ABSTRACT
Background
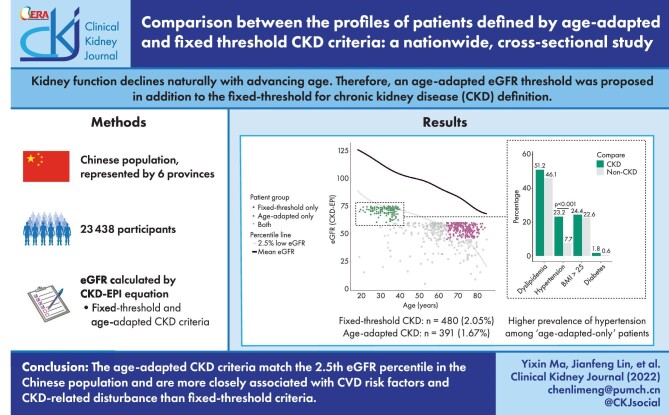
Kidney function declines naturally with advancing age. Therefore an age-adapted estimated glomerular filtration rate (eGFR) threshold has been proposed instead of the fixed threshold for CKD definition. This study aims to describe and compare the profile of CKD patients defined by these two criteria in a Chinese population.
Method
We recruited adult participants with selected biochemical tests from the Chinese Physiological Constant and Health Condition survey conducted from 2007 to 2011, with the GFR estimated by the Chronic Kidney Disease Epidemiology Collaboration formula. The age-adapted threshold of eGFR is 75, 60 and 45 ml/min/1.73 m2 for the population <40 years of age, 40–64 years and >64 years, respectively. The fixed threshold is 60 ml/min/1.73 m2 for all ages.
Results
Among the recruited 23 438 participants, 480 were diagnosed with CKD by fixed threshold criteria, while 391 were diagnosed with CKD by age-adapted criteria. Patients diagnosed by fixed threshold criteria were significantly older (66.4 versus 43.4 years; P < .001) and had a higher prevalence of all CVD risk factors compared with the non-CKD population. In contrast, age-adapted criteria defined a younger patient group and were not significantly associated with diabetes or obesity. When adjusted by age and gender, fixed threshold–defined CKD was not significantly associated with the number of coexisting CVD risk factors, while age-adapted-defined CKD was significantly associated. We also found that the CKD patients defined by age-adapted criteria matched well with the 2.5th percentile of eGFR in Chinese individuals. When compared with their age- and gender-matched controls, patients included by age-adapted criteria but excluded by fixed threshold criteria had a significantly higher prevalence of hypertension (23.2% versus 7.7%; P < .001) and hyperuricaemia (25.0% versus 5.5%; P < .001), while patients included only by the fixed threshold criteria were not significantly different in the prevalence of CVD risk factors and CKD-related disturbance except for hyperuricaemia (41.2% versus 14.0%; P < .001).
Conclusion
An age-adapted criterion is more closely associated with CVD risk factors and CKD-related diseases compared with fixed threshold criteria.
Keywords: CKD, CKD-EPI equation, elderly, hypertension, hyperuricaemia
Graphical Abstract
Graphical Abstract.
INTRODUCTION
In the last decade, chronic kidney disease (CKD) has been gaining increasing attention as a leading public health problem, with high morbidity, mortality and burdens on the healthcare system [1]. The current widely accepted definition of CKD is kidney damage or an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 for >3 months, by the 2012 Kidney Disease: Improving Global Outcomes and the National Kidney Foundation Kidney Disease Outcomes and Quality Initiative guidelines [2–4]. However, there have been arguments that an age-adapted criterion for CKD is needed because eGFR declines with advancing age. Furthermore, the fixed threshold standard of eGFR regardless of age might lead to an overdiagnosis of CKD among the elderly population and an underdiagnosis among young individuals [5–7]. Therefore an age-adapted eGFR threshold of 75, 60and 45 ml/min/1.73 m2 for the population <40 years of age, 40–64 years and >64 years was proposed [8].
A recent study compared the outcomes of these two different CKD criteria–defined cohorts and found a lower risk of death and kidney failure among the conventional fixed threshold criteria compared with the new age-adapted threshold [9]. The finding was not unexpected. In the last 2 decades, several cohort studies have illustrated a weak association between slightly decreased kidney function (eGFR 45–59 ml/min/1.73 m2, or CKD stage 3a) and different adverse outcomes, including all-cause mortality [10–16], cardiovascular mortality [11, 17–19] and progression to end-stage renal disease (ESRD) [16, 20, 21] among the elderly population.
However, detailed profiles of the CKD patients included or excluded by these two criteria have not been studied. The profiles of these patients, such as the prevalence of intervenable CVD risk factors and life quality–determining CKD-related disturbances, may affect the medical approaches to these patients with clinical importance. Questions remain whether the ‘overdiagnosed’ elderly patients defined by the fixed threshold criteria are disease-free and do not need any medical intervention and whether the ‘underdiagnosed’ youngsters defined by the age-adapted threshold were burdened by their kidney conditions.
To address these questions, we used data from a community-based cross-sectional study to identify and characterize adults with incident CKD according to the fixed threshold and age-adapted threshold definitions. We estimated the prevalence of common cardiovascular disease (CVD) risk factors such as hypertension, diabetes, dyslipidaemia and overweight and collected the common CKD-related disturbances of hyperuricaemia, anaemia, hypocalcaemia and hyperphosphataemia among these populations. We observed the impact of age on the association between these disease profiles with two criteria of the CKD definition, illustrated the age and eGFR distribution of the CKD patients and compared the disease profiles with their age- and gender-matched non-CKD controls.
MATERIALS AND METHODS
Study population
The data used in this study are from the Chinese Physiological Constant and Health Condition survey. Details of the study are described elsewhere [22–24]; briefly, it is a population-based, cross-sectional survey conducted from 2007 to 2011. The survey used a random, multistage, stratified sampling method to obtain a nationally representative sample of the general Chinese population of six provinces, with a three-stage cluster sampling method to sample eligible subjects.
We included a total of 23 438 individuals who were >18 years of age with complete age, gender, body measurement (height and weight), blood pressure (BP) measurement and laboratory test data (serum creatinine, lipid profile, blood glucose) (Fig. 1). All the participants had completed a standard questionnaire about demographic characteristics, socio-economic data, lifestyle risk factors and their past medical history.
Figure 1:
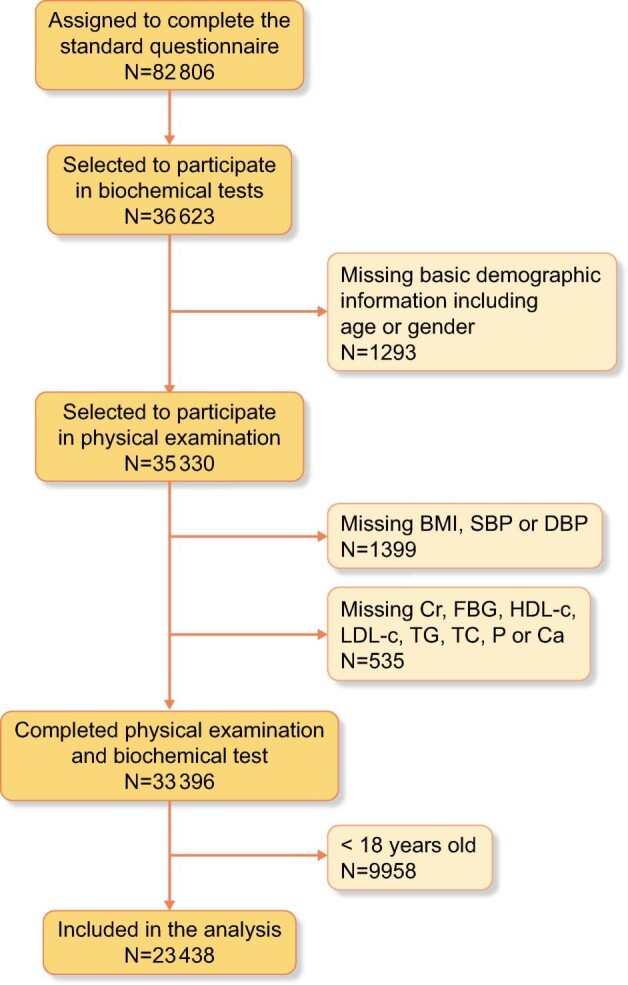
Flow diagram of participants’ inclusion.
The protocol was approved by the Ethics Review Board of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Written informed consent was signed by each participant before data collection.
Clinical and laboratory evaluation
Physical examination included measurement of weight, height and BP. BP was measured by trained medical personnel using an HEM-7000 electronic sphygmomanometer (Omron Healthcare, Muko, Japan) after the participant had rested for at least 10 minutes. The documented BP was the average value of the three-time test, while the body mass index (BMI) was calculated as body weight divided by the square of the height (kg/m2).
We collected the overnight fasting blood by venipuncture with the patient consuming a bland diet before blood testing. The blood was then centrifuged and the serum was stored at −80°C until the laboratory assay test. Serum lipids, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid (UA) and fasting blood glucose (FBG) levels were measured using an AU Series Automatic Biochemical Analyzer (Beckman Coulter, Brea, CA, USA) and Sekisui Medical (Tokyo, Japan) reagents.
The creatinine of the six subcentres in this study was detected by the creatinine oxidase method using regular automatic biochemical analyser and medical reagents for clinical detection. All the centres participated in the proficiency testing (PT) organized by the National Center for Clinical Laboratories of China and processed the appropriate control for the results qualified before and after the sample test. The laboratory of Peking Union Medical College Hospital served as the central laboratory, which is a dual-accredited laboratory by the College of American Pathologists (CAP) and ISO 15189, and the creatinine test also participated in the PT organized by CAP with qualified results.
Before the epidemiological investigation of each subcentre, the central laboratory distributed 20 comparison samples covering high, medium and low concentrations of creatinine to the laboratory of the subcentre to make sure the comparison qualified results before the study. During the investigation, the central laboratory issued another 20 comparison samples. The work could continue only after passing the comparison. Once the comparison results are unqualified, we trace all internal quality control and PT data back to determine the reasons. Except for adopting the appropriate methods such as instrument maintenance and calibration, all the following work stopped until the comparison was qualified. We calculated the eGFR by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) serum creatinine–based equation expressed as follows [25]:
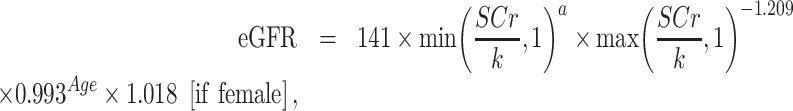 |
where SCr is serum creatinine, k = 0.7 (female) or 0.9 (male) and α = −0.239 (female) or −0.441 (male).
Data interpretation
We defined hypertension as average systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg or current antihypertensive medication use, diabetes as FBG ≥7.0 mmol/l or currently using anti-diabetes medication and dyslipidaemia as at least one of the following: TC ≥5.2 mmol/l, TG ≥1.7 mmol/l, HDL-C <1.0 mmol/l, LDL-C ≥3.4 mmol/l or current lipid-lowering medication use. We diagnosed overweight or obesity based on a BMI ≥25 kg/m2 and calculated the eGFR using the CKD-EPI serum creatinine–based equation [25]. Fixed threshold criteria used an eGFR threshold of 60 ml/min/1.73 m2 regardless of age and age-adapted criteria used an eGFR threshold of 75, 60 and 45 ml/min/1.73 m2 for adults <40, 40–64 and ≥65 years of age, respectively. When excluded by both criteria and without a self-reported history of kidney disease, the non-CKD population was defined.
Statistical analysis
The analysis aimed to compare the characteristics of the CKD population with the non-CKD population. We analysed the difference between the study samples, i.e. the CKD population and the general population, with a t-test for normally distributed continuous variables and chi-squared test for proportions and the association between the diagnosis of CKD and CVD risk factors by logistic regression. The 50th and 2.5th quantile curves of eGFR on age were calculated by quantile regression using the R package ‘quantregGrowth’ without other independent variables included besides age [26]. We conducted the age and gender matching between CKD patients and the non-CKD population using the R package ‘MatchIt’ [27], with nearest neighbour matching with a matching ratio of 1:1.
The database was constructed with Epi Info 3.02 software by two data managers and corrected to guarantee the accuracy and integration of the data. R software version 4.1.2 was used for all statistical analyses (R Foundation for Statistical Computing, Vienna, Austria) and tests with P-values <.05 were considered statistically significant.
RESULTS
The study included 23 438 participants with a mean age of 43.8 years and 53.5% were female. The average eGFR was 99.5 ml/min/1.73 m2; 68.5% has an eGFR >90 ml/min/1.73 m2, 29.4% had an eGFR of 60–89 ml/min/1.73 m2 and 2% had an eGFR of <60 ml/min/1.73 m2. The prevalences of dyslipidaemia, overweight, hypertension and diabetes were 49.4%, 31.8%, 23.8% and 4.5%, respectively. CKD-related disturbances including hyperuricaemia (12.1%), anaemia (6.2%), hyperphosphataemia (2.3%), and hypocalcaemia (2.3%) were observed. The baseline characteristics of the study population are presented in Table 1.
Table 1:
The baseline characteristics of the study population.
| Characteristics | Total |
|---|---|
| Population, N | 23 438 |
| Age (years) | 43.8 (16.19) |
| Age group (years), n (%) | |
| <40 | 10 054 (42.9) |
| 40–64 | 10 563 (45.1) |
| >64 | 2821 (12.0) |
| Female, n (%) | 12 535 (53.5) |
| eGFR (CKD-EPI; ml/min/1.73 m2) | 99.45 (19.47) |
| eGFR category, n (%) | |
| >90 | 16 063 (68.5) |
| 60–89 | 6895 (29.4) |
| 30–59 | 457 (1.9) |
| 15–29 | 18 (0.1) |
| <15 | 5 (0.0) |
| CVD risk factors, n (%) | |
| Diabetes | 1049 (4.5) |
| Hypertension | 5588 (23.8) |
| BMI >25 | 7379 (31.8) |
| Dyslipidaemia | 11 475 (49.4) |
| CKD-related symptoms, n (%) | |
| Hyperphosphataemia | 529 (2.3) |
| Hypocalcaemia | 545 (2.3) |
| Anaemia | 1242 (6.2) |
| Hyperuricaemia | 2836 (12.1) |
Values presented as mean (SD) unless stated otherwise.
Diabetes was defined as FBG ≥7.0 mmol/l or currently on anti-diabetes medication. Hypertension was defined as an average SBP ≥140 mmHg, DBP ≥90 mmHg or currently on antihypertensive medication. Dyslipidaemia was defined as at least one of the following: TC ≥5.2 mmol/l, TG ≥1.7 mmol/l, HDL-C <1.0 mmol/l, LDL-C ≥3.4 mmol/l or currently on lipid-lowering medication. Hyperuricaemia was serum UA ≥420 μmol/l for males and UA ≥360 μmol/l for females. Anaemia was haemoglobin <136 g/l for males and <119 g/l for females. Hyperphosphataemia was defined as serum phosphate >1.53 mmol/l. Hypocalcaemia was defined as serum calcium <2.13 mmol/l.
A total of 480 (2.05%) participants fulfilled the criteria of fixed threshold CKD (eGFR <60 ml/min/1.73 m2 for all ages) and 391(1.67%) participants fulfilled the criteria for age-adapted CKD (<40 years old: eGFR <75 ml/min/1.73 m2; 40–64 years old: eGFR <60 ml/min/1.73 m2; >64 years old: eGFR <45 ml/min/1.73 m2). The baseline profiles of the CKD patients defined by these two criteria and the non-CKD population are presented in Table 2. The average age of the CKD patients defined by the fixed threshold criteria was significantly older than the non-CKD population (66.4 versus 43.4 years; P < .001), with a higher prevalence of all four CVD risk factors. In contrast, the CKD patients defined by age-adapted criteria were slightly older than the non-CKD population (47.9 versus 43.8 years; P < .001). The prevalences of hypertension and dyslipidaemia were significantly higher when compared with the non-CKD population, while the difference between diabetes and overweight (BMI >25) was not significant. Among the CKD-related symptoms, anaemia and hyperuricaemia were significantly higher in CKD patients defined by both criteria. No difference between hypocalcaemia and hyperphosphataemia was observed.
Table 2:
Comparison of disease profile between CKD and non-CKD patients diagnosed by two criteria.
| Fixed threshold CKD | Age-adapted CKD | |||||
|---|---|---|---|---|---|---|
| Characteristics | Non-CKD (n = 22 958) | CKD [n = 480 (2.05%)] | P-value | Non-CKD (n = 23 047) | CKD [n = 391 (1.67%)] | P-value |
| Age (years), mean (SD) | 43.4 (15.94) | 66.4 (11.17) | <.001 | 43.8 (16.18) | 47.9 (16.13) | <.001 |
| Female, n (%) | 12 264 (53.4) | 271 (56.5) | .202 | 12 309 (53.4) | 226 (57.8) | .094 |
| eGFR (CKD-EPI; ml/min/1.73 m2), mean (SD) | 100.46 (18.30) | 50.90 (9.40) | <.001 | 100.17 (18.73) | 57.21 (15.01) | <.001 |
| CVD risk factors, n (%) | ||||||
| Diabetes | 1004 (4.4) | 45 (9.4) | <.001 | 1024 (4.4) | 25 (6.4) | .084 |
| Hypertension | 5328 (23.2) | 260 (54.2) | <.001 | 5427 (23.5) | 161 (41.2) | <.001 |
| BMI >25 | 7169 (31.5) | 210 (44.1) | <.001 | 7242 (31.7) | 137 (35.2) | .158 |
| Dyslipidaemia | 11 149 (49.0) | 326 (68.3) | <.001 | 11 230 (49.2) | 245 (63.0) | <.001 |
| CKD-related disturbance, n (%) | ||||||
| Hyperuricaemia | 2620 (11.4) | 216 (45.3) | <.001 | 2684 (11.7) | 152 (39.2) | <.001 |
| Anaemia | 1176 (6.0) | 66 (15.7) | <.001 | 1206 (6.1) | 36 (10.6) | .001 |
| Hyperphosphataemia | 519 (2.3) | 10 (2.1) | .921 | 518 (2.3) | 11 (2.8) | .56 |
| Hypocalcaemia | 532 (2.3) | 13 (2.7) | .672 | 533 (2.3) | 12 (3.1) | .406 |
For age and eGFR (CKD-EPI), P-values were calculated by an independent t-test. For other categorical variables, P-values were calculated by the chi-squared test.
We then analysed the relationship between the number of existing CVD risk factors (hypertension, dyslipidaemia, BMI >25 and diabetes) and CKD diagnosed by two different criteria (Table 3). When not adjusted by age and gender, the diagnosis of CKD by either criterion was significantly associated with several classic CVD risk factors. Specifically, fixed threshold criteria had the highest odds ratio (OR) with ‘risk number ≥1’ [OR 4.367 (95% CI 3.27–5.97)], while the age-adapted threshold had the highest OR with ‘risk number = 4’ [OR 2.14 (95% CI 1.1–3.7)]. After adjusting for age and gender, the significant association with CVD risk factors was abolished by fixed threshold criteria, while the positive relationship still existed in the age-adapted criteria.
Table 3:
Association of CVD risk factor number with different CKD definitions.
| Fixed threshold CKD | Age-adapted CKD | |||||
|---|---|---|---|---|---|---|
| CVD risk factor count category | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Risk number ≥1 | ||||||
| CKD only | 4.367 | 3.272–5.967 | <.00 | 1.87 | 1.468–2.41 | <.001 |
| Adjusted | 1.112 | 0.817–1.546 | .512 | 1.674 | 1.291–2.192 | <.001 |
| Risk number ≥2 | ||||||
| CKD only | 3.051 | 2.527–3.696 | <.001 | 1.771 | 1.448–2.166 | <.001 |
| Adjusted | 1.11 | 0.907–1.361 | .316 | 1.655 | 1.331–2.057 | <.001 |
| Risk number ≥3 | ||||||
| CKD only | 3.054 | 2.497–3.719 | <.001 | 1.947 | 1.520–2.467 | <.001 |
| Adjusted | 1.203 | 0.973–1.481 | .084 | 1.757 | 1.353–2.260 | <.001 |
| Risk number = 4 | ||||||
| CKD only | 3.113 | 1.877–4.857 | <.001 | 2.137 | 1.093–3.744 | .015 |
| Adjusted | 1.053 | 0.626–1.672 | .835 | 1.707 | 0.866–3.023 | .09 |
CVD risk factor count indicates the number of positive findings among hypertension, diabetes, dyslipidaemia and BMI >25.
The CKD only model takes only CKD as independent variable, the adjusted model was further adjusted for CKD, age and gender as independent variables.
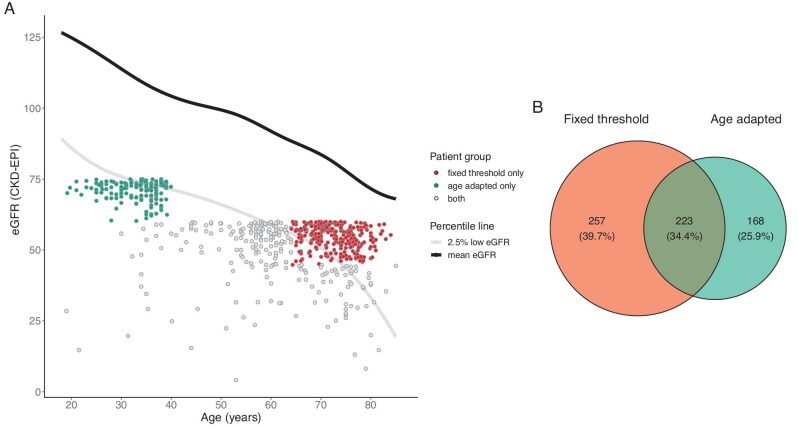
In Fig. 2, the Venn plot demonstrates the separation of the CKD patients defined by the two CKD criteria. There were 223 patients diagnosed by both CKD criteria (0.95%)—257 by fixed threshold criteria only (1.1%) and 168 by age-adapted criteria only (0.72%). The eGFR of the community-based populations decreased steadily with increasing age in Fig. 2A, illustrated by the 2.5th and 50th percentile curves on the plot. The CKD patients defined by age-adapted criteria matched well with the 2.5th percentile of eGFR in the Chinese individuals. Patients diagnosed by the age-adapted threshold but not the fixed threshold criteria were <40 years of age with an eGFR >60 ml/min/1.73 m2, below the 2.5th quantile of the respective age group (Fig. 2A, highlighted by scattered green points). The patients included by the fixed threshold but not the age-adapted criteria were >64 years of age with an eGFR <60 ml/min/1.73 m2 and above the 2.5th eGFR quantile (Fig. 2A, highlighted by scattered red points).
Figure 2:
Age and eGFR distribution of suspected CKD and total population. (A) Estimated GFR by age in the whole population with scatter plot of suspected CKD population. (B) Venn plot of CKD patients defined by fixed threshold and age-adapted criteria.
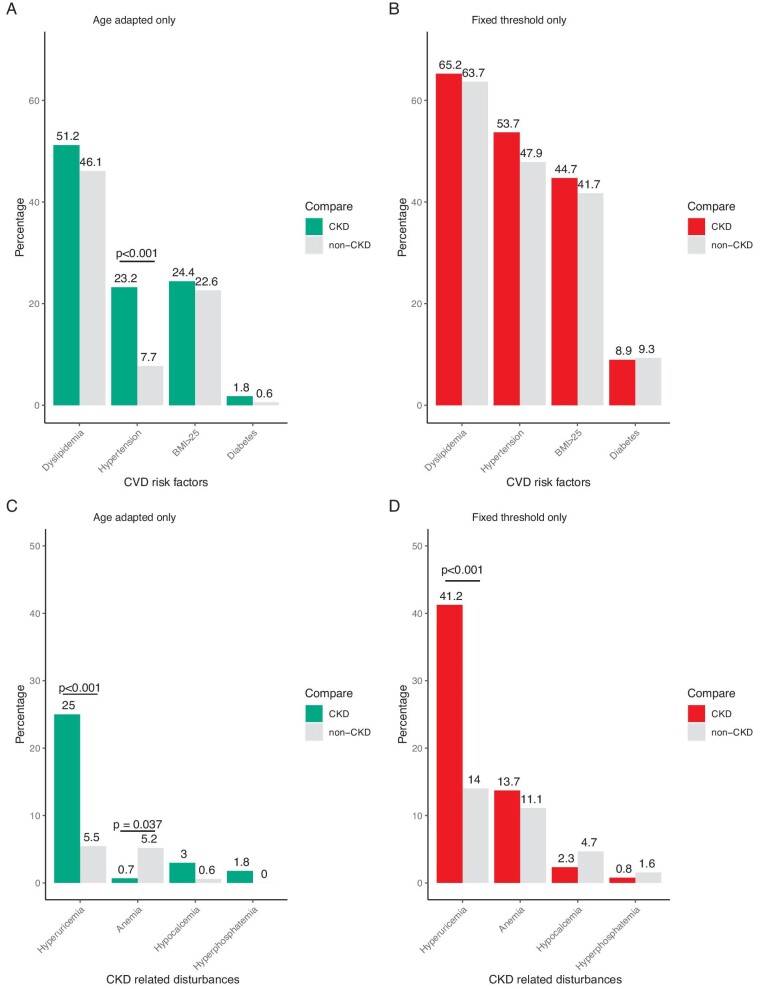
We further compared the prevalence of both CVD risk factors and CKD-related disturbance of CKD patients diagnosed by age-adapted criteria only or fixed threshold criteria only with age- and gender-matched non-CKD populations (Fig. 3). The prevalence of hypertension (23.2% versus 7.7%; P < .001) and hyperuricaemia (25.0% versus 5.5%; P < .001) was significantly higher in the age-adapted only CKD group compared with the age- and gender-matched healthy controls. Significance was seen only in hyperuricaemia of the CKD patients in the fixed threshold criteria only group (41.2% versus 14.0%; P < .001) than healthy controls. When comparing the levels of all the directly measured variables (i.e. serum calcium instead of hypocalcaemia), significantly lower serum calcium was found in age-adapted only CKD patients (Supplementary Tables S1 and S2).
Figure 3:
Comparison of disease profiles between patients and non-CKD controls. (A) CVD risk factors profile of patients diagnosed with age-adapted criteria alone compared with their matched non-CKD population. (B) Risk factors profile of patients diagnosed with fixed threshold criteria alone compared with their matched non-CKD population. (C) CKD-related symptoms of patients diagnosed with age-adapted criteria alone compared with their matched non-CKD population. (D) CKD-related symptoms of patients diagnosed with fixed threshold criteria alone compared with their matched non-CKD population. P-values of significance were annotated directly on the plot. P-values were calculated by chi-squared test elsewhere. The mean age of patients diagnosed by age-adapted criteria only was 33.0 years and the mean age of their matched controls was 33.1 years. The mean age of patients diagnosed by fixed threshold criteria only was 72.7 years and the mean age of their matched controls was 72.8 years.
DISCUSSION
In this study, the age-adapted criteria defined a group of CKD patients more evenly distributed across all age groups and more closely associated with the number of CVD risk factors adjusted by age and gender, with an eGFR approximately below the 2.5th percentile of the respective age. Young people with an eGFR of 60–75 ml/min/1.73 m2 are more likely to have CKD. Except for hyperuricaemia, older people with an eGFR of 45–60 ml/min/1.73 m2 showed similar profiles in CVD risk factors and CKD-related analytical disturbance as those with an eGFR >60 ml/min/1.73 m2.
The natural decline of kidney function with advancing age has been observed in previous studies [28–32]. Among older people between 70 and 79 years, the incidence of eGFR <60 ml/min/1.73 m2 was as high as 23.7%, reaching 52.5% in those >80 years of age [33]. It is thought that an eGFR <60 ml/min/1.73 m2 might be physiological among the elderly [34–36]. In this large community-based Chinese population, the 2.5th percentile of eGFR falls below 60 ml/min/1.73 m2 at 60 years old and below 45 ml/min/1.73 m2 for those >78 years old. Since the 2.5th percentile of a continuous test result in the general population is a commonly used cut-off point when defining normal and disease status, the recently proposed age-adapted eGFR threshold showed good correspondence.
The ‘normal’ value for kidney function is just one consideration for a CKD diagnosis, while another consideration is the risk of future adverse outcomes. In 2012, Hallan et al. [37] published a meta-analysis including >2 million participants, indicating a crucial conclusion that mortality and ESRD risk increased in lower eGFR populations at different ages. For those >75 years of age, the adjusted HR for all-cause mortality at an eGFR of 45 ml/min/1.73 m2 versus 80 ml/min/1.73 m2 was 1.35 (95% CI 1.23–1.48). The absolute risk difference was the highest among all age groups at 27.2 excess deaths per 1000 person-years. However, later in 2016, from the published data of the same paper, Delanaye et al. [5, 8] concluded that the eGFR range for the lowest HR in each age category was different. For example, among participants 18–54 years of age, the eGFR range associated with low mortality is >75 ml/min/1.73 m2, but the value decreases to 45–104 ml/min/1.73 m2 for those >65 years of age. We also noticed in Hallan et al.’s [37] paper that the steepest slope for increased mortality risk by eGFR decline was among participants 18–54 years of age. For this group of young participants, the mortality risk increase began to be significant immediately below an eGFR of 80 ml/min/1.73 m2 and the HR for all-cause mortality rose to >1.5 at an eGFR of 60 ml/min/1.73 m2. The result was consistent with our data and can be partially explained by our observation that young people with an eGFR of 60–70 ml/min/1.73 m2 have a higher prevalence of CVD risk factors, especially hypertension.
Among young people <40 years of age, who generally have a lower mortality rate, the incident rate of CVD events was therefore poorly represented in prior cohort studies. We observed that an eGFR of 60–75 ml/min/1.73 m2 was strongly associated with hypertension. The relationship between hypertension and kidney function is rather complex. First, hypertension is a well-recognized cause of CKD, with the risk ratio for CKD estimated to be 2.8 for a pretreatment SBP of 166–180 mmHg and 7.6 for a pretreatment SBP >180 mmHg compared with the population with a pretreatment SBP <165 mmHg [38]. In China, the percentage of CKD caused by hypertension among all other causes has risen from 11.5% in 2011 to 15.9% in 2015, based on the nationwide Hospital Quality Monitoring System [39]. In contrast, decreased kidney function may also be the underlying cause of hypertension. Recently a bidirectional Mendelian randomization study showed a causal effect of better kidney function on lower BP [40]. In this study, the significantly higher prevalence of hypertension among young people with an eGFR of 60–75 ml/min/1.73 m2 might indicate that hypertension, especially among young people, can be the early flag signal of impaired kidney function.
Hyperuricaemia was another closely related risk factor of CKD patients defined by either criterion. Although the idea of applying uricaemia-lowering therapies to alleviate eGFR decline among CKD patients has not been widely accepted [41–43], hyperuricaemia is common among CKD patients and paralleled the progress of CKD [44]. It might also be a sensitive indicator of early kidney function decline. There is no significant difference in hyperphosphataemia or hypocalcaemia between populations with or without CKD. Since chronic kidney disease–mineral and bone disorder more often occurs among patients with advanced CKD and severely impaired kidney function, the community-based study included mainly early-stage CKD patients.
We also observed an unexpected lower anaemia prevalence among young patients with an eGFR of 60–75 ml/min/1.73 m2. In fact, these patients, both males and females, showed a higher level of blood haemoglobin compared with their age- and-gender matched controls or the normal haemoglobin reference range (male: age-adapted only group 171.3 g/l versus matched controls 158.4 g/l; female: age-adapted group 146.9 g/l versus matched controls 134.2 g/l). One possible explanation for this phenomenon is that the higher haemoglobin level in this patient group is due to their higher prevalence of hypertension [45, 46]. It has been observed in large cohorts of healthy individuals that haemoglobin level is positively associated with BP. Approximately 1 mmol/l increase in haemoglobin level predicts a 1.3 mmHg increase in SBP in males and 1.8 mmHg SBP increase in females [45]. The supplementation of erythropoietin in CKD patients may also lead to the occurrence of hypertension [47–49]. Possible mechanisms include increased responsiveness to catecholamines and angiotensin II in vascular tissue and an impaired hypoxia-induced vasodilation response [47].
To our knowledge, this is the first cross-sectional study to describe the detailed disease profile of patients included by different CKD criteria. Our findings showed that young people with an eGFR of 60–75 ml/min/1.73 m2 are more likely to be ‘CKD burdened’ and elderly people with an eGFR of 45–60 ml/min/1.73 m2 showed similar CVD risk factors as patients with an eGFR >60 ml/min/1.73 m2, except the hyperuricaemia burden. The result was consistent with prior cohort studies [9].
Limitations
Limitations existed in this cross-sectional study and we did not enrol patients with albuminuria. In addition, urinalysis, ultrasound examination of the kidneys and other biological markers indicating existing kidney damage may provide more information but was not available in this study.
CONCLUSION
An age-adapted criterion of CKD is more closely associated with CVD risk factors and CKD-related diseases, especially among people <40 years of age and >65 years.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all of the participants, primary care doctors, and nurses who took part in this survey.
Contributor Information
Yixin Ma, Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Jianfeng Lin, Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Peng Xia, Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Hua Zheng, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Xinqi Cheng, Department of Laboratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Peili Ji, Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Wei Wu, Department of Laboratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Lian Hou, Department of Laboratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Li Wang, Department of Epidemiology and Biostatistics, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Guangjin Zhu, Department of Pathophysiology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Ling Qiu, Department of Laboratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Yali Zheng, Department of Nephrology, Affiliated Ningxia People's Hospital of Ningxia Medical University, Yinchuan, China.
Limeng Chen, Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
FUNDING
This work was partially supported by grants from the National Natural Scientific Foundation of China (82170709 and 81970607 to C.L.), CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-A-001 and CIFMS 2021-1-I2M-003 to C.L.), Capital's Funds for Health Improvement and Research (2020-2-4018 to C.L.) and Beijing Natural Science Foundation (L202035 to C.L). The funders had no role in the study design, data collection, analysis, decision to publish or manuscript preparation.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Romagnani P, Remuzzi G, Glassock Ret al. Chronic kidney disease. Nat Rev Dis Primers 2017;3:17088. 10.1038/nrdp.2017.88 [DOI] [PubMed] [Google Scholar]
- 2. Inker LA, Astor BC, Fox CHet al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 3. Jha V, Garcia-Garcia G, Iseki Ket al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 4. Thomas B, Matsushita K, Abate KHet al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 2017;28:2167–79. 10.1681/ASN.2016050562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delanaye P, Glassock RJ, Pottel Het al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev 2016;37:17–26. [PMC free article] [PubMed] [Google Scholar]
- 6. Rule AD, Glassock RJ. GFR estimating equations: getting closer to the truth? Clin J Am Soc Nephrol 2013;8:1414–20. 10.2215/CJN.01240213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Hare AM, Rodriguez RA, Rule AD. Overdiagnosis of chronic kidney disease in older adults—an inconvenient truth. JAMA Intern Med 2021;181:1366–8. 10.1001/jamainternmed.2021.4823 [DOI] [PubMed] [Google Scholar]
- 8. Delanaye P, Jager KJ, Bökenkamp Aet al. CKD: a call for an age-adapted definition. J Am Soc Nephrol 2019;30:1785–805. 10.1681/ASN.2019030238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu P, Quinn RR, Lam NNet al. Accounting for age in the definition of chronic kidney disease. JAMA Intern. Med. 2021;181:1359–66. 10.1001/jamainternmed.2021.4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roderick PJ, Atkins RJ, Smeeth Let al. CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis 2009;53:950–60. 10.1053/j.ajkd.2008.12.036 [DOI] [PubMed] [Google Scholar]
- 11. van der Velde M, Bakker SJ, de Jong PEet al. Influence of age and measure of eGFR on the association between renal function and cardiovascular events. Clin J Am Soc Nephrol 2010;5:2053–9. 10.2215/CJN.08851209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maaravi Y, Bursztyn M, Hammerman-Rozenberg Ret al. Glomerular filtration rate estimation and mortality in an elderly population. QJM 2007;100:441–9. 10.1093/qjmed/hcm043 [DOI] [PubMed] [Google Scholar]
- 13. Raymond NT, Zehnder D, Smith SCet al. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant 2007;22:3214–20. 10.1093/ndt/gfm396 [DOI] [PubMed] [Google Scholar]
- 14. O'Hare AM, Bertenthal D, Covinsky KEet al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol 2006;17:846–53. 10.1681/ASN.2005090986 [DOI] [PubMed] [Google Scholar]
- 15. Muntner P, Bowling CB, Gao Let al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol 2011;6:2200–7. 10.2215/CJN.02030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh SW, Kim S, Na KYet al. Glomerular filtration rate and proteinuria: association with mortality and renal progression in a prospective cohort of a community-based elderly population. PLoS One 2014;9:e94120. 10.1371/journal.pone.0094120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallan S, Astor B, Romundstad Set al. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II study. Arch Intern Med 2007;167:2490–6. 10.1001/archinte.167.22.2490 [DOI] [PubMed] [Google Scholar]
- 18. Stengel B, Metzger M, Froissart Met al. Epidemiology and prognostic significance of chronic kidney disease in the elderly—the Three-City prospective cohort study. Nephrol Dial Transplant 2011;26:3286–95. 10.1093/ndt/gfr323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Pottelbergh G, Vaes B, Adriaensen Wet al. The glomerular filtration rate estimated by new and old equations as a predictor of important outcomes in elderly patients. BMC Med 2014;12:27. 10.1186/1741-7015-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minutolo R, Lapi F, Chiodini Pet al. Risk of ESRD and death in patients with CKD not referred to a nephrologist: a 7-year prospective study. Clin J Am Soc Nephrol 2014;9:1586–93. 10.2215/CJN.10481013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malmgren L, McGuigan FE, Berglundh Set al. Declining estimated glomerular filtration rate and its association with mortality and comorbidity over 10 years in elderly women. Nephron 2015;130:245–55. 10.1159/000435790 [DOI] [PubMed] [Google Scholar]
- 22. Wu J, Yan WH, Qiu Let al. High prevalence of coexisting prehypertension and prediabetes among healthy adults in northern and northeastern China. BMC Public Health 2011;11:794. 10.1186/1471-2458-11-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu T, Zhu G, Liu Jet al. Gender-specific prevalence and associated risk factors of high normal blood pressure and hypertension among multi-ethnic Chinese adolescents aged 8–18 years old. Blood Press 2015;24:189–95. 10.3109/08037051.2015.1025474 [DOI] [PubMed] [Google Scholar]
- 24. Lin J, Zheng H, Xia Pet al. Long-term ambient PM2.5 exposure associated with cardiovascular risk factors in Chinese less educated population. BMC Public Health 2021;21:2241. 10.1186/s12889-021-12163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muggeo VMR, Torretta F, Eilers PHCet al. Multiple smoothing parameters selection in additive regression quantiles. Stat Model 2021;21:428–48. 10.1177/1471082X20929802 [DOI] [Google Scholar]
- 27. Ho D, Imai K, King Get al. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28. 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 28. Delanaye P, Glassock RJ. Glomerular filtration rate and aging: another longitudinal study—a long time coming! Nephron 2015;131:1–4. 10.1159/000439147 [DOI] [PubMed] [Google Scholar]
- 29. Pottel H, Hoste L, Yayo Eet al. Glomerular filtration rate in healthy living potential kidney donors: a meta-analysis supporting the construction of the full age spectrum equation. Nephron 2017;135:105–19. 10.1159/000450893 [DOI] [PubMed] [Google Scholar]
- 30. Yue L, Fan L, Du X. Age- and sex-specific reference values of estimated glomerular filtration rate in Chinese population. Gerontology 2021;67:397–402. 10.1159/000513451 [DOI] [PubMed] [Google Scholar]
- 31. Ma Y, Zhan J, Xu G. Reference values of glomerular filtration rate for healthy adults in southern China: a cross-sectional survey. Ther Adv Chronic Dis 2021;12:20406223211035287. 10.1177/20406223211035287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu P, Quinn RR, Lam NNet al. Progression and regression of chronic kidney disease by age among adults in a population-based cohort in Alberta, Canada. JAMA Netw Open 2021;4:e2112828. 10.1001/jamanetworkopen.2021.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebert N, Jakob O, Gaedeke Jet al. Prevalence of reduced kidney function and albuminuria in older adults: the Berlin Initiative Study. Nephrol Dial Transplant 2017;32:997–1005. [DOI] [PubMed] [Google Scholar]
- 34. Wesson DE. Does eGFR by any number mean the same? J Am Soc Nephrol 2019;30:1806–7. 10.1681/ASN.2019070749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glassock RJ, Delanaye P, Rule AD. Should the definition of CKD be changed to include age-adapted GFR criteria? YES. Kidney Int 2020;97:34–7. 10.1016/j.kint.2019.08.033 [DOI] [PubMed] [Google Scholar]
- 36. Rovin BH. Do kidneys grow old gracefully? Kidney Int 2020;97:40–41. 10.1016/j.kint.2019.08.031 [DOI] [PubMed] [Google Scholar]
- 37. Hallan SI, Matsushita K, Sang Yet al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012;308:2349–60. 10.1001/jama.2012.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Udani S, Lazich I, Bakris GL. Epidemiology of hypertensive kidney disease. Nat Rev Nephrol 2011;7:11–21. 10.1038/nrneph.2010.154 [DOI] [PubMed] [Google Scholar]
- 39. Yang C, Wang H, Zhao Xet al. CKD in China: evolving spectrum and public health implications. Am J Kidney Dis 2020;76:258–64. 10.1053/j.ajkd.2019.05.032 [DOI] [PubMed] [Google Scholar]
- 40. Yu Z, Coresh J, Qi Get al. A bidirectional Mendelian randomization study supports causal effects of kidney function on blood pressure. Kidney Int 2020;98:708–16. 10.1016/j.kint.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kimura K, Hosoya T, Uchida Set al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018;72:798–810. 10.1053/j.ajkd.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 42. Stack AG, Dronamraju N, Parkinson Jet al. Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: a randomized trial. Am J Kidney Dis 2021;77:481–9. 10.1053/j.ajkd.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sircar D, Chatterjee S, Waikhom Ret al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 2015;66:945–50. 10.1053/j.ajkd.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 44. Sato Y, Feig DI, Stack AGet al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 2019;15:767–75. 10.1038/s41581-019-0174-z [DOI] [PubMed] [Google Scholar]
- 45. Atsma F, Veldhuizen I, de Kort Wet al. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension 2012;60:936–41. 10.1161/HYPERTENSIONAHA.112.193565 [DOI] [PubMed] [Google Scholar]
- 46. Son M, Park J, Park Ket al. Association between hemoglobin variability and incidence of hypertension over 40 years: a Korean national cohort study. Sci Rep 2020;10:12061. 10.1038/s41598-020-69022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agarwal R. Mechanisms and mediators of hypertension induced by erythropoietin and related molecules. Nephrol Dial Transplant 2018;33:1690–8. 10.1093/ndt/gfx324 [DOI] [PubMed] [Google Scholar]
- 48. van de Borne P, Tielemans C, Vanherweghem JLet al. Effect of recombinant human erythropoietin therapy on ambulatory blood pressure and heart rate in chronic haemodialysis patients. Nephrol Dial Transplant 1992;7:45–9. [PubMed] [Google Scholar]
- 49. Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol 2009;4:470–80. 10.2215/CJN.05040908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.