Abstract
Post-traumatic stress disorder is a prevalent disorder within the USA and worldwide with a yearly diagnosis rate of 2–4% and affecting women more than men. One of the primary methods for study of this stress disorder relies on animal models as there are few noninvasive methods and few replicated peripheral biomarkers for use in humans. One area of active research in psychiatric neuroscience is the field of epigenetics − how the chemical modifications of the genetic code regulate behavior. The dynamic changes in histone acetylation and deacetylation in the brain are not fully reflected by the study of peripheral biomarker. In this review, we aim to examine the role of histone acetylation and deacetylation in memory formation and fear memory learning. The studies discussed here focus largely on the role of histone deacetylases (HDACs) in animal models of trauma and fear response. Many studies used HDAC inhibitors to elucidate the effects after inhibition of these enzymes after trauma or stress. These studies of memory processing and cued fear extinction in animal can often shed light on human disorders of cued fear responses and memory dysregulation after stress or trauma such as in PTSD. These results provide strong evidence for a role of these enzymes in PTSD in humans. The few clinical studies that exist with HDAC inhibitors also suggest a fundamental role of these enzymes in the neurobiology of the stress response. Further study of these enzymes in both clinical and pre-clinical settings may help elucidate the neurobiology of stress-related pathology like PTSD and provide a foundation for novel therapy to treat these disorders.
Keywords: Post-traumatic stress disorder, Histone deacetylases, Positron emission tomography, Epigenetics, Stress disorder, Anxiety
Introduction
Post-traumatic stress disorder [1, 2] is a clinical disorder associated with intrusive fear and fear-conditioned behaviors that have been ascribed to an inability to extinguish fear-based memories [3]. However, broader views of the pathophysiology of PTSD are emerging from initial transcriptomic studies of postmortem tissue comparisons from individuals with and without PTSD [4]. PTSD has a high prevalence rate within the USA at roughly 2.5–3.5% per year and more than 1 in 11 Americans experiencing this illness during their lifetime. Women are diagnosed at a rate more than two times that of men, with a prevalence of 10–12% prevalence, as compared to 4–5% prevalence in men after an equivalent traumatic event [5]. As with many psychiatric illnesses, PTSD is diagnosed and treated based solely on clinical symptoms [6] as no diagnostic testing exists. Furthermore, despite its prevalence, the neurobiology of PTSD remains poorly understood.
It is hypothesized that PTSD arises from a failure in memory consolidation and reconsolidation processes after a traumatic event that renders these memories inextinguishable from an individual's long-term memory and cued fear responses. Four major centers of the brain have been particularly implicated in the processing of PTSD-related fear memories: the hippocampus, insula, amygdala, and prefrontal cortex (PFC). When a traumatic stressor occurs, it is processed through the hippocampus and amygdala into a stable memory, and when this memory is brought up through similar stimuli (cue), the memory becomes destabilized [7]. At this time, it may be reconsolidated through a CREB-mediated gene expression pathway within the hippocampus and amygdala or undergo extinction through a similar process in the PFC and amygdala [7, 8]. This conceptual framework suggests that destabilization of a memory represents an important point where a conditioned fear response and fear memory may either begin to extinguish through PFC or become solidified as a new memory via the limbic system. The neuroplasticity required for these processes within the amygdala, hippocampus (predominantly CA1), and PFC is primarily driven through glutamatergic signaling changes as demonstrated through pre-clinical ketamine-related studies [9]. On a long-term basis, these pathological memories may be stored through changes to regulation of gene expression, including via epigenetics [10]. While others have reviewed DNA methylation (DNAm) as a key epigenetic mechanism relevant to PTSD [11, 12], this review aims to elucidate the specific implications of histone acetylation and deacetylation for both the neurobiology of PTSD and novel therapeutic drug discovery.
Overview of Epigenetics in PTSD
Epigenetics refers to changes in genomic expression without changing the DNA code itself, quite literally, “on top (epi) of genetics.” These changes include DNAm, long noncoding RNA, small interfering RNA, microRNA (miRNA), piwi-interacting RNA, histone acetylation, and transcription factor acetylation. Thus far, studies have demonstrated a role for epigenetics in fear learning and memory as well as in PTSD through genomic studies of peripheral tissue and in limited cases ex vivo brain tissue analysis [1, 10, 11, 13, 14, 15, 16, 17, 18, 19]. However, despite this progress, there are still gaps in the knowledge of epigenetic regulation disruptions in PTSD [10, 15]. Epigenome-wide association studies (EWASs) [1] have been conducted on individuals with PTSD and trauma-exposed controls to study DNAm. One of the largest EWASs of military cohorts recently found the gene with the highest impact to be MAD1L1 which is a key component to the mitotic spindle assembly necessary for cell replication [1]. Another EWAS study demonstrated positive associations for DNAm in individuals with PTSD at cg19534438 in the gene G0S2. Another significant association (cg04130728 in CHST11) was found between peripheral tissue (blood) and brain tissue (PFC) though not genome wide [16]. Another study has reported 20 methylation site changes in PTSD but two with the highest level of significance: 5-methylcytosine [20] and 5-hydroxymethylcytosine [21]. Among individuals with PTSD, the biggest changes in DNAm were noted among immune system regulatory genes [10]. These studies suggest strongly that DNAm changes are involved in the development of trauma-associated PTSD, particularly in the military setting.
Other areas with significant methylation changes are within the genes of the aryl-hydrocarbon receptor repressor in individuals with PTSD and associated with decreased kynurenine levels leading to immune system dysregulation through the kynurenine pathway [22]. To this point, significant RNA changes have not been correlated with PTSD symptomatology in human clinical studies [10]. However, there is growing body of evidence in both pre-clinical and clinical models that miRNA changes to decrease substance P and interleukin-1α are implicated in PTSD and depression [23]. Heritable trauma has also been associated with RNA changes in sperm [24].
Importantly, trauma has been associated with shortened life span and an increased epigenetic age, derived from blood markers, as compared to chronological age [25]. Among veterans with PTSD, their epigenetic age was directly correlated with their PTSD symptom severity [17]. The increase in DNAm sites indicates advanced epigenetic age [26] also correlated with decreased telomere length, which can correlate with a shortened life span [17]. Additionally, these changes have effects on the offspring of the trauma-affected individuals and may impact their mental health through hereditary methylation changes [27]. It should be noted one study found changes in offspring methylation within FKBP5 to be opposite of their parents, which has yet to be completely understood, but may be indicative of a compensatory reaction [18]. These findings demonstrate the importance of understanding the epigenetic changes associated with PTSD and developing effective treatment for PTSD symptoms and trauma-exposed individuals.
Histone Deacetylase Overview
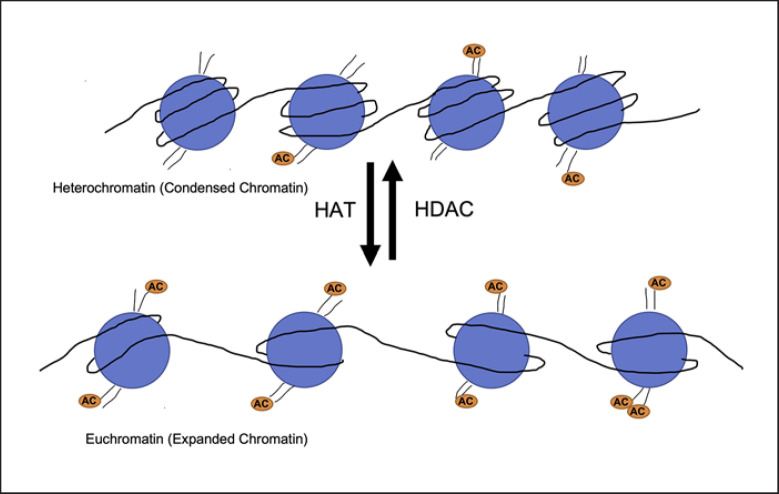
One type of epigenetic regulation within the human genome includes modifications to the histone core proteins that package the DNA. Histone core proteins are composed of four subunits termed H2A, H2B, H3, and H4, and together with coiled DNA, create chromatin. The DNA is uncoiled when needed for “gene expression” through transcription of mRNA and translation into proteins. Histone core protein modifications involve numerous chemical modifications including acetylation and deacetylation when an acetyl moiety (-COOH) is added or removed from an amino acid such as a lysine in the histone core protein tails. Enzymatic linkage through de novo amide bond formation attaches the acetyl to the lysine-free amino terminus. This creates dynamic changes in the DNA/histone binding structure through changes in the polarity of the histone/DNA interaction. Thus, the density of DNA coiled around these histone core proteins regulates whether transcription may occur (Fig. 1). Tighter chromatin (heterochromatin) does not allow for transcription of DNA into mRNA, whereas looser chromatin (euchromatin) does allow for transcription and ultimately gene expression. Acetylation of these residues imparts a negatively charged acetyl group which is repulsed by the negatively charged DNA and promotes opening of DNA to facilitate transcription, thus giving histone acetyl transferases (HATs) the nickname of “writers,” as they promote gene transcription. Histone deacetylases (HDACs) functionally silence areas of the genome, preventing transcription, by removing the negatively charged acetyl groups, such that the positive histone tail is more closely attracted to the negative DNA and closes the DNA (Fig. 1). While the HATs are made up of a wide variety of proteins and activators working closely with RNA polymerase and transcription factors [28], the HDACs are comprised of a much smaller and more focused family of enzymes. Since the 1990s, the number and type of distinct HDACs identified have increased. To date, 18 HDAC enzymes (Fig. 2) have been identified in humans and are typically divided into four major classes [29]. Class I HDACs include HDAC1, HDAC2, HDAC3, and HDAC8, all of which have significant sequence and domain organizational similarity. Class I HDACs typically localize to the nucleus [30] and are involved in the regulation of cellular proliferative activity [31, 32]. Class II HDACs, including HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10, shuttle between the cytoplasm and nucleus and are broken down further into Class IIa (HDACs 4, 5, 7, 9) and Class IIb (HDACs 6, 10). The primary distinction between Classes IIa and IIb is defined as the central domain of Class IIb containing a zinc finger motif, while Class IIa contains a structural regulatory zinc-binding domain [33, 34]. Class IIa is typically able to shuttle more readily between the nucleus and the cytoplasm, whereas Class IIb is primarily found in the cytoplasm. Class IV HDACs are comprised of a single HDAC protein, HDAC11, which is primarily nuclear localized and possesses the unique ability to act as a long-chain fatty acid deacetylase and as a regulator of miRNAs and long noncoding RNAs [35, 36, 37]. All the HDAC enzymes, Classes I, II, and IV, are dependent on Zn2+ or catalytic activity.
Fig. 1.
Pictorial description of the role for HDACs within the cell. HDAC removing acetyl moiety to condense the chromatin further, while HATs add the acetyl moiety to histone protein tails to expand the chromatin.
Fig. 2.
Description of HDAC enzymes and abbreviations used within the manuscript.
HDACs Class III is comprised of HDAC enzymes termed “silent information regulators” (sirtuins, SIRTs) [38], representing a family of closely related deacetylases that are Zn2+-independent but NAD+-dependent. This family was first discovered as SIR2 (silent information regulator) in mouse [38], and thus far, seven sub-types of SIRTs (SIRT 1–7) have been identified in humans [29]. Sirtuins are implicated in a variety of cellular processes including gene silencing, cell cycle regulation, metabolism, apoptosis, life span extension, and the effects of calorie restriction, as well as circadian rhythms [39, 40, 41, 42, 43, 44, 45, 46, 47]. Each of the SIRT isoforms plays a unique role in the regulation of cellular processes through SIRT-mediated epigenetics. Accordingly, the SIRT isoforms vary in their cellular localization, with SIRTs 1, 2, 6, and 7 being primarily located in the nucleus and SIRTs 3, 4, and 5 located in mitochondria. While SIRT enzymes cleave the acetyl moiety from a lysine residue, much like their fellow HDAC counterparts, it has recently been recognized that the SIRT isoforms are capable of cleaving other chemical groups (i.e., succinylation, myristoylation, etc.) [48]. Furthermore, these enzymes act on other target proteins outside of histone core proteins including TNF-a, FOXO, p53, a-tubulin, and others [38] and thus act far outside the traditional scope of histone deacetylases.
The contributions of histone acetylation and deacetylation to the pathophysiology of psychiatric disorders are not well understood and understudied. Histone modifications regulate memory formation and fear memory processing. However, it has been extremely difficult to study these enzymes in humans due to their plasticity and lack of surrogate peripheral biomarker. Therefore, the gold standard for studying these enzymes is pathologic analysis of human brain tissue. Much of what is known about the role of HDACs in PTSD is based on animal studies of PTSD-like models and fear memory processing models. These pre-clinical data provide a starting point to understand the role that these enzymes may play in stress and PTSD.
For example, it is known that histone acetylation changes aid in forming strong fear conditioning in the early stages of fear memory consolidation processes; therefore, many pre-clinical studies have demonstrated improved fear responses through HDAC inhibition [49, 50]. Multiple HDAC inhibitors such as trichostatin A (TSA), tubastatin A, sodium butyrate (NaBu), suberoylanilide hydroxamic acid (SAHA/vorinostat), MS275, and others have been studied for possible treatments to decrease cued fear extinction and improve fear response. This work is supported by evidence that memory formation positively correlates with increased levels of H3 acetylation in CA1 and in lateral amygdala during times of fear conditioning [51]. Furthermore, common clinical psychiatric treatments of PTSD, such as selective serotonin reuptake inhibitors (SSRIs), also influence levels of histone acetylation within the hippocampus and amygdala [52]. HDACs are primary contributors to dynamic acetylation change during periods of memory formation and cued fear response. Therefore, investigation into the roles of each HDAC may help to promote further treatments for primary fear response and memory disorders, such as PTSD.
Herein, we will focus on HDACs within Classes I, II, and IV and their role within memory formation and fear extinction, as they are known to play a role in the pathology of PTSD as evidenced primarily from animal studies of PTSD models. We will discuss implications from inhibitor studies demonstrating significant influences of HDAC inhibition on memory and fear extinction and provide updates and expansion from a similar review in 2014 [12]. Last, we will provide a brief overview of the current state for epigenetic imaging in neuropsychiatric illness and why development in this area is so critical for PTSD.
HDAC Class I: HDACs 1, 2, 3, 8
Within HDAC Class I, the four isoforms have many similarities in their structure and function, reducing the within-class selectivity of HDAC inhibitors, particularly for HDACs 1–3. HDAC 1 was originally thought to deacetylate lysine residues on all four histone core proteins (H1–H4). However, it was later established that HDAC1 deacetylates H4K5, and K12 as well as H2aK5 [53] preferentially. HDAC2 deacetylates H4K12 and H3K9 particularly within the hippocampus [54], while HDAC3 deacetylates H2bK12, H3K14, H3K9, and H4K8, while also functioning as co-regulators to transcription factors such as CREB and P300.
HDAC1 functions in memory consolidation can be difficult to tease apart because few HDAC-1-specific blocking agents exist. Thus, we must rely on data obtained from blocking multiple HDACs. Overall, HDAC1 appears to be closely tied to H3 and H4 acetylation levels, whether through direct deacetylation or co-deacetylation with another HDAC Class I enzymes [55, 56]. It has been noted that levels of HDAC1 decrease following fear conditioning and increase again after extinction in normal animals [57]. Furthermore, HDAC1 and HDAC3 appear to co-regulate H3K9 acetylation and hippocampal Sox-2 expression changes, leading to astriogliogenesis in neuronal development [56].
HDAC2 is primarily expressed within glutamatergic neurons of the hippocampus and is activated during stress states [58]. It acts as a histone and protein deacetylase, regulating transcription factors, such as COX-2 and JNK [54, 57, 59]. HDAC2 is upregulated in periods of neuronal stress and is proposed to directly regulate cognitive and memory impairments following stress [58]. Therefore, HDAC2 selective inhibition (BRD6688 and BRD4884) can rescue memory deficits in impaired mice, primarily through increased H4K12 and H3K9 acetylation [54]. Interestingly, this inhibition has no effect on episodic memory. It is possible that the increased rate of fear extinction occurring after HDAC2 inhibition is due in part to increased COX-2 activation and phosphorylated JNK levels, rather than histone acetylation alone [59]. Data suggests that selective HDAC2 inhibition, particularly after periods of stress, improves memory and cued fear extinction without altering episodic memory consolidation [59].
HDAC3 deacetylates specific histone lysine amino acids to regulate key areas of the genome within the infralimbic cortex, hippocampus, and nucleus accumbens which may help regulate memory formation [60]. However, inhibition studies with a pan-HDAC Class I inhibitor demonstrated significant blunting of the cued fear response in traumatized animals, whereas inhibition of HDAC3 shows a significantly less robust response. Therefore, it is more likely that HDAC1 and 2 are mediating this response [61]. While HDAC3 inhibition does significantly impact levels of H4K8 acetylation [62], it does not correlate with large changes in fear extinction behaviors [61].
HDAC8 is a member of HDAC Class I, though its role within neuronal pathology is less clear than that of its counterparts. Furthermore, HDAC8 is disproportionately uninhibited by pan-HDAC class drugs such as SAHA [63], with the IC50 significantly higher for HDAC8 than HDACs 1, 2, 3, or even 6. Interestingly, HDAC6 (HDAC Class IIB) and HDAC8 share catalytic site activity with inhibitors often unable to differentiate between the two. This is further supported by different patterns of hyperacetylation seen when HDAC-specific inhibitors were used versus a pan-HDAC inhibitor, TSA [64]. Due to the similarity between HDAC8 and 6, several independently selective inhibitors of these isoforms have been developed, such as BRD73954 [65] and C4-benzyl SAHA [63] analogues. However there have yet to be many published studies using these inhibitors in models of neuropsychiatric disorders.
Unfortunately, it is not possible to differentiate the effects of individual HDAC isoforms in memory formation and fear learning based on many of the inhibitor results due to their nonspecificity. The following is an overview for the role of HDAC Class I in memory formation, as interpreted through analysis of studies using HDAC inhibitors. Increased levels of H3 and H4 acetylation have been identified in the hippocampus after treatment with HDAC Class I inhibitor (pre-clinical, Compound 60) [66]. This change causes increased levels of CREB and phosphorylated (activated) CREB to promote new gene transcription for cued fear extinction [50]. CREB itself is an important regulator of long-term potentiation and hippocampal-dependent learning, at least in part through HDAC-mediated pathways [50, 67]. Increased transcription through CREB promoter sites can promote fear memory extinction and decrease cued fear response [68]. Therefore, increased H3 and H4 acetylation levels are correlated with neuronal activation and decreased fear memory consolidation (promoting long-term extinction) when increased specifically in the amygdala [57, 67]. The converse also holds true with increased HDAC expression and downregulation of CREB promoting fear memory consolidation and decreased extinction, specifically with increased HDAC8 expression [12]. Additionally, genes involved in the cAMP-PKA-CREB pathway for long-term memory learning and encoding are upregulated via acetylation of histone tails at their key promoter sites [50]. These genes include NR4A1 and NR4A2, the nuclear receptor subunits, which are responsible for increasing expression of target genes including brain-derived neurotrophic factor production (BDNF) and tyrosine protein kinases, which are all necessary for long-term memory formation [69]. Therefore, inhibition of HDACs Class I may help promote CREB downstream effects, including cued fear extinction.
One of the primary downstream effects of CREB activation is NMDA receptor expression through its sub-classes NR2b transcription. One of the most robust cellular responses to stress, particularly within the hippocampus, includes increased glutamatergic transmission and NMDA receptor upregulation [70]. Typically, when a cell responds to stress activation, the NMDA receptor undergoes restructuring to increase expression and activation [71]. SAHA (pan-HDAC inhibitor) administration within the hippocampus leads to increased levels of activated CREB bound to the promoter site for NR2B, thus promoting transcription for the NMDA receptor genes [72] through HDAC inhibition. Overall, this suggests that increased histone acetylation is associated with increased NR2B levels within the hippocampus, thus promoting long-term extinction or reconsolidation [68, 72].
HDAC Class II
HDAC Class II, comprised of HDACs 4, 5, 7, 9 and 6, 10, plays a large role in the limbic system. In fact, many studies point to HDAC Class II as the most influential HDACs within the hippocampus and amygdala due to their prevalence and multifactorial roles [73]. Of these enzymes, HDAC4 and HDAC5 are most abundant within the hippocampus, PFC, and amygdala [74] and interact with transcription factors of MEF2, SRF, and CREB. HDAC4 is a potent deacetylase for H3K9, 14, 18, 23 and H4K5, 8, 12, 16 within the brain, and it is most well known for its contribution to acetylation levels of H4K5, 8, 12, 16 within the PFC during memory extinction and H3K9 and 14 within the hippocampus during memory reconsolidation [75]. Furthermore, HDAC4 and 5 were downregulated following stress induction in adolescent rats, with a specific protocol modeling adolescent stress in young adults [76]. This may indicate that these enzymes play a role in neurobiological changes occurring in early life stress − a strong predictor of future PTSD diagnosis in humans (need refs) [76].
HDACs 4 and 5 also influence BDNF and subsequent neuroplasticity. BDNF production is also a key component to memory regulation and fear extinction as it allows for neuronal growth and plasticity during these critical periods [73, 77]. This may indicate a link to the changes seen in HDAC expression following SSRI use. HDAC inhibition with valproic acid (VPA) (relatively nonselective HDAC Classes I and II inhibitor) enhances histone acetylation at the transcription site for BDNF to promote increased synaptic plasticity, thereby facilitating fear extinction learning [72]. When comparing four different inhibitors head to head to look at their in vitro effects on BDNF production, SAHA (Class I/IIb), MS275 (Class I only), and MC1568 (Class II only) and tubacin (HDAC6 inhibitor), it was found all upregulated BDNF production, but the temporal distribution and quantity of mRNA upregulation was not equivalent [73]. MS1568 produced the most robust early increase in BDNF transcription, while SAHA produced quantitatively the largest difference with MS275 producing a significantly smaller difference than MC1568 [73]. Lastly, HDAC6 inhibition produced the smallest change in mRNA transcription. Overall, this study concluded that the HDAC II inhibitors exhibited larger and faster effects on BDNF than Class I inhibitor, MS275 [73].
HDAC7 (HDAC Class IIA) is neuroprotective through inhibition of c-jun expression to avoid neuronal death [78], and therefore, inhibition of HDAC7 may be detrimental to neuron health. Within the hippocampus, HDAC7 plays a role in contextual fear learning through deacetylation of Nur77 protein and inhibition of contextual fear memory formation [78, 79]. As a result, increased ubiquitination and degradation of HDAC7 within the hippocampus are linked to long-term fear memory consolidation [79]. While selective HDAC7 inhibition effects have not been extensively studied, it is likely that inhibition of this enzyme contributes to results seen when using HDAC Class II inhibitors. However, it stands to reason that HDAC7 plays a smaller role than other Class II enzymes, as it is less abundant within the limbic system.
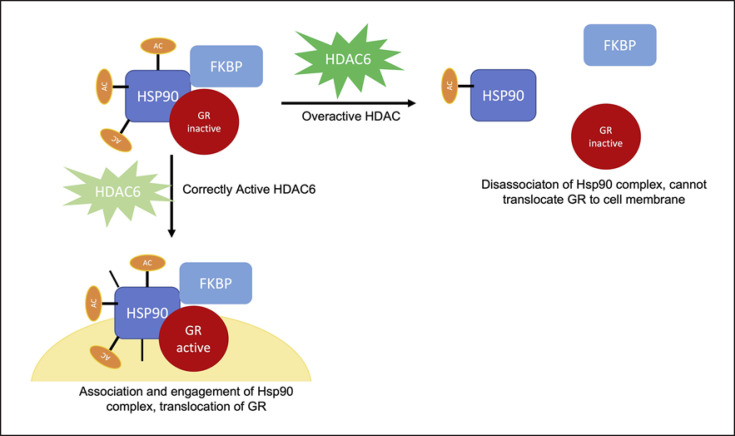
Within the human brain, HDAC6 (HDAC Class IIB) is largely expressed in the temporal cortex [80], amygdala, hippocampus, and PFC [81]. In the murine brain, the highest expression of HDAC6 is within serotonergic neurons, where genetic and molecular evidence demonstrates HDAC6 plays an important role in regulation of glucocorticoid receptor (GR)-heat shock protein 90 (Hsp90) [82, 83] interaction, thereby mediating the stress response within key areas of the brain (i.e., amygdala and hippocampus) [84, 85]. Overall, GR function has been closely linked to stress resilience in both humans [86] and animals [85], with HDAC6 implicated as a player in this pathway (Fig. 3) [84]. There is a well-described interplay between HDAC6 and Hsp90, whereby HDAC6 functions as a deacetylase for Hsp90. The acetylation of Hsp90 at k294 enables GR translocation from the nucleus after transcription [87]. HDAC6 then deacetylates Hsp90 to activate the chaperone complex for mobilization of GR. Therefore, overactivity or underactivity of HDAC6 leads to Hsp90 dysregulation and interferes with the assembly or activation of the GR chaperone complex (Fig. 3) [82]. Consequently, HDAC6 inhibition causes changes in GR expression which improves neuron excitability and serotonergic activity within the dorsal raphe neurons of the hippocampus [84]. As a result, the interplay of HDAC6 and the GR might contribute to the dysregulation of GR expression in stress responses such as in PTSD [88].
Fig. 3.
Visual representation of the complex formed with HDAC6 and Hsp90 to translocate the GR within the cell. When HDAC6 does not function appropriately, the GR complex is disabled.
In rodents, maternal licking of offspring causes increased levels of GR expression through a variety of epigenetic mechanisms, including histone acetylation, and results in greater nerve growth factor inducible protein A within the hippocampus. This change can be reversed, thus decreasing the nerve growth factor, through use of HDAC inhibitor TSA [89]. Furthermore, selective inhibition of HDAC6 using tubastatin A has also resulted in rescuing memory loss in rodents [90]. In animal studies, knockout of the HDAC6 gene prevented the expression of stress-related behaviors including avoidance of social and nonsocial anxiogenic stimuli [84, 91]. HDAC6 deletion does not interfere with normal mouse development, and it promotes an antidepressant response to chronic stress and PTSD-like models, such as social defeat [84]. Increased nuclear levels of HDAC3, HDAC6, and GR have been found in animals more susceptible to a depressive-like feature after social defeat and in fact are reversed with the use of HDAC inhibitor, SAHA [92]. Therefore, in animal stress models, HDAC6 may contribute to the expression of stress-related behavior and these stress responses are attenuated by HDAC6-selective inhibitors [93].
TSA is a pan-HDAC inhibitor, which has demonstrated efficacy in enhancing memory consolidation resulting from H4 hyperacetylation in the hippocampus and amygdala after administration [94]. TSA demonstrated an ability to enhance memory consolidation and increase BDNF within the hippocampus when injected into the basolateral amygdala [95] with concurrent effects on CRE-dependent transcription [96]. Additionally, this inhibitor has demonstrated anti-anxiolytic effects within animals pre-disposed to anxiety from lack of maternal care in the young through hippocampal-mediated effects [97]. However, this “pan-HDAC” inhibitor contains two enantiomers, R and S, where the S-enantiomer shows moderate specificity to HDAC6 [98]. Therefore, the effects of TSA may disproportionately represent HDAC6 inhibition over other HDAC Class I and II enzymes. While it is difficult to say how much of these effects can be attributed to inhibition of HDAC6, it is possible that mixed enantiomer TSA may be disproportionately affecting HDAC6 more than other Class I and II HDAC isoforms.
Class I and IIA Inhibitor Studies
NaBu, a short-chain fatty acid, inhibits Class I and II HDACs, with nanomolar potency [99]. In animal studies, NaBu mitigated the negative impact of single prolonged stress (SPS) on the spatial memory testing (i.e., Morris water maze) [100]. Similarly, NaBu produced neuroprotective effects in mice after cerebral ischemia event, suggesting that increased acetylation of H3K9 was neuroprotective [101]. Animals with increased H3 acetylation levels within the hippocampus have improved memory after ischemia or provocation of inflammation by administration of lipopolysaccharide [101]. While these studies indirectly support HDACs as key players in mediating memory reserves following neurologic insults, they indicate the importance of mediating acetylation levels to reduce neuronal cell death via apoptosis.
VPA targets Class I and Class IIa HDACs. Although it is often studied as a “pan-HDAC” inhibitor, it only reaches the catalytic site in HDAC1-3 and 7. VPA binds but does not catalytically inhibit HDAC4 and 8 [102]. Furthermore, VPA has no inhibitory action on HDAC5, 6, or 10 [103]. Therefore, the epigenetic effects of this inhibitor are likely occurring only through inhibition of HDAC Class I. Use of VPA further supports evidence of a significant role for HDAC Class I in memory reconsolidation and extinction within the PFC [104]. VPA may enhance fear memory consolidation and potentiate fear memories if used immediately prior to the first conditioned fear exposure [105]. However, when administered during fear extinction learning, within the PFC there is a decrease in HDAC Class I enzyme expression and may promote long-term fear extinction [57]. VPA also may reduce stress-related apathy in rodents, as measured in response to the novel object recognition test and sucrose consumption, possibly through an HDAC3-dependent mechanism [106]. Furthermore, a meta-analysis for four small clinical trials conducted using VPA to treat PTSD symptoms demonstrated decreased symptoms burden (i.e., hyperarousal, irritability, mood lability, improved mood) [2]. Given the small number of studies and relatively limited number of patients, it is difficult to say this is a robust effect but suggests that targeting epigenetic mechanisms may be useful in PTSD therapeutic intervention.
Other HDAC inhibitors were tested for their ability to alter behaviors and memory processes that are typically associated with PTSD and MDD. MS275 a selective Class I inhibitor with little HDAC Class II activity [107] demonstrates significantly improved ability to potentiate long-term memory formation within the hippocampus over pan-HDAC inhibitor such as TSA [108]. This may indicate that HDAC Class I is preferentially involved in LTP over HDAC Class II, like the findings in the PFC [108]. An HDAC Class I and II inhibitor, SAHA, also shows antidepressant-like effects in mice [92], coinciding with significant increases in H3 and H4 acetylation levels. However, these results are difficult to interpret mechanistically, as SAHA acts on many HDAC isoforms. Similarly, chronic acetate treatment lowers the levels of HDACs 2, 5, 7, and 8 while notably increasing the transcription level of HAT and P300. This combined with increased acetyl coenzyme A levels produced increased H3 and H4 global acetylation [109]. These changes produced antidepressant-like effects and improved synaptic plasticity within the hippocampus [109].
Neuropeptide-Y (NPY) is a peptide neurotransmitter highly regulated within the amygdala and contributes to stress resilience and response pathway in part through changes in histone acetylation [110]. Animals with alcohol use disorder and anxiety had increased levels of histone acetylation, particularly H3K9 and H4K8, in the amygdala and in concordance with NPY levels. These changes were correlated to the animal's anxiolytic response [111, 112]. However, animals with reduced NPY after trauma-associated traumatic brain injury also had lower H3K9 acetylation in arcuate nucleus and subsequent deacetylation (silencing) of the NPY promoter gene. This is hypothesized to be a primary cause of decreased NPY expression following brain trauma [113]. This may provide direct evidence for feeding changes seen after traumatic events through a histone deacetylation-driven mechanism. In humans, plasma NPY levels correlate positively with stress resilience and negatively with PTSD-like symptoms in a study of US soldiers [114]. Further, reductions in basal and yohimbine-stimulated plasma NPY levels are found in association with PTSD within clinical studies [115]. These effects may be due in part to modifications of histone acetylation. Based on these data, NPY, itself, is being studied as a potential treatment for PTSD and major depression [116, 117].
HDAC Class IV
HDAC Class IV is comprised of HDAC11, which has received relatively little study. Recent studies suggest that HDAC11 may play an important role in fatty acid deacetylase and cellular metabolism functions in the body and the brain [37]. HDAC11 is inhibited by SAHA (pan-HDAC inhibitor) and possibly MS275 (HDAC Class IIa inhibitor) albeit with less potency than other HDAC isoforms [118]. Therefore, reported findings using these HDAC inhibitors may demonstrate effects due to HDAC11 inhibition as well as other Class I and II HDACs [118]. HDAC11 is important to neuronal survival and has been associated with other markers linked to neuronal plasticity and hippocampal-dependent memory formation through NeuN-dependent mechanism in neuroprogenitor cells [119]. When studying memory enhancement using vagal nerve stimulation, there is an inverse relationship between HDAC11 with hippocampal memory formation [120]. Further study of HDAC11 may provide insight into these physiological mechanisms and the role that this enzyme plays in memory formation and dysregulation of fear response network.
Need for Imaging the Epigenetic-Related Stress Response in Living Humans
Currently, the study of in vivo neurochemical changes and neuronal plasticity following stressful events relies primarily on animal models and peripheral tissue analysis in patients. While multiple animal models resemble the sequelae of PTSD behaviorally and biochemically, there are limitations to these models. An extensive review and comparison between animal and human stress disorders demonstrate that most models reliably reproduce anxiety and depressive-like behaviors of PTSD, as well as hyperactive fear circuits and neuronal stress response, but it is more difficult to elucidate nuances of the disease including gender differences, though this is beginning to improve [121, 122]. One of the primary models, SPS in rats, is widely used for pre-clinical studies of stress-induced fear behaviors and fear extinction [123]. Specifically, the SPS model has been validated for its similarity to humans in the robust neuroendocrine response following stressful events [124] and in the hippocampal glutamate receptor response [125]. One limitation of SPS is that sex differences are difficult to measure as only males appear to elicit a stress response in this situation, which does not accurately reflect the human experience where females are twice as likely as males to develop PTSD after an equivalently traumatizing event [126].
An alternative mouse model of PTSD, chronic social defeat stress (CSDS), captures the GR and cortisol changes observed in PTSD [77]. The primary phenotypes include avoidance, anxiety, decreased grooming, hyperactivity, and susceptibility to addiction, all of which fit well within the DSM-5 diagnostic criteria for PTSD in humans [127]. CSDS has been proven to be an excellent PTSD model and displays a mixture of PTSD/MDD phenotype in many cases, which often reflects clinical symptoms as well [85, 86]. Similarly, chronic mild stress for mice has also been experimented with as a possible mechanism for inducing PTSD-like symptomatology; however, this stress appears to promote anhedonia, memory impairment, and appetite suppression [128]. Additionally, these symptoms were partially reversed through treatment with an antidepressant and thus the model is used primarily to study MDD rather than PTSD. CSDS produces a robust sympathetic nervous system response in mice, which can last for days to weeks from a single traumatic event, thereby mimicking much of the robust stress response that occurs in humans [129]. Although this model is also limited in its ability to elicit sex-specific differences, it is improving with wider use of female animals and alteration of the odorants and aggression models used for stress induction [130, 131]. These improvements have demonstrated an ability to achieve similar neuroendocrine effects and stress response behaviors in females to that of males [132].
The predator scent model has been used reliably with rats to produce PTSD symptomatology with robust reliability [133]. This model has also begun to explore the sex differences and has successfully reproduced the percentage of males versus females that go on to develop PTSD after stress, with about 30% of male rats and almost 50% of female rats developing PTSD symptoms [133]. This is different compared to the predator stress model where almost all stressed animals exhibit PTSD-like behaviors [134]. Multiple recent full reviews exist depicting the differences and similarities between animal PTSD models in both mice and rats, with benefits and drawbacks of each clearly laid out [134, 135] but both summarize the need for further work particularly in sex differentiation.
Animal models do relatively well at modeling stress response behaviors and have made great strides toward understanding physiologic and behavioral response to trauma and subsequent treatment. But the dynamic nature of PTSD and tremendous variation between phenotypes is difficult to replicate or account for in an animal model nor can coping styles and mechanisms be adequately captured. Future studies of PTSD phenotype in humans are needed to better understand the full course of stress-related pathology within the human brain. To do this, in vivo noninvasive imaging modalities are necessary. One possible modality for live human imaging of neurochemistry is the use of Positron emission tomography (PET) with radiotracers designed to target specific proteins within the human brain. Currently, multiple HDAC-targeted imaging agents have been developed for PET, with encouraging results, both clinically and pre-clinically.
To date, several PET epigenetic imaging studies have been conducted. The radiotracer [11C]Martinostat was developed to understand HDAC Class I expression [136], and studies have found higher levels of HDAC Class I expression in patients with bipolar disorder and schizophrenia as compared to controls [137, 138]. A promising new imaging agent for HDAC6 has also been developed and tested in humans, [18F]Bavarostat; though clinical studies have not yet been undertaken, this will be an exciting area of study for memory-related pathologies such as PTSD, Alzheimer's disease, and frontotemporal dementia. Additional HDAC selective radiotracers exist and have demonstrated promise pre-clinically in studying psychiatric and neurologic pathologies. Other 11C-labeled HDAC inhibitors (VPA and butyric acid) were tested in nonhuman primates but had minimal blood-brain barrier penetrance and thus were not fully effective [139]. In addition to inhibitor studies, radiotracers developed as ligands selective to HDAC Class IIa have been tested pre-clinically and in nonhuman primates, [18F]FAHA [140] and [18F]TFAHA [141] although thus far, they have only been used to demonstrate HDAC Class IIA activity in glioblastoma and gliosarcoma [142]. Along these lines, [18F]fluoroacetate has also been studied in the brain for imaging cerebral ischemia and demonstrated wide uptake among HDAC enzymes of all classes [143]. Though many of these imaging agents show promise to better decipher the role of HDACs within neuropsychiatric illness, few have been thoroughly studied and significantly more work is needed.
Understanding Epigenetic Response in Stress Disorders May Lead to the Development of Novel Therapeutics
Current treatment of stress and anxiety disorders involves cognitive behavioral therapy, prolonged exposure therapy, or cognitive processing therapy. These therapies are often combined with pharmacologic therapy. Additional pharmacologic agents are often used to control nightmares such as the alpha-1 antagonist, prazosin, but there are currently no effective pharmacologic agents to treat all symptoms of PTSD effectively. The first-line pharmacologic therapy is treatment with SSRIs that only have a 50–60% response rate [144]. Typically, these medications manage the symptoms of PTSD, but do not treat the disease. Developing agents which target histone modifications to “prime” memories for extinction is an exciting promise for drug development in psychiatric illness. Therefore, epigenetics offers a new target for pharmacologic intervention.
One possible innovation inferred from these studies is that pre-treatment with an HDAC modulator (i.e., inhibitor) prior to anticipated trauma or immediately following trauma could improve fear extinction. This method of “priming” epigenetic regulation with HDAC inhibitors may provide a better outcome with nonpharmacologic therapy as well. A relatively recent review discusses the idea that priming with HDAC inhibitors may prove useful in cognitive enhancement as well [12]. Research demonstrated pre-treatment with Class I HDAC inhibitors may improve memory reconsolidation and be most effective at selectively downregulating HDACs within the hippocampus and amygdala for increased H3 and H4 acetylation. SAHA demonstrated efficacy in this role, promoting remote fear attenuation when used prior to memory reconsolidation [145]. Though the effects may also be partially counterproductive as other studies demonstrated selective and potent inhibition of HDAC Class I with MS275 leading to potentiation of fear memory formation due to increased H4K8ac levels within the cortical hippocampal circuit [146]. VPA, although also nonselective for HDAC inhibition, produces different inhibitory levels than SAHA and has demonstrated possible utility as well, when used in adjunct with psychotherapy [57], thus demonstrating improved fear extinction during exposure-based cognitive behavioral therapy when co-treated with VPA administration [147]. With further investigation, alternative novel pharmacologic therapies might be possible, such as using an HDAC6 selective inhibitor prior to trauma exposure, if possible, to increase GR expression and promote stress resilience.
Conclusion
The literature supports a robust role for HDACs in stress pathology and neurobiology within the human and murine brain. HDACs Classes I and II are intricately involved in deacetylation of histones, transcription factors as well as other proteins, such as Hsp90, to regulate transcription of genes for memory consolidation and cued fear extinction. This largely pre-clinical body of work demonstrates exciting promise for better understanding the pathophysiology of stress-trauma disorders, but promise for novel therapeutics is cautionary. HDAC modulators offer great promise for novel targeted therapy but are not widely studied for safety in vivo and in many cases may have wide ranging effects that are not fully characterized. Better understanding the role of each individual class or isoform of HDACs would allow for more selective therapies to be used. Therefore, further study of individual HDAC isoforms, as discussed above, may provide treatment opportunities for PTSD either prior to a known stressful event or immediately following the event to decrease the cued fear response.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Department of Veterans Affairs, National Center for PTSD, Veteran Health Administration, VISN1 Career Development Award, NIH R01MH110674, and the Brain and Behavior Research Foundation. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs (VA) or the US government.
Author Contributions
R.E.B. − primary writer of manuscript and figures. M.G., J.H.K., and K.C. reviewed, edited, and contributed to writing of manuscript.
Funding Statement
This work was supported by the Department of Veterans Affairs, National Center for PTSD, Veteran Health Administration, VISN1 Career Development Award, NIH R01MH110674, and the Brain and Behavior Research Foundation. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs (VA) or the US government.
References
- 1.Snijders C, Maihofer AX, Ratanatharathorn A, Baker DG, Boks MP, Geuze E, et al. Longitudinal epigenome-wide association studies of three male military cohorts reveal multiple CpG sites associated with post-traumatic stress disorder. Clin Epigenetics. 2020;12((1)):11. doi: 10.1186/s13148-019-0798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamou M, Puchalska S, Plummer W, Hale AS. Valproate in the treatment of PTSD: systematic review and meta analysis. Curr Med Res Opin. 2007;23((6)):1285–1291. doi: 10.1185/030079907X188116. [DOI] [PubMed] [Google Scholar]
- 3.Davidson JR, Stein DJ, Shalev AY, Yehuda R. Posttraumatic stress disorder: acquisition, recognition, course, and treatment. J Neuropsychiatry Clin Neurosci. 2004;16((2)):135–147. doi: 10.1176/jnp.16.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Girgenti MJ, Wang J, Ji D, Cruz DA, Traumatic Stress Brain Research Group. Stein MB, et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci. 2021;24((1)):24–33. doi: 10.1038/s41593-020-00748-7. [DOI] [PubMed] [Google Scholar]
- 5.Olff M. Sex and gender differences in post-traumatic stress disorder: an update. Eur J Psychotraumatol. 2017;8((Suppl 4)):1351204. [Google Scholar]
- 6.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26((5)):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kida S. Reconsolidation/destabilization, extinction and forgetting of fear memory as therapeutic targets for PTSD. Psychopharmacology. 2019;236((1)):49–57. doi: 10.1007/s00213-018-5086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29((2)):402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duek O, Kelmendi B, Pietrzak RH, Harpaz-Rotem I. Augmenting the treatment of PTSD with ketamine: a review. Curr Treat Options Psychiatry. 2019;6((2)):143–153. [Google Scholar]
- 10.Blacker CJ, Frye MA, Morava E, Kozicz T, Veldic M. A review of epigenetics of PTSD in comorbid psychiatric conditions. Genes. 2019;10((2)):140. doi: 10.3390/genes10020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim GS, Smith AK, Nievergelt CM, Uddin M. Neuroepigenetics of post-traumatic stress disorder. Prog Mol Biol Transl Sci. 2018;158:227–253. doi: 10.1016/bs.pmbts.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittle N, Singewald N. HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: where do we stand? Biochem Soc Trans. 2014;42((2)):569–581. doi: 10.1042/BST20130233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agís-Balboa RC, Pinheiro PS, Rebola N, Kerimoglu C, Benito E, Gertig M, et al. Formin 2 links neuropsychiatric phenotypes at young age to an increased risk for dementia. Embo J. 2017;36((19)):2815–2828. doi: 10.15252/embj.201796821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daskalakis NP, Rijal CM, King C, Huckins LM, Ressler KJ. Recent genetics and epigenetics approaches to PTSD. Curr Psychiatry Rep. 2018;20((5)):30. doi: 10.1007/s11920-018-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howie H, Rijal CM, Ressler KJ. A review of epigenetic contributions to post-traumatic stress disorder. Dialogues Clin Neurosci. 2019;21((4)):417–428. doi: 10.31887/DCNS.2019.21.4/kressler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logue MW, Miller MW, Wolf EJ, Huber BR, Morrison FG, Zhou Z, et al. An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clin Epigenetics. 2020;12((1)):46. doi: 10.1186/s13148-020-0820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhoeven JE, Yang R, Wolkowitz OM, Bersani FS, Lindqvist D, Mellon SH, et al. Epigenetic age in male combat-exposed war veterans: associations with posttraumatic stress disorder status. Mol Neuropsychiatry. 2018;4((2)):90–99. doi: 10.1159/000491431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, et al. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry. 2016;80((5)):372–380. doi: 10.1016/j.biopsych.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Zovkic IB, Meadows JP, Kaas GA, Sweatt JD. Interindividual variability in stress susceptibility: a role for epigenetic mechanisms in PTSD. Front Psychiatry. 2013;4:60. doi: 10.3389/fpsyt.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korlach J, Turner SW. Going beyond five bases in DNA sequencing. Curr Opin Struct Biol. 2012;22((3)):251–261. doi: 10.1016/j.sbi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Khare T, Pai S, Koncevicius K, Pal M, Kriukiene E, Liutkeviciute Z, et al. 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat Struct Mol Biol. 2012;19((10)):1037–1043. doi: 10.1038/nsmb.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AK, Ratanatharathorn A, Maihofer AX, Naviaux RK, Aiello AE, Amstadter AB, et al. Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nat Commun. 2020;11((1)):1–9. doi: 10.1038/s41467-020-19615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giridharan VV, Thandavarayan RA, Fries GR, Walss-Bass C, Barichello T, Justice NJ, et al. Newer insights into the role of miRNA a tiny genetic tool in psychiatric disorders: focus on post-traumatic stress disorder. Transl Psychiatry. 2016;6((11)):e954. doi: 10.1038/tp.2016.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gapp K, van Steenwyk G, Germain PL, Matsushima W, Rudolph KLM, Manuella F, et al. Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Mol Psychiatry. 2020;25((9)):2162–2174. doi: 10.1038/s41380-018-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katrinli S, Stevens J, Wani AH, Lori A, Kilaru V, van Rooij SJH, et al. Evaluating the impact of trauma and PTSD on epigenetic prediction of lifespan and neural integrity. Neuropsychopharmacology. 2020;45((10)):1609–1616. doi: 10.1038/s41386-020-0700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf EJ, Logue MW, Stoop TB, Schichman SA, Stone A, Sadeh N, et al. Accelerated DNA methylation age: associations with posttraumatic stress disorder and mortality. Psychosom Med. 2018;80((1)):42–48. doi: 10.1097/PSY.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youssef NA, Lockwood L, Su S, Hao G, Rutten BPF. The effects of trauma, with or without PTSD, on the transgenerational DNA methylation alterations in human offsprings. Brain Sci. 2018;8((5)):83. doi: 10.3390/brainsci8050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64((2)):435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10((1)):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JH, Jung Y, Kim TY, Kim SG, Jong H-S, Lee JW, et al. Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Cancer Res. 2004;10((15)):5271–5281. doi: 10.1158/1078-0432.CCR-03-0709. [DOI] [PubMed] [Google Scholar]
- 31.de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdel A, Curtet S, Brocard MP, Rousseaux S, Lemercier C, Yoshida M, et al. Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr Biol. 2000;10((12)):747–749. doi: 10.1016/s0960-9822(00)00542-x. [DOI] [PubMed] [Google Scholar]
- 33.Yang XJ, Grégoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol. 2005;25((8)):2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bottomley MJ, Lo Surdo P, Di Giovine P, Cirillo A, Scarpelli R, Ferrigno F, et al. Structural and functional analysis of the human HDAC4 catalytic domain reveals a regulatory structural zinc-binding domain. J Biol Chem. 2008;283((39)):26694–704. doi: 10.1074/jbc.M803514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem. 2002;277((28)):25748–55. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- 36.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338((1)):17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Núñez-Álvarez Y, Suelves M. HDAC11: a multifaceted histone deacetylase with proficient fatty deacylase activity and its roles in physiological processes. FEBS J. 2021 doi: 10.1111/febs.15895. [DOI] [PubMed] [Google Scholar]
- 38.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404((1)):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM. Structure and biochemical functions of SIRT6. J Biol Chem. 2011;286((16)):14575–87. doi: 10.1074/jbc.M111.218990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3((3)):e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6((4)):298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 42.Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol. 2012;8((5)):287–296. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009;12((4)):431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- 44.Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123((3)):437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5((3)):344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci. 2013;33((41)):16088–98. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson D, Shao N, Heller E, Feng J, Neve R, Kim H-D, et al. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J Neurosci. 2015;35((7)):3100–3111. doi: 10.1523/JNEUROSCI.4012-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao YL, Yang WM. Beyond histone and deacetylase: an overview of cytoplasmic histone deacetylases and their nonhistone substrates. J Biomed Biotechnol. 2011;2011:146493. doi: 10.1155/2011/146493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279((39)):40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 50.Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27((23)):6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS One. 2011;6((5)):e19958. doi: 10.1371/journal.pone.0019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ookubo M, Kanai H, Aoki H, Yamada N. Antidepressants and mood stabilizers effects on histone deacetylase expression in C57BL/6 mice: brain region specific changes. J Psychiatr Res. 2013;47((9)):1204–1214. doi: 10.1016/j.jpsychires.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 53.Johnson CA, White DA, Lavender JS, O'Neill LP, Turner BM. Human class I histone deacetylase complexes show enhanced catalytic activity in the presence of ATP and co-immunoprecipitate with the ATP-dependent chaperone protein Hsp70. J Biol Chem. 2002;277((11)):9590–9597. doi: 10.1074/jbc.M107942200. [DOI] [PubMed] [Google Scholar]
- 54.Wagner FF, Zhang Y-L, Fass DM, Joseph N, Gale JP, Weïwer M, et al. Kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci. 2015;6((1)):804–815. doi: 10.1039/c4sc02130d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassig CA, Tong JK, Fleischer TC, Owa T, Grable PG, Ayer DE, et al. A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc Natl Acad Sci U S A. 1998;95((7)):3519–3524. doi: 10.1073/pnas.95.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Večeřa J, Bártová E, Krejčí J, Legartová S, Komůrková D, Rudá-Kučerová J, et al. HDAC1 and HDAC3 underlie dynamic H3K9 acetylation during embryonic neurogenesis and in schizophrenia-like animals. J Cell Physiol. 2018;233((1)):530–548. doi: 10.1002/jcp.25914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siddiqui SA, Singh S, Ugale R, Ranjan V, Kanojia R, Saha S, et al. Regulation of HDAC1 and HDAC2 during consolidation and extinction of fear memory. Brain Res Bull. 2019;150:86–101. doi: 10.1016/j.brainresbull.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Sun X-Y, Zheng T, Yang X, Liu L, Gao S-S, Xu H-B, et al. HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J Neuroinflammation. 2019;16((1)):249. doi: 10.1186/s12974-019-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM. Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci. 2013;33((15)):6401–6411. doi: 10.1523/JNEUROSCI.1001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McQuown SC, Wood MA. HDAC3 and the molecular brake pad hypothesis. Neurobiol Learn Mem. 2011;96((1)):27–34. doi: 10.1016/j.nlm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowers ME, Xia B, Carreiro S, Ressler KJ. The Class I HDAC inhibitor RGFP963 enhances consolidation of cued fear extinction. Learn Mem. 2015;22((4)):225–231. doi: 10.1101/lm.036699.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 2013;110((7)):2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Negmeldin AT, Knoff JR, Pflum MKH. The structural requirements of histone deacetylase inhibitors: C4-modified SAHA analogs display dual HDAC6/HDAC8 selectivity. Eur J Med Chem. 2018;143:1790–1806. doi: 10.1016/j.ejmech.2017.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krennhrubec K, Marshall BL, Hedglin M, Verdin E, Ulrich SM. Design and evaluation of “Linkerless” hydroxamic acids as selective HDAC8 inhibitors. Bioorg Med Chem Lett. 2007;17((10)):2874–2878. doi: 10.1016/j.bmcl.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 65.Olson DE, Wagner FF, Kaya T, Gale JP, Aidoud N, Davoine EL, et al. Discovery of the first histone deacetylase 6/8 dual inhibitors. J Med Chem. 2013;56((11)):4816–4820. doi: 10.1021/jm400390r. [DOI] [PubMed] [Google Scholar]
- 66.Schroeder FA, Lewis MC, Fass DM, Wagner FF, Zhang Y-L, Hennig KM, et al. A selective HDAC 1/2 inhibitor modulates chromatin and gene expression in brain and alters mouse behavior in two mood-related tests. PLoS One. 2013;8((8)):e71323. doi: 10.1371/journal.pone.0071323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12((11)):1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5((4)):348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 69.Hawk JD, Abel T. The role of NR4A transcription factors in memory formation. Brain Res Bull. 2011;85((1)):21–29. doi: 10.1016/j.brainresbull.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nisticò R, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci U S A. 2015;112((48)):14960–5. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol. 2005;484((1)):43–56. doi: 10.1002/cne.20445. [DOI] [PubMed] [Google Scholar]
- 72.Fujita Y, Morinobu S, Takei S, Fuchikami M, Matsumoto T, Yamamoto S, et al. Vorinostat, a histone deacetylase inhibitor, facilitates fear extinction and enhances expression of the hippocampal NR2B-containing NMDA receptor gene. J Psychiatr Res. 2012;46((5)):635–643. doi: 10.1016/j.jpsychires.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 73.Koppel I, Timmusk T. Differential regulation of Bdnf expression in cortical neurons by class-selective histone deacetylase inhibitors. Neuropharmacology. 2013;75:106–115. doi: 10.1016/j.neuropharm.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. Distribution of histone deacetylases 1-11 in the rat brain. J Mol Neurosci. 2006;31((31)):47–58. doi: 10.1007/BF02686117. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Qin G, Zhao TC. HDAC4: mechanism of regulation and biological functions. Epigenomics. 2014;6((1)):139–150. doi: 10.2217/epi.13.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaby LE, Sadik N, Burson NA, Lloyd S, O'Donnel K, Winters J, et al. Repeated stress exposure in mid-adolescence attenuates behavioral, noradrenergic, and epigenetic effects of trauma-like stress in early adult male rats. Sci Rep. 2020;10((1)):17935. doi: 10.1038/s41598-020-74481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erburu M, Muñoz-Cobo I, Domínguez-Andrés J, Beltran E, Suzuki T, Mai A, et al. Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur Neuropsychopharmacol. 2015 Nov;25((11)):2036–2048. doi: 10.1016/j.euroneuro.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Ma C, D'Mello SR. Neuroprotection by histone deacetylase-7 (HDAC7) occurs by inhibition of c-jun expression through a deacetylase-independent mechanism. J Biol Chem. 2011;286((6)):4819–4828. doi: 10.1074/jbc.M110.146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jing X, Sui W-H, Wang S, Xu X-F, Yuan R-R, Chen X-R, et al. HDAC7 ubiquitination by the E3 ligase CBX4 is involved in contextual fear conditioning memory formation. J Neurosci. 2017;37((14)):3848–3863. doi: 10.1523/JNEUROSCI.2773-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Odagiri S, Tanji K, Mori F, Miki Y, Kakita A, Takahashi H, et al. Brain expression level and activity of HDAC6 protein in neurodegenerative dementia. Biochem Biophys Res Commun. 2013;430((1)):394–399. doi: 10.1016/j.bbrc.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 81.De Carvalho LM, Wiers CE, Sun H, Wang G-J, Volkow ND. Increased transcription of TSPO, HDAC2, and HDAC6 in the amygdala of males with alcohol use disorder. Brain Behav. 2020:e01961. doi: 10.1002/brb3.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kovacs JJ, Murphy PJM, Gaillard S, Zhao X, Wu J-T, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18((5)):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 83.Govindan MV. Recruitment of cAMP-response element-binding protein and histone deacetylase has opposite effects on glucocorticoid receptor gene transcription. J Biol Chem. 2010;285((7)):4489–4510. doi: 10.1074/jbc.M109.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Espallergues J, Teegarden SL, Veerakumar A, Boulden J, Challis C, Jochems J, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci. 2012;32((13)):4400–4416. doi: 10.1523/JNEUROSCI.5634-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jochems J, Teegarden SL, Chen Y, Boulden J, Challis C, Ben-Dor GA, et al. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol Psychiatry. 2015;77((4)):345–355. doi: 10.1016/j.biopsych.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McClung CA. Glucocorticoid receptor function and resilience: a tale of mice and men. Biol Psychiatry. 2015;77((4)):310–311. doi: 10.1016/j.biopsych.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 87.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25((1)):151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szeszko PR, Lehrner A, Yehuda R. Glucocorticoids and hippocampal structure and function in PTSD. Harv Rev Psychiatry. 2018;26((3)):142–157. doi: 10.1097/HRP.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 89.Weaver IC. Epigenetic effects of glucocorticoids. Semin Fetal Neonatal Med. 2009;14((3)):143–150. doi: 10.1016/j.siny.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Selenica ML, Benner L, Housley SB, Manchec B, Lee DC, Nash KR, et al. Histone deacetylase 6 inhibition improves memory and reduces total tau levels in a mouse model of tau deposition. Alzheimers Res Ther. 2014;6((1)):12. doi: 10.1186/alzrt241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukada M, Hanai A, Nakayama A, Suzuki T, Miyata N, Rodriguiz RM, et al. Loss of deacetylation activity of Hdac6 affects emotional behavior in mice. PLoS One. 2012;7((2)):e30924. doi: 10.1371/journal.pone.0030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kv A, Madhana RM, Js IC, Lahkar M, Sinha S, Naidu VGM. Antidepressant activity of vorinostat is associated with amelioration of oxidative stress and inflammation in a corticosterone-induced chronic stress model in mice. Behav Brain Res. 2018;344:73–84. doi: 10.1016/j.bbr.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, et al. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014;39((2)):389–400. doi: 10.1038/npp.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vigushin DM, Ali S, Pace PE, Mirsaidi N, Ito K, Adcock I, et al. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin Cancer Res. 2001;7((4)):971–976. [PubMed] [Google Scholar]
- 95.Valiati FE, Vasconcelos M, Lichtenfels M, Petry FS, de Almeida RMM, Schwartsmann GS, et al. Administration of a histone deacetylase inhibitor into the basolateral amygdala enhances memory consolidation, delays extinction, and increases hippocampal BDNF levels. Front Pharmacol. 2017;8:415. doi: 10.3389/fphar.2017.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fass DM, Butler JE, Goodman RH. Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J Biol Chem. 2003;278((44)):43014–9. doi: 10.1074/jbc.M305905200. [DOI] [PubMed] [Google Scholar]
- 97.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103((9)):3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyake Y, Keusch JJ, Wang L, Saito M, Hess D, Wang X, et al. Structural insights into HDAC6 tubulin deacetylation and its selective inhibition. Nat Chem Biol. 2016;12((9)):748–754. doi: 10.1038/nchembio.2140. [DOI] [PubMed] [Google Scholar]
- 99.Heyward FD, Gilliam D, Coleman MA, Gavin CF, Wang J, Kaas G, et al. Obesity weighs down memory through a mechanism involving the neuroepigenetic dysregulation of Sirt1. J Neurosci. 2016;36((4)):1324–1335. doi: 10.1523/JNEUROSCI.1934-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohammadi-Farani A, Pourmotabbed A, Ardeshirizadeh Y. Effects of HDAC inhibitors on spatial memory and memory extinction in SPS-induced PTSD rats. Res Pharm Sci. 2020;15((3)):241–248. doi: 10.4103/1735-5362.288426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol. 2017;54((8)):6391–6411. doi: 10.1007/s12035-016-0149-z. [DOI] [PubMed] [Google Scholar]
- 102.Sixto-Lopez Y, Bello M, Correa-Basurto J. Exploring the inhibitory activity of valproic acid against the HDAC family using an MMGBSA approach. J Comput Aided Mol Des. 2020 doi: 10.1007/s10822-020-00304-2. [DOI] [PubMed] [Google Scholar]
- 103.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64((3)):1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 104.Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14((4)):268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15((1)):39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goudarzi M, Nahavandi A, Mehrabi S, Eslami M, Shahbazi A, Barati M. Valproic acid administration exerts protective effects against stress-related anhedonia in rats. J Chem Neuroanat. 2020;105:101768. doi: 10.1016/j.jchemneu.2020.101768. [DOI] [PubMed] [Google Scholar]
- 107.Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409((2)):581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 108.Hawk JD, Florian C, Abel T. Post-training intrahippocampal inhibition of class I histone deacetylases enhances long-term object-location memory. Learn Mem. 2011;18((6)):367–370. doi: 10.1101/lm.2097411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang W, Hu W, Cai L, Zeng G, Fang W, Dai X, et al. Acetate supplementation produces antidepressant-like effect via enhanced histone acetylation. J Affect Disord. 2021;281:51–60. doi: 10.1016/j.jad.2020.11.121. [DOI] [PubMed] [Google Scholar]
- 110.Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, et al. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol. 2014;17((8)):1207–1220. doi: 10.1017/S1461145714000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palmisano M, Pandey SC. Epigenetic mechanisms of alcoholism and stress-related disorders. Alcohol. 2017;60:7–18. doi: 10.1016/j.alcohol.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36((1)):61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balasubramanian N, Sagarkar S, Jadhav M, Shahi N, Sirmaur R, Sakharkar AJ. Role for histone deacetylation in traumatic brain injury-induced deficits in neuropeptide Y in arcuate nucleus: possible implications in feeding behavior. Neuroendocrinology. 2020 doi: 10.1159/000513638. [DOI] [PubMed] [Google Scholar]
- 114.Reichmann F, Holzer P, Neuropeptide Y. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99–109. doi: 10.1016/j.npep.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47((6)):526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 116.Mathé AA, Michaneck M, Berg E, Charney DS, Murrough JW. A randomized controlled trial of intranasal neuropeptide Y in patients with major depressive disorder. Int J Neuropsychopharmacol. 2020;23((12)):783–790. doi: 10.1093/ijnp/pyaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164–169. doi: 10.1016/j.neulet.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 118.Tian Y, Lv W, Li X, Wang C, Wang D, Wang PG, et al. Stabilizing HDAC11 with SAHA to assay slow-binding benzamide inhibitors. Bioorg Med Chem Lett. 2017;27((13)):2943–2945. doi: 10.1016/j.bmcl.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 119.Yu P, McKinney EC, Kandasamy MM, Albert AL, Meagher RB. Characterization of brain cell nuclei with decondensed chromatin. Dev Neurobiol. 2015;75((7)):738–756. doi: 10.1002/dneu.22245. [DOI] [PubMed] [Google Scholar]
- 120.Sanders TH, Weiss J, Hogewood L, Chen L, Paton C, McMahan RL, et al. Cognition-enhancing vagus nerve stimulation alters the epigenetic landscape. J Neurosci. 2019;39((18)):3454–3469. doi: 10.1523/JNEUROSCI.2407-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bangasser DA, Cuarenta A. Sex differences in anxiety and depression: circuits and mechanisms. Nat Rev Neurosci. 2021;22((11)):674–684. doi: 10.1038/s41583-021-00513-0. [DOI] [PubMed] [Google Scholar]
- 123.Lisieski MJ, Eagle AL, Conti AC, Liberzon I, Perrine SA. Single-prolonged stress: a review of two decades of progress in a rodent model of post-traumatic stress disorder. Front Psychiatry. 2018;9:196. doi: 10.3389/fpsyt.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett. 2010;480((1)):16–20. doi: 10.1016/j.neulet.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eagle AL, Knox D, Roberts MM, Mulo K, Liberzon I, Galloway MP, et al. Single prolonged stress enhances hippocampal glucocorticoid receptor and phosphorylated protein kinase B levels. Neurosci Res. 2013;75((2)):130–137. doi: 10.1016/j.neures.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keller SM, Schreiber WB, Staib JM, Knox D. Sex differences in the single prolonged stress model. Behav Brain Res. 2015;286:29–32. doi: 10.1016/j.bbr.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131((2)):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 128.Elizalde N, Gil-Bea FJ, Ramírez MJ, Aisa B, Lasheras B, Rio JD, et al. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology. 2008;199((1)):1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- 129.Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- 130.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology. 2018;43((6)):1276–1283. doi: 10.1038/npp.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Newman EL, Covington HE, 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF, et al. Fighting females: neural and behavioral consequences of social defeat stress in female mice. Biol Psychiatry. 2019;86((9)):657–668. doi: 10.1016/j.biopsych.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Doeselaar L, Yang H, Bordes J, Brix L, Engelhardt C, Tang F, et al. Chronic social defeat stress in female mice leads to sex-specific behavioral and neuroendocrine effects. Stress. 2021;24((2)):168–180. doi: 10.1080/10253890.2020.1864319. [DOI] [PubMed] [Google Scholar]
- 133.Albrechet-Souza L, Gilpin NW. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav Pharmacol. 2019;30((2 and 3-Spec Issue)):105–114. doi: 10.1097/FBP.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Verbitsky A, Dopfel D, Zhang N. Rodent models of post-traumatic stress disorder: behavioral assessment. Transl Psychiatry. 2020;10((1)):132. doi: 10.1038/s41398-020-0806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richter-Levin G, Stork O, Schmidt MV. Animal models of PTSD: a challenge to be met. Mol Psychiatry. 2019;24((8)):1135–1156. doi: 10.1038/s41380-018-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wey HY, Gilbert TM, Zürcher NR, She A, Bhanot A, Taillon BD, et al. Insights into neuroepigenetics through human histone deacetylase PET imaging. Sci Transl Med. 2016;8((351)):351ra106. doi: 10.1126/scitranslmed.aaf7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilbert TM, Zürcher NR, Wu CJ, Bhanot A, Hightower BG, Kim M, et al. PET neuroimaging reveals histone deacetylase dysregulation in schizophrenia. J Clin Invest. 2019;129((1)):364–372. doi: 10.1172/JCI123743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tseng CJ, Gilbert TM, Catanese MC, Hightower BG, Peters AT, Parmar AJ, et al. In vivo human brain expression of histone deacetylases in bipolar disorder. Transl Psychiatry. 2020;10((1)):224. doi: 10.1038/s41398-020-00911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim SW, Hooker JM, Otto N, Win K, Muench L, Shea C, et al. Whole-body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4-phenylbutyric acid measured with carbon-11 labeled analogs by PET. Nucl Med Biol. 2013;40((7)):912–918. doi: 10.1016/j.nucmedbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yeh H-H, Tian M, Hinz R, Young D, Shavrin A, Mukhapadhyay U, et al. Imaging epigenetic regulation by histone deacetylases in the brain using PET/MRI with 18F-FAHA. NeuroImage. 2013;64((0)):630–639. doi: 10.1016/j.neuroimage.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bonomi R, Mukhopadhyay U, Shavrin A, Yeh H-H, Majhi A, Dewage SW, et al. Novel histone deacetylase class IIa selective substrate radiotracers for PET imaging of epigenetic regulation in the brain. PLoS One. 2015;10((8)):e0133512. doi: 10.1371/journal.pone.0133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laws MT, Bonomi RE, Kamal S, Gelovani DJ, Llaniguez J, Potukutchi S, et al. Molecular imaging HDACs class IIa expression-activity and pharmacologic inhibition in intracerebral glioma models in rats using PET/CT/(MRI) with [18 F] TFAHA. Sci Rep. 2019;9((1)):1–11. doi: 10.1038/s41598-019-40054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ouyang Y, Tinianow JN, Cherry SR, Marik J. Evaluation of 2-[18F]fluoroacetate kinetics in rodent models of cerebral hypoxia-ischemia. J Cereb Blood Flow Metab. 2014;34((5)):836–844. doi: 10.1038/jcbfm.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schoenfeld FB, Marmar CR, Neylan TC. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr Serv. 2004;55((5)):519–531. doi: 10.1176/appi.ps.55.5.519. [DOI] [PubMed] [Google Scholar]
- 145.Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156((1–2)):261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29((37)):11451–60. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kuriyama K, Honma M, Soshi T, Fujii T, Kim Y. Effect of D-cycloserine and valproic acid on the extinction of reinstated fear-conditioned responses and habituation of fear conditioning in healthy humans: a randomized controlled trial. Psychopharmacology. 2011;218((3)):589–597. doi: 10.1007/s00213-011-2353-x. [DOI] [PubMed] [Google Scholar]