Abstract
Patients with Post-COVID syndrome (PCS) are frequently referred for cardiologic evaluation. We assessed cardiac function and biomarkers in relation to functional status and fatigue in patients with PCS. This prospective single-center cohort study included 227 patients with persisting symptoms after COVID-19 infection. Most frequent complaints were fatigue (70%), dyspnea (56%), neurocognitive symptoms (34%) and chest pain (28%). Standardized questionnaires were used to assess Post-COVID-Functional-Scale (PCFS) and fatigue (MFI-20). The fatigue severity was inversely related to age and did not correlate with cardiovascular diseases, echocardiographic findings, or biomarkers. Similarly, mild to moderate functional impairment (PCFS 1–3) did not correlate with cardiovascular alterations. However, the subgroup of patients with significant functional impairment (PCFS = 4) had more frequent cardiovascular comorbidities, biomarkers and impaired global longitudinal strain (GLS). Patients with elevated troponin T showed abnormal GLS, reduced left ventricular ejection fraction and impaired tricuspid annular plane systolic excursion. The majority of patients with PCS shows a normal cardiac function. Only the small subgroup of patients with severe functional impairment and patients with elevated troponin T is at risk for impaired cardiac function and likely to benefit from specialized care by a cardiologist.
Subject terms: Diagnosis, Medical imaging, Cardiology, Health care, Biomarkers, Diagnostic markers
Introduction
The post-acute sequelae of SARS-CoV-2 infection (PACS) encompass persistent symptoms beyond four weeks from COVID-191,2. The World Health Organization defines the Post-COVID syndrome (PCS) as symptoms that persist three months after infection, last > two months and are not explained by another disease3,4. PACS and PCS are summarized as long-COVID syndrome (LCS). The reported prevalence of PCS differs with the studied population and study design5–9. A tendency towards the female sex was observed, but further risk factors remain unknown10.
Chronic fatigue is the most frequent symptom among PCS patients11. Fatigue is a subjectively perceived exhaustion that follows disproportionately after exertion and does not improve adequately after sleep or rest (post-exertional malaise, PEM). Cardiologic diseases can also cause fatigue and several additional symptoms that are associated with PCS, such as dyspnea, exercise intolerance, palpitations, chest pain, anxiety and depression11–13. The risk of cardiovascular events is increased during the acute phase of COVID-19 infection14 and there are alterations of cardiovascular function and biomarkers in PACS patients2,15–17. However, the effects on ventricular ejection fraction (LVEF), global longitudinal strain (GLS), high sensitive (hs) troponin T and n-terminal pro-brain natriuretic peptide (NT-proBNP) are small and may be primarily observed in individuals with severe courses of COVID-1914,17. The association of clinical symptoms with cardiac findings in patients after mild COVID-19 infection referred to cardiology has not been reported. Therefore, this study aimed to assess the correlation of cardiac function including cardiac biomarkers with clinical complaints like fatigue in patients with PCS.
Methods
This prospective single-center cohort study included 234 patients between April and December 2021 at the outpatient clinic. The inclusion criteria were presentation at least 3 months after the acute course of COVID-19, confirmed COVID-19 infection by SARS-CoV-2 polymerase chain reaction (PCR) test, and at least one persisting symptom 12 weeks after acute course of COVID-19. Individuals below age 18 years were excluded. An initial assessment during the acute course of COVID-19 was not carried out. 2 patients did not have positive PCR and 5 patients withdraw consent, the remaining 227 patients were included and analysed. The clinical course of acute COVID-19 was categorized based on self-reported symptom severity: asymptomatic, mild symptoms (treatment out of hospital), moderate symptoms (treatment in hospital) and severe symptoms (intensive care treatment). All Patients underwent a standardized assessment of functional status, fatigue, depression, anxiety, somatic complaints as well as laboratory testing and echocardiographic examination. All methods were carried out in accordance with current guidelines18,19. The study was approved by the local ethical review committee (Ethik-Kommission Leipzig, 431/20-ek), and written informed consent was obtained from all patients.
Assessment of functional status and fatigue
The five point Post-COVID-Functional-Scale (PCFS) was used to assess the severity of functional impairment and long-term effects of COVID-19 in PCS patients20. The scale reaches from 0 (no limitation) to 4 (severe limitation)21. Assessment of fatigue severity was performed using the Multidimensional Fatigue Inventory-20 (MFI-20)22. The MFI-20 is a self-reporting questionnaire and contains twenty items categorized in five domains with four items in each subscale: general fatigue, mental fatigue, physical activity, motivation and reduced activity. The score in each domain ranges from 4 to 20 points, while higher values indicate more fatigue. The expression of fatigue depends on age and gender22–24. The domains were defined positive above the third quartile considering the mean values in the general population22. The MFI-20 is validated in healthy individuals and several different disease cohorts7,23–25. Additional questionnaires were used to assess depression (Patient Health Questionnaire-9 / PHQ-9), anxiety (Generalized Anxiety Disorder Assessement-7 / GAD-7), somatic complaints Patient Health Questionnaire-15 / PHQ-15)26–29.
Laboratory parameters
Laboratory tests were performed on the day of sample collection at Leipzig University hospital (accredited for ISO 17025 and 15189). Serum hs troponin T and NT-proBNP analyses were performed using the electrochemiluminescence immunoassays “hs troponin T” (REF 09315357190) and “NT-proBNP II” (REF 09315284190) on a cobas® 8000 e801 module, (Roche Diagnostics, Mannheim, Germany). Subgroup analyses were performed in patients with elevated hs troponin T (cut off 14 pg/ml) and elevated NT-proBNP (cut off 125 pg/ml)30,31.
To provide information on the immune status the concentration of antibodies against the receptor-binding domain (RBD) of the spike protein and the nucleocapsid protein of SARS-CoV-2 were determined using the commercially available Abbott SARS-CoV-2 IgG II Quant (REF 6R86-22) and SARS-CoV-2 IgG (REF 6S60-22) assays, respectively. Both assays were performed using an ARCHITECT i2000SR system (Abbott, Chicago, USA). To obtain the values for WHO binding antibody units (BAU/ml), the test specific values in arbitrary units (AU/ml) were multiplied by a correction factor of 0.142.
Echocardiography
Transthoracic echocardiography examinations were performed using GE Vivid E95. Left ventricular (LV) and right ventricular (RV) dimensions, volumes as well as LV and RV function, were measured according to the recommendations of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI)32. LV assessment included LV end-diastolic diameter (LVEDD), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), left atrial diameter (LAD), left atrial volume index (LAVI) and left ventricular ejection fraction (LVEF). RV assessment included RV end-diastolic diameter (RVEDD), right atrial area (RAA), tricuspid annular plane systolic excursion (TAPSE) and systolic pulmonary artery pressure (sPAP). LVEF > 50% was defined as preserved LV-function according to current heart failure ESC guidelines31. 2-D speckle tracking analysis was performed in the three standard apical planes and global longitudinal strain (GLS) was calculated33. As cut-off for GLS we used -16,7% according to the normal ranges for strain measurement34.
Statistical analysis
Statistical analyses were performed using SPSS statistics for windows, version 27.0 Armonk, NY: IBM Corp. Descriptive analyses present continuous data as mean / standard deviation and categorical data as absolute numbers / percentage. Subgroup analyses were performed according to PCFS, MFI-20 as well as LVEF and GLS quartiles. Parameters were tested for normal distribution using Shapiro–Wilk-Test. All parameters without normal distribution were analysed by nonparametric test. Mann–Whitney-U-Test and Kruskal–Wallis-Test were used to test continuous variables. Chi-squared test were used to test categorical variables. A P value < 0.05 was considered significant.
Results
Patient characteristics
The characteristics of the 227 patients are depicted in Table 1 (PCFS) and Table 2 (MFI-20). 64.3% of the population was female. The mean age was 50 ± 15.1 years. 5.7% of the patients had an asymptomatic, 82.8% a mild, 10.1% a moderate course of COVID 19 with hospitalization, while 1.3% required intensive care. The mean time between infection (date of the first positive PCR) and consultation for PCS was seven months (range: three months to eighteen months). Vaccination became widely available in Germany in the summer 2021. Therefore, only two of the patients in this study had been vaccinated at the time of SARS-CoV2 infection. Approximately 20% of the patients received a vaccination after their SARS-CoV2 infection. No patient had more than two vaccinations. There were no statistical differences between vaccination and any of the parameters assessed. The antibody titer after COVID-19 are shown in Tables 1 and 2.
Table 1.
Cardiac characteristics and post-COVID-functional-scale (PCFS).
| Variables | Overall cohort n = 227 | PCFS 0 n = 52 | PCFS 1 n = 29 | PCFS 2 n = 87 | PCFS 3 n = 50 | PCFS 4 n = 9 | P-Value |
|---|---|---|---|---|---|---|---|
| Clinical Course of Covid-19 | |||||||
| Asymptomatic | 13 (5.7) | 10 (19.2) | 0 (0) | 2 (2.3) | 1 (2.0) | 0 (0) | |
| Mild | 188 (82.8) | 38 (73.1) | 26 (89.7) | 78 (89.7) | 42 (84) | 4 (44.4) | |
| Moderate (hospital) | 23 (10.1) | 4 (7.7) | 3 (10.3) | 7 (8) | 7 (14) | 2 (22.2) | |
| Severe (ICU) | 3 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (33.3) | < 0.001* |
| Baseline | |||||||
| Female [%] | 146 (64.3) | 31 (59.6) | 17 (58.6) | 59 (67.8) | 34 (68) | 5 (55.6) | 0.741 |
| Age [y] | 48.6 ± 15.1 | 58.1 ± 13.9 | 41.9 ± 15.3 | 44.1 ± 13.8 | 48.2 ± 11.4 | 62.2 ± 18.9 | < 0.001* |
| Body Mass Index [kg/m2] | 27.2 ± 5.4 | 26.2 ± 4.3 | 26.2 ± 4.5 | 27.1 ± 5.7 | 28.8 ± 5.6 | 28.1 ± 7.6 | 0.123 |
| Clinical presentation | |||||||
| NYHA I | 99 (43.6) | 41 (78.9) | 17(58.6) | 24 (27.6) | 15 (30) | 2 (22.2) | |
| NYHA II | 101 (44.5) | 10 (19.2) | 12 (41.4) | 54 (62.1) | 22 (44) | 3 (33.3) | |
| NYHA III | 27 (11.9) | 1 (1.9) | 0 (0) | 9 (10.3) | 13 (26.0) | 4 (44.4) | < 0.001* |
| Systolic blood pressure [mmHg] | 142.7 ± 20.1 | 146.1 ± 19.7 | 144.0 ± 22.3 | 141.2 ± 21.3 | 141.8 ± 17.6 | 138.8 ± 17.5 | 0.646 |
| Diastolic heart pressure [mmHg] | 85.4 ± 11.2 | 84.3 ± 11.5 | 85.6 ± 10.8 | 86.4 ± 11.8 | 85.8 ± 10.5 | 79.7 ± 10.0 | 0.47 |
| O2-Saturation [%] | 97.7 ± 2.3 | 97.4 ± 3.9 | 98.0 ± 1.5 | 98.0 ± 1.5 | 97.7 ± 1.8 | 96.3 ± 1.8 | 0.25 |
| Heart rate [/min] | 78.8 ± 14.7 | 73.6 ± 14.3 | 82.3 ± 13.6 | 80.3 ± 14.2 | 79.6 ± 15.7 | 78.0 ± 14.0 | 0.052 |
| Comorbidities | |||||||
| Hypertension | 68 (30.1) | 16 (30.8) | 8 (27.6) | 24 (27.6) | 15 (30) | 5 (55.6) | 0.535 |
| Dyslipidaemia | 134 (59) | 34 (65.4) | 12 (41.4) | 50 (57.5) | 31 (6.2) | 7 (77.8) | 0.188 |
| Diabetes mellitus | 12 (5.3) | 4 (7.7) | 0 (0) | 1 (1.1) | 4 (8.2) | 3 (33.3) | < 0.001* |
| Coronary artery disease | 6 (2.6) | 1 (1.9) | 1 (3.4) | 1 (1.1) | 2 (0.4) | 1 (11.1) | 0.434 |
| Obesity | 60 (27.5) | 7 (15.9) | 6 (20.7) | 22 (25.6) | 21 (10.5) | 4 (44.4) | 0.023* |
| Neuropsychiatric assessement | |||||||
| PHQ-9 Depression | 10.1 ± 4.8 | 3.9 ± 3.0 | 7.3 ± 3.5 | 8.9 ± 4.5 | 12.2 ± 4.9 | 13.5 ± 4.6 | < 0.001* |
| GAD-7 Anxiety | 7.5 ± 4.7 | 3.3 ± 3.0 | 6.1 ± 4.0 | 7.9 ± 4.5 | 8.8 ± 5.2 | 9.4 ± 4.9 | < 0.001* |
| PHQ-15 Somatization | 13.7 ± 5.0 | 6.1 ± 4.4 | 11.9 ± 4.3 | 12.7 ± 4.6 | 16.1 ± 4.9 | 14.4 ± 4.5 | < 0.001* |
| MFI-20 Fatigue | 4.0 ± 1.3 | 1.3 ± 1.7 | 3.4 ± 1.6 | 3.8 ± 1.4 | 4.2 ± 1.0 | 4.4 ± 1.4 | < 0.001* |
| Laboratory | |||||||
| Hs troponin T [pg/ml] | 5.3 ± 8.3 | 7.6 ± 7.9 | 3.8 ± 5.0 | 3.8 ± 5.1 | 3.8 ± 4.0 | 20.7 ± 27.7 | < 0.001* |
| Hs troponin T > 14 pg/ml in [%] | 15 (6.6) | 3 (5.8) | 2 (6.9) | 4 (4.7) | 1 (2.0) | 5 (55) | < 0.001* |
| NT-proBNP [pg/ml] | 98.7 ± 209.2 | 98.8 ± 87.5 | 100.9 ± 155.9 | 68.8 ± 66.0 | 81.8 ± 95.1 | 468.6 ± 908.9 | < 0.001* |
| NT-proBNP > 125 pg/ml in [%] | 40 (17.7) | 13 (25) | 4 (13.8) | 11 (12.8) | 6 (12) | 6 (66.7) | < 0.001* |
| Anti-Nucleocapside [S/CO] | 1.7 ± 2.3 | 1.2 ± 1.6 | 1.2 ± 1.7 | 1.8 ± 2.2 | 1.6 ± 2.2 | 3.4 ± 4.5 | 0.189 |
| Anti-RBD [BAU/ml] | 1111 ± 1619 | 1660 ± 1783 | 1022 ± 1842 | 1128 ± 1527 | 1016 ± 1663 | 457 ± 591 | 0.447 |
| Echocardiography | |||||||
| LV-function/dimension | |||||||
| LVEF [%] | 62.2 ± 5.4 | 62.9 ± 5.8 | 62.9 ± 5.0 | 61.9 ± 5.4 | 62.1 ± 5.6 | 60.0 ± 3.5 | 0.58 |
| GLS [%] | − 19.7 ± 2.2 | − 20.6 ± 1.9 | − 19.3 ± 1.9 | − 19.5 ± 2.3 | − 19.4 ± 2.3 | − 17.5 ± 1.7 | 0.001 |
| LAVI [ml/m2] | 20.7 ± 7.8 | 22.2 ± 6.8 | 21.1 ± 8.0 | 19.4 ± 8.7 | 20.8 ± 7.2 | 21.8 ± 5.5 | 0.411 |
| LVEDV index [ml/m2] | 52.6 ± 20.9 | 52.8 ± 10.4 | 54.1 ± 9.2 | 54.7 ± 27.7 | 48.7 ± 12.0 | 49.5 ± 6.4 | 0.595 |
| RV-function/dimension | |||||||
| RVEDD [mm] | 29.2 ± 4.8 | 28.9 ± 5.7 | 29.4 ± 3.7 | 28.6 ± 4.9 | 28.6 ± 4.2 | 35.4 ± 4.7 | 0.007* |
| TAPSE [mm] | 21.6 ± 3.0 | 21.8 ± 2.6 | 22.6 ± 3.0 | 21.2 ± 2.9 | 21.4 ± 3.1 | 20.3 ± 4.7 | 0.232 |
| sPAP [mmHg] | 28.6 ± 8.1 | 31.1 ± 6.6 | 27.9 ± 4.8 | 27.3 ± 5.2 | 29.3 ± 5.8 | 38.5 ± 25.0 | 0.007* |
| RAA [cm2] | 12.1 ± 3.6 | 12.4 ± 4.3 | 12.7 ± 2.7 | 11.7 ± 3.8 | 11.9 ± 3.7 | 14.1 ± 4.0 | 0.227 |
| Diastolic function | |||||||
| E/e ‘ | 7.1 ± 2.1 | 7.6 ± 1.9 | 7.1 ± 1.9 | 6.7 ± 1.8 | 7.8 ± 2.6 | 7.3 ± 2.0 | 0.048* |
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as absolute numbers and percentages.
PCFS post COVID functional scale, ICU intensive care unit, NYHA New York Heart Association, Obesity defined as body mass index > 30 kg/m2, O2 oxygen, PHQ-9 patient health questionnaire-9, GAD-7 generalized anxiety disorder-7, PHQ-15 patient health questionnaire-15, MFI-20 multidimensional fatigue inventory-20, hs troponin T high sensitive troponin T, NT-proBNP N-terminal pro brain natriuretic peptide, Anti-RBD antibody against receptor binding domain / spike protein, LV left ventricular, LVEF left ventricular ejection fraction, GLS global longitudinal strain, TAPSE tricuspid annular plain systolic excursion, LAVI left atrial volume index, LVEDV index left ventricular end diastolic volume indexed, RVEDD right ventricular end diastolic diameter, RAA right atrial area, sPAP systolic pulmonary artery pressure.
Table 2.
Cardiac characteristics and multidimensional fatigue inventory.
| Variables | Overall cohort n = 226 | MFI 0/5 n = 32 | MFI 1/5 n = 18 | MFI 2/5 n = 14 | MFI 3/5 n = 27 | MFI 4/5 n = 50 | MFI 5/5 n = 85 | P-value |
|---|---|---|---|---|---|---|---|---|
| Clinical Course of Covid-19 | ||||||||
| Asymptomatic | 13 (5.8) | 7 (21.9) | 2 (11.1) | 0 (0) | 1 (3.7) | 0 (0) | 3 (3.5) | |
| Mild | 188 (83.2) | 23 (71.9) | 12 (66.7) | 14 (100) | 24 (88.9) | 45 (90.0) | 69 (81.2) | |
| Moderate (hospitalisation) | 23 (10.2) | 2 (6.3) | 2 (11.1) | 0 (0) | 2 (7.4) | 4 (8.0) | 12 (14.1) | |
| Severe (ICU) | 3 (1.3) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 1 (2.0) | 1 (1.2) | 0.016* |
| Baseline | ||||||||
| Female [%] | 145 (64.2) | 20 (62.5) | 11 (61.1) | 10 (71.4) | 16 (59.3) | 36 (72) | 52 (61.2) | 0.794 |
| Age [y] | 48.6 ± 15.1 | 61.5 ± 11.3 | 60.1 ± 14.4 | 46.1 ± 10.8 | 48.3 ± 13.9 | 41.9 ± 15.5 | 45.8 ± 13.4 | < 0.001* |
| Body Mass Index [kg/m2] | 27.2 ± 5.4 | 26.7 ± 5.2 | 26.2 ± 3.3 | 28.3 ± 5.6 | 28.0 ± 5.2 | 26.7 ± 5.5 | 27.4 ± 5.8 | 0.783 |
| Clinical presentation | ||||||||
| NYHA I | 98 (43.4) | 26 (81.3) | 9 (50) | 7 (50) | 11 (40.7) | 15 (30.0) | 30 (35.3) | |
| NYHA II | 101 (44.7) | 6 (18.8) | 8 (44.4) | 5 (35.7) | 13 (48.1) | 26 (52.0) | 43 (50.6) | |
| NYHA III | 27 (11.9) | 0 (0) | 1 (5.6) | 2 (14.3) | 3 (11.1) | 9 (18.0) | 12 (14.1) | 0.003* |
| Systolic blood pressure [mmHg] | 142.7 ± 20.1 | 149.3 ± 22.8 | 146.1 ± 19.5 | 147.1 ± 17.3 | 143.4 ± 19.8 | 140.1 ± 17.3 | 140.0 ± 21.1 | 0.229 |
| Diastolic blood pressure [mmHg] | 85.4 ± 11.2 | 85.3 ± 11.4 | 81.2 ± 10.5 | 87.2 ± 7.9 | 87.0 ± 11.7 | 84.9 ± 10.3 | 85.8 ± 12.3 | 0.601 |
| O2-Saturation [%] | 97.7 ± 2.3 | 98.2 ± 1.1 | 97.8 ± 1.3 | 97.7 ± 2.1 | 96.4 ± 5.2 | 98.0 ± 1.6 | 97.8 ± 1.6 | 0.055 |
| Heart rate [/min] | 78.8 ± 14.7 | 75.5 ± 10.8 | 71.3 ± 13.0 | 82.9 ± 12.4 | 75.9 ± 16.9 | 83.2 ± 15.7 | 79.2 ± 14.5 | 0.022 |
| Cardiovascular Comorbidities | ||||||||
| Hypertension [%] | 68 (30.1) | 13 (40.6) | 5 (27.8) | 5 (35.7) | 9 (33.3) | 8 (16.0) | 28 (34) | 0.208 |
| Dyslipidaemia [%] | 133 (59) | 21 (65.6) | 13 (72.2) | 7 (50) | 16 (59.3) | 25 (50) | 51 (60) | 0.542 |
| Diabetes mellitus [%] | 12 (5.3) | 2 (6.3) | 2 (5.6) | 0 (0) | 4 (14.8) | 1 (2.0) | 3 (3.5) | 0.130 |
| Coronary artery disease [%] | 6 (2.6) | 1 (3.1) | 0 (0) | 0 (0) | 2 (7.4) | 1 (2.0) | 2 (2.4) | 0.643 |
| Obesity [%] | 59 (27.1) | 5 (20) | 2 (11.8) | 6 (42.8) | 10 (37.0) | 12 (24) | 24 (28.6) | 0.147 |
| Neuropsychiatric assessement | ||||||||
| PHQ-9 Depression | 11.3 ± 4.5 | 2.8 ± 3.1 | 4.1 ± 2.4 | 5.5 ± 1.9 | 6.8 ± 3.5 | 8.8 ± 3.7 | 12.5 ± 4.5 | < 0.001* |
| GAD-7 Anxiety | 8.5 ± 4.6 | 2.4 ± 3.1 | 3.4 ± 2.1 | 5.1 ± 2.9 | 4.6 ± 3.3 | 5.9 ± 4.6 | 10.3 ± 3.8 | < 0.001* |
| PHQ-15 Somatization | 14.8 ± 4.7 | 5.1 ± 3.7 | 7.2 ± 4.0 | 9.3 ± 3.3 | 11.1 ± 5.1 | 12.9 ± 4.0 | 15.6 ± 4.8 | < 0.001* |
| PCFS Functional Impairment | 2.2 ± 0.9 | 0.2 ± 0.6 | 1.1 ± 1.3 | 1.0 ± 1.1 | 1.8 ± 1.0 | 2.0 ± 0.9 | 2.2 ± 1.0 | < 0.001* |
| Laboratory | ||||||||
| Hs troponin T [pg/ml] | 5.3 ± 8.3 | 7.6 ± 6.3 | 6.5 ± 5.0 | 4.1 ± 3.9 | 5.8 ± 9.0 | 5.1 ± 12.8 | 4.4 ± 6.4 | 0.526 |
| Hs troponin T > 14 pg/ml in [%] | 15 (6.6) | 2 (6.3) | 1 (5.6) | 0 (0) | 2 (7.4) | 3 (6.0) | 7 (8.2) | 0.928 |
| NT-proBNP [pg/ml] | 98.7 ± 209.2 | 105.6 ± 76.0 | 126 ± 119.5 | 65.3 ± 52.2 | 77.4 ± 109.6 | 138 ± 411.9 | 80.7 ± 94.7 | 0.685 |
| NT-proBNP > 125 pg/ml in [%] | 40 (17.7) | 9 (28.1) | 7 (38.9) | 1 (7.1) | 3 (11.1) | 9 (18.0) | 11 (13.1) | 0.051 |
| Anti-Nucleocapside [S/CO] | 1.6 ± 2.2 | 1.5 ± 2.0 | 1.4 ± 1.5 | 1.9 ± 2.7 | 1.6 ± 1.8 | 1.4 ± 2.0 | 1.8 ± 2.6 | 0.186 |
| Anti-RBD [BAU/ml] | 1178 ± 1656 | 1593 ± 1761 | 1846 ± 1999 | 1589 ± 1963 | 1222 ± 1962 | 824 ± 1107 | 1025 ± 1612 | 0.058 |
| Echocardiography | ||||||||
| LV-function/dimension | ||||||||
| LVEF [%] | 62.2 ± 5.4 | 62.3 ± 5.1 | 63.6 ± 5.5 | 62.7 ± 4.9 | 60.7 ± 5.5 | 62.7 ± 6.3 | 62.0 ± 4.9 | 0.64 |
| GLS [%] | − 19.7 ± 2.2 | − 21.2 ± 2.1 | − 19.2 ± 2.0 | − 19.5 ± 1.8 | − 19.8 ± 2.4 | − 19.6 ± 2.4 | − 19.1 ± 1.9 | 0.005 |
| LAVI [ml/m2] | 20.7 ± 7.8 | 22.6 ± 6.7 | 21.9 ± 6.9 | 22.1 ± 7.1 | 21.2 ± 8.1 | 19.7 ± 7.9 | 19.8 ± 8.3 | 0.550 |
| LVEDV index [ml/m2] | 54.2 ± 22.6 | 49.9 ± 9.1 | 51.5 ± 14.1 | 53.2 ± 13.2 | 50.0 ± 12.3 | 52.3 ± 9.4 | 55.6 ± 28.4 | 0.748 |
| RV-function/dimension | ||||||||
| TAPSE [mm] | 21.6 ± 3.0 | 21.3 ± 2.3 | 22.8 ± 3.2 | 22.3 ± 4.6 | 21.4 ± 2.7 | 21.6 ± 3.3 | 21.2 ± 2.8 | 0.472 |
| RAA [cm2] | 11.9 ± 3.7 | 12.2 ± 4.1 | 13.2 ± 5.2 | 12,5 ± 4.1 | 12.6 ± 2.3 | 12.3 ± 4.2 | 11.6 ± 3.4 | 0.508 |
| RVEDD [mm] | 29.1 ± 5.3 | 28.3 ± 5.3 | 30.0 ± 5.5 | 29.9 ± 4.1 | 29.0 ± 3.6 | 28.9 ± 5.4 | 29.3 ± 5.2 | 0.900 |
| sPAP [mmHg] | 29.0 ± 8.6 | 30.0 ± 5.0 | 28.9 ± 8.0 | 28.6 ± 6.1 | 30.1 ± 8.2 | 29.9 ± 11.8 | 28.5 ± 5.8 | 0.924 |
| Diastolic function | ||||||||
| E/e’ | 7.0 ± 2.0 | 7.8 ± 1.9 | 7.3 ± 1.6 | 7.3 ± 2.0 | 7.5 ± 2.5 | 6.9 ± 1.9 | 7.1 ± 2.1 | 0.366 |
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as absolute numbers and percentages.
MFI-20 multidimensional Fatigue inventory-20, ICU intensive care unit, NYHA New York Heart Association, Obesity defined as Bbody mass index > 30 kg/m2, O2 oxygen, PHQ-9 patient health questionnaire-9, GAD-7 generalized anxiety disorder-7, PHQ-15 patient health questionnaire-15, PCFS post COVID functional scale, hs troponin T high sensitive Troponin T, NT-proBNP N-terminal pro brain natriuretic peptide, Anti-RBD antibody against receptor binding domain/spike protein, LV left ventricular, LVEF left ventricular ejection fraction, GLS global longitudinal strain, TAPSE tricuspid annular plain systolic excursion, LAVI left atrial volume index, LVEDV index left ventricular end diastolic volume indexed, RVEDD right ventricular end diastolic diameter, RAA right atrial area, sPAP systolic pulmonary artery pressure.
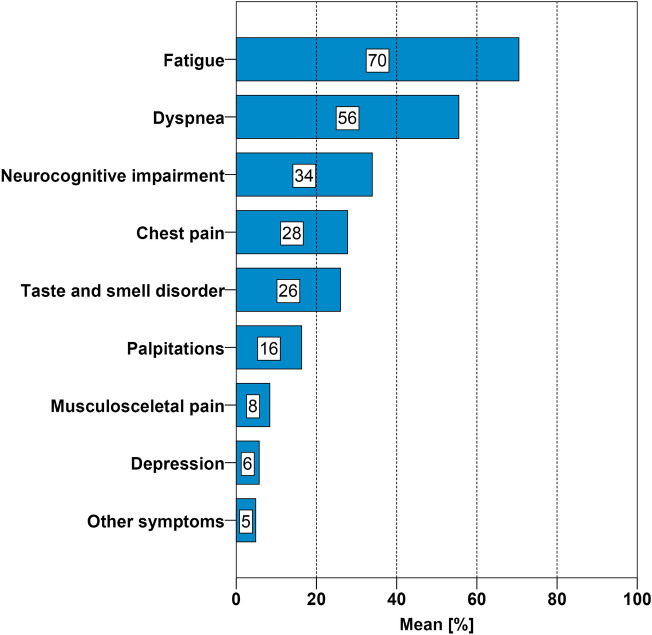
The most frequently self-reported symptoms were fatigue (70%) and dyspnea (56%) (Fig. 1). Higher scores in PHQ-9 (depression), GAD-7 (anxiety), and PHQ-15 (somatic symptoms) correlated with functional limitation / PCFS and fatigue / MFI-20 (P < 0.001).
Figure 1.
Symptoms at consultation. Prevalence of self-reported symptoms of ambulatory patients with post-COVID-syndrome, n = 227.
Cardiac characteristics and post-COVID-functional-scale (PCFS)
Patients with higher PCFS scores were more likely to report a severe clinical course of COVID-19 (P < 0.001). The functional impairment in PCFS did not correlate with cardiac comorbidities except for diabetes mellitus type 2 and obesity (Table 1). More comorbidities were predictive for higher functional impairment according to PCFS (P < 0.013). Patients in the PCFS groups 0 to 3 exhibited laboratory parameters within normal range (Fig. 1). Only 4% of the study population (n = 9) showed significant functional impairment with PCFS category 4. Significantly higher markers of myocardial stress, hs troponin T and NT-proBNP characterized this subgroup. Patients in the group with PCFS 4 had more often diabetes mellitus (P < 0.001).
Echocardiography showed no relevant differences for LVEF, TAPSE, LVEDV, LVESV, LAVI, RVEDD, RAA, and sPAP between the PCFS groups. However, patients in PCFS group 4 had higher GLS, RVEDD and sPAP (P < 0.001) (Table 1 and Fig. 2) compared to patients of PCFS group 0–3.
Figure 2.
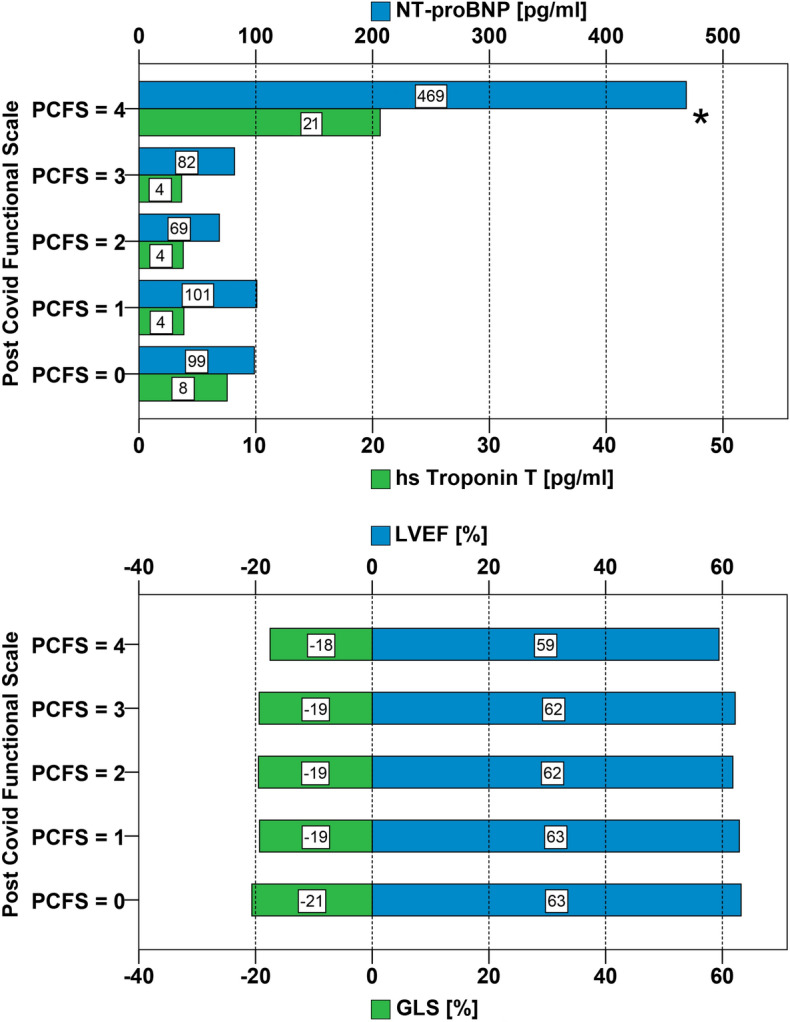
Cardiac biomarkers, echocardiographic parameters and Post-COVID-Functional-Scale. Upper panel: Mean serum concentrations of high sensitive troponin T and N-terminal-pro brain natriuretic peptide stratified by the five point PCFS (*P < 0.001). Lower panel: Mean measurements of LVEF and GLS stratified by PCFS (P < 0.001*). PCFS = Post COVID Functional Scale, hs troponin T = high sensitive troponin T; NT-proBNP = N-terminal pro brain natriuretic peptide; LVEF = left ventricular ejection fraction; GLS = global longitudinal strain.
Cardiac characteristics and multidimensional fatigue inventory-20 (MFI-20)
Patients with more positive domains in fatigue were younger (P < 0.001). The level of fatigue did not correlate with cardiac comorbidities such as hypertension, dyslipideamia, obesity and diabetes mellitus type 2 in MFI-20 (Table 2). More comorbidities were not predictive for higher scores of the MFI-20 ( P = 0.223).
There was no correlation of positive domains of the MFI-20 with laboratory parameters. Hs troponin T and NT-proBNP were not increased in the highest MFI-20 category (Table 2). The analysed echocardiographic parameters LVEF, TAPSE, LVEDV, LVESV, LAVI, RVEDD, RAA, sPAP showed no significant difference in between the fatigue categories. GLS was marginally higher with -19% in patients with 5/5 pos. domains versus -20% in patients with 0 to 4 pos. domains (P = 0.012).
Characteristics of patients with signs of myocardial pathology
LVEF
Patients with lower LVEF were more likely to be male (P = 0.004) and have obesity (P = 0.013), lower GLS (P = 0.001) and lower TAPSE (P = 0.004) (supplementary Table 1). Mean values of hs troponin T (P = 0.015) as well as the percentage of patients with hs troponin T above the cut off > 14 pg/ml (P = 0.003) were higher in the lowest quartile of LVEF.
GLS
Patients in the lowest quartile of GLS were more likely to be male (P < 0.001), to have obesity (P = 0.004) and coronary artery disease (P = 0.005). Patients with lower GLS have lower LVEF (P = 0.002) and TAPSE (P = 0.002) (supplementary Table 2). Hs troponin T and NT-proBNP showed no significant difference concerning GLS quartiles.
High sensitive troponin T
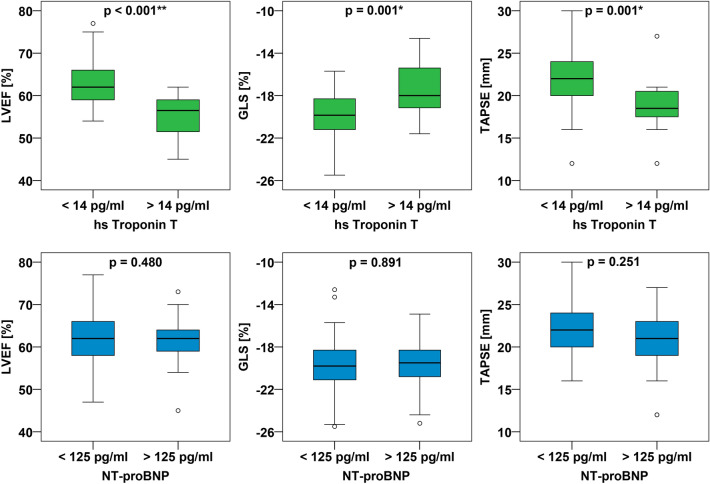
15% of the patients showed a hs troponin above 14 pg/ml, 55.6% of these patients had PCFS 4. Patients with hs troponin T levels above 14 pg/ml were older (P < 0.001) and more likely to have comorbidities such as hypertension, dyslipidemia, diabetes mellitus, coronary artery disease and obesity (P < 0.001). They showed a higher GLS (P = 0.008), sPAP (P = 0.01), LVEDV (P = 0.04) as well as a lower LVEF, (P < 0.001) and TAPSE (P = 0.001) (Fig. 3). Of note, none of the patients with normal hs troponin T had impaired LVEF or GLS.
Figure 3.
Association between echocardiographic parameters and cardiac biomarkers. Upper panel Boxplots of LVEF, GLS, TAPSE according to hs troponin T cut off 14 pg/ml29. Lower panel Boxplots of LVEF, GLS, TAPSE according to NT-proBNP cut off 125 pg/ml30. Hs troponin T = high sensitive troponin T; NT-proBNP = N-terminal pro brain natriuretic peptide; LVEF = left ventricular ejection fraction; GLS = global longitudinal strain; TAPSE = tricuspid annular plain systolic excursion.
N-terminal pro-brain natriuretic peptide
40% of the patients had an NT-proBNP above 125 pg/ml, 15% of these patients had PCFS 4. Patients with NT-proBNP serum concentrations > 125 pg/ml were more likely to have hypertension (P = 0.003), diabetes mellitus (P = 0.042) and coronary artery disease (P = 0.01). Echocardiographic quantifications of GLS, LVEF or TAPSE were not different in individuals with NT-proBNP > 125 pg/ml (Fig. 3).
Discussion
The appropriate approach to the cardiologic evaluation of the large population of patients with post COVID-19 syndrome remains challenging since the reported symptoms, specifically fatigue, dyspnea and chest pain, can be caused by cardiac, pulmonary, muscular and neuropsychiatric diseases35–37. This prospective study provides a quantitative characterization of the functional status (PCFS) and fatigue (MFI-20) in relation to cardiac biomarkers and detailed echocardiographic assessment of a typical PCS patient population presenting for cardiologic examination. The main finding of the study is that the majority of PCS patients show cardiac biomarkers and echocardiographic parameters within normal limits despite symptoms of fatigue, dyspnea or chest pain. Importantly, normal hs troponin T levels virtually ruled out abnormal cardiac function in this population. Only a small subgroup of patients (< 5%) showed signs of impaired cardiac function. These patients can be identified by severe functional impairment and / or elevated hs troponin T.
The heterogenous symptoms of the PCS widely overlap with symptoms of cardiovascular disease12. Due to the large and growing prevalence of PCS, the challenge for the consulted cardiologist is to allocate the optimal diagnostic work-up avoiding both over- and under-testing. The difficulty is the discrepancy between measurable organ dysfunction and subjectively perceived symptoms in PCS38. Our data show that symptoms related to an impaired functional status but not symptoms related to fatigue predict cardiac pathologies. Persisting high scores of PCFS have been observed after initial hospitalization and hypoxemia inducing pneumonia39–41. Longitudinal studies of PCS show that an impaired functional status reduces the quality of life regardless to severity of COVID-1942–45. In our study, patients with the highest functional impairment (PCFS grade 4) showed evidence for cardiac involvement with elevated cardiac biomarkers and abnormal echocardiographic findings consistent with literature14,46. The most likely explanation for the cardiac pathologies are pre-existing cardiac diseases and effects caused by the significantly more severe acute phase of COVID-19 in this subgroup. This small and easily identifiable subgroup of patients are therefore likely to benefit from the specialized care of a cardiologist.
Our study confirms consistent with many observational studies that fatigue is the most common symptom in patients with PCS1,6,10. The pathogenesis of fatigue in PCS is multifactorial. A post viral and immunological etiology for chronic fatigue syndrome (CFS) has been proposed for several viral disease including coxsackie, ebstein barr, influenza and varicella virus47. However, there is a gap in laboratory and imaging diagnostic in CFS patients. Thus, CFS remains a clinical diagnosis48. To date there is no evidence that fatigue is caused by cardiovascular sequelae of COVID-1949. As confirmed in our study, the incidence of neuropsychiatric disease including depression, anxiety disorder, and somatization in PCS is high and may explain neurocognitive symptoms like fatigue50,51. Therefore a systematical screening for CFS should be performed and neuropsychiatric differential diagnosis should be considered in PCS patients. Our study confirms the importance of fatigues for the symptoms reported by patients with PCS, however, fatigue does not appear to be associated with cardiac pathologies and per se does not require cardiology examinations.
Cardiovascular sequalae, defined as acute or subacute perimyocarditis, acute coronary syndrome, arrhythmias and pulmonary embolisms are not limited to the acute course of COVID-199,52. Hs troponins and natriuretic peptides are sensitive biomarkers of cardiomyocyte damage and cardiac wall stress, respectively. Higher concentrations of troponin and NT-proBNP were also found in PCS patients after mild to moderate infection of SARS-COV2 compared with matched healthy controls17. In COVID-19 patients, higher concentrations of hs troponin T are potent markers for cardiac injury and predictive for higher mortality53. As a result with practical importance, our study shows that hs troponin is valuable to distinguish between PCS patients with and without impaired cardiac function. Hs troponin T may be used to detect cardiac involvement and simplifies the decision to refer a PCS patient to a cardiologist.
Several cohort studies have characterized echocardiographic findings during acute and post-acute SARS-COV2 infection16,54,55. Speckle tracking deformation abnormalities, especially reduced GLS are highly prevalent in the acute phase of the infection56,57. Lower GLS are also evident in PCS patients compared to healthy controls58. As an additional observation, our study shows an association between GLS and obesity, higher age and high functional impairment. Abnormal global longitudinal strain is also associated with impaired prognosis56. These data support speckle tracking analysis as a sensitive tool to detect cardiac damage in PCS patients that may be missed by the assessment of the left ventricular ejection fraction alone.
Limitations
This is a single-center study and the majority of the population was caucasian. The data need to be confirmed in other ethnicities and health care systems. The median observational period was seven months. Therefore, very long-term effects cannot be excluded. Effects may be under- or overestimated due to convalescence or potential disease aggravation of PCS. We do not have a healthy control group, therefore non-viral effects of the pandemic are difficult to quantitate. The data set the stage and provide the baseline for a long-time follow-up in PCS that cannot be available at this time and for important comparison with other viral infections, e.g. influenza.
Conclusion
The study shows that the majority (> 95%) of patients with PCS after COVID-19 infection has normal cardiac function assessed by echocardiography without any discernable cardiovascular disease—despite symptoms of fatigue, dyspnea or chest pain. The distinct subgroups of patients with a history of complicated COVID-19 infection requiring hospitalization, patients with severe functional impairment, a higher number of comorbidities and patients with elevated high sensitivity troponin are at risk for impaired cardiac function and are therefore likely to benefit from specialized examination and treatment by a cardiologist.
Supplementary Information
Author contributions
L.D., P.B. and U.L. wrote the main manuscript text. P.B. prepared figs. 1, 2, 3. L.D. and P.B. prepared all tables. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Paul Baum and Lisa Do.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24038-3.
References
- 1.Nalbandian A, et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman TJ, et al. ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: Myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. College Cardiol. 2022 doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing the long-term effects of COVID-19. [PubMed]
- 4.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. [DOI] [PMC free article] [PubMed]
- 5.Dennis A, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. The Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nacul L, et al. European network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina (Kaunas, Lithuania) 2021;57:510. doi: 10.3390/medicina57050510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022 doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitaker, M. et al. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people (Cold Spring Harbor Laboratory, 2021).
- 11.Sudre CH, et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis HE, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrin R, et al. Into the looking glass: Post-viral syndrome post COVID-19. Med. Hypotheses. 2020;144:110055. doi: 10.1016/j.mehy.2020.110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022 doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puntmann VO, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotecha T, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen EL, et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: The Hamburg City Health Study COVID programme. Eur. Heart J. 2022 doi: 10.1093/eurheartj/ehab914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koczulla AR, et al. S1-Leitlinie post-COVID/Long-COVID. Chirurg. 2022;93:101–102. doi: 10.1007/s00104-021-01543-1. [DOI] [Google Scholar]
- 19.Barker-Davies RM, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020;54:949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klok FA, et al. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020 doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed HAA, et al. Post-COVID-19 functional status: Relation to age, smoking, hospitalization, and previous comorbidities. Ann. Thoracic Med. 2021;16:260–265. doi: 10.4103/atm.atm_606_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smets E, Garssen B, Bonke B, de Haes J. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psych. Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26:140–144. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 24.Gecaite-Stonciene J, et al. Validation of the multidimensional fatigue inventory with coronary artery disease patients. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17218003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan KA, et al. Systematic review of the multidimensional fatigue symptom inventory-short form. Supp. Care Cancer Off. J. Multinatl. Assoc. Supp. Care Cancer. 2015;23:191–212. doi: 10.1007/s00520-014-2389-7. [DOI] [PubMed] [Google Scholar]
- 26.Berth, H., Löwe, B., Spitzer, R. L., Zipfel, S., & Herzog, W. PHQ-D. gesundheitsfragebogen für patienten (2003).
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Internal Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psych. Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Internal Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 30.Collet J-P, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 31.McDonagh TA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 32.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–3914. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Voigt J-U, et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto T, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging. 2017;18:833–840. doi: 10.1093/ehjci/jex140. [DOI] [PubMed] [Google Scholar]
- 35.Pavlovic NV, et al. Fatigue in persons with heart failure: A systematic literature review and meta-synthesis using the biopsychosocial model of health. J. Cardiac. Failure. 2022;28:283–315. doi: 10.1016/j.cardfail.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashif A, et al. Follow-up of COVID-19 recovered patients with mild disease. Sci. Rep. 2021;11:13414. doi: 10.1038/s41598-021-92717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menges D, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PloS One. 2021;16:e0254523. doi: 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kersten J, et al. Long COVID: Distinction between organ damage and deconditioning. J. Clin. Med. 2021;10:3782. doi: 10.3390/jcm10173782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benkalfate N, et al. Evaluation of the Post-COVID-19 Functional Status (PCFS) Scale in a cohort of patients recovering from hypoxemic SARS-CoV-2 pneumonia. BMJ Open Respir. Res. 2022;9:e001136. doi: 10.1136/bmjresp-2021-001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giurgi-Oncu C, et al. Cardiovascular abnormalities and mental health difficulties result in a reduced quality of life in the post-acute COVID-19 syndrome. Brain Sci. 2021;11:1456. doi: 10.3390/brainsci11111456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machado FVC, et al. Construct validity of the Post-COVID-19 functional status scale in adult subjects with COVID-19. Health Qual. Life Outcomes. 2021;40:555. doi: 10.1186/s12955-021-01691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaes AW, et al. Recovery from COVID-19: A sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021 doi: 10.1183/23120541.00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betschart M, et al. One year follow-up of physical performance and quality of life in patients surviving COVID-19: A prospective cohort study. Swiss Med. Week. 2021;151:w30072. doi: 10.4414/smw.2021.w30072. [DOI] [PubMed] [Google Scholar]
- 44.Pizarro-Pennarolli C, et al. Assessment of activities of daily living in patients post COVID-19: A systematic review. PeerJ. 2021;9:e11026. doi: 10.7717/peerj.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu G, et al. Health-related quality of life of COVID-19 patients after discharge: A multicenter follow-up study. J. Clin. Nurs. 2021;30:1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: A cohort study. The Lancet Diabetes Endocrinol. 2022 doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Archer MI. The post-viral syndrome: A review. J. R. Coll. Gen. Pract. 1987;37:212–214. [PMC free article] [PubMed] [Google Scholar]
- 48.Bateman L, et al. Myalgic encephalomyelitis/chronic Fatigue syndrome: Essentials of diagnosis and management. Mayo Clinic Proc. 2021;96:2861–2878. doi: 10.1016/j.mayocp.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Newman M. Chronic fatigue syndrome and long covid: Moving beyond the controversy. BMJ. 2021;373:n1559. doi: 10.1136/bmj.n1559. [DOI] [PubMed] [Google Scholar]
- 50.Blomberg B, et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham EL, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunal S, et al. Emerging spectrum of post-COVID-19 syndrome. Postgraduate Med. J. 2022;98:633–643. doi: 10.1136/postgradmedj-2020-139585. [DOI] [PubMed] [Google Scholar]
- 53.Giustino G, et al. Characterization of myocardial injury in patients with COVID-19. J. Am. College Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnweber T, et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall J, et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax. 2021;76:408–411. doi: 10.1136/thoraxjnl-2020-215861. [DOI] [PubMed] [Google Scholar]
- 56.Minhas AS, et al. Myocardial work efficiency, a novel measure of myocardial dysfunction, is reduced in COVID-19 patients and associated with in-hospital mortality. Front. Cardiovasc. Med. 2021;8:667721. doi: 10.3389/fcvm.2021.667721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stöbe S, et al. Echocardiographic characteristics of patients with SARS-CoV-2 infection. Clin. Res. Cardiol. Off. J. German Cardiac. Soc. 2020;109:1549–1566. doi: 10.1007/s00392-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikonomou E, et al. Impaired left ventricular deformation and ventricular-arterial coupling in post-COVID-19: Association with autonomic dysregulation. Heart Vessels. 2022 doi: 10.1007/s00380-022-02180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.