Abstract
Babesia bovis, an intraerythrocytic parasite of cattle, is sequestered in the host microvasculature, a behavior associated with cerebral and vascular complications of this disease. Despite the importance of this behavior to disease etiology, the underlying mechanisms have not yet been investigated. To study the components involved in sequestration, B. bovis parasites that induce adhesion of the infected erythrocytes (IRBCs) to bovine brain capillary endothelial cells (BBEC) in vitro were isolated. Two clonal lines, CD7A+I+ and CE11A+I−, were derived from a cytoadherent, monoclonal antibody 4D9.1G1-reactive parasite population. This antibody recognizes a variant, surface-exposed epitope of the variant erythrocyte surface antigen 1 (VESA1) of B. bovis IRBCs. Both clonal lines were cytoadhesive to BBEC and two other bovine endothelial cell lines but not to COS7 cells, FBK-4 cells, C32 melanoma cells, or bovine brain pericytes. By transmission electron microscopy, IRBCs were observed to bind to BBEC via the knobby protrusions on the IRBC surface, indicating involvement of components associated with these structures. Inhibition of protein export in intact, trypsinized IRBCs ablated both erythrocyte surface reexpression of parasite protein and cytoadhesion. IRBCs allowed to recover surface antigen expression regained the ability to bind endothelial cells, demonstrating that parasite protein export is required for cytoadhesion. We propose the use of this assay as an in vitro model to study the components involved in B. bovis cytoadherence and sequestration.
Infection with the intraerythrocyte protozoal parasite Babesia bovis stimulates an immune response that controls but fails to eradicate the parasite, resulting in long-term, chronic infections (7, 13, 29). One mechanism that enables the parasite to hide from the host immune defenses is the ability of infected erythrocytes (IRBCs) carrying mature parasites to become sequestered in the capillaries (15, 47, 49), presumably avoiding splenic clearance (4). However, the mechanisms used by B. bovis to achieve this sequestration of IRBCs in the capillaries have not yet been determined.
It is probable that IRBCs sequester in the capillaries by receptor-like interactions between the IRBCs and the endothelium, such as those that occur in the related protozoan, Plasmodium falciparum (30, 44). In many aspects, these parasites have similar effects on their host RBCs. Each parasite induces the formation of knobby protrusions on the surface of the IRBC (3, 8, 27, 47), which mediate adhesion to the endothelium (2, 27, 45, 47, 49). Parasite-derived antigens associated with the IRBC surface, and potentially with cytoadhesion, are subject to rapid antigenic variation (7, 12). Both parasites are sequestered in the capillaries of many host tissues, an activity thought to contribute significantly to the pathologic effects of both malaria and babesiosis (1, 15, 28, 37, 38, 47–49), particularly the cerebral complications that are the hallmark of these two diseases (1, 2, 15, 28, 47). It has been clearly demonstrated that P. falciparum-infected erythrocytes achieve sequestration via specific interactions between endothelial cell receptors (10, 21, 22, 25, 32, 44) and knob-associated components, in particular PfEMP1 (11, 22, 42), although other receptors, such as sequestrin (31) and pfalhesin (19), may also be involved. In B. bovis infections, such interactions have been inferred for laminin and thrombospondin (36) and for heparin (23), but these observations remain to be independently confirmed. Electron micrographs of in vivo-sequestered IRBCs revealed that the cells contacted the endothelial cells via the knobs (2, 47, 49), but interpretation of the data is compromised by the localized coagulopathy that accompanies acute disease (47–49).
To test the hypothesis that IRBCs are sequestered by specific interactions with endothelial cell receptors, an in vitro assay of B. bovis cytoadherence is required. We observed that minor populations of the B. bovis lines maintained in our laboratory bound to bovine brain capillary endothelial cells (BBEC) in vitro. By repeatedly selecting for the binding populations, we have established several new parasite lines that bind in vitro to BBEC at high densities. In this paper, we present data indicating that this binding represents an acceptable model for the interactions that occur between IRBCs and endothelial cells during in vivo sequestration of the parasite. The ability to investigate IRBC-endothelial cell binding in vitro will facilitate the identification and characterization of the components involved in B. bovis sequestration.
MATERIALS AND METHODS
Parasites.
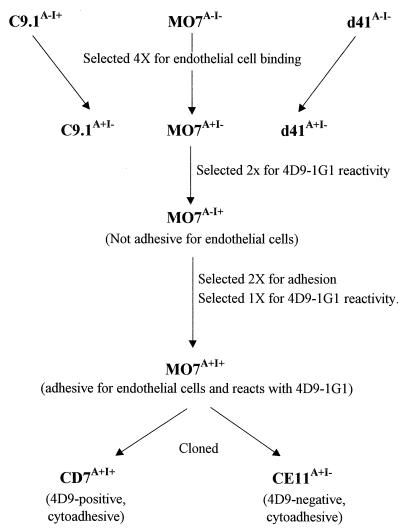
For purposes of clarity, the phenotype of each of the parasite clones and lines described in this paper is indicated in superscript, with A+ denoting the in vitro cytoadhesive phenotype, A− denoting the nonadhesive phenotype, and I+ and I− denoting IRBC surface immunoreactivity and nonreactivity, respectively, with monoclonal antibody (MAb) 4D9.1G1. MAb 4D9.1G1 identifies a variant, surface-exposed epitope on the B. bovis variant erythrocyte surface antigen 1 (VESA1), initially found only on the C9.1 clonal parasite line (34) but subsequently also found on some phenotypic revertants (35). The derivations of the B. bovis clones MO7A−I− and C9.1A−I+ have been described previously (7, 24). The uncloned parasite line d41A−I− was established from parasites present in the peripheral circulation of a calf inoculated 41 days previously with C9.1A−I+ (7). Clonal lines CD7A+I+ and CE11A+I− were derived in this study as described in Results. All parasite cultures were maintained under microaerophilous stationary-phase conditions (26).
Cells.
The BBEC used in the initial development of the cytoadhesion assay were the kind gift of Richard Weiner, University of California San Francisco, and are referred to herein as BBECUCSF. A second BBEC culture line, referred to herein as BBECUF, was isolated from bovine brain by a previously described procedure (17), modified as follows. Brain tissue was collected, minced, and sieved in Dulbecco’s modified Eagle’s medium (DMEM). Nylon net filters (140, 80, and 20 μm) were used to sieve the tissue, and tissue materials were collected from the two finer filters. The growth medium used was DMEM containing 10% heat-inactivated fetal bovine serum (FBS), 100 μg of heparin ml−1, and 25 μg of endothelial cell growth supplement (Collaborative Biomedical Products, Bedford, Mass.) ml−1. Half the growth medium had previously been conditioned on BBECUCSF cultures. The cells were seeded in T25 flasks coated with collagen (10 mg ml−1; Sigma Chemical Co., St Louis, Mo.) and fibronectin (10 μg ml−1; Sigma Chemical Co.), and the BBEC were separated from contaminating cell types (mostly pericytes) by selective release from the substrate with pancreatin (Gibco BRL, Grand Island, N.Y.) (17). Pericytes were separately maintained in culture for use as controls in cytoadhesion experiments.
To confirm the identity of the BBECUF, cultures were incubated in the presence of 20 μg of acetylated low-density lipoprotein ml−1 labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil-Ac-LDL; Biomedical Technologies, Stoughton, Mass.) (46) for 4 h. At the end of the incubation, the excess probe was removed and the cells were examined by fluorescence microscopy, with excitation at 545 nm and observation of emissions at >590 nm. Bovine brain pericytes were used as negative controls. Expression of von Willebrand factor (vWF) was assessed by a fixed-cell indirect immunofluorescence assay (IFA). Endothelial cells, pericytes, and calf pulmonary artery endothelial cells (CPAE; ATCC CCL209) were grown on tissue culture-treated coverslips (Thermonox; Nalge Nunc International, Milwaukee, Wis.) in 24-well plates, fixed in acetone for 10 min, air dried, and incubated with sheep anti-human vWF (The Binding Site, San Diego, Calif.) or normal sheep immunoglobulin G (IgG) diluted to 11.8 μg ml−1 in phosphate-buffered saline (PBS) containing 1:100 (vol/vol) normal donkey serum. After an overnight incubation at 4°C, the primary antibody was removed by three 10-min washes in PBS. The primary reaction was detected with fluorescein isothiocyanate-labeled donkey anti-sheep IgG diluted 1:150 (vol/vol) in PBS plus normal donkey serum. The final washes were performed as described above, and labeled cells were identified by fluorescence microscopy.
Bovine adrenal capillary endothelial cells (EJG; ATCC CRL8659), human amelanotic melanoma cells (C32; ATCC CRL1585), and CPAE were purchased from American Type Culture Collection (Rockville, Md.). African green monkey kidney cells (COS-7) were obtained from Ayalew Mergia, University of Florida, and fetal bovine kidney cells (FBK-4) were obtained from William Castleman, University of Florida.
Selection of BBEC-binding parasite lines.
Tissue culture plates were seeded with BBECUCSF at a density of approximately 5 × 104 cells cm−2 in endothelial cell growth medium (90% [vol/vol] DMEM, 10% [vol/vol] supplemented normal calf serum [HyClone Laboratories, Logan, Utah]; 25 μg of endothelial cell growth supplement ml−1). Two to three days later, the medium was removed, and parasite cultures at 1% packed-cell volume and 2 to 5% parasitized erythrocytes in parasite growth medium (26) were added to the endothelial cells. The plates were incubated at 37°C under microaerophilous conditions for 90 min, with agitation every 15 min to resuspend the RBCs. At the end of the incubation period, noncytoadhering cells were removed by three washes with Hanks balanced salt solution (HBSS) and fresh uninfected RBCs in parasite growth medium were added to each well. After overnight incubation, the RBC suspension was removed and the parasites were cultured under normal conditions until they reached detectable levels.
Selection of MAb-reactive parasites.
The procedure for selection of MAb-reactive parasites has been described elsewhere (35). Briefly, intact IRBCs were reacted with MAb 4D9.1G1. The reaction was amplified with rabbit anti-mouse IgG plus IgM (Pierce, Rockford, Ill.), and the antibody-coated cells were recovered from the suspension with goat anti-rabbit IgG-conjugated magnetic beads (PerSeptive Biosystems, Framingham, Mass.). The captured cells were placed back into culture. Parasites selected for both MAb reactivity and BBEC binding were cloned by two rounds of limiting dilution (41).
Cytoadhesion assay.
For the cytoadhesion assay, 24-well plates containing Thermonox coverslips were seeded with BBECUF, BBECUCSF, EJG, FBK-4, COS-7, C32, CPAE, or pericytes in the growth medium appropriate to the cell type. These media were DMEM containing 10% (vol/vol) FBS or normal calf serum for BBEC and COS-7, DMEM with 20% (vol/vol) FBS for CPAE, and Eagle’s minimal essential medium with 10% (vol/vol) FBS for FBK-4 and EJG. After the cells were established in the wells, the parasites were added at 1% packed-cell volume and 2 to 5% IRBCs and incubated with the endothelial cells as described for the selection of the BBEC-binding parasites. At the end of the incubation, noncytoadherent RBCs were removed by three washes with HBSS. For the washes, HBSS was added to the wells, the coverslips were dislodged from the bottom of the wells, and the plate was shaken vigorously. After the washes, methanol was added to the wells for 10 min and removed, and the coverslips were air dried while still in the plate. The cells were then stained with Giemsa, coverslips were mounted on slides with Permount (Fisher Scientific, Pittsburgh, Pa.), cell side up, and a glass coverslip was mounted over the cells for observation. For counting, a 1-cm2 grid containing nine smaller squares of equal size was marked on the coverslip. The numbers of IRBCs adhering to 16 to 20 endothelial cells was determined for each of the nine sections, and the results are expressed as the number of IRBCs/100 endothelial cells. Each experiment was repeated twice, and within each experiment duplicate samples were read. To avoid reader bias, the slides were coded and read blindly.
Electron microscopy.
IRBCs were allowed to cytoadhere as described above, except that nonadherent cells were removed by three washes in PBS. The cells were fixed in 2% (wt/vol) glutaraldehyde in 0.1 M sodium phosphate (pH 7.4) containing 4% (wt/vol) sucrose and 2 × 10−5 M calcium chloride and then dehydrated through an ascending ethanol series and embedded in EMbed (Electron Microscopy Sciences, Fort Washington, Pa.). The cells were poststained with 2% (wt/vol) aqueous uranyl acetate and Reynolds lead citrate (40) and viewed on a Hitatchi H7000 transmission electron microscope. Embedding and subsequent steps were conducted by the University of Florida Interdisciplinary Center for Biotechnology Electron Microscopy Core Laboratory.
Cytoadhesion of trypsinized IRBCs.
To half the export of new polypeptides to the IRBC surface, CD7A+I+-infected IRBCs (see Fig. 3 for derivation of this parasite line) were cultured for 1 h at 37°C in parasite growth medium containing 10 μg of brefeldin A (BFA; Sigma Chemical Co.) ml−1. The intact IRBCs were then digested for 20 min at 23°C with 10 mg of bovine pancreatic trypsin-tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK) (Sigma Chemical Co.) ml−1 in M199 containing 20 mM (N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid) (M199-TES). The reaction was stopped with an equal volume of 25 mg of soybean trypsin inhibitor (Boehringer Mannheim, Indianapolis, Ind.) ml−1. The trypsinized cells were sedimented at 1000 × g for 1 min at room temperature, resuspended in parasite culture medium, and divided into three aliquots. BFA was added back to two of the three aliquots to a final volume of 10 μg ml−1, and all three samples were then cultured for 4 h under microaerophilous conditions to allow the recovery of surface antigen, if possible. BFA was removed from one of the two BFA-containing samples after 2 h. Thus, samples had recovered for 0, 2, or 4 h in the absence of BFA. Aliquots of nontrypsinized cells were run in parallel as controls. At the end of the 4-h incubation, all the samples were pelleted and resuspended in fresh medium without BFA. One-quarter of each sample was used to evaluate the ability of the cells to adhere to BBECUCSF. The remaining portion of each sample remained under culture conditions for the duration of the cytoadhesion assay (1 h), after which the cells were collected and expression of VESA1 was evaluated by a live-cell immunofluorescence assay (IFA) with MAb 4D9.1G1 (5, 34). The results were analyzed by a two-way analysis of variance procedure.
FIG. 3.
Schematic diagram of the derivation of B. bovis cytoadhesive lines and clones.
[3H]hypoxanthine incorporation was used to evaluate parasite viability after trypsin and BFA treatment. For this experiment, cells were treated with BFA for 1 h and then divided into two aliquots. One aliquot was treated with trypsin as described above. Two aliquots of each sample were distributed into the wells of a 96-well plate and incubated at 37°C for 4 h in the presence or absence of 10 μg of BFA ml−1. At the end of the incubation, the cells were washed into fresh medium containing 15 μCi of [3H]hypoxanthine ml−1 and incubated for 2 h at 37°C under microaerophilous conditions. The cells were resuspended, and 5-μl aliquots were dotted onto fiberglass filters. These were air dried and then submerged in ice-cold 5% (wt/vol) trichloracetic acid for 10 min. The filters were washed sequentially with water, 95% (vol/vol) ethanol, and acetone. After being dried, the filters were placed in Scintisafe scintillation fluid (Fisher Scientific) for liquid scintillation counting.
RESULTS
When initially assayed, only uncloned d41A−I− parasites were observed to bind to BBEC at detectable levels (ca. 10 IRBCs/100 BBEC). In contrast, after MO7A−I−, C9.1A−I+, and d41A−I− parasites were taken through three rounds of selection for binding, the numbers of IRBCs binding per endothelial cell increased significantly, to 400 to 750 IRBCs/100 BBEC depending on the parasite line (data not shown). This binding was not increased further by a fourth round of selection. Although parasites were added to the endothelial cells at 2 to 5% of IRBCs, the cells which remained attached to BBEC after three washes were essentially 100% infected and contained either trophozoite or mature-stage parasites (Fig. 1). There was no difference in the density of IRBCs that adhered when the starting percentage of IRBCs was in the range of 2 to 5% (data not shown). Uninfected RBCs or IRBCs containing ring-stage parasites were observed only rarely and did not increase in proportion with selection.
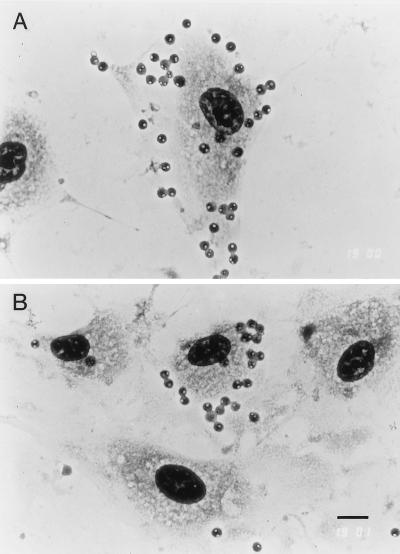
FIG. 1.
D41A+I− parasites cytoadhere to BBECUCSF in vitro. Note that all of the IRBC contain either trophozoites or two merozoites, the parasite stages that are sequestered in vivo, and that the density of IRBCs adhering per endothelial cell varies (A and B). Bar, 10 μm.
Since it was possible that in vitro binding of these parasites to BBEC was an artifact of the selection procedure, attempts were made to select BBEC-binding parasites from Babesia bigemina cultures. B. bigemina also infects cattle but does not sequester in the microvasculature (16, 39) and therefore would not be expected to adhere to endothelial cells in vitro. In initial experiments, stringent washing removed all B. bigemina from the endothelial cells. Therefore, to select for adhesive B. bigemina parasites, only one wash was performed before fresh, uninfected RBCs were added to the cultures. By using this modified procedure, selections were performed on the B. bigemina Puerto Rico isolate and the B. bovis MO7A−I− clone. With each selection, the density of cytoadhering MO7A+I− parasites increased, despite the lower stringency of washes that resulted in the isolation of a high percentage of nonadhesive parasites with each selection. In contrast, repeated selection failed to enrich for BBEC-binding parasites from the B. bigemina cultures (Fig. 2).
FIG. 2.
Selection of cytoadhesive parasites from a noncytoadhesive B. bovis line and from B. bigemina. The Puerto Rico isolate of B. bigemina and the B. bovis MO7A−I− clone were selected concurrently for binding to BBEC by using a modified selection procedure to allow for recovery of B. bigemina parasites after each selection. After three rounds of selection, the numbers of IRBC binding per 100 endothelial cells was determined for all eight populations. Error bars indicate the standard error of the mean for two experiments.
To characterize both parasite and endothelial cell ligands, the availability of clonal parasite lines that express the binding phenotype and are recognized by available immunoreagents would be beneficial. Previous work had identified a parasite-synthesized VESA1 on the C9.1A−I+ clonal line that is recognized by MAbs 4D9.1G1 and 3F7.1H11 (34). Since none of the parasite lines that bound BBEC initially were reactive with these MAbs, one of the BBEC-binding parasite lines, MO7A+I−, was subjected to further selection for both MAb 4D9.1G1 reactivity and BBEC binding. The series of selections used to derive a parasite population that expresses both phenotypes is diagrammed in Fig. 3. When the MO7A+I− population was selected for reactivity with the MAb, the ability to bind BBEC disappeared. When this population was reselected for the adhesive phenotype, the proportion of MAb-reactive cells dropped to 10%. The MAb-reactive parasites in this population were again isolated, yielding the MO7A+I+ line, from which two cytoadhesive parasite clones, CD7A+I+ and CE11A+I−, were derived. CD7A+I+, which is reactive with MAb 4D9.1G1, and CE11A+I−, which is not recognized by the MAb, were used in all subsequent experiments.
The CD7A+I+ and CE11A+I− lines were tested for their ability to bind to cell lines other than BBECUCSF (Table 1). The two parasite lines did not bind at significant levels to any of the nonendothelial cell lines. Very low levels of binding to FBK-4 cells, consisting of rare bound IRBCs scattered on the cell monolayer, were observed. However, CD7A+I+ and CE11A+I− bound very well to CPAE (Table 1, experiment 3) and bound at high density to rare EJG cells (experiment 2), demonstrating that other bovine endothelial cell lines expressed the ligand(s) recognized by CD7A+I+ and CE11A+I− IRBCs, albeit at highly divergent levels.
TABLE 1.
Adhesion of CD7A+I+- and CE11A+I−-infected RBCs to various cell types
| Expt | Cell line | No. of IRBCs/100 endothelial cellsa
|
||
|---|---|---|---|---|
| CD7A+I+ | CE11A+I− | C9.1A−I+ | ||
| 1 | BBECUCSF | 307 (10.8) | 309 (22.1) | 0.15 (0.12) |
| FBK-4 | 3.4 (0.4) | 1.6 (0.5) | 0 (0) | |
| C32 | 0 (0) | 0 (0) | 0 (0) | |
| 2 | BBECUCSF | 313 (23.2) | 313 (19.3) | 0.15 (0.12) |
| COS-7 | 0.28 (0.14) | 0 (0) | 0.3 (0.13) | |
| EJG | 6.8 (2.9) | 2.4 (1.2) | 0.53 (0.3) | |
| 3 | BBECUCSF | 639.8 (110) | 447.8 (49.4) | 0.375 (0.2) |
| CPAE | 537 (163) | 408 (118.8) | 0 (0) | |
| 4 | BBECUF | 340.5 (36.4) | 530.8 (81.9) | 0 (0) |
| Pericytes | 8.3 (3.7) | 10 (3.3) | 0 (0) | |
Values are means (standard errors of the means for two experiments.)
In cytoadherence assays with BBECUCSF cells, the numbers of IRBCs that could be bound decreased with increasing passage number of the endothelial cells (data not shown). Because this created difficulties when performing the assay, new BBEC were isolated from bovine brain by a procedure that allowed the separation and culture of both BBEC and pericytes, a contaminating cell type found in endothelial cell preparations isolated by this method. After four passages with pancreatin, BBECUF cultures appeared essentially free of contaminating cell types, as indicated by the uniform ability of the cells to retain the endothelial cell marker Dil-Ac-LDL (46) (Fig. 4A and B). Pericyte cultures that had been treated four times with pancreatin and passaged twice with trypsin still contained a few endothelial cell colonies (Fig. 4C and D). CD7A+I+ and CE11A+I− IRBCs adhered to the BBECUF cultures and to the BBEC colonies present in the pericyte cultures but not to the pericytes, confirming the endothelial cell-binding specificity of these parasite clones (Table 1, experiment 4). C9.1A−I+, a B. bovis clonal line that is noncytoadhesive for BBECs, was included in these experiments as a negative control and did not adhere at detectable levels to any of the cell types tested (Table 1).
FIG. 4.
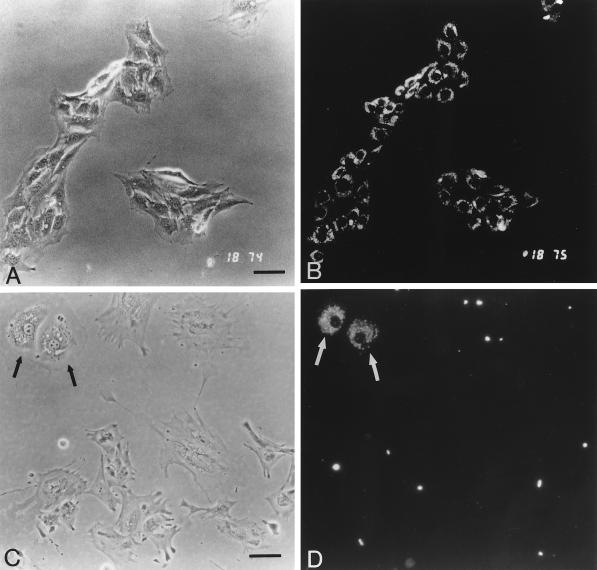
Uptake of the endothelial cell marker Dil-Ac-LDL by BBEC and bovine brain pericytes. BBEC cultures (passage 5) (A and B) and pericytes (passage 2) (C and D) were labeled with Dil-Ac-LDL and examined by phase-contrast (A and C) and fluorescence (B and D) microscopy. Although the pericytes were treated four times with pancreatin to remove endothelial cells, some endothelial cell colonies remained (C and D, arrows). Bar, 50 μm.
To further confirm cell identity, BBECUF cells were also assayed for the expression of another endothelial cell marker, vWF. Surprisingly, only 30% of the BBECUF expressed detectable levels of vWF after five passages. However, CPAE (passage 20) and BBECUCSF (passage 15), included in the assay as positive controls, reacted at even lower levels (20 and 0%, respectively). Other investigators have reported the disappearance of endothelial cell characteristics when capillary endothelial cells were not maintained on extracellular matrices (20). Given that BBECUF cultures were more highly reactive with anti-vWF than were the positive control cultures, it is also possible that the assay as performed was not sensitive enough to detect low-level expression of vWF. Regardless, the morphology of the cells, the ability to retain Dil-Ac-LDL, and the presence of some vWF-positive cells in these cultures all support the premise that BBECUF are cells of endothelial origin.
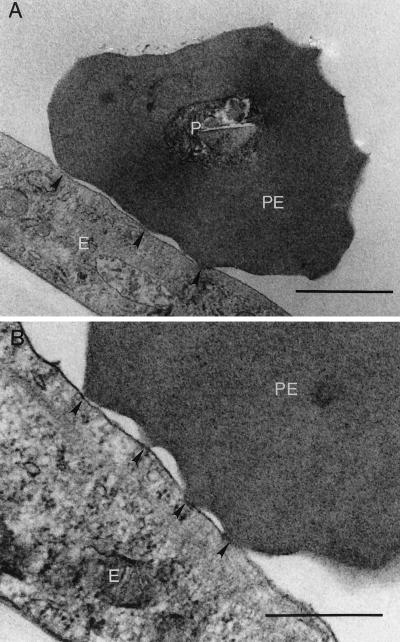
Transmission electron micrographs of B. bovis CD7A+I+-infected RBCs bound to endothelial cells revealed that the parasitized cells bound via the parasite-induced ridge-like protrusions on the IRBC surface (Fig. 5). To explore whether synthesis and export of parasite protein to the IRBC surface was necessary for IRBCs to bind to endothelial cells, proteins were removed from the IRBC surface by trypsinization, and recovery of surface antigen expression was controlled by incubation in the presence or absence of BFA. IRBCs were evaluated for the ability to adhere to BBEC and for the reappearance of the VESA1 protein as a marker for surface antigen expression. The results of these experiments were analyzed by a two-way analysis of variance procedure. The statistical analysis of the cytoadhesion assays was performed on log-normal-transformed data because the raw data failed to meet the assumptions for normality and equality of variance. Since there was a significant interaction between trypsin treatment and BFA treatment for both sets of data, post hoc t-tests with the Bonferroni correction for significance were used to test for differences between control and trypsinized samples for each recovery period. The results of these experiments are shown in Fig. 6. Recovery of VESA1 antigen expression on the surface of trypsinized cells, as detected by immunoreactivity with MAb 4D9.1G1, was dependent upon the length of recovery in the absence of BFA (Fig. 6A). Although the percentage of cells expressing the VESA1 marker on the surface did not directly correlate with the absolute densities of bound IRBCs, the binding densities relative to the control samples closely followed the same qualitative pattern (Fig. 6B). Cells that were trypsinized and allowed to recover VESA1 expression for 4 h bound to endothelial cells as well as did nontrypsinized controls treated in the same manner (Fig. 6B). Conversely, trypsinized IRBCs prevented from exporting new protein by the continued presence of BFA could not cytoadhere to endothelial cells. Trypsinized cells allowed to recover from BFA treatment for only 2 h, however, bound almost as well as did the nontrypsinized samples (Fig. 6B), despite significantly lower surface antigen expression (Fig. 6A). Parasite viability, as assessed by [3H]hypoxanthine incorporation, was unaffected by trypsin treatment but was reduced nearly 50% by BFA treatment in both control and trypsinized samples (data not shown).
FIG. 5.
Transmission electron micrograph of CD7A+I+-infected RBCs adhering to BBEC. The parasites adhere to the endothelial cells via the knob-like structures on the IRBC surface (arrowheads). P, parasite; PE, parasitized RBC; E, endothelial cell. Bars, 1 μm (A) and 0.5 μm (B).
FIG. 6.
Effect of BFA on parasite protein export and cytoadhesion to BBEC. CD7A+I+-infected RBCs were cultured for 1 h in BFA and then trypsinized and cultured in the presence of BFA for 4 h without recovery or in BFA for 2 h with recovery for 2 h or recovered for 4 h in the absence of BFA. Parasites were then assessed for surface antigen expression (A) and cytoadhesion (B). Nontrypsinized controls were run in parallel. Error bars indicate standard error of the mean for three experiments in panel A and four experiments in panel B. The line drawn above the bars gives the level of binding of trypsinized IRBCs, expressed as a percentage of the control values. P values for the post hoc t tests comparing differences between control and treated samples are indicated above the bars.
DISCUSSION
The accumulation of IRBCs in the microvasculature of many tissues is a hallmark of infection with B. bovis (15, 47, 49). Much of the pathology associated with the most severe forms of acute disease has been attributed to sequestration of the IRBCs (15), particularly in the brain. At present it is unclear whether variations in cytoadhesive properties fully explain differences in overall levels of virulence observed in B. bovis isolates or if loss of this adhesive phenotype explains the attenuation that occurs by rapid passage through splenectomized animals (14).
Despite the apparent importance of sequestration to disease etiology, little is known of the mechanisms underlying this phenomenon. It was previously reported that IRBCs appeared to contact the endothelium via the ridge-like protrusions on the IRBC surface (2, 47) and that B. bovis-infected RBCs bind to purified laminin and thrombospondin (36) and to heparin (23). However, these observations have not yet been independently confirmed and the in vitro experiments necessary to demonstrate the relevance of these observations to putative IRBC-endothelial cell interactions have not been reported. Without direct observations of IRBC cytoadhesion to bovine endothelial cells, it was not clear if sequestration of the parasites was attributable to a cell-cell interaction such as occurs in P. falciparum (45) or to some combination of poor deformability of the infected erythrocyte and the localized coagulation associated with severe babesiosis (47, 49).
In this paper we report the first in vitro demonstration of B. bovis cytoadherence by using BBEC. Initially, only one of the parasite lines, d41A−I−, bound to BBEC at detectable levels. Although the number of cells binding per endothelial cell was very small, only infected cells were adhesive, and these cells contained the mature parasite stages that are sequestered in the host animal. Significant levels of cytoadhesion were observed after the parasite populations were repeatedly selected for binding to endothelial cells, indicating that only a minor proportion of the in vitro-cultured parasite population could cytoadhere to the BBECs. This also raised the possibility that the observed binding was an artifactual result of the in vitro manipulations. However, repeated selection of B. bigemina, a nonsequestering Babesia species (16, 39), never yielded endothelial cell-binding parasites. Furthermore, the B. bigemina parasites used in the experiment were of the uncloned Puerto Rico isolate and might be expected to contain a more diverse population of parasites than the B. bovis clonal lines used to select BBEC-binding parasites. This observation indicated that isolation of B. bovis parasites that adhere to BBEC was not due to the selection procedure itself but, rather, was indicative of the unique ability of this species to adhere to endothelial cells.
In a limited comparison of various cell types, the two clones derived from the selection for MAb reactivity and BBEC cytoadhesion bound significantly only to bovine endothelial cell lines. One of these lines, CPAE, is derived from pulmonary artery endothelium that has not been reported to support cytoadhesion in vivo. These cytoadhesion assays, however, were performed under static conditions, and different results might be obtained if the IRBCs were required to bind to these cells under the flow conditions that exist in vivo. For example, in studies of P. falciparum cytoadhesion, the importance of organized knob structures to adhesion became apparent only when the assays were performed under flow conditions (18). CD7A+I+ and CE11A+I− also adhered at high density to rare cells present in the EJG cultures. These adrenal medulla capillary endothelial cells are of unknown passage and could have supported cytoadhesion well during early passage. On the other hand, B. bovis-infected RBCs did not adhere to the pericyte population that copurified with the BBEC during establishment of primary cultures or to any of the nonendothelial cell types. IRBCs were observed bound to the FBK-4 cells (Table 1) at very low densities. The significance of this is unclear but may reflect low-level expression of the receptor(s) to which CD7A+I+ and CE11A+I− bind on endothelial cells, the presence of undifferentiated cells expressing numerous markers, cross-recognition of surface markers unique to kidney epithelial cells, or the presence of a subpopulation of variant parasites capable of binding to epithelial cells. Identification of the receptors mediating cytoadhesion of CD7A+I+- and CE11A+I−-infected RBCs could resolve this issue. Regardless, these results indicate that the binding of B. bovis-infected RBCs to bovine endothelial cells is a specific interaction, and they support the premise that the cytoadhesion assay described here reflects specific host-parasite interactions that occur in vivo during infection.
If cytoadherence of IRBCs to the endothelium represents a specific mechanism of immune system evasion, it is predicted that parasite synthetic activities should be necessary for development of the adhesive state during parasite development. To investigate this hypothesis, intact IRBCs were trypsinized and allowed to recover in the presence or absence of BFA. In eukaryotic cells, BFA inhibits protein transport both from the endoplasmic reticulum to the Golgi apparatus and across the Golgi apparatus (43). The mechanism of action of BFA on B. bovis protein trafficking has not yet been investigated, but live-cell IFA of trypsinized cells clearly demonstrated that protein export to the IRBC surface is severely limited by treatment with BFA (Fig. 6A). When protein transport was allowed to proceed, the IRBCs recovered both surface expression of the VESA1 antigen and the ability to cytoadhere. In contrast, if prevented from transporting protein to the surface by the continued presence of BFA, the cells could not recover the adhesive phenotype. Since the BFA treatment did not destroy parasite viability, these results can be interpreted to indicate that cytoadhesion of IRBC to endothelial cells requires export of parasite proteins to the infected-cell surface.
When expressed as a percentage of control values, the densities of adherent trypsinized IRBCs correlated with the percentage of IRBCs expressing VESA1. However, control samples treated with BFA bound to endothelial cells at reduced densities despite the high percentage of cells expressing VESA1. These results suggest that while protein export may be necessary for adherence, it is not sufficient to achieve normal levels of binding, and that BFA treatment alone affected the capacity of IRBCs to bind. Although BFA treatment did not affect viability, it appeared to halt development of the parasites at the trophozoite stage, as evidenced by a shift in the treated parasite cultures to a high percentage of trophozoite-infected RBCs relative to merozoite-infected RBCs (data not shown). In addition, evaluation of surface antigen expression by live-cell IFA with bovine infection serum revealed that a high percentage of trypsinized cells exhibited constant, low-level fluorescence regardless of BFA treatment. In contrast, control samples appeared to undergo a recovery of surface antigen expression dependent upon the length of BFA treatment. These results may reflect the exposure of normally cryptic parasite-altered host components or nonproteinaceous surface epitopes, but they do not correlate with the recovery of the cytoadhesive phenotype. Thus, BFA treatment alone may affect the surface of the IRBC and IRBC-binding capacities. However, trypsinized cells held on ice to prevent recovery displayed patterns of VESA1 and cytoadhesion recovery that were qualitatively identical to those observed with BFA treatment. Clearly, more than one factor, and probably several, are affecting cytoadherence in this system, including also likely effects of trypsinization on RBC membrane zeta potential and membrane microviscosity. These quantitative anomalies, however, do not alter the conclusions that removal of proteins from the IRBC surface abrogates cytoadhesion and that recovery of the adhesive phenotype is associated with export of parasite proteins to the IRBC surface.
The identities of the parasite receptors and endothelial cell ligands that interact to mediate B. bovis cytoadhesion are still unknown. Previous reports that B. bovis IRBCs may bind to purified thrombospondin, laminin, and heparin in vitro (23, 36) suggest that these materials may act to mediate endothelial cell-IRBC interactions in vivo. Observations made while developing the assay and isolating cytoadhesive parasites indicated that different parasite populations probably use different combinations of endothelial cell receptors and ligands. For example, when testing the adhesive properties of parasites derived from the first round of cloning of the MO7A+I+ population, we found that some of the primary clones bound to all endothelial cells whereas others bound only to minor subpopulations of the endothelial cells (data not shown). Within these two categories, the binding per endothelial cell could be either low (2 to 5 IRBCs/endothelial cell) or high (10 to 15 IRBCs/endothelial cell). These phenotypes were consistent on repeated screening (data not shown). In addition, altering the assay conditions had different effects on the various parasite populations. For example, the adhesion-selected MO7A+I−, C9.1A+I−, and d41A+I− IRBC populations all retained the ability to bind when the assay was performed at 4°C or when the endothelial cells were fixed with 2% glutaraldehyde. In contrast, these treatments ablated cytoadhesion by the clonal lines CD7A+I+ and CE11A+I−. Isolation of these various parasite clones should aid the identification of the endothelial cell ligands which act as receptors for B. bovis-infected RBCs. However, due to the loss of receptor expression observed during extended culture, complete identification of all endothelial cell components that participate in the interactions resulting in sequestration would probably require the use of primary endothelial cell cultures and/or in vivo studies.
Electron micrographs of the endothelial cell-IRBC interaction during cytoadherence revealed attachment of IRBCs via the ridge-like projections, confirming previous in vivo observations of sequestered IRBCs in the capillaries (2, 47, 49). These results indicate that components localized to the knob structures, whether parasite-derived or altered host proteins, are responsible at least in part for adhesion of IRBCs to endothelial cells. In addition, in vitro cytoadhesion could be blocked by immune sera reactive with the IRBC surface (33). VESA1 is one candidate for the B. bovis cytoadherence ligand, since it is expressed on the surface of the IRBC (7, 9, 34) on the ridges of the knobs (33). This antigen, like the P. falciparum cytoadherence ligand, PfEMP1, undergoes clonal antigenic variation within the host (7, 34). Involvement of a variant antigen in cytoadherence could account for the apparent variation in binding phenotype of individual parasite populations. Precedent for this scenario is well established for PfEMP1 in P. falciparum (10, 11, 22, 42). Analysis of B. bovis parasites which bind to BBEC and express VESA1 isoforms containing the epitopes recognized by VESA1a-specific MAbs should facilitate exploration of this hypothesis.
Bovine babesiosis has been proposed as a potential model for cerebral malaria (48) and for antigenic variation (6), because of the similarities between the two diseases. The study of host-parasite interactions in B. bovis infections, however, has lagged far behind the investigation of P. falciparum host-parasite relationships. With the development of an in vitro assay for B. bovis cytoadherence, the mechanics of this interaction can now be dissected and this information can be used to design in vivo experiments in the bovine host. Such experiments could improve the understanding of the pathogenesis of both diseases, and contribute to the development of strategies to alleviate the pathology associated with these parasites.
ACKNOWLEDGMENTS
We thank Suzanne Stroup, Ryan Satcher, and Paula Patterson for excellent technical assistance.
This work was supported by USDA grants 95-37204-2140 and 97-35204-4768 and American Heart Association (Florida affiliate) grant 9810077FL.
REFERENCES
- 1.Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M, Pongponratn E, Tegoshi T, Nakamura K, Nagatake T, Cochrane A, Ozaki L S. A study on the pathogenesis of human cerebral malaria and cerebral babesiosis. Mem Inst Oswaldo Cruz. 1992;87(Suppl. III):297–301. doi: 10.1590/s0074-02761992000700051. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa M, Rabbege J, Uni S, Ristic M, Miller L H. Structural alteration of the membrane of erythrocytes infected with Babesia bovis. Am J Trop Med Hyg. 1985;34:45–49. doi: 10.4269/ajtmh.1985.34.45. [DOI] [PubMed] [Google Scholar]
- 4.Allred D. Immune evasion by Babesia bovis and Plasmodium falciparum: cliff-dwellers of the parasite world. Parasitol Today. 1995;11:100–105. doi: 10.1016/0169-4758(95)80166-9. [DOI] [PubMed] [Google Scholar]
- 5.Allred D R. Immunochemical methods for identification of Babesia bovis antigens expressed on the erythrocyte surface. Methods. 1997;13:177–189. doi: 10.1006/meth.1997.0510. [DOI] [PubMed] [Google Scholar]
- 6.Allred D R. Antigenic variation in Babesia bovis: how similar is it to that in Plasmodium falciparum? Ann Trop Med Parasitol. 1998;92:461–472. doi: 10.1080/00034989859438. [DOI] [PubMed] [Google Scholar]
- 7.Allred D R, Cinque R M, Lane T J, Ahrens K P. Antigenic variation of parasite-derived antigens on the surface of Babesia bovis-infected erythrocytes. Infect Immun. 1994;62:91–98. doi: 10.1128/iai.62.1.91-98.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allred D R, Gruenberg J E, Sherman I W. Dynamic rearrangements of erythrocyte membrane internal architecture induced by infection with Plasmodium falciparum. J Cell Sci. 1986;81:1–16. doi: 10.1242/jcs.81.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Allred D R, Hines S A, Ahrens K P. Isolate-specific parasite antigens of the Babesia bovis-infected erythrocyte surface. Mol Biochem Parasitol. 1993;60:121–132. doi: 10.1016/0166-6851(93)90035-v. [DOI] [PubMed] [Google Scholar]
- 10.Baruch D, Gormley J I, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baruch D, Pasloske B, Singh H, Bi X, Ma X, Feldman M, Taraschi T, Howard R. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 12.Biggs B A, Gooze L, Wycherley K, Wollish W, Southwell B, Leech J H, Brown G V. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calder J A M, Reddy G R, Chieves L, Courtney C H, Littell R, Livengood J R, Norval R A I, Smith C, Dame J B. Monitoring Babesia bovis infections in cattle by using PCR-based tests. J Clin Microbiol. 1996;34:2748–2755. doi: 10.1128/jcm.34.11.2748-2755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callow C, Mellors L, McGregor W. Reduction in virulence of Babesia bovis due to rapid passage in splenectomized cattle. Int J Parasitol. 1979;9:333–338. doi: 10.1016/0020-7519(79)90083-3. [DOI] [PubMed] [Google Scholar]
- 15.Callow L, McGavin M. Cerebral babesiosis due to Babesia argentina. Aust Vet J. 1963;39:15–21. [Google Scholar]
- 16.Callow L L, Johnston L A Y. Babesia spp. in the brains of clinically normal cattle and their detection by a brain smear technique. Aust Vet J. 1963;39:25–31. [Google Scholar]
- 17.Carson M P, Haudenschild C C. Microvascular endothelium and pericytes: high yield, low passage cultures. In Vitro Cell Dev Biol. 1986;22:344–354. doi: 10.1007/BF02623409. [DOI] [PubMed] [Google Scholar]
- 18.Crabb B S, Cooke B M, Reeder J C, Waller R F, Caruana S R, Davern K M, Wickham M E, Brown G V, Coppel R L, Cowman A F. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 19.Crandall I, Land K M, Sherman I W. Plasmodium falciparum: pfalhesin and CD36 form an adhesin/receptor pair that is responsible for the pH-dependent portion of cytoadherence/sequestration. Exp Parasitol. 1994;78:203–209. doi: 10.1006/expr.1994.1020. [DOI] [PubMed] [Google Scholar]
- 20.Doron D A, Jacobowitz D M, Heldman E, Feuerstein G, Pollard H B, Hallenbeck J M. Extracellular matrix permits the expression of von Willebrand factor, uptake of di-I-acetylated low density lipoprotein, and secretion of prostocyclin in cultures of endothelial cells from rat brain microvessels. In Vitro Cell Dev Biol. 1991;27A:689–697. doi: 10.1007/BF02633213. [DOI] [PubMed] [Google Scholar]
- 21.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 22.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodger B, Mahoney D F, Wright I G. Babesia bovis: attachment of infected erythrocytes to heparin-sepharose columns. J Parasitol. 1983;69:248–249. [PubMed] [Google Scholar]
- 24.Hines S A, McElwain T F, Buening G M, Palmer G H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989;37:1–10. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- 25.Ho M, Schollaardt T, Niu X, Looareesuwan S, Patel K, Kubes P. Characterization of Plasmodium falciparum-infected erythrocyte and P-selectin interaction under flow conditions. Blood. 1998;91:4803–4809. [PubMed] [Google Scholar]
- 26.Levy M J, Ristic M. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science. 1980;207:1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- 27.Luse S A, Miller L H. Plasmodium falciparum malaria: ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971;20:655–660. [PubMed] [Google Scholar]
- 28.Macpherson G G, Warrell M J, White N J, Looareesuwan S, Warell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 29.Mahoney D F, Wright I, Mirre G. Bovine babesiasis: the persistence of immunity to Babesia argentina and B. bigemina in calves (Bos taurus) after naturally acquired infection. Ann Trop Med Parasitol. 1973;67:197–203. doi: 10.1080/00034983.1973.11686877. [DOI] [PubMed] [Google Scholar]
- 30.Newbold C I, Craig A G, Kyes S, Berendt A R, Snow R W, Peshu N, Marsh K. PfEMP1, polymorphism and pathogenesis. Ann Trop Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 31.Ockenhouse C, Klotz F, Tandon N, Jamieson G. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malarial-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci USA. 1991;88:3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connor R M. Cytoadhesion of Babesia bovis-infected erythrocytes to endothelial cells is linked to antigenic variation of the infected erythrocyte surface. Ph.D. dissertation. Gainesville: University of Florida; 1999. [Google Scholar]
- 34.O’Connor R M, Lane T J, Stroup S E, Allred D R. Characterization of a variant erythrocyte surface antigen (VESA1) expressed by Babesia bovis during antigenic variation. Mol Biochem Parasitol. 1997;89:259–270. doi: 10.1016/s0166-6851(97)00125-4. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor R M, Long J A, Allred D R. Selection and recovery of minor parasite populations expressing unique infected-erythrocyte phenotypes. Mol Biochem Parasitol. 1999;100:125–129. doi: 10.1016/s0166-6851(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 36.Parrodi F, Wright I, Bourne A, Dobson C. In vitro adherence of bovine erythrocytes infected with Babesia bovis to thrombospondin and laminin. Int J Parasitol. 1989;19:567–569. doi: 10.1016/0020-7519(89)90088-x. [DOI] [PubMed] [Google Scholar]
- 37.Pasloske B L, Howard R J. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. doi: 10.1146/annurev.med.45.1.283. [DOI] [PubMed] [Google Scholar]
- 38.Pongponratn E, Riganti M, Ponpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 39.Rees C W. Characteristics of the piroplasms Babesia argentina and Babesia bigemina in the United States. J Agric Res. 1934;48:427–438. [Google Scholar]
- 40.Reynolds E S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–213. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez S D, Buening G M, Green T J, Carson C A. Cloning of Babesia bovis by in vitro cultivation. Infect Immun. 1983;42:15–18. doi: 10.1128/iai.42.1.15-18.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith J, Chitnis C, Craig A, Roberts D, Hudson-Taylor D, Peterson D, Pinches R, Newbold C, Miller L. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strous G J, van Kerkhof P, van Meer G, Rijnboutt S, Stoorvogel W. Differential effects of brefeldin A on transport of secretory and lysosomal proteins. J Biol Chem. 1993;268:2341–2347. [PubMed] [Google Scholar]
- 44.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 45.Udeinya I J, Schmidt J A, Aikawa M, Miller L H, Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981;213:555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- 46.Voyta J C, Via D P, Butterfield C E, Zetter B R. Identification and isolation of endothelial cells based on their increased uptake of acetylated low density lipoprotein. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright I G. An electron microscopic study of intravascular agglutination in the cerebral cortex due to Babesia argentina infection. Int J Parasitol. 1972;2:209–215. doi: 10.1016/0020-7519(72)90008-2. [DOI] [PubMed] [Google Scholar]
- 48.Wright I G, Goodger B V, Clark I A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988;4:214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]
- 49.Wright I G, Goodger B V, McKenna R V, Mahoney D F. Acute Babesia infection: a study of the vascular lesions in kidney and lung. Z Parasitenkd. 1979;60:19–27. [Google Scholar]