Abstract
Uromodulin (or Tamm-Horsfall protein) is a glycoprotein uniquely produced in the kidney by tubular cells of the thick ascending limb of the loop of Henle and early distal tubules. This protein exhibits bidirectional secretion in the urine and in the renal interstitium and circulation. The role of this protein in maintaining renal and systemic homeostasis is becoming increasingly appreciated. Furthermore, perturbations of its functions may play a role in various diseases affecting the kidney and distant organs. In this review, we will discuss important advances in understanding its biology, highlighting the recent discoveries of its secretion and differential precursor processing that generates two forms: a) a highly polymerizing form that is apically excreted in the urine and generates filaments, and b) a non-polymerizing form that retains a polymerization inhibitory pro-peptide and is released basolaterally in the kidney interstitium and circulation, but can also be found in the urine. We will also discuss factors regulating its production and release, taking into account its intricate physiology, and propose best practices to report its levels. We also discuss breaking advances in its role in hypertension, acute kidney injury and progression to chronic disease, immunomodulation and regulating renal and systemic oxidative stress. We anticipate that this work will be a great resource for researchers and clinicians. This review will highlight the importance of defining what regulates the two forms of uromodulin, so that modulation of uromodulin levels and function could become a novel tool in our therapeutic armamentarium against kidney disease.
Keywords: Uromodulin, Tamm-Horsfall Protein, renal physiology, hypertension, kidney injury
Introduction
Uromodulin or Tamm-Horsfall protein (gene name UMOD) is a protein uniquely expressed in the kidney by cells of the thick ascending limbs (TAL) and early distal convoluted tubules (DCT). This protein is secreted in the urine as one of the most abundant urinary proteins that is uniquely made and released by the kidney. Therefore, its levels in the urine reflect the ability of the kidney to produce this protein. The name Tamm-Horsfall is given after Drs. Igor Tamm and Frank Horsfall Jr., who initially discovered this protein in 1950 as an inhibitor of viral hemagglutination in the urine1. This protein was rediscovered and named Uromodulin in 1985, after Muchmore and Decker isolated a protein with immunomodulatory properties from the urine of pregnant patients2. Uromodulin was also found to be released basolaterally into the renal interstitium and circulation3. Our understanding of the biology of uromodulin, its production, bidirectional release in the urine and blood, and its functions has evolved over the years. The association of uromodulin levels, polymorphisms and mutations with acute and chronic forms of renal disease argues for important regulatory functions of this glycoprotein in maintaining a healthy renal milieu4–7. Missense mutations in the UMOD gene cause autosomal dominant tubulo-interstitial disease (ADTKD-UMOD), an inherited disease characterized by progressive kidney disease with interstitial fibrosis, tubular atrophy and lack of significant glomerular pathology8. Many single nucleotide polymorphisms (SNPs) around the UMOD gene are also associated with kidney disease9.
A form of urinary uromodulin can polymerize into highly organized filaments that are released in the urine10. There have been significant advancements into the mechanisms of polymerization, the structure of these filaments and the role of the polymerized form in the urine11–17. However, recent discoveries underscore the presence of a non-polymerized form of uromodulin that is also released in the urine and is likely the main form released in the renal interstitium and circulation18. The cellular paths governing the release of these two forms are distinct from each other and appear to be independently regulated. Therefore, what was thought to be a single biological process appears to be more complex. In addition, the functions of highly conserved proteins such as uromodulin are likely to be pleotropic. Uromodulin has been implicated in regulating salt reabsorption, cation homeostasis, hypertension, acute kidney injury, chronic kidney disease, urinary defense, systemic inflammation and stress, immunomodulation and kidney stone disease17,19. What uromodulin does will depend on its location, its form (polymerizing vs. non-polymerizing) and its interaction with specific molecules, cells, and structures. In this review, we will discuss important advances in our understanding of uromodulin biology, what regulates the production of its two forms, its functions, and its potential as a theragnostic molecule. There have been additional published resources that could fill in gaps or areas not addressed here and give a complementary interpretation of the literature17,19,20.
Uromodulin Biology
Cellular trafficking and polarized secretion
The biosynthesis and structure of uromodulin are discussed in detail in the online supplemental material and depicted in Figure 1. During its intracellular trafficking to the apical side of the TAL tubular cells, uromodulin is kept in a polymerization-incompetent state by the hydrophobic interaction of an internal (IHP) and external hydrophobic patch (EHP)21. The pathway for apical release of polymerizing uromodulin has been well documented10. However, the basolateral release of uromodulin to the renal interstitium and circulation is poorly understood22. The concentrations of circulating uromodulin are around 1000 times less than those in the urine23. Serum uromodulin originates from the kidney and is practically undetectable in anephric patients3. Unlike the urinary uromodulin that polymerizes, circulating protein does not polymerize and has a higher molecular weight than its urinary counterpart on SDS-PAGE24. This suggest that there are two forms of uromodulin released by TAL cells, which are likely to have separate pathways for cellular processing and release.
Figure 1: Primary structure of Uromodulin and three-dimensional model from Alphafold database.
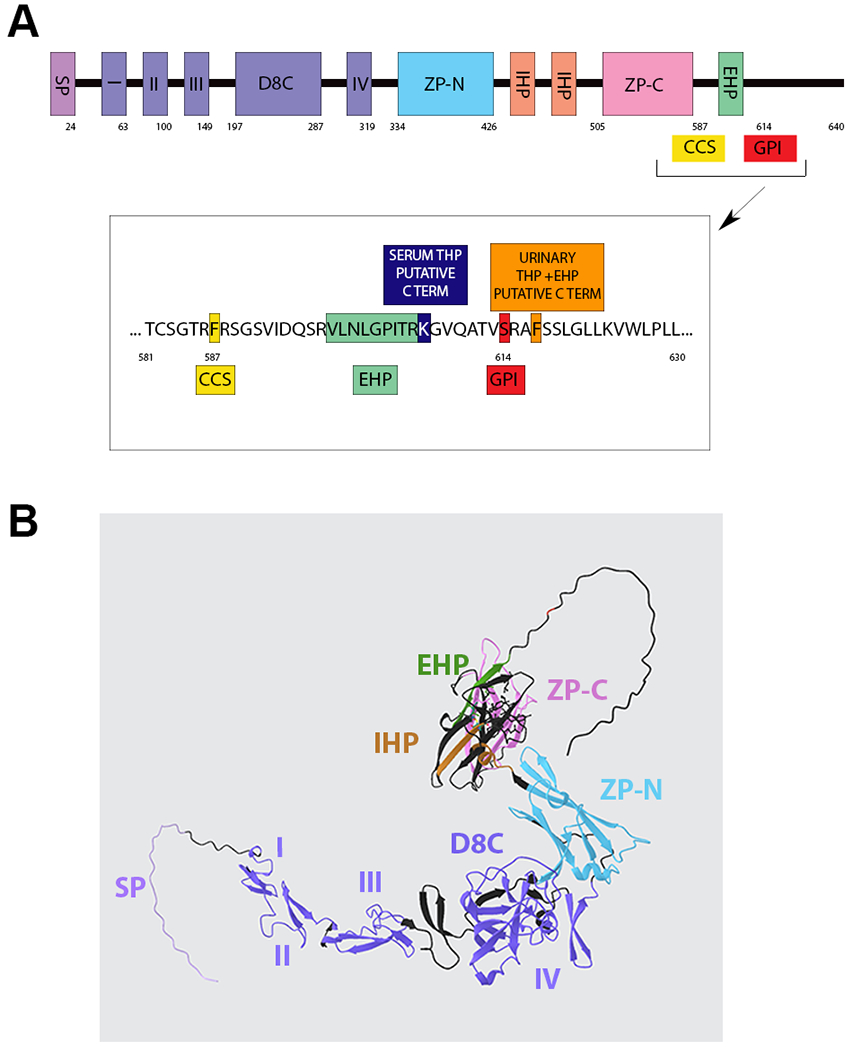
(A) Schematic of uromodulin domains and cleavage sites. SP, signal peptide; I, II, III, and IV, EGF domains; D8C, cysteine-rich domain; ZP, zona pellucida domain; GPI, glycosylphosphatidylinositol anchor at 614; CCS, consensus cleavage site at 587; IHP, internal hydrophobic patch (430–436 and 456–462); EHP, external hydrophobic patch (598–607). (B) 3D protein structure model of uromodulin (AlphaFold) precursor using with domains labeled and color corresponding to (A).
Uromodulin forms
Polymerizing urinary Uromodulin
Polymerizing uromodulin single molecules are anchored on the apical membrane on the urine lumen side at a glycophosphatidylinositol (GPI) addition site (S614). Proteolysis by the type II transmembrane serine protease hepsin at a conserved residue F587 located at the C-terminal end of the split zona pellucida (ZP) domain (consensus cleavage site or CCS, Figure 1) removes the EHP motif, permitting correct orientation of ZP N-terminal (ZP-N) domains for polymerization to occur in the urine10. An extensive hydrophobic interface mediates ZP-N domain homodimerization, whereas a structured interdomain linker between ZP-N and the ZP C-terminal domain (ZP-C) directs that self-association25. These uromodulin homodimers are intermediates in the polymerization process that is mandatory for the formation of uromodulin filaments13,14. The three-dimensional structure of native uromodulin polymers has been recently described by cryo-electron tomography13,14. The intricate assembly of uromodulin into highly organized filaments (discussed in online supplement) suggests that the production of polymerizing uromodulin is a highly regulated process.
Non-polymerizing urinary and circulating uromodulin
Using the fact that the EHP domain makes uromodulin incompetent for polymerization, a polyclonal antibody that specifically recognizes uromodulin with retained EHP was used to characterize both serum and urinary uromodulin species18. The presence of a non-polymerizing form of uromodulin with retained EHP was then demonstrated in the human urine. Proteomic characterization of this form revealed its C-terminus ends at F617, past the S614 site of GPI anchor addition18. In the human kidney, uromodulin with retained EHP was detected in TAL tubules and to a lesser degree in the renal parenchyma. Immunoprecipitated serum uromodulin also retained EHP, with proteomic characterization disclosing its C-terminus at K607, beyond the hepsin cleavage consensus site for urinary polymerizing uromodulin (F587)18. Evidence of non-polymerizing urinary uromodulin in rodents has also been described recently26. Non-polymerizing uromodulin is consistently observed to be a dimer in its native form. The mechanism of such dimerization is not yet defined.
In view of these recent discoveries, we therefore conclude that the kidney produces and secretes two distinct forms of uromodulin (Figure 2): polymerizing uromodulin, lacking the polymerization inhibitory EHP sequence, released by hepsin proteolysis into the urine, and non-polymerizing uromodulin with retained EHP sequence, released in the urine and as the major form in the circulation (serum). The release of this form in humans likely originates from an alternative pathway independent of GPI anchoring and hepsin cleavage. Such a mechanism has been described for other proteins that exist in both membrane-bound (GPI anchored) and soluble forms27,28. This divergence in C-terminal signals for processing may provide an adaptive advantage when there is a need for functional protein both at the cell surface and in the extracellular space, a case that applies to uromodulin.
Figure 2: Pathways for Uromodulin release in the urine and circulation.
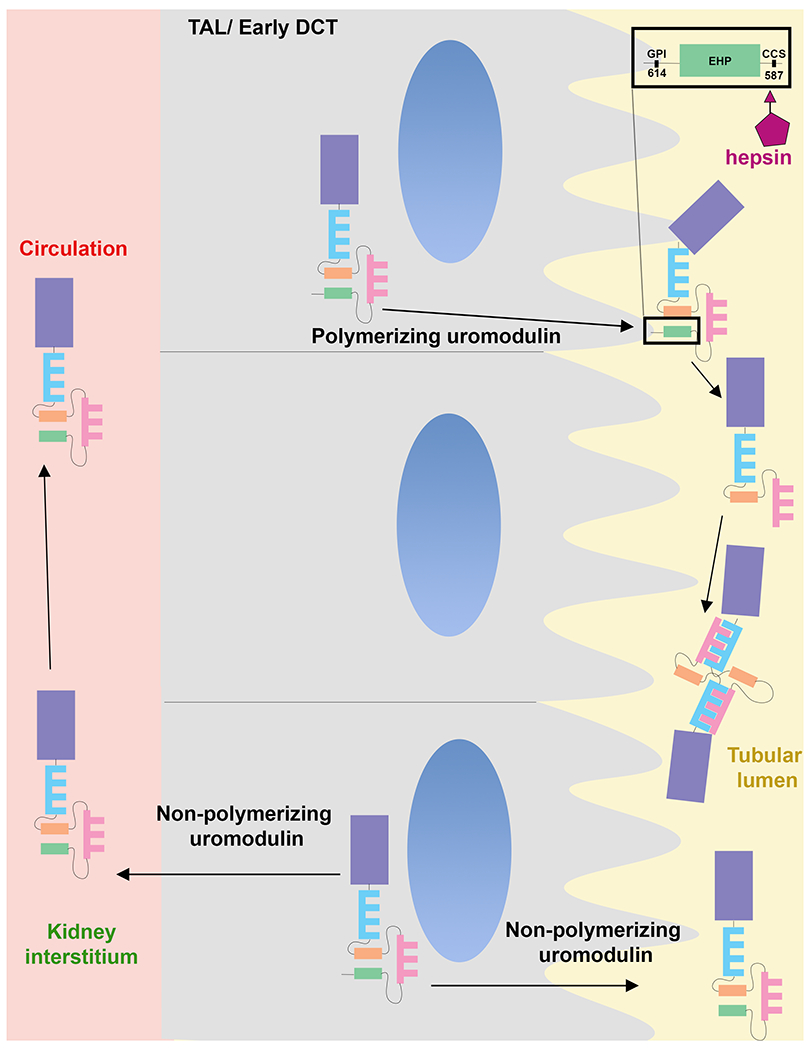
Polymerizing uromodulin is targeted towards the apical membrane, where it is GPI anchored (inset). It is cleaved by hepsin at the consensus cleavage site, which releases the inhibitory external hydrophobic patch (EHP) site, thereby inducing conformational changes that allow the zona pellucida domains to polymerize. Non-polymerizing uromodulin is not cleaved by hepsin and is released in the urine without GPI anchoring. Non-polymerizing uromodulin is also the main form released from the basolateral domain to the kidney interstium and circulation
The two forms of uromodulin likely have different physiological roles and are independently regulated as discussed below. The TAL cells are expected to use the same hepsin-independent alternative processing pathway for producing both urinary non-polymerizing and the interstitial/systemic uromodulin. The fact that the primary structure of recovered serum uromodulin is slightly shorter than the urinary non-polymerizing form (K607 vs F617) could indicate that the former undergoes further proteolytic cleavage in the kidney or systemically. This proteolytic cleavage occurring at double basic residues R606-K607 could be executed by a trypsin-like serine protease, which is reminiscent of the processing of many hormones29.
Because of increasing interest in serum uromodulin, its roles in disease, and its potential as a biomarker, understanding the mechanisms of its release is of paramount importance. In addition, non-polymerizing urinary uromodulin could have separate roles compared to the polymerizing form and may offer some advantages for use as a biomarker18. The distinct functions of the two forms of uromodulin can be inferred from effects attribute to the interstitial/circulating from (e.g. protection from kidney injury, immunomodulation and inhibition of systemic oxidative injury 22,24,30) vs. urinary polymerizing uromodulin (e.g. protection from urinary tract infection and regulating salt homeostasis5,15). However, the function of non-polymerizing uromodulin in the urine is still unclear. In vitro studies of urinary uromodulin have been mostly performed using purified protein from salt precipitation, i.e., polymerization. Therefore, most of the data derived from this process are applicable to polymerizing uromodulin31. We recently showed that a truncated form of uromodulin can be isolated from the urine and can be useful for studying the non-polymerizing or circulating form24. This discovery will help elucidate the functional differences between the two forms.
Uromodulin expression and production
Overview
Based on a unique expression pattern, uromodulin has a distinct vantage point in its spatial distribution in the kidney, spanning from the cortex to medulla (Figure 3). Because of this distinct zonation, the density of uromodulin expression is high in the inner stripe of the outer medulla. This area is also very rich in immune cells, particularly resident mononuclear phagocytic cells (MPCs). Therefore, it is possible that this area is key to immune sensing, particularly because uromodulin has a key role in regulating the abundance and distribution of renal MPCs in this renal area24,32. The outer medulla is very sensitive to changes in perfusion and ischemic injury. Therefore, it is not surprising that a modulator of injury such as uromodulin would be highly abundant in that area33. Of interest also is the distribution of uromodulin along the thick ascending limb and early DCT, which is the free water generating segment of the nephron. Within each nephron segment, the expression for uromodulin increases moving from medullary to cortical TAL (~8 fold higher in cTAL vs mTAL, which correlates with more dilution of the filtrate and an increasing osmotic gradient). This is consistent with what is commonly proposed that uromodulin may play an important role in the impermeability of the free water generating segment of the nephron34. This could also fit if increased sodium uptake is linked to the production and polymerization of uromodulin, for example via hepsin activity35.
Figure 3: Expression of Uromodulin in mouse and Human kidney by large-scale confocal immunofluorescence imaging.
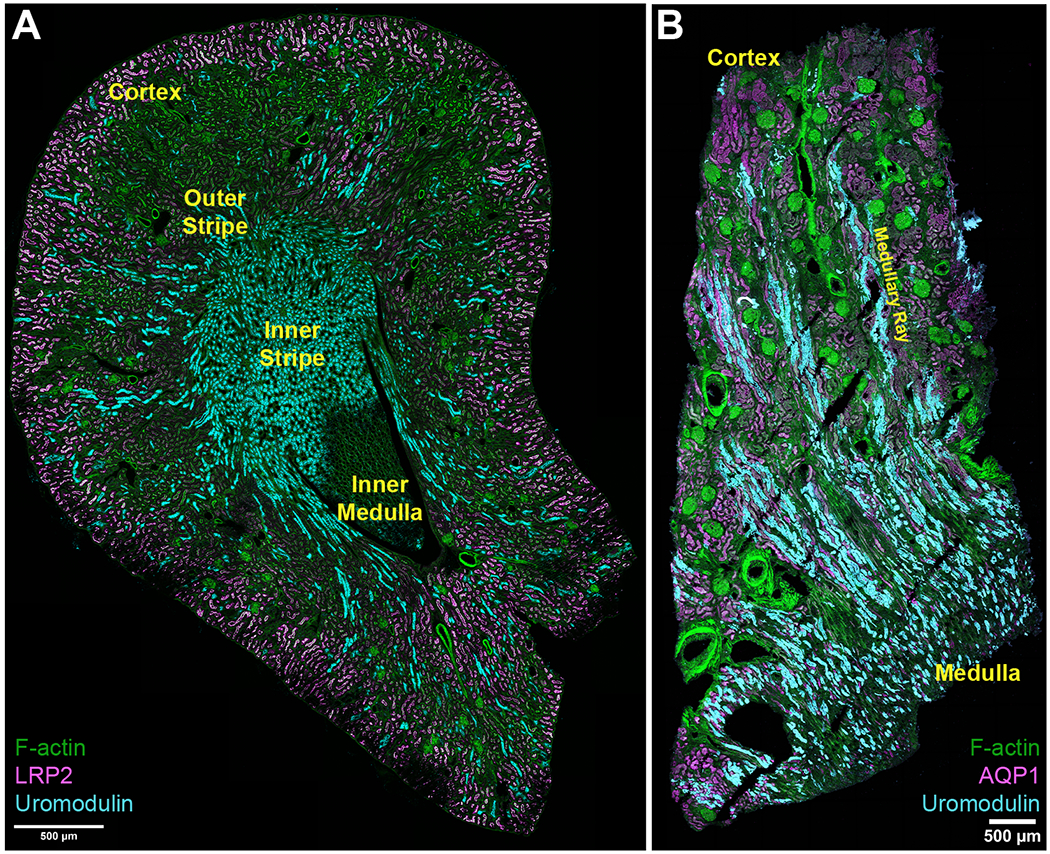
(A) large scale confocal fluorescence imaging of a mouse kidney showing the distribution of uromodulin. The density of thick ascending limbs in the inner stripe increases the abundance of uromodulin in this specific renal zone. (B) Large scale imaging of a human nephrectomy tissue specimen showing the distribution of uromodulin in the cortex and upper medulla.
Understanding key physiological concepts of urinary uromodulin will improve its reporting and interpretation.
Reporting and interpreting urinary uromodulin as biomarkers for disease risk, activity and prognosis are subjects of growing interest for researchers and clinicians. However, there are several areas of unclarity and misconceptions that exist and have been perpetuated in the literature. A contributing factor to this unclarity is the lack of standardized approaches to measure and report uromodulin. A detailed discussion and recommendations on how to report uromodulin expression and production by the kidney is found in the online supplemental material. Other factors are related to its unique physiology. Unlike other molecules that are filtered and cleared by the kidney, uromodulin is in effect produced by the kidney and secreted in the urine. Therefore, measuring random urinary concentration depends on, but does not directly measure, its rate of production. For a conserved protein such as uromodulin, one would expect that its range of urine concentration to remain stable for individual persons in steady states and to be less susceptible to changes in urine volumes. This contrasts with a molecule like creatinine (Cr), the production of which is relatively stable because it is linked to muscle mass, and its urinary levels then will vary depending on urinary concentration and dilution of the urine. For example, in situations of volume depletion and reduced urine volumes, it is expected to observe an increase in urine creatinine concentrations. However, it will be deleterious if concentrations for uromodulin will also increase in that situation, as this may precipitate urine cast formation and predispose most healthy people to obstructive acute tubular injury at the slight occurrence of dehydration. Conversely, in states of excessive hydration and high volumes of diluted urine, the osmotic gradient will be in favor of water reabsorption, and the TAL “impermeability factor” needs to be at its maximum. Therefore, it may be harmful for uromodulin, a molecule that may protect TAL from water entry, to get too diluted in this setting. Consequently, physiological reasoning supports that in steady states, uromodulin production (which is the rate of production per unit of time, independent of urine volume) will vary in a manner to keep the concentration of uromodulin (the amount per urine volume) in a relatively stable range, independently of the volume of urine produced. It is then not surprising to find that interventions such as water loading and administration of anti-diuretic hormone (ADH or its analogues) regulate uromodulin production. In several reported experimental settings, water loading (which suppresses ADH) induces the rate of secretion of urinary uromodulin (Table S1). Conversely, ADH administration reduces uromodulin secretion and its protein levels in the kidney, although it can acutely cause a burst of urinary release36. However, not all studies show a consistent response to ADH or water loading, and this could be related to underappreciated differences in methodology or reporting parameters (Table S1).
Polymerizing and non-polymerizing (including circulating) uromodulin are independently regulated
Experimental determinants of urinary uromodulin expression and secretion are summarized in Table S1 and are described in detail in the supplement. Clinical parameters and disease factors associated with uromodulin levels and rate of secretion are summarized in Table S2. Laboratory values and indicators associated with uromodulin levels are summarized in Table S3. Of note that most of these parameters were studied before the recent discoveries of the distinct forms of uromodulin. Direct evidence from cell culture data and indirect evidence from observational data support that the basolaterally released uromodulin (and circulating uromodulin, by extension) does not respond to the same stimuli as apically released uromodulin. In particular, the study by Nanamatsu et al. showed using a combination of mouse models and in vitro approaches that cyclic AMP-dependent protein kinase A signaling can regulate the release of polymerizing apical but not basolateral uromodulin36. In concordance with this, a clinical study showed that correlates of serum and urine uromodulin concentrations differ substantially, thereby also supporting a differential regulation for apical vs. basolateral secretion37. For example, serum uromodulin was more strongly associated with GFR, female sex and inflammatory indices, whereas urinary uromodulin concentrations were inversely associated with female sex, presence of diabetes and hypertension. This is consistent with the data from Micanovic et al. showing that the non-polymerizing form has different cellular processing than polymerizing uromodulin18. The marked increase of urinary non-polymerizing uromodulin in a rat model of maternal hypertension also suggests differential disease-driven regulation26. In addition, there is evidence that UMOD mutations leading to profound reductions in the apical release of uromodulin may not affect its basolateral release4, which raises an exciting possibility of differential regulation, possibly at the gene expression level, such as alternative splicing. Since the current complete knockout models prevent genetic studies of these distinct forms, the development of new animal models would shed light on the various forms of uromodulin and their function. Recent work from GWAS studies suggest that SNPs linked to circulating uromodulin are different compared to urine uromodulin7. A more detailed description of the genetic factors determining uromodulin expression and secretion is found in the supplemental and summarized in Table S4.
Is uromodulin a marker for nephron mass?
Uromodulin is linked to kidney function in the setting of CKD, but the association is weak in the setting of normal kidney function38. The synthesis of uromodulin is dynamic and likely varies based on physiological and pathological stressors. For example, the levels of urinary uromodulin increase in early diabetes even without evidence of kidney injury39. There is also important variation in uromodulin production even with physiological stimuli (Table S1). Although a link has been described between uromodulin and estimated nephron mass, this link does not hold in several situations (Table S2). In females, higher levels of uromodulin are frequently reported compared to males (serum and urine40). This is despite the fact that women have smaller kidneys than men41 and a decreased total number of nephrons42. In the setting of injury, the production of uromodulin per functioning nephron unit (estimated by adjusting uromodulin to GFR) is increased, likely as a protective mechanism22,30,43. Thornley et al. showed that uromodulin excretion per milliliter of creatinine clearance had a higher range in patients with CKD compared to normal subjects. Thulsen showed increased production of uromodulin per unit weight (ug/g) with experimentally induced water diuresis or diabetes44. These are clear situations demonstrating that uromodulin is not reflecting nephron mass.
The confusion happens here: despite increased production by intact nephrons in the setting of injury, ongoing insults and fibrosis will cause more nephrons to drop out, leading to an absolute decrease in uromodulin production in progressive CKD. The results will be that total uromodulin levels drop as CKD progresses and GFR declines and CKD will become a state of uromodulin deficiency. A similar phenomenon will happen with nephrectomies, and the reduction of uromodulin levels does not establish that uromodulin is a surrogate for renal mass, particularly in settings where other regulatory factors may have more impact. Therefore, uromodulin depends on, but does not reflect renal mass. To give a mathematical example, nephron mass is one variable in a complex equation. The effect of nephron mass will be dominant only if other factors are constant, which is frequently not the case (particularly between individuals). We propose to use the nomenclature of: “functional tubular response”, which in our opinion reflects the physiological significance of uromodulin expression and its urinary secretion. Circulating uromodulin, which predominantly reflects the release of non-polymerizing uromodulin, provides another measure of the functional tubular response, but also reflects the activity of uromodulin within the kidney and systemically. There could be added value in reporting uromodulin levels adjusted to kidney function as performed by Thornley et al.23 or what we proposed previously as uromodulin index45, which will further normalize uromodulin measures across patients and may better reflect the actual physiology of the kidney and the functional tubular response.
Overview of functions and sites of action of uromodulin
Overview
Ongoing discoveries strongly support that uromodulin is a multifaceted protein, the function of which will depend on many factors including: 1) which form of uromodulin (polymerizing/non-polymerizing), 2) where it is acting (within the TAL, on the apical membrane, in the urinary space or bladder, in the interstitium, or systemically, 3) with what cell (neighboring tubules, immune or endothelial cells) and 4) at what concentration.
The functions of uromodulin are summarized in Figure 4. We and other have previously reviewed the various functions of uromodulin 19,20,45. In the remainder of this discussion, we will focus on the role of uromodulin in hypertension and vascular biology. In the online supplementary material, we discuss evolving novel concepts in other important areas such as renal and systemic stress signaling, acute kidney injury (AKI), chronic kidney disease (CKD), AKI to CKD transition, urinary defense and immunity. Table S2 summarizes the association of specific diseases with measurements of uromodulin levels. Table S5 highlights the association of uromodulin levels with clinical outcomes.
Figure 4: Role of polymerizing and non-polymerizing uromodulin in context of site of action.
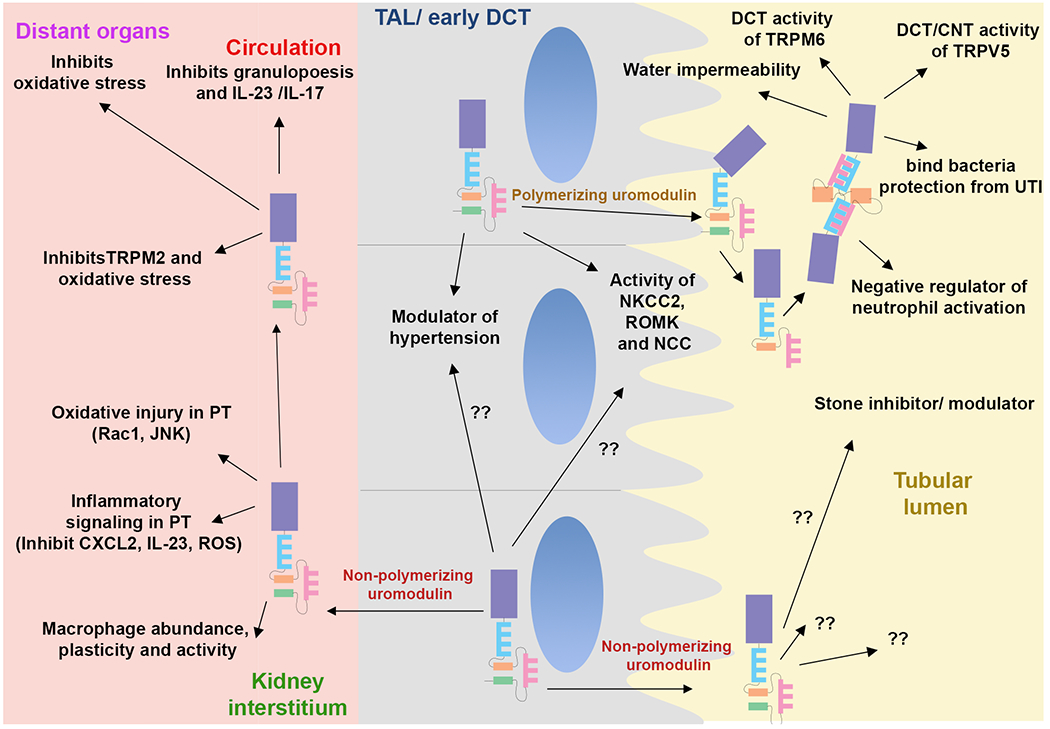
These functions are based on a summary of the literature in experimental and clinical setting. The role of uromodulin likely depends on the form and abundance of uromodulin, the site of interaction and the cell type(s) involved. Thick ascending limb, TAL; Distal convoluted tubule (DCT); Connecting tubule (CNT).
Uromodulin, hypertension and vascular calcification
Uromodulin is emerging as an important regulator of hypertension (HTN). This has been reviewed by Padmanabhan and colleagues46. SNP variants in the UMOD promoter area have been linked to risk of HTN47,48, but such association may be dependent on other factors, including ethnicity (SNP variants association with HTN may be different in patients of African descent compared to European or Chinese49).Uromodulin regulation of salt handling, NKCC2 and NCC activity could be the link to HTN. Uromodulin facilitates the activation of the Na-K-Cl cotransporter (NKCC2) and the sodium chloride cotransporter (NCC)5,50,51. Overexpression of uromodulin in the kidney can lead to salt-sensitive HTN5. The modulatory functions of uromodulin on NKCC2 and NCC activity are present only when uromodulin is co-expressed in the same cell as each cotransporter, suggesting common cellular processing pathways between uromodulin and ion cotransporters in TAL and early DCT. Uromodulin knockout mice have decreased blood pressure at baseline and do not respond to salt intake by increasing blood pressure as in wild-type animals5,50. In another uromodulin knockout model, young mice had evidence of salt and water wasting due to decreased activation of NKCC252. However, compensatory proximal tubular salt reabsorption develops with age, with prolonged activation of the renin-angiotensin-aldosterone system leading to HTN. Therefore, a prolonged state of uromodulin deficiency will lead to tubular dysfunction and HTN driven by compensatory mechanisms52. Interestingly, salt intake decreases secretion of uromodulin and increases intracellular accumulation of uromodulin in rats, but this effect may be more complex in humans (Table S1), and the interaction of uromodulin with salt intake may depend on multiple factors. For example, in a randomized trial of different levels of salt intake, higher urine uromodulin levels were not associated with a greater increase in blood pressure in response to high salt intake53. This is an exciting and intriguing area that requires additional studies and is the subject of ongoing trials to determine whether uromodulin genotype could guide treatment strategies with loop diuretics that target NKCC2 in patients with HTN54. It has also been proposed that the effect of uromodulin on systemic HTN could be also due to an effect of inhibiting vascular calcification by interfering with pro-inflammatory cytokine signaling by binding to and inhibiting the activity of the pro-calcific TNFα and IL-1β55, or an inhibitory effect on vasoactive hormones56. It is also possible that the interaction of uromodulin with salt regulation and HTN may be different between the urinary polymerizing and non-polymerizing circulating form. Serum uromodulin was associated with lower risk of HTN48 and plasma uromodulin levels were significantly lower on a high-salt diet48. Also, pregnancy in a rodent model of maternal chronic HTN differentially increases non-polymerizing uromodulin, and treatment may shift the production to the polymerizing form26. It is also unclear whether uromodulin expression and production is influenced by the circadian rhythm, which could impact blood pressure regulation, knowing that the circadian rhythm interacts with HTN57.
Conclusion and future research
This review highlights the importance of understanding the physiology of uromodulin and its key role in regulating several diseases, with a focus on hypertension. We discussed new insights, showing the existence of two forms of uromodulin, that are likely differentially regulated and may have separate functions. Therefore, there is value in devising standardized methods to measure both concurrently in the urine. The various works presented strongly support a protective role for interstitial/circulating uromodulin, particularly in setting of kidney injury, disease progression, systemic complications and cardiovascular disease. There is therefore solid rationale and preliminary evidence to pursue strategies to replenish systemic uromodulin for a therapeutic purpose in the setting of deficiency. In AKI, this could be administered at the onset of acute injury to improve the course and favor healthy recovery24. It is also possible to envision periodic systemic uromodulin administration in CKD with severe uromodulin deficiency, particularly as prophylaxis prior to high stress/risk situations for renal and cardiovascular complications such as major surgeries or illness30. Systemic administration of uromodulin may also have a positive impact on the function of the immune system in the setting of severe infections58. Based on the discussion above, it is possible that systemic administration of uromodulin may also influence HTN.
Supplementary Material
Acknowledgements
The authors acknowledge all current and past collaborators and also other researchers in this field that could not be cited because of space considerations.
Funding:
This work was funded by the National Institute of Health- National Institute for Diabetes and Digestive and Kidney Diseases (R01DK111651 for TME and K99DK127216-01 for KAL) and a VA Merit Award for TME.
Footnotes
Conflicts of Interest:
The authors have a patent for using modified Uromodulin (Tamm-Horsfall protein) in therapeutic applications: Modified Tamm Horsfall protein and related compositions and methods of use-US11053290B2, and have another patent application for developing new regents to measure non-polymerizing Uromodulin.
References
- 1.Tamm I, Horsfall FL Jr. Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950;74:106–108. [PubMed] [Google Scholar]
- 2.Muchmore AV, Decker JM. Uromodulin. An immunosuppressive 85-kilodalton glycoprotein isolated from human pregnancy urine is a high affinity ligand for recombinant interleukin 1 alpha. J Biol Chem. 1986;261:13404–13407. [PubMed] [Google Scholar]
- 3.Dawnay AB, Cattell WR. Serum Tamm-Horsfall glycoprotein levels in health and in renal disease. Clin Nephrol. 1981;15:5–8. [PubMed] [Google Scholar]
- 4.Jennings P, Aydin S, Kotanko P, Lechner J, Lhotta K, Williams S, Thakker RV, Pfaller W. Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J Am Soc Nephrol. 2007;18:264–273. doi: 10.1681/ASN.2006020158 [DOI] [PubMed] [Google Scholar]
- 5.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, Citterio L, Demaretz S, Trevisani F, Ristagno G, et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med. 2013;19:1655–1660. doi: 10.1038/nm.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellwege JN, Velez Edwards DR, Giri A, Qiu C, Park J, Torstenson ES, Keaton JM, Wilson OD, Robinson-Cohen C, Chung CP, et al. Mapping eGFR loci to the renal transcriptome and phenome in the VA Million Veteran Program. Nat Commun. 2019;10:3842. doi: 10.1038/s41467-019-11704-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Cheng Y, Consolato F, Schiano G, Chong MR, Pietzner M, Nguyen NQH, Scherer N, Biggs ML, Kleber ME, et al. Genome-wide studies reveal factors associated with circulating uromodulin and its relations with complex diseases. JCI Insight. 2022. doi: 10.1172/jci.insight.157035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaFavers KA, El-Achkar TM. Autosomal dominant tubulointerstitial kidney disease: a new tool to guide genetic testing. Kidney Int. 2020;98:549–552. doi: 10.1016/j.kint.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devuyst O, Pattaro C. The UMOD Locus: Insights into the Pathogenesis and Prognosis of Kidney Disease. J Am Soc Nephrol. 2018;29:713–726. doi: 10.1681/ASN.2017070716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng J, Santambrogio S, et al. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife. 2015;4:e08887. doi: 10.7554/eLife.08887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava R, Micanovic R, El-Achkar TM, Janga SC. An intricate network of conserved DNA upstream motifs and associated transcription factors regulate the expression of uromodulin gene. J Urol. 2014;192:981–989. doi: 10.1016/j.juro.2014.02.095 [DOI] [PubMed] [Google Scholar]
- 12.Garimella PS, Bartz TM, Ix JH, Chonchol M, Shlipak MG, Devarajan P, Bennett MR, Sarnak MJ. Urinary Uromodulin and Risk of Urinary Tract Infections: The Cardiovascular Health Study. Am J Kidney Dis. 2017;69:744–751. doi: 10.1053/j.ajkd.2016.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanisich JJ, Zyla DS, Afanasyev P, Xu J, Kipp A, Olinger E, Devuyst O, Pilhofer M, Boehringer D, Glockshuber R. The cryo-EM structure of the human uromodulin filament core reveals a unique assembly mechanism. Elife. 2020;9. doi: 10.7554/eLife.60265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stsiapanava A, Xu C, Brunati M, Zamora-Caballero S, Schaeffer C, Bokhove M, Han L, Hebert H, Carroni M, Yasumasu S, et al. Cryo-EM structure of native human uromodulin, a zona pellucida module polymer. EMBO J. 2020;39:e106807. doi: 10.15252/embj.2020106807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss GL, Stanisich JJ, Sauer MM, Lin CW, Eras J, Zyla DS, Truck J, Devuyst O, Aebi M, Pilhofer M, et al. Architecture and function of human uromodulin filaments in urinary tract infections. Science. 2020;369:1005–1010. doi: 10.1126/science.aaz9866 [DOI] [PubMed] [Google Scholar]
- 16.Jovine L Using machine learning to study protein-protein interactions: From the uromodulin polymer to egg zona pellucida filaments. Mol Reprod Dev. 2021;88:686–693. doi: 10.1002/mrd.23538 [DOI] [PubMed] [Google Scholar]
- 17.Schaeffer C, Devuyst O, Rampoldi L. Uromodulin: Roles in Health and Disease. Annu Rev Physiol. 2021;83:477–501. doi: 10.1146/annurev-physiol-031620-092817 [DOI] [PubMed] [Google Scholar]
- 18.Micanovic R, LaFavers KA, Patidar KR, Ghabril MS, Doud EH, Mosley AL, Sabo AR, Khan S, El-Achkar TM. The kidney releases a nonpolymerizing form of uromodulin in the urine and circulation that retains the external hydrophobic patch domain. Am J Physiol Renal Physiol. 2022;322:F403–F418. doi: 10.1152/ajprenal.00322.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micanovic R, LaFavers K, Garimella PS, Wu XR, El-Achkar TM. Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis. Nephrol Dial Transplant. 2020;35:33–43. doi: 10.1093/ndt/gfy394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol. 2017;13:525–544. doi: 10.1038/nrneph.2017.101 [DOI] [PubMed] [Google Scholar]
- 21.Schaeffer C, Santambrogio S, Perucca S, Casari G, Rampoldi L. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol Biol Cell. 2009;20:589–599. doi: 10.1091/mbc.E08-08-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F1066–1075. doi: 10.1152/ajprenal.00543.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond). 1985;68:529–535. doi: 10.1042/cs0680529 [DOI] [PubMed] [Google Scholar]
- 24.Micanovic R, Khan S, Janosevic D, Lee ME, Hato T, Srour EF, Winfree S, Ghosh J, Tong Y, Rice SE, et al. Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney. J Am Soc Nephrol. 2018;29:841–856. doi: 10.1681/ASN.2017040409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bokhove M, Nishimura K, Brunati M, Han L, de Sanctis D, Rampoldi L, Jovine L. A structured interdomain linker directs self-polymerization of human uromodulin. Proc Natl Acad Sci U S A. 2016;113:1552–1557. doi: 10.1073/pnas.1519803113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mary S, Small HY, Siwy J, Mullen W, Giri A, Delles C. Polymerization-Incompetent Uromodulin in the Pregnant Stroke-Prone Spontaneously Hypertensive Rat. Hypertension. 2017;69:910–918. doi: 10.1161/HYPERTENSIONAHA.116.08826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Shen F, Yan W, Wu M, Ratnam M. Proteolysis of the carboxyl-terminal GPI signal independent of GPI modification as a mechanism for selective protein secretion. Biochemistry. 1997;36:14583–14592. doi: 10.1021/bi970845w [DOI] [PubMed] [Google Scholar]
- 28.Bufler P, Stiegler G, Schuchmann M, Hess S, Kruger C, Stelter F, Eckerskorn C, Schutt C, Engelmann H. Soluble lipopolysaccharide receptor (CD14) is released via two different mechanisms from human monocytes and CD14 transfectants. Eur J Immunol. 1995;25:604–610. doi: 10.1002/eji.1830250244 [DOI] [PubMed] [Google Scholar]
- 29.Gainer H, Russell JT, Loh YP. The enzymology and intracellular organization of peptide precursor processing: the secretory vesicle hypothesis. Neuroendocrinology. 1985;40:171–184. doi: 10.1159/000124070 [DOI] [PubMed] [Google Scholar]
- 30.LaFavers KA, Macedo E, Garimella PS, Lima C, Khan S, Myslinski J, McClintick J, Witzmann FA, Winfree S, Phillips CL, et al. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med. 2019;11. doi: 10.1126/scitranslmed.aaw3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaggi M, Nakagawa Y, Zipperle L, Hess B. Tamm-Horsfall protein in recurrent calcium kidney stone formers with positive family history: abnormalities in urinary excretion, molecular structure and function. Urol Res. 2007;35:55–62. doi: 10.1007/s00240-007-0083-7 [DOI] [PubMed] [Google Scholar]
- 32.Winfree S, Khan S, Micanovic R, Eadon MT, Kelly KJ, Sutton TA, Phillips CL, Dunn KW, El-Achkar TM. Quantitative Three-Dimensional Tissue Cytometry to Study Kidney Tissue and Resident Immune Cells. J Am Soc Nephrol. 2017;28:2108–2118. doi: 10.1681/ASN.2016091027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Achkar TM, Dagher PC. Tubular cross talk in acute kidney injury: a story of sense and sensibility. Am J Physiol Renal Physiol. 2015;308:F1317–1323. doi: 10.1152/ajprenal.00030.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011;80:338–347. doi: ki2011134 [pii] 10.1038/ki.2011.134 [DOI] [PubMed] [Google Scholar]
- 35.Olinger E, Lake J, Sheehan S, Schiano G, Takata T, Tokonami N, Debaix H, Consolato F, Rampoldi L, Korstanje R, et al. Hepsin-mediated Processing of Uromodulin is Crucial for Salt-sensitivity and Thick Ascending Limb Homeostasis. Sci Rep. 2019;9:12287. doi: 10.1038/s41598-019-48300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanamatsu A, Mori T, Ando F, Furusho T, Mandai S, Susa K, Sohara E, Rai T, Uchida S. Vasopressin Induces Urinary Uromodulin Secretion By Activating PKA (Protein Kinase A). Hypertension. 2021;77:1953–1963. doi: 10.1161/HYPERTENSIONAHA.121.17127 [DOI] [PubMed] [Google Scholar]
- 37.Steubl D, Buzkova P, Ix JH, Devarajan P, Bennett MR, Chaves PHM, Shlipak MG, Bansal N, Sarnak MJ, Garimella PS. Association of serum and urinary uromodulin and their correlates in older adults-The Cardiovascular Health Study. Nephrology (Carlton). 2020;25:522–526. doi: 10.1111/nep.13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enko D, Meinitzer A, Scherberich JE, Marz W, Herrmann M, Artinger K, Rosenkranz AR, Zitta S. Individual uromodulin serum concentration is independent of glomerular filtration rate in healthy kidney donors. Clin Chem Lab Med. 2021;59:563–570. doi: 10.1515/cclm-2020-0894 [DOI] [PubMed] [Google Scholar]
- 39.Zimmerhackl LB, Pfleiderer S, Kinne R, Manz F, Schuler G, Brandis M. Tamm-Horsfall-Protein excretion as a marker of ascending limb transport indicates early renal tubular damage in diabetes mellitus type I. J Diabet Complications. 1991;5:112–114. doi: 10.1016/0891-6632(91)90037-p [DOI] [PubMed] [Google Scholar]
- 40.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechere-Bertschi A, Vogt B, Mohaupt MG, Martin PY, et al. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin J Am Soc Nephrol. 2016;11:70–80. doi: 10.2215/CJN.04230415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moell H Size of normal kidneys. Acta radiol. 1956;46:640–645. [PubMed] [Google Scholar]
- 42.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, Poggio E, Glassock RJ, Rule AD. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N Engl J Med. 2017;376:2349–2357. doi: 10.1056/NEJMoa1614329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puthumana J, Thiessen-Philbrook H, Xu L, Coca SG, Garg AX, Himmelfarb J, Bhatraju PK, Ikizler TA, Siew ED, Ware LB, et al. Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest. 2021;131. doi: 10.1172/JCI139927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thulesen J, Jorgensen PE, Torffvit O, Nexo E, Poulsen SS. Urinary excretion of epidermal growth factor and Tamm-Horsfall protein in three rat models with increased renal excretion of urine. Regul Pept. 1997;72:179–186. doi: 10.1016/s0167-0115(97)01058-6 [DOI] [PubMed] [Google Scholar]
- 45.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis. 2012;59:452–461. doi: 10.1053/j.ajkd.2011.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padmanabhan S, Graham L, Ferreri NR, Graham D, McBride M, Dominiczak AF. Uromodulin, an emerging novel pathway for blood pressure regulation and hypertension. Hypertension. 2014;64:918–923. doi: 10.1161/HYPERTENSIONAHA.114.03132 [DOI] [PubMed] [Google Scholar]
- 47.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, Hastie CE, Menni C, Monti MC, Delles C, et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 2010;6:e1001177. doi: 10.1371/journal.pgen.1001177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du MF, Yao S, Zou T, Mu JJ, Zhang XY, Hu GL, Chu C, Jia H, Liao YY, Chen C, et al. Associations of plasma uromodulin and genetic variants with blood pressure responses to dietary salt interventions. J Clin Hypertens (Greenwich). 2021;23:1897–1906. doi: 10.1111/jch.14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nqebelele NU, Dickens C, Dix-Peek T, Duarte R, Naicker S. Urinary Uromodulin Levels and UMOD Variants in Black South Africans with Hypertension-Attributed Chronic Kidney Disease. Int J Nephrol. 2019;2019:8094049. doi: 10.1155/2019/8094049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graham LA, Padmanabhan S, Fraser NJ, Kumar S, Bates JM, Raffi HS, Welsh P, Beattie W, Hao S, Leh S, et al. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension. 2014;63:551–558. doi: 10.1161/HYPERTENSIONAHA.113.01423 [DOI] [PubMed] [Google Scholar]
- 51.Tokonami N, Takata T, Beyeler J, Ehrbar I, Yoshifuji A, Christensen EI, Loffing J, Devuyst O, Olinger EG. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int. 2018;94:701–715. doi: 10.1016/j.kint.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Goldfarb DS, El-Achkar TM, Lieske JC, Wu XR. Tamm-Horsfall protein/uromodulin deficiency elicits tubular compensatory responses leading to hypertension and hyperuricemia. Am J Physiol Renal Physiol. 2018;314:F1062–F1076. doi: 10.1152/ajprenal.00233.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakhoum CY, Anderson CAM, Juraschek SP, Rebholz CM, Appel LJ, Miller ER, Parikh CR, Obeid W, Rifkin DE, Ix JH, et al. The Relationship Between Urine Uromodulin and Blood Pressure Changes: The DASH-Sodium Trial. Am J Hypertens. 2021;34:154–156. doi: 10.1093/ajh/hpaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCallum L, Brooksbank K, McConnachie A, Aman A, Lip S, Dawson J, MacIntyre IM, MacDonald TM, Webb DJ, Padmanabhan S. Rationale and Design of the Genotype-Blinded Trial of Torasemide for the Treatment of Hypertension (BHF UMOD). Am J Hypertens. 2021;34:92–99. doi: 10.1093/ajh/hpaa166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alesutan I, Luong TTD, Schelski N, Masyout J, Hille S, Schneider MP, Graham D, Zickler D, Verheyen N, Estepa M, et al. Circulating uromodulin inhibits vascular calcification by interfering with pro-inflammatory cytokine signalling. Cardiovasc Res. 2021;117:930–941. doi: 10.1093/cvr/cvaa081 [DOI] [PubMed] [Google Scholar]
- 56.Then C, Thorand B, Then HL, Meisinger C, Heier M, Peters A, Koenig W, Rathmann W, Bidlingmaier M, Lechner A, et al. Serum uromodulin is inversely associated with arterial hypertension and the vasoconstrictive prohormone CT-proET-1 in the population-based KORA F4 study. PLoS One. 2020;15:e0237364. doi: 10.1371/journal.pone.0237364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costello HM, Gumz ML. Circadian Rhythm, Clock Genes, and Hypertension: Recent Advances in Hypertension. Hypertension. 2021;78:1185–1196. doi: 10.1161/HYPERTENSIONAHA.121.14519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LaFavers KA, Hage CA, Gaur V, Micanovic R, Hato T, Khan S, Winfree S, Doshi S, Moorthi RN, Twigg H, et al. The kidney protects against sepsis by producing systemic uromodulin. Am J Physiol Renal Physiol. 2022. doi: 10.1152/ajprenal.00146.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


