Abstract
Lifestyle factors are modifiable behavioral factors that have a significant impact on health and longevity. Diet-induced obesity and physical activity/exercise are two prevalent lifestyle factors that have strong relationships to overall health. The mechanisms linking obesity to negative health outcomes and the mechanisms linking increased participation in physical activity/exercise to positive health outcomes are beginning to be elucidated. Chronic inflammation, due in part to overproduction of myeloid cells from hematopoietic stem cells (HSCs) in the bone marrow, is an established mechanism responsible for the negative health effects of obesity. Recent work has shown that exercise training can reverse the aberrant myelopoiesis present in obesity in part by restoring the bone marrow microenvironment. Specifically, exercise training reduces marrow adipose tissue, increases HSC retention factor expression, and reduces pro-inflammatory cytokine levels in the bone marrow. Other, novel mechanistic factors responsible for these exercise-induced effects, including intercellular communication using extracellular vesicles (EVs), is beginning to be explored. This review will summarize the recent literature describing the effects of exercise on hematopoiesis in individuals with obesity and introduce the potential contribution of EVs to this process.
Keywords: exercise, physical activity, obesity, hematopoiesis, myelopoiesis, extracellular vesicles
Introduction
Acute stresses are typically consequent to foreign body intrusion (e.g., puncture wounds), internal injury (e.g., muscle strain), or by infection (e.g., bacterial or viral). However, diet-induced obesity induces a chronic, low-grade, systemic inflammatory condition. This chronic inflammatory state has far-reaching consequences with long-term health effects, including an increased risk of developing high blood pressure, heart disease, type 2 diabetes, and many cancers. Immune cells comprise white blood cells that originate from the bone marrow. Inflammatory cells of the innate immune system include monocytes, macrophages, and granulocytes. These cells are a part of the myeloid cell lineage that is one of two lineages that develop from rare, primitive hematopoietic stem cells (HSCs). HSCs are located in the bone marrow and are responsible for the formation of all blood cells, a process termed hematopoiesis. HSCs proliferate and differentiate when subject to stressed conditions to replenish the blood system. Under chronic inflammatory conditions, HSCs proliferate and differentiate more quickly than they would during steady-state conditions. This leads to preemptive HSC pool exhaustion and myeloid-skewed hematopoiesis (i.e., myelopoiesis), resulting in the overproduction of inflammatory cells.1-3
Whereas obesity induces a chronic inflammatory condition that promotes myeloid cell development, exercise promotes opposing effects. Previous work from our lab showed that exercise reduces myelopoiesis within a high-fat, diet-induced (HFD) obesity mouse model, where peripheral monocyte populations decreased.4-7 These effects were seen independent of weight loss, suggesting the effects were specific to exercise. Obesity and exercise also have opposing impacts on the bone marrow environment. Obesity promotes marrow adipose tissue formation that physically replaces the space available for red marrow, where hematopoiesis occurs.5 Adipocytes also secrete pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and free fatty acids, thereby promoting HSC differentiation into myeloid cells.8 Exercise can reverse these effects by offsetting leptin signaling in the marrow and promoting HSC quiescence factor signaling through leptin receptor-positive (LEPR+) mesenchymal stromal cells.9
The opposing effects obesity and exercise impart on the bone marrow environment are consequent to altered intra- and intercellular signaling. Most of the literature to date has focused on the role of soluble proteins (i.e., cytokines, growth factors, and neurotransmitters) as the language for intercellular communication in the bone marrow.5 Although important insights have been derived from these studies, the role of other mediators of intercellular communication, such as extracellular vesicles (EVs), has received relatively less attention. EVs are small, membrane-bound nanoparticles (<1000 nm) that often contain cell-specific cargo, including proteins and microRNAs (miRs). When irradiated mouse donor EVs are injected into healthy mice, subsequent sample analysis shows hematopoietic injury similar to those seen in the irradiated mice.10 Similarly, EV injection from healthy donor mice into irradiated mice promotes hematopoietic recovery.11 Furthermore, an emerging body of literature has described the EV response to acute exercise and exercise training with these studies, implicating exercise-induced EVs as potential mediators of the systemic effects of exercise.12 These data demonstrate a role for EVs in affecting HSC activity; however, the role of exercise-induced EVs in regulating hematopoiesis has not been previously explored. The purpose of this review is to provide an updated overview of the current literature describing the role of obesity and exercise on hematopoiesis and the bone marrow microenvironment and introduce exercise-induced EVs as novel potential mediators of these effects.
Obesity and Systemic Inflammation
Obesogenic conditions promote systemic inflammation that persists in a chronic, low-grade fashion. During chronic caloric excess, adipocytes respond by undergoing hypertrophy and hyperplasia.13 Lowered vascular density surrounding the enlarged adipocytes can eventually lead to hypoxia and increased inflammatory cytokine release (i.e., IL-6 and TNF-α).14,15 In response to the inflammatory environment, circulating monocytes enter the tissue and differentiate into macrophages through C-C chemokine ligand type 2 (CCL2) chemokine interactions with its receptor, CCR2, on the monocyte cell surface.16,17 Of these macrophages, pro-inflammatory macrophages (M1) propagate the inflammatory condition through IL-6 and TNF-α cytokine release.18,19 Together, adipocyte and M1 macrophage cytokine release promote a positive feedback loop.20,21 Interestingly, adipose tissue in lean individuals contains a greater proportion of anti-inflammatory macrophages (M2) that act as a mediator for resolving inflammation.22 Nevertheless, the chronic low-grade inflammatory state seen in obesity has far-reaching effects on multiple organ systems, where TNF-α and IL-6 affect nuclear factor kappa B (NF-κB) and Janus kinase-signal transducer and activator of transcription proteins (JAK-STAT) signaling.23,24 Chronic inflammation, characterized by both an upregulation of pro-inflammatory cytokines and overproduction of myeloid lineage cells, including macrophages, termed myelopoiesis has detrimental effects on several tissues that contribute to the long-term health effects of obesity.
Bone Marrow—The Home for Hematopoiesis
All erythrocytes, leukocytes, and thrombocytes originate from a select set of cells termed HSCs. The majority of these cells reside in the bone marrow and represent the most primitive cells in the hematopoietic hierarchy.25-28 HSCs were originally discovered by Ernest McCullogh and James Till in 1961 by their unique capability to self-renew and produce pluripotent progenitors.29,30 They make up a rare cell population, accounting for approximately 1 in every 10,000 bone marrow cells.30,31
As a foundational cell for the entire blood system, maintenance of HSC genetic and functional integrity is critical for proper immune system health. Consequently, HSCs largely remain quiescent, with less than 5% activated at a given time.32 Cell population maintenance occurs through the asymmetric division of HSCs, with the division rate occurring once per year up to a rate of once every three months.32 This asymmetric division gives rise to HSC subpopulations, including long-term HSCs (LT-HSCs) that give rise to short-term HSCs (ST-HSCs), which further differentiate into multipotent progenitor cells (MPPs).33 Of the HSC subpopulations, LT-HSCs have the greatest self-renewal capacity, and the MPPs the lowest. Conversely, LT-HSCs contain the lowest proliferative capacity and MPPs the highest. HSCs can be identified through specific cell surface markers. In mice, HSCs can be identified through the presence or absence of the lineage, Sca-1, and c-kit (LSK) surface markers. The LT-HSCs, ST-HSCs, and MPPs can be individually identified by the signaling lymphocytic activation molecule (SLAM) markers cluster of differentiation 48 (CD48), CD150, CD229, and CD244.28 The interplay between these cell populations underlies the importance of hematopoietic maintenance throughout the life span, leading to complex bone marrow organization, where distinct locations for each cell type can be found.34,35
The MPPs are set in a juxtaposition for differentiation into either the myeloid or lymphoid lineages. Myeloid cells form most blood cell populations, including erythrocytes, megakaryocytes, dendritic cells, and cells that form the innate immune system (granulocytes, monocytes, and macrophages). Lymphoid cells are responsible for adaptive immunity, whose cells include T lymphocytes, B lymphocytes, and natural killer (NK) cells.
Bone Marrow—Regulation of Hematopoiesis by the HSC Niche
Hematopoiesis is affected by the local tissue microenvironment in the bone marrow, termed the HSC niche, where all components required for HSC function and regulation are located.36 The HSC niche is a highly complex organization of many cell populations that work together to give rise to all mature blood cells. These other cell groups include, but are not limited to, mesenchymal stem cells (MSCs), osteoblasts, adipocytes, endothelial cells, and mature hematopoietic cells. With respect to hematopoiesis, two distinct HSC niches have been described: the arteriolar and sinusoidal regions. Quiescent HSCs are found in close proximity to arterioles, whereas less-quiescent HSCs are found close to sinusoids.37,38
Common secreted factors affecting the HSC pool involve complex interplay between C-X-C motif chemokine ligand 4 (CXCL4), CXCL12, transforming growth factor beta 1 (TGF-β1), and stem cell factor (SCF).38-41 The MSCs, similar to HSCs, are relatively rare cells capable of self-renewal and differentiation into chondrocytes, adipocytes, and osteoblasts.42-44 They are located in the vicinity of arterioles and sinusoids38,45-47 and can be identified as nestin+, CXCL12-abundant reticular (CAR), platelet-derived growth factor receptor-α (PDGFR-α), and LEPR+ cells.48,49 The importance of these cells in HSC maintenance was shown through the ablation of CXCL12+ bone marrow cells in mice, which led to HSC pool and osteogenic cell reductions.50,51 Osteoblasts were once thought to play a direct role in hematopoietic control, but recent developments suggest their role may be indirect.52,53 Unlike osteoblasts, adipocytes appear to directly affect HSC regulation. Adipocytes are derived mainly from LEPR+ cells and can alter the HSC niche by producing marrow adipose tissue.54 Throughout life, progressive marrow adipose tissue accumulation has a demonstrated effect on lowering hematopoietic activity and bone regeneration.55-57 Marrow adipose tissue accumulation negatively affects hematopoiesis through physical replacement of red marrow, thereby decreasing the space available for hematopoiesis.55 Adipocytes also promote premature HSC exhaustion through pro-inflammatory cytokine secretion, including TNF-α, IL-6, and free fatty acids.8,58,59
Mature leukocytes also influence HSC activity. Macrophages can regulate MSC CXCL12 expression through liver X receptor (LXR) when clearing aged neutrophils within the bone marrow, thereby promoting HSC retention.60 Neutrophils affect osteogenic cells through prostaglandin E2 to reduce HSC release from the marrow into the circulation.61 Lymphocytes are widely distributed within the HSC niche and appear to play several roles in HSC maintenance.62 Natural killer cells may play an inhibitory role in HSC differentiation through NK cell-derived colony-inhibiting activity soluble factor.63 The CD4+ regulatory T cells deletion similarly depresses HSC myeloid differentiation in addition to reduced HSC colony growth.64 However, regulatory T cells may also provide a protective effect on HSC survival through IL-10.65-67 Finally, endothelial cells surrounding sinusoids play a pivotal role in HSC maintenance as Butler and colleagues68 found an expansion of the HSC pool following the disruption of vascular endothelial growth factor 2 (VEGFR2) and vascular endothelial (VE)-cadherin-dependent angiogenic signaling both in vivo and in vitro through notch signaling. Coculture experiments have further demonstrated a promotional effect of arteriolar and sinusoidal endothelial cells on HSC maintenance, with arteriolar endothelial cells being the major producer of SCF.69 Altogether, the HSC niche undergoes complex cell intercommunication to control HSC production and differentiation. Exercise and obesity affect this cell communication to induce downstream changes in myeloid and lymphoid cell production and, by extension, alterations to systemic inflammatory responses.
Bone Marrow—Obesity and Exercise’s Effects on Bone Marrow Structure and HSC Function
Chronic inflammation, as seen in obesity, has a demonstrated role in affecting hematopoiesis, the HSC niche, and circulating blood cell populations, whereas exercise opposes these effects (Figure 1).5 Chronic inflammatory conditions are linked to marrow adipose tissue accumulation and elevated circulating leukocyte content in humans.2 Under obesogenic conditions, these leukocytes are predominantly myeloid in origin.3 The observed alterations to blood cell populations are consequent to upregulated HSC proliferation that can lead to HSC pool exhaustion and myeloid cell overproduction.6,70 In mice, myeloid skewing has been documented to occur at as early as 6 weeks following a HFD regimen.71 Within the bone marrow, obesity promotes the inflammatory state.72 Even following subsequent weight loss, the chronic inflammatory state placed on the HSC niche leads to the accumulation of myeloid progenitors and LT- and ST-HSC depletion.4,73,74 However, exercise mitigates these alterations in HSC activity. Circulating HSC concentrations have a noted increase following acute and endurance exercise training in mice.75-77 These elevations have also been observed in healthy, well-trained humans following an acute exercise bout.78 In mice, treadmill exercise increases bone marrow HSC proportion in healthy mice,79 whereas exposing mice to voluntary wheel running did no change in bone marrow HSC frequency in healthy mice.9 Discrepant findings in healthy mice may be due to the exercise model used. Although voluntary wheel running is generally considered less stressful, increases in stress hormones have been observed.80 Furthermore, given that mice provided with a running wheel will run 8 to 15 km/day9 while treadmill exercise allows for a translatable dose of exercise to be administered, the treadmill exercise intervention is more relevant to human populations. In disease models, atherosclerotic mice exposed to a running wheel have reduced HSC frequency.9 Similarly, in adults with obesity, exercise training reduces the content of inflammatory primed-HSCs in circulation.81 Furthermore, exercise training in previously obese mice who had undergone weight loss and were at increased risk of carcinogenesis restored the HSC frequency to levels in never obese mice.4 Thus, the effects of exercise appear to be dependent on exercise dose and model in healthy conditions and to reduce aberrant HSC expansion in disease.
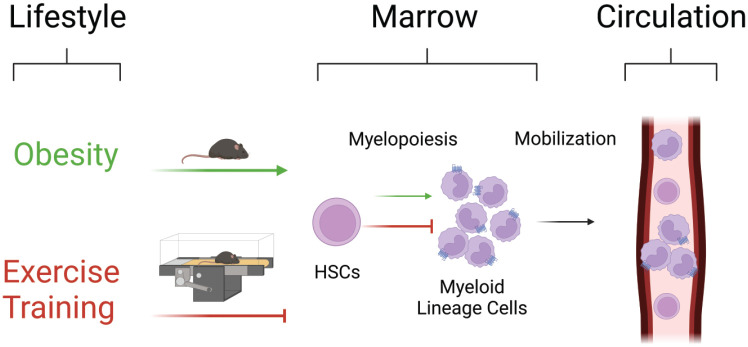
Figure 1.
Exercise training reverses aberrant myelopoiesis in obesity. In obesity, hematopoietic stem cell (HSC) differentiation skews toward myelopoiesis, resulting in an overproduction of pro-inflammatory myeloid lineage cells in circulation and HSC exhaustion. Exercise reverses these effects by promoting HSC maintenance and inhibiting aberrant myelopoiesis. Green lines indicate the effects of obesity and red lines indicate the effects of exercise. Figure created with BioRender.com.
Functionally, Baker and colleagues82 reported increased HSC colony-forming unit capacity with exercise training. In vivo, exercise training does not reduce HSC activity in a transplantation assay79 but improves hematopoietic recovery in stress hematopoiesis7 and in response to infection.9,83 Importantly, this increased hematopoietic activity in exercise-trained mice in the context of stress hematopoiesis does not appear to result in increased risk of hematological malignancies; however, the risk of hematological malignancies is increased in experimental models of obesity.84 With respect to HSC fate decisions, exercise training induces a notable decrease in HSC myeloid-skewing under obesogenic conditions4 and an increase in lymphopoiesis.83 Collectively, these results suggest that, regardless of the effects of exercise training on HSC content, exercise training improves the functional capacity of HSCs to respond to hematopoietic stress and may restore HSC lineage commitment to reverse the inflammatory phenotype in obesity.
New technologies have allowed for dissection of molecular alterations induced by exercise that may explain the effects of exercise training on hematopoiesis at the single-cell level. Using single-cell RNA-sequencing, Liu and colleagues characterized molecular changes in bone marrow, following 5 weeks of voluntary wheel running.85 The authors found that HSCs had the fewest number of differentially expressed genes in response to wheel running, whereas mature monocytes had the greatest number and that this effect was diminished in aged animals.85 Interestingly, a separate study, also using 4-weeks of wheel running as their exercise model, observed an increase in common lymphoid progenitors in aged animals with no effect on HSCs or other hematopoietic progenitor cell subsets.86 Perhaps unsurprisingly, pathways involved in oxidative phosphorylation were among the most upregulated by exercise, whereas cytokine signaling pathways were among the most downregulated.85 These findings aligned with cellular analyses, which indicated that exercise directs immune cell composition toward an anti-inflammatory profile.85 Combined, these findings led the authors to conclude that wheel running led to anti-inflammatory adaptations in hematopoietic cells.85
Within the HSC niche, chronic elevations of IL-6 and TNF-α in obesity promote MSC differentiation away from the osteogenic lineage and toward the adipogenic lineage, thereby enhancing marrow adipose tissue formation.87-89 In a TNF-α knockout mouse model, there was an increase in bone density and a reduction in marrow adipose tissue despite being on a long-term HFD regimen.88,89 There was also a decrease in bone density and a tendency toward adipogenic differentiation of MSCs with elevated monocyte chemoattractant protein-1 (MCP-1) content, an inflammatory chemokine that is elevated in obesity.90,91 Combined, these results suggest a strong relationship between the content of pro-inflammatory markers and detrimental effects on homeostasis in individuals with obesity. Exercise, however, increases bone mineral density and decreases marrow adipose tissue formation, even in conditions where peroxisome proliferator-activated receptor gamma (PPARγ) agonists are provided to promote marrow adipose tissue formation.6 Exercise decreases TNF-α and IL-1β signaling in the bone marrow, which may be responsible for mitigated marrow adipose tissue formation and increase bone density compared with sedentary obesogenic conditions.23 In addition, exercise preconditioning of the HSC niche prior to bone marrow transplantation accelerates hematopoietic recovery in mice92 and perhaps also in humans.93 These effects are due, in part, to preservation of the HSC niche (Figure 2).92,94
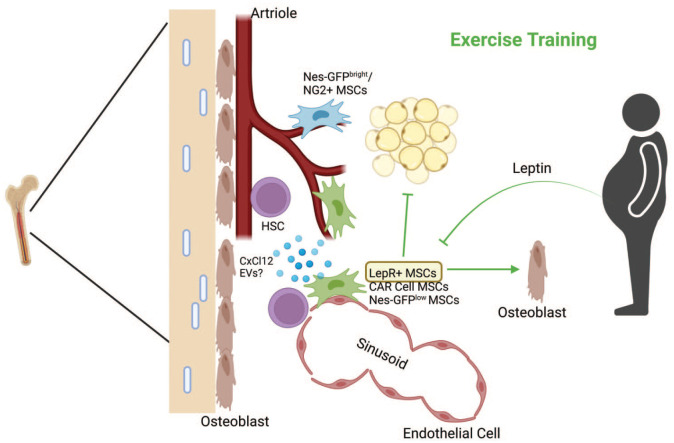
Figure 2.
Exercise training remodels the HSC niche. Exercise training reduces marrow adipose tissue and stimulates osteogenic differentiation of MSCs. Furthermore, exercise training promotes the expression and release of HSC retention factors (i.e., CxCl12), specifically from LepR+ MSCs, and reduces leptin production from peripheral adipose tissue. A relatively unexplored mechanism of the effects of exercise involves the release of EVs from other sources that contribute to the restoration of hematopoiesis in obesity. Green lines indicate the effects of exercise training. Figure created with BioRender.com. CxCl12, C-X-C motif chemokine ligand; EV, extracellular vesicle; LepR+, leptin receptor-positive; MSC, mesenchymal stem cell.
Several groups have investigated the effects of exercise training on the HSC niche. It has been well-established that exercise training reduces marrow adipose tissue content.4,6,7 These effects can be traced back to exercise-induced alterations in cell fate decisions of bone marrow-MSCs that favor osteogenic over adipogenic differentiation.6,82 Bone marrow-MSCs proliferate in response to acute exercise75 and recent work has demonstrated that chronic wheel running increased the content of osteoprogenitors in the bone marrow of aged mice, whereas unloading reduced their content.86 Furthermore, chronic wheel running increases the expression of Cxcl12 in LepR+ bone marrow-MSCs through reducing leptin release from adipocytes.9 Recently, Peng and colleagues used a 5-week treadmill exercise intervention to determine that exercise training increased osteoblast and common lymphoid progenitor cell content.83 Mechanistically, mechanical stimulation induced by running resulted in reticulocalbin-2 (RCN2) release from bone marrow macrophages that stimulated fatty acid release from marrow adipocytes that fueled osteogenic and lymphopoietic fate decisions by mesenchymal stromal cells and HSCs, respectively.83 Collectively, these reports suggest cells within the marrow alter their communication in response to obesity- and exercise-induced stressors. A full understanding of mediators of this cell communication within the bone with obesity and exercise, however, is lacking.
Extracellular Vesicles—An Overview
First discovered in 1946, EVs were initially thought of as procoagulant particles due to their release from platelets.95 EVs are now appreciated to be membrane-bound particles released by cells for intercellular communication and waste.96,97 The term “EV” is an umbrella term for membrane-bound particles that are most commonly referred to as exosomes and microvesicles, among other less-commonly used names.97-100 Exosomes are small EVs that form within multivesicular endosomes, whereas microvesicles are larger than exosomes and are a product of direct budding of the cell membrane. To address inconsistent nomenclature in the literature, a group of EV experts formed the International Society for Extracellular Vesicles (ISEV) along with an all-encompassing document in 2014 titled, Minimal Information for Studies of Extracellular Vesicles 2014 (MISEV2014), which outlined rationale for EV isolation recommendations and established standards that should be met for a sound study design.101 These recommendations were updated and refined in 2018.99
Challenges remain in identifying EVs from other particles due to unknown surface marker characteristics.102,103 Defining EV presence in a solution has therefore been commonly attributed to particle size and ubiquitous protein expression. The first step in identifying EV presence is to isolate them from a tissue sample. When choosing an EV isolation method, important considerations must be made regarding recovery capabilities, sample purity, volume, and protocol duration.104 Four isolation methods are typically used: ultracentrifugation, immunoaffinity capture, ultrafiltration, and precipitation, each with their own drawbacks and advantages.104,105 Once isolated, EVs can be identified by high-resolution flow cytometry, nanoparticle tracking analysis, transmission electron microscopy, and western blot. Cargo can be analyzed by transcriptomics and proteomics techniques.106
Extracellular Vesicles—Vehicles for Cell-Cell Communication in the Bone Marrow
The EV release from cells is a highly conserved cell-cell communication method that is present in all cells of the body.107 Wen and colleagues11 examined the role of bone marrow-derived EVs on irradiated hematopoietic cell injury recovery in vitro. Specifically, exosomes, microvesicles, and their combination were introduced to murine HSCs following irradiation. The microvesicle-exosome collection showed a 6-fold increase in cell proliferation compared with the vehicle control, suggesting a positive role for EVs in radiation injury hematopoietic recovery.11 When observed individually, exosomes doubled cell proliferation compared with control, whereas microvesicles quadrupled cell proliferation compared with control.11 These results suggest microvesicles substantially facilitate hematopoietic cell recovery from injury, whereas exosomes have a relatively minor effect on recovery. Despite these findings, discriminating possible differences between exosome and microvesicle-driven influences in cell activity across the literature remains a challenge due to nomenclature inconsistencies. However, growing evidence suggests EVs can invoke HSC activity alterations, which may be useful for understanding disease development and creating therapeutic options.107-109
Studies are continually finding that EVs carry cell-specific contents originating from the source cell, which may be used to understand the influence of specific cell populations on their surroundings.110,111 These findings extend to pathological or healthy cells.112,113 For example, Haraszti and colleagues110 examined the surface content and cargo of exosomes and microvesicles of U87 glioblastoma cells, Huh7 hepatocellular carcinoma cells, and healthy human bone marrow-derived MSCs. They found similar proteomes in U87- and Huh7-derived EVs, whereas healthy MSC cell-derived EV proteomes were significantly different, suggesting cells curate their cargo based on internal cues.110 The ability for EVs to bind and release cargo into a target cell is dependent upon surface adhesion protein expression on both the EVs and the target cells. Understanding the interplay between pathological cell EV release, target cell adhesion and uptake, and target cell activity alterations will provide great insight in mitigating or halting negative effects.
Within the context of exercise, EV cargo and release from skeletal muscle has been primarily studied with little information available for other tissues. In general, exercise alters circulating blood EV content. For example, Whitham and colleagues114 showed a single 1-hour cycling exercise bout altered circulating EV protein content and increased total circulating EV count, thereby corroborating the findings of others.115 In muscle-derived EVs, there have been notable alterations to EV cargo in response to exercise. For example, miR-486 is downregulated in muscle-derived EVs following an acute bout of exercise.116 The miR-486 has been linked to promoting erythroid cell differentiation, indicating a potential role for exercise-induced EV signaling in affecting distant hematopoietic cells.117 Furthermore, downregulated miRNA linked to inhibited mesenchymal stromal cell osteogenic differentiation has been observed in circulating blood of rats following exercise,118,119 suggesting that exercise-induced EVs may alter the HSC niche. Collectively, these data suggest exercise alters EV cargo in a manner that can affect cell activity of distant tissues. However, the impacts of exercise on bone marrow-derived EV release and cargo, and their role in hematopoiesis, have not been directly investigated.
Conclusions and Future Directions
Obesity and exercise have opposing effects on hematopoiesis and the bone marrow microenvironment that contributes to their divergent effects on health. Obesity skews hematopoiesis to favor myelopoiesis, increases marrow adipose tissue, creates a pro-inflammatory HSC niche, and impairs HSC regenerative potential, whereas these effects are generally reversed by exercise. However, the effects of exercise training on the HSC niche do not appear to be durable, suggesting that consistent participation in exercise training interventions is necessary. The role of other signaling factors as regulators of hematopoiesis, including but not limited to EVs and their cargo, are beginning to be appreciated; however, their role in regulating hematopoiesis in the context of obesity and exercise remains understudied. Furthermore, the exercise prescription that can be translated to humans, which induces the beneficial effects of exercise on bone marrow remodeling in obesity remains unknown. Making progress toward these two key gaps in literature will improve both our understanding as well as application of exercise training in the context of obesity-associated pathologies.
Footnotes
Author Contribution: Authors contributed to conception of the topic of this review (MD); and writing and editing the manuscript (MD, JV). All authors gave final approval and agree to be accountable for all aspects of work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funds were provided by the National Sciences and Engineering Council (NSERC) and the American Institute for Cancer Research (AICR) to Michael De Lisio. James J. Vanhie received support from the uOttawa/CHEO-RI Doctoral Fellowship and an Ontario Graduate Scholarship.
ORCID iD: Michael De Lisio  https://orcid.org/0000-0002-6462-2955
https://orcid.org/0000-0002-6462-2955
References
- 1. Niemiro GM, Raine LB, Khan NA, et al. Circulating progenitor cells are positively associated with cognitive function among overweight/obese children. Brain Behav Immun. 2016;57:47-52. doi: 10.1016/j.bbi.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci USA. 2012;109(20):7622-7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664-675. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emmons R, Xu G, Hernández-Saavedra D, et al. Effects of obesity and exercise on colon cancer induction and hematopoiesis in mice. Am J Physiol-Endocrinol Metab. 2018;316(2):E210-E220. doi: 10.1152/ajpendo.00237.2018. [DOI] [PubMed] [Google Scholar]
- 5. Emmons R, Niemiro GM, De Lisio M. Hematopoiesis with obesity and exercise: role of the bone marrow niche. Exerc Immunol Rev. 2017;23:82-95. [PubMed] [Google Scholar]
- 6. Styner M, Thompson WR, Galior K, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39-46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emmons R, Ngu M, Xu G, Hernández-Saavedra D, Chen H, De Lisio M. Effects of obesity and exercise on bone marrow progenitor cells after radiation. Med Sci Sports Exerc. 2019;51(6):1126-1136. doi: 10.1249/MSS.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 8. Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21(12):1162-1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frodermann V, Rohde D, Courties G, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25(11):1761-1771. doi: 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szatmári T, Kis D, Bogdándi EN, et al. Extracellular vesicles mediate radiation-induced systemic bystander signals in the bone marrow and spleen. Front Immunol. 2017;8:347. doi: 10.3389/fimmu.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen S, Dooner M, Cheng Y, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30(11):2221-2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular vesicles and exosomes: insights from exercise science. Front Physiol. 2020;11:604274. doi: 10.3389/fphys.2020.604274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37(3):753-768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347-2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 15. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347-355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 16. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujimura N, Xu B, Dalman J, Deng H, Aoyama K, Dalman RL. CCR2 inhibition sequesters multiple subsets of leukocytes in the bone marrow. Sci Rep. 2015;5(1):11664. doi: 10.1038/srep11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49(7):1562-1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 19. Revelo XS, Luck H, Winer S, Winer DA. Morphological and inflammatory changes in visceral adipose tissue during obesity. Endocr Pathol. 2014;25(1):93-101. doi: 10.1007/s12022-013-9288-1. [DOI] [PubMed] [Google Scholar]
- 20. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796-1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87-91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 22. Lauterbach MA, Wunderlich FT. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch. 2017;469(3-4):385-396. doi: 10.1007/s00424-017-1955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kern L, Mittenbühler M, Vesting A, Ostermann A, Wunderlich C, Wunderlich F. Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation—driven liver and colorectal cancers. Cancers. 2018;11(1):24. doi: 10.3390/cancers11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jais A, Brüning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017;127(1):24-32. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gazit R, Weissman IL, Rossi DJ. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45(4):218-224. doi: 10.1053/j.seminhematol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 26. Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation: hematopoietic stem cell. Wiley Interdiscip Rev Syst Biol Med. 2010;2(6):640-653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108(50):20012-20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102-116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siminovitch L, Mcculloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Comp Physiol. 1963;62:327-336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 30. Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 2011;175(2):145-149. doi: 10.1667/RRXX28.1. [DOI] [PubMed] [Google Scholar]
- 31. Spangrude G, Heimfeld S, Weissman I. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58-62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 32. Catlin SN, Busque L, Gale RE, Guttorp P, Abkowitz JL. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117(17):4460-4466. doi: 10.1182/blood-2010-08-303537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto R, Morita Y, Ooehara J, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154(5):1112-1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 34. Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109-1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 35. Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621-2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598-611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Itkin T, Gur-Cohen S, Spencer JA, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532(7599):323-328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637-643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruns I, Lucas D, Pinho S, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315-1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146-1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 41. Hérault A, Binnewies M, Leong S, et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 2017;544(7648):53-58. doi: 10.1038/nature21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324-336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 43. Chan CKF, Chen CC, Luppen CA, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490-494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31(1):285-316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 45. Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407-421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 46. Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3(4):364-366. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442-447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 48. Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370(1):82-96. doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303-320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marie P. Strontium as therapy for osteoporosis. Curr Opin Pharmacol. 2005;5(6):633-636. doi: 10.1016/j.coph.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 51. Roberts EW, Deonarine A, Jones JO, et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210(6):1137-1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841-846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 53. Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836-841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 54. Scheller EL, Troiano N, Vanhoutan JN, et al. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 2014;537:123-139. doi: 10.1016/B978-0-12-411619-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ambrosi TH, Scialdone A, Graja A, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771-784.e6. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14(1):10-19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 57. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259-263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aaron N, Costa S, Rosen CJ, Qiang L. The implications of bone marrow adipose tissue on inflammaging. Front Endocrinol. 2022;13:853765-853710. doi: 10.3389/fendo.2022.853765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hermetet F, Buffière A, Aznague A, et al. High-fat diet disturbs lipid raft/TGF-β signaling-mediated maintenance of hematopoietic stem cells in mouse bone marrow. Nat Commun. 2019;10(1):523. doi: 10.1038/s41467-018-08228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chow A, Huggins M, Ahmed J, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429-436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kawano Y, Fukui C, Shinohara M, et al. G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood. 2017;129(5):587-597. doi: 10.1182/blood-2016-07-725754. [DOI] [PubMed] [Google Scholar]
- 62. Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12(1):49-60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Degliantoni G, Murphy M, Kobayashi M, Francis MK, Perussia B, Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor: relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985;162(5):1512-1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Urbieta M, Barao I, Jones M, et al. Hematopoietic progenitor cell regulation by CD4+CD25+ T cells. Blood. 2010;115(23):4934-4943. doi: 10.1182/blood-2009-04-218826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gandy KL, Domen J, Aguila H, Weissman IL. CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11(5):579-590. doi: 10.1016/s1074-7613(00)80133-8. [DOI] [PubMed] [Google Scholar]
- 66. Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216-219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hirata Y, Furuhashi K, Ishii H, et al. CD150high bone marrow Tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22(3):445-453.e5. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251-264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu C, Gao X, Wei Q, et al. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat Commun. 2018;9(1):2449. doi: 10.1038/s41467-018-04726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van den Berg SM, Seijkens TT, Kusters PJ, et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J. 2016;30(5):1779-1788. doi: 10.1096/fj.201500175. [DOI] [PubMed] [Google Scholar]
- 71. Liu A, Chen M, Kumar R, et al. Bone marrow lympho-myeloid malfunction in obesity requires precursor cell-autonomous TLR4. Nat Commun. 2018;9:708. doi: 10.1038/s41467-018-03145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10(12):737-748. doi: 10.1038/nrendo.2014.169. [DOI] [PubMed] [Google Scholar]
- 73. Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22(6):492-505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- 74. Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies: physical activity and colorectal cancer mortality. Int J Cancer. 2013;133(8):1905-1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 75. Emmons R, Niemiro GM, Owolabi O, De Lisio M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J Appl Physiol. 2016;120(6):624-632. doi: 10.1152/japplphysiol.00925.2015. [DOI] [PubMed] [Google Scholar]
- 76. Mooren FC, Krüger K. Apoptotic lymphocytes induce progenitor cell mobilization after exercise. J Appl Physiol. 2015;119(2):135-139. doi: 10.1152/japplphysiol.00287.2015. [DOI] [PubMed] [Google Scholar]
- 77. Marycz K, Mierzejewska K, Śmieszek A, et al. Endurance exercise mobilizes developmentally early stem cells into peripheral blood and increases their number in bone marrow: implications for tissue regeneration. Stem Cells Int. 2016;2016:5756901-5756910. doi: 10.1155/2016/5756901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kröpfl JM, Beltrami FG, Gruber HJ, Stelzer I, Spengler CM. Exercise-induced circulating hematopoietic stem and progenitor cells in well-trained subjects. Front Physiol. 2020;11:308-310. doi: 10.3389/fphys.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. De Lisio M, Parise G. Characterization of the effects of exercise training on hematopoietic stem cell quantity and function. J Appl Physiol 1985. 2012;113(10):1576-1584. doi: 10.1152/japplphysiol.00717.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fuss J, Ben Abdallah NM, Vogt MA, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20(3):364-376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- 81. Niemiro GM, Allen JM, Mailing LJ, et al. Effects of endurance exercise training on inflammatory circulating progenitor cell content in lean and obese adults. J Physiol. 2018;596(14):2811-2822. doi: 10.1113/JP276023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baker JM, De Lisio M, Parise G. Endurance exercise training promotes medullary hematopoiesis. FASEB J. 2011;25(12):4348-4357. doi: 10.1096/fj.11-189043. [DOI] [PubMed] [Google Scholar]
- 83. Peng H, Hu B, Xie LQ, et al. A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis. Cell Metab. 2022;34(8):1168-11822.e6. doi: 10.1016/j.cmet.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 84. Farber E, Kwiecien JM, Bojic D, et al. Exercise improves cancer-free survival and health span in a model of radiation-induced cancer. Med Sci Sports Exerc. 2021;1(53):2254-2263. doi: 10.1249/MSS.0000000000002711. [DOI] [PubMed] [Google Scholar]
- 85. Liu L, Buckley MT, Reyes JM, et al. Exercise reprograms the inflammatory landscape of multiple stem cell compartments during mammalian aging [Published online ahead of print January 12, 2022]. bioRxiv Preprint. doi: 10.1101/2022.01.12.475145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shen B, Tasdogan A, Ubellacker JM, et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591(7850):438-444. doi: 10.1038/s41586-021-03298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kaneki H, Guo R, Chen D, et al. Tumor necrosis factor promotes runx2 Degradation through up-regulation of Smurf1 and Smurf2 in Osteoblasts. J Biol Chem. 2006;281(7):4326-4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu W, Konermann A, Guo T, Jäger A, Zhang L, Jin Y. Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions. Biochim Biophys Acta. 2014;1840(3):1125-1134. doi: 10.1016/j.bbagen.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 89. Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NFκB pathways. J Biol Chem. 2006;281(10):6297-6306. doi: 10.1074/jbc.M507804200. [DOI] [PubMed] [Google Scholar]
- 90. Sul OJ, Ke K, Kim WK, et al. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. J Cell Physiol. 2012;227(4):1619-1627. doi: 10.1002/jcp.22879. [DOI] [PubMed] [Google Scholar]
- 91. Sullivan CB, Porter RM, Evans CH, et al. TNFα and IL-1β influence the differentiation and migration of murine MSCs independently of the NF-κB pathway. Stem Cell Res Ther. 2014;5(4):104. doi: 10.1186/scrt492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. De Lisio M, Baker JM, Parise G. Exercise promotes bone marrow cell survival and recipient reconstitution post-bone marrow transplantation, which is associated with increased survival. Exp Hematol. 41(2):143-154. doi: 10.1016/j.exphem.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 93. Aziz JA, Smith C, Slobodian M, et al. Impact of exercise training on hematological outcomes following hematopoietic cell transplantation: a scoping review. Clin Investig Med Med Clin Exp. 2021;44(2):E19-E26. doi: 10.25011/cim.v44i2.36369. [DOI] [PubMed] [Google Scholar]
- 94. De Lisio M, Phan N, Boreham DR, Parise G. Exercise-induced protection of bone marrow cells following exposure to radiation. Appl Physiol Nutr Metab. 2011;36(1):80-87. doi: 10.1139/H10-087. [DOI] [PubMed] [Google Scholar]
- 95. Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189-197. doi: 10.1016/S0021-9258(17)34997-9. [DOI] [PubMed] [Google Scholar]
- 96. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783-797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ratajczak MZ, Ratajczak J. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future. Leukemia. 2020;34(12):3126-3135. doi: 10.1038/s41375-020-01041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019;8(1):1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stein JM, Luzio JP. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J. 1991;274(2):381-386. doi: 10.1042/bj2740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364-372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 104. Doyle L, Wang M. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Peterson MF, Otoc N, Sethi JK, Gupta A, Antes TJ. Integrated systems for exosome investigation. Methods. 2015;87:31-45. doi: 10.1016/j.ymeth.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 106. Kormelink TG, Arkesteijn GJA, Nauwelaers FA, van den Engh G, Nolte-’t Hoen ENM, Wauben MHM. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry: sorting extracellular vesicle subpopulations. Cytometry A. 2016;89(2):135-147. doi: 10.1002/cyto.a.22644. [DOI] [PubMed] [Google Scholar]
- 107. Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, EL, Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Machhi J, Shahjin F, Das S, et al. A role for extracellular vesicles in SARS-CoV-2 therapeutics and prevention. J Neuroimmune Pharmacol. 2021;16(2):270-288. doi: 10.1007/s11481-020-09981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439-464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Haraszti RA, Didiot MC, Sapp E, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968-E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kitamura Y, Kojima M, Kurosawa T, et al. Proteomic profiling of exosomal proteins for blood-based biomarkers in Parkinson’s disease. Neuroscience. 2018;392:121-128. doi: 10.1016/j.neuroscience.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 113. Ohshima K, Hatakeyama K, Kanto K, et al. Comparative proteomic analysis identifies exosomal Eps8 protein as a potential metastatic biomarker for pancreatic cancer. Oncol Rep. 2019;41(2):1019-1034. doi: 10.3892/or.2018.6869. [DOI] [PubMed] [Google Scholar]
- 114. Whitham M, Parker BL, Friedrichsen M, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27(1):237-251e4. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 115. Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Aoi W, Ichikawa H, Mune K, et al. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol. 2013;4:80. doi: 10.3389/fphys.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shi XF, Wang H, Kong FX, et al. Exosomal miR-486 regulates hypoxia-induced erythroid differentiation of erythroleukemia cells through targeting Sirt1. Exp Cell Res. 2017;351(1):74-81. doi: 10.1016/j.yexcr.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 118. Oliveira GP, Jr, Porto WF, Palu CC, et al. Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Front Physiol. 2018;9:532. doi: 10.3389/fphys.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sol Kim D, Young Lee S, Hee Lee J, Chan Bae Y, Sup Jung J. MicroRNA-103a-3p controls proliferation and osteogenic differentiation of human adipose tissue-derived stromal cells. Exp Mol Med. 2015;47(7):e172. doi: 10.1038/emm.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]