Abstract
Epithelial ovarian cancer (EOC) is a gynecologic malignancy with a poor prognosis due to resistance to first-line chemotherapeutic agents. Some cancer cells are primarily dependent on glycolysis, but others favor mitochondrial oxidative phosphorylation (OXPHOS) over glycolysis. Changes in metabolic reprogramming have been reported to be involved in cancer cell survival. In this review, we summarize the metabolic profiles (e.g., metabolic heterogeneity, plasticity, and reprogramming) and adaptation to the dynamic tumor microenvironment and discuss potential novel therapeutic strategies. A literature search was performed between January 2000 and March 2022 in the PubMed and Google Scholar databases using a combination of specific terms. Ovarian cancer cells, including cancer stem cells, depend on glycolysis, OXPHOS, or both for survival. Several environmental stresses, such as nutrient starvation or glucose deprivation, hypoxic stress, acidification, and excessive reactive oxygen species (ROS) generation, reprogram the metabolic pathways to adapt. The interaction between tumors and adjacent stromal cells allows cancer cells to enhance mitochondrial energy metabolism. The metabolic reprogramming varies depending on genomic and epigenetic alterations of metabolism-related genes and the metabolic environment. Developing accurate and non-invasive methods for early identification of metabolic alterations could facilitate optimal cancer diagnosis and treatment. Cancer metabolism research has entered an exciting era where novel strategies targeting metabolic profiling will become more innovative.
Keywords: Glycolysis, Metabolic plasticity, Metabolic reprogramming, Ovarian cancer, Oxidative phosphorylation, Stem cells, Warburg effect
Glycolysis; Metabolic plasticity; Metabolic reprogramming; Ovarian cancer; Oxidative phosphorylation; Stem cells; Warburg effect.
1. Introduction
Epithelial ovarian cancer (EOC) is a highly lethal gynecologic malignancy. It is the fifth leading cause of cancer deaths in women worldwide, with a high mortality rate [1]. Cytoreductive surgery followed by a combination of platinum and taxane-based chemotherapy and targeted therapy, such as anti-angiogenesis and poly-ADP ribose polymerase (PARP) inhibitors, has been the standard therapy in advanced EOC [2]. In recent years, clinical trials demonstrated that PARP inhibitor treatment is a rapidly changing therapeutic option for EOC patients with homologous recombination deficiency [1]. However, patients often develop resistance to chemotherapeutic agents [2].
The tumor contains a small subfraction of cells with stemness features (i.e., cancer stem cells, CSCs). Ovarian CSCs exhibit chemoresistant properties [3]. Chemoresistance of CSCs may be involved in various biological processes, such as an increase in drug efflux, drug inactivation, detoxification, epigenetic modification of relevant genes, and tumor heterogeneity due to an altered microenvironment [3]. Recent studies have revealed that changes in metabolic reprogramming are involved in increased stemness activity and chemoresistance [4]. Furthermore, ovarian cancer cells and CSCs acquire the energy necessary for their survival through the regulation of several metabolic pathways (e.g., glucose metabolism). Metabolic reprogramming in the tumor or its microenvironment (i.e., cancer-host crosstalk) may affect cancer cell survival. The original hypothesis demonstrated that cancer cells are primarily dependent on glycolysis, known as the Warburg effect, while recent evidence suggests that some cancer cells use mitochondrial oxidative phosphorylation (OXPHOS) over glycolysis [5, 6]. These findings indicate that OXPHOS is also the energy source for EOC growth and survival. Metabolic preferences vary due to intertumoral and intratumoral heterogeneity, such as histological types and the hypoxic and acidic niches, or study design (in vitro, in vivo, or animal model) [4]. To date, there are no reports on adaptive strategies for energy metabolism comparing ovarian cancer cells and CSCs. In addition, malignant niches are formed by cancer cells and their adjacent host cells in the tumor microenvironment. However, metabolic reprogramming of these cells has not yet been fully elucidated. Therefore, the development of therapeutic strategies based on metabolic reprogramming that affects cell survival and growth is a significant challenge. We believe that targeting metabolic reprogramming could help to potentially overcome chemoresistance.
This review aims to discuss the significant molecules involved in metabolic reprogramming and summarize metabolic profiles in ovarian CSCs and cancer non-stem cells, the metabolic plasticity of ovarian cancer cells, adaptation to the dynamic tumor microenvironment, and potential novel therapeutic strategies.
2. Methods
2.1. Search strategy and selection criteria
A computerized literature search was performed to identify the relevant studies. The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines updated in 2020 [7] (see Supplementary Table). Combining the keywords, electronic databases PubMed and Google Scholar published between January 2000 and December 2021 were searched. The search strategy is shown in Table 1. The inclusion criteria were publications of original studies, review papers, and reference lists of the included studies. The exclusion criteria were duplicates, studies in languages other than English, letters to the editor, poster presentations, and literature unrelated to the research topic. The first identification phase included records identified through a database search (Figure 1), and the identified titles and abstracts were screened in the first stage. Duplicates were removed during the second screening phase, and titles, abstracts, and full-text articles were read to remove inappropriate papers. Citation tracking was conducted manually to identify additional relevant articles. The final eligibility phase included the full-text articles for analysis after excluding those for which detailed data could not be extracted. The author assessed the identified articles for eligibility, inclusion and exclusion, and full-text articles. The last computerized literature search was conducted on April 1, 2022.
Table 1.
The search strategy.
| Search mode | The keyword and search term combinations |
|---|---|
| Search term 1 | Ovarian cancer OR Epithelial ovarian cancer OR Ovarian neoplasm |
| Search term 2 | Stem cells OR non-Stem cells |
| Search term 3 | Metabolic shift OR Metabolic reprogramming |
| Search term 4 | Warburg effect OR Reverse Warburg effect |
| Search term 5 | Treatment OR Therapy |
| Search | Search term 1 AND Search term 2 |
| Search term 1 AND Search term 3 | |
| Search term 1 AND Search term 4 | |
| Search term 1 AND Search term 5 | |
| Search term 1 AND Search term 2 AND Search term 3 | |
| Search term 1 AND Search term 2 AND Search term 4 | |
| Search term 1 AND Search term 2 AND Search term 3 AND Search term 5 | |
| Search term 1 AND Search term 2 AND Search term 4 AND Search term 5 |
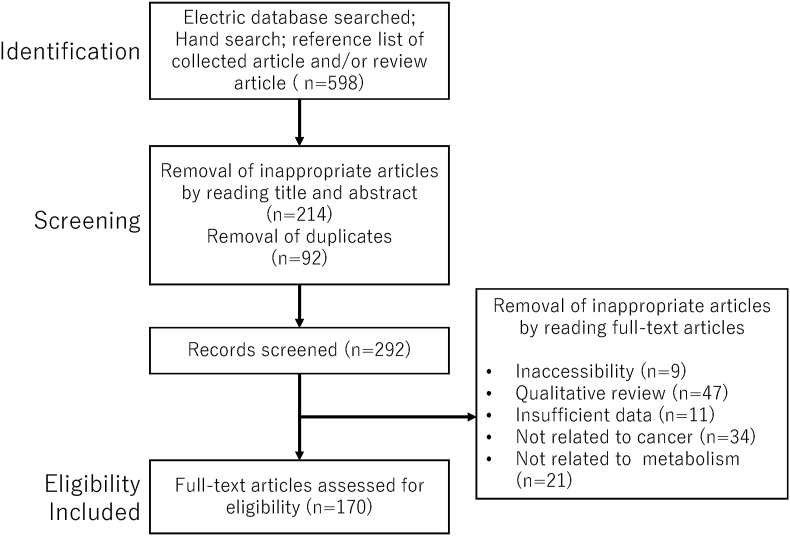
Figure 1.
The number of articles identified by searching for keyword combinations. Figure shows the number of articles identified by keyword combinations and the number of records identified through database searching, records after duplicate removal, records screened, removal of inappropriate articles by reading full-text articles, and full-text articles assessed for eligibility.
3. Results
3.1. Selection of studies
Figure 1 shows the 598 literature citations searched in the PubMed and Google Scholar electronic databases. The overlaps were removed, and 292 records were obtained, of which 122 were excluded, and 170 met the inclusion and exclusion criteria.
3.2. Metabolism in ovarian cancer
3.2.1. Overview of glucose metabolism: glycolysis and the oxidative phosphorylation pathway
Catabolic pathways (e.g., glycolysis) are linked to anabolic pathways (e.g., mitochondrial OXPHOS) for energy (adenosine triphosphate (ATP)) production in a human cell [8]. Glycolysis provides rapid ATP but a lower yield compared with OXPHOS [8]. The key glycolysis-related metabolic enzymes include hexokinase II (HKII), glucose-6-phosphate dehydrogenase (G6PD), lactate dehydrogenase A (LDHA), pyruvate dehydrogenase kinase (PDK), and pyruvate dehydrogenase (PDH) [9, 10]. The PDK-PDH pathway plays a crucial role as the central enzyme in the glycolysis network [9]. PDH converts pyruvate to acetyl-CoA, which provides the link between glycolysis and the tricarboxylic acid (TCA) cycle [11]. PDK inhibits the PDH enzymatic activity. Hence, a decreased expression of PDK alters cell metabolism from glycolysis toward OXPHOS. Mitochondria produce not only ATP but also building blocks for macromolecules such as DNA, lipids, proteins, and antioxidants [12]. OXPHOS is regulated by complex biochemical systems to maintain cellular homeostasis, including electron transport and ATP synthesis.
3.2.2. Metabolic profiles in ovarian cancer
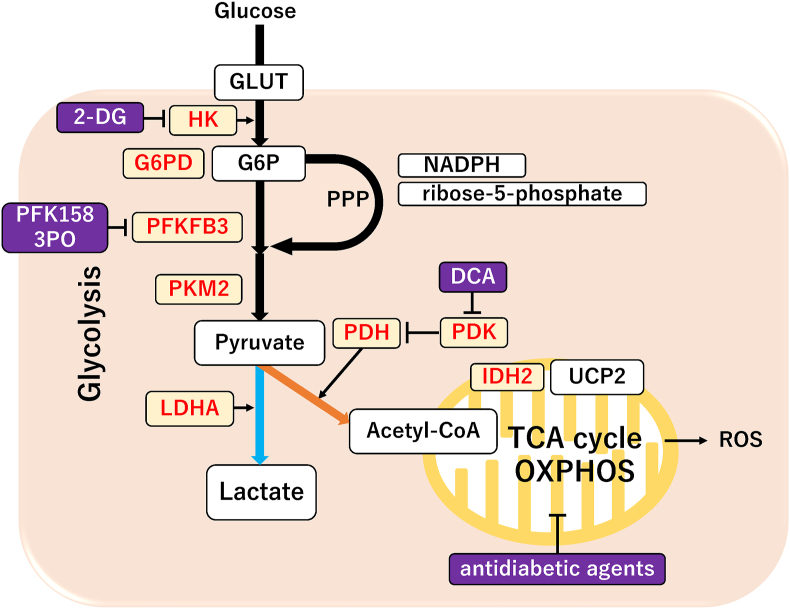
Cancer cells can adapt to the harsh milieu, such as hypoxia and nutrient starvation, by altering their metabolic profiles and their pathways through genetic and epigenetic alterations of various metabolism-related enzymes (i.e., metabolic plasticity) [13]. Altered energy metabolism and its metabolic phenotype represent the remarkable features of cancer [14]. Highly proliferating cancer cells require an altered glycolysis pathway, unlike normal cells, which catabolize glucose by OXPHOS (blue line from pyruvate to lactate, Figure 2) [15]. Furthermore, one of the most prominent hallmarks of cancer cells includes aerobic glycolysis, a phenomenon known as the “Warburg effect,” in which cells rely on glycolysis instead of OXPHOS as the primary energy source even under aerobic conditions [11, 16, 17]. However, recent studies have shown that specific cancer cells display increased mitochondrial respiration rate and OXPHOS activity as their energy supply (orange line from pyruvate to acetyl-CoA, Figure 2) [17]. Additionally, several studies have shown that glycolysis serves as a primary energy source [11, 16, 17] and others indicate that OXPHOS is the principal site of ATP production [17]. Cancer cells may affect alterations in metabolism toward OXPHOS to support their high energy needs by changes in mitochondrial fission and fusion and then adapt to the constantly changing microenvironments. Furthermore, emerging evidence reveals that metabolic reprogramming plays a critical role in malignant biological behaviors, such as the initiation and progression of multiple cancers, including ovarian cancer [18] (Figure 1).
Figure 2.
The key regulators of metabolic profiles in ovarian cancer. A schematic diagram of the glycolysis pathway with metabolites (square boxes) [60]. The chief regulatory molecules that either promote (yellow box and red letters) or suppress (purple box and white letters) glycolytic enzymes are shown [60]. The blue and orange arrows indicate the metabolic shift toward glycolysis and OXPHOS, respectively.
3.3. Metabolism-related target molecules in ovarian cancer
The altered expression of many metabolism-related genes and proteins exhibits a metabolic shift to favor glycolysis in cancer cells, such as:
Glucose transporters (GLUT1): Upregulation of GLUT1 expression results in an increase in glucose uptake, supplying energy to various cancer cells. A high level of GLUT1 expression is observed in high-grade serous ovarian cancer (HGSC) [18]. Also, GLUT1 plays a functional role in glucose metabolism in ovarian clear cell carcinoma (CCC) [19]. GLUT1, a downstream target of hypoxia-inducible factor-1alpha (HIF-1α), has been demonstrated to play a critical role in the Warburg effect [18].
Hexokinase (HK): Hexokinase II (HKII), the first rate-limiting enzyme of glycolysis and the HIF-1α target gene, converts glucose to glucose 6-phosphate (G6P) [11]. HKII contributes to tumor aggressiveness and is associated with cancer cell initiation, growth, maintenance, and chemoresistance in ovarian cancer cells [20]. Deletion of HKII impairs tumor burden in experimental mice models, suggesting that HKII is a critical enzyme involved in tumor progression [21].
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3): 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) is involved in both the synthesis and degradation of fructose-2,6-bisphosphate, a regulatory molecule that controls glycolysis [18]. PFKFB3 is upregulated in ovarian cancer and is a potential biomarker for chemoresistance, recurrence, and prognosis [22].
Pyruvate kinase (PK): PK, the last rate-limiting enzyme in glycolysis, converts phosphoenolpyruvate to pyruvate. High levels of PKM2 are associated with tumor growth, leading to poor prognosis in patients with ovarian cancer [23].
Pyruvate dehydrogenase kinase (PDK)/Pyruvate dehydrogenase (PDH): PDK1 promotes tumor initiation and progression via the HIF-1α [24] and Akt signaling axis [25]. High expression of PDK1 has been correlated to tumor size, the International Federation of Gynecology and Obstetrics (FIGO) stage, metastases, and overall survival [26]. Moreover, PDK2 [27] and PDK4 [28] have been reported to promote cisplatin resistance in ovarian CCC by suppressing mitochondrial OXPHOS. Additionally, their high levels are associated with a poor prognosis in patients with ovarian cancer. In contrast, Lohneis et al. [29] found that patients with ovarian cancer who were positive for PDK1 expression had more prolonged survival than those who were negative.
Lactate dehydrogenase (LDH): LDHA converts pyruvate to lactate, but LDHB favors the reverse reaction. A high expression of LDHA triggers protumorigenic acidification through excessive lactate [30]. Lactate is transported intracellularly and intercellularly by monocarboxylate transporters (MCT) [31]. LDHA plays a pivotal role in glycolysis and promotes tumor growth, enhancing the Warburg effect [32]. A high level of LDHA is a poor prognostic biomarker for patients with ovarian cancer [33]. Oncoproteins c-Myc and HIF-1 transcriptionally upregulate glycolysis through direct activation of glycolysis-related target genes, including LDHA [10].
Cancer cells are frequently subjected to hypoxic milieu. Glycolysis-related molecules, such as GLUT1, HKII, PFK1 (phosphofructokinase 1), PDK, LDHA, phosphoglycerate kinase 1 (PGK1), fructose-bisphosphate aldolase A (ALDOA), and PKM2, are HIF-1α target genes and are activated in a hypoxic environment [34]. HIF-1 activates these glycolysis molecules, which block the flux of pyruvate into mitochondria [10]. Moreover, the tumor suppressor p53 inhibits glycolysis via suppressing HKII expression and stimulates OXPHOS. Hence, the loss of functional p53 shifts ovarian cancer cells toward glycolysis from the OXPHOS phenotype [10].
The role of mitochondria: Metabolic changes may be associated with mitochondrial dysfunction through mutations in mitochondrial DNA detected in ovarian cancer [35]. Moreover, dysregulation of the TCA cycle enzymes (e.g., succinate dehydrogenase (SDH) [36] and fumarate hydratase (FH) [35]) has been identified in serous ovarian cancer [37]. SDH is located in the inner membrane of the mitochondria and converts succinate to fumarate in the TCA cycle. FH converts fumarate to malate [12]. Thus, mutations or lack of function of SDH and FH result in increased levels of fumarate and succinate [12]. The loss-of-function mutations or dysregulation in most mitochondrial enzymes can lead to the stabilization of HIF-1α, which primarily affects the metabolic profiles, shifting tumor cells toward glycolysis from the OXPHOS phenotype [12]. These cancer cells are forced to rely mainly on glycolysis as the main energy source [16]. Cancer cells that have acquired a metabolic adaptation to meet the energy demand can ensure proliferation. However, Lim et al. [38] reported that aberrant gene expression and a loss of function of the mitochondria-related enzymes are rare in human ovarian and peritoneal cancer tissues. Furthermore, some ovarian cancers have been reported to show metabolic reprogramming toward OXPHOS [39, 40]. It remains unclear whether ovarian cancer inherently retains OXPHOS competent or dysfunctional mitochondria.
In addition, mitochondrial function is known to be affected by fission-fusion dynamics. Increased mitochondrial fission has been reported in several types of cancer cells, including ovarian cancer, and promotes glycolysis over OXPHOS for energy generation [41]. Furthermore, unopposed mitochondrial fission plays a role in conferring pluripotency on stem-like cells [42]. Therefore, cancer cells can adapt to unfavorable environments for survival by modulating mitochondrial morphologies and metabolic functions.
3.4. The metabolic profile of ovarian cancer
3.4.1. The metabolic profile of ovarian cancer stem cells (CSCs)
EOC is characterized by intertumoral (i.e., tumor subtype [histological and molecular subtyping]) and intratumoral (i.e., within the tumor microenvironment) heterogeneity. Intratumoral changes in the metabolic profile of ovarian cancer cells are discussed separately for cancer stem cells (CSCs) and non-stem cancer cells (hereafter referred to as non-CSCs; i.e., differentiated cancer cells) (Figure 3). The identification of CSCs is crucial for studying tumor biology, such as cancer initiation, progression, metastasis, recurrence, chemoresistance, and tumor metabolism [3]. Several surface markers for CSC have been identified, including Cluster of differentiation 44 (CD44), CD24, CD117, CD133, aldehyde dehydrogenase (ALDH), the G subfamily of ATP-binding cassette transporters (ABCG), and epithelial-specific antigen (ESA) [3]. The CSC markers used vary between studies. Cancer metabolic changes are considered to be closely linked with CSCs. However, there is still a lack of understanding of the metabolic characteristics of CSCs.
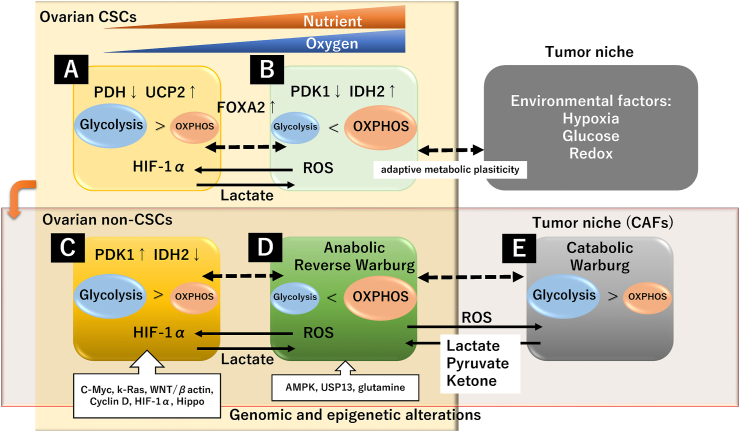
Figure 3.
Metabolic profile of ovarian cancer in the tumor microenvironment. The upper row (A and B) shows ovarian CSCs, and the lower row (E, D, and E) shows non-CSCs. A, C, and E cells favor glycolysis over OXPHOS, and B and D cells predominantly prefer OXPHOS. Dotted arrows indicate adaptive metabolic plasticity.
CD44, a cell surface transmembrane glycoprotein, has been widely implicated as a CSC marker in several cancers. Myeloid differentiation response gene 88 (MyD88) expression is downregulated during retinoic acid-induced differentiation of pluripotent cells. Therefore, CD44 and MyD88 are thought to be the molecular characterization of ovarian CSCs [43]. In the CD44+/MyD88+ ovarian CSCs, PDH was downregulated and uncoupling protein 2 (UCP2) was upregulated [44]. Inactivation of PDH limits pyruvate to acetyl-CoA conversion in mitochondria and is critical for metabolic adaptation to hypoxia through converting pyruvate to lactate [44]. PDH expression and activity are reduced by HIF-1α, a master regulator of CSC adaptation to hypoxia. Moreover, UCP2 is an inner mitochondrial membrane protein and plays a regulatory role in lowering mitochondrial OXPHOS and facilitating glycolysis [45]. Based on the above, ovarian CSCs cannot switch from glycolysis to OXPHOS and rely on glycolysis as the means to maintain quiescence and the self-renewal potential through these two molecular phenotypes (CSCs are marked “A” in Figure 3) [44]. In general, CSCs in many tumor types, including breast cancer, lung cancer, colon cancer, osteosarcoma, and glioblastoma, rely primarily on glycolysis and exhibit impairment of OXPHOS than their differentiated cancer cells [46, 47]. Therefore, ovarian CSCs are thought to be highly sensitive to cell death triggered by glucose deprivation.
In contrast, a growing body of evidence shows that CSCs are more dependent on mitochondrial OXPHOS [46]. In particular, lung and pancreatic CSCs exhibited increased mitochondrial membrane potential and decreased glucose consumption compared with non-CSCs [48, 49]. Moreover, leukemia stem cells exhibit a metabolic profile, dependent on OXPHOS [50]. Additionally, there is an obvious difference in the metabolic profile among glioblastoma cells with different passage numbers [51]. Early-passage glioblastoma stem cells were found to rely more on OXPHOS than glycolysis [51]. Indeed, the involvement of mitochondrial oxidative metabolism in ovarian CSCs has been reported (CSCs are marked “B” in Figure 3). CD44 and CD117 were used as stem cell markers for EOC in a mouse model [52]. The CD117 gene is known as c-kit or stem cell factor receptor. CD44+/CD117+ CSCs isolated from the peritoneal fluid of HGSC patients are a subset of tumor cells with the ability to form new tumors in mice. These ovarian CSCs showed decreased expression of PDK1 and increased expression of isocitrate dehydrogenase (IDH2) than the non-CSC population [52]. IDH2 catalyzes the formation of α-ketoglutarate (2-oxoglutarate) from isocitrate during the TCA cycle. Changes in these enzyme activities upregulate OXPHOS activity to fuel tumorigenesis. Upon activation of OXPHOS, ovarian CSCs also exhibited an increased mitochondrial ROS production [53]. ROS created as a byproduct of the mitochondrial OXPHOS play a key role in inflammation and oxidative injury; however, the endogenous antioxidants exert protective roles against ROS injuries [53]. Collectively, some ovarian CSCs show metabolic reprogramming toward OXPHOS and fulfill the energy demands, possibly through the downregulation of PDK1 and upregulation of IDH2. There is an ongoing debate about whether ovarian CSCs uniquely depend on glycolysis or OXPHOS for survival, or adapt to environmental challenges by alternating between glycolysis and OXPHOS [46].
3.4.2. The metabolic profile of ovarian non-CSCs
Several studies have shown that ovarian non-CSCs rely predominantly on the glycolytic phenotype [39, 54]. GLUT1 and HKII are significantly upregulated in HGSC compared with non-HGSC [54] (non-CSCs are marked “C” in Figure 3). Moreover, a serous/non-CCC cell line, OVCA420, exhibited reduced mitochondrial membrane potential, oxygen consumption rate, and ATP production and triggered a metabolic switch to glycolysis, indicating mitochondrial impairment [39]. This was accompanied by changes in mitochondrial morphology and increased mitochondrial fission via increased expression of the mitochondrial fission protein dynamin-related protein 1 (Drp1) [39].
However, there is growing evidence that HGSC and CCC undergo metabolic reprogramming toward OXPHOS that allows them to survive [55, 56] (non-CSCs are marked “D” in Figure 3). Notably, CCC cell lines (ES-2 and TOV-21-G) were highly metabolically active, with increased expression of glycolysis-related genes [39]. Furthermore, CCC overexpressing the transcription factor hepatocyte nuclear factor-1beta (HNF-1β) gene produced energy by glycolysis rather than mitochondrial OXPHOS for energy production [4]. HNF-1β is involved in glucose metabolism and facilitates glucose uptake and glycolysis [57]. However, total OXPHOS activities were significantly increased in EOC, including HGSC and CCC, compared with normal cells, as indicated by the higher levels of indicators of mitochondrial respiration (e.g., upregulation of OXPHOS enzymes, increased oxygen consumption rate, and increased respiratory capacity) [39, 40]. Therefore, these studies suggest that EOC is dependent on both glycolysis and OXPHOS and harbors the ability to metabolic shift between glycolysis and OXPHOS in both CSCs and non-CSCs [4, 55]. EOC has the metabolic capacity to regulate energy production for survival but displays metabolic heterogeneity and plasticity. Collectively, the metabolic profile could vary depending on ovarian cancer subtype, genomic and epigenetic alterations of metabolism-related genes, or technical limitations (e.g., early or late passages).
3.5. The metabolic plasticity of ovarian cancer
3.5.1. The metabolic plasticity of cancer stem cells
The metabolic phenotype of ovarian cancer depends on metabolic plasticity, i.e., the ability to optimize metabolic pathways and adapt to different environmental conditions. Metabolic plasticity occurs due to active metabolic reprogramming under normoxic and hypoxic, quiescent and proliferative, or nutrient supply and starvation conditions [46]. Furthermore, some CSCs can switch from OXPHOS to glycolysis to compensate for inadequate mitochondrial functions through decreased expression of PDH and increased expression of UCP2 [4] during cell differentiation under hypoxic conditions (“A” in Figure 3). Although, CSCs exert cancer metabolic plasticity through decreased expression of PDK1, favoring mitochondrial OXPHOS over glycolysis under normoxic conditions [4] (“B” in Figure 3). IDH2 was found to cause ROS-dependent destabilization of HIF-1α in normoxia [58]. Thus, tumor microenvironmental conditions, such as hypoxia, may drive the plasticity of CSCs [59]. In addition, glutamine as a nutrient is essential for maintaining the TCA cycle, cellular redox homeostasis, and the survival of ovarian cancer cells [60, 61]. Its deficiency leads to metabolic reprogramming to glycolysis and increases the CSC population [60]. These CSCs can adapt to starvation and hypoxia by upregulating glucose transporters and glycolytic enzymes and switching from OXPHOS to a glycolytic phenotype [62]. Additionally, the autophagy process has been reported to have a significant role in maintaining the CSC population [63]. Ovarian CSCs presented higher basal autophagy compared with their non-stem counterpart [63]. Autophagy maintains the characteristics of ovarian CSCs by the forkhead box A2 (FOXA2) gene, a transcription factor that controls cell differentiation [64]. FOXA2 can differentiate pluripotent stem cells into progenitors for human tissues. The dynamic plasticity model revealed that ovarian cancer cells harbor the ability to shift between non-CSC and CSC states in certain tumor milieu [65]. Therefore, ovarian CSCs can respond and adapt to dynamic microenvironmental conditions, including limited nutrient supplies, hypoxia, inflammation, and oxidative stress. High metabolic plasticity can drive CSCs to switch between glycolysis and OXPHOS to acquire energy. Hence, intratumoral heterogeneity promotes metabolic plasticity.
3.5.2. The metabolic plasticity of ovarian non-CSCs
Cell differentiation requires an adaptive metabolic shift from glycolysis to OXPHOS for sustained proliferation [66]. However, rapidly growing cancer cells also need glycolysis metabolism that provides ATP and building blocks, such as intermediate metabolites necessary for the synthesis of nucleic acids, proteins, and lipids. Ovarian cancer has revealed bioenergetic diversity [17]. Smolkova et al. demonstrated that the (epi)genetic program influences metabolic choices of different cell types during the process of tumorigenesis by dynamically controlling glycolysis and OXPHOS activities [67] (“C”, “D”, and “E” in Figure 3). Furthermore, oncogenic mutations, such as amplification and increased oncogene expression, and tumor suppressor deletions (e.g., c-Myc, K-Ras, mutant p53, cyclin D1, HIF-1α, and the WNT/β-catenin signaling pathway) contribute to tumor metabolic reprogramming [68, 69]. Oncogenic signaling pathways, such as phosphoinositide 3-kinase (PI3K)/AKT, Myc, and the Hippo pathway mediated upregulation and hyperactivation of metabolism-related genes [70]. K-Ras and c-Myc are responsible for increased glycolysis [71, 72]. PIK3CA and ubiquitin-specific peptidase 13 (USP13) genes have been reported to be co-amplified in about 30% of HGSC [73]. USP13 activates mitochondrial energy metabolism, autophagy, DNA damage response, glutaminolysis, and fatty acid synthesis [73]. Moreover, glutaminase (GLS), a key enzyme in TCA glutaminolysis, is a downstream target of the c-Myc and mTOR oncogenes [74]. Additionally, the Hippo pathway plays a vital role in inducing glycolysis-dependent growth and reducing mitochondrial respiration [75]. In addition, HIF-1α-mediated PDK1 expression acts as a shutdown switch of OXPHOS. However, incomplete shutdown allows cancer cells to induce a metabolic shift to OXPHOS [55]. In particular, HIF-1 positively regulates glycolysis in cancer cells and promotes tumor growth, while adenosine monophosphate-activated protein kinase (AMPK) negatively regulates glycolysis and, in turn, enhances mitochondrial function [55]. HIF-1α and AMPK levels are higher in some cancer cells, which activate aerobic glycolysis and mitochondrial OXPHOS [55]. Therefore, EOC exhibits intratumoral subclonal heterogeneity at the genomic, epigenetic, and phenotypic levels, which may determine metabolic diversity [17]. Metabolic stresses that arise during tumor initiation and progression may ultimately select subclones with desired bioenergetics.
3.6. Metabolic phenotypes in the tumor microenvironment
Tumor cells have been reported to be influenced by their surrounding normal stroma, cancer-associated fibroblasts, local immune cells, endothelial cells, and adipocytes within the tumor microenvironment [76]. Metabolic stress (e.g., genetically or epigenetically predisposed, environmental factors, and therapy response) also affects the energy homeostasis in host cells within the tumor. Ovarian cancer cells with the metabolic shift toward OXPHOS induce oxidative stress, which in turn upregulates glycolysis in adjacent cancer-associated fibroblasts (CAFs) through upregulating HIF-1α expression [12] (non-CSCs marked “D” and “E” in Figure 3). CAFs fuel the proliferation of anabolic cancer cells by supplying several catabolites such as pyruvate, lactate, ketones, glutamine, glutamate, alanine, and fatty acids [77]. MCT4 in CAFs plays a crucial role in transporting fuels such as lactates and expelling them into the tumor microenvironment [13]. These fuels are utilized by cancer cells (“D” in Figure 3) as a critical energy source for mitochondrial OXPHOS [12]. Moreover, the tumor milieu may suppress the activity of immune cells due to an increased accumulation of lactate (reviewed in [78]). Lactate inhibited the cytolytic function of natural killer (NK) cells through lower expression of perforin and granzyme [79]. Tregs promote tumor growth by relying more on OXPHOS than glycolysis [80]. The hypoxia-induced upregulation of the HIF-1α gene skews macrophage polarization toward an immunosuppressive phenotype [81]. The interaction between cancer and stromal cells allows cancer cells to enhance mitochondrial energy metabolism through the reverse Warburg effect (“D” in Figure 3) [77]. On the other hand, in some cancer cells (“C” in Figure 3) that produce high levels of lactate in the tumor microenvironment, lactate fuels the adjacent cancer cell proliferation (“D” in Figure 3) [55]. In addition, like non-CSCs, lactate production in glycolytic CSCs (“A” in Figure 3) was reported to fuel non-glycolytic CSCs (“B” in Figure 3), providing evidence to support the reverse Warburg effect [82]. There seems to be no difference between ovarian CSC and non-CSC in terms of the metabolic stress response. The metabolic phenotype of malignant cells and non-malignant cells in the tumor microenvironment is also heterogeneous, and such crosstalk is bidirectional. Collectively, metabolic reprogramming in cancer cells and adjacent host cells results in the spatiotemporal fine-tuning of the phenotypes of “the Warburg effect” and “the Reverse Warburg effect” [82].
3.7. Therapeutic strategies
Targeted therapeutic agents for glycolysis and OXPHOS interventions in human cancers have been previously summarized [3, 4]. This section briefly summarizes recent advances in molecular-based therapies focused on metabolic profiles in ovarian cancer.
3.7.1. Targeted therapeutic agents for glycolysis
The outlines of therapeutic drugs targeting glycolysis are as follows:
2-DG 2-deoxy-D-glucose: treatment with 2-deoxy-D-glucose (2-DG), an HKII inhibitor, reduced tumor burden in a mouse xenograft model of human ovarian cancer [44]. CCC also displayed strong sensitivity to 2-DG in vitro [39].
PFKFB3: PFKFB3 contributed to metabolic reprogramming and chemoresistance, possibly through the nuclear factor-kappaB (NF-κB) signaling pathway [22]. Potent small-molecule inhibitors of PFKFB3 (e.g., PFK158 and 3PO) reduced lactate levels, sensitized ovarian cancer cells to cisplatin, or impaired the cancer stemness features of ALDH+/CD44+ ovarian CSCs [22].
DCA: Dichloroacetic acid (DCA) reversed the Warburg effect by inhibiting the PDK1-induced metabolic switch from mitochondrial OXPHOS toward glycolysis [9, 11, 24, 83, 84]. Furthermore, inhibition of PDKs (e.g., dicumarol, a coumarin compound) reversed cisplatin resistance by increasing the production of mitochondrial ROS [9, 27].
HIF-1α inhibition: Hypoxia drives a metabolic switch favoring glycolysis over OXPHOS via upregulating the HIF-1α/PDK1 axis and then induced CSC enrichment [4]. Knockdown of HIF-1α leads to ovarian cancer cell death through overproduction of mitochondrial ROS and improved cisplatin resistance [85].
These findings suggest that glycolysis inhibition may be a potential therapeutic target for ovarian cancer if cancer cells are solely reliant on glucose for survival [86].
3.7.2. Targeted therapeutic agents for OXPHOS
In this section, we provide a brief overview of treatments targeting OXPHOS. Nayak et al. [55] provided an overview of the metabolic function in HGSC, summarized the role of small-molecule inhibitors of OXPHOS, elucidated the mechanism of action of the drugs, and suggested ways to overcome the clinical problem. Furthermore, antidiabetic agents (e.g., metformin and thiazolidinediones) and anti-malaria agents (e.g., atovaquone) have been reported to suppress cancer cell proliferation by inhibiting the mitochondrial electron transport chain [55, 56]. In addition, treatment strategies with OXPHOS inhibition to eradicate chemoresistant cancer stem cells have also been studied [2]. The failure of platinum treatment in ovarian cancer is due to an increase in ALDH+ ovarian CSCs through activating mitochondrial OXPHOS [2]. Therefore, OXPHOS inhibition blocks the platinum-dependent enrichment of CSCs and inhibits the development of chemoresistance. Recently, the metabolic pathways not only in tumors and cancer stem cells but also in stromal cells and immune cells in the tumor microenvironment have been recognized as targets for the development of novel anti-cancer therapies.
4. Discussion
In this review, we summarize the metabolic profiles of EOC (ovarian CSCs and non-CSCs), the key molecules involved in metabolic reprogramming, metabolic heterogeneity and plasticity, and adaptation to the dynamic tumor microenvironment, and discuss potential novel therapeutic strategies. The review article covers papers through December 2021. From January 2022 to September 2022, more than 30 papers related to “metabolic reprogramming in ovarian cancer” were published, making it an area of deep interest for many researchers.
EOC is characterized by clinical, morphological, and molecular heterogeneity at the genomic, epigenetic, and phenotypic levels. Highly proliferating ovarian cancer cells rely mainly on glycolysis to supply energy [87]. Glycolysis supports NADPH generation and increases macromolecular biosynthesis by activation of the pentose phosphate pathway [87]. Increased lactate production from glycolysis results in cellular acidification and promotes HIF-1α stabilization, which upregulates glycolysis [87]. The expression of the glycolytic genes is upregulated by activation of oncogenes (e.g., c-Myc and k-Ras), transcription factors (e.g., HIF1α and AKT), and loss of tumor suppressor genes (e.g., BRCA1/2 and mutated p53) [68, 69]. Several studies have identified different expressions of two metabolism-related genes, PDH and UCP2 that favor glycolysis over OXPHOS [44]. Furthermore, changes in expression patterns of PDK1 and IDH2 are involved in molecular mechanisms shifting ovarian cancer cells to OXPHOS, indicating that OXPHOS is the primary source of ATP in some ovarian cancer cells [88, 89]. Cancer cells adapt to unfavorable conditions or variations in the tumor environment, such as nutrient starvation or glucose deprivation, hypoxic stress, acidification, excessive ROS generation, and fine-tuned metabolic reprogramming [61, 69]. The metabolic pathways (e.g., glycolysis, OXPHOS, glutaminolysis, or lipid metabolism) are reprogrammed to adapt to a variety of environmental stresses [61, 69]. Therefore, not all ovarian cancer cells exhibit the Warburg effect [44].
Furthermore, not only non-CSCs but also CSCs have adaptive strategies for survival. Both populations adapt to the tumor microenvironment and evade host immune attacks. Indeed, breast CSCs have been found to comprise a hierarchy of quiescent and proliferating CSCs [87]. Quiescent breast CSCs prefer OXPHOS rather than glycolysis to generate ATP [87]. In breast cancer, CSCs marked “A” (Figure 3) and CSCs marked “B” (Figure 3) correspond to proliferating and quiescent CSCs, respectively [87]. On the other hand, some ovarian CSCs (CSCs marked “A” in Figure 3) favor glycolytic metabolism over OXPHOS, while others (CSCs marked “B” in Figure 3) prefer OXPHOS through decreased levels of PDK1 and increased expression of IDH2 [52]. Both ovarian CSCs and non-CSCs can predominantly utilize catabolites from adjacent host cells through the reverse Warburg effect [87]. Specifically, cancer cells secrete ROS into the tumor microenvironment through OXPHOS-dominant metabolic reprogramming and sensitize neighboring CAFs to oxidative stress [82]. CAFs undergo glycolysis and provide fuel to cancer cells (i.e., lactate, pyruvate, ketone, and fatty acids) [34, 82, 90]. Survival strategies for cancer cells themselves and for neighboring host stromal cells include metabolic plasticity and hijacking [91].
It is crucial to select certain patients for a specific therapeutic option. Cancer cells often display a hybrid glycolysis/OXPHOS phenotype, regulated by genomic and epigenetic variations in metabolism-related genes [92]. However, the extent of the dependence of cancer cells and CAFs on glycolysis or OXPHOS in an individual patient's tumor remains largely unknown. Consequently, the influence of metabolic heterogeneity should be considered before cancer treatment. Developing non-invasive techniques for the fast and accurate identification of metabolic alterations and metabolic reprogramming status could facilitate optimal cancer treatment. The cancer metabolism field is entering an exciting era with the advent of novel treatments targeting metabolic reprogramming, shifting from basic research to clinical validation.
5. Current and future perspectives
In this review, we have focused on controlling the spatiotemporal fine-tuning of the metabolic phenotypes and heterogeneity in cancer cells and host cells and presented current and future perspectives on treatment. Ovarian cancer generally favors glycolysis over OXPHOS [15, 44]. However, cancer cells induce mitochondrial energy production by cross-talking with surrounding host cells during the different stages of cancer (e.g., cancer initiation, progression, metastasis, or chemoresistance). First, inhibition of the glycolysis pathway can induce cancer cell death if early-stage ovarian cancer is largely unaffected by surrounding host cells and is heavily dependent on glycolysis for survival. Glycolysis inhibitors (e.g., 2-DG, DCA, PFKFB3 inhibitors, and HIF-1α inhibitors) reverse the Warburg effect and induce cell death by switching cytoplasmic glucose metabolism toward mitochondrial OXPHOS [11]. Second, advanced, recurrent, or metastatic ovarian cancer can survive in an unfavorable environment with the help of the metabolic capacities of the surrounding host cells, such as CAFs [91]. CAFs promote the proliferation of anabolic cancer cells through the reverse Warburg effect [55]. Thus, OXPHOS may serve as a potential therapeutic target for ovarian cancer with upregulated mitochondrial respiration [55, 93]. However, a distinctive metabolic property of cancer cells themselves and adjacent host cells changes dynamically during tumor progression. Thus, the fast and accurate detection of changes in metabolic reprogramming is a major challenge. In first-line therapy, the differential expression of genes, proteins, and enzymes related to glycolysis and OXPHOS can be analyzed by transcriptome data-based gene expression analysis, immunohistochemistry, and enzyme-linked immunosorbent assay in surgically removed biopsies. Dynamic changes in intratumoral and intertumoral metabolism eventually led to therapeutic resistance [94]. After the failure of first-line therapy, liquid biopsy can offer a less invasive approach for repeated sampling of tumor markers (e.g., circulating tumor cells, circulating tumor DNA (ctDNA), circulating cell-free DNA (cfDNA), extracellular vesicles, and exosomes) and selection of therapy targeted to genomic and molecular alterations within an individual's tumor [94]. Characterization of genomic and mutational heterogeneity using ctDNA profiling could track metabolic patterns [94]. Technological advances in ctDNAs characterization (e.g., the development of single-cell metabolic phenotyping or single-cell transcriptomic analysis) can provide clinicians with novel information on metabolic alterations of the entire tumor [94]. Furthermore, using single-cell on-chip metabolic cytometry and fluorescent metabolic probes, Li et al. [14] identified extensive metabolic heterogeneity of tumor cells in pleural effusions in lung adenocarcinoma patients. Therefore, examples of the diagnostics platforms may include liquid biopsy using ctDNA and serologic detection of bioactive metabolites or exosomes containing RNAs and microRNAs [61, 90]. Changes in metabolic phenotype are highly complex but can predict patient survival and their responses to therapies [14]. Understanding the fine-tuning of metabolic reprogramming is essential for developing novel therapeutic strategies for inhibiting tumor progression.
6. Conclusion
We summarize the metabolic profiles and adaptation to the tumor microenvironment and discuss potential novel therapeutic strategies. The metabolic profiles of ovarian cancer cells, including CSCs and non-CSCs, are influenced by the tumor microenvironment (such as normoxic and hypoxic, quiescent and proliferative, or nutrient supply and starvation conditions). The energy homeostasis in cancer cells and host cells within the tumor may be regulated by metabolic stress, including genomic and epigenetic alterations of metabolism-related genes. Ovarian cancer cells may evolve by adapting to environmental challenges by alternating between glycolysis and OXPHOS. Significant advances in ctDNA-based liquid biopsy technology are essential for analyzing ever-changing metabolic patterns. Therefore, there is a dire need for the development of new therapies that take into account the influence of metabolic heterogeneity.
Declarations
Author contribution statement
Hiroshi Kobayashi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The author thanks Mrs. Toyomi Kobayashi for creating the figures.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Tew W.P., Lacchetti C., Ellis A., Maxian K., Banerjee S., Bookman M., et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J. Clin. Oncol. 2020;38(30):3468–3493. doi: 10.1200/JCO.20.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sriramkumar S., Sood R., Huntington T.D., Ghobashi A.H., Vuong T.T., Metcalfe T.X., et al. Platinum-induced mitochondrial OXPHOS contributes to cancer stem cell enrichment in ovarian cancer. J. Transl. Med. 2022;20(1):246. doi: 10.1186/s12967-022-03447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S.S., Ma J., Wong A.S.T. Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. J. Gynecol. Oncol. 2018;29(2):e32. doi: 10.3802/jgo.2018.29.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nantasupha C., Thonusin C., Charoenkwan K., Chattipakorn S., Chattipakorn N. Metabolic reprogramming in epithelial ovarian cancer. Am. J. Transl. Res. 2021;13(9):9950–9973. [PMC free article] [PubMed] [Google Scholar]
- 5.LeBleu V.S., O'Connell J.T., Gonzalez Herrera K.N., Wikman H., Pantel K., Haigis M.C., et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014;16(10):992–1003. doi: 10.1038/ncb3039. 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan A.S., Baty J.W., Dong L.F., Bezawork-Geleta A., Endaya B., Goodwin J., et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metabol. 2015;21(1):81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander H.M., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W., Su J., Xu H., Yu S., Liu Y., Zhang Y., et al. Dicumarol inhibits PDK1 and targets multiple malignant behaviors of ovarian cancer cells. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X.B., Gu J.D., Zhou Q.H. Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac. Cancer. 2015;6(1):17–24. doi: 10.1111/1759-7714.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L., Liu L., Chai W., Zhao T., Jin X., Guo X., et al. Dichloroacetic acid upregulates apoptosis of ovarian cancer cells by regulating mitochondrial function. OncoTargets Ther. 2019;12:1729–1739. doi: 10.2147/OTT.S194329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pustylnikov S., Costabile F., Beghi S., Facciabene A. Targeting mitochondria in cancer: current concepts and immunotherapy approaches. Transl. Res. 2018 Dec;202:35–51. doi: 10.1016/j.trsl.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitaker-Menezes D., Martinez-Outschoorn U.E., Lin Z., Ertel A., Flomenberg N., Witkiewicz A.K., et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z., Wang Z., Tang Y., Lu X., Chen J., Dong Y., et al. Liquid biopsy-based single-cell metabolic phenotyping of lung cancer patients for informative diagnostics. Nat. Commun. 2019;10(1):3856. doi: 10.1038/s41467-019-11808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Duan W., Li X., Liu J., Li D., Ye L., et al. PTTG regulates the metabolic switch of ovarian cancer cells via the c-myc pathway. Oncotarget. 2015;6(38):40959–40969. doi: 10.18632/oncotarget.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Han G., Li X., Kan Q., Fan Z., Li Y., et al. Mitochondrial pyruvate carrier function determines cell stemness and metabolic reprogramming in cancer cells. Oncotarget. 2017;8(28):46363–46380. doi: 10.18632/oncotarget.18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dar S., Chhina J., Mert I., Chitale D., Buekers T., Kaur H., Rattan R. Bioenergetic adaptations in chemoresistant ovarian cancer cells. Sci. Rep. 2017;7:8760. doi: 10.1038/s41598-017-09206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han C.Y., Patten D.A., Richardson R.B., Harper M.E., Tsang B.K. Tumor metabolism regulating chemosensitivity in ovarian cancer. Genes Cancer. 2018;9(5-6):155–175. doi: 10.18632/genesandcancer.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto T., Mandai M., Matsumura N., Yamaguchi K., Kondoh H., Amano Y., et al. Hepatocyte nuclear factor-1β (HNF-1β) promotes glucose uptake and glycolytic activity in ovarian clear cell carcinoma. Mol. Carcinog. 2015;54(1):35–49. doi: 10.1002/mc.22072. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X.Y., Zhang M., Cong Q., Zhang M.X., Zhang M.Y., Lu Y.Y., et al. Hexokinase 2 confers resistance to cisplatin in ovarian cancer cells by enhancing cisplatin-induced autophagy. Int. J. Biochem. Cell Biol. 2018;95:9–16. doi: 10.1016/j.biocel.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W., et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y.X., Siu M.K.Y., Wang J.J., Leung T.H.Y., Chan D.W., Cheung A.N.Y., et al. PFKFB3 regulates chemoresistance, metastasis and stemness via IAP proteins and the NF-κB signaling pathway in ovarian cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.748403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao T.K., Huang T.S., Liao Y.P., Huang R.L., Su P.H., Shen H.Y., et al. Pyruvate kinase M2 is a poor prognostic marker of and a therapeutic target in ovarian cancer. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K., Kong X., Feng G., Xiang W., Chen L., Yang F., et al. Investigation of hypoxia networks in ovarian cancer via bioinformatics analysis. J. Ovarian Res. 2018;11(1):16. doi: 10.1186/s13048-018-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han C.Y., Patten D.A., Lee S.G., Parks R.J., Chan D.W., Harper M.E., et al. p53 Promotes chemoresponsiveness by regulating hexokinase II gene transcription and metabolic reprogramming in epithelial ovarian cancer. Mol. Carcinog. 2019;58(11):2161–2174. doi: 10.1002/mc.23106. [DOI] [PubMed] [Google Scholar]
- 26.Yao S., Shang W., Huang L., Xu R., Wu M., Wang F. The oncogenic and prognostic role of PDK1 in the progression and metastasis of ovarian cancer. J. Cancer. 2021;12(3):630–643. doi: 10.7150/jca.47278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura S., Yamaguchi K., Murakami R., Furutake Y., Higasa K., Abiko K., et al. PDK2 leads to cisplatin resistance through suppression of mitochondrial function in ovarian clear cell carcinoma. Cancer Sci. 2021;112(11):4627–4640. doi: 10.1111/cas.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Qian Y., Gao M. Overexpression of PDK4 is associated with cell proliferation, drug resistance and poor prognosis in ovarian cancer. Cancer Manag. Res. 2018;11:251–262. doi: 10.2147/CMAR.S185015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohneis P., Darb-Esfahani S., Dietel M., Braicu I., Sehouli J., Arsenic R. PDK1 is expressed in ovarian serous carcinoma and correlates with improved survival in high-grade tumors. Anticancer Res. 2015;35(11):6329–6334. [PubMed] [Google Scholar]
- 30.Gatenby R.A., Gawlinski E.T., Gmitro A.F., Kaylor B., Gillies R.J. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 31.San-Millan I., Brooks G.A. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38:119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie H., Valera V.A., Merino M.J., Amato A.M., Signoretti S., Linehan W.M., et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol. Cancer Therapeut. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boran N., Kayikcioglu F., Yalvac S., Tulunay G., Ekinci U., Kose M.F. Significance of serum and peritoneal fluid lactate dehydrogenase levels in ovarian cancer. Gynecol. Obstet. Invest. 2000;49:272–274. doi: 10.1159/000010258. [DOI] [PubMed] [Google Scholar]
- 34.Schiliro C., Firestein B.L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021;10(5):1056. doi: 10.3390/cells10051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Dong L., Cui H., Shen D.H., Wang Y., Chang X.H., et al. Up-regulation of mitochondrial antioxidation signals in ovarian cancer cells with aggressive biologic behavior. J. Zhejiang Univ. - Sci. B. 2011;12(5):346–356. doi: 10.1631/jzus.B1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aspuria P.P., Lunt S.Y., Väremo L., Vergnes L., Gozo M., Beach J.A., et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metabol. 2014;2:21. doi: 10.1186/2049-3002-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selak M.A., Armour S.M., MacKenzie E.D., Boulahbel H., Watson D.G., Mansfield K.D., et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Lim H.Y., Ho Q.S., Low J., Choolani M., Wong K.P. Respiratory competent mitochondria in human ovarian and peritoneal cancer. Mitochondrion. 2011;11(3):437–443. doi: 10.1016/j.mito.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Dier U., Shin D.H., Hemachandra L.P., Uusitalo L.M., Hempel N. Bioenergetic analysis of ovarian cancer cell lines: profiling of histological subtypes and identification of a mitochondria-defective cell line. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0098479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N., Zhan X., Zhan X. The lncRNA SNHG3 regulates energy metabolism of ovarian cancer by an analysis of mitochondrial proteomes. Gynecol. Oncol. 2018;150(2):343–354. doi: 10.1016/j.ygyno.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Huang Q., Long X., Guo X., Sun X., Jin X., et al. Mitochondrial elongation-mediated glucose metabolism reprogramming is essential for tumour cell survival during energy stress. Oncogene. 2017;36:4901–4912. doi: 10.1038/onc.2017.98. [DOI] [PubMed] [Google Scholar]
- 42.Prieto J., Leon M., Ponsoda X., Sendra R., Bort R., Ferrer-Lorente R., et al. Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nat. Commun. 2016;7 doi: 10.1038/ncomms11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y., Zhang H., Zhang G., Shi Y., Huang J. Co-expression of CD44/MyD88 is a poor prognostic factor in advanced epithelial ovarian cancer. Ann. Transl. Med. 2019;7(5):91. doi: 10.21037/atm.2019.01.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvero A.B., Montagna M.K., Sumi N.J., Joo W.D., Graham E., Mor G. Multiple blocks in the engagement of oxidative phosphorylation in putative ovarian cancer stem cells: implication for maintenance therapy with glycolysis inhibitors. Oncotarget. 2014;5(18):8703–8715. doi: 10.18632/oncotarget.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J., Khvorostov I., Hong J.S., Oktay Y., Vergnes L., Nuebel E., et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30(24):4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peiris-Pagès M., Martinez-Outschoorn U.E., Pestell R.G., Sotgia F., Lisanti M.P. Cancer stem cell metabolism. Breast Cancer Res. 2016;18(1):55. doi: 10.1186/s13058-016-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng W., Gentles A., Nair R.V., Huang M., Lin Y., Lee C.Y., et al. Targeting unique metabolic properties of breast tumor initiating cells. Stem Cells. 2014;32(7):1734–1745. doi: 10.1002/stem.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye X.Q., Li Q., Wang G.H., Sun F.F., Huang G.J., Bian X.W., et al. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int. J. Cancer. 2011;129(4):820–831. doi: 10.1002/ijc.25944. [DOI] [PubMed] [Google Scholar]
- 49.Viale A., Pettazzoni P., Lyssiotis C.A., Ying H., Sánchez N., Marchesini M., et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagadinou E.D., Sach A., Callahan K., Rossi R.M., Neering S.J., Minhajuddin M., et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlashi E., Lagadec C., Vergnes L., Matsutani T., Masui K., Poulou M., et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108(38):16062–16067. doi: 10.1073/pnas.1106704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasto A., Bellio C., Pilotto G., Ciminale V., Silic-Benussi M., Guzzo G., et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5:4305–4319. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastò A., Bellio C., Pilotto G., Ciminale V., Silic-Benussi M., Guzzo G., et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5(12):4305–4319. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xintaropoulou C., Ward C., Wise A., Queckborner S., Turnbull A., Michie C.O., Williams A.R.W., Rye T., Gourley C., Langdon S.P. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC Cancer. 2018;18(1):636. doi: 10.1186/s12885-018-4521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nayak A.P., Kapur A., Barroilhet L., Patankar M.S. Oxidative phosphorylation: a target for novel therapeutic strategies against ovarian cancer. Cancers. 2018;10(9):337. doi: 10.3390/cancers10090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapur A., Mehta P., Simmons A.D., Ericksen S.S., Mehta G., Palecek S.P., et al. Atovaquone: an inhibitor of oxidative phosphorylation as studied in gynecologic cancers. Cancers. 2022;14(9):2297. doi: 10.3390/cancers14092297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mandai M., Amano Y., Yamaguchi K., Matsumura N., Baba T., Konishi I. Ovarian clear cell carcinoma meets metabolism; HNF-1β confers survival benefits through the Warburg effect and ROS reduction. Oncotarget. 2015;6(31):30704–30714. doi: 10.18632/oncotarget.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Agarwal E., Bertolini I., Ghosh J.C., Seo J.H., Altieri D.C. IDH2 reprograms mitochondrial dynamics in cancer through a HIF-1α-regulated pseudohypoxic state. FASEB J. 2019;33(12):13398–13411. doi: 10.1096/fj.201901366R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heddleston J.M., Li Z., Lathia J.D., Bao S., Hjelmeland A.B., Rich J.N. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer. 2010;102(5):789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasad P., Ghosh S., Roy S.S. Glutamine deficiency promotes stemness and chemoresistance in tumor cells through DRP1-induced mitochondrial fragmentation. Cell. Mol. Life Sci. 2021;78(10):4821–4845. doi: 10.1007/s00018-021-03818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang C., Qin Y., Zhang B., Ji S., Shi S., Xu W., et al. Energy sources identify metabolic phenotypes in pancreatic cancer. Acta Biochim. Biophys. Sin. 2016;48(11):969–979. doi: 10.1093/abbs/gmw097. [DOI] [PubMed] [Google Scholar]
- 62.Flavahan W.A., Wu Q., Hitomi M., Rahim N., Kim Y., Sloan A.E., et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat. Neurosci. 2013;16(10):1373–1382. doi: 10.1038/nn.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao S., Fortier T.M., Baehrecke E.H. Autophagy promotes tumor-like stem cell niche occupancy. Curr. Biol. 2018;28(19):3056–3064. doi: 10.1016/j.cub.2018.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng Q., Qin J., Zhang Y., Cheng X., Wang X., Lu W., et al. Autophagy maintains the stemness of ovarian cancer stem cells by FOXA2. J. Exp. Clin. Cancer Res. 2017;36(1):171. doi: 10.1186/s13046-017-0644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dirks P. Cancer stem cells: invitation to a second round. Nature. 2010;466:40–41. doi: 10.1038/466040a. [DOI] [PubMed] [Google Scholar]
- 66.Shetty D.K., Kalamkar K.P., Inamdar M.S. OCIAD1 controls electron transport chain complex I activity to regulate energy metabolism in human pluripotent stem cells. Stem Cell Rep. 2018;11(1):128–141. doi: 10.1016/j.stemcr.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smolková K., Plecitá-Hlavatá L., Bellance N., Benard G., Rossignol R., Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int. J. Biochem. Cell Biol. 2011;43(7):950–968. doi: 10.1016/j.biocel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Yang M., Su H., Soga T., Kranc K.R., Pollard P.J. Prolyl hydroxylase domain enzymes: important regulators of cancer metabolism. Hypoxia. 2014;2:127–142. doi: 10.2147/HP.S47968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hon K.W., Zainal Abidin S.A., Othman I., Naidu R. The crosstalk between signaling pathways and cancer metabolism in colorectal cancer. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.768861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park J.H., Pyun W.Y., Park H.W. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9(10):2308. doi: 10.3390/cells9102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neuzil J., Rohlena J., Dong L.F. K-Ras and mitochondria: dangerous liaisons. Cell Res. 2012;22(2):285–287. doi: 10.1038/cr.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., Zuo X., Sun X., Tian X., Teng Y. Hexokinase 2 promotes cell proliferation and tumor formation through the Wnt/β-catenin pathway-mediated cyclin D1/c-myc upregulation in epithelial ovarian cancer. J. Cancer. 2022;13(8):2559–2569. doi: 10.7150/jca.71894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han C., Yang L., Choi H.H., Baddour J., Achreja A., Liu Y., et al. Amplification of USP13 drives ovarian cancer metabolism. Nat. Commun. 2016;7 doi: 10.1038/ncomms13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen Y.A., Hong J., Asaka R., Asaka S., Hsu F.C., Suryo Rahmanto Y., et al. Inhibition of the MYC-regulated glutaminase metabolic Axis is an effective synthetic lethal approach for treating chemoresistant ovarian cancers. Cancer Res. 2020;80(20):4514–4526. doi: 10.1158/0008-5472.CAN-19-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White S.M., Avantaggiati M.L., Nemazanyy I., Di Poto C., Yang Y., Pende M., et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2-deficient tumor cells. Dev. Cell. 2019;49(3):425–443. doi: 10.1016/j.devcel.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng W., Dean D.C., Hornicek F.J., Shi H., Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol. Cancer. 2019;18(1):124. doi: 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lisanti M.P., Martinez-Outschoorn U.E., Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and "fibroblast addiction" are new therapeutic targets for drug discovery. Cell Cycle. 2013;12(17):2723–2732. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrot A., da Fonseca L.M., Salustiano E.J., Gentile L.B., Conde L., Filardy A.A., et al. Metabolic symbiosis and immunomodulation: how tumor cell-derived lactate may disturb innate and adaptive immune responses. Front. Oncol. 2018;8:81. doi: 10.3389/fonc.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Husain Z., Huang Y., Seth P., Sukhatme V.P. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol. 2013;191(3):1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 80.Wang T., Liu G., Wang R. The intercellular metabolic interplay between tumor and immune cells. Front. Immunol. 2014;5:358. doi: 10.3389/fimmu.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams C.B., Yeh E.S., Soloff A.C. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2 doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang L., Li W., Li X., Jin X., Liao Q., Li Y., et al. Reverse Warburg effect' of cancer-associated fibroblasts (Review) Int. J. Oncol. 2022;60(6):67. doi: 10.3892/ijo.2022.5357. [DOI] [PubMed] [Google Scholar]
- 83.Venturoli C., Piga I., Curtarello M., Verza M., Esposito G., Venuto S., et al. Genetic perturbation of pyruvate dehydrogenase kinase 1 modulates growth, angiogenesis and metabolic pathways in ovarian cancer Xenografts. Cells. 2021;10(2):325. doi: 10.3390/cells10020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olszewski U., Poulsen T.T., Ulsperger E., Poulsen H.S., Geissler K., Hamilton G. In vitro cytotoxicity of combinations of dichloroacetate with anticancer platinum compounds. Clin. Pharmacol. 2010;2:177–183. doi: 10.2147/CPAA.S11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ai Z., Lu Y., Qiu S., Fan Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016;373(1):36–44. doi: 10.1016/j.canlet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tyagi K., Mandal S., Roy A. Recent advancements in therapeutic targeting of the Warburg effect in refractory ovarian cancer: a promise towards disease remission. Biochim. Biophys. Acta Rev. Canc. 2021;1876(1) doi: 10.1016/j.bbcan.2021.188563. [DOI] [PubMed] [Google Scholar]
- 87.Patra S., Elahi N., Armorer A., Arunachalam S., Omala J., Hamid I., et al. Mechanisms governing metabolic heterogeneity in breast cancer and other tumors. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.700629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodriguez-Enriquez S., Carreno-Fuentes L., Gallardo-Perez J.C., Saavedra E., Quezada H., Vega A., et al. Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. Int. J. Biochem. Cell Biol. 2010;42(10):1744–1751. doi: 10.1016/j.biocel.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 89.Bellance N., Benard G., Furt F., Begueret H., Smolková K., Passerieux E., et al. Bioenergetics of lung tumors: alteration of mitochondrial biogenesis and respiratory capacity. Int. J. Biochem. Cell Biol. 2009;41(12):2566–2577. doi: 10.1016/j.biocel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Gaál Z. MicroRNAs and metabolism: revisiting the Warburg effect with emphasis on epigenetic background and clinical applications. Biomolecules. 2021;11(10):1531. doi: 10.3390/biom11101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitchell M.I., Engelbrecht A.M. Metabolic hijacking: a survival strategy cancer cells exploit? Crit. Rev. Oncol. Hematol. 2017;109:1–8. doi: 10.1016/j.critrevonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Jia D., Park J.H., Jung K.H., Levine H., Kaipparettu B.A. Elucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic states. Cells. 2018;7(3):21. doi: 10.3390/cells7030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ashton T.M., McKenna W.G., Kunz-Schughart L.A., Higgins G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018;24(11):2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 94.Xu C., Cao H., Shi C., Feng J. The role of circulating tumor DNA in therapeutic resistance. OncoTargets Ther. 2019;12:9459–9471. doi: 10.2147/OTT.S226202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.