Abstract
Transcription factors E26 transformation-specific-1 (Ets-1) and Friend leukemia insertion site-1 (Fli-1) and type I interferon (IFN) have been implicated in systemic lupus erythematosus (SLE). We examined the expression of these genes in peripheral blood mononuclear cells (PBMCs) from Japanese patients with SLE and analyzed their association with SLE. We enrolled 53 Japanese patients with SLE, 42 patients with rheumatoid arthritis (RA), and 30 healthy donors (HDs) (as controls) in this study. PBMCs were collected from all participants, and the expressions of Ets-1, Fli-1, and three interferon-inducible genes (IFIGs) (interferon-inducible protein with tetratricopeptide 1 [IFIT1], interferon-inducible protein 44 [IFI44], and eukaryotic translation initiation factor 2 alpha kinase 2 [EIF2AK2]) were measured using real-time polymerase chain reaction (PCR). The relationships of each molecule with clinical symptoms, laboratory data, and treatments were analyzed. The expression of Ets-1 and Fli-1 was significantly lower in the PBMCs from patients with SLE than that in the PBMCs from patients with RA and HDs. The expression of the three IFIGs was significantly higher in the PBMCs from patients with SLE than that in the PBMCs from patients with RA and HDs. For patients with SLE, significantly positive correlations were found between Ets-1 and three IFIGs; a similar trend was observed between Fli-1 and IFIGs. IFIG expression in the PBMCs was significantly higher in patients with SLE than that in other participants, and the expression of Ets-1 and Fli-1 was positively associated with IFN expression. Therefore, it was suggested that Ets-1 and Fli-1 were associated with the pathophysiology of SLE by regulating the type I IFN pathway.
Keywords: Ets-1, Fli-1, interferon-inducible gene, systemic lupus erythematosus, type I interferon
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with a wide spectrum of clinical and immunological abnormalities.[1,2] SLE is characterized by arthritis, glomerulonephritis, vasculitis, and autoantibody production.[1,2] The pathogenesis of SLE is multifactorial and includes contributions from the environment, stochastic factors, and genetic susceptibility.[3] The immunological abnormalities of SLE are believed to include a mechanism in which nuclear antigens are exposed to the immune system for a prolonged period due to phagocytosis of macrophages and other cells, resulting in autoantibody induction, as well as a mechanism in which immune complexes are taken up by plasmacytoid dendritic cells (PDCs) and bind to toll-like receptors 7 and 9, inducing type I interferon (IFN) production, which in turn induces inflammation and autoantibody production.[3] The production of type I IFNs (with IFN-α as the dominant mediator) was reported to increase in SLE and play an important role in its pathogenesis.[4–6] Several IFN-targeted therapies for SLE have been examined in clinical trials,[7–9] and anifrolumab, a fully human immunoglobulin G1 kappa monoclonal antibody that targets several type I IFNs through binding of the type I IFN receptor subunit 1, improved the pathogenesis of SLE and has started to be used as a therapeutic agent.[10–13]
E26 transformation-specific-1 (Ets-1) and Friend leukemia insertion site-1 (Fli-1) belong to the Ets family of transcription factors, which share a common 85-amino-acid DNA binding domain.[14,15] Ets transcription factors control a wide variety of cellular processes including cell proliferation and differentiation.[14,15] The gene expression of Ets-1 was reported to be lower and the gene expression of Fli-1 higher in the peripheral blood mononuclear cells (PBMCs) of patients with SLE.[16,17] Ets-1 mutant mice were also reported to spontaneously develop SLE-like disease.[18] The involvement of Fli-1 in lupus was established by Fli-1 transgenic mice with 2- to 3-fold Fli-1 overexpression that developed a lupus-like disease with a progressive immune complex-mediated kidney disease and ultimately died of renal failure.[19] Fli-1 heterozygous knockout mice of lupus model mice showed significantly reduced SLE-like disease.[20,21] Two genome-wide association studies of Chinese patients with SLE independently found that genetic variants in Ets-1 are associated with susceptibility to SLE.[22,23] Ets-1 has also been implicated in numerous cellular abnormalities that are known to participate in the pathogenesis of SLE, playing an inhibitory role in T helper 17 (Th17) cell, B-cell, and T follicular helper type 2 cell differentiation.[24–27] Based on the results from Fli-1 hetero-knockout and functional domain knockout lupus model mice, Fli-1 modulates T cells, including a cluster of differentiation 3 (CD3+), CD4+, regulatory T cells, and B cells. Fli-1 also modulates mononuclear phagocyte development, including dendritic cells and macrophages.[15,28–30] Fli-1 also regulates the production of inflammatory cytokines and chemokines including interleukin-6, C-C motif chemokine ligand 5, and monocyte chemoattractant protein-1, which contribute to the pathogenesis of SLE and lupus nephritis.[15,31–33]
Although type I IFNs and corresponding modulating systems are important in the pathogenesis of SLE, the relationship between Ets-1/Fli-1 and type I IFNs is still unclear. Furthermore, few reports on the expression of Ets-1 and Fli-1 in SLE in clinical practice exist. In this report, we measured the gene expression of Ets-1 and Fli-1 in the PBMCs of patients with SLE and those with rheumatoid arthritis (RA) followed-up by Fukushima Medical University (Japan). We investigated the differential expression of Ets-1, Fli-1, and interferon-inducible genes (IFIGs) among patients with SLE, those with RA, and healthy donors (HDs) and analyzed the associations between those genes and the clinical manifestations including symptoms, disease activity, laboratory results, and IFIGs.
2. Materials and Methods
2.1. Patients and controls
Our cohort included 53 patients with SLE (4 men, 49 women, median age 36.0 years, interquartile range [IQR] 28.0–46.0 years), 42 patients with RA (14 men, 28 women, median age 63.5 years, IQR 57.8–69.3), and 30 HDs (6 men, 24 women, median age 33.5 years, IQR 27.8–40.3). Among the 53 SLE patients, 48 were receiving treatment, and 5 were not. The SLE and RA patients were followed-up at Fukushima Medical University Hospital. The patients with SLE met the American College of Rheumatology revised criteria for the classification of SLE,[34] and the patients with RA met the American Rheumatism Association 1987 classification of RA[35] or the 2010 RA classification criteria (American College of Rheumatology/European League Against Rheumatism).[36] The SLE disease activity score was calculated based on the SLE Disease Activity Index (SLEDAI)-2K instrument.[37] Written informed consent was obtained from all participants, and the study was approved by the appropriate ethics committee.
2.2. Samples and complementary DNA
Peripheral blood was obtained from all participants. PBMCs were isolated from 10 mL of heparinized peripheral blood via Ficoll–Paque Plus (Amersham Biosciences, Uppsala, Sweden), following the protocol recommended by the manufacturer. One million cells of each sample were spun down to pellets, and total RNA was extracted from the cell pellets using Isogen (Nippon Gene, Tokyo, Japan). Complementary DNA was synthesized using SuperScript™ II Reverse Transcriptase (Thermo Fisher Scientific, Yokohama, Japan), following the instructions provided by the manufacturer. Complementary DNA was used to quantify the expression of several genes. Residual cells were suspended in Cellbanker 2 (Takara Bio Inc., Kusatsu, Japan) and stored at -80 °C before use for flow cytometry.
2.3. Real-time reverse transcriptase-PCR
The complementary DNA samples were amplified with specific primers and fluorescence-labeled probes for the target genes. Amplified product genes were monitored on a StepOne Real-Time PCR system (Thermo Fisher Scientific). TaqMan Fast Universal PCR Master Mix (2×) was purchased from Thermo Fischer Scientific. Mixtures of one pair of primer and fluorescent probe (TaqMan Gene Expression Assays) for Ets-1 (Hs 00428293_m1), Fli-1 (Hs 00956709_m1), IFIT1 (Hs 01911452_s1), IFI44 (Hs 00951349_m1), EIF2AK2 (Hs 00169345_m1), and GAPDH (Hs 02786624_g1) were purchased from Applied Biosystems (South San Francisco, CA). The thermal cycle conditions were as follows: 95 °C for 2 minutes, then 40 cycles at 95 °C for 1 second and 60 °C for 20 seconds. In all measurements, a specific sample was used to adjust the relative amount of gene expression in each measurement. All measurements were performed in triplicate. Each gene expression was corrected via GAPDH gene expression.
2.4. Flow cytometry
Single-cell suspensions were prepared from the PBMCs. The cells were stained with fluorochrome-conjugated antibodies and analyzed on a FACSCanto II flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Data were analyzed via FlowJo software (Tomy Digital Biology Co., Ltd, Tokyo, Japan). The antibodies were purchased from BioLegend (San Diego, CA) and eBioscience (San Diego, CA, USA). The following specific antibodies were used to characterize the cell subsets: PDCs [CD45 + lineage (CD2, CD3, CD14, CD16, CD19, CD56, CD235a) − HLA-DR + CD123+].
2.5. Statistical analysis
To determine statistically significant differences between the two groups, we employed the Mann–Whitney U test and Fisher exact test when comparing the two categories. A P value of <.05 was considered statistically significant. GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA) was applied to perform all of the statistical analyses.
2.6. Ethical approval and consent to participate
The study was approved by the Ethics Committee of Fukushima Medical University (No. 1648), and written informed consent was obtained from all participants.
3. Results
3.1. Patient characteristics
Table 1 lists the participants’ demographics. All participants in this study were Japanese; the patients with SLE had a median age of 36.0 years (interquartile rate [IQR] 28.0–46.0) and were significantly younger than the patients with RA (36 years vs 63.5 years; P < .001). No statistically significant difference was found between the patients with SLE and the HDs. Of the patients with SLE, 92.5% were women, a significantly larger proportion than for the patients with RA (66.7%; P = .003). For the study patients with SLE, the median disease duration was 8.0 years (IQR 3.0–17.0), the median prednisolone dose was 10.0 mg (IQR 5.0–15.0), and the median SLEDAI-2K score was 4 (IQR 2.0–8.0). Table 2 lists the clinical characteristics of SLE patients. Since the majority of these patients were receiving treatment at the time of the study’s admission, their SLEDAI-2K scores were often low.
Table 1.
Baseline characteristics of the participants.
| SLE | RA | HD | SLE vs RAP | SLE vs HDP | |
|---|---|---|---|---|---|
| Number of subjects | 53 | 42 | 30 | ||
| Median age (IQR), year | 36.0 (28.0, 46.0) | 63.5 (57.8, 69.3) | 33.5 (27.8, 40.3) | <.001† * | .29† |
| Number of woman (%) | 49 (92.5) | 28 (66.7) | 24 (80) | .003†† * | .16†† |
| Median disease duration (IQR), year | 8.0 (3.0, 17.0) | 7.0 (2.0, 12.8) | NA | .45† | - |
| Median prednisolone dosage (IQR), mg/day | 10.0 (5.0, 15.0) | 0 (0, 2.3) | NA | <.001† * | - |
| Median SLEDAI-2K (IQR) | 4.0 (2.0, 8.0) | NA | NA | - | - |
| Median ESR (IQR), mm/h | 7.0 (4.0, 14.0) | 8.0 (4.0, 19.0) | NA | .65† | - |
ESR = erythrocyte sedimentation rate, HD = healthy donor, IQR = interquartile range, NA = not applicable, RA = rheumatoid arthritis, SLE = systemic lupus erythematosus, SLEDAI-2K = systemic lupus erythematosus disease activity index 2000.
Mann–Whitney U test.
Fisher exact test.
indicates P < .05.
Table 2.
Characteristics of patients of SLE at study initiation.
| Total number of SLE patients | 53 |
| Median age (IQR), year | 36.0 (28.0, 46.0) |
| Number of women (%) | 49 (92.5) |
| Median disease duration (IQR), year | 8.0 (3.0, 17.0) |
| Number of untreated patients (%) | 5 (9.4) |
| Median SLEDI-2K (IQR) | 4 (2, 8) |
| Clinical manifestations during the disease period, number (%) | |
| Skin rash | 32 (60.4) |
| Oral ulcer | 8 (15.0) |
| Arthritis | 35 (66.0) |
| Serositis | 16 (30.2) |
| Nephritis | 28 (52.8) |
| Neurological disorder | 17 (32.1) |
| Hematological disorder | 48 (90.6) |
| Laboratory characteristics, number (%) | |
| Leukopenia (<4000/μL) | 10 (18.9) |
| Low serum complement level (C3, C4) | 37 (69.8) |
| Increased titer of anti-DNA antibodies | 31 (58.5) |
| Treatment, number (%) (in 48 treated patients) | |
| No therapy | 2 (4.2) |
| Prednisolone ≦ 10 mg/day | 26 (49.1) |
| Prednisolone > 10 mg/day | 20 (41.7) |
| Use of immunosuppressants | 14 (26.4) |
IQR = interquartile range, SLE = systemic lupus erythematosus, SLEDAI = SLE disease activity index 2000.
3.2. Ets-1/Fli-1 expression in the PBMCs of the HDs and patients with SLE or RA
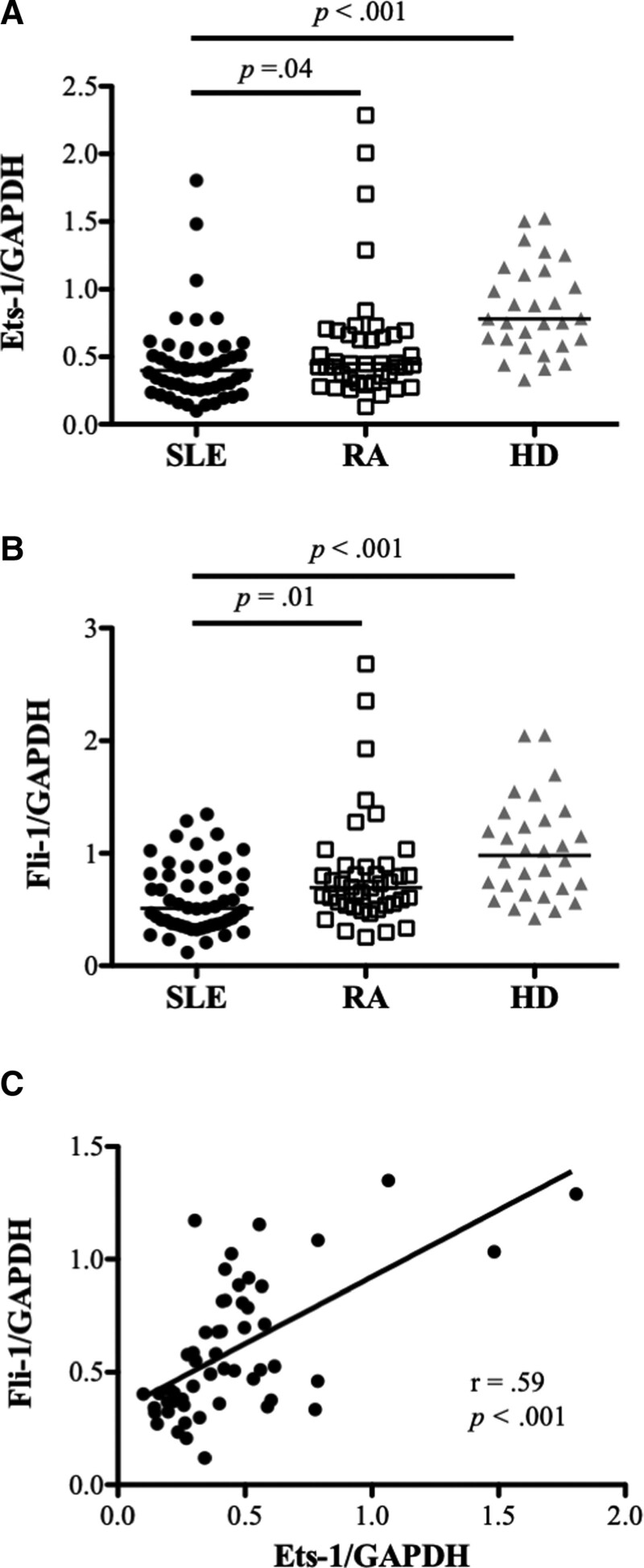
We measured the gene expression of Ets-1 and Fli-1 in the PBMCs from the HDs and patients with SLE or RA. Ets-1 expression in the patients with SLE was significantly lower than in the HDs and patients with RA (P = .04 for SLE vs RA; P < .001 for SLE vs HD). Fli-1 expression in the patients with SLE was significantly lower than in the HDs and patients with RA (P = .01 for SLE vs RA; P < .001 for SLE vs HD). A significantly positive correlation was found between the gene expression of Ets-1 and Fli-1 in the patients with SLE (r = .59, P < .001) (Fig. 1).
Figure 1.
The expression of Ets-1 and Fli-1 in peripheral blood mononuclear cells (PBMCs) from patients with systemic lupus erythematosus (SLE), patients with rheumatoid arthritis (RA), and healthy donors (HDs). Each symbol represents an individual sample; horizontal lines show median values. (A) Relative expression levels of Ets-1 in PBMCs were significantly lower in the patients with SLE (n = 53) than in the patients with RA (n = 42) and the HDs (n = 30). (B) Relative Fli-1 expression levels in PBMCs were significantly lower in the patients with SLE than in the patients with RA and the HDs. (C) Relative Fli-1 expression levels were positively correlated with the relative expression of Ets-1 in the patients with SLE.
3.3. Association between Ets-1 or Fli-1 and clinical findings in the patients with SLE
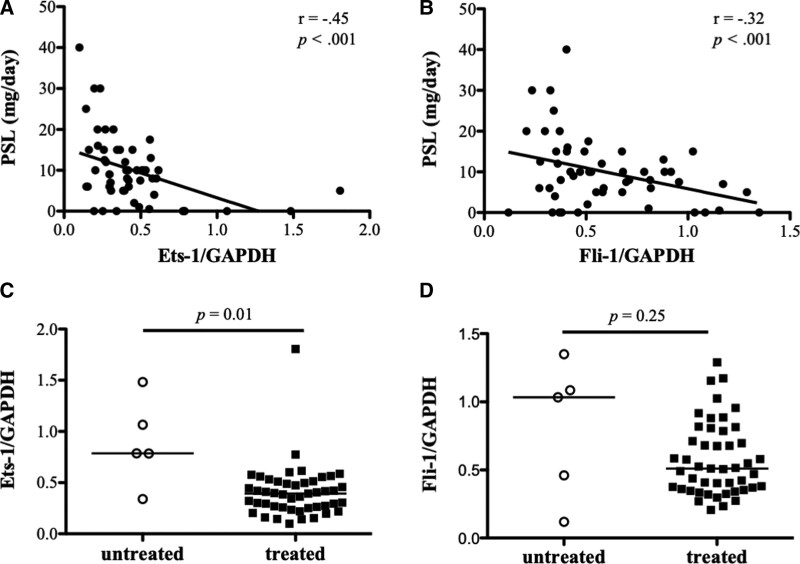
We analyzed the association between the expression of Est-1/Fli-1 and clinical findings including symptoms, laboratory data, and treatments for patients with SLE. Significantly negative correlations were found between the expression of Ets-1 and Fli-1 and the amount of prednisolone administered (Ets-1, r = −.45, P < .001; Fli-1, r = −.32, P < .001) (Fig. 2A and B). Ets-1 expression was considerably higher in the untreated group than the treatment group (Est-1: P = .01 for untreated vs. treated; Fli-1: P = .25 for untreated vs treated) according to a comparison of Ets-1 and Fli-1 expression in the untreated and treated groups (Fig. 2C and D). In the other items (e.g., skin rash, arthritis, renal involvement, neuropsychiatric symptoms, hematological abnormalities, and anti-DNA antibodies), no statistically significant correlations were found with Ets-1 or Fli-1.
Figure 2.
Association between Ets-1/Fli-1 and prednisolone dose in the patients with systemic lupus erythematosus (SLE). (A) The relative expression levels of Ets-1 in peripheral blood mononuclear cells (PBMCs) of the patients with SLE (n = 53) were negatively correlated with the prednisolone (PSL) dose. (B) The relative expression levels of Fli-1 in the PBMCs of the patients with SLE were negatively correlated with the prednisolone dose. (C) Ets-1 gene expression was considerably greater in the untreated group (n = 5) than in the treated group (n = 48). (D) No statistically significant difference between the treated and untreated groups in the Fli-1 gene expression.
3.4. Association of PDCs in PBMCs of patients with SLE and Ets-1 or Fli-1
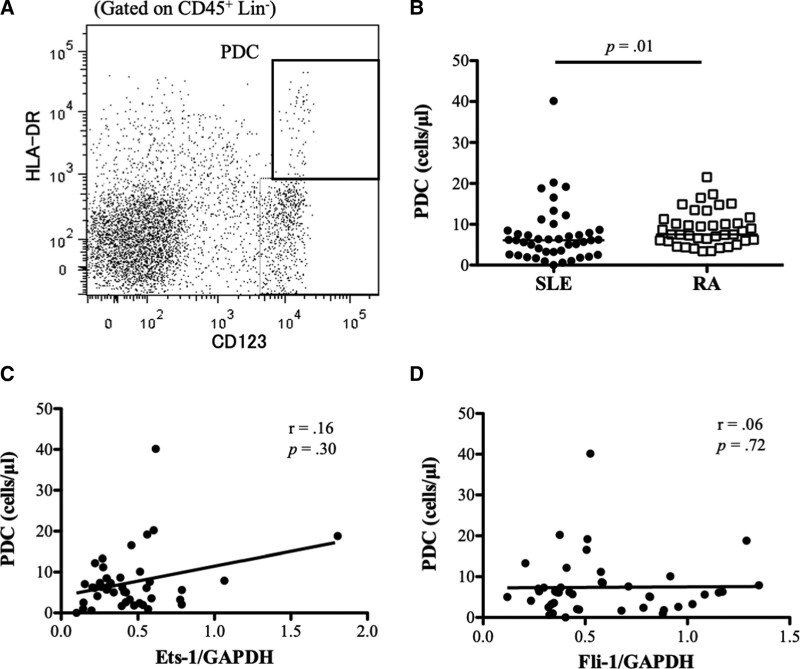
The PDCs in the PBMCs were detected via flow cytometry and were defined as CD45+ lineage (CD2, CD3, CD14, CD16, CD19, CD56, CD235a)−HLA-DR+CD123+ cells. Figure 3A shows a representative result. Significantly fewer PDCs were found in the PBMCs of the patients with SLE than in the patients with RA (6.1/μL [IQR 2.6–8.5] vs 7.5/μL [IQR 6.0–11.3]; P = .01) (Fig. 3B). No statistically significant correlation was found between Ets-1 or Fli-1 and the number of PDCs (Ets-1, r = .16, P = .29; Fli-1, r = .06, P = .72) (Fig. 3C and D).
Figure 3.
Association between Ets-1/Fli-1 and the plasmacytoid dendritic cells (PDCs) in the peripheral blood mononuclear cells (PBMCs) of patients with systemic lupus erythematosus (SLE). (A) Flow cytometry analysis of PDCs in PBMCs was performed. PDCs were defined as CD45+ lineage (CD2, CD3, CD14, CD16, CD19, CD56, CD235a)− HLA-DR+CD123+. (A) Representative result is shown. (B) Significantly fewer PDCs were found in the PBMCs of the patients with SLE than in patients with rheumatoid arthritis (RA). (C) No correlation was found between Ets-1 and the number of PDCs. (D) No correlation was found between Fli-1 and the number of PDCs.
3.5. Gene expression of IFIGs and the association with Ets-1 or Fli-1
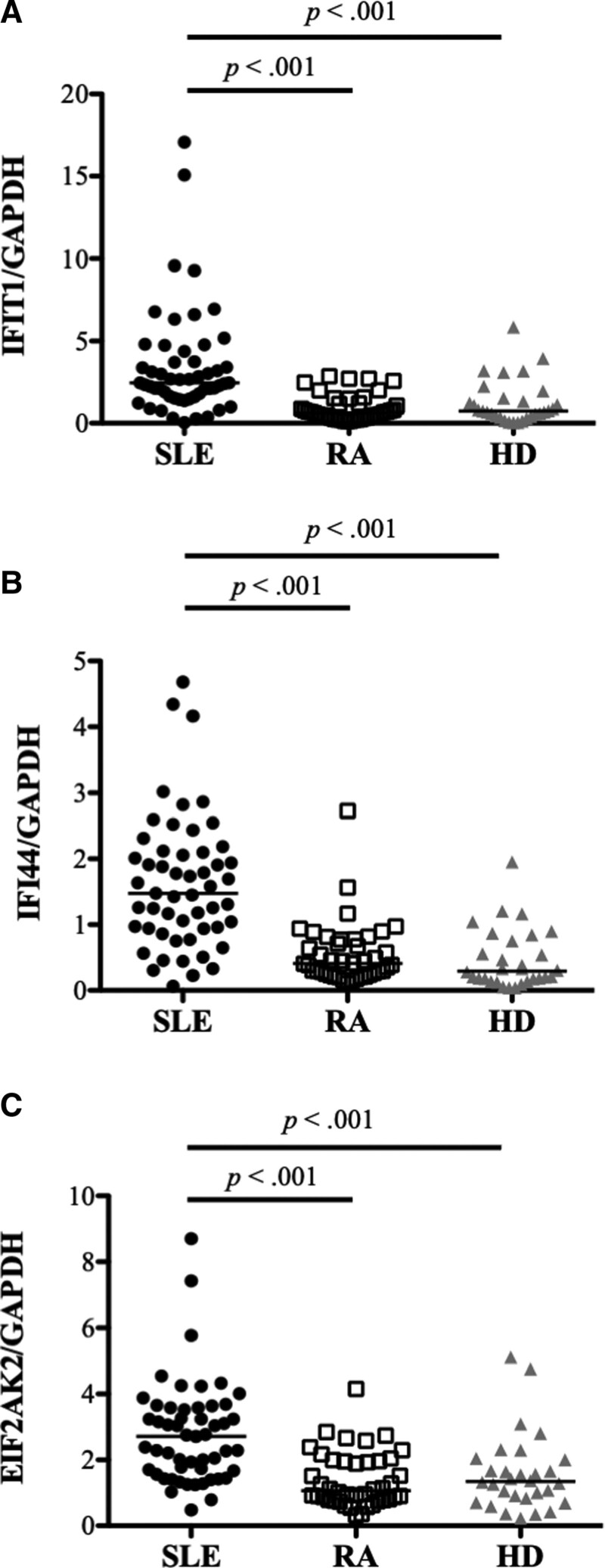
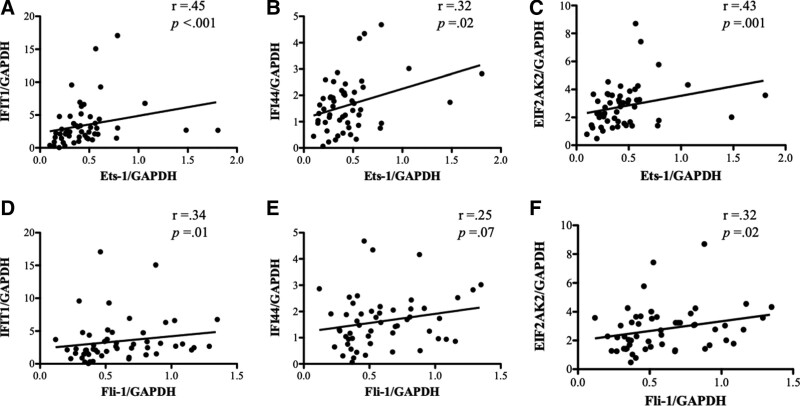
The gene expression of representative IFIGs (interferon-inducible protein with tetratricopeptide 1 [IFIT1], interferon-inducible protein 44 [IFI44], and eukaryotic translation initiation factor 2 alpha kinase 2 [EIF2AK2]) from PBMCs was measured using real-time PCR. These three IFIGs were selected based on a study conducted by Kirou et al[4] In the patients with SLE, the gene expression of IFIT1, IFI44, and EIF2AK2 was significantly higher than that in the HDs and patients with RA (Fig. 4A–C). In the patients with SLE, significantly positive correlations were found between Ets-1 and IFIGs (IFIT1, r = .45, P < .001; IFI44, r = .32, P = .02; EIF2AK2, r = .43, P = .001) (Fig. 5A–C), and significantly positive correlations were found between Fli-1 and IFIGs except for IFI44 (IFIT1, r = .34, P = .01; IFI44, r = .25, P = .07; EIF2AK2, r = .32, P = .02) (Fig. 5D–F).
Figure 4.
The gene expression of interferon-inducible genes in the peripheral blood mononuclear cells (PBMCs) of patients with systemic lupus erythematosus (SLE), patients with rheumatoid arthritis (RA), and healthy donors (HDs). Each symbol represents an individual sample; horizontal lines show median values. (A) In the patients with SLE, the gene expression of interferon-inducible protein with tetratricopeptide 1 (IFIT1) was significantly higher than that in the HDs and patients with RA. (B) In the patients with SLE, the gene expression of interferon-inducible protein 44 (IFI44) was significantly higher than in the HDs and patients with RA. (C) In the patients with SLE, the gene expression of eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2) was significantly higher than in the HDs and patients with RA.
Figure 5.
Gene expression of interferon-inducible genes (IFIGs) and association with Ets-1 or Fli-1. (A–C) In the patients with systemic lupus erythematosus (SLE), significantly positive correlations were found between Ets-1 and all IFIGs (interferon-inducible protein with tetratricopeptide 1 [IFIT1], interferon-inducible protein 44 [IFI44], and eukaryotic translation initiation factor 2 alpha kinase 2 [EIF2AK2]). (D‐F) In the patients with SLE, significantly positive correlations were found between Ets-1 and IFIGs, except for IFI44.
4. Discussion
In this study, we found that the expression of type I IFN-induced genes was significantly higher in the PBMCs from patients with SLE than from HDs or patients with RA. The gene expression of Ets-1 and Fli-1 was significantly lower in patients with SLE than in the HDs or patients with RA. A positive correlation was found between the gene expression of Ets-1/Fli-1 and several IFIGs in the patients of SLE.
Among the SLE-associated genes that have undergone genome-wide association studies, Ets-1 has been identified along with molecules related to DNA modification and IFN-related genes.[22,23,38,39] The identification of a large number of IFN-related genes in the pathogenesis of SLE reaffirms the importance of type I IFNs in the pathogenesis of SLE. Previous studies have also reported that Ets-1 is involved in the pathogenesis of SLE. Ets-1 had been reported as a negative regulator of B-cell differentiation and Th 17 cell proliferation, and patients with SLE have demonstrated reduced Est-1 expression, which might contribute to abnormal B-cell differentiation into immunoglobulin-secreting plasma cells and an increased number of Th 17 cells.[40] Ets-1 was also reported to inhibit the differentiation of T follicular helper type 2 cells and regulate the development of SLE.[26] These reports; therefore, suggest that Ets-1 might be involved in the pathogenesis of SLE by regulating the function of B and T cells. Few reports describe a direct relationship between type I IFN and Ets-1. The positive correlation between the expression levels of Ets-1 and the three IFIGs in the patients with SLE in this study suggests that Ets-1 might be associated with the expression of type I IFN (Fig. 5A–C). The results for increased production of IFN signatures include PDC activation, retention of risk alleles, and dysfunction of B cells and natural killer cells.[41] In this study, we were unable to correlate Ets-1 expression with the number of PDCs, and we did not investigate PDC activation in this report. Previous reports have found an association between Ets-1 and B-cell activation, and Ets-1 is also required for natural killer cell differentiation.[42] In terms of risk alleles, an association has been suggested between single nucleotide polymorphisms and IFN regulatory factor 5 and Ets-1, molecules involved in innate immunity that are thought to be important in the development of SLE.[43,44] Rutherford et al showed that Ets-1 competes for the binding site of IFN-stimulated gene factors 3 and IFN-stimulated response element and mentioned the possibility that Ets transcription factors negatively regulate IFN transcriptional activity via an IFN-stimulated response element.[45] Wen et al reported that in patients with SLE, the DNA containing immune complex induced anti-dsDNA antibodies through the Toll-like receptor 2/MyD88/microRNA-155 pathway, that is, microRNA-155 inhibited the expression of Ets-1, resulting in the reduction of anti-dsDNA antibodies through activating Blip-1.[46] This mechanism may lead to an increased type I IFN production via activation of PDC by immune complexes containing anti-ds-DNA antibodies. Ets-1 might also be involved in regulating type I IFNs through direct and indirect mechanisms.
In this study, we found that Fli-1 expression in the PBMCs from the patients with SLE was significantly lower than that in the HDs and patients with RA, which contradicts a previous report showing higher Fli-1 expression in PBMCs from patients with SLE compared with healthy controls.[16] Several possible explanations exist for this difference. First, we found that Ets-1 had significantly lower expression in the PBMCs of our patients with SLE, and Ets-1 was reported to regulate Fli-1 expression.[47] Thus, the lower Fli-1 expression in the PBMCs in our study might be partially due to Ets-1 expression, which, in a previous report, did not differ between patients with SLE and healthy controls.[16] Second, we demonstrated that Fli-1 regulates the expression of inflammatory cytokines through type I IFN stimulation. Reduced Fli-1 expression in human mesangial cells resulted in significantly less inflammatory cytokine production following type I IFN stimulation.[48] High type I IFN in patients with SLE might have a negative feedback loop to lower Fli-1 expression. Another explanation is the difference between the patients with SLE enrolled in our study and those in previous studies. Conclusively, the various methods utilized in our study and the prior publication to evaluate gene expression may have contributed to the discrepancy in the results. We found that although the level of Fli-1 expression is important for regulating inflammatory cytokines, reduced Fli-1 expression by specific Fli-1 siRNA or Fli-1 inhibitors resulted in significantly decreased inflammatory cytokine production in renal cells and immune cells. Fli-1 is largely activated through posttranslational modifications following stimulation rather than by transcription levels.[49] (Zhang et. al., unpublished data) Interpreting the collaboration between Ets-1 and Fli-1 and prednisolone dose is equally challenging. Furthermore, it was challenging to determine whether the decrease in Ets-1 and Fli-1 expression with increasing doses of prednisone was more of a direct effect of prednisolone or a secondary effect of improved SLE status even though gene expression levels of both were high in the untreated group (Fig. 2C and D).
In terms of IFIG expression, the gene expression of IFIT1, IFI44, and EIF2KA2 was predominantly higher in Japanese patients with SLE than that in HDs or patients with RA. These results were similar to those of a previous report.[4] The dataset used in this study was taken from a study by Panwar et al[50] In this study by Panwar et al, the expression of Ets-1 in the PDCs of patients with SLE was low, whereas the expression of Fli-1 was high. The relationship between IFIGs and the expression of Ets-1 or Fli-1 tended to be often negative. The results of Panwar et al study differed from those of our study. There are several explanations for the differences in the results between our study and Panwar et al study, including racial differences, disease condition of patients with SLE (many patients in this study were treated and were relatively less severe), different cell types and PBMCs used, and the effects of steroid and other therapeutic drugs used. Type I IFN and IFIG blood levels are higher in SLE patients, according to previous reports.[51,52] Studies have reported that Japanese people have distinct genetic polymorphisms than people of other racial groups in relation to IFN regulatory factor 5, a crucial molecule in type I IFN regulation.[53] The findings imply that ethnic disparities in IFN synthesis and IFIG expression may exist in SLE. Additionally, we were able to demonstrate that patients with SLE had considerably fewer PDCs in their PBMCs than patients with RA. Owing to the fact that activated PDCs appeared to infiltrate inflammatory tissue, the frequency of circulating PDCs appeared to decrease in patients with SLE when compared to healthy controls.[54,55] Additionally, the PDC frequency in early RA patients is known to be lower than that of healthy controls.[56] Our search has not yet brought up any reports presenting a comparison of articles comparing PDC counts in patients with SLE and RA’s peripheral blood. According to these findings, PDC may play a larger role in the pathogenesis of SLE in the tissues.
The present study has several limitations that should be acknowledged. First, the sample size was relatively small. It is therefore difficult to perform a sufficient statistical analysis. Second, due to the study’s retrospective nature, heterogeneity among the patients with SLE could not be ruled out. Owing to differences in the timing of treatments, the treatment regimen varied based on time and pathogenesis. At the time of entry into this study, many of the patients were in remission after a period of high disease activity. Third, we could not rule out the possibility that the high median dose of prednisolone (10 mg) in patients with SLE affected the results for each gene expression. Fourth, although it might be due to the disease characteristics, differences in the sex and age distribution of the patients with RA were found, which might be a potential bias in the comparison. Lastly, the small volume of specimens from each individual did not allow the measurement of the expression of each gene on a segmented PBMC or the measurement of the gene expression of different IFIGs. We were unable to examine the expression levels of various IFIGs and genes after subdividing the PBMCs. A large, multicenter, prospective study of patients before treatments and of patients with relatively similar treatments needs to be conducted to reveal more detailed and accurate information on the mechanisms.
In summary, we found that IFIG expression was significantly higher in the PBMCs from patients with SLE than that from HDs and patients with RA. IFIT1 expression was positively related to the expression of Ets-1 and Fli-1 in the PBMCs from patients with SLE. In addition to B and T cell-mediated pathways as previously reported, Ets-1 and Fli-1 could regulate the pathogenesis of SLE through type I IFNs. The role of Ets-1 and Fli-1 in SLE needs further investigation.
Acknowledgments
We would like to thank Chikako Saito and Kyoko Onuma for the excellent technical support, Dr Hideharu Sekine and Dr Takeshi Machida (Department of Immunology, Fukushima Medical University School of Medicine) for their accurate advice and technical assistance, and Dr Ei Wakamatsu (Department of Immunology, Tokyo Medical University) for his appropriate advises and verification of this paper. We would like to thank Enago (www.enago.jp) for the English language review. Lastly, we would like to thank all of the participants in this study.
Author contributions
Conceptualization: Eiji Suzuki, Makiko Yashiro-Furuya, Xian K Zhang.
Data curation: Eiji Suzuki, Makiko Yashiro-Furuya.
Formal analysis: Eiji Suzuki, Kiyoshi Migita, Makiko Yashiro-Furuya, Xian K Zhang.
Funding acquisition: Eiji Suzuki, Kiyoshi Migita, Hiromasa Ohira, Takashi Kanno.
Investigation: Eiji Suzuki, Hiroko Obayashi, Makiko Yashiro-Furuya, Tomoyuki Asano.
Methodology: Eiji Suzuki, Kiyoshi Migita, Xian K Zhang.
Project administration: Eiji Suzuki, Hiromasa Ohira, Kiyoshi Migita.
Resources: Eiji Suzuki, Hiroko Obayashi, Hiromasa Ohira, Kiyoshi Migita, Makiko Yashiro-Furuya, Tomoyuki Asano, Xian K Zhang.
Software: Eiji Suzuki, Kiyoshi Migita, Xian K Zhang.
Supervision: Kiyoshi Migita, Hiromasa Ohira, Xian K Zhang.
Validation: Eiji Suzuki, Kiyoshi Migita, Xian K Zhang.
Visualization: Eiji Suzuki, Kiyoshi Migita, Makiko Yashiro-Furuya, Xian K Zhang.
Writing – original draft: Eiji Suzuki, Xian K Zhang.
Writing – review & editing: Eiji Suzuki, Kiyoshi Migita, Xian K Zhang.
Abbreviations:
- CD =
- cluster of differentiation
- EIF2AK2 =
- eukaryotic translation initiation factor 2 alpha kinase 2
- Ets-1 =
- E26 transformation-specific-1
- Fli-1 =
- Friend leukemia insertion site-1
- HDs =
- healthy donors
- IFI44 =
- interferon-inducible protein 44
- IFIGs =
- interferon-inducible genes
- IFIT1 =
- interferon-inducible protein with tetratricopeptide 1
- IFN =
- interferon
- IQR =
- interquartile range
- PBMCs =
- peripheral blood mononuclear cells
- PCR =
- polymerase chain reaction
- PDCs =
- plasmacytoid dendritic cells
- RA =
- rheumatoid arthritis
- SLE =
- systemic lupus erythematosus
- SLEDAI =
- SLE Disease Activity Index
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflict of interests to disclose.
This work was supported by JSPS KAKENHI [Grant Number 25860814].
This study was approved by the Ethics Committee of Fukushima Medical University (No. 1648).
The corresponding author will provide the datasets produced during and/or analyzed during the current research upon reasonable request.
How to cite this article: Suzuki E, Zhang XK, Yashiro-Furuya M, Asano T, Kanno T, Kobayashi H, Migita K, Ohira H. The expression of Ets-1 and Fli-1 is associated with interferon-inducible genes in peripheral blood mononuclear cells from Japanese patients with systemic lupus erythematosus. Medicine 2022;101:45(e31522).
Contributor Information
Xian K. Zhang, Email: zhangjo@musc.edu.
Makiko Yashiro-Furuya, Email: myashiro@fmu.ac.jp.
Tomoyuki Asano, Email: asanovic@fmu.ac.jp.
Takashi Kanno, Email: kanno-t@ohta-hp.or.jp.
Hiroko Kobayashi, Email: hkoba@fmu.ac.jp.
Kiyoshi Migita, Email: migita@fmu.ac.jp.
Hiromasa Ohira, Email: h-ohira@fmu.ac.jp.
References
- [1].Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. [DOI] [PubMed] [Google Scholar]
- [2].Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsokos GC, Lo MS, Costa Reis PC, et al. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–30. [DOI] [PubMed] [Google Scholar]
- [4].Kirou KA, Lee C, George S, et al. Coordinate overexpression of interferon-α-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–67. [DOI] [PubMed] [Google Scholar]
- [5].Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192:5459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crow MK. Advances in understanding the role of type I interferons in systemic lupus erythematosus. Curr Opin Rheumatol. 2014;26:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Msaasrotti EM, Allore HG, Costenbader K. Interferon-targeted therapy for systemic lupus erythematosus: are the trials of target? Arthritis Rheum. 2017;69:245–8. [DOI] [PubMed] [Google Scholar]
- [8].Yasuda S. Emerging targets for the treatment of lupus erythematosus: there is no royal road to treating lupus. Mod Rheumatol. 2019;29:60–9. [DOI] [PubMed] [Google Scholar]
- [9].Furie R, Werth VP, Merola JF, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesion in systemic lupus erythematosus. J Clin Invest. 2019;129:1359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Furie RA, Morand EF, Bruce IN, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomized, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:e208–19. [DOI] [PubMed] [Google Scholar]
- [11].Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- [12].Tanaka Y, Tummala R. Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Mod Rheumatol. 2021;31:1–12. [DOI] [PubMed] [Google Scholar]
- [13].Chatham WW, Furie R, Saxena A, et al. Long-term safety and efficacy on anifrolumab in adults with systemic lupus erythematosus: results of phase II open-label extension study. Arthritis Rheumatol. 2021;73:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Leng RX, Pan HF, Chen GM, et al. The dual nature of Ets-1: focuses on the pathogenesis of systemic lupus erythematosus. Autiummun Rev. 2011;10:439–43. [DOI] [PubMed] [Google Scholar]
- [15].Xu WD, Zhang M, Zhao Y, et al. Fli-1, a functional factor performed in autoimmune lupus. Inflammation. 2016;39:493–8. [DOI] [PubMed] [Google Scholar]
- [16].Georgiou P, Maroulakou I, Green J, et al. Expression of ets family of genes in systemic lupus erythematosus and Sjogren’s syndrome. Int J Oncol. 1996;9:9–18. [PubMed] [Google Scholar]
- [17].Li Y, Sun LD, Lu WS, et al. Expression analysis of Ets1 gene in peripheral blood mononuclear cells with systemic lupus erythematosus by real-time reverse transcription PCR. Chin Med J (Engl). 2010;123:2287–8. [PubMed] [Google Scholar]
- [18].Wang D, John SA, Clements JL, et al. Ets-1 deficiency leads to altered B cell differentiation, hyperresponsiveness to TLR 9, and autoimmune disease. Int Immunol. 2005;17:1179–91. [DOI] [PubMed] [Google Scholar]
- [19].Zhang L, Eddy A, Teng YT, et al. An immunological renal disease in transgenic mice that overexpress Fli-1, a member of the ets family of transcription factor genes. Mol Cell Biol. 1995;15:6961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang XK, Gallant S, Molano I, et al. Decreased expression of the Ets family transcription factor Fli-1 markedly prolongs survival and significantly reduces renal disease in MRL/lpr mice. J Immunol. 2004;173:6481–9. [DOI] [PubMed] [Google Scholar]
- [21].Mathenia J, Reyes-Cortes E, Williams S, et al. Impact of Fli-1 transcription factor on autoantibody and lupus nephritis in NZM2410 mice. Clin Exp Immunol. 2010;162:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Han JW, Zheng HF, Cui Y, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–7. [DOI] [PubMed] [Google Scholar]
- [23].Yang W, Shen N, Ye DQ, et al. Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genet. 2010;6:e1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moisan J, Grenningloh R, Bettelli E, et al. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bories JC, Willerford DM, Grévin D, et al. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature. 1995;377:635–8. [DOI] [PubMed] [Google Scholar]
- [26].Kim CJ, Lee CG, Jung JY, et al. The transcription factor Ets1 suppresses T follicular helper type 2 cell differentiation to halt the onset of systemic lupus erythematosus. Immunity. 2018;49:1034–1048.e8. [DOI] [PubMed] [Google Scholar]
- [27].Pan HF, Leng RX, Tao JH, et al. Ets-1: a new player in the pathogenesis of systemic lupus erythematosus? Lupus. 2011;20:227–30. [DOI] [PubMed] [Google Scholar]
- [28].Smeets MF, Chan AC, Dagger S, et al. Fli-1 overexpression in hematopoietic progenitors deregulates T cell development and induces pre-T cell lymphoblastic leukemia/lymphoma. PLoS One. 2013;8:e62346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang XK, Moussa O, LaRue A, et al. The transcription factor Fli-1 modulates marginal zone and follicular B cell development in mice. J Immunol. 2008;181:1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suzuki E, Williams S, Sato S, et al. The transcription factor Fli-1 regulates monocyte, macrophage, and dendritic cell development in mice. Immunology. 2013;139:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sato S, Lennard Richard M, Brandon D, et al. A critical role of the transcription factor fli-1 in murine lupus development by regulation of interleukin-6 expression. Arthritis Rheumatol. 2014;66:3436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lennard Richard ML, Sato S, Suzuki E, et al. The Fli-1 transcription factor regulates the expression of CCL5/RANTES. J Immunol. 2014;193:2661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suzuki E, Karam E, Williams S, et al. Fli-1 transcription factor affects glomerulonephritis development by regulating the expression of monocyte chemoattractant protein-1 in endothelial cells in the kidney. Clin Immunol. 2012;145:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- [35].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised the criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [36].Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [37].Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- [38].Molineros JE, Yang W, Zhou XJ, et al. Confirmation of five novel susceptibility loci for systemic lupus erythematosus (SLE) and integrated network analysis of 82 SLE susceptibility loci. Hum Mol Genet. 2017;26:1205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yin X, Kim K, Suetsugu H, et al. Meta-analysis of 208370 East Asians identifies 113 susceptibility loci for systemic lupus erythematosus. Ann Rheum Dis. 2021;80:632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guerra SG, Vyse TJ, Cunninghame Graham DS. The genetics of lupus: a functional perspective. Arthritis Res Ther. 2012;14:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rönnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol. 2013;25:248–53. [DOI] [PubMed] [Google Scholar]
- [42].Barton K, Muthusamy N, Fischer C, et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–63. [DOI] [PubMed] [Google Scholar]
- [43].Ban T, Kikuchi M, Sato GR, et al. Genetic and chemical inhibition of IRF5 suppresses pre-existing mouse lupus-like disease. Nat Commun. 2021;12:4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dang J, Shan S, Li J, et al. Gene-gene interactions of IRF5, STAT4, IKZF1 and ETS1 in systemic lupus erythematosus. Tissue Antigens. 2014;83:401–8. [DOI] [PubMed] [Google Scholar]
- [45].Rutherford MN, Kumar A, Haque SJ, et al. Specific binding of the ETS-domain protein to the interferon-stimulated response element. J Interferon Cytokine Res. 1997;17:1–10. [DOI] [PubMed] [Google Scholar]
- [46].Wen Z, Xu L, Chen X, et al. Autoantibody induction by DNA-containing immune complexes require HMGB1 with the TLR2/microRNA-155 pathway. J Immunol. 2013;190:5411–22. [DOI] [PubMed] [Google Scholar]
- [47].Lelièvre E, Lionneton F, Mattot V, et al. Ets-1 regulates fli-1 expression in endothelial cells. Identification of ETS binding sites in the fli-1 gene promoter. J Biol Chem. 2002;277:25143–51. [DOI] [PubMed] [Google Scholar]
- [48].Wang X, Oates JC, Helke KL, et al. Camptothecin and topotecan, inhibitors of transcription factor Fli-1 and topoisomerase, markedly ameliorate lupus nephritis in (NZB × NZW)F1 mice and reduce the production of inflammatory mediators in human renal cells. Arthritis Rheumatol. 2021;73:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lennard Richard ML, Brandon D, Lou N, et al. Acetylation impacts Fli-1-driven regulation of granulocyte colony-stimulating factor. Eur J Immunol. 2016;46:2322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Panwar B, Schmiedel BJ, Liang S, et al. Multi-cell type gene coexpression network analysis reveals coordinated interferon response and cross-cell type correlation in systemic lupus erythematosus. Genome Res. 2021;31:659–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kirou KA, Lee C, George S, et al. Activation of the interferon-alpha pathway identified a subgroup of systemic lupus erythematosus patients with distinct serological features and active disease. Arthritis Rheum. 2005;52:1491–503. [DOI] [PubMed] [Google Scholar]
- [52].Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kawasaki A, Kyogoku C, Ohashi J, et al. Association of IRF5 polymorphisms with systemic lupus erythematosus in Japanese population: support for a crucial role of intron 1 polymorphisms. Arthritis Rheum. 2008;58:826–34. [DOI] [PubMed] [Google Scholar]
- [54].Blomberg S, Eloranta ML, Magnusson M, et al. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2524–32. [DOI] [PubMed] [Google Scholar]
- [55].Tucci M, Quatraro C, Lombardi L, et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–62. [DOI] [PubMed] [Google Scholar]
- [56].Cooles FAH, Anderson AE, Skelton A, et al. Dendritic cells in early drug naïve rheumatoid arthritis. Front Immunol. 2018;9:755. [DOI] [PMC free article] [PubMed] [Google Scholar]