Abstract
The primary kratom alkaloid mitragynine is proposed to act through multiple mechanisms, including actions at µ-opioid receptors (MORs) and adrenergic-α2 receptors (Aα2Rs), as well as conversion in vivo to a MOR agonist metabolite (i.e., 7-hydroxymitragynine). Aα2R and MOR agonists can produce antinociceptive synergism. Here, contributions of both receptors to produce mitragynine-related effects were assessed by measuring receptor binding in cell membranes and, in rats, pharmacological behavioral effect antagonism studies. Mitragynine displayed binding affinity at both receptors, whereas 7-hydroxymitragynine only displayed MOR binding affinity. Compounds were tested for their capacity to decrease food-maintained responding and rectal temperature and to produce antinociception in a hotplate test. Prototypical MOR agonists and 7-hydroxymitragynine, but not mitragynine, produced antinociception. MOR agonist and 7-hydroxymitragynine rate-deceasing and antinociceptive effects were antagonized by the opioid antagonist naltrexone but not by the Aα2R antagonist yohimbine. Hypothermia only resulted from reference Aα2R agonists. The rate-deceasing and hypothermic effects of reference Aα2R agonists were antagonized by yohimbine but not naltrexone. Neither naltrexone nor yohimbine antagonized the rate-decreasing effects of mitragynine. Mitragynine and 7-hydroxymitragynine increased the potency of the antinociceptive effects of Aα2R but not MOR reference agonists. Only mitragynine produced hypothermic effects. Isobolographic analyses for the rate-decreasing effects of the reference Aα2R and MOR agonists were also conducted. These results suggest mitragynine and 7-hydroxymitragynine may produce antinociceptive synergism with Aα2R and MOR agonists. When combined with Aα2R agonists, mitragynine could also produce hypothermic synergism.
SIGNIFICANCE STATEMENT
Mitragynine is proposed to target the µ-opioid receptor (MOR) and adrenergic-α2 receptor (Aα2R) and to produce behavioral effects through conversion to its MOR agonist metabolite 7-hydroxymitragynine. Isobolographic analyses indicated supra-additivity in some dose ratio combinations. This study suggests mitragynine and 7-hydroxymitragynine may produce antinociceptive synergism with Aα2R and MOR agonists. When combined with Aα2R agonists, mitragynine could also produce hypothermic synergism.
Introduction
Prescription µ-opioid receptor (MOR) agonists are a primary medication class to treat severe pain (Haq et al., 2021; Montgomery, 2022). However, due to the current high incidents of opioid overdose in the United States (Mattson et al., 2021), there is the need for novel analgesics that are equally effective as MOR agonists but are safer. One of the adverse effects of MOR agonists is the development of dependence and withdrawal. The current medications to treat opioid dependence and withdrawal are either MOR or adrenergic-α2 receptor (Aα2R) agonists.
Mitragyna speciosa (kratom), a plant native to Southeast Asia, is used as a self-remedy to alleviate opioid withdrawal symptoms in countries such as Malaysia and Thailand (Singh et al., 2014). The use of kratom has increased significantly in the West where kratom products are used for pain reduction and opioid dependence, as well as recreationally (Lydecker et al., 2016; Sharma et al., 2019). Mitragynine (MG), the primary alkaloid in kratom, has received much attention due to its MOR activity (Matsumoto et al., 1996; Shamima et al., 2012; Harun et al., 2015; Varadi et al., 2016; Kruegel et al., 2019; Obeng et al., 2020; Chakraborty et al., 2021; Obeng, Wilkerson et al., 2021). However, MG appears to have a complex pharmacology that may include Aα2R activity. For example, the antinociceptive effects of MG were reversed by both opioid (naloxone) and Aα2R (yohimbine and idazoxan) antagonists (Matsumoto et al., 1996; Kruegel et al., 2019; Foss et al., 2020).
Decreased overreliance on prescription MOR agonists for pain management could be achieved by combining MOR agonists with nonopioid analgesics, thereby reducing the analgesic dose of the prescribed MOR agonist (i.e., opioid-sparing effect) (Wilkerson et al., 2016; Wilkerson et al., 2017; Wilkerson et al., 2019; Obeng, Hiranita et al., 2021). Although the antinociceptive effectiveness of Aα2R agonists is generally lower than that of MOR agonists, Aα2R agonists have well-established opioid-sparing effects and have been safely used (Crassous et al., 2007; Giovannoni et al., 2009; Tonner, 2017; Valverde and Skelding, 2019). It has been hypothesized that the basis of Aα2R agonist opioid-sparing effects is due to antinociceptive synergism (supra-additivity) between agonists at these receptors. For example, an inactive dose of the Aα2R agonist clonidine (0.016 mg/kg) increased the antinociceptive potency of morphine four- to fivefold without producing tolerance in the mouse tail flick assay (Spaulding et al., 1979). The opioid-sparing effects of Aα2R agonists have been demonstrated regardless of rodent species (i.e., mouse and rat), antinociceptive assays (e.g., hotplate, tail pressure, formalin), and combinations of agonists at these receptors (Drasner and Fields, 1988; Ossipov, Lozito et al., 1990; Plummer et al., 1992; Meert and de Kock, 1994; Stone et al., 1997; Hao et al., 2000; Tajerian et al., 2012; Stone et al., 2014). Importantly, antinociceptive synergism was not accompanied with nonspecific motor (rotarod and open field tests) or cardiovascular (pulse oximetry) disruptions (Tajerian et al., 2012; Stone et al., 2014). Additionally, the adverse effects of the Aα2R agonists are far less severe than those of the MOR agonists (Walker et al., 2002). In marked contrast to the MOR agonists, Aα2R agonists have low, if any, potential for development of abuse and dependence (Arnsten and Li, 2005; Clemow and Walker, 2014; Gowing et al., 2016) which suggests that Aα2R agonists may be ideal for reducing opioid use and overdose. Given the capacity of the Aα2R agonists to reduce opioid use as well as the agonistic activity of MG at MOR and Aα2R as previously mentioned (Matsumoto et al., 1996; Kruegel et al., 2019; Foss et al., 2020; Chakraborty et al., 2021), we hypothesized that MG mitigates opioid withdrawal through dual agonism at these receptors.
Herein, we first assessed preclinical interaction profiles of reference agonists at MOR (methadone and morphine) and Aα2R (lofexidine and clonidine) in rats by measuring effects of drugs on schedule-controlled responding for food, response latency in the hotplate test, and rectal temperature (Boxwalla et al., 2010). Interactions between agonists at the κ-opioid receptor [KOR, (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide (U69,593)] and Aα2R were also investigated. The mechanism underlying the activity of these compounds was further investigated using antagonists at the MOR (naltrexone) and Aα2R (yohimbine). Isobolographic analyses were conducted to investigate synergism between MOR and Aα2R agonists. In addition, we compared the contribution of MOR and Aα2R to the activity of MG and 7-hydroxymitragynine (7-OH-MG), a MOR active metabolite of MG (Kruegel et al., 2019). A receptor binding assay was employed to assess affinity of test compounds at these receptors.
Methods and Materials
Compounds
The following are sources of compounds: [3H][D-Ala2, D-Leu5]-enkephalin ([3H][D-Ala2, D-Leu5]-enkephalin) (PerkinElmer, Boston, MA), [3H][D-Ala2, N-MePhe4, Gly-ol]-enkephalin ([3H][D-Ala2, N-MePhe4, Gly-ol]-enkephalin) (PerkinElmer), [3H]2-(2,3-dihydro-2-methoxy-1,4-benzodioxin-2-yl)-4,5-dihydro-1H-imidazole (PerkinElmer), [3H]U69,593 (PerkinElmer), clonidine hydrochloride (XGen Pharmaceuticals DJB, Inc., Horseheads, NY), lofexidine hydrochloride (Sigma-Aldrich Co., St. Louis, MO), (-)-methadone hydrochloride (National Institute on Drug Abuse, Drug Supply Program, Rockville, MD), (-)-MG hydrochloride [extracted as described in Hiranita et al. (2019)], (-)-7-OH-MG [semisynthesized from MG as in Obeng, Wilkerson et al. (2021)], (-)-morphine sulfate pentahydrate (National Institute on Drug Abuse), (-)-naltrexone hydrochloride (Sigma-Aldrich), U69,593 (Sigma-Aldrich), and yohimbine hydrochloride (Sigma-Aldrich). Dose/concentration is expressed as the weight of the previously listed salt form or as a base if no salt form is noted. For in vitro studies, compounds were dissolved in dimethyl sulfoxide (Sigma-Aldrich) to form stock concentrations of 10 mM. For behavioral studies, a vehicle consisting of sterile water containing 5% Tween 80 (polyoxyethylenesorbitanmonooleate; Sigma-Aldrich) and 5% propylene glycol (Sigma-Aldrich) was used. Compounds and vehicle were administered intraperitoneally in a volume of 1.0 to 10 mL/kg per body weight. MG and vehicle were also administered subcutaneously and orally via gavage in volumes of 1.0 to 10 mL/kg.
In Vitro Receptor Binding Assay
[3H]2-(2,3-dihydro-2-methoxy-1,4-benzodioxin-2-yl)-4,5-dihydro-1H-imidazole (PerkinElmer) was used to label both the human Aα2AR and adrenergic-α2C receptor (Aα2CR) (O'Rourke et al., 1994). These two Aα2R subtypes were chosen because they are involved in antinociception (Brede et al., 2004). L-α-2A (ATCC CRL11180) and L-α-2C (ATCCCRL-11181) L-cells (American Type Culture Collection, Manassas, VA) were used for the Aα2AR and Aα2CR, respectively. [3H][D-Ala2, D-Leu5]-enkephalin, [3H]U69,593, and [3H][D-Ala2, N-MePhe4, Gly-ol]-enkephalin were used to label the human δ-opioid receptor (DOR), KOR, MOR, respectively, as described previously (Obeng, Wilkerson et al., 2021). The binding assay at the opioid receptor subtypes was conducted using monoclonal opioid receptors expressed in Chinese hamster ovary cell lines for the DOR (a generous gift from Dr. Stephen J. Cutler, University of South Carolina) and MOR (PerkinElmer). The KORs (a generous gift from Dr. Stephen J. Cutler, University of South Carolina) were expressed in human embryonic kidney cells. The Kd and Bmax values for the radioligands at each receptor subtype were first determined using a saturation assay (Supplementary Table 1). The Bradford protein assay was used to determine and adjust the concentration of protein required for the assay (Tal et al., 1985). Ten micrograms of each membrane protein was separately incubated with one of the radioligands in the presence of different concentrations of test compounds in Tris, MgCl2, and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid [(50 mM Tris (Sigma-Aldrich), 3 mM MgCl2 (Sigma-Aldrich), and 0.2 mM EGTA (Sigma-Aldrich), pH 7.7)] buffer for 60 minutes at room temperature. The bound radioligand was separated by filtration using the Connectorate filtermat harvester for 96-well microplates (Inotech, Dietikon, Switzerland) and counted for radioactivity using a MicroBeta2 microplate counter (PerkinElmer). Specific binding at each Aα2R subtype was determined as the difference in binding obtained in the absence and presence of 10 µM lofexidine (Supplementary Table 1). Specific binding at the DOR, KOR, and MOR was determined as the difference in binding obtained in the absence and presence of 10 µM (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide, U69,593, and naltrexone, respectively.
Subjects
Adult female and male Sprague Dawley rats at 10 weeks old upon arrival (Taconics, Germantown, NY; n = 4 per sex) were housed individually and acclimated for at least three days to a temperature- (21.9°C ± 1.9°C) and humidity-controlled (53% ± 14%) vivarium with a 12-hour light/dark cycle (lights on at 7:00 a.m. EST in the daylight saving time period) during which food (2918 Teklad global 18% protein rodent diets, Envigo, Frenchtown, NJ) and reverse osmosis water were available at all times. After the acclimation period, individual body weights were maintained at no less than 85% of free-feeding body weight as well as no less than 2.5 of the Body Conditioning Score (Ullman-Culleré and Foltz, 1999) by adjusting daily food rations. The free-feeding body weight was redetermined as requested by the veterinary staff at University of Florida. Access to chow (Dustless Precision Pellets Grain-Based Rodent Diet, Bio-Serv, Frenchtown, NJ) was provided in the rats’ home cages approximately 30 minutes following daily experimental sessions. In addition to chow consumption, rats consumed a maximum of fifty 45-mg sucrose pellets (Dustless Precision Pellets 45 mg, Sucrose, Bio-Serv) available during experimental sessions for schedule-controlled responding as described in the following text. The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Florida and was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Apparatus
The apparatus and procedures for the operant-conditioning and hotplate experiments were as previously described (Hiranita et al., 2019; Wilkerson et al., 2019; Obeng, Wilkerson et al., 2021).
Operant Conditioning Apparatus
Eight operant-conditioning chambers (Model ENV-008; Med Associates Inc., Fairfax, VT) were used, each enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two retractable, 5-cm-long response levers, 5 cm from the midline and 9 cm above the grid floor. A response was defined as a downward displacement of the right lever with a force approximating 0.20 N whereas the left lever was not used. Two amber light-emitting diodes (LEDs) were aligned horizontally above two levers (one LED/lever); however, only the right LED and lever were activated for the correct study. A receptacle for the delivery of 45-mg sucrose pellets (Dustless Precision Pellets 45 mg, Sucrose, Bio-Serv) via a pellet dispenser (Model ENV-203-20; Med Associates Inc.) was mounted on the midline of the front wall between the levers and 2 cm above the floor. Each operant conditioning chamber was connected to a Dell desktop computer (Intel Core i7-7700 3.60 GHz processor, 16.0 GB of RAM, Microsoft Windows 10) through an interface (MED-SYST-8, Med Associates Inc.). Med-PC software version V (Med Associates Inc.) controlled experimental events and recorded responses. The chamber assignments remained the same for each subject throughout the study.
Hotplate
A square plate (Hot Plate Analgesia Meter, 1440 Analgesia Hot Plate with RS-232 Port and Software, Columbus Instruments, Columbus, OH) was surrounded by a clear acrylic cubicle with a lid. The stability of temperature on the plate surface was verified at 52°C ± 0.1°C 30 minutes prior to each use.
Rectal Thermometer
An uninsulated microprobe (50313 Rat Rectal Probe, Stoelting, Wood Dale, IL) and a digital thermometer (50315 Body Temperature Thermometer, Stoelting) were used to measure rectal temperature. Veterinary ophthalmic ointment (Puralube, Dechra Veterinary Products, Overland Park, KS) was applied to the tip of the microprobe prior to each use.
In Vivo Procedures
The temperature, humidity, and light/dark cycle in the experimental room were equivalent to those in the vivarium. After the acclimation period to the vivarium, schedule-controlled responding experiments were conducted in the light cycle (8:00 a.m. to 11:00 a.m. E.S.T. in the daylight-saving time period) at the same time each day seven days per week. On drug test days, temperature and hotplate experiments were also conducted in that order (Fig. 1). Prior to the start of each daily experiment, body weight was measured. The sample size of each experimental group per treatment was eight using a within-subject design (n = 4 per sex). The doses of each test compound per injection were incremented sequentially at approximately 20-minute intervals (Fig. 1).
Fig. 1.
Schematic presentation of experimental timelines on test and intertest sessions. The rate-decreasing, hypothermic, and antinociceptive effects of test compounds were repeatedly assessed in eight rats (four rats per sex) by measuring schedule-controlled responding (SCR) for presentation of food pellets, rectal temperature (RT), and hotplate (HP) response latency, respectively. RT and HP response latency were measured manually in this order only on test days. RT was measured using a microprobe. HP response latency was measured by placing each rat on a heated hotplate at 52°C and using a stopwatch. The experimental session consisted of six 20-minute experimental cycles and lasted for 120 minutes. On the test days, baseline values of RT and HP response latency were measured before the experimental session. After each rat received an injection (intraperitoneally, orally by gavage, or subcutaneously; T = 0 minutes), the first experimental cycle commenced by placing the rat in the operant-conditioning chamber. Each experimental cycle consisted of the 15-minute timeout period and then a 5-minute period for data collection of lever-pressing responses for presentations of food pellets using an automated system. Immediately following each 20-minute cycle, RT and HP response latency were measured in this order. Then, each rat received an injection of a dose of test compound, and the second cycle commenced by placing the rat in the operant-conditioning chamber. Doses of each test compound were administered cumulatively. The experimental procedures on intertest days were basically identical to those on test sessions. However, RT and HP response latency were not measured on intertest days. In addition, only the vehicle was administered on intertest days. The intertest sessions were conducted consecutively at least twice. See the Methods and Material section for more details.
Within-Session, Six-Cycle Schedule-Controlled Responding
Lever-response shaping
Each experimental session commenced by placing an experimental subject in an individually assigned chamber daily up to 120 minutes. Each session started with the presentation of the right retractable lever and the illumination of the LED above the right lever. Each downward deflection of the right lever turned off the LEDs and activated the pellet dispenser for 0.1 seconds [fixed ratio (FR) 1 schedule] followed by a 0.1-second time-out period during which LEDs were turned off and responding had no scheduled consequences; the retractable lever remained presented during this time-out time. After 50 reinforcers per session were presented within 20 minutes for two consecutive sessions under the terminal FR10 schedule of reinforcement, and daily sessions were divided into multiple, discrete cycles.
Training
Each session consisted of six, 20-minute cycles with each cycle consisting of a 15-minute pretest phase and a 5-minute test phase in the operant-conditioning chambers (Fig. 1). Immediately prior to each cycle, vehicle was injected intraperitoneally, and each animal was placed in the assigned chamber. Upon commencement of each session and at the beginning of each pretest phase, the right response lever was extended into the chamber, but the stimulus light remained off. Responses on the lever had no scheduled consequences. Upon commencement of each test phase of the cycle, the stimulus light was illuminated. Thereafter, all the experimental variables for the stimulus changes and response time-out conditions under the FR10 schedule of reinforcement were identical to those for response shaping except that the maximal number of food reinforcers delivered was fixed at 10 per cycle. When 10 food reinforcers were delivered during each test phase, the stimulus light was turned off and lever responding had no scheduled consequences. Upon completion of the last test phase, the lever was retracted, and the stimulus light was turned off. Then, each animal was placed back to their home cages. Training continued until overall response rates (responses per second) across six cycles for two consecutive sessions were stably maintained with less than 25% variation, as determined per individual subject.
Testing
All the experimental variables were identical to those for the training period. However, a dose of a test compound was also injected per cycle other than vehicle. The first injection received was either vehicle or the pretreatment compound (i.e., antagonists naltrexone or yohimbine). The subsequent five injections were either vehicle or test compound. Each test compound was dosed cumulatively such that each dose per cycle was a subtraction from a summation of all the previous doses administered to achieve the target dose. The doses of the compounds administered (mg/kg) increased by either quarter or half log unit increments. Each test session was separated by a minimum of 72 hours and was studied with a nonsystematic order of compounds and doses. During the intertest maintenance sessions, all the experimental variables were identical to those for the training period, without any determination of the hotplate latency and rectal temperature as described next. Vehicle was injected at the beginning of each pretest phase.
Among food-maintained behavior, hotplate response latency, and rectal temperature, only analyses of food-maintained behavior allowed us to determine ED50 values of all the reference agonists at MOR, KOR, and Aα2R (see Data Analysis). For the combinations of reference agonists, the cumulative doses in quarter log units in the mixtures per animal were determined based on the ED50 values of the rate-decreasing effects of reference agonist alone (Table 1), (Wilkerson et al., 2019). To determine the pharmacological influence of each drug on the observed effects, three ED50 ratios of drug mixtures were used. The order of testing was determined randomly. All dose-effect functions for drug mixtures were singly determined.
TABLE 1.
Inhibition of binding of the radioligands labeling Aα2R and opioid receptor subtypes
Values are Ki values for displacement of the radioligands (see Supplementary Table 1). Values in parentheses are 95% CIs unless noted. Values listed from previous studies were also added as reference.
| Compound | Aα2AR Ki Value (nM) | Aα2CR Ki Value (nM) | DOR Ki Value (nM) | KOR Ki Value (nM) | MOR Ki Value (nM) | Aα2C/Aα2A | Aα2A/MOR | Aα2C/MOR |
|---|---|---|---|---|---|---|---|---|
| Clonidine | 5.97 (3.66, 10.4) | 60.8 (33.7, 115) | No inhibition up to 10 µM | No inhibition up to 10 µM | No inhibition up to 10 µM | 10.2 | NA | NA |
| 7-OH-MG | No inhibition up to 10 µM | No inhibition up to 10 µM | 243 (168, 355) | 220 (162, 302) | 77.9 (45.8, 152) | NA | NA | NA |
| Lofexidine | 1.21 (0.60, 2.43) | 7.62 (3.96, 14.8) | No inhibition up to 10 µM | No inhibition up to 10 µM | No inhibition up to 10 µM | 6.30 | NA | NA |
| Methadone | No inhibition up to 10 µM | No inhibition up to 10 µM | No inhibition up to 10 µM | 481 (294, 816) | 6.61 (5.27, 8.32) | NA | NA | NA |
| MG | 4,420 (2,720, 7,670)a 4,720 (S.E.M.: 120)b 2.3 µMc |
4,040 (1,880, 6,820)a 2,320 (S.E.M.: 140)b 3.5 µMc |
6,800 (2,980, 15,900)a | 1,700 (1,090, 2,710)a | 709 (451, 1,130)a | 0.914 | 6.23 | 5.70 |
| Morphine | No inhibition up to 10 µM | No inhibition up to 10 µM | 250 (177, 346)a | 40.4 (23.7, 70.9)a | 4.19 (2.03, 11.1)a | NA | NA | NA |
| Naltrexone | No inhibition up to 10 µM | No inhibition up to 10 µM | 37.2 (26.3, 53.0)a | 1.19 (0.803, 1.79)a | 1.84 (1.14, 3.03)a | NA | NA | NA |
| U69,593 | No inhibition up to 10 µM | No inhibition up to 10 µM | 6,700 (2,160, 28,000)a | 1.62a (1.02, 2.64)a | 3,180 (1,050, 11,600)a | NA | NA | NA |
| Yohimbine | 8.24 (5.40, 12.8) | 7.77 (4.76, 12.8) | No inhibition up to 10 µM | No inhibition up to 10 µM | No inhibition up to 10 µM | 0.943 | NA | NA |
Ki, Inhibition constant. NA, Not applicable.
aHuman recombinant Chinese hamster ovary cells using [3H]2-(2,3-dihydro-2-methoxy-1,4-benzodioxin-2-yl)-4,5-dihydro-1H-imidazole conducted at Eurofins Cerep (Celle l’Evescault, France) (Obeng et al., 2020).
bBinding at human opioid receptor cell lines (Obeng et al., 2021b).
cBinding at adrenergic receptors (Aα2A and Aα2C) conducted at the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH, PDSP) (Ellis et al., 2020).
Hotplate and Rectal Temperature
On drug test days, the microprobe tip was inserted approximately 2.0-cm into each subject’s rectum, and individual baseline temperature was measured within 10 seconds. Immediately after the baseline measurement of rectal temperature, each subject was manually placed on the heated plate, and baseline hotplate response latency was determined manually using a stopwatch (Martin Stopwatch, Martin Sports, Carlstadt, NJ) by trained and experimentally blinded raters. Hotplate response latency was measured until the subject jumped, licked or shook the back paws, or up to 60 seconds to avoid tissue damage, whichever occurred first.
Immediately following the determination of the baseline values, each subject underwent an injection of a dose of a test compound or vehicle and was placed in their respective operant conditioning chamber. Immediately after each cycle of the schedule-controlled responding experiment (cycles 1–6), rectal temperature and hotplate response latency were measured followed by an injection of a dose of the test compound or vehicle in this order.
Data Analysis
The dependent variables in each figure are shown as mean values ± S.E.M. Mean and S.E.M. values per group of eight subjects were calculated as a function of compound doses, cycles, or dose ratios of combined compounds. Statistical analyses were conducted using GraphPad Prism version 9 for Windows (GraphPad Software, Inc., San Diego, CA), SigmaPlot version 14.0 (Systat Software Inc., San Jose, CA), or R-4.1/RStudio Desktop (R Core Team, 2017). Comparisons were considered significant when a P value < 0.05. A one-, two-, or three-way (repeated-measures) ANOVA followed by post hoc Bonferroni t tests was used as appropriate to analyze the effects of the compound dose, cycle, sex, dose ratio, or tolerance (assessment order: first or last dose-effect assessment for morphine, U69,593, and lofexidine) (Supplementary Fig. 2; Supplementary Tables 5–7). For the three-way repeated measures ANOVA, GraphPad Prism software was used for all 2 × 2 by X design, and the RStudio Desktop software was used for all others.
For rectal temperature and hotplate latency, each mean baseline value was determined per animal from all the baseline values determined on the drug sessions used in the following analyses. Hotplate latency values were converted to percent maximum possible antinociceptive effect (%MPE) with the following equation: (100 × [(experimental test latency value – the averaged baseline latency value)/(60 seconds – the averaged baseline latency value)]). Changes in rectal temperature were calculated individually as the test value subtracted from the averaged baseline value. Rates of responding maintained by presentations of food pellets (responses/second) were expressed as a percentage of control, defined as the mean baseline rates across six daily cycles during all sessions one day prior to each test session. There was no increased or decreased trend for either hotplate latency, rectal temperature, or response rate baseline values (P values > 0.05). The dose-effect functions of morphine, U69,593, and lofexidine were determined twice, once at the start and once at the end of the within-subjects drug assessments. Only when the mean effect of a compound to reduce schedule-controlled responding or to increase %MPE was >50% of maximum effects were the ED50 and slope values calculated using multiple linear regression (Snedecor and Cochran, 1967) and GraphPad Prism version 9 for Windows (GraphPad Software), where slopes were allowed to vary (Tallarida, 2000). Because only α2R agonists produced ≥2°C hypothermia, ED-2°C values were also individually calculated to compare the hypothermic potency. Only points on the linear part of the ascending (%MPE) and descending (response rate and rectal temperature) limbs of the dose–effect functions were used. If the 95% confidence intervals (CIs) of the ED50, ED-2°C, and slope values did not overlap or the potency or slope ratio of the compound alone or in combination with another compound did not include 1, potencies or slopes of the compounds were deemed statistically different. Among food-maintained behavior, hotplate response latency, and rectal temperature, only analyses of food-maintained behavior allowed us to determine ED50 values of all the reference agonists at MOR, KOR, and Aα2R. For the mixture studies, the cumulative doses in quarter log units in the mixtures per animal were determined based on the ED50 values of the rate-decreasing effects of reference agonist alone (Wilkerson et al., 2019). That is, a within-subjects design was used, and each subject received dose combinations that were equivalent to the dose ratio based upon the individual ED50 of a drug to decrease response rates in that subject. The theoretical additive ED50 value of the combined drugs was calculated from the individual dose-effect functions to determine synergistic, additive, or subadditive interactions as previously described (Wilkerson et al., 2016, 2017, 2019). The combination was assumed to equal the sum of the effects of each drug. The experimentally derived ED50 values (Zmix) from the dose-effect functions of the ratios were compared with the predicted additive ED50 values (Zadd) via a Fisher’s exact test (Wilkerson et al., 2016, 2017, 2019). If the empirically derived value and the theoretical value did not significantly differ, the interaction was considered additive (Tallarida, 2001, 2006). For the in vitro studies, the assays were conducted in triplicate and repeated at least three times, and the IC50 values were determined using a nonlinear, least-squares regression analysis (Prism 9; GraphPad Software, Inc.) and then converted to the inhibition constant (Ki) values using the Cheng–Prusoff equation (Cheng and Prusoff, 1973). The 95%CI (asymptotic) was calculated using Prism 9.
Results
Only the primary findings are shown here. Full details are described in the supplemental materials.
Receptor Binding
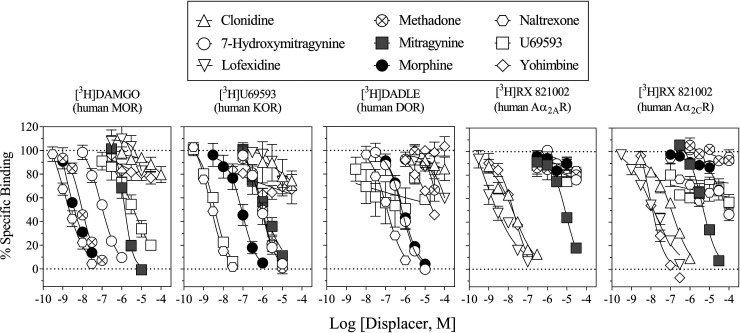
The Ki (nM) values of reference Aα2R ligands clonidine, lofexidine, and yohimbine were 5.97, 1.21, and 8.24 at the Aα2AR, and 60.8, 7.62, and 7.77 at the Aα2CR, respectively (Table 1). The Ki values of reference Aα2R ligands at opioid receptor subtypes and of reference opioid receptor ligands (methadone, morphine, naltrexone, and U69,593) at Aα2R subtypes were not determined due to lack of inhibition up to 10 µM (Table 1). The Ki values of MG were 4420 and 4040 nM at the Aα2AR and Aα2CR, respectively, whereas those of 7-OH-MG at these receptors were not determined due to lack of inhibition up to 10 µM. Both MG and 7-OH-MG had higher affinities at the MOR than at the DOR and KOR; however, 7-OH-MG had a ninefold higher affinity at the MOR than MG (Fig. 2; Table 1). A summary of scintillation counting conditions employed for assessing affinity at various binding sites in competition for the radioligands labeling human Aα2R and opioid receptor subtypes can be found in Supplementary Table 1.
Fig. 2.
Displacement of radioligands at opioid receptor and Aα2R subtypes. Ordinates: percentage of specific radiotracer bound to membrane preparations. Abscissae: concentrations of each competing compound (log scale). Each data point represents the mean results of three repeated experiments; vertical bars represent S.E.M. (n = 3) from at least three independent triplicate replications per sample. Ki and 95% CI values from curve-fitting analyses of these data are shown in Table 1. Note that affinity of MG at the MOR and Aα2R was approximately equal whereas no considerable affinity of 7-OH-MG was found at the Aα2R.
Reference MOR Agonists Alone
Repeated vehicle injections did not alter response rates, rectal temperature, or nociceptive responding (Supplementary Fig. 1; Supplementary Tables 2 and 3). Morphine dose-dependently and significantly decreased response rates and rectal temperature, as well as produced antinociception (Fig. 3, upper panels, upward triangles; Supplementary Table 4). The ED50 values of morphine to decrease response rates and to produce antinociception are shown in Table 2. The potency of morphine to produce the rate-decreasing effects was fourfold more potent than that for antinociception (Table 2).
Fig. 3.
The rate-decreasing, antinociceptive, and hypothermic effects of various compounds alone in rats. Abscissae: Vehicle and cumulative dose of compound in mg/kg (log scale). Ordinates: Left panels, percentage of mean rates of responding after repeated administration of vehicle during intertest sessions; middle panels, percentage of %MPE in the hotplate assay; right panels, changes in rectal temperature from mean baselines. Each point represents the mean ± S.E.M. (n = 4 per sex per data point). All compounds were administered intraperitoneally 15 minutes before each 5-minute period for data collection for food-maintained behavior, and MG was also administered orally by gavage and subcutaneously (lower panels). The data for morphine, U69,593, and lofexidine on the first assessment were plotted. Upper left: The rate-decreasing effects of vehicle, the reference MOR agonists (morphine and methadone), reference Aα2R agonists (lofexidine and clonidine), and reference KOR agonist U69,593. Filled circles represent repeated vehicle (intraperitoneal) administration. Morphine dose (intraperitoneal, upward triangles); vehicle, 5.6, 10, 17.8, 32, and 56 mg/kg. Methadone dose (intraperitoneal, downward triangles); vehicle, 0.32, 0.56, 1.0, 1.78, and 3.2 mg/kg. Lofexidine doses (intraperitoneal, diamonds); vehicle, 0.056, 0.1, 0.178, 0.32, and 0.56 mg/kg. Clonidine doses (intraperitoneal, squares); vehicle, 0.0178, 0.032, 0.056, 0.1, and 0.178 mg/kg. U69,593 doses (intraperitoneal, open circles); 0.56, 1.0, 1.78, 3.2, and 5.6 mg/kg. Upper middle: The antinociceptive effects of reference compounds. Upper right: The hypothermic effects of reference compounds. Lower left: The rate-decreasing effects of MG and 7-OH-MG. MG dose (intraperitoneal, circles); vehicle, 5.6, 10, 17.8, 32, and 56 mg/kg. MG dose (orally by gavage, circles); vehicle, 17.8, 32, 56, 100, and 178 mg/kg. MG dose (subcutaneously, triangles); vehicle, 17.8, 32, 56, 100, and 178 mg/kg. 7-OH-MG dose (intraperitoneal, squares); vehicle, 0.32, 1.0, 3.2, 10, and 32 mg/kg. Lower middle: The antinociceptive effects of MG and 7-OH-MG. Lower right: The hypothermic effects of MG and 7-OH-MG. Each gray symbol indicates a significant difference from vehicle per corresponding cycle. Note that all test compounds decreased food-maintained behavior. Robust antinociception was produced by the reference MOR agonists but not by the reference Aα2R agonists whereas robust hypothermia was produced by the reference Aα2R agonists but not by the reference MOR agonists. Regardless of the route of administration, MG did not produce robust antinociception or hypothermia. As with the reference MOR agonists, 7-OH-MG produced robust antinociception but did not produce significant hypothermia.
TABLE 2.
ED50 and E-2C° values in mg/kg for the rate-decreasing, antinociceptive, hypothermic effects of various compounds as shown in Figs. 3 to 6 and Supplementary Figs. 2 to 4
The sample sizes are described in each figure legend. Each value is a combination of females and males unless otherwise noted. Potency ratios (S.E.M.s) are calculated by dividing the ED50 or E-2C° values for producing the antinociceptive or hypothermic effects, respectively, and by the ED50 values for producing the rate-decreasing effects. Values in parentheses are 95% CIs. Significant differences are bold.
| Morphine Dose | |||||
|---|---|---|---|---|---|
| Combination | ED50 or E-2C° (S.E.M.) | Potency Ratio | |||
| Decrease in Response Rate (ED50) |
Antinociception (ED50) | Hypothermia (E-2C°) | Antinociception/ Decrease in Response Rate |
Hypothermia/ Decrease in Response Rate | |
| Morphine dose | |||||
| Morphine alone | 9.81 (7.32, 12.30) | 39.30 (37.18, 41.43) | NA | 4.00 (3.02, 5.66) | NA |
| Morphine + 0.032 mg/kg naltrexone | 43.8 (41.6, 46.0) | 210 (188, 232) | NA | 4.79 (4.09, 5.58) | NA |
| Morphine + 1.0 mg/kg naltrexone | 309 (257, 361) | NA | NA | NA | NA |
| Morphine + 3.2 mg/kg yohimbine | 13.5 (11.1, 15.9) | 24.3 (19.0, 29.6) | NA | 1.80 (1.20, 2.67) | NA |
| Morphine + 17.8 mg/kg MG | 9.29 (6.55, 12.03) | 35.9 (33.8, 38.0) | NA | 3.86 (2.81, 5.80) | NA |
| Morphine + 0.32 mg/kg 7-OH-MG | 19.5 (15.8, 23.2) | 33.1 (29.6, 36.6) | NA | 1.70 (1.28, 2.32) | NA |
| Methadone dose | |||||
| Methadone alone | 0.70 (0.48, 0.92) | 2.22 (1.74, 2.70) | NA | 3.17 (1.89, 5.63) | NA |
| Methadone + 0.032 mg/kg naltrexone | 2.87 (2.65, 3.09) | 25.3 (24.3, 26.3) | NA | 8.81 (7.86, 9.92) | NA |
| Methadone + 3.2 mg/kg yohimbine | 1.19 (1.00, 1.40) | 2.28 (1.80, 2.76) | NA | 1.91 (1.29, 2.76) | NA |
| Methadone + 17.8 mg/kg MG | 1.04 (0.92, 1.16) | 2.25 (2.05, 2.45) | NA | 2.16 (1.77, 2.66) | NA |
| Methadone + 0.32 mg/kg 7-OH-MG | 1.16 (0.86, 1.50) | 1.93 (1.86, 2.00) | NA | 1.66 (1.24, 2.33) | NA |
| U69,593 dose | |||||
| U69,593 alone | 2.17 (1.70, 2.65) | 3.17 (2.39, 3.95) | NA | 1.46 (0.90, 2.32) | NA |
| U69,593 + 0.032 mg/kg naltrexone | NA | 1.86 (1.52, 2.20) | NA | NA | NA |
| U69,593 + 1.0 mg/kg naltrexone | 14.58 (11.87, 17.29) | 49.07 (47.0, 51.20) | NA | 3.36 (2.72, 4.31) | NA |
| U69,593 + 3.2 mg/kg yohimbine | 2.28 (1.80, 2.80) | 2.62 (1.84, 3.40) | NA | 1.15 (0.657, 1.89) | NA |
| U69,593 + 17.8 mg/kg MG | 3.10 (2.74, 3.46) | 4.66 (4.15, 5.17) | NA | 1.50 (1.19, 1.89) | NA |
| U69,593 + 0.32 mg/kg 7-OH-MG | 5.61 (4.67, 6.55) | 16.0 (15.0, 16.9) | NA | 2.85 (2.29, 3.62) | NA |
| Lofexidine dose | |||||
| Lofexidine alone | 0.153 (0.121, 0.185) | NA | 0.294 (0.267, 0.321) | NA | 1.92 (1.44, 2.65) |
| Lofexidine + 1.0 mg/kg naltrexone | 0.107 (0.085, 0.129) | NA | 0.395 (0.332, 0.458) | NA | 3.69 (2.57, 5.39) |
| Lofexidine + 1.0 mg/kg yohimbine | 0.788 (0.683, 0.893) | NA | 1.06 (0.887, 1.23) | NA | 1.35 (0.993, 1.80) |
| Lofexidine + 3.2 mg/kg yohimbine | 1.89 (1.60, 2.18) | NA | 3.69 (2.84, 4.54) | NA | 1.95 (1.30, 2.84) |
| Lofexidine + 17.8 mg/kg MG | 0.019 (0.014, 0.024) | 0.168 (0.161, 0.175) | 0.037 (0.027, 0.046) | 8.84 (6.71, 12.5) | 1.95 (1.13, 3.29) |
| Lofexidine + 0.32 mg/kg 7-OH-MG | NA | 0.472 (0.457, 0.487) | 0.208 (0.181, 0.235) | NA | NA |
| Clonidine dose | |||||
| Clonidine alone | 0.048 (0.038, 0.058) | NA | 0.094 (0.088, 0.100) | NA | 1.96 (1.52, 2.63) |
| Clonidine + 1.0 mg/kg naltrexone | 0.054 (0.044, 0.064) | NA | 0.105 (0.087, 0.123) | NA | 1.94 (1.36, 2.80) |
| Clonidine + 1.0 mg/kg yohimbine | 0.186 (0.159, 0.213) | NA | 0.544 (0.474, 0.614) | NA | 2.92 (2.23, 3.86) |
| Clonidine + 17.8 mg/kg MG | NA (no more than 50% data point) |
0.042 (0.039, 0.186) | 0.0633 (0.0501, 0.045) | NA | NA |
| Clonidine + 0.32 mg/kg 7-OH-MG | NA (no more than 50% data point) |
NA (up to 47.5% MPE) | 0.093 (0.089, 0.097) | NA | NA |
| MG dose | |||||
| MG alone (i.p.) | 27.2 (21.0, 33.4) | NA | NA | NA | NA |
| MG (i.p.) + 1.0 mg/kg naltrexone | 33.8 (22.7, 45.0) | NA | NA | NA | NA |
| MG (i.p.) + 3.2 mg/kg yohimbine | 32.0 (27.0, 37.0) | NA | NA | NA | NA |
| MG alone (p.o.) | 89.3 (69.8, 108) | NA | NA | NA | NA |
| MG alone (s.c.) | 161 (118, 204) | NA | NA | NA | NA |
| 7-OH-MG dose | |||||
| 7-OH-MG alone | 1.82 (1.22, 2.42) | 9.13 (7.41, 10.9) | NA | 5.02 (3.06, 8.93) | NA |
| 7-OH-MG + 0.032 mg/kg naltrexone | 17.5 (14.4, 20.7) | 41.8 (38.2, 45.5) | NA | 2.39 (1.85, 3.16) | NA |
| 7-OH-MG + 3.2 mg/kg yohimbine | 3.07 (2.53, 3.61) | 15.7 (14.1, 17.3) | NA | 5.11 (3.91, 6.84) | NA |
NA, not applicable.
Methadone significantly decreased response rates and rectal temperature and produced antinociception (Fig. 3, upper panels, downward triangles; Supplementary Tables 4 and 5). Relative to morphine, methadone was 7- and fivefold more potent to produce rate-decreasing and antinociceptive effects, respectively (Table 2).
Reference KOR Agonist Alone
U69,593 significantly decreased response rates and rectal temperature and produced antinociception (Fig. 3, upper panels, circles; Supplementary Table 6). Relative to morphine, U69,593 was two- and fourfold more potent to produce the rate-decreasing and antinociceptive effects, respectively (Table 2). U69,593 was equipotent to decrease response rates and produce antinociception, as measured by increased %MPE (Table 2). There was no significant change in potency across the rates of responding, antinociception, or rectal temperature (Table 2; Supplementary Fig. 2; Supplementary Table 6).
Reference Aα2R Agonists Alone
Lofexidine significantly decreased response rates and rectal temperature and significantly increased %MPE; the antinociceptive effects of lofexidine reached statistical significance, but the maximum effects of lofexidine were a mean of 17.3% and significantly less than those of reference MOR agonists (F1,6 = 361, P < 0.001, two-way repeated measures ANOVA) (Fig. 3, upper panels, diamonds; Supplementary Table 7). In contrast, as compared with the reference MOR agonists, the hypothermic effects of lofexidine were significantly greater (e.g., 4.1°C decrease in rectal temperature at 0.56 mg/kg) (Fig. 3). Lofexidine was 38-fold more potent than morphine to produce the rate-decreasing effects (Table 2). The potency of lofexidine to reduce response rates was threefold greater than its potency to decrease rectal temperature (Table 2).
Clonidine significantly decreased response rates and rectal temperature; however, statistically significant antinociception was not obtained (Fig. 3, upper panels, squares; Supplementary Table 7). Clonidine was four- and threefold more potent than lofexidine to produce the rate-decreasing and hypothermic effects, respectively (Table 2). The potency of clonidine to produce the rate-decreasing effects was fourfold more potent than that for the hypothermic effects (Table 2).
MG and 7-OH-MG Alone
When administered intraperitoneally, MG significantly decreased response rates; however, neither statistically significant antinociception nor altered rectal temperature was obtained (Fig. 3, lower panels, circles; Supplementary Table 8). MG (intraperitoneally) was fourfold more potent than intraperitoneal morphine to produce the rate-decreasing effects (Table 2). MG had been expected to produce antinociceptive and hypothermic effects because other effects produced by MG are antagonized by MOR and A2R antagonists (Foss et al., 2020; Obeng, Wilkerson et al., 2021). Thus, the route of administration of MG was varied, and the effects of 7-OH-MG, an active metabolite of MG at the MOR, were assessed.
Both oral and subcutaneous MG significantly decreased rates of responding, and no significant antinociception was observed; there were relatively small yet significant increases in rectal temperature (Fig. 3, lower panels, downward and upward triangles, respectively; Supplementary Table 8). MG administered orally by gavage and subcutaneously was three- and sixfold less potent, respectively, than intraperitoneal MG to produce the rate-decreasing effects (Table 2).
In contrast to MG, intraperitoneal 7-OH-MG significantly decreased response rates and produced hot plate antinociception; however, no significant effects on rectal temperature were obtained (Fig. 3, lower panels, squares; Supplementary Table 8). The potency of 7-OH-MG to reduce response rates was approximately fourfold more potent than its potency to produce antinociception (Table 2).
Reference MOR Agonists in Combination with Naltrexone or Yohimbine
By themselves, naltrexone (0.032, 1 mg/kg i.p.) and yohimbine (1, 3.2 mg/kg i.p.), did not alter food-maintained behavior, antinociception, or rectal temperature (Supplementary Fig. 3; Supplementary Table 9). Naltrexone dose-dependently and significantly shifted to the right the dose-effect functions of the rate-decreasing and antinociceptive effects of morphine (Fig. 4; Table 2; Supplementary Table 4). The lower dose of naltrexone (0.032 mg/kg) produced significant antagonism of the rate-decreasing and antinociceptive effects of morphine (Table 2). Yohimbine (3.2 mg/kg) did not significantly change the effects of morphine on rates of responding, antinociception, or changes in rectal temperature (Fig. 4; Table 2; Supplementary Table 4).
Fig. 4.
The rate-decreasing, antinociceptive, and hypothermic effects of reference agonists in the presence of naltrexone (NLT; opioid receptor antagonist) or yohimbine (YHM; Aα2R antagonist). Abscissae: Vehicle and cumulative dose of reference agonist in mg/kg (intraperitoneal, log scale). Ordinates: Top row, percentage of mean rates of responding after repeated administration of vehicle during intertest sessions; middle row, percentage of maximum possible effects in the hotplate assay; bottom row, changes in rectal temperature from mean baselines. Each point represents the mean ± S.E.M. (n = 4 per sex per data point). Naltrexone and yohimbine were administered intraperitoneally immediately before each session, and all reference agonists were administered intraperitoneally 15 minutes before each 5-minute period for data collection for food-maintained behavior. Each data of compound alone (i.e., “None” in each figure key) was replotted from Figure 3. Leftmost panels: The effects of morphine. Morphine dose alone (filled circles) and in the presence of 3.2 mg/kg yohimbine (open squares); vehicle, 5.6, 10, 17.8, 32, and 56 mg/kg. Morphine dose in the presence of 0.032 mg/kg naltrexone (open upward triangles); vehicle, 17.8, 32, 56, 100, and 178 mg/kg. Morphine dose in the presence of 1.0 mg/kg naltrexone (open downward triangles); vehicle, 56, 100, 178, 320, and 560 mg/kg. Second leftmost panels: The effects of methadone. Methadone dose alone (filled circles) and in the presence of 3.2 mg/kg yohimbine (open squares); vehicle, 0.32, 0.56, 1.0, 1.78, and 3.2 mg/kg. Methadone dose in the presence of 1.0 mg/kg naltrexone (open downward triangles); vehicle, 1.0, 1.78, 3.2, 5.6, and 10 mg/kg. Third leftmost panels: The effects of U69,593. U69,593 dose alone (filled circles) and in the presence of 0.032 mg/kg naltrexone (open upward triangles) or 3.2 mg/kg yohimbine (open squares); vehicle, 0.56, 1.0, 1.78, 3.2, and 5.6 mg/kg. U69,593 dose in the presence of 1.0 mg/kg naltrexone (open downward triangles); vehicle, 1.78, 3.2, 5.6, 10, and 17.8 mg/kg. Fourth leftmost panels: The effects of lofexidine. Lofexidine dose alone (filled circles) and in the presence of 1.0 mg/kg naltrexone (open downward triangles); vehicle, 0.056, 0.1, 0.178, 0.32, and 0.56 mg/kg. Lofexidine dose in the presence of 1.0 mg/kg yohimbine (diamonds); vehicle, and 0.178, 0.32, 0.56, 1.0, and 1.78 mg/kg. Lofexidine dose in the presence of 3.2 mg/kg yohimbine (open squares); vehicle, 0.56, 1.0, 1.78, 3.2, and 5.6 mg/kg. Rightmost panels: The effects of clonidine. Clonidine alone and in the presence of 1.0 mg/kg naltrexone (open downward triangles); vehicle, 0.0178, 0.032, 0.056, 0.1, and 0.178 mg/kg. Clonidine dose in the presence of 3.2 mg/kg yohimbine (open squares); vehicle, 0.056, 0.1, 0.178, 0.32, and 0.56 mg/kg. Each gray symbol indicates a significant difference from vehicle per corresponding cycle as shown in Fig. 3. Note that the lower dose of naltrexone antagonized the rate-decreasing and antinociceptive effects of the reference MOR agonists. The higher dose of naltrexone antagonized the rate-decreasing and antinociceptive effects of morphine and U69,593. The lower dose of yohimbine antagonized the rate-decreasing and hypothermic effects of the reference Aα2R agonists.
Naltrexone (0.032 mg/kg) produced a fivefold rightward shift of the methadone rate-decreasing dose-effect function (Fig. 4; Table 2; Supplementary Table 4). Yohimbine (3.2 mg/kg) did not significantly modify the effects of methadone on rates of responding, antinociception, or changes in rectal temperature (Fig. 4; Table 2; Supplementary Table 4).
U69,593 in Combination with Naltrexone or Yohimbine
Naltrexone (0.032 mg/kg) produced a small but statistically significant leftward shift of the U69,593 rate-decreasing dose-effect function but did not modify U69,593 antinociceptive or hypothermic effects (Fig. 4; Table 2; Supplementary Table 6). Naltrexone (1.0 mg/kg) significantly antagonized the rate-decreasing, antinociceptive, and hypothermic effects of U69,593 (Fig. 4; Table 2; Supplementary Table 6). Naltrexone produced a fie- and threefold, respectively, rightward shift of the U69,593 rate-decreasing and antinociceptive dose-effect function (Table 2). Yohimbine (3.2 mg/kg) did not modify U69,593-related rates of responding, antinociception, or rectal temperature (Fig. 4; Table 2; Supplementary Table 6).
Reference Aα2R Agonists in Combination with Naltrexone or Yohimbine
Naltrexone did not modify the effects of lofexidine on rates of responding, hot plate antinociception, or rectal temperature (Fig. 4; Table 2; Supplementary Table 7). Yohimbine dose-dependently and significantly shifted to the right the dose-effect functions of the rate-decreasing and hypothermic effects of lofexidine (Fig. 4; Table 2; Supplementary Table 7). The lower dose of yohimbine (1.0 mg/kg) produced a fourfold shift to the right of the lofexidine dose-effect functions to decrease response rates and rectal temperature (Supplementary Table 7).
Naltrexone did not modify the effects of clonidine on rates of responding, antinociception, or rectal temperature (Fig. 4; Table 2; Supplementary Table 7). Yohimbine (1.0 mg/kg) produced an eight- and fourfold, respectively, rightward shift of the clonidine rate-decreasing and hypothermic dose-effect function (Fig. 4; Table 2; Supplementary Table 7).
MG (Intraperitoneal) and 7-OH-MG in Combination with Naltrexone or Yohimbine
Because the intraperitoneal route was most potent of the three routes of administration tested in decreasing the response rates, the intraperitoneal route was used to assess the pharmacological impact of naltrexone (1.0 mg/kg) or yohimbine (3.2 mg/kg) on MG-related behaviors and physiology. Neither naltrexone nor yohimbine significantly modified the dose-effect function of MG to decrease responding (Fig. 5; Table 2; Supplementary Table 8). Naltrexone (0.032 mg/kg) significantly shifted the dose-effect functions of 7-OH-MG threefold rightward for both rate-decreasing and antinociceptive effects (Fig. 5; Table 2; Supplementary Table 8). In contrast, yohimbine (3.2 mg/kg) did not significantly modify the rate-decreasing or antinociceptive 7-OH-MG dose-effect functions (Fig. 5, Table 2; Supplementary Table 8).
Fig. 5.
The rate-decreasing, antinociceptive, and hypothermic effects of MG and 7-OH-MG in the presence of naltrexone (NLT: opioid receptor antagonist) or yohimbine (YHM; Aα2R antagonist). Abscissae: Vehicle and cumulative dose of test compound in mg/kg (intraperitoneal, log scale). Ordinates: Top row, percentage of mean rates of responding after repeated administration of vehicle during intertest sessions; middle row, percentage of maximum possible effects in the hotplate assay; bottom row, changes in rectal temperature from mean baselines. Each point represents the mean ± S.E.M. (n = 4 per sex per data point). Naltrexone and yohimbine were administered intraperitoneally immediately before each session, and all other compounds were administered intraperitoneally 15 minutes before each 5-minute period for data collection for food-maintained behavior. Each data of test compound alone (i.e., “None” in each figure key) was replotted from Fig. 3. Left panels: The effects of MG. MG dose alone (filled circles) and in the presence of 1.0 mg/kg naltrexone (open downward triangles) or 3.2 mg/kg yohimbine (open squares); vehicle, 5.6, 10, 17.8, 32, and 56 mg/kg. Right panels: The effects of 7-OH-MG. 7-OH-MG dose alone (filled circles) and in the presence of 3.2 mg/kg yohimbine (open squares); vehicle, 0.32, 1.0, 3.2, 10, and 32 mg/kg. 7-OH-MG dose in the presence of .032 mg/kg naltrexone (open upward triangles); vehicle, 1.0, 3.2, 10, 32, and 56 mg/kg. Each gray symbol indicates a significant difference from vehicle per corresponding cycle as shown in Fig. 3. Note that each high dose of naltrexone and yohimbine did not significantly antagonize the rate-decreasing effects of MG. The lower dose of naltrexone antagonized the rate-decreasing and antinociceptive effects of 7-OH-MG.
Reference Agonists in Combination with MG or 7-OH-MG
By themselves, MG (17.8 mg/kg i.p.) and 7-OH-MG (0.32 mg/kg i.p.) did not alter food-maintained behavior, antinociception, or rectal temperature (Supplementary Fig. 3; Supplementary Table 9). Pretreatment effects of behaviorally inactive doses of MG (17.8 mg/kg) or 7-OH-MG (0.32 mg/kg) were assessed on the effects of previously tested reference agonists to understand the interaction of MG or its metabolite with the reference agonists (Fig. 6). Neither MG nor 7-OH-MG significantly modified the rate-decreasing and antinociceptive dose-effect functions of morphine and methadone (Fig. 6; Table 2; Supplementary Table 4).
Fig. 6.
The rate-decreasing, antinociceptive, and hypothermic effects of reference agonists in the presence of MG and 7-OH-MG. Abscissae: Vehicle and cumulative dose of reference agonist in mg/kg (intraperitoneal, log scale). Ordinates: Top row, percentage of mean rates of responding after repeated administration of vehicle during intertest sessions; middle row, percentage of maximum possible effects in the hotplate assay; bottom row, changes in rectal temperature from mean baselines. Each point represents the mean ± S.E.M. (n = 4 per sex per data point). MG and 7-OH-MG were administered intraperitoneally immediately before each session, and all reference agonists were administered intraperitoneally 15 minutes before each 5-minute period for data collection for food-maintained behavior. Each data of reference agonists alone (i.e., “None” in each figure key) was replotted from Fig. 3. Leftmost panels: The effects of morphine. Morphine dose alone (filled circles) and in the presence of 17.8 mg/kg MG (open squares) or 0.32 mg/kg 7-OH-MG (open diamonds); vehicle, 5.6, 10, 17.8, 32, and 56 mg/kg. Second leftmost panels: The effects of methadone. Methadone dose alone (filled circles) and in the presence of 17.8 mg/kg MG (open squares) or 0.32 mg/kg 7-OH-MG (open diamonds); vehicle, 0.32, 0.56, 1.0, 1.78, and 3.2 mg/kg. Third leftmost panels: The effects of U69,593. U69,593 dose alone (filled circles) and in the presence of 17.8 mg/kg MG (open squares) or 0.32 mg/kg 7-OH-MG (open diamonds); vehicle, 0.56, 1.0, 1.78, 3.2, and 5.6 mg/kg. Fourth leftmost panels: The effects of lofexidine. Lofexidine dose alone (filled circles) and in the presence of 0.32 mg/kg 7-OH-MG (open diamonds); vehicle, 0.056, 0.1, 0.178, 0.32, and 0.56 mg/kg. Lofexidine dose in the presence of 17.8 mg/kg MG (open squares); vehicle, 0.0178, 0.032, 0.056, 0.1, and 0.178 mg/kg. Rightmost panels: The effects of clonidine. Clonidine alone and in the presence of 0.32 mg/kg 7-OH-MG (open diamonds); vehicle, 0.0178, 0.032, 0.056, 0.1, and 0.178 mg/kg. Clonidine dose in the presence of 17.8 mg/kg MG (open squares); vehicle, 0.0056, 0.01, 0.0178, 0.032, and 0.056 mg/kg. Each gray symbol indicates a significant difference from vehicle per corresponding cycle as shown in Fig. 3. Note that MG potentiated the rate-decreasing and hypothermic effects of the reference Aα2R agonists. In the presence of MG and 7-OH-MG, the reference Aα2R agonists also produced relatively robust antinociception.
MG pretreatment did not significantly modify the rate-decreasing, antinociceptive and hypothermic dose-effect functions of U69,593 (Fig. 6; Table 2; Supplementary Table 6). 7-OH-MG did not significantly alter the dose-effect functions of rates of responding or rectal temperature for U69,593 whereas 7-OH-MG produced a significant fourfold rightward shift in the U69,593 hotplate antinociception dose-effect function (Fig. 6; Table 2; Supplementary Table 6).
MG produced a leftward shift in both lofexidine and clonidine rate-decreasing and hypothermic effect dose-effect functions (Fig. 6; Table 2; Supplementary Table 7). When combined with MG, lofexidine and clonidine produced significantly greater hotplate antinociception than either lofexidine alone or clonidine alone (Fig. 6; Table 2; Supplementary Table 7). The mean hotplate antinociceptive values, expressed as %MPE, of lofexidine alone and clonidine alone were <20% (Fig. 6). As with MG, 7-OH-MG shifted to the left the dose-effect functions of the rate-decreasing effects of lofexidine and clonidine and rendered lofexidine and clonidine antinociceptive (Fig. 6; Table 2; Supplementary Table 7). However, in contrast to MG, 7-OH-MG did not significantly modify either lofexidine or clonidine hypothermic dose-effect functions (Fig. 6; Table 2; Supplementary Table 7).
Combinations of the Reference Agonists
Among food-maintained behavior, hotplate response latency, and rectal temperature, only analyses of food-maintained behavior were used to determine the ED50 values of all the reference agonists at MOR, KOR, and Aα2R (Table 3). Based on the calculated rate decreasing ED50 values of each reference compound alone, doses for the mixtures in ED50 ratios of 3:1, 1:1, and 1:2 parts morphine to lofexidine were administered cumulatively in quarter log units (Table 3). Each drug combination produced dose-related decreases in response rates (Supplementary Fig. 4; Supplementary Table 10). Hotplate antinociception and hypothermia were also assessed. All morphine dose ratios produced similar leftward antinociceptive morphine dose-effect function shifts. As the morphine dose ratio increased (i.e., 1:2, 1:1, 3:1 morphine to lofexidine) the hypothermia dose-effect functions shifted further to the left (Supplementary Fig. 4; Supplementary Table 10). As the lofexidine dose ratio decreased (i.e., 1:2, 1:1, 3:1 morphine to lofexidine) the antinociception dose-effect functions shifted further to the left (Supplementary Fig. 4; Supplementary Table 10). All lofexidine dose ratios produced similar leftward lofexidine hyperthermic dose-effect function shifts.
TABLE 3.
Cumulative doses of test compounds (mg/kg) studied in compound mixtures
Values in parentheses are S.E.M.
| Compound | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 |
|---|---|---|---|---|---|---|
| 1 Morphine: 1 Lofexidine | ||||||
| Morphine | Vehicle | 1.79 (0.447) | 3.19 (0.795) | 5.69 (1.42) | 10.1 (2.52) | 18.0 (4.48) |
| Lofexidine | Vehicle | 0.0196 (0.00433) | 0.0348 (0.00771) | 0.0620 (0.0137) | 0.110 (0.0244) | 0.196 (0.0435) |
| 1 Morphine: 2 Lofexidine | ||||||
| Morphine | Vehicle | 0.897 (0.223) | 1.60 (0.398) | 2.84 (0.708) | 5.06 (1.26) | 9.01 (2.24) |
| Lofexidine | Vehicle | 0.0293 (0.00650) | 0.0522 (0.0116) | 0.0930 (0.0206) | 0.165 (0.0367) | 0.295 (0.0652) |
| 3 Morphine: 1 Lofexidine | ||||||
| Morphine | Vehicle | 2.69 (0.670) | 4.79 (1.19) | 8.53 (2.12) | 15.2 (3.78) | 27.0 (6.73) |
| Lofexidine | Vehicle | 0.00978 (0.00217) | 0.0174 (0.00386) | 0.0310 (0.00686) | 0.0552 (0.0122) | 0.0982 (0.0217) |
| 2 Morphine: 1 Clonidine | ||||||
| Morphine | Vehicle | 1.79 (0.447) | 3.19 (0.795) | 5.69 (1.42) | 10.1 (2.52) | 18.0 (4.48) |
| Clonidine | Vehicle | 0.00379 (0.000994) | 0.00675 (0.00177) | 0.0120 (0.00315) | 0.0214 (0.00561) | 0.0381 (0.00998) |
| 1 Morphine: 2 Clonidine | ||||||
| Morphine | Vehicle | 0.897 (0.223) | 1.60 (0.398) | 2.84 (0.708) | 5.06 (1.26) | 9.01 (2.24) |
| Clonidine | Vehicle | 0.00569 (0.00149) | 0.0101 (0.00266) | 0.0180 (0.00473) | 0.0321 (0.00841) | 0.0571 (0.0150) |
| 3 Morphine: 1 Clonidine | ||||||
| Morphine | Vehicle | 2.69 (0.670) | 4.79 (1.19) | 8.53 (2.12) | 15.2 (3.78) | 27.0 (6.73) |
| Clonidine | Vehicle | 0.00190 (0.000497) | 0.00338 (0.000885) | 0.00601 (0.00158) | 0.0107 (0.00280) | 0.0190 (0.00499) |
| 1 Methadone: 1 Lofexidine | ||||||
| Methadone | Vehicle | 0.144 (0.0429) | 0.257 (0.0764) | 0.457 (0.136) | 0.813 (0.242) | 1.45 (0.431) |
| Lofexidine | Vehicle | 0.0196 (0.00433) | 0.0348 (0.00771) | 0.0620 (0.0137) | 0.110 (0.0244) | 0.196 (0.0435) |
| 1 Methadone: 2 Lofexidine | ||||||
| Methadone | Vehicle | 0.0721 (0.0215) | 0.128 (0.0382) | 0.228 (0.0680) | 0.407 (0.121) | 0.724 (0.215) |
| Lofexidine | Vehicle | 0.0293 (0.00650) | 0.0522 (0.0166) | 0.0930 (0.0206) | 0.165 (0.0367) | 0.295 (0.0652) |
| 3 Methadone: 1 Lofexidine | ||||||
| Methadone | Vehicle | 0.216 (0.0644) | 0.385 (0.115) | 0.685 (0.204) | 1.22 (0.363) | 2.17 (0.646) |
| Lofexidine | Vehicle | 0.00978 (0.00217) | 0.0174 (0.00386) | 0.0310 (0.00686) | 0.0552 (0.0122) | 0.0982 (0.0217) |
| 2 Methadone: 1 Clonidine | ||||||
| Methadone | Vehicle | 0.144 (0.0429) | 0.257 (0.0764) | 0.457 (0.136) | 0.813 (0.242) | 1.45 (0.431) |
| Clonidine | Vehicle | 0.00379 (0.000994) | 0.00675 (0.00177) | 0.0120 (0.00315) | 0.0214 (0.00561) | 0.0381 (0.00998) |
| 1 Methadone: 1 Clonidine | ||||||
| Methadone | Vehicle | 0.0721 (0.0215) | 0.128 (0.0382) | 0.228 (0.0680) | 0.406 (0.121) | 0.724 (0.215) |
| Clonidine | Vehicle | 0.00569 (0.00149) | 0.0101 (0.00266) | 0.0180 (0.00473) | 0.0321 (0.00841) | 0.0571 (0.0150) |
| 4 Methadone: 1 Clonidine | ||||||
| Methadone | Vehicle | 0.216 (0.0644) | 0.385 (0.115) | 0.685 (0.204) | 1.22 (0.363) | 2.17 (0.646) |
| Clonidine | Vehicle | 0.00190 (0.000467) | 0.00338 (0.000885) | 0.00601 (0.00158) | 0.0107 (0.00280) | 0.0190 (0.00499) |
| 1 U69,593: 1 Lofexidine | ||||||
| U69,593 | Vehicle | 0.346 (0.0471) | 0.616 (0.0839) | 1.10 (0.149) | 1.95 (0.266) | 3.47 (0.473) |
| Lofexidine | Vehicle | 0.0177 (0.00346) | 0.0316 (0.00616) | 0.0562 (0.0110) | 0.100 (0.0195) | 0.178 (0.0348) |
| 1 U69,593: 2 Lofexidine | ||||||
| U69,593 | Vehicle | 0.173 (0.0236) | 0.308 (0.0420) | 0.548 (0.0747) | 0.975 (0.133) | 1.74 (0.237) |
| Lofexidine | Vehicle | 0.0339 (0.00757) | 0.0603 (0.135) | 0.107 (0.0240) | 0.191 (0.0427) | 0.340 (0.760) |
| 2 U69,593: 1 Lofexidine | ||||||
| U69,593 | Vehicle | 0.519 (0.0707) | 0.923 (0.126) | 1.64 (0.224) | 2.93 (0.399) | 5.21 (0.710) |
| Lofexidine | Vehicle | 0.0113 (0.00252) | 0.0201 (0.00449) | 0.0358 (0.00800) | 0.0637 (0.0142) | 0.113 (0.0253) |
| 2 U69,593: 1 Clonidine | ||||||
| U69,593 | Vehicle | 0.346 (0.0471) | 0.616 (0.0839) | 1.10 (0.149) | 1.95 (0.266) | 3.47 (0.473) |
| Clonidine | Vehicle | 0.00382 (0.000956) | 0.00680 (0.00170) | 0.0121 (0.00303) | 0.0215 (0.00539) | 0.0384 (0.00960) |
| 1 U69,593: 2 Clonidine | ||||||
| U69,593 | Vehicle | 0.173 (0.0236) | 0.308 (0.0420) | 0.548 (0.0747) | 0.975 (0.133) | 1.74 (0.237) |
| Clonidine | Vehicle | 0.00761 (0.00215) | 0.0135 (0.00383) | 0.0241 (0.00681) | 0.0429 (0.0121) | 0.0764 (0.0216) |
| 3 U69,593: 1 Clonidine | ||||||
| U69,593 | Vehicle | 0.519 (0.0707) | 0.923 (0.126) | 1.64 (0.224) | 2.93 (0.399) | 5.21 (0.710) |
| Clonidine | Vehicle | 0.00254 (0.000717) | 0.00451 (0.00128) | 0.00803 (0.00227) | 0.0143 (0.00404) | 0.0255 (0.00720) |
We also examined, based upon the ED50 doses to decrease response rates, 2:1, 1:2, and 3:1 morphine to clonidine dose mixtures. Each drug combination produced dose-related decreases in response rates. We found similar shifts as seen with morphine and lofexidine, in the morphine and clonidine antinociceptive and hypothermia dose-effect relationships (Supplementary Fig. 5; Supplementary Table 10). A similar trend for inverse opioid and adrenergic receptor agonist antinociceptive and hypothermic dose-effect function shifts, based on the relative opioid to adrenergic receptor agonist dose ratio were also consistently observed with 1:2, 1:1, and 3:1 methadone to lofexidine (Supplementary Fig. 6; Supplementary Table 10); 4:1, 2:1, and 1:1 methadone to clonidine (Supplementary Fig. 7; Supplementary Table 10); 1:2, 1:1, and 2:1 U69,593 to lofexidine (Supplementary Fig. 8; Supplementary Table 10); and 1:2, 2:1, and 3:1 U69,593 to clonidine (Supplementary Fig. 9; Supplementary Table 10) ED50 ratios.
Interactive Effects of Reference Compounds
Subadditivity for drug combination rate decreasing effects was not observed in any of the previously discussed morphine to lofexidine, morphine to clonidine, methadone to lofexidine, methadone to clonidine, U69,593 to lofexidine, or U69,593 to clonidine drug combinations (Fig. 7; Table 4). Additive effects were generally observed, with a few exceptions where supra-additivity was found. Supra-additivity was observed under the following dose ratios 1:1 and 1:2 morphine to lofexidine; 2:1 and 1:2 morphine to clonidine; 2:1 methadone to clonidine; 1:1, 1:2, and 2:1 U69,593 to lofexidine; and 2:1 and 1:2 U69,593 to clonidine (Fig. 7; Table 4).
Fig. 7.
Isobolographic analysis of reference Aα2R agonists combined with MOR or KOR reference agonists. Ordinates, ED50 values of morphine (left panels), methadone (middle panels), and U69,593 (right panels) in mg/kg. Abscissae, ED50 values of lofexidine (upper panels) and clonidine (lower panels) in mg/kg. Each point represents the ED50 value and error bars represent 95% CIs. The points at which the line of additivity crosses the ordinates and abscissae represent the ED50 values of each compound alone. The line of additivity (dashed line) represents combinations of doses that would be predicted to produce a 50% effect if the compounds were strictly dose-additive. The vertical and horizontal lines around each data point represent the 95% CIs. *Indicates at least P < 0.05 difference between Zmix and Zadd for a respective dose combination, denoting supra-additivity.
TABLE 4.
Theoretical Zadd (mg/kg), experimental Zmix (mg/kg), with CIs, and observed interactive effects of studied compound mixtures
| Combination | Zadd | Zmix | Interactive Effect |
| 1 Morphine: 1 Lofexidine | 9.13 (6.55–11.71) | 2.88 (1.94–3.81) | Supra-additive |
| 1 Morphine: 2 Lofexidine | 9.00 (6.45–11.54) | 1.42 (0.932–1.91) | Supra-additive |
| 3 Morphine: 1 Lofexidine | 9.22 (3.14–15.31) | 4.00 (2.23–6.23) | Additive |
| 2 Morphine: 1 Clonidine | 9.26 (6.64–11.84) | 2.90 (1.15–4.66) | Supra-additive |
| 1 Morphine: 2 Clonidine | 9.24 (6.63–11.86) | 1.57 (1.06–2.08) | Supra-additive |
| 3 Morphine: 1 Clonidine | 9.26 (6.65–11.88) | 5.73 (3.50–7.95) | Additive |
| 1 Methadone: 1 Lofexidine | 0.604 (0.541–0.668) | 0.514 (0.084–0.944) | Additive |
| 1 Methadone: 2 Lofexidine | 0.540 (0.159–0.922) | 0.237 (0.170–0.304) | Additive |
| 3 Methadone: 1 Lofexidine | 0.660 (0.279–1.04) | 0.767 (0.488–1.05) | Additive |
| 2 Methadone: 1 Clonidine | 0.683 (0.608–0.757) | 0.280 (0.137–0.424) | Supra-additive |
| 1 Methadone: 1 Clonidine | 0.672 (0.415–0.930) | 0.183 (0.0261–0.340) | Supra-additive |
| 4 Methadone: 1 Clonidine | 0.688 (0.590–0.786) | 0.680 (0.224–1.14) | Additive |
| 1 U69,593: 1 Lofexidine | 2.033 (1.71–2.35) | 1.01 (0.907–1.108) | Supra-additive |
| 1 U69,593: 2 Lofexidine | 1.91 (1.25–2.57) | 0.484 (0.395–0.573) | Supra-additive |
| 2 U69,593: 1 Lofexidine | 2.102 (1.73–2.47) | 1.23 (0.903–1.56) | Supra-additive |
| 2 U69,593: 1 Clonidine | 2.16 (1.82–2.50) | 0.735 (0.189–1.28) | Supra-additive |
| 1 U69,593: 2 Clonidine | 2.12 (1.73–2.51) | 0.567 (0.462–0.672) | Supra-additive |
| 3 U69,593: 1 Clonidine | 2.17 (1.82–2.51) | 1.63 (1.38–1.87) | Additive |
Discussion
In this study, we observed several novel findings. MG had comparable binding affinities at Aα2R and MOR whereas 7-OH-MG, an active metabolite of MG, had relatively high affinity at MOR and negligible affinity at Aα2R. Among three experimental assays employed in this study, we examined drug–drug schedule-controlled responding interactions via isobolar analysis. MG and 7-OH-MG potentiated the rate-decreasing effects of Aα2R agonists, but not MOR agonists, and increased the potency of Aα2R agonists to produce antinociception. MG, but not 7-OH-MG, potentiated the hypothermic effects of the Aα2R agonists. Neither naltrexone nor yohimbine antagonized the rate-decreasing effects of MG, whereas naltrexone, but not yohimbine, antagonized the rate-deceasing effects of 7-OH-MG. Thus, these isobolar analyses suggest that to produce the opioid-sparing effects of Aα2R agonists a specific dose combination is required. In addition, these results suggest that MG and 7-OH-MG may produce antinociceptive synergism with both Aα2R and MOR agonists. Furthermore, MG, but not 7-OH-MG, when combined with Aα2R agonists may produce hypothermic synergism.
The supra-additive interactions between MOR and Aα2R on schedule-controlled responding was observed at various dose ratios (i.e., 2:1, 1:1, 1:2), and these interactive effects may be specific to schedule-controlled responding. For example, in several mouse and rat antinociception studies, others have found supra-additive interactions between MOR and Aα2R only when mixtures included low proportions of the MOR agonist relative to an Aα2R agonist based on their individual potencies (Spaulding et al., 1979; Drasner and Fields, 1988; Tajerian et al., 2012; Stone et al., 2014). Additionally, our findings demonstrate that schedule-controlled responding supra-additive interactions at Aα2R were not pharmacologically specific for MOR, as supra-additive interactions with Aα2R agonists were observed with the KOR agonist U69,593. These results highlight the importance of the proportions of MOR agonists in complex drug mixtures on observed behavior. An additional consideration for these studies is that here we only examine schedule-controlled responding drug–drug interactions via isobolar analysis. Although we additionally studied hotplate antinociception and hypothermia in these animals, we are unable to determine whether these observed dose-response function shifts were subadditive, additive, or supra-additive. Additional experiments beyond the scope of the current study would identify antinociceptive and hypothermic drug-drug additivity interactions.
Although not explicitly examined in the present study, supra-additive antinociception resulting from combinations of Aα2R and KOR agonists has been reported (Ossipov, Harris et al., 1990; Roerig, 1995). Specifically, supra-additive antinociception was produced in rats using a tail withdrawal assay when three parts of clonidine and one part of U69,593 were administered intrathecally (Ossipov, Harris et al., 1990). Further, supra-additive antinociception was produced in mice using the tail withdrawal assay when one part of clonidine and one part of the KOR agonist U50-488H were administered intrathecally (Roerig, 1995). When compared with our additive KOR and Aα2R schedule-controlled responding behavioral findings in rats, there are a number of differences across the present and previous studies that may contribute to the observed differences in additive versus supra-additive drug effects (Ossipov, Harris et al., 1990; Roerig, 1995) including assays employed (i.e., antinociception versus schedule-controlled responding), the routes of administration of compounds (i.e., intraperitoneally vs. intrathecally), and drug history (i.e., a complex drug history vs. naive). These differences may individually and combined yield different receptor densities and receptor pools that mediate the underlying observed behavioral results.
The affinities of MG at both MOR and Aα2R were approximately equal whereas the affinity of 7-OH-MG was high at the MOR (77.9 nM) and negligible at the Aα2R. In our studies, MG failed to mimic the antinociceptive effects of MOR agonists or the hypothermic effects of Aα2R agonists. These findings are in contrast to previously reported results that demonstrated that MG produced antinociceptive effects in C57BL/6J mice (Chakraborty et al., 2021). Additionally, neither naltrexone nor yohimbine antagonized MG-induced decreases in food-maintained behavior. Under the same experimental conditions, naltrexone antagonized the effects of MOR agonists, and yohimbine antagonized the effects of Aα2R agonists. In contrast to MG, 7-OH-MG mimicked the effects of morphine and methadone. Superficially, these MG results suggest no contribution of the MOR or Aα2R to the pharmacological effects of MG in rats. However, as the discriminative-stimulus effects of MG in rats were antagonized by naltrexone, our current results do not broadly apply to all in vivo pharmacological assessments (Obeng, Wilkerson et al., 2021). Additionally, in a neuropathic pain model, the antiallodynic effects of MG in rats were antagonized by yohimbine (Foss et al., 2020). The inability of naltrexone to antagonize the rate-decreasing effects of MG has previously been reported (Hiranita et al., 2019; Obeng, Wilkerson et al., 2021). Naltrexone was 3.2-fold less potent in antagonizing the rate-decreasing effects of morphine than in antagonizing the discriminative-stimulus effects of morphine in rats (Obeng, Wilkerson et al., 2021). Thus, the sensitivity to the pharmacological activity of interest differs across experimental assays employed.
Both MG and 7-OH-MG potentiated the rate-decreasing effects of lofexidine and clonidine, but not those of morphine and methadone, and increased the maximum antinociceptive effects of the Aα2R agonists. However, MG, but not 7-OH-MG, potentiated the hypothermic effects of the reference Aα2R agonists. The MG-induced potentiation of the hypothermic and antinociceptive effects of the reference Aα2R agonists might suggest positive allosteric effects of MG at the Aα2R; however, there is currently no such published report or supportive evidence. Nonetheless, there are clinical implications in that MG can be used to enhance the clinical effects of Aα2R agonists such as pain relief as well as the ability to block the acute withdrawal symptoms in chronic opioid users. Additionally, the in vivo “apparent” positive allosteric effects of MG at the Aα2R might indicate a challenging hypothesis that MG could mitigate opioid withdrawal (Wilson et al., 2020; Wilson et al., 2021) primarily due to allosteric agonism at the Aα2R rather than dual agonism at the MOR and Aα2R (Chakraborty et al., 2021). It is worth noting that MG is metabolized by CYP3A4 to 7-OH-MG (Kamble et al., 2020; Basiliere and Kerrigan, 2020; Chakraborty et al., 2021). It was recently reported that metabolic conversion of 7-OH-MG does not contribute to MG pharmacological activity (Berthold et al., 2022). However, other studies showed that 7-OH MG does contribute to the analgesic and respiratory depressive effects of MG, albeit its contribution was found to be limited by metabolic saturation (Kruegel et al., 2019; Chakraborty et al., 2021; Hill et al., 2022). Berthold et al. (2022) demonstrated that in mice treated with MG doses, which produced significant hotplate antinociception, 7-OH-MG brain levels remained significantly below the observed 7-OH-MG brain levels found in 7-OH-MG treated mice that were dosed sufficiently to produce acute antinociception. In this study, the pharmacological activity of 7-OH-MG was quite different from that of MG, which contradicts the hypothesis that 7-OH-MG is responsible for the “apparent” antinociceptive effects of MG in mice (Kruegel et al., 2019). The inconsistency between the present and previous (Kruegel et al., 2019) studies might simply be due to a difference in species (i.e., rat vs. mouse, respectively).
To assess the therapeutic utility of these kratom alkaloids, future studies should examine the subadditive and additive versus supra-additive effects of MG, 7-OH-MG, and MOR as well as Aα2R agonists in relevant pathologic pain and drug dependence models. In conclusion, supra-additive interaction between agonism at the MOR and Aα2R depend on the dose combination ratio and MOR agonist used. Affinity of MG at these receptors was approximately equal whereas no considerable affinity of 7-OH-MG was found at the Aα2R.
Acknowledgments
The authors would like to thank Ms. Danielle M. Sevier and Samantha N. Hart at the College of Pharmacy, University of Florida, for administrative assistance.
Abbreviations
- Aα2R
adrenergic-α2 receptor
- Aα2CR
adrenergic-α2C receptor
- CI
confidence interval
- DOR
δ-opioid receptor
- FR
fixed ratio
- i.p.
intraperitoneally
- Ki
inhibition constant
- KOR
κ-opioid receptor
- LED
light-emitting diode
- MG
mitragynine
- MOR
µ-opioid receptor
- %MPE
percent maximum possible antinociceptive effect
- 7-OH-MG
7-hydroxymitagynine
- p.o.
orally by gavage
- U69, 593
(+)-(5α, 7α, 8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide
Authorship Contributions
Participated in research design: Obeng, McMahon, Hiranita.
Conducted experiments: Obeng, Patel, Restrepo, Gamez-Jimenez, Ho, Guerrero Calvache, Pallares, Helmes.
Contributed new reagents or analytic tools: Leon, McCurdy.
Performed data analysis: Obeng, Zuarth Gonzalez, Chaves Da Silva, Restrepo, Guerrero Calvache, Shiomitsu, Soto, Wilkerson, Hiranita.
Wrote or contributed to the writing of the manuscript: Obeng, Leon, Zuarth Gonzalez, Chaves Da Silva, Shiomitsu, Soto, McCurdy, McMahon, Wilkerson, Hiranita.
Footnotes
The present study was supported by National Institutes of Health National Institute on Drug Abuse [Grants R01-DA025267 and UG3-DA048353-01] (to C.R.M. and L.R.M.), University of Florida Foundation and University of Florida Department of Pharmacodynamics Funding.
No author has an actual or perceived conflict of interest with the contents of this article.
Current affiliation: Department of Pharmacology, Joe R. and Teresa Lozano Long School of Medicine, University of Texas Health San Antonio, San Antonio, Texas.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Arnsten AF, Li B-M (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384. [DOI] [PubMed] [Google Scholar]
- Basiliere S, Kerrigan S (2020) CYP450-mediated metabolism of mitragynine and investigation of metabolites in human urine. J Anal Toxicol 44:301–313. [DOI] [PubMed] [Google Scholar]
- Berthold ECKamble SHRaju KSKuntz MASenetra ASMottinelli MLeón FRestrepo LFPatel AHo NP, et al. (2022) The lack of contribution of 7-hydroxymitragynine to the antinociceptive effects of mitragynine in mice: a pharmacokinetic and pharmacodynamic study. Drug Metab Dispos 50:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxwalla M, Matwyshyn G, Puppala BL, Andurkar SV, Gulati A (2010) Involvement of imidazoline and opioid receptors in the enhancement of clonidine-induced analgesia by sulfisoxazole. Can J Physiol Pharmacol 88:541–552. [DOI] [PubMed] [Google Scholar]
- Brede M, Philipp M, Knaus A, Muthig V, Hein L (2004) α2-adrenergic receptor subtypes - novel functions uncovered in gene-targeted mouse models. Biol Cell 96:343–348. [DOI] [PubMed] [Google Scholar]
- Chakraborty SUprety RSlocum STIrie TLe Rouzic VLi XWilson LLScouller BAlder AFKruegel AC, et al. (2021) Oxidative metabolism as a modulator of kratom’s biological actions. J Med Chem 64:16553–16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- Clemow DB, Walker DJ (2014) The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med 126:64–81. [DOI] [PubMed] [Google Scholar]
- Crassous P-A, Denis C, Paris H, Sénard JM (2007) Interest of α2-adrenergic agonists and antagonists in clinical practice: background, facts and perspectives. Curr Top Med Chem 7:187–194. [DOI] [PubMed] [Google Scholar]
- Drasner K, Fields HL (1988) Synergy between the antinociceptive effects of intrathecal clonidine and systemic morphine in the rat. Pain 32:309–312. [DOI] [PubMed] [Google Scholar]
- Ellis CR, Racz R, Kruhlak NL, Kim MT, Zakharov AV, Southall N, Hawkins EG, Burkhart K, Strauss DG, Stavitskaya L (2020) Evaluating kratom alkaloids using PHASE. PLoS One 15:e0229646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss JD, Nayak SU, Tallarida CS, Farkas DJ, Ward SJ, Rawls SM (2020) Mitragynine, bioactive alkaloid of kratom, reduces chemotherapy-induced neuropathic pain in rats through α-adrenoceptor mechanism. Drug Alcohol Depend 209:107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni MP, Ghelardini C, Vergelli C, Dal Piaz V (2009) α2-agonists as analgesic agents. Med Res Rev 29:339–368. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White JM (2016). Alpha2‐adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev 5:CD002024. [DOI] [PubMed] [Google Scholar]
- Hao S, Takahata O, Iwasaki H (2000) Intrathecal endomorphin-1 produces antinociceptive activities modulated by alpha 2-adrenoceptors in the rat tail flick, tail pressure and formalin tests. Life Sci 66:PL195–PL204. [DOI] [PubMed] [Google Scholar]
- Haq N, McMahan VM, Torres A, Santos G-M, Knight K, Kushel M, Coffin PO (2021) Race, pain, and opioids among patients with chronic pain in a safety-net health system. Drug Alcohol Depend 222:108671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M (2015) Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology (Berl) 232:2227–2238. [DOI] [PubMed] [Google Scholar]
- Hill R, Kruegel AC, Javitch JA, Lane JR, Canals M (2022) The respiratory depressant effects of mitragynine are limited by its conversion to 7-OH mitragynine. Br J Pharmacol 179:3875–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita TLeon FFelix JSRestrepo LFReeves MEPennington AEObeng SAvery BAMcCurdy CRMcMahon LR, et al. (2019) The effects of mitragynine and morphine on schedule-controlled responding and antinociception in rats. Psychopharmacology (Berl) 236:2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble SHLeón FKing TIBerthold ECLopera-Londoño CSiva Rama Raju KHampson AJSharma AAvery BAMcMahon LR, et al. (2020) Metabolism of a kratom alkaloid metabolite in human plasma increases its opioid potency and efficacy. ACS Pharmacol Transl Sci 3:1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel ACUprety RGrinnell SGLangreck CPekarskaya EALe Rouzic VAnsonoff MGassaway MMPintar JEPasternak GW, et al. (2019) 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci 5:992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydecker AG, Sharma A, McCurdy CR, Avery BA, Babu KM, Boyer EW (2016) Suspected adulteration of commercial kratom products with 7-hydroxymitragynine. J Med Toxicol 12:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S, Aimi N, Watanabe H (1996) Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol 317:75–81. [DOI] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL (2021) Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep 70:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meert TF, De Kock M (1994) Potentiation of the analgesic properties of fentanyl-like opioids with alpha 2-adrenoceptor agonists in rats. Anesthesiology 81:677–688. [DOI] [PubMed] [Google Scholar]
- Montgomery LS (2022) Pain management with opioids in adults. J Neurosci Res 100:10–18. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Blaxall HS, Iversen LJ, Bylund DB (1994) Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther 268:1362–1367. [PubMed] [Google Scholar]
- Obeng S, Hiranita T, León F, McMahon LR, McCurdy CR (2021) Novel approaches, drug candidates, and targets in pain drug discovery. J Med Chem 64:6523–6548. [DOI] [PubMed] [Google Scholar]
- Obeng SKamble SHReeves MERestrepo LFPatel ABehnke MChear NJYRamanathan SSharma ALeón F, et al. (2020) Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J Med Chem 63:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng SWilkerson JLLeón FReeves MERestrepo LFGamez-Jimenez LRPatel APennington AETaylor VAHo NP, et al. (2021) Pharmacological comparison of mitragynine and 7-hydroxymitragynine: in vitro affinity and efficacy for mu-opioid receptor and opioid-like behavioral effects in rats. J Pharmacol Exp Ther 376:410–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E (1990) An isobolographic analysis of the antinociceptive effect of systemically and intrathecally administered combinations of clonidine and opiates. J Pharmacol Exp Ther 255:1107–1116. [PubMed] [Google Scholar]
- Ossipov MH, Lozito R, Messineo E, Green J, Harris S, Lloyd P (1990) Spinal antinociceptive synergy between clonidine and morphine, U69593, and DPDPE: isobolographic analysis. Life Sci 47:PL71–PL76. [DOI] [PubMed] [Google Scholar]
- Plummer JL, Cmielewski PL, Gourlay GK, Owen H, Cousins MJ (1992) Antinociceptive and motor effects of intrathecal morphine combined with intrathecal clonidine, noradrenaline, carbachol or midazolam in rats. Pain 49:145–152. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Roerig SC (1995) Decreased spinal morphine/clonidine antinociceptive synergism in morphine-tolerant mice. Life Sci 56:PL115–PL122. [DOI] [PubMed] [Google Scholar]
- Shamima AR, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MAM, Arulselvan P (2012) Antinociceptive action of isolated mitragynine from Mitragyna Speciosa through activation of opioid receptor system. Int J Mol Sci 13:11427–11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kamble SH, León F, Chear NJ, King TI, Berthold EC, Ramanathan S, McCurdy CR, Avery BA (2019) Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography-tandem mass spectrometry. Drug Test Anal 11:1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Müller CP, Vicknasingam BK (2014) Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend 139:132–137. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG (1967) Statistical Methods, Iowa State University Press, Ames, IA. [Google Scholar]
- Spaulding TC, Fielding S, Venafro JJ, Lal H (1979) Antinociceptive activity of clonidine and its potentiation of morphine analgesia. Eur J Pharmacol 58:19–25. [DOI] [PubMed] [Google Scholar]
- Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL (2014) Morphine and clonidine combination therapy improves therapeutic window in mice: synergy in antinociceptive but not in sedative or cardiovascular effects. PLoS One 9:e109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL (1997) The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci 17:7157–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajerian M, Millecamps M, Stone LS (2012) Morphine and clonidine synergize to ameliorate low back pain in mice. Pain Res Treat 2012:150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Silberstein A, Nusser E (1985) Why does Coomassie Brilliant Blue R interact differently with different proteins? A partial answer. J Biol Chem 260:9976–9980. [PubMed] [Google Scholar]
- Tallarida RJ (2000) Drug Synergism and Dose-Effect Data Analysis, CRC Press, Boca Raton, FL. [Google Scholar]
- Tallarida RJ (2001) Drug synergism: its detection and applications. J Pharmacol Exp Ther 298:865–872. [PubMed] [Google Scholar]
- Tallarida RJ (2006) An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther 319:1–7. [DOI] [PubMed] [Google Scholar]
- Tonner PH (2017) Additives used to reduce perioperative opioid consumption 1: Alpha2-agonists. Best Pract Res Clin Anaesthesiol 31:505–512. [DOI] [PubMed] [Google Scholar]
- Ullman-Culleré MH, Foltz CJ (1999) Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]
- Valverde A, Skelding AM (2019) Alternatives to opioid analgesia in small animal anesthesia: alpha-2 agonists. Vet Clin North Am Small Anim Pract 49:1013–1027. [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, et al. (2016) Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with Mu agonism and delta antagonism, which do not recruit β-arrestin-2. J Med Chem 59:8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Goudas LC, Cousins MJ, Carr DB (2002) Combination spinal analgesic chemotherapy: a systematic review. Anesth Analg 95:674–715. [DOI] [PubMed] [Google Scholar]
- Wilkerson JL, Felix JS, Restrepo LF, Ansari MI, Coop A, McMahon LR (2019) The effects of morphine, baclofen, and buspirone alone and in combination on schedule-controlled responding and hot plate antinociception in rats. J Pharmacol Exp Ther 370:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, Maldonado RL, Cravatt BF, Lichtman AH (2017) The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology 114:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JLNiphakis MJGrim TWMustafa MAAbdullah RAPoklis JLDewey WLAkbarali HBanks MLWise LE, et al. (2016) The selective monoacylglycerol lipase inhibitor MJN110 produces opioid-sparing effects in a mouse neuropathic pain model. J Pharmacol Exp Ther 357:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LL, Chakraborty S, Eans SO, Cirino TJ, Stacy HM, Simons CA, Uprety R, Majumdar S, McLaughlin JP (2021) Kratom alkaloids, natural and semi-synthetic, show less physical dependence and ameliorate opioid withdrawal. Cell Mol Neurobiol 41:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LLHarris HMEans SOBrice-Tutt ACCirino TJStacy HMSimons CALeón FSharma ABoyer EW, et al. (2020) Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend 216:108310. [DOI] [PubMed] [Google Scholar]