Abstract
Unsubstituted flavone induced CYP1A1, CYP1B1 and UGT1A1 gene expression in Caco2 cells and was characterized as an aryl hydrocarbon receptor (AhR) agonist. The structure-activity relationships among 15 mono- and dihydroxyflavones showed that addition of one or two hydroxyl groups resulted in active (e.g.: 5- and 6- mono- and 5,6-dihydroxyflavones) and inactive (e.g.: 7-mono, 7,4′ and 6,4′-dihydroxyflavones) AhR ligands. Ligand docking studies of flavone, mono- and dihydroxyflavones to the human AhR resulted in similar docking scores that varied from −3.48 to −4.58 kcal/mol and these values did not distinguish between AhR-active and AhR-inactive mono- and dihydroxyflavones. The AhR-inactive flavones were subsequently investigated as AhR antagonists by determining their activities as inhibitors of TCDD-induced expression of CYP1A1, CYP1AA2 and UGT 1A1 gene expression in Caco2 cells. Initial studies with 7,4′-dihydroxyflavone showed that this compound was an AhR antagonist in Caco2 cells and resembled the activity of the classical AhR antagonist CH223191. With few exceptions most of the remaining AhR-inactive compounds in terms of inducing AhR responsive genes were also AhR antagonists. Thus, based on modeling studies, mono- and dihydroxyflavones bind with similar affinities to the AhR and exhibit AhR agonist or antagonist activities, however, the structural requirements (substitution patterns) for predicting these opposing activities were not apparent and could only be determined using bioassays.
Keywords: Flavones, Ah receptor, structure-activity, antagonists
INTRODUCTION
The aryl hydrocarbon receptor (AhR) was initially identified as the intracellular receptor that bound and was necessary for inducing the biochemical and toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) and structurally related halogenated aromatic hydrocarbons (HAHs) (1, 2). AhR-dependent responses include several characteristic toxic effects such as chloracne, immune deficits and also induction of CYP1A1 and other drug metabolizing enzymes. Benzo[a]pyrene and some related polynuclear aromatic hydrocarbons (PAHs) have also been identified as AhR ligands (3). Although PAHs induce CYP1A1 (4) these compounds do not induce many of the dioxin-like toxicities and this may be due, in part, to their rapid metabolism and lack of “persistent” occupation of the AhR (5). Subsequent studies have identified structurally diverse synthetic compounds, endogenous biochemicals such as bilirubin, pharmaceuticals, microbial metabolites and health promoting phytochemicals such as indole-3-carbinol and flavonoids as AhR ligands (6–8). The AhR-active microbial metabolites of tryptophan and other dietary compounds do not induce dioxin-like toxicities and play a role in protecting against various adverse health effects (9–12)
Structure activity studies among HAHs and PAHs have defined the parameters for high affinity binding to the AhR. This includes compound coplanarity for PAHs and for HAHs the chlorine substituents are isosteric with the 2,3,7, and 8 chlorine groups on TCDD. In contrast, structural determinants and substitution patterns for other classes of AhR ligands are less well defined. For example, polyphenolics such as resveratrol, curcumin and flavonoids exhibit both AhR agonist and antagonist activities. However, the structural requirements responsible for these effects have not been determined (13–16). Studies in this laboratory have previously investigated a series of hydroxylated flavones, flavanones and flavonones as AhR agonists in colonic-derived cells and their order of activity based on induction of CYP1A1 in Caco2 cells was pentahydroxy > hexahydroxy ≥ tetrahydroxy flavonoids (17). There was some variability within each group and among the tetrahydroxyflavones some isomers were AhR-inactive and others were AhR antagonists (17). For example, apigenin (5,7,4′-trihydroxyflavone) did not induce CYP1A1 but inhibited 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced CYP1A1 thus acting as an AhR antagonist. In contrast, apigenin induced UGT1A1 (AhR-dependent) acting as an agonist. There is evidence that flavones such as baicalein (5,6,7-trihydroxyflavone) containing less than 4 hydroxyl groups also exhibit AhR activity (13, 18, 19). Therefore, this structure-activity study focused on lower hydroxylated flavones and effects of the number and position of the hydroxyl substituents in determining activities of flavones as inducers of AhR-regulated drug-metabolizing enzymes in Caco2 cells.
MATERIALS AND METHODS
Cell lines, antibodies, and reagents.
Caco-2 human colon cancer cell line was obtained from the American Type Culture Collection (Manassas, VA). Caco-2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) nutrient mixture supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 20% fetal bovine serum (FBS), and 1X antibiotic/antimycotic reagent (Sigma-Aldrich). Cells were maintained at 37°C in the presence of 5% CO2, and the solvent (dimethyl sulfoxide, DMSO) used in the experiments was ≤0.2%. CYP1A1, AHR, and GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and RNA polymerase II antibody were purchased from Active Motif (Carlsbad, CA). All compounds used in this study were purchased from Sigma-Aldrich (St. Louis, MO).
Chromatin immunoprecipitation (ChIP) assay.
The ChIP assay was performed using ChIP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. Caco-2 cells (1 × 107 cells) were treated with 50 μM 4′-hydroxyl flavone or 10 nM TCDD for 2 hr and then cells were fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to desired chromatin length (~200–1500-bp). The sonicated chromatin was immunoprecipitated with primary antibodies and protein A-conjugated magnetic beads at 4 °C for 12 hr. The magnetic beads were extensively washed, protein-DNA crosslinks were reversed and eluted. DNA was extracted from the immunoprecipitate and purified using ChIP DNA Purification Kit (Active Motif, Carlsbad, CA) followed to the manufacturer’s protocol. RT-qPCR was used to analyze ChIP-qPCR. The primers for detecting the CYP1A1 promoter were 5′-TCA ATC AAG AGG CGC GAA CCT C-3′, and 5′-CTA CAG CCT ACC AGG ACT CG-3′.
Quantitative real-time PCR.
cDNA was prepared from the total RNA of cells using High capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). Each PCR was carried out in triplicate in a 20 μL volume using SYBR Green Mastermix (Applied Biosystems) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min in the Bio-Rad iCycler (MyiQ™2) real-time PCR System. The comparative CT method was used for relative quantitation of samples. Values for each gene were normalized to expression levels of TATA-binding protein (TBP). The sequences of the primers used for real-time PCR were as follows: CYP1A1 sense 5′-CCC AGC TCA GCT CAG TAC CT-3′, antisense 5′-GAG GCC AGA AGA AAC TCC GT-3′; CYP1B1 sense 5′- TAT CAC TGA CAT CTT CGG CG-3′, antisense 5′- ACC TGA TCC AAT TCT GCC TG-3′; UGT1A1 sense 5′-GAA TCA ACT GCC TTC ACC AAA AT-3′, antisense 5′-AGA GAA AAC CAC AAT TCC ATG TTC T-3′; and TBP sense 5′-GAT CAG AAC AAC AGC CTG CC-3′, antisense 5′-TTC TGA ATA GGC TGT GGG GT-3′.
Western blot analysis.
Cells (3 × 105) were plated in six-well plates in DMEM media containing 2.5% FBS for 16 hours and then treated with different concentrations of the compounds. Cellular lysates were prepared in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.5% deoxycholate, 0.1% sodium dodecylsulfate (SDS), each 10 μL/ml Protease and phosphatase inhibitor cocktail (GenDEPOT, Barker, TX) and 1% NP-40. The cells were disrupted and extracted at 4°C for 30 min. After centrifugation, the supernatant was obtained as the cell lysate. Protein concentrations were measured using the Bio-Rad protein assay. Aliquots of cellular proteins were electrophoresed on 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). The membrane was allowed to react with a specific antibody, and detection of specific proteins was carried out by enhanced chemiluminescence. Loading differences were normalized using a polyclonal GAPDH antibody.
Statistics.
All of the experiments were repeated a minimum of three times. The data are expressed as the means ± SEM. Statistical significance was analyzed using either Student’s t-test or analysis of variance (ANOVA) with Scheffe’s test and a P value of less than 0.05 was considered statistically significant.
Computation-based molecular modeling.
Human aryl hydrocarbon receptor (hAhR) (amino acid residues 247 through 406) was modeled as described previously (12, 20–22) using I-TASSER (23, 24). The I-TASSER TM-align structural alignment program was used to match the generated AhR model from I-TASSER to all protein structures in the Protein Data Bank (PDB) library. The identified structural analog to AhR with highest TM-score (0.924) was the human endothelial PAS domain-containing protein-1 (PDB ID 4XT2) with associated ligand 43L: (5S, 7R)-5,7-bis (3-bromophenyl)-4,5,6,7-tetrahydrotetrazolo [1,5-a] pyrimidine. Ligand docking studies were conducted using Maestro (Schrödinger, LLC, New York, NY, 2020). The version of Maestro used for these studies is licensed to the Laboratory for Molecular Simulation (LMS), a Texas A&M University core user facility for molecular modeling, and is associated with the Texas A&M University High Performance Research Computing (HPRC) facility (College Station, TX). All Maestro-associated applications were accessed using the graphical user interface (GUI) VNC interactive application through the HPRC Terra OnDemand portal. The modeled PBD TD4XT2 protein structures coordinated from I-TASSER were imported into Maestro. Maestro Quick Align was used to superimpose the two proteins in order to align binding domains of the proteins. Each PDB ID4XT2 was used for ligand docking utilizing the Maestro Protein Preparation Wizard; restrained minimization of the protein structure was performed utilizing the OPLS3e force field. The three-dimensional structure of each ligand was prepared for docking utilizing the Maestro LigPrep and the OPLS3e force field. Maestro Glide (25–27) was utilized with the default settings to dock each prepared ligand to each prepared protein in order to predict the lowest energy ligand binding orientation, and calculate the predicted binding energy in units of kcal/mol.
RESULTS
The major objectives of this study were to investigate the AhR activity of mono- and dihydroxyflavones in Caco2 cells and to determine whether the pattern of hydroxyl substituents can be predictive. Since there are 8 possible mono- and 55 possible di-hydroxy- flavones, and characterizing them all is intractable, a representative subset of the compounds was included in this study. Cells were treated with 10, 50 and 100 μM concentrations of the flavones. In several cases the higher doses (100 and 50 μM) were toxic and the induced drug-metabolizing enzyme mRNA levels are not reported. Figure 1A illustrates the structure of flavone, the parent compound and treatment of Caco2 cells with flavone significantly induces CYP1A1, CYP1B1 and UGT1A1 mRNAs (Figs. 1B–1D). The magnitude of flavone-induced CYP1A1 gene expression was > 50% of that observed for 10 nM TCDD. Robust induction of CYP1B1 and UGT1A1 (Figs. 1C and 1D) was also observed in cells treated with 10 or 50 μM flavone. Western blot analysis showed that compared to 10 nM TCDD, 50 μM flavone was a weak inducer of CYP1A1 protein and less effective than TCDD in downregulation of the AhR protein which is observed for some AhR ligands.
Figure 1:

Flavone is an AhR agonist. A. Structure of flavone. Caco2 cells were treated for 24 hours with DMSO, 10 nM TCDD and different concentrations of flavone and expression of (B) CYP1A1, (C) CYP1B1 and (D) UGT1A1 were determined by real-time PCR. Results are expressed as means ± SE for at least 3 replicate determinations and significant (p<0.05) induction is indicated (*). E. Cells were treated as described above and after 24 hours whole cell lysates were obtained and analyzed by western blots as outlined in the Methods.
The effects of adding a single hydroxyl group on the AhR activity of flavone were also investigated in Caco2 cells treated with 3-, 5-, 6- and 7- hydroxyflavone (chromen-4-one ring substituents) and 3′- and 4′- hydroxyflavone (phenyl ring substituents). Among the six hydroxyflavone isomers, the 5-, 6-, 3′- and 4′- hydroxyflavones (10 or 50 μM) induced CYP1A1 mRNA levels that were ≥ 50% of those observed for 10 nM TCDD (Fig. 2A). 3-Hydroxyflavone also induced CYP1A1 gene expression (< 50% of TCDD-induced responses) whereas only minimal induction was observed for 7-hydroxyflavone. A similar pattern was observed for induction of CYP1B1 (Fig. 2B) and UGT1A1 (Fig. 2C) for the isomeric hydroxyflavones. Thus, introduction of a single hydroxyl group at (5-, 6-, 3′-, and 4′- positions) had minimal effects on the AhR activity of the parent hydrocarbon (flavone) whereas a 3-hydroxyl group decreased and a 7-hydroxyl group eliminated the AhR activity of flavone. A ChIP assay showed that 10 nM TCDD and the AhR-active 4′-hydroxyflavone induced interactions of the AhR with the dioxin-responsive element (DRE) regions of the CYP1A1 promoter (Fig. 2D) and also recruit Pol II to the CYP1A1 promoter (Fig. 2E) which was consistent with the activity of 4′-hydroxyflavone as an AhR agonist.
Figure 2:

Monohydroxy flavones as AhR agonists. Caco2 cells were treated with DMSO, 10 nM TCDD and 10 or 50 μM 3-, 5-, 6-, 7-, 3′- and 4′- hydroxyflavone and expression of (A) CYP1A1, (B) CYP1B1 and (C) UGT1A1 was determined by real-time PCR from Figures (D and E). Results are expressed as means ± SE for at least 3 determinations and significant (p<0.05) induction is indicated (*). D. ChIP assays on the effects of 10 nM TCDD and 4′-hydroxyflavone induced interactions with the DRE region of the CYP1A1 promoter and (E) pol II interactions with the CYP1A1 promoter were determined as outlined in the Methods. Relative signals were replicated 3 times and results are means ± SE for 3 determinations.
Since 6-hydroxyflavone has the highest AhR activity and induction of CYP1A1 in Caco2 cells we further investigated the effects of adding a second hydroxyl group to this molecule (Fig. 3A). The results show that at least two of these compounds 6,3′- and 5,6- dihydroxyflavone (10 μM) induced CYP1A1 > 50% observed for 10 nM TCDD (Fig. 3A) and this was comparable to the high activity observed not only for 6-hydroxyflavone but also 3′-, 4 and 5- hydroxyflavone (Fig. 2). Higher concentrations of these compounds were cytotoxic to Caco2 cells. We observed that 6,4′-dihydroxyflavone (10 μM) exhibited relatively low activity as an inducer of CYP1A1 even through both the 6- and 4′- hydroxyflavones were active as CYP1A1 inducers. 3,6-Dihydroxyflavone was minimally active as a CYP1A1 inducer indicating the introduction of a 3-hydroxyl group into the 6-hydroxyflavone backbone decreased AhR activity. A similar pattern of structure-dependent induction of CYP1B1 (Fig. 3B) and UGT1A1 (Fig. 3C) was observed for this series of dihydroxyflavones.
Figure 3:
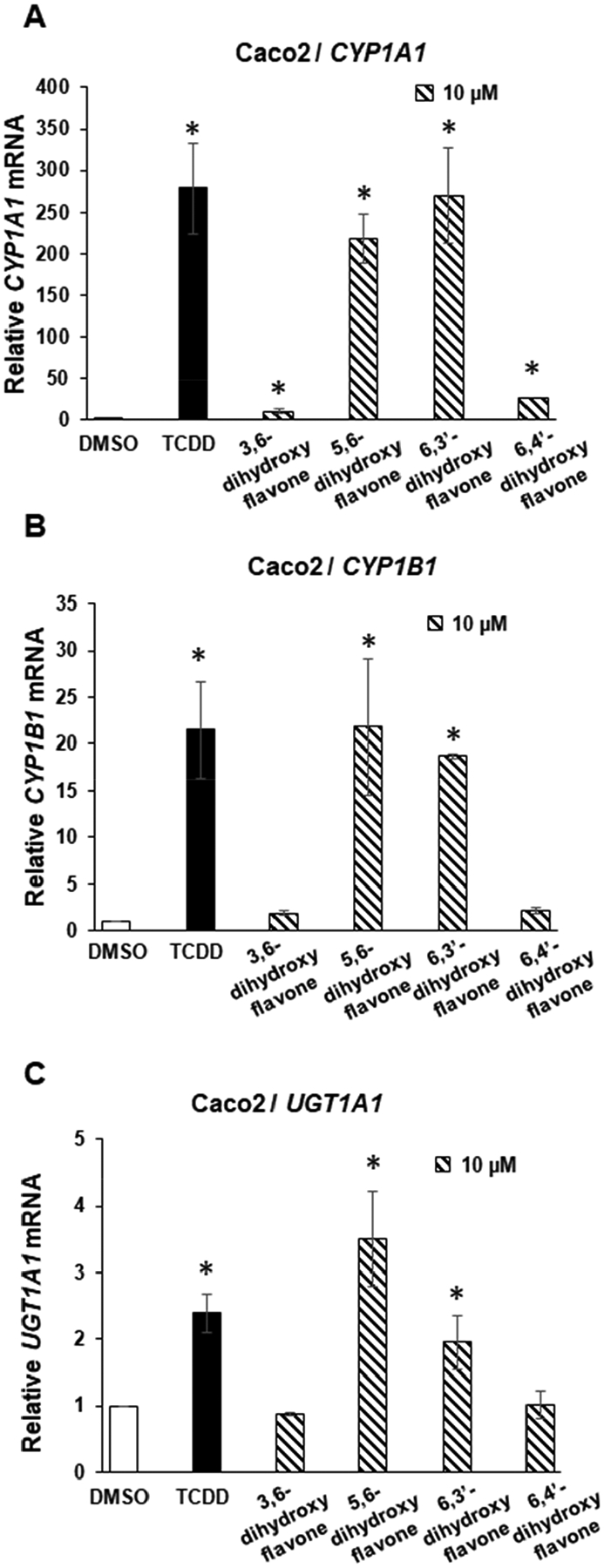
AhR agonist activity of 6-hydroxy substituted dihydroxyflavone isomers. Caco2 cells were treated for 24 hours with DMSO 10 nM TCDD and μM 3,6-, 5,6-, 6,3′- and 6,4′-dihydroxy flavones and expression of (A) CYP1A1, (B) CYP1B1 and (C) UGT1A1 were determined by real-time PCR. Results are expressed as means ± SE for at least 3 determinations and significant (p<0.05) induction is indicated (*).
7-Hydroxyflavone exhibited minimal AhR activity (Fig. 2) and we further investigated effects of an additional hydroxyl group on the activity of 3,7-, 5,7-, 7,8-, 7,3′-, and 7,4′-dihydroxyflavones as inducers of AhR-responsive drug metabolizing enzymes. All of these dihydroxyflavones containing a 7-hydroxyl substituent exhibited low to non-detectable activity as inducers of CYP1A1 (Fig. 4A), CYP1B1 (Fig. 4B) and UGT1A1 (Figure 4C). This was observed with compounds containing an “activating” 3′-, and 4′- hydroxyl substituents suggesting that the 7-hydroxyl group suppressed AhR activity of the dihydroxyflavones. We also investigated the activities of several AhR-active compounds including the 6-,4′ and 3′-hydroxyflavone and 6, 3′- and 5,6-dihydroxyflavones as inducers of CYP1A1, CYP1B1 and UGT1A1 (Fig. 5A – 5C) in CRISPR/Cas9-derived Caco2-AhRKO cells which do not express AhR (17). Loss of the AhR significantly inhibited induction responses for all flavonoids compared to that observed in wild-type cells (Figures 2 and 3). 6-Hydroxyflavone induced CYP1A1 by approximately 2-fold, however, this was significantly lower from the > 190-fold induction observed in wild-type Caco2 cells (Fig. 2).
Figure 4:

AhR agonist activity of 7-hydroxy substituted dihydroxyflavone isomers. Caco2 cells were treated for 24 hours with DMSO, 10 nM TCDD and 3,7-, 5,7-, 7,8-, 7,3′- and 7,4′-dihydroxyflavone and induction of (A) CYP1A1, (B) CYP1B1 and (C) UGT1A1 were determined. Results are expressed as means ± SE for at least 3 determinations and significant (p<0.05) induction is indicated.
Figure 5:

Induction of drug-metabolizing enzymes in Caco2-AhRKO cells. Caco2-AhRKO cells were treated 24 hours with 6-,4′- and 3′- hydroxyflavone and 6,3′- and 5,6-dihydroxyflavone. Induction of (A) CYP1A1, (B) CYP1B1 and (C) UGT1A1 were determined by real-time PCR. Results are expressed as means ± SE for at least 3 replicate determinations and significant (p<0.01) induction is indicated (*).
Based on the wide divergence of activity of the mono- and dihydroxyflavones we used a computational modeling approach targeting human AhR sequences (aa 247 through 500). I-TASSER-TM (24, 25) to align the protein structures from the protein data bank library. The best match was the human endothelial PAS domain-containing protein 1 which was used for protein docking studies summarized in Table 1. The docking scores varied from −4.84 kcal/mol for 7,3′-dihydroxyflavone to −3.48 kcal/mol for 5,6-dihydroxyflavone and did not correlate with the potency of hydroxyflavones as inducers of CYP1A1 gene expression. Figure 6A and 6B show the interactions of 6- and 7- hydroxyflavone isomers which exhibit high and low to non-detectable induction of CYP1A1 respectively. Thus, both AhR-active (6-hydroxyflavone) and inactive (7-hydroxyflavone) isomers interact with the binding pocket of the AhR. Furthermore, their docking scores (−4.0 and −4.3 kcal/mol respectively) show higher interactions for the AhR-inactive 7-hydroxyflavone (−4.34 kcal/mol) isomer.
Table 1:
Mono- and dihydroxyflavones: Induction of CYP1A1 in Caco2 cells and computational-based molecular modeling docking scores.
| Induction Response | Docking Score (kcal/mol) | |
|---|---|---|
| Flavone | +++ | −3.77 |
| 3-hydroxy | ++ | −3.76 |
| 5-hydroxy | +++ | −3.66 |
| 6-hydroxy | +++ | −3.95 |
| 7-hydroxy | − | −4.34 |
| 3′-hydroxy | +++ | −4.36 |
| 4′-hydroxy | +++ | −3.88 |
| 3,6-dihydroxy | − | −3.88 |
| 5,6-dihydroxy | +++ | −3.48 |
| 6,3′-dihydroxy | +++ | −4.02 |
| 6,4′-dihydroxy | − | −3.52 |
| 3,7-dihydroxy | − | −4.21 |
| 5,7-dihydroxy | +/− | −4.50 |
| 7,8-dihydroxy | − | −4.58 |
| 7,3′-dihydroxy | − | −4.84 |
| 3′,7-dihydroxy | − | −3.96 |
Figure 6:

Modeling studies. Modeling of (A) 6- and (B) 7-hydroxyflavone to the AhR was carried out as previously described (23, 24) and the figure illustrates compound specific interactions with amino acid (aa) side chains.
Since modeling studies of ligand interactions with the AhR are measures of interactions with the binding pocket and not agonist activity (i.e.: CYP1A1 induction) we hypothesized that some of the AhR-inactive ligands that fit well in the AhR binding pocket may be AhR antagonists that block ligand-dependent AhR-mediated transactivation. Figure 7 summarize the effects of 7,4′-dihydroxyflavone as an AhR antagonist in Caco2 cells. This compound exhibited minimal AhR agonist activity (Figure 4) however, incubation of 50 μM 7,4′-dihydroxyflavone plus 10 nM TCDD resulted in potent inhibition of TCDD-induced CYP1A1, CYP1B1 and UGT1A1 in Caco2 cells and the inhibitory effects were similar to those observed for the classical AhR antagonist CH223191 (Fig. 7A). Since the molecular modeling values for receptor ligand interactions of flavones do not correlate with their AhR agonist activity (Table 1) we hypothesized that other AhR-inactive flavonoids might also be antagonists and resemble effects observed for 7,4′-dihydroxyflavone (Fig.7A). Results in Figure 7B show that a series of 7-substituted AhR-inactive hydroxyflavone isomers all inhibited induction of CYP1A1 by TCDD and acted as AhR antagonists for this response. Many of these same compounds also inhibited induction of CYP1B1 by TCDD (Fig.7C). However, 7-hydroxyflavone was inactive as an AhR agonist or antagonist of CYP1B1 induction (Fig. 7C) and all of the compounds inhibited TCDD-induced UGT1A1 (Fig. 7C). Thus, a possible explanation of favorable docking scores of some flavonoids to the AhR may be due to their activities as AhR antagonists which for some compounds such as 7-hydroxyflavone are response-specific.
Figure 7:

Hydroxylated flavones as AhR antagonists. A. Caco2 cells were treated with DMSO, 10 nM TCDD and 10 nM TCDD plus 50 μM 7,4′-dihydroxyflavone or 10 μM CH223191 and induction of CYP1A1, CYP1B1 and UGT1A1 were determined by real-time PCR. Caco2 cells were treated with DMSO, 10 nM TCDD and 10 nM TCDD plus 50 μM 3,7-, 5,7-, 7,8- and 7,3′-dihydroxyflavone and 7-hydroxyflavone, and induction of (B) CYP1A1, (C) CYP1A1 and (D) UGT1A1 were determined by real-time PCR. Results are expressed as means ± SE for 3 determinations and significant (p<0.05) induction (*) or inhibition (**) of TCDD-induced responses are indicated.
DISCUSSION
Dietary consumption of polyphenolic phytochemicals such as flavonoids have been associated with multiple health benefits including increased lifespan, decreased metabolic disease, decreased cardiovascular problems, enhanced neuronal health and decreased rates of cancer (28–38). These health promoting activities of polyphenolics represents their effects on both chemoprevention or inhibition of adverse health conditions. There are also many examples of the activities of flavonoids as chemotherapeutic agents. The combined chemopreventive and chemotherapeutic effects of flavonoids have contributed to their extensive and increasing uses as nutraceuticals and dietary supplements (28–38). The mechanisms of action of flavonoids are diverse and include their antioxidant activities, inhibition of tissue-specific enzymes/pathways, immunomodulatory effects and interactions with multiple intracellular and membrane proteins and receptors (rev. in (39)). However, despite the multiple activities of flavonoids, their underlying mechanisms of action as therapeutic agents for specific adverse responses and effects of structural modifications on activity are not well defined.
Studies in this laboratory have investigated the AhR activity of flavonoids including flavones, isoflavones and chalcones (17, 20–22) since there is evidence that dietary AhR active flavonoids may exhibit enhanced protection against intestinal inflammation (18, 39, 40). Several prior studies demonstrate that the AhR and its ligands exhibit anti-inflammatory activity (9–11) and that inhibition of gut inflammation by the flavonoids baicalen and cardamonin are AhR-dependent (18, 40). Our initial studies with flavones indicated that their AhR activity was, with few exceptions, structure-dependent on the overall number of hydroxyl substituents (17). Pentahydroxyflavones were more active than hexahydroxyflavones whereas the tri- and tetrahydroxyflavones were either inactive or weakly active or partial AhR antagonists based on CYP1A1 induction as the measure of AhR activity in Caco2 cells. These results contrast with studies showing that 5,6,7-trihydroxyflavone (baicalein) is AhR active. A recent review on flavonoids as AhR agonists/antagonists also shows that other “lightly” hydroxylated flavones activate or inactivate the AhR (13). Thus, our approach in this study was to initially investigate flavone and monohydroxyflavones and determine their activity as AhR agonists in Caco2 cells in terms of their induction of AhR-dependent CYP1A1, CYP1B1 and UGT1A1. These initial studies coupled with determining the effects of additional hydroxyl groups at specific positions would indicate if there were obvious structure-activity relationships for hydroxylated flavones as AhR agonists as previously observed for dioxin-like compounds (2). This would facilitate selection of “optimal” AhR-active flavonoids for potential clinical applications in treating intestinal inflammation and carcinogenesis.
The initial screening of flavone and hydroxyflavone isomers showed that flavone itself induced CYP1A1, CYP1B1 and UGT1A1 in Caco2 cells (Fig. 1) and similar results were observed for 5-, 6-, 3′- and 4′-hydroxyflavone whereas low to minimal AhR activity was observed for 3- and 7- hydroxyflavone (Fig. 2). Since flavone is AhR-active in Caco2 cells these results do not define a specific hydroxyl group that is required for activity. However, our studies (Fig. 2) show that introduction of hydroxyl groups at specific positions (5, 6, 3′, or 4′) either maintains or decreases (3- and 7-) AhR activity of the resulting hydroxyflavone compared to flavone (Fig. 3). 6-Hydroxyflavone was one of the more active AhR agonists and introduction of an additional hydroxyl group at the 3-, or 4- position decreased activity whereas the 5,6- and 6,4′- dihydroxylflavones exhibited activity comparable to 6-hydroxyflavone (Fig. 3). Using 7-hydroxyflavone as prototypical AhR-inactive compound, the addition of a second hydroxyl group at the 3, 5, 8, 3′ or 4′ positions gave dihydroxyflavones with minimal to non-detectable AhR activity. Previous studies showed that several tri- and tetrahydroxyflavones containing a 7-hydroxyl group also exhibit minimal to non-detectable AhR activity. These include galangin (3,5,7-), apigenin (4′, 5, 7-), luteolin (5, 7; 3′,4′-), kaempferol (3, 5, 7, 4′-), fisetin (3,7,3′,4′-), naringenin (5,7,4′-; 2,3 – dihydro) and apigenin (5,7,4′-) (17). In contrast, many of the AhR-active penta- and hexahydroxyflavones also contain a 7-hydroxyl group including quercetin (3,5,7,3′,4′-), (5,7,3′,4′,5′), robinetin (3,7,3′,4′,5′-), myricetin (3,5,7,3′,4′,5′-) and gossypetin (3,5,7,8,3′,4′) (31). These funding indicate that the 7-hydroxyl group was not an inhibitor of AhR activity for the penta- and hexahydroxyflavones.
For the mono- and dihydroxyflavones examined in this study, we also observed that compound-induced responses were similar for induction of CYP1A1, CYP1B1 and UGT1A1 mRNA levels. For the tri- and tetra- hydroxyflavones which exhibited minimal induction of CYP1A1 mRNA, both apigenin and luteolin induced levels of UGT1A1 mRNA levels similar to that observed for TCDD (17). Structure-activity relationships among 15 mono- and dihydroxyflavones demonstrate that some of these compounds were AhR agonists whereas others were inactive. We therefore used modeling studies to determine possible structure-dependent differences in their interactions within the AhR binding pocket. Docking of AhR-active 6-hydroxyflavone to human AhR resulted in a predicted docking score of −4.0 kcal/mol. Analysis of the 6-hydroxyflavone/AhR model (Fig. 6A) yielded three specific good interactions between 6-hydroxyflavone and AhR. These include an aromatic H-bond between Pro 29 (Pro 275 in full-length sequence) and the C-8 H of the ligand; a H-bond between Lys 44 (Lys 290) and the 6-OH group of the ligand, and a H-bond between Tyr 132 (Tyr 378) -OH and the -C=O moiety of the ligand. No unfavorable molecular interactions between ligand and protein were predicted. Docking of AhR-inactive 7-hydroxyflavone to human AhR resulted in a predicted docking score of −4.3 kcal/mol, very similar to that of 6-hydroxyflavone (Table 1). Analysis of the 7-hydroxyflavone/AhR model (Fig. 6B) yielded the same aromatic H-bond interaction between Pro 29 (Pro 275) and the C-8 H of 7-OH flavone as predicted for 6-hydroxyflavone. The model predicted a H-bond between Tyr 132 (Tyr 378) -OH and the -C=O group of 7-hydroxyflavone. The remaining two positive interactions predicted for 7-hydroxy flavone were not the same interactions predicted for 6-hydroxyflavone. These were a predicted H-bond between Pro 29 (Pro 275) and the 7-OH group of the ligand, and second an H-bond was predicted between Arg 42 (Arg 288) and the 7-OH group of the ligand. Unlike the predicted protein-ligand interactions of AhR and 6-OH flavone, modeling of 7-hydroxyflavone yielded one predicted unfavorable protein-ligand interaction: an unfavorable H-H interaction between Arg 42 (Arg 288) and the C-6 H of 7-hydroxyflavone. Other comparisons of AhR-active vs. AhR-inactive hydroxyflavones showed only minor differences in their interactions within the AhR binding site. This observation is consistent with our results showing that the mono- and dihydroxyflavones exhibit both AhR agonist and antagonist activities. However, inspection of both the modeling and functional activities of these compounds does not reveal obvious structure-functional relationships that predict AhR agonist or antagonist activity. Difficulties in assigning AhR agonist or antagonist activity to individual flavonoids also has been observed in a recent comprehensive review (13) which shows that agonist/antagonist activities of flavones are also cell context dependent. This suggests that optimizing flavonoids for a specific therapeutic response that acts through the AhR cannot be readily predicted and will require a response-specific screening assays to identify optimal drug candidates.
Funding:
Texas AgriLife Research; the Sid Kyle Chair Endowment; Allen Endowed Chair in Nutrition and Chronic Disease Prevention; and the National Institutes of Health (R35-CA197707, R01-AT010282, and P30-ES029067).
Abbreviations:
- AhR
Aryl hydrocarbon receptor
- CYP
cytochrome P450
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- UDP
uridine 5′-phosphate
- UGT
UDP-glucuronosyltransferase
Footnotes
Competing Interests: The authors have no conflict of interest to declare.
Data availability statement:
All data generated or analyzed during this study are included either in this article or in the Supplementary Data files.
REFERENCES
- 1.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. The Journal of biological chemistry. 1976;251:4936–46. [PubMed] [Google Scholar]
- 2.Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annual review of pharmacology and toxicology. 1982;22:517–54. Epub 1982/01/01. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 3.Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicology letters. 1986;34(1):67–74. [DOI] [PubMed] [Google Scholar]
- 4.Bigelow SW, Nebert DW. The Ah regulatory gene product. Survey of nineteen polycyclic aromatic compounds′ and fifteen benzo[a]pyrene metabolites’ capacity to bind to the cytosolic receptor. Toxicology letters. 1982;10(1):109–18. Epub 1982/01/01. doi: 10.1016/0378-4274(82)90276-4. [DOI] [PubMed] [Google Scholar]
- 5.Dolciami D, Ballarotto M, Gargaro M, Lopez-Cara LC, Fallarino F, Macchiarulo A. Targeting Aryl hydrocarbon receptor for next-generation immunotherapies: Selective modulators (SAhRMs) versus rapidly metabolized ligands (RMAhRLs). Eur J Med Chem. 2020;185:111842. Epub 2019/11/16. doi: 10.1016/j.ejmech.2019.111842. [DOI] [PubMed] [Google Scholar]
- 6.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annual review of pharmacology and toxicology. 2003;43:309–34. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 7.Safe S, Han H, Goldsby J, Mohankumar K, Chapkin RS. Aryl Hydrocarbon Receptor (AhR) Ligands as Selective AhR Modulators: Genomic Studies. Curr Opin Toxicol. 2018;11–12:10–20. Epub 2019/08/28. doi: 10.1016/j.cotox.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denison MS, Faber SC. And Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr Opin Toxicol. 2017;2:124–31. Epub 2017/08/29. doi: 10.1016/j.cotox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez-Vazquez C, Quintana FJ. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity. 2018;48(1):19–33. Epub 2018/01/19. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67(2):259–79. Epub 2015/02/07. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 11.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32. Epub 2014/03/25. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich AK, Kerkvliet NI. Is chronic AhR activation by rapidly metabolized ligands safe for the treatment of immune-mediated diseases? Curr Opin Toxicol. 2017;2:72–8. Epub 2017/09/26. doi: 10.1016/j.cotox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goya-Jorge E, Jorge Rodriguez ME, Veitia MS, Giner RM. Plant Occurring Flavonoids as Modulators of the Aryl Hydrocarbon Receptor. Molecules. 2021;26(8). Epub 2021/05/01. doi: 10.3390/molecules26082315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Molecular pharmacology. 1999;56(4):784–90. Epub 1999/09/25. [PubMed] [Google Scholar]
- 15.Yang T, Feng YL, Chen L, Vaziri ND, Zhao YY. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Critical reviews in toxicology. 2019;49(5):445–60. Epub 2019/08/23. doi: 10.1080/10408444.2019.1635987. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi-Bardbori A, Bengtsson J, Rannug U, Rannug A, Wincent E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chemical research in toxicology. 2012;25(9):1878–84. Epub 2012/08/08. doi: 10.1021/tx300169e. [DOI] [PubMed] [Google Scholar]
- 17.Jin UH, Park H, Li X, Davidson LA, Allred C, Patil B, Jayaprakasha G, Orr AA, Mao L, Chapkin RS, Jayaraman A, Tamamis P, Safe S. Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. Toxicological sciences : an official journal of the Society of Toxicology. 2018;164(1):205–17. Epub 2018/03/28. doi: 10.1093/toxsci/kfy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Li Y, Chen Y, Huang S, Wang X, Luo S, Su Y, Zhou L, Luo X. Baicalein Restores the Balance of Th17/Treg Cells via Aryl Hydrocarbon Receptor to Attenuate Colitis. Mediators Inflamm. 2020;2020:5918587. Epub 2020/10/22. doi: 10.1155/2020/5918587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur M, Badhan RK. Phytochemical mediated-modulation of the expression and transporter function of breast cancer resistance protein at the blood-brain barrier: An in-vitro study. Brain Res. 2017;1654(Pt A):9–23. Epub 2016/10/25. doi: 10.1016/j.brainres.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Park H, Jin UH, Orr AA, Echegaray SP, Davidson LA, Allred CD, Chapkin RS, Jayaraman A, Lee K, Tamamis P, Safe S. Isoflavones as Ah Receptor Agonists in Colon-Derived Cell Lines: Structure-Activity Relationships. Chemical research in toxicology. 2019;32(11):2353–64. Epub 2019/10/18. doi: 10.1021/acs.chemrestox.9b00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y, Jin UH, Davidson LA, Chapkin RS, Jayaraman A, Tamamis P, Orr A, Allred C, Denison MS, Soshilov A, Weaver E, Safe S. Editor’s Highlight: Microbial-Derived 1,4-Dihydroxy-2-naphthoic Acid and Related Compounds as Aryl Hydrocarbon Receptor Agonists/Antagonists: Structure-Activity Relationships and Receptor Modeling. Toxicological sciences : an official journal of the Society of Toxicology. 2017;155(2):458–73. Epub 2016/11/12. doi: 10.1093/toxsci/kfw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Jin UH, Karki K, Allred C, Davidson LA, Chapkin RS, Orr AA, Nowshad F, Jayaraman A, Tamamis P, Safe S. Hydroxylated Chalcones as Aryl Hydrocarbon Receptor Agonists: Structure-Activity Effects. Toxicological sciences : an official journal of the Society of Toxicology. 2021;180(1):148–59. Epub 2020/12/03. doi: 10.1093/toxsci/kfaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucleic acids research. 2015;43(W1):W174–81. Epub 2015/04/18. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Freddolino PL, Zhang Y. COFACTOR: improved protein function prediction by combining structure, sequence and protein-protein interaction information. Nucleic acids research. 2017;45(W1):W291–W9. Epub 2017/05/05. doi: 10.1093/nar/gkx366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49(21):6177–96. Epub 2006/10/13. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 26.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47(7):1750–9. Epub 2004/03/19. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- 27.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739–49. Epub 2004/03/19. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 28.Alseekh S, Perez de Souza L, Benina M, Fernie AR. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry. 2020;174:112347. Epub 2020/03/24. doi: 10.1016/j.phytochem.2020.112347. [DOI] [PubMed] [Google Scholar]
- 29.Pei R, Liu X, Bolling B. Flavonoids and gut health. Curr Opin Biotechnol. 2020;61:153–9. Epub 2020/01/19. doi: 10.1016/j.copbio.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Tohge T, Fernie AR. Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function. Nat Protoc. 2010;5(6):1210–27. Epub 2010/06/12. doi: 10.1038/nprot.2010.82. [DOI] [PubMed] [Google Scholar]
- 31.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. Epub 2017/06/18. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients. 2016;8(9). Epub 2016/09/13. doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Bi X, Yu B, Chen D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients. 2016;8(6). Epub 2016/06/15. doi: 10.3390/nu8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez de Souza L, Garbowicz K, Brotman Y, Tohge T, Fernie AR. The Acetate Pathway Supports Flavonoid and Lipid Biosynthesis in Arabidopsis. Plant Physiol. 2020;182(2):857–69. Epub 2019/11/14. doi: 10.1104/pp.19.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. Journal of the National Cancer Institute. 2004;96(21):1577–84. Epub 2004/11/04. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi H, Yuan B, Hu X, Okazaki M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am J Cancer Res. 2019;9(8):1517–35. Epub 2019/09/10. [PMC free article] [PubMed] [Google Scholar]
- 37.Krizova L, Dadakova K, Kasparovska J, Kasparovsky T. Isoflavones. Molecules. 2019;24(6). Epub 2019/03/22. doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12(2). Epub 2020/02/16. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safe S, Jayaraman A, Chapkin RS. Ah receptor ligands and their impacts on gut resilience: structure-activity effects. Critical reviews in toxicology. 2020;50(6):463–73. Epub 2020/07/01. doi: 10.1080/10408444.2020.1773759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Lv Q, Miao YM, Qiao SM, Dai Y, Wei ZF. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochemical pharmacology. 2018;155:494–509. Epub 2018/08/03. doi: 10.1016/j.bcp.2018.07.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included either in this article or in the Supplementary Data files.


