Abstract
Purpose:
This study aimed to assess the antitumor activity and safety of neoadjuvant chemotherapy combined with PD-1 inhibitor camrelizumab in patients with locally advanced head and neck squamous cell carcinoma (HNSCC).
Patients and Methods:
In this single-center, single-arm, phase 2 trial, patients with resectable stage III–IVB HNSCC received chemotherapy [albumin-bound paclitaxel 260 mg/m2 (or docetaxel 75 mg/m2) plus cisplatin 75 mg/m2] and camrelizumab 200 mg on day 1 of each 21-day cycle for three cycles, followed by surgery, and adjuvant radiotherapy. Co-primary end points were pathological complete response (pCR) rate and safety.
Results:
Thirty patients were enrolled and completed the neoadjuvant therapy, with an objective response rate (ORR) of 96.7% (29/30). Twenty-seven patients underwent surgery without delay, with an R0 resection rate of 92.6% (25/27). The clinical to pathological downstaging rate was 100% (27/27). The pCR rate was 37.0% [95% confidence interval (CI), 19.4%–57.6%], and the major pathological response (MPR) rate was 74.1% (95% CI, 53.7%–88.9%). The median follow-up duration was 16.1 months (range, 8.3–28.5), and the disease-free survival rate at 12 months was 95.8% (95% CI, 73.9%–99.4%). Grade 3 neoadjuvant therapy–related adverse events included rash (1; 3.3%), pruritis (1; 3.3%), and thrombocytopenia (1; 3.3%), and no grade 4 or 5 treatment-related events occurred. The most common surgical complication was delayed wound healing (5; 18.5%).
Conclusions:
Neoadjuvant chemotherapy plus camrelizumab for locally advanced HNSCC showed high ORR, pCR, and MPR rates, with an acceptable safety profile. These data support further evaluation of neoadjuvant chemoimmunotherapy for the treatment of locally advanced HNSCC.
Translational Relevance.
Immune-checkpoint inhibitors in combination with platinum-based chemotherapy have been shown to improve survival in the first-line setting of recurrent/metastatic head and neck squamous cell carcinoma (HNSCC). We aimed to assess the antitumor activity and safety of PD-1 inhibitor camrelizumab plus platinum-based chemotherapy in patients with resectable locally advanced HNSCC in the neoadjuvant setting. In this phase 2 single-arm clinical trial, including 30 patients with HNSCC, camrelizumab combined with neoadjuvant chemotherapy showed a favorable safety profile without surgical delay and exhibited encouraging efficacy results, including high rates of objective response rate (96.7%) and pathological response. The pathological complete response (pCR) rate (37.0%) or major pathological response (MPR) rate (74.1%) was higher than that previously reported with PD-1 inhibitor alone (0%–6.7%) or in combination with the CTLA4 antibody (3%–20%). These results provide initial evidence for the safety and efficacy of neoadjuvant chemoimmunotherapy for the treatment of locally advanced HNSCC.
Introduction
Current standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is either surgical resection followed by risk-adapted adjuvant radiotherapy with or without platinum-based chemotherapy, or definitive concurrent chemoradiotherapy. With this aggressive combined modality therapy, the risk of recurrence, distant metastases, and death (5-year survival rate) for patients with locally advanced human papillomavirus (HPV)–negative HNSCC remains unsatisfactorily high (1, 2). Although HPV-positive HNSCC has been identified as a distinct clinical entity with better prognosis than HPV-negative HNSCC, distant metastases remain a problem and usually occur later (3).
Immunotherapy in HNSCC is a rapidly evolving field. The role of anti–programmed cell death-1 (PD-1) antibody has been well established for second-line treatment of recurrent/metastatic (R/M) HNSCC (4, 5). Recently, data from KEYNOTE-048 support use of pembrolizumab with or without chemotherapy for first-line treatment of R/M HNSCC (6). The successful application of immune checkpoint inhibitors in R/M HNSCC has fueled widespread interest in their neoadjuvant use. Several ongoing trials of neoadjuvant immunotherapy (mainly mono- or dual immunotherapy) in HNSCC showed promising results (7, 8). However, data on neoadjuvant chemoimmunotherapy in HNSCC are lacking.
Pathological complete response (pCR) after neoadjuvant therapy has been recognized as a surrogate endpoint to predict long-term outcome, such as disease-free survival (DFS), and overall survival (OS) in several cancers (9–12). Although earlier studies failed to confirm the survival benefit of neoadjuvant chemotherapy in patients with locally advanced HNSCC, some of them reported pCR rates ranging from 10% to 27% and showed that patients with a pCR had improved OS (13–15). Furthermore, previous neoadjuvant studies of immune check point blockade in locally advanced HNSCC have shown low pCR rates with some variation up to 6.7% (7, 8), suggesting that more effective treatment approaches, such as combinatorial neoadjuvant therapies, are needed to achieve more rapid and deeper tumor regression during the neoadjuvant window. Chemotherapy can enhance antitumor immunity by inducing immunogenic cell death, upregulating tumor neo-antigen expression and disturbing the immunosuppressive tumor microenvironment (16). Incorporation of neoadjuvant chemoimmunotherapy in potentially resectable locally advanced HNSCC might attain favorable pathological response, enhance systemic immunity to prevent tumor recurrence and metastasis, and increase the possibility of tumor shrinkage to facilitate more limited surgery and de-escalate adjuvant therapy.
On the basis of these rationales, we hypothesized that neoadjuvant chemoimmunotherapy would be safe and increase the proportion of patients who achieved a pCR. Camrelizumab is a high-affinity, fully humanized, IgG4-κ PD-1 monoclonal antibody with promising clinical activity and tolerable safety across multiple cancers (17–25). It has also recently been approved in combination with chemotherapy as a first-line treatment for patients with recurrent or metastatic nasopharyngeal carcinoma in China (26). Here, we reported the results of a phase 2 study evaluating neoadjuvant camrelizumab in combination with chemotherapy in patients with locally advanced HNSCC.
Patients and Methods
Study Design and Patient Eligibility
This single-arm phase 2 study was conducted at a tertiary hospital in China. Eligible patients aged 18–70 years, had pathologically confirmed HNSCC (oral cavity, oropharynx, hypopharynx, or larynx), had stage III–IVB disease for non-oropharyngeal cancer and HPV-negative oropharyngeal cancer or stage II–III disease for HPV-positive oropharyngeal cancer according to the 8th Edition of American Joint Committee on Cancer (AJCC) guideline, had resectable tumor evaluated by a head and neck surgeon before enrollment, had no evidence of distant metastases, had no prior anti-cancer therapy for HNSCC, had at least one measurable target lesion according to the RECIST 1.1, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and had normal hematological, hepatic, and renal function. HPV status for oropharyngeal cancer was determined using p16 IHC. Samples were considered p16 positive if >70% of tumor cells showed strong and diffuse nuclear and cytoplasmic staining (27). Tested samples were derived from the primary tumor or a lymph node metastasis. The full trial protocol is available in the Supplementary Data. The study was done in accordance with the Good Clinical Practice and Declaration of Helsinki. All patients provided written informed consent before enrollment. Ethics approval was obtained from the Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. This trial is registered with Chictr.org.cn, ChiCTR1900025303.
Procedures
All patients received albumin-bound paclitaxel 260 mg/m2 (or docetaxel 75 mg/m2), cisplatin 75 mg/m2, and camrelizumab 200 mg on day 1 of each 3-week cycle for 3 cycles. All patients received routine antiemetic prophylaxis during each cycle, including 12 mg of dexamethasone (intravenous) on day 1, and 8 mg on days 2–4. Surgery was performed at one to four weeks after the completion of 9-week neoadjuvant therapy. Surgical delay was defined as the delay in the planned surgery of no more than 49 days after the first day of the third treatment cycle. All patients were evaluated by a head and neck surgeon before enrollment. Surgical plan and resection margins were defined by baseline evaluation before neoadjuvant therapy and did not change in the case of treatment response. Adjuvant radiotherapy was given at four weeks after surgery if the patients were recovered from surgery.
End Points
Co-primary end points were pCR rate and safety. Secondary end points included objective response rate (ORR), major pathological response (MPR) rate, 2-year DFS rate, and 2-year and 5-year OS rates. ORR was defined as the proportion of patients with best response of complete or partial response as per RECIST 1.1 before surgery. All imaging examinations were reviewed by a board-certified radiologist and subsequently confirmed by 100% agreement of another two masked board-certified radiologists. A local board-certified head and neck pathologist (with at least 10 years of experience) evaluated the pathological response to the percentage of residual viable tumor in resected tumor specimens. Subsequently, two independent masked pathologists who were blinded to patients’ clinical information verified the results with 100% consistency. The entire primary tumor and all sampled lymph nodes were examined for pathological analysis. Subsequently, two independent pathologists who were blinded to patients’ clinical information verified the results with 100% consistency. The entire primary tumor and all sampled lymph nodes were examined for pathological analysis. The median number of sections reviewed for pathological response for each patient was 8 (ranging from 4 to 15). pCR was defined as no residual viable tumor cells in the resected primary tumor and all sampled lymph nodes. MPR was defined as ≤10% residual viable tumor cells in the resected tumor specimens (28). Incomplete pathological response (IPR) was defined as the presence of >10% viable tumor cells in the resected tumor specimens. Clinical to pathological downstaging was defined as a reduction in T stage or N stage of pathologic staging (ypTNM) compared with clinical staging (cTNM) based on the 8th edition of the AJCC cancer staging manual. DFS was defined as the time from the completion of screening for eligibility to disease recurrence, progression, second tumor, or death from any cause, whichever came first. OS was defined as the time the completion of screening for eligibility to death from any cause. Adverse events (AE) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Surgical complications were assessed according to the Clavien–Dindo classification system (29). Patients’ quality of life (QOL) was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30; ref. 30). Patients’ depression was measured by the self-rating depression scale (31).
Pretreatment peripheral blood samples were collected and examined for lymphocyte subsets (CD3+ T cells, CD4+ T cells, CD8+ T cells, CD19+ B cells, and CD56+ NK cells) by flow cytometry (lymphocyte test kit, Beckman Coulter Inc.) and plasma cytokines (IL2, IL4, IL6, IL10, TNFα, and IFNγ) by ELISA (human Th1/2 cytokine kit II, BD Biosciences Ltd.). To detect the expression of PD-ligand 1 (PD-L1), immunohistochemical staining was carried out using PD-L1 IHC 22C3 pharmDx assay (Agilent) by the Dako Autostainer Link 48. Appropriate positive and negative controls were used. PD-L1 expression was evaluated using the combined positive score [CPS; the number of PD-L1–staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumors and multiplied by 100].
Statistical analysis
Historical data showed that the pCR rate after neoadjuvant chemotherapy (docetaxel, cisplatin, and 5-fluorouracil) in patients with locally advanced HNSCC was 13.4% (14). We estimated that a sample size of 27 patients would detect approximately 20% improvement (34%) in pCR rate with a power of 80%, using a one-sided alpha of 0.025. Assuming a 10% drop-out rate, a total of 30 patients were planned to accrue in our study.
Statistical analysis was conducted using SAS statistical software (V.9.4, SAS Institute). Continuous variables were expressed as median (range), and compared between groups using the Mann–Whitney test. Categorical variables were expressed as frequency (percentage). Survival was estimated using the Kaplan–Meier method.
Data availability
The data generated in this study are available upon request from the corresponding author.
Results
Patient characteristics
From July 2019 to May 2021, a total of 30 patients were enrolled (Fig. 1). Demographics and clinical characteristics are shown in Table 1. The median age was 58 years (range, 41–69), and 27 (90.0%) patients were males. There were 12 (40.0%) patients with cancer at tonsil, 9 (30.0%) at hypopharynx, 6 (20.0%) at larynx, 2(6.7%) at base of tongue, and 1 (3.3%) at soft palate. Five (38.5%) out of 13 patients with oropharyngeal cancer had HPV-positive disease as determined by p16 IHC. The majority of patients (17; 56.7%) had stage IVA disease.
Figure 1.
Trial flow diagram.
Table 1.
Baseline characteristics.
| Characteristics | Patients (n = 30) |
|---|---|
| Age, median (range; years) | 58 (41–69) |
| Sex, n (%) | |
| Male | 27 (90.0) |
| Female | 3 (10.0) |
| Smoker, n (%) | |
| No | 16 (53.3) |
| Former, ≤10 pack-year | 1 (3.3) |
| Former, >10 pack-year | 13 (43.3) |
| Alcohol use history, n (%) | |
| Ever | 21 (70.0) |
| Never | 9 (30.0) |
| HPV status (oropharynx), n (%) | |
| Positive | 5 (38.5) |
| Negative | 8 (61.5) |
| Primary tumor site, n (%) | |
| Tonsil | 12 (40.0) |
| Soft palate | 1 (3.3) |
| Larynx | 6 (20.0) |
| Hypopharynx | 9 (30.0) |
| Base of tongue | 2 (6.7) |
| Pretreatment clinical T-stage, n (%) | |
| T1 | 1 (3.3) |
| T2 | 7 (23.3) |
| T3 | 14 (46.7) |
| T4 | 8 (26.7) |
| Pretreatment clinical N-stage, n (%) | |
| N0 | 3 (10.0) |
| N1 | 4 (13.3) |
| N2 | 18 (60) |
| N3 | 5 (16.7) |
| AJCC stage, n (%) | |
| II | 4 (13.3) |
| III | 4 (13.3) |
| IV | 22 (73.3) |
| IVA | 17 (56.7) |
| IVB | 5 (16.7) |
| PD-L1 combined positive score, n (%) | |
| <1 | 2 (6.7) |
| 1–19 | 12 (40.0) |
| ≥20 | 8 (26.7) |
| Not available | 8 (26.7) |
Safety and feasibility
All 30 patients completed three cycles of neoadjuvant chemoimmunotherapy and thus were included in the intention-to-treat (ITT) population. All patients had treatment-related AEs (TRAE) during neoadjuvant therapy and 2 (6.7%) of them had grade 3 or worse TRAEs. The most common TRAEs were alopecia (30; 100%), neurotoxicity (13; 43.3%), fatigue (9; 30.0%), and nausea/vomiting (9; 30.0%; Table 2). None of the TRAEs led to discontinuation of the whole study treatment, dose reduction, surgery delay, or death; however, 1 (3.3%) patient had grade 3 rash and pruritus leading to discontinuation of camrelizumab treatment after the first cycle.
Table 2.
Treatment-related adverse events during neoadjuvant therapy.
| Patients (n = 30) | |||
|---|---|---|---|
| Events | Grade 1–2 | Grade 3 | Grade 4 |
| Alopecia | 30 (100) | 0 | 0 |
| Neurotoxicity | 13 (43.3) | 0 | 0 |
| Fatigue | 9 (30.0) | 0 | 0 |
| Nausea/vomiting | 9 (30.0) | 0 | 0 |
| Hypothyroidism | 8 (26.7) | 0 | 0 |
| Leukopenia | 7 (23.3) | 0 | 0 |
| Pruritus | 6 (20.0) | 1 (3.3) | 0 |
| Anemia | 5 (16.7) | 0 | 0 |
| RCCEP | 4 (13.3) | 0 | 0 |
| Myalgia | 4 (13.3) | 0 | 0 |
| Arthralgia | 3 (10.0) | 0 | 0 |
| Constipation | 3 (10.0) | 0 | 0 |
| Diarrhea | 2 (6.7) | 0 | 0 |
| Increased aminotransferases | 2 (6.7) | 0 | 0 |
| Rash | 1 (3.3) | 1 (3.3) | 0 |
| Increased creatinine | 1 (3.3) | 0 | 0 |
| Tuberculosis recurrence | 1 (3.3) | 0 | 0 |
| Thrombocytopenia | 0 | 1 (3.3) | 0 |
Abbreviation: RCCEP, reactive cutaneous capillary endothelial proliferation.
Two patients (P04 and P10) with HPV-negative tonsil carcinoma achieved stable disease and partial response (33.2% target lesion reduction) after three cycles of chemoimmunotherapy, respectively. They both refused surgery and received definitive concurrent chemoradiotherapy. Another patient (P30) with laryngeal carcinoma who achieved partial response refused surgery or chemoradiotherapy. The remaining 27 (90.0%) patients underwent surgery without delay. The median time to surgery from the first day of the third treatment cycle was 29 days (range, 20–48). No grade III or IV surgical complications were observed. The most common complications were delayed wound healing (5; 18.5%), intraoperative blood loss >50 mL (3; 11.1%), and pharyngeal fistula (3; 11.1%; Supplementary Table S1). No surgery-related deaths occurred.
Of the 27 patients who underwent surgery, 23 received planned adjuvant radiotherapy and thus were included in the per-protocol population. The detailed reasons for the patients who did not receive or complete planned radiotherapy were listed in Fig. 1.
Efficacy outcomes
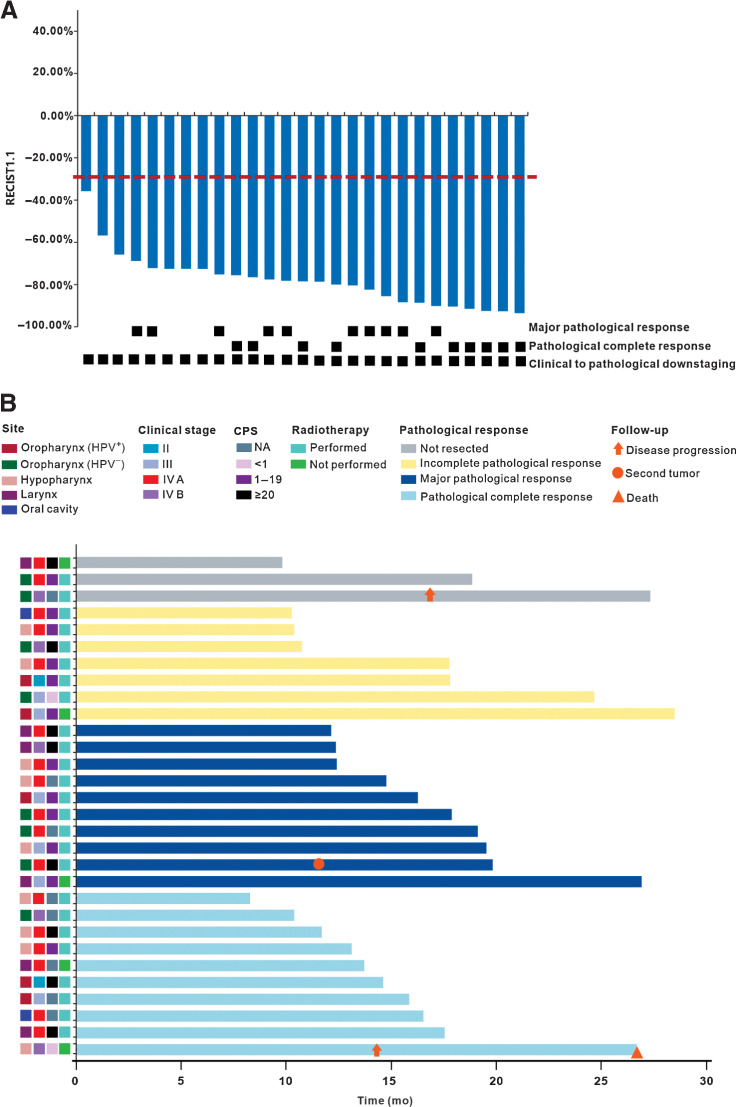
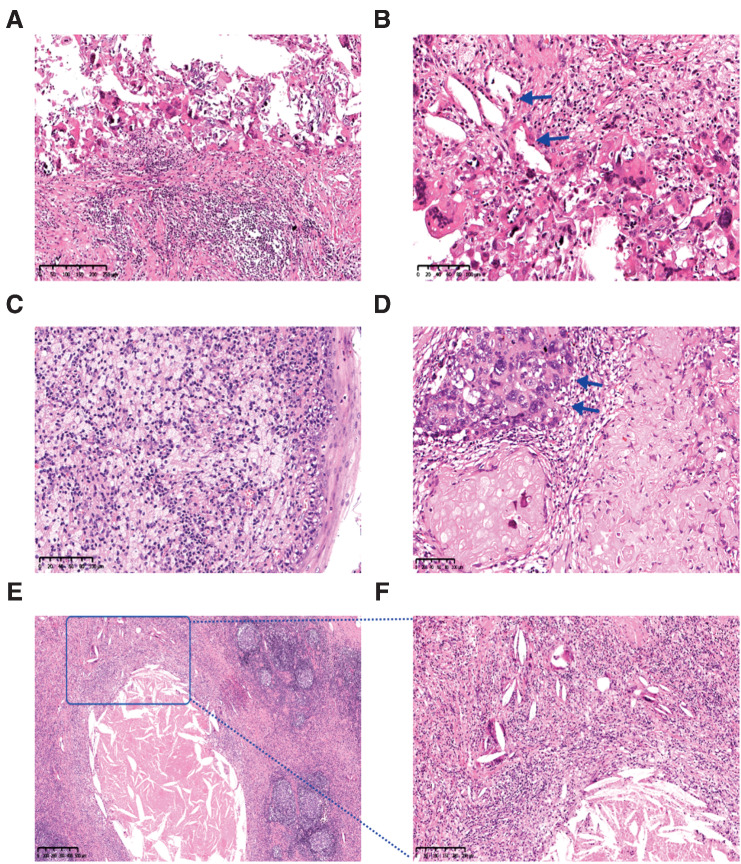
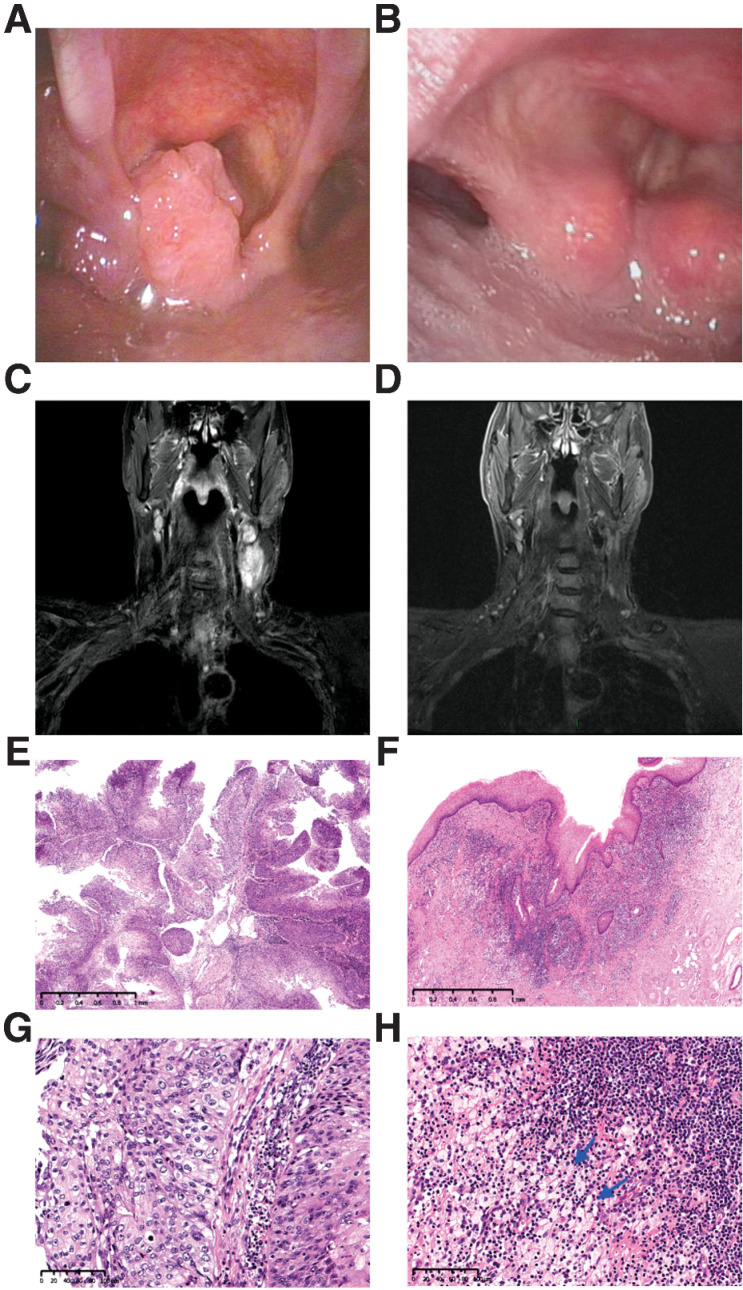
After neoadjuvant therapy, 29 of 30 patients had a partial response, with an ORR of 96.7% [95% confidence interval (CI), 82.8%–99.9%]. One (3.3%; 95% CI, 0.1%–17.2%) patient had stable disease. Clinical to pathological downstaging was observed in all 27 (100%; 95% CI, 87.2%–100%) patients who underwent surgery. The R0 resection rate was 92.6% (25/27; 95% CI, 75.7%–99.1%), and the throat and hypopharyngeal function retention rate was 85.7% (12/14; 95% CI, 57.2%–98.2%). Three patients (11.1%) had high-risk pathology (two with positive margins and one with extranodal extension) and were treated with post-operative adjuvant cisplatin and radiotherapy. Twenty of 27 (74.1%; 95% CI, 53.7%–88.9%) patients achieved an MPR, including 10 (37.0%; 95% CI, 19.4%–57.6%) with a pCR. The MPR and pCR rates were 60.0% (95% CI, 14.7%–94.7%) and 40.0% (95% CI, 5.3%–85.3%) among 5 HPV-positive oropharyngeal patients, and 66.7% (95% CI, 22.3%–95.7%) and 16.7% (95% CI, 0.4%–64.1%) among 6 HPV-negative oropharyngeal patients. The waterfall plot of radiographic and pathological response for individual patients is shown in Fig. 2A. The detailed treatment outcomes for individual patients are listed in Supplementary Table S2. Regardless of HPV status, pathological features observed in patients with MPR included mixed inflammatory infiltration, visible tumor regression, fibrosis, aggregation of macrophages into the regression bed, cholesterol clefts, giant cell reaction, hemosiderin deposition, calcification, and acellular keratin (Fig. 3A–F). Figure 4A–H shows a representative example of striking response in a patient (P21) with T2N2cM0 squamous cell carcinoma involving the left pyriform sinus. His primary tumor bulk disappeared by the time of surgery, and subsequent pathogenic analysis demonstrated pCR.
Figure 2.
Tumor response. A, Treatment response of individual patients who underwent surgery (n = 27). The red horizontal line represents 30% target lesion reduction. B, Swimming plot of disease-free survival for individual patients (n = 30). CPS, combined positive score; HPV, human papilloma virus; NA, not available.
Figure 3.
Significant tumor response was observed in the radical specimens. A and B, Fibrosis, lymphocyte infiltration, necrosis, cholesterol clefts (arrow), giant cell reaction, and calcification in primary tumor with pathological complete response (pCR). Hematoxylin–eosin, magnifications, ×100 (A) and ×200 (B). C, An aggregation of macrophages into the regression bed of HPV+ subject with pCR. Hematoxylin–eosin, magnifications, ×200. D, Acellular keratin and residual viable tumor (arrow) in the primary tumor with major pathological response (MPR). Hematoxylin–eosin, magnifications, ×200. E and F, Necrosis, histiocytes, cholesterol clefts, and giant cell reaction in metastatic lymph node with pCR. Hematoxylin–eosin, magnifications, ×40 (E) and ×100 (F). F is an enlargement within the square of E.
Figure 4.
Tumor regression in a 61-year-old man with stage IVA hypopharyngeal cancer (T2N2cM0, P21). The tumor was PD-L1 positive (combined positive score = 10) and originated in the left pyriform sinus. A, Pretreatment image showed a cT2 squamous cell carcinoma of the left pyriform sinus. B, Pretreatment MR imaging showed bilateral cervical lymph nodes. C and D, After three cycles of neoadjuvant chemoimmunotherapy, image at the time before surgery demonstrates near-complete clinical regression of primary lesion as well as cervical lymph nodes. E and F, Pathological findings of biopsy specimens of hypopharyngeal masses before treatment. The tumor is non-keratinizing squamous cell carcinoma with papillary features. Hematoxylin–eosin, magnifications, ×30 (E) and ×200 (F). G and H, Histopathologic images of the resection specimen after treatment. Fibrosis, lymphocyte infiltration, and histiocytes aggregation (arrow) were found in the regression bed, and no cancer residue was found (pathological complete response).Hematoxylin–eosin, magnifications, ×30 (G) and ×200 (H).
By the cutoff value date on January 25, 2022, the median follow-up duration was 16.1 months (range, 8.3–28.5). The median duration of DFS was not reached in the ITT population or in the per-protocol population. Twenty-seven (90.0%) of 30 patients in the ITT population and 22 (95.7%) of 23 patients in the per-protocol population remained alive, without evidence of disease (Fig. 2B). In the ITT population, the DFS rate at 12 months was 95.8% (95% CI, 73.9%–99.4%). In the per-protocol population, the DFS rate at 12 months was 94.4% (95% CI, 66.6%–99.2%). One patient (P04) with HPV-negative tonsil carcinoma (T3N3bM0) who refused surgery received definitive chemoradiotherapy. However, chemoradiotherapy was stopped at 23 fractions due to the impact of coronavirus disease 2019 (COVID-19). After 17 months of follow-up, he had lymph nodes relapse in his right neck, refused additional therapy, and was alive at the time of data cutoff. One patient (P02) with hypopharyngeal carcinoma (T1N3bM0) who had pCR after surgery did not receive adjuvant radiotherapy due to COVID-19 outbreak. He developed a disease relapse at the primary site 14 months later and died. One patient (P07) with HPV-negative tonsil carcinoma (T2N2bM0) developed a second laryngeal carcinoma with T1N0M0 stage at 7 months after completing adjuvant radiotherapy. He received cordectomy later, and is currently without evidence of disease.
Quality of life
The global health status, physical function, social function, emotional function, and dyspnea were improved during neoadjuvant therapy and surgery, but showed deterioration after adjuvant radiotherapy. These deteriorations were improved during the subsequent follow-up. Fatigue, pain, nausea/vomiting, diarrhea, constipation, insomnia, and depression showed deterioration during the neoadjuvant therapy, surgery, and adjuvant radiotherapy, but improved during the subsequent follow-up (Supplementary Fig. S1A–S1L).
Biomarker analysis
Twenty pretreatment samples were evaluable for PD-L1 analysis. CPS ranged from 0 to 100. Eight patients (26.7%) had CPS higher than 20. There was no significant difference in CPS between patients with pCR and IPR, and patients with MPR and IPR (Supplementary Fig. S2A–S2C). To explore potential biomarkers that can predict pathological responses to chemoimmunotherapy, we examined the levels of a panel of lymphocyte subsets (Supplementary Fig. S3A–S3E) and plasma cytokines (Supplementary Fig. S4A–S4F) in pretreatment blood samples from the patients. We found that the pretreatment levels of IL-6 in patients with MPR or pCR + MPR was significantly higher than those with IPR (both P = 0.01; Supplementary Fig. S4C). Patients with pCR also had a trend toward higher pretreatment levels of IL-6 than those with IPR (P = 0.09).
Discussion
To the best of our knowledge, this is the first report of neoadjuvant chemoimmunotherapy before surgery as a therapeutic strategy in patients with resectable locally advanced HNSCC. Camrelizumab combined with neoadjuvant chemotherapy in locally advanced HNSCC showed a favorable safety profile without surgical delay and exhibited encouraging efficacy results, including relatively high rates of radiological response, pathological response, and clinical to pathological downstaging.
There is a lack of evidence to support the routine use of neoadjuvant chemotherapy in resectable locally advanced HNSCC patients (14, 15, 32). We hypothesized that neoadjuvant chemotherapy in combination with checkpoint inhibitors can have a synergistic effect and provide long-term survival benefit in locally advanced HNSCC, which is built on the success of combination of chemotherapy and PD-1 inhibitors in the recurrent and metastatic setting. In the KEYNOTE-048 study, long-term follow-up confirmed the significant improvement in OS. Pembrolizumab combined with chemotherapy was associated with a significant increased 4-year survival rate than the EXTREME regimen (19.4% vs. 4.5% in the total population). In addition, previous neoadjuvant studies of immune check point blockade in locally advanced HNSCC have shown low pathological response rate. In our study, the pCR rate (37.0%) that represents a potential surrogate end point in locally advanced HNSCC, was higher than that previously reported with pembrolizumab alone (0%; ref. 7), nivolumab alone (0%), or in combination with ipilimumab (6.7%; ref. 8). The MPR rate observed in our study (74.1%) was also much higher than pembrolizumab alone (3%; ref. 7), nivolumab alone (7%), or in combination with ipilimumab (20%; ref. 8). A recent study reported a high rate of biopsy-proven pCR (52%) of the patients with locally advanced HNSCC after combined durvalumab plus tremelimumab and platinum-based chemotherapy. However, it is possible that this rate is overestimated, because viable tumors in other regions were not taken into consideration (33). Further follow-up will be conducted to determine whether pCR or MPR in our study are associated with DFS and OS. In our study, a high ORR (96.7%) after neoadjuvant treatment was also observed, suggesting a strong combinatorial effect of chemoimmunotherapy. Another possible reason for the high ORR might be that East Asian patients with HSNCC seem to have a relatively higher response rate (67.3%–86.5%) to neoadjuvant/induction chemotherapy alone (14, 34–36).
In general, the neoadjuvant chemoimmunotherapy regimen in our study was well tolerated. No delays to surgical resection or severe TRAEs were observed. Half of patients had mild surgical complications. Similar to the recent NIRT trial using neoadjuvant nivolumab, a small proportion of patients in our study had wound healing problems (37). However, a previous study using neoadjuvant nivolumab or nivolumab plus ipilimumab in locally advanced oral cavity squamous cell carcinoma, did not report any concerning events, including wound healing after surgery (8). Future trials with larger sample size are needed to confirm the postoperative safety following chemoimmunotherapy.
With a median follow-up of 16.1 months, in patients in the per-protocol population, only one developed a second cancer in the larynx. We observed two patients with recurrence, but both of them did not follow major protocol. The patient (P02) with very advanced hypopharyngeal cancer (T1N3bM0) achieved pCR after surgery, but did not receive adjuvant radiotherapy due to COVID-19 outbreak. He recurred at the primary site after 14 months and died soon after. This case might highlight the necessity for the role of adjuvant radiotherapy in locally advanced HNSCC with high-risk features. The second patient (P04) with stage IVB tonsil cancer refused surgery, and did not complete chemoradiotherapy either because of COVID-19 outbreak.
Cytokines are considered as immune-signaling molecules, and can reflect the systemic immune conditions of patients. We measured six plasma cytokines and only found that pretreatment IL-6 level was associated with pathological response in our cohort. Recent similar studies have shown that baseline plasma IL-6 levels or their changes during treatment correlated to checkpoint inhibitors responses in non–small cell lung cancer (38, 39). The role of IL-6 as a potential biomarker in the immunotherapy era needs to be explored further.
In addition to our trial, immunotherapy is being combined with chemotherapy or radiotherapy before surgery in several neoadjuvant combinations. In a phase II trial, nivolumab plus chemotherapy followed by transoral robotic surgery or radiotherapy/chemoradiotherapy is being investigated in patients with HPV-positive oropharyngeal cancer (NCT03107182). Several ongoing clinical trials are also aimed at deintensifying the radiotherapy treatment impact by incorporating immunotherapy into radiation-based therapy. A phase II/III trial is currently underway to determine whether radiotherapy combined with immunotherapy (nivolumab or durvalumab) can be used to deintensify patients with HPV-positive HNSCC (NCT03952585 and NCT03410615). The purpose of these trials is to establish whether there is a synergistic effect of immunotherapy, radiotherapy, and/or chemotherapy in combination.
There are several limitations in our study. First, this was a single-arm study with relatively small sample size and short postoperative follow-up time. Second, a validated end point to define a clinically meaningful pathological response following neoadjuvant therapy in locally advanced HNSCC was lacking. Long-term follow-up is necessary to determine whether the observed pathological response in our trial translates into improved survival. Third, the treatment algorithms for HPV-negative and HPV-positive oropharyngeal cancer were identical in our trial. As the prevalence of HPV-positive oropharyngeal cancer in China is much lower than that in western countries (40, 41), there is a lack of high-level evidence to support the prognostic value of HPV as well as the role of treatment deintensification for HPV-positive oropharyngeal cancer in the Chinese population. Our trial started in 2019, and according to the Chinese Society of Clinical Oncology guidelines for HNSCC at that time, there was no difference regarding the treatment strategies between HPV-positive and HPV-negative oropharyngeal cancer. The long-term QOL and functional outcomes of our trial participants, especially those who had HPV-positive disease, will help us better understand the impact of treatment and will be reported in the future.
In conclusion, we reported the first neoadjuvant study using chemotherapy combined with PD-1 inhibitor before surgery in patients with HNSCC. This regimen was safe and resulted in promising rates of radiographic and pathological response. Further evaluation in large-scale randomized clinical trials of this combination strategy is warranted.
Supplementary Material
Acknowledgments
This work was supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd. and the National Natural Science Foundation of China (grant no. 81874222 to K. Yang). We thank the participants and their families for participating in this trial. We thank Du Wang (Statistics Manager at Jiangsu Hengrui Pharmaceuticals Co., Ltd.), Cheng Zhang (Medical Manager at Hengrui), and Can Zhang for their input into data analysis, and Fangzhou Xia (Medical Writer at Hengrui) for the medical writing assistance.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 3171
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
K. Yang reports grants from National Natural Science Foundation of China during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
Z. Zhang: Conceptualization, resources, data curation, software, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. B. Wu: Conceptualization, resources, data curation, software, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. G. Peng: Conceptualization, resources, data curation, supervision, investigation. G. Xiao: Data curation, formal analysis, validation, investigation, writing–original draft. J. Huang: Resources, data curation. Q. Ding: Resources, data curation. C. Yang: Supervision, investigation. X. Xiong: Resources, data curation. H. Ma: Supervision, methodology. L. Shi: Investigation. J. Yang: Investigation. X. Hong: Investigation. J. Wei: Investigation. Y. Qin: Investigation. C. Wan: Software, formal analysis. Y. Zhong: Investigation. Y. Zhou: Investigation. X. Zhao: Investigation. Y. Leng: Investigation. T. Zhang: Supervision. G. Wu: Supervision. M. Yao: Supervision. X. Zhang: Conceptualization, resources, data curation, supervision, funding acquisition, validation, investigation, methodology, writing–original draft, project administration, writing–review and editing. K. Yang: Conceptualization, resources, data curation, supervision, funding acquisition, validation, investigation, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44. [DOI] [PubMed] [Google Scholar]
- 2. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–52. [DOI] [PubMed] [Google Scholar]
- 3. Trosman SJ, Koyfman SA, Ward MC, Al-Khudari S, Nwizu T, Greskovich JF, et al. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA 2015;141:457–62. [DOI] [PubMed] [Google Scholar]
- 4. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- 6. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 7. Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin Cancer Res 2020;26:5140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoenfeld JD, Hanna GJ, Jo VY, Rawal B, Chen YH, Catalano PS, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol 2020;6:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. [DOI] [PubMed] [Google Scholar]
- 10. Berruti A, Amoroso V, Gallo F, Bertaglia V, Simoncini E, Pedersini R, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883–91. [DOI] [PubMed] [Google Scholar]
- 11. He J, Blair AB, Groot VP, Javed AA, Burkhart RA, Gemenetzis G, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg 2018;268:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 2020;26:2838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leon X, Quer M, Orus C, Sancho FJ, de Juan M, Lopez-Pousa A. Histologically negative specimens after induction therapy: frequency and impact on survival. Head Neck 2000;22:808–13. [DOI] [PubMed] [Google Scholar]
- 14. Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Jr, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol 2013;31:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bossi P, Lo Vullo S, Guzzo M, Mariani L, Granata R, Orlandi E, et al. Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Ann Oncol 2014;25:462–6. [DOI] [PubMed] [Google Scholar]
- 16. Judd J, Borghaei H. Combining immunotherapy and chemotherapy for non–small cell lung cancer. Thorac Surg Clin 2020;30:199–206. [DOI] [PubMed] [Google Scholar]
- 17. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571–80. [DOI] [PubMed] [Google Scholar]
- 18. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832–42. [DOI] [PubMed] [Google Scholar]
- 19. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non–small cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305–14. [DOI] [PubMed] [Google Scholar]
- 20. Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu K, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res 2021;27:1296–304. [DOI] [PubMed] [Google Scholar]
- 21. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 2021;326:916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res 2021;27:3069–78. [DOI] [PubMed] [Google Scholar]
- 23. Lan C, Shen J, Wang Y, Li J, Liu Z, He M, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): a multicenter, open-label, single-arm, phase II trial. J Clin Oncol 2020;38:4095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen J, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): a multicenter, two-stage, phase 2 trial. J Thorac Oncol 2021;16:299–309. [DOI] [PubMed] [Google Scholar]
- 25. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol 2022;17:544–57. [DOI] [PubMed] [Google Scholar]
- 26. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2021;22:1162–74. [DOI] [PubMed] [Google Scholar]
- 27. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck 2012;34:459–61. [DOI] [PubMed] [Google Scholar]
- 28. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non–small cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 31. Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- 32. Marta GN, Riera R, Bossi P, Zhong LP, Licitra L, Macedo CR, et al. Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: systematic review and meta-analysis. Eur J Cancer 2015;51:2596–603. [DOI] [PubMed] [Google Scholar]
- 33. Hecht M, Eckstein M, Rutzner S, von der Grun J, Illmer T, Klautke G, et al. Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer. J Immunother Cancer 2022;10:e003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang XR, Liu ZM, Liu XK, Wang FH, Li Q, Li H, et al. Influence of pathologic complete response to neoadjuvant chemotherapy on long-term survival of patients with advanced head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:218–23. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Fang Q, Du W, Zhang X, Dai L, Qiao Y. Induction chemotherapy combined with immunotherapy in locally advanced head and neck squamous cell carcinoma. BMC Cancer 2021;21:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Won HS, Lee YS, Jeon EK, Hong SH, Kang JH, Kim YS, et al. Clinical outcome of induction chemotherapy in locally advanced head and neck squamous cell carcinoma. Anticancer Res 2014;34:5709–14. [PubMed] [Google Scholar]
- 37. Leidner R, Crittenden M, Young K, Xiao H, Wu Y, Couey MA, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer 2021;9:e002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang DH, Park CK, Chung C, Oh IJ, Kim YC, Park D, et al. Baseline serum interleukin-6 levels predict the response of patients with advanced non–small cell lung cancer to PD-1/PD-L1 inhibitors. Immune Netw 2020;20:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keegan A, Ricciuti B, Garden P, Cohen L, Nishihara R, Adeni A, et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J Immunother Cancer 2020;8:e000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Y, Liu S, Yi H, Wang J, Luo Y, Yin S. Low prevalence of human papillomavirus in head and neck squamous cell carcinoma in Chinese patients. J Med Virol 2015;87:281–6. [DOI] [PubMed] [Google Scholar]
- 41. Guo L, Yang F, Yin Y, Liu S, Li P, Zhang X, et al. Prevalence of human papillomavirus type-16 in head and neck cancer among the Chinese population: a meta-analysis. Front Oncol 2018;8:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.