Abstract
Helicobacter pylori has been widely recognized as an important human pathogen responsible for chronic gastritis, peptic ulcers, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma. Little is known about the natural history of this infection since patients are usually recognized as having the infection only after years or decades of chronic disease. Several animal models of H. pylori infection, including those with different species of rodents, nonhuman primates, and germ-free animals, have been developed. Here we describe a new animal model in which the clinical, pathological, microbiological, and immunological aspects of human acute and chronic infection are mimicked and which allows us to monitor these aspects of infection within the same individuals. Conventional Beagle dogs were infected orally with a mouse-adapted strain of H. pylori and monitored for up to 24 weeks. Acute infection caused vomiting and diarrhea. The acute phase was followed by polymorphonuclear cell infiltration, interleukin 8 induction, mononuclear cell recruitment, and the appearance of a specific antibody response against H. pylori. The chronic phase was characterized by gastritis, epithelial alterations, superficial erosions, and the appearance of the typical macroscopic follicles that in humans are considered possible precursors of MALT lymphoma. In conclusion, infection in this model mimics closely human infection and allows us to study those phases that cannot be studied in humans. This new model can be a unique tool for learning more about the disease and for developing strategies for treatment and prevention.
Chronic infection of human gastroduodenal mucosae by Helicobacter pylori is associated with chronic gastritis and peptic ulcers and increases the risk of occurrence of gastric malignancies such as adenocarcinoma and low-grade B-cell lymphoma (5, 20, 55, 56). The occurrence of these pathologies correlates epidemiologically with infection by a particular subset of H. pylori strains, called type I strains (6, 11, 12, 21, 67). This subset of strains is endowed with increased virulence due to the expression of a biologically active toxin (VacA), which is cytopathic to gastric epithelial cells in vitro and in vivo (27, 31, 63), and also due to the acquisition of a pathogenicity island, called cag, which contains a set of genes encoding several virulence factors (8) that are involved in the induction of tyrosine phosphorylation, pedestal formation, NF-κB activation, and synthesis of the neutrophil chemotactic cytokine interleukin 8 (IL-8) in gastric epithelial cells (8).
Several animal models of H. pylori infection and disease, including those with several rodent species and nonhuman primates, have been proposed. Some of these models also employ species that are kept under gnotobiotic conditions. Among these are gnotobiotic piglets (41), specific-pathogen-free cats (24, 30), gnotobiotic beagle pups (59), and athymic nu/nu or germ-free mice (39). However, the need to maintain these animals under germ-free conditions for long periods of time renders these models impractical, also because they are technologically sophisticated and particularly expensive. Furthermore, the peculiar immunological status of the gnotobiotic or immunodeficient hosts employed may jeopardize the physiology of infection and the outcome of the immune response.
More recently, a euthymic, not germ-free, mouse model of H. pylori infection has been developed. In this model, H. pylori freshly isolated from human gastroduodenal biopsies have been adapted to persistently colonize the gastric mucosae of mice (47). This model has proven particularly useful for assessing the feasibility of either preventive (46–48, 58) or therapeutic (28) vaccination, as well as for the in vivo screening of anti-H. pylori antimicrobials (43) and for studying the pathogenesis of infection (22, 60). However, infected mice do not develop symptoms and they need to be sacrificed in order to evaluate gastric infection. Thus, the pathological changes induced by chronic infection and/or the effects of therapeutic or immunizing regimens cannot be followed up in the same individual.
A more physiologically relevant animal model in which infection resembles closely human H. pylori infection would certainly be desirable. Nonhuman primates have been proposed as a model of experimental infection with H. pylori. However, several factors, including cost and housing, do not allow the extensive application of this model. Furthermore, most of these monkeys (e.g., rhesus monkeys) are usually spontaneously infected with Helicobacter (19).
Conventional beagle dogs have already been used to reproduce experimental infections with human pathogens such as Yersinia enterocolitica (32), Borrelia burgdorferi (9), and Leishmania infantum (4). Furthermore, it has been reported that the gastroduodenal mucosae of conventional dogs may be naturally colonized by some gastrospirilla (35, 36), which may occasionally cause mild gastritis, but not by H. pylori (36).
In this study we have assessed the feasibility of establishing H. pylori infection in conventional dogs, using a strain of H. pylori previously adapted to the mouse (47). We report that H. pylori can colonize the gastric mucosae of conventional beagle dogs, causing both acute symptoms and long-term chronic infection. The animal model described here is unique because it is the only model in which the animals show acute symptoms that resemble some of those described during experimental infection of humans.
MATERIALS AND METHODS
H. pylori strain.
SPM326s, a streptomycin-resistant derivative of the mouse-adapted H. pylori type I (CagA+ VacA+) strain SPM326 (47), was obtained by allelic exchange of the S12 gene with a mutated gene sequence harbored by a naturally occurring streptomycin-resistant H. pylori strain. This strain has been shown to be as infective and virulent in mice as its parental strain. Details of the experimental procedure followed to obtain this strain and of the strain’s infectivity in mice will be described elsewhere (47a).
Animals and experimental design.
Three 4- to 6-month-old conventional beagle dogs, one male and two females (Morini SpA, San Polo D’Enza, Reggio Emilia, Italy), were selected on the basis of the absence of detectable immunoglobulin G (IgG) against H. pylori in serum in Western blot (WB) analysis with total bacterial lysate as the antigen (see below). The three dogs selected were housed under standard conditions and maintained on a diet of dry food (MIL; Morini SpA) and tap water ad libitum. Upon arrival of the dogs in our animal facilities, an additional WB analysis of sera confirmed their H. pylori status. The dogs were housed in individual boxes and allowed to adapt for a month to their new environment. During the month of adaptation, two tests were carried out on fecal samples to assess the possible presence of intestinal parasites or common enteric pathogenic bacteria.
The dogs were then challenged every other day over a 1-week period, for a total of three times, with the mouse-adapted strain H. pylori SPM326s as follows. Twenty-four hours before each challenge the dogs were not given food. Two hours before bacterial inoculation the dogs received 10 mg of cimetidine (Tagamet 200; Smith Kline & French, Philadelphia, Pa.) intramuscularly per kg of body weight. At the moment of challenge, the dogs were intravenously anesthetized with a mixture of 40 μg of medetomidine chloridrate (Domitor; Centralvet-Vetem SpA, Milano, Italy) per kg and 5 mg of ketamine (Ketavet; Gellini, Latina, Italy) per kg, and then a gastric lavage was performed with 100 ml of sterile 0.2 M NaHCO3, followed by oral challenge with 3 ml of a freshly prepared suspension in sterile saline of 109 CFU of H. pylori SPM326s, grown under microaerobic conditions (see below). At the end of the bacterial inoculation, 200 μg of the anesthetic antagonist atipamezole (Antisedan; Centralvet-Vetem SpA) was administered, and then dogs were again treated with cimetidine and fed after 2 h.
Dogs were observed daily for the appearance of any signs, with particular attention being paid to gastrointestinal signs, such as vomiting and diarrhea. Prior to endoscopic examinations, blood samples, salivary samples, and oropharyngeal and rectal swabs were collected under sterile conditions immediately before the challenge and then at 1, 2, 4, 8, 12, 18, and 24 weeks. At the same times gastric endoscopy was performed, following anesthesia carried out as described above, with a 4.9-mm-diameter pediatric bronchoscope (Pentax Technologies, Zaventem, Belgium). Gastric biopsies were taken during the endoscopy with flexible pinch biopsy forceps at the antrum, corpus, fundus, and cardia before the challenge for urease testing and for microbiological, PCR, histopathological, and immunohistochemical analyses. Before each endoscopy the whole instrument and the flexible forceps were soaked in 4% glutaraldehyde for 45 min and then rinsed in sterile saline. To avoid cross-contamination among biopsies taken at different sites, the forceps were washed with tap water and lightly flame sterilized before the collection of each biopsy sample.
In a second experiment, three 4- to 6-month-old female beagle dogs were infected and followed up as described above; three additional dogs were kept uninfected, as a control, for at least 5 months. Also in this experiment, dogs were housed in single boxes.
The experimental protocol was approved by the Scientific and Ethical Committee of the University of Pisa and received official authorization by the Italian Ministry of Health (Department of Veterinary Health, Food and Nutrition, DM no. 21/97-C).
Rapid urease test.
Antral biopsies were incubated for up to 24 h in 1 ml of a 10% urea solution in distilled water to which 2 drops of a 1% phenol red solution (Sigma) in sodium phosphate buffer (pH 6.5) had been added. A positive test is indicated by change of color (from orange to dark pink) in the medium; the time necessary for the color change is recorded. The time to positivity of this test has been shown to be proportional to the number of bacteria present at the biopsy site (33).
Histopathology and transmission electron microscopy (TEM).
Samples for histological, immunohistochemical, and ultrastructural examinations were taken from the biopsies at sites adjacent to those used for microbiological analysis and for PCR. The samples were fixed in 10% buffered formalin and embedded in paraffin. Three-micrometer-thick sections were stained with hematoxylin-eosin (HE) and Alcian and periodic acid-Schiff (PAS) stain by standard procedures for histopathological examination.
Similar sections were also employed for immunohistochemical analyses by the ABC-peroxidase technique with a monoclonal antibody (MAb) specific for H. pylori (Biogenesis Ltd., Poole, England), a mouse MAb specific for VacA (clone C1G9) obtained by immunizing BALB/c mice with purified native H. pylori VacA (7a), or a mouse MAb specific for human IL-8 (clone DM/C7; Genzyme Diagnostics, Cambridge, Mass.). For IL-8 detection, sections were placed onto pretreated slides (Bio-Optica, Milan, Italy) to promote adhesion and dried overnight at 37°C. After being dewaxed, sections were immersed in citrate buffer (10 mM, pH 6.0) and processed in a microwave oven at 650 W for 10 min. Slides were then allowed to cool at room temperature for at least 20 min before being further processed for immunostaining by standard procedures. Biotinylated horse anti-mouse antibody (Dako, Milan, Italy) was used as secondary antibody. The reaction was developed with 3-1-diaminobenzidine-chlorhydrate (Sigma Chemical Co., St. Louis, Mo.) for the identification and localization of bacterial antigen and for the detection of IL-8.
For electron microscopy, samples were fixed in Karnowsky, postfixed in OsO4, and embedded in Epon-Araldite (Polysciences Inc., Warrington, Pa.). Semithin sections were stained with toluidine blue for evaluation of cell damage, whereas ultrathin sections were stained with uranyl acetate and lead citrate and then examined with a model EM 301 TEM (Philips, Eindhoven, The Netherlands) operating at 80 kV.
Bacterial growth conditions.
Gastric biopsies taken from the different gastric sites, as well as specimens from rectal and oropharyngeal swabs, were streaked onto Columbia agar plates (Gibco BRL, Paisley, United Kingdom) containing 5% horse blood supplemented with Dent’s or Skirrow’s antibiotic mixture (Oxoid, Basingstoke, United Kingdom) plus 400 μg of streptomycin (Sigma) per ml. Plates were incubated under microaerobic conditions with an Anaerojar system and a Campygen atmosphere-generating system (Oxoid) for 7 to 12 days. Growing H. pylori colonies were identified by their typical morphology, as assessed by visual inspection of the plates, and then confirmed by Gram staining, urease testing, and PCR amplification of the cagA gene.
PCR.
Bacteria or gastric biopsy samples were incubated at 37°C for 30 min with lysozyme at 100 μg/ml in 0.1 M NaCl–1 mM EDTA–10 mM Tris-HCl (pH 8). Then sodium dodecyl sulfate was added (at a final concentration of 1%, wt/vol), and the mixture was heated to 65°C. Proteinase K (25 μg/ml) was added before incubation at 50°C for 2 h. Then the mixture was extracted with phenol-chloroform. After extraction, the DNA solution was mixed with absolute ethanol at −20°C for 1 h, washed with 75% ethanol, and dried. The DNA pellet was suspended in distilled water (11).
Oligonucleotides were synthesized on an automatic synthesizer (Applied Biosystems Inc., Foster City, Calif.) by the automated phosphoramidite coupling method and purified by standard protocols. The H. pylori CagA primers R008 (5′-TTAGAATAATCAACAAACATCACGCCAT-3′) and D008 (5′-AATGCTAAATTAGACAACTTGAGCGA-3′) were derived from the sequenced cagA gene (11), giving an amplified product of 298 bp. The PCR mixture contained 50 mM KCl, 10 mM Tris, 200 mM deoxynucleoside triphosphates, 30 pmol of each primer, 0.1 μg of bovine serum albumin, 2.5 U of AmpliTaq (Perkin-Elmer, Norwalk, Conn.), and 80 ng of H. pylori chromosomal DNA. Amplifications were performed on a PCR system 9600 thermocycler (Perkin-Elmer) with the following cycling profile: 94°C for 30 s, 65°C for 30 s, and 72°C for 30 s for 38 cycles and then extension at 72°C for 7 min.
Detection of anti-H. pylori antibodies.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of H. pylori (strain SPM326s) and WB analysis of sera were performed according to previously published procedures (47). Briefly, dog sera were diluted 1:200 and incubated for 2 h at room temperature. Then, horseradish peroxidase-conjugated rabbit anti-dog IgG antibody (Nordic Immunological Laboratories, Tilburg, The Netherlands) was added at a 1:2,000 dilution for 2 h and the reaction mixture was developed with 4-α-chloronaphtol as the substrate. Detection of antibody against H. pylori by enzyme-linked immunosorbent assay was carried out on 96-well plates coated overnight at 4°C with SPM326s lysate (10 μg/well) or with purified native VacA or CagA (0.2 μg/well). Coated wells were blocked with phosphate-buffered saline containing 5% nonfat milk. Twofold serial dilutions of the sera were incubated at 37°C for 2 h and then washed with phosphate-buffered saline. Antigen-specific IgG, IgG1, and IgG2 titers were determined with appropriate dilutions of horseradish peroxidase-conjugated rabbit anti-dog IgG (Nordic), goat anti-dog IgG1, or sheep anti-dog IgG2 (Bethyl Laboratories Inc., Montgomery, Tex.) polyclonal antibody for 2 h at 37°C. Antigen-bound antibodies were revealed by adding o-phenylenediamine dihydrochloride (Sigma) as a substrate. Antibody titers were determined as previously described (28).
For the detection of H. pylori-specific antibodies in the saliva, we used a similar procedure and an SPM326s lysate (10 μg/well) to coat the plates. Specific alkaline-phosphatase-conjugated anti-dog IgG and IgA antibodies (Nordic) were added at an appropriate dilution. Results were expressed as optical densities at 405 nm of the salivary samples tested at a 1:20 dilution.
RESULTS
Acute symptoms following H. pylori infection.
Three conventional beagle dogs were inoculated three times with 109 CFU of a virulent H. pylori strain and then closely followed up for the appearance of signs and for histopathological lesions over time. During the first week after the third challenge with H. pylori, the three dogs experienced at least one episode per day of loose stools, a condition which persisted, but occurred less frequently, in the second week. Furthermore, during the first week postinfection (p.i.), the dogs had episodes of mucous and foamy vomiting, often containing gastric juices and not associated with food intake. These symptoms disappeared spontaneously without specific treatment, and no other symptoms were observed. All three dogs remained alert and ate normally throughout the whole study. The same symptoms appeared in two of the three dogs infected in the second experiment and again disappeared spontaneously. Uninfected control dogs did not show any vomiting or diarrhea.
Histopathology and endoscopic findings.
Results of endoscopic and histological examinations of the gastric mucosae performed at time zero were normal for all the dogs used in the two experiments (not shown). In the histological sections of biopsies taken at 1 week p.i. we observed a marked hyperemia and edema of the mucosal lamina propria, particularly in the corpus and in the antrum (Fig. 1A). At this time acute antral gastritis was evident and was characterized histologically by the presence of numerous polymorphonuclear leukocytes infiltrating the laminae propriae among the glands, the structure of which was in some cases significantly altered (Fig. 1B). Notably, several polymorphonuclear leukocytes were interspersed in the epithelial cell layer and were also found in the mucus (not shown), suggesting that neutrophil transcytosis was elicited by the infection.
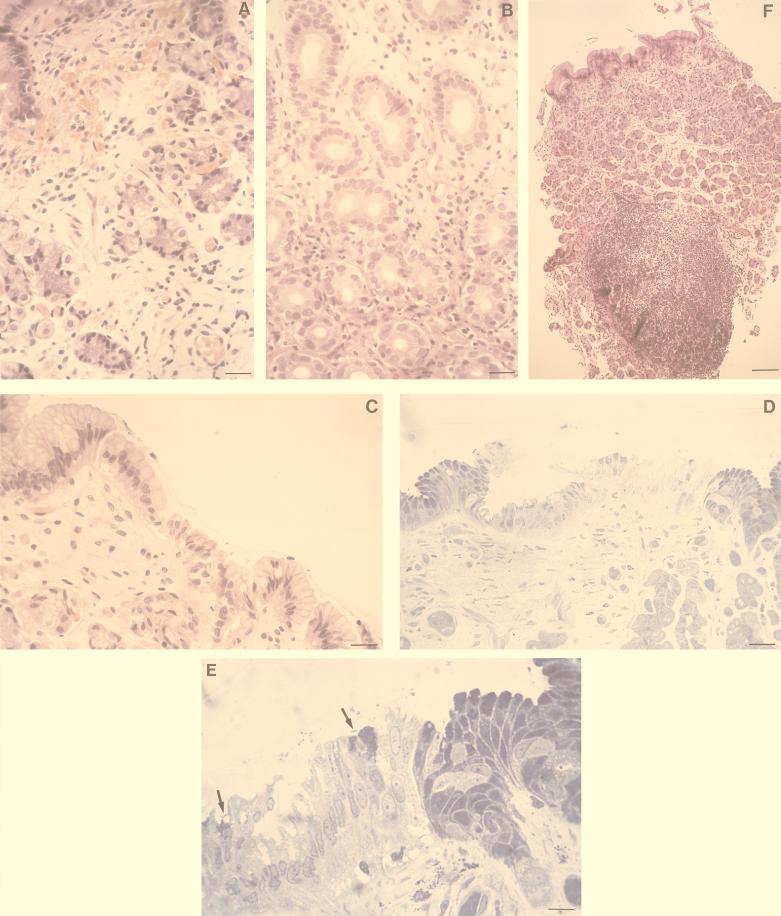
FIG. 1.
Histopathological findings with infected dogs. (A) HE stain of gastric mucosa at 1 week p.i. showing hyperemia and edema of the mucosal lamina propria (bar = 25 μm); (B) HE staining of antral mucosa at 1 week p.i. showing numerous polymorphonuclear granulocytes infiltrating the laminae propriae among the glands (bar = 25 μm); (C) HE stain of antral mucosa at 4 weeks p.i. showing vacuolar degeneration of the epithelium (bar = 25 μm); (D) evident epithelium damage in the antral mucosa at 4 weeks p.i. after toluidine blue staining of a semithin section (bar = 100 μm); (E) high-power magnification (bar = 12.5 μm) of the field in panel D showing some curved bacteria (arrows) close to epithelial cells which are heavily vacuolized and show loss of the apical secreting portion; (F) HE stain of a biopsy taken 8 weeks p.i. showing a characteristic lymphoplasmacytic follicle associated with chorion edema and displacement of glands (bar = 400 μm).
Upon endoscopic examination performed 1 and 2 weeks p.i., a gastric hyperemia was observed in all infected dogs. Edema of the mucosa and catarrh adhering to the mucosae of the antrum and corpus were present, conferring a congested aspect to the gastric wall. At 2 weeks p.i., histology of the gastric biopsies evidenced a decrease in the level of neutrophilic infiltration into the lamina propria and a concomitant increase in the level of mononuclear cells. Edema and hyperemia of the lamina propria were still present (not shown).
After 4 weeks, clear signs of superficial erosions were endoscopically evident in the antrum. Histological sections showed vacuolar degeneration, loss of the apical secreting portion of the cells, piknosis, and rhexis of epithelial cell nuclei (Fig. 1C to E).
At 8 weeks p.i., chronic follicular gastritis, with the characteristic “bumpy” aspect of the gastric wall, was easily detected by endoscopy in the infected animals. Histological examination of gastric biopsies revealed the presence of small lymphoplasmacytic aggregates among the glands of the corpus and fundus; lymphoid follicles (1.5 to 2 mm) appeared in the antrum (Fig. 1F). Degenerative processes in the epithelial cells, the presence of scattered neutrophilic granulocytes, and an increase in the exocytosis of mononuclear cells were associated with these follicles. All these signs have been reported as being typical of chronic active gastritis in infected humans (13, 26, 29, 54, 62, 66). These macroscopic and microscopic signs remained unchanged at later times (i.e., at 12, 18, and 24 weeks p.i.). At this advanced stage of infection, marked modifications in the mucin composition, as detected by Alcian-PAS staining of histological sections, was also observed, with a progressive depletion of PAS-positive cells in the antrum, suggestive of a functional gastric atrophy (not shown).
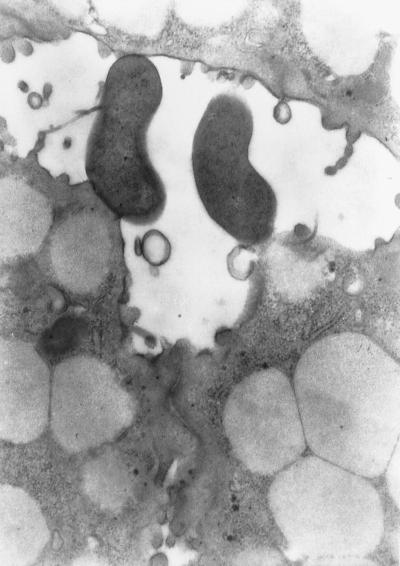
H. pylori was identified in the biopsies immunohistochemically with the C1G9 MAb specific for VacA. H. pylori was also observed by TEM in the lumens of antral glands, in close contact with the luminal surfaces of epithelial cells (Fig. 2).
FIG. 2.
TEM showing the ultrastructural aspect of antral mucosa 18 weeks p.i. Typical H. pylori bacteria are visible in a glandular lumen. Magnification, ×7,920.
Control dogs, which were not infected, always showed normal mucosae upon endoscopical, histological, and immunohistochemical examination for the entire period of the follow-up.
Immunohistochemical detection of IL-8 in gastric biopsies.
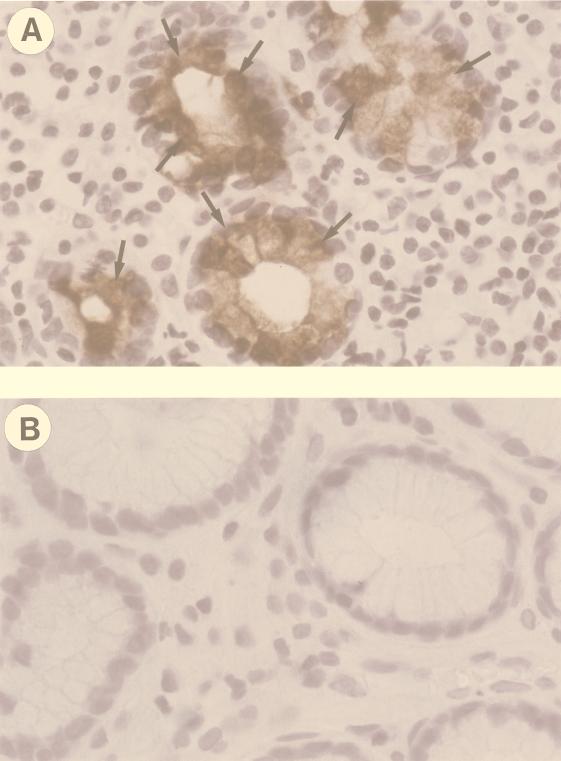
One of the most striking findings of the present work was the demonstration of a strong infiltration of neutrophils within the lamina propria and the superficial epithelia during the first weeks of infection, which then became much less evident. To investigate whether this early infiltration was mediated by IL-8, as reported for humans (14), the presence of this chemokine was investigated immunohistochemically. Histological sections from biopsies taken before infection were consistently negative for the presence of IL-8. At the first and second weeks after infection, IL-8 was expressed by gastric epithelial cells, particularly in areas corresponding to a more pronounced neutrophilic infiltration in the underlying lamina propria or in areas where neutrophil transcytosis was more detectable (Fig. 3). IL-8-positive mononuclear cells were also found interspersed in the lamina propria in correspondence with neutrophilic infiltrates (not shown). IL-8 became undetectable in sections from biopsies taken 8 weeks after infection (Fig. 3B), at a time when neutrophilic infiltration was much less prominent than in the early phases of infection.
FIG. 3.
Detection of IL-8 expression by immunohistochemistry. (A and B) Representative fields of sections obtained from antral biopsies collected at 2 and 8 weeks p.i., respectively. Sections were stained with anti-IL-8 MAb and counterstained with hematoxylin. In panel A, IL-8-positive epithelial cells are indicated by arrows. Magnification, ×100.
Isolation and identification of H. pylori.
The presence of H. pylori in the biopsies taken from the gastric mucosae of experimentally infected dogs was first assessed by the rapid urease test. The test, which gave negative results for the gastric biopsies taken before the oral challenge with H. pylori and for samples from the control, uninfected dogs, showed strongly and quickly positive results (a few minutes to 2 h) for the infected dogs in the two experiments from the first sampling, 1 week after infection, and continued to show strongly positive results at all time points during the follow-up of the study.
Antral biopsies from each dog were also applied to microbiological culture for H. pylori identification. In samples taken 1, 2, and 4 weeks p.i., H. pylori was identified by culture, although at these time points the growth of bacteria from the selective culture plates was rather scanty. From the eighth week onward, the growth of H. pylori from all the gastric biopsy samples was more evident, and 50 to 200 H. pylori colonies were easily recovered from each biopsy. At later times (i.e., at 18 and 24 weeks) the bacterium was easily isolated from the corpus and antrum only, whereas a marked decrease in the growth efficiency was observed in biopsies taken from cardias and fundi.
Starting from the first week p.i., H. pylori could also be cultured from rectal swabs. These microbiological findings were always confirmed by PCR performed on the bacterial colonies grown in the plates.
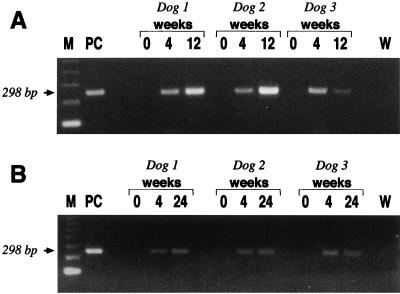
PCR was carried out on antral biopsy samples taken at time zero and at each endoscopic examination with cagA-specific primers. The presence of H. pylori was confirmed in all p.i. samples. Figure 4A shows the PCR products that confirmed the presence of H. pylori in antral gastric biopsies taken at 4 and 12 weeks p.i. from each of the three dogs of the first experiment. Although all attempts to culture H. pylori from oropharyngeal swab specimens were unsuccessful at any time during the follow-up, H. pylori DNA was detectable by PCR amplification of the cagA gene at all times p.i., even 24 weeks p.i. (Fig. 4B).
FIG. 4.
Detection of H. pylori in gastric biopsies and oropharyngeal swab specimens by PCR. (A) H. pylori DNA amplification of the cagA sequence showing the expected PCR product of 298 bp in gastric biopsies of dogs collected at 4 and 12 weeks p.i. but not in gastric samples collected before infection. (B) cagA amplification obtained in material extracted from oropharyngeal swab specimens of dogs taken at 0, 4, and 24 weeks. Lanes: M, markers; PC, positive controls; W, water.
Anti-H. pylori antibody response.
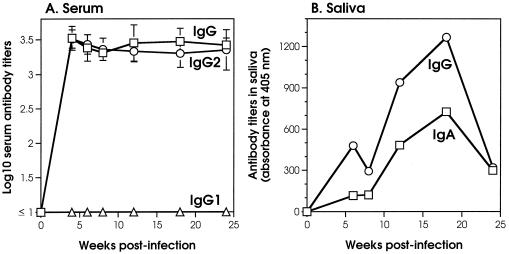
The induction of a specific H. pylori antibody response was followed up with serum and salivary samples taken before and at different times after infection and analyzed by enzyme-linked immunosorbent assay. Infected dogs mounted a fast serum IgG antibody response to H. pylori antigens when we used both a crude bacterial lysate (Fig. 5A) and purified recombinant CagA and VacA (not shown). This antibody response was already easily detectable in the serum samples 2 to 4 weeks after infection and persisted for the entire period of the study. It is noteworthy that most of the H. pylori-specific IgG in serum belonged to the IgG2 isotype, with IgG1 being undetectable for the entire follow-up of the study (Fig. 5A). Furthermore, and interestingly, both IgA and IgG antibodies binding to H. pylori lysate were also detected in the samples of saliva collected after infection (Fig. 5B). In the saliva, H. pylori-specific antibodies exhibited a slower kinetic compared to that observed in the sera. In fact, salivary IgA and IgG reached their peaks 12 to 15 weeks after infection and then declined, although they were still detectable at 24 weeks.
FIG. 5.
Anti-H. pylori antibody responses in infected dogs. (A) Serum IgG and IgG subclass antibody responses to H. pylori lysate. Each curve represents the mean titers at the different time points. (B) Salivary IgA and IgG antibody responses to H. pylori lysate. Each curve represents the mean titers at the different time points obtained with salivary samples diluted 1:20.
DISCUSSION
The increasing importance of H. pylori in the induction of a wide variety of gastric pathologies has stressed the need for a physiological animal model to test the pathophysiology of the infection and to aid in the development of new drugs and vaccines. In this paper we describe the experimental infection of conventional dogs with H. pylori and the long-term outcome of this infection. We show that a mouse-adapted H. pylori type I strain persistently colonizes the stomachs of conventional beagle dogs, causes acute symptoms and gastric pathology, and elicits specific immune responses.
A remarkable feature of the model described here is that the infected dogs presented evident clinical signs, such as vomiting and diarrhea, at the time of the onset of infection, which lasted for 1 to 2 weeks. This is the first animal model in which acute symptoms were observed as a consequence of gastric inoculation of H. pylori. In a study carried out with 7-day-old gnotobiotic beagle pups, Radin et al. (59) did not report any acute symptoms, with the pups remaining asymptomatic for the duration (30 days) of the experiment. There may be several explanations for the occurrence of evident clinical signs in our study. First, in the present study we used a phenotypically characterized cytotoxic type I strain which had been adapted to a nonhuman host by several in vivo passages. Second, we used a larger dose of bacteria (three inocula of 109 bacteria) than that (one single bolus of 3 × 108 bacteria) employed by Radin et al. (59). Third, the animals used in our study had a normal gastrointestinal commensal microbial flora, which may have contributed to amplification of the pathological consequences of infection. The possibility that the acute symptoms were induced by the experimental manipulation of the dogs (i.e., anesthesia, endoscopy, etc.) is highly unlikely because uninfected control dogs did not show any symptom, despite the fact that they were subjected to the same treatments as infected dogs. Furthermore, these symptoms were never seen at later stages of infection.
We believe that infection of conventional beagle dogs reproduces the human situation and allows us to study also the acute phase, a step that is never noticed in humans and is not reproduced in any of the other animal models. Although the natural history of H. pylori infection is poorly known and, as a consequence, the documentation of the clinical picture of the early stages of acute infection in humans is scarce (49, 51, 53, 61), it is noteworthy that in self-infection experiments, vomiting (49, 53), as well as an increase in intestinal peristalsis and a transient softening of feces (49), has been reported. Furthermore, VacA toxic activity was also found in a proportion of diarrheal feces from children (44, 45). It is then logical to hypothesize that the acute symptoms observed in the dogs of the present study may reproduce those which can be encountered during the early stages of H. pylori infection in humans.
One of the advantages of the conventional dog model presented here is the ease of follow-up of the infection over a long period by endoscopy, at variance with small animal models and with the time limitations imposed by the germ-free animal models (59). This advantage has been exploited in the present investigation, where it was possible to follow up for at least 24 weeks the occurrence and progression of histopathological lesions induced by the infection.
Indeed, the dogs developed early superficial gastritis, with the appearance of mucosal erosions, which progressed to follicular gastritis. It is remarkable that at early stages of infection the gastric lamina propria was heavily infiltrated by polymorphonuclear cells. In other H. pylori animal models, this neutrophil infiltration is usually much less pronounced (41, 47) or has been described only at later stages of infection (42, 59). This infiltration, accompanied by symptoms like vomiting, mimics that observed in infected humans with acute gastritis (49, 53, 61), which has been hypothesized to be mediated by proinflammatory chemokines, such as IL-8, specifically induced by products of cytotoxic (type I) H. pylori strains (2, 14, 38, 57, 65).
In the present study, where we used a well-characterized type I strain to infect dogs, IL-8 could be easily detected by immunohistochemistry in early stages of infection. Interestingly, the time course of expression of this chemokine paralleled that of infiltration by neutrophils. In fact, it was more evident when neutrophils were more abundant in the lamina propria and rapidly decreased at later stages, when the infiltrates were more characterized by mononuclear cells. The detection of IL-8 in the gastric epithelia of infected dogs with a MAb directed against human IL-8 was clearly not due to nonspecific binding. In fact, human IL-8 and dog IL-8 have 75% identity both at the nucleotide and at the amino acid level (50). Furthermore, the absence of detectable positivity for IL-8 in sections taken 8 weeks p.i. speaks in favor of the specificity of our results. Previous studies of humans have clearly shown that IL-8 can be detected immunochemically (14) and immunohistochemically (15) in gastric biopsies from individuals infected with H. pylori. In humans, however, gastric epithelia from normal, noninfected individuals can be reactive with anti-IL-8 antibodies (15). In the present study, normal gastric mucosa was always negative. This difference may be explained by the fact that most of the human studies are carried out with adult individuals, in which IL-8 induction may be mediated by exogenous (e.g., bacterial lipopolysaccharide) and/or endogenous factors not related to H. pylori. On the contrary, in this study, we used young (4- to 6-month-old) conventional beagle dogs, which had had less time and opportunity to have contacts with other IL-8-inducing factors. On the other hand, a recent study has clearly shown that 1 month after successful eradication of H. pylori infection patients experience a significant reduction of infiltrating neutrophils and normalization of the mucosal IL-8 levels (1). These data are in full agreement with the kinetics of appearance of neutrophil infiltration and IL-8 detection in the gastric mucosae of infected dogs in the present study.
With the progression of infection, clear signs of epithelial erosions appeared both macroscopically and microscopically, with characteristic cellular vacuolization and loss of the apical portions of the epithelial cells. We have previously observed similar lesions in the gastric mucosae of H. pylori-infected mice (28, 47). One of the most striking features at later stages of infection was the presence of well-structured lymphoid follicles both in the corpus and in the antrum. This is a finding typical of chronic stages of infection by Helicobacter species and has been extensively reported both for chronically infected patients (26, 54) and for experimentally infected animals (24, 28, 30, 59). These organized lymphoid structures may be the consequence of an active and persistent stimulation of the immune system at the local mucosal level, with recruitment in situ of specific T lymphocytes (37). It has been suggested that in some individuals such lymphoid structures in the gastric mucosa may precede the development of low-grade B-cell lymphoma (56). More recently this hypothesis has been proven by showing B-cell clonality at the site of chronic gastritis in patients with chronic H. pylori gastritis and subsequent gastric mucosa-associated lymphoid tissue lymphoma (68). Thus, the present model of chronic infection with H. pylori may turn out to be useful also in investigating the kinetics of the organization of lymphoid follicles in the gastric mucosa and eventually in testing the development of gastric cancer.
During the entire follow-up, the persistence of gastric colonization by H. pylori in the gastric biopsies was assessed by urease testing, PCR, immunohistochemistry, and TEM. Furthermore, it was also possible to culture the bacteria from the gastric biopsies. These facts clearly show that the evolution of the pathology in this model is constantly associated with the presence of H. pylori. It should be stressed that viable H. pylori was also recovered from rectal swabs at every time of observation, even at 24 weeks after infection. The ability to culture strain SPM326s from the fecal swabs of conventional animals is likely to be a consequence of the fact that, since this strain bears a gene for streptomycin resistance, it is possible to efficiently use selective culture conditions to isolate it from contaminating commensal flora normally present in these samples. Bacterial isolation from feces has been reported for experimental Helicobacter mustelae infection of ferrets (25). These findings demonstrate that, at least in some animal models, a considerable quantity of viable H. pylori organisms is eliminated in the feces. Should this be applicable to human infections, as claimed by some researchers (40, 64), it is tempting to hypothesize that infected individuals can spread viable H. pylori with stools and that, under certain epidemiological conditions (3, 34), this may represent a source of dissemination to other subjects. It is, however, highly unlikely that oral-fecal transmission caused the persistence of the chronic infection in our experiments, since the dogs were constantly kept under isolation in separated individual boxes. In fact, control dogs, kept under the same conditions, never exhibited any signs of infection with H. pylori.
Our data show that H. pylori DNA could also be detected in the oropharyngeal swabs of infected dogs during the entire follow-up. The presence of bacterial DNA was not due to contamination from endoscopic manipulation, since all samples, including orophraryngeal swab specimens, were taken before endoscopic examination, or a result of vomiting, since specific PCR products were still detectable 24 weeks after infection. It is possible that H. pylori or H. pylori material reaches the oral cavity through episodes of gastroesophageal regurgitation. Nevertheless, since it was not possible in the present study to isolate viable (i.e., culturable) forms of the bacterium, it is not possible to conclude whether this might represent another possible route of transmission of infection to other individuals, as has been suggested by others (3).
The experimental infection with H. pylori induced early detectable antibody responses to different antigens, including CagA and VacA, which persisted during the entire period of follow-up. Interestingly, the majority of the anti-H. pylori IgG antibodies detected in the serum belonged to the IgG2 isotype. A predominant production of serum IgG2 has been reported for dogs in association with chronic infections, such as with Toxoplasma gondii and Leishmania infantum (7, 18), whereas preferential production of IgG1 has been associated with helminthic infections or allergies (16, 18, 62). Our data, thus, suggest that experimental H. pylori infection in dogs is associated with a preferential activation of Th1-type CD4+ T-cell subsets, in agreement with data previously obtained with mice (28, 52) and humans (17). It is remarkable that infected dogs exhibited H. pylori-specific IgA and IgG antibody responses in their saliva, which peaked 12 to 15 weeks after infection. Taken together, these data clearly show that experimental infection of conventional dogs with H. pylori induces specific seroconversion and mucosal (salivary) antibody response. Once again, these findings are in full agreement with those amply reported for humans in terms of both serum (20) and salivary antibodies (10, 23) specifically induced after infection with H. pylori.
In conclusion, the H. pylori infection of conventional dogs described here mimics clinical, pathological, microbiological, and serological aspects of the infection in humans. In fact, acute infection induces symptoms (vomiting and diarrhea) that disappear spontaneously and acute gastritis, with early recruitment of neutrophils, possibly mediated by H. pylori-induced IL-8, followed by the appearance of superficial erosion and of lymphoid follicles and chronic gastritis. Bacterial colonization is chronic, inducing an early and sustained immune response.
Unlike most of the animal models of H. pylori infection, in which animals are sacrificed at given time points to evaluate both infection and pathology, this model has allowed us to monitor the evolution of the consequences of H. pylori infection at both acute and chronic stages in the same individuals. It is likely that this animal model will permit a better investigation of the pathogenesis of the bacterium and will also allow us to better assess the feasibility of vaccination. In particular, it will now be possible to investigate the effect of both preventive and therapeutic vaccination regimens on the evolution of infection and disease in the same individual.
ACKNOWLEDGMENTS
We thank A. Covacci and M. Marchetti for H. pylori SPM326s, M. Carlucci for revising the manuscript, and N. Figura and S. Censini for helpful advice. We are also grateful to V. Mazzoncini (Abiogen Pharma, Migliarino Pisano, Pisa, Italy) for kindly providing the housing for the dogs used in this study and A. Fornaciari (Morini SpA) for allowing us free access to the breeding of the beagle dogs for the selection of the dogs used in this study.
E.T. was supported in part by the University of Pisa (Fondi di Ateneo).
REFERENCES
- 1.Ando T, Kusugami K, Ohsuga M, Ina K, Shinoda M, Konagaya T, Sakai T, Imada A, Kasuga N, Nada T, Ichiyama S, Blaser M J. Differential normalization of mucosal interleukin-8 and interleukin-6 activity after Helicobacter pylori eradication. Infect Immun. 1998;66:4742–4747. doi: 10.1128/iai.66.10.4742-4747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, Fukatsu A, Ichiyama S, Ohta M. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150–1156. [PubMed] [Google Scholar]
- 3.Axon A T R. The transmission of Helicobacter pylori: which theory fits the facts? Eur J Gastroenterol Hepatol. 1995;8:1–2. doi: 10.1097/00042737-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Binhazim A A, Chapman W L, Shin S S, Hanson W L. Determination of virulence and pathogenesis of a canine strain of Leishmania infantum in hamsters and dogs. Am J Vet Res. 1993;54:113–121. [PubMed] [Google Scholar]
- 5.Blaser M J, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Investig. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 7.Bourdoiseau G, Bonnefont C, Hoareau E, Boehringer C, Stolle T, Chabanne L. Specific IgG1 and IgG2 antibody and lymphocyte subset levels in naturally Leishmania infantum-infected treated and untreated dogs. Vet Immunol Immunopathol. 1997;59:21–30. doi: 10.1016/s0165-2427(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 7a.Burroni, D., and J. Telford. Unpublished data.
- 8.Censini S, Lange C, Xiang Z Y, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerri D, Farina R, Andreani E, Nuvoloni R, Pedrini A, Cardini G. Experimental infection of dogs with Borrelia burgdorferi. Res Vet Sci. 1994;57:256–258. doi: 10.1016/0034-5288(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 10.Christie J M, McNulty C A, Shepherd N A, Valori R M. Is saliva serology useful for the diagnosis of Helicobacter pylori? Gut. 1996;39:27–30. doi: 10.1136/gut.39.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covacci A, Falkow S, Berg D E, Rappuoli R. Did the inheritance of a pathogenicity island modify the virulence of Helicobacter pylori? Trends Microbiol. 1997;5:205–208. doi: 10.1016/S0966-842X(97)01035-4. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree J E. Gastric mucosal inflammatory responses in Helicobacter pylori. Aliment Pharmacol Ther. 1996;10(Suppl. 1):29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree J E, Peichl P, Wyatt J I, Stachl U, Lindley I J. Gastric interleukin-8 and IgA IL-8 antibodies in Helicobacter pylori infection. Scand J Immunol. 1993;37:65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree J E, Wyatt J I, Trejdosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J D. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day M J, Corato A, Shaw S E. Subclass profile of allergen-specific IgG antibodies in atopic dogs. Res Vet Sci. 1996;61:136–142. doi: 10.1016/s0034-5288(96)90088-0. [DOI] [PubMed] [Google Scholar]
- 17.D’Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 18.Deplazes P, Smith N C, Arnold P, Lutz H, Eckert J. Specific IgG1 and IgG2 antibody responses of dogs to Leishmania infantum and other parasites. Parasite Immunol. 1995;17:451–458. doi: 10.1111/j.1365-3024.1995.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 19.Dubois A, Berg D E. The nonhuman primate model for H. pylori infection. In: Clayton C L, Mobley H L T, editors. Methods in molecular medicine. Helicobacter pylori protocols. Totowa, N.J: Humana Press Inc.; 1997. pp. 253–269. [DOI] [PubMed] [Google Scholar]
- 20.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eck M, Schmausser B, Haas R, Greiner A, Czub S, Muller-Hermelink H K. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the Cag-A protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 22.Elizalde J I, Gomez J, Panes J, Lozano M, Casadevall M, Ramirez J, Pizcueta P, Marco F, Rojas F D, Granger D N, Pique J M. Platelet activation in mice and human Helicobacter pylori infection. J Clin Investig. 1997;100:996–1005. doi: 10.1172/JCI119650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallone C A, Elizov M, Cleland P, Thompson J A, Wild G E, Lough J, Faria J, Barkun A N. Detection of Helicobacter pylori infection by saliva IgG testing. Am J Gastroenterol. 1996;91:1145–1149. [PubMed] [Google Scholar]
- 24.Fox J C, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward J, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox J G, Paster B J, Dewhirst F E, Taylor N S, Yan L L, Macuch P J, Chmura L M. Helicobacter mustelae isolation from feces of ferrets: evidence to support fecal-oral transmission of a gastric Helicobacter. Infect Immun. 1992;60:606–611. doi: 10.1128/iai.60.2.606-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genta R M, Hamner H W, Graham D Y. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24:577–583. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- 27.Ghiara P, Marchetti M, Blaser M J, Tummuru M K R, Cover T L, Segal E D, Tompkins L S, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, De Magistris M T, Pizza M, Rappuoli R, Del Giudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillanders I A, Scott P J W, Smith G D. Helicobacter pylori and chronic antral gastritis in elderly patients. Age Ageing. 1994;23:277–279. doi: 10.1093/ageing/23.4.277. [DOI] [PubMed] [Google Scholar]
- 30.Handt L K, Fox J G, Stalis I H, Rufo R, Lee G, Linn J, Li X T, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995;33:2280–2289. doi: 10.1128/jcm.33.9.2280-2289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris P R, Cover T L, Crowe D R, Orenstein J M, Graham M F, Blaser M, Smith P D. Helicobacter pylori cytotoxin induces vacuolation of primary human mucosal epithelial cells. Infect Immun. 1996;64:4867–4871. doi: 10.1128/iai.64.11.4867-4871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashidani H, Kaneko K, Sakurai K, Ogawa M. Experimental infection with Yersinia enterocolitica serovar 0:8 in beagle dogs. Vet Microbiol. 1995;47:71–77. doi: 10.1016/0378-1135(95)00052-c. [DOI] [PubMed] [Google Scholar]
- 33.Hazell S L, Borody T J, Gal A, Lee A. Campylobacter pyloridis gastritis. I. Detection of urease as a marker of bacterial colonization and gastritis. Am J Gastroenterol. 1987;82:292–296. [PubMed] [Google Scholar]
- 34.Hazell S L, Mitchell H M, Hedges M, Shi X, Hu P J, Li Y Y, Lee A, Reiss-Levy E. Hepatitis A and evidence against community dissemination of Helicobacter pylori via feces. J Infect Dis. 1994;170:686–689. doi: 10.1093/infdis/170.3.686. [DOI] [PubMed] [Google Scholar]
- 35.Heilmann K L, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry G A, Long P H, Burns J L, Charbonneau D L. Gastric spirillosis in beagles. Am J Vet Res. 1987;48:831–836. [PubMed] [Google Scholar]
- 37.Hussell T, Isaacson P G, Crabtree J E, Spencer J. Helicobacter pylori-specific tumour infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Husson M O, Gottrand F, Vachee A, Dhaenens L, de la Salle E M, Turck D, Houche M, Leclerc H. Importance in diagnosis of gastritis of detection by PCR of the cagA gene in Helicobacter pylori strains isolated from children. J Clin Microbiol. 1995;33:3300–3303. doi: 10.1128/jcm.33.12.3300-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karita M, Kouchiyama T, Okita K, Nakazawa T. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol. 1991;86:1596–1603. [PubMed] [Google Scholar]
- 40.Kelly S M, Pitcher M C, Farmery S M, Gibson G R. Isolation of Helicobacter pylori from feces of patients with dyspepsia in the United Kingdom. Gastroenterology. 1994;107:1671–1674. doi: 10.1016/0016-5085(94)90806-0. [DOI] [PubMed] [Google Scholar]
- 41.Krakowska S, Morgan D R, Kraft W G, Leunk R D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A, Fox J C, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 43.Lee A, O’Rourke J, DeUngria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 44.Luzzi I, Covacci A, Censini S, Pezzella C, Crotti D, Facchini M, Giammanco A, Guglielmetti P, Piersimoni C, Bonamico M, Mariani P, Rappuoli R, Caprioli A. Detection of a vacuolating cytotoxin in stools from children with diarrhea. Clin Infect Dis. 1996;23:101–106. doi: 10.1093/clinids/23.1.101. [DOI] [PubMed] [Google Scholar]
- 45.Luzzi I, Pezzella C, Caprioli A, Covacci A, Bugnoli M, Censini S. Detection of vacuolating toxin of Helicobacter pylori in human faeces. Lancet. 1993;341:1348. doi: 10.1016/0140-6736(93)90857-d. [DOI] [PubMed] [Google Scholar]
- 46.Manetti R, Massari P, Marchetti M, Magagnoli C, Nuti S, Lupetti P, Ghiara P, Rappuoli R, Telford J L. Detoxification of the Helicobacter pylori cytotoxin. Infect Immun. 1997;65:4615–4619. doi: 10.1128/iai.65.11.4615-4619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchetti M, Aricò B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 47a.Marchetti, M., and A. Covacci. Unpublished data.
- 48.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non toxic mutant of E. coli heat labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 49.Marshall B J, Armstrong J A, McGechie D B, Glancy R J. Attempt to fulfill Koch’s postulate for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto Y, Mohamed A, Onodera T, Kato H, Ohashi T, Goitsuka R, Tsujimoto H, Hasegawa A, Furusawa S, Yoshihara K. Molecular cloning and expression of canine interleukin 8 cDNA. Cytokine. 1994;6:455–461. doi: 10.1016/1043-4666(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell J D, Mitchell H M, Tobias V. Acute Helicobacter pylori infection in an infant, associated with gastric ulceration and serological evidence of intra-familial transmission. Am J Gastroenterol. 1992;87:382–386. [PubMed] [Google Scholar]
- 52.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 53.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised resting gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 54.Owen D A. The morphology of gastritis. Yale J Biol Med. 1996;69:51–60. [PMC free article] [PubMed] [Google Scholar]
- 55.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1231. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 56.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 57.Peek R M, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Investig. 1995;73:760–770. [PubMed] [Google Scholar]
- 58.Radcliff F J, Hazell S L, Kolesnikow T, Doidge C, Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect Immun. 1997;65:4668–4674. doi: 10.1128/iai.65.11.4668-4674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radin M J, Eaton K A, Krakowka S, Morgan D R, Lee A, Otto G, Fox J. Helicobacter pylori gastric infection in gnotobiotic beagle dogs. Infect Immun. 1990;58:2606–2612. doi: 10.1128/iai.58.8.2606-2612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakagami T, Dixon M, O’Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sobala G M, Crabtree J E, Dixon M F, Schorah C J, Taylor J D, Rathbone B J, Heatley R V, Axon A T. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stolte M, Stadelmann O, Bethke B, Burkard G. Relationship between the degree of Helicobacter pylori colonization and the degree of activity of gastritis, surface, epithelial degeneration, and mucus secretion. Z Gastroenterol. 1995;33:89–93. [PubMed] [Google Scholar]
- 63.Telford J L, Ghiara P, Dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z Y, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas J E, Gibson G R, Darboe M K, Dale A, Weaver L T. Isolation of Helicobacter pylori from human feces. Lancet. 1992;340:1194–1195. doi: 10.1016/0140-6736(92)92894-l. [DOI] [PubMed] [Google Scholar]
- 65.Uemura N, Oomoto Y, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Sumii K, Haruma K, Kajiyama G. Gastric corpus IL-8 concentration and neutrophil infiltration in duodenal ulcer patients. Aliment Pharmacol Ther. 1997;11:793–800. doi: 10.1046/j.1365-2036.1997.00218.x. [DOI] [PubMed] [Google Scholar]
- 66.Wyatt J I, Dixon M F. Chronic gastritis—a pathogenic approach. J Pathol. 1988;154:113–123. doi: 10.1002/path.1711540203. [DOI] [PubMed] [Google Scholar]
- 67.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vaculating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid tissue lymphoma of the stomach. N Engl J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]