Highlights
-
•
We characterized the relationship between obstructive sleep apnea and medial temporal structures, while considering the potential impact of individual characteristics on these associations (sex, cognitive status, and age).
-
•
We showed that obstructive sleep apnea is associated with increased cerebral medial temporal volume, specifically in women and participants with amnestic mild cognitive impairment.
-
•
Using free-water fraction highlighted that these hypertrophies may be explained in part by cerebral edema and neuroinflammation.
Keywords: Sleep-disordered breathing, Entorhinal cortex, Hippocampus, Sex differences, Mild cognitive impairment
Abstract
Medial temporal structures, namely the hippocampus, the entorhinal cortex and the parahippocampal gyrus, are particularly vulnerable to Alzheimer’s disease and hypoxemia. Here, we tested the associations between obstructive sleep apnea (OSA) severity and medial temporal lobe volumes in 114 participants aged 55–86 years (35 % women). We also investigated the impact of sex, age, cognitive status, and free-water fraction correction on these associations. Increased OSA severity was associated with larger hippocampal and entorhinal cortex volumes in women, but not in men. Greater OSA severity also correlated with increased hippocampal volumes in participants with amnestic mild cognitive impairment, but not in cognitively unimpaired participants, regardless of sex. Using free-water corrected volumes eliminated all significant associations with OSA severity. Therefore, the increase in medial temporal subregion volumes may possibly be due to edema. Whether these structural manifestations further progress to neuronal death in non-treated OSA patients should be investigated.
1. Introduction
Obstructive sleep apnea (OSA) is possibly the most harmful sleep disorder for brain health, as it fragments sleep chronically and provokes intermittent episodes of hypoxemia that lead to oxidative stress, neuroinflammation and neuronal death (Lim and Pack, 2014). OSA is associated with increased amyloid and tau burden, two hallmarks of Alzheimer’s disease (AD) (Bubu et al., 2019). In addition, most epidemiological studies have shown an increased risk of cognitive decline and dementia in apneic adults (Leng et al., 2017).
Neuroimaging studies have investigated whether neurodegenerative changes occur in middle-aged and older adults with OSA. The entorhinal cortex, the hippocampi and the parahippocampal gyri are particularly important to investigate, as they are early affected in AD (Braak and Braak, 1991) and vulnerable to hypoxemia (Bartsch et al., 2015). Previous studies found a loss of grey matter volume in these regions (Marchi et al., 2020, Owen et al., 2019, Shi et al., 2017), others did not report changes (André et al., 2020, Baril et al., 2017), and a few observed increased volumes (Cross et al., 2018, Macey et al., 2018, Rosenzweig et al., 2013). The latter phenomenon is intriguing and might be due to intra- or extracellular fluid accumulation (cerebral edema) affecting grey matter volume estimates (André et al., 2020, Baril et al., 2017, Baril et al., 2020, Rosenzweig and Morrell, 2017). In accordance with this hypothesis, experimental models showed reactive gliosis and higher brain water content in mice exposed to intermittent hypoxia compared to non-exposed mice (Baronio et al., 2013, Wang et al., 2018). In humans, an abnormally low whole brain free-water (FW) fraction (Baril. et al., 2020) has been observed in adults with OSA compared to non-apneic participants, suggesting intracellular edema. FW fraction is a neuroimaging algorithm applied to diffusion magnetic resonance imaging (MRI) that measures water diffusing freely in the extracellular space, providing an estimate of edema and neuroinflammation. Abnormally low FW fraction suggests intracellular edema or reactive gliosis (Anderova et al., 2011, Montal et al., 2018), while a high FW fraction suggests extracellular edema, and is observed concomitantly with age-related atrophy (Edde et al., 2020), mild cognitive impairment (MCI) and AD (Dumont et al., 2019, Montal et al., 2018).
Individual characteristics may also impact how OSA severity increases risks of neurodegeneration. These characteristics include being a woman, younger age (middle-aged in comparison with the elderly) and ongoing neurodegenerative processes (Bubu et al., 2020, Legault et al., 2021). Most OSA and neuroimaging studies have controlled for these variables but have not tested whether they interact with OSA severity to predict changes in brain structure. These factors have the potential to explain part of the heterogeneity observed in previous studies and could orient clinical strategies regarding OSA screening and treatment.
The present study aimed at 1) characterizing the links between OSA severity and grey matter volume in the hippocampi, the entorhinal cortex and the parahippocampal gyri, 2) testing how sex, age, and cognitive status affect these associations and 3) verify whether associations between OSA and medial temporal grey matter volumes could be partly explained by changes in water content by applying a FW fraction correction to volumes. We hypothesized that OSA severity, particularly hypoxemia, would be associated with medial temporal lobe hypertrophy and that these associations would be stronger in women, younger participants and those with amnestic MCI (aMCI). We also expected that part of the hypertrophy would be explained by cerebral edema.
2. Methods
We included participants aged 55 to 86 years old, recruited between August 2012 and March 2020. Most participants included in this study were from a project on OSA and MCI (n = 91) and were also included in past studies from our group (Baril et al., 2015, Baril., Gagnon, 2018, Baril et al., 2017, Baril et al., 2020, Gosselin et al., 2016, L'Heureux et al., 2021). The remaining participants included in the sample were part of a recent multicenter study on sleep and MCI (n = 45). These participants were recruited in Montreal (n = 25), or Sherbrooke (n = 20). Exclusion criteria were inability to communicate in French or English, diagnosed neurological or psychiatric diseases, sleep disorders other than OSA, treated OSA, cerebrovascular or pulmonary diseases, body mass index > 40 kg/m2, < 7 years of education, uncontrolled diabetes or hypertension, drugs or alcohol addiction or abuse, contraindications for MRI, dementia suspicion and use of psychoactive medication. We also excluded non-amnestic MCI participants from our sample, as medial temporal lobe changes are more central to the pathophysiology of participants with aMCI. Data from four protocols were used, all approved by institutional ethics committees (#2012–697, #12–13-008, #2010–468 and #MP-32–2018-1537), and participants gave their written consent.
All potential participants were scheduled for an initial visit, which allowed obtaining information regarding their eligibility for the study. Eligible participants proceeded to a second visit, which included an overnight polysomnography, a neuropsychological assessment, and questionnaires. Participants were then asked to return for a 3T MRI session (range: 3 days to 13 months; mean delay: 111 days ± 72 days).
2.1. Polysomnography
The polysomnography included a 12 to 19-channel electroencephalography montage based on the international 10–20 system and used mastoid references. Participants were recorded using a Grass system and digitized using Harmonie software (n = 91 participants; bandpass 0.3–100 Hz; sampling rate 256 Hz), or with Natus system (Brain Monitor, Trex or Embla NDx) (n = 45 participants; bandpass 0.3–300 Hz; sampling rate 512 Hz). The montage included an electrooculogram, an electrocardiogram, as well as chin and anterior tibialis electromyogram. An oronasal canula, an oronasal thermistor and a thoraco-abdominal strain gauge were used in addition to transcutaneous finger pulse oximeter. Apneas were defined as a reduction of airflow ≥ 90 % for at least 10 s, and hypopneas as a diminution of airflow ≥ 30 % for at least 10 s, ending with an oxygen desaturation ≥ 3 % or with an arousal (Berry et al., 2012). Sleep stage scoring was done according to the American Academy of Sleep Medicine criteria by an experienced sleep technologist (Berry et al., 2012).
2.2. Principal components analysis of OSA severity
Based on the methodology of past OSA neuroimaging studies (Cross et al., 2018, Baril et al., 2017), we used a principal components analysis (PCA) to extract independent variables of OSA severity. This allowed to include multiple markers of OSA severity into our analyses, while limiting multicollinearity and the number of statistical tests done. Briefly, we used a criterion of eigenvalues higher than 1 and a varimax rotation, and obtained two components (sleep fragmentation and hypoxemia; see Table 1).
Table 1.
Results from the principal component analysis of respiratory and sleep variables.
| Component |
||
|---|---|---|
| Sleep fragmentation | Hypoxemia | |
| Number of arousals associated with a respiratory event | 0.92 | 0.16 |
| Number of stage transitions from NREM1 and wakefulness | 0.81 | −0.05 |
| Apnea-hypopnea index, events/h | 0.73 | 0.41 |
| Micro-arousal index, events/h | 0.64 | 0.52 |
| Inverted minimum oxygen saturation, % | 0.22 | 0.81 |
| Inverted mean oxygen saturation, % | −0.02 | 0.82 |
| 3 % oxygen desaturation index, events/h | 0.57 | 0.66 |
| Accounted variance, % | 40.5 | 31.9 |
NREM1: stage 1 of non rapid-eye movement sleep.
2.3. MRI acquisition
All neuroimaging data were acquired between December 2012 and December 2019. The sample included 136 participants which underwent neuroimaging acquisition at the Neuroimaging Functional Unit of the Montreal Geriatric University Institute, with either the Siemen Magneton Trio Tim (n = 91) or the upgraded Siemen Prisma Fit scanner (n = 25), or at University Institute of Geriatrics of Sherbrooke with a 3T Ingenia Philips Scanner (n = 20). 91 participants completed the scanning protocol between December 2012 and July 2016 with the Magneton 3T Trio Tim Siemens Scanner with a 32-channel head coil. The parameters used were those of the Massachusetts General Hospital (Boston, Massachusetts, USA). A three-dimensional (3D-T1) T1-weighted Turbo Flash multi-echo Magnetization-prepared rapid gradient-echo (MPRAGE) sequence was acquired first using the following parameters: repetition time = 2,530 ms/root mean square of four echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms; inversion time: 1200 ms; matrix size = 256 × 256; field of view = 256 × 256 mm; voxel size = 1.0 mm isotropic; flip angle = 7°; and 176 sagittal orientations. In addition, a pulsed spin echo diffusion-weighted imaging sequence was acquired, as well as a reference image without diffusion (b = 0 s/mm2). The following parameters were used: 64 uniformly distributed directions; b value of 1000 s/mm2; repetition time = 9,100 ms; echo time = 89 ms; 72 slices; matrix size = 120 × 120 mm; field of view = 240 × 240 mm; voxel size = 2.0 mm isotropic; flip angle = 90°.
The other 45 participants were tested between August 2019 and December 2019 in Montreal and Sherbrooke research sites. For this study, The Canadian Dementia Imaging Protocol (https://www.cdip-pcid.ca) developed by the Canadian Consortium on Neurodegeneration in Aging was used to harmonize acquisition parameters between sites and minimize the impact of using different scanners. Overall, 25 participants were tested with the upgraded scanner, the 3T Prisma Tim Siemen Scanner. First, a T1-weighted MPRAGE sequence was acquired following these parameters: repetition time = 2300 ms; echo time = 2.98 ms; inversion time: 900 ms; matrix size = 256 × 256; field of view = 256 × 256 mm; voxel size = 1.0 mm isotropic; flip angle = 9°; and 192 sagittal orientations. The acquisition of a diffusion sequence was done with the following parameters: 32 uniformly distributed directions; b value 1 of 0 s/mm2; b value 2 of 1000 s/mm2; repetition time = 6900 ms; echo time = 64 ms; 70 slices; field of view = 256 × 256 mm; voxel size = 2.0 mm isotropic. The remaining 20 participants were tested using a 3T Ingenia Philips Scanner. The MPRAGE sequence was acquired with these parameters: repetition time = 7.1 ms; echo time = 3.2 ms; inversion time: 944.55 ms; matrix size = 256 × 256; field of view = 256 × 256 mm; voxel size = 1.0 mm isotropic; flip angle = 9°; and 192 sagittal orientations. The acquisition of a diffusion sequence was done with the following parameters: 32 uniformly distributed directions; b value 1 of 0 s/mm2; b value 2 of 1000 s/mm2; repetition time = 10,185 ms; echo time = 109 ms; 70 slices; field of view = 256 × 256 mm; voxel size = 2.0 mm isotropic.
2.4. MRI analyses
Volumes of regions of interest (ROIs) were obtained using Freesurfer 7.1 (https://surfer.nmr.mgh.harvard. edu/). T1-weighted imaging scans were processed using ‘Recon-all’ automatic segmentation, which allowed computing volumes of the entorhinal cortex and the parahippocampal gyri. The steps included motion correction, registration, intensity normalisation, removal of non-brain tissues, smoothing and inflating, tracing of white/grey matter boundaries and a cortical parcellation and labelling based on a probabilistic atlas. The ‘Segmentation of hippocampal subfields and nuclei of the amygdala module’ was used to obtain whole hippocampal volume, as it is expected to provide a more accurate volume estimation than the standard Freesurfer pipeline by using a statistical atlas constructed with both ex vivo and in vivo MRI data (Iglesias et al., 2015). These volumes were then normalized with the estimated total intracranial volume (TIV) obtained with Computational Anatomy Toolbox version 12.7 (CAT12, Jena University Hospital, Germany; release 1742; https://www.neuro.uni-jena.de/cat/index.html), as this estimation is considered superior to the one provided by FreeSurfer in the context of neurodegeneration and when multiple scanners are used (Malone et al., 2015, Tavares et al., 2019). To obtain TIV values, standard segmentation preprocessing was done using CAT12 for SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK; release 6906) (https://www.fil.ion.ucl.ac.uk/spm/), under MATLAB R2018b ([MathWorks, Natick, MA, USA]; https://www.mathworks.com/).
Thereafter, a FW fraction ranging from 0 to 1 per voxel was extracted. To obtain these values, a T1 resampled (from FreeSurfer output), a diffusion sequence, as well as bval and bvec files were all imported into TractoFlow Atlas Based Segmentation pipeline (TractoFlow-ABS; (Theaud et al., 2020a, Theaud et al., 2020), running under Compute Canada HPC Beluga node (beluga.computecanada.ca) for initial preprocessing. Adapted parameters were selected for diffusion image preprocessing using TractoFlow-ABS (DTI shells: 0 1000; fODF shells: 0 1000; FRF value: 10, 3, 3; SH order: 6; Algo: prob; Seeding type: npv; Number of seeds: 10; see https://github.com/scilus/tractoflow-ABS for more details). These adapted parameters allowed to obtain valid estimations for both the 32 and 64 direction sequences. From these outputs, brain-mask and diffusion-weighted images were analysed with FreeWater Flow (https://github.com/scilus/freewater_flow) using python 3.8 and AMICO wheels module available on HPC. Parameters to run these analyses were as follows: axial diffusivity in the corpus callosum = 0.001; mean diffusivity in ventricles = 0.0025; radial diffusivity minimum = 0.0001; radial diffusivity maximum = 0.0065; first regularization parameter = 0; second regularization parameter = 0.25. The output from this step was a whole brain free-water nifty card for each subject. Next, either the head-body-tail (HBT) or the aseg + aparc atlas from Freesurfer output for each participant were used to extract FW mean values for each ROI using customized codes from the Sherbrooke Connectivity Lab (https://scilpy.readthedocs.io/en/latest/scripts). First, for the hippocampi, the HBT templates from the right and left hemisphere of each subject were registered on their respective T1-warped computed from Tractoflow using the antsApplyTransforms python code with the NearestNeighbor function. Thereafter, these warped images were transformed to the same data type (e.g., uint8), and FW fractions for each ROI were identified (e.g., scil_split_volume_by_ids.py) and then extracted (e.g., scil_compute_metrics_stats_in_ROI.py). To extract FW values for the remaining regions, the same steps were performed using the aseg + aparc atlas from the right and left hemispheres of the entorhinal cortex (e.g., 1006 ctx-lh-entorhinal; 2006-ctx-rh-entorhinal) and parahippocampal gyrus (e.g., 1016-ctx-lh-parahippocampal; 2016-ctx-rh-parahippocampal). TIV-corrected bilateral volumes were normalized for FW fractions and are referred to as FW-corrected volumes.
2.5. Neuropsychological evaluation and questionnaires
An extensive neuropsychological battery evaluating multiple cognitive domains (i.e., attention, executive functioning, memory, language and visuospatial skills) was implemented. Additionally, global cognition, depressive symptoms, anxiety symptoms, sleep quality, and daily activities were assessed in all participants included in this study. However, specific neuropsychological tests and questionnaires used varied between cohorts (see supplementary table 1). aMCI diagnoses were established when participants had 1) at least two z-scores below −1.5 standard deviations compared to normative data in the memory cognitive domain, or 2) a Montreal Cognitive Assessment score < 26 and at least two z-scores below −1.5 standard deviations, including at least one from the memory cognitive domain. To evaluate the presence or absence of aMCI in participants, we used as many scores per domain as available in the cohorts. We also obtained a self-reported measure of autonomy using the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL) for 78 % of our participants (Galasko et al., 1997). Based on previously established cut-offs on the 18-item version (Pedrosa et al., 2010), we considered that participants with a score below 34 might present with dementia. No participant who completed this inventory met this cut-off.
Because questionnaires assessing depressive and anxiety symptoms varied between cohorts, we dichotomized these variables as ‘present’ or ‘absent’. The presence of significant depressive symptoms was concluded using cut-offs of ≥ 14 on the Beck Depression Inventory II, which usually represents mild symptoms of depression (Beck et al., 1996); or ≥ 5 on the Geriatric Depression Scale, which is considered suggestive of depression (Yesavage et al., 1982). For the presence of anxiety symptoms, we used cut-offs of ≥ 8 on the Beck Anxiety Inventory, which is used to assess the presence of mild symptoms of anxiety (Beck et al., 1988); or ≥ 8 on the Geriatric Anxiety Scale, which is suggestive of anxiety symptoms (Johnco et al., 2015, Pachana and Byrne, 2012). Additionally, the Epworth Sleepiness Scale was administered to all participants to assess daytime sleepiness.
2.6. Statistical analysis
Normality was assessed using the Kolmogorov-Smirnov test and variables were log-transformed when necessary. As we did not have a lateralization hypothesis, we combined volumes from both hemispheres analyses to obtain a total bilateral volume for each ROI. To obtain FW-corrected volumes, ROI volumes were extracted from FreeSurfer and divided by FW-fraction and TIV. One-way ANOVAs or chi-square tests were performed to assess differences between controls (AHI < 5), participants with mild OSA (AHI ≥ 5 to < 15) and with moderate-to-severe OSA (≥15) on demographic, sleep and clinical variables. Multiple linear regressions were performed with measures of OSA severity (namely hypoxemia and sleep fragmentation components) as independent variables and bilateral volumes of the hippocampi, the entorhinal cortex and parahippocampal gyri as dependent variables, adjusted for age, sex and education. Multiple linear regressions were also performed with FW-corrected ROI volumes. These analyses were performed in the whole sample and according to sex (adjusted for age and education), age (<68 versus ≥ 68 years old; adjusted for sex and education) and cognitive status (aMCI versus cognitively unimpaired; adjusted for sex, age and education). The 68 years old cut-off was chosen as it represented the median in the sample. Analyses were also performed for hippocampal subfields in the whole sample and in each subgroup. P was set at < 0.05 with a Family-Wise Error (FWE) correction. In addition, group differences were assessed for FW fraction for subgroups of participants as defined previously using ANCOVAs, with age, sex and education as covariates (when applicable). All statistical analyses were performed using SPSS 27 (IBM SPSS Statistics, New York, USA).
3. Results
3.1. Study participants
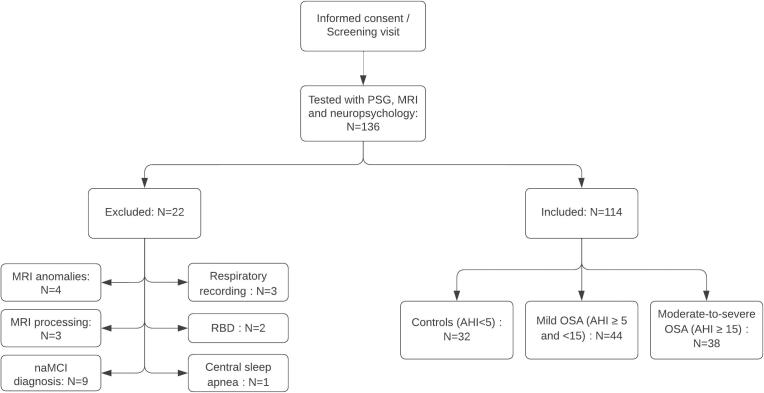
The final sample included 114 participants (68.0 ± 7.9 years old; 40 women) (see Fig. 1; Table 2). Groups did not differ in terms of age, sex, education, sleepiness, depressive and anxiety symptoms, and diagnosis of aMCI. However, moderate-to-severe OSA participants had higher BMI than control and mild OSA participants. We characterized sex, age, and cognitive status subgroups in the supplementary material (see supplementary tables 2-4).
Fig. 1.
Study participants flow chart. PSG = Polysomnography; MRI = Magnetic resonance imaging; RBD = Rapid eye movement sleep behavior disorder; OSA = Obstructive sleep apnea. naMCI: non-amnestic mild cognitive impairment.
Table 2.
Demographic, clinical and polysomnographic characteristics of participants according to OSA severity.
| Variables | Whole group | Controls (A) | Mild OSA (B) | Moderate-to-severe OSA (C) | F/X2 | P values | Post-Hoc Analyses |
|---|---|---|---|---|---|---|---|
| Total N | 114 | 32 | 44 | 38 | |||
| Age (years) | 68.0 ± 7.9 | 67.2 ± 8.4 | 68.6 ± 7.9 | 68.21 ± 7.7 | 0.5 | 0.6 | |
| Women, N (%) | 40 (35 %) | 13 (41 %) | 19 (43 %) | 8 (21 %) | 2.5 | 0.1 | |
| Education (years) | 15.1 ± 3.6 | 14.4 ± 3.5 | 15.4 ± 3.6 | 15.3 ± 3.8 | 1.0 | 0.4 | |
| Body mass index (kg/m2) | 26.5 ± 3.7 | 25.3 ± 3.6 | 25.6 ± 3.5 | 28.4 ± 3.4 | 8.9 | < 0.001 | A, B < C |
| Epworth Sleepiness Scale score | 7.1 ± 4.5 | 6.1 ± 4.0 | 6.7 ± 4.7 | 8.5 ± 4.5 | 2.5 | 0.1 | |
| Depressive symptoms (% with) | 7.3 | 6.5 | 4.5 | 11.4 | 1.5 | 0.5 | |
| Anxiety symptoms (% with) | 18.2 | 22.6 | 13.6 | 20.0 | 1.4 | 0.5 | |
| Montreal Cognitive Assessment score | 26.7 ± 2.6 | 26.7 ± 2.6 | 26.8 ± 2.5 | 26.6 ± 2.8 | 0.02 | 1.0 | |
| Amnestic mild cognitive impairment, N (%) | 46 (40 %) | 15 (47 %) | 19 (43 %) | 12 (32 %) | 3.2 | 0.5 | |
| Apnea-hypopnea index (events/h) | 13.9 ± 14.3 | 2.2 ± 1.5 | 8.7 ± 2.8 | 29.7 ± 14.3 | 92.5 | < 0.001 | A < B < C |
| 3 % oxygen desaturation index (events/h) | 8.6 ± 10.6 | 1.6 ± 2.2 | 4.5 ± 3.8 | 19.2 ± 12.4 | 57.3 | < 0.001 | A, B < C |
| Mean oxygen saturation (%) | 94.6 ± 1.3 | 95.2 ± 0.9 | 94.8 ± 1.3 | 93.9 ± 1.3 | 9.5 | < 0.001 | A, B < C |
| Minimal oxygen saturation (%) | 85.9 ± 4.9 | 89.4 ± 2.8 | 86.8 ± 3.2 | 81.9 ± 5.2 | 32.9 | < 0.001 | A > B > C |
| Sleep time with oxygen saturation < 90 % (min) | 6.9 ± 18.8 | 0.3 ± 0.5 | 2.8 ± 5.3 | 17.0 ± 30.4 | 9.5 | < 0.001 | A, B < C |
| Total sleep time (min) | 364.3 ± 58.2 | 363.7 ± 61.5 | 368.2 ± 65.6 | 360.6 ± 46.8 | 0.2 | 0.8 | |
| Total wake time after sleep onset (min) | 91.9 ± 51.4 | 94.9 ± 48.2 | 91.0 ± 56.6 | 90.4 ± 49.0 | 0.04 | 1.0 | |
| Sleep efficiency (%) | 79.8 ± 10.9 | 79.2 ± 10.7 | 79.8 ± 12.3 | 80.1 ± 9.7 | 0.01 | 1.0 | |
| Micro-arousal index (events/h) | 15.0 ± 8.2 | 10.6 ± 4.6 | 12.6 ± 5.6 | 21.4 ± 9.1 | 24.0 | < 0.001 | A, B < C |
| N1 (min) | 71.3 ± 37.3 | 50.4 ± 28.3 | 64.7 ± 25.6 | 96.4 ± 41.9 | 14.9 | < 0.001 | A, B < C |
| N2 (min) | 204.7 ± 51.5 | 215.0 ± 45.7 | 211.3 ± 52.1 | 185.4 ± 53.3 | 3.2 | 0.05 | A,B < C |
| N3 (min) | 29.1 ± 31.9 | 34.1 ± 34.4 | 30.9 ± 35.7 | 22.9 ± 24.0 | 0.8 | 0.5 | |
| REM sleep (min) | 60.2 ± 22.2 | 64.0 ± 20.5 | 61.2 ± 24.3 | 55.9 ± 20.7 | 2.4 | 0.1 |
OSA = obstructive sleep apnea; REM: rapid-eye movement.
Results for continuous variables are reported as means ± standard deviation. Controls: AHI < 5, Mild OSA: AHI ≥ 5 to < 15, Moderate-to-severe OSA: ≥ 15.
3.2. OSA severity and medial temporal lobe volumes in the whole sample
Positive associations were found between hypoxemia and entorhinal cortex volume (see Table 3A; β (95% CI) = 0.09 (0.02–0.16), P = 0.045). No association was found between sleep fragmentation and medial temporal grey matter volumes.
Table 3.
Significant multiple linear regression results for analyses with OSA severity markers and medial temporal subregional volumes.
| Brain regions | Unstandardized β coefficient (95% CI) | Standard error | P values, FWE corrected |
|---|---|---|---|
| A. Whole sample1 | |||
| Entorhinal cortex volume | |||
| Hypoxemia | 0.09 (0.02–0.16) | 0.04 | 0.045 |
| B. Older participants2 | |||
| Entorhinal cortex volume | |||
| Hypoxemia | 0.16 (0.06–0.27) | 0.05 | 0.006 |
| C. Women3 | |||
| Hippocampal volume | |||
| Sleep fragmentation | 0.39 (0.08–0.70) | 0.06 | 0.048 |
| Entorhinal cortex volume | |||
| Hypoxemia | 0.20 (0.09–0.31) | 0.05 | 0.002 |
| D. aMCI participants1 | |||
| Hippocampal volume | |||
| Hypoxemia | 0.18 (0.05–0.31) | 0.06 | 0.024 |
CI = confidence interval, FWE: Family-Wise Error, aMCI = amnestic mild cognitive impairment; OSA = obstructive sleep apnea.
Analyses adjusted for age, sex and education.
Analyses adjusted for sex and education.
Analyses adjusted for age and education.
3.3. Analyses by age groups
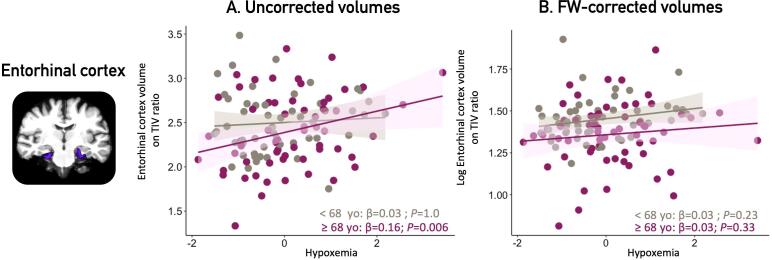
In older individuals (≥68 years old), more severe hypoxemia was associated with higher entorhinal cortex volumes (β (95% CI) = 0.16 (0.06–0.27); P = 0.006, see Fig. 2, Table 3B). No association was found in younger participants, except with hippocampal subfields (see below).
Fig. 2.
Associations between hypoxemia severity and entorhinal cortex by age groups. Legend: Panel A presents uncorrected grey matter volumes of the entorhinal cortex in younger (<68 yo) and older participants (≥68 yo) obtained at p < 0.05 corrected for multiple comparisons with a family-wise error correction. Panel B presents the FW-corrected volumes. All volumes were corrected for TIV (volume/TIV*1000). Covariates included in the multiple linear regressions were sex and education. OSA = obstructive sleep apnea; FW = free-water; TIV = total intracranial volume; yo = years old.
3.4. Sex-specific analyses
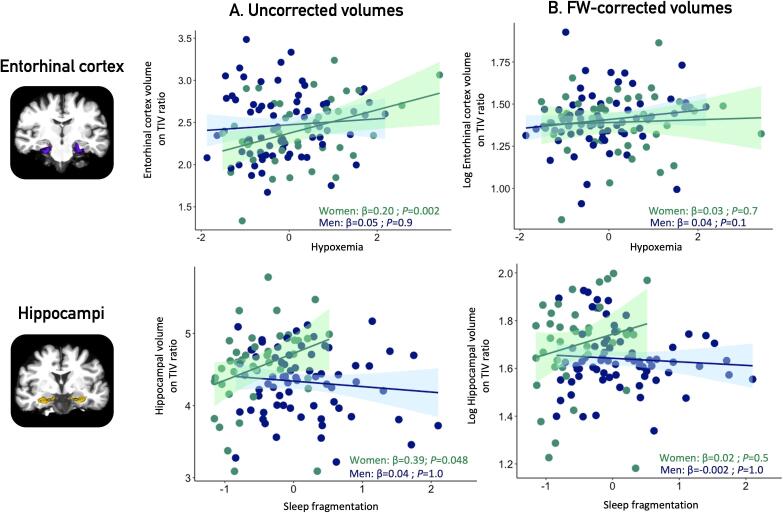
In women, more severe OSA was associated with larger medial temporal lobe structures (Fig. 3, Table 3C). Higher sleep fragmentation was associated with larger hippocampal volumes (β (95% CI) = 0.39 (0.08–0.70), P = 0.048). High hypoxemia levels were also associated with increased entorhinal cortex volumes (β (95% CI) = 0.20 (0.09–0.31); P = 0.002). No significant association was found in men.
Fig. 3.
Associations between OSA severity markers and medial temporal volumes in women and men. Legend: Panel A shows uncorrected grey matter volumes of the hippocampi (upper) and entorhinal cortex (lower) in women and men, obtained at p < 0.05 corrected for multiple comparisons with a family-wise error correction. Panel B presents the FW-corrected volumes. All volumes were corrected for TIV (volume/TIV*1000). Covariates included in the multiple linear regressions were age and education. OSA = obstructive sleep apnea; FW = free-water; TIV = total intracranial volume.
3.5. Analyses by cognitive status
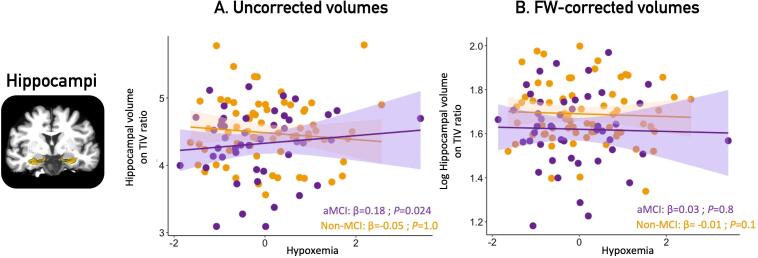
In aMCI participants, higher hypoxemia levels were associated with increased bilateral hippocampal volumes (Fig. 4, Table 3D): (β (95% CI) = 0.18 (0.05–0.31); P = 0.024). No association was found in cognitively healthy participants -.
Fig. 4.
Associations between hypoxemia and hippocampal volumes in aMCI participants and controls. Legend: Panel A shows uncorrected volumes of the bilateral hippocampus, obtained at p < 0.05 corrected for multiple comparisons with a family-wise error correction in participants with aMCI only. No association remained significant between any markers of OSA severity and FW-corrected volumes (panel B). All volumes are TIV-normalized (volume/TIV*1000). Covariates included in the multiple linear regressions were age, education and sex. OSA = obstructive sleep apnea; FW = free-water; aMCI = amnestic mild cognitive impairment, TIV = total intracranial volume.
3.6. Hippocampal subfields
We performed additional analyses on the associations between OSA severity markers and hippocampal subfield volumes extracted from FreeSurfer. In the whole sample, no association was found. When stratifying our sample (by sex, age and cognitive status), we found that increased sleep fragmentation was associated with higher CA2-CA3-CA4-DG volumes (β (95% CI) = 0.06 (0.01–0.1), P = 0.048) in younger participants (<68 years old). In aMCI participants, higher hypoxemia levels were associated with higher subiculum volumes (β (95% CI) = 0.01(0.05–0.02), P = 0.012). No association was found when stratifying by sex. When using FW-corrected volumes, no association remained significant.
3.7. FW fraction and FW-corrected volumes
FW fraction values were extracted for all ROIs and showed no association with OSA severity in the whole sample and in subgroups. Regardless of OSA severity, older individuals displayed a higher entorhinal cortex FW fraction than younger participants (F = 9.4; P = 0.009), men displayed a higher FW fraction than women in parahippocampal gyri (F = 15.5; P = 0.0004) and aMCI participants had a higher FW fraction in all three medial temporal lobe subregions (F = 6.9–9.2; P = 0.009–0.030) compared to cognitively healthy participants. Finally, although there were no differences in the medial temporal lobe subregions between aMCI and non-MCI participants when comparing uncorrected volumes, FW-corrected volumes revealed atrophy in all three subregions in aMCI participants (F = 6.3–9.8; P = 0.006–0.039).
We then investigated the association between OSA severity and FW-corrected grey matter volumes of medial temporal lobe regions. No association between OSA severity markers and medial temporal volumes remained significant, neither in the whole sample nor when stratified for age, sex or cognitive status.
4. Discussion
Our study showed that increased OSA severity, particularly hypoxemia, was associated with higher volumes in the entorhinal cortex and the hippocampus, and that these associations were mostly observed in women, older participants and those with aMCI. While few neuroimaging studies have investigated the impact of individual characteristics in OSA, these findings represent a significant step toward personalized medicine and could guide treatment decision for patients who might be at greater risk of pathological changes when OSA is left untreated. In addition, when we applied a FW correction to grey matter volumes, no association between OSA severity and medial temporal subregional volume remained significant, while this correction unveiled atrophy of the medial temporal lobe in participants with aMCI regardless of OSA severity. This suggests that the grey matter hypertrophies were likely due to increased brain water content. To our knowledge, this is the first study investigating the potential impact of edema and neuroinflammation on grey matter volumes using FW-corrected volumes in OSA participants. Applying FW correction on grey matter volumes could therefore lead to more valid neuroimaging results in participants with suspected edema and neuroinflammation.
4.1. Edema as an early response to hypoxia
Medial temporal lobe subregions require a high oxygen supply as demonstrated by their dense vascularity, which makes them especially vulnerable to hypoxemia (Spallazzi et al., 2019). In animal models, regions affected by hypoxemia first show reactive or adaptive mechanisms that include edema, reactive gliosis, oxidative stress, neuroinflammation and ischemic preconditioning (Baril et al., 2021, Gosselin et al., 2019, Rosenzweig et al., 2015). These mechanisms are followed by apoptosis when episodes of hypoxemia are repeated (Fung et al., 2007). We (Baril. et al., 2021) and others (Rosenzweig et al., 2015) have suggested that structural neuroimaging changes follow a biphasic evolution in OSA, with a first stage characterized by edema and increased grey matter volume, and a second stage characterized by neuronal death, grey matter loss and cognitive decline. In support of this hypothesis, André et al. (André et al., 2020) tested 127 cognitively unimpaired adults (mean age: 69.1 years old), and found increased amyloid burden and metabolism, but also larger posterior cingulate cortex, cuneus and precuneus volumes in OSA participants compared to controls. Interestingly, a study by Cross et al. included 83 participants (mean age: 67.4 years old) with subjective and/or objective cognitive impairment and found both thicker and thinner cerebral volumes associated with OSA severity (Cross et al., 2018), notably in hippocampal and temporo-parietal areas. Our results further support a biphasic hypothesis in all three medial temporal lobe subregions, in which both atrophy and hypertrophy have been previously underlined (André et al., 2020, Cross et al., 2018, Owen et al., 2019, Shi et al., 2017).
4.2. Effects of age
When stratifying our sample in two age groups (<68 versus ≥ 68 years old), we found associations between OSA severity and medial temporal subregional volumes in both groups. We expected to see changes in the younger group, as stronger links between OSA and cognitive function in young and middle-aged adults compared to older adults have been previously reported (Bubu et al., 2020). Accordingly, in the < 68 years old group, we found associations between sleep fragmentation and higher hippocampal subfield (CA2-CA3-CA4-DG) volume. However, we also found that more hypoxemia was associated with larger entorhinal cortex volume in the ≥ 68 years old group, which is in line with previous findings in the elderly suggesting that chronic intermittent hypoxemia might be the central mechanism linking OSA to risk of cognitive impairment (Zimmerman and Aloia, 2012). Elderly adults with OSA can present with multiple comorbidities (e.g. obesity, hypertension, diabetes and cardiovascular diseases) (Bonsignore et al., 2019), that are also risk factors for cognitive impairment and dementia. Separating the effects of these conditions from the effects of OSA can thus be challenging, which may explain why some previous studies have observed associations between OSA and cognitive decline in “young elderly” only.
4.3. Sex modulates the association between OSA severity and medial temporal structures
Sex-specific pathophysiology, clinical presentation, and health consequences of OSA have been suggested (Huang et al., 2018). We highlighted noticeable differences on how OSA severity is associated with medial temporal grey matter volumes in men and women, where OSA severity was associated with increased volumes of the hippocampus and entorhinal cortex in women only. In accordance with these observations, epidemiological studies reported that the association between OSA and cognitive decline might be stronger in women compared to men (Thompson et al., 2022, Zhu and Zhao, 2018). This sex effect may however not be specific to OSA. For example, a recent study found that women with aMCI or dementia present higher tau burden measured with positron emission tomography in several regions (e.g., entorhinal cortex) compared to men who had similar cognitive functioning (Edwards et al., 2021). Women may tolerate higher pathology burden with less cognitive consequences; however, this can be followed by marked atrophy and steeper cognitive decline than men. Based on these findings, a particular attention should be paid to women with OSA as they may report being asymptomatic, while their brains may show significant pathological changes.
4.4. Mild cognitive impairment, OSA and neuroimaging findings
Regarding the impact of cognitive status, only participants with aMCI showed a link between increased OSA severity and higher hippocampal volumes. These results suggest that the larger volumes observed in relation to OSA severity are not an adaptive process, as they are associated with poorer cognition among participants with OSA. Interestingly, MCI and AD studies (regardless of OSA) report cortical thickening in the early stages of neurodegeneration, which is hypothesized to be linked to edema, as amyloid deposition is a pro-inflammatory process that can lead to changes in water content (Montal et al., 2018). As aMCI participants with increased OSA severity showed significant structural changes in the present study, they likely are at an early stage of neurodegeneration, and the OSA-related sleep disturbances could therefore enhance ongoing neurodegenerative processes and cognitive decline if left untreated (Lucey et al., 2021).
4.5. Hippocampal subfields
Studies have shown that medial temporal lobe subregions are not homogenously affected in the first stages of AD. In fact, the entorhinal cortex is the first region affected, followed by specific hippocampal subfields, more specifically the CA1, which is also increasingly sensitive to hypoxia, and the subiculum (Small et al., 2011). Additional analyses on hippocampal subfields (CA1, subiculum, combined CA2-CA3-CA4-DG) showed that the associations between OSA severity and grey matter volumes were more prominent in the subiculum (in aMCI participants) and combined CA2-CA3-CA4-DG (younger participants), but not in the CA1. It was previously shown that CA1 could particularly benefit from ischemic preconditioning (Levchenkova et al., 2021), which could explain the absence of association with OSA. However, due to our MRI resolution, this finding must be interpreted with caution and future studies in OSA should investigate hippocampal subfields with high resolution MRI.
4.6. Using FW fraction to understand medial temporal lobe structure
A gradual increase in FW fraction has been shown in participants with MCI or AD compared to controls (Bergamino et al., 2021, Dumont et al., 2019, Montal et al., 2018). One study also noted a decrease in FW fraction in asymptomatic amyloid-positive but tau-negative patients, and higher FW fraction in further stages (Montal et al., 2018). In this study, we showed that using FW-corrected volumes might allow more precise estimates of grey matter volumes by limiting the impact of artificial hypertrophies caused by water content changes and edema. This is consistent with the atrophy revealed in all three medial temporal lobe regions in our aMCI compared to non-MCI participants, regardless of OSA severity. When investigating associations between OSA and FW-corrected volumes, none remained significant, suggesting that the structural changes in medial temporal lobe subregions could precede future atrophy, thus representing an interesting therapeutic window. Edema could also be due to a combination of many factors, such as ongoing neurodegenerative processes or OSA-related medical comorbidities, as no clear relationship between OSA severity and FW fraction was established.
4.7. Are structural changes reversible in treated OSA?
Another important question to answer is whether structural changes to medial temporal lobe subregions are reversible. Longitudinal studies suggest that treating OSA surgically or using continuous positive airway pressure therapy has a positive impact on medial temporal lobe structure and function by increasing regional cerebral blood flow distribution in the hippocampus and parahippocampal gyri over time (L'Heureux et al., 2021) or decreasing grey matter volumes in the hippocampus (Lin et al., 2016), leading to improved cognitive performance and levels of systemic inflammation markers. These findings highlight the need to follow cohorts of treated and untreated OSA patients over time, and this should be done while considering individual characteristics that might significantly impact outcomes.
4.8. Limitations
The present study has some limitations. Globally, the severity of symptomatology (sleepiness, depression, anxiety and aMCI) was not associated with OSA severity suggesting that the functional consequences of OSA were relatively mild. Although we did follow a protocol aimed at limiting the impact of using multiple scanners, this could still have an impact on neuroimaging analyses. In addition, as FW imaging is a bi-tensor model, it relies on the fact that there is no exchange of liquid between the two compartments (intra/extracellular), and therefore represents a limitation of this model. In the future, FW fraction could be even more robust using a multi-shell diffusion MRI sequence.
4.9. Conclusion
In this study, OSA severity is associated with increased volumes in medial temporal subregions, particularly in women and in participants presenting aMCI. These changes are likely linked to maladaptive processes in the form of extracellular fluid accumulation, which usually precedes or is seen with neuronal death. This study is of clinical importance, as pulmonologists, geriatricians, and family physicians need to know which OSA patients are more vulnerable to cognitive decline and should be treated.
Financial disclosures
This research was supported by the Canadian Institutes of Health Research [Grant No FDN154291, MOP123294, MOP102631, PJT153259], the Fonds de la Recherche du Québec – Santé and the Quebec Bio-Imaging Network.
CRediT authorship contribution statement
Marie-Ève Martineau-Dussault: Methodology, Investigation, Formal analysis, Visualization, Writing – original draft. Claire André: Methodology, Investigation, Formal analysis, Writing – original draft. Véronique Daneault: Formal analysis, Investigation, Writing – review & editing. Andrée-Ann Baril: Investigation, Writing – review & editing. Katia Gagnon: Investigation, Writing – review & editing. Hélène Blais: Formal analysis, Writing – review & editing. Dominique Petit: Conceptualization, Project administration, Investigation, Writing – review & editing. Jacques Y. Montplaisir: Conceptualization, Writing – review & editing. Dominique Lorrain: Conceptualization, Project administration, Writing – review & editing. Célyne Bastien: Conceptualization, Project administration, Writing – review & editing. Carol Hudon: Conceptualization, Project administration, Writing – review & editing. Maxime Descoteaux: Conceptualization, Software, Writing – review & editing. Arnaud Boré: Software, Writing – review & editing. Guillaume Theaud: Software, Writing – review & editing. Cynthia Thompson: Investigation, Writing – review & editing. Julie Legault: Investigation, Writing – review & editing. Guillermo E. Martinez Villar: Investigation, Writing – review & editing. Alexandre Lafrenière: Investigation, Writing – review & editing. Chantal Lafond: Conceptualization, Writing – review & editing. Danielle Gilbert: Conceptualization, Writing – review & editing. Julie Carrier: Conceptualization, Project administration, Supervision, Writing – review & editing, Funding acquisition. Nadia Gosselin: Conceptualization, Project administration, Supervision, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank Sonia Frenette, Caroline d’Aragon, Joëlle Robert, Sarah-Hélène Julien, Anne-Sophie Deshaies-Rugama, Tyna Paquette, Pauline Brayet, Maxime Fortin, Marc-André D. Gareau, and Dr Maria Tuineag for their help with data acquisition.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103235.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Anderova M., Vorisek I., Pivonkova H., Benesova J., Vargova L., Cicanic M., Chvatal A., Sykova E. Cell death/proliferation and alterations in glial morphology contribute to changes in diffusivity in the rat hippocampus after hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2011;31:894–907. doi: 10.1038/jcbfm.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André, C., Rehel, S., Kuhn, E., Landeau, B., Moulinet, I., Touron, E., Ourry, V., Le Du, G., Mezenge, F., Tomadesso, C., de Flores, R., Bejanin, A., Sherif, S., Delcroix, N., Manrique, A., Abbas, A., Marchant, N.L., Lutz, A., Klimecki, O.M., Collette, F., Arenaza-Urquijo, E.M., Poisnel, G., Vivien, D., Bertran, F., de la Sayette, V., Chetelat, G., Rauchs, G., Medit-Ageing Research, G., 2020. Association of Sleep-Disordered Breathing With Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. [DOI] [PMC free article] [PubMed]
- Baril., Gagnon, K., Arbour, C., Soucy, J.P., Montplaisir, J., Gagnon, J.F., Gosselin, N., 2015. Regional Cerebral Blood Flow during Wakeful Rest in Older Subjects with Mild to Severe Obstructive Sleep Apnea. Sleep 38, 1439-1449. [DOI] [PMC free article] [PubMed]
- Baril., Martineau-Dussault, M.-È., Sanchez, E., André, C., Thompson, C., Legault, J., Gosselin, N., 2021. Obstructive Sleep Apnea and the Brain: a Focus on Gray and White Matter Structure. Current neurology and neuroscience reports, p. 11. [DOI] [PubMed]
- Baril., Gagnon, K., Brayet, P., Montplaisir, J., De Beaumont, L., Carrier, J., Lafond, C., L'Heureux, F., Gagnon, J.F., Gosselin, N., 2017. Gray Matter Hypertrophy and Thickening with Obstructive Sleep Apnea in Middle-aged and Older Adults. Am J Respir Crit Care Med 195, 1509-1518. [DOI] [PubMed]
- Baril., Gagnon, K., Brayet, P., Montplaisir, J., Carrier, J., Soucy, J.P., Lafond, C., Blais, H., d'Aragon, C., Gagnon, J.F., Gosselin, N., 2018. Obstructive sleep apnea during REM sleep and daytime cerebral functioning: A regional cerebral blood flow study using high-resolution SPECT. J Cereb Blood Flow Metab, 271678X18814106. [DOI] [PMC free article] [PubMed]
- Baril., Gagnon, K., Descoteaux, M., Bedetti, C., Chami, S., Sanchez, E., Montplaisir, J., De Beaumont, L., Gilbert, D., Poirier, J., Pelleieux, S., Osorio, R.S., Carrier, J., Gosselin, N., 2020. Cerebral white matter diffusion properties and free-water with obstructive sleep apnea severity in older adults. Hum Brain Mapp. [DOI] [PMC free article] [PubMed]
- Baronio D., Martinez D., Fiori C.Z., Bambini-Junior V., Forgiarini L.F., Pase da Rosa D., Kim L.J., Cerski M.R. Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respir. Physiol. Neurobiol. 2013;185:217–221. doi: 10.1016/j.resp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Bartsch T., Döhring J., Reuter S., Finke C., Rohr A., Brauer H., Deuschl G., Jansen O. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J. Cereb. Blood Flow Metab. 2015;35:1836–1845. doi: 10.1038/jcbfm.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. The Psychological Corporation; San Antonio, Texas: 1996. Beck Depression Inventory-II. [Google Scholar]
- Bergamino M., Walsh R.R., Stokes A.M. Free-water diffusion tensor imaging improves the accuracy and sensitivity of white matter analysis in Alzheimer's disease. Sci. Rep. 2021;11:6990. doi: 10.1038/s41598-021-86505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C., Kapur V.K., Marcus C.L., Mehra R., Parthasarathy S., Quan S.F., Redline S., Strohl K.P., Davidson Ward S.L., Tangredi M.M. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore M.R., Baiamonte P., Mazzuca E., Castrogiovanni A., Marrone O. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidisciplinary Respiratory Med. 2019;14:8. doi: 10.1186/s40248-019-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bubu, O.M., Pirraglia, E., Andrade, A.G., Sharma, R.A., Gimenez-Badia, S., Umasabor-Bubu, O.Q., Hogan, M.M., Shim, A.M., Mukhtar, F., Sharma, N., Mbah, A.K., Seixas, A.A., Kam, K., Zizi, F., Borenstein, A.R., Mortimer, J.A., Kip, K.E., Morgan, D., Rosenzweig, I., Ayappa, I., Rapoport, D.M., Jean-Louis, G., Varga, A.W., Osorio, R.S., Alzheimer's Disease Neuroimaging, I., 2019. Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep 42. [DOI] [PMC free article] [PubMed]
- Bubu, O.M., Andrade, A.G., Umasabor-Bubu, O.Q., Hogan, M.M., Turner, A.D., de Leon, M.J., Ogedegbe, G., Ayappa, I., Jean-Louis G, G., Jackson, M.L., Varga, A.W., Osorio, R.S., 2020. Obstructive sleep apnea, cognition and Alzheimer's disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med Rev 50, 101250. [DOI] [PMC free article] [PubMed]
- Cross N.E., Memarian N., Duffy S.L., Paquola C., LaMonica H., D'Rozario A., Lewis S.J.G., Hickie I.B., Grunstein R.R., Naismith S.L. Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur. Respir. J. 2018;52 doi: 10.1183/13993003.00740-2018. [DOI] [PubMed] [Google Scholar]
- Dumont M., Roy M., Jodoin P.M., Morency F.C., Houde J.C., Xie Z., Bauer C., Samad T.A., Van Dijk K.R.A., Goodman J.A., Descoteaux M. Free Water in White Matter Differentiates MCI and AD From Control Subjects. Front. Aging Neurosci. 2019;11:270. doi: 10.3389/fnagi.2019.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde M., Theaud G., Rheault F., Dilharreguy B., Helmer C., Dartigues J.F., Amieva H., Allard M., Descoteaux M., Catheline G. Free water: A marker of age-related modifications of the cingulum white matter and its association with cognitive decline. PLoS ONE. 2020;15:e0242696. doi: 10.1371/journal.pone.0242696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L., La Joie R., Iaccarino L., Strom A., Baker S.L., Casaletto K.B., Cobigo Y., Grant H., Kim M., Kramer J.H., Mellinger T.J., Pham J., Possin K.L., Rosen H.J., Soleimani-Meigooni D.N., Wolf A., Miller B.L., Rabinovici G.D. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer's continuum: greater tau-PET retention in females. Neurobiol. Aging. 2021;105:86–98. doi: 10.1016/j.neurobiolaging.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S.J., Xi M.C., Zhang J.H., Sampogna S., Yamuy J., Morales F.R., Chase M.H. Apnea promotes glutamate-induced excitotoxicity in hippocampal neurons. Brain Res. 2007;1179:42–50. doi: 10.1016/j.brainres.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M., Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 1997;11 Suppl 2:S33–S39. [PubMed] [Google Scholar]
- Gosselin N., De Beaumont L., Gagnon K., Baril A.A., Mongrain V., Blais H., Montplaisir J., Gagnon J.F., Pelleieux S., Poirier J., Carrier J. BDNF Val66Met Polymorphism Interacts with Sleep Consolidation to Predict Ability to Create New Declarative Memories. J. Neurosci. 2016;36:8390–8398. doi: 10.1523/JNEUROSCI.4432-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N., Baril A.A., Osorio R.S., Kaminska M., Carrier J. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am. J. Respir. Crit. Care Med. 2019;199:142–148. doi: 10.1164/rccm.201801-0204PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Lin B.M., Markt S.C., Stampfer M.J., Laden F., Hu F.B., Tworoger S.S., Redline S. Sex differences in the associations of obstructive sleep apnoea with epidemiological factors. Eur. Respir. J. 2018;51 doi: 10.1183/13993003.02421-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K., Alzheimer's Disease Neuroimaging I. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnco C., Knight A., Tadic D., Wuthrich V.M. Psychometric properties of the Geriatric Anxiety Inventory (GAI) and its short-form (GAI-SF) in a clinical and non-clinical sample of older adults. Int. Psychogeriatr. 2015;27:1089–1097. doi: 10.1017/S1041610214001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault, J., Thompson, C., Martineau-Dussault, M.-È., André, C., Baril, A.-A., Martinez Villar, G., Carrier, J., Gosselin, N., 2021. Obstructive Sleep Apnea and Cognitive Decline: A Review of Potential Vulnerability and Protective Factors. 11, 706. [DOI] [PMC free article] [PubMed]
- Leng Y., McEvoy C.T., Allen I.E., Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74:1237–1245. doi: 10.1001/jamaneurol.2017.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenkova O.S., Novikov V.E., Korneva Y.S., Dorosevich A.E., Parfenov E.A. Combined preconditioning reduces the negative influence of cerebral ischemia on the morphofunctional condition of CNS. Bull. Exp. Biol. Med. 2021;171:489–493. doi: 10.1007/s10517-021-05257-6. [DOI] [PubMed] [Google Scholar]
- L'Heureux F., Baril A.A., Gagnon K., Soucy J.P., Lafond C., Montplaisir J., Gosselin N. Longitudinal changes in regional cerebral blood flow in late middle-aged and older adults with treated and untreated obstructive sleep apnea. Hum. Brain Mapp. 2021 doi: 10.1002/hbm.25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.C., Pack A.I. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med. Rev. 2014;18:35–48. doi: 10.1016/j.smrv.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.-C., Huang C.-C., Chen H.-L., Chou K.-H., Chen P.-C., Tsai N.-W., Chen M.-H., Friedman M., Lin H.-C., Lu C.-H. Longitudinal brain structural alterations and systemic inflammation in obstructive sleep apnea before and after surgical treatment. J. Transl. Med. 2016;14 doi: 10.1186/s12967-016-0887-8. 139-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey B.P., Wisch J., Boerwinkle A.H., Landsness E.C., Toedebusch C.D., McLeland J.S., Butt O.H., Hassenstab J., Morris J.C., Ances B.M., Holtzman D.M. Sleep and longitudinal cognitive performance in preclinical and early symptomatic Alzheimer’s disease. Brain. 2021;144:2852–2862. doi: 10.1093/brain/awab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Prasad J.P., Ogren J.A., Moiyadi A.S., Aysola R.S., Kumar R., Yan-Go F.L., Woo M.A., Albert Thomas M., Harper R.M. Sex-specific hippocampus volume changes in obstructive sleep apnea. Neuroimage Clin. 2018;20:305–317. doi: 10.1016/j.nicl.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone I.B., Leung K.K., Clegg S., Barnes J., Whitwell J.L., Ashburner J., Fox N.C., Ridgway G.R. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N.A., Ramponi C., Hirotsu C., Haba-Rubio J., Lutti A., Preisig M., Marques-Vidal P., Vollenweider P., Kherif F., Heinzer R., Draganski B. Mean Oxygen Saturation during Sleep Is Related to Specific Brain Atrophy Pattern. Ann. Neurol. 2020 doi: 10.1002/ana.25728. [DOI] [PubMed] [Google Scholar]
- Montal V., Vilaplana E., Alcolea D., Pegueroles J., Pasternak O., Gonzalez-Ortiz S., Clarimon J., Carmona-Iragui M., Illan-Gala I., Morenas-Rodriguez E., Ribosa-Nogue R., Sala I., Sanchez-Saudinos M.B., Garcia-Sebastian M., Villanua J., Izagirre A., Estanga A., Ecay-Torres M., Iriondo A., Clerigue M., Tainta M., Pozueta A., Gonzalez A., Martinez-Heras E., Llufriu S., Blesa R., Sanchez-Juan P., Martinez-Lage P., Lleo A., Fortea J. Cortical microstructural changes along the Alzheimer's disease continuum. Alzheimers Dement. 2018;14:340–351. doi: 10.1016/j.jalz.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Owen J.E., BenediktsdOttir B., Gislason T., Robinson S.R. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep. 2019;42 doi: 10.1093/sleep/zsy199. [DOI] [PubMed] [Google Scholar]
- Pachana N.A., Byrne G.J. The Geriatric Anxiety Inventory: international use and future directions. Australian Psychologist. 2012;47:33–38. [Google Scholar]
- Pedrosa H., De Sa A., Guerreiro M., Maroco J., Simoes M.R., Galasko D., de Mendonça A. Functional evaluation distinguishes MCI patients from healthy elderly people — The ADCS/MCI/ADL scale. J. Nutri. Health Aging. 2010;14:703–709. doi: 10.1007/s12603-010-0102-1. [DOI] [PubMed] [Google Scholar]
- Rosenzweig I., Kempton M.J., Crum W.R., Glasser M., Milosevic M., Beniczky S., Corfield D.R., Williams S.C., Morrell M.J. Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning? PLoS ONE. 2013;8:e83173. doi: 10.1371/journal.pone.0083173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig I., Glasser M., Polsek D., Leschziner G.D., Williams S.C., Morrell M.J. Sleep apnoea and the brain: a complex relationship. Lancet Respir. Med. 2015;3:404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- Rosenzweig I., Morrell M.J. Hypotrophy versus Hypertrophy: It's Not Black or White with Gray Matter. Am. J. Respir. Crit. Care Med. 2017;195:1416–1418. doi: 10.1164/rccm.201701-0109ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Chen L., Chen T., Li L., Dai J., Lui S., Huang X., Sweeney J.A., Gong Q. A meta-analysis of voxel-based brain morphometry studies in obstructive sleep apnea. Sci. Rep. 2017;7:10095. doi: 10.1038/s41598-017-09319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallazzi M., Dobisch L., Becke A., Berron D., Stucht D., Oeltze-Jafra S., Caffarra P., Speck O., Düzel E. Hippocampal vascularization patterns: a high-resolution 7 Tesla time-of-flight magnetic resonance angiography study. NeuroImage. Clinical. 2019;21 doi: 10.1016/j.nicl.2018.11.019. 101609-101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares V., Prata D., Ferreira H.A. Comparing SPM12 and CAT12 segmentation pipelines: a brain tissue volume-based age and Alzheimer's disease study. J. Neurosci. Methods. 2019;334 doi: 10.1016/j.jneumeth.2019.108565. [DOI] [PubMed] [Google Scholar]
- Theaud, G., Houde, J.-C., Boré, A., Rheault, F., Morency, F., Descoteaux, M., 2020a. TractoFlow-ABS (Atlas-Based Segmentation). 2020.2008.2003.197384.
- Theaud G., Houde J.-C., Boré A., Rheault F., Morency F., Descoteaux M. TractoFlow: A robust, efficient and reproducible diffusion MRI pipeline leveraging Nextflow & Singularity. Neuroimage. 2020;218 doi: 10.1016/j.neuroimage.2020.116889. [DOI] [PubMed] [Google Scholar]
- Thompson C., Legault J., Moullec G., Martineau-Dussault M., Baltzan M., Cross N., Dang-Vu T.T., Gervais N., Einstein G., Hanly P., Ayas N., Lorrain D., Kaminska M., Gagnon J.F., Lim A., Carrier J., Gosselin N. Association between risk of obstructive sleep apnea, inflammation and cognition after 45 years old in the Canadian Longitudinal Study on Aging. Sleep Med. 2022;91:21–30. doi: 10.1016/j.sleep.2022.02.006. [DOI] [PubMed] [Google Scholar]
- Wang B., Li W., Jin H., Nie X., Shen H., Li E., Wang W. Curcumin attenuates chronic intermittent hypoxia-induced brain injuries by inhibiting AQP4 and p38 MAPK pathway. Respir. Physiol. Neurobiol. 2018;255:50–57. doi: 10.1016/j.resp.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., Leirer V.O. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhao Y. Sleep-disordered breathing and the risk of cognitive decline: a meta-analysis of 19,940 participants. Sleep Breath. 2018;22:165–173. doi: 10.1007/s11325-017-1562-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M.E., Aloia M.S. Sleep-disordered breathing and cognition in older adults. Curr. Neurol. Neurosci. Reports. 2012;12:537–546. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.