Abstract
Helicobacter pylori strains that contain the cag pathogenicity island (PAI) elicit increased synthesis of gastric C-X-C chemokines, promote neutrophilic infiltration into the gastric epithelium, and stimulate the synthesis of interleukin-8 (IL-8) in cultured gastric epithelial cells. To investigate the effects of cag PAI genes on the transcription of the IL-8 gene, the Kato-3 gastric epithelial cell line was stably transfected with plasmid DNA containing the IL-8 gene promoter fused to a luciferase reporter gene. The resulting reporter cell line, L5F11, was used to monitor the effects of infection in cell culture by H. pylori 26695 and isogenic derivatives with null mutations in genes in the cag PAI on transcription of the IL-8 gene. We found that null mutations in eight open reading frames, including homologs of the Agrobacterium virB9, virB10, and virB11 genes, in the left half of the cag PAI abrogated the induction of IL-8 gene transcription. Further studies with the L5F11 cell line showed that IL-8 gene transcription induced by H. pylori was blocked by the protein tyrosine kinase inhibitor herbimycin A but not by the protein kinase C inhibitor calphostin C or by the protein kinase G inhibitor KT5823. IL-8 gene transcription in L5F11 cells could also be induced by the cytokine tumor necrosis factor alpha (TNF-α) without exposure to H. pylori. This TNF-α-induced IL-8 transcription was inhibited by the protein kinase A inhibitor H7, which had no significant effect on H. pylori-induced IL-8 transcription. These studies show that multiple genes in the left half of the cag PAI are essential for the transcription of the IL-8 gene in gastric epithelial cells and that this depends on protein tyrosine kinase activation.
Human infection by strains of the gastric pathogen Helicobacter pylori that carry a discrete 40-kb DNA segment called the cag pathogenicity island (PAI) (termed cag+ strains) is associated with the development of chronic active gastritis (12, 39, 53), peptic ulceration (11, 12, 24, 51), and atrophic gastritis (52) and with an increased risk of developing distal gastric cancer (7, 14, 38, 47). In contrast, infections by strains that lack this PAI are associated with less severe clinical outcomes.
A chronic inflammatory response that is characterized by polymorphonuclear cell infiltration into the surface epithelium (21) is thought to play a critical role in disease pathogenesis elicited by cag+ H. pylori strains (12, 19, 39, 53). H. pylori infection can induce neutrophil chemotaxis and activation directly (22, 36, 37, 56). However, host inflammatory mediators, such as the C-X-C chemokine family, which includes the neutrophil chemoattractant interleukin-8 (IL-8) (4, 5, 41), are also considered important in the immunopathogenesis of chronic gastritis. H. pylori infection is associated with increased gastric mucosal levels of members of the C-X-C chemokine family, such as IL-8 (3, 13, 15, 33, 39, 48, 53, 54), and levels of gastric C-X-C chemokines are increased in persons with cag+ H. pylori infection (39, 48, 53, 54). A major source of IL-8 in the gastric mucosa is the epithelium (15).
Critical early steps in this inflammatory pathway, in particular the induction of IL-8 synthesis and secretion in epithelial cells, can be modelled by using established gastric epithelial cell cultures (16, 17, 45). Such studies have shown that the response depends on activation of protein tyrosine kinase (1, 6, 43) and NF-κB (1, 28, 35, 46). Initial mutational studies showed that inactivation of several of the genes in the cag PAI (2, 8, 50) markedly reduced IL-8 protein secretion in gastric epithelial cells. As expected, several of these cag mutants tested were also deficient in the ability to activate NF-κB (23, 46) or tyrosine phosphorylation (43). However, additional mutational tests showed that three genes in the cag PAI, including cagA (18, 45), which encodes an immunodominant protein of unknown function (11), and cagF (23) and cagN (8), were not needed for this cell signalling response.
To examine in molecular genetic detail how H. pylori infection induces epithelial IL-8 far more efficiently than has been possible by reverse transcription-PCR and enzyme-linked immunosorbent assays (ELISAs) of mRNA and protein levels, respectively, we constructed a derivative of the Kato-3 gastric epithelial cell line containing an added IL-8 promoter sequence joined to a luciferase reporter gene. We used the resulting stably transfected cell line, L5F11, (i) to investigate the ability of H. pylori strains with null mutations in previously unstudied genes in the left half of the cag PAI to stimulate IL-8 transcription and (ii) to investigate the effects of tyrosine kinase inhibitors on H. pylori- and cytokine-induced IL-8 transcription and IL-8 secretion.
MATERIALS AND METHODS
Bacteria.
Bacteria were grown on blood agar base no. 2 (Oxoid, Basingstoke, United Kingdom) supplemented with 7% fresh horse blood and incubated under microaerobic conditions at 37°C. Bacteria were harvested on day 3 into antibiotic-free RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Sera Lab, Crawley, United Kingdom). The bacterial preparations were adjusted to an optical density at 625 nm (OD625) of 0.35, corresponding to 2.5 × 107 CFU/ml, and used immediately. The strains used included NCTC 11637; the cag+ H. pylori type strain; cag G50, which lacks the entire cag PAI; and 26695, the strain whose genome has been entirely sequenced (49).
Construction of cag locus isogenic mutants.
Isogenic mutants of strain 26695 were constructed by DNA transformation with plasmid clones containing insertions of a chloramphenicol resistance cassette (Table 1) as described previously (2). Strain 8-1 was a mutant in which the whole cag PAI was deleted (2). In all cases, Camr transformants of strain 26695 were confirmed to contain the cassette in the appropriate site and orientation and to lack the wild-type allele by PCR and/or DNA hybridization. Because of possible polar effects on the expression of downstream cotranscribed genes in the cag PAI, in most open reading frames (ORFs) tested, insertions with the chloramphenicol resistance cassette in each orientation were constructed and used (Table 1).
TABLE 1.
cag locus isogenic mutants of strain 26695
| Strain | Allele | Orientation of resistance cassettea |
|---|---|---|
| 8-1 | Total cag deletion | |
| 14-1 | ORF7 | F |
| 20-1 | ORF7 | R |
| 12-1 | ORF11 | R |
| 13-1 | ORF11 | F |
| 17-1 | ORF13 | R |
| 22-1 | ORF13 | F |
| 23-1 | ORF14 | F |
| 9-1 | ORF14 | R |
| 27-1 | ORF15 | F |
| 10-1 | ORF15 | R |
| 21-1 | ORF16 | F |
| 11-1 | ORF16 | R |
| 18-1 | ORF16–ORF18 | F16/R18 |
| 25-1 | ORF18 | F |
| 26-1 | ORF19–ORF20 | F19/R20 |
F, forward; R, reverse.
IL-8 promoter-luciferase plasmid construction.
The 5′ upstream regulatory sequence which comprises the IL-8 promoter was isolated as a 1,200-bp PCR fragment from EcoRI-digested human placental genomic DNA (Clontech, Palo Alto, Calif.) with primers derived from the published sequence (34). After ligation into pCR 1000 (TA cloning kit; Invitrogen, San Diego, Calif.) and sequencing, the fragment was excised and cloned into the pGL2 basic luciferase vector (Promega, Madison, Wis.) cut with KpnI and HindIII. The construct carries a neomycin resistance cartridge, comprising the Neor gene from Tn5 under control of the herpes simplex virus type 1 thymidine kinase promoter between the BamHI and SalI sites. The promoter DNA and its correct relationship to the luciferase coding region were verified by sequencing. The resulting plasmid was then used to generate the reporter cell line.
Generation of the L5F11 stable cell line.
A total of 4.5 × 107 Kato-3 cells (European Collection of Animal Cell Cultures, Salisbury, United Kingdom) in 300 μl of saccharose buffer containing 5 μg of the plasmid construct were electroporated in a 0.4-cm cuvette at 250 μF and 300 V in a Bio-Rad gene pulser. After a 72-h culture at 37°C in a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% FCS, 1% glutamine, and 40 μg of gentamicin per ml, the cells were harvested, centrifuged, washed, and resuspended in 2 ml of medium supplemented with 400 μg of G418 (Calbiochem, San Diego, Calif.) per ml before being distributed into a 96-well plate at 2 × 104 cells/well and incubated at 37°C in a 5% CO2 atmosphere. The clones which developed were expanded sequentially through 48-, 24-, and 6-well plates before being transferred into tissue culture flasks.
Stimulation of the L5F11 epithelial cell line with H. pylori.
The L5F11 cell line was routinely maintained in RPMI 1640 medium supplemented with 10% FCS, 40 μg of gentamicin per ml, 400 μg of Geneticin (Life Technologies) per ml, and 2 mM glutamine (Life Technologies). After reaching confluency, the cells were resuspended in gentamicin- and Geneticin-free medium and cultured in 96-well plates (Corning Costar, High Wycombe, United Kingdom) at a density of 5 × 105/ml. The cells were cocultured with H. pylori (2.5 × 107 organisms/ml) in quadruplicate for 10 h at 37°C in a 95% air–5% CO2 humidified incubator. In all experiments, H. pylori wild-type strains cag PAI positive (NCTC 11637) and cag PAI negative (G50) were included. Tumor necrosis factor alpha (TNF-α) (5 ng/ml; R&D Systems, Abingdon, United Kingdom) was included as a positive control. The cell culture supernatant was harvested for the IL-8 protein assay, and the cell lysates were used for assessment of luciferase activity.
Luciferase assay.
After coculture with H. pylori for 10 h, the above cells were washed twice with cold phosphate-buffered saline (pH 7.4). Cell lysates were prepared by using cell culture lysis reagent (Promega). Before the luciferase assay, 100 μl of lysis reagent was added to each well. Luciferase concentrations were measured with a luciferase assay kit (Promega). Luciferase activity was measured in a scintillation counter (Canberra Packard, Pangbourne, United Kingdom), and concentrations were expressed as counts per minute. A blank control, cell lysis buffer control, and cell control were included in each assay.
IL-8 ELISA.
IL-8 was assayed in duplicate by ELISA as described previously (13, 16–18) with a murine monoclonal antibody to IL-8 (Novartis, Vienna, Austria) and phosphatase-conjugated goat anti-IL-8 antibody (Novartis). The concentrations of IL-8 were determined from a standard curve of recombinant IL-8 (Novartis) and expressed as nanograms per milliliter.
Kinase inhibition assay.
To determine the effect of kinase inhibitors on IL-8 transcription and IL-8 protein secretion, L5F11 cells were seeded as above and preincubated with herbimycin A (Gibco BRL, Paisley, United Kingdom), H7 (Sigma, Poole, United Kingdom), calphostin C (Sigma) and KT5823 (Affiniti Research Products Ltd., Exeter, United Kingdom) for 1 h before the addition of H. pylori NCTC 11637 or TNF-α. The inhibitors were solubilized in dimethyl sulfoxide, and carrier controls were included in all experiments. The supernatants and the cells were harvested at 10 h for assessment of secreted IL-8 protein and luciferase activity as above. None of the inhibitors used had any antimicrobial activity against H. pylori in vitro.
Statistical analysis.
Data are expressed as means ± 1 standard error (SEM). The statistical significance of observed differences between the isogenic mutants and parent strain 26695 were analyzed by Student’s t test. The correlation of luciferase and IL-8 protein was analyzed by the Pearson correlation. A probability (P) of <0.05 was considered statistically significant.
RESULTS
H. pylori- and TNF-α-induced IL-8 gene transcription in L5F11 cells.
A significant (fourfold) increase in luciferase activity, a reporter for IL-8 transcription, was evident 2 h after infection of L5F11 gastric epithelial cells with H. pylori NCTC 11637. Luciferase levels continued to increase for a further 6 h, with the peak value at 8 h, after which the luciferase activity began to decrease, with the lowest level being measured at 24 h (Fig. 1). In subsequent studies (see below), luciferase activity was assayed in the plateau phase at 10 h postinfection. Addition of TNF-α (5 ng/ml) to L5F11 cells resulted in a more rapid stimulation of luciferase synthesis, which reached a peak level at 4 h (Fig. 1).
FIG. 1.
Time course of luciferase production by L5F11 cells (5 × 105/ml) exposed to H. pylori (NCTC 11637, 2.5 × 107 organisms/ml) or TNF-α (5 ng/ml). Data shown are expressed as means and SEMs (n = 3).
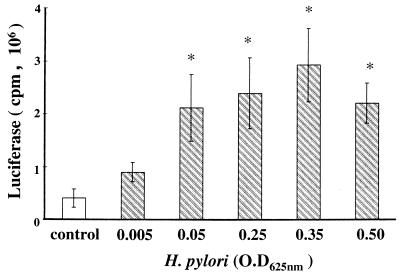
To assess the effect of bacterial density on IL-8 transcription, L5F11 gastric epithelial cells were cocultured with various concentrations of H. pylori NCTC 11637. Luciferase activity increased dose dependently with increasing bacterial density in the range from approximately 3.5 × 105 to 2.5 × 107/ml. The luciferase activity was evident even at a low bacterial density of approximately 3.5 × 105/ml (Fig. 2). TNF-α stimulation of IL-8 transcription in L5F11 cells was maximal at 5 ng/ml (data not shown).
FIG. 2.
H. pylori infection increases L5F11 cell luciferase production in a dose-dependent manner. L5F11 cell monolayers were infected with different concentrations of H. pylori ranging from an OD625 of 0.005 to 0.5, and luciferase levels were measured in cell lysates after 10 h. An OD625 of 0.35 corresponds to a bacterial concentration of 2.5 × 107/ml. Data shown are expressed as means and SEMs (n = 3). ∗, P < 0.05 with respect to control.
Comparison of IL-8 transcription and IL-8 protein secretion in L5F11 cells.
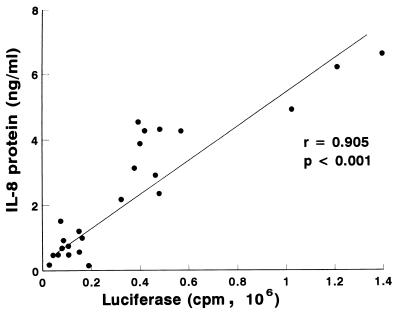
We sought to assess if (i) increased IL-8 gene transcription necessarily led to increased IL-8 protein secretion or (ii) additional posttranscriptional controls were also likely to modulate the levels of IL-8 secretion. A total of 24 H. pylori strains (both wild types and isogenic mutants of cag PAI genes) were assayed in five independent experiments for their ability to induce increased luciferase activity in L5F11 cells and increased IL-8 protein secretion from them. Figure 3 shows that there was a strong correlation between increased luciferase activity and IL-8 protein secretion (r = 0.905, P < 0.001) in response to coculture of L5F11 cells with H. pylori.
FIG. 3.
Correlation of the luciferase activity and IL-8 protein secretion in L5F11 following stimulation with H. pylori strains. Results from five independent experiments performed with 24 H. pylori strains (both wild type and mutants) are shown.
Effect of mutations in the cag PAI on IL-8 transcription in L5F11 gastric epithelial cells.
The role of genes in the left half of the cag PAI, the so-called cagII region (2), in induction of IL-8 transcription in gastric epithelial cells was assessed by comparing the luciferase activity induced by wild-type strain 26695 and isogenic derivatives carrying null insertion mutations in each of seven individual cagII locus ORFs or deletions that removed segments extending from ORF16 to ORF18 and from ORF19 to ORF20 (Table 1). Negative controls, included in all assays, consisted of a 26695 derivative lacking the entire cag PAI (strain 8-1) and a clinical isolate naturally lacking the entire cag PAI (strain G50).
Strain 26695 induced only one-third as much IL-8 gene transcription as strain NCTC 11637 did (Table 2). This is in keeping with the previously observed diversity among cag+ strains in their ability to induce IL-8 production (16, 17, 45). Despite this, null mutations in each of the seven individual ORFs in the cag PAI (ORF7, ORF11, ORF13, ORF14, ORF15, ORF16, and ORF18) resulted in a further significant reduction in induction of IL-8 gene transcription, as did the multi-ORF deletions (ORF16 to ORF18 and ORF19 to ORF20; P < 0.01 and P < 0.001, respectively [Table 2]). The reduction caused by insertion in six of the seven ORFs was equivalent to that caused by deletion of the total cag PAI. Where tested (ORF7, ORF11, ORF13, ORF14, ORF15, and ORF16), this reduction was also independent of the orientation of the chloramphenicol resistance cassette, suggesting that the observed reductions reflected inactivation of the targeted gene, not an effect on downstream bacterial gene transcription (Table 2).
TABLE 2.
IL-8 transcription induced by wild-type and cag locus isogenic mutant H. pylori strains in L5F11 gastric epithelial cellsa
| Strain | Allele | n | Luciferase activity (cpm, 106)b |
|---|---|---|---|
| 26695 | Parent | 18 | 0.59 ± 0.05 |
| 8-1 | Total cag | 18 | 0.08 ± 0.02** |
| 14-1 | ORF7 | 8 | 0.05 ± 0.02** |
| 20-1 | ORF7 | 8 | 0.11 ± 0.02** |
| 12-1 | ORF11 | 9 | 0.15 ± 0.04** |
| 13-1 | ORF11 | 10 | 0.21 ± 0.04** |
| 17-1 | ORF13 | 8 | 0.06 ± 0.03** |
| 22-1 | ORF13 | 8 | 0.06 ± 0.04** |
| 23-1 | ORF14 | 9 | 0.15 ± 0.05** |
| 9-1 | ORF14 | 10 | 0.09 ± 0.03** |
| 27-1 | ORF15 | 8 | 0.07 ± 0.02** |
| 10-1 | ORF15 | 10 | 0.16 ± 0.03** |
| 21-1 | ORF16 | 10 | 0.13 ± 0.02** |
| 11-1 | ORF16 | 9 | 0.1 ± 0.04** |
| 18-1 | ORF16–ORF18 | 9 | 0.26 ± 0.08* |
| 25-1 | ORF18 | 9 | 0.1 ± 0.02** |
| 26-1 | ORF19–ORF20 | 9 | 0.19 ± 0.05** |
| G50 | Negative control | 18 | 0.13 ± 0.02 |
| NCTC 11637 | Positive control | 18 | 1.77 ± 0.2 |
Comparison of the ability of H. pylori isogenic mutant strains and the parent strain 26695 to induce luciferase activity in L5F11.
Luciferase activity is expressed as mean ± SEM of n determinations after subtraction of background cell control values. *, P < 0.01; **, P < 0.001.
Kinase inhibition studies.
To investigate the involvement of kinases in H. pylori-elicited induction of IL-8 gene transcription, L5F11 cells were preincubated with a protein tyrosine kinase inhibitor (herbimycin A, 1 μM) (25), a protein kinase A (PKA) inhibitor (H7, 50 μM) (25), a protein kinase C (PKC) inhibitor (calphostin C, 1 μM) (29), and a protein kinase G (PKG) inhibitor (KT5823, 100 μM) (43). One hour later, H. pylori (NCTC 11637) or TNF-α was added. Control experiments with L5F11 cells cultured without H. pylori or TNF-α showed that these inhibitors had no effect on basal levels of luciferase activity (IL-8 gene transcription) or IL-8 secretion (as monitored by ELISA).
The protein tyrosine kinase inhibitor herbimycin A significantly (P < 0.05) reduced both H. pylori-induced IL-8 gene transcription and IL-8 protein production (Table 3) but had no effect on the TNF-α induction of these events (Table 4). The PKA inhibitor H7 caused only a mild (ca. 30%) reduction in H. pylori-induced IL-8 gene transcription, whereas it caused a more significant reduction in IL-8 protein secretion (71%; P < 0.01) (Table 3). However, this inhibitor did reduce TNF-α-induced IL-8 gene transcription significantly (72%; P < 0.001), and it also significantly reduced IL-8 protein secretion (P < 0.01) (Table 4). Neither the PKG inhibitor KT5823 nor the PKC inhibitor calphostin C had any effect on TNF-α- or H. pylori-induced IL-8 transcription or IL-8 protein secretion (Tables 3 and 4).
TABLE 3.
Effect of kinase inhibitors on H. pylori-induced IL-8 transcription and IL-8 protein secretion in L5F11 gastric epithelial cells
| Inhibitor | Inhibitor concn (μM) | Luciferase activity (cpm, 106) (n = 5)a | IL-8 secretion (ng/ml) (n = 3–5)a |
|---|---|---|---|
| None (H. pylori NCTC 11637) | 1.51 ± 0.23 | 3.22 ± 0.59 | |
| Herbimycin A | 1 | 0.71 ± 0.18* | 0.83 ± 0.41* |
| H7 | 50 | 1.05 ± 0.42 | 0.91 ± 0.3** |
| Calphostin C | 1 | 1.63 ± 0.33 | 2.55 ± 0.24 |
| KT5823 | 100 | 1.62 ± 0.33 | 2.9 ± 0.48 |
Both luciferase activity and IL-8 protein concentrations are expressed as means ± SEMs after subtraction of the respective background cell controls. *, P < 0.05; **, P < 0.01 (compared with H. pylori stimulation in the absence of kinase inhibitors).
TABLE 4.
Effect of kinase inhibitors on TNF-α-induced IL-8 transcription and IL-8 protein secretion in L5F11 gastric epithelial cells
| Inhibitor | Inhibitor concn (μM) | Luciferase activity (cpm, 106) (n = 5)a | IL-8 secretion (ng/ml) (n = 5)a |
|---|---|---|---|
| None (TNF-α, 5 ng/ml) | 2.62 ± 0.26 | 4.12 ± 0.43 | |
| Herbimycin A | 1 | 2.27 ± 0.53 | 3.43 ± 0.55 |
| H7 | 50 | 0.74 ± 0.16** | 1.45 ± 0.38* |
| Calphostin C | 1 | 3.02 ± 0.29 | 4.00 ± 0.12 |
| KT5823 | 100 | 2.47 ± 0.21 | 3.06 ± 0.36 |
Both luciferase activity and IL-8 protein concentrations are expressed as means ± SEMs after subtraction of the respective background cell controls. *, P < 0.01; **, P < 0.001 (compared with TNF-α stimulation in the absence of kinase inhibitors).
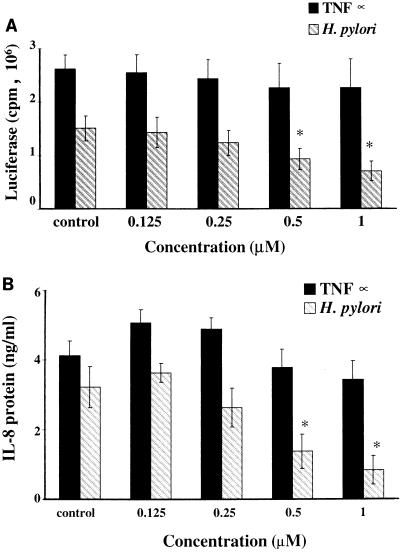
The effects of the herbimycin A and H7 (protein tyrosine kinase and PKA inhibitors, respectively) were examined over a range of concentrations (Fig. 4 and 5). Herbimycin A reduced H. pylori-induced IL-8 gene transcription and IL-8 protein secretion in proportion to the dose but, as noted above (Table 4), was without effect on TNF-α induction of these processes. Similarly, H7 reduced TNF-α-induced IL-8 gene transcription and IL-8 protein secretion in proportion to the dose. Significant inhibition of the H. pylori-induced IL-8 protein secretion by H7 was observed only at the highest doses (50 μM H7 [Fig. 5B]).
FIG. 4.
Effect of the protein tyrosine kinase inhibitor herbimycin A on H. pylori (2.5 × 107 organisms/ml)- and TNF-α (5 ng/ml)-induced IL-8 transcription (A) and IL-8 protein (B) secretion by L5F11 cells. Results are shown as means and SEMs, after subtraction of background cell control values, of five independent experiments. ∗, P < 0.05.
FIG. 5.
Effect of the PKA inhibitor H7 on H. pylori (2.5 × 107 organisms/ml)- and TNF-α (5 ng/ml)-induced IL-8 transcription (A) and IL-8 protein (B) secretion by L5F11 cells. Results are shown as means and SEMs, after subtraction of background cell control values, of five independent experiments. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
DISCUSSION
The ability of H. pylori to stimulate IL-8 protein secretion in gastric epithelial cell lines in vitro (16, 17, 45), a process involving tyrosine kinase activation (1, 6, 43) and NF-κB activation (1, 28, 35, 46), has been extensively studied. Early experiments suggested that the induction of IL-8 secretion by cag+ H. pylori strains involved complex bacterium–epithelial-cell interactions. It depended, for example, on the use of whole viable H. pylori and was abrogated by sonicating, fixing, freeze-thawing, or heat killing the bacteria (16, 45) or by their conversion by growth into stationary phase into the coccoid form (10). Recent mutational studies have implicated several genes in the cag PAI (2, 8, 50) in this induction process and have also shown that some, most strikingly cagA, are not involved (18, 45).
Five of the genes in the 40-kb cag PAI are homologs of genes that in Agrobacterium, Bordetella pertussis, and several conjugative plasmids are needed for the assembly of pili on the bacterial cell surface and for the delivery of proteins or protein-nucleic acid complexes to target bacterial or eukaryotic cells (9). DNA sequence inspection suggested that the products of several other cag PAI genes might also be involved in membrane assembly or secretion (2, 8), although some of the signature motifs are quite weak (49). It is thus parsimonious to imagine an equivalent bacterial membrane complex in H. pylori target cell signalling. However, another five or six of the genes that are rather highly conserved in the Agrobacterium, Bordetella, and R-factor systems and whose products are also needed for delivery of macromolecules to target cells are conspicuously absent from the cag PAI.
To facilitate analyses of the mechanisms of the H. pylori-induced epithelial-cell response and cell signalling processes that underlie it, we engineered an IL-8 gene promoter-luciferase reporter fusion in cells derived from the highly responsive Kato-3 gastric epithelial cell line (2, 8, 16–18). This allowed IL-8 gene transcription to be monitored by simple luciferase assays far more efficiently and sensitively than before and permitted us to study transcriptional versus posttranscriptional events, including secretion, in a cost-effective manner. The strong correlation observed between H. pylori-induced luciferase activity and IL-8 protein secretion permits a direct comparison of the results on IL-8 transcription with our laboratory’s earlier studies (2, 8, 16–18) and those of others (1, 10, 35) on H. pylori-induced IL-8 protein secretion in Kato-3 gastric epithelial cells.
The cag region mutants we studied were constructed from strain 26695, the strain whose genome has been fully sequenced (49), before we learned that this strain is actually a relatively poor inducer of IL-8 transcription compared to the type strain, NCTC 11637. Nevertheless, because of the sensitivity of the luciferase assay, our data showed that the homologs of the Agrobacterium genes virB9, virB10, and virB11 (ORF15, ORF13, and ORF11), as well as the four “function-unknown” ORFs that we tested, were each needed for H. pylori to induce IL-8 transcription. Thus, our findings reinforce and extend earlier observations that loss of function of the picA1 gene and picB/cagE (an Agrobacterium virB4 homolog) close to the right end of the cag PAI abrogates the induction of IL-8 gene transcription in gastric epithelial cells (46). Although the possibility of polar effects on downstream genes as a result of the inserted Camr cassette cannot be totally excluded, the extent of reduction in IL-8 transcription was independent of orientation in each of the six cases tested. The loss of the ability to induce IL-8 transcription caused by insertions in each of seven individual genes or by a deletion removing two or three genes was generally similar to that seen with an isogenic total cag PAI deletion strain. The low basal levels of IL-8 transcription seen with these cag region mutants were, in turn, equivalent to that seen during infection with the clinical isolate G50, which lacks the entire cag PAI.
The basis of quantitative differences in the ability of nominally cag+ H. pylori strains to elicit IL-8 gene expression, as exemplified here with 26695 and NCTC 11637, is not known. However, cag+ H. pylori strains are an extremely diverse group genetically, perhaps needing to induce inflammatory responses and the attendant host cell damage to create a niche in which they can proliferate but also to downregulate these responses to ensure that they (the bacteria themselves) are not eradicated. We suggest that some of the diversity among cag+ strains in the extent of induction of IL-8 gene transcription observed is due to point mutations or possibly larger mutational differences in the cag PAI. The possibility that the relatively small effects on IL-8 levels seen with some strains, such as 26695, reflect a modulatory “anti-inflammatory” cag PAI determinant, rather than a relatively inactive (recessive) allele of one of the proinflammatory genes, will now be much easier to examine by using the luciferase reporter gene assay system described here.
Induction of IL-8 secretion by H. pylori has been blocked by protein tyrosine kinase inhibitors in AGS (6, 43) and MKN45 (1) gastric epithelial cells. However, different results have been obtained for other protein kinase inhibitors, even in what is nominally the same gastric epithelial cell line (6, 43). Such seeming conflicts might reflect the several steps in IL-8 production that are affected quantitatively by specific protein phosphorylation and the possible divergence in the fine-tuned regulation of this process in different lineages of cultured cells. In any event, the possible importance of protein phosphorylation in H. pylori-induced gastric epithelial IL-8 gene transcription versus IL-8 secretion has not been studied previously, and no data are available on the effects of protein kinase inhibitors in the Kato-3 gastric epithelial cell line. Here we examined these processes in the Kato-3-derived L5F11 cells and showed that both IL-8 gene transcription and protein secretion induced by H. pylori are blocked by the protein tyrosine kinase inhibitor herbimycin A. This is in accord with other recent studies showing that herbimycin A inhibited H. pylori-induced IL-8 secretion in MKN45 (1) and AGS (6) gastric epithelial cells and that H. pylori binding stimulated tyrosine phosphorylation of two large proteins in AGS cells (42).
Highlighting the possibility of significant genetic differences among established eukaryotic cell lines, however, we found that hermimycin A did not affect TNF-α-induced IL-8 gene transcription in L5F11 cells but that this transcription could be blocked by the protein kinase A inhibitor H7, in contrast to the results obtained by others studying AGS cells (6). Some reduction in H. pylori-induced IL-8 secretion was also observed with the PKA inhibitor but only at high concentrations. These results suggest that in L5F11 cells, as in MKN45 cells (1), the signal transduction pathways for the transcription of IL-8 induced by H. pylori and cytokines differ.
The PKG inhibitor KT5834, previously reported to reduce H. pylori-induced IL-8 secretion in AGS (43), had no effect on H. pylori-induced IL-8 transcription or IL-8 protein secretion in L5F11 cells in our study. These results might reflect differences in cell-specific signalling pathways in response to H. pylori infection. However, the maximal secretion of IL-8 in AGS cells occurs 8 to 12 h after infection with H. pylori (28), but the previous studies with PKG inhibitors (43) relied on samples taken after just 2 h of coculture. Therefore, the seemingly different effects of PKG inhibitors in these two cell lineages might also reflect differences in experimental design. Very divergent effects of the broad-range kinase inhibitor staurosporine on H. pylori-induced IL-8 secretion have also been reported. No inhibitory effects were found in MKN45 cells by one group (1), whereas others have reported that staurosporine increased (6) or decreased (43) H. pylori-induced IL-8 secretion in AGS cells. Since our initial studies showed that staurosporine increased H. pylori-induced IL-8 gene transcription in L5F11 cells (data not shown), further studies were undertaken with the more specific PKC inhibitor calphostin C (29). In contrast to data obtained by others (43), we found that PKC inhibition had no effect on H. pylori-induced IL-8 transcription or protein secretion. The evidence from our study shows that H. pylori-stimulated IL-8 transcription and protein secretion in L5F11 cells are dependent predominantly on protein tyrosine kinase activity, and we could find no evidence of signal transduction via a PKG- or PKC-dependent pathway. Recent studies on Salmonella typhimurium have shown that the bacterially induced IL-8 secretion by intestinal cells results from the activation of mitogen-activated protein kinases ERK, JNK, and p28 (26). Whether similar epithelial signal transduction pathways are stimulated in gastric epithelial cells, which are developmentally distinct, by cag PAI-positive H. pylori remains to be determined.
In vitro studies with gastrointestinal cell lines have been useful in evaluating the functional importance of enteric pathogens and their virulence factors in the induction of chemokine responses (1, 2, 8, 16–18, 26, 27, 45, 55) and the role of epithelial-cell-derived chemoattractants in intestinal neutrophilic migration (30–32). Importantly, there is now also increasing evidence from in vivo experiments that gastrointestinal epithelial cells produce C-X-C chemokines in response to pathogens (15, 40, 44) and that this response is likely to be critical for neutrophil-mediated tissue injury (19, 20). In the human gastrointestinal mucosa, infection with cag PAI-positive strains of H. pylori has been associated with enhanced gastric C-X-C chemokine responses and neutrophilic infiltration (39, 48, 54) as well as a more severe clinical outcome (7, 12, 14, 38, 39, 53). Therefore, in vitro observations on the functional importance of the cag PAI in IL-8 chemokine induction have in vivo correlates.
In conclusion, the present in vitro studies emphasize the importance of multiple cag PAI gene products in the transcription of IL-8 in gastric epithelial cells. Our kinase inhibition studies suggest some interesting and potentially important differences between H. pylori- and TNF-α-induced signal transduction pathways for IL-8 transcription in L5F11 gastric epithelial cells, with only the former being dependent on tyrosine kinase activation. Further studies should reveal the molecular components of these pathways and show whether H. pylori itself also has mechanisms that quantitatively affect these cag PAI-dependent epithelial-cell signalling responses.
ACKNOWLEDGMENTS
These studies were supported by the British Digestive Foundation, the European Commission (contract IC18CT95OO24), Yorkshire Cancer Research, and the National Institutes of Health (grants A138166 and DK48029).
We thank Philip Chambers for expert technical assistance.
REFERENCES
- 1.Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN 45. Infect Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek S E, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–54. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 3.Ando T, Kusugami K, Ohsuga M, Ina K, Shinoda M, Konagaya T, Sakai T, Imada A, Kasuga N, Nada T, Ichiyama S, Blaser M J. Differential normalization of mucosal interleukin-8 and interleukin-6 activity after Helicobacter pylori eradication. Infect Immun. 1998;66:4742–4747. doi: 10.1128/iai.66.10.4742-4747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 5.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 6.Beales I L P, Calam J. Stimulation of IL-8 production in human gastric epithelial cells by Helicobacter pylori, IL-1β and TNF-α requires tyrosine kinase activity, but not protein kinase C. Cytokine. 1997;9:514–520. doi: 10.1006/cyto.1996.0195. [DOI] [PubMed] [Google Scholar]
- 7.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 8.Censini S, Lange N, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole S, Cirillo D, Kagnoff M F, Guiney D G, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997;65:843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree J E, Peichl P, Wyatt J I, Stachl U, Lindley I J D. Gastric IL-8 and IL-8 IgA autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993;37:65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree J E, Wyatt J I, Sobala G M, Miller G, Tompkins D S, Primrose J N, Morgan A G. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut. 1993;34:1339–1343. doi: 10.1136/gut.34.10.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree J E, Wyatt J I, Trejdosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree J E, Farmery S M, Lindley I J D, Figura N, Peichl P, Tompkins D S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cells. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J D, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree J E, Xiang Z, Lindley I J D, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree J E. The role of cytokines in Helicobacter pylori induced mucosal damage. Dig Dis Sci. 1998;43:46S–55S. [PubMed] [Google Scholar]
- 20.Dallegri F, Ottonello L. Tissue injury in neutrophilic infiltration. Inflamm Res. 1997;46:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 21.Dixon M F, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Enders G, Brooks W, von Jan N, Lehn N, Bayerdorffer E, Hatz R. Expression of adhesion molecules on human granulocytes after stimulation with Helicobacter pylori membrane proteins: comparison with membrane proteins from other bacteria. Infect Immun. 1995;63:2473–2477. doi: 10.1128/iai.63.7.2473-2477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glocker E, Lange C, Covacci A, Bereswill S, Kist M, Pahl H L. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect Immun. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham D Y. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983–1991. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 25.Gross V, Andus T, Daig R, Aschenbrenner E, Scholmerich J, Falk W. Regulation of interleukin-8 production in a human colon epithelial cell line (HT-29) Gastroenterology. 1995;108:653–661. doi: 10.1016/0016-5085(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 26.Hobbie S, Chen L M, Davis R J, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 27.Kagnoff M F, Eckmann L. Epithelial cells as sensors of microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keates S, Hitti Y S, Upton M, Kelly C P. Helicobacter pylori infection activates NF-κB in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 30.McCormick B, Hofman P, Kim J, Carnes D, Miller S, Madara J. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick B, Miller S, Carnes D, Madara J. Transepithelial signalling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick B, Parkos C A, Colgan S P, Carnes D K, Madara J L. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 33.Moss S F, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 35.Munzenmaier A, Lange C, Glocker E, Covacci A, Moran A, Bereswill S, Baeuerle P A, Kist M, Pahl H L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-κB. J Immunol. 1997;159:6140–6147. [PubMed] [Google Scholar]
- 36.Neilsen H, Andersen L P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992;33:738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noraard A, Andersen L P, Nielsen H. Neutrophil degranulation by Helicobacter pylori proteins. Gut. 1995;36:354–357. doi: 10.1136/gut.36.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsonnet J, Friedman G D, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive and CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peek R M, Jr, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to CagA+ Helicobacter pylori strains. Lab Investig. 1995;73:760–770. [PubMed] [Google Scholar]
- 40.Raqib R, Lindberg A A, Wretland B, Bardhan P K, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 42.Segal E D, Falkow S, Tompkins L S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal E D, Lange C, Covacci A, Tompkins L S, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seydel K B, Li E, Swanson P E, Stanley S L. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S A, Tummuru M K R, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 47.Shimoyama T, Fukada S, Tanaka M, Mikami T, Munakata A, Crabtree J E. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51:225–228. doi: 10.1136/jcp.51.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimoyama T, Everett S M, Dixon M F, Axon A T R, Crabtree J E. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol. 1998;51:765–770. doi: 10.1136/jcp.51.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, et al. The complete genome sequence of the gastric pathogen. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 50.Tummuru M K R, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 51.Walker M M, Crabtree J E. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann N Y Acad Sci. 1998;859:96–111. doi: 10.1111/j.1749-6632.1998.tb11114.x. [DOI] [PubMed] [Google Scholar]
- 52.Webb P, Crabtree J E, Forman D the Eurogast Study Group. Gastric cancer, cytotoxin associated gene A positive Helicobacter pylori and serum pepsinogens: an international study. Gastroenterology. 1999;116:269–276. doi: 10.1016/s0016-5085(99)70122-8. [DOI] [PubMed] [Google Scholar]
- 53.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 54.Yamaoka Y, Kita M, Kodama T, Sawai N, Tanahashi T, Kashima K, Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S K, Eckmann L, Panja A, Kagnoff M F. Differential and regulated expression of C-X-C, C-C and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida N, Granger D N, Evans D J, Evans D G, Graham D Y, Anderson D C, Wolf R E, Kvietys P R. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]