Abstract
Background
The purpose of this study is to undertake a comprehensive systematic review to describe multilevel factors (barriers and facilitators) that may influence the implementation of low-dose chest computed tomography for lung cancer screening in the United States.
Methods
Systematic literature searches were performed using 6 online databases and citation indexes for peer-reviewed studies, for articles published from 2013 to 2021. Studies were classified into 3 perspectives, based on the study’s unit of analysis: system, health-care provider, and patient. Barriers and facilitators identified for each study included in our final review were then coded and categorized using the Consolidate Framework for Implementation Research domains.
Results
At the system level, the 2 most common constructs were external policy and incentives and executing the implementation process. At the provider level, the most common constructs were evidence strength and quality of the intervention characteristics, patient needs and resources, implementation climate, and an individual’s knowledge and beliefs about the intervention. At the patient level, the most common constructs were patient needs and resources, individual’s knowledge and beliefs about the intervention, and engaging in the implementation process. These constructs can act as facilitators or barriers to lung cancer screening implementation.
Conclusions
Applying the Consolidate Framework for Implementation Research domains and constructs to understand and specify factors facilitating uptake of lung cancer screening as well as cataloging the lessons learned from previous efforts helps inform the development and implementation processes of lung cancer screening programs in the community setting.
Registration
PROSPERO, CRD42021247677.
With more than half of lung cancer cases being diagnosed at the distant stage where the 5-year survival rate is only 6% (1), lung cancer is the leading cause of cancer mortality in the United States. Early detection is imperative because pathological stage at diagnosis is associated with prognosis, where the most effective treatment options are available for early stage tumors. In 2011, results from the National Lung Screening Trial demonstrated that screening for lung cancer using low-dose chest computed tomography (LDCT) reduced mortality by 20% in high-risk patients compared with using chest radiography (eg, x-ray) (2). As a result, the US Preventive Services Task Force (USPSTF), along with a number of professional organizations, endorsed annual lung cancer screening using LDCT for high-risk adults since 2013 (3). Two years later, the Centers for Medicare and Medicaid Services (CMS) announced sufficient evidence to support coverage of a lung cancer screening counseling and shared decision-making (SDM) visit for eligible patients (4). Despite national organizational guidelines, health insurance coverage, and scientific evidence of screening, the implementation of lung cancer screening with LDCT (hereafter, referred to as lung cancer screening) has remained suboptimal, particularly in the community settings (5-8). Moreover, the slow uptake of evidence-based interventions is not unique to lung cancer screening (9).
Implementation science, anchored in organizational theories, can inform the translation from research and practice. Although current literature, including Cochrane systematic reviews, has attributed the suboptimum lung cancer screening uptake to differences in adherence rates among subgroups of patients (10,11), no reviews have examined the complex implementation process, which is often driven by multilevel factors at the patient, provider, and health-care system level. A clear understanding of barriers and facilitators, using an evidence-informed theoretical framework, that influences implementation is urgently needed. Moreover, recognizing there are interactions between context and the implementation process that occur throughout the continuum of care (eg, patient to system), it is important to capture these interactions to further illuminate the implementation process. To date, no existing work has applied a theoretical framework to guide the systematic review (using core standards that are generally expected of Cochrane reviews) and analyze the data. A comprehensive overview of available evidence would derive lessons learned and enable a deeper understanding of drivers of implementation, which would in turn facilitate the implementation of successful, effective, and sustainable screening programs.
To bridge this gap, this study aimed to conduct a comprehensive systematic review to synthesize multilevel factors that influence the implementation of lung cancer screening in the United States, guided by the Consolidated Framework for Implementation Research (CFIR). Utilization of the CFIR, a metatheoretical framework, enables the systematic and comprehensive aggregation of findings across studies and context to maximize generalizability to different settings and improve implementation of interventions (12).
Methods
Protocol
We developed a systematic review protocol a priori and registered it with the International Prospective Register of Ongoing Systematic Reviews (No. CRD42021247677). We followed the standards of the Cochrane Handbook for Systematic Reviews of Interventions and reported our results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guideline (13,14). The selection of data sources and search strategy were developed in consultation with the lead health science librarian. The study was approved by the University of Oklahoma institutional review board (IRB No. 10187), although this portion of the study is exempt with no human subject involvement.
Data Sources and Management
Systematic literature searches were performed using the following electronic databases: 3 Ovid databases (MEDLINE, Embase, PsycINFO), Cumulative Index to Nursing and Allied Health Literature, PubMed, and Web of Science. Retrieval was limited to English language literature published from 2013 to present; all searches were conducted in April 2021. EndNote X9 (Clarivate Analytics, Boston, MA, USA) was used to manage the records retrieved from searches. The EndNote library containing the search results was then imported into a web-based software platform that streamlines the production of systematic reviews, Covidence (Veritas Health Innovation, Melbourne, VIC, Australia; available at www.covidence.org), and duplicates were automatically removed. Covidence was used for title and abstract screening, full-text review, reference management, and data extraction (15–17).
Search Strategies and Inclusion Criteria
Search terms aimed to represent primary concepts of implementation, lung cancer screening, and barriers or facilitators. Keywords were generated for each of these concepts by examining the terminology used in similar review papers.
A population, intervention, comparison, outcome, and time format was used to structure the research question, eligibility criteria, and study selection (18). A study was eligible for inclusion in the review if the study met the following criteria: 1) data collection in the United States between 2013 (after USPSTF recommendation) and 2021; 2) identified perceived barriers and/or facilitators to LDCT for lung cancer screening; 3) data from the health-care professionals’, health system or clinics’, or patients’ perspective; 4) reported on original collection of data; and 5) full-text article available.
The following exclusion criteria were applied. First, all non–US-based studies were excluded as the US health-care system differs from those of most comparable developed nations thus making our implementation process unique. Second, because our analysis focused on articles that identified barriers and facilitators of the implementation process, rather than solely characteristics of the intervention (eg, lung cancer screening), studies that only reported intervention outcomes (including effectiveness data) were excluded. Factors are considered as facilitators if their presence promoted (eg, enabled) the implementation of, or adherence to, the guideline-indicated screenings. Factors are considered as barriers if they impeded (eg, hindered) implementation of, or adherence to, the guideline. Third, only full-length articles were retained. Reviews, abstracts, posters, and commentaries were excluded.
Conceptual Framework: CFIR
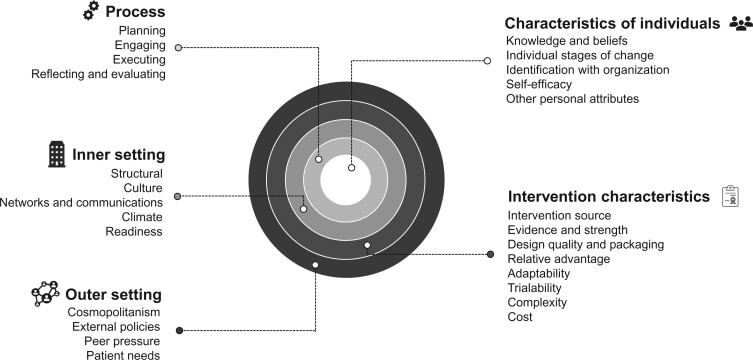
This review is guided by CFIR, an integrated conceptual framework for implementation. We categorized study findings to 26 CFIR main constructs across 5 domains and stratified by the study unit of analysis (eg, patient, provider, and system level). The 5 major domains of CFIR are 1) intervention characteristics, which are the features of an intervention; 2) outer setting, which includes the features of the external context or environment; 3) inner setting, which includes features of the implementing organization; 4) characteristics of the individuals involved; and 5) process of implementation (see Figure 1) (12). Each of these domains includes constructs based on a comprehensive review of literatures and models that have informed implementation science since its inception. Applying the CFIR constructs across these domains enabled the systematic and comprehensive aggregation of findings across studies using standardized terminology (19). Further information and detailed descriptions of each domain are available at http://www.cfirguide.org/.
Figure 1.
The Consolidated Framework for Implementation Research (CFIR) framework.
Data Extraction and Synthesis
Studies were initially screened by title and abstract by 2 independent reviewers based on the eligibility criteria. Disagreements were resolved through discussion or by a third reviewer when no decision was reached. The same screening process was repeated for full-text screening and data extraction. During the full-text screening process, duplicates were assessed again, and only the most recent publication was included.
Synthesis of the evidence was carried out in 3 stages. First, preliminary synthesis was conducted using a data extraction template created in Covidence. The following relevant information from each article were extracted from the articles: title, first author, study aim(s), setting, perspective level, study design, data collection time period, sample size, data collection methods, location, descriptive characteristics of the sample, barriers and/or facilitators, key findings, limitations, funding sources, conflicts of interest, and additional notes. Factors were identified as a barrier or a facilitator based on evidence in the studies. If the study did not explicitly state a factor as a barrier or a facilitator, the 2 independent reviewers based identification on logical reasoning.
Second, data synthesis was done synergistically by combining qualitative, quantitative, and mixed-methods studies to classify them into 3 perspectives, based on the study’s unit of analysis: 1) system (eg, health system or clinic), 2) health-care provider, and 3) patient. For each perspective, thematic synthesis was conducted to identify barriers and facilitators of implementation. They were then coded and categorized into 1 of the 5 CFIR domains then further defined by one of the constructs. The coding process involved moving backward and forward between the data and emerging concepts.
Barriers and facilitators reported in the studies were tabulated, and top themes were compiled and reported for each perspective. In this stage, a matrix was created with reviews in rows and contexts in columns. This allowed us to compare the extracted descriptions across the included reviews within each perspective and across all studies. This process permitted the identification of frequent and recurrent barriers and facilitators in the literature. To ensure that data were not being inappropriately forced into a construct, an additional column category was created for data that did not neatly fit into one of the constructs and warranted further discussion between reviewers. Finally, we built a general interpretation of the most reported factors for each perspective according to the findings in the previous stage.
Assessment of Risk of Bias in Individual Studies
Two reviewers independently appraised the methodological quality (risk of bias) of the included studies using the Mixed Methods Appraisal Tool (MMAT version 2018, available at http://mixedmethodsappraisaltoolpublic.pbworks.com/) (20). Disagreements were resolved through discussion or by a third reviewer when no decision was reached. Although several tools and methodologies are available to appraise primary evidence, this tool was selected because of its ability to report on the quality of varying study designs and definitions and detailed instructions included in its manual. The checklist consists of 2 screening questions and a set of 5 questions related to the validity methodology that varies by study design. Responses include the following: “no,” “can’t tell,” or “yes.” The manual discourages the calculation of an overall score from the ratings of each criterion and instead advises individuals to provide a more detailed narrative explaining the ratings for each criterion to better inform the quality of included studies (20).
Results
Study Selection
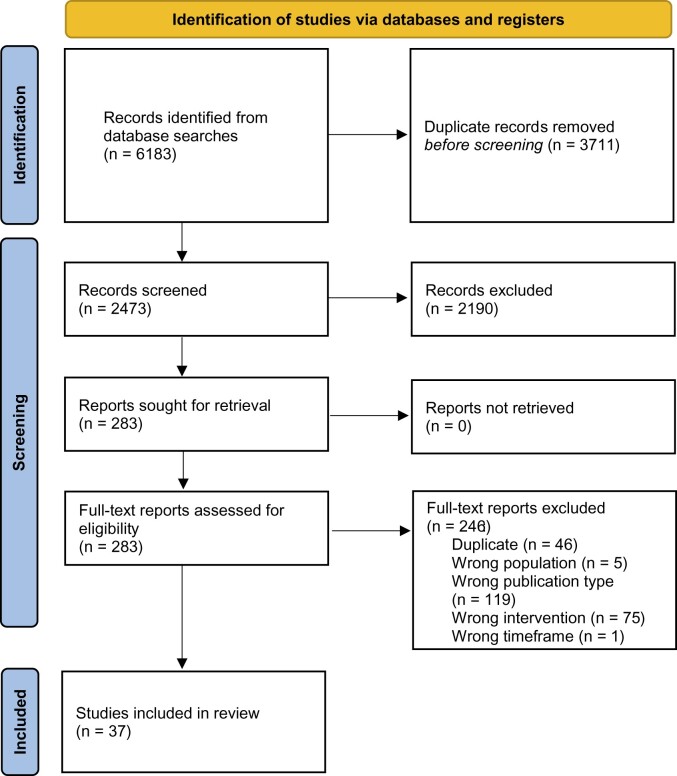
Figure 2 shows a Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flow diagram with the results of the search and selection process. The initial search identified 6183 titles. After eliminating duplicates in Covidence, 2473 titles underwent title and abstract screen. Interrater reliability was assessed via the Cohen kappa statistic and was 0.73 for title and abstract screening. A total of 283 articles were selected for full-text review, and 37 were selected for inclusion (21–57).
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flow diagram for new systematic reviews.
Study Characteristics
Table 1 presents the main characteristics of studies included in this analysis. Of the 37 studies, 33 provided the time of data collection, with the data collection periods ranging from 2013 to 2019 and publication period ranging from 2014 to 2021; 17 studies either started or were completed before the release of the CMS memo indicating Medicare coverage of annual screening for eligible individuals (22,26,27,32,36–39,45,47,50,53,55). The majority of studies were conducted using quantitative methodology, specifically cross-sectional studies using surveys (n = 23) (23,24,28,29,32–37,39,41–43,45,48–52,57). Ten were qualitative studies (21,25–27,31,38,40,47,53–55), and 4 used mixed-methods approach (22,30,44,46,56). Of the studies, 31 provided information on study sites; 25 studies were single state or city studies, covering 14 US states (California [n = 3], Florida [n = 1], Illinois [n = 3], Iowa [n = 1], Louisiana [n = 1], Massachusetts [n = 1], Minnesota [n = 1], New Mexico [n = 2], New York [n = 3], North Carolina [n = 2], Pennsylvania [n = 1], South Carolina [n = 2], Tennessee [n = 1], and Washington [n = 3]); 6 articles were multistate studies (21,23,24,28,30,33) (23,24,28,30,33). Most of the articles were at the provider-level (n = 16) (26,29,31–34,36–43,46,49–51,54). Five studies were at the system level (21–25), 13 at the patient level, and 3 focused on more than 1 level (eg, both patient and provider) (32,40,54). The sample size in the studies varied by unit of analysis and study design. The sample sizes for studies examining factors at the system level ranged from 2 to 165 sites (21–25); for providers, 11 to 970; and for patients, 15 to 12 801 participants. Of the studies that included information on the health system setting, 8 studies were conducted in an academic setting, 17 in a nonacademic (eg, community-based) setting, and 11 in academic and community settings. Six studies were conducted in integrated health-care systems including 4 within the Veterans Affairs health-care system (39,40,43,45) and 2 within Kaiser Permanente (52,53).
Table 1.
Characteristics of studies selected for inclusion (n = 37)
| Citation | Purpose of study | Study period, y | Study design | Study participants (n = sample size) | Location | Setting |
|---|---|---|---|---|---|---|
| Ahsan et al. (26) | To understand attitudes and beliefs among PCPs regarding LCS as well as to assess gaps in knowledge. | 2015-2016 | Qualitative research | Provider | New Yorkb | Community |
| (n = 38) | ||||||
| Allen et al. (25)a | To identify the critical facilitators and barriers to LCS program implementation. | 2017-2018 | Qualitative research | System | Not specified | Community |
| (n = 2) | ||||||
| Carter-Harris and Gould (27) | To explore the reasons for eligible patients’ decisions to opt out of LCS after receiving a provider recommendation. | 2015-2016 | Qualitative research | Patient | Washington | Community |
| (n = 18) | ||||||
| Cataldo (28)a | To identify demographics, smoking history, health risk perceptions, knowledge, and attitude factors of older smokers related to LCS agreement. | 2014 | Cross-sectional | Patient | 46 states | Community |
| (n = 388) | ||||||
| Copeland et al. (24) | To assess LCS implementation during the first full year of CMS coverage. | 2017 | Cross-sectional | System | 34 states | Both |
| (n = 165) | ||||||
| Coughlin et al. (29) | To measure PCPs’ knowledge of current guidelines for LCS after approval by CMS and to gain insight into the barriers to implementation of LCS at 5 hospitals. | Not Specified | Cross-sectional | Provider | Illinois | Both |
| (n = 96) | ||||||
| Draucker et al. (30) | To describe how current and former long-term smokers explain their decisions regarding participation in LCS. | Not Specified | Mixed methods | Patient | 20 states | Not Specified. |
| (n = 39) | ||||||
| Dukes et al. (31) | To investigate cancer specialists and PCPs’ practices and attitudes toward LCS with patients who have survived head and neck cancer. | 2016-2017 | Qualitative research | Provider | Iowa | Academic |
| (n = 11) | ||||||
| Duong et al. (32) | To identify PCP and patient facilitators and barriers to LCS utilization within the Stanford Medical Center. | 2015-2016 | Cross-sectional | Patient (n = 80) & provider (n = 31) | California | Academic |
| Eberth et al. (23) | To determine the availability and characteristics of LCS programs and to identify barriers to program development and implementation among members of the Society of Thoracic Radiology. | 2013 | Cross-sectional | System | 36 states | Both |
| (n = 82) | ||||||
| Eberth et al. (33) | To assess PCP members of the American Medical Association’s knowledge of current LCS guidelines and insurance reimbursement, perceptions of screening effectiveness and cost, screening referral practices, and associated barriers. | 2016-2017 | Cross-sectional | Provider | All 50 states | Both |
| (n = 293) | ||||||
| Ersek et al. (34) | To evaluate the knowledge, attitudes, and practice patterns related to LCS among South Carolina Academy of Family Physicians members. | 2015 | Cross-sectional | Provider | South Carolina | Both |
| (n = 101) | ||||||
| Hall et al. (35) | To identify correlates of LCS utilization among LCS patients. | 2014-2016 | Cross-sectional | Patient | Massachusetts | Community |
| (n = 169) | ||||||
| Henderson et al. (36) | To understand and compare perceptions of LCS among attending and resident PCPs at one academic medical center. | 2015-2016 | Cross-sectional | Provider | North Carolina | Academic |
| (n = 72) | ||||||
| Henderson et al. (37) | To understand the LCS practices and attitudes of pulmonologists and PCPs in a large academic medical center. | 2015 | Cross-sectional | Provider | North Carolina | Academic |
| (n = 89) | ||||||
| Hoffman et al. (38) | To characterize PCPs’ knowledge, attitudes, and beliefs about LCS. | 2014 | Qualitative research | Provider | New Mexico | Community |
| (n = 10) | ||||||
| Iaccarino et al. (39) | To assess pulmonologists’ attitudes about LCS, propensity for recommending screening, and perceived barriers to implementing LCS programs at Veterans Health Administration pulmonary clinics. | 2013-2014 | Cross-sectional | Provider | Not specified | Academic |
| (n = 286) | ||||||
| Kanodra et al. (40) | To identify perceptions of and perspectives on LCS and implementation among PCPs and high-risk eligible veteran patients at the Ralph H. Johnson VA Medical Center. | Not Specified | Qualitative research | Patient (n = 28) and provider (n = 13) | South Carolina | Academic |
| Khairy et al. (41) | To evaluate practice patterns and assess facilitators and barriers to LCS by surveying FQHC providers and comparing these responses to a previous study examining those of academic providers. | 2016 | Cross-sectional | Provider | Californiab | Community |
| (n = 36) | ||||||
| Leng et al. (42) | To assess PCPs’ knowledge, attitudes, beliefs, and practice related to LCS and the recent USPSTF guidelines in 5 high-risk Chinese immigrant communities in New York City. | 2016-2018 | Cross-sectional | Provider | New Yorkb | Both |
| (n = 83) | ||||||
| Lewis et al. (43) | To test the hypothesis that low provider knowledge of LCS guideline recommendations would be associated with less provider-reported LCS. | 2017 | Cross-sectional | Provider | Tennessee | Both |
| (n = 378) | ||||||
| Li et al. (44)a | To measure knowledge and attitudes regarding LCS among Chinese Americans with a history of smoking in Chicago. | 2018-2019 | Mixed methods | Patient | Illinoisb | Community |
| (n = 50) | ||||||
| Lillie et al. (45) | To identify the factors patients consider important in making LCS decisions and explore variations by LCS participation at the Minneapolis VA Health Care System. | 2014 | Cross-sectional | Patient | Minnesota | Community |
| (n = 588) | ||||||
| McDonnell et al. (46) | To examine knowledge, attitudes, and practices regarding LCS among nurse practitioners who work in primary care settings. | 2016 | Mixed methods | Provider | Not specified | Both |
| (n1 = 380; n2 = 15) | ||||||
| Mishra et al. (47) | To characterize patient knowledge and attitudes about LCS and smoking cessation and their views on supporting decision making for LCS. | 2014 | Qualitative research | Patient | New Mexico | Both |
| (n = 22) | ||||||
| Monu et al. (48) | To characterize knowledge, attitudes, and beliefs regarding LCS among individuals at high risk for lung cancer. | 2018 | Cross-sectional | Patient | Not specified | Community |
| (n = 240) | ||||||
| Mukthinuthalapati et al. (49) | To assess the knowledge pertaining to LCS guidelines, providers’ experience with LCS, and their recommendations for quality improvement among PCPs in Cook County Health. | 2019 | Cross-sectional | Provider | Illinoisb | Community |
| (n = 152) | ||||||
| Qiu et al. (22) | To describe the characteristics and program implementation barriers experienced by LCS programs. | 2013 | Mixed methods | System | Not specified | Both |
| (n1 = 65; n2 = 13) | ||||||
| Rajupet et al. (50) | To assess the knowledge and attitudes of PCPs vs specialists (oncologists, pulmonologists, radiologists) toward LCS as well as their likelihood to recommend LCS. | 2014 | Cross-sectional | Provider | New Yorkb | Academic |
| (n = 103) | ||||||
| Randhawa et al. (51)a | To identify barriers to adoption of LCS in the Einstein Healthcare Network in Philadelphia, Pennsylvania. | 2016 | Cross-sectional | Provider | Pennsylvaniab | Community |
| (n = 19) | ||||||
| Raz et al. (52) | To assess LCS health beliefs, including perceived barriers to screening among current smokers who were enrolled in a tobacco cessation program at Kaiser Permanente Medical Centers in Southern California. | 2017 | Cross-sectional | Patient | Californiab | Community |
| (n = 185) | ||||||
| Roth et al. (53) | To explore patients’ motivations for agreeing to recieve LCS. | 2015 | Qualitative research | Patient | Washington | Community |
| (n = 20) | ||||||
| Simmons et al. (54) | To examine the barriers to screening, including knowledge and attitudes about LCS among an ethnically and racially diverse sample of high-risk community members and PCPs. | Not Specified | Qualitative research | Patient (n = 38) & provider (n = 23) | Florida | Both |
| Sin et al. (55) | To explore facilitators of and barriers to lung cancer prevention and LCS among Korean immigrant men. | 2015 | Qualitative research | Patient | Washingtonb | Community |
| (n = 24) | ||||||
| Tseng et al. (56) | To investigate knowledge, attitudes, and smoking cessation needs for African Americans who receive LCS. | 2018 | Mixed methods | Patient | Louisianaa | Academic |
| (n = 15) | ||||||
| Watson et al. (21) | To describe the key facilitators and barriers to implementation of LCS at 2 FQHCs. | 2016-2018 | Qualitative research | System | Tennessee; West Virginia | Community |
| (n = 2) | ||||||
| Williams et al. (57) | To describe knowledge and awareness about LCS, personal values about screening, and uncertainty about the test, as well as decisional control and resources, among high-risk African American adults. | Not Specified | Cross-sectional | Patient | Not specified | Community |
| (n = 119) |
Only included portion of the study, information based on portion of study we included. FQHC = federally qualified health centers; LCS = (low-dose CT) lung cancer screening; PCP = primary care provider; USPSTF = US Preventive Services Task Force; VA = Veterans Affairs.
Included specific city or region of the state.
Barriers and Facilitators
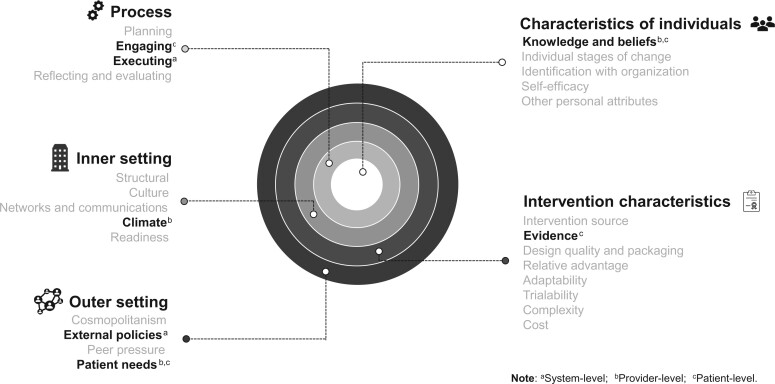
Overall, more studies emphasized barriers than facilitators. Figure 3 and Table 2 summarize themes illustrating key barriers and facilitators that emerged from the analysis by levels (systems, provider, individual). Across the 3 perspective levels, barriers and facilitators reported in the studies can be described by 20 of the 39 CFIR constructs in all 5 domains. All data could be coded to one of the CFIR constructs. A summary of classified barriers and facilitators by article are available in Supplementary Figure 1 (available online). The following sections will report specific results for each perspective.
Figure 3.
Key barriers and facilitators to lung cancer screening implementation within the CFIR. aSystem level; bProvider level; cPatient level. Bold type indicates most common constructs in a given level.
Table 2.
Summary of Consolidated Framework for Implementation Research (CFIR) domain and construct by perspective
| CFIR domain | CFIR construct | No. of studies |
|---|---|---|
| System (n = 5) | ||
| Intervention characteristics | Evidence strength and quality | 1 |
| Relative advantage | 1 | |
| Complexity | 2 | |
| Cost | 2 | |
| Outer setting | External policy and incentives | 5 |
| Patient needs and resources | 2 | |
| Inner setting | Structural characteristics | 2 |
| Networks and communications | 2 | |
| Implementation climate | 1 | |
| Readiness for implementation | 2 | |
| Characteristics of individuals | Knowledge and beliefs about the intervention | 2 |
| Process | Planning | 2 |
| Engaging | 2 | |
| Executing | 3 | |
| Reflecting and evaluating | 1 | |
| Provider (n = 19) | ||
| Intervention characteristics | Evidence strength and quality | 16 |
| Relative advantage | 6 | |
| Complexity | 3 | |
| Cost | 5 | |
| Outer setting | Patient needs and resources | 12 |
| Inner setting | External policy and incentives | 5 |
| Structural characteristics | 2 | |
| Culture | 1 | |
| Implementation climate | 13 | |
| Readiness for implementation | 8 | |
| Characteristics of individuals | Knowledge and beliefs about the intervention | 19 |
| Other personal attributes | 2 | |
| Process | Engaging | 2 |
| Executing | 2 | |
| Reflecting and evaluating | 1 | |
| Patient (n = 16) | ||
| Intervention characteristics | Design quality and packaging | 1 |
| Outer setting | Patient needs and resources | 10 |
| External policy and incentives | 2 | |
| Characteristics of individuals | Knowledge and beliefs about the intervention | 16 |
| Individual stage of change | 3 | |
| Individual identification with organization | 5 | |
| Other attributes | 2 | |
| Process | Engaging | 14 |
At the system level, all 5 studies identified barriers, but only 3 described facilitators. Findings were categorized into 15 constructs (Table 3). The 2 most common constructs were external policy and incentives in the outer setting domain, describing strategies such as policy and regulations, benchmark reporting, and so forth, to spread the intervention, and executing in the process domain, which is achieved by carrying out or accomplishing the implementation according to plan. The environment in which the organizations operated showed impact on lung cancer screening uptake, referencing the external policy and incentives construct (outer setting domain), specifically reimbursement policies and systems (21–25). This included barriers with receiving prompt or correct reimbursement from government agencies (CMS) and private insurance groups, as well as new billing codes requiring continuous engagement with insurance and reimbursement experts. One study, for example, referenced that substantial referrals coincided with the date of the final decision on CMS coverage–facilitated implementation (25).
Table 3.
Evidence table summarizing implementation facilitators and barriers at the system level
| Citation | Target population | Summary points (CFIR construct) |
|
|---|---|---|---|
| Facilitators | Barriers | ||
| Allen et al. (25) | FQHC and partner American College of Radiology–accredited screening facility | Understanding the patient population (patient needs and resources); Medicare reimbursement (external policy and incentives); frequent, standing meetings (network and communications); relative priority (implementation climate); front end planning the process and flow (planning) | Speaking with radiologists, billing component, registration component, education for staff, required paperwork (relative advantage); complexity of referrals—too many hand offs (complexity); costs required to implement and sustain an intervention, high cost of time, and staff resources (costs); patient trust among current smokers and difficultly providing clear understandable information about the screening process because of low health literacy among population (patient needs and resources); insurance and reimbursement challenges; Medicare requirements (external policy and incentives); missing training piece (planning); numerous competing demands at the organization, leadership—capacity to change (implementation climate) |
| Copeland et al. (24) | LCS centers that have been designated Screening Centers of Excellence | None provided | Complex CMS regulations including data requirements, complexity of smoking cessation, integrating SDM visits (complexity); insurance and billing issues (external policy and incentives); staffing shortage and turnover (structural characteristics); lack of patient awareness about screening availability (knowledge and beliefs); lack of provider referral (executing) |
| Eberth et al. (23) | Members of the Society of Thoracic Radiology, an international organization of radiologists | None provided | Lack of reimbursement (external policy and incentives); staffing shortage and turnover (structural characteristics) |
| Qiu et al. (22) | LCS centers that have been designated Screening Centers of Excellence | The results of research, particularly NLST (evidence strength and quality); front end planning the process and flow (planning); LCS nurse navigators were involved in the development, implementation, and surveillance of many programs (engaging; formally appointed internal implementation leaders) | Costs of the intervention and costs associated with implementing the intervention (cost); transportation, cost for patients (patient needs and resources); not receiving prompt or correct reimbursement from some insurance companies (external policy and incentives); time limitations, chief financial officer or other administrator concerned with cost or other financial concerns (readiness for implementation; available resources and leadership engagement); lack of awareness and knowledge about LCS among the public and physician (knowledge and beliefs); a wide spectrum of adherence to the guidelines, leniency of guidelines (executing) |
| Watson et al. (21) | FQHCs | Regular meetings to proactively address challenges (network and communications); leadership buy-in and support (readiness for implementation); enthusiastic project champion who conducted internal pilot and provider education; strong champion support at project outset (engaging; champions) | Lack of reimbursement, challenges with individual payer groups (external policy and incentives); tense relationship after screening partner halted project temporarily, inconsistent meeting schedule (networks and communications); low buy-in from leadership; lack of health information technology (readiness for implementation; available resources and leadership engagement); champions left (engaging); rapid rollout leads to numerous implementation challenges simultaneously (executing); minimal communication of lessons learned when expanding to additional clinic sites (reflecting and evaluating) |
ACR = American College of Radiology; CFIR = Consolidated Framework for Implementation Research; CMS = Centers for Medicare and Medicaid Services; FQHC = federally qualified health centers; LCS = lung cancer screening; NLST = National Lung Screening Trial; SDM = shared decision making.
In the implementation process domain, 3 (60%) articles reported internal workflow issues, differing levels of adherence to screening guidelines among providers, and lack of patient referrals as barriers regarding the executing construct (21,22,24). Ways to negate some of the barriers included (categorized throughout the process domain), front-end planning of workflows (25), multidisciplinary teams (22), use of enthusiastic and strong project champions (21), and formally appointed internal implementation leaders (22).
At the provider level, all 19 of the included studies identified barriers, and 15 discussed facilitators. Findings were categorized into 15 constructs (Table 4). The most common constructs were evidence strength and quality in the intervention characteristics domain, patient needs and resources in the outer setting domain, implementation climate in the inner setting domain, and knowledge and beliefs about the intervention in the individual characteristics domain.
Table 4.
Evidence table summarizing implementation facilitators and barriers at the provider level
| Citation | Target population | Facilitators | Barriers |
|---|---|---|---|
| Ahsan et al. (26) | PCPs (physicians, NPs, and PAs) working in both internal and family medicine specialties | Familiarity with LDCT LCS (knowledge and beliefs about the intervention) | Lack of efficacy and/or evidence, unnecessary diagnostic procedures, unnecessary exposure to radiation; potential for emotional harm (evidence strength and quality); patients’ knowledge deficits—limited awareness of LCS among patients (patient needs and resources); lack of insurance coverage (external policy and incentives); time limitations (implementation climate); limited awareness of LCS among patients, embarrassment about smoking, lower perceived usefulness (knowledge and belief) |
| Coughlin et al. (29) | PCPs (physicians, PAs, and advanced practice nurse who specialize in internal medicine or family practice) in 3 different types of health-care settings in the United States: 1) a university tertiary care center, 2) a public safety net hospital, and 3) 3 community hospitals | Familiarity with LCS guidelines and ability to identify appropriate patients for screening (knowledge and beliefs about the intervention) | Perceived high false-positive rate leading to unnecessary procedures (evidence strength and quality); lack of insurance coverage (external policy and incentives); time constraints (implementation climate); failure of the EHR to notify providers of eligible patients (readiness for implementation; available resources); provider uncertainty of patient eligibility, patient refusal or declining screening (knowledge and beliefs) |
| Dukes et al. (31) | PCPs and cancer specialists for HNC survivors | None provided | Concerns about the high false-positive rate, potential overdiagnosis or overtreatment, and potentially heightened patient anxiety because of false-positives (evidence strength and quality); complexity of the SDM component, clinical challenge of scheduling and monitoring CTs and follow-ups within a typical yearly appointment (complexity); costs of screening and treatment (cost); patient understanding (patient needs and resources); lack of reimbursement for SDM, LCS reimbursement criteria (external policy and incentives); time constraints (implementation climate); provider uncertainty about its benefit for patient population, lack of patient adherence to screening over time (knowledge and belief); potential HNC treatment-related health issues that could complicate screening (executing) |
| Duong et al. (32) | PCPs from the Stanford Health Care System | Being aware or influenced by USPSTF LCS guidelines (evidence strength and quality); believed current screening guidelines were at least moderately effective (knowledge and belief) | Patient can’t afford or lacks insurance (patient needs and resources); time constraints during a patient encounter (implementation climate); lack of patient awareness of LDCT screening, low provider awareness of appropriate screening guidelines (knowledge and belief) |
| Eberth et al. (33) | PCP (primary specialty as general medicine, family medicine, or internal medicine) members of American Medical Association | Believed that the benefits of LDCT outweigh the risks for patients at high risk for lung cancer (relative advantage); provider able to identify the appropriate screening recommendation (knowledge and belief) | Did not think that there is substantial evidence that LDCT screening reduces lung cancer mortality (evidence strength and quality); complexity of the topic (complexity); coverage denials, authorization was required by health insurance companies, lack of insurance coverage, lack of reimbursement to engage in SDM, SDM requirements—a separate office visit (external policy and incentives); out-of-pocket costs are a problem for patients, transportation or financial challenges for the patient, patients’ health literacy (patient needs and resources); not yet making screening discussions a routine part of practice (culture); time limitations (implementation climate; relative priority); difficulty ordering screening in the EHR, lacking decision aids (readiness for implementation); providers uncertain about how to document patient eligibility in the EHR, not knowing where to refer patients for screening, patient refusal/declining screening, fear that screening may undermine smoking cessation efforts (knowledge and belief); institutional requirements that screening be ordered by a pulmonologist, time to document SDM using decision aids (executing) |
| Ersek et al. (34) | Family physicians from the South Carolina chapter of the American Academy of Family Physicians (South Carolina Academy of Family Physicians) | Scientific evidence is strong enough to warrant screening guidelines (evidence strength and quality); LDCT screening benefits outweigh the potential harms for high-risk patients (relative advantage); knowledge of appropriate screening guidelines, knowledge of the closest CT machine available (knowledge and belief) | Concerns about the number of false-positives leading to unnecessary diagnostic procedures, psychological stress and anxiety, and unnecessary exposure to radiation (evidence strength and quality); concern of cost-effectiveness of LDCT (cost); unsure whether CMS covers LDCT LCS, unsure about whether LDCT is offered at facilities (knowledge and belief) |
| Henderson et al. (36) | PCPs (internal and family medicine physicians—attendings and residents) | Providers felt that they had enough knowledge to explain the pros and cons of LCS to patients, belief that screening is beneficial for patients (knowledge and belief) | Lack of efficacy/evidence, too many false-positives—potential for complications and emotional harm (evidence strength and quality); cost to the health-care system (cost); cost to patients (patient needs and resources) |
| Henderson et al. (37) | Pulmonologists and PCPs (physicians in family medicine, internal medicine, and pulmonary medicine) at an academic setting | Providers felt they had enough knowledge to explain the pros and cons of LCS to patients, belief that screening is beneficial for patients (knowledge and belief); provider specialty—pulmonologists were more likely than PCPs to report LCS as beneficial for patients (other attributes) | Too many false-positives—potential for complications and emotional harm (evidence strength and quality); cost to the health-care system (cost); cost to the patient (patient needs and resources); inconsistent recommendations about LCS (knowledge and belief) |
| Hoffman et al. (38) | PCPs in New Mexico clinics for underserved minority populations | None provided | Cost to patient for follow-up testing and cancer treatment, cost to patient for travel and missing work (patient needs and resources); time limitations—providers facing competing patient demands (implementation climate); lack of infrastructure to support the high-quality screening program required by guidelines, lack of technology in rural areas (readiness for implementation; available resources); providers not as confident in their abilities to decide on an appropriate workup of patients with abnormal or positive findings (knowledge and belief) |
| Iaccarino et al. (39) | Pulmonologists (attending physicians) who were active in outpatient pulmonary medicine in VA medical centers | Believed that the evidence for LDCT screening is strong, clinical trial evidence (evidence strength and quality); reduction in mortality with LDCT screening (relative advantage) | High false-positive rate—detection of incidental findings and radiation exposure (evidence strength and quality); high costs (cost); insufficient personnel (structural characteristics); insufficient infrastructure (readiness for implementation; available resources); lack of patient interest, physicians’ belief that screening is not cost effective (knowledge and belief); lack of buy-in (engaging; staff member engagement) |
| Kanodra et al. (40) | PCPs at the Ralph H. Johnson VA Medical Center and outpatient clinics | Evidence for screening—that there is substantial scientific evidence (evidence strength and quality); EHR reminders and system (readiness for implementation; available resources); awareness of USPSTF guidelines (knowledge and belief); screening coordinator—dissemination and dedicated personnel to review screening findings and offer smoking cessation would be needed to operationalize LCS (engaging; formally appointed internal implementation leaders) | Multiple screening guidelines, hard to keep up with (complexity); cost of co-pay (patient needs and resources); time limitations (implementation climate; relative priority) |
| Khairy et al. (41) | Community providers (physicians, NPs, and PAs) at FQHC “look-alike” centers and PCP physicians at an academic setting (Stanford) | LDCT effective in reducing mortality (evidence strength and quality); knowledgeable of LDCT screening based on the NLST and NCCN criteria (knowledge and belief) | False-positive rate—potential harm (evidence strength and quality); patient can’t afford or lacks insurance (patient needs and resources); shortage of trained providers (structural characteristics); complex comorbidities (implementation climate; relative priority); patient unaware of screening (knowledge and belief) |
| Leng et al. (42) | PCPs serving the NYC Chinese community | Viewed screening as effective (relative advantage); use of EHR to refer patients, ease of making referrals (readiness for implementation); correctly stated they would recommend LDCT for the scenario in which it was clinically indicated (knowledge and belief) | Vague screening criteria, patient being worried about radiation exposure (evidence strength and quality); patient’s lack of insurance or patient insurance doesn’t cover the cost of the LDCT, patient’s lack of time for screening (patient needs and resources); patient’s fear of screening, not believing that they’re at risk, patients think they should have symptoms before screening (knowledge and belief) |
| Lewis et al. (43) | Providers who practiced within general internal medicine or family medicine, pulmonology, hematology/oncology, and gynecology within an academic medical center and its affiliated VA hospital and community practices | Higher knowledge associated with accurate referral (knowledge and belief); ordering/referring for LDCT were highest among general internal medicine/PCPs followed by pulmonologists (other personal attributes) | Providers with low guideline knowledge were less likely to perform LDCT LCS (knowledge and belief) |
| McDonnell et al. (46) | NPs practicing in primary care settings | There is substantial evidence that LDCT screening saves lives (evidence strength and quality); the benefits of LDCT outweigh the risks for patients at risk of lung cancer (relative advantage); the ability to bill for SDM visits was a financial incentive to complete the screening (external policy and incentives); EHR reminders and systems to identify appropriate patients (readiness for implementation; available resources); selected LDCT for the correct vignette that reflected the USPSTF criteria (knowledge and belief) | Acknowledged that the false-positive rate is unacceptable (evidence strength and quality); financial constraints—transportation, uninsured patients (patient needs and resources); greatest barrier is that previous authorization was required by health insurance companies (external policy and incentives); experienced tension with clinic colleagues who opted for a different approach to LCS, complex comorbidities, time limitations (implementation climate; tension for change and relative priority); lack of EHR reminders and system, lack of education/training—individual provider knowledge deficits (readiness for implementation; available resources and access to knowledge and information); patients’ knowledge deficits; patient’s fear and the psychological consequences of waiting for follow-up if screening results revealed abnormal results that did not warrant immediate intervention, outright denial, or unwillingness to change behaviors (knowledge and belief) |
| Mukthinuthalapati et al. (49) | PCPs (residents, mid-level providers, and attending physicians) in safety net health-care system (Cook County Health) | Evidence applied to their population (evidence strength and quality); EHR prompts (readiness for implementation; available resources); belief that screening is cost-effective (knowledge and belief); referral rates varied by provider specialty—highest among general internal medicine/PCPs (other personal attributes); receiving statistics about their LCS practices (reflecting and evaluating) | Potential for complications (evidence strength and quality); complex comorbidities (implementation climate; relative priority); inefficient follow-up process, unclear patient smoking history record (readiness for implementation; available resources); providers felt they lacked knowledge regarding the SDM discussion, forgetting to mention screening to patients (knowledge and belief) |
| Rajupet et al. (50) | Physicians in PCP and specialists (oncologists, pulmonologists, radiologists) | Familiarity with the USPSTF LCS guidelines and ability to identify appropriate patients for screening, felt confident and comfortable (knowledge and belief); having sufficient time to counsel about LDCT screening (implementation climate; relative priority) | Concerns with false-positives (evidence strength and quality); insufficient time to counsel patients about screening (implementation climate; relative priority); belief that screening is not cost-effective, not confident in their abilities to decide on an appropriate workup of patients with positive findings (knowledge and belief) |
| Randhawa et al. (51) | PCPs (physicians) in the network offering care to indigent patient population with limited access to health care | None provided | Time constraints (implementation climate; relative priority); unaware that LDCT was recommended by the USPSTF on par with colonoscopy and mammography (knowledge and belief) |
| Simmons et al. (54) | PCPs (active license as a physician, NP or PA) working in a primary care setting in the state of Florida | Knowledge and current LCS recommendation, felt even resistant patients could be swayed with education and a tailored discussion (knowledge and belief) | False-positives (evidence strength and quality); lack of time (implementation climate; relative priority); uncertain of recommendations (knowledge and belief); cost to patient, patient resistance (patient needs and resources) |
CMS = Centers for Medicare and Medicaid Services; CT = computed tomography; EHR = electronic health record; FQHC= federally qualified health centers; HNC = head and neck cancer; LCS = lung cancer screening; LDCT = low-dose chest computed tomography; NCCN = National Comprehensive Cancer Network; NLST = National Lung Screening Trial; NP = nurse practitioner; NYC = New York City; PA = physician assistant; PCP = primary care provider; SDM = shared decision making; USPSTF = US Preventive Services Task Force; VA = Veterans Affairs.
Pertaining to intervention characteristics, nearly all studies (n = 16) made reference to the evidence strength and quality construct (26,29,31,34,36,37,39,41,42,46,49,50,54). Concerns cited the most, reported by 14 studies, were those related to the efficacy and effectiveness, specifically the high false-positive rate and the potential physical and emotional harm (26,29,31,33,34,36,37,39,41,42,46,54). Possible physical harm included potential for complications, overdiagnosis or overtreatment; detection of incidental findings; unnecessary diagnostic procedures; and exposure to radiation. Providers were also concerned with the potential for emotional harm to the patient, including potentially heightened patient anxiety because of false-positives. However, 6 studies referenced providers’ beliefs in substantial scientific evidence that LDCT lung cancer screening saves lives (32,34,39,40,46,49).
Providers also frequently (n = 12) noted barriers related to their perception of their patients’ needs and resources, a reflection of the outer setting domain (26,31–33,36–38,40–42,46,54). Specifically, patients’ needs and resources included monetary costs (36–38,40–42,46); access to convenient care (31,33,46), such as transportation barriers (31,33,46); communication (eg, language barriers, general health literacy) (26,31–33,41,46,54); and priority level (33,42,46).
In the inner setting domain, 13 studies had content regarding the implementation climate construct as an important bottleneck to implementation (26,29,31–33,38,40,41,46,49–51,54). One main subconstruct (ie, relative priority) absorbed a majority of the items’ in which providers mentioned barriers related to time limitations (26,29,31–33,38,40,46,51,54) and patients’ complex comorbidities (33). This was related to what providers perceived as patients’ needs (eg, patients’ competing health priorities), as well as the complexity of the intervention including the SDM discussion even noting that SDM discussions take too much time during patient encounters, scheduling and monitoring CTs, and explaining the process to patients.
The most prominent factor to lung cancer screening implementation from the health-care providers’ perspective was the construct describing knowledge and beliefs about the intervention in the characteristics of individuals domain (26,29,31–34,36–43,46,49–51,54). Knowledge and beliefs were reported to act as barriers (n = 17) and facilitators (n = 14). Differences were not observed by the health system setting (academic vs community based). Specific examples of barriers included lack of self-efficacy; low to no awareness and knowledge about lung cancer screening and guidelines; and low levels of belief in the cost effectiveness, usefulness, and criteria of screening (26,31,32,39,41–43,51,54). Moreover, providers reported uncertainty of patient eligibility, documentation of screening in the medical record, scheduling CTs, ability to decide on appropriate workup of patients with abnormal or positive results, CMS reimbursement, where to refer patients for screening, and SDM discussions. Providers also referenced patient knowledge and beliefs, mentioning that patients were not interested, did not believe their risk status, or refused or declined screening. On the other hand, 14 studies described familiarity with the USPSTF lung screening guidelines and ability to identify appropriate patients for screening facilitated implementation (26,29,32–34,40–43,46,50,54).
At the patient-level, among the 16 included studies, 11 addressed barriers and 15 discussed facilitators. Factors were categorized into 8 constructs within 4 domains: intervention characteristics, outer setting, characteristics of individuals, and process (Table 5). The most common constructs were patient needs and resources, knowledge and beliefs about the intervention, and engaging.
Table 5.
Evidence table summarizing implementation facilitators and barriers at the patient level
| Citation | Target population | Facilitators | Barriers |
|---|---|---|---|
| Carter-Harris and Gould (27) | Patients aged 55 to 77 years within primary care clinics in Washington state | None provided | Practical barriers represented time and logistical issues related to the inconvenience associated with the screening location (patient needs and resources); knowledge avoidance—fear of the disease and treatment and perceived low value—wasted effort and skepticism (knowledge and beliefs) |
| Cataldo (28) | US current and former smokers aged older than 55 years | Perceive accuracy of the LDCT as an important factor in the decision to have a LDCT scan, believe that early detection will result in a good prognosis, believe that they are at high risk for lung cancer; are not afraid of CT scans (knowledge and beliefs) | None provided |
| Draucker et al. (30) | Long-term current and former smokers in the United States, eligible for LCS per USPSTF guidelines | Screening covered by their insurance as a preventative tool (patient needs and resources); required by Medicaid or was part of a research study (external policy and incentives); valuing early detection (knowledge and beliefs); family history of lung cancer, receiving a recommendation from a physician (engaging; external change agents and champions) | Cost of the scan, no time to get screened (patient needs and resources); felt it was unnecessary, knew little about LDCT (knowledge and beliefs); physician who was not supportive of screening and/or recommended against it, physician did not mention it (engaging) |
| Duong et al. (32) | Patients referred for LDCT screening through Stanford’s Lung Cancer Screening Program | Have insurance (patient needs and resources); worried about their health, believe screening is useful and accurate (knowledge and beliefs); former smoker (individual stage of change); trust in provider(s) (individual identification with organization) | None provided |
| Hall et al. (35) | Participants undergoing a LDCT scan | Medicare insurance (external policy and incentives); patients understood the reason for their screening referral and perceived LDCT to be moderately accurate (knowledge and beliefs); having higher education levels (other personal attributes) | None provided |
| Kanodra et al. (40) | Veterans meeting USPSTF criteria for LCS who had been offered LDCT screening by their PCPs | Identified smoking as a risk factor, identified previous inhalational exposures as increasing their risk of lung cancer (knowledge and beliefs); trusting relationship with PCP (individual identification with organization); found the conversation with the screening coordinator, brochures, posters, or videos in the waiting room helpful (engaging; formally appointed internal implementation leaders) | Screening isn’t convenient (patient needs and resources); did not think they were at risk of disease, perceived their own health to be good, very good, or excellent compared with their age cohort, test accuracy concerns (knowledge and beliefs); PCP did not discuss LDCT screening with them, did not read brochure when it was mailed to them (engaging) |
| Li et al. (44) | Aged 55 years and older current or former smoker Chinese Americans (can read and write in Chinese) living in the greater Chicago Metropolitan area | Program that provides transportation to clinics and covers cost of screening (patient needs and resources); adequate knowledge about the screening procedure (knowledge and beliefs); high levels of trust in their physicians (individual identification with organization); providing older adults with adequate information about the screening procedure, physician recommendation, patient navigator, or some other type of assistance (engaging; formally appointed internal implementation leaders and champions) | Lack of insurance, out-of-pocket costs, lack of transportation, entire health care process—due to language barriers difficult to navigate (patient needs and resources); poor English language (other personal attributes); lack of specific lung cancer symptoms, perceived lack of provider time to discuss screening during appointment (knowledge and beliefs) |
| Lillie et al. 2017 (45) | Patients meeting the USPSTF LCS criteria at the time of an appointment with their PCP at Minneapolis VA Health Care System | Viewed LCS as convenient (design quality and packaging); fear of getting lung cancer, LCS knowledge (knowledge and beliefs) | Anxiety waiting for results, fear of incidental findings (knowledge and beliefs) |
| Mishra et al. (47) | Patients who were receiving care at an FQHC Clinic in Albuquerque, New Mexico, or the University of New Mexico Comprehensive Cancer Center | Concern for one’s personal health—knowing consequences of long-term tobacco use (knowledge and beliefs); doctor referral (engaging; champions) | Lack of transportation, cost of purchasing insurance, low health literacy, distance to care, cost of treatment (patient needs and resources); uncertainty about the value of screening (in terms of the benefits and necessity) and logistics of the screening procedure, fear of radiation exposure, concerns about psychological distress (knowledge and beliefs); the challenge of being able to trust health-care providers (individual identification with organization) |
| Monu et al. (48) | Registered participants in the Amazon MTurk crowdsourcing marketplace living in the United State, at high risk for lung cancer who met the USPSTF eligibility criteria for screening | Efficacy of early detection, knowledge of a screening test, believe they were at high risk for lung cancer (knowledge and beliefs); former smoker (individual stage of change); if recommended by their physician (engaging) | Did not think a screening test exists (knowledge and beliefs); current smoker (individual stage of change) |
| Raz et al. (52) | Current smokers who were enrolled in a tobacco cessation program at Kaiser Permanente medical centers in Southern California | Patient perceptions that LCS decreases worry for patients and families, gives peace of mind, and helps future planning (knowledge and beliefs) | High cost of test (patient needs and resources); worrying about being blamed for having smoked, worrying about feeling like a social outcast for smoking (knowledge and beliefs); current smoker (individual stage of change); no family history of lung cancer (engaging; external change agents) |
| Roth et al. (53) | Members of Kaiser Permanente, Washington, who met USPSTF screening criteria | Low or limited harm from LDCT scan perception, benefit of early detection of lung cancer (knowledge and beliefs); trust in providers (individual identification with organization); experiences of friends or family with advanced cancer (engaging; external change agents) | None provided |
| Simmons et al. (54) | Community members in Florida who met the USPSTF LCS screening criteria and had not undergone LDCT screening for lung cancer | Believes screening is beneficial, values early detection (knowledge and beliefs); informational and discussions and shared decision making (engaging) | Cost, inconvenient (patient needs and resources); confusion and misunderstanding of screening, concerns related to accuracy of the screening test (knowledge and beliefs); not brought up by provider (engaging; champions) |
| Sin et al. (55) | Korean men in Washington who were immigrants to the United States and who met the USPSTF eligibility criteria for screening | Interest in health, perceptions about the importance of seeking preventive health care in relation to the consequences of aging, perceptions concerning positive aspects of the health-care system in South Korea (knowledge and beliefs); doctor referral, recommendations from others—family members and the Korean Women’s Association (engaging; champions and external change agents) | Costs of health care in the United States, lack of time—competing demands (patient needs and resources); lack of knowledge about lung cancer, attitudes about prevention (knowledge and beliefs); lack of physician recommendation (engaging; champions) |
| Tseng et al. (56) | African American smokers aged 55-77 years who have had either 30 or more pack-years of smoking or had received an LDCT exam in the past year | Believes screening is beneficial, values early detection, concern about lung health (knowledge and beliefs); doctoral referral (engaging; champions) | Screening cost (patient needs and resources); moderate to low knowledge regarding LDCT screening (knowledge and beliefs). |
| Williams et al. (57) | African Americans who met the USPSTF eligibility criteria for LDCT screening | Felt they had all the information they needed to make an informed decision (knowledge and beliefs) | Had not heard of LDCT, uncertain of their decision to be screened (knowledge and beliefs) |
FQHC = federally qualified health centers; LCS = lung cancer screening; LDCT = low-dose chest computed tomography; PCP = primary care provider; VA = Veterans Affairs; USPSTF = US Preventive Services Task Force.
Similar to those identified at the provider level, patients’ needs and resources, in the outer setting domain, were also seen as major barriers in many papers (n = 10) (27,30,40,44,47,52,54–56). Patients raised concerns and reported challenges regarding monetary costs (30,44,47,52,54–56) and access to convenient care, including transportation barriers (27,30,40,47,54,55). Costs included those related not only to the screening test but to treatment if diagnosed with cancer, as well as health-care insurance costs in general. In contrast, 3 studies mentioned facilitators within this construct (30,32,44). Patients mentioned that having insurance (32) and coverage for lung cancer screening facilitated screening adherence (30). Practical barriers represented time and logistical issues including inconvenience associated with the screening location and time it would take to travel to and from the facility to have the scan and not having a routine provider. One study’s focus group identified that providing transportation to clinics and covering cost of screening would be an effective facilitating strategy to meet some of patients’ needs (44).
At a patient level, all 16 papers referenced patients’ knowledge and beliefs about the intervention as an important factor, both as barriers or facilitators to lung cancer screening implementation (27,28,30,32,35,40,44,45,47,48,52–57). Despite differences in study populations, low knowledge and understanding of lung cancer screening and lung cancer was largely cited as a barrier in 10 studies (27,30,40,45,47,48,52,54–56). Patients mentioned not knowing screening tests existed and subsequently not knowing eligibility requirements thus feeling that they’re not at risk for lung cancer. Even among those who had knowledge about lung cancer screening, screening was perceived to be low value and therefore low priority. Some even mentioned that they had fears regarding exposure; positive results; waiting for results, if the results are inconclusive or false-positive; invasive testing procedures; and feeling vulnerable or even being blamed for contributing to their own health problems (eg, smoking). However, knowledge and understanding of lung cancer and lung cancer screening, concern for one’s personal health, and high value (ie, interest) in prevention and/or lung cancer screening were identified as facilitators in 15 studies (28,30,32,35,40,44,45,47,48,52–57). Additionally, some studies found that demographic factors, such as level of educational attainment, current smoking status, English-speaking ability, and chronic pulmonary disease diagnosis may influence attitudes toward lung cancer screening (32,35,44,48,52).
The second most referenced factor by studies from the patient perspective was the engaging construct in the process’ domain, with 9 studies identifying it as a facilitator (30,40,44,47,48,52–56). The engaging construct was further broken down into 3 subconstructs: champions, formally appointed implementation leaders, and external change agents (30,40,44,47,48,54–56). Patients frequently acknowledged their health-care providers as champions and being a key facilitating factor for lung cancer screening (30,44,47,48,55,56). However, providers’ lack of enthusiasm and, in some cases, opposition of screening were viewed as barriers (30,40,54,55). Patients also identified patient navigators or screening coordinators (eg, formally appointed internal implementation leaders) and friends and family (eg, external change agents) as important factors that facilitated screening (30,40,44,52,53,55). Despite trust and value placed in their relationships with primary care providers (PCPs) and conversations with screening coordinators, patients in one focus group indicated that there would be added value in “educational content by way of brochures, posters, or videos” in the waiting area prior to a clinic visit, explaining that “when the brochure was mailed to them, they paid less attention to it” (40).
Risk of Bias in Individual Studies
A large proportion of ratings overall were “cannot tell.” Recurrent concerns among quantitative study designs were rooted in a lack of information regarding methods. Conversely, the majority of qualitative studies met all of the methodological quality criterion of MMAT. All mixed-methods studies met the methodological quality criterion of MMAT. Further details are available in Supplementary Tables 1-3 (available online).
Discussion
This Cochrane systematic review of 37 studies identified facilitators and barriers to implementing lung cancer screening. This study bridges an important gap in implementation literature by categorizing and cataloging implementation barriers and facilitators guided by a multilevel theoretical framework. Using the CFIR framework ensured that the key barriers and facilitators to implementation at the system, provider, and patient levels were examined systematically. Moreover, examining constructs that describe barriers and facilitators across the 5 CFIR domains and stratified by 3 levels of analysis allowed us to observe and capture interactions across constructs and levels of analysis. Many factors do not occur in a vacuum, many of which interact and possibly reinforce one another in a dynamic implementation process. Although confirming some barriers and facilitators presented in current literature, several noteworthy themes emerged when comparing findings of this review with those of other cancer screenings. Reinforcing lessons learned from breast, cervical, and colorectal cancer prevention can help inform lung cancer screening implementation.
Applying the CFIR framework provides strategic direction that may help specify next steps for effective implementation and subsequently increase lung cancer screening. Despite different target populations across varying contexts, this study demonstrates some interrelationships of common factors that influence lung cancer screening. Among the CFIR constructs that appeared across levels, the most referenced construct that affects care delivery at patient, provider, and systems levels was knowledge and beliefs about the intervention, where the substantial knowledge deficit warrants concern. To our surprise, this was observed not only in community-based settings but in academic settings as well. For providers to be able to recommend screening, they first must be knowledgeable of the recommendations and able to effectively communicate eligibility criteria, risks, benefits, and potential costs to their patients. Gaps in provider knowledge may impede the ability of providers to assist patients in SDM thus leading to lower referral rates, as shown across multiple health domains. Although patients reported fears toward screening and cancer, many also reported viewing screening as important. Additionally, they placed a large emphasis of their decision on their provider. Providers should be aware of the influence of their endorsement and recommendation and the importance of bidirectional communication (58–60). Unfortunately, our review found that a substantial number of providers were more hesitant toward recommending lung cancer screening and placed greater emphasis on potential harms.
Given the dynamic nature of the implementation process, and as mentioned previously, the constructs would often interact- either reinforcing or inhibiting one another. An example is the relationship between provider concerns about evidence strength and quality and their knowledge about the intervention. Based on these results, educational interventions targeting such knowledge deficits among providers and high-risk individuals may be imperative. Implementation strategies should be carefully designed to increase provider knowledge and subsequently focus on improving the quantity and quality of SDM. Achieving high-quality SDM in practice remains challenging (61) especially depending on practice size. Compared with other cancer screening tests, the requirements for SDM discussions and coordinating with specialists place new and greater demands on the PCP. Additionally, strategies focused on increased and improved integration of complex decision aids in electronic health records has the potential to positively impact implementation.
On the basis of this review, we found that knowledge alone is insufficient to implement a successful screening program. Most of the perceived barriers identified at the patient and provider level were within the construct of patients’ needs and resources. Providers identified barriers in communication (eg, language barriers, general health literacy), time constraints, and complexity of patients’ comorbidities. At the patient level, this included multiple components of access to care. Differential access may occur throughout the process of obtaining care and subsequently benefiting from care (62–64). Findings from this construct can be seen as an overarching accessibility concern, emphasizing that accessibility, characterized as approachability, acceptability, availability and accommodation, affordability, and appropriateness (62), represents a critical step toward improving implementation. These fundamental issues need to be addressed to maintain effective interventions over time. Deploying mobile screening units to communities has been shown to reduce structural barriers attributed to location, distance, and hours of operation and demonstrated success in other cancer screening areas (65–67). It may also be important for specific screening programs to understand the unique accessibility needs of their service population. This is particularly important, as eligible individuals constitute a unique cancer screening population with smoking-related health inequalities, including racial and ethnicity, socioeconomic, geographical, and sexual and gender minorities. Moreover, accessibility may be further ameliorated by raising awareness and meeting the information needs of patients, especially among those who do not have a PCP. Patient-directed information will likely require tailoring to ensure that it can be readily understood by the intended audience.
There are several key limitations that warrant consideration when interpreting results of this review. One of the primary limitations was the heterogeneity of the study designs, analyses, context and subpopulations represented, and the quality of studies. Based on the assessment using the modified MMAT, the quality of the studies included in our review was variable. The majority of studies at times did not provide significant detail, highlighting methodological concerns. On the other hand, some studies scored well on the MMAT scale, despite shortcomings in design and reporting. Despite these differences, all studies were synthesized equally to investigate the diversity of facilitators and barriers to implementation throughout the continuum of care, thereby gaining as broad an interpretation of the topic as possible. Although using the CFIR as a guiding framework is a strength of our review, the significant number of constructs meant that some areas with few facilitators and/or barriers identified received less consideration. As the aim of this work was to provide an inventory of factors acting as barriers and/or facilitators, information on the magnitude of the effect was beyond the scope of this review. Therefore, evidence from studies may have been given undue weight and others underemphasized. Lastly, despite a vigorous and comprehensive search strategy and review processes, some studies may have been overlooked, and our findings may exclude potentially important factors. By choosing to include only original research published in full, it is possible that there is a risk of publication bias (due to the exclusion of grey literature and unpublished research) in the findings presented (68). Nevertheless, we believe that this review summarizes a diverse body of current literature and identifies prominent factors to lung cancer screening implementation in the community setting in the United States.
Although CMS coverage was established in 2015, procedural and reimbursement issues took longer to resolve. A greater number of studies from 2020 onward are urgently needed to illustrate the current landscape of lung cancer screening. More research needs to be conducted in high-burden areas across different contexts, particularly among the populations at higher risk of death from lung cancer or populations that are socially and economically disadvantaged. These groups are often noted in literature as populations with low screening adherence; however, most of these groups are historically left out of biomedical research. Among these groups are females, racial and ethnic minorities, and rural residents.
This high-quality comprehensive systematic review synthesized the available knowledge regarding factors related to lung cancer screening implementation throughout the continuum of care using a metatheoretical framework. Applying the CFIR domains and constructs to understand and specify factors related to implementation of lung cancer screening, as well as cataloging the lessons learned from previous efforts, helps inform the development and implementation processes of lung cancer screening programs in the community setting. Although it may not be possible to target and change all of the aforementioned barriers identified in this review, there are some clear next steps for effective implementation to increase lung cancer screening, such as increasing knowledge and beliefs about the intervention among providers and patients and understanding the unique accessibility needs of high-risk patients. Further work is needed to develop best practices and address structural barriers to reduce the burden of suffering from lung cancer. Strategies may likely need to be population and context specific, but findings from this study may serve as a framework to guide the planning process.
Funding
AFC was supported by the National Cancer Institute (grant number R01CA225439).
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors do not report any conflicts of interest.
Author contributions: AES and AFC: conceptualization, methodology. AES, OCD, and SCC: Investigation. AES, OCD, and AFC: Formal Analysis, Validation. AES: Project administration, Writing—original draft, Visualization. AES, OCD, SCC, JEC, and AFC: Writing—review & editing.
Supplementary Material
Contributor Information
Ami E Sedani, Department of Biostatistics and Epidemiology, Hudson College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Olivia C Davis, College of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Shari C Clifton, Robert M. Bird Health Sciences Library, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Janis E Campbell, Department of Biostatistics and Epidemiology, Hudson College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Ann F Chou, Department of Family and Preventive Medicine, College of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72(1):7-33. [DOI] [PubMed] [Google Scholar]
- 2. Aberle DR, Adams AM, Berg CD, et al. ; for the National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Preventive Services Task Force. Final update summary: lung cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening. Published March 9, 2021. Accessed April 4, 2022.
- 4. Centers for Medicare & Medicaid Services. National Coverage Determination (NCD). Lung cancer screening with low dose computed tomography (LDCT). https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=364&ncdver=1&bc=AAAAgAAAAAAAAA. Published February 10, 2022. Accessed April 4, 2022.
- 5. Jemal A, Fedewa SA.. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3(9):1278-1281. doi: 10.1001/jamaoncol.2016.6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sedani AE, Ford LA, James SA, Beebe LA.. Factors associated with low-dose CT lung cancer screening participation in a high burden state: results from the 2017-2018 BRFSS. J Cancer Policy. 2021;28:100284. [DOI] [PubMed] [Google Scholar]
- 7. Pham D, Bhandari S, Oechsli M, Pinkston CM, Kloecker GH.. Lung cancer screening rates: data from the lung cancer screening registry. J Clin Oncol. 2018;36(suppl 15):6504. doi:10.1200/J Clin Oncol.2018.36.15_suppl.6504. [Google Scholar]
- 8. Zahnd WE, Eberth JM.. Lung cancer screening utilization: a behavioral risk factor surveillance system analysis. Am J Prev Med. 2019;57(2):250-255. doi: 10.1016/j.amepre.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 9. Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM.. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez-Olivo MA, Maki KG, Choi NJ, et al. Patient adherence to screening for lung cancer in the US: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(11):e2025102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam AC, Aggarwal R, Cheung S, et al. Predictors of participant nonadherence in lung cancer screening programs: a systematic review and meta-analysis. Lung Cancer. 2020;146:134-144. [DOI] [PubMed] [Google Scholar]
- 12. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC.. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kellermeyer L, Harnke B, Knight S.. Covidence and Rayyan. J Med Libr Assoc. 2018;106(4):580. [Google Scholar]
- 16. Harrison H, Griffin SJ, Kuhn I, Usher-Smith JA.. Software tools to support title and abstract screening for systematic reviews in healthcare: an evaluation. BMC Med Res Methodol. 2020;20(1):7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covidence. Reviewers. https://www.covidence.org/reviewers/. Published 2021. Accessed May 2022.
- 18. Richardson WS, Wilson MC, Nishikawa J, Hayward RS.. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123(3):A12-A13. [PubMed] [Google Scholar]
- 19. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L.. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 2015;11(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong QN, Fàbregues S, Bartlett G, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educa Info. 2018;34(4):285-291. [Google Scholar]
- 21. Watson L, Cotter MM, Shafer S, Neloms K, Smith RA, Sharpe K.. Implementation of a lung cancer screening program in two federally qualified health centers. Public Health Rep. 2021;136(4):397-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu R, Copeland A, Sercy E, Porter NR, McDonnell KK, Eberth J.. Planning and implementation of low-dose computed tomography lung cancer screening programs in the United States. Clin J Oncol Nurs. 2016;20(1):52-58. [DOI] [PubMed] [Google Scholar]
- 23. Eberth JM, Qiu R, Linder SK, Gallant NR, Munden RF.. Computed tomography screening for lung cancer: a survey of Society of Thoracic Radiology members. J Thorac Imaging. 2014;29(5):289-292. [DOI] [PubMed] [Google Scholar]
- 24. Copeland A, Criswell A, Ciupek A, King JC.. Effectiveness of lung cancer screening implementation in the community setting in the United States. J Oncol Pract. 2019;15(7):e607-e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen CG, Cotter MM, Smith RA, Watson L.. Successes and challenges of implementing a lung cancer screening program in federally qualified health centers: a qualitative analysis using the Consolidated Framework for Implementation Research. Transl Behav Med. 2021;11(5):1088-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahsan A, Zimmerman E, Rodriguez EM, et al. Examining lung cancer screening behaviors in the primary care setting: a mixed methods approach. J Cancer Treat Res. 2019;7(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carter-Harris L, Gould MK.. Multilevel barriers to the successful implementation of lung cancer screening: why does it have to be so hard? Ann Am Thorac Soc. 2017;14(8):1261-1265. [DOI] [PubMed] [Google Scholar]
- 28. Cataldo JK. High‐risk older smokers’ perceptions, attitudes, and beliefs about lung cancer screening. Cancer Med. 2016;5(4):753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coughlin JM, Zang Y, Terranella S, et al. Understanding barriers to lung cancer screening in primary care. J Thorac Dis. 2020;12(5):2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Draucker CB, Rawl SM, Vode E, Carter‐Harris L.. Understanding the decision to screen for lung cancer or not: a qualitative analysis. Health Expect. 2019;22(6):1314-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dukes K, Seaman AT, Hoffman RM, et al. Attitudes of clinicians about screening head and neck cancer survivors for lung cancer using low-dose computed tomography. Ann Otol Rhinol Laryngol. 2020;129(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duong DK, Shariff-Marco S, Cheng I, et al. Patient and primary care provider attitudes and adherence towards lung cancer screening at an academic medical center. Prev Med Rep. 2017;6:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eberth JM, McDonnell KK, Sercy E, et al. A national survey of primary care physicians: perceptions and practices of low-dose CT lung cancer screening. Prev Med Rep. 2018;11:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ersek JL, Eberth JM, McDonnell KK, et al. Knowledge of, attitudes toward, and use of low‐dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324-2331. [DOI] [PubMed] [Google Scholar]
- 35. Hall DL, Lennes IT, Carr A, Eusebio JR, Yeh GY, Park ER.. Lung cancer screening uncertainty among patients undergoing LDCT. Am J Health Behav. 2018;42(1):69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henderson LM, Jones LM, Marsh MW, et al. Opinions, practice patterns, and perceived barriers to lung cancer screening among attending and resident primary care physicians. Risk Manag Healthc Policy. 2017;10:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henderson LM, Marsh MW, Benefield TS, et al. Opinions and practices of lung cancer screening by physician specialty. N C Med J. 2019;80(1):19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffman R, Sussman A, Getrich C, et al. Attitudes and beliefs of primary care providers in New Mexico about lung cancer screening using low-dose computed tomography. Prev Chronic Dis. 2019;16:E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iaccarino JM, Clark J, Bolton R, et al. A national survey of pulmonologists’ views on low-dose computed tomography screening for lung cancer. Ann Am Thorac Soc. 2015;12(11):1667-1675. [DOI] [PubMed] [Google Scholar]
- 40. Kanodra NM, Pope C, Halbert CH, Silvestri GA, Rice LJ, Tanner NT.. Primary care provider and patient perspectives on lung cancer screening. A qualitative study. Ann Am Thorac Soc. 2016;13(11):1977-1982. [DOI] [PubMed] [Google Scholar]
- 41. Khairy M, Duong DK, Shariff-Marco S, et al. An analysis of lung cancer screening beliefs and practice patterns for community providers compared to academic providers. Cancer Control. 2018;25(1):1073274818806900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leng J, Lei SF, Lei L, et al. Primary care providers’ knowledge, attitudes, beliefs, and practice related to lung cancer screening in five high-risk communities in New York City. J Cancer Educ. 2022;37(3):631-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lewis JA, Chen H, Weaver KE, et al. Low provider knowledge is associated with less evidence-based lung cancer screening. J Natl Compr Canc Netw. 2019;17(4):339-346. [DOI] [PubMed] [Google Scholar]
- 44. Li C-C, Matthews AK, Wu T.. Adaptation and preliminary evaluation of a lung cancer screening decision tool for older Chinese American populations. J Natl Med Assoc. 2020;112(4):433-444. [DOI] [PubMed] [Google Scholar]
- 45. Lillie SE, Fu SS, Fabbrini AE, et al. What factors do patients consider most important in making lung cancer screening decisions? Findings from a demonstration project conducted in the Veterans Health Administration. Lung Cancer. 2017;104:38-44. [DOI] [PubMed] [Google Scholar]
- 46. McDonnell KK, Estrada RD, Dievendorf AC, et al. Lung cancer screening: practice guidelines and insurance coverage are not enough. J Am Assoc Nurse Pract. 2019;31(1):33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mishra SI, Sussman AL, Murrietta AM, et al. Peer reviewed: patient perspectives on low-dose computed tomography for lung cancer screening, New Mexico, 2014. Prev Chronic Dis. 2016;13:E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monu J, Triplette M, Wood DE, et al. Evaluating knowledge, attitudes, and beliefs about lung cancer screening using crowdsourcing. Chest. 2020;158(1):386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mukthinuthalapati VPK, Putta A, Farooq MZ, Singh SR, Gupta S, Smith S.. Knowledge, attitudes, and practices pertaining to lung cancer screening among primary care physicians in a public urban health network. Clinical Lung Cancer. 2020;21(5):450-454. [DOI] [PubMed] [Google Scholar]
- 50. Rajupet S, Doshi D, Wisnivesky JP, Lin JJ.. Attitudes about lung cancer screening: primary care providers versus specialists. Clinical Lung Cancer. 2017;18(6):e417-e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Randhawa S, Drizin G, Kane T, Song GY, Reilly T, Jarrar D.. Lung cancer screening in the community setting: challenges for adoption. Am Surg. 2018;84(9):1415-1421. [PubMed] [Google Scholar]
- 52. Raz DJ, Wu G, Nelson RA, et al. Perceptions and utilization of lung cancer screening among smokers enrolled in a tobacco cessation program. Clin Lung Cancer. 2019;20(1):e115-e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roth JA, Carter-Harris L, Brandzel S, Buist DS, Wernli K.. A qualitative study exploring patient motivations for screening for lung cancer. PLoS One. 2018;13(7):e0196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simmons VN, Gray JE, Schabath MB, Wilson LE, Quinn GP.. High-risk community and primary care providers knowledge about and barriers to low-dose computed topography lung cancer screening. Lung Cancer. 2017;106:42-49. [DOI] [PubMed] [Google Scholar]
- 55. Sin M-K, Ha A, Taylor V.. Sociocultural barriers to lung cancer screening among Korean immigrant men. J Community Health. 2016;41(4):790-797. [DOI] [PubMed] [Google Scholar]
- 56. Tseng T-S, Gross T, Celestin MD, et al. Knowledge and attitudes towards low dose computed tomography lung cancer screening and smoking among African Americans—a mixed method study. Transl Cancer Res. 2019;8(suppl 4):S431-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams RM, Beck KH, Butler IIJ, et al. Lung cancer screening decisional needs among African American smokers of lower socioeconomic status. Ethn Health. 2022;27(3):565-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McLachlan S-A, Clements A, Austoker J.. Patients’ experiences and reported barriers to colonoscopy in the screening context—a systematic review of the literature. Patient Educ Couns. 2012;86(2):137-146. [DOI] [PubMed] [Google Scholar]
- 59. Zapka JG, Lemon SC.. Interventions for patients, providers, and health care organizations. Cancer. 2004;101(suppl 5):1165-1187. [DOI] [PubMed] [Google Scholar]
- 60. Rutten LJF, Nelson DE, Meissner HI.. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987-2000). Prev Med. 2004;38(3):258-268. [DOI] [PubMed] [Google Scholar]
- 61. Elwyn G, Frosch DL, Kobrin S.. Implementing shared decision-making: consider all the consequences. Implementation Sci. 2015;11(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Levesque J-F, Harris MF, Russell G.. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013;12(1):18-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aday LA, Andersen R.. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208. [PMC free article] [PubMed] [Google Scholar]
- 64. Gulliford M, Figueroa-Munoz J, Morgan M, et al. What does ‘access to health care’ mean? J Health Serv Res Policy. 2002;7(3):186-188. [DOI] [PubMed] [Google Scholar]
- 65. Baron RC, Rimer BK, Coates RJ, et al. ; for the Task Force on Community Preventive Services. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening: a systematic review. Am J Prev Med. 2008;35(suppl 1):S56-S66. [DOI] [PubMed] [Google Scholar]
- 66. Sabatino SA, Lawrence B, Elder R, et al. ; for the Community Preventive Services Task Force. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97-118. [DOI] [PubMed] [Google Scholar]
- 67. Greenwald ZR, El-Zein M, Bouten S, Ensha H, Vazquez FL, Franco EL.. Mobile screening units for the early detection of cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1679-1694. [DOI] [PubMed] [Google Scholar]
- 68. Hopewell S, McDonald S, Clarke MJ, Egger M; for the Cochrane Methodology Review Group. Grey literature in meta‐analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;2010(1):MR000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.