Abstract
Objective
Recent studies revealed the neuroprotective effects of hyperbaric oxygen (HBO) on spinal cord injury (SCI). Meanwhile, the use of methylprednisolone (MP) is one of the current protocols with limited effects in SCI patients. Accordingly, the aim of the present study was to investigate the effect of combined HBO and MP treatment on SCI.
Design
The present study was conducted on five groups of rats each as follows: Sham group (underwent laminectomy alone at T9 level vertebra); SCI group (underwent moderate contusive SCI); MP group (underwent SCI and received MP); HBO group (underwent SCI and received HBO); HBO + MP group (underwent SCI and simultaneously received MP and HBO). Blood serum and Spinal cord tissue samples were taken 48 h after SCI for analysis of serum ferric reducing antioxidant power (FRAP) and tissue malodialdehyde (MDA) levels as well as immunohistochemistry of caspase-3 and tumor necrosis factor-alpha (TNF-α). Neurological function was evaluated by the Basso–Beattie–Bresnehan (BBB) locomotion scores until the end of experiments. Additionally, histopathology was assessed at the end of the study.
Setting
Mazandaran University of Medical Sciences, Sari, Iran.
Results
Combination therapy with HBO and MP in the HBO + MP group significantly decreased MDA as well as increased FRAP levels compared to other treatment groups. Meanwhile, attenuated TNF-α and Caspase-3 expression could be significantly detected in the HBO + MP group. At the end of treatment, the neurological outcome was significantly improved and the extent of injured spinal tissue was also significantly reduced in the HBO + MP compared to other treatment groups.
Conclusion
The results suggest that combined therapy with MP and HBO has synergistic effects on SCI treatment.
Keywords: Methylprednisolone, Hyperbaric oxygen, Spinal cord injury, Inflammation, Apoptosis
Introduction
Traumatic spinal cord injury (SCI) is a debilitating neurological disease with a complex multifactorial process that is caused first by mechanical trauma and then by various secondary injury mechanisms which leads to changes in motor, sensory or autonomic function.1 The outcome of SCI is highly correlated with the extent of secondary damage mediated by a set of molecular and cellular events such as apoptosis, free radical-induced lipid peroxidation, inflammation, and exitotoxicity.2–4 Despite advances in care, patients suffering from severe SCI are less likely to actively contribute to the economy.5 In recent years, much attention has been focused on secondary injury to preserve or restore neurological function after injury, because it appears to be susceptible to therapeutic interventions that may include the use of anti-apoptotic, free radical scavenger, and anti-inflammatory agents.
To date, there are no effective and acceptable pharmacological interventions for SCI. The only current protocol against acute SCI is the use of high doses of methylprednisolone (MP), a synthetic corticosteroid with anti-inflammatory effects and inhibition of lipid peroxidation.6 Nevertheless, pooled evidence from multiple randomized controlled trials and observational studies does not demonstrate a significant long-term benefit for patients with acute traumatic spinal cord injuries and suggests that MP may be associated with increased gastrointestinal bleeding.7,8 On the other hand, because SCI involves a variety of molecular and biochemical events, it is unlikely that the use of a single drug can significantly reduce lesions and promote functional recovery. Therefore, it seems necessary to find new and or complementary protocols.
Hyperbaric oxygen (HBO) therapy, treatment providing 100% oxygen at a pressure greater than that at sea level, can be considered as one of these methods. In this regard, experimental and clinical trial studies documented that HBO have some neuroprotective effects against spinal cord injuries.9–11 There is accumulating evidence that attributed the beneficial effects of HBO after SCI to a variety of biological activities such as anti-inflammatory,12,13 anti-apoptotic,14,15 and anti-oxidative,16,17 as well as enhancement of neurotrophic factors and autophagy along with the acceleration of cell repair rate.18–20 Also, in vitro studies have shown that HBO can protect cultured spinal cord neurons against oxidative stress.21,22
Although, the neuroprotective effects of HBO or MP therapy alone are considered, these methods do not provide the desired motor improvement. Accordingly, this study has been conducted on the synergistic neuroprotective effects of combination of HBO and MP against contusive spinal cord injury in rat.
Materials and methods
Animals
Female adult Wistar rats were used (200–225 g) (Laboratory Animal Research Center, Sari, Iran) in this study. They were kept in the laboratory under constant conditions of temperature (23 ± 1 °C) and light/dark cycle (12 h/12 h) for at least 7 days before and over the course of the experiment. All procedures were performed according to the guidelines of the university’s animal care codes (IR.MAZUMS..REC.1399.7641) to minimize the animal’s suffering.
Induction of SCI and experimental design
The standard New York University (NYU) impactor device was used to induce a spinal cord contusion injury model as described previously.23 Briefly, the rats were anesthetized intraperitoneal (IP) using ketamine (90 mg/kg, Bremer Pharma GmbH) and xylazine (10 mg/kg, Alfasan) before the surgical procedure. After removing the T9 lamina; the dorsal surface of the spinal cord at the midline of T9 was then subjected to weight drop impact using a long 10 g metal rod with a 2 mm diameter from a height of 25 mm in order to produce moderate contusive SCI. After the surgery, the wound was closed by suturing the muscles and skin and then the recovery of the animals was assisted by administering lactated Ringer’s solution (IPPC, Tehran) (12–25 ml) subcutaneously immediately after surgery and cefazolin (Jaber Ibn Hayan, Tehran) (50 μg/kg) which was administered twice a day for 3 days.24 In order to maintain bladder function, they were drained manually three times a day. HBO therapy was performed in the oxygen chamber, the pressure was gradually raised to and maintained at 2 atmosphere absolute (ATA) and then allowed to breathe 100% oxygen for 60 min per day.13,16
The animals were randomly allocated in five groups, each containing 15 rats: (1) Sham group, which underwent laminectomy alone at T9 level vertebra; (2) SCI group, which underwent laminectomy followed by SCI and received saline; (3) MP group, which received a single dose of 30 mg/kg MP (Caspian Tamin pharmaceutical company, Tehran) via the lateral tail vein immediately post-SCI and followed by 10 mg/kg/day MP via the lateral tail vein until the end of experiments according to the protocol of Saker et al.;25 (4) HBO group, which allowed to breath HBO (60 min/d) at 6 h after SCI and then daily until the end of experiments;16 (5) HBO plus MP group, which simultaneously received MP and HBO similar to groups 3 and 4.
Each group of animals was divided into three subgroups: (A) treatment performed up to 48 h after SCI (a single dose of 30 mg/kg MP immediately after SCI and followed by 10 mg/kg/day for 2 days; a 60 min HBO session 6 h after SCI and then a 60 min HBO session daily for 2 days) and finally the rats (n = 5) were euthanized for biochemical analysis of the total antioxidant capacity of plasma and lipid peroxidation of spinal tissue (n = 5), (B) treatment performed up to 48 h after SCI (a single dose of 30 mg/kg MP immediately after SCI and followed by 10 mg/kg/day for 2 days; a 60 min HBO session 6 h after SCI and then a 60 min HBO session daily for 2 days) and finally the rats (n = 5) were euthanized for immunohistochemistry of TNF-α and Caspase-3 (n = 5), and (C) treatment performed along with neurological examination up to 14 days after SCI and finally the rats (n = 5) were euthanized for histopathological assessment.
Studies showed that 24–48 h after SCI in rat are the appropriate time to investigate the factors involved in the secondary stage of SCI, including lipid peroxidation, apoptosis, and inflammation.26,27 While, neurological evaluation requires a longer time. Accordingly, in the present study, the samples for biochemical and immunohistochemical studies obtained 48 h after SCI. Also, neurological assessment performed up to 14 days after SCI and then spinal tissue sample obtained for histopathological assessment. All the histological and neurological studies were performed in a blinded fashion.
Biochemistry
Forty-eight hours after SCI induction, the rats of subgroup A were euthanized with an injection of sodium pentobarbital. Blood samples were obtained immediately by cardiac puncture; the serum was separated by centrifuge and finally stored at −20°C for total antioxidant capacity (TAC) assay. Also, traumatized spinal cord in T9 vertebra removed from vertebral column and frozen at −80°C for lipid peroxidation assay. Total antioxidant capacity of plasma was evaluated using ferric reducing antioxidant power (FRAP) method.28 This method is based on the reduction of colorless ferric complex (Fe3+ tripyridyltriazine) to blue-colored ferrous complex (Fe2+ tripyridyltriazine) by the action of electron-donating antioxidants at low pH. The reduction was monitored by measuring the change of absorbance at 593 nm. FRAP is expressed as umol/L Fe2+.
Malondialdehyde (MDA) as a toxic aldehydic end product of lipid peroxidation was measured in the spinal cord tissue according to Ohkawa et al.29 The meninges were removed using a scalpel and then the blood was cleaned with isotonic saline. The samples were homogenized in ice-cold KcL solution (1.15%) for 2 min and then centrifuged at 3500×g (4C) for 15 min. The tissue MDA levels were determined on the reaction of MDA with thiobarbituric acid (TBA) at 95 °C which produces a pink color with an absorption maximum of 532 nm. The results were expressed as nmol/mg-protein.
Immunohistochemistry
Forty-eight hours after SCI induction, the rats of subgroup B were euthanized with an injection of sodium pentobarbital and then traumatized spinal cord in T9 vertebra removed from vertebral column. The obtained spinal cord samples were fixed in neutral buffered formalin solution (10% v/v), embedded in paraffin wax, and finally serial transverse sections (5 micrometer thickness) prepared by a conventional rotary microtome. Some sections of the lesion epicenter were primarily incubated with anti-caspase-3 rabbit polyclonal antibody (1:100 in PBS, v/v, Abcam) or with anti-TNF-α rabbit polyclonal antibody (1:100 in PBS, v/v, Elabscience) overnight at 4°C, secondarily incubated with mouse and rabbit specific HRP/DAB (ABC) detection IHC kit (Abcam), and finally counterstained through hematoxylin. Ten sections of the lesion epicenter (the distance between the samples was 48 μm) were selected and then densitometry analysis was performed on the immunohistochemical photographs as a percentage of total tissue area using ImageJ software (MacBiophotonics ImageJ 1.41a).
Neurological function
The Basso–Beattie–Bresnehan (BBB) test was used to evaluate hind limb motor function in subgroup C,30 which was carried out before SCI and then 6 h, 1, 3, 7, and 14 days after SCI.31 The rats were placed for 4 min in an open-field box and two independent examiners studied their locomotor ability. During open-field testing, rats were encouraged to locomote continuously. All hindlimb movements were recorded, except for those that were part of a reflex or elicited by contact with an examiner or another animal. The maximum possible BBB score (normal) was 21 and a score of 0 was used to indicate complete hind limb paralysis after SCI.
Histopathology
At the end of the experiment (14 days after SCI), the rats of subgroup C were euthanized by IP sodium pentobarbital injection and then traumatized spinal cord in T9 vertebra removed from vertebral column. The obtained spinal cord samples were fixed in neutral buffered formalin solution (10% v/v), embedded in paraffin wax, and finally serial transverse sections (5 micrometer thickness) prepared by a conventional rotary microtome. Ten sections of the lesion epicenter (the distance between the selected samples was 48 μm) were selected and stained through hematoxylin and eosin (H&E). The extent of the lesion area was measured as a percentage of total tissue area using ImageJ software (MacBiophotonics ImageJ 1.41a).
Statistical analysis
GraphPad Prism 6.01 (GraphPad Software, San Diego, CA, USA) program was used for statistical analyses and graphic drawings. Results were presented as mean values (±SD) whereby a significance level of <0.05 was considered significant. Normality of the data evaluated with Kolmogorov–Smirnov test. Also, one-way or two-way analysis of variance was also used to compare data among the groups.
Results
Biochemical analysis
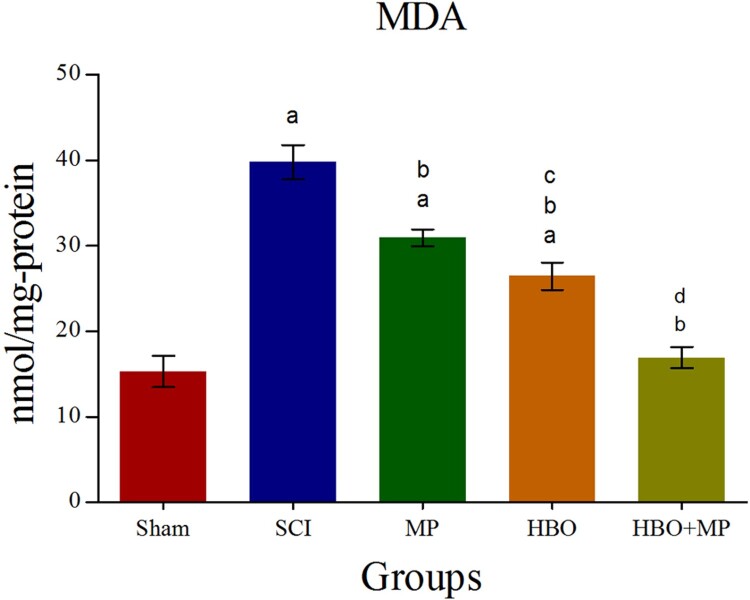
Biochemical analysis of the tissue MDA levels for all groups is shown in Figure 1. Trauma in the SCI group produced a significant elevation (P < 0.001) in MDA level compared to the sham group. The MDA levels in the MP (P < 0.001), HBO (P < 0.001), and HBO + MP (P < 0.001) groups were significantly lower than that those in the SCI group. Combined therapy with HBO and MP in the HBO + MP group significantly decreased MDA levels compared to the MP (P < 0.001) and HBO (P < 0.001) groups. Meanwhile, the differences between HBO and MP groups were significant (P < 0.01). The difference between groups HBO + MP and sham was not significant (P > 0.05).
Figure 1.
Effects of HBO and MP on lipid peroxidation. Histogram shows the levels of MDA in the traumatized spinal tissue 48 h after SCI. Values are expressed as nanomole per milligram of protein (nmol/mg-protein). aP < 0.001 versus sham group; bP < 0.001 versus SCI group; cP < 0.01 versus MP group; dP < 0.001 versus MP and HBO groups.
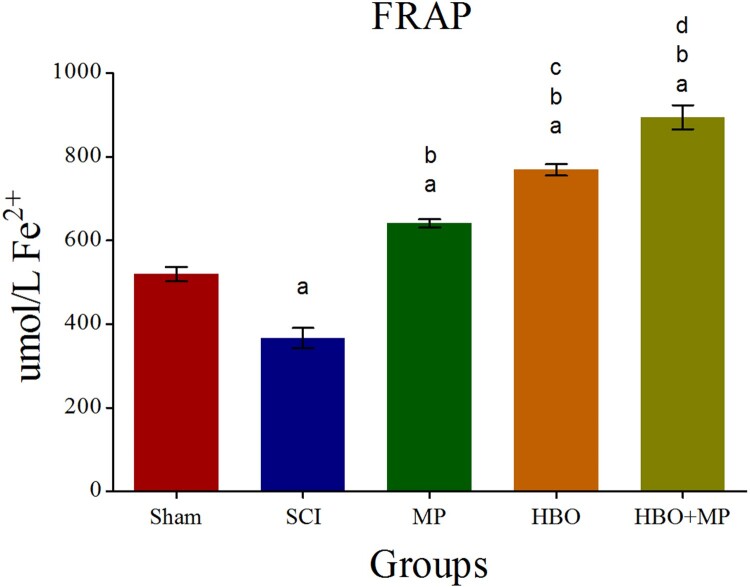
Biochemical analysis of the serum FRAP levels for all groups is shown in Figure 2. Trauma in the SCI group significantly (P < 0.001) decreased FRAP level compared to the sham group. The FRAP levels in the MP (P < 0.001), HBO (P < 0.001), and HBO + MP (P < 0.001) groups were significantly upper than that those in the SCI group. Combined therapy with HBO and MP in the HBO + MP group significantly increased FRAP levels compared to the MP (P < 0.001) and HBO (P < 0.001) groups. Meanwhile, the differences between HBO and MP groups were significant (P < 0.001). The difference between groups HBO + MP and sham was significant (P < 0.001).
Figure 2.
Effects of HBO and MP on total antioxidant capacity. Histogram shows the serum levels of FRAP 48 h after SCI. Values are expressed as unit mole per liter Fe2+ (umol/LFe2+). aP < 0.001 versus sham group; bP < 0.001 versus SCI group; cP < 0.001 versus MP group; dP < 0.001 versus MP and HBO groups.
Immunohistochemical evaluation
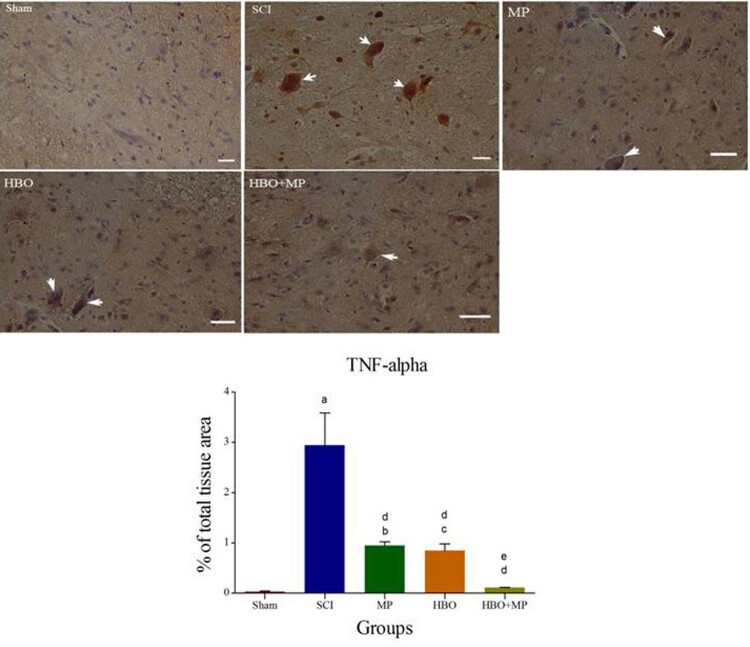
Immunohistochemical staining of TNF-α and its quantitative analysis are shown in Figure 3. Trauma in the SCI group significantly (P < 0.001) increased the expression of TNF-α in spinal cord compared to the sham group. The levels of TNF-α expression in the MP (P < 0.001), HBO (P < 0.001), and HBO + MP (P < 0.001) groups were significantly lower than that those in the SCI group. Combined therapy with HBO and MP in the HBO + MP group significantly reduced the degree of positive staining for TNF-α compared to the MP (P < 0.05) and HBO (P < 0.05) groups. Meanwhile, the differences between HBO and MP groups were not significant (P > 0.05). The difference between groups HBO + MP and sham was not significant (P > 0.05).
Figure 3.
Light Photomicrographs show immunohistochemical expression of TNF-α in the horizontal sections of traumatized spinal tissue. The positive staining of TNF-α is presented by a brown color of cytoplasm (arrows) (Scale bar = 100 µm). Densitometry analysis was performed for immunohistochemical photomicrographs of TNF-α. Data are expressed as a percentage of total tissue area. aP < 0.001 versus sham group; bP < 0.01 versus sham group; cP < 0.05 versus sham group; dP < 0.001 versus SCI group; eP < 0.05 versus MP and HBO groups.
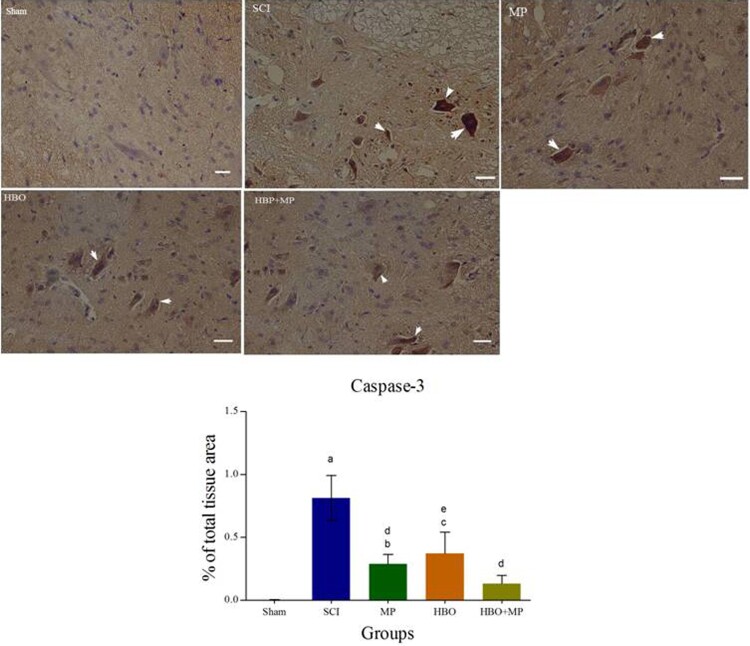
Immunohistochemical staining of caspase-3 and its quantitative analysis are shown in Figure 4. Trauma in the SCI group significantly (P < 0.001) increased the expression of caspase-3 in spinal cord compared to the sham group. The levels of caspase-3 expression in the MP (P < 0.001), HBO (P < 0.01), and HBO + MP (P < 0.001) groups were significantly lower than that those in the SCI group. Combined therapy with HBO and MP in the HBO + MP group did not significantly reduce the degree of positive staining for caspase-3 compared to the MP (P > 0.05) and HBO (P > 0.05) groups. Also, the differences between HBO and MP groups were not significant (P > 0.05). The difference between groups HBO + MP and sham was not significant (P > 0.05).
Figure 4.
Light Photomicrographs show immunohistochemical expression of caspase-3 in the horizontal sections of traumatized spinal tissue. The positive staining of caspase-3 is presented by a brown color of cytoplasm (arrows) (Scale bar = 100 µm). Densitometry analysis was performed for immunohistochemical photomicrographs of caspase-3. Data are expressed as a percentage of total tissue area. aP < 0.001 versus sham group; bP < 0.05 versus sham group; cP < 0.01 versus sham group; dP < 0.001 versus SCI group; eP < 0.01 versus SCI group.
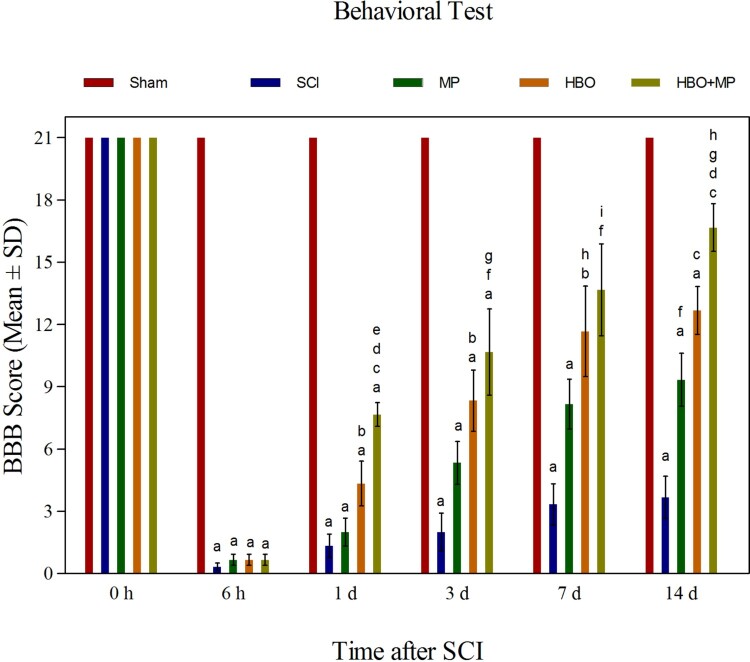
Neurological outcome
The BBB locomotor rating scores in the five groups have been presented as mean value ± SD (Figure 5). All rats of the sham group had a normal score (21). Trauma in the SCI group significantly (P < 0.001) decreased the BBB locomotor rating scores compared to the sham group. At the end of the study (14th day), the BBB locomotor rating scores in the MP (P < 0.01), HBO (P < 0.001), and HBO + MP (P < 0.001) groups were significantly upper than that those in the SCI group. Combined therapy with HBO and MP in the HBO + MP group significantly increased the BBB locomotor rating scores compared to the MP (P < 0.001) and HBO (P < 0.05) groups. Meanwhile, the differences between HBO and MP groups were not significant (P > 0.05). The difference between groups HBO + MP and sham was significant (P < 0.01).
Figure 5.
Effects of HBO and MP on neurologic function. Histogram shows the neurologic outcome before SCI and then 6 h, 1, 3 days, 7, and 14 days after SCI in all groups as a BBB score. Values are mean ± SD. aP < 0.001 versus sham group; bP < 0.05 versus SCI group; cP < 0.001 versus SCI group; dP < 0.001 versus MP group; eP < 0.05 versus HBO group; fP < 0.01 versus SCI group; gP < 0.05 versus MP group; hP < 0.01 versus sham group; iP < 0.05 versus sham group.
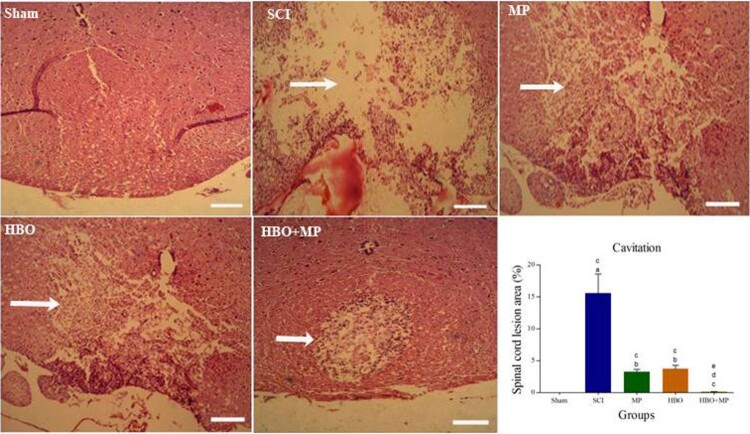
Histopathologic assessment
Results of the histopathological assessment are shown in Figure 6. Spinal cord section obtained from the sham group was normal. Characteristic necrosis, complete loss of neurons, and extensive vacuolation in the epicenter of injured spinal cord are detectable in the SCI group. The sections of injured spinal cord obtained from the MP, HBO, and HBO + MP groups retained more myelin tissue and neurons in gray matter than those in the SCI group. Calculation of the area of lesion as a percentage of the whole spinal cord area presented in the histogram. The spinal cord lesion epicenter of the MP (P < 0.001), HBO (P < 0.001), and HBO + MP (P < 0.001) groups had smaller cavities than those of the SCI group. Combined therapy with HBO and MP in the HBO + MP group significantly decreased the size of the cavities compared to the MP (P < 0.05) and HBO (P < 0.01) groups. Meanwhile, the differences between HBO and MP groups were not significant (P > 0.05). The difference between groups HBO + MP and sham was not significant (P > 0.05).
Figure 6.
Effects of HBO and MP on tissue protection. Photomicrographs taken from the lesion epicenter indicate the destruction of the spinal tissue (arrows) 14 days after SCI in all groups (stained with H&E; magnification, ×40). Histogram shows the mean lesion area as a percentage of the whole spinal cord. Values are mean ± SD. aP < 0.001 versus sham group; bP < 0.01 versus sham group; cP < 0.001 versus SCI group; dP < 0.05 versus HBO + MP group; eP < 0.01 versus HBO + MP group.
Discussion
The main findings of the present study showed that combination therapy with HBO and MP attenuates SCI-induced lipid peroxidation, apoptosis, inflammation, tissue loss, and motor dysfunction as well as increasing the total antixoxidant capacity. Secondary autodestructive process of SCI is a highly debilitating pathology that has irreversible impacts and results in functional loss.2–4 Each treatment that interrupts the secondary processes can improve SCI. Free radical-induced lipid peroxidation is an important pathologic event in post-traumatic neurodegeneration that peaks immediately after SCI.32 High pressure oxygen, as a nondrug and noninvasive therapy, increases the amount of dissolved oxygen in the plasma as well as saturated hemoglobin with oxygen, leading to greater oxygen availability to the organs.33,34 Accumulating evidence indicates that in addition to improving of oxygen supply and neural metabolism, there is an association between the beneficial effects of HBO therapy and a variety of biological properties, including anti-oxidative, anti-inflammatory, and anti-apoptotic effects.35–39 In this regard, a comparative study on the effects of MP and HBO showed that HBO, but not MP, diminished oxidative stress indicators such as thiobarbituric acid reactive substances (TBARS) and glutathione peroxidase (GSH-Px) after SCI in rat.40 Another study suggested that increasing oxygen-free radical scavenging and reducing lipid oxidation are among the mechanisms of the protective effects of HBO following SCI in rat.41 According to the results of our present study, HBO and MP alone could reduce the secondary damage of SCI through decreasing free radical-induced lipid peroxidation and increasing total antioxidant capacity. Although, the results showed that antioxidant properties of HBO significantly better than MP. On the other hand, the simultaneous use of HBO and MP significantly improved these properties. In fact, they have synergistic effects in inhibiting free radicals.
The inflammatory reaction is one of the major causes of the secondary autodestructive process of SCI. In this regard, Yang et al.42 documented that HBO intervention reduced secondary SCI via nuclear factor-κB (NF-κB) and high-mobility group protein B1 (HMGB1) downregulation in rats with acute SCI. Also, it was found that HBO via Toll-like receptor (TLR)2/ NF-кB signaling induced protective effects against rat SCI.43 Meanwhile, HBO significantly attenuated SCI-induced tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β overproduction, which in turn significantly increased the number of both vascular endothelial growth factor (VEGF)-positive cells and glial cell line-derived neurotrophic factor-positive cells.19 According to the results of our present study, HBO and MP alone could reduce the secondary damage of SCI through decreasing TNF-α as an inflammatory cytokine produced by macrophages/monocytes during acute inflammation. However, there was no significant difference between HBO and MP groups. On the other hand, the simultaneous use of HBO and MP significantly improved this property. In fact, they have synergistic effects in reducing inflammation. Of course, there are other studies on the anti-inflammatory effects of HBO following SCI, suggesting that other factors may play a role. Kang et al.44 documented that HBO intervention by regulation of NF-κB, TLR4, and HMGB1 signaling pathways reduces inflammatory process after SCI in rats. Hyperbaric oxygen improves functional recovery through inhibiting inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), glial fibrillary acidic protein (GFAP), and neuron-glial antigen 2; meanwhile, this process may be due to inhibiting Akt and NF-κB pathways.12 Results of another study suggested that HBO modified the inflammatory environment through shifting M1 macrophage phenotype to M2 macrophage phenotype.45
Apoptosis is an important mediator of secondary damage after SCI. In this regard, Liang et al.46 demonstrated that HBO compromised NALP3-inflammasome and caspase-1 expression in the SCI model rats. HBO has a protective effect on SCI by reducing neuronal cell apoptosis and matrix metalloproteinase (MMP)-9/2 gene expression in rats, thus improving motor function scores and increasing myelin nerve fibers.47 Studies emphasize the key role of endoplasmic reticulum stress in the induction of neuronal apoptosis following SCI. It was documented that HBO by inhibiting endoplasmic reticulum stress-induced apoptosis alleviated secondary SCI and thereby improved the neurological function.16 Also, it was documented that HBO through iNOS mRNA-iNOS-nitric oxide signaling pathway can promote the neuroprotection following SCI.48 Liu et al.31 found that HBO reduces SCI-induced caspase-3 activation and promotes the recovery of neurological function by inhibiting the endoplasmic reticulum stress (ERS) response. Recently, it was found that HBO improves neurological disorders after SCI by amelioration of apoptosis and suppressing dendritic/synaptic degeneration via upregulating the BDNF/TrkB signaling pathways in the anterior horn of spinal cord.14 Our immunohistochemical results showed that HBO and MP alone could reduce the secondary damage of SCI through decreasing Caspase-3, which plays a critical role in apoptosis. However, there was no significant difference between HBO and MP groups. It should be noted that the simultaneous use of HBO and MP does not significantly improve the anti-apoptotic property of these compounds. In fact, they have no synergistic effect in reducing apoptosis.
Regulation of autophagy improves neural function after SCI. In this regard, it was documented that enhancement of autophagy expression and acceleration of cell repair rate after SCI may be another mechanism of action of HBO.20 Yang et al.49 also found that HBO stabilizes the blood–spinal cord barrier via downregulation of MMP-2 and -9, and upregulation of VEGF.
Conclusion
Overall, the biochemical, histopathological and functional findings of the present study indicate the synergistic neuroprotective effects of hyperbaric oxygen and methylprednisolone on spinal cord injury. This synergy can provide the basis for further improvement of the disabilities caused by SCI, as well as compensate the limitations of the effectiveness of methylprednisolone as a current protocol in SCI patients.
Funding Statement
This work was supported by Mazandaran University of Medical Sciences [grant number 7641].
Disclaimer statements
Contributors None.
Conflicts of interest The authors declare no conflict of interest.
References
- 1.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S.. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 2019;10:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Ashwell KW, Waite P.. Advances in secondary spinal cord injury: role of apoptosis. Spine 2000;25(14):1859–66. [DOI] [PubMed] [Google Scholar]
- 3.Jorge A, Taylor T, Agarwal N, Hamilton DK.. Current agents and related therapeutic targets for inflammation following acute traumatic spinal cord injury. World Neurosurg 2019;132:138–47. [DOI] [PubMed] [Google Scholar]
- 4.Hall ED, Wang JA, Bosken JM, Singh IN.. Lipid peroxidation in brain or spinal cord mitochondria after injury. J Bioenerg Biomembr 2016;48(2):169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lidal IB, Huynh TK, Biering-Sørensen F.. Return to work following spinal cord injury: a review. Disabil Rehabil 2007;29(17):1341–75. [DOI] [PubMed] [Google Scholar]
- 6.Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2015;76(Suppl 1):S71–83. [DOI] [PubMed] [Google Scholar]
- 7.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med 1990;322(20):1405–11. [DOI] [PubMed] [Google Scholar]
- 8.Evaniew N, Belley-Côté EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF.. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a systematic review and meta-analysis. J Neurotrauma 2016;33(5):468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel NP, Huang JH.. Hyperbaric oxygen therapy of spinal cord injury. Med Gas Res 2017;7(2):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Zhao L, Li P, Liu X, Liang F, Jiang Y, et al. Effect of hyperbaric oxygen therapy on HMGB1/NF-κB expression and prognosis of acute spinal cord injury: a randomized clinical trial. Neurosci Lett 2019;692:47–52. [DOI] [PubMed] [Google Scholar]
- 11.Glover M, Smerdon GR, Andreyev HJ, Benton BE, Bothma P, Firth O, et al. Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol 2016;17(2):224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Dong Q, Pan Z, Song Y, Su P, Niu Y, et al. Hyperbaric oxygen improves functional recovery of the injured spinal cord by inhibiting inflammation and glial scar formation. Am J Phys Med Rehabil 2019;98(10):914–20. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Li C, Gao C, Li Z, Yang J, Liu X, et al. Effects of hyperbaric oxygen therapy on RAGE and MCP-1 expression in rats with spinal cord injury. Mol Med Rep 2016;14(6):5619–25. [DOI] [PubMed] [Google Scholar]
- 14.Ying X, Tu W, Li S, Wu Q, Chen X, Zhou Y, et al. Hyperbaric oxygen therapy reduces apoptosis and dendritic/synaptic degeneration via the BDNF/TrkB signaling pathways in SCI rats. Life Sci 2019;229:187–99. [DOI] [PubMed] [Google Scholar]
- 15.Pan JY, Cai RX, Chen Y, Li Y, Lin WW, Wu J, et al. Analysis the effect of hyperbaric oxygen preconditioning on neuronal apoptosis, Ca2+ concentration and caspases expression after spinal cord injury in rats. Eur Rev Med Pharmacol Sci 2018;22(11):3467–73. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Yang J, Li Z, Liang F, Wang Y, Su Q, et al. Hyperbaric oxygen treatment protects against spinal cord injury by inhibiting endoplasmic reticulum stress in rats. Spine 2015;40(24):E1276–83. [DOI] [PubMed] [Google Scholar]
- 17.Topuz K, Colak A, Cemil B, Kutlay M, Demircan MN, Simsek H, et al. Combined hyperbaric oxygen and hypothermia treatment on oxidative stress parameters after spinal cord injury: an experimental study. Arch Med Res 2010;41(7):506–12. [DOI] [PubMed] [Google Scholar]
- 18.Meng XL, Hai Y, Zhang XN, Wang YS, Liu XH, Ma LL, et al. Hyperbaric oxygen improves functional recovery of rats after spinal cord injury via activating stromal cell-derived factor-1/CXC chemokine receptor 4 axis and promoting brain-derived neurothrophic factor expression. Chin Med J (Engl) 2019;132(6):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai PA, Chang CK, Niu KC, Lin MT, Chiu WT, Lin CM.. Attenuating experimental spinal cord injury by hyperbaric oxygen: stimulating production of vasculoendothelial and glial cell line-derived neurotrophic growth factors and interleukin-10. J Neurotrauma 2010;27(6):1121–7. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Liu D, Su P, Lin F, Tang Q.. Changes in autophagy in rats after spinal cord injury and the effect of hyperbaric oxygen on autophagy. Neurosci Lett 2016;618:139–45. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Li J, Zhang L, Wang B, Xiong L.. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci 2007;80(12):1087–93. [DOI] [PubMed] [Google Scholar]
- 22.Huang G, Xu J, Xu L, Wang S, Li R, Liu K, et al. Hyperbaric oxygen preconditioning induces tolerance against oxidative injury and oxygen-glucose deprivation by up-regulating heat shock protein 32 in rat spinal neurons. PLoS One 2014;9(1):e85967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basso DM, Beattie MS, Bresnahan JC.. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996;139(2):244–56. [DOI] [PubMed] [Google Scholar]
- 24.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 2000;11(13):3001–5. [DOI] [PubMed] [Google Scholar]
- 25.Şaker D1, L1 S, Yılmaz DM2, S1 P.. Relationships between microRNA-20a and microRNA-125b expression and apoptosis and inflammation in experimental spinal cord injury. Neurol Res 2019;41(11):991–1000. [DOI] [PubMed] [Google Scholar]
- 26.Christie SD, Comeau B, Myers T, Sadi D, Purdy M, Mendez I.. Duration of lipid peroxidation after acute spinal cord injury in rats and the effect of methylprednisolone. Neurosurg Focus 2008;25(5):E5. [DOI] [PubMed] [Google Scholar]
- 27.Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, et al. Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 2000;166(2):213–26. [DOI] [PubMed] [Google Scholar]
- 28.Benzie IF, Strain JJ.. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–6. [DOI] [PubMed] [Google Scholar]
- 29.Ohkawa H, Ohishi N, Yagi K.. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351–8. [DOI] [PubMed] [Google Scholar]
- 30.Basso DM, Beattie MS, Bresnahan JC.. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12:1–21. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Li C, Liang F, Wang Y, Li Z, Yang J.. Effects of hyperbaric oxygen on glucose-regulated protein 78 and c-Jun N-terminal kinase expression after spinal cord injury in rats. Int J Clin Exp Med 2015;8(3):3309–17. [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR.. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 2004;4:451–64. [DOI] [PubMed] [Google Scholar]
- 33.Thom SR. Hyperbaric oxygen-its mechanisms and efficacy. Plast Reconstr Surg 2011;127:131S–141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalski D, Hartig W, Schneider D, Hobohm C.. Use of normobaric and hyperbaric oxygen in acute focal cerebral ischemia-a preclinical and clinical review. Acta Neurol Scand 2011;123:85–97. [DOI] [PubMed] [Google Scholar]
- 35.Yang ZY, Xie Y, Bosco GM, Chen C, Camporesi EM.. Hyperbaric oxygenation alleviates MCAO-induced brain injury and reduces hydroxyl radical formation and glutamate release. Eur J Appl Physiol 2010;108:513–22. [DOI] [PubMed] [Google Scholar]
- 36.Lin KC, Niu KC, Tsai KJ, Kuo JR, Wang LC, Chio CC, et al. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg 2012;72:650–9. [DOI] [PubMed] [Google Scholar]
- 37.Wee HY, Lim SW, Chio CC, Niu KC, Wang CC, Kuo JR.. Hyperbaric oxygen effects on neuronal apoptosis associations in a traumatic brain injury rat model. J Surg Res 2015;197:382–9. [DOI] [PubMed] [Google Scholar]
- 38.Sunami K, Takeda Y, Hashimoto M, Hirakawa M.. Hyperbaric oxygen reduces infarct volume in rats by increasing oxygen supply to the ischemic periphery. Crit Care Med 2000;28:2831–6. [DOI] [PubMed] [Google Scholar]
- 39.Calvert JW, Cahill J, Zhang JH.. Hyperbaric oxygen and cerebral physiology. Neurol Res 2007;29:132–41. [DOI] [PubMed] [Google Scholar]
- 40.Kahraman S, Düz B, Kayali H, Korkmaz A, Oter S, Aydin A, et al. Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: a comparative study in rats. Neurochem Res 2007;32:1547–51. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Liu D, Wang Q, Su P, Tang Q.. Hyperbaric oxygen treatment of spinal cord injury in rat model. BMC Neurol 2017;17:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Liu X, Zhou Y, Wang G, Gao C, Su Q.. Hyperbaric oxygen alleviates experimental (spinal cord) injury by downregulating HMGB1/NF- κ B expression. Spine 2013;38(26):E1641–8. [DOI] [PubMed] [Google Scholar]
- 43.Tan J, Zhang F, Liang F, Wang Y, Li Z, Yang J, et al. Protective effects of hyperbaric oxygen treatment against spinal cord injury in rats via toll-like receptor 2/nuclear factor-κB signaling. Int J Clin Exp Pathol 2014;7:1911–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Kang N, Hai Y, Yang J, Liang F, Gao CJ.. Hyperbaric oxygen intervention reduces secondary spinal cord injury in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int J Clin Exp Pathol 2015;8(2):1141–53. [PMC free article] [PubMed] [Google Scholar]
- 45.Geng CK, Cao HH, Ying X, Zhang HD, Yu HJ.. The effects of hyperbaric oxygen on macrophage polarization after rat spinal cord injury. Brain Res 2015;1606:68–76. [DOI] [PubMed] [Google Scholar]
- 46.Liang F, Li C, Gao C, Li Z, Yang J, Liu X, et al. Effects of hyperbaric oxygen therapy on NACHT domain-leucine-rich-repeat- and pyrin domain-containing protein 3 inflammasome expression in rats following spinal cord injury. Mol Med Rep 2015;11:4650–6. [DOI] [PubMed] [Google Scholar]
- 47.Hou YN, Ding WY, Shen Y, Yang DL, Wang LF, Zhang P.. Effect of hyperbaric oxygen on MMP9/2 expression and motor function in rats with spinal cord injury. Int J Clin Exp Med 2015;8:14926–34. [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H, Xue L, Zhang X, Weng Q, Chen H, Gu J, et al. Hyperbaric oxygen therapy provides neuroprotection following spinal cord injury in a rat model. Int J Clin Exp Pathol 2013;6:1337–42. [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Wang G, Gao C, Shao G, Kang N.. Effects of hyperbaric oxygen on MMP-2 and MMP-9 expression and spinal cord edema after spinal cord injury. Life Sci 2013;93:1033–8. [PubMed] [Google Scholar]