Abstract
Background
Durvalumab consolidation is associated with improved survival following concurrent chemoradiotherapy (CCRT) in patients with stage III non‐small cell lung cancer (NSCLC). Given the heterogeneity of stage III NSCLC patients, in this study we evaluated the efficacy and safety of durvalumab in the real‐world setting.
Method
Unresectable stage III NSCLC patients were retrospectively studied: one cohort received CCRT, another had CCRT‐durvalumab. Primary endpoints were progression‐free survival (PFS) and overall survival (OS), secondary endpoints were relapse rate and safety. In CCRT‐durvalumab cohort, association between blood markers with survival and pneumonitis risk were analyzed.
Results
A total of 84 patients were enrolled: 45 received CCRT, and 39 received CCRT‐durvalumab.
Median PFS was 17.5 months for CCRT‐durvalumab and 8.9 months for CCRT‐alone (HR 0.47, p = 0.038). Median OS was not‐reached for CCRT‐durvalumab and 22.3 months for CCRT‐alone (HR 0.35, p = 0.024). Both EGFR‐positive and wild‐type (WT) patients had numerically improved PFS with durvalumab consolidation compared to CCRT‐alone, 17.5 versus 10.9 months and 11.8 versus 6.63 months, respectively (interaction p‐value = 0.608). Grade 2+ pneumonitis was detected in 25% of patients in the durvalumab cohort. Most pneumonitis occurred at 3.5 weeks after durvalumab initiation. Baseline neutrophil‐to‐lymphocyte ratio (NLR) ≥ 3 and ≥5 were associated with shorter PFS with durvalumab. Week 6 platelet‐lymphocyte‐ratio ≥ 180 was associated with a lower risk of pneumonitis.
Conclusion
In this real‐world study, durvalumab consolidation post CCRT was associated with a statistically significant improvement in PFS and OS. Effect of durvalumab on PFS was not modified by EGFR status. Active surveillance for pneumonitis is crucial. Baseline NLR may help to predict the benefit of treatment with durvalumab.
Keywords: consolidation, Durvalumab, non‐small cell lung cancer, real‐world, stage III
Durvalumab consolidation following definitive chemoradiation was associated with statistically significant improvement in progression‐free and overall survival. Pneumonitis occurred early at 3.5 weeks following durvalumab initiation. Baseline neutrophil‐lymphocyte‐ratio may predict a benefit from treatment with durvalumab. Patients with EGFR positive and wild‐type NSCLC had a numerically longer PFS with durvalumab consolidation compared to chemoradiation alone, though benefit was greater in wild‐type NSCLC.

INTRODUCTION
Despite multiple earlier efforts, 1 , 2 , 3 , 4 very little progress has been made in the treatment of unresectable stage III non‐small cell lung cancer (NSCLC) and prognosis remains poor with 5‐year overall survival (OS) of 15% 5 . More recently, results from the PACIFIC study, a randomized phase III study, showed the addition of consolidation durvalumab following concurrent platinum based chemoradiotherapy was associated with an improvement in progression‐free survival (PFS) (16.9 vs. 5.6 months, HR 0.55) and overall survival (OS) (5‐year OS 42.9% vs. 33.4%, HR 0.72) compared to placebo at 5 years. 6 , 7 Based on results from the PACIFIC study, durvalumab is now approved for stage III NSCLC in many countries including Singapore.
Given the heterogeneity of stage III NSCLC patients, 8 , 9 it is crucial to evaluate the efficacy and safety of durvalumab when used in the real‐world setting. The benefit of consolidation durvalumab is uncertain in epidermal growth factor receptor (EGFR) mutation positive patients as they constituted only 6% in the PACIFIC study. Presently, real‐world data is scarce on the efficacy of consolidation durvalumab in NSCLC with EGFR mutations.
Systemic inflammation plays a critical role in tumor development and it is associated with prognosis in solid tumors due to its effect on the immune response to the disease. 10 , 11 The neutrophil‐to‐lymphocyte ratio (NLR) is a marker for the general immune response to stress stimuli. 12 , 13 Recent studies have reported that elevated neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) are associated with poorer outcomes in NSCLC patients treated with immune‐checkpoint inhibitors (ICIs). 14 , 15 , 16 , 17 , 18 , 19 Low PLR at baseline has also been reported to be associated with the development of immune‐related adverse events (IrAEs). 17
On this background, we sought to determine the efficacy and safety of durvalumab consolidation in unresectable stage III NSCLC patients in a tertiary institution in Singapore. Outcomes of EGFR positive and wild‐type patients were also evaluated. In addition, for patients who received durvalumab consolidation, the association between clinical outcomes (PFS, OS and the risk of pneumonitis) and NLR and PLR at baseline and at 6 weeks 20 after durvalumab initiation were analyzed.
METHOD
Study design
This was an institutional review board approved retrospective cohort study (DSRB 2021/00221).
Study population
We describe the outcomes of two cohorts of unresectable stage III NSCLC patients treated at the National University Cancer Institute Singapore: one cohort received definitive concurrent platinum‐based chemoradiation (CCRT) alone between January 2013 to December 2017 before the availability of durvalumab; another cohort were treated with consolidation durvalumab at 10 mg/kg IV every 2 weeks for up to 12 months following CCRT between January 2018 to August 2020, following the approval of durvalumab in stage III NSCLC. Patients in the earlier cohort were staged according to the AJCC seventh edition of staging, while in the latter cohort, they were staged according to the eighth edition. All patients in the CCRT‐durvalumab cohort received positron emission tomography‐computed tomography (PET‐CT) and magnetic resonance imaging (MRI) brain as part of pretreatment work‐up. In the CCRT‐alone cohort, more than half (57.7%, 26 out of 45 patients) underwent PET‐CT and MRI brain as pretreatment work‐up. The remaining 19 patients had CT thorax/abdomen, bone scan and contrasted brain imaging as routine staging. Patients who received sequential chemoradiotherapy and those who progressed within 42 days after CCRT were excluded.
Thoracic irradiation therapy
Radiation technique was similar between the two cohorts. Radiation therapy was delivered at 2 Gy per fraction daily, five fractions per week. The total prescribed dose ranged from 60 to 66 Gy. All patients underwent CT simulation‐based planning. 4D‐CT simulation was utilized based on physicians' discretion, typically for lower lobe tumors. Tumor volumes were delineated using PET‐CT diagnostic imaging. The quantitative analyses of normal tissue effects in the clinic (QUANTEC) dose constraints were adopted for treatment planning. Radiation therapy was delivered via either intensity modulated radiation therapy or Arc therapy. Image‐guided radiation therapy using cone beam CT was used for all cases.
Covariates
Clinical data were collected from the institutional electronic medical records. These data included age at diagnosis, gender, race, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, tumor histology, EGFR status and programmed death‐ligand 1 (PD‐L1) tumor proportion score (TPS). In the durvalumab cohort, full blood count at baseline and at 6 weeks after treatment initiation were used to calculate baseline neutrophil‐to‐lymphocyte ratio (NLR) (absolute neutrophil count/absolute lymphocyte count) and platelet‐to‐lymphocyte ratio (PLR) (platelet count/lymphocyte count).
Outcomes
The primary outcomes were progression‐free survival (PFS) and overall survival (OS). Progression‐free survival was defined from the end of CCRT to the date of the first documented event of tumor progression or death. Overall survival was defined from the end of CCRT until death from any cause. In the CCRT‐alone cohort, for patients who received consolidation chemotherapy post CCRT, PFS was defined from the end of consolidation chemotherapy until tumor progression or death, and OS defined from end of consolidation chemotherapy until death from any cause. The secondary outcomes were locoregional relapse rate at 1 year, distant relapse rate at 1 year, pattern of relapse, objective response rate to CCRT and adverse events. Locoregional relapse was defined from end of CCRT until progression of disease in the primary tumor and mediastinal, supraclavicular lymph nodes. Distant relapse was defined from the end of CCRT to development of disease in the contralateral lung, pleural, pericardium, brain, bones, or other organs. Objective response rate was assessed by independent radiological review according to RECIST 1.1. Adverse events were graded using the Common Terminology Criteria for Adverse Events version 4.0 and 5.0 for events before and after November 2017, respectively. In the definitive CCRT cohort, CT scan was done at the end of CCRT and 3–6 monthly thereafter in the next 5 years. Response was assessed 3 months after the end of CCRT. For the cohort that received consolidation durvalumab, CT scan was done at the end of CCRT, every 3‐monthly during 1 year of consolidation durvalumab, and 3–6 monthly thereafter for the next 5 years. In this cohort, response was assessed at the end of CCRT, as patients were initiated on durvalumab consolidation within 42 days from the end of CCRT as per the PACIFIC trial.
Statistical analysis
Frequency with percentage and median with interquartile range were used to describe the baseline characteristics, patterns of relapse and adverse events of the two cohorts in this study. The differences in the proportions for baseline characteristics, patterns of relapse and adverse events between the two cohorts were analyzed using the Chi‐square test or Mann–Whitney U test where appropriate. The Kaplan Meier curves was used to describe the time to event data. The log rank test was used to compare the time to event intervals between the two cohorts. The Cox proportional hazards regression model was used for the analysis of the progression‐free and overall survival outcomes. The Fine and Gray proportional subhazards model was used for the analysis of the time to locoregional relapse and distant relapse outcomes with death as a competing risk. The logistic regression model was used for the analysis of pneumonitis (any grade) as a dichotomous outcome. Time to pneumonitis (any grade) was modeled within a Royston Parmar spline model, where changes in hazard rates were described by time‐varying hazards.
NLR data were analyzed as a continuous variable dichotomized into prespecified cutoffs for ≥3 versus <3 and ≥5 versus <5. 17 PLR data were analyzed as a continuous variable or dichotomized into prespecified cutoffs for ≥180 versus <180. 17 A univariable logistic regression model was used to analyze the effect of NLR on the odds of pneumonitis while a univariable was used to analyze the effect of NLR on PFS.
For all analyses, two‐sided p‐values of less than 0.05 were considered statistically significant. All analyses were performed using R‐4.0.0 (with packages “survival”, “survRM2”, “rstpm2”, and “ggplot2”).
RESULTS
Between January 2013 to August 2020, 84 patients were enrolled: 45 received CCRT alone and 39 received durvalumab consolidation following CCRT. The median age was 64.8 years. The majority were male (82.1%), Chinese (75.0%), and of Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 (95.3%). A total of 36% were current smokers, 44.0% had stage IIIA disease and about half (56%) had adenocarcinoma histology. About a fifth of patients (21.4%) harbored EGFR mutations. For those with known PD‐L1 TPS, 65.5% had PD‐L1 TPS of ≥1%. Baseline characteristics were generally well balanced between the two cohorts, except for higher proportion of stage IIIA disease in CCRT‐alone cohort compared to the durvalumab cohort (Table 1). Cisplatin and etoposide was the most common chemotherapy regimen administered, and median number of chemotherapy cycles was 2 (Table 1). In the durvalumab cohort, the median time to starting durvalumab after CCRT was 38 days, and the median number of cycles of durvalumab was 13. The median duration of follow‐up was 21.6 months for CCRT‐alone cohort and 15.06 months for the durvalumab cohort, respectively.
TABLE 1.
Patient demographics
| Overall | CCRT‐only | CCRT with durvalumab | p‐value | ||
|---|---|---|---|---|---|
| N | 84 | 45 | 39 | ||
| Age at diagnosis (median [IQR]) | 64.76 [58.52, 70.00] | 65.50 [59.50, 71.54] | 64.00 [58.00, 69.00] | 0.326 | |
| Gender (%) | Female | 15 (17.9) | 7 (15.6) | 8 (20.5) | 0.554 |
| Male | 69 (82.1) | 38 (84.4) | 31 (79.5) | ||
| Race (%) | Chinese | 63 (75.0) | 32 (71.1) | 31 (79.5) | 0.412 |
| Malay | 5 (6.0) | 2 (4.4) | 3 (7.7) | ||
| Indian | 10 (11.9) | 6 (13.3) | 4 (10.3) | ||
| Others | 6 (7.1) | 5 (11.1) | 1 (2.6) | ||
| Smoking history (%) | Current smoker | 30 (35.7) | 18 (40.0) | 12 (30.8) | 0.574 |
| Ex‐smoker | 36 (42.9) | 17 (37.8) | 19 (48.7) | ||
| Never‐smoker | 18 (21.4) | 10 (22.2) | 8 (20.5) | ||
| ECOG (%) | 0 | 35 (41.7) | 15 (33.3) | 20 (51.3) | 0.224 |
| 1 | 45 (53.6) | 28 (62.2) | 17 (43.6) | ||
| 2 | 4 (4.8) | 2 (4.4) | 2 (5.1) | ||
| Clinical stage (%) | IIIA | 37 (44.0) | 24 (53.3) | 13 (33.3) | <0.001 |
| IIIB | 36 (42.9) | 21 (46.7) | 15 (38.5) | ||
| IIIC | 11 (13.1) | 0 (0.0) | 11 (28.2) | ||
| Histological subtype (%) | Adenocarcinoma | 47 (56.0) | 22 (48.9) | 25 (64.1) | 0.208 |
| Squamous cell carcinoma | 25 (29.8) | 14 (31.1) | 11 (28.2) | ||
| Others | 12 (14.3) | 9 (20.0) | 3 (7.7) | ||
| EGFR mutation status (%) | Yes | 18 (21.4) | 13 (28.9) | 5 (12.8) | 0.181 |
| No | 29 (34.5) | 15 (33.3) | 14 (35.9) | ||
| Unknown | 37 (44.0) | 17 (37.8) | 20 (51.3) | ||
| PDL‐1 TPS (%) | <1 | 9 (10.7) | 1 (2.2) | 8 (20.5) | <0.001 |
| ≥1 | 18 (21.4) | 1 (2.2) | 17 (43.6) | ||
| Unknown | 57 (67.9) | 43 (95.6) | 14 (35.9) | ||
| Chemotherapy regimen (%) | Cisplatin and etoposide | 32 (38.1) | 19 (42.2) | 13 (33.3) | 0.006 |
| Carboplatin and paclitaxel | 22 (26.2) | 16 (35.6) | 6 (15.4) | ||
| Cisplatin and pemetrexed | 21 (25.0) | 5 (11.1) | 16 (41.0) | ||
| Carboplatin and pemetrexed | 2 (2.4) | 0 (0.0) | 2 (5.1) | ||
| Others | 7 (8.3) | 5 (11.1) | 2 (5.1) | ||
| Chemotherapy cycles (median [IQR]) | 2.00 [2.00, 3.75] | 2.00 [2.00, 4.00] | 2.00 [2.00, 3.00] | 0.901 |
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD‐L1, programmed death‐ligand 1; TPS, tumor proportion score.
The median PFS was 17.5 months (95% CI: 11.3 to NR) for the durvalumab cohort, and 8.9 months (95% CI: 5.8 to 16.3) for the CCRT‐alone cohort (HR 0.47, 95% CI: 0.23–0.96, p = 0.038) (Figure 1). The median OS was not reached at time of analysis for the durvalumab cohort and 22.3 months (95% CI: 15.2‐NR) for the CCRT‐alone cohort (HR 0.35, 95% CI: 0.14–0.87, p = 0.024). The two‐year overall survival rate was 75.2% versus 42.8%, respectively (Figure 2).
FIGURE 1.
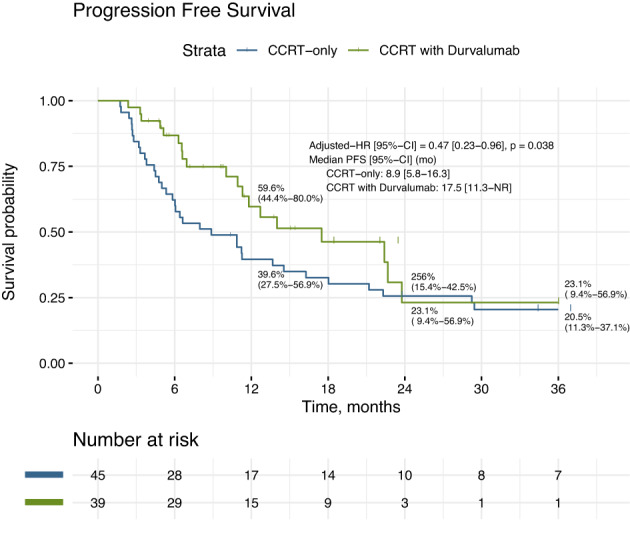
Progression‐free survival (PFS) of CCRT‐durvalumab versus CCRT‐alone. The median PFS was 17.5 months (95% CI: 11.3 to NR) for CCRT‐durvalumab cohort, and 8.9 months (95% CI: 5.8 to 16.3) for CCRT‐alone cohort (HR 0.47, 95% CI: 0.23 to 0.96, p = 0.038)
FIGURE 2.
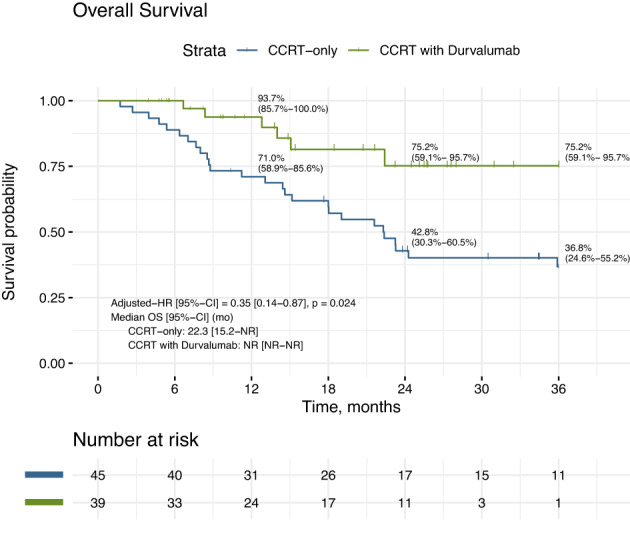
Overall survival of CCRT‐durvalumab versus CCRT‐alone. Median OS was not reached (NR) at time of analysis for durvalumab cohort and 22.3 months (95% CI: 15.2 to NR) for CCRT‐alone cohort (HR 0.35, 95% CI: 0.14 to 0.87, p = 0.024). The two‐year overall survival rate was 75.2% versus 42.8%, respectively
In the durvalumab cohort, 74.4% had a partial response after CCRT, and none had progressive disease. In the CCRT‐alone cohort, the response rate was lower at 57.8%, and 4.4% of patients had disease progression. Distant relapse was the most common pattern of relapse in both cohorts (Table S1). Locoregional relapse rate at 1 year was 44.6% versus 16% (HR 0.40, p = 0.020), and distant relapse rate at 1 year was 46.9% versus 26.2% (HR 0.54, p = 0.059) in the CCRT‐alone cohort and durvalumab cohort, respectively (Figures S1 and S2). Eight out of 39 (20.5%) patients in the durvalumab cohort had distant‐only failure and amongst them, five patients (62.5%) had solitary sites of distant metastases. The central nervous system (CNS) was the most common site of distant relapse (n = 2), for which both patients underwent surgical resection. Other sites included bone, adrenal and lymph node, for which one patient each developed recurrence in these sites and received high dose radiotherapy.
On subset analysis, both EGFR mutation positive and EGFR wild‐type (WT) patients had longer PFS with durvalumab consolidation compared to CCRT alone, 17.5 versus 10.9 months (log‐rank p = 0.907) and 11.8 versus 6.63 months (log‐rank p = 0.419), respectively (Table S2). There was a statistically significant improvement in OS and locoregional relapse with consolidation durvalumab for patients with EGFR WT NSCLC but not for EGFR‐mutated NSCLC (Table S2). However, subgroup analysis showed that there was no significant effect modification on PFS, OS, local relapse and distant relapse by EGFR mutation status (interaction p‐value >0.05) (Table S2).
Safety
Adverse event of any cause and grade occurred in 88.9% of the CCRT alone cohort and 87.2% of the durvalumab cohort, grade 3 or more adverse events occurred in 20.0 and 12.8%, respectively (Table 2). Odynophagia, fatigue, dermatitis, esophagitis and pneumonitis were the most common any‐grade AE, while pneumonitis was the most common high grade (grade 3 or more) AE (7.7% in the durvalumab cohort, and 4.4% in the CCRT‐alone cohort). Overall, there was a higher incidence of dermatitis (33.3% vs. 11.1%, p = 0.017) and pneumonitis (28.2% vs. 4.4%, p = 0.005) in the durvalumab cohort compared to the CCRT‐alone cohort (Table 2).
TABLE 2.
Adverse events of any cause
| All grades | Grade 3/4 only | |||||
|---|---|---|---|---|---|---|
| CCRT‐only | CCRT with durvalumab | p‐value | CCRT‐only | CCRT with durvalumab | p‐value | |
| N | 45 | 39 | 45 | 39 | ||
| Any AE | 40 (88.9) | 34 (87.2) | 0.809 | 9 (20.0) | 5 (12.8) | 0.558 |
| Skin | ||||||
| Dermatitis (%) | 5 (11.1) | 13 (33.3) | 0.017 | ‐ | ‐ | ‐ |
| Rash (%) | 7 (15.6) | 5 (12.8) | 0.765 | ‐ | ‐ | ‐ |
| Dry skin (%) | 0 (0.0) | 2 (5.1) | 0.213 | ‐ | ‐ | ‐ |
| Eczema (%) | 0 (0.0) | 2 (5.1) | 0.213 | ‐ | ‐ | ‐ |
| Gastrointestinal | ||||||
| Odynophagia (%) | 24 (53.3) | 15 (38.5) | 0.194 | 0 (0.0) | 2 (5.1) | 0.213 |
| Esophagitis (%) | 10 (22.2) | 6 (15.4) | 0.579 | 3 (6.7) | 0 (0.0) | 0.245 |
| Dysphagia (%) | 4 (8.9) | 2 (5.1) | 0.681 | ‐ | ‐ | ‐ |
| Mucositis (%) | 1 (2.2) | 2 (5.1) | 0.595 | 0 (0.0) | 1 (2.6) | 0.464 |
| Nausea (%) | 7 (15.6) | 5 (12.8) | 0.765 | ‐ | ‐ | ‐ |
| Diarrhea (%) | 3 (6.7) | 1 (2.6) | 0.620 | 1 (2.2) | 0 (0.0) | 1.000 |
| Constipation (%) | 2 (4.4) | 1 (2.6) | 1.000 | ‐ | ‐ | ‐ |
| Dysgeusia (%) | 1 (2.2) | 1 (2.6) | 1.000 | ‐ | ‐ | ‐ |
| Gastritis (%) | 1 (2.2) | 0 (0.0) | 1.000 | ‐ | ‐ | ‐ |
| Colitis (%) | 1 (2.2) | 0 (0.0) | 1.000 | 1 (2.2) | 0 (0.0) | 1.000 |
| Vomiting (%) | 2 (4.4) | 1 (2.6) | 1.000 | 1 (2.2) | 0 (0.0) | 1.000 |
| Pulmonological | ||||||
| Pneumonitis (%) | 2 (4.4) | 11 (28.2) | 0.005 | 2 (4.4) | 3 (7.7) | 0.660 |
| Cough (%) | 10 (22.2) | 3 (7.7) | 0.078 | ‐ | ‐ | ‐ |
| Pneumonia (%) | 3 (6.7) | 1 (2.6) | 0.620 | 3 (6.7) | 0 (0.0) | 0.245 |
| Hematological | ||||||
| Bicytopenia (%) | 1 (2.2) | 1 (2.6) | 1.000 | ‐ | ‐ | ‐ |
| Neutropenia (%) | 1 (2.2) | 1 (2.6) | 1.000 | ‐ | ‐ | ‐ |
| Others | ||||||
| Fatigue (%) | 12 (26.7) | 10 (25.6) | 1.000 | ‐ | ‐ | ‐ |
| Lethargy (%) | 4 (8.9) | 1 (2.6) | 0.366 | ‐ | ‐ | ‐ |
| Myositis (%) | 0 (0.0) | 2 (5.1) | 0.213 | 0 (0.0) | 1 (2.6) | 0.464 |
| Myalgia (%) | 0 (0.0) | 2 (5.1) | 0.213 | ‐ | ‐ | ‐ |
| Rheumatological | ||||||
| Arthralgia (%) | 0 (0.0) | 1 (2.6) | 0.464 | ‐ | ‐ | ‐ |
| Spondyloarthritis (%) | 0 (0.0) | 1 (2.6) | 0.464 | ‐ | ‐ | ‐ |
| Neurological | ||||||
| Neuropathy (%) | 2 (4.4) | 1 (2.6) | 1.000 | ‐ | ‐ | ‐ |
| Electrolytes | ||||||
| Hyponatremia (%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Endocrine | ||||||
| Thyroiditis (%) | 0 (0.0) | 3 (7.7) | 0.096 | ‐ | ‐ | ‐ |
Abbreviations: AE, adverse events; CCRT, concurrent chemoradiotherapy.
In the durvalumab cohort, 59% of patients experienced immune‐related adverse events (IrAEs) of any grade. Dermatitis (33.3%) and pneumonitis (28.2%) were the most common organ‐specific IrAEs reported. A total of 25% of patients (10 out of 39) experienced grade 2 or higher pneumonitis. These patients all received a course of immunosuppressants and all recovered except one patient who died from complications. One patient developed grade 4 pneumonitis at the end of one‐year durvalumab consolidation. He required a course of steroids and prolonged used of adjunct with mycofenolate mofetil but eventually recovered. Five patients were subsequently rechallenged with durvalumab with no recurrence of pneumonitis. The overall incidence rate of pneumonitis was 26 events per 100 person‐years. Most pneumonitis events develop early at 3.5 weeks post durvalumab initiation (Figure 3).
FIGURE 3.
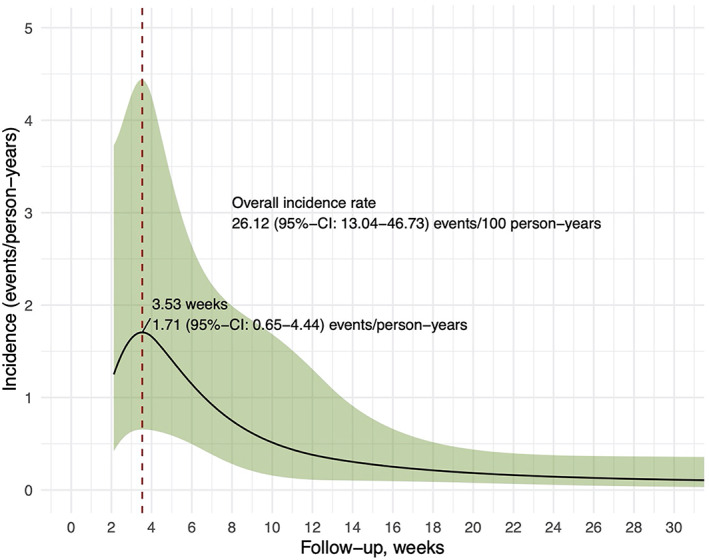
Time‐varying incidence of pneumonitis. The overall incidence rate of pneumonitis was 26 events per 100 person‐years. Most pneumonitis events develop early at 3.5 weeks post durvalumab initiation
NLR and PLR as predictors for progression‐free survival and pneumonitis
In the durvalumab cohort, patients with baseline NLR ≥3 and ≥5 were predictive of shorter PFS (HR 6.775, 95% CI: 0.88–52.12, p = 0.018; HR 2.845, 95% CI: 1.071–7.558, p = 0.041). Week 6 PLR ≥180 was predictive of lower odds of developing pneumonitis (OR = 0.135, 95% CI: 0.016–0.832, p = 0.038) (Table S3).
DISCUSSION
In this real‐world experience (RWE), durvalumab consolidation post CCRT was associated with a statistically significant improvement in PFS and OS, a finding similar to the PACIFIC trial. Our reported PFS of 17.5 months was consistent with that reported in the PACIFIC trial and other RWEs. 6 , 7 , 21 To date, there have been a few published data on the efficacy of durvalumab in Korea, Japan, Germany and United States, demonstrating improved PFS and local control. 21 , 22 , 23 , 24 A German study reported a median PFS of 20.1 months, and 2‐year OS of 66% with consolidation durvalumab, while a Japanese study reported a 1‐year local control rate of 86% and 1‐year PFS of 58%. 21 , 23 Similarly, experience from the US showed a 1‐year PFS and OS of 65 and 85%, respectively. 24
The impact of consolidation durvalumab post CCRT on locoregional control is unknown. In our study, the locoregional relapse at 1 year was 44.6% versus 16% (HR 0.40, p = 0.020), in the durvalumab and CCRT alone cohort, respectively. This finding is similar to that reported from two retrospective studies. 23 , 24 Abe and colleagues reported a 1‐year local control rate of 86% versus 62% (p = 0.005) in the durvalumab cohort, compared to the CCRT cohort. 23 Similarly, Offin et al. reported that local control was improved in stage III NSCLC patients treated with durvalumab and chemoradiation compared with historical records with CCRT alone. 24
A follow‐up exploratory analysis of the PACIFIC study reported that most patients with progression had limited sites of extrathoracic disease, suggesting the potential role for ablative, oligometastasis‐directed therapies. 25 Similarly, a US study of 62 locally advanced NSCLC patients treated with CCRT and durvalumab also reported that nearly half of the patients with distant relapse were potential candidates for metastasis‐directed therapy at first progression. 24 A previous study has shown that stereotactic ablative radiotherapy can significantly improve disease outcome in oligometastatic NSCLC patients with primary local control. 26 In our study, five out of eight patients (62.5%) in the durvalumab cohort with distant relapse had oligometastasis, for which they received surgery or high dose radiotherapy. Findings from our study are consistent with results from the PACIFIC trial and a US study, 24 , 25 suggesting that in patients with distant relapse following durvalumab consolidation, the majority had limited extra‐thoracic disease amenable to local directed therapies.
The toxicities of durvalumab in our study was consistent with that of the PACIFIC trial, with pneumonitis and skin toxicity being two of the most common IrAEs reported. Most adverse events were low grade. In the PACIFIC trial, pneumonitis was the most frequent adverse event leading to treatment discontinuation, occurring in 33.9% and 24.8% of patients administered with durvalumab and placebo, respectively. 6 Grade 3 or 4 pneumonitis was seen in 3.4% of the durvalumab cohort, compared to 2.6% in the placebo arm. In our study, 28% (11 out of 39) in the durvalumab cohort developed pneumonitis, and the majority (10 out of 11 cases) were grade 2 or higher in severity. These patients were all symptomatic, required bronchoscopic assessment and received prolonged duration of steroids. These findings highlight that whilst consolidation durvalumab can improve survival in stage III NSCLC, complications of pneumonitis can be debilitating in a significant proportion of patients, underscoring the need for close surveillance and early multidisciplinary management of pneumonitis. In addition, pneumonitis occurred early in our study, with its peak of onset occurring at 3.5 weeks following durvalumab initiation. The PACIFIC trial also reported a median time to onset of pneumonitis at about 8 weeks after the first durvalumab dose. 27 Similarly, a Korean study reported a median radiation pneumonitis‐free survival of 3.1 months following durvalumab initiation. 22 Taken together, pneumonitis most frequently develops within the first 3 months of durvalumab initiation. Our study is one of the few studies that evaluated the overall incidence rate and time‐varying incidence of pneumonitis from durvalumab.
The benefit of durvalumab consolidation in EGFR mutation positive patients remains unclear. A recent post hoc exploratory analysis of 35 EGFR mutation positive patients from the PACIFIC trial revealed a similar survival benefit of durvalumab treatment versus placebo. 28 In addition, a retrospective study of 37 patients with EGFR mutated NSCLC reported that these patients did not benefit from consolidation durvalumab and experienced a high frequency of IrAEs. 29 Several retrospective studies have also demonstrated smaller benefits of durvalumab consolidation in EGFR positive NSCLC compared to wild‐type patients following chemoradiation. 30 , 31 Recently, a multicenter retrospective analysis involving 323 stage III NSCLC patients across Europe and America reported limited activity of consolidation durvalumab in those harboring EGFR mutation, BRAF mutation and ALK rearrangement, but not for those harboring KRAS mutation. 32 In our study, both EGFR‐mutation positive and WT patients had longer PFS and OS with durvalumab consolidation compared to CCRT alone. The magnitude of benefit appeared greater in the WT patients. As our sample size was relatively small, we were unable to draw definitive conclusion in this group of patients. Future larger studies are needed to clarify the role of consolidation durvalumab in this patient group.
In recent years, there has been emerging data on the prognostic value of inflammation‐related peripheral blood markers such as NLR, PLR in NSCLC patients receiving immunotherapy. 14 , 15 , 16 , 18 , 19 In a multicenter retrospective study of 466 NSCLC patients across Europe, Mezquita and colleagues reported that derived NLR >3 correlated with worse outcome for ICIs, but not for chemotherapy. 18 A recent meta‐analysis of 21 studies involving 1845 NSCLC patients showed that high pretreatment NLR and PLR were associated with poorer outcomes in patients treated with ICIs. 19 In our study, we showed that baseline NLR ≥3 and ≥5 were predictive of shorter PFS with durvalumab, a finding similar to previous reports. In a recent Taiwan study of 31 patients, Chu and colleagues also reported that patients with low baseline NLR (<3.8) had significantly longer post‐CCRT PFS and time to distant metastasis or death compared to patients with high NLR on durvalumab. 33 Taken together, baseline NLR may help predict benefit with durvalumab in stage III NSCLC.
On a parallel note, several retrospective studies have reported NLR and PLR as predictive factors for irAEs in patients with advanced NSCLC treated with ICIs. 16 , 17 The exact mechanisms of irAEs have remained unclear. Postulated mechanisms include the production of autoantibodies and that ICIs may unmask low level self‐reacting T cells. 34 An earlier study in pancreatic cancer revealed that an elevated NLR level was associated with an elevated level of peripheral blood regulatory T cells. 35 These observations lay the basis for studying the predictive role of NLR and PLR in IrAEs. Pavan and colleagues reported that low baseline NLR and PLR were associated with the development of IrAEs in advanced NSCLC treated with ICIs. 17 Similarly, Lee and colleagues also reported that low NLR <3 at baseline was associated with higher occurrence of IrAEs in a case–control study. 16 While our study did not demonstrate an association between baseline PLR or NLR and toxicity, we found that an elevated PLR of ≥180 at week 6 was associated with a lower risk of pneumonitis. This finding is novel and warrants further larger studies in the future. NLR and PLR are readily available, inexpensive biomarkers for outcomes of patients receiving ICIs, and future studies involving larger cohort of patients are needed to validate their application.
Several limitations are acknowledged. First, this was a retrospective single institution study. Second, the sample size was relatively small. Third, there was a significant proportion of patients with unknown or untested EGFR mutation status. This was largely attributed to the patients with squamous histology (29.8%) for whom EGFR mutation was not tested. The percentage of EGFR mutation was also higher in the CCRT‐alone cohort compared to the CCRT‐durvalumab cohort, representing a potential selection bias whereby, following durvalumab approval, patients with known EGFR mutations could not have been offered durvalumab consolidation. If so, this may compromise the analysis and impact the results of durvalumab treatment in this group of patients. Lastly, there was a significant proportion of patients with unknown PD‐L1 status, and an imbalance in PD‐L1 status between the two arms. This was largely driven by the CCRT‐alone cohort which were recruited between January 2013 to December 2017, during which PD‐L1 testing was not readily available in the earlier years between 2013 to 2015, and not routinely tested in stage III NSCLC. Nonetheless, this is one of the few RWEs of consolidation durvalumab that has been reported and all patients who received durvalumab in our study fulfilled the criteria as per the PACIFIC study. Although the sample size of patients with EGFR mutations was small, our study highlighted that durvalumab may be beneficial in this group of patients.
Despite the survival benefit with consolidation durvalumab post CCRT, efforts are required to improve outcomes in patients with locally advanced unresectable NSCLC. A phase II trial of concurrent pembrolizumab with chemoradiation in stage III NSCLC have reported promising results. 36 A recent phase II trial of durvalumab in combination with oleclumab or monalizumab after CCRT has shown improved response rates and PFS compared to durvalumab alone. 37 Ongoing studies include concurrent durvalumab with chemoradiation, 38 consolidation durvalumab following stereotactic radiotherapy in early stage unresected NSCLC, 39 consolidation durvalumab following sequential chemoradiation 40 and M7824 with CCRT (NCT03840902). In patients with stage III NSCLC with EGFR mutations, a phase II study reported concurrent gefitinib with chemoradiation was tolerable, 41 and a phase III study of maintenance osimertinib following chemoradiation is ongoing. 42
In conclusion, in this RWE, durvalumab consolidation post CCRT was associated with a statistically significant improvement in PFS and OS. Pneumonitis, a common and debilitating complication, occurred early following durvalumab initiation. This finding highlights to oncologists the need for close surveillance especially in the early phase of treatment. Further studies are needed to validate the role of NLR and PLR in unresectable stage III NSCLC.
CONFLICT OF INTEREST
All authors have no conflict of interest to declare except BC Goh and Ross Soo. Ross Soo received research funding from AstraZeneca and Boehringer Ingelheim, and received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, Merck, Roche, Takeda, Yuhan, Novartis, Lilly, Amgen and Puma. BC Goh received honorarium from Novartis, MSD, Bayer Healthcare and provided consultancy for Bayer Healthcare, MSD and Adagene. He also has stock ownership in Gilead Sciences, Merus Therapeutics.
Supporting information
Table S1. Response to CCRT and Patterns of relapse
Table S2. Subgroup analysis of EGFR mutation positive and EGFR wild type patients
Table S3. Association of NLR and PLR with survival and risk of pneumonitis
Figure S1. Local relapse rate of CCRT‐Durvalumab vs CCRT alone
Figure S2. Distant relapse rate of CCRT‐Durvalumab vs CCRT alone
ACKNOWLEDGMENTS
We would like to acknowledge the patients and families that have participated in this study, as well as Ms Felly Ng, Mr Kyaw Zin Thant, Ms Lena Kwok and Ms Priscilla Chong for their administrative support to the study. This study was supported by National Research Foundation, Singapore and National Medical Research Council (NMRC), Singapore under its NMRC Centre Grant Program (NMRC/CG/M005/2017_NCIS).
Huang Y, Zhao JJ, Soon YY, Wong A, Aminkeng F, Ang Y, et al. Real‐world experience of consolidation durvalumab after concurrent chemoradiotherapy in stage III non‐small cell lung cancer. Thorac Cancer. 2022;13(22):3152–3161. 10.1111/1759-7714.14667
This study has been presented as a poster presentation at the World Conference on Lung Cancer 2021.
Funding information National Research Foundation, Singapore and National Medical Research Council (NMRC), Grant/Award Number: NMRC Centre Grant Programme (NMRC/CG/M005/2017_NCI
REFERENCES
- 1. Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non‐small‐cell lung cancer: the Hoosier oncology group and U.S. Oncology. 2008;26(35):5755–60. [DOI] [PubMed] [Google Scholar]
- 2. Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): a randomised, two‐by‐two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vokes EE, Herndon JE, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non‐small‐cell lung cancer: cancer and leukemia group B. J Clin Oncol. 2007;25(13):1698–704. [DOI] [PubMed] [Google Scholar]
- 4. Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L‐BLP25) versus placebo after chemoradiotherapy for stage III non‐small‐cell lung cancer (START): a randomised, double‐blind, phase 3 trial. Lancet Oncol. 2014;15(1):59–68. [DOI] [PubMed] [Google Scholar]
- 5. Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol Off. 2010;28(13):2181–90. [DOI] [PubMed] [Google Scholar]
- 6. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 7. Spigel DR, Faivre‐Finn C, Gray JE, Vicente D, Planchard D, Paz‐Ares L, et al. Five‐year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. J Clin Oncol. 2022;40(12):1301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jazieh AR, Onal HC, Tan DSW, Soo RA, Prabhash K, Kumar A, et al. Real‐world treatment patterns and clinical outcomes in patients with stage III NSCLC: results of KINDLE, a multicountry observational study. J Thorac Oncol. 2021;16(10):1733–44. [DOI] [PubMed] [Google Scholar]
- 9. Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev. 2019;28(152):190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 11. Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016–36. [PMC free article] [PubMed] [Google Scholar]
- 12. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol. 2016;17(12):e542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Granot Z, Jablonska J. Distinct functions of neutrophil in cancer and its regulation. Mediators Inflamm. 2015;2015:701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune‐related adverse events in advanced non‐small cell lung cancer treated with PD‐1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. [DOI] [PubMed] [Google Scholar]
- 16. Lee PY, Oen KQX, Lim GRS, Hartono JL, Muthiah M, Huang DQ, et al. Neutrophil‐to‐lymphocyte ratio predicts development of immune‐related adverse events and outcomes from immune checkpoint blockade: a case‐control study. Cancer. 2021;13(6):1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune‐related toxicity in advanced non‐small cell lung cancer treated with immune‐checkpoint inhibitors. Oncologist. 2019;24(8):1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in patients with advanced non‐small cell lung cancer. JAMA Oncol. 2018;4(3):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio in non‐small cell lung cancer patients treated with immune checkpoint inhibitors: a meta‐analysis. Int Immunopharmacol. 2020;85:106677. Available from: https://pubmed.ncbi.nlm.nih.gov/32531712/ [DOI] [PubMed] [Google Scholar]
- 20. Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, et al. Post‐treatment neutrophil‐to‐lymphocyte ratio at week 6 is prognostic in patients with advanced non‐small cell lung cancers treated with anti‐PD‐1 antibody. Cancer Immunol Immunother. 2018;67(3):459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faehling M, Schumann C, Christopoulos P, Hoffknecht P, Alt J, Horn M, et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non‐small cell lung cancer (NSCLC): real‐world data on survival and safety from the German expanded‐access program (EAP). Lung Cancer. 2020;150:114–22. [DOI] [PubMed] [Google Scholar]
- 22. Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS, Ahn MJ, et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non‐small‐cell lung cancer. Lung Cancer. 2020;146:23–9. [DOI] [PubMed] [Google Scholar]
- 23. Abe T, Saito S, Iino M, Aoshika T, Ryuno Y, Ohta T, et al. Effect of durvalumab on local control after concurrent chemoradiotherapy for locally advanced non‐small cell lung cancer in comparison with chemoradiotherapy alone. Thorac Cancer. 2021;12(2):245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Offin M, Shaverdian N, Rimner A, Lobaugh S, Shepherd AF, Simone CB, et al. Clinical outcomes, local‐regional control and the role for metastasis‐directed therapies in stage III non‐small cell lung cancers treated with chemoradiation and durvalumab. Radiother Oncol. 2020;149:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raben D, Rimner A, Senan S, Broadhurst H, Pellas T, Dennis PA, et al. Patterns of disease progression with Durvalumab in stage III non‐small cell lung cancer (PACIFIC). Int J Rad Oncol Biol Phys. 2019;105:683. [Google Scholar]
- 26. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR‐COMET): a randomised, phase 2, open‐label trial. Lancet. 2019;393(10185):2051–8. [DOI] [PubMed] [Google Scholar]
- 27. Vansteenkiste J, Naidoo J, Faivre‐Finn C. PACIFIC subgroup analysis: pneumonitis in stage III, Unresectable NSCLC patients treated with durvalumab vs placebo after CRT. Pneumologie. 2018;13(10):S370–1. [Google Scholar]
- 28. Naidoo J, Antonia SJ, Wu YL, Cho BC. Durvalumab after chemoradiotherapy in unresectable stage III EGFR mutation positive NSCLC: a post hoc subgroup analysis from PACIFIC. J Clin Oncol. 2022;40(suppl 16):8541. [DOI] [PubMed] [Google Scholar]
- 29. Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, et al. Durvalumab for stage III EGFR‐mutated NSCLC after definitive Chemoradiotherapy. J Thorac Oncol. 2021;16(6):1030–41. [DOI] [PubMed] [Google Scholar]
- 30. Hellyer JA, Aredo JV, Das M, Ramchandran K, Padda SK, Neal JW, et al. Role of consolidation Durvalumab in patients with EGFR‐ and HER2‐mutant Unresectable stage III NSCLC. J Thorac Oncol. 2021;16(5):868–72. [DOI] [PubMed] [Google Scholar]
- 31. Wang CC, Chiu LC, Ju JS, Lin YC, Fang YF, Yang CT, et al. Durvalumab as consolidation therapy in post‐concurrent Chemoradiation (CCRT) in Unresectable stage III non‐small cell lung cancer patients: a multicenter observational study. Vaccine. 2021;9(10):1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riudavets M, Auclin E, Mosteiro M, Dempsey N, Majem M, Lobefaro R, et al. Durvalumab consolidation in patients with unresectable stage III non‐small cell lung cancer with driver genomic alterations. Eur J Cancer. 2022;1990(167):142–8. [DOI] [PubMed] [Google Scholar]
- 33. Chu CH, Chiu TH, Wang CC, Chang WC, Huang ACC, Liu CY, et al. Consolidation treatment of durvalumab after chemoradiation in real‐world patients with stage III unresectable non‐small cell lung cancer. Thorac Cancer. 2020;11(6):1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity. 2013;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 35. Chen L. Co‐inhibitory molecules of the B7‐CD28 family in the control of T‐cell immunity. Nat Rev Immunol. 2004;4(5):336–47. Available from: https://pubmed.ncbi.nlm.nih.gov/15122199/ [DOI] [PubMed] [Google Scholar]
- 36. KEYNOTE‐799: phase 2 trial of pembrolizumab plus platinum chemotherapy and radiotherapy for unresectable, locally advanced, stage 3 NSCLC J Clin Oncol. 2021;39(suppl 15):8512. 10.1200/JCO.2021.39.15_suppl.8512 [DOI] [Google Scholar]
- 37. Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: an open‐label, phase II, multidrug platform study of Durvalumab alone or in combination with Oleclumab or Monalizumab in patients with Unresectable, stage III non‐small‐cell lung cancer. J Clin Oncol. 2022;JCO2200227. 10.1200/JCO.22.00227. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38. AstraZeneca . A Phase III, Randomized, Placebo‐controlled, Double‐blind, Multi‐center, International Study of Durvalumab Given Concurrently With Platinum‐based Chemoradiation Therapy in Patients With Locally Advanced, Unresectable NSCLC (Stage III) (PACIFIC2) [Internet]. clinicaltrials.gov; 2021 Jul [cited 2021 Aug 18]. Report No.: NCT03519971. Available from: https://clinicaltrials.gov/ct2/show/NCT03519971
- 39. AstraZeneca . A Phase III, Randomized, Placebo‐controlled, Double‐blind, Multi‐center, International Study of Durvalumab With Stereotactic Body Radiation Therapy (SBRT) for the Treatment of Patients With Unresected Stage I/II, Lymph‐node Negative Non‐small Cell Lung Cancer (PACIFIC‐4/RTOG‐3515) [Internet]. clinicaltrials.gov; 2021 Apr [cited 2021 Aug 18]. Report No.: NCT03833154. Available from: https://clinicaltrials.gov/ct2/show/NCT03833154
- 40. AstraZeneca . A Phase II, Open‐Label, Multi‐Centre, International Safety Study of Durvalumab Following Sequential Chemotherapy and Radiation Therapy in Patients With Stage III, Unresectable Non‐Small Cell Lung Cancer (PACIFIC 6). [Internet]. clinicaltrials.gov; 2021. Aug [cited 2021 Aug 18]. Report No.: NCT03693300. Available from: https://clinicaltrials.gov/ct2/show/NCT03693300
- 41. Akamatsu H, Murakami H, Harada H, Shimizu J, Hayashi H, Daga H, et al. Gefitinib with concurrent thoracic radiotherapy in unresectable locally advanced NSCLC with EGFR mutation; West Japan oncology group 6911L. J Thorac Oncol. 2021;16(21):1745–52. [DOI] [PubMed] [Google Scholar]
- 42. Lu S, Casarini I, Kato T, Cobo M, Özgüroğlu M, Hodge R, et al. Osimertinib maintenance after definitive chemoradiation in patients with unresectable EGFR mutation positive stage III non‐small‐cell lung cancer: LAURA trial in progress. Clin Lung Cancer. 2021. Jul;22(4):371–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Response to CCRT and Patterns of relapse
Table S2. Subgroup analysis of EGFR mutation positive and EGFR wild type patients
Table S3. Association of NLR and PLR with survival and risk of pneumonitis
Figure S1. Local relapse rate of CCRT‐Durvalumab vs CCRT alone
Figure S2. Distant relapse rate of CCRT‐Durvalumab vs CCRT alone


