Keywords: cancer, inflammation, involution, macrophages, pregnancy
Abstract
Normal developmental processes, such as those seen during embryonic development and postpartum mammary gland involution, can be reactivated by cancer cells to promote immune suppression, tumor growth, and metastatic spread. In mammalian embryos, paternal-derived antigens are at risk of being recognized as foreign by the maternal immune system. Suppression of the maternal immune response toward the fetus, which is mediated in part by the trophoblast, is critical to ensure embryonic survival and development. The postpartum mammary microenvironment also exhibits immunosuppressive mechanisms accompanying the massive cell death and tissue remodeling that occurs during mammary gland involution. These normal immunosuppressive mechanisms are paralleled during malignant transformation, where tumors can develop neoantigens that may be recognized as foreign by the immune system. To circumvent this, tumors can dedifferentiate and co-opt immune-suppressive mechanisms normally utilized during fetal tolerance and postpartum mammary involution. In this review, we discuss those similarities and how they can inform our understanding of cancer progression and metastasis.
INTRODUCTION
Pregnancy and postpartum breast/mammary gland involution exemplify unique immunological conditions whereby a critical immune balance is necessary. During pregnancy, cells at the maternal-fetal interface must support fetal growth and development while also developing immune tolerance to the fetus and simultaneously maintaining the ability to mount an immune response if infection occurs. It has been long appreciated that the maternal immune system can recognize paternal antigens in the partially allogeneic fetus, yet it is critical that the fetus is not rejected by the maternal immune system (1). This dynamic immune state is mediated in part by the uterine microenvironment, which adapts as the immunologically distinct fetus develops. Although it has been postulated that pregnancy creates a chronic state of immunological suppression, a successful pregnancy in fact cannot exist without select immune cells and factors (2–4), highlighting the need for appropriate and well-controlled communication and balance between the developing fetus and immune cells at the maternal-fetal interface. Similarly, after lactation, the mammary gland must remodel to regenerate a lactation-competent epithelium for subsequent rounds of pregnancy; this process, termed postpartum mammary gland involution, involves death of the milk-secreting mammary epithelial cells, which results in release of self-antigens. Therefore, activation of immune-suppressive mechanisms that promote tolerance occurs in the mammary microenvironment. Select immune cells are also required for proper execution of involution (5, 6). In addition, tumor progression is mediated not only by the tumor itself but also by the tumor microenvironment (TME), which comprises immune cells, extracellular matrix (ECM) proteins, and soluble factors, highlighting the ongoing cross talk between tumor and immune cells.
The immune system comprises two main components: the innate and adaptive immune systems. Innate immunity refers to cells that generate a quick response to immune threats; these cells include natural killer (NK) cells, macrophages, dendritic cells (DCs), and neutrophils, as well as others. Innate immune responses are generally independent of antigens, which are molecular structures that are recognized as foreign and bound by antibodies or cells of the adaptive immune system. The adaptive immune system comprises cells, including B-cell and T-cell lymphocytes, that destroy immune threats, either directly through cytotoxicity or indirectly via the production of antibodies and cytokines. The adaptive immune system also assists in remembering foreign antigens to protect against future threats. During an infection, antigen-specific B cells and T cells become activated and undergo clonal expansion. B cells produce foreign antigen-targeted antibodies, whereas T cells can expand into multiple effector subtypes, including cytotoxic T cells, T-helper cells, and memory T cells. Effector T cells include two primary subtypes: Th1 and Th2 cells, which are proinflammatory and anti-inflammatory, respectively (7). Immune activation is heavily regulated by regulatory immune cells like Th2 cells, as well as T-regulatory (Treg) cells. Tregs function to suppress the expansion of cytotoxic T cells, reduce cytokine production, and promote cytotoxic T-cell exhaustion to result in resolution of the immune response (8–11). The balance between immune-suppressive regulatory cells, cytotoxic cells, and cytokines controls immune attack versus an equilibrium state.
Successful maternal-fetal tolerance, postpartum mammary gland involution, and tumor progression are all dependent on mechanisms of immunoediting, whereby dynamic cross talk between multiple cell types affects immune cell interactions and impacts the overall immune response. Because tumor growth and metastasis, which largely occurs through the blood and lymphatic vasculature, are highly reliant on avoiding destruction by the immune system, tumors evolve multiple mechanisms of immune tolerance to avoid immune clearance (12). During tumorigenesis, there is initial immune activation and elimination of tumor cells, followed by immune and tumor equilibrium, and finally tumor cell escape from the immune system (referred to as the “three Es” of immunoediting; reviewed in Refs. 13 and 14). Importantly, tumor immunoediting has been implicated in poor clinical response to multiple immunotherapy regimens (reviewed in Ref. 15). The equilibrium phase of tumor immunoediting reflects the balance between immune suppression and activation that is observed during maternal-fetal tolerance and postpartum mammary gland involution. During fetal development, physiological maternal immune suppression is tightly regulated by an “immune clock of human pregnancy,” where the composition and function of immune cells are temporally controlled (16). Likewise, during mammary gland involution, composition, and function of immune cells in the mammary tissue are initially increased, followed by a resolution phase (6, 17, 18). In this review, we will discuss normal mechanisms of immunoediting such as those in fetal tolerance and mammary gland involution that can be co-opted by pathologies, with a focus on breast cancer.
Trophoblast Mimicry
After fertilization, a human zygote travels down the fallopian tube to the uterus and develops into a blastocyst. The blastocyst further develops into two distinct compartments: the embryoblast, which comprises the inner mass of cells that will become the embryo, and the trophoblast, which comprises the outermost layer of cells. The trophoblast mediates attachment to the uterine wall—a process called implantation—and later forms part of the placenta. After initial implantation, the trophoblast begins to invade the maternal endometrial decidua (a thick mucous membrane described in Parallels to the Decidua section) and cytotrophoblast cells surround the embryo. The cytotrophoblast cells then differentiate into two distinct subtypes. The first is the syncytiotrophoblast, which mediates nutrient uptake and hormone production. The second is the extravillous trophoblast (EVT), which invades through the syncytiotrophoblast to attach to the uterine wall (reviewed in Ref. 19). Notably, tumor cells share multiple phenotypes with the trophoblast, including proliferation, migration/invasion, and vasculature recruitment—a concept sometimes referred to as “trophoblast mimicry” (20, 21). Importantly, the trophoblast also mediates immune protection for the developing embryo—another phenotype similarly observed during tumorigenesis.
Trophoblasts alter the cytokine milieu and directly express immune-altering proteins, such as major histocompatibility complex (MHC; also known as human leukocyte antigen/HLA). HLA proteins are expressed at the cell surface and can present antigens to T cells via interaction with the T-cell receptor (TCR). Trophoblasts repress expression of class I HLA-A, -B, -C, and class II HLA-D antigens, which stimulate immune rejection while they upregulate immune-suppressive HLA-G (22). HLA-G can bind to KIR2DL4 and LILRB1 receptors on decidual NK cells to trigger cell senescence-associated secretory phenotype to promote immune tolerance (23). HLA-G can also ligand the LILRB2 receptor to activate immune-suppressive Tregs and myeloid-derived suppressor cells (MDSCs) to maintain fetal tolerance (24, 25). Similarly, cancers exhibit altered HLA profiles that affect immune activation. Breast tumors and breast cancer cells express HLA-G in a hormone-dependent manner. HLA-G on tumor cells can activate Tregs, and HLA-G expression correlates with increased breast tumor size, lymph node status, and clinical disease stage (26). Like trophoblasts, breast tumors can express HLA-G with corresponding loss of all class I HLA genes (27). Tumors can also secrete extracellular vesicles with high amounts of HLA-G, which correlate with breast cancer progression and increased detectable stem cell-like circulating tumor cells (28). Thus, trophoblast mimicry by breast tumors can promote immune suppression in part via altered expression of antigen presentation receptors.
Parallels to the Decidua
During fetal development, a thick mucous membrane called a decidua develops and lines the uterus. Maternal-fetal interaction is mediated by two distinct interfaces; the first is between the placenta and decidua basalis, and the second is between fetal membranes and the decidua parietalis. These interfaces facilitate immune tolerance to protect the fetus and to promote fetal growth and development. The placenta, which provides oxygen and nutrients to the fetus, is the primary barrier between mother and fetus and interfaces with the decidua basalis. Both placental and decidual cells are important for mediating maternal immune tolerance to the fetus.
After the maturing blastocyst is implanted in the uterus, it is initially exposed to a largely proinflammatory Th1 microenvironment. The immune milieu quickly shifts to an anti-inflammatory Th2 microenvironment, allowing the pregnancy to avoid immune clearance and continue (29, 30). The Th2-skewed state is maintained until after birth (16). Macrophages, NK cells, and DCs infiltrate the decidua and accumulate around the trophoblast (31, 32). Notably, when macrophages, NK cells, and DCs are depleted, placental development is impaired, supporting that these cells are critical for successful fetal development (4, 33–36). Women with a history of spontaneous miscarriages have higher ratios of proinflammatory to anti-inflammatory cytokines (37–39), which is thought to be largely mediated by NK cells (40, 41). Of all the immune cells present at the fetal interface, uterine NK (uNK) cells are the most abundant (42). As opposed to NK cells found in peripheral blood, uNK cells are less cytotoxic, as they do not express CD16—a critical antibody-dependent cellular cytotoxicity (ADCC) receptor (43). Instead, uNK cells are more immunomodulatory, as they secrete galectin 1 to promote tolerogenic DCs (34), and altered ratios of cytotoxic to immunomodulatory uNK cells have been linked to recurrent miscarriages (40).
Similar to the decidua microenvironment, Th2-skewed TMEs have been reported for numerous cancer types, including breast, melanoma, glioma, and lymphoma (44–46). Th2 cytokines, such as IL-4 and IL-10, induce macrophage polarization into a protumor state and suppress antitumor macrophage polarization, thus establishing an immune permissive TME that allows tumor progression (47). In breast cancer, Th2 high tumors are reported to be more proliferative and are correlated with higher tumor stage and grade (48, 49). During ADCC, NK cells are recruited to antibody-dense areas in a coordinated innate and adaptive response to tumorigenesis, which can be exploited therapeutically to induce a cytokine storm and leukocyte infiltration (50). However, soluble factors in the TME, such as TGF-β, adenosine, and PGE2, can suppress ADCC and render NK cells less cytotoxic (51), similar to the minimally cytotoxic uNK cells. Therapeutic approaches to increase ADCC by NK cells are thus currently being investigated and may prove fruitful in increasing immune clearance of tumors.
Mammary Gland Involution
The primary function of the mammary gland is to produce and secrete milk to nourish offspring after birth. As such, terminal differentiation of the mammary epithelium does not occur until postnatally and more specifically when lactation occurs. After lactation ends, postpartum mammary gland involution occurs, which is the highly conserved physiological process that remodels the breast tissue to a prepregnant state. The process of involution is essential for subsequent rounds of lactation for future offspring. A primary characteristic of postpartum involution is massive cell death, which is accompanied by immunosuppressive mechanisms so that the mammary gland can successfully regenerate for future lactation. Although pregnancy has long been thought to be protective against breast cancer in the long term, there is a transient increase in risk for all parous women that can last for upward of 30 yr that is dependent on age at first birth (52, 53).
Forced-weaned mice models have provided details regarding normal postpartum mammary gland physiology. Involution, after ceased lactation, occurs in two stages—reversible and irreversible (54, 55). The first reversible stage is triggered by milk stasis and upregulates cathepsins to mediate lysosomal cell death (56). If suckling resumes during this phase, which lasts for 48 h in mice, lactation can restart. However, in the absence of suckling, the irreversible second phase begins. During this phase, the mammary gland undergoes drastic changes, including anoikis-mediated cell death of ∼80% of the mammary epithelium and significant matrix remodeling (55, 57). The process of involution creates a wound healing-like environment and significant increases in proinflammatory cells, such as Tcells and macrophages, which aid in clearing apoptotic bodies and remodeling (58, 59).
The immune system plays a vital role in mammary gland involution and has been shown to promote an immunosuppressive environment. It is thought that breast cancers may take advantage of this microenvironment to facilitate tumor progression. During Phase I of involution, Signal Transducer and Activator of Transcription 3 (STAT3) upregulates cathepsin and facilitates lysosomal-mediated cell death (60). STAT3 is a transcription factor that is also linked to early breast cancer progression, chemoresistance, proliferation, and metastasis. During malignancy, STAT3 induces the production of VEGF and IL-10 in various immune cells, as well as production of the immunosuppressive factors IL-23 and TGF-β. Furthermore, NF-κB is activated during the early phase of involution, which aids in epithelial cell death and milk loss (61, 62). During Phase II of involution, apoptosis is mediated by intrinsic mitochondrial-mediated pathways (59, 63, 64). There is a peak of macrophage infiltration during Phase II, around involution day 6 in rodent models, to assist with clearing apoptotic bodies and remodeling (65). These macrophages are protumor, based on increased expression of Arginase-1 (Arg-1) and IL-13 (6). Furthermore, mammary epithelial cells become phagocytic to aid in this process (66, 67). During the second phase of involution, there is a peak in lymphatic vessel density, elevated levels of matrix metalloproteases (MMPs), deposition of fibrillar collagen, increased deposition of the oncofetal protein tenascin, and increased fibronectin and laminin fragments (6, 17, 58, 59, 68, 69). Each of these phenotypes has also been linked to stromal attributes of wound healing and inflammation, as well as tumor progression and dissemination (70–72). However, the immune system expands the innate/adaptive natural killer T (NKT) cell population in late-stage involution, which may help prevent oncogenesis via the antigen-presenting molecule CD1d. This suggests the complexity of mammary gland involution and the immune environment. NKT cells can promote an inflammatory environment via Th1 and Th2 cells, characterized by IFN-γ and IL-4. Increased IL-4 expression is significantly increased in breast tumors and promotes chemoresistance in breast cancer models (73, 74). Finally, rodent models have also shown that the mammary gland microenvironment created by postpartum involution is sufficient to drive mammary tumor growth and metastasis (70, 71). The ability of tumors to co-opt immune-suppressive programs observed during pregnancy and involution, combined with an inflammatory microenvironment in advanced-age pregnancies and subsequent involution, may increase tumor-promotional effects and warrants additional investigation.
Immune-Suppressive Leukocytes
T-regulatory cells.
In the decidua, T cells initially constitute ∼20% of total lymphocytes, and this population can increase up to 80% during a successful pregnancy (75) and Tregs are an essential immunosuppressive component in pregnancy (76). Approximately 14% of all decidual CD4+ T cells are CD25+ Treg cells, which peak in the first trimester and then gradually decrease during the remainder of pregnancy (77). Multiple murine studies have confirmed that CD4+ CD25+ Treg cells expand during pregnancy, and that depletion of these Treg cells causes immunological rejection of the fetus (78, 79). Syngeneic and allogeneic murine models have also revealed that mice prone to pregnancy loss exhibit lower CD4+ CD25+ Treg cell frequencies compared with mice with normal pregnancy success rates (80). Adoptive transfer of CD4+ CD25+ cells from other pregnant mice prevents fetal rejection, supporting that pregnancy-induced Treg cells promote allogeneic fetus immune tolerance (80). Interestingly, adoptive transfer of Tregs from nonpregnant mice did not have the same effect on fetal rescue, suggesting that prior exposure to paternal antigens is critical for transferred tolerance. Treg-mediated tolerance transfer is only effective before pregnancy day 4, highlighting the need for early immune tolerance in successful fetal survival and development. Furthermore, depletion of Treg cells increases pregnancy loss in allogeneic mice, but not in syngeneic mice (81), highlighting that immune recognition of paternal antigens is an important component of Treg-mediated immune suppression. Similarly, during involution, the T-cell population is enriched for Foxp3+ Tregs, reflective of an immune-suppressive microenvironment (18).
During tumorigenesis, Tregs suppress the immune system and allow tumor escape and growth. As reported in mechanisms of maternal-fetal tolerance, Treg depletion can cause immune-mediated rejection of leukemia, melanoma, plasmacytoma, and mastocytoma (58, 59). More recent studies have mirrored these results in murine mammary cancers, where Foxp3+ Treg cells accumulate with tumor progression and Treg ablation induces robust antitumor immunity in both primary and metastatic tumors (82). Furthermore, Treg ablation results in increased breast tumor invasion, collagen deposition, an increase in the percentage of cancer stem cells, as well as increased inflammatory cytokines IL-4 and IL-5 and reduced antitumor macrophages, consistent with a Th2 phenotype (83). In human breast tumors, Foxp3+ Treg cells are activated by DCs, reducing activation of effector T cells and promoting immune escape and tumor progression (84). Treg cells can also upregulate cytokine and chemokine receptors, including CCR8 (85). In addition, more aggressive breast cancers, including triple-negative breast cancer (TNBC) and HER2+ breast tumors, have increased numbers of Treg cells compared with less aggressive estrogen receptor-positive (ER+) subtypes. Higher Treg infiltration also correlates with a higher proliferative index, larger tumor size, and more lymph node metastases (86). Furthermore, Treg infiltration in breast cancer metastases correlates with poor patient survival (87). In addition, Tregs can affect breast cancer metastatic organotropism. Specifically, highly immune-suppressive Treg accumulation occurs during primary breast tumorigenesis, but tumors can then transcriptionally reprogram Tregs to render them ineffective at blocking metastasis to lymph nodes, but not to lungs. Tregs normally suppress NK-cell activation in lymph nodes, but this effect is lost after tumor-mediated reprogramming (88). Patients with breast cancer thus have high Treg:NK ratios in sentinel lymph nodes (88). These data support immune regulation of site-specific metastasis and, overall, highlight the importance of Tregs in tumor immune permissiveness.
Cytotoxic T cells.
During pregnancy, cytotoxic T cells that recognize paternal alloantigens in the fetus decrease to facilitate immune tolerance. These T cells become exhausted, and placental trophoblasts induce T-cell death to deplete fetus-directed clones (89). For example, the male H-Y peptide, which is present on a male fetus, can induce T-cell deletion and hyporesponsiveness in a mechanism mediated by a Fas/FasL interaction between the maternal T cells and the fetus (90). Exhausted and senescent CD4+ and CD8+ T cells are present at the maternal-fetal interface and decline in mothers who undergo preterm labor (91), supporting that T-cell exhaustion permits sustained fetal tolerance. Indeed, memory CD8+ T cells generated from a pregnancy are uniquely prone to exhaustion by re-stimulation with fetal antigens during subsequent pregnancies in a PD-1 and LAG-3-mediated mechanism (92). Similarly, during involution, CD4+ and CD8+ T cells in the mammary gland and in the draining lymph node are PD-1+ and accompanied by PD-L1+ lymphatic endothelial cells and macrophages, suggesting active immune checkpoint signaling.
This is mirrored in malignancies, whereby exhaustion of T cells can be a defining feature in many cancers (93). CD8+ T cells in the TME exhibit increased expression of PD-1 and LAG-3 and have impaired effector function with decreased production of IL-2, IFN-y, and TNF-α. The exhausted T-cell state allows for the tumor to escape cytotoxic T cells, and it can also predict clinical outcomes. An exhausted CD8+ T-cell phenotype correlates with poor survival in subset of patients with breast cancer (94). However, in a Phase II clinical trial, it was reported that patients with ER+ tumors were more likely to benefit from immune checkpoint inhibition if they had an exhausted CD8+ T-cell immune signature in both blood and/or tumor samples (95), suggesting that this patient population may be susceptible to select therapeutic interventions. PD-1 blockage can cause a paradoxical increase in PD-1 expression on Tregs and enhance their suppressive activity (96), further suggesting the need for advanced therapeutics for patients with exhausted T cells in the TME, particularly in an advanced, metastatic setting. In addition, in mouse models of postpartum breast cancer (PPBC), where tumor cells are exposed to postpartum involution, the tumors in postpartum mice take on characteristics of the immune milieu observed during postpartum involution including enrichment for PD-1/PD-L1 cells.
The ratio of CD8+ T cells to Treg cells has been implicated in breast cancer progression, with high CD8+/FOXP3+ ratios associated with decreased relapse rates, increased response to chemotherapy, and increased patient survival (97–100). In addition, CD4+ T cells have multifaceted roles in breast cancer. CD4+ “helper” T-cell infiltration corresponds to worse breast cancer patients’ overall and relapse-free survival, as does the intratumor CD4/CD8 ratio (101). However, multiple studies have also shown antitumor roles for CD4+ T cells. In breast cancer xenograft models, inhibition of naive CD4+ recruitment reduces the number of Treg cells and correspondingly decreases tumor growth (102). CD4+ T-helper cells have also been reported to block spontaneous breast cancer development in murine models by inducing epigenetic reprogramming of cancer cells to cause terminal differentiation, thus reducing invasive capacities (103). A subpopulation of CD4+ cells known as follicular helper T cells has a gene signature that positively correlates with patient survival and response to chemotherapy (104). Overall, a more robust characterization of CD4+ helper T cells in tumorigenic contexts is needed. Taken together, these data support that increased Treg infiltration and exhausted cytotoxic T cells are characteristic of maternal-fetal tolerance, postpartum mammary involution, and tumor-induced immune suppression.
Immune-Suppressive Myeloid Cells
Macrophages.
Macrophages can be recruited to the endometrium to contribute to a successful pregnancy (105). Approximately 20% of hematopoietic cells in the decidua are macrophages (106, 107). Decidual macrophages are critical in the clearance of apoptotic cell debris during pregnancy, which prevents the release of maternal self-antigens and paternal alloantigens, as well as a corresponding immune response that could damage the fetus (108). Decidual macrophages suppress T-cell alloreactivity—the ability to recognize allogeneic MHC complexes (109). Although decidual macrophages were previously considered a single population, three distinct populations have recently been characterized: 1) CCR2− CD11cLO, 2) CCR2+ CD11cHI, and 3) CCR2− CD11cHI (110). The CCR2− CD11cHI population resides proximal to EVTs and expresses heme oxygenase-1 (HMOX1), which has immune-suppressive function that is discussed in the Heme oxygenase-1 section. The CCR2+ CD11cHI population expresses interleukin-1β (IL-1β) and prostaglandin synthase 2 (PTGS2/COX2) at the gene and protein level (110). Decidual macrophages can thus promote the secretion of prostaglandin E2 (PGE2), which is converted from arachidonic acid (AA) by COX-2. In addition, in the context of malignancies, macrophage polarization is frequently referred to in terms of tumor-suppressive “M1-like” or tumor-promotional “M2-like” phenotypes, although it is now appreciated that this is a plastic and dynamic spectrum comprised of many unique populations rather than simply two separate states. Notably, the CCR2− decidual macrophage subtypes constitute a majority (>80%) of decidual macrophages and are considered more “M2-like” (i.e., protumor) (110). Decidual macrophages can also decrease the activation of T cells and alloreactivity via inhibition of IL-2 (109). Tissue-resident macrophages are also critical for additional developmental processes, as mice with loss of the colony-stimulating factor (CSF-1) signaling axis exhibit markedly reduced fertility capacity, as well as lactational defects due to incomplete mammary gland development (111).
During mammary gland involution, macrophages are the predominant immune cell infiltrate. Although macrophages were thought to play a secondary phagocytic role to mammary epithelial cells during involution, they are required for proper clearance and tissue remodeling (67). Involution-associated macrophages are mostly immunosuppressive M2-like and secrete Arg-1 and IL-23 (6). This phenotype is conserved in mice, rats, and human mammary tissues (6). ECM proteins that are upregulated during involution, such as procollagen, laminin, and fibronectin, are also sufficient to recruit immunosuppressive macrophages in vitro (6). Recently, involution-associated macrophages also were found to express more podoplanin, a lymphatic cell marker, and incorporate into lymphatic vessels (112)—a phenotype that can subsequently promote breast cancer lymphangiogenesis and lymphatic metastasis (112).
In parallel, tumors can co-opt macrophages and induce immune-suppressive, protumor function. Often termed tumor-associated macrophages (TAMs), these immune cells can support tumor cell growth, invasion, and metastasis. Mechanistically, this is done in a similar manner to macrophages during fetal tolerance—via suppression of cytotoxic T-cell function, as well as degradation of ECM and promotion of angiogenesis. In the context of tumorigenesis, a large meta-analysis revealed that TAM infiltration correlates with worse outcomes in breast and other cancer types (113). TAM infiltration is also associated with tumor progression in murine models and transgenic expression of macrophage CSF-1 induces high macrophage tumor infiltration, accelerated mammary tumor growth, and increased lung metastasis (114).
Cancer cells can impact macrophage phenotypes via cell-derived factors. An example of this is the Janus Kinase (JAK)/STAT pathway, a known oncogenic survival pathway, and the Notch pathway, which are also activated during postpartum involution. In human breast cancer and murine mammary cancer cell lines, conditioned media stimulates the activation of STAT5 and STAT3 in macrophages via GM-CSF (115). Breast cancer cells can also activate signaling of Jagged1, a Notch ligand, which correlates with an M2-like polarization via increased STAT3 activation (116). HER2+ breast cancer cells can also signal to macrophages through the Wnt pathway, which contributes to priming the tumor for early dissemination. Specifically, using an MMTV-HER2+ murine model, it was reported that macrophages infiltrate early lesions of HER2+ breast cancer, in part to HER2+ activation of NF-κB and upregulation of CCL2 to attract macrophages and signaled Wnt activation on the macrophage (117). Activated macrophages correlate with a mesenchymal tumor phenotype and increased metastatic burden. Importantly, M2-like macrophages may play a role in epithelial-to-mesenchymal transition (EMT), a key step for tumor cells to leave their primary site, intravasate, and survive in circulation (118–121). Thus, therapeutic approaches to target TAMs and overcome resistance to chemotherapy and other targeted therapies are an active area of interest (122).
Myeloid-derived suppressor cells.
MDSCs are immature myeloid cells that promote an immunosuppressive environment via interaction with the immune milieu, affecting both the innate and the adaptative immune response. MDSCs can expand during states of chronic infection or inflammation and, as such, they have both physiologic and pathologic roles, including in pregnancy and cancer. The role of MSDCs in maternal-fetal tolerance has only recently been characterized (reviewed in Ref. 123). MDSCs accumulate during tumor metastatic spread in pregnant mice; they impacted NK-cell activity, and depletion of MDSCs reduced metastasis (124). MDSCs can be derived from different precursors to generate unique MDSC subtypes termed GM-MDSCs and PMN-MDSCs. Peripheral granulocytic MDSCs (GM-MDSCs) increase during normal physiologic pregnancies compared with nonpregnant controls (125). Levels were highest during early gestation and dropped a few days postpartum to nonpregnant levels (126). Furthermore, the human placenta was further enriched for maternally derived GM-MDSCs, which were localized to the decidua and intervillous space (127, 128). MDSC function during pregnancy was organ dependent and, in brief, was found to inhibit T-cell production, inhibit Th1 activity, induce Th2 activity, induce Treg production, reduce NK cytotoxicity, and inhibit perforin release (125, 127–131). Finally, during involution, myeloid cells express the IFN-y receptor, which is expressed by immune-suppressive MDSCs (18).
MDSCs can be protumorigenic by having roles linked to immune evasion, EMT, angiogenesis, and metastases (reviewed in Ref. 132). In breast cancer, MDSCs were found to suppress antitumor immune response via IDO, and MDSC expression correlated with lymph node metastases in patients (133). Furthermore, MDSCs are induced by PGE2, a product of COX2 activity, via EP2/4. Inhibition of this pathway led to maturation of effector T cells and suppresses Treg induction (134). IDO is also significantly upregulated in tumor-infiltrating MDSCs compared with periphery MDSCs (133). Monocytic MDSC infiltration is also linked to reduced immunotherapy efficiency and worse patient outcomes. Circulating peripheral MDSCs in patients with breast cancer are elevated in all stages of disease and increase with higher stages. MDSCs are correlated with worse metastatic burden and overall survival in patients with breast cancer (135–137). This phenotype was found to be associated with potent immunosuppressive activity on T cells. Finally, MDSCs directly impact breast cancer cells and contribute to invasion and metastasis via the PTEN/AKT pathway and subsequent recruitment of MMPs, which are known to allow for tumor cell invasion and metastasis (138). Overall, current studies have shown that MDSCs are increased in breast cancer and contribute to an immunosuppressive environment that may impact patients’ response to current immunotherapy. This suggests that targeting MDSCs may be a potential therapeutic for patients with breast cancer.
Cytokines and Chemokines
Cytokines are small, immune regulatory proteins that modulate the strength and duration of an immune response. Chemokines are cytokine family members with specialized promigratory functions. The suppression of proinflammatory cytokines, such as IL-2, tumor necrosis factor α (TNF-α), and interferon-γ (IFN-γ), alongside increased production of anti-inflammatory cytokines, such as IL-4, IL-6, and IL-10, is necessary for the progression of a successful pregnancy. Cytokines and chemokines are detectable in the serum of pregnant women, and these cytokine profiles have characteristic trajectories as pregnancies progress (139). Functionally, IL-15 is required for differentiation of uNK cells, as well as microenvironment remodeling (3). Distinct from peripheral blood NK cells, decidual NK cells produce IL-8 and interferon-inducible protein-10 to regulate trophoblast invasion, as well as angiogenic factors to promote blood vasculature growth at the maternal-fetal interface (34). Multiple cytokines such as IL-4, IL-6, and IL-10 have been linked to miscarriages and preterm delivery (140). Women with preeclampsia have high Th1/Th2 cytokine ratios with elevated IL-6, IL-8, and TNF-α, suggesting a pathogenic proinflammatory state (141). Decidual cells can directly produce proinflammatory cytokines such as IL-6 (142), which is reported to contribute to preeclampsia by causing endothelial cell dysfunction and increased macrophage infiltration during EVT invasion (143). Decreased Tregs are also observed alongside the altered cytokine milieu during preeclampsia, as Tregs can permit fetal tolerance in a heightened inflammatory state (78, 144). In addition, trophoblasts can recruit and modulate immune cells such as monocytes via secretion of chemokines including growth-related oncogene-α, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1β, as well as cytokines including IL-6, IL-8, and TNF-α (145). However, trophoblast-educated monocytes have damped responses to stimulus with lipopolysaccharide (LPS) (145), supporting that trophoblast-secreted factors suppress immune cell function. Trophoblast-secreted factors can also differentiate monocytes into CD14+/CD16+ macrophages that produce more IL-1β and IL-10 and have increased phagocytosis abilities (146). Decidual CD14+ macrophages also express anti-inflammatory and immunosuppressive cytokines, such as IL-10, to mediate fetal tolerance and dampen the inflammatory fetal microenvironment (147). Furthermore, cytokines and chemokines are important during postpartum mammary gland involution. During the first phase of involution, there is an increase in IL-1α, IL-1β, and IL-13 (58, 59). This initial wave of proinflammatory cytokines and neutrophils is followed by an influx of monocyte and macrophage chemoattractants, including CCL6, CCL7, CC8, and CXCL14, followed by increases in CSF-1R and CD68-positive macrophages (58, 59).
Alterations in cytokine profiles are also observed during tumorigenesis (reviewed in Ref. 148) and NF-κB activity in myeloid cells is considered a master regulator of cytokine production in the TME. NF-κB can increase IL-1, IL-6, TNF, IL-8, IL-17, IFN-γ, and CCL-5 to promote cell proliferation (149, 150). IL-6 has a well-characterized role in breast tumorigenesis, where it is expressed by tumor cells and fibroblasts and is associated with tumor growth, metastasis, and therapeutic resistance (151). IL-6 is known to activate JAK/STAT signaling to upregulate proliferative genes, such as CCND1 (152). In murine mammary cancer models, IL-10 expression downregulates proinflammatory MHC class I and enhances NK-mediated tumor cell lysis, thereby suppressing tumor growth and metastasis (153). IL-10 can also affect DCs and macrophage function, although this can be tumor-suppressive or tumor-promotional depending on the context (154). Taken together, these data reveal multiple immune-altering cytokines found during fetal tolerance, involution, and tumorigenesis. However, for metastatic malignancies, we lack comprehensive characterizations of the cytokine milieu in distinct metastatic sites, which warrants additional investigation.
Immune-Suppressive Enzymes and Metabolites
Indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase.
Metabolic enzymes have reported roles in both fetal tolerance and tumorigenesis. The rate-limiting enzymes indoleamine 2,3-dioxygenase 1 and 2 (IDO1/2) and tryptophan 2,3-dioxygenase 2 (TDO2) in the tryptophan catabolism pathway produce kynurenine, which has immune-suppressive effects by decreasing cytotoxic T-cell viability and expanding T-regulatory cells (155–157). CD4+ CD24+ Treg cells treated with the proinflammatory cytokine IFNγ upregulate IDO, which is understood to be immunosuppressive in pregnancy (77). In pregnant women, immune-inhibitory decidual CD14+ macrophages express IDO at the maternal-fetal interface (147, 158). More specifically, IDO is expressed in the syncytiotrophoblast, in the decidua, and in the fallopian tubes. TDO2 is also expressed in decidual stromal cells in murine placenta (159), and the immune-suppressive tryptophan metabolite kynurenine is detectable in cervical mucus (158). Furthermore, DCs produce IDO, and IDO expression is induced by MCSC activity, which can inhibit the proliferation of T cells and contribute to an immune-suppressive state (160). In rodents, inhibition of IDO during pregnancy causes fetal loss, further supporting its critical function in fetal immune tolerance (161, 162). Interestingly, it has been reported that TDO2-KO and double IDO1-TDO2-KO females do not exhibit reduced fertility when mated with allogeneic males (159), although this is likely explained by the activation of compensatory immunosuppression mechanisms. Finally, kynurenine can prevent cytokine-mediated induction of NK-cell-mediated killing (163), and NK killing capacity can be restored by both IDO and PGE2 inhibitors, suggestive of an additional role for COX-2 (164). Given the roles of COX-2 during postpartum involution, kynurenine may also play a role during involution, but to the best of our knowledge, this has not been investigated.
IDO and TDO2 are implicated in tumor-mediated immunosuppression. IDO is expressed in aggressive TNBC and is frequently coexpressed with PD-L1 (165). These data suggest that IDO may be involved in PD-1/PD-L1 regulation and could have a role in immunotherapy resistance. IDO has been reported to promote angiogenesis in preclinical breast cancer models, and IDO expression correlates to blood vasculature in patient tissues (166). IDO- and TDO2-mediated production of kynurenine can reduce viability and function of cytotoxic CD8+ T cells, and TDO2 mRNA expression correlates with poor prognosis of patients with breast cancer (167). TIM-3/Gal-9 can induce NK-cell-mediated production of IFNγ to subsequently increase IDO1 expression, which then downregulates NK-cell degranulation and allows tumor cell to escape from immune clearance (168). Furthermore, a TDO2-mediated signaling axis promotes TNBC-cell survival in anchorage-independent conditions and metastasis (168). Importantly, IDO has an identified role in breast cancer chemoresistance (169), and pharmacological inhibition of IDO increases sensitivity to chemotherapeutic approaches in murine mammary carcinoma models (170) and in a recent Phase II clinical trial in breast cancer (171). The role of IDO in cancer progression may be partially attributed to its ability to increase the expression of M2-like macrophage markers and decrease M1-like markers in cultured THP-1 cells (172). In addition, TDO2 has recently been implicated in ovarian cancer, where gene expression correlates with increased disease stage and recurrence (173). However, additional studies are required to better assess the role of TDO2 in immune suppression and to determine if TDO2 is a therapeutic target in additional cancer types. Additional studies are also needed to test the efficacy of new drugs targeting both IDO and TDO2, which are in the development pipeline. Overall, these data support the role of tryptophan catabolism via TDO or IDO and kynurenine in fetal tolerance and tumor-induced immune suppression.
Cyclooxygenase-2.
PTGS2/COX2 catalyzes the rate-limiting step of AA to prostaglandin conversion. Multiple components of the PGE2 synthesis pathway have been identified in maternal uterine tissue postembryonic attachment, as well as in embryonic tissue itself, supporting that the inflammatory response is cooperatively mediated by both maternal and fetal tissues (174). This pathway is also implicated during involution. COX-2 expression on the mammary epithelium is high during pregnancy and during involution in mice and remains elevated after involution is complete when compared with nulliparous samples (175). PGE2 levels are also increased during involution in a manner that can be reversed by ibuprofen. Involution infiltrating monocyte phenotypes are driven by PGE2, which can also be reduced by ibuprofen (176). PGE2 is the only known prostaglandin that has demonstrated a role in tumor formation, progression, and metastases; it has been shown to act on tumor cells and the surrounding stroma, including macrophages. PGE2 secreted from melanoma cells inhibited macrophage production of proinflammatory cytokines, such as TNF-α and IL-12. Furthermore, pharmaceutical inhibition of COX2, one of the main enzymes responsible for prostaglandin synthesis, reduces tumorigenesis in multiple breast cancer models (reviewed in Refs. 177 and 178). It is hypothesized that COX2 inhibition reduced PGE2 production and its ability to signal mitogenic and antiapoptotic pathways. COX2 overexpression correlates with worse breast cancer stages and outcomes (179–181). Furthermore, COX-2 expression activates VEGF-C/D expression, which promotes lymphangiogenesis and lymphatic metastases in human breast cancer and mouse mammary carcinoma models (182). A similar phenotype is seen by TAMs in a manner dependent on tumor or macrophage PGE2 production (183). Finally, in PPBC models, tumors implanted into involuting mammary glands acquire increased COX-2 expression and fibrillar collagen deposition that was dependent on COX-2 (69), and tumor cells collected from postpartum mice had higher amounts of PGE2 (17). Collectively, these data support an important immune-suppressive role for COX2 in multiple contexts.
Heme oxygenase-1.
The heme-degrading enzymes heme oxygenase-1 and -2 (HO-1; HO-2) convert heme into carbon monoxide, ferrous iron, and biliverdin (which is quickly converted to bilirubin). HO-1 has been implicated in immune suppression during fetal development. HO-1 is expressed in the human placenta (184). Reduced HO-1 activity is associated with increased pregnancy complications in humans (185), and upregulation of HO-1 decreases the rate of fetal rejection in mice (186, 187). HO-1 prevents fetal loss in abortion-prone mice by increasing the Th2/Th1 ratio (187). HO-1 can maintain maternal DCs in an immature state during murine pregnancy, which promotes the expansion Tregs (188). Loss of HO-1 prevents macrophage differentiation and reduces the expression of the macrophage markers CD14 and macrophage colony-stimulating factor receptor (189), which likely affects pregnancy tolerance. In the context of bacterial infection, LPS causes upregulation of HO-1 in macrophages (190) and a corresponding decrease in inflammatory components (191). Collectively, these data suggest that HO-1 is immune suppressive during fetal development to promote maternal-fetal tolerance. Interestingly, Hmox1 (the gene encoding murine HO-1) mRNA is upregulated during involution in mouse mammary glands (Fig. 1A) (58, 59); thus, further investigation regarding its role in involution is warranted.
Figure 1.
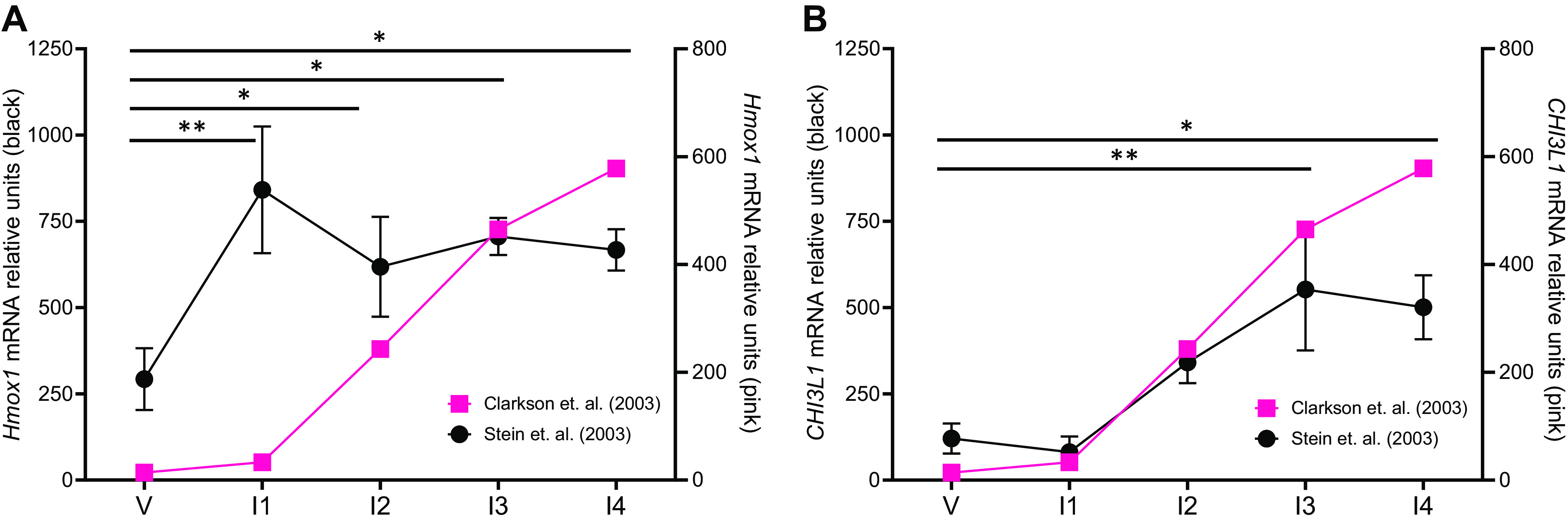
Chi3l1 and Ho-1 during mammary gland involution. Microarray analysis of (A) Hmox1 and (B) Chi3l1 mRNA expression from mice that are virgin [V, (day 8 from the study by Clarkson et al. (59) and day 10 from the study by Stein et al. (58)] or undergoing postpartum mammary gland involution (I; days 1–4). *P < 0.05, **P < 0.005 via ANOVA with Tukey’s multiple-comparisons test. Data from the study by Clarkson et al. (pink) (59) and Stein et al. (black) (58).
Similar HO-1-mediated mechanisms of immune suppression appear to be co-opted by tumor cells. In breast cancer, HO-1 is detectable in the TME and correlates with poor patient prognosis and chemoresistance (192). In preclinical breast cancer cell models, HO-1 reduces chemotherapy-induced cell death (193–195). Inhibition of HO-1 blocks chemotherapy-elicited CD8+ T cell-mediated immune suppression in murine models (196). HO-1 inhibition can resensitize murine TNBC and melanoma models to anti-PD-1 immunotherapy (197, 198) and differentiate M2-like protumor macrophages from antitumor M1-like macrophages (199). TAMs also express HO-1 in an IL-6-mediated manner, and HO-1 activation in TAMs can facilitate tumor cell migration and metastasis (200, 201). TAM expression of HO-1 also induces CD8+ T-cell proliferation and cytotoxicity in lymphoma models (192). In normal CD4+ CD25+ Treg cells, Foxp3 promotes expression of HO-1, which causes downstream suppression of effector T cells (202, 203). HO-1 expression in DCs alters inflammatory function by preventing DC maturation and secretion of proinflammatory cytokines (204, 205). Collectively, these data suggest immune cells in the TME may utilize HO-1 to promote immune suppression in a manner similar to fetal tolerance, but additional studies are needed to assess the full function of HO-1 in tumorigenesis, particularly at distinct metastatic sites. Clinical studies to target HO-1 or its metabolites in metastatic cancers may be a promising future direction.
Granzymes.
Another family of enzymes involved in fetal tolerance is granzymes. Granzyme B (GrB) is a serine protease expressed in NK cells and cytotoxic T cells. It is secreted along with perforin, which forms pores in target cells and promotes apoptosis. Decidual CD8+ effector T cells have lower GrB and perforin compared with peripheral T cells (206), suggesting that they have suppressed effector function. In breast cancer, GrB and perforin are expressed in tumor cells, and high expression levels correlate with lymph node invasion (207). Granzyme B has also been characterized on cells in the TME, including CD4+ Foxp3+ Treg cells, but not naive Treg cells; further, TME-derived Treg cells promote cell death of NK and CD8+ T cell in a GrB-dependent manner (11). In PPBC models, ibuprofen reduces granzyme levels (208) and levels of granzyme B are altered during postpartum involution (59). Collectively, these data support the existence of a GrB-mediated immune-suppressive, protumor response, similar to the role of GrB in maternal-fetal and involution-mediated tolerance.
Immune Checkpoint Molecules
An additional conserved mechanism of immune suppression between pregnancy, mammary gland involution, and malignancy is altered immune checkpoint molecules, such as CTLA-4, PD-L1, PD-1, Tim-3, and LAG-3. The immune regulatory circuit at the maternal-fetal interface is heavily mediated by IFN-β and PD-L1, which polarize macrophages toward an M2-like phenotype and decrease inflammatory responses. Macrophages educated by trophoblasts acquire a unique transcriptional profile, characterized by increased expression of type I IFN-β. IFN-β further increases soluble PD-L1 from trophoblasts, and PD-1 inhibition reduces trophoblast-induced macrophage differentiation (209). Trophoblast-educated macrophages exhibit diminished responses to TLR4 ligation. Soluble PD-L1 is detectable in blood from pregnant women, and soluble PD-L1 increases increased during pregnancy progression (209). PD-L1, as well as the immune-regulatory molecule galectin-9, are increased in the blood of pregnant women, and, interestingly, women with male fetuses had significantly higher galectin-9, but not soluble PD-L1, in their blood (210). Furthermore, it has recently been reported that the decidual microenvironment alters the tolerance-defense balance in CD8+ T cells, as coculture with trophoblasts increased the exhaustion markers PD-1 and CD39 (211). Together, these immune checkpoint molecules promote fetal tolerance by diminishing the maternal immune response.
PD-L1 is expressed on mammary stem cells, and PD-L1 expressing mammary basal cells exhibited robust mammary regeneration (212). PD-L1 and PD-L1 were also found on lymphatic endothelial cells and myeloid cells during involution (5), and PD-L1 blockade during involution in a PPBC model reactivated antitumor responses, such as delayed tumor growth, increased CD8+ T cells, and decreased lymphatic endothelial cells (5). Malignancies exhibit immune suppression via immune checkpoint signaling. Indeed, clinical availability of immune checkpoint inhibitors targeting CTLA-4, PD-L1, and PD-1 in solid tumors has dramatically impacted patient treatment and improved patient outcomes. The CTLA4-blocking antibody ipilimumab was authorized in 2011, and antibodies targeting PD-L1 (atezolizumab and durvalumab) and PD-1 (pembrolizumab and nivolumab) were approved soon after. There are multiple, excellent reviews available on the state of immune checkpoint blockade therapies in cancer treatments (213, 214). Although immune checkpoint-blocking therapies are now approved for ∼50 different cancer types, including TNBC, there are still large numbers of patients whose tumors do not respond to immune checkpoint inhibition. This highlights the need for better understanding of the complexities of the immune system to increase our ability to predict for efficacy and toxicities of these regimens.
Immune-Suppressive Secreted Factors
Semaphorin 7a.
Semaphorin 7a (SEMA7A; also named CDw108) is a glycosylphosphatidylinositol-linked protein that can be cleaved and shed into the extracellular space, allowing autocrine, paracrine, and juxtracrine signaling (215, 216). SEMA7A can signal through two receptors: ß1 integrin and plexin C1 (217–219). SEMA7A was first identified on lymphocytes to aid in wound healing, but it is best known for its role in axonal guidance during development (216, 220, 221). SEMA7A has well-characterized immune modulation capabilities. SEMA7A is expressed in B and T cells, macrophages, DCs, and NK cells, along with immature monocyte and lymphocyte lineages (222–228). SEMA7A is best characterized in T lymphocytes, where SEMA7A−/− mice exhibited hyperactivated T cells after stimulation, suggesting SEMA7A’s role in immunosuppression (226). To further support this, SEMA7A was found to be crucial for severe inflammation resolution. SEMA7A was linked to metabolic reprogramming of macrophages to an M2-like phenotype via IL-10 in a murine colitis model (229). However, SEMA7A also is upregulated on stimulated T cells and signals through ß1 integrin to release proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (224, 230–232). SEMA7A promotes lymphangiogenesis by inducing macrophages to express podoplanin and become more epithelial-like (112). Furthermore, SEMA7A has been recently linked to regulating PD-1/PD-L1 checkpoint blockade (5). The varying immune activating and suppressing roles may be due to SEMA7A’s different receptors and tissue specificity. In the placenta, SEMA7A and plexin C1 expression are significantly increased and SEMA7A expression in the placenta was not found to be cell-specific, consistent with its role in a variety of cell types (233). The exact mechanism of why SEMA7A and plexin C1 are high in placenta tissue has yet to be uncovered; however, it is believed that SEMA7A promotes immune suppression via plexin C1 to prevent maternal-fetal immune responses.
SEMA7A is well known for its role in wound healing and fibrosis—mechanisms that are co-opted in breast cancer to aid in progression and invasion. In breast tissue, SEMA7A is upregulated during postpartum involution and plays a role in fibrosis-like remodeling (69, 234–236). During involution, SEMA7A promotes macrophage-mediated lymphatic remodeling, anoikis resistance, and luminal progenitor cell survival (112, 237). SEMA7A promotes tumorigenesis in breast cancer, lung cancer, glioblastoma, and melanoma (112, 238–241). SEMA7A can promote drug resistance in lung and breast cancer models (241, 242). In addition, SEMA7A is upregulated in PPBC and drives multiple aspects of tumor progression such as proliferation, survival, migration/invasion, and lymphangiogenesis (112, 243). In breast cancer, SEMA7A promotes EMT via TGF-ß signaling, the release of proangiogenic cytokines, and tumor growth and metastases (239, 242–247). Finally, SEMA7A was reported to promote metastasis through the upregulation of Chitinase 3–like-1 (CHI3L1) by signaling via ß1-integrin to activate AKT and MAPK signaling in mammary carcinoma and melanoma models (239). Collectively, these data support that SEMA7A has important roles in multiple immune-suppressive contexts.
Chitinase 3–like-1.
CHI3L1 (also called breast regression protein 39/BRP-39 in mouse and YKL-40 in human) has reported immune-modulatory functions. CHI3L1 is a glycosyl hydrolase family 18 protein member, which binds to chitin but lacks the enzymatic activity necessary to degrade it. CHI3L1 is expressed and secreted by multiple cell types, including tumor cells, macrophages (248, 249), neutrophils (250), and activated T cells (251). Although the role of CHI3L1 during fetal development is not well characterized, it has been reported that pregnant women with gestational diabetes mellitus have an increased inflammatory status, including an enriched neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and high serum CHI3L1 levels (252). In the normal mammary gland, Chi3l1 (the gene encoding murine CHI3L1) is upregulated during postpartum involution, where it is thought to be expressed because of the massive death of mammary epithelial cells on weaning to suppress the immune system during this normal process (Fig. 1B) (58, 59, 253, 254). CHI3L1 suppresses E-cadherin but increases MMP-9 activity and cell motility in mammary epithelial cells (255). It is suggested that STAT3 is regulated by CHI3L1 during involution, suggesting a link between CHI3L1 and the immunosuppressed microenvironment during mammary gland involution (254, 256).
CHI3L1 has been implicated in multiple cancer types where it correlates with poor patient outcomes (239, 257–260). In TNBC, tumor-promotional M2-like macrophages exhibit increased secretion of CHI3L1 compared with tumor-suppressive M1-like macrophages (261). In lung cancer, CHI3L1 suppresses Th1 and cytotoxic T-cell responses to promote immunosuppression and metastasis (251). In the normal lung epithelium, the murine CHI3L1 homolog, BRP-39, prevents cell death of inflammatory cells via increased AKT signaling; CHI3L1 also increased Arg-1 activity and macrophage mannose receptor (MMR) expression, which are characteristic of M2-like macrophages (262). Cancer-associated fibroblasts in mammary tumors also upregulate CHI3L1, which then induces recruitment of macrophages and promotes tumor growth (263). Recently, CHI3L1 was reported to promote the expression of immune checkpoint proteins, including PD-L1, PD-L2, PD-1, LAG3, and TIM3, in melanoma. Genetic ablation of Chi3l1 (the murine gene encoding CHI3L1) increases CD8+ and CD4+ T-cell infiltration and promotes a Th1 phenotype via increased proinflammatory cytokine secretion, further supporting the role of CHI3L1 in protumor immune suppression (264). Collectively, these data suggest that tumor cells upregulate CHI3L1 to block the antitumor immune response, particularly via skewing the immune milieu toward a Th2 phenotype. This is similar to the Th1 to Th2 shift observed during fetal development, further demonstrating how tumor cells can co-opt mechanisms of immune suppression from maternal-fetal tolerance.
Prolactin inducible protein.
Another secreted factor involved in immune suppression is prolactin inducible protein (PIP; also called gross cystic disease fluid protein 15/GCDFP-15 and secretory actin-binding protein/SABP). PIP has multiple receptors, including actin, myosin, keratin, tropomyosin, immunoglobulin (Ig)-G, and human zinc-α2 glycoprotein (ZAG). PIP is a large component of amniotic fluid and breast milk (265), where it likely facilitates immune tolerance. PIP expression is positively regulated by both prolactin and androgen-bound androgen receptors and is negatively regulated by estrogens (266). PIP can also be promoted by the cytokines IL-1α and IL-6 (267, 268). Immune cells express PIP, including a specialized population of T cells, termed invariant natural killer T (iNKT) cells, that are involved in the recognition of lipid antigens and protect against autoimmunity. PIP expression on iNKT cells promotes DC maturation and differentiation in a Toll-like receptor (TLR)2-mediated manner, induces naïve T-cell maturation into Foxp3+ Treg cells, and reduces the number of Th1 and Th17 cells (269). Furthermore, PIP is also described as an antiapoptotic factor and is found to be regulated by STAT5A during mammary gland involution (270). Inactivation of STAT5A via glucocorticoids preserved the expression of PIP and inhibited apoptosis (270). For decades, PIP has been used to identify breast origin of metastatic tumors of unknown origin (271–273) and over 90% of breast cancers express PIP (273). PIP has mitogenic activity in breast and prostate cancers (274, 275). Surprisingly, in murine mammary carcinoma tumors, PIP expression reduces tumor growth and type 2 T-helper cells while simultaneously promoting protumor cytokines and metastasis (276), suggesting that PIP’s effects on tumorigenesis and immune alteration may be complex and at times conflicting. Overall, a more robust characterization of the role of PIP and its receptors during tumor-associated immune suppression is needed.
Invasion and Epithelial to Mesenchymal Transition
During fetal development, EVT cells must invade through the decidua to establish a blood supply by remodeling the spinal arteries. To facilitate this invasive process, EVT cells undergo EMT processes (277). During embryonic development, when epithelial cells invade surrounding tissue (for instance when trophoblasts invade the maternal uterine lining to seek a blood supply), a gene expression program is utilized to facilitate invasion. Our group discovered a common link between this invasive signature, steroid hormone receptor action, and immune suppression, all tightly controlled by small noncoding microRNA family miR-200. This miRNA family regulates trophoblast invasion in the uterus, and it is elevated in pathological conditions such as preeclampsia (278). Conversely, loss or silencing of miR-200 family members in carcinomas renders tumor cells more invasive by relieving repression of its direct targets and permitting expression of proteins mesenchymal-like markers such as ZEB that are not normally expressed in epithelial cells, and an increase in immune-suppressive factors including PD-L1 (279–281) and many of the factors discussed in this review. Since ZEB1 is a direct repressor of E-cadherin and other epithelial markers, these are often decreased or completely repressed when miR-200c is silenced.
EMT plays an important role in mammary gland involution where it aids in tissue remodeling and in the termination of milk production. The main overlap between EMT and involution is TGF-ß, which is known as a central inducer of EMT and contributor to involution (282, 283). TGF-ß is found to be highly expressed during pregnancy and greatly reduced during lactation because it inhibits the synthesis of milk protein (284). TGF-ß3 was found to promote loss of tight junction and adherens reorganization during mammary gland involution, which in turn helped mammary epithelial cell phagocytosis (284). A decrease in lactogenic hormones induced TGF-ß1 and AA, the main substrate of COX-2, expression in mammary epithelial cells (285). This relationship induced a mesenchymal-like phenotype via upregulation of vimentin and TWIST1 gene expression (285). Interestingly, TGF-ß1 was found to induce expression of AA synthesis, but AA did not induce TGF-ß expression in mammary epithelial cells (285). In breast cancer, TGF-ß has been shown to play a multitude of roles in immunomodulation, cancer stem cells, and metastasis. Exosomes secreted from mesenchymal stem cells (MSCs) in breast cancer contain TGF-ß and promote M2-like macrophage phenotypes, contributing to breast cancer progression (286). These MSC-derived exosomes also contain PD-L1 and semaphorins, which contribute to an immunosuppressive microenvironment (286).
SNAI2, an EMT transcription factor, is required for proper mammary gland involution (287). SNAI2-deficient mice had lower levels of activated STAT3, a key molecule during Phase I of involution (287). STAT3 is a key regulator of EMT and is known to be co-opted by tumors to promote progression (reviewed in Ref. 288). However, the link between EMT and the immunosuppressive environment found in involution has yet to be studied. There are underappreciated immune-suppressive characteristics of cancer cells that have undergone EMT to facilitate invasion into surrounding tissues and corresponding metastasis (reviewed in Ref. 289). Many of the aforementioned immunosuppressive phenotypes (e.g., PD-L1 and COX-2) have been linked to the ability of cancer cells to escape the primary tumor, travel in an anchorage-independent manner, and seed a secondary site.
Approaches to manipulate EMT in TNBC models revealed altered immune modulatory factors, including the cytokines GM-CSF and M-CSF, the enzymes TDO2 and HO-1, the checkpoint protein PD-L1, and other secreted proteins including CHI3L1 (281, 290–292). It has been proposed that these factors could work in tandem to facilitate the characteristic immune-suppressed state often observed in mesenchymal-like TNBC and that they could be inhibited to create an antitumor-skewed microenvironment and promote disease clearance (289). Future studies should utilize our understanding of immune suppression observed with these mesenchymal tumor models.
Additional Therapeutic Implications
Despite breast cancers being widely regarded as immunologically quiescent compared with other cancer types, there is a large amount of interest in identifying therapeutic strategies that harness the ability to reactivate the immune system and initiate tumor clearance. These include strategies to improve presentation of tumor antigens, to reduce inhibitory cytokines and immune regulatory cells, and to activate and expand effector T cells, NK cells, and DCs (as reviewed in Ref. 293). Although most current immunotherapy approaches harness the power of the adaptive immune system, lessons from maternal-fetal tolerance suggest that innate immunity is also highly effective in mounting a robust immune response. This could be particularly useful in cancer cases that have acquired resistance to adaptive immunotherapeutic agents, such as those that target PD-L1, PD-1, and CTLA-4. In line with this, novel approaches that harness innate immunity to induce antitumor immunity are being investigated (294). An example of this is anti-CSF-1R therapy, which blocks CSF-1 from binding to CSF-1R and preventing myeloid cell differentiation into immunosuppressive MDSCs and M2 macrophages. Early clinical trials have generated promising reports that reprogramming myeloid responses can effectively enhance immunotherapy (295, 296). Additional clinical trials are ongoing to assess anti-CSF-1R therapy in multiple malignancies, both along and in combination therapy approaches.
Just as the maternal immune system can recognize paternal antigens as foreign and cause fetal rejection, tumors can also express antigens that may be recognized as foreign by the host immune system. Tumor cells exhibit genomic instability and consequently express neoantigens, sometimes referred to as tumor-associated antigens, which can arise from genetic rearrangements, alternative splicing, or viral infections (297). For example, TNBCs produce the largely tumor-specific antigens MAGE-A and NY-ESO-1, which are proposed immunotherapeutic vulnerabilities of these tumors with efforts underway to target these antigens to induce a tumor-specific immune response (298, 299). Interestingly, NY-ESO1 is also expressed in adult and fetal testes (300). T cells recognize neoantigens presented by MHC molecules and can initiate a robust downstream immune response. Indeed, vaccines targeting tumor neoantigens are an emerging and promising area of cancer immunotherapy, and tumor neoantigen-targeted vaccines are now in clinical trials for multiple cancer types (297, 301–303).
It was recently reported that decidual NK cells can selectively target bacteria in infected trophoblasts by transferring the antimicrobial peptide granulysin through nanotubes, allowing decidual NK cells to fight infection and simultaneously preserve maternal-fetal tolerance (304). Soon after this report, it was reported that nanotubes can mediate the transfer of mitochondria from immune cells to cancer cells, which boosts the cancer cell metabolically while simultaneously metabolically depleting the immune cells (305). Although nanotubes were identified over nearly two decades earlier (306), they have only recently been proposed as a novel target for cancer immunotherapy. This is a prime example of immune-related mechanisms reported in the context of fetal tolerance that have soon after been identified as having therapeutic potential during tumorigenesis.
In summary, identification of new therapeutic approaches to activate antitumor immune responses during cancer progression is an active area of interest. Indeed, there are numerous drugs currently in clinical trials targeting different components of the immune system in multiple cancer types. Emerging understandings of fetal tolerance and mammary gland involution should offer insights into next-generation approaches to cancer immunotherapy.
CONCLUSIONS
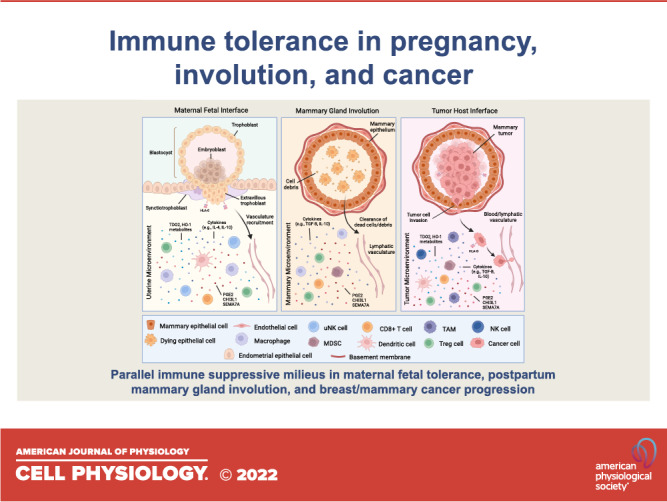
There are numerous parallels between immune suppression during fetal development, mammary gland involution, and tumorigenesis (summarized in Fig. 2, A–C). During pregnancy, tightly regulated, transient immune suppression is a critical process that protects the allogeneic fetus from the maternal immune system until birth. During involution, the mammary gland and breast tissues create an immunosuppressive “wound healing” environment to aid in tissue remodeling and prevent an immune response to dead mammary/breast epithelial cells that have terminally differentiated after lactation. Similar mechanisms can be activated and co-opted by cancer cells, creating a chronic state of immune suppression that promotes tumor progression. We propose that further understanding of the fine-tuned, reversible process of immune suppression that facilitates normal fetal tolerance during pregnancy and involution will allow us to develop novel approaches to prevent tumor immune escape and metastasis.
Figure 2.
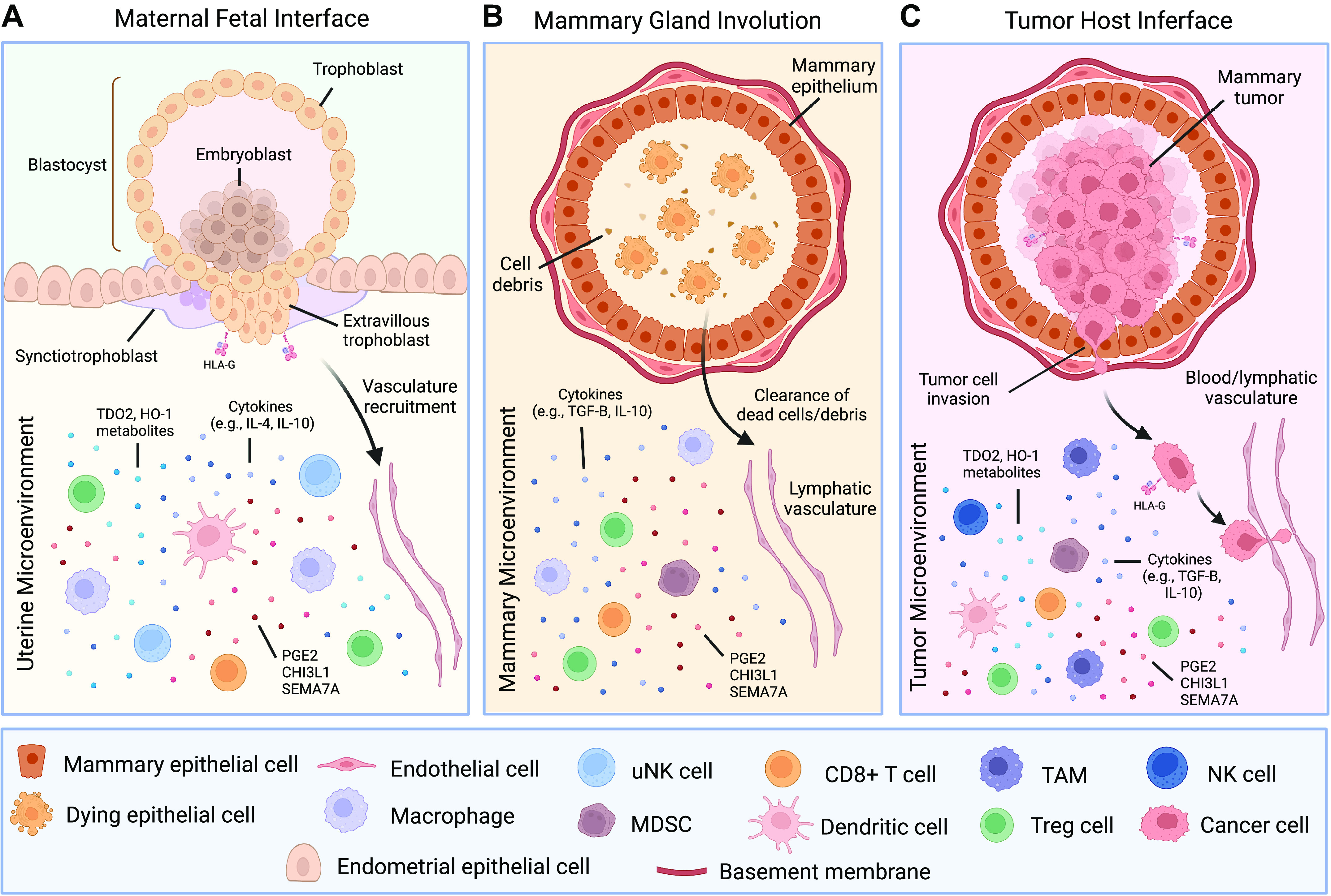
Parallels between the immune-suppressive milieu at the maternal-fetal interface, in the involution mammary microenvironment, and during breast tumorigenesis. Immune cells and immune-suppressive factors (e.g., TDO2 metabolites, HO-1 metabolites, cytokines, and other secreted factors including CHI3L1, SEMA7A, and PGE2) are shown in (A) the maternal endometrial decidua, (B) during mammary gland involution, and (C) in the breast tumor microenvironment. CHI3L1, chitinase 3-like-1; HO-1, heme oxygenase 1; MDSC, myeloid-derived suppressor cell; NK, natural killer; PGE2, Prostaglandin E2; SEMA7A, semaphorin 7a; TAM, tumor-associated macrophage; TDO2, tryptophan 2,3-dioxygenase. This figure was created with BioRender.com.
GRANTS
This work was supported by The Department of Defense W81XWH-19-BCRP-EA (to J.K.R.), HERA Ovarian Cancer Foundation Outside-The-Box grant (to L.S.C.), NIH/NCI R01 CA211696-01A1 (to T.R.L.), American Cancer Society (ACS) RSG-16–171-010CSM (to T.R.L.), The Department of Medicine Outstanding Early Career Scholars Award (to T.R.L.), and The University of Colorado Cancer Center Men For the Cure Award (to T.R.L. and J.K.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.S.C., K.T.K., J.K.R., and T.R.L. conceived and designed research; L.S.C., K.T.K., J.K.R., and T.R.L. drafted manuscript; L.S.C., K.T.K., J.K.R., and T.R.L. edited and revised manuscript; L.S.C., K.T.K., J.K.R., and T.R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Michelle M. Williams for review of the manuscript prior to publication. Graphical abstract created with Biorender.com.
This article is part of the special collection “Tumor Host Interactions in Metastasis.” Dr. Mythreye Karthikeyan and Dr. Nadine Hempel served as Guest Editors of this collection.
REFERENCES
- 1. Tafuri A, Alferink J, Möller P, Hämmerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science 270: 630–633, 1995. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 2. Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 15: 517–535, 2009. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 171: 2937–2944, 2003. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 4. Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 21: 693–702, 2000. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 5. Tamburini BAJ, Elder AM, Finlon JM, Winter AB, Wessells VM, Borges VF, Lyons TR. PD-1 blockade during post-partum involution reactivates the anti-tumor response and reduces lymphatic vessel density. Front Immunol 10: 1313, 2019. doi: 10.3389/fimmu.2019.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, Man Y-G, Borges V, Schedin P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol 176: 1241–1255, 2010. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy K, Weaver C, Janeway C. Janeway's Immunobiology (9th ed.). New York: Garland Science, 2017. [Google Scholar]
- 8. Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 10: 1969–1980, 1998. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 9. Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA 101: 10398–10403, 2004. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gondek DC, Lu L-F, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 174: 1783–1786, 2005. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 11. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27: 635–646, 2007. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32: 1267–1284, 2018. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gubin MM, Vesely MD. Cancer immunoediting in the era of immuno-oncology. Clin Cancer Res 28: 3917–3928, 2022. doi: 10.1158/1078-0432.CCR-21-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360, 2004. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 15. O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16: 151–167, 2019. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 16. Aghaeepour N, Ganio EA, Mcilwain D, Tsai AS, Tingle M, Van Gassen S, Gaudilliere DK, Baca Q, McNeil L, Okada R, Ghaemi MS, Furman D, Wong RJ, Winn VD, Druzin ML, El-Sayed YY, Quaintance C, Gibbs R, Darmstadt GL, Shaw GM, Stevenson DK, Tibshirani R, Nolan GP, Lewis DB, Angst MS, Gaudilliere B. An immune clock of human pregnancy. Sci Immunol 2: eaan2946, 2017. doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, Jindal S, Schedin P. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest 124: 3901–3912, 2014. doi: 10.1172/JCI73777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer 136: 1803–1813, 2015. doi: 10.1002/ijc.29181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol 3: 56, 2005. doi: 10.1186/1477-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc 84: 985–1000, 2009. doi: 10.4065/84.11.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costanzo V, Bardelli A, Siena S, Abrignani S. Exploring the links between cancer and placenta development. Open Biol 8: 180081, 2018. doi: 10.1098/rsob.180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunt JS, Orr HT. HLA and maternal-fetal recognition. FASEB J 6: 2344–2348, 1992. doi: 10.1096/fasebj.6.6.1544544. [DOI] [PubMed] [Google Scholar]
- 23. Rajagopalan S, Long EO. Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proc Natl Acad Sci USA 109: 20596–20601, 2012. doi: 10.1073/pnas.1208248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo M-G. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116: 935–944, 2010. [Erratum in Blood 118: 5060, 2011]. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 25. Kostlin N, Ostermeir AL, Spring B, Schwarz J, Marmé A, Walter CB, Poets CF, Gille C. HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4. Eur J Immunol 47: 374–384, 2017. doi: 10.1002/eji.201646564. [DOI] [PubMed] [Google Scholar]
- 26. He X, Dong D-D, Yie S-M, Yang H, Cao M, Ye S-R, Li K, Liu J, Chen J. HLA-G expression in human breast cancer: implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann Surg Oncol 17: 1459–1469, 2010. doi: 10.1245/s10434-009-0891-9. [DOI] [PubMed] [Google Scholar]
- 27. Palmisano GL, Pistillo MP, Fardin P, Capanni P, Nicolò G, Salvi S, Spina B, Pasciucco G, Ferrara GB. Analysis of HLA-G expression in breast cancer tissues. Hum Immunol 63: 969–976, 2002. doi: 10.1016/S0198-8859(02)00642-0. [DOI] [PubMed] [Google Scholar]
- 28. König L, Kasimir-Bauer S, Hoffmann O, Bittner AK, Wagner B, Manvailer LF, Schramm S, Bankfalvi A, Giebel B, Kimmig R, Horn PA, Rebmann V. The prognostic impact of soluble and vesicular HLA-G and its relationship to circulating tumor cells in neoadjuvant treated breast cancer patients. Hum Immunol 77: 791–799, 2016. doi: 10.1016/j.humimm.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 29. Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 151: 4562–4573, 1993. [PubMed] [Google Scholar]
- 30. Chaouat G, Ledée-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol 134: 93–119, 2004. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 31. Shimada S, Nishida R, Takeda M, Iwabuchi K, Kishi R, Onoé K, Minakami H, Yamada H. Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from sporadic miscarriage. Am J Reprod Immunol 56: 193–200, 2006. doi: 10.1111/j.1600-0897.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 32. Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 192: 259–270, 2000. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Bouteiller P, Piccinni MP. Human NK cells in pregnant uterus: why there? Am J Reprod Immunol 59: 401–406, 2008. doi: 10.1111/j.1600-0897.2008.00597.x. [DOI] [PubMed] [Google Scholar]
- 34. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12: 1065–1074, 2006. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 35. Manaseki S, Searle RF. Natural killer (NK) cell activity of first trimester human decidua. Cell Immunol 121: 166–173, 1989. doi: 10.1016/0008-8749(89)90014-2. [DOI] [PubMed] [Google Scholar]
- 36. Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest 118: 3954–3965, 2008. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol 196: 122–130, 1999. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 38. Polgar K, Hill JA. Identification of the white blood cell populations responsible for Th1 immunity to trophoblast and the timing of the response in women with recurrent pregnancy loss. Gynecol Obstet Invest 53: 59–64, 2002. doi: 10.1159/000049413. [DOI] [PubMed] [Google Scholar]
- 39. Jenkins C, Roberts J, Wilson R, MacLean MA, Shilito J, Walker JJ. Evidence of a T(H) 1 type response associated with recurrent miscarriage. Fertil Steril 73: 1206–1208, 2000. doi: 10.1016/S0015-0282(00)00517-3. [DOI] [PubMed] [Google Scholar]
- 40. Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol 156: 4027–4034, 1996. [PubMed] [Google Scholar]
- 41. Saito S, Shigeru S, Nakashima A, Akitoshi N, Myojo-Higuma S, Subaru M-H, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol 77: 14–22, 2008. [Erratum in J Reprod Immunol 78: 84, 2008]. [DOI] [PubMed] [Google Scholar]
- 42. Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C, Bossi F, Mocci C, Sarazani F, Tedesco F, Santoni A, Gismondi A. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 111: 3108–3115, 2008. doi: 10.1182/blood-2007-08-105965. [DOI] [PubMed] [Google Scholar]
- 43. Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2: 656–663, 2002. [Erratum in Nat Rev Immunol 2: 975, 2002]. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 44. Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, Fisher DC, Kupper TS, Clark RA. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res 19: 3755–3763, 2013. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN; Melanoma Study Group of the Mayo Clinic Cancer Center. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 15: 1931–1939, 2009. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimato S, Maier LM, Maier R, Bruce JN, Anderson RCE, Anderson DE. Profound tumor-specific Th2 bias in patients with malignant glioma. BMC Cancer 12: 561, 2012. doi: 10.1186/1471-2407-12-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555, 2002. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 48. Tokumaru Y, Le L, Oshi M, Katsuta E, Matsuhashi N, Futamura M, Yoshida K, Takabe K. Association of Th2 high tumors with aggressive features of breast cancer. JCO 38: e12584, 2020. doi: 10.1200/JCO.2020.38.15_suppl.e12584. [DOI] [Google Scholar]
- 49. Le L, Tokumaru Y, Oshi M, Asaoka M, Yan L, Endo I, Ishikawa T, Futamura M, Yoshida K, Takabe K. Th2 cell infiltrations predict neoadjuvant chemotherapy response of estrogen receptor-positive breast cancer. Gland Surg 10: 154–165, 2021. doi: 10.21037/gs-20-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu EF, Gai SA, Opel CF, Kwan BH, Surana R, Mihm MC, Kauke MJ, Moynihan KD, Angelini A, Williams RT, Stephan MT, Kim JS, Yaffe MB, Irvine DJ, Weiner LM, Dranoff G, Wittrup KD. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 27: 489–501, 2015. doi: 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol 10: 3038, 2019. doi: 10.3389/fimmu.2019.03038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast cancer risk after recent childbirth: a pooled analysis of 15 prospective studies. Ann Intern Med 170: 22–30, 2019. doi: 10.7326/M18-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Husby A, Wohlfahrt J, Øyen N, Melbye M. Pregnancy duration and breast cancer risk. Nat Commun 9: 4255, 2018. doi: 10.1038/s41467-018-06748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hassiotou F, Geddes D. Anatomy of the human mammary gland: current status of knowledge. Clin Anat 26: 29–48, 2013. doi: 10.1002/ca.22165. [DOI] [PubMed] [Google Scholar]
- 55. Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development 115: 49–58, 1992. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- 56. Lund LR, Rømer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Danø K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 122: 181–193, 1996. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Furth PA, Bar-Peled U, Li M. Apoptosis and mammary gland involution: reviewing the process. Apoptosis 2: 19–24, 1997. doi: 10.1023/A:1026454207398. [DOI] [PubMed] [Google Scholar]
- 58. Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy M-A, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res 6: R75–R91, 2004. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Clarkson RWE, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res 6: R92–R109, 2004. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hennigar SR, Seo YA, Sharma S, Soybel DI, Kelleher SL. ZnT2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci Rep 5: 8033, 2015. doi: 10.1038/srep08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clarkson RW, Heeley JL, Chapman R, Aillet F, Hay RT, Wyllie A, Watson CJ. NF-κB inhibits apoptosis in murine mammary epithelia. J Biol Chem 275: 12737–12742, 2000. doi: 10.1074/jbc.275.17.12737. [DOI] [PubMed] [Google Scholar]
- 62. Connelly L, Barham W, Pigg R, Saint-Jean L, Sherrill T, Cheng D-S, Chodosh LA, Blackwell TS, Yull FE. Activation of nuclear factor κB in mammary epithelium promotes milk loss during mammary development and infection. J Cell Physiol 222: 73–81, 2010. doi: 10.1002/jcp.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arnandis T, Ferrer-Vicens I, García-Trevijano ER, Miralles VJ, García C, Torres L, Viña JR, Zaragozá R. Calpains mediate epithelial-cell death during mammary gland involution: mitochondria and lysosomal destabilization. Cell Death Differ 19: 1536–1548, 2012. doi: 10.1038/cdd.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baxter FO, Neoh K, Tevendale MC. The beginning of the end: death signaling in early involution. J Mammary Gland Biol Neoplasia 12: 3–13, 2007. doi: 10.1007/s10911-007-9033-9. [DOI] [PubMed] [Google Scholar]
- 65. O'Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia 14: 145–157, 2009. doi: 10.1007/s10911-009-9118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim JI, Konishi S, Iwai H, Kohno T, Gouda H, Shimada I, Sato K, Arata Y. Three-dimensional solution structure of the calcium channel antagonist omega-agatoxin IVA: consensus molecular folding of calcium channel blockers. J Mol Biol 250: 659–671, 1995. doi: 10.1006/jmbi.1995.0406. [DOI] [PubMed] [Google Scholar]
- 67. Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod 78: 586–594, 2008. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- 68. Schedin P, O'Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia 12: 71–82, 2007. doi: 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- 69. Lyons TR, O'Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan A-C, Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 17: 1109–1115, 2011. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wallace TR, Tarullo SE, Crump LS, Lyons TR. Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. J Cancer Metastasis Treat 5: 9, 2019. doi: 10.20517/2394-4722.2019.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, Schedin P. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol 168: 608–620, 2006. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1986. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 73. Conticello C, Pedini F, Zeuner A, Patti M, Zerilli M, Stassi G, Messina A, Peschle C, De Maria R. IL-4 protects tumor cells from anti-CD95 and chemotherapeutic agents via up-regulation of antiapoptotic proteins. J Immunol 172: 5467–5477, 2004. doi: 10.4049/jimmunol.172.9.5467. [DOI] [PubMed] [Google Scholar]
- 74. Obiri NI, Siegel JP, Varricchio F, Puri RK. Expression of high-affinity IL-4 receptors on human melanoma, ovarian and breast carcinoma cells. Clin Exp Immunol 95: 148–155, 1994. doi: 10.1111/j.1365-2249.1994.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tilburgs T, Claas FH, Scherjon SA. Elsevier Trophoblast Research Award Lecture: unique properties of decidual T cells and their role in immune regulation during human pregnancy. Placenta 31: S82–S86, 2010. doi: 10.1016/j.placenta.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 76. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112: 38–43, 2004. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Heikkinen J, Möttönen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol 136: 373–378, 2004. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5: 266–271, 2004. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 79. Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett 102: 106–109, 2006. [Erratum in Immunol Lett. 102: 241, 2006. Darasse-Jèze, Guillaume [corrected to Darrasse-Jèze, Guillaume]. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 80. Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk H-D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 166: 811–822, 2005. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol 85: 121–129, 2010. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 82. Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med 210: 2435–2466, 2013. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martinez LM, Robila V, Clark NM, Du W, Idowu MO, Rutkowski MR, Bos PD. Regulatory T Cells Control the Switch From in situ to invasive breast cancer. Front Immunol 10: 1942, 2019. doi: 10.3389/fimmu.2019.01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay J-Y, Ménétrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 69: 2000–2009, 2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 85. Shang B, Liu Y, Jiang S-J, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5: 15179, 2015. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Glajcar A, Szpor J, Hodorowicz-Zaniewska D, Tyrak KE, Okoń K. The composition of T cell infiltrates varies in primary invasive breast cancer of different molecular subtypes as well as according to tumor size and nodal status. Virchows Arch 475: 13–23, 2019. doi: 10.1007/s00428-019-02568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stenström J, Hedenfalk I, Hagerling C. Regulatory T lymphocyte infiltration in metastatic breast cancer-an independent prognostic factor that changes with tumor progression. Breast Cancer Res 23: 27, 2021. doi: 10.1186/s13058-021-01403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kos K, Aslam MA, van de Ven R, Wellenstein MD, Pieters W, van Weverwijk A, Duits DEM, van Pul K, Hau C-S, Vrijland K, Kaldenbach D, Raeven EAM, Quezada SA, Beyaert R, Jacobs H, de Gruijl TD, de Visser KE. Tumor-educated Tregs drive organ-specific metastasis in breast cancer by impairing NK cells in the lymph node niche. Cell Rep 38: 110447, 2022. doi: 10.1016/j.celrep.2022.110447. [DOI] [PubMed] [Google Scholar]
- 89. Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft”. J Immunol 160: 3086–3090, 1998. [PubMed] [Google Scholar]
- 90. Vacchio MS, Hodes RJ. Fetal expression of Fas ligand is necessary and sufficient for induction of CD8 T cell tolerance to the fetal antigen H-Y during pregnancy. J Immunol 174: 4657–4661, 2005. doi: 10.4049/jimmunol.174.8.4657. [DOI] [PubMed] [Google Scholar]
- 91. Slutsky R, Romero R, Xu Y, Galaz J, Miller D, Done B, Tarca AL, Gregor S, Hassan SS, Leng Y, Gomez-Lopez N. Exhausted and senescent T cells at the maternal-fetal interface in preterm and term labor. J Immunol Res 2019: 3128010, 2019. doi: 10.1155/2019/3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kinder JM, Turner LH, Stelzer IA, Miller-Handley H, Burg A, Shao T-Y, Pham G, Way SS. CD8(+) T cell functional exhaustion overrides pregnancy-induced fetal antigen alloimmunization. Cell Rep 31: 107784, 2020. doi: 10.1016/j.celrep.2020.107784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6: e1792, 2015. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Egelston CA, Guo W, Tan J, Avalos C, Simons DL, Lim MH, Huang YJ, Nelson MS, Chowdhury A, Schmolze DB, Yim JH, Kruper L, Melstrom L, Margolin K, Mortimer JE, Yuan Y, Waisman JR, Lee PP. Tumor-infiltrating exhausted CD8+ T cells dictate reduced survival in premenopausal estrogen receptor-positive breast cancer. JCI Insight 7, 2022. doi: 10.1172/jci.insight.153963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Terranova-Barberio M, Pawlowska N, Dhawan M, Moasser M, Chien AJ, Melisko ME, Rugo H, Rahimi R, Deal T, Daud A, Rosenblum MD, Thomas S, Munster PN. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat Commun 11: 3584, 2020. doi: 10.1038/s41467-020-17414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA 116: 9999–10008, 2019. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, Onoda N, Tanaka S, Ohsawa M, Hirakawa K. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 103: 845–854, 2016. doi: 10.1002/bjs.10127. [DOI] [PubMed] [Google Scholar]
- 98. Semeraro M, Adam J, Stoll G, Louvet E, Chaba K, Poirier-Colame V, Sauvat A, Senovilla L, Vacchelli E, Bloy N, Humeau J, Buque A, Kepp O, Zitvogel L, André F, Mathieu M-C, Delaloge S, Kroemer G. The ratio of CD8(+)/FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology 5: e1218106, 2016. doi: 10.1080/2162402X.2016.1218106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, Watanabe G, Tada H, Suzuki A, Ohuchi N, Ishida T. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 17: 124, 2015. doi: 10.1186/s13058-015-0632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Goda N, Sasada S, Shigematsu H, Masumoto N, Arihiro K, Nishikawa H, Sakaguchi S, Okada M, Kadoya T. The ratio of CD8 + lymphocytes to tumor-infiltrating suppressive FOXP3 + effector regulatory T cells is associated with treatment response in invasive breast cancer. Discov Oncol 13: 27, 2022. doi: 10.1007/s12672-022-00482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang Y, Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Hunborg P, Varvares MA, Hoft DF, Hsueh EC, Peng G. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 6: 17462–17478, 2015. doi: 10.18632/oncotarget.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Su S, Liao J, Liu J, Huang D, He C, Chen F, Yang L, Wu W, Chen J, Lin L, Zeng Y, Ouyang N, Cui X, Yao H, Su F, Huang J-D, Lieberman J, Liu Q, Song E. Blocking the recruitment of naive CD4(+) T cells reverses immunosuppression in breast cancer. Cell Res 27: 461–482, 2017. doi: 10.1038/cr.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boieri M, Malishkevich A, Guennoun R, Marchese E, Kroon S, Trerice KE, Awad M, Park JH, Iyer S, Kreuzer J, Haas W, Rivera MN, Demehri S. CD4+ T helper 2 cells suppress breast cancer by inducing terminal differentiation. J Exp Med 219: e20201963, 2022. doi: 10.1084/jem.20201963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothé F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 123: 2873–2892, 2013. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wheeler KC, Jena MK, Pradhan BS, Nayak N, Das S, Hsu C-D, Wheeler DS, Chen K, Nayak NR. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS One 13: e0191040, 2018. doi: 10.1371/journal.pone.0191040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bulmer JN, Johnson PM. Macrophage populations in the human placenta and amniochorion. Clin Exp Immunol 57: 393–403, 1984. [PMC free article] [PubMed] [Google Scholar]
- 107. Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods 132: 181–189, 1990. doi: 10.1016/0022-1759(90)90028-T. [DOI] [PubMed] [Google Scholar]
- 108. Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol 51: 275–282, 2004. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 109. Parhar RS, Yagel S, Lala PK. PGE2-mediated immunosuppression by first trimester human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity. Cell Immunol 120: 61–74, 1989. doi: 10.1016/0008-8749(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 110. Jiang X, Du M-R, Li M, Wang H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol Immunol 15: 1027–1037, 2018. doi: 10.1038/s41423-018-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pollard JW, Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci USA 91: 9312–9316, 1994. doi: 10.1073/pnas.91.20.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Elder AM, Tamburini BAJ, Crump LS, Black SA, Wessells VM, Schedin PJ, Borges VF, Lyons TR. Semaphorin 7A promotes macrophage-mediated lymphatic remodeling during postpartum mammary gland involution and in breast cancer. Cancer Res 78: 6473–6485, 2018. doi: 10.1158/0008-5472.CAN-18-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang Q-W, Liu L, Gong C-y, Shi H-S, Zeng Y-h, Wang X-Z, Zhao Y-W, Wei Y-Q. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One 7: e50946, 2012. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193: 727–740, 2001. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jesser EA, Brady NJ, Huggins DN, Witschen PM, O'Connor CH, Schwertfeger KL. STAT5 is activated in macrophages by breast cancer cell-derived factors and regulates macrophage function in the tumor microenvironment. Breast Cancer Res 23: 104, 2021. doi: 10.1186/s13058-021-01481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tao S, Chen Q, Lin C, Dong H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J Exp Clin Cancer Res 39: 191, 2020. doi: 10.1186/s13046-020-01676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones J, Condeelis J, Merad M, Aguirre-Ghiso JA. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun 9: 21, 2018. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhu L, Fu X, Chen X, Han X, Dong P. M2 macrophages induce EMT through the TGF-β/Smad2 signaling pathway. Cell Biol Int 41: 960–968, 2017. doi: 10.1002/cbin.10788. [DOI] [PubMed] [Google Scholar]
- 119. Hsieh CH, Tai SK, Yang MH. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia 20: 775–788, 2018. doi: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bednarczyk RB, Tuli NY, Hanly EK, Rahoma GB, Maniyar R, Mittelman A, Geliebter J, Tiwari RK. Macrophage inflammatory factors promote epithelial-mesenchymal transition in breast cancer. Oncotarget 9: 24272–24282, 2018. doi: 10.18632/oncotarget.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Neelakantan D, Zhou H, Oliphant MUJ, Zhang X, Simon LM, Henke DM, Shaw CA, Wu M-F, Hilsenbeck SG, White LD, Lewis MT, Ford HL. Publisher Correction: EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat Commun 9: 4720, 2018. doi: 10.1038/s41467-018-07168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mehta AK, Kadel S, Townsend MG, Oliwa M, Guerriero JL. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol 12: 643771, 2021. doi: 10.3389/fimmu.2021.643771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kostlin-Gille N, Gille C. Myeloid-derived suppressor cells in pregnancy and the neonatal period. Front Immunol 11: 584712, 2020. doi: 10.3389/fimmu.2020.584712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mauti LA, Le Bitoux M-A, Baumer K, Stehle J-C, Golshayan D, Provero P, Stamenkovic I. Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. J Clin Invest 121: 2794–2807, 2011. doi: 10.1172/JCI41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Köstlin N, Kugel H, Spring B, Leiber A, Marmé A, Henes M, Rieber N, Hartl D, Poets CF, Gille C. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur J Immunol 44: 2582–2591, 2014. doi: 10.1002/eji.201344200. [DOI] [PubMed] [Google Scholar]
- 126. Nair RR, Sinha P, Khanna A, Singh K. Reduced myeloid-derived suppressor cells in the blood and endometrium is associated with early miscarriage. Am J Reprod Immunol 73: 479–486, 2015. doi: 10.1111/aji.12351. [DOI] [PubMed] [Google Scholar]
- 127. Bartmann C, Junker M, Segerer SE, Häusler SF, Krockenberger M, Kämmerer U. CD33(+)/HLA-DR(neg) and CD33(+)/HLA-DR(+/-) cells: rare populations in the human decidua with characteristics of MDSC. Am J Reprod Immunol 75: 539–556, 2016. doi: 10.1111/aji.12492. [DOI] [PubMed] [Google Scholar]
- 128. Kostlin N, Hofstädter K, Ostermeir AL, Spring B, Leiber A, Haen S, Abele H, Bauer P, Pollheimer J, Hartl D, Poets CF, Gille C. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J Immunol 196: 1132–1145, 2016. doi: 10.4049/jimmunol.1500340. [DOI] [PubMed] [Google Scholar]
- 129. Kropf P, Baud D, Marshall SE, Munder M, Mosley A, Fuentes JM, Bangham CRM, Taylor GP, Herath S, Choi B-S, Soler G, Teoh T, Modolell M, Müller I. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol 37: 935–945, 2007. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ren J, Zeng W, Tian F, Zhang S, Wu F, Qin X, Zhang Y, Lin Y. Myeloid-derived suppressor cells depletion may cause pregnancy loss via upregulating the cytotoxicity of decidual natural killer cells. Am J Reprod Immunol 81: e13099, 2019. doi: 10.1111/aji.13099. [DOI] [PubMed] [Google Scholar]
- 131. Kang X, Zhang X, Liu Z, Xu H, Wang T, He L, Zhao A. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-β/β-catenin pathway. Mol Hum Reprod 22: 499–511, 2016. doi: 10.1093/molehr/gaw026. [DOI] [PubMed] [Google Scholar]
- 132. Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-derived suppressor cells in tumors: from mechanisms to antigen specificity and microenvironmental regulation. Front Immunol 11: 1371, 2020. doi: 10.3389/fimmu.2020.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 190: 3783–3797, 2013. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 134. Ching MM, Reader J, Fulton AM. Eicosanoids in cancer: prostaglandin E2 receptor 4 in cancer therapeutics and immunotherapy. Front Pharmacol 11: 819, 2020. doi: 10.3389/fphar.2020.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, Garrett-Mayer E, Montero AJ, Bronte V, Mandruzzato S. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 118: 2254–2265, 2011. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58: 49–59, 2009. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bergenfelz C, Larsson A-M, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, Jernström H, Janols H, Wullt M, Bredberg A, Rydén L, Leandersson K. Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS One 10: e0127028, 2015. doi: 10.1371/journal.pone.0127028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shou D, Wen L, Song Z, Yin J, Sun Q, Gong W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget 7: 64505–64511, 2016. doi: 10.18632/oncotarget.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jarmund AH, Giskeødegård GF, Ryssdal M, Steinkjer B, Stokkeland LMT, Madssen TS, Stafne SN, Stridsklev S, Moholdt T, Heimstad R, Vanky E, Iversen A-C. Cytokine patterns in maternal serum from first trimester to term and beyond. Front Immunol 12: 752660, 2021. doi: 10.3389/fimmu.2021.752660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Raghupathy R, Makhseed M, Azizieh F, Omu A, Gupta M, Farhat R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod 15: 713–718, 2000. doi: 10.1093/humrep/15.3.713. [DOI] [PubMed] [Google Scholar]
- 141. Szarka A, Rigó J, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11: 59, 2010. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, Ernerudh J, Ekerfelt C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol 70: 83–91, 2006. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 143. Lockwood CJ, Yen C-F, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol 172: 1571–1579, 2008. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJHM. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy 28: 300–311, 2009. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 145. Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, Chavez SL, Romero R, Mor G. Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol 57: 55–66, 2007. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 146. Aldo PB, Racicot K, Craviero V, Guller S, Romero R, Mor G. Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages. Am J Reprod Immunol 72: 270–284, 2014. doi: 10.1111/aji.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Heikkinen J, Möttönen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 131: 498–505, 2003. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014: 149185, 2014. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J, Petzelbauer P, Assinger A, Schmid JA. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol 10: 85, 2019. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res 2: 823–830, 2014. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Masjedi A, Hashemi V, Hojjat-Farsangi M, Ghalamfarsa G, Azizi G, Yousefi M, Jadidi-Niaragh F. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother 108: 1415–1424, 2018. doi: 10.1016/j.biopha.2018.09.177. [DOI] [PubMed] [Google Scholar]
- 152. Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res 66: 2544–2552, 2006. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- 153. Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, and enhances NK lysis. Cell Immunol 180: 55–61, 1997. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- 154. Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat 133: 11–21, 2012. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 155. Greene LI, Bruno TC, Christenson JL, D'Alessandro A, Culp-Hill R, Torkko K, Borges VF, Slansky JE, Richer JK. A role for tryptophan-2,3-dioxygenase in CD8 T-cell suppression and evidence of tryptophan catabolism in breast cancer patient plasma. Mol Cancer Res 17: 131–139, 2019. doi: 10.1158/1541-7786.MCR-18-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ 9: 1069–1077, 2002. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 157. Siska PJ, Jiao J, Matos C, Singer K, Berger RS, Dettmer K, Oefner PJ, Cully MD, Wang Z, QuinnIII WJ, Oliff KN, Wilkins BJ, Christensen LM, Wang L, Hancock WW, Baur JA, Levine MH, Ugele I, Mayr R, Renner K, Zhou L, Kreutz M, Beier UH. Kynurenine induces T cell fat catabolism and has limited suppressive effects in vivo. EBioMedicine 74: 103734, 2021. doi: 10.1016/j.ebiom.2021.103734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sedlmayr P, Blaschitz A, Wintersteiger R, Semlitsch M, Hammer A, MacKenzie CR, Walcher W, Reich O, Takikawa O, Dohr G. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod 8: 385–391, 2002. doi: 10.1093/molehr/8.4.385. [DOI] [PubMed] [Google Scholar]
- 159. Hoffmann D. Tryptophan 2,3-dioxygenase expression identified in murine decidual stromal cells is not essential for feto-maternal tolerance. Front Immunol 11: 601759, 2020. doi: 10.3389/fimmu.2020.601759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297: 1867–1870, 2002. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 161. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193, 1998. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 162. Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol 2: 64–68, 2001. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 163. Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A, Vitale M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108: 4118–4125, 2006. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 164. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111: 1327–1333, 2008. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 165. Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol 31: 1513–1522, 2018. doi: 10.1038/s41379-018-0061-3. [DOI] [PubMed] [Google Scholar]
- 166. Wei L, Zhu S, Li M, Li F, Wei F, Liu J, Ren X. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol 9: 724, 2018. doi: 10.3389/fimmu.2018.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Folgiero V, Cifaldi L, Li Pira G, Goffredo BM, Vinti L, Locatelli F. TIM-3/Gal-9 interaction induces IFNγ-dependent IDO1 expression in acute myeloid leukemia blast cells. J Hematol Oncol 8: 36, 2015. doi: 10.1186/s13045-015-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. D'Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC, Richer JK. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res 75: 4651–4664, 2015. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhao Y, Wei L, Liu J, Li F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharmacol 85: 77–93, 2020. doi: 10.1007/s00280-019-04009-8. [DOI] [PubMed] [Google Scholar]
- 170. Gao J, Deng F, Jia W. Inhibition of indoleamine 2,3-dioxygenase enhances the therapeutic efficacy of immunogenic chemotherapeutics in breast cancer. J Breast Cancer 22: 196–209, 2019. doi: 10.4048/jbc.2019.22.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Mariotti V, Han H, Ismail-Khan R, Tang S-C, Dillon P, Montero AJ, Poklepovic A, Melin S, Ibrahim NK, Kennedy E, Vahanian N, Link C, Tennant L, Schuster S, Smith C, Danciu O, Gilman P, Soliman H. Effect of taxane chemotherapy with or without indoximod in metastatic breast cancer: a randomized clinical trial. JAMA Oncol 7: 61–69, 2021. [Erratum in JAMA Oncol 7: 140, 2021]. doi: 10.1001/jamaoncol.2020.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wang X-F, Wang H-S, Wang H, Zhang F, Wang K-F, Guo Q, Zhang G, Cai S-H, Du J. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Cell Immunol 289: 42–48, 2014. doi: 10.1016/j.cellimm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 173. Smith LP, Bitler BG, Richer JK, Christenson JL. Tryptophan catabolism in epithelial ovarian carcinoma. Trends Cancer Res 14: 1–9, 2019. [PMC free article] [PubMed] [Google Scholar]
- 174. Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci USA 114: E6566–E6575, 2017. doi: 10.1073/pnas.1701129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fornetti J, Jindal S, Middleton KA, Borges VF, Schedin P. Physiological COX-2 expression in breast epithelium associates with COX-2 levels in ductal carcinoma in situ and invasive breast cancer in young women. Am J Pathol 184: 1219–1229, 2014. doi: 10.1016/j.ajpath.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Pennock ND, Martinson HA, Guo Q, Betts CB, Jindal S, Tsujikawa T, Coussens LM, Borges VF, Schedin P. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J Immunother Cancer 6: 98, 2018. doi: 10.1186/s40425-018-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 10: 181–193, 2010. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res 9: 210, 2007. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer 90: 423–429, 2004. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wülfing P, Diallo R, Müller C, Wülfing C, Poremba C, Heinecke A, Rody A, Greb RR, Böcker W, Kiesel L. Analysis of cyclooxygenase-2 expression in human breast cancer: high throughput tissue microarray analysis. J Cancer Res Clin Oncol 129: 375–382, 2003. doi: 10.1007/s00432-003-0459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res 62: 632–635, 2002. [PubMed] [Google Scholar]
- 182. Nandi P, Girish GV, Majumder M, Xin X, Tutunea-Fatan E, Lala PK. PGE2 promotes breast cancer-associated lymphangiogenesis by activation of EP4 receptor on lymphatic endothelial cells. BMC Cancer 17: 11, 2017. doi: 10.1186/s12885-016-3018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Majumder M, Xin X, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci 105: 1142–1151, 2014. doi: 10.1111/cas.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. McLaughlin BE, Lash GE, Smith GN, Marks GS, Nakatsu K, Graham CH, Brien JF. Heme oxygenase expression in selected regions of term human placenta. Exp Biol Med (Maywood) 228: 564–567, 2003. doi: 10.1177/15353702-0322805-28. [DOI] [PubMed] [Google Scholar]
- 185. Denschlag D. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol Hum Reprod 10: 211–214, 2004. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- 186. Sollwedel A, Bertoja AZ, Zenclussen ML, Gerlof K, Lisewski U, Wafula P, Sawitzki B, Woiciechowsky C, Volk H-D, Zenclussen AC. Protection from abortion by heme oxygenase-1 up-regulation is associated with increased levels of Bag-1 and neuropilin-1 at the fetal-maternal interface. J Immunol 175: 4875–4885, 2005. doi: 10.4049/jimmunol.175.8.4875. [DOI] [PubMed] [Google Scholar]
- 187. Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, Gerlof K, Sollwedel A, Volk H-D, Ritter T, Zenclussen AC. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol 69: 35–52, 2006. doi: 10.1016/j.jri.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 188. Schumacher A, Wafula PO, Teles A, El-Mousleh T, Linzke N, Zenclussen ML, Langwisch S, Heinze K, Wollenberg I, Casalis PA, Volk H-D, Fest S, Zenclussen AC. Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS One 7: e42301, 2012. doi: 10.1371/journal.pone.0042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wegiel B, Hedblom A, Li M, Gallo D, Csizmadia E, Harris C, Nemeth Z, Zuckerbraun BS, Soares M, Persson JL, Otterbein LE. Heme oxygenase-1 derived carbon monoxide permits maturation of myeloid cells. Cell Death Dis 5: e1139, 2014. doi: 10.1038/cddis.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Immenschuh S, Tan M, Ramadori G. Nitric oxide mediates the lipopolysaccharide dependent upregulation of the heme oxygenase-1 gene expression in cultured rat Kupffer cells. J Hepatol 30: 61–69, 1999. doi: 10.1016/S0168-8278(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 191. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 192. Alaluf E, Vokaer B, Detavernier A, Azouz A, Splittgerber M, Carrette A, Boon L, Libert F, Soares MP, Le Moine A, Goriely S. Heme oxygenase-1 orchestrates the immunosuppressive program of tumor-associated macrophages. JCI Insight 5: e133929, 2020. doi: 10.1172/jci.insight.133929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Pei L, Kong Y, Shao C, Yue X, Wang Z, Zhang N. Heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to pharmorubicin by promoting autophagy via PI3K/Akt pathway. J Cell Mol Med 22: 5311–5321, 2018. doi: 10.1111/jcmm.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tan Q, Wang H, Hu Y, Hu M, Li X, Ma Y, Wei C, Song L. Src/STAT3-dependent heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to doxorubicin by promoting autophagy. Cancer Sci 106: 1023–1032, 2015. doi: 10.1111/cas.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhu X-F, Li W, Ma J-Y, Shao N, Zhang Y-J, Liu R-M, Wu W-B, Lin Y, Wang S-M. Knockdown of heme oxygenase-1 promotes apoptosis and autophagy and enhances the cytotoxicity of doxorubicin in breast cancer cells. Oncol Lett 10: 2974–2980, 2015. doi: 10.3892/ol.2015.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Muliaditan T, Opzoomer JW, Caron J, Okesola M, Kosti P, Lall S, Van Hemelrijck M, Dazzi F, Tutt A, Grigoriadis A, Gillett CE, Madden SF, Burchell JM, Kordasti S, Diebold SS, Spicer JF, Arnold JN. Repurposing tin mesoporphyrin as an immune checkpoint inhibitor shows therapeutic efficacy in preclinical models of cancer. Clin Cancer Res 24: 1617–1628, 2018. doi: 10.1158/1078-0432.CCR-17-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Khojandi N, Kuehm LM, Piening A, Donlin MJ, Hsueh EC, Schwartz TL, Farrell K, Richart JM, Geerling E, Pinto AK, George SL, Albert CJ, Ford DA, Chen X, Kline J, Teague RM. Oxidized lipoproteins promote resistance to cancer immunotherapy independent of patient obesity. Cancer Immunol Res 9: 214–226, 2021. doi: 10.1158/2326-6066.CIR-20-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kuehm LM, Khojandi N, Piening A, Klevorn LE, Geraud SC, McLaughlin NR, Griffett K, Burris TP, Pyles KD, Nelson AM, Preuss ML, Bockerstett KA, Donlin MJ, McCommis KS, DiPaolo RJ, Teague RM. Fructose promotes cytoprotection in melanoma tumors and resistance to immunotherapy. Cancer Immunol Res 9: 227–238, 2021. doi: 10.1158/2326-6066.CIR-20-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Deng R, Wang S-M, Yin T, Ye T-H, Shen G-B, Li L, Zhao J-Y, Sang Y-X, Duan X-G, Wei Y-Q. Inhibition of tumor growth and alteration of associated macrophage cell type by an HO-1 inhibitor in breast carcinoma-bearing mice. Oncol Res 20: 473–482, 2013. doi: 10.3727/096504013x13715991125684. [DOI] [PubMed] [Google Scholar]
- 200. Muliaditan T, Caron J, Okesola M, Opzoomer JW, Kosti P, Georgouli M, Gordon P, Lall S, Kuzeva DM, Pedro L, Shields JD, Gillett CE, Diebold SS, Sanz-Moreno V, Ng T, Hoste E, Arnold JN. Macrophages are exploited from an innate wound healing response to facilitate cancer metastasis. Nat Commun 9: 2951, 2018. doi: 10.1038/s41467-018-05346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, Turgeon M-O, Fish L, Erard N, Gable AL, Maceli AR, Dickopf S, Papachristou EK, D’Santos CS, Carey LA, Wilkinson JE, Harrell JC, Perou CM, Goodarzi H, Poulogiannis G, Hannon GJ. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554: 378–381, 2018. [Erratum in Nature 556: 135, 2018]. doi: 10.1038/nature25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pae H-O, Oh G-S, Choi B-M, Chae S-C, Chung H-T. Differential expressions of heme oxygenase-1 gene in CD25- and CD25+ subsets of human CD4+ T cells. Biochem Biophys Res Commun 306: 701–705, 2003. doi: 10.1016/S0006-291X(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 203. Choi B-M, Pae H-O, Jeong Y-R, Kim Y-M, Chung H-T. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem Biophys Res Commun 327: 1066–1071, 2005. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 204. Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert F-X, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 106: 1694–1702, 2005. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 205. Rémy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol 182: 1877–1884, 2009. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 206. Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol 69: 395–407, 2013. doi: 10.1111/aji.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Kontani K, Sawai S, Hanaoka J, Tezuka N, Inoue S, Fujino S. Involvement of granzyme B and perforin in suppressing nodal metastasis of cancer cells in breast and lung cancers. Eur J Surg Oncol 27: 180–186, 2001. doi: 10.1053/ejso.2000.1060. [DOI] [PubMed] [Google Scholar]
- 208. Guo Q, Minnier J, Burchard J, Chiotti K, Spellman P, Schedin P. Physiologically activated mammary fibroblasts promote postpartum mammary cancer. JCI Insight 2: e89206, 2017. doi: 10.1172/jci.insight.89206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhang Y-H, Aldo P, You Y, Ding J, Kaislasuo J, Petersen JF, Lokkegaard E, Peng G, Paidas MJ, Simpson S, Pal L, Guller S, Liu H, Liao AH, Mor G. Trophoblast-secreted soluble-PD-L1 modulates macrophage polarization and function. J Leukoc Biol 108: 983–998, 2020. doi: 10.1002/JLB.1A0420-012RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Enninga EAL, Harrington SM, Creedon DJ, Ruano R, Markovic SN, Dong H, Dronca RS. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol 79: e12795, 2018. doi: 10.1111/aji.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Liu L, Huang X, Xu C, Chen C, Zhao W, Li D, Li L, Wang L, Du M. Decidual CD8(+)T cells exhibit both residency and tolerance signatures modulated by decidual stromal cells. J Transl Med 18: 221, 2020. doi: 10.1186/s12967-020-02371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wang R, Huang F, Wei W, Zhou Y, Ye Z, Yu L, Hu J, Cai C. Programmed cell death ligand 1 is enriched in mammary stem cells and promotes mammary development and regeneration. Front Cell Dev Biol 9: 772669, 2021. doi: 10.3389/fcell.2021.772669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11: 3801, 2020. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Liu X, Hogg GD, DeNardo DG. Rethinking immune checkpoint blockade: 'beyond the T cell'. J Immunother Cancer 9: e001460, 2021. doi: 10.1136/jitc-2020-001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Goodman CS, Kolodkin AL, Luo Y, Püschel AW, Raper JA. Unified nomenclature for the semaphorins/collapsins. Cell 97: 551–552, 1999. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- 216. Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci 33: 161–170, 2008. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 217. Liu H, Juo ZS, Shim AH-R, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell 142: 749–761, 2010. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99: 71–80, 1999. [Erratum in Cell 104: following 320, 2001]. doi: 10.1016/S0092-8674(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 219. Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424: 398–405, 2003. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 220. Bobolis KA, Moulds JJ, Telen MJ. Isolation of the JMH antigen on a novel phosphatidylinositol-linked human membrane protein. Blood 79: 1574–1581, 1992. doi: 10.1182/blood.V79.6.1574.1574. [DOI] [PubMed] [Google Scholar]
- 221. Cerný J, Stockinger H, Horejsí V. Noncovalent associations of T lymphocyte surface proteins. Eur J Immunol 26: 2335–2343, 1996. doi: 10.1002/eji.1830261010. [DOI] [PubMed] [Google Scholar]
- 222. Mine T, Harada K, Matsumoto T, Yamana H, Shirouzu K, Itoh K, Yamada A. CDw108 expression during T-cell development. Tissue Antigens 55: 429–436, 2000. doi: 10.1034/j.1399-0039.2000.550505.x. [DOI] [PubMed] [Google Scholar]
- 223. Angelisova P, Drbal K, Ćervý J, Hilgert I, Hořejšf V. Characterization of the human leukocyte GPI-anchored glycoprotein CDw108 and its relation to other similar molecules. Immunobiology 200: 234–245, 1999. doi: 10.1016/S0171-2985(99)80073-4. [DOI] [PubMed] [Google Scholar]
- 224. Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K, Fox J, Deen K, Pettman G, Wattam T, Lewis C. Sema7A is a potent monocyte stimulator. Scand J Immunol 56: 270–275, 2002. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 225. Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol 174: 51–59, 2005. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- 226. Czopik AK, Bynoe MS, Palm N, Raine CS, Medzhitov R. Semaphorin 7A is a negative regulator of T cell responses. Immunity 24: 591–600, 2006. doi: 10.1016/j.immuni.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 227. van Rijn A, Paulis L, Te Riet J, Vasaturo A, Reinieren-Beeren I, van der Schaaf A, Kuipers AJ, Schulte LP, Jongbloets BC, Pasterkamp RJ, Figdor CG, van Spriel AB, Buschow SI. Semaphorin 7A promotes chemokine-driven dendritic cell migration. J Immunol 196: 459–468, 2016. doi: 10.4049/jimmunol.1403096. [DOI] [PubMed] [Google Scholar]
- 228. Ghofrani J, Lucar O, Dugan H, Reeves RK, Jost S. Semaphorin 7A modulates cytokine-induced memory-like responses by human natural killer cells. Eur J Immunol 49: 1153–1166, 2019. doi: 10.1002/eji.201847931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kang S, Okuno T, Takegahara N, Takamatsu H, Nojima S, Kimura T, Yoshida Y, Ito D, Ohmae S, You D-J, Toyofuku T, Jang MH, Kumanogoh A. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via alphavbeta1 integrin. J Immunol 188: 1108–1116, 2012. doi: 10.4049/jimmunol.1102084. [DOI] [PubMed] [Google Scholar]
- 230. Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, Kumanogoh A, Kikutani H. Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1 integrin. Nature 446: 680–684, 2007. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 231. Okuno T, Nakatsuji Y, Kumanogoh A. The role of immune semaphorins in multiple sclerosis. FEBS Lett 585: 3829–3835, 2011. doi: 10.1016/j.febslet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 232. Gras C, Eiz-Vesper B, Seltsam A, Immenschuh S, Blasczyk R, Figueiredo C. Semaphorin 7A protein variants differentially regulate T-cell activity. Transfusion 53: 270–283, 2013. doi: 10.1111/j.1537-2995.2012.03812.x. [DOI] [PubMed] [Google Scholar]
- 233. Saben J, Zhong Y, McKelvey S, Dajani NK, Andres A, Badger TM, Gomez-Acevedo H, Shankar K. A comprehensive analysis of the human placenta transcriptome. Placenta 35: 125–131, 2014. doi: 10.1016/j.placenta.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, Borges VF. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat 138: 549–559, 2013. doi: 10.1007/s10549-013-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Goddard ET, Bassale S, Schedin T, Jindal S, Johnston J, Cabral E, Latour E, Lyons TR, Mori M, Schedin PJ, Borges VF. Association between postpartum breast cancer diagnosis and metastasis and the clinical features underlying risk. JAMA Netw Open 2: e186997, 2019. doi: 10.1001/jamanetworkopen.2018.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol 3: a003228, 2011. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rutherford TR, Elder AM, Lyons TR. Anoikis resistance in mammary epithelial cells is mediated by semaphorin 7a. Cell Death Dis 12: 872, 2021. doi: 10.1038/s41419-021-04133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lazova R, Gould Rothberg BE, Rimm D, Scott G. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am J Dermatopathol 31: 177–181, 2009. doi: 10.1097/DAD.0b013e318196672d. [DOI] [PubMed] [Google Scholar]
- 239. Ma B, Herzog EL, Lee CG, Peng X, Lee C-M, Chen X, Rockwell S, Koo JS, Kluger H, Herbst RS, Sznol M, Elias JA. Role of chitinase 3-like-1 and semaphorin 7a in pulmonary melanoma metastasis. Cancer Res 75: 487–496, 2015. doi: 10.1158/0008-5472.CAN-13-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Scott GA, McClelland LA, Fricke AF, Fender A. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol 129: 954–963, 2009. doi: 10.1038/jid.2008.329. [DOI] [PubMed] [Google Scholar]
- 241. Kinehara Y, Nagatomo I, Koyama S, Ito D, Nojima S, Kurebayashi R, Nakanishi Y, Suga Y, Nishijima-Futami Y, Osa A, Nakatani T, Kato Y, Nishide M, Hayama Y, Higashiguchi M, Morimura O, Miyake K, Kang S, Minami T, Hirata H, Iwahori K, Takimoto T, Takamatsu H, Takeda Y, Hosen N, Hoshino S, Shintani Y, Okumura M, Kumagai T, Nishino K. Semaphorin 7A promotes EGFR-TKI resistance in EGFR mutant lung adenocarcinoma cells. JCI Insight 3: e123093, 2018. doi: 10.1172/jci.insight.123093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Crump LS, Wyatt GL, Rutherford TR, Richer JK, Porter WW, Lyons TR. Hormonal regulation of semaphorin 7a in ER(+) breast cancer drives therapeutic resistance. Cancer Res 81: 187–198, 2021. doi: 10.1158/0008-5472.CAN-20-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Black SA, Nelson AC, Gurule NJ, Futscher BW, Lyons TR. Semaphorin 7a exerts pleiotropic effects to promote breast tumor progression. Oncogene 35: 5170–5178, 2016. doi: 10.1038/onc.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Allegra M, Zaragkoulias A, Vorgia E, Ioannou M, Litos G, Beug H, Mavrothalassitis G. Semaphorin-7a reverses the ERF-induced inhibition of EMT in Ras-dependent mouse mammary epithelial cells. Mol Biol Cell 23: 3873–3881, 2012. doi: 10.1091/mbc.E12-04-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Garcia-Areas R, Libreros S, Simoes M, Castro-Silva C, Gazaniga N, Amat S, Jaczewska J, Keating P, Schilling K, Brito M, Wojcikiewicz EP, Iragavarpu-Charyulu V. Suppression of tumor-derived Semaphorin 7A and genetic ablation of host-derived Semaphorin 7A impairs tumor progression in a murine model of advanced breast carcinoma. Int J Oncol 51: 1395–1404, 2017. doi: 10.3892/ijo.2017.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Donnenberg VS, Zhang JJ, Moravcikova E, Meyer EM, Lu H, Carson CT, Donnenberg AD. Antibody-based cell-surface proteome profiling of metastatic breast cancer primary explants and cell lines. Cytometry A 93: 448–457, 2018. doi: 10.1002/cyto.a.23300. [DOI] [PubMed] [Google Scholar]
- 247. Tarullo SE, Hill RC, Hansen KC, Behbod F, Borges VF, Nelson AC, Lyons TR. Postpartum breast cancer progression is driven by semaphorin 7a-mediated invasion and survival. Oncogene 39: 2772–2785, 2020. doi: 10.1038/s41388-020-1192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Krause SW, Rehli M, Kreutz M, Schwarzfischer L, Paulauskis JD, Andreesen R. Differential screening identifies genetic markers of monocyte to macrophage maturation. J Leukoc Biol 60: 540–545, 1996. doi: 10.1002/jlb.60.4.540. [DOI] [PubMed] [Google Scholar]
- 249. Kirkpatrick RB, Matico RE, McNulty DE, Strickler JE, Rosenberg M. An abundantly secreted glycoprotein from Drosophila melanogaster is related to mammalian secretory proteins produced in rheumatoid tissues and by activated macrophages. Gene 153: 147–154, 1995. doi: 10.1016/0378-1119(94)00756-I. [DOI] [PubMed] [Google Scholar]
- 250. Volck B, Price PA, Johansen JS, Sørensen O, Benfield TL, Nielsen HJ, Calafat J, Borregaard N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians 110: 351–360, 1998. [PubMed] [Google Scholar]
- 251. Kim D-H, Park H-J, Lim S, Koo J-H, Lee H-G, Choi JO, Oh JH, Ha S-J, Kang M-J, Lee C-M, Lee CG, Elias JA, Choi J-M. Regulation of chitinase-3-like-1 in T cell elicits Th1 and cytotoxic responses to inhibit lung metastasis. Nat Commun 9: 503, 2018. doi: 10.1038/s41467-017-02731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Aktulay A, Engin-Ustun Y, Ozkan MS, Erkaya S, Kara M, Kaymak O, Danisman N. Gestational diabetes mellitus seems to be associated with inflammation. Acta Clin Croat 54: 475–478, 2015. [PubMed] [Google Scholar]
- 253. Watson CJ, Kreuzaler PA. Remodeling mechanisms of the mammary gland during involution. Int J Dev Biol 55: 757–762, 2011. doi: 10.1387/ijdb.113414cw. [DOI] [PubMed] [Google Scholar]
- 254. Hughes K, Watson CJ. The role of Stat3 in mammary gland involution: cell death regulator and modulator of inflammation. Horm Mol Biol Clin Investig 10: 211–215, 2012. doi: 10.1515/hmbci-2012-0008. [DOI] [PubMed] [Google Scholar]
- 255. Scully S, Yan W, Bentley B, Cao QJ, Shao R. Inhibitory activity of YKL-40 in mammary epithelial cell differentiation and polarization induced by lactogenic hormones: a role in mammary tissue involution. PLoS One 6: e25819, 2011. doi: 10.1371/journal.pone.0025819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Tran HT, Lee I-A, Low D, Kamba A, Mizoguchi A, Shi HN, Lee CG, Elias JA, Mizoguchi E. Chitinase 3-like 1 synergistically activates IL6-mediated STAT3 phosphorylation in intestinal epithelial cells in murine models of infectious colitis. Inflamm Bowel Dis 20: 835–846, 2014. doi: 10.1097/MIB.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Shao R, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers. Br J Cancer 105: 1203–1209, 2011. doi: 10.1038/bjc.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer 46: 333–340, 2004. doi: 10.1016/j.lungcan.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 259. Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res 9: 4423–4434, 2003. [PubMed] [Google Scholar]
- 260. Ma B, Herzog EL, Moore M, Lee C-M, Na SH, Lee CG, Elias JA. RIG-like helicase regulation of chitinase 3-like 1 axis and pulmonary metastasis. Sci Rep 6: 26299, 2016. doi: 10.1038/srep26299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol 10: 36, 2017. doi: 10.1186/s13045-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, Coyle A, Chupp G, Reed J, Flavell RA, Elias JA. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med 206: 1149–1166, 2009. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L, Erez N. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene 36: 4457–4468, 2017. doi: 10.1038/onc.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ma B, Akosman B, Kamle S, Lee CM, He CH, Koo JS, Lee CG, Elias JA. CHI3L1 regulates PD-L1 and anti-CHI3L1-PD-1 antibody elicits synergistic antitumor responses. J Clin Invest 131: e137750, 2021. doi: 10.1172/JCI137750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Haagensen DE, Gall SA, Brazy JE, Giannola J, Wells SA Jr.. Analysis of amniotic fluid, maternal plasma, and cord blood for a human breast gross cystic disease fluid protein. Am J Obstet Gynecol 138: 25–32, 1980. doi: 10.1016/0002-9378(80)90007-1. [DOI] [PubMed] [Google Scholar]
- 266. Dumont M, Dauvois S, Simard J, Garcia T, Schachter B, Labrie F. Antagonism between estrogens and androgens on GCDFP-15 gene expression in ZR-75-1 cells and correlation between GCDFP-15 and estrogen as well as progesterone receptor expression in human breast cancer. J Steroid Biochem 34: 397–402, 1989. doi: 10.1016/0022-4731(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 267. Blais Y, Sugimoto K, Carriere MC, Haagensen DE, Labrie F, Simard J. Potent stimulatory effect of interleukin-1 alpha on apolipoprotein D and gross cystic disease fluid protein-15 expression in human breast-cancer cells. Int J Cancer 59: 400–407, 1994. doi: 10.1002/ijc.2910590319. [DOI] [PubMed] [Google Scholar]
- 268. Blais Y, Sugimoto K, Carrière MC, Haagensen DE, Labrie F, Simard J. Interleukin-6 inhibits the potent stimulatory action of androgens, glucocorticoids and interleukin-1 α on apolipoprotein D and GCDFP-15 expression in human breast cancer cells. Int J Cancer 62: 732–737, 1995. doi: 10.1002/ijc.2910620614. [DOI] [PubMed] [Google Scholar]
- 269. Lee H-W, Shin J, Wilson BS, Oh J-W. Peripheral immune tolerance by prolactin-induced protein originated from human invariant natural killer T cells. Bioengineered 12: 461–475, 2021. doi: 10.1080/21655979.2021.1875664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Bertucci PY, Quaglino A, Pozzi AG, Kordon EC, Pecci A. Glucocorticoid-induced impairment of mammary gland involution is associated with STAT5 and STAT3 signaling modulation. Endocrinology 151: 5730–5740, 2010. doi: 10.1210/en.2010-0517. [DOI] [PubMed] [Google Scholar]
- 271. de Almeida PC, Pestana CB. Immunohistochemical markers in the identification of metastatic breast cancer. Breast Cancer Res Treat 21: 201–210, 1992. doi: 10.1007/BF01975003. [DOI] [PubMed] [Google Scholar]
- 272. Fiel MI, Cernaianu G, Burstein DE, Batheja N. Value of GCDFP-15 (BRST-2) as a specific immunocytochemical marker for breast carcinoma in cytologic specimens. Acta Cytol 40: 637–641, 1996. doi: 10.1159/000333931. [DOI] [PubMed] [Google Scholar]
- 273. Clark JW, Snell L, Shiu RP, Orr FW, Maitre N, Vary CP, Cole DJ, Watson PH. The potential role for prolactin-inducible protein (PIP) as a marker of human breast cancer micrometastasis. Br J Cancer 81: 1002–1008, 1999. doi: 10.1038/sj.bjc.6690799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Mazoujian G, Pinkus GS, Davis S, Haagensen DE. Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol 110: 105–112, 1983. [PMC free article] [PubMed] [Google Scholar]
- 275. Dauvois S, Simard J, Dumont M, Haagensen DE, Labrie F. Opposite effects of estrogen and the progestin R5020 on cell proliferation and GCDFP-15 expression in ZR-75-1 human breast cancer cells. Mol Cell Endocrinol 73: 171–178, 1990. doi: 10.1016/0303-7207(90)90130-Z. [DOI] [PubMed] [Google Scholar]
- 276. Edechi CA, Ikeogu NM, Akaluka GN, Terceiro LEL, Machado M, Salako ES, Barazandeh AF, Kung SKP, Uzonna JE, Myal Y. The Prolactin Inducible Protein Modulates Antitumor Immune Responses and Metastasis in a Mouse Model of Triple Negative Breast Cancer. Front Oncol 11: 639859, 2021. doi: 10.3389/fonc.2021.639859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Vićovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 156: 202–216, 1996. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 278. Su M-T, Tsai P-Y, Wang C-Y, Tsai H-L, Kuo P-L. Aspirin facilitates trophoblast invasion and epithelial-mesenchymal transition by regulating the miR-200-ZEB1 axis in preeclampsia. Biomed Pharmacother 139: 111591, 2021. doi: 10.1016/j.biopha.2021.111591. [DOI] [PubMed] [Google Scholar]
- 279. Anastasiadou E, Ceccarelli S, Messina E, Gerini G, Megiorni F, Pontecorvi P, Camero S, Onesti MG, Trivedi P, Faenza M, Coscioni E, Nicoletti GF, Napoli C, Marchese C. MiR-200c-3p maintains stemness and proliferative potential in adipose-derived stem cells by counteracting senescence mechanisms. PLoS One 16: e0257070, 2021. doi: 10.1371/journal.pone.0257070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J, Wistuba II, Heymach JV, Weinstein JN, Coombes KR, Wang J, Byers LA. A Patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res 22: 609–620, 2016. doi: 10.1158/1078-0432.CCR-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Rogers TJ, Christenson JL, Greene LI, O'Neill KI, Williams MM, Gordon MA, Nemkov T, D'Alessandro A, Degala GD, Shin J, Tan A-C, Cittelly DM, Lambert JR, Richer JK. Reversal of triple-negative breast cancer EMT by miR-200c decreases tryptophan catabolism and a program of immunosuppression. Mol Cancer Res 17: 30–41, 2019. doi: 10.1158/1541-7786.MCR-18-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proc Natl Acad Sci USA 98: 6686–6691, 2001. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Wakefield LM, Piek E, Bottinger EP. TGF-β signaling in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia 6: 67–82, 2001. doi: 10.1023/A:1009568532177. [DOI] [PubMed] [Google Scholar]
- 284. Fornetti J, Flanders KC, Henson PM, Tan A-C, Borges VF, Schedin P. Mammary epithelial cell phagocytosis downstream of TGF-β3 is characterized by adherens junction reorganization. Cell Death Differ 23: 185–196, 2016. doi: 10.1038/cdd.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Zhang H, Liu Y, Weng J, Usuda K, Fujii K, Watanabe G, Nagaoka K. Decrease of lactogenic hormones induce epithelial-mesenchymal transition via TGFβ1 and arachidonic acid during mammary gland involution. J Reprod Dev 63: 325–332, 2017. doi: 10.1262/jrd.2016-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Biswas S, Mandal G, Roy Chowdhury S, Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X, Conejo-Garcia JR, Bhattacharyya A. Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive M2-polarized macrophages in breast cancer. J Immunol 203: 3447–3460, 2019. doi: 10.4049/jimmunol.1900692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Castillo-Lluva S, Hontecillas-Prieto L, Blanco-Gómez A, Del Mar Sáez-Freire M, García-Cenador B, García-Criado J, Pérez-Andrés M, Orfao A, Cañamero M, Mao JH, Gridley T, Castellanos-Martín A, Pérez-Losada J. A new role of SNAI2 in postlactational involution of the mammary gland links it to luminal breast cancer development. Oncogene 34: 4777–4790, 2015. [Erratum in Oncogene 34: 4797–4798, 2015]. doi: 10.1038/onc.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Wendt MK, Balanis N, Carlin CR, Schiemann WP. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT 3: e28975, 2014. doi: 10.4161/jkst.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Williams MM, Hafeez SA, Christenson JL, O’Neill KI, Hammond NG, Richer JK. Reversing an oncogenic epithelial-to-mesenchymal transition program in breast cancer reveals actionable immune suppressive pathways. Pharmaceuticals (Basel) 14: 1122, 2021. doi: 10.3390/ph14111122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res 77: 3982–3989, 2017. doi: 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal J, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 5: 5241, 2014. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Noman MZ, Janji B, Abdou A, Hasmim M, Terry S, Tan TZ, Mami-Chouaib F, Thiery JP, Chouaib S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology 6: e1263412, 2017. doi: 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Gatti-Mays ME, Balko JM, Gameiro SR, Bear HD, Prabhakaran S, Fukui J, Disis ML, Nanda R, Gulley JL, Kalinsky K, Abdul Sater H, Sparano JA, Cescon D, Page DB, McArthur H, Adams S, Mittendorf EA. If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer 5: 37, 2019. doi: 10.1038/s41523-019-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature 574: 45–56, 2019. [Erratum in Nature 576: E3, 2019]. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 295. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord J-P, Levitsky H, Blay J-Y, Rüttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25: 846–859, 2014. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 296. Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, Murphy M, Schaer D, Wolchok JD, Merghoub T. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology 5: e1151595, 2016. doi: 10.1080/2162402X.2016.1151595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Zhang Z, Lu M, Qin Y, Gao W, Tao L, Su W, Zhong J. Neoantigen: a new breakthrough in tumor immunotherapy. Front Immunol 12: 672356, 2021. doi: 10.3389/fimmu.2021.672356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Raghavendra A, Kalita-de Croft P, Vargas AC, Smart CE, Simpson PT, Saunus JM, Lakhani SR. Expression of MAGE-A and NY-ESO-1 cancer/testis antigens is enriched in triple-negative invasive breast cancers. Histopathology 73: 68–80, 2018. doi: 10.1111/his.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Tessari A, Pilla L, Silvia D, Duca M, Paolini B, Carcangiu ML, Mariani L, de Braud FG, Cresta S. Expression of NY-ESO-1, MAGE-A3, PRAME and WT1 in different subgroups of breast cancer: an indication to immunotherapy? Breast 42: 68–73, 2018. doi: 10.1016/j.breast.2018.08.106. [DOI] [PubMed] [Google Scholar]
- 300. Satie A-P, Rajpert-De Meyts E, Spagnoli GC, Henno S, Olivo L, Jacobsen GK, Rioux-Leclercq N, Jégou B, Samson M. The cancer-testis gene, NY-ESO-1, is expressed in normal fetal and adult testes and in spermatocytic seminomas and testicular carcinoma in situ. Lab Invest 82: 775–780, 2002. doi: 10.1097/01.lab.0000017169.26718.5f. [DOI] [PubMed] [Google Scholar]
- 301. Sahin U, Derhovanessian E, Miller M, Kloke B-P, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547: 222–226, 2017. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 302.Society for Immunotherapy of Cancer. 34th Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2019): part 1: National Harbor, MD, USA. 6-10 November 2019. J Immunother Cancer 7, Suppl 1: 282. 2019. doi: 10.1186/s40425-019-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, Friedlander T, Bushway ME, Balogh KN, Sciuto TE, Kohler V, Turnbull SJ, Besada R, Curran RR, Trapp B, Scherer J, Poran A, Harjanto D, Barthelme D, Ting YS, Dong JZ, Ware Y, Huang Y, Huang Z, Wanamaker A, Cleary LD. A phase Ib trial of personalized neoantigen therapy plus Anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 183: 347–362.e24, 2020. doi: 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 304. Crespo ÂC, Mulik S, Dotiwala F, Ansara JA, Sen Santara S, Ingersoll K, Ovies C, Junqueira C, Tilburgs T, Strominger JL, Lieberman J. Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell 182: 1125–1139.e18, 2020. doi: 10.1016/j.cell.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Saha T, Dash C, Jayabalan R, Khiste S, Kulkarni A, Kurmi K, Mondal J, Majumder PK, Bardia A, Jang HL, Sengupta S. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat Nanotechnol 17: 98–106, 2022. doi: 10.1038/s41565-021-01000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J Immunol 173: 1511–1513, 2004. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]


