Highlights
-
•
Compared with non-image-guided radiotherapy (non-IGRT), IGRT significantly reduced acute and late gastrointestinal (GI) and acute genitourinary (GU) toxicity.
-
•
Compared with non-IGRT, IGRT significantly improved 3-year prostate specific antigen relapse free survival (PRFS) and biochemical failure-free survival (BFFS).
-
•
There was no significant effects of IGRT on acute rectal toxicity, late GU toxicity, 5-year overall survival (OS) and second cancer mortality (SCM).
-
•
Compared with weekly IGRT, high-frequency daily IGRT could lead to greater 3-year biochemical failure-free survival (BFFS) benefit in prostate cancer patients.
-
•
IGRT with reduced PTV margins could further reduce acute GU. However, prescription dose escalation would balance out the decrease in acute GU.
Keywords: Image-guided radiotherapy, Prostate cancer, Meta-analysis, Survival, Gastrointestinal toxicity, Genitourinary toxicity, Second cancer mortality
Abstract
Background
Image-guided radiotherapy (IGRT) has gradually been widely promoted in clinical procedure. However, there has been no consensus on the effects of IGRT on toxicity and survival, and no clear level 1 evidence has even been promulgated.
Methods
Medline, EMBASE, PubMed, Cochrane databases and ClinicalTrials.gov were searched for studies comparing IGRT vs non-IGRT or higher frequency IGRT vs lower frequency IGRT during prostate radiotherapy, indexed from database inception to April 2022.
Results
The review included 18 studies (3 randomized clinical trial and 15 cohort studies) involving 6521 men, with a median duration of patient follow-up of 46.2 months in the IGRT group vs 52.7 months in the control group. The meta-analysis demonstrated that IGRT significantly reduced acute GU (risk ratio [RR], 0.78; 95 % confidence interval [CI], 0.69–0.88; P < 0.001 [9 studies]) and GI toxicity (RR, 0.49; 95 % CI, 0.35–0.68; P < 0.001 [4 studies]) and late GI toxicity (HR, 0.25; 95 % CI, 0.07–0.87; P = 0.03 [3 studies]) compared with non-IGRT. Meanwhile, compared with prospective studies, retrospective studies showed that IGRT had a more significant effect in reducing the late GI toxicity. Compared with non-daily IGRT, daily IGRT significantly improved 3-year PRFS (HR, 0.45; 95 % CI, 0.28–0.72; P = 0.001 [2 studies]) and BFFS (HR, 0.57; 95 % CI, 0.39–0.83; P = 0.003 [3 studies]). Furthermore, high-frequency daily IGRT could lead to greater 3-year BFFS benefit in prostate cancer patients than weekly IGRT. However, no significant effects of IGRT on acute rectal toxicity, late GU toxicity, 5-year OS and SCM were found.
Conclusions
For men receiving prostate radiotherapy, IGRT was associated with an improvement in biochemical tumor control and a reduction in GI and acute GU toxicity, but did not significantly improve 5-year OS or increase 5-year SCM.
Introduction
The essence of radiotherapy is to kill tumor cells with radiation, which has been proved to be greatly effective in practice [1], [2]. The goal of radiotherapy is to deliver high dose to the tumor while sparing adjacent normal healthy tissues. The geometric accuracy of dose deposited to the desired target is critical to ensure high quality of treatments [3], [4]. Under this demand, image-guided radiotherapy (IGRT) was born in 1980 s, which can accurately locate and guide radiotherapy [5], [6]. IGRT also potentially reduces the planning target volume (PTV) margins and increases the prescription dose, helping to decrease toxicity and improve survival, respectively [7], [8], [9]. Over several decades, IGRT has gradually been widely promoted in clinic with its theoretical advantages [10], [11], [12], [13]. However, there has been no consensus on the effects of IGRT on toxicity and survival, and no clear level 1 evidence has even been promulgated.
Prostate cancer is the most frequent cancers in men, accounting for more than 1 in 5 new diagnoses [14]. Encouragingly, with advances in treatment, life expectancy for men with localized prostate cancer can be as high as 99 % over 10-years if diagnosed at an early stage [15]. Radiotherapy contributes significantly to the treatment of prostate cancer, and it can cure 60 % of men with localized prostate cancer, which might benefit from IGRT technology [16]. But what exactly is the role of IGRT in radiotherapy? It has been reported that the clinical target volume (CTV)-PTV margins of prostate cancer can be shrunk from 15 mm to 7 mm with weekly IGRT and higher frequency daily IGRT may further tighten the margins to 5 mm, reducing the PTV volume by approximately 20 mm3 [17], [18], [19]. A smaller radiotherapy volume provided an opportunity to escalate the prescription dose from 75.6 Gy to 79.8 Gy, which mitigated the biochemical recurrence rate by 6 % in patients with electronic portal imaging device (EPID) and 14 % with cone beam computer tomography (CBCT)-guided radiotherapy [20].
Meanwhile, there are some negative reports about IGRT. Kok et al. [21] and Sveistrup et al. [22] pointed out that IGRT with increased prescription dose did not improve biochemical failure-free survival (BFFS). A recent French phase III multicenter randomized trial even reported significantly worse overall survival (OS) when daily IGRT was compared to weekly verification, possibly due to increased second cancer mortality (SCM) [23]. IGRT also imposes a greater financial burden on patients and increases treatment time, especially in radiotherapy rooms with heavy workload [24]. More worryingly, the mainstream IGRT can generate additional doses during radiotherapy, the clinical consequences of which may be deterioration of late onset toxicity or risk of a second cancer [25], [26], [27].
In the absence of definite conclusions about risks and benefits, IGRT is still being used more frequently in the field of radiotherapy [11], [28]. Therefore, it is imperative to urgently find out the impact of IGRT on patient efficacy, toxicity and second cancer, including IGRT frequency, IGRT technology, PTV margins reduction and prescription dose increase brought by IGRT, and the cooperation with radiotherapy technology. This systematic review and meta-analysis is intended to address these questions and help in the decision-making process regarding the clinical application of IGRT.
Methods
We registered the protocol for this systematic review in the International Prospective Register of Systematic Reviews (PROSPERO) public database (CRD42021254752). This systematic review and meta-analysis followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions [29] and reported findings according to the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) reporting guideline [30].
Eligibility Criteria.
We reviewed studies reporting on men with nonmetastatic prostate cancer treated with any commonly-utilized form of IGRT. We excluded review articles and commentaries, studies with surgery or brachytherapy, pre-post dosimetric studies, studies that failed to report a prespecified outcome of this review, and unpublished or gray literature study data. We included randomized controlled trials (RCTs) and cohort studies (CRSs).
Literature Search.
Medline, EMBASE, PubMed, Cochrane databases and ClinicalTrials.gov were searched for studies indexed from database inception to April 2022. We used both subject headings and text-word terms for “prostatic neoplasms”, “image-guided radiotherapy”, “toxicity”, “survival/mortality’’, “second cancer”, and related and exploded terms including medical subject headings terms in combination with keyword searching. A full search strategy is presented in the Supplementary Table 1. No limitations were placed with respect to publication language or publication year.
Table 1.
Studies Characteristics.
| Author (year) | Patient characteristics |
Follow-up (median) |
Study | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion criteria | Radiotherapy | Country | Size | Type | Intervention | Comparator | Survival | GI | GU | ||
| Becker-Schiebe 2016 [49] | Localized (T1-T4) | 3D-CRT/ IMRT |
Germany | 55.4mo | 198 | RCS | Daily IG-S | Non-IG | X | ✓ | ✓ |
| Chung 2009 [50] | High-risk (T1c-T3) | IMRT | Singapore and USA | <1yr | 25 | RCS | Daily IG-R | Non-IG | X | Rectal | X |
| de Crevoisier 2018 [23] | Localized | 3D-CRT/ IMRT |
France | 4.1 yr | 470 | RCT | Daily IG-S | Weekly IG-S | BFFS, OS, SCM | X | X |
| Ghanem 2021 [45] | Intermediate and high-risk localized | IMRT/Arc | Egypt | IG: 3.7 yr Non: 11.2 yr | 257 | PCS | Daily IG-S | Non-IG | X | ✓ | ✓ |
| Gill 2011 [46] | T1-T4 | CRT/IMRT | Australia | 4 yr | 275 | PCS | Daily IG-S | Non-IG | X | ✓ | ✓ |
| Jereczek-fossa 2018 [47] | localized T1-T3 | 3D-CRT | Italy | 85mo | 353 | PCS | Daily IG-S | Non-IG | OS | X | ✓ |
| Kok 2013 [21] | Localized (T1-T3) | 3D-CRT/ IMRT |
Australia | 22mo | 554 | RCS | Daily IG-S | Non-IG | BFFS | ✓ | ✓ |
| Kuo 2021 [51] | Localized (T1-T4) | 3D-CRT/ IMRT |
China | 50mo | 836 | RCS | Daily IG | Non-IG | OS, SCM | X | X |
| Murray 2020 [44] | Localized (pT1b-T3aN0M0) | IMRT | UK | 56.9mo | 293 | RCT | Daily IG-R | Daily IG-S, Non-IG | X | X | ✓ |
| Singh 2013 [18] | localized prostate (T1-T3N0M0) | 3D-CRT | Australia | 17 mo | 266 | RCS | Daily IG-R | Non-IG | X | X | ✓ |
| Stuk 2021 [52] | localized | IMRT | Czech | IG: 31.7mo Non: 60mo |
469 | RCS | Daily IG-R | Non-IG | X | ✓ | ✓ |
| Sveistrup 2014 [22] | high-risk | 3D-CRT/ IMRT/arc |
Denmark | IG: 3.5 yr Non: 8.2 yr |
503 | RCS | Daily IG-R | Non-IG | BFFS | ✓ | ✓ |
| Tøndel 2018 [19] | intermediate or high risk non-metastatic | 3D-CRT | Norway | greater than5yr | 257 | RCT | Daily IG-R | Weekly IG-S | X | ✓ | ✓ |
| Valeriani 2013 [53] | Intermediate-risk (T2b-T2c) | 3D-CRT | Italy | 31mo | 105 | RCS | Daily IG-R | Non-IG | X | Rectal | ✓ |
| Wortel 2015 and 2016 [43], [48] | localized | 3D-CRT/ IMRT |
Netherlands | IG: 57mo Non: 62mo |
431 | PCS | Daily IG-R | Non-IG | X | ✓ | ✓ |
| Zapatero 2017 [54] | Localized (T1c-T4N0M0) | 3D-CRT/ IMRT |
Spain | 75mo | 733 | RCS | IG-S | Non-IG | X | X | ✓ |
| Zelefsky 2012 [55] | Localized (T1-T3) | IMRT | USA | 2.8 yr | 376 | RCS | Daily IG-S | Non-IG | PRFS | Rectal | ✓ |
| Zhong 2014 [56] | Localized (T1-T3) | IMRT | China | 4.8 yr | 127 | RCS | 1–2 times weekly IG-S | Non-IG | PRFS | Rectal | ✓ |
Abbreviations: 3D-CRT = three-dimensional conformal radiotherapy; IMRT = intensity-modulated radiation therapy; Arc = volumetric modulated arc therapy; RCS = retrospective cohort study; RCT = randomized controlled trial; PCS = prospective cohort study; IG-S = image-guided radiotherapy with standard CTV-PTV margins; IG-R = image-guided radiotherapy with reduced CTV-PTV margins; BFFS = biochemical failure-free survival; OS = overall survival; SCM = second cancer mortality; PRFS = prostate specific antigen relapse free survival; GI = gastrointestinal; GU = genitourinary; NR = not reported.
Study Selection and Data Extraction.
Two experienced systematic reviewers (S.L.W. and W.T.) independently screened records for eligibility. After the exclusion of irrelevant records, we obtained the full texts of remaining articles and reviewed them for eligibility. Discrepancies between the reviewers were resolved by discussion. When multiple articles included overlapping series of patients, we preferentially extracted outcome data from the primary article with the largest sample size for early outcomes and from the article with the longest follow-up duration for late outcomes.
Risk of Bias.
A risk of bias assessment was conducted using the Cochrane Risk of Bias Tool for RCTs, and the Newcastle-Ottawa Scale for prospective cohort studies (PCSs) and retrospective cohort studies (RCSs). In the Cochrane Risk of Bias Tool, each domain can score low risk if there is no indication for risk of bias, some concerns if there is potential for risk of bias, or high risk if there is clear indication for risk of bias [31]. Similarly, the Newcastle-Ottawa Scale assesses risk of bias in three domains: [32] (1) selection of the study groups; (2) comparability of groups; and (3) ascertainment of exposure and outcome. We investigated the potential for publication bias by visually inspecting funnel plots for asymmetry and with the Egger regression test [33], [34].
Statistical analysis
The results of late gastrointestinal (GI) toxicity, BFFS, prostate specific antigen relapse-free survival (PRFS), OS and SCM were reported as hazard ratios (HRs) with 95 % confidence interval (CIs), and the most fully adjusted HR were extracted. The results of acute GI toxicity, genitourinary (GU) toxicity and rectal toxicity were recorded as risk ratios (RRs) with 95 % CIs. The following effect modifiers on the end points were tested using subgroup analysis: imaging technology, IGRT frequency, PTV margins, radiotherapy volume, radiotherapy dose, radiotherapy technology and the type of included studies.
Heterogeneity was assessed using the χ2 test and the I2 statistic. Significant heterogeneity was indicated by P < 0.05 in Cochrane Q tests or a ratio greater than 40 % in I2 statistics, which led to the use of random-effects models according to the DerSimonian and Laird method [35], [36]. Otherwise, these tests were negative for heterogeneity, and fixed-effects models were chosen. We performed a 1-study-removed sensitivity analysis, in which the meta-analysis for each outcome was recalculated after removing 1 study at a time to determine the association of individual studies with meta-analysis results. Statistical analyses were performed using the Cochrane Review Manager, version 5.3. A confidence level of 95 % (P < 0.05) was considered statistically significant.
Results
Systematic review results and study identification
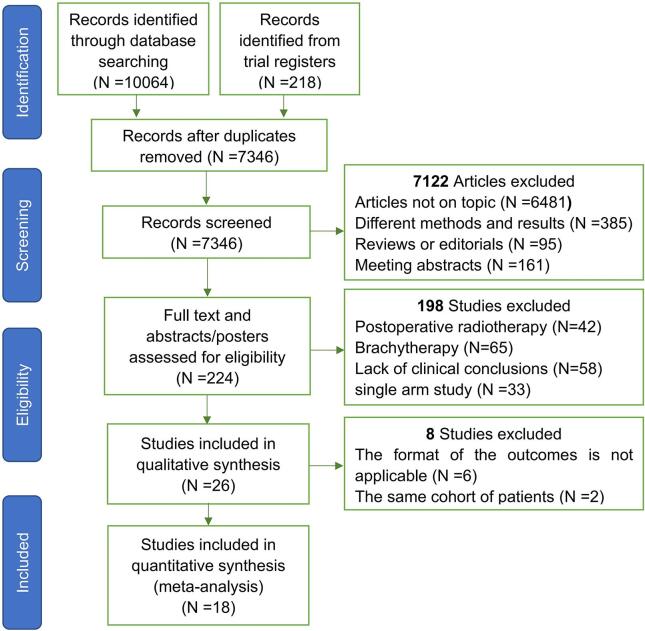
A total of 10,064 publications and 218 registered clinical studies were identified from the literature search. After screening titles and abstracts for eligibility, 224 full-text articles were reviewed. After further exclusion, 26 articles were eligible for inclusion, but 6 of them were excluded due to the inappropriate form of outcomes [17], [37], [38], [39], [40], [41] and 2 were excluded because of the same cohort of patients [42], [43]. Ultimately, 18 studies that met the eligibility criteria were finally selected, including 3 RCTs, [19], [23], [44] 4 PCSs [45], [46], [47], [48] and 11 RCSs [18], [21], [22], [49], [50], [51], [52], [53], [54], [55], [56]. A PRISMA flow diagram depicting the study identification and selection is shown in Fig. 1.
Fig. 1.
Flow Chart of Publication Search and Selection.
Patient and trial characteristics
A total of 6521 participants were enrolled, 3104 men were in the IGRT group and 3107 men were in the control group. The median duration of treatment in the IGRT group was 46.2 months vs 52.7 months in the control group. Trial characteristics including radiotherapy technology, IGRT frequency, IGRT technology, PTV margins, radiotherapy volume, radiation dose, study type and adjuvant therapy are presented in Table 1 and Table 2.
Table 2.
Potential Heterogeneity Factors of Studies.
| Author (year) | CTV-to-PTV margins | Radiation range | Radiation Dose (Gy) | IGRT technology | Adjuvant | ||
|---|---|---|---|---|---|---|---|
| Intervention | Comparator | Intervention | Comparator | ||||
| Becker-Schiebe 2016 [49] | 10 mm circumferentially, except for 6 mm posteriorly; For boost contouring, 5 mm in all directions | Same margins | PORT or WPRT according to the risk | Prostate: 77.4 (1.8 per fraction) Pelvic: 50.4 (1.8 per fraction) |
Prostate: 72 or 73.8 (1.8 per fraction) Pelvic: 50.4 (1.8 per fraction) |
FMs + KV/MV or CBCT | ADT |
| Chung 2009 [50] | 2–3 mm circumferentially | 10 mm circumferentially, except for 5 mm posteriorly | WPRT | Prostate: 73.8 (1.8 per fraction) Pelvic: 48.6 (1.8 per fraction) |
Same dose | FMs + OI | NR |
| de Crevoisier 2018 [23] | 10 mm circumferentially, except for 5 mm posteriorly | Same margins | PORT | 70–80 (2 per fraction) | Same dose | FMs + EPID/KV, CBCT or Ultrasounds | ADT |
| Ghanem 2021 [45] | 10 mm circumferentially, except for 5 mm posteriorly | Same margins | PORT or WPRT according to the risk | 79.2 (1.8–2 per fraction) for prostate | 74 (1.8–2 per fraction) for prostate | CBCT | ADT |
| Gill 2011 [46] | 10 mm circumferentially, except for 7 mm posteriorly | Same margins | PORT | 78 (2 per fraction) | 74 (2 per fraction) | FMs + OI | ADT |
| Jereczek-fossa 2018 [47] | 7 mm and 3 mm, for all but posterior margins | 10 and 5 mm, for all but posterior margins | PORT | 70.2 (2.7 per fraction) | 80 (2 per fraction) | BAT, ExacTrac® or CBCT | ADT |
| Kok 2013 [21] | 10 mm circumferentially, except for 7 mm posteriorly | Same margins | PORT | 78 (2 per fraction) | 74 (2 per fraction) | FMs + KV OI | ADT |
| Kuo 2021 [51] | NR | NR | NR | 72–81 (1.8–2 per fraction) | Same dose | NR | ADT |
| Murray 2020 [44] | In the IGRT and IGRT-S arms, standard CTV to PTV posterior margins of 6 mm/3 mm/0 mm were used. In the IGRT-R arm, posterior margins were 10 mm/5 mm/0 mm | Posterior margins were 10 mm/5 mm/0 mm | PORT | Conventional: 74 (2 per fraction) hypo-fractionated: 60 (3 per fraction) or 57 (3per fraction) |
Same dose | FMs or MVCT | NR |
| Singh 2013 [18] | 7–12 mm circumferentially, except for 5–7 mm posteriorly; For boost contouring, 6–10 mm circumferentially, except for 5–7 mm posteriorly | 10–15 mm circumferentially, except for 7–10 mm posteriorly; For boost contouring, 10 mm circumferentially, except for 7–10 mm posteriorly | PORT | 70–76 (2 per fraction) | Same dose | FMs + MRI | ADT |
| Stuk 2021 [52] | 6–8 mm | 10 mm isotropic margins | PORT | 70–74 (2 per fraction) | Same dose | CBCT or KV-KV | ADT |
| Sveistrup 2014 [22] | 5 mm in the right-left and anterior-posterior planes and 7 mm in the superior-inferior plane | Fourth and fifth fractions were delivered with a margin to the PTV of 1 cm and the remaining 33 fractions with a margin of 2 cm | PORT | 78 (2 per fraction) | 76 (2 per fraction) | FMs + KV OI (ExacTrac®) | ADT |
| Tøndel 2018 [19] | 7 mm in all direction; for boost contouring, 3 mm in all direction | 15 mm in all direction; for boost contouring, 3 mm in all direction | PORT | 78 (2 per fraction) | Same dose | FMs + 2D MV OI or FMs + KV CBCT | ADT |
| Valeriani 2013 [53] | 5 mm expansion in all direction | 8 mm circumferentially, except for 6 mm posteriorly | PORT | 54.75 (3.65 per fraction) | Same dose | KV CBCT | ADT |
| Wortel 2016 [48] | 5–8 mm; for boost contouring, 3–5 mm | 10 mm; for boost contouring, 5 mm | PORT | 78 (2 per fraction) | Same dose | FMs + CBCT | ADT |
| Zapatero 2017 [54] | 1 cm circumferentially and 7 mm at the prostate-rectal interface |
Same margins | PORT | 76–80 (2 per fraction) | Same dose | FMs | ADT |
| Zelefsky 2012 [55] | 1 cm circumferentially and 6 mm at the prostate-rectal interface |
Same margins | PORT | 86.4 (1.8 per fraction) | Same dose | FMs + KV OI | ADT |
| Zhong 2014 [56] | 8–10 cm circumferentially and 6 mm at the prostate-rectal interface; for boost contouring, 3–4 mm circumferentially | Same margins | PORT or WPRT according to the risk | 76–80 (2 per fraction) for prostate | Same dose | CBCT | ADT |
Abbreviations: CTV = clinical target volume; PTV = planning target volume; WPRT = whole-pelvic radiotherapy; PORT = prostate-only radiotherapy; IGRT = image-guided radiotherapy; FMs = fiducial markers; KV = kilovoltage; MV = megavoltage; CBCT = cone beam computer tomography; OI = orthogonal imaging; EPID = electronic portal imaging device; MRI = magnetic resonance imaging; ADT = androgen deprivation therapy; NR = not reported.
Genitourinary (GU) toxicity
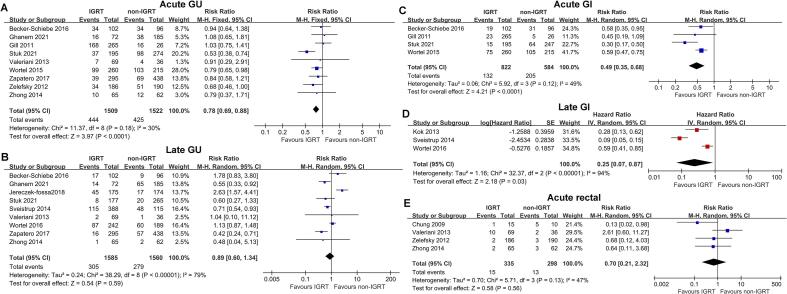
The forest plot depicted that IGRT significantly decreased grade 2 or worse (G2+ ) acute GU toxicity (risk ratio [RR], 0.78; 95 % confidence interval [CI], 0.69–0.88; P < 0.001 [9 studies]; Fig. 2A) compared with non-IGRT. However, there was no difference between IGRT and non-IGRT in the risk of G2+ late GU toxicity (RR, 0.89; 95 % CI, 0.60–1.34; P = 0.59 [9 studies]; Fig. 2B).
Fig. 2.
Forest Plots of the meta-analytic Estimate for Acute Genitourinary (GU), Late GU, Acute Gastrointestinal (GI), Late GI and Acute Rectal Toxicity With vs Without IGRT.
Gastrointestinal (GI) toxicity and acute rectal toxicity
Patients who received IGRT had lower G2+ acute GI (RR, 0.49; 95 % CI, 0.35–0.68; P < 0.001 [4 studies]; Fig. 2C) and late GI toxicity (HR, 0.25; 95 % CI, 0.07–0.87; P = 0.03 [3 studies]; Fig. 2D) than those who did not. Four studies reported the G2+ acute rectal toxicity, and the incidence of G2+ acute rectal toxicity was low overall. According to the forest plot (Fig. 2E), IGRT did not significantly decrease the acute rectal toxicity (RR, 0.70; 95 % CI, 0.21–2.32; P = 0.56 [4 studies]).
Subgroup analysis of the effects of IGRT on toxicity
The heterogeneity of acute and late GU toxicity was I2 = 30 % and I2 = 79 %. Subgroup analyses were performed for possible heterogeneity factors. The results demonstrated that IGRT technology, PTV margins and radiotherapy technology might lead to different outcomes of acute GU toxicity, but the differences were not significant. However, dose-escalation could have a significant adverse effect on the reduction of acute GU toxicity (P = 0.02; I2 = 83 %). And two-dimensional (2D) imaging + fiducial markers (FMs) might be more beneficial for late GU than other types of IGRT although it was also not significant (Table 3). On the other hand, there were no heterogeneity factors significantly associated with acute GI toxicity (Supplementary Table 2). For cooperation of IGRT and radiotherapy technology, IGRT combined with intensity-modulated radiation therapy (IMRT) might be more effective than three-dimensional conformal radiotherapy (3D-CRT) in preventing the occurrence of acute rectal toxicity (P = 0.05; I2 = 74.6 %; Supplementary Table 3).
Table 3.
Subgroup Analysis of Potential Heterogeneity Factors for Acute and Late GU Toxicity.
| Heterogeneity factors | Acute GU toxicity | Late GU toxicity | ||||||
|---|---|---|---|---|---|---|---|---|
| No. Studies | Hazard ratio (95 % CI, P value) |
P Value for Interaction |
I2 | No. Studies | Hazard ratio (95 % CI, P value) |
P Value for Interaction |
I2 | |
| Imaging technology | ||||||||
| 2D imaging + FMs | 2 | 0.81 (0.62–1.05; P = 0.11) | P = 0.28 | 22.1 % | 1 | 0.71 (0.54–0.93; P = 0.01) | P = 0.28 | 22.4 % |
| 3D imaging | 4 | 0.84 (0.70–1.01; P = 0.07) | 4 | 0.82 (0.47–1.42; P = 0.47) | ||||
| Mixed use | 2 | 0.65 (0.51–0.84; P < 0.001) | 3 | 1.46 (0.62–3.44; P = 0.38) | ||||
| Reduced margins in IGRT | ||||||||
| Yes | 6 | 0.87 (0.73–1.03; P = 0.10) | P = 0.07 | 69.9 % | 4 | 0.85 (0.59–1.21; P = 0.36) | P = 0.59 | 0 % |
| No | 3 | 0.69 (0.58–0.82; P < 0.001) | 4 | 0.69 (0.35–1.35; P = 0.28) | ||||
| Radiotherapy volume | ||||||||
| PORT | 6 | 0.74 (0.65–0.85; P < 0.001) | P = 0.12 | 59.5 % | 6 | 0.90 (0.55–1.48; P = 0.68) | P = 0.95 | 0 % |
| WPRT for high risk | 3 | 0.96 (0.72–1.28; P = 0.77) | 3 | 0.87 (0.34–2.26; P = 0.78) | ||||
| Dose escalation in IGRT | ||||||||
| Yes | 3 | 1.01 (0.80–1.27; P = 0.96) | P = 0.02 | 83 % | 3 | 0.81 (0.49–1.34; P = 0.42) | P = 0.69 | 0 % |
| No | 6 | 0.72 (0.62–0.83; P < 0.001) | 5 | 0.69 (0.37–1.28; P = 0.24) | ||||
| Radiotherapy technology | ||||||||
| 3D-CRT | 1 | 0.91 (0.29–2.91; P = 0.88) | P = 0.17 | 44.2 % | 1 | 1.04 (0.10–11.12; P = 0.97) | P = 0.90 | 0 % |
| IMRT or Arc | 4 | 0.67 (0.54–0.83; P < 0.001) | 4 | 0.90 (0.33–2.41; P = 0.83) | ||||
| Mixed use | 4 | 0.86 (0.74–1.00; P = 0.04) | 3 | 0.73 (0.45, 1.19; P = 0.20) | ||||
Abbreviations: GU = genitourinary; FMs = fiducial markers; IGRT = image-guided radiotherapy; WPRT = whole-pelvic radiotherapy; PORT = prostate-only radiotherapy; 3D-CRT = three-dimensional conformal radiotherapy; IMRT = intensity-modulated radiation therapy; Arc = volumetric modulated arc therapy.
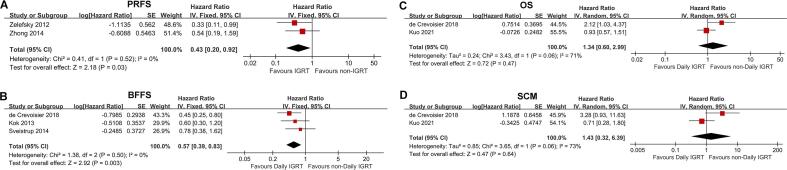
3-year Prostate Specific Antigen Relapse Free Survival (PRFS) and Biochemical Failure-free Survival (BFFS)
The forest plots (Fig. 3A) showed that IGRT significantly improved 3-year PRFS (hazard ratio [HR], 0.45; 95 % CI, 0.28–0.72; P = 0.001 [2 studies]). Moreover, daily IGRT compared with non-daily IGRT (weekly or non-IGRT) also significantly increased 3-year BFFS in prostate cancer patients (HR, 0.57; 95 % CI, 0.39–0.83; P = 0.003 [3 studies]; Fig. 3B).
Fig. 3.
Forest Plots of the meta-analytic Estimate for Prostate Specific Antigen Relapse Free Survival (PRFS), Biochemical Failure-free Survival (BFFS), Overall Survival (OS) and Second Cancer Mortality (SCM).
5-year overall survival (OS) and second cancer mortality (SCM)
Daily IGRT had no significant effect on neither 5-year OS nor SCM (Fig. 3C and Fig. 3D). However, the heterogeneity of both analyses was large, with I2 = 71 % and I2 = 73 %, respectively. Among them, de Crevoisier et al. [23] showed that daily IGRT significantly reduced the 5-year OS of patients and potentially increased the SCM. However, Kuo et al. [51] believed that daily IGRT had no effect on 5-year OS and SCM.
Subgroup analysis of the effects of IGRT on survival
Further subgroup analysis revealed that compared with non-IGRT, daily IGRT resulted in a significant improvement in 3-year PRFS (HR, 0.33; 95 % CI, 0.11–0.99; P = 0.03), whereas weekly IGRT did not (Supplementary Table 4). And daily IGRT also significantly improved 3-year BFFS compared with weekly IGRT (HR, 0.45; 95 % CI, 0.25–0.80; P = 0.007; Supplementary Table 4). However, among the included studies, only one study [23] compared the effects of daily IGRT and weekly IGRT on survival of patients with prostate cancer. On the other hand, reduced PTV margins might have an unfavorable effect on BFFS. IGRT significantly improved BFFS over non-IGRT when PTV margins were the same (HR, 0.51; 95 % CI, 0.32–0.79; P = 0.003). However, when PTV margins in IGRT group reduced, IGRT did not significantly increase BFFS (HR, 0.78; 95 % CI, 0.38–1.62; P = 0.50; Supplementary Table 4).
Subgroup analysis of the type of included studies
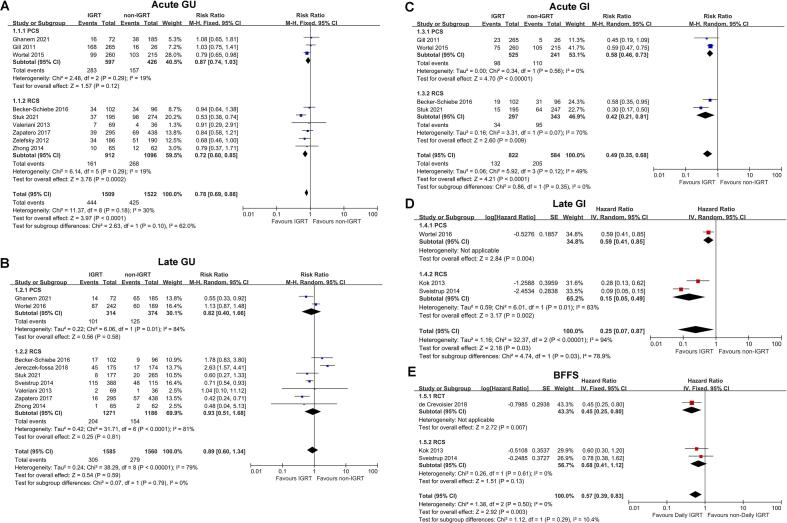
To explore the influence of different studies types on the results, subgroup analyses were conducted according to three subgroups: RCT, PCS and RCS. The results exhibited that there was no significant difference in acute and late GU toxicity, acute GI toxicity and BFFS due to different studies types. However, for late GI toxicity, although PCS and RCS both showed that IGRT had better protection effect than non-IGRT, RCS believed that the effect was more significant (P = 0.03; I2 = 78.9 %; Fig. 4). The conclusions of RCT and RCS on OS and SCM were different, but there were too few studies included for statistical analysis. The results were presented directly in Supplementary Table 4.
Fig. 4.
Subgroup Analysis of the Type of Included Studies. RCTs, randomized controlled trials; PCSs, prospective cohort studies; RCSs, retrospective cohort studies.
Quality Assessment, sensitivity analyses and publication bias
The risk of bias in the included trials was rated as low to moderate (Supplementary Fig. 1 and Supplementary Table 5 in the Supplement). meta-analysis conclusions were largely unchanged in a 1-study-removed sensitivity analysis in which the meta-analysis was recalculated after removing 1 study at a time (Supplementary Table 6). Funnel plot asymmetry was not evident for any outcome (Supplementary Fig. 2), and the results of the Egger regression test did not indicate publication bias (Supplementary Table 7).
Discussion
This meta-analysis examined the effects of the presence or absence of IGRT, IGRT frequency, IGRT technology, PTV margins, prescription dose escalation, and the combination with different radiotherapy technology on the survival, toxicity and second cancer of patients with prostate cancer. The meta-analysis demonstrated that IGRT significantly reduced acute GU and GI toxicity and late GI toxicity compared with non-IGRT. Moreover, compared with non-daily IGRT, daily IGRT significantly improved 3-year PRFS and BFFS. However, no significant effects of IGRT on late GU toxicity, 5-year OS and SCM were found. Further analysis showed that 2D imaging + FMs might be more beneficial for late GU than other types of IGRT, and that high-frequency daily IGRT could lead to greater 3-year BFFS benefit in prostate cancer patients than weekly IGRT. In addition, IGRT with reduced PTV margins could significantly reduce the acute GU toxicity. However, increasing the prescription dose would balance out the decrease in acute GU toxicity. For cooperation of IGRT and radiotherapy technology, IGRT combined with IMRT might be more effective than 3D-CRT in protecting acute GU and rectal toxicity.
One of the most potential benefits of IGRT is to reduce PTV margins and increased prescription dose, due to lower setup errors and higher accuracy [39], [41]. With mainstream IGRT, including CBCT or 2D + FMs imaging, PTV margins in the range of 6–8 mm (3–5 mm posteriorly) were the most commonly reported, much tighter than 10–15 mm margins in non-IGRT [57]. Our meta-analysis showed that compared to the same PTV margins, IGRT with reduced margins could significantly reduce the acute GU toxicity. A reduction in the PTV margins could facilitate further increases in prescription doses [58]. Raziee et al. [20] suggested continuous improvement in biochemical control rates with progressive dose-escalation (DE) when high-precision daily IGRT was used. However, it should be reminded in our meta-analysis that DE could have adverse effects on the reduction of acute GU toxicity produced by IGRT, although it did not alter the reduction in acute GI toxicity.
At present, most researchers have agreed that IGRT is conducive to reduce acute toxicities [43], [49], [50], [52], [55]. Our meta-analysis concluded that IGRT significantly decreased acute GU and GI toxicity compared with non-IGRT. However, the reduction of the PTV margins did not further alleviate acute GI and rectal toxicity. We regarded that this was likely because most non-IGRT patients were also reduced posterior margins to protect the rectum [43], [53]. It was worth mentioning that in our meta-analysis, IGRT combined with IMRT might be more effective than 3D-CRT in reducing acute GU and rectal toxicity, probably benefiting from better bladder and rectal protection with IMRT than with 3D-CRT [59]. Our previous study concluded that the choice of whole pelvic radiotherapy (WPRT) or prostate-only radiotherapy (PORT) significantly affected the toxicity of localized prostate cancer [60]. This meta-analysis further analyzed the impact of radiotherapy region selection on IGRT efficacy. The results suggested that selective WPRT could attenuate the reduction in acute GU toxicity brought about by IGRT. It was possible that the prostatic urethra and part of the bladder neck lay within the PTV due to the large area irradiation, despite the use of more advanced techniques [43].
Late toxicity, especially GU toxicity, is highly controversial in current studies [22], [47], [48], [54]. This meta-analysis showed that IGRT reduced late GI toxicity but had no effect on late GU toxicity. However, there was considerable heterogeneity in both late outcomes, with I2 = 79 % and I2 = 94 %. No further subgroup analysis was performed due to the few published studies of late GI toxicity. Conversely, we did an exhaustive heterogeneity analysis for late GU toxicity, including IGRT technology, PTV margins, radiotherapy volume, radiotherapy dose and radiotherapy technology. Regrettably, no study-level factor was significantly associated with late GU toxicity. Nevertheless, 2D imaging + FMs might be more beneficial for late GU although it was not significant. 2D imaging systems need to be combined with the implantation of FMs to achieve clinically recognized guidance. However, most 3D imaging did not use FMs for alignment. Barney et al. [61] pointed out that although kV portal images using fiducials and CBCT were similar for defining interfraction prostate shifts, 2D imaging + FMs had obvious advantages in alignment. They believed the difficult interpretation and alignment of CBCT required a physician to be at the machine prior to each treatment to perform the match. Finally, by using fiducials for prostate IGRT, they avoided the uncertainty associated with CBCT soft-tissue definition, thus providing their patients with what they believed to be a more reliable, reproducible treatment. Their institution had opted to continue to use fiducials with kV imaging for daily prostate IGRT. However, the clinical effect still needs further confirmation. The acquisition of late toxicity data requires a longer follow-up, and there is a certain risk of loss to follow-up and information distortion, which makes it difficult to obtain compelling late toxicity results. Fully convincing results of late toxicity demand more rigorous studies with longer follow-up times to be published.
There are few studies on the impact of IGRT on survival, and the follow-up time is short. And among the included studies, there was only one study [23] involving the comparison of the effects of daily IGRT and weekly IGRT on the survival, which made it impossible to conduct a comprehensive and quantitative analysis on the survival impact of daily IGRT compared with weekly IGRT. Nevertheless, through this meta-analysis, it could be concluded that daily IGRT significantly improved 3-year PRFS and BFFS, with very low heterogeneity (I2 = 0). The result of the BFFS differed slightly from our previous presentation at the ASTRO conference due to an update of the included literature. On the other hand, the 5-year OS showed large heterogeneity, I2 = 71 %. Kuo et al. disputed a recent French phase III multicenter randomized trial. They argued that IGRT did not have a significant effect on OS, rather than high-frequency IGRT that resulted in a noteworthy reduction in OS, according to a recent RCS [51]. The significantly worse 5-year OS in the multicenter randomized trial might be due to the increased SCM with IGRT [23]. However, its incidence of second cancer had been questioned, as 5 years of follow-up was insufficient to observe such a clear result. The final judgement for OS must require studies with longer follow-up.
Another considerable controversy surrounding IGRT is whether the additional doses produced by the current mainstream IGRT lead to an increase in SCM [26], [27], [62]. The 5-year SCM was also highly heterogeneous in this meta-analysis of published studies (I2 = 73 %). In addition, it is argued that time (lag period) must elapse between the date of exposure to radiation and the development of a secondary cancer for that tumor to be considered induced by radiation [63]. Historically, this has been defined as five years, [64], [65] and more than 5 years of follow-up is required to obtain reliable SCM results. Theoretically, the use of daily standard CBCT for a 35-fraction treatment could result in up to 1.5 to 2 Gy to some critical organs and an effective dose of 600 to 800 mSv to the body, which might induce an additional SCM of 3 % to 4 % [66]. The average dose per image for kilovoltage (KV) or megavoltage (MV) 2D planar was just 1–3 mGy [11]. However, KV and MV 2D imaging systems need to be combined with the implantation of FMs to achieve clinically recognized guidance, which is invasive to patients [67]. CBCT currently accounts for the majority of IGRT, and this proportion is increasing [10], [12], [13]. The publication of studies with longer follow-up is urgently needed to determine whether additional higher doses brought about by CBCT lead to increased SCM.
Limitations
This study has several limitations. First, the quality of included studies varied at a certain extent, but the results remained robust in the sensitivity analyses. Second, it should be emphasized that the comparison of IGRT frequency was based on only two publications. One of them was related to the toxicity of radiotherapy [19] and the other to BFFS and OS [23]. Therefore, the impact of IGRT frequency on survival and toxicity could not be comprehensively and quantitatively analyzed. Third, although no obvious bias was found by funnel plot and Egger regression test, the majority of included studies were retrospective studies that were prone to inherent bias that could not be detected. Fourth, most of the included studies involved the application of 3D-CRT. However, it was more widely recommended to use more advanced radiotherapy methods such as IMRT or VMAT to treat prostate cancer, so as to reduce the radiation dose of organs at risk. Unfortunately, 3D-CRT was also applied in two studies comparing IGRT frequency, so the results of daily IGRT compared with weekly IGRT on survival and toxicity should be viewed critically. Fifth, 5-year follow-up analysis may be underpowered for some survival indicators. More high-quality research publications with longer follow-up are needed for the next longer follow-up level 1 evidence summary.
Conclusions
In this meta-analysis of patients with prostate cancer, IGRT was significantly associated with a reduction in GI and acute GU toxicity and an improvement in biochemical tumor control, but there was no significant effect on 5-year OS and SCM. Furthermore, high-frequency daily IGRT could lead to greater 3-year BFFS benefit in prostate cancer patients than weekly IGRT, and 2D imaging + FMs might be more beneficial for late GU than other types of IGRT. In addition, IGRT with reduced PTV margins could significantly mitigate the acute GU toxicity. However, increasing the prescription dose would balance out the reduction of acute GU toxicity. For cooperation of IGRT and radiotherapy technology, IGRT combined with IMRT might be more effective than 3D-CRT in protecting acute GU and rectal toxicity. Meanwhile, compared with prospective studies, retrospective studies showed that IGRT had a more significant effect in reducing the late GI toxicity. In this meta-analysis, the majority of the analyzed publications are retrospective studies, and more high-quality randomized controlled trials are urgently needed to further verify the role of IGRT in prostate cancer.
Funding
The data analysis was supported by Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) under Grant No. 2022DBXM005.
CRediT authorship contribution statement
Shilin Wang: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Wen Tang: Data curation, Formal analysis, Methodology, Writing – review & editing. Huanli Luo: Supervision, Validation, Writing – review & editing. Fu Jin: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. Ying Wang: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.11.001.
Contributor Information
Fu Jin, Email: jfazj@126.com.
Ying Wang, Email: wangying1967_cq@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ahmad S.S., Duke S., Jena R., et al. Adv Radiother Bmj. 2012;345:e7765. doi: 10.1136/bmj.e7765. [DOI] [PubMed] [Google Scholar]
- 2.Bhide S.A., Nutting C.M. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citrin D.E. Recent Developments in Radiotherapy. N Engl J Med. 2017;377(11):1065–1075. doi: 10.1056/NEJMra1608986. [DOI] [PubMed] [Google Scholar]
- 4.Sun B., Chang J., Rong Y. The more IGRT systems, the merrier? J Appl Clin Med Phys. 2017;18(4):7–11. doi: 10.1002/acm2.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaorsky N.G., Harrison A.S., Trabulsi E.J., et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol. 2013;10(10):565–579. doi: 10.1038/nrurol.2013.185. [DOI] [PubMed] [Google Scholar]
- 6.Sterzing F., Engenhart-Cabillic R., Flentje M., et al. Image-guided radiotherapy: a new dimension in radiation oncology. Dtsch Arztebl Int. 2011;108(16):274–280. doi: 10.3238/arztebl.2011.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Zhang Q., Eisenberg B.L., et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015;33(20):2231–2238. doi: 10.1200/JCO.2014.58.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yock A.D., Garden A.S., Court L.E., et al. Anisotropic margin expansions in 6 anatomic directions for oropharyngeal image guided radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87(3):596–601. doi: 10.1016/j.ijrobp.2013.06.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navran A., Heemsbergen W., Janssen T., et al. The impact of margin reduction on outcome and toxicity in head and neck cancer patients treated with image-guided volumetric modulated arc therapy (VMAT) Radiother Oncol. 2019;130:25–31. doi: 10.1016/j.radonc.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Nabavizadeh N., Elliott D.A., Chen Y., et al. Image Guided Radiation Therapy (IGRT) Practice Patterns and IGRT's Impact on Workflow and Treatment Planning: Results From a National Survey of American Society for Radiation Oncology Members. Int J Radiat Oncol Biol Phys. 2016;94(4):850–857. doi: 10.1016/j.ijrobp.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 11.De Los S.J., Popple R., Agazaryan N., et al. Image guided radiation therapy (IGRT) technologies for radiation therapy localization and delivery. Int J Radiat Oncol Biol Phys. 2013;87(1):33–45. doi: 10.1016/j.ijrobp.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Ariyaratne H., Chesham H., Alonzi R. Image-guided radiotherapy for prostate cancer in the United Kingdom: a national survey. Br J Radiol. 2017;90(1070):20160059. doi: 10.1259/bjr.20160059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batumalai V., Holloway L.C., Kumar S., et al. Survey of image-guided radiotherapy use in Australia. J Med Imaging Radiat Oncol. 2017;61(3):394–401. doi: 10.1111/1754-9485.12556. [DOI] [PubMed] [Google Scholar]
- 14.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 16.Rebello R.J., Oing C., Knudsen K.E., et al. Prostate cancer Nat Rev Dis Primers. 2021;7(1):9. doi: 10.1038/s41572-020-00243-0. [DOI] [PubMed] [Google Scholar]
- 17.Ariyaratne H., Chesham H., Pettingell J., et al. Image-guided radiotherapy for prostate cancer with cone beam CT: dosimetric effects of imaging frequency and PTV margin. Radiother Oncol. 2016;121(1):103–108. doi: 10.1016/j.radonc.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Singh J., Greer P.B., White M.A., et al. Treatment-related morbidity in prostate cancer: a comparison of 3-dimensional conformal radiation therapy with and without image guidance using implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2013;85(4):1018–1023. doi: 10.1016/j.ijrobp.2012.07.2376. [DOI] [PubMed] [Google Scholar]
- 19.Tøndel H., Lund J., Lydersen S., et al. Radiotherapy for prostate cancer - Does daily image guidance with tighter margins improve patient reported outcomes compared to weekly orthogonal verified irradiation? Results from a randomized controlled trial. Radiother Oncol. 2018;126(2):229–235. doi: 10.1016/j.radonc.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Raziee H., Moraes F.Y., Murgic J., et al. Improved outcomes with dose escalation in localized prostate cancer treated with precision image-guided radiotherapy. Radiother Oncol. 2017;123(3):459–465. doi: 10.1016/j.radonc.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Kok D., Gill S., Bressel M., et al. Late toxicity and biochemical control in 554 prostate cancer patients treated with and without dose escalated image guided radiotherapy. Radiother Oncol. 2013;107(2):140–146. doi: 10.1016/j.radonc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Sveistrup J, af Rosenschöld PM, Deasy JO, et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol 2014;9:44. [DOI] [PMC free article] [PubMed]
- 23.de Crevoisier R., Bayar M.A., Pommier P., et al. Daily Versus Weekly Prostate Cancer Image Guided Radiation Therapy: Phase 3 Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys. 2018;102(5):1420–1429. doi: 10.1016/j.ijrobp.2018.07.2006. [DOI] [PubMed] [Google Scholar]
- 24.Perrier L., Morelle M., Pommier P., et al. Cost of prostate image-guided radiation therapy: results of a randomized trial. Radiother Oncol. 2013;106(1):50–58. doi: 10.1016/j.radonc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Dzierma Y., Ames E., Nuesken F., et al. Image quality and dose distributions of three linac-based imaging modalities. Strahlenther Onkol. 2015;191(4):365–374. doi: 10.1007/s00066-014-0798-7. [DOI] [PubMed] [Google Scholar]
- 26.Dzierma Y., Minko P., Ziegenhain F., et al. Abdominal imaging dose in radiology and radiotherapy - Phantom point dose measurements, effective dose and secondary cancer risk. Phys Med. 2017;43:49–56. doi: 10.1016/j.ejmp.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Wu H., Chen Z., et al. Concomitant Imaging Dose and Cancer Risk in Image Guided Thoracic Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):523–531. doi: 10.1016/j.ijrobp.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 28.Das S., Liu T., Jani A.B., et al. Comparison of image-guided radiotherapy technologies for prostate cancer. Am J Clin Oncol. 2014;37(6):616–623. doi: 10.1097/COC.0b013e31827e4eb9. [DOI] [PubMed] [Google Scholar]
- 29.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:Ed000142. [DOI] [PMC free article] [PubMed]
- 30.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7(27):iii-x, 1-173. [DOI] [PubMed]
- 33.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 34.Egger M., Davey Smith G., Schneider M., et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drozdz S., Schwedas M., Salz H., et al. Prostate cancer treated with image-guided helical TomoTherapy® and image-guided LINAC-IMRT : Correlation between high-dose bladder volume, margin reduction, and genitourinary toxicity. Strahlenther Onkol. 2016;192(4):223–231. doi: 10.1007/s00066-015-0935-y. [DOI] [PubMed] [Google Scholar]
- 38.Ghilezan M., Yan D., Liang J., et al. Online image-guided intensity-modulated radiotherapy for prostate cancer: How much improvement can we expect? A theoretical assessment of clinical benefits and potential dose escalation by improving precision and accuracy of radiation delivery. Int J Radiat Oncol Biol Phys. 2004;60(5):1602–1610. doi: 10.1016/j.ijrobp.2004.07.709. [DOI] [PubMed] [Google Scholar]
- 39.Kupelian P.A., Lee C., Langen K.M., et al. Evaluation of image-guidance strategies in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1151–1157. doi: 10.1016/j.ijrobp.2007.07.2371. [DOI] [PubMed] [Google Scholar]
- 40.Pawlowski J.M., Yang E.S., Malcolm A.W., et al. Reduction of dose delivered to organs at risk in prostate cancer patients via image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76(3):924–934. doi: 10.1016/j.ijrobp.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 41.Rudat V., Nour A., Hammoud M., et al. Image-guided intensity-modulated radiotherapy of prostate cancer: Analysis of interfractional errors and acute toxicity. Strahlenther Onkol. 2016;192(2):109–117. doi: 10.1007/s00066-015-0919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munck Af Rosenschold P., Zelefsky M.J., Apte A.P., et al. Image-guided radiotherapy reduces the risk of under-dosing high-risk prostate cancer extra-capsular disease and improves biochemical control. Radiat Oncol. 2018;13(1):64 doi: 10.1186/s13014-018-0978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wortel R.C., Incrocci L., Pos F.J., et al. Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2015;91(4):737–744. doi: 10.1016/j.ijrobp.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Murray J., Griffin C., Gulliford S., et al. A randomised assessment of image guided radiotherapy within a phase 3 trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2020;142:62–71. doi: 10.1016/j.radonc.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghanem A.I., Elsaid A.A., Elshaikh M.A., et al. Volumetric-Modulated Arc Radiotherapy with Daily Image-Guidance Carries Better Toxicity Profile for Higher Risk Prostate Cancer. Asian Pac J Cancer Prev. 2021;22(1):61–68. doi: 10.31557/APJCP.2021.22.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill S., Thomas J., Fox C., et al. Acute toxicity in prostate cancer patients treated with and without image-guided radiotherapy. Radiat Oncol. 2011;6:145. doi: 10.1186/1748-717X-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jereczek-Fossa B.A., Surgo A., Maisonneuve P., et al. Late toxicity of image-guided hypofractionated radiotherapy for prostate: non-randomized comparison with conventional fractionation. Radiol Med. 2019;124(1):65–78. doi: 10.1007/s11547-018-0937-9. [DOI] [PubMed] [Google Scholar]
- 48.Wortel R.C., Incrocci L., Pos F.J., et al. Late Side Effects After Image Guided Intensity Modulated Radiation Therapy Compared to 3D-Conformal Radiation Therapy for Prostate Cancer: Results From 2 Prospective Cohorts. Int J Radiat Oncol Biol Phys. 2016;95(2):680–689. doi: 10.1016/j.ijrobp.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 49.Becker-Schiebe M., Abaci A., Ahmad T., et al. Reducing radiation-associated toxicity using online image guidance (IGRT) in prostate cancer patients undergoing dose-escalated radiation therapy. Rep Pract Oncol Radiother. 2016;21(3):188–194. doi: 10.1016/j.rpor.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung H.T., Xia P., Chan L.W., et al. Does image-guided radiotherapy improve toxicity profile in whole pelvic-treated high-risk prostate cancer? Comparison between IG-IMRT and IMRT. Int J Radiat Oncol Biol Phys. 2009;73(1):53–60. doi: 10.1016/j.ijrobp.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Kuo Y.H., Liang J.A., Chen G.H., et al. Safety of image-guided radiotherapy in definitive radiotherapy for localized prostate cancer: a population-based analysis. Br J Radiol. 2021;94(1121):20200456. doi: 10.1259/bjr.20200456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuk J., Vanasek J., Odrazka K., et al. Image-guided radiation therapy produces lower acute and chronic gastrointestinal and genitourinary toxicity in prostate cancer patients. J Buon. 2021;26(3):940–948. [PubMed] [Google Scholar]
- 53.Valeriani M., Bracci S., Osti M.F., et al. Intermediate-risk prostate cancer patients treated with androgen deprivation therapy and a hypofractionated radiation regimen with or without image guided radiotherapy. Radiat Oncol. 2013;8:137. doi: 10.1186/1748-717X-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zapatero A., Roch M., Büchser D., et al. Reduced late urinary toxicity with high-dose intensity-modulated radiotherapy using intra-prostate fiducial markers for localized prostate cancer. Clin Transl Oncol. 2017;19(9):1161–1167. doi: 10.1007/s12094-017-1655-9. [DOI] [PubMed] [Google Scholar]
- 55.Zelefsky M.J., Kollmeier M., Cox B., et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 56.Zhong Q., Gao H., Li G., et al. Significance of image guidance to clinical outcomes for localized prostate cancer. Biomed Res Int. 2014;2014 doi: 10.1155/2014/860639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yartsev S., Bauman G. Target margins in radiotherapy of prostate cancer. Br J Radiol. 2016;89(1067):20160312. doi: 10.1259/bjr.20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goulet C.C., Herman M.G., Hillman D.W., et al. Estimated limits of IMRT dose escalation using varied planning target volume margins. Phys Med Biol. 2008;53(14):3777–3788. doi: 10.1088/0031-9155/53/14/005. [DOI] [PubMed] [Google Scholar]
- 59.Fenoglietto P., Laliberte B., Allaw A., et al. Persistently better treatment planning results of intensity-modulated (IMRT) over conformal radiotherapy (3D-CRT) in prostate cancer patients with significant variation of clinical target volume and/or organs-at-risk. Radiother Oncol. 2008;88(1):77–87. doi: 10.1016/j.radonc.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Wang S., Tang W., Luo H., et al. Efficacy and Toxicity of Whole Pelvic Radiotherapy Versus Prostate-Only Radiotherapy in Localized Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.796907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barney B.M., Lee R.J., Handrahan D., et al. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT) Int J Radiat Oncol Biol Phys. 2011;80(1):301–305. doi: 10.1016/j.ijrobp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Jin F., Luo H.L., Zhou J., et al. Cancer risk assessment in modern radiotherapy workflow with medical big data. Cancer Manag Res. 2018;10:1665–1675. doi: 10.2147/CMAR.S164980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murray L., Henry A., Hoskin P., et al. Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol. 2014;110(2):213–228. doi: 10.1016/j.radonc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murray E.M., Werner D., Greeff E.A., et al. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys. 1999;45(4):951–961. doi: 10.1016/s0360-3016(99)00279-5. [DOI] [PubMed] [Google Scholar]
- 65.Wallis C.J., Mahar A.L., Choo R., et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352 doi: 10.1136/bmj.i851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kan M.W., Leung L.H., Wong W., et al. Radiation dose from cone beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2008;70(1):272–279. doi: 10.1016/j.ijrobp.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 67.Ng M., Brown E., Williams A., et al. Fiducial markers and spacers in prostate radiotherapy: current applications. BJU Int. 2014;113(Suppl 2):13–20. doi: 10.1111/bju.12624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.