ABSTRACT
Oral commensal streptococci are primary colonizers of the oral cavity. These streptococci produce many adhesins, metabolites, and antimicrobials that modulate microbial succession and diversity within the oral cavity. Often, oral commensal streptococci antagonize cariogenic and periodontal pathogens such as Streptococcus mutans and Porphyromonas gingivalis, respectively. Mechanisms of antagonism are varied and range from the generation of hydrogen peroxide, competitive metabolite scavenging, the generation of reactive nitrogen intermediates, and bacteriocin production. Furthermore, several oral commensal streptococci have been shown to alter the host immune response at steady state and in response to oral pathogens. Collectively, these features highlight the remarkable ability of oral commensal streptococci to regulate the structure and function of the oral microbiome. In this review, we discuss mechanisms used by oral commensal streptococci to interact with diverse oral pathogens, both physically and through the production of antimicrobials. Finally, we conclude by exploring the critical roles of oral commensal streptococci in modulating the host immune response and maintaining health and homeostasis.
KEYWORDS: oral commensal streptococci, bacterial competition, Streptococcus parasanguinis, adhesins, hydrogen peroxide, nitrite, polymicrobial interaction
INTRODUCTION
The oral cavity is remarkably heterogeneous. The teeth, gingiva, and tongue are composed of vastly different tissue types, and nutrients are both transient and variable. As an auxiliary opening for the respiratory system, both oxygen and carbon dioxide are present at different concentrations depending on host behavior and location within the oral cavity. This diverse environment is conducive to microbial colonization, allowing many different microbes to colonize various regions of the oral cavity. Indeed, more than 700 bacterial species, 100 fungal species, and hundreds of viral genotypes are found within the human oral cavity (1–4). Microbe-host interactions within the oral cavity have been well documented, with substantial efforts being made to characterize diseases of bacterial etiology such as caries, endodontic infections, gingivitis, and periodontitis. While single-pathogen–single-disease models, such as Koch’s postulates, explain some diseases within the oral cavity, these models do not incorporate microbe-microbe interactions that can dampen or exacerbate disease progression.
To accommodate the impact of microbe-microbe interactions, Sigmund Socranksy developed a complex theory where bacteria within the oral cavity were broadly grouped into green, orange, and red complex organisms (5, 6). Green complex organisms such as streptococci are early colonizers of the mouth that bind well to the tooth pellicle and produce antimicrobial agents, such as hydrogen peroxide, that prevent colonization by other bacteria (7–15). Bacteria within the orange complex are so-called “bridging species” that physically associate with both green and red complex members (6, 16). Bacteria within the red complex were thought to be overt pathogens that cause disease (6). Interestingly, subsequent microbiome studies indicate that red complex pathogens are present in people free of disease in the oral cavity, illustrating that this complex theory does not integrate all factors that lead to disease (17). Thus, the polymicrobial synergy-dysbiosis model was put forth, which incorporates the importance of the microbial community in health and disease (18). Polymicrobial interactions within the oral cavity are exceedingly complex, with interactions characterized as physical, chemical, synergistic, or antagonistic. Of particular importance, however, are the interactions between oral streptococci and other bacteria. Oral commensal streptococci play a variety of roles within the oral cavity as both early colonizers and accessory pathogens, in which case they may enhance the colonization or metabolic functions of pathogens under certain conditions. As the largest bacterial group within the oral cavity, streptococci are a significant component of oral health (19, 20).
Streptococci are heterogeneous, with some streptococci being cariogenic, others being accessory pathogens of periodontitis, others inhibiting colonization by pathogens, and some that are known to modulate the host immune response. The genus Streptococcus is large, with approximately 72 identified species, of which at least 36 have been isolated from the human body. Of those within the human body, 18 species reside within the oral cavity (Table 1). The genus has been broken into 8 groups, mitis, sanguinis, anginosus, salivarius, downei, mutans, pyogenic, and bovis, although taxonomic changes are continuous where nomenclature, species, and subspecies designations have been variable for the genus (19, 21–28). The genus harbors many commensal organisms as well as pathogens such as S. pneumoniae, S. pyogenes, and S. agalactiae. It is important to note that many oral streptococci that are regarded as commensals have the ability to cause disease, namely, bacteremia and endocarditis, which is seen with several oral species such as S. mitis, S. parasanguinis, and S. salivarius.
TABLE 1.
Classification and species of human-associated streptococci
| Group | Species | Body site(s) | Reference(s) |
|---|---|---|---|
| Anginosus | S. anginosus | Oral cavity, GIb | 160, 161 |
| S. constellatus | Oral cavity | 162, 163 | |
| S. intermedius | Oral cavity | 160, 164 | |
| Bovis | S. equinus | GI | 161 |
| S. gallolyticus (subsp. bovis) | Blood | 165 | |
| S. infantarius | GI | 166, 167 | |
| Downei | S. criceti | Oral cavity | 168 |
| S. downei | Oral cavity | 169 | |
| Mitis | S. australis | Oral cavity | 170 |
| S. dentisani | Oral cavity | 28, 171 | |
| S. infantis | Oral cavity | 172 | |
| S. massiliensis | Blood | 173 | |
| S. mitis | Oral cavity | 161, 174 | |
| S. oralis | Oral cavity | 174, 175 | |
| S. parasanguinis | Oral cavity | 176 | |
| S. peroris | Oral cavity | 172 | |
| S. pneumoniae | Nasopharynx | 177 | |
| S. pseudopneumoniae | Blood | 178 | |
| Mutans | S. mutans | Oral cavity | 179 |
| S. sobrinus | Oral cavity | 180 | |
| Pyogenic | S. agalactiae | Urogenital | 181 |
| S. dysgalactiae | Urogenital | 182 | |
| S. equi | Urogenital | 183 | |
| S. iniae | Zoonotic (cellulitis) | 184 | |
| S. porcinus | Urogenital | 185 | |
| S. pseudoporcinus | Urogenital | 186 | |
| S. pyogenes | Throat | 187 | |
| S. urinalis | Urogenital | 188 | |
| Salivarius | S. salivarius | Oral cavity | 161 |
| S. vestibularis | Oral cavity | 189 | |
| Sanguinis | S. cristatus (S. oligofermentans)a | Oral cavity | 190, 191 |
| S. gordonii | Oral cavity | 174 | |
| S. sanguinis | Oral cavity | 174, 192 | |
| Unresolved | S. acidominimus | Blood | 193 |
| S. pluranimalium | Blood | 194 | |
| S. sinensis | Heart | 195 | |
| S. suis | Zoonotic (meningitis) | 196 | |
S. oligofermentans is a later synonym of S. cristatus.
GI, gastrointestinal.
Given the heterogeneity of tissue, metabolites, and nutrients within the oral cavity, the microbes that reside in the oral cavity are similarly heterogeneous and vary by site within the oral cavity (29, 30). Additionally, person-to-person variation is relatively high within the oral cavity, with some harboring significantly more or fewer microbial species than others. Despite this, core oral microbiota members have been identified. Hall et al. identified 26 core microbes that were present across all tissue sites, time points, and individuals sampled, indicating that while the community composition within the oral cavity can be transient, certain microbes are ubiquitously present (29). Importantly, mitis/oralis group streptococci are constituents of the core oral microbiota.
STREPTOCOCCI OF THE ENAMEL
Streptococci have been shown to be primary colonizers of the tooth surface. These colonizers adhere to the acquired salivary pellicle, which is a thin proteinaceous film that is important for calcium and phosphorous resorption, protects against acid erosion, and initiates the adherence of oral microbes to the tooth surface (10, 31, 32). S. sanguinis, S. gordonii, S. parasanguinis, S. mitis, and S. oralis have been shown to bind to the salivary pellicle that forms on the tooth surface with high affinity (33–39). These streptococci possess adhesins that enable fast and stable attachment to the tooth surface. Once bound, these primary streptococci can act as anchor points for secondary colonizers to bind (19, 40). Importantly, oral streptococci are hardy competitors and are capable of competitive exclusion through the production of hydrogen peroxide and various bacteriocins, nutrient uptake, and competitive binding (41). Thus, the prevalence of certain oral streptococci on the tooth surface can select for secondary colonizers that may shift the biosis of the oral cavity.
The species of streptococci that are present in the biofilm on the enamel surface can dramatically alter tooth health. Cariogenic streptococci such as Streptococcus mutans and S. sobrinus are capable of binding to other microbes, including other streptococci (42, 43). S. mutans establishes binding through the production of glucan, an extremely sticky exopolysaccharide that is produced by extracellular glucosyltransferase (Gtf) enzymes. S. mutans is very aciduric and acidogenic, and this acid lowers the pH and promotes enamel degradation, leading to caries (44, 45). Interestingly, the presence of commensal streptococci has been shown to inhibit S. mutans in both a hydrogen peroxide- and peroxynitrite-dependent manner (46, 47).
In addition to the direct antagonism of S. mutans through hydrogen peroxide activity, commensal-generated hydrogen peroxide has also been shown to inhibit Candida albicans, a fungal pathobiont that synergizes with S. mutans and is widely found in carious lesions with S. mutans during early childhood caries (48, 49). Falsetta et al. first demonstrated that (i) dual-species biofilms with S. mutans and C. albicans are composed of increased biomass compared to the single-species biofilms alone; (ii) C. albicans increases the abundance of S. mutans cells, and C. albicans mannans provide sites for S. mutans GtfB binding and activity; (iii) coculture with C. albicans induces the expression of S. mutans virulence factors such as Gtfs; and (iv) dual infection in a rat model leads to increased caries compared to single-species-infected animals (48). Interestingly, oral commensal streptococci such as S. parasanguinis can inhibit S. mutans and C. albicans synergy independently of both contact and hydrogen peroxide (50). Huffines and Scoffield demonstrated that S. parasanguinis can restrict sucrose utilization by S. mutans, perhaps through inhibiting GtfB binding to C. albicans. Additionally, metabolomics indicated that S. parasanguinis alters metabolites available to both S. mutans and C. albicans, further underscoring that oral commensal streptococci can restrict colonization by other microbes through metabolic competition (50).
STREPTOCOCCI ON THE TONGUE AND GINGIVA
Streptococci have been shown to adhere to the tongue, with S. parasanguinis and S. salivarius being the most dominant streptococci. Both S. parasanguinis and S. salivarius have been cultured from tongue scrape samples. S. mitis can be found on both the tongue surface and the enamel (51). Interestingly, regular tongue scraping has been shown to reduce S. mutans prevalence in saliva (52, 53). Furthermore, streptococci that colonize the tongue routinely mediate interactions with C. albicans, a pathobiont that causes oral candidiasis (54). In a mouse model of oral candidiasis, S. salivarius K12 inhibits hyphal growth and the adhesion of C. albicans to mucosal surfaces (55). However, there are instances where streptococci can potentiate C. albicans pathogenicity. For example, S. oralis has been demonstrated to enhance oral candidiasis disease severity and the dissemination of C. albicans in oral mucosal models (56). Similarly, S. oralis, S. sanguinis, and S. gordonii display enhanced biofilm development during coculture with C. albicans (57). These observations illustrate that interactions between commensal streptococci and other resident oral microbes are often synergistic and dynamic.
Although streptococci are prevalent on the tongue, they have not been demonstrated to bind to gingival cells in vivo; however, they are present in gingival swabs in high numbers (29). Additionally, when cultured with gingival cells, S. sanguinis grows to high numbers and forms a robust biofilm that can complex with other microbes such as Corynebacterium durum and Porphyromonas gingivalis (58). Interestingly, oral commensal streptococci have also been shown to be accessory gingival pathogens that can synergize with the gingival and periodontal pathogens P. gingivalis and Fusobacterium nucleatum. S. gordonii has been shown to bind P. gingivalis through two mechanisms: through surface polypeptides (SspA and SspB) on S. gordonii that bind P. gingivalis short fimbriae (59) and through glyceraldehyde 3-phosphate dehydrogenase on S. gordonii binding FimA on the long fimbriae of P. gingivalis (60). These instances of adherence have been demonstrated both in biofilms and in invasive disease where S. gordonii has been coisolated with P. gingivalis from periodontal abscesses (59, 61). The presence of S. gordonii has been shown to upregulate P. gingivalis adhesin expression through streptococcal 4-aminobenzoate/para-amino benzoic acid (pABA), and the presence of S. gordonii increases periodontal damage (62, 63). Conversely, S. gordonii has also been shown to alter cell signaling to modulate the host immune response to P. gingivalis (64, 65). Both S. sanguinis and S. gordonii have been found to physically associate with F. nucleatum to form so-called corncob aggregates (66, 67). F. nucleatum is a bridging species that is known to adhere to both early commensal streptococci as well as periopathogens such as P. gingivalis, Treponema denticola, and Tannerella forsythia (68). Interestingly, the presence of F. nucleatum and its production of autoinducer-2 (AI-2) have been shown to increase biofilm formation by S. gordonii as well as P. gingivalis, indicating that F. nucleatum can modulate the activity of both streptococcal commensals as well as periodontal pathogens (69, 70). Further, cross-feeding between oral commensal streptococci and periodontal pathogens has also been shown; for example, Aggregatibacter actinomycetemcomitans is capable of metabolizing streptococcus-generated lactic acid (71).
MODULATION OF pH IN THE ORAL CAVITY
As the formation of caries is acid dependent, the role of oral commensal streptococci in altering the pH within the oral cavity and on the tooth surface has been explored. pH is site dependent within the oral cavity, where the pH in healthy individuals ranges from 6.28 to 7.34 on the buccal mucosa and the hard palate, respectively (72). While the mechanism behind these differences in pH between these sites is unknown, proximity to salivary glands, the thickness of saliva that accumulates at each of these sites, and the amount of local blood flow may all play roles. In contrast to healthy sites within the oral sites, carious lesions have a pH value of between 5.5 and 6.2 (44). Many oral streptococci are capable of converting arginine into ammonia via the arginine deiminase system (ADS), which raises the local pH of biofilms and facilitates the antagonism of S. mutans. Arginolytic streptococci such as Streptococcus sp. strain A12, S. salivarius, S. gordonii, and S. dentisani have been shown to raise the local pH, thus restricting acid-induced enamel demineralization that leads to tooth decay (73, 74). Due to the pH-increasing capabilities of these ADS-harboring streptococci, their utility as potential probiotics has been examined. Interestingly, ADS activity is highly heterogeneous among oral commensal species, and high activity does not always correlate with increased S. mutans antagonism (75). Nonetheless, these studies reveal that probing antimicrobial or anti-infection mechanisms by commensal streptococci offers a unique opportunity for developing effective probiotics that target cariogenic pathogens.
EFFECTS OF STREPTOCOCCUS-PRODUCED HYDROGEN PEROXIDE ON MICROBIAL COMMUNITIES
Given that oral commensal streptococci are among the first bacteria to colonize the salivary pellicle and are abundant throughout the oral cavity over time and across individuals, understanding their roles in polymicrobial interactions within the oral cavity is paramount to understanding oral health and disease. One major mechanism through which oral commensal streptococci modulate the activity and colonization of other microbes is through the production of hydrogen peroxide. A potent, nonspecific antimicrobial, hydrogen peroxide is a reactive oxygen species (ROS) that can damage microbial DNA through the formation of hydroxyl radicals (41). When hydrogen peroxide reacts with iron III, extremely reactive hydroxyl radicals are formed through the Fenton reaction. These radicals can damage proteins, reduce protein function, and damage DNA to kill bacterial cells. While it is known that oral commensal streptococci produce hydrogen peroxide, hydrogen peroxide has never been detected in saliva, primarily due to ROS scavengers such as thiocyanate, hypothiocyanite, salivary peroxidase, and/or myeloperoxidase, all of which have been shown to be produced in the oral cavity (76, 77). Interestingly, while hypothiocyanite can sequester ROS, its combination with ROS and salivary peroxidase is also antimicrobial (78). While these ROS scavengers are present in saliva, it is clear that there are likely local effects within the biofilm and in proximity to streptococci through which hydrogen peroxide is produced at concentrations high enough to inhibit colonization and/or growth of other microbes. This phenomenon was first described by Thompson and Johnson where inhibition of Corynebacterium by oral streptococci was noted (13). Subsequent studies have shown robust hydrogen peroxide production by streptococci grown in biofilms, where Liu et al. found concentrations of up to 1.4 mM 100 μm away from S. gordonii biofilms (79). Hydrogen peroxide production is known to shape the microbial community within the oral cavity and has even been shown to inhibit nonoral bacteria such as Escherichia coli from colonizing the oral cavity in the first place (80, 81). Furthermore, E. coli lipopolysaccharide (LPS) was shown to induce hydrogen peroxide production, indicating that the presence of specific microbes or microbial components can influence hydrogen peroxide production, suggesting that oral commensal streptococci can fine-tune hydrogen peroxide production to ward off foreign invaders. Additionally, the presence of hydrogen peroxide can induce extracellular DNA (eDNA) release from these streptococci, contributing to both biofilm formation and the uptake and exchange of DNA, allowing dynamic genetic and physiological changes on the community level (82).
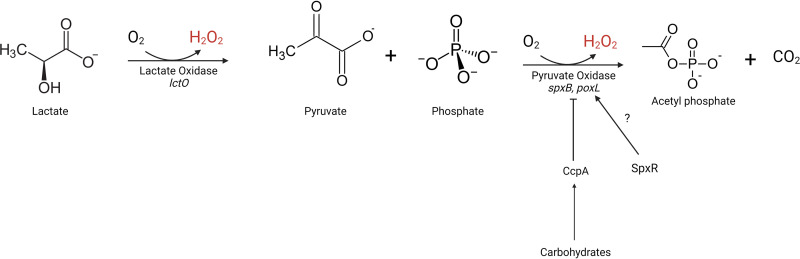
All streptococci within the mitis group, which is the most prevalent streptococcal group within the oral cavity, produce hydrogen peroxide. Hydrogen peroxide production is largely attributed to pyruvate oxidase activity through which pyruvate, oxygen, and inorganic phosphate are converted to acetyl phosphate, hydrogen peroxide, and carbon dioxide (Fig. 1). However, S. oligofermentans uses lactate oxidase for hydrogen peroxide production. Lactate oxidase catalyzes the conversion of lactate, oxygen, and l-amino acids into pyruvate and hydrogen peroxide, and this alternate pathway of hydrogen peroxide production has been demonstrated to influence other microbes (83, 84) (Fig. 1).
FIG 1.
Metabolic pathway for hydrogen peroxide production in streptococci. Lactate is converted into pyruvate through lactate oxidase activity, producing hydrogen peroxide. Pyruvate is converted into acetyl phosphate through pyruvate oxidase activity, producing hydrogen peroxide (S. gordonii, S. sanguinis, S. parasanguinis, and S. pneumoniae). The regulation of pyruvate oxidase is species dependent. S. gordonii represses pyruvate oxidase expression through catabolite control protein A (CcpA) in the presence of carbohydrates such as galactose, maltose, and lactose. S. sanguinis basally represses pyruvate oxidase activity via CcpA regardless of the carbohydrates present. Pyruvate oxidase has been shown to be regulated by SpxR in S. pneumoniae. The role of SpxR in oral commensal streptococci has not been elucidated.
REGULATION OF HYDROGEN PEROXIDE PRODUCTION
The mitis and sanguinis streptococcal groups are heterogeneous, with species within the groups having varied conservation of metabolic and enzymatic processes. While hydrogen peroxide production has not been explored in every streptococcus capable of producing hydrogen peroxide within the oral cavity, efforts to understand the mechanisms and regulation of hydrogen peroxide production in S. gordonii, S. mitis, S. oralis, S. sobrinus, S. oligofermentans, S. sanguinis, and S. parasanguinis have been undertaken. Hydrogen peroxide production has been found to differ not only among strains within species but also in solid versus liquid media, with different carbohydrate sources, and with various oxygen concentrations. García-Mendoza et al. found that S. sanguinis and S. oralis produced more hydrogen peroxide on solid media than on liquid media and that the addition of carbohydrates to base media reduced hydrogen peroxide production in general (85). Additionally, multiple groups have demonstrated that the addition of carbohydrates to media reduces hydrogen peroxide production in S. gordonii (86, 87). Zheng et al. demonstrated that this is due to catabolite repression in S. gordonii, where the pyruvate oxidase gene’s promoter region has a binding site for catabolite control protein A (CcpA), resulting in the reduced expression of pyruvate oxidase in the presence of many carbohydrates (88). This mechanism has also been demonstrated in S. pneumoniae, where there is an increase in hydrogen peroxide production during colonization of the nasopharynx, where carbohydrates are scarce, and a decrease in hydrogen peroxide production in the blood, where glucose is readily available (89, 90). Pyruvate oxidase expression is not modulated by the presence of carbohydrates in S. sanguinis; however, the deletion of CcpA increases pyruvate oxidase expression and hydrogen peroxide production (91). Additionally, SpxA1, a global regulator of S. sanguinis, has been found to positivity regulate pyruvate oxidase and is crucial for tolerance to both oxidative and acid stress (92). However, the regulation of pyruvate oxidase in S. pneumoniae has been better characterized where the regulator of pyruvate oxidase is SpxR (93). The regulation and behavior of SpxR as well as pyruvate oxidase remain to be elucidated in the majority of oral streptococcal species. To resist hydrogen peroxide damage, oral commensal streptococci secrete pyruvate through Nox, an NADH dehydrogenase, to (i) reduce the substrates available to generate hydrogen peroxide and (ii) sequester ROS (94).
HYDROGEN PEROXIDE-MEDIATED ANTAGONISM OF S. MUTANS
Observational studies indicate that a high prevalence of oral commensals such as S. sanguinis is correlated with a lower abundance of S. mutans (95, 96). Conversely, a high prevalence of S. mutans has been correlated with reduced colonization by commensal streptococci, suggesting that oral commensal streptococci and S. mutans antagonize one another to compete for space and nutrients on the enamel surface (45). Animal studies demonstrate that if S. sanguinis is dosed before S. mutans, S. sanguinis dominates; however, if S. mutans is given before S. sanguinis, S. mutans outcompetes S. sanguinis (97). More recent in vitro studies have indicated that S. sanguinis and S. oligofermentans antagonize S. mutans through the production of hydrogen peroxide, while S. mutans antagonizes commensal streptococci through the production of bacteriocins (mutacins), and that whichever species is established first can inhibit the other through the respective mechanisms (Fig. 2) (98, 99). Additional studies indicate that S. sanguinis isolated from individuals with carious lesions has reduced hydrogen peroxide production compared to that in individuals without caries (100). This antagonism of S. mutans by hydrogen peroxide can be altered by Veillonella species expressing catalase (101). Veillonella, a bridging species, is known to bind to both commensal streptococci as well as oral buccal cells and the periopathogens F. nucleatum and P. gingivalis (102, 103).
FIG 2.
Microbial mechanisms of caries inhibition and formation. (Left) Oral commensal bacteria colonize the salivary pellicle. The production of hydrogen peroxide prevents colonization by cariogenic microbes. Commensal streptococci, such as S. parasanguinis, dominate the metabolomic profile, reducing the concentration of sucrose that is available, blunting S. mutans growth and Gtf activity. Arginolytic streptococci produce ammonia to raise the pH, preventing caries formation. (Right) S. mutans establishes glucan-rich biofilms to promote colonization. Once the biofilm is established, copious amounts of lactic acid are produced, which decreases the local pH, leading to caries formation. Mutacin production prevents colonization by oral commensal streptococci (OCS). The presence of C. albicans allows increased glucan binding and production and increased microbial biomass.
IMPACT OF COMMENSAL-MEDIATED HYDROGEN PEROXIDE PRODUCTION ON PERIODONTAL PATHOGENS
Periodontal pathogens vary in their sensitivities to oxidative stress and hydrogen peroxide. As many periodontal pathogens are anaerobic bacteria, oxygen is toxic and often leads to cell death; however, these pathogens have evolved various strategies to tolerate oxidative stress. Nonetheless, hydrogen peroxide antagonism has been documented to reduce periodontal pathogen viability.
P. gingivalis is an obligate anaerobic bacterium that is widely associated with adult periodontal disease. P. gingivalis infection is associated with increased bone resorption and nonproductive inflammation, which often leads to tooth loss and subsequent infection. During infection, P. gingivalis often encounters atmospheric oxygen as well as ROS from the host immune response. Additionally, P. gingivalis encounters hydrogen peroxide from host commensal streptococci, where the hydrogen peroxide antagonism of P. gingivalis has been documented for a variety of mitis group streptococci (65, 104–107). Thus, mechanisms to tolerate oxidative stress are paramount for the survival of P. gingivalis in the oral cavity. P. gingivalis is known to tolerate oxidative stress by (i) acquiring heme and sequestering it on its cell surface and (ii) upregulating the expression of iron-binding proteins that limit the Fenton reaction (108–111). While oxidative stress by hydrogen peroxide does not seem to inhibit P. gingivalis directly, hydrogen peroxide from S. gordonii has been shown to decrease gingipain activity (65).
Another periodontal pathogen, F. nucleatum, has differing sensitivity to hydrogen peroxide that is dependent on binding with oral commensal bacteria. When F. nucleatum is introduced into an oral community, hydrogen peroxide production increases (68). However, this hydrogen peroxide production is decreased if F. nucleatum is allowed to form aggregates with S. sanguinis, indicating that oral commensal streptococci can modulate hydrogen peroxide production depending on the microbes present within that community and that F. nucleatum can mask this phenomenon if it binds directly to S. sanguinis. Furthermore, F. nucleatum has been found to synergize with Veillonella spp., where the presence of Veillonella increases the viability of F. nucleatum. When grown in a trispecies coculture containing Veillonella parvula, F. nucleatum, and S. gordonii, catalase produced by Veillonella parvula protects F. nucleatum from death by S. gordonii hydrogen peroxide (112).
Aggregatibacter actinomycetemcomitans has also been found to be sensitive to commensal streptococcus-generated hydrogen peroxide (40). Coculture of A. actinomycetemcomitans with S. parasanguinis revealed that A. actinomycetemcomitans decreases the protein levels of pyruvate oxidase and consequently reduces the amount of hydrogen peroxide generated by the commensal (113). Furthermore, the presence of A. actinomycetemcomitans increased S. parasanguinis cell numbers, but the loss of hydrogen peroxide production altogether decreased the biofilm biomass, indicating that the presence of subinhibitory concentrations of hydrogen peroxide is beneficial for the ecology of these two organisms. While A. actinomycetemcomitans is sensitive to streptococcus-generated hydrogen peroxide, it encodes a catalase gene, where catalase activity has been associated with reduced concentrations of hydrogen peroxide in dual culture with S. sanguinis (107). Trispecies cultures with S. sanguinis, P. gingivalis, and A. actinomycetemcomitans indicate that the presence of A. actinomycetemcomitans protects P. gingivalis from S. sanguinis hydrogen peroxide-dependent killing through the expression of its catalase. Additionally, Stacy et al. demonstrated that the viability of A. actinomycetemcomitans is increased in an abscess model in the presence of S. gordonii due to the ability of A. actinomycetemcomitans to aerobically respire using hydrogen peroxide (114). Furthermore, Ramsey and Whiteley demonstrated that streptococcus-generated hydrogen peroxide induces the expression of the A. actinomycetemcomitans oxidative stress regulator OxyR, which induces the expression of ApiA, a complement resistance protein, promoting A. actinomycetemcomitans growth in serum (115). Taken together, A. actinomycetemcomitans modulates the production and availability of hydrogen peroxide and can aerobically respire using hydrogen peroxide but is sensitive to oxidative stress at high concentrations of hydrogen peroxide.
EFFECTS OF STREPTOCOCCUS-GENERATED REACTIVE NITROGEN SPECIES ON MICROBIAL COMMUNITIES
Nitrate and its derivatives are found in the oral cavity at various concentrations. Nitrate is taken in through the consumption of food with nitrate, such as leafy green vegetables. While human cells are not capable of reducing nitrate, many anaerobic bacteria within the oral cavity, such as Prevotella, Veillonella, Neisseria, and Rothia, are capable of reducing nitrate to nitrite, nitric oxide, and nitrous oxide (Fig. 3). This nitrate reduction has been shown to increase the pH of oral community biofilms (116–118). Ex vivo studies have indicated that the addition of nitrate to saliva biofilm communities increases ammonium concentrations while decreasing lactate concentrations, raising the overall pH. The addition of nitrate was also found to reduce the prevalence of both cariogenic and periodontal pathogens while increasing the number of nitrate-reducing commensals in the oral cavity (117, 119).
FIG 3.

Microbial nitrate reduction and hydrogen peroxide production contribute to RNS production. Dietary nitrates (NO3) are reduced by oral bacteria into nitrite (NO2) and nitric oxide (NO). Salivary nitric oxide enters the bloodstream and regulates vascular tone and blood pressure. The remaining nitrite is available to react with streptococcus-generated hydrogen peroxide to form RNS such as peroxynitrite (ONOO−). Hydrogen peroxide, nitric oxide, and peroxynitrite are antimicrobials that inhibit oral pathogens.
The reduction of nitrate also leads to the generation of nitric oxide, a reactive nitrogen species (RNS) that has been shown to disperse bacterial biofilms, alter bacterial physiology, and reduce bacterial viability. As nitric oxide is a short-lived but potent antimicrobial, nitric oxide-releasing nanoparticles have been developed to reduce periodontal burden, where nitric oxide reduces the viability of A. actinomycetemcomitans and P. gingivalis (120). Nitric oxide alone is not effective at reducing streptococci, including S. mutans, indicating that nitric oxide treatments would be less effective for caries prevention and/or treatment. In addition to nitric oxide acting as an antimicrobial RNS, the hydrogen peroxide generated by oral commensal streptococci can react with nitrite, an intermediate in nitrate reduction, to form other RNS such as peroxynitrite (121) (Fig. 3). This generation of RNS by oral commensal streptococci has been shown to inhibit S. mutans, A. actinomycetemcomitans, and Enterococcus faecalis. In rat infection studies, the presence of S. parasanguinis and nitrite significantly reduced S. mutans viability, biofilm formation, as well as caries (47). Furthermore, in the presence of nitrite in a trispecies coculture model with S. parasanguinis, S. mutans and C. albicans were both inhibited within biofilms (122).
The effects of RNS generated in the oral cavity are not restricted to the oral cavity. The reduction of nitrate leads to available nitrite and nitric oxide, which are important regulators of blood pressure. Multiple studies have indicated that the use of mouthwash increases blood pressure due to the loss of nitrate reduction by oral commensals (123–127). Additionally, the effect of oral commensals is not restricted to the oral cavity. Multiple studies have indicated that oral commensal streptococci can translocate to the lung in people with cystic fibrosis (CF). The presence of these oral commensal streptococci has been associated with increased lung function (128, 129). The generation of RNS through streptococcus-produced hydrogen peroxide has been shown to inhibit the major CF pathogen Pseudomonas aeruginosa, with increased inhibition being seen in CF isolates of P. aeruginosa compared to acute non-CF or environmental isolates (121, 130).
BACTERIOCIN PRODUCTION BY ORAL STREPTOCOCCI
Specific Streptococcus species in the oral cavity produce bacteriocins, which are antimicrobial peptides that inhibit the growth of genetically related species in many cases. These can be classified into lantibiotic and nonlantibiotic bacteriocins. Lantibiotic bacteriocins are small (<5-kDa) peptides that are characterized by containing the unusual amino acids lanthionine and β-methyllanthionine as well as a number of dehydrated amino acids (131, 132). This class of bacteriocins is produced by Gram-positive bacteria and inhibits the growth of other Gram-positive species by targeting the cell wall (133). A high-BLIS (bacteriocin-like inhibitory substance)-producing S. salivarius strain, K12, produces lantibiotics known as salivaricin A2 and salivaricin B (134). Through numerous studies and clinical trials, S. salivarius strain K12 has been shown to inhibit the growth of Streptococcus pyogenes, the causative agent of streptococcal pharyngitis and tonsillitis, through bacteriocin production. Additionally, S. salivarius strain K12 is used as a commercially developed probiotic against S. pyogenes infection (135). Another BLIS strain of S. salivarius, M18, produces the lantibiotics salivaricin A2, salivaricin 9 (136), and salivaricin M (137) and the nonlantibiotic salivaricin MPS the non-lantibiotic bacteriocin (salivaricin-MPS) (138). Salivaricin 9 has an inhibitory effect against Corynebacterium sp. strain GH17 as well as S. pyogenes at higher concentrations (139). Salivaricin D, a lantibiotic identified in S. salivarius strain 5M6c, isolated from a healthy infant, displays a broad spectrum of inhibition against members of the genera Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, Micrococcus, Bacillus, and Clostridium (140). While many S. salivarius bacteriocins have been identified, further research into their target species and effects on the oral polymicrobial community is warranted. S. dentisani (designated S. oralis subsp. dentisani in this study) produces bacteriocins that inhibit the growth of many Streptococcus mutans strains (141). Streptococcus anginosus, a commensal of the oral cavity and other bodily sites, produces a bacteriocin called angicin, which is inhibitory against Gram-positive species, including the oral commensal Streptococcus constellatus, other strains of S. anginosus, vancomycin-resistant E. faecalis, and multiple Listeria species (142).
S. mutans produces multiple bacteriocins, termed mutacins, which have demonstrated inhibitory effects against many other Streptococcus species, including species belonging to the pyogenic, mitis, salivarius, anginosus, and bovis subgroups (143). Mutacins have been categorized into lantibiotic and nonlantibiotic mutacins. Specifically, S. mutans has been shown to inhibit the growth of S. sanguinis through the production of the lantibiotic mutacin I and the nonlantibiotic mutacin IV (98). This inhibition of S. sanguinis is contingent upon initial colonization by S. mutans before S. sanguinis; simultaneous colonization by both species leads to coexistence, while colonization by S. sanguinis before S. mutans leads to the inhibition of S. mutans growth via S. sanguinis-mediated hydrogen peroxide production (98). In a trispecies model with S. mutans, S. sanguinis, and S. gordonii, mutacin IV gene expression was shown to be coordinated with S. mutans competence genes, and the expression of both sets of genes was elevated under aerobic conditions (46). The coordination of these two systems is a proposed mechanism by which S. mutans acquires transforming DNA from the surrounding environment; mutacin IV production is increased, causing S. sanguinis and S. gordonii cells to lyse and release eDNA, which can be more effectively taken up by S. mutans competent cells under aerobic conditions (144). The novel oral commensal Streptococcus sp. strain A12 inhibits S. mutans mutacin production via the protease degradation of competence-stimulating peptide (CSP), which is essential for bacteriocin production by S. mutans (74).
MODULATION OF IMMUNE RESPONSES BY ORAL STREPTOCOCCI
Inflammatory responses in the oral cavity play a critical role in tissue damage associated with periodontitis. Periodontal pathogens stimulate the release of proinflammatory cytokines, including interleukin-1α (IL-1α), IL-1β, IL-6, and IL-8, which facilitate immune cell recruitment, tissue destruction, and bone resorption that contribute to periodontitis (145). Many commensal streptococci residing within the oral cavity have been shown to downregulate proinflammatory pathways in host cells and are associated with a healthy state of the oral cavity (Fig. 4). This modulation of the host immune response by commensal streptococci may help sustain their own colonization while modulating the growth of pathogens.
FIG 4.
Oral commensal streptococci decrease inflammation. The production of both hydrogen peroxide (H2O2) and small molecules by oral commensal streptococci has been shown to modulate NF-κB activity both basally and during pathogen induction of NF-κB activity. The presence of oral commensal streptococci also promotes the induction of β-defensin 2, to which oral pathogens are sensitive.
Modulation of the proinflammatory NF-κB pathway by commensal streptococci has been extensively studied. Several studies have demonstrated the ability of S. salivarius to downregulate NF-κB activity and downstream proinflammatory cytokines in gingival fibroblasts, human bronchial epithelial cells, primary keratinocytes, and intestinal epithelial cells (135, 146, 147). This phenomenon is consistent among multiple strains of S. salivarius (135, 148). One study concluded that S. salivarius downregulates NF-κB activation by inhibiting the NF-κB transcription factor p65 from entering the nucleus for transcription initiation. This inhibition is dependent on an S. salivarius protein with a molecular weight of less than 3 kDa (146). Transcriptomics performed in the same study on bronchial epithelial cells incubated with S. salivarius revealed an overall upregulation of host genes involved in homeostatic activities and anti-inflammatory responses as well as a general downregulation or baseline maintenance of inflammatory gene expression (146). Therefore, S. salivarius may ensure tolerance by the host while also potentially protecting the host against pathogen-induced inflammation.
While hydrogen peroxide produced by oral streptococci has been shown to modulate the growth of polymicrobial communities in the oral cavity, including the inhibition of S. mutans, hydrogen peroxide also has a complex role in the development of the host immune response. Hydrogen peroxide-producing oral streptococci, including S. mitis and S. oralis, have been shown to downregulate the NF-κB response in a hydrogen peroxide-dependent manner. Hydrogen peroxide produced by S. mitis and S. oralis activates nuclear factor erythroid 2-related factor 2 (Nfr2), which subsequently inhibits NF-κB transcription (149). Additionally, hydrogen peroxide produced by the oral commensals S. sanguinis and S. mitis has been shown to induce cell death of a THP-1 human macrophage cell line despite being associated with a healthy state in the oral cavity. This killing of macrophages by hydrogen peroxide is dependent on the pyruvate oxidase gene spxB, which is critical for H2O2 production in many streptococcal species (150).
A unique mechanism of pathogen inhibition by an oral streptococcus has been described, in which host cells are stimulated to produce antimicrobial products. Streptococcus mitis colonization of gingival epithelial cells induces the production of human β-defensin 2 (hβd-2), an antimicrobial peptide known to kill oral pathogens, including S. mutans and S. sobrinus (151). S. mitis is highly tolerant to hβd-2 and other antimicrobial peptides (152); therefore, S. mitis stimulation of hβd-2 produced by host cells is a mechanism by which S. mitis can persist in the oral cavity while modulating the oral polymicrobial community, including the inhibition of oral pathogens.
MODULATION OF PATHOGEN-INDUCED INFLAMMATION
Oral streptococci have also been shown to modulate inflammation induced during pathogenic infection. S. salivarius colonizing human bronchial epithelial cells and primary keratinocytes inhibits IL-8 production that is induced by the respiratory pathogen P. aeruginosa (146). S. salivarius strains K12 and M18 downregulate the proinflammatory cytokines IL-6 and IL-8 in gingival fibroblasts that have been challenged with oral pathogens, including P. gingivalis, A. actinomycetemcomitans, and F. nucleatum (147). While the anti-inflammatory factor produced by S. salivarius that downregulates IL-6 and IL-8 was not identified, it was demonstrated to be a small molecule of <10 kDa that is heat stable and proteinaceous (147). Another oral commensal, Streptococcus cristatus, was shown to downregulate IL-8 production induced by F. nucleatum in an oral epithelial cell infection model via the inhibition of NF-κB transcription (153, 154). F. nucleatum induced the degradation of the NF-κB-inhibitory protein IκB-α, while the presence of S. cristatus during F. nucleatum infection stabilized IκB-α (154). S. gordonii was shown to significantly impact mitogen-activated protein kinase (MAPK) and Toll-like receptor pathway genes, which are involved in host cell proliferation as well as cytokine expression. Additionally, S. gordonii was shown to downregulate gingival epithelial cell IL-6 and IL-8 secretion induced by the oral pathogens F. nucleatum, P. gingivalis, and A. actinomycetemcomitans (104). Conversely, S. sanguinis was shown to induce IL-6 and IL-8 expression in oral keratinocytes and primary periodontal ligament cells despite being a health-associated commensal (58). In a trispecies biofilm model with P. gingivalis and the commensal species Corynebacterium durum in the same study, the cytokine expression profiles resembled that of single-species S. sanguinis-colonized cells, demonstrating the ability of oral streptococci to govern the host response to polymicrobial communities (58). Finally, S. salivarius was shown to alleviate radiation-induced oral mucositis in a mouse model by preventing the overgrowth of oral anaerobes commonly observed in oral mucositis (155).
THE PATHOBIONT STATUS OF ORAL STREPTOCOCCI
Oral streptococci tend to behave as commensals at steady state, producing mediators such as hydrogen peroxide and bacteriocins that can antagonize other bacteria and prevent colonization. However, in the presence of pathogenic bacteria that have successfully colonized, oral streptococci can act as accessory pathogens and develop associations with these pathogens. For example, S. gordonii binds to and is commonly coisolated with P. gingivalis and has been shown to increase bone loss when present with P. gingivalis (63). Furthermore, outside the oral cavity, oral streptococci can initiate inflammatory immune responses in the bloodstream and the heart. In rare cases, S. mitis, S. oralis, S. gordonii, S. parasanguinis, and S. sanguinis can cause infectious bacteremia and endocarditis (156, 157). This entry into the bloodstream and the heart is typically associated with poor dental health, i.e., periodontitis, which provides a leaky, inflammatory environment that promotes translocation into the bloodstream (158, 159). Overall, it is important to note that while oral commensal streptococci are generally associated with health, there are unique instances where these microbes influence or potentiate the virulence of pathogens or directly cause disease themselves.
CONCLUSION
Commensal streptococci comprise the vast majority of the oral polymicrobial community and therefore play a complex but important role in oral health. In this review, we discuss mechanisms by which commensal streptococci modulate the homeostasis of polymicrobial communities within the oral cavity. Some commensal streptococci are thought to be accessory periodontal pathogens by interacting with and forming robust biofilms with these pathogens. However, commensal streptococci also employ multiple mechanisms to inhibit pathogen growth and maintain oral health. Many streptococcal species produce hydrogen peroxide, which can directly kill a wide variety of oral and nonoral pathogens. Additionally, several oral commensals can reduce dietary nitrate in the oral cavity down to nitric oxide, an RNS that has antimicrobial properties and has been shown to inhibit the growth of specific periodontal pathogens. Nitrite, another nitrate reduction intermediate, can react with hydrogen peroxide produced by commensal streptococci to form the RNS peroxynitrite, which has been shown to kill the oral pathogens S. mutans and A. actinomycetemcomitans. Certain streptococcal species, such as A12, S. salivarius, S. gordonii, and S. dentisani, can counteract the acidification caused by S. mutans that leads to the progression of dental caries via the conversion of arginine to ammonia, thereby raising the oral cavity pH. S. salivarius produces a variety of bacteriocins that have the potential to influence the microbial makeup of the oral cavity and have been shown to effectively inhibit S. pyogenes growth in the oropharynx. Finally, S. salivarius, S. gordonii, S. mitis, and S. oralis downregulate inflammatory cytokines that have been shown to contribute to bone loss and periodontal disease progression during infection with periodontal pathogens. In summation, oral commensal streptococci play dynamic roles in the maintenance of oral health and homeostasis by structuring the composition of the oral microbiota through microbial competition and the regulation of the host immune response.
ACKNOWLEDGMENTS
This work is supported by grants awarded to J.A.S. from the National Institute of Dental and Craniofacial Research (NIDCR) (R00DE025913) and the National Institute of General Medical Sciences (R35GM142748). This work is also supported by startup funds from the UAB Department of Microbiology and a James A. Pittman, Jr., M.D., Scholars grant from the UAB Heersink School of Medicine (J.A.S.). J.J.B. was supported by the NIDCR Dental Academic Research Training Program (T90DE022736) and is now supported by an NIH NIDCR National Research Service award (1F31DE031508-01A1). S.N.S. is supported by an NIH NHLBI National Research Service award (1F31HL162487-01).
Contributor Information
Jessica A. Scoffield, Email: jscoff@uab.edu.
George O’Toole, Geisel School of Medicine at Dartmouth.
REFERENCES
- 1.Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. 2010. The Human Oral Microbiome Database: a Web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010:baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. 2010. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pride DT, Salzman J, Haynes M, Rohwer F, Davis-Long C, White RA, Loomer P, Armitage GC, Relman DA. 2012. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. ISME J 6:915–926. doi: 10.1038/ismej.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuy AK, David MS, Li L, Heider TN, Peterson JD, Montano EA, Dongari-Bagtzoglou A, Diaz PI, Strausbaugh LD. 2014. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One 9:e90899. doi: 10.1371/journal.pone.0090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socransky SS, Smith C, Haffajee AD. 2002. Subgingival microbial profiles in refractory periodontal disease. J Clin Periodontol 29:260–268. doi: 10.1034/j.1600-051x.2002.290313.x. [DOI] [PubMed] [Google Scholar]
- 6.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 7.Pratt-Terpstra IH, Weerkamp AH, Busscher HJ. 1989. The effects of pellicle formation on streptococcal adhesion to human enamel and artificial substrata with various surface free-energies. J Dent Res 68:463–467. doi: 10.1177/00220345890680030501. [DOI] [PubMed] [Google Scholar]
- 8.Ericson T. 1967. Adsorption to hydroxyapatite of proteins and conjugated proteins from human saliva. Caries Res 1:52–58. doi: 10.1159/000259499. [DOI] [PubMed] [Google Scholar]
- 9.Ericson T, Magnusson I. 1976. Affinity for hydroxyapatite of salivary substances inducing aggregation of oral streptococci. Caries Res 10:8–18. doi: 10.1159/000260185. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RJ, Etherden I. 1983. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun 41:1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu JP, Dabbous MK. 1986. Interaction of salivary fibronectin with oral streptococci. J Dent Res 65:1094–1100. doi: 10.1177/00220345860650081001. [DOI] [PubMed] [Google Scholar]
- 12.Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ. 1992. Adherence of oral streptococci to salivary glycoproteins. Infect Immun 60:31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson R, Johnson A. 1951. The inhibitory action of saliva on the diphtheria bacillus: hydrogen peroxide, the inhibitory agent produced by salivary streptococci. J Infect Dis 88:81–85. doi: 10.1093/infdis/88.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg K, Hallander HO. 1973. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol 18:423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- 15.McLeod JW, Gordon J. 1922. Production of hydrogen peroxide by bacteria. Biochem J 16:499–506. doi: 10.1042/bj0160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. 2006. Bacterial interactions and successions during plaque development. Periodontol 2000 42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 17.Riep B, Edesi-Neuss L, Claessen F, Skarabis H, Ehmke B, Flemmig TF, Bernimoulin J-P, Göbel UB, Moter A. 2009. Are putative periodontal pathogens reliable diagnostic markers? J Clin Microbiol 47:1705–1711. doi: 10.1128/JCM.01387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh PD. 1994. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 19.Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ, Jr, Kolenbrander PE. 2006. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol 72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, Wen ZT, Richards VP, Brady LJ, Lemos JA. 2018. Biology of oral streptococci. Microbiol Spectr 6:GPP3-0042-2018. doi: 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein MI. 2022. Oral streptococci, p 125–137. In de Filippis I (ed), Molecular typing in bacterial infections, 2nd ed, vol 1. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 23.Jensen A, Scholz CFP, Kilian M. 2016. Re-evaluation of the taxonomy of the mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int J Syst Evol Microbiol 66:4803–4820. doi: 10.1099/ijsem.0.001433. [DOI] [PubMed] [Google Scholar]
- 24.Richards VP, Palmer SR, Pavinski Bitar PD, Qin X, Weinstock GM, Highlander SK, Town CD, Burne RA, Stanhope MJ. 2014. Phylogenomics and the dynamic genome evolution of the genus Streptococcus. Genome Biol Evol 6:741–753. doi: 10.1093/gbe/evu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köhler W. 2007. The present state of species within the genera Streptococcus and Enterococcus. Int J Med Microbiol 297:133–150. doi: 10.1016/j.ijmm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Facklam R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skerman VBD, McGowan V, Sneath PHA (ed). 1989. Approved lists of bacterial names, amended ed. American Society for Microbiology, Washington, DC. [PubMed] [Google Scholar]
- 28.Oren A, Garrity GM. 2014. Notification that new names of prokaryotes, new combinations and new taxonomic opinions have appeared in volume 64, part 1, of the IJSEM. Int J Syst Evol Microbiol 64:1075–1076. doi: 10.1099/ijs.0.062372-0. [DOI] [PubMed] [Google Scholar]
- 29.Hall MW, Singh N, Ng KF, Lam DK, Goldberg MB, Tenenbaum HC, Neufeld JD, Beiko RG, Senadheera DB. 2017. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microbiomes 3:2. doi: 10.1038/s41522-016-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaioannou W, Gizani S, Haffajee AD, Quirynen M, Mamai-Homata E, Papagiannoulis L. 2009. The microbiota on different oral surfaces in healthy children. Oral Microbiol Immunol 24:183–189. doi: 10.1111/j.1399-302X.2008.00493.x. [DOI] [PubMed] [Google Scholar]
- 31.Hannig M, Fiebiger M, Güntzer M, Döbert A, Zimehl R, Nekrashevych Y. 2004. Protective effect of the in situ formed short-term salivary pellicle. Arch Oral Biol 49:903–910. doi: 10.1016/j.archoralbio.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Hannig M, Hess NJ, Hoth-Hannig W, De Vrese M. 2003. Influence of salivary pellicle formation time on enamel demineralization—an in situ pilot study. Clin Oral Investig 7:158–161. doi: 10.1007/s00784-003-0219-2. [DOI] [PubMed] [Google Scholar]
- 33.Fachon-Kalweit S, Elder BL, Fives-Taylor P. 1985. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun 48:617–624. doi: 10.1128/iai.48.3.617-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okahashi N, Nakata M, Terao Y, Isoda R, Sakurai A, Sumitomo T, Yamaguchi M, Kimura RK, Oiki E, Kawabata S, Ooshima T. 2011. Pili of oral Streptococcus sanguinis bind to salivary amylase and promote the biofilm formation. Microb Pathog 50:148–154. doi: 10.1016/j.micpath.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Hamada T, Kawashima M, Watanabe H, Tagami J, Senpuku H. 2004. Molecular interactions of surface protein peptides of Streptococcus gordonii with human salivary components. Infect Immun 72:4819–4826. doi: 10.1128/IAI.72.8.4819-4826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demuth DR, Duan Y, Brooks W, Holmes AR, McNab R, Jenkinson HF. 1996. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol Microbiol 20:403–413. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 37.Garnett JA, Simpson PJ, Taylor J, Benjamin SV, Tagliaferri C, Cota E, Chen Y-YM, Wu H, Matthews S. 2012. Structural insight into the role of Streptococcus parasanguinis Fap1 within oral biofilm formation. Biochem Biophys Res Commun 417:421–426. doi: 10.1016/j.bbrc.2011.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chahal G, Quintana-Hayashi MP, Gaytán MO, Benktander J, Padra M, King SJ, Linden SK. 2022. Streptococcus oralis employs multiple mechanisms of salivary mucin binding that differ between strains. Front Cell Infect Microbiol 12:889711. doi: 10.3389/fcimb.2022.889711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scannapieco FA, Solomon L, Wadenya RO. 1994. Emergence in human dental plaque and host distribution of amylase-binding streptococci. J Dent Res 73:1627–1635. doi: 10.1177/00220345940730100701. [DOI] [PubMed] [Google Scholar]
- 40.Hillman JD, Socransky SS, Shivers M. 1985. The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Arch Oral Biol 30:791–795. doi: 10.1016/0003-9969(85)90133-5. [DOI] [PubMed] [Google Scholar]
- 41.Imlay JA, Chin SM, Linn S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 42.Russell RR. 1979. Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol 112:197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- 43.Shah DSH, Russell RRB. 2004. A novel glucan-binding protein with lipase activity from the oral pathogen Streptococcus mutans. Microbiology (Reading) 150:1947–1956. doi: 10.1099/mic.0.26955-0. [DOI] [PubMed] [Google Scholar]
- 44.Kuribayashi M, Kitasako Y, Matin K, Sadr A, Shida K, Tagami J. 2012. Intraoral pH measurement of carious lesions with qPCR of cariogenic bacteria to differentiate caries activity. J Dent 40:222–228. doi: 10.1016/j.jdent.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Loesche WJ, Rowan J, Straffon LH, Loos PJ. 1975. Association of Streptococcus mutants with human dental decay. Infect Immun 11:1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scoffield J, Michalek S, Harber G, Eipers P, Morrow C, Wu H. 2019. Dietary nitrite drives disease outcomes in oral polymicrobial infections. J Dent Res 98:1020–1026. doi: 10.1177/0022034519855348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai C-H, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, Koo H. 2014. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun 82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du Q, Ren B, He J, Peng X, Guo Q, Zheng L, Li J, Dai H, Chen V, Zhang L, Zhou X, Xu X. 2021. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J 15:894–908. doi: 10.1038/s41396-020-00823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huffines JT, Scoffield JA. 2020. Disruption of Streptococcus mutans and Candida albicans synergy by a commensal streptococcus. Sci Rep 10:19661. doi: 10.1038/s41598-020-76744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilbert SA, Mark Welch JL, Borisy GG. 2020. Spatial ecology of the human tongue dorsum microbiome. Cell Rep 30:4003–4015.e3. doi: 10.1016/j.celrep.2020.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White GE, Armaleh MT. 2004. Tongue scraping as a means of reducing oral mutans streptococci. J Clin Pediatr Dent 28:163–166. doi: 10.17796/jcpd.28.2.n18275821658263v. [DOI] [PubMed] [Google Scholar]
- 53.Park O-J, Kim J, Kim HY, Kwon Y, Yun C-H, Han SH. 2019. Streptococcus gordonii induces bone resorption by increasing osteoclast differentiation and reducing osteoblast differentiation. Microb Pathog 126:218–223. doi: 10.1016/j.micpath.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, Abe S. 2003. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol 47:321–326. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 55.Ishijima SA, Hayama K, Burton JP, Reid G, Okada M, Matsushita Y, Abe S. 2012. Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect in an oral candidiasis model. Appl Environ Microbiol 78:2190–2199. doi: 10.1128/AEM.07055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz PI, Dongari-Bagtzoglou A. 2014. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol 16:214–231. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, Ikonomou L, Dongari-Bagtzoglou A. 2012. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun 80:620–632. doi: 10.1128/IAI.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redanz U, Redanz S, Treerat P, Prakasam S, Lin L-J, Merritt J, Kreth J. 2021. Differential response of oral mucosal and gingival cells to Corynebacterium durum, Streptococcus sanguinis, and Porphyromonas gingivalis multispecies biofilms. Front Cell Infect Microbiol 11:686479. doi: 10.3389/fcimb.2021.686479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks W, Demuth DR, Gil S, Lamont RJ. 1997. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun 65:3753–3758. doi: 10.1128/iai.65.9.3753-3758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park Y, Simionato MR, Sekiya K, Murakami Y, James D, Chen W, Hackett M, Yoshimura F, Demuth DR, Lamont RJ. 2005. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun 73:3983–3989. doi: 10.1128/IAI.73.7.3983-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. 2012. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuboniwa M, Houser JR, Hendrickson EL, Wang Q, Alghamdi SA, Sakanaka A, Miller DP, Hutcherson JA, Wang T, Beck DAC, Whiteley M, Amano A, Wang H, Marcotte EM, Hackett M, Lamont RJ. 2017. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat Microbiol 2:1493–1499. doi: 10.1038/s41564-017-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daep CA, Novak EA, Lamont RJ, Demuth DR. 2011. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect Immun 79:67–74. doi: 10.1128/IAI.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mans JJ, von Lackum K, Dorsey C, Willis S, Wallet SM, Baker HV, Lamont RJ, Handfield M. 2009. The degree of microbiome complexity influences the epithelial response to infection. BMC Genomics 10:380. doi: 10.1186/1471-2164-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitzsimonds ZR, Liu C, Stocke KS, Yakoumatos L, Shumway B, Miller DP, Artyomov MN, Bagaitkar J, Lamont RJ. 2021. Regulation of olfactomedin 4 by Porphyromonas gingivalis in a community context. ISME J 15:2627–2642. doi: 10.1038/s41396-021-00956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lancy P, Dirienzo JM, Appelbaum B, Rosan B, Holt SC. 1983. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect Immun 40:303–309. doi: 10.1128/iai.40.1.303-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lima BP, Shi W, Lux R. 2017. Identification and characterization of a novel Fusobacterium nucleatum adhesin involved in physical interaction and biofilm formation with Streptococcus gordonii. Microbiologyopen 6:e00444. doi: 10.1002/mbo3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He X, Hu W, Kaplan CW, Guo L, Shi W, Lux R. 2012. Adherence to streptococci facilitates Fusobacterium nucleatum integration into an oral microbial community. Microb Ecol 63:532–542. doi: 10.1007/s00248-011-9989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang Y-J, Sim J, Jun H-K, Choi B-K. 2013. Differential effect of autoinducer 2 of Fusobacterium nucleatum on oral streptococci. Arch Oral Biol 58:1594–1602. doi: 10.1016/j.archoralbio.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Jang Y-J, Choi Y-J, Lee S-H, Jun H-K, Choi B-K. 2013. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol 58:17–27. doi: 10.1016/j.archoralbio.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Ramsey MM, Rumbaugh KP, Whiteley M. 2011. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog 7:e1002012. doi: 10.1371/journal.ppat.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aframian DJ, Davidowitz T, Benoliel R. 2006. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis 12:420–423. doi: 10.1111/j.1601-0825.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 73.Ferrer MD, López-López A, Nicolescu T, Salavert A, Méndez I, Cuñé J, Llena C, Mira A. 2020. A pilot study to assess oral colonization and pH buffering by the probiotic Streptococcus dentisani under different dosing regimes. Odontology 108:180–187. doi: 10.1007/s10266-019-00458-y. [DOI] [PubMed] [Google Scholar]
- 74.Huang X, Palmer SR, Ahn S-J, Richards VP, Williams ML, Nascimento MM, Burne RA. 2016. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol 82:2187–2201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velsko IM, Chakraborty B, Nascimento MM, Burne RA, Richards VP. 2018. Species designations belie phenotypic and genotypic heterogeneity in oral streptococci. mSystems 3:e00158-18. doi: 10.1128/mSystems.00158-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pruitt KM, Tenovuo J, Mansson-Rahemtulla B, Harrington P, Baldone DC. 1986. Is thiocyanate peroxidation at equilibrium in vivo? Biochim Biophys Acta 870:385–391. doi: 10.1016/0167-4838(86)90245-1. [DOI] [PubMed] [Google Scholar]
- 77.Ashby MT. 2008. Inorganic chemistry of defensive peroxidases in the human oral cavity. J Dent Res 87:900–914. doi: 10.1177/154405910808701003. [DOI] [PubMed] [Google Scholar]
- 78.Thomas EL, Milligan TW, Joyner RE, Jefferson MM. 1994. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect Immun 62:529–535. doi: 10.1128/iai.62.2.529-535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Ramsey MM, Chen X, Koley D, Whiteley M, Bard AJ. 2011. Real-time mapping of a hydrogen peroxide concentration profile across a polymicrobial bacterial biofilm using scanning electrochemical microscopy. Proc Natl Acad Sci USA 108:2668–2673. doi: 10.1073/pnas.1018391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He X, Tian Y, Guo L, Ano T, Lux R, Zusman DR, Shi W. 2010. In vitro communities derived from oral and gut microbial floras inhibit the growth of bacteria of foreign origins. Microb Ecol 60:665–676. doi: 10.1007/s00248-010-9711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He X, Tian Y, Guo L, Lux R, Zusman DR, Shi W. 2010. Oral-derived bacterial flora defends its domain by recognizing and killing intruders—a molecular analysis using Escherichia coli as a model intestinal bacterium. Microb Ecol 60:655–664. doi: 10.1007/s00248-010-9708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Itzek A, Zheng L, Chen Z, Merritt J, Kreth J. 2011. Hydrogen peroxide-dependent DNA release and transfer of antibiotic resistance genes in Streptococcus gordonii. J Bacteriol 193:6912–6922. doi: 10.1128/JB.05791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tong H, Chen W, Shi W, Qi F, Dong X. 2008. SO-LAAO, a novel l-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J Bacteriol 190:4716–4721. doi: 10.1128/JB.00363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L, Tong H, Dong X. 2012. Function of the pyruvate oxidase-lactate oxidase cascade in interspecies competition between Streptococcus oligofermentans and Streptococcus mutans. Appl Environ Microbiol 78:2120–2127. doi: 10.1128/AEM.07539-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.García-Mendoza A, Liébana J, Castillo AM, de la Higuera A, Piédrola G. 1993. Evaluation of the capacity of oral streptococci to produce hydrogen peroxide. J Med Microbiol 39:434–439. doi: 10.1099/00222615-39-6-434. [DOI] [PubMed] [Google Scholar]
- 86.Barnard JP, Stinson MW. 1999. Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii. Infect Immun 67:6558–6564. doi: 10.1128/IAI.67.12.6558-6564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng L, Itzek A, Chen Z, Kreth J. 2011. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl Environ Microbiol 77:4318–4328. doi: 10.1128/AEM.00309-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng L, Itzek A, Chen Z, Kreth J. 2011. Oxygen dependent pyruvate oxidase expression and production in Streptococcus sanguinis. Int J Oral Sci 3:82–89. doi: 10.4248/IJOS11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Im H, Kruckow KL, D’Mello A, Ganaie F, Martinez E, Luck JN, Cichos KH, Riegler AN, Song X, Ghanem E, Saad JS, Nahm MH, Tettelin H, Orihuela CJ. 2022. Anatomical site-specific carbohydrate availability impacts Streptococcus pneumoniae virulence and fitness during colonization and disease. Infect Immun 90:e00451-21. doi: 10.1128/IAI.00451-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carvalho SM, Kloosterman TG, Kuipers OP, Neves AR. 2011. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS One 6:e26707. doi: 10.1371/journal.pone.0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng L, Chen Z, Itzek A, Ashby M, Kreth J. 2011. Catabolite control protein A controls hydrogen peroxide production and cell death in Streptococcus sanguinis. J Bacteriol 193:516–526. doi: 10.1128/JB.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen L, Ge X, Wang X, Patel JR, Xu P. 2012. SpxA1 involved in hydrogen peroxide production, stress tolerance and endocarditis virulence in Streptococcus sanguinis. PLoS One 7:e40034. doi: 10.1371/journal.pone.0040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramos-Montañez S, Tsui H-CT, Wayne KJ, Morris JL, Peters LE, Zhang F, Kazmierczak KM, Sham L-T, Winkler ME. 2008. Polymorphism and regulation of the spxB (pyruvate oxidase) virulence factor gene by a CBS-HotDog domain protein (SpxR) in serotype 2 Streptococcus pneumoniae. Mol Microbiol 67:729–746. doi: 10.1111/j.1365-2958.2007.06082.x. [DOI] [PubMed] [Google Scholar]
- 94.Redanz S, Treerat P, Mu R, Redanz U, Zou Z, Koley D, Merritt J, Kreth J. 2020. Pyruvate secretion by oral streptococci modulates hydrogen peroxide dependent antagonism. ISME J 14:1074–1088. doi: 10.1038/s41396-020-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nyvad B, Kilian M. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res 24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- 96.Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. 2000. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun 68:4018–4023. doi: 10.1128/IAI.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mikx FH, van der Hoeven JS, Plasschaert AJ, König KG. 1976. Establishment and symbiosis of Actinomyces viscosus, Streptococcus sanguis and Streptococcus mutans in germ-free Osborne-Mendel rats. Caries Res 10:123–132. doi: 10.1159/000260196. [DOI] [PubMed] [Google Scholar]
- 98.Kreth J, Merritt J, Shi W, Qi F. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol 187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tong H, Chen W, Merritt J, Qi F, Shi W, Dong X. 2007. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: a possible counteroffensive strategy for interspecies competition. Mol Microbiol 63:872–880. doi: 10.1111/j.1365-2958.2006.05546.x. [DOI] [PubMed] [Google Scholar]
- 100.Giacaman RA, Torres S, Gómez Y, Muñoz-Sandoval C, Kreth J. 2015. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults. Arch Oral Biol 60:154–159. doi: 10.1016/j.archoralbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Liu J, Wu C, Huang I-H, Merritt J, Qi F. 2011. Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology (Reading) 157:2433–2444. doi: 10.1099/mic.0.048314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chalmers NI, Palmer RJ, Jr, Cisar JO, Kolenbrander PE. 2008. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J Bacteriol 190:8145–8154. doi: 10.1128/JB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Periasamy S, Kolenbrander PE. 2010. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J Bacteriol 192:2965–2972. doi: 10.1128/JB.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hasegawa Y, Mans JJ, Mao S, Lopez MC, Baker HV, Handfield M, Lamont RJ. 2007. Gingival epithelial cell transcriptional responses to commensal and opportunistic oral microbial species. Infect Immun 75:2540–2547. doi: 10.1128/IAI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohshima J, Wang Q, Fitzsimonds ZR, Miller DP, Sztukowska MN, Jung Y-J, Hayashi M, Whiteley M, Lamont RJ. 2019. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc Natl Acad Sci USA 116:8544–8553. doi: 10.1073/pnas.1900101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herrero ER, Slomka V, Bernaerts K, Boon N, Hernandez-Sanabria E, Passoni BB, Quirynen M, Teughels W. 2016. Antimicrobial effects of commensal oral species are regulated by environmental factors. J Dent 47:23–33. doi: 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Zhu B, Macleod LC, Newsome E, Liu J, Xu P. 2019. Aggregatibacter actinomycetemcomitans mediates protection of Porphyromonas gingivalis from Streptococcus sanguinis hydrogen peroxide production in multi-species biofilms. Sci Rep 9:4944. doi: 10.1038/s41598-019-41467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smalley JW, Silver J, Marsh PJ, Birss AJ. 1998. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem J 331(Part 3):681–685. doi: 10.1042/bj3310681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smalley JW, Birss AJ, Silver J. 2000. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the mu-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett 183:159–164. doi: 10.1111/j.1574-6968.2000.tb08951.x. [DOI] [PubMed] [Google Scholar]
- 110.McKenzie RME, Johnson NA, Aruni W, Dou Y, Masinde G, Fletcher HM. 2012. Differential response of Porphyromonas gingivalis to varying levels and duration of hydrogen peroxide-induced oxidative stress. Microbiology (Reading) 158:2465–2479. doi: 10.1099/mic.0.056416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McKenzie RME, Henry LG, Boutrin M-C, Ximinies A, Fletcher HM. 2016. Role of the Porphyromonas gingivalis iron-binding protein PG1777 in oxidative stress resistance. Microbiology (Reading) 162:256–267. doi: 10.1099/mic.0.000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou P, Li X, Huang I-H, Qi F. 2017. Veillonella catalase protects the growth of Fusobacterium nucleatum in microaerophilic and Streptococcus gordonii-resident environments. Appl Environ Microbiol 83:e01079-17. doi: 10.1128/AEM.01079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duan D, Scoffield JA, Zhou X, Wu H. 2016. Fine-tuned production of hydrogen peroxide promotes biofilm formation of Streptococcus parasanguinis by a pathogenic cohabitant Aggregatibacter actinomycetemcomitans. Environ Microbiol 18:4023–4036. doi: 10.1111/1462-2920.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. 2016. A commensal bacterium promotes virulence of an opportunistic pathogen via cross-respiration. mBio 7:e00782-16. doi: 10.1128/mBio.00782-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramsey MM, Whiteley M. 2009. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci USA 106:1578–1583. doi: 10.1073/pnas.0809533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. 2020. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep 10:12895. doi: 10.1038/s41598-020-69931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burleigh MC, Liddle L, Monaghan C, Muggeridge DJ, Sculthorpe N, Butcher JP, Henriquez FL, Allen JD, Easton C. 2018. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic Biol Med 120:80–88. doi: 10.1016/j.freeradbiomed.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 118.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, Bryan NS. 2014. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One 9:e88645. doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosier BT, Moya-Gonzalvez EM, Corell-Escuin P, Mira A. 2020. Isolation and characterization of nitrate-reducing bacteria as potential probiotics for oral and systemic health. Front Microbiol 11:555465. doi: 10.3389/fmicb.2020.555465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Backlund CJ, Sergesketter AR, Offenbacher S, Schoenfisch MH. 2014. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J Dent Res 93:1089–1094. doi: 10.1177/0022034514529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scoffield JA, Wu H. 2015. Oral streptococci and nitrite-mediated interference of Pseudomonas aeruginosa. Infect Immun 83:101–107. doi: 10.1128/IAI.02396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huffines JT, Stoner SN, Baty JJ, Scoffield JA. 2022. Nitrite triggers reprogramming of the oral polymicrobial metabolome by a commensal streptococcus. Front Cell Infect Microbiol 12:833339. doi: 10.3389/fcimb.2022.833339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joshipura K, Muñoz-Torres F, Fernández-Santiago J, Patel RP, Lopez-Candales A. 2020. Over-the-counter mouthwash use, nitric oxide and hypertension risk. Blood Press 29:103–112. doi: 10.1080/08037051.2019.1680270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. 2013. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, Hodgson JM. 2015. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens 28:572–575. doi: 10.1093/ajh/hpu192. [DOI] [PubMed] [Google Scholar]
- 126.McDonagh STJ, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM. 2015. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med 36:1177–1185. doi: 10.1055/s-0035-1554700. [DOI] [PubMed] [Google Scholar]
- 127.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. 2008. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O’Toole GA. 2012. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol 194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cuthbertson L, Walker AW, Oliver AE, Rogers GB, Rivett DW, Hampton TH, Ashare A, Elborn JS, De Soyza A, Carroll MP, Hoffman LR, Lanyon C, Moskowitz SM, O’Toole GA, Parkhill J, Planet PJ, Teneback CC, Tunney MM, Zuckerman JB, Bruce KD, van der Gast CJ. 2020. Lung function and microbiota diversity in cystic fibrosis. Microbiome 8:45. doi: 10.1186/s40168-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baty JJ, Huffines JT, Stoner SN, Scoffield JA. 2022. A commensal streptococcus dysregulates the Pseudomonas aeruginosa nitrosative stress response. Front Cell Infect Microbiol 12:817336. doi: 10.3389/fcimb.2022.817336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guder A, Wiedemann I, Sahl H-G. 2000. Posttranslationally modified bacteriocins—the lantibiotics. Biopolymers 55:62–73. doi:. [DOI] [PubMed] [Google Scholar]
- 132.van Kraaij C, de Vos WM, Siezen RJ, Kuipers OP. 1999. Lantibiotics: biosynthesis, mode of action and applications. Nat Prod Rep 16:575–587. doi: 10.1039/a804531c. [DOI] [PubMed] [Google Scholar]
- 133.Chatterjee C, Paul M, Xie L, van der Donk WA. 2005. Biosynthesis and mode of action of lantibiotics. Chem Rev 105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 134.Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. 2007. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol 73:1107–1113. doi: 10.1128/AEM.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaci G, Lakhdari O, Doré J, Ehrlich SD, Renault P, Blottière HM, Delorme C. 2011. Inhibition of the NF-κB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl Environ Microbiol 77:4681–4684. doi: 10.1128/AEM.03021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wescombe PA, Upton M, Renault P, Wirawan RE, Power D, Burton JP, Chilcott CN, Tagg JR. 2011. Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius. Microbiology (Reading) 157:1290–1299. doi: 10.1099/mic.0.044719-0. [DOI] [PubMed] [Google Scholar]
- 137.Heng NCK, Haji-Ishak NS, Kalyan A, Wong AYC, Lovric M, Bridson JM, Artamonova J, Stanton J-AL, Wescombe PA, Burton JP, Cullinan MP, Tagg JR. 2011. Genome sequence of the bacteriocin-producing oral probiotic Streptococcus salivarius strain M18. J Bacteriol 193:6402–6403. doi: 10.1128/JB.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wescombe PA, Heng NCK, Burton JP, Tagg JR. 2010. Something old and something new: an update on the amazing repertoire of bacteriocins produced by Streptococcus salivarius. Probiotics Antimicrob Proteins 2:37–45. doi: 10.1007/s12602-009-9026-7. [DOI] [PubMed] [Google Scholar]
- 139.Barbour A, Philip K, Muniandy S. 2013. Enhanced production, purification, characterization and mechanism of action of salivaricin 9 lantibiotic produced by Streptococcus salivarius NU10. PLoS One 8:e77751. doi: 10.1371/journal.pone.0077751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Birri DJ, Brede DA, Nes IF. 2012. Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy infant. Appl Environ Microbiol 78:402–410. doi: 10.1128/AEM.06588-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Conrads G, Westenberger J, Lürkens M, Abdelbary MMH. 2019. Isolation and bacteriocin-related typing of Streptococcus dentisani. Front Cell Infect Microbiol 9:110. doi: 10.3389/fcimb.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vogel V, Bauer R, Mauerer S, Schiffelholz N, Haupt C, Seibold GM, Fändrich M, Walther P, Spellerberg B. 2021. Angicin, a novel bacteriocin of Streptococcus anginosus. Sci Rep 11:24377. doi: 10.1038/s41598-021-03797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hossain MS, Biswas I. 2011. Mutacins from Streptococcus mutans UA159 are active against multiple streptococcal species. Appl Environ Microbiol 77:2428–2434. doi: 10.1128/AEM.02320-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Merritt J, Qi F. 2012. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol 27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okada H, Murakami S. 1998. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med 9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 146.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock REW. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76:4163–4175. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.MacDonald KW, Chanyi RM, Macklaim JM, Cadieux PA, Reid G, Burton JP. 2021. Streptococcus salivarius inhibits immune activation by periodontal disease pathogens. BMC Oral Health 21:245. doi: 10.1186/s12903-021-01606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guglielmetti S, Taverniti V, Minuzzo M, Arioli S, Stuknyte M, Karp M, Mora D. 2010. Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl Environ Microbiol 76:3948–3958. doi: 10.1128/AEM.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tang YL, Sim TS, Tan KS. 2022. Oral streptococci subvert the host innate immune response through hydrogen peroxide. Sci Rep 12:656. doi: 10.1038/s41598-021-04562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Okahashi N, Nakata M, Sumitomo T, Terao Y, Kawabata S. 2013. Hydrogen peroxide produced by oral streptococci induces macrophage cell death. PLoS One 8:e62563. doi: 10.1371/journal.pone.0062563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eberhard J, Pietschmann R, Falk W, Jepsen S, Dommisch H. 2009. The immune response of oral epithelial cells induced by single-species and complex naturally formed biofilms. Oral Microbiol Immunol 24:325–330. doi: 10.1111/j.1399-302X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 152.Nishimura E, Eto A, Kato M, Hashizume S, Imai S, Nisizawa T, Hanada N. 2004. Oral streptococci exhibit diverse susceptibility to human beta-defensin-2: antimicrobial effects of hBD-2 on oral streptococci. Curr Microbiol 48:85–87. doi: 10.1007/s00284-003-4108-3. [DOI] [PubMed] [Google Scholar]
- 153.Zhang G, Chen R, Rudney JD. 2008. Streptococcus cristatus attenuates Fusobacterium nucleatum-induced interleukin-8 expression in oral epithelial cells. J Periodontal Res 43:408–416. doi: 10.1111/j.1600-0765.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- 154.Zhang G, Chen R, Rudney JD. 2011. Streptococcus cristatus modulates the Fusobacterium nucleatum-induced epithelial interleukin-8 response through the nuclear factor-kappa B pathway. J Periodontal Res 46:558–567. doi: 10.1111/j.1600-0765.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- 155.Wang Y, Li J, Zhang H, Zheng X, Wang J, Jia X, Peng X, Xie Q, Zou J, Zheng L, Li J, Zhou X, Xu X. 2021. Probiotic Streptococcus salivarius K12 alleviates radiation-induced oral mucositis in mice. Front Immunol 12:684824. doi: 10.3389/fimmu.2021.684824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Douglas CW, Heath J, Hampton KK, Preston FE. 1993. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol 39:179–182. doi: 10.1099/00222615-39-3-179. [DOI] [PubMed] [Google Scholar]
- 157.Kitten T, Munro CL, Zollar NQ, Lee SP, Patel RD. 2012. Oral streptococcal bacteremia in hospitalized patients: taxonomic identification and clinical characterization. J Clin Microbiol 50:1039–1042. doi: 10.1128/JCM.06438-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Šutej I, Peroš K, Trkulja V, Rudež I, Barić D, Alajbeg I, Pintarić H, Stevanović R, Lepur D. 2020. The epidemiological and clinical features of odontogenic infective endocarditis. Eur J Clin Microbiol Infect Dis 39:637–645. doi: 10.1007/s10096-019-03766-x. [DOI] [PubMed] [Google Scholar]
- 159.Moby V, Millot S, Erpelding M-L, Euvrard E, Bourgeois G, Martin-Thomé H, Chirouze C, Tattevin P, Strady C, Agrinier N, Alla F, Iung B, Selton-Suty C, Hoen B, Duval X, EI-dents Association pour lȉEtude et la Prévention de l’Endocardite Infectieuse (AEPEI) Study Group . 2022. Poor oral health and hygiene habits in patients with infective endocarditis and previously identified predisposing cardiac condition: a prospective cohort study. J Infect 84:e58–e61. doi: 10.1016/j.jinf.2022.02.028. [DOI] [PubMed] [Google Scholar]
- 160.Whiley RA, Beighton D. 1991. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int J Syst Bacteriol 41:1–5. doi: 10.1099/00207713-41-1-1. [DOI] [PubMed] [Google Scholar]
- 161.Andrewes FW, Horder TJ. 1906. A study of the streptococci pathogen to man. Lancet 168:708–713. doi: 10.1016/S0140-6736(01)31538-6. [DOI] [Google Scholar]
- 162.Whiley RA, Hall LM, Hardie JM, Beighton D. 1999. A study of small-colony, beta-haemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int J Syst Bacteriol 49(Part 4):1443–1449. doi: 10.1099/00207713-49-4-1443. [DOI] [PubMed] [Google Scholar]
- 163.Holdeman LV, Moore WEC. 1974. New genus, Coprococcus, twelve new species, and emended descriptions of four previously described species of bacteria from human feces. Int J Syst Bacteriol 24:260–277. doi: 10.1099/00207713-24-2-260. [DOI] [Google Scholar]
- 164.Prevot AR. 1925. Les streptocoques anaerobies. Ann Inst Pasteur 39:417–447. [Google Scholar]
- 165.Osawa R, Fujisawa T, Sly LI. 1995. Streptococcus gallolyticus sp. nov.; gallate degrading organisms formerly assigned to Streptococcus bovis. Syst Appl Microbiol 18:74–78. doi: 10.1016/S0723-2020(11)80451-0. [DOI] [Google Scholar]
- 166.Bouvet A, Grimont F, Collins MD, Benaoudia F, Devine C, Regnault B, Grimont PA. 1997. Streptococcus infantarius sp. nov. related to Streptococcus bovis and Streptococcus equinus. Adv Exp Med Biol 418:393–395. doi: 10.1007/978-1-4899-1825-3_94. [DOI] [PubMed] [Google Scholar]
- 167.Schlegel L, Grimont F, Collins MD, Régnault B, Grimont PA, Bouvet A. 2000. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov., isolated from humans and food. Int J Syst Evol Microbiol 50(Part 4):1425–1434. doi: 10.1099/00207713-50-4-1425. [DOI] [PubMed] [Google Scholar]
- 168.Coykendall AL. 1977. Proposal to elevate the subspecies of Streptococcus mutans to species status, based on their molecular composition. Int J Syst Bacteriol 27:26–30. doi: 10.1099/00207713-27-1-26. [DOI] [Google Scholar]
- 169.Whiley RA, Russell RRB, Hardie JM, Beighton D. 1988. Streptococcus downei sp. nov. for strains previously described as Streptococcus mutans serotype h. Int J Syst Bacteriol 38:25–29. doi: 10.1099/00207713-38-1-25. [DOI] [Google Scholar]
- 170.Willcox MD, Zhu H, Knox KW. 2001. Streptococcus australis sp. nov., a novel oral streptococcus. Int J Syst Evol Microbiol 51:1277–1281. doi: 10.1099/00207713-51-4-1277. [DOI] [PubMed] [Google Scholar]
- 171.Camelo-Castillo A, Benítez-Páez A, Belda-Ferre P, Cabrera-Rubio R, Mira A. 2014. Streptococcus dentisani sp. nov., a novel member of the mitis group. Int J Syst Evol Microbiol 64:60–65. doi: 10.1099/ijs.0.054098-0. [DOI] [PubMed] [Google Scholar]
- 172.Kawamura Y, Hou XG, Todome Y, Sultana F, Hirose K, Shu SE, Ezaki T, Ohkuni H. 1998. Streptococcus peroris sp. nov. and Streptococcus infantis sp. nov., new members of the Streptococcus mitis group, isolated from human clinical specimens. Int J Syst Bacteriol 48(Part 3):921–927. doi: 10.1099/00207713-48-3-921. [DOI] [PubMed] [Google Scholar]
- 173.Glazunova OO, Raoult D, Roux V. 2006. Streptococcus massiliensis sp. nov., isolated from a patient blood culture. Int J Syst Evol Microbiol 56:1127–1131. doi: 10.1099/ijs.0.64009-0. [DOI] [PubMed] [Google Scholar]
- 174.Kilian M, Mikkelsen L, Henrichsen J. 1989. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906). Int J Syst Bacteriol 39:471–484. doi: 10.1099/00207713-39-4-471. [DOI] [Google Scholar]
- 175.Bridge PD, Sneath PHA. 1982. Streptococcus gallinarum sp. nov. and Streptococcus oralis sp. nov. Int J Syst Bacteriol 32:410–415. doi: 10.1099/00207713-32-4-410. [DOI] [Google Scholar]
- 176.Whiley RA, Fraser HY, Douglas CWI, Hardie JM, Williams AM, Collins MD. 1990. Streptococcus parasanguis sp. nov., an atypical viridans Streptococcus from human clinical specimens. FEMS Microbiol Lett 56:115–121. doi: 10.1111/j.1574-6968.1990.tb04133.x. [DOI] [PubMed] [Google Scholar]
- 177.Chester FD. 1901. A manual of determinative bacteriology. The MacMillan Co, New York, NY. [Google Scholar]
- 178.Arbique JC, Poyart C, Trieu-Cuot P, Quesne G, Carvalho MDGS, Steigerwalt AG, Morey RE, Jackson D, Davidson RJ, Facklam RR. 2004. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J Clin Microbiol 42:4686–4696. doi: 10.1128/JCM.42.10.4686-4696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Clarke JK. 1924. On the bacterial factor in the aetiology of dental caries. Br J Exp Pathol 5:141–147. [Google Scholar]
- 180.Coykendall AL. 1983. Streptococcus sobrinus nom. rev. and Streptococcus ferus nom. rev.: habitat of these and other mutans streptococci. Int J Syst Bacteriol 33:883–885. doi: 10.1099/00207713-33-4-883. [DOI] [Google Scholar]
- 181.Lehmann KB, Neumann R. 1896. Atlas und Grundriss der Bakteriologie und Lehrbuch der speziellen bakteriologische Diagnostik, 1st ed. JF Lehmann, Munich, Germany. [Google Scholar]
- 182.Garvie EI, Farrow JAE, Bramley AJ. 1983. Streptococcus dysgalactiae (Diernhofer) nom. rev. Int J Syst Bacteriol 33:404–405. doi: 10.1099/00207713-33-2-404. [DOI] [Google Scholar]
- 183.Sand G, Jensen CO. 1888. Die Aetiologie der Druse. Dtsch Z Tiermed Vgl Pathol 13:437–464. [Google Scholar]
- 184.Pier GB, Madin SH. 1976. Streptococcus iniae sp. nov., a beta-hemolytic streptococcus isolated from an Amazon freshwater dolphin, Inia geoffrensis. Int J Syst Bacteriol 26:545–553. doi: 10.1099/00207713-26-4-545. [DOI] [Google Scholar]
- 185.Collins MD, Farrow JAE, Katic V, Kandler O. 1984. Taxonomic studies on streptococci of serological groups E, P, U and V: description of Streptococcus porcinus sp. nov. Syst Appl Microbiol 5:402–413. doi: 10.1016/S0723-2020(84)80041-7. [DOI] [Google Scholar]
- 186.Bekal S, Gaudreau C, Laurence RA, Simoneau E, Raynal L. 2006. Streptococcus pseudoporcinus sp. nov., a novel species isolated from the genitourinary tract of women. J Clin Microbiol 44:2584–2586. doi: 10.1128/JCM.02707-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rosenbach FJ. 1884. Microorganismen bei den Wund-Infections-Krankheiten des Menschen. JF Bergmann, Wiesbaden, Germany. [Google Scholar]
- 188.Collins MD, Hutson RA, Falsen E, Nikolaitchouk N, LaClaire L, Facklam RR. 2000. An unusual Streptococcus from human urine, Streptococcus urinalis sp. nov. Int J Syst Evol Microbiol 50(Part 3):1173–1178. doi: 10.1099/00207713-50-3-1173. [DOI] [PubMed] [Google Scholar]
- 189.Whiley RA, Hardie JM. 1988. Streptococcus vestibularis sp. nov. from the human oral cavity. Int J Syst Bacteriol 38:335–339. doi: 10.1099/00207713-38-4-335. [DOI] [PubMed] [Google Scholar]
- 190.Handley P, Coykendall A, Beighton D, Hardie JM, Whiley RA. 1991. Streptococcus crista sp. nov., a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int J Syst Bacteriol 41:543–547. doi: 10.1099/00207713-41-4-543. [DOI] [PubMed] [Google Scholar]
- 191.Tong H, Gao X, Dong X. 2003. Streptococcus oligofermentans sp. nov., a novel oral isolate from caries-free humans. Int J Syst Evol Microbiol 53:1101–1104. doi: 10.1099/ijs.0.02493-0. [DOI] [PubMed] [Google Scholar]
- 192.White JC, Niven CF. 1946. Streptococcus S.B.E.: a streptococcus associated with subacute bacterial endocarditis. J Bacteriol 51:717–722. doi: 10.1128/jb.51.6.717-722.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ayers SH, Mudge CS. 1922. The streptococci of the bovine under: IV. Studies of the streptococci. J Infect Dis 31:40–50. doi: 10.1093/infdis/31.1.40. [DOI] [Google Scholar]
- 194.Devriese LA, Vandamme P, Collins MD, Alvarez N, Pot B, Hommez J, Butaye P, Haesebrouck F. 1999. Streptococcus pluranimalium sp. nov., from cattle and other animals. Int J Syst Bacteriol 49(Part 3):1221–1226. doi: 10.1099/00207713-49-3-1221. [DOI] [PubMed] [Google Scholar]
- 195.Woo PCY, Tam DMW, Leung K-W, Lau SKP, Teng JLL, Wong MKM, Yuen K-Y. 2002. Streptococcus sinensis sp. nov., a novel species isolated from a patient with infective endocarditis. J Clin Microbiol 40:805–810. doi: 10.1128/JCM.40.3.805-810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kilpper-Balz R, Schleifer KH. 1987. Streptococcus suis sp. nov., nom. rev. Int J Syst Bacteriol 37:160–162. doi: 10.1099/00207713-37-2-160. [DOI] [Google Scholar]