. Rev. Endocrinol. 2014;10(10):592–602. doi: 10.1038/nrendo.2014.125. [DOI] [PubMed] [Google Scholar]
- 4.Reid I.R. A broader strategy for osteoporosis interventions. Nat. Rev. Endocrinol. 2020;16(6):333–339. doi: 10.1038/s41574-020-0339-7. [DOI] [PubMed] [Google Scholar]
- 5.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Wu X., Li J., Jiang Y., Xu K., Su J. Bone-targeted nanoparticle drug delivery system: an emerging strategy for bone-related disease. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.909408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niu Y., Cao L., Wei J., Ma Y., Song S., Weng W., Li H., Liu C., Su J. Development of a bioactive composite of nano fluorapatite and poly(butylene succinate) for bone tissue regeneration. J. Mater. Chem. B. 2014;2(9):1174–1181. doi: 10.1039/c3tb21371d. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y., Li X., Zhang Q., Gu Z., Luo Y., Guo J., Wang X., Jing Y., Chen X., Su J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact. Mater. 2021;6(9):2905–2913. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Hu Y., Geng Z., Su J. The "three in one" bone repair strategy for osteoporotic fractures. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.910602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal R., Garcia A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren X., Chen X., Geng Z., Su J. Bone-targeted biomaterials: strategies and applications. Chem. Eng. J. 2022;446 [Google Scholar]
- 12.Huang Q., Zou Y., Arno M.C., Chen S., Wang T., Gao J., Dove A.P., Du J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017;46(20):6255–6275. doi: 10.1039/c6cs00052e. [DOI] [PubMed] [Google Scholar]
- 13.Xue X., Hu Y., Deng Y., Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021;19(31) [Google Scholar]
- 14.Amiri M., Khazaeli P., Salehabadi A., Salavati-Niasari M. Hydrogel beads-based nanocomposites in novel drug delivery platforms: recent trends and developments. Adv. Colloid Interface Sci. 2021;288 doi: 10.1016/j.cis.2020.102316. [DOI] [PubMed] [Google Scholar]
- 15.Yue S., He H., Li B., Hou T. Hydrogel as a biomaterial for bone tissue engineering: a review. Nanomaterials (Basel) 2020;10(8):1511. doi: 10.3390/nano10081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Q., Li D., Li Q., Cao X., Dong H. Microgel assembly: fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact. Mater. 2022;9:105–119. doi: 10.1016/j.bioactmat.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Guo L., Dong S., Cui J., Hao J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019;266:1–20. doi: 10.1016/j.cis.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Tiemeijer B.M., Tel J. Hydrogels for single-cell microgel production: recent advances and applications. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.891461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kittel Y., Kuehne A.J.C., De Laporte L. Translating therapeutic microgels into clinical applications. Adv. Healthc. Mater. 2022;11(6) doi: 10.1002/adhm.202101989. [DOI] [PubMed] [Google Scholar]
- 20.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 21.Lepage S., Robson N., Gilmore H., Davis O., Hooper A., St J.S., Kamesan V., Gelis P., Carvajal D., Hurtig M., Koch T.G. Beyond cartilage repair: the role of the osteochondral unit in joint health and disease. Tissue Eng. B Rev. 2019;25(2):114–125. doi: 10.1089/ten.teb.2018.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., Chen X., Wang S., Jing Y., Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decker R.S., Koyama E., Pacifici M. Articular cartilage: structural and developmental intricacies and questions. Curr. Osteoporos. Rep. 2015;13(6):407–414. doi: 10.1007/s11914-015-0290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Findlay D.M., Kuliwaba J.S. Bone–cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016;4(1) doi: 10.1038/boneres.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health: A Multidisciplinary Approach. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyde A. The bone cartilage interface and osteoarthritis. Calcif. Tissue Int. 2021;109(3):303–328. doi: 10.1007/s00223-021-00866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madry H., van Dijk C.N., Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010;18(4):419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 28.Loret B., Simões F.M.F. Effects of pH on transport properties of articular cartilages. Biomech. Model. Mechanobiol. 2010;9(1):45–63. doi: 10.1007/s10237-009-0158-1. [DOI] [PubMed] [Google Scholar]
- 29.Arbabi V., Pouran B., Weinans H., Zadpoor A.A. Transport of neutral solute across articular cartilage: the role of zonal diffusivities. J. Biomech. Eng. 2015;137(7) doi: 10.1115/1.4030070. [DOI] [PubMed] [Google Scholar]
- 30.Brown S.B., Wang L., Jungels R.R., Sharma B. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 2020;101:469–483. doi: 10.1016/j.actbio.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M., Hu W., Cai C., Wu Y., Li J., Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio. 2022;14 doi: 10.1016/j.mtbio.2022.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta S., He T., Bajpayee A.G. Recent advances in targeted drug delivery for treatment of osteoarthritis. Curr. Opin. Rheumatol. 2021;33(1):94–109. doi: 10.1097/BOR.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Q., Li X., Li Y., Wu Z., Xu C., Chen Z., He W. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm. Sin. B. 2021;11(4):941–960. doi: 10.1016/j.apsb.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue X., Hu Y., Wang S., Chen X., Jiang Y., Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022;12:327–339. doi: 10.1016/j.bioactmat.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin X.F., Wang L.L., Chu X.C. A novel chondroitin sulfate decorated nano platinum for the treatment of osteoarthritis. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017;78:452–456. doi: 10.1016/j.msec.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Liu H., Zhang Q., Wang S., Weng W., Jing Y., Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact. Mater. 2022;14:169–181. doi: 10.1016/j.bioactmat.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J., Yin Z., Wang X., Su J. Exosome-laden hydrogels: a novel cell-free strategy for in-situ bone tissue regeneration. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.866208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedingfield S.K., Colazo J.M., Yu F., Liu D.D., Jackson M.A., Himmel L.E., Cho H., Crofford L.J., Hasty K.A., Duvall C.L. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 2021;5(9):1069–1083. doi: 10.1038/s41551-021-00780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G., Lee W.J., Tan C.P., Lai O.M., Wang Y., Qiu C. Tailored rigidity of W/O Pickering emulsions using diacylglycerol-based surface-active solid lipid nanoparticles. Food Funct. 2021;12(23):11732–11746. doi: 10.1039/d1fo01883c. [DOI] [PubMed] [Google Scholar]
- 40.Jain A., Mishra S.K., Vuddanda P.R., Singh S.K., Singh R., Singh S. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine (Lond) 2014;10(5):1031–1040. doi: 10.1016/j.nano.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Sobal G., Velusamy K., Kosik S., Menzel J., Hacker M., Pagitz M. Preclinical evaluation of (99m)Tc labeled chondroitin sulfate for monitoring of cartilage degeneration in osteoarthritis. Nucl. Med. Biol. 2016;43(6):339–346. doi: 10.1016/j.nucmedbio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Safaei-Yaraziz A., Akbari-Birgani S., Nikfarjam N. Porous scaffolds with the structure of an interpenetrating polymer network made by gelatin methacrylated nanoparticle-stabilized high internal phase emulsion polymerization targeted for tissue engineering. RSC Adv. 2021;11(37):22544–22555. doi: 10.1039/d1ra03333f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebada H.M.K., Nasra M.M.A., Nassra R.A., Abdallah O.Y. Chondroitin sulfate-functionalized lipid nanoreservoirs: a novel cartilage-targeting approach for intra-articular delivery of cassic acid for osteoarthritis treatment. Drug Deliv. 2022;29(1):652–663. doi: 10.1080/10717544.2022.2041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z., Chen J., Wu L., Li W., Chen J., Cheng H., Pan J., Cai B. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int. J. Nanomed. 2013;8:3843–3853. doi: 10.2147/IJN.S50721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laroui H., Grossin L., Léonard M., Stoltz J., Gillet P., Netter P., Dellacherie E. Hyaluronate-covered nanoparticles for the therapeutic targeting of cartilage. Biomacromolecules. 2007;8(12):3879–3885. doi: 10.1021/bm700836y. [DOI] [PubMed] [Google Scholar]
- 46.Gao W., Zhang Y., Zhang Q., Zhang L. Nanoparticle-hydrogel: a hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016;44(6):2049–2061. doi: 10.1007/s10439-016-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narayanaswamy R., Torchilin V.P. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24(3) doi: 10.3390/molecules24030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira I.M., Fernandes D.C., Maia F.R., Canadas R.F., Reis R.L., Oliveira J.M. Bioengineered nanoparticles loaded-hydrogels to target TNF alpha in inflammatory diseases. Pharmaceutics. 2021;13(8) doi: 10.3390/pharmaceutics13081111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao S.C., Huang J.Y., Gao Y., Li Z.X., Wei Z.Y., Dawes H., Guo S.C. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021;6(12):4455–4469. doi: 10.1016/j.bioactmat.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin N., Tan X., Liu H., He F., Ding N., Gou J., Yin T., He H., Zhang Y., Tang X. A novel indomethacin/methotrexate/MMP-9 siRNA in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale. 2020;12(15):8546–8562. doi: 10.1039/d0nr00454e. [DOI] [PubMed] [Google Scholar]
- 51.Garcia J.P., Stein J., Cai Y., Riemers F., Wexselblatt E., Wengel J., Tryfonidou M., Yayon A., Howard K.A., Creemers L.B. Fibrin-hyaluronic acid hydrogel-based delivery of antisense oligonucleotides for ADAMTS5 inhibition in co-delivered and resident joint cells in osteoarthritis. J. Contr. Release. 2019;294:247–258. doi: 10.1016/j.jconrel.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 52.Lolli A., Sivasubramaniyan K., Vainieri M.L., Oieni J., Kops N., Yayon A., van Osch G. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J. Contr. Release. 2019;309:220–230. doi: 10.1016/j.jconrel.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 53.Chen P., Zhu S., Wang Y., Mu Q., Wu Y., Xia Q., Zhang X., Sun H., Tao J., Hu H., Lu P., Ouyang H. The amelioration of cartilage degeneration by ADAMTS-5 inhibitor delivered in a hyaluronic acid hydrogel. Biomaterials. 2014;35(9):2827–2836. doi: 10.1016/j.biomaterials.2013.12.076. [DOI] [PubMed] [Google Scholar]
- 54.Seror J., Zhu L., Goldberg R., Day A.J., Klein J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015;6:6497. doi: 10.1038/ncomms7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao W., Wang H., Han Y., Wang H., Sun Y., Zhang H. Dopamine/phosphorylcholine copolymer as an efficient joint lubricant and ROS scavenger for the treatment of osteoarthritis. ACS Appl. Mater. Interfaces. 2020;12(46):51236–51248. doi: 10.1021/acsami.0c14805. [DOI] [PubMed] [Google Scholar]
- 56.Yu M., Liu M., Zhang L., Li M., Hou Y., Wang D., Fu S. Liquid-repellent and self-repairing lubricant-grafted surfaces constructed by thiol-ene click chemistry using activated hollow silica as the lubricant reservoir. J. Colloid Interface Sci. 2021;586:279–291. doi: 10.1016/j.jcis.2020.10.091. [DOI] [PubMed] [Google Scholar]
- 57.Duan Y., Liu Y., Li J., Feng S., Wen S. AFM study on superlubricity between Ti6Al4V/polymer surfaces achieved with liposomes. Biomacromolecules. 2019;20(4):1522–1529. doi: 10.1021/acs.biomac.8b01683. [DOI] [PubMed] [Google Scholar]
- 58.Lei Y., Wang Y., Shen J., Cai Z., Zhao C., Chen H., Luo X., Hu N., Cui W., Huang W. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022;8(5):l6449. doi: 10.1126/sciadv.abl6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madry H., Gao L., Rey-Rico A., Venkatesan J.K., Muller-Brandt K., Cai X., Goebel L., Schmitt G., Speicher-Mentges S., Zurakowski D., Menger M.D., Laschke M.W., Cucchiarini M. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv. Mater. 2020;32(2) doi: 10.1002/adma.201906508. [DOI] [PubMed] [Google Scholar]
- 60.Xue X.L.H.W.S. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Compos. B Eng. 2022:237–109855. [Google Scholar]
- 61.Jiang S., Liu Y., Xu B., Zhang Y., Yang M. Noncoding RNAs: new regulatory code in chondrocyte apoptosis and autophagy. Wiley Interdiscip Rev RNA. 2020;11(4):e1584. doi: 10.1002/wrna.1584. [DOI] [PubMed] [Google Scholar]
- 62.Brown S., Kumar S., Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–257. doi: 10.1016/j.actbio.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopes E.B.P., Filiberti A., Husain S.A., Humphrey M.B. Immune contributions to osteoarthritis. Curr. Osteoporos. Rep. 2017;15(6):593–600. doi: 10.1007/s11914-017-0411-y. [DOI] [PubMed] [Google Scholar]
- 64.Deng Y., Lei G., Lin Z., Yang Y., Lin H., Tuan R.S. Engineering hyaline cartilage from mesenchymal stem cells with low hypertrophy potential via modulation of culture conditions and Wnt/beta-catenin pathway. Biomaterials. 2019;192:569–578. doi: 10.1016/j.biomaterials.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abraham R., Malkani A.L., Lewis J., Beck D. An anatomical study of tibial metaphyseal/diaphyseal mismatch during revision total knee arthroplasty. J. Arthroplasty. 2007;22(2):241–244. doi: 10.1016/j.arth.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 66.Wuelling M., Vortkamp A. The fountain of bone growth. Nature (London) 2019;567(7747):1–2. doi: 10.1038/d41586-019-00527-w. [DOI] [PubMed] [Google Scholar]
- 67.White J.R., Wilsman N.J., Leiferman E.M., Noonan K.J. Histomorphometric analysis of an adolescent distal tibial physis prior to growth plate closure. J. Children's Orthopaed. 2008;2(4):315–319. doi: 10.1007/s11832-008-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3(Supplement 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oftadeh R., Perez-Viloria M., Villa-Camacho J.C., Vaziri A., Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J. Biomech. Eng. 2015;137(1) doi: 10.1115/1.4029176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue S., Fujikawa K., Matsuki-Fukushima M., Nakamura M. Effect of ovariectomy induced osteoporosis on metaphysis and diaphysis repair process. Injury. 2021;52(6):1300–1309. doi: 10.1016/j.injury.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Arcos D., Vallet-Regi M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B. 2020;8(9):1781–1800. doi: 10.1039/c9tb02710f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rico-Llanos G.A., Borrego-Gonzalez S., Moncayo-Donoso M., Becerra J., Visser R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers (Basel) 2021;13(4) doi: 10.3390/polym13040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonseca H., Moreira-Gonçalves D., Coriolano H.A., Duarte J.A. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 74.Arnett T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008;138(2):415S–418S. doi: 10.1093/jn/138.2.415S. [DOI] [PubMed] [Google Scholar]
- 75.Wang L., Zhang H., Wang S., Chen X., Su J. Bone marrow adipocytes: a critical player in the bone marrow microenvironment. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.770705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black D.M., Rosen C.J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2016;374(3):254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 77.Marshall J.K., Rainsford K.D., James C., Hunt R.H. A randomized controlled trial to assess alendronate-associated injury of the upper gastrointestinal tract. Aliment. Pharmacol. Ther. 2000;14(11):1451–1457. doi: 10.1046/j.1365-2036.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 78.Ossipov D.A. Bisphosphonate-modified biomaterials for drug delivery and bone tissue engineering. Expet Opin. Drug Deliv. 2015;12(9):1443–1458. doi: 10.1517/17425247.2015.1021679. [DOI] [PubMed] [Google Scholar]
- 79.Zhao Z., Li G., Ruan H., Chen K., Cai Z., Lu G., Li R., Deng L., Cai M., Cui W. Capturing magnesium ions via microfluidic hydrogel microspheres for promoting cancellous bone regeneration. ACS Nano. 2021 doi: 10.1021/acsnano.1c02147. [DOI] [PubMed] [Google Scholar]
- 80.Farbod K., Diba M., Zinkevich T., Schmidt S., Harrington M.J., Kentgens A.P., Leeuwenburgh S.C. Gelatin nanoparticles with enhanced affinity for calcium phosphate. Macromol. Biosci. 2016;16(5):717–729. doi: 10.1002/mabi.201500414. [DOI] [PubMed] [Google Scholar]
- 81.Chen W., Zhou Z., Chen D., Li Y., Zhang Q., Su J. Bone regeneration using MMP-cleavable peptides-based hydrogels. Gels. 2021;7(4) doi: 10.3390/gels7040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1(12) doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Posadowska U., Parizek M., Filova E., Wlodarczyk-Biegun M., Kamperman M., Bacakova L., Pamula E. Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int. J. Pharm. 2015;485(1):31–40. doi: 10.1016/j.ijpharm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Yu P., Xie J., Chen Y., Liu J., Liu Y., Bi B., Luo J., Li S., Jiang X., Li J. A thermo-sensitive injectable hydroxypropyl chitin hydrogel for sustained salmon calcitonin release with enhanced osteogenesis and hypocalcemic effects. J. Mater. Chem. B. 2020;8(2):270–281. doi: 10.1039/c9tb02049g. [DOI] [PubMed] [Google Scholar]
- 85.Yu P., Liu Y., Jin R., Zhang P., Ding C., Jiang X., Xing J., Bi B., Xie J., Li J. Thermosensitive polysaccharide hydrogel as a versatile platform for prolonged salmon calcitonin release and calcium regulation. ACS Biomater. Sci. Eng. 2020;6(7):4077–4086. doi: 10.1021/acsbiomaterials.0c00591. [DOI] [PubMed] [Google Scholar]
- 86.Gacanin J., Kovtun A., Fischer S., Schwager V., Quambusch J., Kuan S.L., Liu W., Boldt F., Li C., Yang Z., Liu D., Wu Y., Weil T., Barth H., Ignatius A. Spatiotemporally controlled release of rho-inhibiting C3 toxin from a protein-DNA hybrid hydrogel for targeted inhibition of osteoclast formation and activity. Adv. Healthc. Mater. 2017;6(21) doi: 10.1002/adhm.201700392. [DOI] [PubMed] [Google Scholar]
- 87.Gilarska A., Hinz A., Bzowska M., Dyduch G., Kaminski K., Nowakowska M., Lewandowska-Lancucka J. Addressing the osteoporosis problem-multifunctional injectable hybrid materials for controlling local bone tissue remodeling. ACS Appl. Mater. Interfaces. 2021;13(42):49762–49779. doi: 10.1021/acsami.1c17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J., Yin Z., Huang B., Xu K., Su J. Stat3 signaling pathway: a future therapeutic target for bone-related diseases. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.897539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mei Z., Dong X., Qian Y., Hong D., Xie Z., Yao G., Qin A., Gao S., Hu J., Liang L., Zheng Y., Su J. Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li D., Zhou J., Zhang M., Ma Y., Yang Y., Han X., Wang X. Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomater. Sci. 2020;8(11):3138–3146. doi: 10.1039/d0bm00376j. [DOI] [PubMed] [Google Scholar]
- 91.Lee B., Iwaniec U.T., Turner R.T., Lin Y.W., Clarke B.L., Gingery A., Wei L.N. RIP140 in monocytes/macrophages regulates osteoclast differentiation and bone homeostasis. JCI Insight. 2017;2(7) doi: 10.1172/jci.insight.90517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng Y., Zhou M., Chen L., Fang H., Liu S., Zhou C., Sun J., Wang Z. Alendronate loaded graphene oxide functionalized collagen sponge for the dual effects of osteogenesis and anti-osteoclastogenesis in osteoporotic rats. Bioact. Mater. 2020;5(4):859–870. doi: 10.1016/j.bioactmat.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inoue S., Takito J., Nakamura M. Site-specific fracture healing: comparison between diaphysis and metaphysis in the mouse long bone. Int. J. Mol. Sci. 2021;22(17):9299. doi: 10.3390/ijms22179299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao Q., Wang L., Wang S., Huang B., Jing Y., Su J. Bone marrow mesenchymal stromal cells: identification, classification, and differentiation. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.787118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang B., Liu X. Osteon: structure, turnover, and regeneration. Tissue Eng. B Rev. 2022;28(2):261–278. doi: 10.1089/ten.teb.2020.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buck D.W., Dumanian G.A. Bone biology and physiology. Plast. Reconstr. Surg. 2012;129(6):1314–1320. doi: 10.1097/PRS.0b013e31824eca94. [DOI] [PubMed] [Google Scholar]
- 97.Chen J., Hendriks M., Chatzis A., Ramasamy S.K., Kusumbe A.P. Bone vasculature and bone marrow vascular niches in health and disease. J. Bone Miner. Res. 2020;35(11):2103–2120. doi: 10.1002/jbmr.4171. [DOI] [PubMed] [Google Scholar]
- 98.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y., Guan Z., Dai X., Shen Y., Wei Q., Ren L., Jiang J., Xiao Z., Jiang Y., Liu D., Huang Z., Xu X., Luo Y., Zhao C. Engineered macrophages as near-infrared light activated drug vectors for chemo-photodynamic therapy of primary and bone metastatic breast cancer. Nat. Commun. 2021;12(1):4310. doi: 10.1038/s41467-021-24564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang J., Liang J., Zhu Y., Hu M., Deng L., Cui W., Xu X. Fullerol-hydrogel microfluidic spheres for in situ redox regulation of stem cell fate and refractory bone healing. Bioact. Mater. 2021;6(12):4801–4815. doi: 10.1016/j.bioactmat.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuang Y., Cui W. Biomaterial-based delivery of nucleic acids for tissue regeneration. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113885. [DOI] [PubMed] [Google Scholar]
- 102.Witzler M., Büchner D., Shoushrah S.H., Babczyk P., Baranova J., Witzleben S., Tobiasch E., Schulze M. Polysaccharide-based systems for targeted stem cell differentiation and bone regeneration. Biomolecules. 2019;9(12) doi: 10.3390/biom9120840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davis H.E., Binder B.Y., Schaecher P., Yakoobinsky D.D., Bhat A., Leach J.K. Enhancing osteoconductivity of fibrin gels with apatite-coated polymer microspheres. Tissue Eng. A. 2013;19(15–16):1773–1782. doi: 10.1089/ten.tea.2012.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi B., Cui Z.K., Kim S., Fan J., Wu B.M., Lee M. Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J. Mater. Chem. B. 2015;3(31):6448–6455. doi: 10.1039/C5TB00843C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCaffrey J., McCrudden C.M., Ali A.A., Massey A.S., McBride J.W., McCrudden M.T., Vicente-Perez E.M., Coulter J.A., Robson T., Donnelly R.F., McCarthy H.O. Transcending epithelial and intracellular biological barriers; a prototype DNA delivery device. J. Contr. Release. 2016;226:238–247. doi: 10.1016/j.jconrel.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 106.Sathy B.N., Olvera D., Gonzalez-Fernandez T., Cunniffe G.M., Pentlavalli S., Chambers P., Jeon O., Alsberg E., McCarthy H.O., Dunne N., Haut Donahue T.L., Kelly D.J. RALA complexed α-TCP nanoparticle delivery to mesenchymal stem cells induces bone formation in tissue engineered constructs in vitro and in vivo. J. Mater. Chem. B. 2017;5(9):1753–1764. doi: 10.1039/c6tb02881k. [DOI] [PubMed] [Google Scholar]
- 107.Huang C.C., Kang M., Shirazi S., Lu Y., Cooper L.F., Gajendrareddy P., Ravindran S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021;126:199–210. doi: 10.1016/j.actbio.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xia W., Xie J., Cai Z., Liu X., Wen J., Cui Z.K., Zhao R., Zhou X., Chen J., Mao X., Gu Z., Zou Z., Zou Z., Zhang Y., Zhao M., Mac M., Song Q., Bai X. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 2021;12(1):6043. doi: 10.1038/s41467-021-26302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li D., Yang Z., Luo Y., Zhao X., Tian M., Kang P. Vol. 11. 2022. Delivery of MiR335-5p-pendant tetrahedron DNA nanostructures using an injectable heparin lithium hydrogel for challenging Bone Defects in Steroid-Associated Osteonecrosis. 1. [DOI] [PubMed] [Google Scholar]
- 110.Chen X., Tan B., Bao Z., Wang S., Tang R., Wang Z., Chen G., Chen S., Lu W.W., Yang D., Peng S. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121117. [DOI] [PubMed] [Google Scholar]
- 111.Posritong S., Flores Chavez R., Chu T.G., Bruzzaniti A. A Pyk2 inhibitor incorporated into a PEGDA-gelatin hydrogel promotes osteoblast activity and mineral deposition. Biomed. Mater. 2019;14(2) doi: 10.1088/1748-605X/aafffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamashita S., Katsumi H., Hibino N., Isobe Y., Yagi Y., Kusamori K., Sakane T., Yamamoto A. Development of PEGylated carboxylic acid-modified polyamidoamine dendrimers as bone-targeting carriers for the treatment of bone diseases. J. Contr. Release. 2017;262:10–17. doi: 10.1016/j.jconrel.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 113.Wang B., Liu J., Niu D., Wu N., Yun W., Wang W., Zhang K., Li G., Yan S., Xu G., Yin J. Mussel-inspired bisphosphonated injectable nanocomposite hydrogels with adhesive, self-healing, and osteogenic properties for bone regeneration. ACS Appl. Mater. Interfaces. 2021;13(28):32673–32689. doi: 10.1021/acsami.1c06058. [DOI] [PubMed] [Google Scholar]
- 114.Wang R., Che L., Feng Q., Cai K. Tough, flexible, and bioactive amphoteric copolymer-based hydrogel for bone regeneration without encapsulation of seed cells/simulating cues. ACS Appl. Mater. Interfaces. 2022;14(10):12038–12049. doi: 10.1021/acsami.1c23017. [DOI] [PubMed] [Google Scholar]
- 115.Datta S., Rameshbabu A.P., Bankoti K., Jana S., Roy S., Sen R., Dhara S. Microsphere embedded hydrogel construct - binary delivery of alendronate and BMP-2 for superior bone regeneration. J. Mater. Chem. B. 2021;9(34):6856–6869. doi: 10.1039/d1tb00255d. [DOI] [PubMed] [Google Scholar]
- 116.Lan Y., Xie H., Jin Q., Zhao X., Shi Y., Zhou Y., Hu Z., Ye Y., Huang X., Sun Y., Chen Z., Xie Z. Extracellular vesicles derived from neural EGFL-Like 1-modified mesenchymal stem cells improve acellular bone regeneration via the miR-25-5p-SMAD2 signaling axis. Bioact. Mater. 2022;17:457–470. doi: 10.1016/j.bioactmat.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zou Y., Huang B., Cao L., Deng Y., Su J. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering. Adv. Mater. 2021;33(2) doi: 10.1002/adma.202005215. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Z., Cui J., Wu S., Geng Z., Su J. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics. 2022;12(11):5103–5124. doi: 10.7150/thno.74548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 120.Chen S., Chen X., Geng Z., Su J. The horizon of bone organoid: a perspective on construction and application. Bioact. Mater. 2022;18:15–25. doi: 10.1016/j.bioactmat.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iordachescu A., Hughes E., Joseph S., Hill E.J., Grover L.M., Metcalfe A.D. Trabecular bone organoids: a micron-scale 'humanised' prototype designed to study the effects of microgravity and degeneration. NPJ Microgravity. 2021;7(1):17. doi: 10.1038/s41526-021-00146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iordachescu A., Williams R.L., Hulley P.A., Grover L.M. Organotypic culture of bone-like structures using composite ceramic-fibrin scaffolds. Curr. Protoc. Stem Cell Biol. 2019;48(1):e79. doi: 10.1002/cpsc.79. [DOI] [PubMed] [Google Scholar]
- 123.O'Connor S.K., Katz D.B., Oswald S.J., Groneck L., Guilak F. Formation of osteochondral organoids from murine induced pluripotent stem cells. Tissue Eng. A. 2021;27(15–16):1099–1109. doi: 10.1089/ten.tea.2020.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]