Abstract
Introduction
Necrotising enterocolitis (NEC) remains a major contributor to preterm mortality and morbidity. Prolonged duration of antibiotic therapy after delivery is associated with later NEC development but recent evidence suggests that absence of antibiotic treatment after delivery may also increase NEC risk. We will explore this controversy using a large pre-existing dataset of preterm infants in the UK.
Methods and analysis
This is a retrospective cohort study using data from UK National Neonatal Research Database (NNRD) for infants born 1 January 2012 to 31 December 2020. Eligible infants will be <32 weeks gestation, alive on day 3. Primary outcome is development of severe NEC, compared in infants receiving early antibiotics (days 1–2 after birth) and those not. Subgroup analysis on duration of early antibiotic exposure will also occur. Secondary outcomes are: late onset sepsis, total antibiotic use, predischarge mortality, retinopathy of prematurity, intraventricular haemorrhage, bronchopulmonary dysplasia, focal intestinal perforation and any abdominal surgery. To address competing risks, incidence of death before day 7, 14 and 28 will be analysed. We will perform logistic regression and propensity score matched analyses. Statistical analyses will be guided by NEC risk factors, exposures and outcome presented in a causal diagram. These covariates include but are not limited to gestational age, birth weight, small for gestational age, sex, ethnicity, delivery mode, delivery without labour, Apgar score, early feeding and probiotic use. Sensitivity analyses of alternate NEC definitions, specific antibiotics and time of initiation will occur.
Ethics and dissemination
We will use deidentified data from NNRD, which holds permissions for the original data, from which parents can opt out and seek study-specific research ethics approval. The results will help to determine optimal use of early antibiotics for very preterm infants.
Implications
This data will help optimise early antibiotic use in preterm infants.
Trial registration number
ISRCTN55101779.
Keywords: neonatal intensive & critical care, paediatric gastroenterology, bacteriology
Strengths and limitations of this study.
Use of the National Neonatal Research Database gives access to a very large dataset of preterm infants.
The primary outcome (necrotising enterocolitis (NEC)) and the many contributory covariates are routinely recorded in this dataset.
Analysis by both regression and using propensity matching optimises learning from this large dataset.
Data entry may not always be as accurate as that collected specifically within a trial.
The diagnosis of NEC has no gold standard to allow standardisation across units.
Introduction
Around 3% of all babies are born very preterm (VPT, <32 weeks’ gestation) and they require prolonged hospital stay, commonly including intensive care. Survival in these VPT infants (VPTI) has increased dramatically in recent years, but death is still common (~10% overall) as are life-long physical and cognitive impairment.1 In the UK around 10 000 VPTI are born every year, representing an annual cost to the National Health Service (NHS) of ~£3 billion.2 The most common cause of death or serious illness in preterm infants after the first few days are gut or infectious complications such as necrotising enterocolitis (NEC) or late onset sepsis.3 Although knowledge around NEC, and preventive practices such as use of mothers own milk, donor human milk and probiotics are increasing, there has been little reduction in NEC incidence over recent years,4 5 and mechanisms underlying the development of NEC are poorly understood. Antibiotic use as part of neonatal intensive care is common, particularly immediately after birth when infection is implicated in preterm delivery—studies show more than half of infants weighing <1000 g routinely received more than 5 days antibiotics at birth.6 Antibiotic use in VPTI has been implicated in NEC development in several ways. Studies show an increase in NEC incidence with increased duration of empirical early antibiotics7 8 and alteration of the gut microbiotia (dysbiosis) has been mechanistically linked to NEC development.9 However, recent observational data from 13 Neonatal Intensive Care Units (NICUs) from 5 continents (n=2831) identified that NEC incidence was higher in infants who did not receive empirical antibiotics early after birth, despite higher gestational age, compared with those receiving them (OR: 1.8 (95% CI 1.1 to 2.9)), with even higher OR when adjusted for relevant confounders (OR: 4.0 (95% CI 2.1 to 7.3)).10 In contrast, results from a very recent study in preterm infants with low risk of infection shows opposite trends of lower odds in those not treated, but is underpowered for NEC as outcome (n=641, OR: 0.7 (95% CI 0.3 to 1.5)).11 There is an increasing focus on antibiotic stewardship, and it can be expected that the proportion of infants that are not given antibiotics after preterm birth will increase in the coming years. Therefore, it is important to know if lower early usage of antibiotics will increase the incidence of NEC. While there have been calls for a trial of routine early antibiotic treatment12 in VPT babies, and a single trial has so far attempted to do this, there are important logistical difficulties13 with such an approach.
The National Neonatal Research Database (NNRD) provides a large, population level dataset that can be used to further test the hypothesis that early empiric antibiotic treatment reduces the incidence of NEC in preterm infants, and allows adjustment for confounding through the large number of patient level covariates recorded in the NNRD.
Methods and analysis
Design
Retrospective cohort study using routinely recorded clinical data held in the NNRD.
Data source
NNRD holds data from all infants admitted to NHS neonatal units in England and Wales around 90 000 infants annually. Neonatal units in England and Wales have contributed data since 2012. Data are entered by contributing units to a point-of-care electronic dataset and a defined dataset is extracted by NNRD. Data are extracted quarterly and sent to the Neonatal Data Analysis Unit, based at Imperial College, London.14 The data include variables pertinent to the present analysis, including demographics, exposure and outcome variables.
Eligibility criteria
Eligible infants must have been born at <32 weeks gestation, be cared for in a unit contributing data to NNRD, and be alive at day 3. Infants will be excluded if they have a known severe congenital or gastrointestinal anomaly (excluding the presence of a patent ductus arteriosus, online supplemental etables 1 and 2) or have had abdominal surgery before day 3.
bmjopen-2022-065934supp003.pdf (186.2KB, pdf)
Time period
Infants born between 1 January 2012 and 31 December 2020 will be included.
Setting
UK neonatal units in England and Wales contributing to NNRD.
Definitions
Exposure (primary)
Receipt of any intravenous antibiotic drug (online supplemental appendix 1) for any of the first 2 days after birth.
bmjopen-2022-065934supp001.pdf (213.2KB, pdf)
Comparator: did not receive any antibiotics for any of the first 2 days after birth.
Primary outcomes
Severe NEC resulting in death or surgery as defined by Battersby et al.4
Secondary outcomes
Secondary outcomes for analysis are the effects of early antibiotic exposure on:
Late onset sepsis (blood stream or cerebrospinal fluid (CSF) confirmed pure growth in culture (National Neonatal Audit Programme (NNAP) definition) after first 3 days and/or treatment with 5 days of antibiotics and a concurrent diagnosis of infection after the first 3 days).
Total antibiotic use (number of days with any treatment of antibiotics during admission).
Length of stay (postnatal age at discharge or death).
Time to reach full feeding (first day of 3 consecutive days where parenteral nutrition or intravenous fluid are not recorded.
Growth (change in SD score between birth and 36 weeks and discharge).
Further, we will analyse effects on some relevant adverse outcomes:
Total predischarge mortality.
Death prior to day 7, day 14, day 28.
Bronchopulmonary dysplasia (respiratory support given at 36 weeks).
Retinopathy of prematurity (ROP) (received treatment for ROP, according to NNRD definition).
Brain injury (intraventricular haemorrhage grade 3 or above or cystic leukomalacia diagnoses recorded).
Need for surgical procedures (online supplemental appendix 1).
Comparison of different durations of early antibiotic exposure will be performed based on the following categories:
Antibiotic duration no longer than 3 days.
Antibiotic duration 3–5 days.
Antibiotic duration longer than 5 days without positive culture (blood stream or CSF confirmed pure growth in culture (NNAP definition) in the first 3 days.
For the above analyses, infants with a positive blood or CSF culture in the first 3 days will be excluded.
A specific subgroup of interest are the infants that are considered to have low risk of early onset sepsis (EOS), specified as fulfilling all of the following prenatal characteristics: no premature rupture of membranes, no labour and no (suspected) chorioamnionitis. Additional subgroup analyses will be performed for infants with gestation age <28 weeks and birth weight <1000 g.
Sample size
Observed NEC incidence noted in a previous study on a total 2831 infants from five different continents, using criteria for NEC diagnosis in keeping with pragmatically defined NEC, was 9% when early antibiotic treatment was absent and 4% when antibiotic was provided in the first 3 days.10 We hypothesise to find a similar antibiotic related proportional reduction in incidence of severe NEC in this study, based on data collected over 9 years (2012–2020) from around 45 000 infants. In an earlier report based on an NNRD subgroup, the incidence of severe NEC was 3.2% for infants born <32 weeks.4 The cohort event estimate is 1440 cases.
Data required
Online supplemental appendix 1 carries the full list of variables considered relevant for extraction from NNRD including definitions of constructed items/variables.
Potential confounders
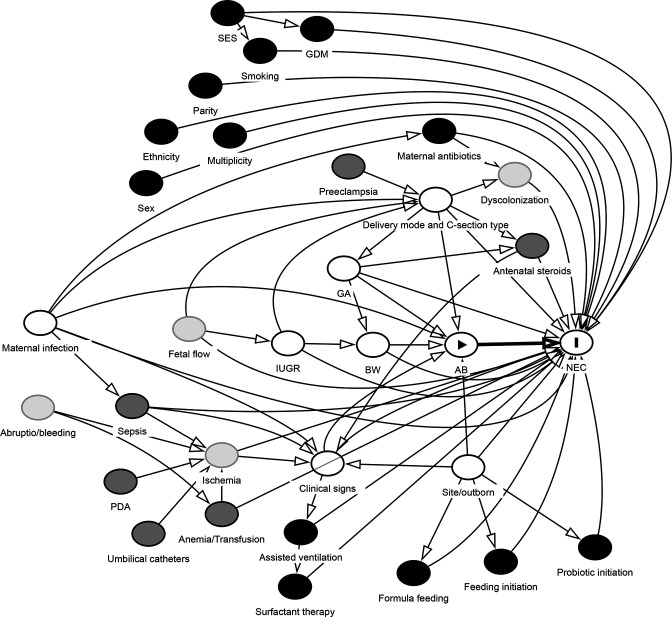
Several covariates are relevant to include in the analysis as potential confounders. We will take a hypothesis driven approach to the selection of covariates. A causal diagram (directed acyclic graphs, DAG, figure 1) is drawn and analysed with relevant variables and potential confounders related to antibiotic exposure and NEC outcome. Nodes and edges are determined based on literature and subject matter knowledge. The selected covariates are considered to reflect conditions prior to the defined exposure (ie, within day 1–2 after birth). For several variables, only proxies will be available (table 1).
Figure 1.
Directed acyclic graphs (DAG) diagram of causal assumptions related to the hypothesis based on subject-matter knowledge, used for confounder selection. Model code text for figure and interactive diagram analysis on dagitty.net is available in online supplemental appendix 2. Node with arrowhead: exposure; node with I: outcome; black nodes: ancestor of outcome; dark grey nodes: ancestor of exposure and outcome; white nodes: adjusted variables (primary analysis); thick arrow: causal path; thin arrows: non-biasing paths. AB, early antibiotics; BW, birth weight; GA, gestational age; GDM, gestational diabetes mellitus; IUGR, intrauterine growth restriction; NEC, necrotising enterocolitis; PDA, patent ductus arteriosus; SES, socioeconomic status.
Table 1.
Overview of variables
| Variable | Class/type | Expected availability in NNRD | Importance for effect estimation |
| AB | Exposure | Available (definable) | |
| NEC | Outcome | Available (definable) | |
| Site | Confounder | Available | Minimal sufficient adjustment set to model the direct and total effect of AB on NEC according to proposed DAG |
| BW | Confounder | Available | |
| Delivery mode* | Confounder | Available (categories) | |
| Clinical signs† | Confounder | Available (proxies) | |
| Maternal infection | Confounder | Available (clinical) | |
| GA | Confounder | Available | |
| IUGR | Confounder | Available (definable) | |
| Fetal flow | Ancestor of exposure and outcome (indirect) | Unobserved | Blocked by IUGR and delivery mode |
| Ischaemia | Ancestor of exposure and outcome (indirect) | Unobserved | Blocked by clinical signs |
| Pre-eclampsia | Ancestor of exposure and outcome (indirect) | Available | Blocked by delivery mode |
| Antenatal steroids | Ancestor of outcome | Available | Blocked by clinical signs |
| Sepsis | Ancestor of exposure and outcome (indirect) | Available (definable/proxy) | Blocked by clinical signs |
| PDA | Ancestor of exposure and outcome (indirect) | Available (definable) | Blocked by clinical signs |
| Umbilical catheters | Ancestor of exposure and outcome (indirect) |
Available | Blocked by clinical signs |
| Anaemia/transfusion | Ancestor of exposure and outcome (indirect) | Available (proxy, that is, transfusions) | Blocked by clinical signs |
| Sex | Ancestor of outcome | Available | Precision variable |
| Ethnicity | Ancestor of outcome | Available | Precision variable |
| Multiparity | Ancestor of outcome | Available | Precision variable |
| Smoking | Ancestor of outcome | Available | Precision variable |
| GDM | Ancestor of outcome | Available | Precision variable |
| Socioeconomic status | Ancestor of outcome | Available (proxy that is, deprivation score) | Precision variable |
| Maternal antibiotics | Ancestor of outcome | Available (intra partum) | Precision variable |
| Dyscolonisation | Ancestor of outcome | Unobserved | Precision variable |
| Assisted ventilation | Ancestor of outcome | Available | Precision variable |
| Surfactant therapy | Ancestor of outcome | Available | Precision variable |
| Formula feeding | Ancestor of outcome | Available | Precision variable |
| Feeding initiation | Ancestor of outcome | Available | Precision variable |
| Probiotic initiation | Ancestor of outcome | Available | Precision variable |
*Specification of different clinical conditions with important impact on decision to treat with AB, categorised as: vaginal AND spontaneous, vaginal AND induced, emergency caesarean AND labour, emergency caesarean AND no labour, elective caesarean AND labour, elective caesarean AND no labour.
†Respiratory/circulatory/unspecific signs/symptoms/parameters used clinical assessment and decision making related to decision to treat with antibiotics.
AB, early antibiotics; BW, birth weight; DAG, directed acyclic graphs; GA, gestational age; GDM, gestational diabetes mellitus; IUGR, intrauterine growth restriction; NEC, necrotising enterocolitis; NNRD, National Neonatal Research Database; PDA, patent ductus arteriosus.
bmjopen-2022-065934supp002.pdf (127.5KB, pdf)
Statistical analyses
Primary analyses
Previous work using logistic regression included the following covariates in the model for the hypothesis: NICU (random effect)+gestational age+birthweight+sex+delivery mode+APGAR scores+antenatal steroids+feeding type. We aim to test the hypothesis with data from NNRD using the same regression model as used in the previous work (variables 1–8 in table 2) and also an expanded regression model with inclusion of all potentially relevant variables (table 2). Results will be presented as adjusted ORs with 97.5% CIs and Bonferroni-adjusted p values (unadjusted p values multiplied by 2). To better quantify the causal effect of antibiotics, standardised risk differences with 97.5% bootstrap CIs will also be presented.
Table 2.
Priority of covariates to include in model based on DAG and availability from NNRD
| Influence on NEC27–29 | Influence on AB-start (decision to treat based on sepsis risk)30 31 | Potential repetition/ redundancy | Relation to node in DAG | Structure (continuous or number of categories) | ||
| 1 | Neonatal Intenisve Care Unit/site | Yes | Yes | Site | Random | |
| 2 | GA | Yes | Yes | GA | Continuous | |
| 3 | BW | Yes | Yes | BW | Continuous | |
| 4 | Sex | Yes | No? | Sex | Dichotomous | |
| 5 | APGAR5 | Yes | Yes | Clinical signs | 11 categories (0–10) | |
| 6 | Delivery mode+expanded (6 categories) | Yes | Yes | Delivery mode and type | 6 (see table 1) | |
| 7 | Maternal antenatal steroids | Yes | Yes? (indicator of fetal status/delivery conditions) | Antenatal steroids | None/incomplete/complete | |
| 8 | Feeding first day | Yes | No | Feeding | 1: Enteral feeding on day 1–2, human milk only 2: Enteral feeding on day 1–2, formula only 3: Enteral feeding on day 1–2, mix 3: No enteral feeding on day 1–2 |
|
| 9 | IUGR | Yes | Yes | IUGR | Dichotomous (less than −2SDS) | |
| 10 | APGAR1 | Yes | Yes? | 11 categories (0–10) | ||
| 11 | APGAR10 | Yes | Yes | Clinical signs | 11 categories (0–10) | |
| 12 | EOS | Yes? | No | Sepsis | Dichotomous | |
| 13 | Birth year (epoch) | Yes | Yes | (Similar to site/standards) | 4–5 | |
| 14 | Transfer on first day | Yes | Yes | Site/outborn | Dichotomous | |
| 15 | Level of initial unit | Yes | Yes | Site | Dichotomous | |
| 16 | Maternal pre-eclampsia requiring preterm birth | Yes? | Yes | Pre-eclampsia | Dichotomous | |
| 17 | Prolonged ROM | Yes? | Yes | Maternal infection | Dichotomous | |
| 18 | Maternal suspected chorioamnionitis | Yes? | Yes | Defined by antibiotics and fever | Maternal infection | Dichotomous |
| 19 | Intrapartum antibiotics | Yes? | Yes (in relation to chorioamnionitis) | Maternal antibiotics | Dichotomous | |
| 20 | Maternal fever | Yes? | Yes (untreated chorioamnionitis) | Maternal infection | Dichotomous | |
| 21 | Maternal GBS | Yes? | Yes | Maternal infection | Dichotomous | |
| 22 | Umbilical cord pH | Yes | Yes | Clinical signs | Dichotomous: <7.00 yes or no | |
| 23 | Umbilical cord lactate | Yes | Yes | Resembles pH | Clinical signs | Cont/Di/tri? |
| 24 | Base excess 12 hours worst | Yes | Yes | Clinical signs | Dichotomous: <−5 yes/no | |
| 25 | Umbilical cord base excess | Yes | Yes | Resembles BE 12 hours worst | Clinical signs | Dichotomous: <−5 yes/no |
| 26 | Blood transfusion day 1–2 | Yes | Yes? | Anaemia | Dichotomous | |
| 27 | Chest compressions | Yes | Yes? | Clinical signs | Dichotomous | |
| 28 | Resuscitation drugs at delivery | Yes | Yes? | Clinical signs | Dichotomous | |
| 29 | Ventilation at delivery | Yes? | Yes? (clinical status at birth) | Assisted ventilation | Dichotomous | |
| 30 | Spontaneous respiration time | Yes? | Yes? | Clinical signs | 3 categories: <1 min, 1–5 min, >5 min |
|
| 31 | Admission temp | Yes | Yes? | Clinical signs | 3 categories: <36.5, 36.5–37.5, >37.5 |
|
| 32 | Admission oxygen SAT | Yes | Yes | Clinical signs | 3 categories: >94, 90–94, <90 |
|
| 33 | Inotropes on first day | Yes | Yes? | Clinical signs | Dichotomous | |
| 34 | Admission mean BP | Yes? | Yes/no? | Resembles inotropes | Clinical signs | Dichotomous: below GA yes/no |
| 35 | Ethnicity | Yes | Yes? (risk of inf) | Ethnicity | 4 categories as suggested in appendix | |
| 36 | Maternal deprivation score | Yes? | Yes? (risk of inf) | SES | Deprivation centiles? | |
| 37 | Intubation first day | ? | Yes? | Assisted ventilation | Dichotomous | |
| 38 | Intubation at delivery | ? | Yes? | Resembles intubation d1 | Assisted ventilation | Dichotomous |
| 39 | Surfactant first day | Yes? | Yes? | Surfactant therapy | Dichotomous | |
| 40 | Surfactant at delivery | ? | Yes? | Resembles intubation d1 | Surfactant therapy | Dichotomous |
| 41 | Time of cord clamp | Yes/No?? | Yes? (clinical status at birth) | Clinical signs | Dichotomous: >60 s yes/no | |
| 42 | Probiotics | Yes | No | Probiotic initiation | Dichotomous | |
| 43 | PDA identified day 1–2 | Yes | No | PDA | Dichotomous | |
| 44 | PDA treatment day 1–2 | Yes | No | PDA | Dichotomous | |
| 45 | Multiplicity | ? | No? | Multiplicity | Dichotomous | |
| 46 | Smoking | Yes? | No? | Smoking | Dichotomous | |
| 47 | Parity | ? | No? | Parity | Dichotomous | |
| 48 | Umbilical catheters | Yes? | No | Umbilical catheters | Dichotomous | |
| 49 | Parenteral nutrition d1-2 | ? | ? | Dichotomous | ||
| 50 | Admission heart rate | ? | ? | Clinical signs | 3 categories: >200, 100–200, <100 | |
| 51 | Maternal antenatal magnesium sulphate | No? | Yes/no ? | Resembles pre-eclampsia | Pre-eclampsia | Dichotomous |
| 52 | Maternal gestational hypertension | No? | No | Dichotomous | ||
| 53 | Maternal diabetes | No? | No | GDM | Dichotomous |
BW, birth weight; DAG, directed acyclic graphs; EOS, early onset sepsis; GA, gestational age; GDM, gestational diabetes mellitus; IUGR, intrauterine growth restriction; NEC, necrotising enterocolitis; PDA, patent ductus arteriosus; ROM, rupture of membranes; SES, socioeconomic status.
Priority of covariates
Covariates to include in the model are listed and prioritised in table 2. Confounders are ranked higher based on importance, that is, variables which arguably have effect on both outcome (NEC) as well as exposure (decision to start antibiotic treatment, which relates to infection risk/concern). Assessment of covariate importance is based on subject matter knowledge and scientific literature (references in table 2). For several variables, it is unclear whether there is a relevant effect on NEC and a conservative approach is employed to include potential confounders in the model.15 Similar considerations apply for assessment of variables with relevant effect on decision to treat with early antibiotics. These variables will be included in the regression for propensity score calculation and subsequent matching. For highly similar variables, the lower priority or quality variables may be omitted if necessary (eg, multi-collinearity issues). Variables with very low quality (eg, too many missing values) will be omitted. For categorical variables, groups with very few observations will be removed (eg, separation issues). Estimated effects of each variable included in the model included will be reported. Based on the recommendation to have at least 10 events per variable,16 with the event estimate approximately 1500 cases, this will provide 150 df in the model. Based on the proposed covariates listed in table 2, the required df for analysis is 108. If the actual number of cases in the obtained dataset is much lower than expected, thus providing insufficient df, covariates may be excluded in reverse order of priority. See detailed specifications of listed covariates/items in online supplemental appendix 1.
Sensitivity analyses
The following sensitivity analyses will be performed:
Early antibiotic exposure only with ampicillin or penicillin plus gentamicin, early antibiotic exposure defined by other timings after birth (later initiation and lasting until 4–6 days after birth) and alternative methods for diagnosing NEC (as standards for NEC diagnosis are unclear). For the latter analyses, we will define and reanalyse NEC diagnosis as ‘pragmatic NEC’ (5 days of nil by mouth and antibiotics and a diagnostic code of NEC) and NEC including focal intestinal perforation diagnosis (FIP). This condition is sometimes difficult to separate from NEC. We will also record infants with laparotomy-confirmed FIP (intestinal perforation, classified as non-NEC) in addition to the primary NEC (Battersby et al). definition. The statistical analyses will be repeated using propensity score matching (with propensity scores based on exposure regression), as an alternative approach to logistic regression.
Secondary analyses
We intend to use the same logistic regression models for secondary outcomes, as those specified for the primary outcome. The most important confounders (or proxies) for the secondary outcomes are included in this model. Detailed model specification for each specific secondary outcome as done for the primary outcome is beyond the aim and scope of this study (focusing on NEC). With propensity score matching, direct comparison between antibiotic exposure vs controls can in principle be performed for any outcome, assuming correct model specification for the propensity score.
Exploratory analyses
Additional non-defined exploratory analyses based on findings from the dataset may be performed.
Missing data
We assume that missing data occur randomly between groups and will be imputed 10-fold using multiple imputation by chained equations. Results will be pooled according to Rubin’s rule.
Multiple testing
Adjusted p values will be reported with Bonferroni correction of the two primary analyses (along with corresponding 97.5% CIs) and Benjamini-Hochberg adjusted p values from the secondary analyses. Post hoc exploratory analyses will be reported without adjustment of p values and should be interpreted with corresponding caution.
Ethics and dissemination
The study will be registered with International Standard Randomised Controlled Trials Number before opening and is sponsored by Newcastle Hospitals NHS Foundation Trust and the protocol with statistical analysis plan will be uploaded to the Open Science Framework website osf.io prior to data analysis initiation. We will apply for HRA/REC approvals. The study is observational and uses deidentified data that is already collected. Dissemination will be by presentation and publication in peer-reviewed journals.
Patient public involvement and importance to the NHS
We have worked closely with parents on all our studies. The NEC UK parent group and other parent groups and representatives continue to assert that better understanding of NEC is a key priority. The NHS, parents and babies experience significant burden from NEC in terms of adverse outcome, prolonged hospitalisation, developmental impact and NHS costs. There is a significant concern related to use of antibiotics in the neonatal population and it is important that studies help optimal use of early antibiotics.
Discussion
This study aims to add relevant scientific information to an important clinical decision made for every preterm infant admitted to a neonatal unit: the use and duration of antibiotics in the absence of clear signs of bacteraemia or EOS. Cases of culture-proven EOS are relatively few, with rates being one to seven per 1000 live births in high-income countries.17 There are potentially large numbers of infants where a clinical choice is available to withhold early antibiotic treatment. Data are currently conflicting as to the overall impact on NEC of receiving (or withholding) antibiotics in the first days of life. Early bacterial nature and load in the preterm gut have been linked to NEC development.18 19 Use of intravenous antibiotics shortly after birth may slow colonisation, allowing the gut immune system a short period of adaption that reduces the risk of TLR4 mediated NEC.20 The integrity of the mucosal barrier has been shown to improve significantly in the first days after preterm birth in humans.21 Thus, potentially only short duration of very early antibiotic treatment may be relevant for such effect, in contrast to prolonged treatment which have been shown to cause persistent gut dysbiosis22 that may instead increase NEC risk.7 8 Data from a piglet model of NEC suggests that antibiotic use is mechanistically linked to preterm NEC development23 and preterm immune development.24 However, no difference was seen in total bacterial load of stool in preterm infants who did and did not go on to develop NEC.25 Given the conflicting data Clinicians need better information to help guide early antibiotic treatment in relation to NEC, especially important as NEC rates in premature infants may actually be increasing.26 The proposed study using NNRD benefits from access to large numbers of infants with recorded relevant risk factors and outcomes. Large datasets offer the advantage of including many NEC cases, and we anticipate around 1500 informative cases of NEC. These data are increasingly well-validated by individual units at the point of data entry, but are potentially less well-validated than infants with trial data collected within specific trials.
We have in this study given careful thought to handling confounding factors. Analysis of the current understanding of NEC and the use of directed acyclic graph to guide analysis have been undertaken to attempt to control for what are highly complex clinical factors.27–29 As demonstrated in the DAG many factors, including those on a causal pathway to NEC, impact the decision to administer early antibiotics.30 31 The aim to analyse this data using both propensity scoring and logistic regression is a major strength for this study and for future analyses using large databases to address complex questions. Propensity scoring has recently been used to address feeding during hypothermia32 and the impact of early parenteral nutrition on preterm outcomes33 using the NNRD, but without alternate statistical approach. While both propensity scoring and regression analysis have strengths and weaknesses to the best of our knowledge direct comparison of these methodologies has not been undertaken within large neonatal datasets, and is important methodologically for future neonatal studies. The data generated by this study will thus inform important aspects of wider neonatal care and in relation to early neonatal use of antibiotics and later occurrence of NEC.
Supplementary Material
Footnotes
Twitter: @NeoResearch_Net, @DrCGale
Correction notice: This article has been corrected since it published online to reflect the correct author name and affiliations for author Gorm Greisen.
Contributors: NE had the original idea for the study. RS undertook the DAG. RS, JLF, JB, PTS planned statistical analysis. RS, NE, JLF, JB, PTS, CG, GG and SU all contributed to overall study design, protocol development and the writing and review of this paper. JB submitted for registration and approvals.
Funding: We will use institutional co-funding to cover the cost of data extraction from the NNRD. Work to plan and carry out the analytical work of this study was supported by a grant from the Novo Nordic Foundation (postdoctoral fellowship to René Shen, BRIDGE Translational Excellence Programme, grant no. NNF18SA0034956).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Howson CP, Kinney MV, Lawn JE, eds. Born too soon. Born Too Soon, Glob Action Rep Preterm Birth. Vol. 13. Geneva: World Heal Organ, 2012. [Google Scholar]
- 2.Khan KA, Petrou S, Dritsaki M, et al. Economic costs associated with moderate and late preterm birth: a prospective population-based study. BJOG 2015;122:1495–505. 10.1111/1471-0528.13515 [DOI] [PubMed] [Google Scholar]
- 3.Berrington JE, Hearn RI, Bythell M, et al. Deaths in preterm infants: changing pathology over 2 decades. J Pediatr 2012;160:49–53. 10.1016/j.jpeds.2011.06.046 [DOI] [PubMed] [Google Scholar]
- 4.Battersby C, Santhalingam T, Costeloe K, et al. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed 2018;103:F182–9. 10.1136/archdischild-2017-313880 [DOI] [PubMed] [Google Scholar]
- 5.Jones IH, Hall NJ. Contemporary outcomes for infants with necrotizing Enterocolitis-A systematic review. J Pediatr 2020;220:86–92. 10.1016/j.jpeds.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 6.Greenberg RG, Chowdhury D, Hansen NI, et al. Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr Res 2019;85:994–1000. 10.1038/s41390-019-0300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuppala VS, Meinzen-Derr J, Morrow AL, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 2011;159:720–5. 10.1016/j.jpeds.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123:58–66. 10.1542/peds.2007-3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pammi M, Cope J, Tarr PI, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017;5:31. 10.1186/s40168-017-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Shen RL, Ayede AI, et al. Early use of antibiotics is associated with a lower incidence of necrotizing enterocolitis in preterm, very low birth weight infants: the NEOMUNE-NeoNutriNet cohort study. J Pediatr 2020;227:128–34. 10.1016/j.jpeds.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letouzey M, Lorthe E, Marchand-Martin L, et al. Early antibiotic exposure and adverse outcomes in very preterm infants at low risk of early-onset sepsis: the EPIPAGE-2 cohort study. J Pediatr 2022;243:91–8. 10.1016/j.jpeds.2021.11.075 [DOI] [PubMed] [Google Scholar]
- 12.Cantey JB. Early antibiotic therapy and adverse outcomes in preterm infants: time for a trial! J Pediatr 2020;227:13–14. 10.1016/j.jpeds.2020.07.046 [DOI] [PubMed] [Google Scholar]
- 13.Ruoss J, Bazacliu C, Russell J. Routine early antibiotic use in symptomatic preterm neonates (reason): a prospective randomized controlled trial. MedRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer A, Modi N. National neonatal data to support specialist care and improve infant outcomes. Arch Dis Child Fetal Neonatal Ed 2013;98:F175–80. 10.1136/archdischild-2011-300872 [DOI] [PubMed] [Google Scholar]
- 15.VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol 2019;34:211–9. 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and COX regression. Am J Epidemiol 2007;165:710–8. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 17.Lukacs SL, Schrag SJ. Clinical sepsis in neonates and young infants, United States, 1988-2006. J Pediatr 2012;160:960–5. 10.1016/j.jpeds.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 18.Choi Y-S, Song IG. Fetal and preterm infant microbiomes: a new perspective of necrotizing enterocolitis. Korean J Pediatr 2017;60:307–11. 10.3345/kjp.2017.60.10.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brower-Sinning R, Zhong D, Good M, et al. Mucosa-Associated bacterial diversity in necrotizing enterocolitis. PLoS One 2014;9:e105046. 10.1371/journal.pone.0105046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihi B, Good M. Impact of Toll-like receptor 4 signaling in necrotizing enterocolitis: the state of the science. Clin Perinatol 2019;46:145–57. 10.1016/j.clp.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Elburg RM, Fetter WPF, Bunkers CM, et al. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed 2003;88:52F–5. 10.1136/fn.88.1.F52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwittink RD, Renes IB, van Lingen RA, et al. Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants. Eur J Clin Microbiol Infect Dis 2018;37:475–83. 10.1007/s10096-018-3193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen ML, Thymann T, Cilieborg MS, et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am J Physiol Gastrointest Liver Physiol 2014;306:G59–71. 10.1152/ajpgi.00213.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen DN, Fuglsang E, Jiang P, et al. Oral antibiotics increase blood neutrophil maturation and reduce bacteremia and necrotizing enterocolitis in the immediate postnatal period of preterm pigs. Innate Immun 2016;22:51–62. 10.1177/1753425915615195 [DOI] [PubMed] [Google Scholar]
- 25.Abdulkadir B, Nelson A, Skeath T, et al. Stool bacterial load in preterm infants with necrotising enterocolitis. Early Hum Dev 2016;95:1–2. 10.1016/j.earlhumdev.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 26.Juhl SM, Gregersen R, Lange T, et al. Incidence and risk of necrotizing enterocolitis in Denmark from 1994-2014. PLoS One 2019;14:e0219268. 10.1371/journal.pone.0219268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels N, van de Graaf RA, de Jonge RCJ, et al. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr 2017;17:105. 10.1186/s12887-017-0847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med 2018;23:374–9. 10.1016/j.siny.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gephart SM, McGrath JM, Effken JA, et al. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care 2012;12:77–87. 10.1097/ANC.0b013e31824cee94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puopolo KM, Benitz WE, Zaoutis TE, et al. Management of neonates born at ≤34 6/7 weeks' gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018;142:e20182896. 10.1542/peds.2018-2896 [DOI] [PubMed] [Google Scholar]
- 31.Martius JA, Roos T, Gora B, et al. Risk factors associated with early-onset sepsis in premature infants. Eur J Obstet Gynecol Reprod Biol 1999;85:151–8. 10.1016/S0301-2115(99)00018-4 [DOI] [PubMed] [Google Scholar]
- 32.Gale C, Jeyakumaran D, Longford N, et al. Administration of parenteral nutrition during therapeutic hypothermia: a population level observational study using routinely collected data held in the National neonatal research database. Arch Dis Child Fetal Neonatal Ed 2021;106:608–13. 10.1136/archdischild-2020-321299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webbe JWH, Longford N, Battersby C, et al. Outcomes in relation to early parenteral nutrition use in preterm neonates born between 30 and 33 weeks' gestation: a propensity score matched observational study. Arch Dis Child Fetal Neonatal Ed 2022;107:131–6. 10.1136/archdischild-2021-321643 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065934supp003.pdf (186.2KB, pdf)
bmjopen-2022-065934supp001.pdf (213.2KB, pdf)
bmjopen-2022-065934supp002.pdf (127.5KB, pdf)