ABSTRACT
Chronic kidney disease (CKD) is a risk factor for premature cardiovascular disease. As kidney function declines, the presence of left ventricular abnormalities increases such that by the time kidney replacement therapy is required with dialysis or kidney transplantation, more than two-thirds of patients have left ventricular hypertrophy. Historically, much research in nephrology has focussed on the structural and functional aspects of cardiac disease in CKD, particularly using echocardiography to describe these abnormalities. There is a need to translate knowledge around these imaging findings to clinical outcomes such as unplanned hospital admission with heart failure and premature cardiovascular death. Left ventricular hypertrophy and cardiac fibrosis, which are common in CKD, predispose to the clinical syndrome of heart failure with preserved left ventricular ejection fraction (HFpEF). There is a bidirectional relationship between CKD and HFpEF, whereby CKD is a risk factor for HFpEF and CKD impacts outcomes for patients with HFpEF. There have been major improvements in outcomes for patients with heart failure and reduced left ventricular ejection fraction as a result of several large randomized controlled trials. Finding therapy for HFpEF has been more elusive, although recent data suggest that sodium-glucose cotransporter 2 inhibition offers a novel evidence-based class of therapy that improves outcomes in HFpEF. These observations have emerged as this class of drugs has also become the standard of care for many patients with proteinuric CKD, suggesting that there is now hope for addressing the combination of HFpEF and CKD in parallel. In this review we summarize the epidemiology, pathophysiology, diagnostic strategies and treatment of HFpEF with a focus on patients with CKD.
Keywords: cardiovascular, chronic kidney disease, heart failure, mineralocorticoid receptor antagonism, sodium-glucose cotransporter 2
BACKGROUND
It is well recognized that chronic kidney disease (CKD) is a risk factor for premature cardiovascular disease (CVD), and that CVD is the leading cause of death in patients with all stages of CKD [1, 2]. Although CKD increases the risk of all subtypes of CVD, as the estimated glomerular filtration rate (eGFR) declines, the risk of heart failure (HF) and cardiac death increases compared with that of myocardial infarction (MI) and stroke [3]. This aligns with the increasing prevalence of left ventricular hypertrophy (LVH) as eGFR declines such that more than two-thirds of patients with eGFR <30 mL/min/1.73 m2 have been reported to have LVH [4]. While left ventricular systolic dysfunction certainly is common, affecting 16% of patients at the commencement of dialysis, with LVH as the dominant ventricular lesion, it stands to reason that the driver of HF events in patients with CKD, including those on dialysis, is the syndrome of heart failure with preserved ejection fraction (HFpEF) [5, 6]. Effective HF treatments have not been assessed reliably in advanced CKD and dialysis. Patients with advanced CKD are often excluded from trials in HF [7–9]. There have been significant developments in our understanding of risk factors for and outcomes of HFpEF in patients with and without CKD. In this review, we describe the epidemiology, pathophysiology, diagnostic strategies and new evidence for therapies in managing HFpEF, with a particular focus on patients with CKD.
HFpEF in the general population
The European Society of Cardiology (ESC) clinical practice guidelines recommend categorizing HF into distinct phenotypes based on the left ventricular ejection fraction (LVEF) [10]. Historically, the basis for this is the original landmark treatment trials that showed improved outcomes in patients with HF and LVEF <40% and this group is described as heart failure with reduced ejection fraction (HFrEF). Patients with heart failure and mildly reduced ejection fraction (HFmrEF; LVEF 41–49%) resemble patients with HFrEF in terms of outcomes and response to therapies [11, 12]. Patients with HFpEF (LVEF ≥50%) have essentially a ‘normal’ ejection fraction. Table 1 highlights the differences between HFrEF and HFpEF.
Table 1.
Summary of demographic, pathophysiological, diagnostic and therapeutic differences between HFrEF and HFpEF
| Characteristics | HFpEF | HFrEF | |
|---|---|---|---|
| Demographics | Older age, female predominance | Younger age, male predominance | |
| Risk factors | Ageing, hypertension, obesity, physical inactivity | Ischaemic heart disease, smoking | |
| Pathophysiology | Coronary microvascular inflammation | Direct cardiomyocyte injury and loss | |
| Natriuretic peptides | Lower levels | Higher levels | |
| LV morphology and other properties | LVEF | ≥50% | <50% |
| LV remodelling | Concentric remodelling | Eccentric remodelling | |
| LV volumes | Normal | Increased | |
| Systolic dysfunction (other than EF) | + | ++ | |
| Diastolic dysfunction | ++ | + | |
| Aortic stiffness | ++ | + | |
| Disturbance of LV relaxation or compliance | ++ | + | |
| Response to HF therapy | ACEia | − | ++ |
| ARBa | − | ++ | |
| SGLT2 inhibitorsb | ++ | ++ | |
| ARNI | − | ++ | |
| Bteablockers | − | ++ | |
| MRA | + | ++ | |
| Cause of death | Predominantly non-CV death | Predominantly CV death | |
aTrials have not demonstrated clinically meaningful benefit in HFrEF but ARBs/ACEis have proven long-term benefits in the progression of CKD.
bLower risk of HF hospitalization in HFpEF.
The diagnosis of HF requires the presence of typical signs (e.g. pulmonary crackles, raised jugular venous pressure) and symptoms (e.g. fatigue, breathlessness) in the context of cardiac structural or functional abnormality and further corroborated by elevated concentrations of circulating natriuretic peptides or evidence of cardiogenic pulmonary or systemic congestion [13]. Myocardial dysfunction is the most common cause of HF and can be divided into systolic, diastolic or both [10].
The diagnosis of HFpEF can be challenging, especially in the context of coexisting non-cardiac causes of dyspnoea, including obesity and chronic lung disease. There is also substantial phenotypic heterogeneity. Approximately three-quarters of unrecognized cases of HF are in patients with HFpEF [14], hence the true prevalence of HFpEF may be underestimated.
Epidemiology of HFpEF and HFrEF and associated outcomes
HFpEF accounts for ∼50–60% of HF cases in the community [15–17] and similar proportions are reported in hospitalized patients [18–20]. Compared with patients with HFrEF or HFmrEF, those with HFpEF are more often female and tend to be older [20, 21]. Table 1 highlights some clinical and epidemiological differences between HFrEF and HFpEF. Relative to HFrEF, the incidence and prevalence of HFpEF have been growing by 10% every 10 years [21, 22] and this widening gap is thought to reflect the ageing population and the growing prevalence of obesity, diabetes, hypertension and other conditions linked to the development of HFpEF [21]. The prevalence of atrial fibrillation, CKD and other non-cardiovascular comorbidities is higher in patients with HFpEF than in HFrEF. There is growing evidence for the central role of inflammation [19, 23] as well as coronary microvascular and macrovascular disease [24] in the pathogenesis of HFpEF.
Although some studies report that mortality rates for patients with HFpEF and HFrEF are similar [25–27], others report lower mortality in HFpEF [15, 17, 19, 28]. Consistent with the observation that the prevalence of non-cardiovascular comorbidities is higher in patients with HFpEF than it is in HFrEF, patients with HFpEF tend to have higher non-cardiovascular mortality than patients with HFrEF. Conversely, cardiovascular mortality is higher in patients with HFrEF. These findings are observed both in clinical trial populations [29] and in epidemiological studies [30]. Rates of hospitalization and the duration of hospitalizations [31] are similar for HFpEF and HFrEF and the decrease in quality of life in the two groups appears similar and substantial [32].
Diagnosis of HFpEF
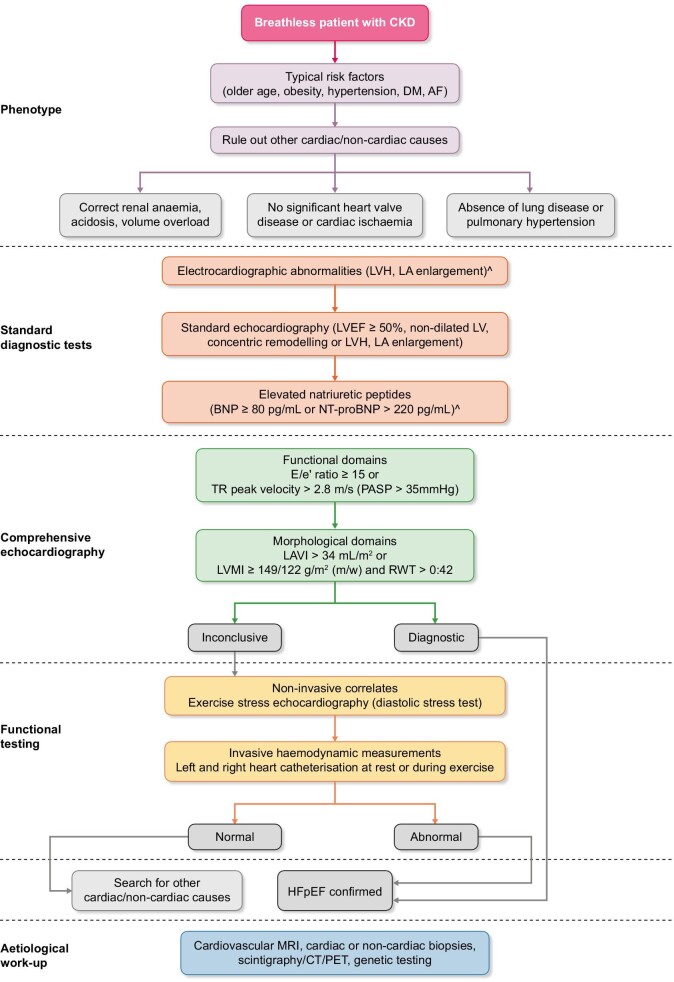
Diagnostic algorithms based on clinical assessment, electrocardiogram (ECG) and echocardiography have been produced by the Heart Failure Association (HFA) to diagnose HF in the presence of preserved ejection fraction and to document whether diastolic dysfunction is present. Fig. 1 highlights diagnostic algorithms for HFpEF, which have been tailored to the assessment of suspected HF in CKD.
FIGURE 1:
Diagnostic algorithm for approaching a patient with CKD and suspected HFpEF. DM, diabetes mellitus; AF, atrial fibrillation; TR, tricuspid regurgitation; PASP, pulmonary artery systolic pressure; RWT, relative wall thickness.
More advanced imaging techniques (stress testing, invasive haemodynamic measurements, cross-sectional imaging) may assist in the workup [33]. The first step is pretest assessment (‘P’) involving clinical assessment, ECG, basic echocardiography and functional/anatomical ischaemia testing (if indicated). This step excludes patients with significant coronary artery disease, valvular heart disease, chronic lung disease or anaemia. This is followed by a diagnostic workup (‘E’) centring on echocardiography and natriuretic peptide quantification. If ‘E’ is inconclusive, then F1 should be considered invasive or non-invasive stress testing for diastolic dysfunction. An aetiological search should be carried out (F2) [33, 34].
Echocardiography is advised to assess the breathless patient with a clinical suspicion of HF [35, 36]. Table 2 summarizes echocardiographic parameters and their relevance to diagnosing HFpEF, while Fig. 2 demonstrates imaging indicators of HFpEF graphically.
Table 2.
Summary of echocardiographic indices and their role in diagnosis of HFpEF and HFrEF
| Evaluation systolic function | Interpretation | |
|---|---|---|
| LVEF | Contour endocardial borders in end-diastole and systole on apical 4 and 2 chamber | Systolic function—preserved in HFpEF [1, 6, 33, 37] |
| Left ventricular end diastolic volume (LVEDV) | Contour LV endocardium in end diastole | Chamber size—normal or decreased in HFpEF |
| Global longitudinal strain | Contour endocardial borders in end diastole and systole on apical 4, 3 and 2 chamber | Systolic function—may be decreased in HFpEF [41, 42, 43]. |
| Diastolic function | ||
| E′ septum and E′ lateral wall | Tissue Doppler sample volume at septal and lateral basal LV regions | Early diastolic velocity of mitral annular motion—decreased in HFpEF |
| Mitral flow velocities (E/A) | PW Doppler sample volume at the tips of the mitral valve leaflets to gain peak velocity in early diastole (E wave), peak velocity in late diastole (A wave) | Progressive diastolic dysfunction: delayed relaxation (E/A <0.8), pseudonormalization (E/A 0.8–1.5) and restrictive pattern (E/A ≥2) [39, 40, 44] |
| E:e′ ratio | Ratio of peak mitral valve inflow velocity during early diastole (E wave) to the average septal/lateral mitral annular early diastolic velocity (e′) | Increased in HFpEF and when >15, diagnostic of increased LV filling pressures [39, 45, 46] |
| Peak TR jet velocity | Peak velocity through tricuspid valve during systole, measured by using continuous wave (CW) Doppler aligned over colour flow to obtain the highest velocity | TR velocity >2.8 m/s is an indirect marker of LV diastolic dysfunction and indicates increased pulmonary artery systolic pressure (PASP) [39] |
| LAVI, or the LA maximal volume indexed to body surface area | Measured using two orthogonal long-axis views | Increased in HFpEF [39, 47] |
| LVH [LVMI, regional wall thickness (RWT)] | LVMI uses 2-dimensional measurements in one view while RWT allows classification into concentric (>0.42) or eccentric hypertrophy (≤0.42). | May be increased in HFpEF |
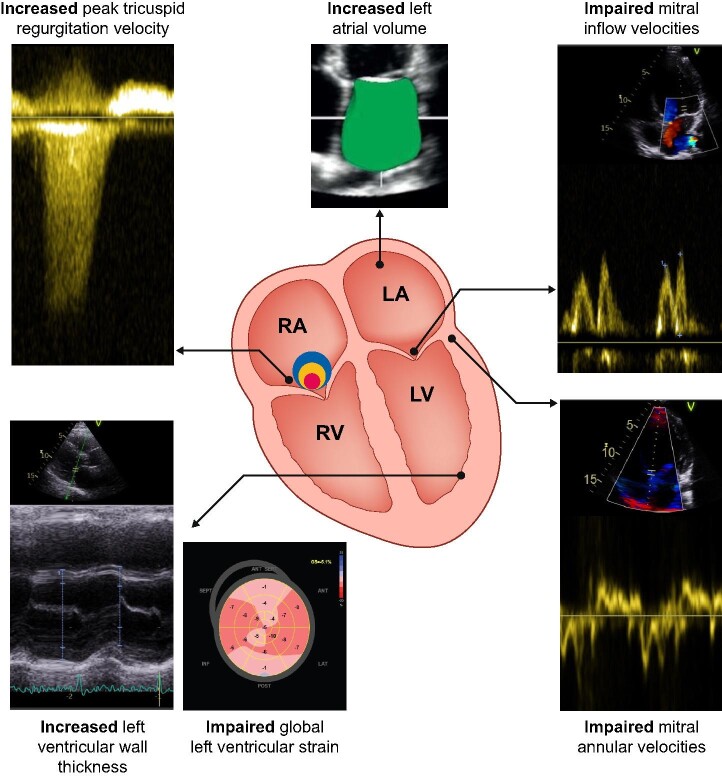
FIGURE 2:
Imaging findings non echocardiography to consider in assessment of HFpEF.
The LVEF should be quantified using three-dimensional [5] or Simpson's biplane and a cut-off of ≥50% (HFA) used to identify preserved LVEF [1, 6, 33, 37] and measurements made to ensure the left ventricle (LV) is not dilated (LV end-diastolic volume, LV end-diastolic dimension). The so-called HFpEF phenotype is a non-dilated LV, with preserved LVEF, LVH and a dilated left atrium (LA). These findings support but do not exclude HFpEF [38].
The additional echocardiographic measurements recommended to support a diagnosis of HFpEF [33] are identical to the recommendations for the identification of LV diastolic dysfunction [39, 40]—mitral flow velocities, mitral annular e′ velocity, E:e′ ratio, peak velocity of tricuspid regurgitation (TR) jet and left atrial maximum volume index (LAVI). Through a combination of measurements, one can identify if LV filling pressure is normal or abnormal and using cut-offs specified by the HFA can diagnose HFpEF if ≥5 points are present. Besides LVEF, global longitudinal strain (GLS) measurement is a more subtle measure of LV dysfunction [41] in HFpEF [42] and is a marker of adverse outcomes [43]. Diastolic metrics include the early diastolic velocity of mitral annular motion (e′) and in HFpEF the values are decreased. Mitral flow velocities (E/A) represent the pressure gradient between the left atrium (LA) and LV and are affected by changes in LV relaxation, LA pressure (E wave) and LV compliance and LA contractile function (A wave) [44]. The E:e′ ratio is an indirect estimate of mean pulmonary capillary wedge pressure [39] and associates with LV fibrosis [45]. Correlation between E:e′ and invasive filling pressure in HFpEF vary widely in correlation strength (r = 0.02–0.87) but has prognostic utility [46].
A peak tricuspid regurgitation jet >2.8 m/s is an indirect marker of LV diastolic dysfunction and indicates increased pulmonary artery systolic pressure (PASP) [39].
LAVI or the LA maximal volume indexed to body surface area is associated with LV filling pressures [39] and increases with worsening measures of diastolic dysfunction [47].
Diastolic stress testing
Patients with diastolic dysfunction may not be able to increase LV relaxation with exercise compared with healthy controls and hence have increased LV filling pressures to achieve the required cardiac output, i.e. exercise unmasks diastolic dysfunction. Guidelines are available for further investigation of suspected HFpEF [39, 48, 49] utilizing stress echocardiography.
Invasive measurements
Catheter measurements provide direct measurements of LV diastolic pressures and can be done at rest or with exercise [10, 50]. Utilizing left heart catheterization, demonstration of impaired LV relaxation at rest, tau (the time constant of LV relaxation τ > 48 ms) or elevated LV filling pressures at rest (LV end diastolic pressure ≥16 mmHg) confirms definite evidence of HFpEF [33]. With right heart catheterization, demonstration of elevated mean pulmonary capillary wedge pressure confirms HFpEF [10].
Cardiac magnetic resonance imaging (CMRI)
CMRI is a non-invasive diagnostic test for cardiac anatomy, function and pathology [33, 51, 52]. CMRI is more accurate at measuring LVEF, LV mass and LAVI [53] and has utility in the context of patients with suboptimal acoustic windows. CMRI can identify the presence of coronary syndromes by identifying subendocardial scarring and perfusion defects due to epicardial or microvascular disease [24]. Several pathologies can present with HFpEF. CMRI has utility in differentiating between these pathologies, including hypertrophic, infiltrative, restrictive cardiomyopathies [54, 55], myocarditis [51] and other aetiologies [10, 33].
HFpEF in patients with CKD
HF is highly prevalent in patients with CKD, with HFpEF accounting for half of these cases [56]. CKD and HF occur in a bidirectional fashion, with 55% of patients with HFrEF and HFpEF having CKD stage G3a or higher in a large meta-analysis [57]. The prevalence of HF increases with the severity of CKD and it is present in up to 44% of patients on haemodialysis (HD; 10% with HFpEF, 13% with HFrEF and 21% with unspecified) [58]. In patients with a kidney transplant, HF risk decreases compared with dialysis but the exact prevalence of HFpEF is unknown, as HF is most commonly reported using administrative data rather than diagnostic testing.
The diagnosis of HFpEF in patients with CKD is challenging. Typical HF manifestations overlap with symptoms of fluid overload due to sodium and water retention as a result of CKD itself. Serum brain natriuretic peptide (BNP) levels require different threshold levels in CKD and typical echocardiographic findings (such as concentric remodelling or LVH and left atrial enlargement) are common but might not represent an incident HF event [59].
CKD and HFpEF share common risk factors, such as older age, diabetes, hypertension and cardiovascular disease. Although they may evolve independently of one another, the presence of one condition appears to accelerate the presentation and progression of an other. Experimental studies suggest a common pathway of endothelial dysfunction and inflammation leading to both cardiac and renal fibrosis [60, 61]. More interestingly, several observations suggest CKD in the pathogenic processes leading to HFpEF [62]. In the Prevention of Renal and Vascular End-stage Disease (PREVEND) trial [63], baseline elevations in urinary albumin excretion and cystatin C (in addition to atrial fibrillation and female sex) increased the risk of new-onset HFpEF but not HFrEF. In another study from the Swedish Heart Failure Registry, CKD was more common in HFpEF than in HFrEF but less strongly associated with mortality, suggesting that it represents one of many comorbidities and may have more of a bystander role than it does in HFrEF [64]. Decreased eGFR is independently associated with an increased risk of all-cause mortality, cardiovascular mortality and hospitalization in patients with HFpEF [64–66].
Imaging the heart in CKD
Early studies with echocardiography showed that at the time of commencing renal replacement therapy, the majority of CKD patients had normal LVEF and cardiac dimensions, but 32% had LV dilation with preserved systolic function and 74% had concentric LVH [67, 68]. The association of CKD with abnormalities in cardiac structure is now well established [4]. Cardiac structural abnormalities common to CKD include LVH, ventricular dilation and myocardial fibrosis [6]. The development of these features, in association with decreased cardiac function, often without coronary artery disease, is consistent with cardiomyopathy specifically related to CKD—sometimes termed ‘uraemic cardiomyopathy’ [69]. Decreased LVEF is a late finding of uraemic cardiomyopathy [70], however, earlier abnormalities in myocardial function can be demonstrated by echocardiographic assessment of GLS. In CKD, echocardiographic assessment of GLS has been shown to associate with histologically confirmed myocardial fibrosis [71] and to be predictive of clinical outcomes [71, 72]. So echocardiography is of great utility in CKD. Echocardiography has limitations, requiring suitable windows for ultrasound and, in advanced CKD, dialysis associated fluid shifts may lead to overestimation of ventricular indices [73]. CMRI is an invaluable tool in the assessment of cardiac structural and functional changes in CKD.
CMRI can detect altered GLS in patients with CKD, which has been associated with outcomes [74]. In a study of HD patients, CMRI-GLS was associated with left ventricular mass index (LVMI) and negatively correlated with LVEF [75]. Improvements in cardiac strain have been demonstrated following kidney transplantation [76]. In contrast, acute deteriorations in cardiac strain, myocardial perfusion and segmental LV dysfunction were demonstrated during dialysis in an impressive study that scanned people during an HD session [77]. Gadolinium contrast-enhanced CMRI previously demonstrated gadolinium enhancement consistent with diffuse myocardial fibrosis that is associated with LVH in patients with kidney failure [78, 79]. Gadolinium contrast agents are no longer routinely used in advanced CKD because of their association with nephrogenic systemic fibrosis [80]. However, non-contrast CMRI techniques have rapidly developed. T1 mapping is a promising non-invasive surrogate marker of myocardial fibrosis. T1 times have been demonstrated to be abnormally long in HD populations when compared with controls [75, 81]. Although T1 times correlate with histological findings of myocardial fibrosis in other conditions, such as aortic stenosis [82], recent studies have increased uncertainty about the influence of myocardial oedema and fluid shifts on their reproducibility in people requiring dialysis [83].
The use of N-terminal pro-brain natriuretic peptide (NT-proBNP) for assessing cardiac function in CKD
The diagnosis of HFrEF has been made considerably easier by the use of serum levels of natriuretic peptides, in particular, NT-proBNP to indicate patients who are likely to have LV systolic dysfunction [84]. Current ESC guidelines suggest measurement of NT-proBNP in patients with symptoms of HF and proceeding to echocardiography if NT-proBNP is >125 pmol/L. Below this value, HF is unlikely [10]. Furthermore, NT-proBNP has prognostic value, with patients with the highest NT-proBNP at greatest risk of mortality, irrespective of the presence of HFrEF or HFpEF [85].
In patients with CKD not on dialysis, NT-proBNP (or BNP) can be used in the diagnosis of LV systolic dysfunction with similar accuracy to non-CKD controls, although higher diagnostic threshold values are required [86, 87]. It is less clear how well NT-proBNP performs as a diagnostic tool for HFpEF in the setting of CKD. There is clear evidence that NT-proBNP correlates with LV mass in people with CKD, so it is likely to highlight patients at risk of developing clinical HF [88], irrespective of whether there is a specific diagnostic cut point for NT-proBNP as a diagnostic tool in the breathless patient [89]. NT-proBNP has been shown to correlate with CKD-specific changes on CMRI [86]. In people on dialysis, BNP and NT-proBNP have prognostic value but a limited role in the diagnosis of HF [90, 91].
Fluid status assessment to improve HF outcomes in CKD
Bioimpedance body composition monitoring uses electrodes to pass electrical currents through the body to derive the proportions of fluid, lean tissue mass and fat. Two randomized controlled trials (RCTs) failed to show meaningful clinical benefit from using bioimpedance to guide fluid management in patients on peritoneal dialysis [92] and HD, respectively [93]. The BioImpedance Spectroscopy To maintain Renal Output (BISTRO) trial [94] will assess the impact of bioimpedance-guided fluid management on residual renal function in >500 dialysis patients and will also report on cardiovascular events as secondary outcomes.
Point-of-care lung ultrasound is a promising tool in the assessment of fluid status in CKD but does not yet have hard clinical outcome data supporting its use [95–97]. Lung ultrasound has been shown to more frequently detect interstitial fluid than traditional clinical assessment in dialysis cohorts [98], and these findings (presence of ‘B-lines’) are associated with increased mortality in this population [99]. In a trial of 71 dialysis patients, ultrasound-guided lung dry weight assessment led to improved blood pressure [100] and improved LV dimensions (but not function) [101]. In a separate trial in dialysis patients at high cardiovascular risk, lung ultrasound successfully relieved lung congestion compared with standard care, but without an improvement in the primary outcome (composite of all-cause death, non-fatal myocardial infarction and decompensated HF) [102].
RCTs addressing HFpEF not targeting CKD populations
There has been dramatic progress in improving outcomes in patients with HFrEF with renin–angiotensin system inhibitors (RASis), beta-blockers, mineralocorticoid receptor antagonists (MRAs) and most recently sodium-glucose cotransporter 2 inhibitors (SGLT2is), all based on large RCTs [103]. The same has not been the case for HFpEF. Table 3 summarizes the major clinical trials in HFpEF and clinical trials relevant to CKD. One of the first large RCTs to address HFpEF showed no benefit in outcomes with irbesartan compared with placebo [104], and similarly, no overall benefit was seen with spironolactone compared with placebo in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) [105]. There were major regional differences observed in TOPCAT, with a definite significant benefit with MRAs in many countries [106]. The angiotensin receptor–neprilysin inhibitor sacubitril–valsartan showed no statistically significant benefit compared with valsartan alone in a large RCT in HFpEF despite the benefit with this agent in HFrEF [107, 108].
Table 3.
Summary of significant RCTs in addressing HFpEF and/or surrogates of HFpEF in patients with CKD and/or treated with dialysis
| Trial [reference] | Population | N | Intervention | Duration | Primary outcome(s) | Comments |
|---|---|---|---|---|---|---|
| I-PRESERVE [104] | HF and LVEF ≥45% and creatinine <221 µmol/L | 4128 | Irbesartan 300 mg or placebo | Mean 49.5 months | Death and cardiovascular hospitalization | No improvement in primary outcome with irbesartan |
| TOPCAT [105] | HF and LVEF ≥45% and eGFR >30 mL/min/1.73 m2 | 3445 | Spironolactone 15–45 mg or placebo | Mean 3.3 years | Cardiovascular death and HF hospitalization | No difference in primary outcome between groups |
| PARAGON-HF [107] | HF and LVEF ≥ 45% and eGFR > 30 mL/min/1.73 m2 | 4822 | Sacubitril–valsartan or valsartan | Median 35 months | Cardiovascular death and HF hospitalization | No difference in primary outcome between groups |
| SOLOIST [109] | Patients with type 2 DM recently hospitalized with HF and eGFR >30 mL/min/1.73 m2 | 1222 | Sotagliflozin 200–400 mg or placebo | Median 9.0 months | Cardiovascular death and HF hospitalization | Significant benefit [HR 0.69 (95% CI 0.52–0.85)] with sotagliflozin. Benefit in subgroup LVEF ≥50% [HR 0.48 (95% CI 0.27–0.86)] |
| EMPEROR-Preserved [110] | Symptomatic HF and LVEF ≥40% and eGFR >20 mL/min/1.73 m2 | 5988 | Empagliflozin 10 mg or placebo | Median 26.2 months | Cardiovascular death and HF hospitalization | Significant benefit with empagliflozin [HR 0.79 (95% CI 0.69–0.90)] |
| EMPULSE [111] | HF regardless of LVEF and eGFR >20 mL/min/1.73 m2 | 530 | Empagliflozin 10 mg or placebo | 90 days | Clinical benefit, composite of death, HF events and QoL | Empagliflozin clinical benefit compared with placebo-stratified win ratio [1.36 (95% CI 1.09–1.68)] |
| SPIRO-CKD [137] | Non-diabetic CKD eGFR 30–89 mL/min/1.73 m2 | 154 | Spironolactone 25 mg or chlorthalidone 25 mg | 40 weeks | LVM on CMR | No difference in LVM between groups |
| CREDENCE [129] | Diabetes and eGFR 30–89 mL/min/1.73 m2 and albuminuria | 4401 | Canagliflozin 100 mg or placebo | Median 2.62 years | ESKD, doubling creatinine, death from renal or CV causes | Reduction in primary outcome with canagliflozin [HR 0.70 (95% CI 0.59–0.82)] HF hospitalization [HR 0.61 (95% CI 0.47–0.80)] |
| DAPA-CKD [130] | CKD eGFR 25–75 mL/min/1.73 m2 and albuminuria | 4304 | Dapagliflozin 10 mg or placebo | Median 2.4 years | ESKD, decline in eGFR ≥50%, death from renal or CV causes | Reduction in primary outcome with dapagliflozin [HR 0.61 (95% CI 0.51–0.72)], HF hospitalization [HR 0.51 (95% CI 0.34–0.76)] |
| SPIN-DIAL [115, 116] | Patients on HD | 129 | Spironolactone (12.5–50 mg) or placebo | 36 weeks | Assess safety and tolerability of intervention | No difference in diastolic function on echocardiography between groups |
| Hammer et al. [115] | Patients on HD | 97 | Spironolactone 50 mg or placebo | 40 weeks | LVMI on CMRI | No difference in LVMI between groups |
| PIVOTAL [118] | Patients on HD >3 months and treated with ESA | 2141 | Proactive or reactive intravenous iron | Median 2.1 years | Death, myocardial infarction, stroke, HF hospitalisation | Proactive iron fewer primary end point events that reactive iron. Significant reduction in HF events with proactive iron [HR 0.66 (95% CI 0.46–0.94)] |
| PRIMO [121] | CKD, mild-moderate LVH, LVEF ≥50%, eGFR 15–60 mL/min/1.73 m2 | 227 | Paricalcitol or no therapy | 48 weeks | LVMI on CMRI | No difference in LVMI between groups |
| EVOLVE [122] | Patients on HD with hyperparathyroidism | 3883 | Cinacalcet or placebo | Mean 21.2 months | Death or cardiovascular event | No difference in primary outcome between groups. Fewer non-atherosclerotic CV events (including HF, sudden death) with cinacalcet [HR 0.84 (95% CI 0.74–0.96)] |
| Dörr et al. [124] | Patients on HD with hyperparathyroidism and LVH | 62 | Intravenous etelcalcetide or alfacalcidol | 12 months | LVMI on CMRI | Significantly less progression LVMI with etelcalcetide |
| Odudu et al. [132] | Patients on HD | 73 | Dialysate 37°C or 0.5°C below body temperature | 12 months | LVEF at CMRI | No change in primary endpoint but decreased LVMI with cool dialysis |
| FHN [131] | Patients on HD | 245 | Six times HD/week versus three times HD/week | 12 months | Composite of mortality, QoL and LV mass | Decreased LV mass with frequent HD |
CV: cardiovascular; DM: diabetes mellitus; ESKD: end-stage kidney disease; QoL: quality of life.
The advent of SGLT2is offers a new therapeutic paradigm in HFpEF. Early insights into the potential benefit of SGLT2is in HFpEF came from the Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST) RCT where, compared with placebo, sotagliflozin was associated with a significantly lower incidence of the primary outcome of cardiovascular mortality or hospitalization for HF patients with baseline LVEF >50% but not those with LVEF <50% [109]. In the landmark Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) trial, empagliflozin was associated with a lower incidence of combined cardiovascular death or hospitalization for HF [110]. These results have prompted the licencing of empagliflozin as a treatment for HF in the USA regardless of LVEF and demonstrate that SGLT2is represent the first therapy to demonstrate efficacy in HFpEF. Finally, the EMPULSE trial demonstrated that in patients with acute HF, the addition of empagliflozin 10 mg daily was associated with a combined improvement in HF outcomes and/or quality of life compared with placebo, with no differences between subgroups with HFpEF or HFrEF [111].
Interventions to alleviate HFpEF in CKD
There are few trials that have directly investigated interventions for HFpEF specific to CKD. Most RCTs have employed changes in LV parameters, namely LVMI and LVEF, as an outcome that is a reasonable surrogate for the risk of developing HFpEF. A number of pharmacological interventions often used in HFrEF have been investigated in CKD. In dialysis patients with dilated cardiomyopathy, the beta-blocker carvedilol shows significant improvement in LV function and dilatation with an associated improvement in survival after 2 years [112]. A meta-analysis of five RCTs exploring the effect of RASis with angiotensin-converting enzyme inhibition or angiotensin receptor blockers on LVMI in HD patients demonstrated a significant reduction in LVMI {mean difference 15.4 g/m2 [95% confidence interval (CI) 7.4–23.3]; P < .001} but no statistical improvement in cardiovascular morbidity or mortality [113]. Spironolactone has also been shown to decrease LV mass in early CKD (stages 1 and 2) [114], but had no significant effect on LVMI in HD patients in one RCT or diastolic dysfunction in another similarly sized RCT [115, 116].
Other strategies that have theoretical benefits on cardiac outcomes have been tested. A number of studies have investigated the effect of anaemia correction in CKD. A meta-analysis of 15 studies including CKD patients demonstrated significant reductions in LVMI [−33.7 g/m2 (95% CI −49.4 to −16.1), P < .05] in patients with anaemia (haemoglobin <100 g/L at baseline) given erythropoietin and aiming for a level ≤ 120 g/L. The effect was not altered by dialysis status and did not show significant regression of LVMI in patients with milder anaemia.
In one of the few outcome studies to demonstrate a benefit on HF outcomes in dialysis, the Proactive IV IrOn Therapy in HaemodiALysis Patients (PIVOTAL) study demonstrated high-dose intravenous iron to be safe and efficacious [117], with post hoc analyses demonstrating significant improvements in HF events [hazard ratio (HR) 0.66 (95% CI 0.46–0.94)] [118]. An RCT investigating the effect of intravenous iron in patients with chronic HF and CKD demonstrated significant improvement in LV systolic and diastolic diameters and LV function. LV wall thickness was decreased in the treatment arm but did not reach statistical significance [119].
Dysregulation of bone mineral metabolism with subsequent hyperparathyroidism and vascular calcification are associated with LVH [120]. However, interventions to decrease its effect on cardiac function have demonstrated variable effects. The Paricalcitol Capsule Benefits in Renal Failure–Induced Cardiac Morbidity (PRIMO) study was an RCT investigating the effect of paricalcitol on cardiac structure and function in CKD patients and showed no effect on LVMI or other echocardiographic measures of diastolic function [121]. Post hoc analysis of the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial demonstrated use of the calcimimetic, cinacalcet for 64 months significantly decreased the risk of sudden cardiac death and HF [122]. Additionally, cinacalcet was associated with improvement of LVH on CMRI in a small RCT of 36 months, in HD patients [123]. More recently, suppression of fibroblast growth factor-23 by the calcimimetic etelcalcetide was associated with a significant reduction in CMRI assessed LVMI [124].
Other agents with putative benefits on LVMI have been tried with variable results. An RCT investigating the effect of allopurinol in CKD patients demonstrated that 9 months of treatment was statistically associated with regression of CMRI-measured LVMI [125]. An association was not shown in a similar study in HD patients [126]. In a different study looking at the effect of treatment with levocarnitine in patients with biochemical evidence of deficiency, replacement therapy for 12 months was associated with regression of LVH compared with no supplementation [127].
Although baseline echocardiography was not performed in the large Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trials, which showed the benefit of SGLT2is on cardiorenal outcomes in CKD, there was a clear benefit on HF incidence with SGLTis in both RCTs [128, 129]. Further subgroups analysis of DAPA-CKD demonstrated no difference in benefits of dapagliflozin in patients with or without prior HF [130].
In patients on dialysis, there is a need to test non-pharmacological interventions to improve LV changes by modifying the doses and characteristics of dialysis. The Frequent Haemodialysis Network daily trial showed that dialysis six times a week was associated with improved LV volumes, measured by CMRI, compared with thrice-weekly treatments [131]. In an RCT of patients starting maintenance HD, reducing dialysate temperature by 1.2 ± 0.3°C was also associated with slowing progression of LVH and echocardiographic markers of diastolic dysfunction [132], but it did not have a statistically significant effect on LV function. Successful kidney transplantation is associated with improved fluid status and blood pressure and is considered the gold standard treatment for kidney failure given its benefits on morbidity and mortality. Regression of LVH has been demonstrated after successful transplantation using echocardiography [133], but this may have been confounded by improved fluid status and its effect on measuring LV mass. Studies using CMRI have not demonstrated a similar significant reduction in LV mass after transplantation but have shown a decrease in CMRI-measured markers of myocardial fibrosis [134, 135]. Nevertheless, HF remains a significant clinical concern post-transplant, occurring in 10–20% of patients in the first year [136].
CONCLUSIONS
Although it has long been recognized that CKD is a risk factor for HF and that LVH is more prevalent in patients with advanced CKD than impaired LV function, it is now clear that HFpEF is one of, if not the dominant subtype of cardiovascular disease in patients with advanced CKD. Recent evidence from positive clinical trials in HFpEF demonstrated that progress is finally being made in improving outcomes in this condition. Greater awareness of HFpEF combined with widespread implementation of evidence-based therapy with SGLT2is should be a priority. Emerging evidence for adjunctive strategies such as lung ultrasound and bioimpedance specifically to address cardiovascular outcomes give further hope that we are entering a new era for addressing HFpEF in patients with CKD, including those requiring dialysis.
Acknowledgements
A.R. is personally funded by a Clinical Academic Training Fellowship from the Chief Scientist Office (Scotland; CAF/18/02). E.R. is supported by a post-doctoral Clinical Lectureship from the Chief Scientist Office (Scotland; PCL/03/18). The work was additionally funded by British Heart Foundation Clinical Research Training Fellowships to K.M. (FS/15/54/31639), R.P. and P.B.M.
Contributor Information
Patrick B Mark, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; Glasgow Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK.
Kenneth Mangion, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Alastair J Rankin, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Elaine Rutherford, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; Department of Nephrology, NHS Dumfries and Galloway, Dumfries, UK.
Ninian N Lang, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Mark C Petrie, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Sokratis Stoumpos, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; Glasgow Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK.
Rajan K Patel, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; Glasgow Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK.
CONFLICT OF INTEREST STATEMENT
P.B.M. reports personal fees and/or non-financial support from Vifor, Napp, Pharmacosmos, AstraZeneca, Astellas and Novartis and grants from Boehringer Ingelheim (paid to the University of Glasgow, his employing institution). N.N.L. reports personal fees and non-financial support from Roche, Pfizer, Novartis, AstraZeneca, Pharmacosmos and Vifor Pharma and grant support from Roche Diagnostics, AstraZeneca and Boehringer Ingelheim (paid to the University of Glasgow, his employing institution). M.C.P. reported receiving lecture fees from AstraZeneca and Eli Lilly and personal fees from AstraZeneca, Napp, Novo Nordisk and Takeda (paid to the University of Glasgow, his employing institution). All others report no conflicts of interest.
REFERENCES
- 1. Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde Met al.. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. . Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 3. Matsushita K, Coresh J, Sang Yet al. . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. lancet. Lancet Diabetes Endocrinol 2015; 3: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paoletti E, De Nicola L, Gabbai FBet al. . Associations of left ventricular hypertrophy and geometry with adverse outcomes in patients with CKD and hypertension. Clin J Am Soc Nephrol 2016; 11: 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. House AA, Wanner C, Sarnak Met al. . Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 1304–1317 [DOI] [PubMed] [Google Scholar]
- 6. Parfrey PS, Foley RN, Harnett JDet al. . Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 1996; 11: 1277–1285 [PubMed] [Google Scholar]
- 7. Rossignol P, Agarwal R, Canaud Bet al. . Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. Eur Heart J 2019; 40: 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konstantinidis I, Nadkarni GN, Yacoub Ret al. . Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern Med 2016; 176: 121–124 [DOI] [PubMed] [Google Scholar]
- 9. Damman K, Tang WH, Felker GMet al. . Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol 2014; 63: 853–871 [DOI] [PubMed] [Google Scholar]
- 10. McDonagh TA, Metra M, Adamo Met al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726 [DOI] [PubMed] [Google Scholar]
- 11. Koh AS, Tay WT, Teng THKet al. . A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail 2017; 19: 1624–1634 [DOI] [PubMed] [Google Scholar]
- 12. Kapoor JR, Kapoor R, Ju Cet al. . Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC: Heart Failure 2016; 4: 464–472 [DOI] [PubMed] [Google Scholar]
- 13. Bozkurt B, Coats AJ, Tsutsui Het al. . Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021; 23: 352–380 [Google Scholar]
- 14. van Riet EE, Hoes AWet al. . Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016; 18: 242–252 [DOI] [PubMed] [Google Scholar]
- 15. Vasan RS, Larson MG, Benjamin EJet al. . Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999; 33: 1948–1955 [DOI] [PubMed] [Google Scholar]
- 16. Kitzman DW, Gardin JM, Gottdiener JSet al. . Importance of heart failure with preserved systolic function in patients ≥65 years of age. Am J Cardiol 2001; 87: 413–419 [DOI] [PubMed] [Google Scholar]
- 17. Gottdiener JS, McClelland RL, Marshall Ret al. . Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002; 137: 631–639 [DOI] [PubMed] [Google Scholar]
- 18. Tribouilloy C, Rusinaru D, Mahjoub Het al. . Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J 2008; 29: 339–347 [DOI] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res 2019; 124: 1598–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lenzen MJ, Scholte op Reimer WJ, Boersma Eet al. . Differences between patients with a preserved and a depressed left ventricular function: a report from the Euroheart Failure Survey. Eur Heart J 2004; 25: 1214–1220 [DOI] [PubMed] [Google Scholar]
- 21. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 2020; 17: 559–573 [DOI] [PubMed] [Google Scholar]
- 22. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602 [DOI] [PubMed] [Google Scholar]
- 23. Mooney L, Goodyear CS, Chandra Tet al. . Clonal haematopoiesis of indeterminate potential: intersections between inflammation, vascular disease and heart failure. Clin Sci (Colch) 2021; 135: 991–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rush CJ, Berry C, Oldroyd KGet al. . Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021; 6: 1130–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bursi F, Weston SA, Redfield MMet al. . Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216 [DOI] [PubMed] [Google Scholar]
- 26. Owan TE, Hodge DO, Herges RMet al. . Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259 [DOI] [PubMed] [Google Scholar]
- 27. Bhatia RS, Tu JV, Lee DSet al. . Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355: 260–269 [DOI] [PubMed] [Google Scholar]
- 28. Kupari M, Lindroos M, Iivanainen AMet al. . Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med 1997; 241: 387–394 [DOI] [PubMed] [Google Scholar]
- 29. Solomon SD, Anavekar N, Skali Het al. . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744 [DOI] [PubMed] [Google Scholar]
- 30. Gerber Y, Weston SA, Redfield MMet al. . A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015; 175: 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loop MS, Van Dyke MK, Chen Let al. . Comparison of length of stay, 30-day mortality, and 30-day readmission rates in Medicare patients with heart failure and with reduced versus preserved ejection fraction. Am J Cardiol 2016; 118: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis EF, Lamas GA, O'Meara Eet al. . Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007; 9: 83–91 [DOI] [PubMed] [Google Scholar]
- 33. Pieske B, Tschöpe C, de Boer RAet al. . How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317 [DOI] [PubMed] [Google Scholar]
- 34. Smiseth OA, Morris DA, Cardim Net al. . Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2022; 23: e34–e61 [DOI] [PubMed] [Google Scholar]
- 35. Lang RM, Badano LP, Mor-Avi Vet al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–271 [DOI] [PubMed] [Google Scholar]
- 36. Lang RM, Badano LP, Tsang Wet al. . EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012; 25: 3–46 [DOI] [PubMed] [Google Scholar]
- 37. Galderisi M, Cosyns B, Edvardsen Tet al. . Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017; 18: 1301–1310 [DOI] [PubMed] [Google Scholar]
- 38. Reddy YNV, Carter RE, Obokata Met al. . Evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagueh SF, Smiseth OA, Appleton CPet al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314 [DOI] [PubMed] [Google Scholar]
- 40. Smiseth OA. Evaluation of left ventricular diastolic function: state of the art after 35 years with Doppler assessment. J Echocardiogr 2018; 16: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Voigt JU, Pedrizzetti G, Lysyansky Pet al. . Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 1–11 [DOI] [PubMed] [Google Scholar]
- 42. Kraigher-Krainer E, Shah AM, Gupta DKet al. . Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014; 63: 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morris DA, Ma X-X, Belyavskiy Eet al. . Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: a meta-analysis. Open Heart 2017; 4: e000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schirmer H, Lunde P, Rasmussen K. Mitral flow derived Doppler indices of left ventricular diastolic function in a general population. The Tromsø Study. Eur Heart J 2000; 21: 1376–1386 [DOI] [PubMed] [Google Scholar]
- 45. Kasner M, Westermann D, Lopez Bet al. . Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol 2011; 57: 977–985 [DOI] [PubMed] [Google Scholar]
- 46. Obokata M, Reddy YNV, Borlaug BA. The role of echocardiography in heart failure with preserved ejection fraction: what do we want from imaging? Heart Fail Clin 2019; 15: 241–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pritchett AM, Mahoney DW, Jacobsen SJet al. . Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 2005; 45: 87–92 [DOI] [PubMed] [Google Scholar]
- 48. Lancellotti P, Pellikka PA, Budts Wet al. . The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2016; 17: 1191–1229 [DOI] [PubMed] [Google Scholar]
- 49. Ha JW, Andersen OS, Smiseth OA. Diastolic stress test: invasive and noninvasive testing. JACC Cardiovasc Imaging 2020; 13: 272–282 [DOI] [PubMed] [Google Scholar]
- 50. Obokata M, Kane GC, Reddy YNet al. . Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017; 135: 825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferreira VM, Schulz-Menger J, Holmvang Get al. . Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176 [DOI] [PubMed] [Google Scholar]
- 52. Cosyns B, Lochy S, Luchian MLet al. . The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging 2020; 21: 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grothues F, Smith GC, Moon JCet al. . Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90: 29–34 [DOI] [PubMed] [Google Scholar]
- 54. Seferović PM, Polovina M, Bauersachs Jet al. . Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 553–576 [DOI] [PubMed] [Google Scholar]
- 55. Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 2 of a 2 part series. J Am Coll Cardiol 2018; 71: 1149–1166 [DOI] [PubMed] [Google Scholar]
- 56. Saran R, Robinson B, Abbott KCet al. . US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McAlister FA, Ezekowitz J, Tarantini Let al. . Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ Heart Fail 2012; 5: 309–314 [DOI] [PubMed] [Google Scholar]
- 58. United States Renal Data System . Annual Data Report 2016. Chapter 9: Cardiovascular Disease in Patients with ESRD. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 59. Stewart GA, Gansevoort RT, Mark PBet al. . Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int 2005; 67: 217–226 [DOI] [PubMed] [Google Scholar]
- 60. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271 [DOI] [PubMed] [Google Scholar]
- 61. Ter Maaten JM, Damman K, Verhaar MCet al. . Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail 2016; 18: 588–598 [DOI] [PubMed] [Google Scholar]
- 62. Fang JC. Heart failure with preserved ejection fraction: a kidney disorder? Circulation 2016; 134: 435–437 [DOI] [PubMed] [Google Scholar]
- 63. Smink PA, Lambers Heerspink HJ, Gansevoort RTet al. . Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular End-Stage Disease) study. Am J Kidney Dis 2012; 60: 804–811 [DOI] [PubMed] [Google Scholar]
- 64. Löfman I, Szummer K, Dahlström Uet al. . Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail 2017; 19: 1606–1614 [DOI] [PubMed] [Google Scholar]
- 65. Hillege HL, Nitsch D, Pfeffer MAet al. . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678 [DOI] [PubMed] [Google Scholar]
- 66. Smith DH, Thorp ML, Gurwitz JHet al. . Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes 2013; 6: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32(5 Suppl 3): S112–S119 [DOI] [PubMed] [Google Scholar]
- 68. Foley RN, Parfrey PS, Harnett JDet al. . Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995; 47: 186–192 [DOI] [PubMed] [Google Scholar]
- 69. Kunz K, Dimitrov Y, Muller Set al. . Uraemic cardiomyopathy. Nephrol Dial Transplant 1998; 13: 39–43 [DOI] [PubMed] [Google Scholar]
- 70. London GM, Pannier B, Guerin APet al. . Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol 2001; 12: 2759–2767 [DOI] [PubMed] [Google Scholar]
- 71. Kramann R, Erpenbeck J, Schneider RKet al. . Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J Am Soc Nephrol 2014; 25: 2351–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu YW, Su CT, Sung JMet al. . Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin J Am Soc Nephrol 2013; 8: 1564–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stewart GA, Foster J, Cowan Met al. . Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int 1999; 56: 2248–2253 [DOI] [PubMed] [Google Scholar]
- 74. Rankin AJ, Zhu L, Mangion Ket al. . Global longitudinal strain by feature-tracking cardiovascular magnetic resonance imaging predicts mortality in patients with end-stage kidney disease. Clin Kidney J 2021; 14: 2187–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rutherford E, Talle MA, Mangion Ket al. . Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int 2016; 90: 845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gong IY, Al-Amro B, Prasad GVRet al. . Cardiovascular magnetic resonance left ventricular strain in end-stage renal disease patients after kidney transplantation. J Cardiovasc Magn Reson 2018; 20: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Buchanan C, Mohammed A, Cox Eet al. . Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol 2017; 28: 1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mark PB, Johnston N, Groenning BAet al. . Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 2006; 69: 1839–1845 [DOI] [PubMed] [Google Scholar]
- 79. Schietinger BJ, Brammer GM, Wang Het al. . Patterns of late gadolinium enhancement in chronic hemodialysis patients. JACC Cardiovasc Imaging 2008; 1: 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Collidge TA, Thomson PC, Mark PBet al. . Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology 2007; 245: 168–175 [DOI] [PubMed] [Google Scholar]
- 81. Graham-Brown MP, March DS, Churchward DRet al. . Novel cardiac nuclear magnetic resonance method for noninvasive assessment of myocardial fibrosis in hemodialysis patients. Kidney Int 2016; 90: 835–844 [DOI] [PubMed] [Google Scholar]
- 82. Bull S, White SK, Piechnik SKet al. . Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013; 99: 932–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rankin AJ, Mangion K, Lees JSet al. . Myocardial changes on 3T cardiovascular magnetic resonance imaging in response to haemodialysis with fluid removal. J Cardiovasc Magn Reson 2021; 23: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McDonagh TA, Holmer S, Raymond Iet al. . NT-proBNP and the diagnosis of heart failure: a pooled analysis of three European epidemiological studies. Eur J Heart Fail 2004; 6: 269–273 [DOI] [PubMed] [Google Scholar]
- 85. Salah K, Stienen S, Pinto YMet al. . Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019; 105: 1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Arcari L, Engel J, Freiwald Tet al. . Cardiac biomarkers in chronic kidney disease are independently associated with myocardial edema and diffuse fibrosis by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2021; 23: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mark PB, Stewart GA, Gansevoort RTet al. . Diagnostic potential of circulating natriuretic peptides in chronic kidney disease. Nephrol Dial Transplant 2006; 21: 402–410 [DOI] [PubMed] [Google Scholar]
- 88. Bansal N, Hyre Anderson A, Yang Wet al. . High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol 2015; 26: 946–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vickery S, Price CP, John RIet al. . B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 2005; 46: 610–620 [DOI] [PubMed] [Google Scholar]
- 90. Cheng YJ, Yao FJ, Liu LJet al. . B-type natriuretic peptide and prognosis of end-stage renal disease: a meta-analysis. PLoS One 2013; 8: e79302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. deFilippi CR, Herzog CA. Interpreting cardiac biomarkers in the setting of chronic kidney disease. Clin Chem 2017; 63: 59–65 [DOI] [PubMed] [Google Scholar]
- 92. Tian N, Yang X, Guo Qet al. . Bioimpedance guided fluid management in peritoneal dialysis: a randomized controlled trial. Clin J Am Soc Nephrol 2020; 15: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sommerer C, Felten P, Toernig Jet al. . Bioimpedance analysis is not superior to clinical assessment in determining hydration status: a prospective randomized-controlled trial in a Western dialysis population. Hemodial Int 2021; 25: 380–390 [DOI] [PubMed] [Google Scholar]
- 94. Davies SJ, Caskey FJ, Coyle Det al. . Rationale and design of BISTRO: a randomized controlled trial to determine whether bioimpedance spectroscopy-guided fluid management maintains residual kidney function in incident haemodialysis patients. BMC Nephrol 2017; 18: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Koratala A, Ronco C, Kazory A. The promising role of lung ultrasound in assessment of volume status for patients receiving maintenance renal replacement therapy. Blood Purif 2020; 49: 643–646 [DOI] [PubMed] [Google Scholar]
- 96. Zoccali C. Lung ultrasound in the management of fluid volume in dialysis patients: potential usefulness. Semin Dial 2017; 30: 6–9 [DOI] [PubMed] [Google Scholar]
- 97. Covic A, Siriopol D, Voroneanu L. Use of lung ultrasound for the assessment of volume status in CKD. Am J Kidney Dis 2018; 71: 412–422 [DOI] [PubMed] [Google Scholar]
- 98. Torino C, Gargani L, Sicari Ret al. . The agreement between auscultation and lung ultrasound in hemodialysis patients: the LUST Study. Clin J Am Soc Nephrol 2016; 11: 2005–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zoccali C, Torino C, Tripepi Ret al. . Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 2013; 24: 639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Loutradis C, Sarafidis PA, Ekart Ret al. . The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: a randomized controlled trial. Kidney Int 2019; 95: 1505–1513 [DOI] [PubMed] [Google Scholar]
- 101. Loutradis C, Papadopoulos CE, Sachpekidis Vet al. . Lung ultrasound-guided dry weight assessment and echocardiographic measures in hypertensive hemodialysis patients: a randomized controlled study. Am J Kidney Dis 2020; 75: 11–20 [DOI] [PubMed] [Google Scholar]
- 102. Zoccali C, Torino C, Mallamaci Fet al. . A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int 2021; 100: 1325–1333 [DOI] [PubMed] [Google Scholar]
- 103. McDonagh TA, Metra M, Adamo Met al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021; 42: 3599–372634447992 [Google Scholar]
- 104. Massie BM, Carson PE, McMurray JJet al. . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467 [DOI] [PubMed] [Google Scholar]
- 105. Pitt B, Pfeffer MA, Assmann SFet al. . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392 [DOI] [PubMed] [Google Scholar]
- 106. Pfeffer MA, Claggett B, Assmann SFet al. . Regional variation in patients and outcomes in the Treatment Of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42 [DOI] [PubMed] [Google Scholar]
- 107. Solomon SD, McMurray JJV, Anand ISet al. . Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620 [DOI] [PubMed] [Google Scholar]
- 108. McMurray JJV, Packer M, Desai ASet al. . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004 [DOI] [PubMed] [Google Scholar]
- 109. Bhatt DL, Szarek M, Steg PGet al. . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117–128 [DOI] [PubMed] [Google Scholar]
- 110. Anker SD, Butler J, Filippatos Get al. . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451–1461 [DOI] [PubMed] [Google Scholar]
- 111. Voors AA, Angermann CE, Teerlink JRet al. . The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022; 28: 568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cice G, Ferrara L, D'Andrea Aet al. . Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol 2003; 41: 1438–1444 [DOI] [PubMed] [Google Scholar]
- 113. Tai DJ, Lim TW, James MTet al. . Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol 2010; 5: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Edwards NC, Steeds RP, Stewart PMet al. . Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol 2009; 54: 505–512 [DOI] [PubMed] [Google Scholar]
- 115. Hammer F, Malzahn U, Donhauser Jet al. . A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int 2019; 95: 983–991 [DOI] [PubMed] [Google Scholar]
- 116. Charytan DM, Himmelfarb J, Ikizler TAet al. . Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int 2019; 95: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Macdougall IC, Bhandari S, White Cet al. . Intravenous iron dosing and infection risk in patients on hemodialysis: a prespecified secondary analysis of the PIVOTAL trial. J Am Soc Nephrol 2020; 31: 1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jhund PS, Petrie MC, Robertson Met al. . Heart failure hospitalization in adults receiving hemodialysis and the effect of intravenous iron therapy. JACC Heart Fail 2021; 9: 518–527 [DOI] [PubMed] [Google Scholar]
- 119. Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ 2015; 24: 686–695 [DOI] [PubMed] [Google Scholar]
- 120. Patel RK, Oliver S, Mark PBet al. . Determinants of left ventricular mass and hypertrophy in hemodialysis patients assessed by cardiac magnetic resonance imaging. Clin J Am Soc Nephrol 2009; 4: 1477–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Thadhani R, Appelbaum E, Pritchett Yet al. . Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307: 674–684 [DOI] [PubMed] [Google Scholar]
- 122. Wheeler DC, London GM, Parfrey PSet al. . Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc 2014; 3: e001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Eddington H, Chinnadurai R, Alderson Het al. . A randomised controlled trial to examine the effects of cinacalcet on bone and cardiovascular parameters in haemodialysis patients with advanced secondary hyperparathyroidism. BMC Nephrol 2021; 22: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dörr K, Kammer M, Reindl-Schwaighofer Ret al. . Randomized trial of etelcalcetide for cardiac hypertrophy in hemodialysis. Circ Res 2021; 128: 1616–1625 [DOI] [PubMed] [Google Scholar]
- 125. Kao MP, Ang DS, Gandy SJet al. . Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2011; 22: 1382–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rutherford E, Ireland S, Mangion Ket al. . A randomized, controlled trial of the effect of allopurinol on left ventricular mass index in hemodialysis patients. Kidney Int Rep 2021; 6: 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Higuchi T, Abe M, Yamazaki Tet al. . Levocarnitine improves cardiac function in hemodialysis patients with left ventricular hypertrophy: a randomized controlled trial. Am J Kidney Dis 2016; 67: 260–270 [DOI] [PubMed] [Google Scholar]
- 128. Heerspink HJL, Stefansson BV, Correa-Rotter Ret al. . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 129. Perkovic V, Jardine MJ, Neal Bet al. . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 130. McMurray JJV, Wheeler DC, Stefánsson BVet al. . Effects of dapagliflozin in patients with kidney disease, with and without heart failure. JACC Heart Fail 2021; 9: 807–820 [DOI] [PubMed] [Google Scholar]
- 131. Chan CT, Greene T, Chertow GMet al. . Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clin J Am Soc Nephrol 2013; 8: 2106–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Odudu A, Eldehni MT, McCann GPet al. . Randomized controlled trial of individualized dialysate cooling for cardiac protection in hemodialysis patients. Clin J Am Soc Nephrol 2015; 10: 1408–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Montanaro D, Gropuzzo M, Tulissi Pet al. . Effects of successful renal transplantation on left ventricular mass. Transplant Proc 2005; 37: 2485–2487 [DOI] [PubMed] [Google Scholar]
- 134. Contti MM, Barbosa MF, del Carmen Villanueva Mauricio Aet al. . Kidney transplantation is associated with reduced myocardial fibrosis. A cardiovascular magnetic resonance study with native T1 mapping. J Cardiovasc Magn Reson 2019; 21: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Patel RK, Mark PB, Johnston Net al. . Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol 2008; 3: 1807–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lentine KL, Schnitzler MA, Abbott KCet al. . De novo congestive heart failure after kidney transplantation: a common condition with poor prognostic implications. Am J Kidney Dis 2005; 46: 720–733 [DOI] [PubMed] [Google Scholar]
- 137. Edwards NC, Price AM, Mehta Set al. . Effects of spironolactone and chlorthalidone on cardiovascular structure and function in chronic kidney disease: a randomized, open-label trial. Clin J Am Soc Nephrol 2021; 16: 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]