Abstract
Objective:
To evaluate the association of primary infertility with subsequent bilateral oophorectomy and hysterectomy, using a population-based cohort of women with primary infertility and age-matched referent women.
Methods:
The Rochester Epidemiology Project record-linkage system was used to assemble a population-based cohort of women with primary infertility diagnosed between 1980-1999 (index date). Women were age-matched (±1 y) 1:1 to a referent woman without a history of infertility or hysterectomy at the index date. Cox proportional hazards models were fit to compare long-term risks of bilateral oophorectomy and hysterectomy, respectively, between infertility cases and referents.
Results:
Among both groups of 1,001 women, the mean age at index date was 29.2 ± 4.4 years. Median duration of follow-up was 23.7 years for both groups. Women with primary infertility were 1.7 times (adjusted hazard ratio (aHR) 1.69, 95% CI 1.22-2.33) more likely to have a bilateral oophorectomy compared to referent women. In a sensitivity analysis excluding women with a diagnosis of infertility related to endometriosis and their matched referents, this association persisted (aHR 1.50, 95% CI 1.06-2.14). Women with primary infertility did not have a significant increased risk of hysterectomy (aHR 0.98, 95% CI 0.79-1.23). However, risk of hysterectomy was increased in those with primary infertility related to endometriosis (aHR 1.94, 95% CI 1.12-3.34). We observed that women with primary infertility were more likely to have a hysterectomy with bilateral oophorectomy. Referent women were more likely to have a hysterectomy with ovarian conservation. Few women in either group had an isolated bilateral oophorectomy.
Conclusion:
Primary infertility, with and without a diagnosis of endometriosis, is associated with an increased risk of bilateral oophorectomy. In women with endometriosis-related infertility, there is an association with future hysterectomy. These findings represent important confounders in the evaluation of long-term health outcomes related to primary infertility.
Precis:
Women with a history of primary infertility have an increased risk of bilateral oophorectomy, and women with endometriosis-associated infertility have an increased risk of hysterectomy.
Introduction:
There is growing interest in the impact of infertility on long-term health.1 Prior studies have shown an association between infertility and risk of mental health disorders, diabetes, renal disease, cerebrovascular disease and cardiovascular disease.2-4 However, these studies are often limited by length of follow-up and the ability to include patient specific data including baseline health characteristics and the type and length of infertility and associated treatments. Importantly, a full understanding of long-term health outcomes associated with infertility requires adequate identification of confounding variables.
Given the frequency of hysterectomy with or without oophorectomy and known long-term health risks of these procedures, we sought to evaluate the association of primary infertility with the subsequent occurrence of these common surgical procedures. In the general population, women undergoing hysterectomy and oophorectomy have increased risks of cardiovascular disease5, osteoporosis, psychiatric illness6, and all-cause mortality.7, 8 The association between primary infertility and future gynecologic surgery has not been previously investigated, and if present, may identify a compounded risk profile for women with primary infertility and their long-term health.
In this study, we utilized the Mayo Clinic Primary Infertility (MPIC) cohort, a population-based sample of women with primary infertility, and age-matched referents to evaluate the association between primary infertility and subsequent risk of gynecologic surgery, specifically hysterectomy and oophorectomy.9 This association, if present, was hypothesized to differ by underlying infertility diagnosis and whether a woman was ever pregnant.
Methods:
The Rochester Epidemiology Project (REP) medical records-linkage system was used to create the population-based MPIC cohort of women with primary infertility.10, 11 The establishment of the REP and its reliability have been previously published with enumerated validity when compared to other sources.12 This study was deemed IRB exempt as it included secondary use of information (chart review). A detailed description of the cohort utilized in this study has been previously published9 and is summarized here. A total of 3,489 women aged 18-50 with at least one diagnosis code of infertility were identified in the REP. By manual chart review, a cohort of 1,001 women had a confirmed diagnosis of primary infertility. Primary infertility was defined as an inability to conceive after 12 months of attempted conception in women aged <35 and after 6 months of attempted conception in women ≥ age 35, that was first diagnosed between January 1, 1980 and December 31, 1999 while the woman was a resident of Olmsted County, Minnesota. Secondary infertility was not included to reduce potential confounding variables, such as effects of prior treatment and prior parity on long-term outcomes. The date at their first evaluation for infertility was termed the “index date” and each woman was 1:1 age-matched (±1 y) to a randomly selected woman (“referent”) residing in Olmsted County without a history of infertility or hysterectomy at index date. The matching process was made possible by the availability of a complete enumeration of the Olmsted County population using the REP Personal Timelines (i.e., the REP Census).12 For each case with primary infertility, all age-matched (±1 y) women residing in Olmsted County on the index date were electronically identified. From that pool of potential referents, one age-matched woman was randomly selected based on a random number generator. Referent women were reviewed and replaced if they had a history of infertility at the time of index date. Referent women may have been parous or nulliparous at the time of matching.
Baseline demographic data, as of the index date, was manually collected and included age, length of attempted conception prior to evaluation, level of education, body mass index (BMI), and gynecologic surgeries prior to index date. The medical records of both groups were manually reviewed between September 2019 and November 2020. Three individuals (AA, ES and LKR) completed all chart reviews. To ensure consistency and refine the data collection tool, AA reviewed the first 50 charts in duplicate with ES and LKR. Any charts with unclear data were subsequently reviewed by AA. All data was entered into a secure electronic database. Outliers were re-reviewed by AA after the full dataset was reviewed.
The primary outcomes were subsequent hysterectomy and bilateral oophorectomy. For women undergoing hysterectomy, the date, preoperative indication, pathologic diagnosis, and uterine weight were recorded. Among women with oophorectomies, we identified the date at which both ovaries had been removed, meaning upon completion of either two separate unilateral procedures or a single bilateral procedure, which we have collectively termed bilateral oophorectomy. Complete removal of both ovaries, rather than a single unilateral oophorectomy, was deemed most important to potential long-term health risks in a premenopausal woman. The date of the last clinical visit to a REP-affiliated health care professional at the time of the chart review was also abstracted.
Patient characteristics at the time of the index date were compared between the two groups using the chi-square test or Fisher’s exact test. For the analysis of each primary outcome (i.e. subsequent hysterectomy and bilateral oophorectomy, respectively), duration of follow-up was calculated from the index date to the date of procedure of interest, or the last clinical visit to a REP-affiliated health care professional for those without the procedure of interest. Cumulative incidence curves for each primary outcome, were estimated using the Kaplan-Meier method using age as the time scale. In addition, the cumulative incidence was estimated for each of the three competing outcomes (or risks) based on the first subsequent procedure per woman using a nonparametric method: i) concurrent hysterectomy and bilateral oophorectomy, ii) hysterectomy with ovarian conservation, and iii) isolated bilateral oophorectomy.13 Cox proportional hazards models were fit to estimate the hazard ratio (HR) and 95% confidence interval for the association between primary infertility status and each primary outcome.
Multivariable Cox models were fit evaluating these associations after adjusting for the following covariates at the time of the index date, each coded as yes, no, or unknown: White race, married, 4-years of college or more, current or former tobacco use, and obesity defined as a BMI > 30 kg/m2. Race was self-reported on patient intake forms. The Cox models were fit using age as the time scale with women entering the risk set at their respective index ages in order to more completely adjust for age. Robust sandwich covariance estimates were used in the Cox models to account for women included in both cohorts. Analyses were performed for all women combined and stratified by type of primary infertility. A sensitivity analysis was performed excluding the matched sets involving women with primary infertility related to endometriosis to evaluate whether endometriosis alone explained the associations described above.
Lastly, ever being pregnant was evaluated as a time-dependent covariate in Cox models (using time since birth) to evaluate its association with the risk of each primary outcome, respectively. Initially we included this time-dependent covariate along with primary infertility status and their interaction in each model. In addition, this covariate was evaluated in Cox models for each primary outcome, separately for primary infertility cases and referents.
All calculated p-values were two-sided. Data was analyzed using SAS version 9.4 statistical software (SAS Institute, NC; Cary, NC) and version 1.4.1103-4 of RStudio (RStudio, MA; Boston, MA).
Results:
Among both the 1,001 women with primary infertility and the 1,001 matched referents the mean age at index date was 29.2 ± 4.4 years. Sociodemographic characteristics at the time of the index date were compared between women with primary infertility and their age-matched referents (Table 1). At the time of the index date, a higher proportion of those with primary infertility were married (96.5% [966/1001] vs. 65.1% [646/993], p<0.001), had at least 4-years of college (54.6% [499/914] vs. 38.9% [361/929], p<0.001), never smoked (77.4% [770/995] vs. 64.0% [632/988], p<0.001), and were less likely to be obese (11.6% [115/988] vs. 14.9% [146/979], p=0.03) compared to the referents.
Table 1.
Sociodemographic characteristics and gynecologic surgery history at the time of the index date of women diagnosed with primary infertility between 1980 and 1999 and age-matched female referents.
| Characteristic at the index date† | Primary infertility (N=1001) |
Referent (N=1001) |
p value‡ |
|---|---|---|---|
| Age (years) | --^ | ||
| Mean ± SD | 29.2 ± 4.4 | 29.2 ± 4.4 | |
| Range | (18.4-45.9) | (18.2-46.1) | |
| Race | 0.045 | ||
| American Indian or Alaskan Native | 1 (0.1%) | 6 (0.6%) | |
| Asian | 28 (2.8%) | 23 (2.3%) | |
| Black | 11 (1.1%) | 5 (0.5%) | |
| Native Hawaiian or Pacific Islander | 0 (0.0%) | 1 (0.1%) | |
| Unknown or chose not to disclose | 151 (15.1%) | 191 (19.1%) | |
| White | 797 (79.6%) | 761 (76.0%) | |
| None of the Above | 13 (1.3%) | 14 (1.4%) | |
| Ethnicity | 0.016 | ||
| Hispanic or Latino | 25 (2.5%) | 9 (0.9%) | |
| Not Hispanic or Latino | 726 (72.5%) | 722 (72.1%) | |
| Unknown or chose not to disclose | 250 (25.0%) | 270 (27.0%) | |
| Marital Status | <0.001 | ||
| Single | 15 (1.5%) | 337 (33.7%) | |
| Married | 966 (96.5%) | 646 (64.5%) | |
| Partnered (not Married) | 20 (2.0%) | 10 (1.0%) | |
| Not documented | 0 (0.0%) | 8 (0.8%) | |
| Level of education | <0.001 | ||
| Less than high school | 12 (1.2%) | 33 (3.3%) | |
| High school graduate | 129 (12.9%) | 201 (20.1%) | |
| Some college | 274 (27.4%) | 335 (33.5%) | |
| College (4-yr) graduate | 340 (34.0%) | 226 (22.6%) | |
| Beyond college | 159 (15.9%) | 135 (13.5%) | |
| Not documented | 87 (8.7%) | 71 (7.1%) | |
| Tobacco use | <0.001 | ||
| Current | 153 (15.3%) | 262 (26.2%) | |
| Former | 72 (7.2%) | 94 (9.4%) | |
| Never | 770 (76.9%) | 632 (63.1%) | |
| Not documented | 6 (0.6%) | 13 (1.3%) | |
| BMI (kg/m2) | 0.014 | ||
| Mean ± SD | 24.1 ± 5.7 | 24.8 ± 5.9 | |
| Less than 18.5 | 49 (4.9%) | 26 (2.6%) | |
| 18.5 - 24.9 | 645 (64.4%) | 617 (61.6%) | |
| 25 - 29.9 | 179 (17.9%) | 190 (19.0%) | |
| 30 - 39.9 | 88 (8.8%) | 118 (11.8%) | |
| 40 or more | 27 (2.7%) | 28 (2.8%) | |
| Not documented | 13 (1.3%) | 22 (2.2%) | |
| Gynecologic surgery history | |||
| At least one of the following | 91 (9.1%) | 120 (12.0%) | 0.035 |
| Laparoscopic drilling | 1 (0.1%) | 0 | >.99 |
| Cystectomy | 24 (2.4%) | 15 (1.5%) | 0.15 |
| Unilateral oophorectomy | 13 (1.3%) | 6 (0.6%) | 0.11 |
| Salpingectomy | 11 (1.1%) | 44 (4.4%) | <0.001 |
| Dilation and curettage | 31 (3.1%) | 58 (5.8%) | 0.003 |
| Myomectomy | 7 (0.7%) | 0 | 0.016 |
| Endometriosis resection | 33 (3.3%) | 8 (0.8%) | <0.001 |
The index date for each matched pair (case and referent) was defined as the date when the infertility case was first diagnosed with primary infertility.
Comparisons between the two groups were evaluated using the two-sample t-test for BMI and the chi-square test or Fisher’s exact test for all other characteristics.
Age was not statistically compared as each infertility case was 1:1 age-matched (±1 y) to a referent woman.
Among women with primary infertility, 883 (88.2%) had a single etiology for infertility and 118 had 2 or more. The indications included unexplained infertility (37.4%), ovulatory dysfunction (31.6%), male factor (24.6%), endometriosis (10.4%), tubal factor (6.2%), and uterine factor (2.9%). Endometriosis was diagnosed surgically. Uterine factor included known uterine fibroids, prior myomectomy, or congenital uterine anomalies. Women with primary infertility were more likely to have had a prior endometriosis surgery than referent women (3.3% vs. 0.8%, p<0.001). However, referent women were more likely to have had gynecologic surgery prior to the index date overall, specifically prior salpingectomy or dilation and curettage (D&C), either for miscarriage or abnormal bleeding (Table 1).
The median duration of follow-up after the index date was 23.7 years (interquartile range (IQR), 9.1-31.4) and 23.7 years (IQR, 9.3-30.8) for the primary infertility cases and referents, respectively. Seventeen referents were diagnosed with primary infertility after the index date for which they were selected as matched referents: 5 were diagnosed during 1985-1995 while a resident of Olmsted County and therefore also included as cases in this study, 4 were diagnosed between 1992-1998 but were not Olmsted County residents at the time of diagnosis, and 8 were diagnosed after 2000 which was outside the time period of this study. The follow-up of these 17 women in the referent group was censored at their subsequent primary infertility diagnosis date as they were no longer at risk for the outcomes as a referent woman.
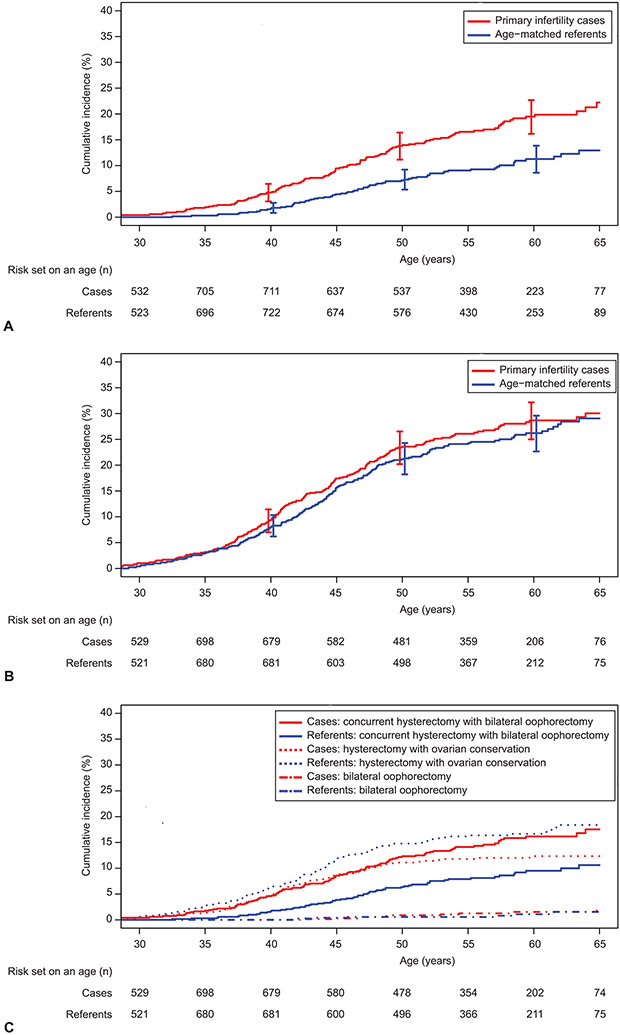
The cumulative incidence of having a bilateral oophorectomy is depicted in Figure 1a. Women with primary infertility were 1.8 times (HR 1.81, 95% CI 1.36-2.42; p<0.001) more likely to have bilateral oophorectomy compared to referent women. This association persisted (adjusted HR 1.69, 95% CI 1.22-2.33; p=0.001) after adjustment for the following covariates at index date: race, marital status, education, smoking history, and obesity (Table 2). Among women who had either ovulatory dysfunction or endometriosis as the indication for primary infertility, the risk of bilateral oophorectomy was significantly increased compared to referent women, with the highest hazard ratio observed for endometriosis (Table 2). Additionally, excluding women with a diagnosis of infertility related to endometriosis and their matched referents as a sensitivity analysis confirmed the positive association between primary infertility and bilateral oophorectomy (HR 1.64, 95% CI 1.20-2.45; p = 0.002) (Appendix 1, available online at http://links.lww.com/xxx) and this persisted in the adjusted analysis (adjusted HR 1.50, 95% CI 1.06-2.14; p = 0.024). Among all women in the cohort with a bilateral oophorectomy, 7.0% had two sequential oophorectomies, including 11 of 126 (8.7%) in the primary infertility group and 3 of 73 (4.1%) in the referent group. The mean ± SD age when both ovaries were completely removed was 45.4±7.9 and 47.1±7.3 years, respectively, for the women with primary infertility compared to referent women.
Figure 1:
Cumulative incidence of subsequent bilateral oophorectomy (A), hysterectomy (B), and the competing risks (C) of which procedure occurs first in women with primary infertility compared to age-matched referent women, using age as the time scale. A. Case vs referent: hazard ratio (HR) 1.81, 95% CI 1.36–2.42. B. Case vs referent: HR 1.08, 95% CI 0.88–1.32. C. Concurrent hysterectomy with bilateral oophorectomy; case vs referent HR 1.73, 95% CI 1.27–2.37. Hysterectomy with ovarian conservation; case vs referent HR 0.73, 95% CI 0.55–0.96.
Table 2.
Comparison of the cumulative incidence of bilateral oophorectomy and hysterectomy between women with primary infertility and age-matched referent woman, overall and within type of primary infertility strata
| Outcome | Primary infertility group | Referent group | Unadjusted analysis† | Adjusted analysis‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. at risk |
Total person- years |
No. of events |
No. at risk |
Total person- years |
No. of events |
Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | ||||
| Overall | |||||||||||||
| Bilateral oophorectomy | 1,001 | 19,760 | 126 | 1,001 | 20,458 | 73 | 1.81 (1.36 - 2.42) |
<0.001 | 1.69 (1.22 - 2.33) |
0.001 | |||
| Hysterectomy | 1,001 | 18,707 | 190 | 1,001 | 18,889 | 179 | 1.08 (0.88 - 1.32) |
0.46 | 0.98 (0.79 - 1.23) |
0.88 | |||
| Type of infertility= Ovulatory dysfunction | |||||||||||||
| Bilateral oophorectomy | 316 | 6300 | 35 | 316 | 6445 | 16 | 2.27 (1.26 - 4.08) |
0.006 | 2.25 (1.14 - 4.41) |
0.019 | |||
| Hysterectomy | 316 | 5932 | 58 | 316 | 6041 | 51 | 1.18 (0.81 - 1.71) |
0.39 | 1.02 (0.68 - 1.51) |
0.93 | |||
| Type of infertility= Male factor | |||||||||||||
| Bilateral oophorectomy | 246 | 4864 | 35 | 246 | 5048 | 19 | 1.94 (1.11 - 3.40) |
0.020 | 1.59 (0.87 - 2.90) |
0.13 | |||
| Hysterectomy | 246 | 4598 | 51 | 246 | 4642 | 44 | 1.19 (0.80 - 1.78) |
0.39 | 1.08 (0.71 - 1.64) |
0.71 | |||
| Type of infertility= Endometriosis | |||||||||||||
| Bilateral oophorectomy | 104 | 1828 | 27 | 104 | 2028 | 10 | 2.95 (1.45 - 6.01) |
0.003 | 2.77 (1.32 - 5.81) |
0.007 | |||
| Hysterectomy | 104 | 1749 | 33 | 104 | 1838 | 20 | 1.72 (1.00 - 2.98) |
0.051 | 1.94 (1.12 - 3.34) |
0.017 | |||
| Type of infertility= Unexplained | |||||||||||||
| Bilateral oophorectomy | 374 | 7506 | 36 | 374 | 7881 | 29 | 1.33 (0.82 - 2.16) |
0.25 | 1.25 (0.70 - 2.22) |
0.45 | |||
| Hysterectomy | 374 | 7082 | 61 | 374 | 7272 | 65 | 0.97 (0.68 - 1.37) |
0.86 | 0.84 (0.56 - 1.26) |
0.40 | |||
Hazard ratios were estimated using Cox proportional hazards models with age as the time scale.
Hazard ratios were estimated using Cox proportional hazards models with age as the time scale and adjusted for the following covariates at the time of the index date, each coded as yes, no, or unknown: White race, married, 4-years of college or more, current or former tobacco use, and obesity defined as a BMI > 30 kg/m2
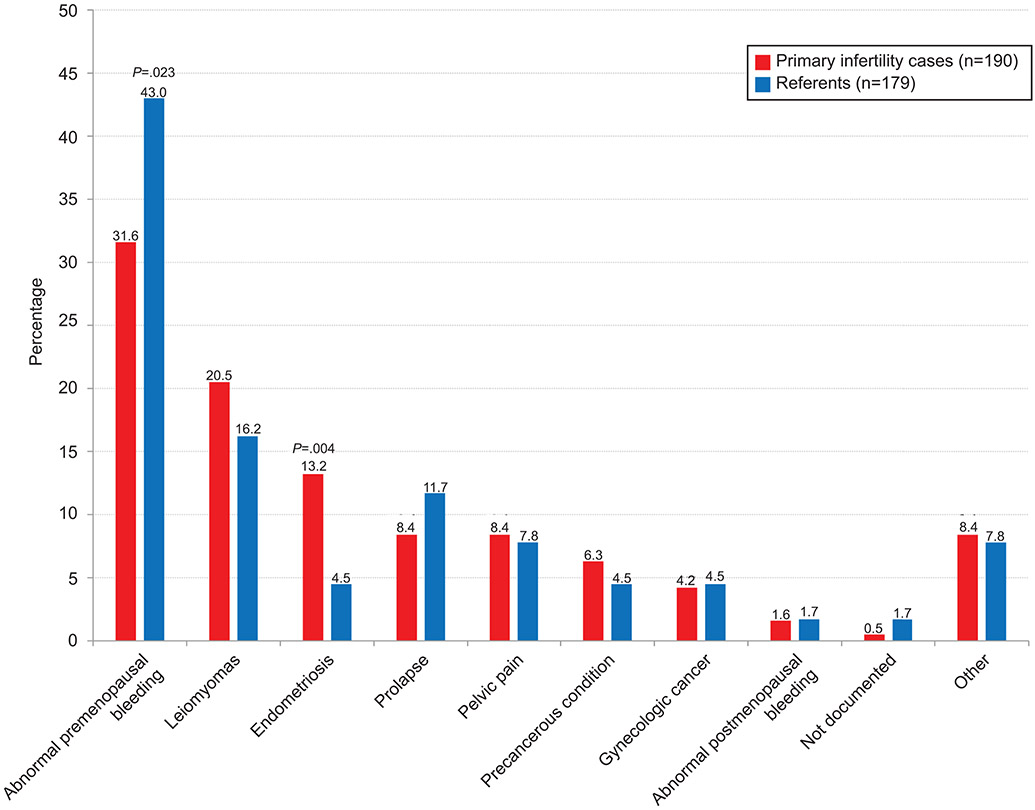
Figure 1b depicts the cumulative incidence of subsequent hysterectomy. Women with primary infertility did not have a significantly increased risk of hysterectomy compared to referent women (adjusted HR 0.98, 95% CI 0.79-1.23, p=0.88; Table 2). The age at the time of hysterectomy was similar with mean±SD age of 43.2±6.9 and 43.9±7.1 years, respectively, for the women with primary infertility compared to referent women. Although the two most common primary indications for hysterectomy were the same in both groups, abnormal pre-menopausal bleeding was more common in referent women and uterine fibroids were more common in women with primary infertility. Importantly, a higher proportion of those with primary infertility underwent a hysterectomy due to endometriosis compared to the referent women (13.2% [25/190] vs. 4.5% [8/179]; p=0.003; Figure 2). The indications of tubal or uterine factor were not evaluated due to the small numbers.
Figure 2:
Primary indication for hysterectomy.
When excluding the matched sets involving women with endometriosis-associated infertility for the sensitivity analysis, there was no statistically significant difference in the risk of subsequent hysterectomy (adjusted HR 0.87, 95% CI 0.68-1.10, p=0.24) between women with primary infertility and referent women (Appendix 1, http://links.lww.com/xxx). The median uterine weights were similar, 130 (IQR, 95-220) grams in women with primary infertility compared to 145 (IQR, 110-205) grams in the referent group.
The two primary outcomes of interest were further categorized into three event types based on the first subsequent procedure per woman: i) concurrent hysterectomy and bilateral oophorectomy, ii) hysterectomy with ovarian conservation, and iii) isolated bilateral oophorectomy. As shown in Figure 1c, we observed that the cumulative incidence of subsequent concurrent hysterectomy with bilateral oophorectomy was significantly higher in primary infertility cases compared to referent women (HR 1.73, 95% CI 1.27-2.37; p<0.001; adjusted HR 1.51, 95% CI 1.07-2.14; p=0.019). This association was attenuated and no longer significant in the sensitivity analysis (adjusted HR 1.25, 95% CI 0.86-1.81; p=0.25). In contrast, the cumulative incidence of subsequent hysterectomy with ovarian conservation was significantly lower in primary infertility cases compared to referent women (HR 0.73, 95% CI 0.55-0.96, p=0.026; adjusted HR 0.68, 95% CI 0.50-0.92; p=0.011) and this association persisted in the sensitivity analysis (adjusted HR 0.66, 0.49-0.90; p=0.009). Only a few women in both groups had the third event type, an isolated bilateral oophorectomy.
Hysterectomy with ovarian conservation has become more prevalent over time among the primary infertility cases. Among the 455 primary infertility cases diagnosed in 1980-1989, 105 have had a subsequent procedure including 59 (56.2%) with a concurrent hysterectomy with bilateral oophorectomy, 40 (38.1%) with hysterectomy and ovarian conservation, and 6 (5.7%) with an isolated bilateral oophorectomy. Comparatively, among the 546 primary infertility cases diagnosed in 1990-1999, 95 have had a subsequent procedure including 46 (48.4%) with a concurrent hysterectomy with bilateral oophorectomy, 44 (46.3%) with hysterectomy and ovarian conservation, and 5 (5.3%) with an isolated bilateral oophorectomy (Appendix 2, available online at http://links.lww.com/xxx).
Of the 1,001 women with primary infertility, 704 were noted to have at least one pregnancy during the available follow-up, of which 698 had a documented year reported for first pregnancy. In women with primary infertility who were ever pregnant, 42.2% (422/1,001) conceived their first pregnancy with use of fertility medications and 28.2% (282/1,001) conceived spontaneously. Of the 1,001 referent women, 794 were noted to have at least one pregnancy, of which 762 had a documented year reported for first pregnancy.
Ever being pregnant was evaluated as a time-dependent covariate in a Cox model for an association with the risk of hysterectomy and bilateral oophorectomy, respectively. For both outcomes there was evidence of an interaction effect between ever being pregnant and primary infertility status, although not statistically significant (p=0.06 for hysterectomy, p=0.08 for bilateral oophorectomy), in that the magnitude of the association between ever being pregnant and the subsequent occurrence of each outcome was different for women with primary infertility compared to the referents. Among women with a history of primary infertility, ever being pregnant was significantly associated with a decreased risk of oophorectomy (HR 0.69, 95% CI, 0.47-1.00; p=0.05) but not hysterectomy (HR 0.9, 95% CI, 0.66-1.25; p=0.54). Whereas among the referent women, ever being pregnant was not significantly associated with an increased risk of hysterectomy (HR 1.60, 95% CI 0.97-2.64; p=0.07) or oophorectomy (HR 1.42, 95% CI 0.65-3.10; p=0.38).
Discussion:
In this study, we identified an increased risk of bilateral oophorectomy for women with primary infertility compared to referent women. This association persisted when excluding women with primary infertility related to endometriosis. While there was no increased risk of hysterectomy observed overall, women with primary infertility related to endometriosis were more likely to undergo subsequent hysterectomy compared to referent women. Upon evaluating the primary outcomes as three competing outcomes or risks (based on the first subsequent procedure per woman: i) concurrent hysterectomy and bilateral oophorectomy, ii) hysterectomy with ovarian conservation, and iii) isolated bilateral oophorectomy), we observed that women with primary infertility were more likely to have a subsequent hysterectomy with bilateral oophorectomy compared to referent women. To our knowledge, there are limited data on the association of infertility and subsequent gynecologic surgery. The associations of oophorectomy and hysterectomy identified here, in women with a history of primary infertility, represent a meaningful mediator and possible confounder of long-term health risks in this population.
The long-term risks of oophorectomy have been well-described and as such, a general trend toward decreased rates of oophorectomy at the time of hysterectomy in younger women continues.14 While oophorectomy has been shown to be associated with a decreased risk of death from ovarian cancer and breast cancer, in younger women, there is an increased risk of all-cause mortality across all age ranges.15 All-cause mortality is likely influenced most by the presence of coronary artery disease and associated cardiovascular death, especially in women less than 45 years of age at oophorectomy and not treated with hormone therapy.16 In our study, the relative risk of bilateral oophorectomy was highest in women with a history of primary infertility related to endometriosis or ovulatory dysfunction. However, even in the sensitivity analysis excluding women with a diagnosis of primary infertility related to endometriosis and their matched referents, this association persisted indicating an underlying diagnosis of primary infertility has an association with increased risk of bilateral oophorectomy. Importantly, age at bilateral oophorectomy was lower for women with primary infertility compared to referent women, which has shown to increase long-term health risks.17-19
The risk of subsequent hysterectomy did not differ between women with primary infertility and referent women, except when evaluating women with primary infertility related to endometriosis. This finding highlights a unique population with known long-term health risks ranging from increased rates of gynecologic surgery to gynecologic cancer.20 This relationship is important as there is increasing recognition of the long-term health effects of hysterectomy, with or without ovarian conservation. Women with a history of hysterectomy and bilateral oophorectomy have a 1.12 to 1.29 hazard ratio for all-cause mortality compared to women with ovarian conservation or without prior surgery, respectively.7, 21 Similarly, hysterectomy with ovarian conservation has been associated with increased rates of de novo hyperlipidemia, hypertension, obesity and osteoporosis.5, 22 Lastly, hysterectomy with ovarian conservation at a young age (<40 years) has been associated with high rates of poor psychologic health, including increased rates of de novo depression and anxiety.6, 23
The increased risk of subsequent gynecologic surgery in women with a history of primary infertility may have multiple explanations. First and most contributory is the underlying pathology associated with infertility that is also associated with increased rates of hysterectomy and oophorectomy.20, 24, 25 Primary infertility related to endometriosis is one obvious example. However, it is notable that the association of bilateral oophorectomy persisted even in the sensitivity analysis excluding the matched sets involving women with primary infertility related to endometriosis.
Less well-studied, the psychologic impact of primary infertility on personal perception of reproductive organ utility may provide an additional hypothesis for this association. This hypothesis is an extension of prior studies showing increased rates of body image distress and feelings of ‘brokenness’ shared by some patients with infertility.26, 27 Additionally, a history of adverse childhood outcomes has been associated with increased rates of hysterectomy and oophorectomy, with many women reporting distress from their ‘sexual or reproductive life’.28, 29 Adverse childhood outcomes have not previously been evaluated in a population with infertility, and if increased, this may be a relevant contributor to our findings.
Finally, the association between infertility and infertility treatments on future cancer risk must be considered. The association between fertility treatments and ovarian tumors, specifically the extended use of clomiphene citrate and incidence of borderline tumors has been previously published.30 However, the association between infertility and subsequent ovarian cancer is low, lacking differentiation between primary and secondary infertility and with low-certainty evidence.31, 32 More convincingly, increased rates of endometrial cancer have been seen in women with infertility, largely driven by the shared pathophysiology of polycystic ovary syndrome (PCOS).33, 34 In our study, the indications for hysterectomy for both gynecologic pre-cancerous or cancerous conditions did not differ between primary infertility cases and referent women, although numbers of these indications were small.
The incidence of hysterectomy in our study is comparable to prior reports; however, the primary indication for hysterectomy differed. The Oxford Family Planning Study found fibroids as the leading indication for hysterectomy followed by abnormal bleeding,35 as did a more contemporary studies from Lombardo, Italy and from the US.36, 37 Our study had a higher incidence of abnormal pre-menopausal bleeding followed by fibroids as the primary indication for hysterectomy. This may reflect our predominantly White population with a lower incidence of fibroids, although the prior studies did not report race, so our explanation cannot be formally explained. The quality of ultrasound imaging during this time period may have limited use and interpretation of pre-operative imaging. Additionally, the rate of myomectomy was not assessed after index data and myomectomy rather than hysterectomy may have been more commonly pursued in women with primary infertility than referent women. This is an opportunity for future study and a limitation of our findings.
The difference in incidence of oophorectomy and hysterectomy in this cohort may be best explained by a difference in decision making regarding oophorectomy at the time of hysterectomy. Interestingly, trends in oophorectomy and hysterectomy differed for women who were ever pregnant. Although not statistically significant, women with a history of primary infertility who were ever pregnant were less likely to undergo subsequent hysterectomy or oophorectomy. Conversely, referent women who were ever pregnant were more likely to undergo either hysterectomy or oophorectomy. The protective effect of pregnancy on growth of uterine fibroids, endometriosis, gynecologic cancer rates may partially explain this, while the psychologic perspective on reproductive organs may also contribute. This cohort represents one of the few historical infertility cohorts which allows for assessment of long-term health outcomes and risks associated with primary infertility. Our previous study highlighted that characteristics of our cohort of women with primary infertility are consistent with other studies of women with primary infertility, supporting its use as a representative sample for future study9.
While the length of follow-up and number of women included in our cohort contribute to the strength and impact of our findings, our study is not without limitations. In our analysis stratified by the primary infertility indication, particularly for those with endometriosis as the indication, some models may be considered overfit using the rule of thumb of 10-15 events per each covariate included in the regression model. This overfit is evident by the wider 95% CIs and therefore some results should be cautiously interpreted. Our cohort includes a primarily White population which limits the generalizability of these associations and may weaken the risks of future hysterectomy, which are higher in African American women.38 Future work in other historical cohort studies is needed to confirm our findings in a more racially diverse cohort.
In summary, the effects of oophorectomy and hysterectomy on overall health and all-cause mortality may present an important confounding variable in studies assessing long-term risks of infertility and should be included in future studies evaluating long-term health outcomes.
Supplementary Material
Acknowledgements:
The authors thank Dr. Lauren Kendall Rauchfuss, who contributed to chart review for this cohort.
Financial Disclosure:
Elizabeth A Stewart has the following conflicts of interest: Consultant for AbbVie, Bayer, ObsEva, and Myovant; Research support from National Institutes for Health (P50HS023418); Royalties from UpToDate; Consulting for the development of educational content from the Med Learning Group, PER, Massachusetts Medical Society, MedIQ and Peer View.
This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
Footnotes
The other authors did not report any potential conflicts of interest.
Presented at the American Society of Reproductive Medicine, held virtually, October 17-21, 2020.
References:
- 1.Cedars MI, Taymans SE, DePaolo LV, Warner L, Moss SB, Eisenberg ML. The sixth vital sign: what reproduction tells us about overall health. Proceedings from a NICHD/CDC workshop. Hum Reprod Open. 2017;2017(2):hox008. doi: 10.1093/hropen/hox008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of incident chronic medical conditions in infertile women: analysis of US claims data. American journal of obstetrics and gynecology. 2019/05/January/ 2019;220(5):473.e1–473.e14. doi: 10.1016/j.ajog.2019.01.214 [DOI] [PubMed] [Google Scholar]
- 3.Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self-reported infertility. Fertility research and practice. 2017;3:7. doi: 10.1186/s40738-017-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman's long-term health using data linkage. The Journal of clinical endocrinology and metabolism. Mar 2015;100(3):911–9. doi: 10.1210/jc.2014-3886 [DOI] [PubMed] [Google Scholar]
- 5.Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause. May 2018;25(5):483–492. doi: 10.1097/gme.0000000000001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laughlin-Tommaso SK, Satish A, Khan Z, Smith CY, Rocca WA, Stewart EA. Long-term risk of de novo mental health conditions after hysterectomy with ovarian conservation: a cohort study. Menopause. Jan 2020;27(1):33–42. doi: 10.1097/gme.0000000000001415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstetrics and gynecology. Apr 2013;121(4):709–16. doi: 10.1097/AOG.0b013e3182864350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart EA, Missmer SA, Rocca WA. Moving Beyond Reflexive and Prophylactic Gynecologic Surgery. Mayo Clinic proceedings. 2021;96(2):291–294. doi: 10.1016/j.mayocp.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadecki E, Weaver A, Zhao Y, Stewart EA, Ainsworth AJ. Fertility trends and comparisons in a historical cohort of US women with primary infertility. Reproductive health. Jan 18 2022;19(1):13. doi: 10.1186/s12978-021-01313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, Grossardt BR, Brue SM, et al. Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). International journal of epidemiology. 2018/April// 2018;47(2):368–368j. doi: 10.1093/ije/dyx268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. Dec 2012;41(6):1614–24. doi: 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. May 1 2011;173(9):1059–68. doi: 10.1093/aje/kwq482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. Mar 30 1999;18(6):695–706. doi: [DOI] [PubMed] [Google Scholar]
- 14.Asante A, Whiteman MK, Kulkarni A, Cox S, Marchbanks PA, Jamieson DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998-2006. Obstetrics and gynecology. Nov 2010;116(5):1088–95. doi: 10.1097/AOG.0b013e3181f5ec9d [DOI] [PubMed] [Google Scholar]
- 15.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstetrics and gynecology. May 2009;113(5):1027–37. doi: 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans EC, Matteson KA, Orejuela FJ, et al. Salpingo-oophorectomy at the Time of Benign Hysterectomy: A Systematic Review. Obstetrics and gynecology. Sep 2016;128(3):476–85. doi: 10.1097/aog.0000000000001592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clinic proceedings. Nov 2016;91(11):1577–1589. doi: 10.1016/j.mayocp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. Jan-Feb 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. Sep 11 2007;69(11):1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6 [DOI] [PubMed] [Google Scholar]
- 20.Saraswat L, Ayansina D, Cooper KG, Bhattacharya S, Horne AW, Bhattacharya S. Impact of endometriosis on risk of further gynaecological surgery and cancer: a national cohort study. BJOG : an international journal of obstetrics and gynaecology. Jan 2018;125(1):64–72. doi: 10.1111/1471-0528.14793 [DOI] [PubMed] [Google Scholar]
- 21.Tuesley KM, Protani MM, Webb PM, et al. Hysterectomy with and without oophorectomy and all-cause and cause-specific mortality. American journal of obstetrics and gynecology. Nov 2020;223(5):723.e1–723.e16. doi: 10.1016/j.ajog.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 22.Choi HG, Jung YJ, Lee SW. Increased risk of osteoporosis with hysterectomy: A longitudinal follow-up study using a national sample cohort. American journal of obstetrics and gynecology. Jun 2019;220(6):573.e1–573.e13. doi: 10.1016/j.ajog.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 23.Cooper R, Mishra G, Hardy R, Kuh D. Hysterectomy and subsequent psychological health: findings from a British birth cohort study. Journal of affective disorders. May 2009;115(1-2):122–30. doi: 10.1016/j.jad.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 24.Whynott RM, Vaught KCC, Segars JH. The Effect of Uterine Fibroids on Infertility: A Systematic Review. Seminars in reproductive medicine. //03.November.2017 2017;35(06):523–532. doi: 10.1055/s-0037-1607295 [DOI] [PubMed] [Google Scholar]
- 25.Treloar SA, Do K-A, O’Connor VM, O’Connor DT, Yeo MA, Martin NG. Predictors of hysterectomy: An Australian study. American journal of obstetrics and gynecology. 1999/04/January/ 1999;180(4):945–954. doi: 10.1016/S0002-9378(99)70666-6 [DOI] [PubMed] [Google Scholar]
- 26.CLARKE LH, MARTIN-MATTHEWS A, MATTHEWS R. The Continuity and Discontinuity of the Embodied Self in Infertility*. Canadian Review of Sociology/Revue canadienne de sociologie. 2006;43(1):95–113. doi: 10.1111/j.1755-618X.2006.tb00856.x [DOI] [Google Scholar]
- 27.Alur-Gupta S, Chemerinski A, Liu C, et al. Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertility and Sterility. 2019;112(5):930–938.e1. doi: 10.1016/j.fertnstert.2019.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzuola Rocca L, Smith CY, Stewart EA, Rocca WA. Adverse childhood experiences and adult abuse are predictors of hysterectomy and oophorectomy. Maturitas. Dec 2017;106:95–96. doi: 10.1016/j.maturitas.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 29.Gazzuola Rocca L, Smith CY, Grossardt BR, et al. Adverse childhood or adult experiences and risk of bilateral oophorectomy: a population-based case-control study. BMJ open. Jun 7 2017;7(5):e016045. doi: 10.1136/bmjopen-2017-016045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fertility drugs and cancer: a guideline. Fertil Steril. Dec 2016;106(7):1617–1626. doi: 10.1016/j.fertnstert.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 31.Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database of Systematic Reviews. 2019;(6)doi: 10.1002/14651858.CD008215.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinton LA, Lamb EJ, Moghissi KS, et al. Ovarian cancer risk associated with varying causes of infertility. Fertility and Sterility. 2004/August/01/ 2004;82(2):405–414. doi: 10.1016/j.fertnstert.2004.02.109 [DOI] [PubMed] [Google Scholar]
- 33.Ding DC, Chen W, Wang JH, Lin SZ. Association between polycystic ovarian syndrome and endometrial, ovarian, and breast cancer: A population-based cohort study in Taiwan. Medicine. Sep 2018;97(39):e12608. doi: 10.1097/md.0000000000012608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female infertility, infertility-associated diagnoses, and comorbidities: a review. Journal of assisted reproduction and genetics. Feb 2017;34(2):167–177. doi: 10.1007/s10815-016-0836-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vessey MP, Villard-Mackintosh L, McPherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. British journal of obstetrics and gynaecology. May 1992;99(5):402–7. doi: 10.1111/j.1471-0528.1992.tb13758.x [DOI] [PubMed] [Google Scholar]
- 36.Parazzini F, Ricci E, Bulfoni G, et al. Hysterectomy rates for benign conditions are declining in Lombardy, Italy: 1996-2010. European journal of obstetrics, gynecology, and reproductive biology. Jul 2014;178:107–13. doi: 10.1016/j.ejogrb.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 37.Jacobson GF, Shaber RE, Armstrong MA, Hung YY. Hysterectomy rates for benign indications. Obstetrics and gynecology. Jun 2006;107(6):1278–83. doi: 10.1097/01.AOG.0000210640.86628.ff [DOI] [PubMed] [Google Scholar]
- 38.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. Feb 2009;99(2):300–7. doi: 10.2105/ajph.2008.133702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.