Abstract
Older adults represent a vulnerable population with elevated risk for numerous morbidities. To explore the association of the microbiome with aging and age-related susceptibilities including frailty and infectious disease risk, we conducted a longitudinal study of the skin, oral, and gut microbiota in 47 community- or skilled nursing facility-dwelling older adults vs. younger adults. We found that microbiome changes were not associated with chronological age so much as frailty: we identified prominent changes in microbiome features associated with susceptibility to pathogen colonization and disease risk, including diversity, stability, heterogeneity, and biogeographic determinism, which were moreover associated with a loss of Cutibacterium (C.) acnes in the skin microbiome. Strikingly, the skin microbiota were also the primary reservoir for antimicrobial resistance, clinically important pathobionts, and nosocomial strains, suggesting a potential role particularly for the skin microbiome in disease risk and dissemination of multidrug resistant pathogens.
One Sentence Summary
A metagenomic study of gut, oral, and skin microbiota describes a pattern of microbial dysbiosis in more frail institutionalized older adults and identifies the skin as the major reservoir of pathogenicity.
Introduction
Aging is the predominant risk factor for most healthspan-limiting diseases1. A complex interplay of environmental, lifestyle and genetic factors contributes to the development of chronic diseases and geriatric syndromes that decrease length and quality of life2. An emerging risk factor for aging-related diseases, based on its ubiquity and central role in immunity and metabolism, is the microbiome - the diversity of bacteria, archaea, viruses, and fungi inhabiting the human body. The microbiome has mechanistic roles in virtually every dimension of human health overlapping considerably with aging, including cardiovascular health3, 4, cancer, infection risk, and numerous other morbidities linked to metabolic and immune senescence5, 6. An improved understanding of its dynamics in older adults offers opportunities for developing interventions to reduce the public health burden of aging and improve quality of life for older adults.
However, we must first gain a baseline understanding of how the microbiota of older adults differ from younger adults and how these differences associate with chronic conditions, including frailty. Frailty comprises a state of decreased function and physiological reserves and increased risk of morbidity and mortality resulting from an accumulation of deficits7-9. Notably, there is considerable heterogeneity in frailty, health status, and function among individuals of the same chronological age due to the compounding and interacting effects of genetic and environmental factors10-12. We hypothesized that the microbiome would associate more strongly with frailty rather than chronological age, and that heterogeneity in the microbiome would be similarly increased.
While including older adults in biomedical research is essential, it presents challenges especially in institutionalization13, which has emerged as an important variable for microbiome studies and for its clinical relevance. Recent studies identified nursing-home specific microbiota, a byproduct of the residents’ age, frailty, location, diet, and other factors14, 15. In addition, skilled nursing facility dwellers (SNFD) are at a particularly elevated risk for infection with antimicrobial resistant organisms given elevated antibiotic use and prolonged exposure to health care environments16-19. The vulnerability of SNFD to infectious diseases has been especially evident during the COVID-19 pandemic20. As SNFD are an easily exploited group with a high rate of adverse events, involving them in microbiome research requires special attention and human subjects research expertise. Furthermore, SNFD are inherently a more frail population, making it difficult to draw direct comparisons to community dwellers (CD).
The gut microbiome has been the focus of aging research to date21, 22, but major knowledge gaps remain in its relationship with frailty, the contribution of subspecies, or strain-level diversity, and links with other body sites. Insights into the role of the oral and skin microbiota in aging are even fewer despite known functions in local and systemic health though immune modulation, pathogen resistance, and cross-colonization23-26. A recent meta-analysis found that the skin microbiome was a better predictor of chronological age than the oral or gut microbiomes27. However, the few available studies rarely interrogate multiple skin sites despite the known skin site specificity of the microbiome and skin disease predilection28-30. They also use 16S rRNA gene sequencing31-34, which lacks the resolution needed for discovery of pathogenic species, strains, antibiotic resistance genes, and virulence factors35, 36. A high-resolution, longitudinal metagenomic study of multiple body sites would provide significant advantages to deciphering the microbiome’s potential relationships with health and disease in older adults.
To address these knowledge gaps, we performed such a study of the skin, oral, and gut microbiomes of older adults dwelling in the community and skilled nursing facilities, compared with younger adults. To make clinically relevant associations, we also performed frailty assessments and collected detailed dietary, medication, and lifestyle data for an association analysis of each individual’s microbiota down to the strain level. Strikingly, the most dramatic differences were found in the skin microbiome, which associated with frailty rather than chronological age. In addition, the skin, rather than the mouth or gut, was the primary reservoir for clinically important pathogens, including nosocomial strains, and antimicrobial resistance genes. Our study provides a high resolution baseline of the older adult microbiome that may be built upon to reduce infection risk and improve healthspan in older and frailer adults.
Results
Study Design
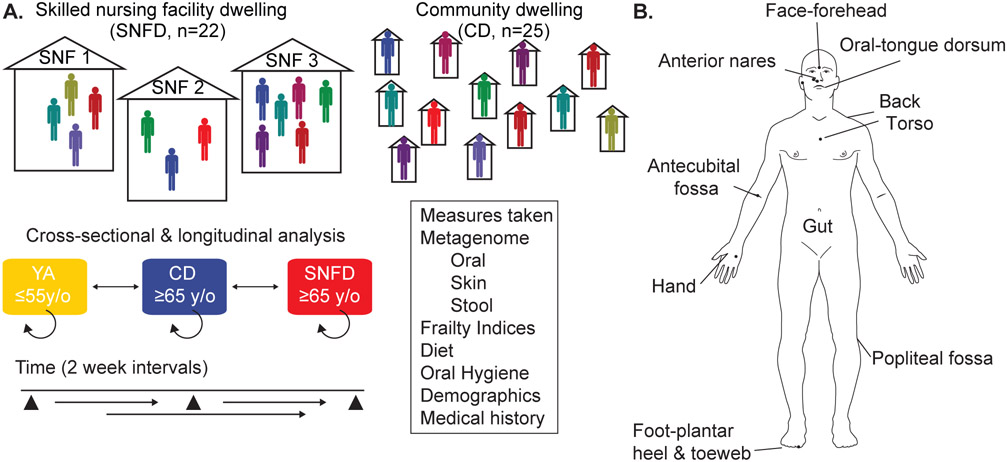
We collected skin, oral, and gut microbiome samples and clinical data from two cohorts - SNF dwelling/dwellers (SNFD), recruited from three SNFs; and age-matched community dwelling/dwellers (CD), not living in long-term care, but privately in the community (Fig. 1A, Table 1). All participants were aged ≥65, had no evidence of active skin, oral, or gastrointestinal illness, and were sampled at 3 timepoints over a month with no antibiotic or antifungal use in the past 30 days. Participants were sampled at eight skin sites and the tongue dorsum, and performed stool self-sampling (Fig. 1B). At each visit, we also collected medical history, dietary and hygiene surveys, and assessed frailty using the Rockwood Frailty Index (RFI) and the Fried Frailty Phenotype and the Physical Activity Scale for the Elderly (PASE), which collectively gauges physical and cognitive abilities and medical conditions37-39.
Fig. 1. Study Design.
(A) SNFD cohort was recruited from three different SNFs. CD cohort was recruited from older adults living privately outside of a nursing home. In addition to metagenomic sampling, we collected medical histories, conducted PASE, Fried, and Rockwood Frailty Indices, administered dietary and oral hygiene surveys. Our major comparisons were within cohorts, between cohorts, and with a young adult (YA) cohort derived from our biorepository obtained with identical methods, and our and others’ previously published longitudinal YA cohorts (skin: Oh et al. 2014 & 2016, Zhou et al., 2020; oral and gut samples: Human Microbiome Project 28-30, 42. (B) Sites obtained by swab at each subject visit: face (forehead), anterior nares, oral (tongue dorsum), upper torso, upper back, antecubital fossa (Af), palmar hand, popliteal fossa (Pf), and foot (plantar surface and toe web space). Participants performed stool self-sampling. Figure adapted from Oh et al., 2014.
Table 1. Cohort Demographics.
SD: Standard deviation; MET: Oh 2014-2016 cohort; HMP: Human Microbiome Project cohort. Grey indicates no data available.
| YA | CD | SNFD | |||
|---|---|---|---|---|---|
| Demographic Characteristics | MET | HMP | in-house | ||
| Total No. | 12 | 260 | 95 | 25 | 22 |
| Age range, y | 18-55 | 18-55 | 18-55 | 65-91 | 65-97 |
| Age, y, Mean (SD) | 78.2 (7.59) | 82.9 (8.46) | |||
| Sex, No. (Self-Reported) | |||||
| Male Participant | 7 | 136 | 50 | 11 | 2 |
| Female Participant | 5 | 124 | 45 | 14 | 20 |
| Race | |||||
| American Indian or Alaska Native | 1 | 0 | |||
| White | 24 | 20 | |||
| More than one race | 0 | 2 | |||
| Ethnicity | |||||
| Hispanic or Latino | 1 | 2 | |||
| Non-Hispanic or Latino | 24 | 18 | |||
| Height, mean (SD), cm | 167.0 (11.9) | 160.0 (8.5) | |||
| Weight, mean (SD), kg | 69.0 (15.4) | 78.7 (23.9) | |||
We examined the distributions of clinical data obtained, both for their relevance and their potential to stratify cohort comparisons (table S1). As designed, our age distribution (65-74, 75-84, and 85+ years old) allowed us to consider chronological age independently from frailty or place of residence (fig. S1A). Although the age distribution of the two groups were relatively matched, the frailty of SNFD was substantially greater than CD. This allowed us to use SNFD status as a proxy for frailty in certain comparisons, but limited our ability to deconvolute these two variables. Our SNFD cohort was also comparatively enriched for women, consistent with SNFD demographics, and had on average higher BMIs (fig. S1B-E). Diets between SNFs were relatively homogenous with few meaningful differences in diet between CD and SNFD with regard to sweets, whole grain, or red meat consumption, factors that alter the gut microbiome40, 41, but SNFD more often reported “never consuming fresh fruits or vegetables” (fig. S2). Oral hygiene habits, which included denture use, use of tobacco products, frequency and time since last brushing, and mouthwash use, were comparable between cohorts, except denture use, which we considered as a potential variate (fig. S3).
Of 47 enrolled, four volunteers had to withdraw after their first or second visit due to infection requiring antibiotic treatment, highlighting challenges of studying older adult cohorts. In total, we collected 1,385 samples (table S2; 1,072 skin, 159 oral, 154 stool). To identify differences between our older cohorts vs. younger adults (YA), we performed parallel analyses with two YA cohorts. Our “in-house” YA cohort comprised 219 samples (107 matched skin, 32 oral, and 80 stool samples) from n=95 healthy younger adults (18-55yo), collected and processed identically to the CD/SNFD cohorts. To further support the robustness of our conclusions, we merged the “in-house” data with data from two published datasets (“expanded” YA cohort). This included our longitudinal skin microbiome dataset (247 samples from three timepoints spanning ~1 month and ~1-3 year, n=12)29, 30, and 1,090 longitudinal stool, tongue dorsum, and anterior nares samples (1-3 timepoints ~2 weeks apart) from the Human Microbiome Project, n=260 healthy YAs42, 43. Our rationale was that these external data would increase power and external validity, but could present potential confounders due to methodological differences (assessed in fig. S4 and Methods). Select analyses, like longitudinal analysis, were performed only with the “expanded” YA dataset, as ample longitudinal data was lacking from the “in-house” cohort. Henceforth, we referred primarily to conclusions derived from the “in-house” YA cohort and reported, where significant or significantly discrepant, the results from the “expanded” YA analysis (full “expanded” analysis available in the supplement).
Diversity, stability, and heterogeneity of the older adult microbiome
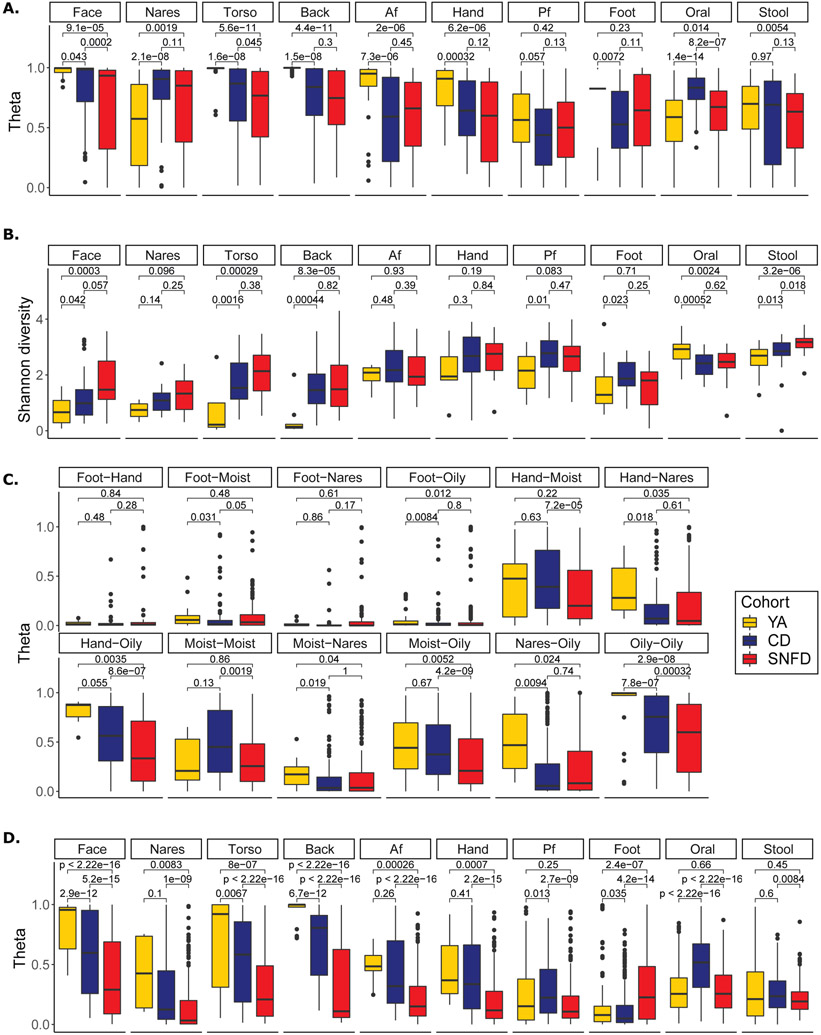
We first examined community-level metrics, which can provide insights into the collective resilience of the ecosystem. Stability is a hallmark of healthy microbiomes, implying a resistance to perturbation or pathogen colonization30, 44, 45. We calculated θ and Bray-Curtis dissimilarity, metrics of similarity between communities based on the proportion of species shared, for samples taken from the same individual over time. Here, we used the “expanded” cohort for an improved representation of body sites over time. The microbiome of SNFD at oral, torso, and face sites were significantly less stable compared to CD (Fig. 2A, bidirectional Wilcoxon p < 0.05, fig. S5A). Strikingly, in contrast to the stable skin microbiome of YA over both short and long timespans30, we observed that SNFDs had substantially decreased stability in oily skin sites compared to YA and CD.
Fig. 2. Instability, Hyperdiversification, Heterogeneity, and Biogeographic Divergence in the Aging Microbiome.
(A) Stability, as measured by Yue-Clayton theta index (θ) comparing samples from an individual at different timepoints. θ=1 represents 100% similarity in shared species and their relative abundance; θ=0 indicates no (0%) similarity. The expanded YA cohort – including the in-house, MET and HMP samples -- was used to estimate stability. YA samples from Oh et al. 2016 (face, torso, back, Af, Hand, Pf, foot) had three timepoints, 10-30 months between timepoint 1 and 2, and 5-10 weeks between timepoints 2 and 3. YA samples from Zhou et al. 2021 had 2-4 time points, 2-4 weeks apart. 58 unpublished in-house YA stool samples had 2 time points, approximately 1 year apart (Table S2). HMP samples (YA nares, oral, stool) were taken at ~2 week intervals, as our SNFD and CD cohorts. Additional YA samples were cross-sectional. The analyses were conducted using n=406 YA skin, n=421 YA gut, n=275 YA oral, n=498 CD skin, n=64 CD gut, n= 62 CD oral, n=522 SNFD skin, n=53 SNFD gut, n= 66 SNFD oral time series samples. (B) Shannon Diversity Index represents the number and evenness of different taxa. Diversity of YA was estimated using only the in-house YA cohort. (C) Biogeographic divergence, as measured by θ comparing samples from different skin sites on the same individual at the same timepoint. Divergence of YA was estimated using only the in-house YA cohort. Hand sampling in this study included both dry and moist sites, so we treated hand samples as a distinct site type, as are feet. (D) Inter-individual similarity, as measured by θ comparing samples between individuals within each cohort. Similarity of YA was estimated using only the in-house YA cohort. Lower θ within a cohort ~ higher heterogeneity. The analyses for B, C, and D were conducted using n=83 YA skin, n=53 YA gut, n=28 YA oral, n=195 CD skin, n=25 CD gut, n=25 CD oral, n=176 SNFD skin, n=20 SNFD gut, and n=22 SNFD oral biologically independent (i.e. averaged across repeated measurements) samples. Boxplot edges represent the lower and upper quartile, center lines represent the median, whiskers are extended to the most extreme data point that is no more than 1.5 times the interquartile range from the edges. Benjamini-Hochberg-adjusted two-sided Wilcoxon tests p values are indicated for each comparison. Analogous Bray-Curtis dissimiliarities, analyses using the expanded YA cohort, and analyses using data rarefied to 500,000 and 100,000 reads are reported in fig. S5-S7.
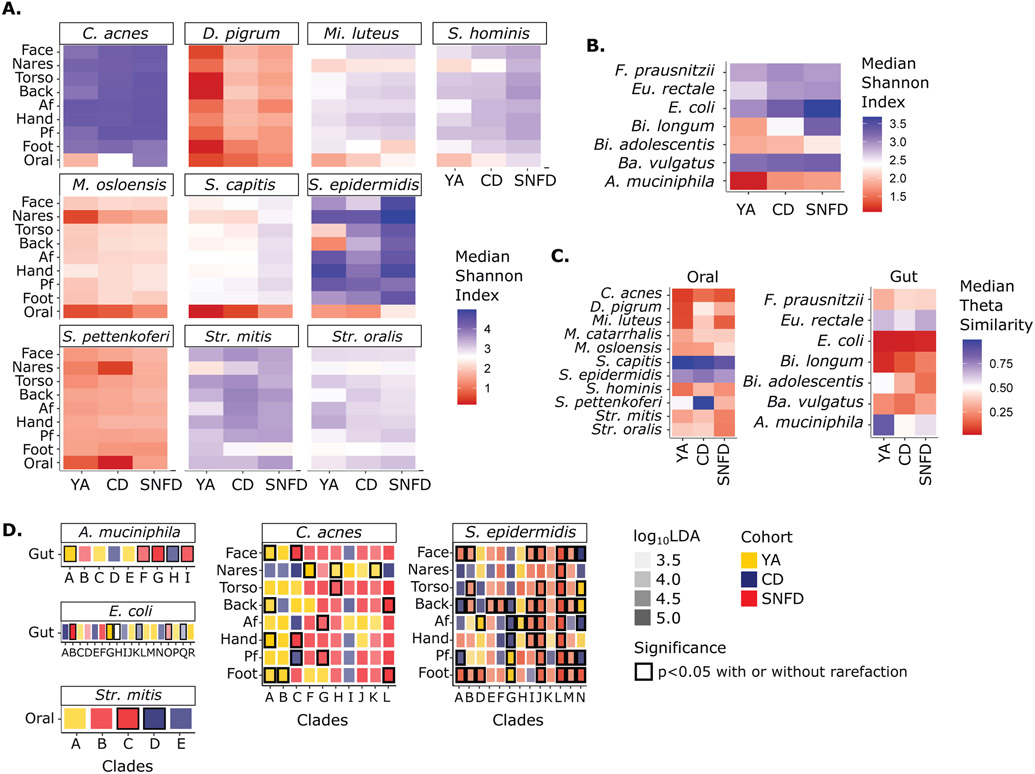
Diversity is also commonly attributed to a healthy microbiota, where reduced diversity often represents pro-inflammatory or infectious states44. A healthy microbiome can also be low diversity, e.g., oily skin of healthy individuals is dominated by lipophile Cutibacterium (C.) acnes29, 30. In such cases, hyperdiversification is associated with disease due to a loss of immune surveillance or selectivity of the ecosystem, leading to higher pathogen susceptibility46. We observed broad hyperdiversification at oily skin sites and in the gut of the older vs. younger adults (face, torso, back, and gut, Fig. 2B, bidirectional Wilcoxon, p < 0.05, fig. S6A & S7), with SNFD diversity also exceeding CD diversity in the gut. Increasing gut microbial diversity with age is consistent with previous reports and is thought to result from the accumulation of taxa due to higher colonization susceptibility with age47. Conversely, the oral diversity of CD and SNFD was reduced compared to YA, but not significantly different between CD and SNFD. These results suggest a broad remodeling of microbial diversity in older adults that is body-site specific.
Concurrently, we observed loss of biogeographic differences in the microbiota of older adults. Physiologic differences in skin type (oily like the face, torso, and back, moist like the antecubital and popliteal fossa, and dry like the volar forearm and hypothenar palm) are associated with different microbiome compositions29, 30. Loss of such biogeographic determination has been associated with primary immunodeficiency46, was recently reported in older adults in the upper respiratory tract48, and could potentially alter the site-specificity of microbe-associated skin diseases49. To test this, we calculated θ and Bray-Curtis dissimilarity between body sites within individuals. Surprisingly, biogeographic determinism was increased for older adults, with most skin sites (except the foot) becoming even less similar (Fig. 2C, fig. S5C & S6B).
This skin site specificity and hyperdiversification is unlikely to be driven by a single microbe, but rather by many different microbes. We quantified heterogeneity of the microbiome with θ and Bray-Curtis dissimilarity between individuals within a cohort. For all skin sites except the foot and popliteal fossa, heterogeneity was significantly and substantially increased in SNFD compared to YA, and also SNFD compared to CD (Fig. 2D, bidirectional Wilcoxon, p < 0.05, fig. S5B & S6C). This suggests that the skin microbiome diverges with frailty from a central core structure seen in YA and CD adults. With the increased statistical power provided by the expanded YA cohort, this decrease in heterogeneity comparing YA to CD was also statistically significant (fig. S6C), suggesting that aging-related changes may support a greater variety of skin microbiome compositions.
Composition of the skin, gut, and oral microbiome of older adults
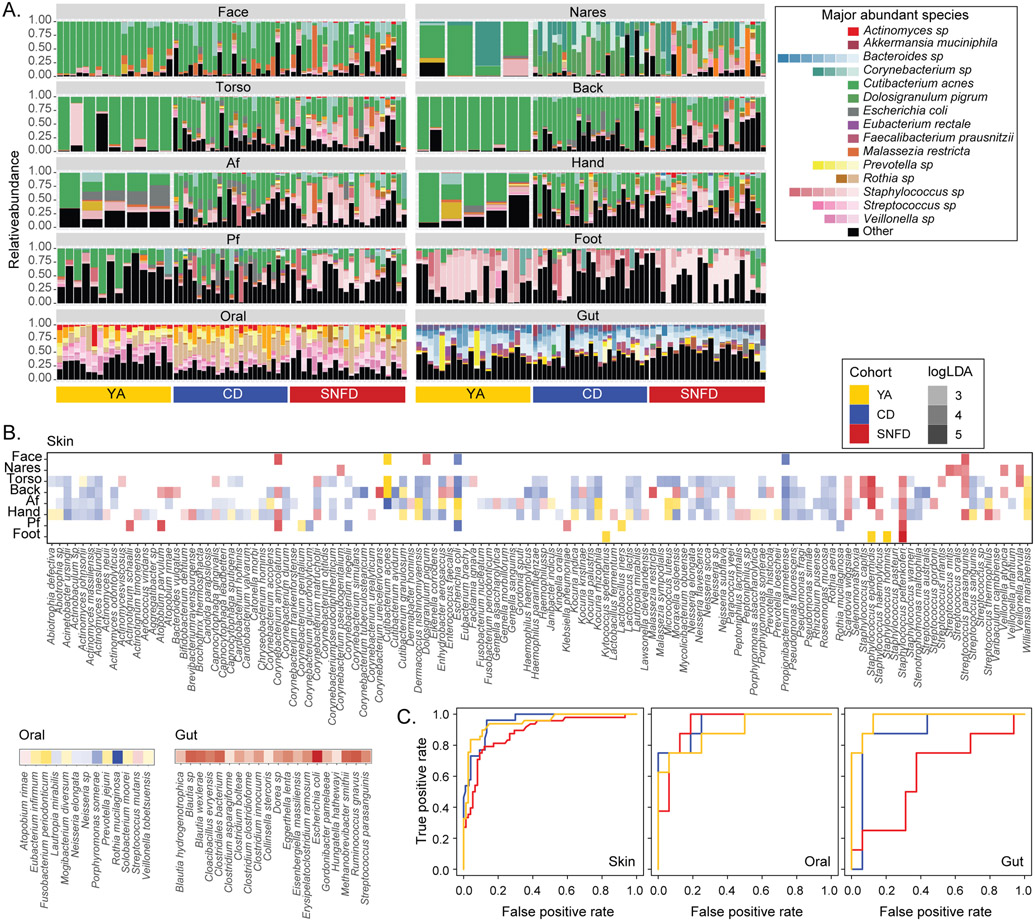
We then examined species-level differences between cohorts. Identifying increases in the prevalence or relative abundance of pathobionts, or the loss of commensal microbes might reflect risk factors for infection or inflammatory disease. We observed the most striking compositional differences between cohorts in the skin microbiome (Fig. 3A, fig. S8A, and table S3). Notably, CD and SNFD skin showed a substantial depletion of C. acnes at oily sites (dry sites also significant with the expanded cohort; Fig. 3A-B, fig. S8A-B, table S4, Kruskal-Wallis test p < 0.05 and log10LDA > 2.0). C. acnes is a ubiquitous commensal skin microbe, dominant in oily skin sites in younger adults29 with key roles in immunomodulation, epithelial barrier maintenance, and protecting the host from pathogens as well as acne vulgaris50-53. At oily sites, Streptococcus (Str.) parasanguinis was significantly enriched in SNFD, while Propionibacterium (P.) namnetense was significantly enriched in CD (Fig. 3B, fig. S8B, table S4, Kruskal-Wallis test p < 0.05 and log10LDA > 2.0). Coagulase-negative staphylococci, also essential in cutaneous health but also a major cause of skin infections and nosocomial sepsis54, were frequently enriched in SNFD skin, particularly Staphylococcus (S.) epidermidis and S. capitis at torso, back and antecubital fossa, and S. pettenkoferi at all sites except for face and nares (Fig. 3B, fig. S8B, table S4, Kruskal-Wallis test p < 0.05 and log10LDA > 2.0; most associations also significant using the expanded YA).
Fig. 3. Compositional Differences in the Microbiota of Older Adults.
Only the in-house YA cohort was used. (A) Relative abundance plots of microbial species across body sites. The top 10 most abundant species in the skin, oral, and gut samples for YA, CD, SNFD cohorts were shown. Each bar represents an individual with relative abundance values from all timepoints averaged. See table S3 for full classifications and fig. S8A for full legend. ~20 YA subjects were shown for each body site. (B) Microbial species significantly enriched in YA, CD, or SNFD cohort. Only significant and strongly enriched species (Kruskal-Wallis test p < 0.05 and log10LDA > 2.0) are shown. Color opacity indicates the corresponding log10LDA value. (C) Receiver operating characteristic (ROC) curves for random forests classifiers assigning individuals into YA (orange), CD (blue), or SNFD (red) based on species-level taxonomic composition. Repeated measurements were averaged for each subject-body site combination. Analogous analyses using the expanded YA cohort are reported in fig. S8 and fig. S9A.
We then examined if these species could differentiate older adults from YAs, or CD from SNFD to identify potential biomarkers of aging. We trained a random forest model on a randomly selected 60% of subjects and tested it on the remaining 40%, accounting for repeated measurements and subject-specific patterns (Fig. 3C, fig. S8C & S9A-B). Notably, S. pettenkoferi contributed most strongly to differentiation of cohorts (fig. S9C, AUC ≥ 0.87), supporting our assessment that SNFD cohorts are distinguished by the relative abundance of certain coagulase-negative staphylococci.
Differences in the oral microbiota were less extensive than in skin (Fig. 3B, fig. S8B & S9, table S4, Kruskal-Wallis test p < 0.05 and log10LDA > 2.0. Among SNFDs, subjects without denture use had enriched Actinobaculum sp., Selenomonas sputigena, and Alloprevotella tannerae; those without showed enriched Granulicatella adiacens (fig. S10A, Kruskal-Wallis test p < 0.05 and log10LDA > 2.0). There was insufficient denture use among CDs to investigate correlations within that cohort. Mouthwash-microbe associations were inconsistent for CD and SNFD (fig. S10B-C), and none of these associations involved taxa differentially enriched in CD or SNFD (Fig. 3B, fig. S8B & S10B-C).
In the gut, Clostridium species were significantly enriched in SNFD (Fig. 3B and fig. S8B, Kruskal-Wallis test p < 0.05 and log10LDA > 2.0) and were also among the most important differentiating features between cohorts (Fig. 3C, fig. S8C & S9, AUC ≥ 0.61 for “ in-house” and AUC > 0.85 with the expanded YA cohort). Compared to CDs, SNFDs had a lower Bacteroidetes:Firmicutes ratio (fig. S11A, bidirectional Wilcoxon test, p < 0.05), which has been implicated with metabolic syndrome and gut dysbiosis55. However, this ratio is also associated with obesity56, thus this difference may be related to the modestly higher BMI of SNFD (fig. S1). We also classified stool samples into ‘enterotypes’ based on their microbiome composition: “R”– Ruminococcus-, “B”–Bacteroides-, and “P”–Prevotella-rich (fig. S11B-D). Interestingly, no SNFD samples were classified as Prevotella-rich (fig. S11D). This enterotype is believed to be associated with a long-term carbohydrate-rich diet57, potentially reflecting lifestyle impacts on the gut microbiome.
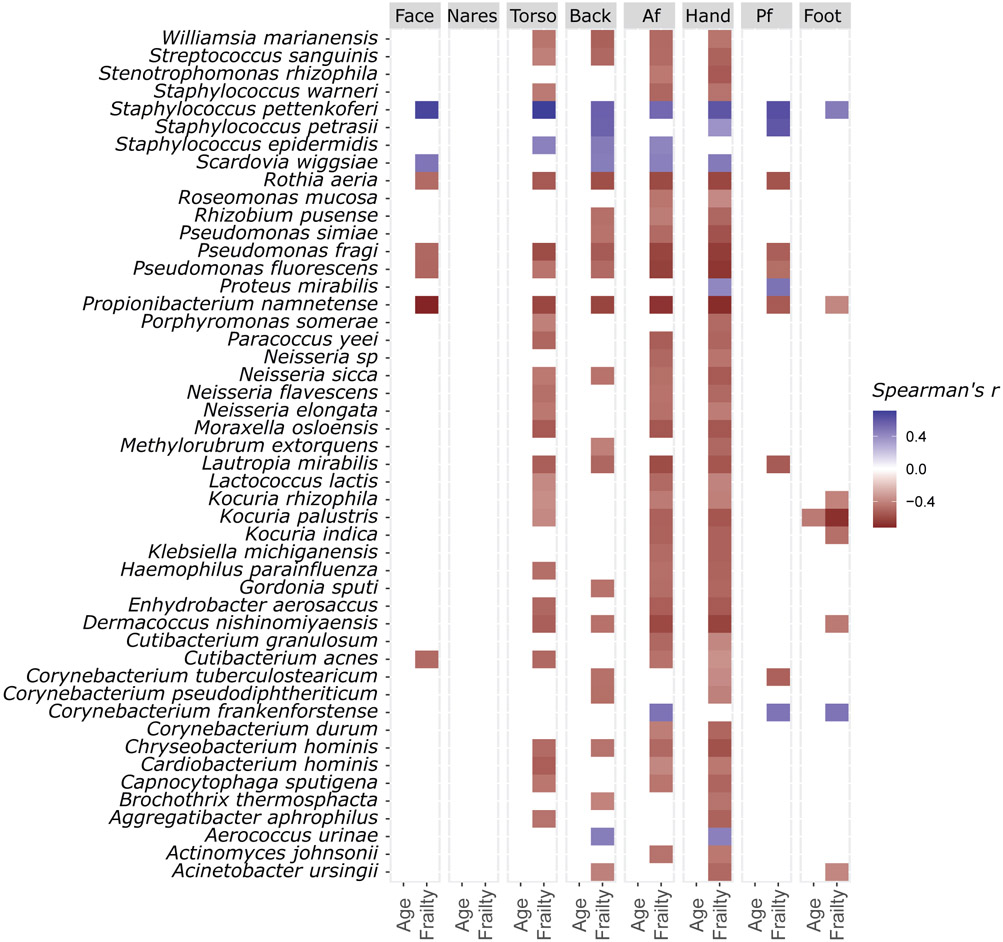
We then examined whether these taxonomic differences associated with clinical features of aging. Surprisingly, chronological age alone did not consistently correlate with any species (Fig. 4), stability, inter-individual heterogeneity, diversity, or biogeographic divergence (fig. S12). On the other hand, the Rockwood Frailty Index was positively associated with the relative abundance of C. acnes at face, torso, antecubital fossa and hands (Fig. 4, p < 0.05). S. pettenkoferi and P. namnetense, both poorly studied opportunistic pathogens58, 59, were most consistently associated with frailty, with the former positively and the latter negatively correlated with frailty at all sites except the nares (Fig. 4, p < 0.05). Other significant correlations included R. aeria and two Pseudomonas (Ps.) species: Ps. fluorescens and fragi, all exhibiting negative correlations with frailty at all sites except the nares and foot (Fig. 4, p < 0.05).
Fig. 4. Associations between Age, Frailty, and the Skin Microbiome.
Association between species relative abundance, age, and frailty (Rockwood Index), tested using the hierarchical all-against-all association testing method (HAllA). Only significant (fdr adjusted p < 0.05) Spearman’s coefficients are shown. Sample sizes: back/face/foot/af/nares n=46, hand/pf/torso N=47.
Finally, earlier studies suggested that the gut microbiota of SNFD may be driven by their place of residence14. We investigated if facility predicted SNFD microbiota versus individual factors. In our cohorts, facility did not coincide with the first principal components of gut, oral, or skin metagenome composition (fig. S13). In addition, no microbiome could differentiate SNFs, even with a random forests classifier that can account for nonlinear relationships (fig. S14). These findings suggest that a particular SNF is not a substantial confounder of the SNF-associated signatures we observed.
Strain Level Composition
Microbial species can encompass genetically diverse strains with profoundly different implications for the host60. For example, E. coli encompasses probiotic and enterohemorrhagic strains61, 62; in the skin, S. epidermidis is both a keystone commensal and opportunistic pathogen. We recently determined that within-individual strain diversity of S. epidermidis is extensive and relevant for skin health, suppressing population-level expression of virulence factors28. Thus, strain-level investigations can add clinical implications to species-level inferences.
Using a reference-based approach leveraging curated sets of reference genomes of different species29, we identified phylogenetically ‘most similar’ strains (table S5, fig. S15). As in species-level analyses, we found strain- and site- specific differences in diversity and heterogeneity (Fig. 5, fig. S16 & S17A-F, table S6). Interestingly, while C. acnes was more abundant in YA skin (Fig. 5A, fig. S16A), it did not exhibit higher strain diversity, suggesting that species-level abundance cannot predict strain-level diversity. In contrast, S. epidermidis strain diversity increased in SNFD versus YA and CD (Fig. 5A, fig. S16A). Many species, including Dolosigranulum (D.) pigrum, Micrococcus (Mi.) luteus, and S. hominis, also exhibited moderately increased strain diversity across multiple skin sites in SNFD, although the elevation was less prominent. Gut strain diversity differences were also species-specific (Fig. 5B, fig. S16B, table S6). Heterogeneity of A. muciniphila and Bifidobacterium (Bi.) longum tended to be larger in YA versus older adults, while heterogeneity of Bacteroides (Ba.) vulgatus and E. coli tended to be larger in SNFD versus YA and CD. Finally, oral strain diversity was notably higher in SNFD for most species (Fig. 5A, fig. S16A, table S6). However, heterogeneity was generally lowest in SNFD, most notably for Str. mitis and Str. oralis, with the prominent exception of Moraxella (M.) osolensis (Fig. 5C, fig. S16C, table S6).
Fig. 5. Strain-Level Diversity, Heterogeneity, and Differential Abundance of Clades.
Only the in-house YA cohort was used. Strain diversity of select oral & skin (A), and gut (B) species. Previously unabbreviated species: Dolosigranulum (D.), Micrococcus (Mi.), Faecalibacterium (F.), Eubacterium (Eu.) Bifidobacterium (Bi.), Bacteroides (Ba.). Median Shannon Index of strains within species grouped by body site and cohort. (C) Heterogeneity as represented by median θ similarity of strain composition within a cohort. θ=1 represents 100% similarity and thus minimal heterogeneity. θ=0 represents no (0%) similarity, maximum heterogeneity. For A-C, Analogous Bray-Curtis dissimiliarities and analyses using data rarefied to 500,000 reads are reported in fig. S14. (D) Strain clades significantly enriched in YA, CD, or SNFD cohort. Outline color indicates significance (Kruskal-Wallis test p < 0.05). Opacity of the fill color indicates the corresponding log10LDA value. Clade assignments in D are arbitrary letters assigned to primary branches of unrooted phylogenic trees of all genomes for that species (fig S15, table S5-7). Analogous analyses using the expanded YA cohort are reported in fig. S16.
We then examined if these population-level differences could be attributed to specific pathogenic or health-associated strains, focusing on a subset of clinically relevant and ubiquitous species. Associations between cohort and phylogenetic clade was observed for A. muciniphila, C. acnes, or E. coli only with the in-house, not expanded YA cohort (Fig. 5D, table S7, fig. S16D). Unlike the discrepancy between YA cohorts for gut strains, S. epidermidis phylogenetic clade “L” was consistently enriched in the SNFD cohort (Kruskal-Wallis test p < 0.05 and log10LDA > 2.0). “L” contains strains associated with nosocomial infections63, suggesting that the increased S. epidermidis diversity observed in SNFDs may represent an increased acquisition of healthcare-associated pathogenic strains. For Str. mitis, a ubiquitous oral pathobiont64, clade “D” and clade “C” was significantly and consistently enriched in CD and SNFD, respectively (Kruskal-Wallis test p < 0.05 and log10LDA > 2.0), demonstrating cohort-specific distributions of phylogenetic clades. Taken together, we observed notable differences in strain composition of important commensal species in older and younger adults, with potential implications for disease predilection in the skin.
Pathogenicity Reservoirs
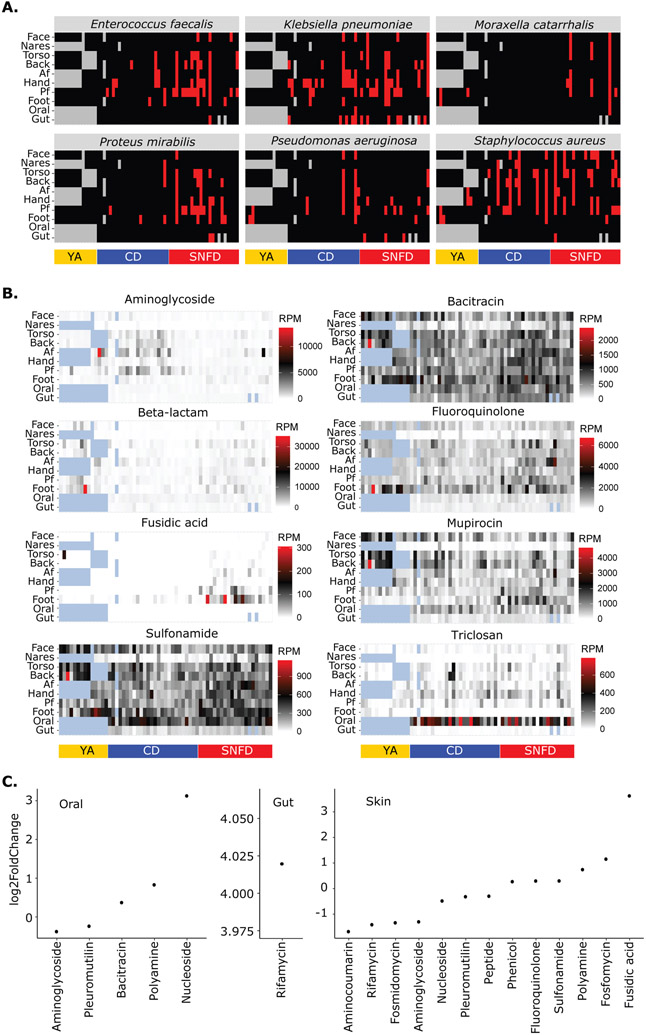
Colonization is an independent risk factor for infection for numerous pathogens, hence described as pathogenicity ‘reservoir’16-19. Though metagenomics can be less sensitive than culture-based detection, we visualized the presence/absence of some clinically important pathobionts. Strikingly, in addition to skin pathobiont S. aureus, skin sites were the primary reservoir for Klebsiella (K.) pneumoniae, Ps. aeruginosa, M. catarrhalis, Proteus (Pro.) mirabilis, and Enterococcus faecalis (Fig. 6A, fig. 18A & S19). Notably, these organisms were rarely found in oral samples, and only K. pneumoniae was prevalent in stool samples. This was surprising because the skin is not commonly considered a reservoir, and given the higher biomass of gut and oral samples, colonization is more likely to be detected at those sites. We also noted that pathobiont carriers were most often colonized at multiple body sites, including the high-transmission hand site. In addition, Pro. mirabilis and M. catarrhalis were more prevalent among SNFDs than CDs (p < 0.05 assessed using body site-restricted permutation and adjusted using the Benjamini-Hochberg procedure), reflecting potential differential exposure in the SNF environment.
Fig. 6. The Skin is a Major Reservoir of Pathobionts and Plasmid-borne Antimicrobial Resistance in Older Adults.
Only the in-house YA cohort was used. (A) Presence (red) or absence (black) of pathobiont. None of these organisms were detected at any level in our environmental or reagent negative controls. (B) Abundance heatmap of plasmid antimicrobial resistance gene (ARG) class. Each column represents one individual, with one timepoint plotted per volunteer. For (A) and (B), blue and gray patterned tiles, respectively, are samples not represented because they were not collected as part of this study or had insufficient sequencing depth to be included in the analysis. Actual relative abundances are shown in table S4. (C) Differential abundance of plasmid ARG classes between CD and SNFD cohorts. Log2fold change in relative abundance is shown, with positive values representing enrichment in SNFD. Only significant differences (fdr adjusted p < 0.05) were plotted. RPM = reads mapped per million. Analogous analyses using the expanded YA cohort are reported in fig. S18.
Similarly, reservoirs of virulence factors or antibiotic resistance genes (ARGs) can affect relative infection risk65. To examine gene-level pathogenicity reservoirs, we mapped metagenomic reads to the Virulence Factor Database (VFDB), a curated database of genes involved in bacterial pathogenesis. We trained a random forests model (fig. S20A-C) to examine if, like taxonomic composition, such genes could differentiate cohorts. Indeed, all cohorts could be identified based on virulence gene abundances in skin, oral, or stool (AUC > 0.74 for any cohort-body site combinations, with AUC > 0.94 for the expanded cohort). Interestingly, the most discriminatory features were not the most differentially abundant, so discriminatory ability was likely driven by subsets of individuals with markedly different virulence gene abundance patterns (fig. S20D-F & S21). More virulence genes were enriched for SNFD versus CD in the gut microbiome (167 enriched vs. 9 depleted, p < 0.01). The most enriched genes include operon iucABCD, which encodes aerobactin biosynthesis, a siderophore necessary for colonization, penetration, and translocation66-69. Interestingly, SNFD had more virulence genes depleted rather than enriched in the skin (204 vs. 661, p < 0.01) and oral microbiome (92 vs. 151, p < 0.01). Nonetheless, certain virulence genes were substantially enriched in these sites, such as EF0149 (log2FoldChange=5.0), an aggregation substance important for host cell adhesion and internalization70. This was concordant with the increased prevalence of E. faecalis in SNFD skin.
Finally, we hypothesized that the SNF environment could enrich for ARGs. We focused on plasmid-borne ARGs as the most likely facilitator of horizontal gene transfer of these elements between organisms71, 72 (examining chromosomally predicted ARGs had similar results, fig. S22). First, we visualized select clinically relevant ARG classes within-individual and between-cohort to convey prevalence and potential body-site specificity (Fig. 6B). Like other pathogenicity reservoirs, ARG classes were notable for high prevalence and relative abundance in skin compared to oral and gut microbiota (Fig. 6B, fig. S18B). Triclosan (banned in hand soaps but not toothpaste) resistance genes in the oral microbiome was an exception. Surprisingly, a canonical reservoir for S. aureus73, the nares, had relatively few ARGs identified. Differential abundance analysis then showed that ARG classes with the largest effect sizes (log2FoldChange) between SNFD and CD were all enriched in SNFDs (Fig. 6C, fig. S18C & S23). For example, rifamycin resistance, substantially enriched in SNFD fecal samples (log2FoldChange = 4.01), was the only ARG class differentially abundant in the gut microbiota (p < 0.05). For oral microbiota, nucleoside, polyamine, and bacitracin ARGs were significantly enriched in SNFD, while pleuromutilin and aminoglycoside ARGs were enriched in CD (p < 0.05). The skin microbiota of SNFD were enriched for multiple ARG classes, including fusidic acid (log2FoldChange=3.63), fosfomycins, polyamines, sulfonamides, fluoroquinolones, and phenicols (p < 0.05), suggesting that the SNF environment does enrich for clinically important ARG classes, and such enrichments are often manifested in the skin microbiome. These data underscore that the skin is an important reservoir for both pathobionts and antimicrobial resistance genes.
Discussion
We present here a high-resolution metagenomic study comparing the continuum of gut, oral, and skin microbiome differences in younger and healthy vs. institutionalized older adults. The patterns of dysbiosis associated with frailty, strain-level differences between our cohorts, and skin-specific patterns of pathogenicity reservoirs identified in this study may represent potential targets to improve or surveil the health of older adults.
Most surprising was that the broadest differences in functional and taxonomic features of older adults were found in the skin microbiota, with implications for skin, whole-body, and public health. The skin, rather than the oral or gut microbiota, was the primary reservoir of a diversity of healthcare-associated pathogens and ARGs (note though, that the skin’s biomass is approximated at ~1/100th of stool74, which can affect the concept of the skin as a reservoir). This may be because the skin is most readily exposed to environmental sources of multi-drug resistant organisms, is subject to different hygiene habits (e.g., the hand, a major transmission route28, had the highest prevalence of pathogen colonization), or that the skin microbiota of frailer adults are inherently more susceptible to pathogen colonization. Indeed, this finding was consistent within the context of the broader differences observed in older adult skin, like high heterogeneity centered around the reduction of dominant organisms like C. acnes, elevated abundance and diversity of staphylococci, hyperdiversification at the species and strain level suggesting a loss of immune or nutritive selectivity of the skin niche, and increased instability compared to younger adults. This implies a system that is susceptible to perturbation, including colonization by exogenous microbes.
Another important potential factor mediating pathogen colonization is the competition provided by the native flora. In particular, C. acnes was significantly depleted at most skin sites, associating with changes in their heterogeneity, instability, hyperdiversification, and biogeographic divergence. C. acnes can inhibit colonization and infection by staphylococci via secreted factors and lowering the local pH6, 75. We suspect that aging-related changes in skin physiology, such as follicular atrophy and decreased sebum production49, 50, 76, result in a decreasingly hospitable environment for this lipophile. The subsequent loss of C. acnes may then precipitate other changes in the environment, such as increased pH and decreased inhibitory factors, facilitating the colonization and proliferation of opportunistic staphylococci51 as observed enriched in SNFD. Interestingly, while C. acnes relative abundance decreased in older adults, its strain diversity did not, suggesting that bioburden is a salient feature. On the contrary, S. epidermidis strain diversity was elevated among SNFDs, though this diversity was likely due in part to the accumulation of nosocomial strains.
Taken together, our findings and others77 call attention to the skin as a potential beacon for general health. We propose a pattern characteristic of frail older adults; a Frailty-Associated Dysbiosis of the Skin (FADS), defined by instability and biogeographic divergence in the context of a depletion of C. acnes, but omitting heterogeneity and diversity because the former is a population-specific trait and the latter was older adult- but not frailty-associated. We note that as an association analysis, this study is limited in its ability to test the health implications of FADS, such as increased risk of pathogen colonization or infection. However, this concept could inform future laboratory and clinical studies seeking to understand how FADS and its co-morbidities predispose to disease predilection.
Finally, we note limitations of our dataset inbuilt with the difficulties of geriatric research. First, our SNFD versus CD cohort was 1) inherently more frail, limiting our ability to decompose SNF residence and frailty, 2) more female-predominant, and 3) had a slightly higher BMI. These, in addition to other SNF-specific factors like environmental microbial reservoirs and hygiene practices present potential confounders that we are insufficiently powered to address. Future studies may address these factors with increased cohort sizes; sampling of younger SNF residents healthcare workers, and environmental reservoirs; detailed skin clinical metrics and hygiene and behavioral surveys; or following volunteers as they transition into and out of SNFs.
In conclusion, our findings raise hypotheses of interest on the role of the microbiota in infection risk and antibiotic resistance dissemination in older adults, particularly in the skin. Correspondingly, this dataset may inform potential therapeutic targets and prophylactic strategies leveraging the microbiota to reduce infection risk. This dataset builds a foundational understanding of the strain-level and functional dynamics of the gut, oral, and skin microbiome of older adults.
Methods
All human subjects methods in this study were reviewed and approved by both the UConn Health and Jackson Laboratory Institutional Review Boards (UConn Health IRB #18-086JS-1).
Subject recruitment and sampling.
Human subject research volunteers were recruited into either the SNF or CD cohort. To minimize age as a confounder between cohorts, we recruited the SNF cohort first, then recruited the CD cohort in an age-group matched fashion (65-74, 75-84, and 85+ years old). In addition, while we sought to recruit both men and women in this study, there were only three eligible male volunteers at the time in our partner SNFs. We capped our CD cohort at 11 men to maintain a female majority, but we acknowledge that the SNFD cohort was still comparatively enriched for women. We partnered with three CT SNFs to recruit residents from each of their facilities. Volunteers were recruited to the CD cohort from the UConn Center on Aging research volunteer database. Inclusion criteria for either cohort were: 1) 65 years of age or older; 2) independently able to provide written informed consent; 3) English or Spanish speaking. Exclusion criteria were: 1) presence of visible skin or oral lesions at or near the sites of sampling; 2) self-reported gastrointestinal distress within the past 30 days; 3) antibiotic use within the past 30 days; 4) Rockwood Frailty Index of > 0.5; 5) hospice protocol or terminal illness diagnosis. Inclusion in the SNF cohort additionally required subjects have resided in an SNF for at least 30 days and to eat meals within a SNF five or more days a week. Designated study contacts among the healthcare staff at the partnering SNFs identified potential participants who meet the eligibility criteria. These individuals were given the IRB-approved informational form to briefly describe the study and then the SNF contact person asked about their interest in participating in the study. SNF staff provided a list of interested residents to the UConn Center on Aging study coordinator who then met with each person to further explain the study and complete the informed consent process. CD inclusion required permanently residing out of a nursing home setting and volunteers were excluded if they spent more than two days out of a week in a nursing home in the past 6 months. Designated study research staff at the Center on Aging sent recruitment letters to individuals listed in the Center on Aging Recruitment Registry, set up UConn Health broadcast message notices and distributed flyers. Interested participants called the Center on Aging study coordinator who conducted a brief telephone screen and then scheduled an in-person visit to the Center on Aging clinical research area. All volunteers except one consented to having their de-identified metagenomic or survey data made publicly available. Their data is specifically omitted from supplementary tables and data repositories.
Volunteers were visited at three timepoints separated by two-week intervals. To assure consistency in skin sampling, volunteers were asked to refrain from showering or applying topical products 24 hours prior to sampling. Eight skin sites and the tongue dorsum were swabbed rigorously using PurFlock Ultra buccal swabs (Puritan Medical Products) dry (oral) or pre-moistened with water for twenty seconds before the swab was submerged into a sterile, nuclease-free 2.0 ml Eppendorf Safe-Lock tube containing 100 μg of autoclaved 0.1 mm zirconia beads (BioSpec Products) and 0.3 ml of sterile Tissue & Cell Lysis Solution (Lucigen). An environmental (air swab) controls was collected for each participant visit. After collection, samples were immediately placed on dry ice and transported back to the Jackson Laboratory for Genomic Medicine for storage at −80°C until processing. For stool sampling, volunteers were issued Omnigene Gut kits for use within two days of each visit.
At each visit, we also collected medical histories including current and past medication use. As a summary metric for frailty conceptualized as the accumulation of aging-associated deficits, we used the Rockwood Frailty Index (RFI), which encompasses deficits as varied as cognitive ability, mobility, level of independence in activities of daily living, medical conditions, and medication use. We supplemented the RFI with the Fried Frailty Phenotype and the Physical Activity Scale for the Elderly (PASE) to better gauge activity level and physical ability 37-39, 78. Since diet and hygiene have demonstrated influences on the gut and oral microbiome 41, 79, we administered dietary, oral, and basic skin hygiene surveys to identify potential lifestyle confounders.
For the “in-house” YA cohort, 107 skin, 32 oral, and 80 stool samples from body sites matching those of the SNFD/CD cohorts were obtained from our IRB-approved biorepository. All participants were 55 years of age or younger, had no co-morbidities, and fulfilled the same minimum exclusion criteria of no antibiotic or antifungal use within the past thirty days. With the exception of 48 skin samples from Zhou et al. 2020 (2-4 timepoints taken 2-4 weeks apart) and 29 previously unpublished stool samples (~one year apart, table S2), YA samples were cross-sectional. In all, the “in-house” cohort comprised 22 stool, 32 oral, and 59 cross-sectional skin samples and an additional 58 longitudinal stool and 48 skin samples deriving from n=95 healthy younger adults, collected and processed identically to the SNFD/CD cohorts.
In addition, we downloaded from the Short Read Archive 1090 longitudinal samples from the Human Microbiome Project (“HMP”, 367 oral, 236 nares, 487 gut from a total of 260 individuals, 1-3 timepoints ~2 weeks apart) 42, 43, 80 and our previously published 247 longitudinal skin samples from 12 adults aged 18-55, 1-33 timepoints 1 month and 1-3 years apart 29, 30 (“MET”), which was collected using comparable methodology. Because these external datasets had methodological differences (e.g., sample collection, extraction, sequencing prep, or having been sequenced on HiSeq), we performed a PERMANOVA analysis to assess if there were significant differences between the YA cohorts.
All raw data were processed (or re-processed) as described in the Analysis section. After we stratified the YA recruitments into oral, skin, and gut samples and adjusted for skin site differences, we found that microbiome variation between the “in-house” and the HMP or MET cohorts was very small, albeit significant (fig. S4, PERMANOVA, skin: p=0.001, R2=0.01, oral: p=0.001, R2=0.03, and gut: p=0.001, R2=0.008). We concluded that the methodological differences should not substantially affect major findings, and thus performed two analyses in parallel. First, we compared the SNF and CD cohorts with the “in-house” YA cohort only, for the most methodologically consistent rigorous analysis. Second, our results suggest that the HMP and MET datasets provide additional statistical power without qualitatively skewing biological interpretations given their similarity. Thus, we have provided in the supplement the parallel analysis merging the “in-house” YA dataset with the HMP and MET datasets (“expanded” cohort).
Metagenomic Sample Extraction, Library Prep, and Sequencing
Samples were processed according to our previously published methods 28. Briefly, metagenomic DNA was extracted using GenElute Bacterial DNA Isolation kits (MilliporeSigma) according to manufacturer protocol with the following modifications: each sample was digested with 50 μg of lysozyme, 5 units lysostaphin, and 5 units mutanolysin for 30 minutes prior to bead beating in the TissueLyser II (QIAGEN) for 2 x 3 minutes at 30 Hz. Samples were centrifuged for 1 minute at 15000 x g prior to loading onto the GenElute column. Environmental, reagent controls, and positive (defined ‘mock’ community of characterized skin, oral, and gut bacteria) controls were included with each extraction, library, and sequencing batch to screen for contamination or batch effects.
DNA samples were diluted to 1ng/μl after quantification with Qubit HS Assay (Thermofisher Scientific). Sequencing libraries were made according to an optimized reaction Nextera XT (Illumina Inc.) protocol where all reagents for library preparation were taken in 1/4th amount 28. The dual indexed paired-end libraries of genomic DNA were generated with an average insert size of 400bp using 200pg DNA from each sample. Tagmentation and PCR reactions were carried out according to the manufacturer's instructions. Resulting Nextera XT libraries were sequenced on an Illumina Novaseq with 2x150bp paired end reads to a sequencing depth up to 127 million reads/sample. Libraries from the SNF cohort were initially sequenced on an Illumina HiSeq2500, but were resequenced on the Novaseq with the CD samples to avoid a potential batch effect. However, after analyzing both runs and finding no appreciable differences, we pooled the SNFD sample reads from both runs to maximize sequencing depth.
Analysis
Metagenomic sequence data quality control:
Demultiplexed Illumina reads were trimmed and quality checked with Cutadapt (v0.4.1) 81, requiring a minimum length of 50 base pairs and default Phred quality score 20. Human reads were then removed with Bowtie2 (v2.2.9) on “very-sensitive” mode 82, mapping to a human reference genome our group previously published for this purpose 83. Median human-dehosted metagenomic read depths for CD/SNFD were 5.56E6, 4.46E6, and 4.16E6 microbial reads/sample for stool, oral, and skin, respectively (additional metrics in table S2).
Species-level taxonomy:
Quality controlled, dehosted reads were then used to estimate the relative abundance of microbial species in the samples using MetaPhlAn 3.0 84. To validate patterns in community-level statistics, taxonomic analyses were also conducted using PathSeq (a tool suite in GATK v4.2.1.0) and our previously described ReprDB 83-85. The species-level taxonomic trends we report were consistent between all three classifiers. We selected MetaPhlAn 3.0 to present here and for our downstream analyses because although ReprDB and Pathseq have larger databases for classifying rarer microbes, our study was more about microbial community structure than low-abundance organisms and MetaPhlAn 3.0 was the highest accuracy classifier, making it the most appropriate tool for this particular line of inquiry because it minimizes noise when comparing communities between groups. For further quality control, we examined our positive controls for each extraction and sequencing batch, and environmental and reagent negative controls were screened for potential contaminants. Several negative controls associated with skin and oral samples contained E. coli, and although these represented less than 500 reads. E. coli can sometimes be found natively at low abundance in the human skin and thus could be of scientific interest in frail older adults, but out of caution to protect the integrity of our conclusions, in addition to the original profiles, we constructed a second set of taxonomic profiles by setting the relative abundance of E. coli in our low biomass (skin) samples to 0 and renormalizing the relative abundance table to confirm that the potential contaminant did not alter our conclusions (Fig. S4 and S5). No other concern for contamination was identified. To estimate microbial community diversity, we calculated the Shannon diversity index 86 and bidirectional Wilcoxon test (α= 0.05) for comparing groups. We additionally rarefied each sample to 500,000, or 100,000 reads to account for differences in read depth, showing that our results were consistent when rarefying reads to account for sequencing depth (subsampled to 500,000 or 100,000 reads, fig. S7A-B). The Yue-Clayton theta index (θ) was calculated between indicated samples to assess stability (pairwise between each timepoint, within an individual), heterogeneity (pairwise between individuals), or biogeographic divergence (pairwise between different sites on the same individual) 87, with bidirectional Wilcoxon test (a= 0.05) for comparing groups, averaging within-individual measurements to avoid pseudoreplication. We also computed the Bray-Curtis dissimilarity to confirm the robustness of θ. Differential enrichment of species was evaluated using LEfSe (v1.0). Principal components were calculated using the prcomp function from R stats package (v4.0.2) at the species level. Random forests classifiers were implemented with R randomForest package (v4.6-14) and individuals were randomized in a 3:2 ratio into training and testing datasets, plotting feature importance and receiver operating characteristic curves from validation test to visualize the robustness of the model 88. To ensure that each subject-body site combination was represented only once, we either averaged the repeated measurements (e.g., Fig. 3C and fig. S8C) or used only the first measurement (e.g., fig. S9A-B). To account for subject-specific patterns, no subject was present in both the training and testing site.
“Enterotypes” were inferred for stool samples by clustering the MetaPhlAn profiles using PAM clustering implemented in the R cluster package (v. 2.1.3) with argument k=3. HAllA was used to investigate correlation between microbial signature and subject data. To assess the carriage of specific pathobionts in our samples, we converted the relative abundance data into presence/absence, defining carriage as any relative abundance value no smaller than 0.00001. To account for the effect of sequencing depth, we also performed the same analyses using samples rarefied to 500,000 reads. Significance for differential prevalence was assessed by permuting the cohort labels (i.e. CD and SNFD) among samples at the same body site. Consequently, the number of SNFD samples with a specific pathobiont present was used as the test statistics. The permutation analyses were repeated 1000 time to estimate the p-values.
Strain analysis:
To estimate strain diversity, we used a modification on our previous pipeline which leverages SNPs and genic differences between strains to approximate nearest neighbor strains based on a set of reference genomes for a given species 29. For gut species (Faecalibacterium prausnitzii, Eubacterium rectale, E. coli, Akkermansia muciniphila, Bifidobacterium longum, Bifidobacterium adolescentis, Bacteroides vulgatus), we used databases that our group previously curated for strain level classification 89. For skin and oral species, new reference databases were generated by compiling all Refseq genomes for Cutibacterium acnes, Micrococcus luteus, Moraxella (M.) osloensis, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus pettenkoferi, Streptococcus mitis, and Streptococcus oralis (as of 10/28/2020) 90. Information for these genomes was available at https://github.com/ohlab/Strain_collection. Phylogenic trees were generated using Parsnp (v1.2, default parameters), and visualized with iToL (v3) 91, 92. Strains were assigned into clades based on their primary branch from an unrooted tree.
Reads were then mapped to the genome databases for each species with Bowtie2 (v2.3.4.3) using k=10 and “very-sensitive” mode. We then used Pathoscope (v2.0.6) on the resulting SAM files using default parameters 93 for reassignment to nearest neighbor strains. Relative abundance of species used in strain analyses are included in table S4. Strain diversity and heterogeneity were calculated as for species level analyses. To account for read depth differences, the analyses were repeated after each sample was rarefied to 500,000 reads. We assessed where genomes of strains with known properties fell in our trees to make comparisons to published literature. Clades enriched in each cohort were identified using LEfSe (v1.0). NIH06004 and NIH5001 were strains previously associated with nosocomial infections from 63.
Functional analyses:
To calculate the relative abundance of virulence associated genes reads were mapped to the VFDB (retrieved 4/8/2020) using DIAMOND (v0.9.30.131, blastx mode) 94, 95. To characterize the general metabolic and functional composition of our dataset, reads were classified using HUMAnN2 (v2.8.0, diamond mode), referencing nucleotide database ChocoPhlAn (v0.1.1), protein database UniRef90, and MetaPhlAn 2.0 96. Gene hits were aggregated by class to facilitate interpretation. For plasmid antibiotic resistance gene (ARG) identification, we assembled metagenomic reads into contigs, then predicted ARGs on predicted plasmid contigs. Contigs generated from quality-controlled reads using MEGAHIT (v1.2.9, kmin-1pass mode) and were classified as plasmid or chromosomal using Plasflow (v1.1, default parameters, threshold 0.7) 97, 98. Plasmid genes were then identified from contigs with Prodigal (v2.6.3, meta procedure), from which antimicrobial resistance genes were annotated using DeepARG (v1.0.1, align mode for genes) 99, 100. All annotated antimicrobial resistance genes were clustered at 95% nucleotide sequence identity using USEARCH (v8.0.1517) to generate a gene catalog, and reads were mapped to the centroids of the clusters using Bowtie2 (v2.3.4.3, --very-sensitive mode) to measure gene abundance. For full-genome ARG differential abundance analyses, ARGs from both the plasmid contigs and the chromosomal contigs were included. VFDB and ARG count tables were normalized by reads per kilobase million to avoid bias due to differences in reference gene length. Differentially abundant genes were identified using DESeq2 (v1.26.0). Random forests analyses were performed as for species-level classifications.
Statistics and reproducibility
No statistical method was used to predetermine sample size. No data were excluded from the analyses. The experiments were not randomized. The Investigators were not blinded to allocation during experiments and outcome assessment.
All statistical analyses were performed in R (v3.6.3)101 unless noted otherwise. All p-values in this study are false discovery rate (fdr) adjusted unless noted elsewhere. The Yue-Clayton theta index was implemented as previously described 87, and Bray-Curtis dissimilarities were used to support trends identified by the Yue-Clayton theta index, which can be affected by sequencing depth. To account for missing data (e.g., early termination of study because of antifungal/antibiotic treatment, or different numbers of timepoints), for all analyses excepted the stability test, we averaged timeseries to represent each subject-site combination evenly. For the stability test, subject-site combinations sampled at only one time point were excluded from the test. 125, 126 For boxplots, center lines represent the median and the edges represent first and third quartiles. 113 For random forests model for taxonomic composition, we trained a random forest model on a randomly selected 60% of subjects and tested it on the remaining 40% (Fig. 3C and fig. S8C). To ensure that each subject-body site combination was represented only once, we either averaged the repeated measurements (Fig. 3C and fig. S8C) or used only the first measurement (fig. S9A-B). To account for subject-specific patterns, no subject was present in both the training and testing site.
Supplementary Material
Acknowledgements:
Funding for this project were provided by internal UConn support via the UConn Research Excellence Program and the UConn Microbiome Research Seed Grant. Investigator salaries were additionally supported by NIA R56 AG060746 and P30 AG067988, Claude D. Pepper Older Americans Independence Center at UConn. JO is additionally supported by the NIH (DP2 GM126893-01, K22 AI119231-01, 1U54NS105539, 1 U19 AI142733, 1 R21 AR075174), the NSF (1853071), the American Cancer Society, the Leo Foundation, and the Mackenzie Foundation.
Footnotes
Data and materials availability: Metagenomic sequence files from participants who consented to making their de-identified metagenomic data available in public access data can be accessed in National Center for Biotechnology Information (NCBI) BioProject PRJNA699281. HMP data can be accessed from https://www.hmpdacc.org/hmp/; SRS IDs of the samples used in this study were detailed in Table S2. Oh et al. 2014 & 2016 data can be accessed from NCBI BioProject PRJNA46333.
Competing interests: The authors report no competing interests for this study.
References
- 1.Franceschi C et al. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne) 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye SK, Studenski S, Tinetti ME & Kuchel GA Geriatric Syndromes: Clinical, Research and Policy Implications of a Core Geriatric Concept. Journal of the American Geriatrics Society 55, 780 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WHW et al. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. The New England journal of medicine 368, 1575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koren O et al. Colloquium Paper: Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America 108, 4592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickard JM, Zeng MY, Caruso R & Núñez G Gut Microbiota: Role in Pathogen Colonization, Immune Responses and Inflammatory Disease. Immunol Rev 279, 70–89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd AL, Belkaid Y & Segre JA The human skin microbiome. Nature Reviews Microbiology 16, 143 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Xue Q The Frailty Syndrome: Definition and Natural History. Clin Geriatr Med 27, 1–15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meehan CJ, Langille MGI & Beiko RG Frailty and the Microbiome. Top Gerontol Geriatr 41, 54–65 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K et al. A Frailty Index Based On Deficit Accumulation Quantifies Mortality Risk in Humans and in Mice. Scientific reports 7, 43068 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitnitski A, Howlett SE & Rockwood K Heterogeneity of Human Aging and Its Assessment. J Gerontol A Biol Sci Med Sci 72, 877–884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L & Kuchel GA Heterogeneity of Aging: Individual Risk Factors, Mechanisms, Patient Priorities, and Outcomes. Journal of the American Geriatrics Society n/a (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen QD et al. Health Heterogeneity in Older Adults: Exploration in the Canadian Longitudinal Study on Aging. Journal of the American Geriatrics Society n/a (2020). [DOI] [PubMed] [Google Scholar]
- 13.Kuchel GA Inclusion of Older Adults in Research: Ensuring Relevance, Feasibility, and Rigor. Journal of the American Geriatrics Society 67, 203–204 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Haran JP, Bucci V, Dutta P, Ward D & McCormick B The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol 67, 40–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLAESSON MJ et al. Gut microbiota composition correlates with diet and health in the elderly. Nature (London) 488, 178–184 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Zorrilla S et al. Prospective Observational Study of Prior Rectal Colonization Status as a Predictor for Subsequent Development of Pseudomonas aeruginosa Clinical Infections. Antimicrobial agents and chemotherapy 59, 5213–5219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbier F et al. Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum β-lactamase-producing Enterobacteriaceae. Intensive Care Med. 44, 616–626 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Grasselli G et al. Gastrointestinal colonization with multidrug-resistant Gram-negative bacteria during extracorporeal membrane oxygenation: effect on the risk of subsequent infections and impact on patient outcome. Annals of Intensive Care 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson RE et al. Methicillin-resistant Staphylococcus aureus Colonization and Pre- and Post-hospital Discharge Infection Risk. Clin Infect Dis 68, 545–553 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Gmehlin Cameron G. & Munoz-Price L. Silvia. Coronavirus disease 2019 (COVID-19) in long-term care facilities: A review of epidemiology, clinical presentations, and containment interventions. Infection control and hospital epidemiology, 1–6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aleman FDD & Valenzano DR Microbiome evolution during host aging. PLOS Pathogens 15, e1007727 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu C, Zhu H & Qiu P Aging progression of human gut microbiota. BMC Microbiology 19, 236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dréno B et al. Microbiome in healthy skin, update for dermatologists. Journal of the European Academy of Dermatology and Venereology 30, 2038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prescott SL et al. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. The World Allergy Organization Journal 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire M & Maguire G The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermatol Res 309, 411–421 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Willis JR & Gabaldón T The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 8, 308. doi: 10.3390/microorganisms8020308 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S et al. Human Skin, Oral, and Gut Microbiomes Predict Chronological Age. mSystems 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W et al. Host-Specific Evolutionary and Transmission Dynamics Shape the Functional Diversification of Staphylococcus epidermidis in Human Skin. Cell 180, 454–470.e18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh J et al. Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh J, Byrd AL, Park M, Kong HH & Segre JA Temporal Stability of the Human Skin Microbiome. Cell 165, 854–866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z et al. New Insights Into the Skin Microbial Communities and Skin Aging. Front Microbiol 11, 565549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.N S et al. Aging-related changes in the diversity of women's skin microbiomes associated with oral bacteria. Scientific reports 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roghmann M et al. Comparison of the Microbiota of Older Adults Living in Nursing Homes and the Community. mSphere 2, 210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagase S et al. Distinct Skin Microbiome and Skin Physiological Functions Between Bedridden Older Patients and Healthy People: A Single-Center Study in Japan. Front. Med 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranjan R, Rani A, Metwally A, McGee HS & Perkins DL Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun 469, 967–977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brumfield KD, Huq A, Colwell RR, Olds JL & Leddy MB Microbial resolution of whole genome shotgun and 16S amplicon metagenomic sequencing using publicly available NEON data. PLOS ONE 15, e0228899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried LP et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci 56, 146 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Washburn RA, Smith KW, Jette AM & Janney CA The physical activity scale for the elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology 46, 153–162 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Rockwood K et al. A Frailty Index Based On Deficit Accumulation Quantifies Mortality Risk in Humans and in Mice. Scientific reports 7, 43068 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hills RD et al. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turnbaugh PJ et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1, 6ra14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson J et al. The NIH Human Microbiome Project. Genome Res. 19, 2317–2323 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A framework for human microbiome research. Nature 486, 215–221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK & Knight R Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickson I Stability and individuality of adult microbiota. Nature Research (2019). [Google Scholar]
- 46.Oh J et al. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 23, 2103–2114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagpal R et al. Gut microbiome and aging: Physiological and mechanistic insights. Nutrition and healthy aging 4, 267–285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whelan FJ et al. The Loss of Topography in the Microbial Communities of the Upper Respiratory Tract in the Elderly. Annals ATS 11, 513–521 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Kerns ML, Chien AL & Kang S in Fitzpatrick's Dermatology, 9e (ed Kang S et al.) (McGraw-Hill Education, New York, NY, 2019). [Google Scholar]
- 50.Dréno B et al. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. Journal of the European Academy of Dermatology and Venereology 32, 5–14 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Claesen J et al. A Cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci Transl Med 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolla BS et al. Cutibacterium acnes regulates the epidermal barrier properties of HPV-KER human immortalized keratinocyte cultures. Scientific Reports 10, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jasson F et al. Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Experimental Dermatology 22, 587–592 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Otto M Staphylococcus epidermidis – the “accidental” pathogen. Nature reviews. Microbiology 7, 555 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magne F et al. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castaner O et al. The Gut Microbiome Profile in Obesity: A Systematic Review. International journal of endocrinology 2018, 4095789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu GD et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 334, 105–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park S, Chung H & Lee M Clinical and Microbiological Characteristics of Six Staphylococcus pettenkoferi Isolates From Blood Samples. Annals of Laboratory Medicine 35, 250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aubin GG et al. Propionibacterium namnetense sp. nov., isolated from a human bone infection. Int J Syst Evol Microbiol 66, 3393–3399 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Yan Y, Nguyen LH, Franzosa EA & Huttenhower C Strain-level epidemiology of microbial communities and the human microbiome. Genome Medicine 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnenborn U Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett 363 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Lim JY, Yoon JW & Hovde CJ A Brief Overview of Escherichia coli O157:H7 and Its Plasmid O157. Journal of microbiology and biotechnology 20, 5 (2010). [PMC free article] [PubMed] [Google Scholar]
- 63.Conlan S et al. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biology 13, R64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell J Streptococcus mitis: walking the line between commensalism and pathogenesis. Molecular Oral Microbiology 26, 89–98 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Brinkac L, Voorhies A, Gomez A & Nelson KE The Threat of Antimicrobial Resistance on the Human Microbiome. Microbial ecology 74, 1001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keller R, Pedroso MZ, Ritchmann R & Silva RM Occurrence of virulence-associated properties in Enterobacter cloacae. Infect Immun 66, 645–649 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.S Y & S S [Iron uptake mechanisms of pathogenic bacteria]. Nihon saikingaku zasshi. Japanese journal of bacteriology 51 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Saffrey M & Saffrey M Aging of the mammalian gastrointestinal tract: a complex organ system. AGE 36, 1019–1032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sovran B et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Scientific Reports 9, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller C et al. The Intraperitoneal Transcriptome of the Opportunistic Pathogen Enterococcus faecalis in Mice. PLOS ONE 10, e0126143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vrancianu CO, Popa LI, Bleotu C & Chifiriuc MC Targeting Plasmids to Limit Acquisition and Transmission of Antimicrobial Resistance. Front Microbiol 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orlek A et al. Plasmid Classification in an Era of Whole-Genome Sequencing: Application in Studies of Antibiotic Resistance Epidemiology. Front. Microbiol 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kao K et al. Risk Factors of Methicillin-Resistant Staphylococcus aureus Infection and Correlation With Nasal Colonization Based on Molecular Genotyping in Medical Intensive Care Units: A Prospective Observational Study. Medicine (Baltimore) 94, e1100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sender R, Fuchs S & Milo R Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flowers L & Grice EA The Skin Microbiota: Balancing Risk and Reward. Cell host & microbe 28, 190–200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Proliferative Activity of the Sebaceous Glands of the Aged. Journal of Investigative Dermatology 70, 314–317 (1978). [DOI] [PubMed] [Google Scholar]
- 77.Luna PC Skin Microbiome as Years Go By. American journal of clinical dermatology 21, 12–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only References
- 78.Rockwood K, Theou O & Mitnitski A What are frailty instruments for? Age Ageing 44, 545–547 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Wade WG The oral microbiome in health and disease. Pharmacological Research 69, 137–143 (2013). [DOI] [PubMed] [Google Scholar]
- 80.McInnes P & Cutting M Human Microbiome Project Core Microbiome Sampling Protocol A HMP Protocol # 07-001. (2010). [Google Scholar]
- 81.Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10–12 (2011). [Google Scholar]
- 82.Langmead B & Salzberg SL Fast gapped-read alignment with Bowtie 2. Nature methods 9, 357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou W, Gay N & Oh J ReprDB and panDB: minimalist databases with maximal microbial representation. Microbiome 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segata N et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nature Methods 9, 811–814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kostic AD et al. PathSeq: A comprehensive computational tool for the identification or discovery of microorganisms by deep sequencing of human tissue. Nat Biotechnol 29, 393–396 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morris EK et al. Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecology and evolution 4, 3514–3524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yue JC & Clayton MK A Similarity Measure Based on Species Proportions. Communications in Statistics - Theory and Methods 34, 2123–2131 (2005). [Google Scholar]
- 88.Liaw A & Weiner M Classification and Regression by randomForest. (2002). [Google Scholar]
- 89.Emiola A, Zhou W & Oh J Metagenomic growth rate inferences of strains in situ. Science Advances 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Leary NA et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44, 733 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Letunic I & Bork P Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic acids research 44, W242–W245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Treangen TJ, Ondov BD, Koren S & Phillippy AM The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biology 15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong C et al. PathoScope 2.0: a complete computational framework for strain identification in environmental or clinical sequencing samples. Microbiome 2, 33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 33, 325 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buchfink B, Xie C & Huson DH Fast and sensitive protein alignment using DIAMOND. Nature methods 12, 59–60 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Franzosa EA et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Methods 15, 962–968 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li D, Liu C, Luo R, Sadakane K & Lam T MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Krawczyk PS, Lipinski L & Dziembowski A PlasFlow: predicting plasmid sequences in metagenomic data using genome signatures. Nucleic Acids Res 46, e35–e35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hyatt D et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC bioinformatics 11, 119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arango-Argoty G et al. DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 6, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.R Core Team. R: A language and environment for statistical computing. 3.4.0 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.