Abstract
Background:
Despite recent therapeutic advances, osteoarthritis continues to be a challenging health problem, especially in the elderly population. Opioids, potent analgesics, have shown extraordinary ability to reduce intense pain in many osteoarthritic clinical trials, but there is an increased need for a study to integrate the reported outcomes and utilize them to achieve a better understanding. Herein, efficacy and safety aspects of opioids used to manage osteoarthritic pain were assessed and compared using a model-based meta-analysis (MBMA).
Methods:
To perform the analysis, a comprehensive database consisting of pain relief compounds with information on summary-level of efficacy over time, adverse events and dropout rates was compiled from multiple sources. MBMA was conducted using a nonlinear mixed-effects modeling approach.
Results:
The results of primary efficacy endpoint analysis indicated that the dose producing 50% of the maximum effect of oxycodone, oxymorphone, and tramadol were 47, 84, and 247 mg per day, respectively. Efficacy-time course analysis showed that opioids had rapid time to efficacy onset, suggesting potential powerful pain relief effects. It was also found that gastrointestinal adverse events were the most opioid-associated and dose-dependent adverse effects. In addition, the analysis revealed that opioids are well-tolerable at low to moderate doses.
Conclusions:
MBMA provides clinically meaningful insights into the efficacy and safety profiles of oxycodone, oxymorphone, and tramadol. Resultantly, the presented framework analysis has a clinical impact on drug development where it can help in optimizing the dose of opioids to manage osteoarthritic pain, making precise key decisions for positioning of new drugs, and designing more efficient trials.
Keywords: Opioids, Osteoarthritis, Model-based Meta-analysis, Efficacy, Efficacy-time course, Safety, Tolerability
Graphical Abstract
1. INTRODUCTION
Osteoarthritis (OA) is a common degenerative disorder that affects articular cartilages and underlying bones, particularly weight-bearing joints such as knee, hip, and spine. This ultimately leads to joint signs and symptoms such as pain and swelling 1-2. OA is the leading cause of disability among adults 3, and it represents one of the most prevalent form of chronic musculoskeletal pain disorders in the United State and worldwide 4. Approximately, 50% of the worldwide population aged 65 years or older are experiencing OA 1. In the US alone, chronic diseases such as back pain and arthritis (OA and rheumatoid) cost more than $200 billion annually 5. The incidence of OA is estimated to be tripled over the coming decades. It is expected that by the year 2030, around 70 million Americans would be at risk of OA 6. Pain is the primary and preponderant symptom of OA. In addition to the pain, patients who are experiencing OA usually suffer from morning stiffness, swelling, reduced range of motion and decrease in the physical activity, and OA can result in disability 2.
Acetaminophen (paracetamol), cyclooxygenase-2 (COX-2) inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), and opioids are the most commonly used pain relievers for the management of chronic OA pain 3. Although acetaminophen, COX-2 inhibitors, and NSAIDs have been frequently used, they are usually associated with several unwanted drawbacks such as cardiovascular effects, hepatic and renal toxicities especially in elderly people 7. It was reported that NSAIDs alone were responsible for 29.6% of hospitalizations due to adverse drug effects such as gastrointestinal bleeding, peptic ulcer, hemorrhagic cerebrovascular stroke, kidney failure, wheezing, and rash 8. Therefore, the potential adverse events (AEs) may limit their applicability. In addition to the serious potential adverse effects, these compounds may fail to accomplish adequate pain management in advanced OA and, in turn, patients continue to experience the pain and function disability 9. Therefore, more potent analgesic compounds, such as opioids, are required in situations involving sever pain such as postoperative pain. According to the American Pain Society guidelines, opioids are analgesic compounds that have been recommended to be used as effective and safe therapeutic agents for the management of moderate to severe chronic OA pain when other medications fail to achieve effective pain relief 10. Also, opioids are among the pain management medications for osteoarthritis according to the Arthritis Foundation 11. In addition, the Centers for Disease Control and Preventions published guidelines for opioids for chronic pain based on information obtained from observational studies and expert opinions (i.e., not based on meta-analysis). The report has a list of 12 recommendations about the benefits and risks of opioids for chronic pain 12. Over the past 20 years, opioid prescriptions become more frequently due to the prevalence of pain-associated chronic diseases 13. However, opioids are also associated with some complications that are related to their AEs and/or the abuse 14.
Several clinical trials reported the efficacy and safety of opioids for the management of OA pain. However, there are very few studies documenting the assessment and utilization of efficacy and safety data to support clinical decision-making. To date, only one published study has compared the efficacy and tolerability of two opioids (tramadol and tapentadol) for the treatment of several chronic non-malignant pain conditions (such as OA pain, back pain, neuropathic pain, and other chronic nonmalignant pain) 15. The lack of data from direct comparisons between opioid compounds for OA results in an increased demand for indirect comparisons through leveraging the data. Herein, summary level data collected from multiple sources were integrated and analyzed using a model-based meta-analysis framework, and our primary focus was to compare the efficacy, efficacy-time course, safety, and tolerability of the opioid compounds used in the treatment of OA pain. Meta-analysis is a well-established statistical practice that allows collecting and combining the results from several individual clinical studies for integrating findings 16. An example of meta-analysis is a model-based meta-analysis (MBMA) which is an emerging approach that becomes increasingly important in the field of drug development. Generally, MBMA employs pharmacologic principles such as treatment effects, dose, time, and patient population characteristics on the outcomes. Also, MBMA is widely used to compare treatments that have never been studied together in one clinical trial 17. Other advantages of MBMA include: (i) optimizing dose selection through characterization of dose-response and efficacy-time course relationships, (ii) predicting the response for doses that have not been clinically studied, (iii) bridging data across studies to optimize clinical trials for future studies, (iv) comparing the new treatments and other emerging drugs with existing compounds, (v) making clinical and/or marketing decisions based on efficacy and safety profiles, and (vi) mitigating the heterogeneity in the management effect due to combining data from multiple clinical trials and accounting for trial-to-trial covariates (random and fixed effects) 17. The purpose of this MBMA on opioids is to combine and reanalyze multiple trials to (a) investigate the opioids dose-response relationship at specific endpoint of OA clinical trials, (b) assess the efficacy-time course relationship for opioids that have been used in the management of OA pain, (c) evaluate the safety of opioids in patients with OA pain and (d) determine the tolerability of opioid analgesia for OA pain management.
2. METHODS
2.1. Literature Search and Database Construction
A systematic review of publicly available data was conducted, and a comprehensive database of therapeutic compounds approved for the management of chronic OA pain was collected and combined according to a prespecified database-building protocol and based on the relevant identification, screening, and assessment steps described in the Cochrane Handbook for Systematic Review of Interventions and reporting items in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Figure S1, Supplementary Material). The search used the following keywords: ‘osteoarthritis’ and ‘randomized controlled trial’ (publication type) and ‘English’ (language). Summary-level of efficacy over time, AEs and dropouts (DOs) longitudinal data were compiled from publicly available randomized, controlled clinical trials, literature, United States Food and Drug Administration (FDA), company websites, PubMed and clinicalstudyresults.org for unpublished clinical trials. The database included clinical trials up to 2015. Majority of the clinical trials were double-blinded and placebo (PLC) controlled. The compiled database included a total of 33 compounds belong to several pharmacological classes that approved for the management of OA pain. Particularly, the database had 11 NSAIDs, 8 opioids, 6 COX-2 inhibitors, 2 serotoninnorepinephrine reuptake inhibitor (SNRI), and 6 miscellaneous compounds: acetaminophen (aniline analgesic), tanezumab (anti-nerve growth factor), hyaluronate (glycosaminoglycan), willow bark extract (herbal remedy), glucosamine and chondroitin (OA supplements). The database also comprised of approximately 150 clinical trials, 500 arms, and 121,000 patients. In addition, all trials involved patients who were only diagnosed with moderate to severe OA. Moreover, the clinical outcomes in the database consisted of various primary efficacy endpoints such as Western Ontario and McMaster Universities OA index (WOMAC), pain intensity, and patient/physician global assessment of disease status.
2.2. Data Selection Criteria for Primary Efficacy Endpoints and Efficacy-Time Course Analyses
To evaluate the efficacy of competing compounds and ensure the validity of our estimates, trials that have been designed with similar criteria were selected. To explain, data filtration steps have been applied to the raw database and final dataset was created to perform MBMA. Since the aim of this study was to compare the opioids, the first inclusion criterion was to select opioid trials only. In addition, only clinical trials that their primary focus was efficacy were chosen. This was followed by selecting WOMAC as an endpoint. WOMAC generally measures pain, stiffness, and physical function, but only mean change in WOMAC pain-subscale was chosen as an endpoint to evaluate the efficacy of opioids. To account for the differences in the patients WOMAC pain-subscale scores at the beginning of each clinical study, the mean change in WOMAC pain score was considered and it was derived from the difference between change from final mean values (pain values after giving the medication) and baseline (BL) mean values (pain values at the start of trials, i.e., before giving the medication). The reason for selecting WOMAC as a primary endpoint is because the fact that it is originally designed to assess patients with OA, and has been widely reported in the clinical trials 18. All WOMAC data were normalized on a scale ranging from 0 to 100 (where 0 = no pain while 100 = worst pain). In case of missing data, the last observation carried forward was set as an imputation. In addition, only primary clinical trial endpoints were selected for this analysis. Finally, only compounds that at least have two trials were selected to successfully determine the brunt of covariance effects. Similarly, the filtration steps were applied to the raw database to create a dataset for efficacy-time course analysis. The only difference was that not only the primary endpoints but also data points at other time points (i.e., efficacy over a course of time) were included in the final dataset.
2.3. Data Selection Criteria for Safety and Tolerability Analyses
Two filtration steps were applied to the raw database to generate a dataset for safety analysis. First, only opioid compounds evaluated for their primary efficacy endpoint and efficacy-time course (i.e., compounds were chosen after filtration steps) were advanced for further assessment in terms of their safety and tolerability. Second, AEs were selected as the endpoint and the analysis was performed based on the number of patients experiencing the event (at least once) during the treatment course. The most common reported AEs in the clinical trials were classified by the body’s systems (Table 1). It is of note that only high incident AEs associated with opioids for each system were selected and analyzed. The dataset for tolerability analysis was similarly created by applying the above-mentioned filtration steps, but with one exception that the number of DOs was chosen as an endpoint. The analysis was carried out using the number of patients who failed to complete the clinical trial (i.e., dropout rate). The reasons underlying DOs can be broadly categorized into (i) DOs due to AEs, and (ii) DOs due to lack of efficacy (LoE). Also, in some clinical trials, investigators reported the total dropouts (TDOs), which refers to DOs due to any reason (i.e., DOs due AEs, DOs due to LoE, and DOs due to other reasons such as protocol violation, patient or investigator decision, noncompliance with study medication, and lost to follow-up). Since the total dropout included reasons that may not be related to the drug itself (i.e., nonspecific), we primarily focused on the two main reasons (i.e., due to AEs and LoE) which represent the most commonly reported causes of DOs in the clinical trials. A summary of the DOs reasons with their DOs rates as reported in the clinical trials is provided in the Table S1, Supplementary Material.
Table 1.
Summary of the most common AEs reported in the clinical trials.
| AEs | PLC Ntotal, NAE (%) |
Oxycodone Ntotal, NAE (%) |
Oxymorphone Ntotal, NAE (%) |
Tramadol Ntotal, NAE (%) |
|---|---|---|---|---|
| Digestive system | ||||
| • Nausea | 2505, 206 (8.22) | 1566, 518 (33.1) | 674, 277 (41.1) | 3175, 642 (20.2) |
| • Constipation | 2168, 104 (4.80) | 1224, 332 (27.1) | 674, 158 (23.2) | 3175, 508 (16.0) |
| • Vomiting | 2291, 47 (2.05) | 1566, 267 (17.1) | 674, 150 (22.3) | 2743, 219 (7.98) |
| • Diarrhea | 1464, 67 (4.58) | 1055, 69 (6.54) | 432, 11 (2.55) | 1853, 105 (5.67) |
| Central nervous system | ||||
| • Dizziness | 2505, 129 (5.15) | 1441, 330 (22.9) | 674, 144 (21.3) | 3175, 522 (16.4) |
| • Somnolence | 2505, 89 (3.55) | 1566, 305 (19.5) | 674, 129 (19.1) | 3175, 326 (10.3) |
| • Headache | 2101, 221 (10.5) | 1495, 190 (12.7) | 674, 63 (9.35) | 2590, 256 (9.88) |
| • Insomnia | 786, 22 (2.80) | 310, 21 (6.77) | no reported data. | 1740, 131 (7.53) |
| Integumentary system | ||||
| • Pruritus | 1791, 30 (1.68) | 1497, 199 (13.3) | 674, 102 (15.1) | 2417, 167 (6.91) |
| • Hyperhidrosis | 869, 6 (0.69) | 366, 23 (6.28) | 279, 21 (7.53) | 1331,60 (4.51) |
| Respiratory system | ||||
| • Xerostomia | 1396, 30 (2.15) | 1023, 103 (10.1) | 521, 52 (9.98) | 1870, 124 (6.63) |
| • Hypoventilation | 125, 0 (0) | 125, 0 (0) | 242, 0 (0) | no reported data. |
| Locomotor system | ||||
| • Fatigue | 1354, 32 (2.36) | 1156, 92 (7.96) | 432, 21 (4.86) | 1573, 86 (5.47) |
| • Asthenia | 271, 3 (1.11) | 310, 11 (3.56) | no reported data. | 211, 10 (4.88) |
Ntotal: the total number of patients involved in the clinical trials.
NAE: the total number of patient experienced AEs.
%: percentage of patients with AEs.
2.4. Model Development and Data Analysis
MBMA was conducted using nonlinear mixed-effects modeling (NONMEM® 7.3) interfaced with PDx-Pop®5.2 software. For primary efficacy endpoint and efficacy-time course analyses, a simple pharmacodynamic model (Emax model) using first-order conditional estimation method was employed (time variable was introduced to the general Emax model for time course analysis). Emax model is a nonlinear model frequently used in dose-response analyses, and it is a typical representation of MBMA 19. This model is beneficial when there are multiple observations per arm as it considers the variability of the within-arm responses. For safety and tolerability analyses, the number of patients experiencing the AEs assumed to follow a binomial distribution with a probability (P) since the final dataset had only binary outcomes (i.e., the patients simply had or had not AEs). The probability of patients having AEs was modeled using logistic regression model, and the log-likelihood function of the binomial distribution with twice the differences in the log-likelihoods (i.e., −2LL) using second-order conditional (Laplacian) estimation method was employed to express how likely the patients experience AEs. Several hierarchical models were tested starting with the simplest model and gradually moved to more complicated models through incorporating new individual relevant parameters such as placebo effect, between-arm and within-arm variability to capture the observed data appropriately.
2.5. Model Evaluation Criteria
The improvement in the model performance was evaluated using several criteria including statistical and graphical methods. Since hierarchical models were compared, log-likelihood ratio test was primarily used where the drop in the objective function (OF) value (obtained from NONMEM output) by 3.84 (for adding one parameter, i.e., one degree of freedom) or 5.99 (for adding two parameters, i.e., two degrees of freedom) were considered statistically significant difference at α = 0.05. Maximum likelihood estimation is a widely used approach because it avoids the need for data weighting, and the minimum value of the OF value for a particular model and data set is associated with the “best fit” parameter values. In addition to the log-likelihood ratio test, a graphical analysis was performed using visual goodness-of-fit plots including scatter plots of conditional weighted residuals vs population predicted values/time to check for model accuracy. Precise and plausible parameters estimates were also considered to judge model validity.
3. RESULTS
3.1. Primary Efficacy Endpoint Analysis
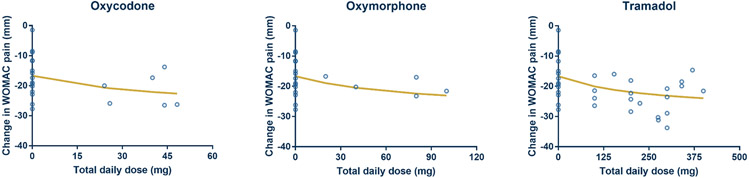
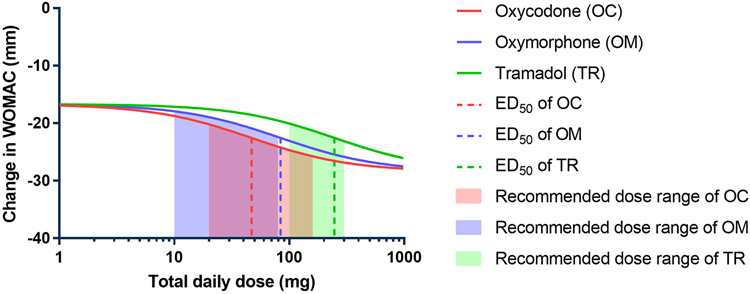
The final dataset for primary efficacy endpoint analysis consisted of three opioids (oxycodone, oxymorphone, and tramadol; controlled/extended release formulations) with a total of 15 clinical trials, 26 arms, and approximately 3835 patients 9, 20-33. A network of comparisons in the final dataset is depicted in the Figure S2-A (Supplementary Material) where direct (e.g. oxycodone vs oxymorphone) and indirect (oxycodone vs placebo trials and tramadol vs placebo trials) evidence exist. Thus, MBMA provides a means of comparing the treatments. As described in the model development process, multiple hierarchical models were tested and compared. The list of models with their OF values is provided in the Table S2 (Supplementary Material). It was observed that model #3 had the smallest OF value (204) which was significantly smaller than other tested models (ΔOF > −3.84). Therefore, model #3 was selected to perform analysis on primary efficacy endpoints. The structure of the selected model is described in Equation 1 where Emax is the maximum effect of the drug, PLC represents placebo response, dose represents the total daily dose of the drug, ED50 is the dose of the drug that achieves 50% of Emax, η is the random residual due to between-arm variability, and Ɛ is the random residual due to within-arm variability. In NONMEM parlance, η is a random variability with a mean of zero and a variance of omega-squared (ω2 (i.e., η = N(0, ω2)). In our study, we reported ω2 values (obtained from the NONMEM software output) as unbiased measures of the variance component. Primary efficacy endpoint analysis showed that PLC response was −16.7 points relative to the BL with η of 28.2%. The Emax (i.e., maximum pain relief) to the active treatment was −28.4 points relative to the BL (i.e., −11.7 points relative to PLC response). Also, ED50 values for oxycodone, oxymorphone, and tramadol were 47.0, 83.9, and 247 mg per day, respectively. The estimated ED50 with other model parameters for the tested opioids with their recommended dose ranges (according to FDA guidelines) 34 are summarized in Table 2. It is of note that the estimated ED50 values were employed in the efficacy-time course analysis and treated as fixed values. The fit of the selected dose-response model to the combined observed data from all selected trials of opioids showed that the fitting line successfully captured the observed data points (Figure 1). This was also supported by visual goodness-of-fit plots where our model predicted values well-explained the variation in the observed WOMAC data. Also, scatter plots of conditional weighted residuals vs population predicted values/time exhibited random distribution (Figure S3, Supplementary Material). To compare the relative potency of oxycodone, oxymorphone, and tramadol, the dose-response relationship was estimated and depicted (Figure 2). The relationship displayed that the rank order of potency was as following: oxycodone > oxymorphone > tramadol.
Table 2.
Estimated parameters of primary efficacy endpoint analysis.
| Parameter | Value | %RSE | 95% CI | Recommended dose |
|---|---|---|---|---|
| Oxycodone ED50 (mg/day) | 47.0 | 17.7 | (12.3, 179) | 20–160 mg/day |
| Oxymorphone ED50 (mg/day) | 83.9 | 15.0 | (22.9, 308) | 10–80 mg/day |
| Tramadol ED50 (mg/day) | 247 | 15.4 | (47.0, 1299) | 100–300 mg/day |
| Emax (mm) | −28.4 | 16.0 | (−37.3, −19.5) | - |
| PLC (mm) | −16.7 | 8.26 | (−19.4, −14.0) | - |
| ω2 of PLC | 0.0797 | 41.3 | (0.0152, 0.144) | - |
| σ2 | 0.0482 | 36.3 | (0.0139, 0.0825) | - |
%RSE = relative standard error.
95% CI = 95% confidence interval estimated by PDx-Pop®.
Fig. 1. MBMA of the primary efficacy endpoints.
The black circles represent the observed data whilst the best fitting analysis is represented by the solid line (curve). The change in WOMAC responses at daily dose = 0 represents the placebo arm in each study.
Fig. 2. Dose-response relationship of opioid compounds accompanied by model predicted ED50 and recommended dose range.
Solid lines represent the predicted response while the dashed lines represent the estimated ED50 value. Shaded areas refer to the recommended dose range of opioid compounds.
| (1) |
3.2. Efficacy-Time Course Analysis
For efficacy-time course analysis, multiple hierarchical models were also tested and compared, and the OF values are reported (Table S3, Supplementary Material). The results indicated that model #3 (described in Equation 2 where PLCmax is the maximum response in the placebo arm over time, and PLC50 is the time for PLC response to drop to 50% of PLCmax) had the smallest OF value (433) which was significantly smaller than other analyzed models (ΔOF value > −3.84). This was also supported by visual goodness-of-fit plots (Figure S4, Supplementary Material). This model was chosen to perform further analysis on the data of the efficacy-time course. The results from selected model displayed that PLC response was −22.3 points relative to the BL with η of 13.3% while Emax response was −9.07 points relative to PLC response (i.e., −31.4 relative to the BL). In addition, the results showed that PLC50 value was less than two weeks (Table S4, Supplementary Material). The fit of the dose-response model over time course for oxycodone, oxymorphone, and tramadol to the combined data from all trials stratified by time for each tested dose is provided in the Figure S5 (Supplementary Material).
| (2) |
3.3. Safety Analysis
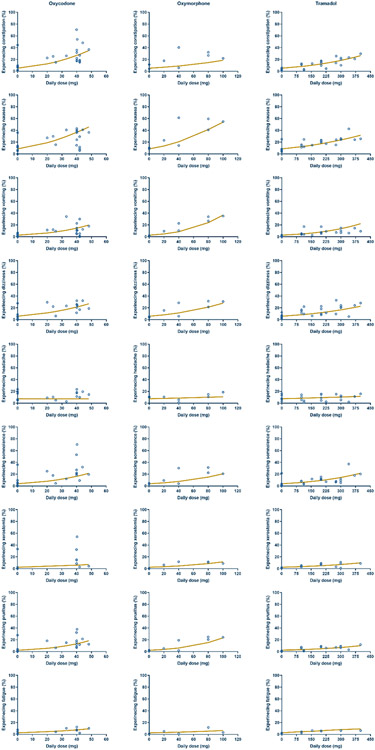
It is of note that final dataset of safety analysis consisted of 23 trials, 39 arms, and approximately 5415 patients 9, 20-33, 35-42. A network of comparisons in the final dataset is depicted in the Figure S2-B (Supplementary Material). Herein, three hierarchical models were tested and compared, and the OF values were reported (Table S5, Supplementary Material). Based on the OF values, it was found that the data of five AEs (constipation, dizziness, somnolence, pruritus, and xerostomia) were best fitted by model #2. In contrast, three AEs (nausea, vomiting, and headache) were best represented by model #3. Surprisingly, model #2 and #3 did not display any significant improvement in the OF values for fatigue AE. Since model #3 (described in Equations 3 and 4) was built on the top of model #2 and significantly improved the fitting for some AEs as demonstrated by the significant drop in OF values, it was selected to perform further analysis on the safety data for opioid compounds. In the model structure, the dependent variable represents the number of patients with AEs while N refers to the total number of patients enrolled in the clinical trial. Model parameters for each AE are summarized in Table 3 while a summary of predicted percentages of AEs at ED50 and recommended dose range of opioids is given in Table 4. The fit of the selected model to the combined AEs data is depicted in the Figure 3.
Table 3.
Estimated model parameters of safety analysis.
| AEs | Intercept | ω2 of intercept |
Slope | ω2 | ||||
|---|---|---|---|---|---|---|---|---|
| Oxycodone | Oxymorphone | Tramadol | Oxycodone | Oxymorphone | Tramadol | |||
| Constipation | 5.32 | 0.0602 | 0.0470 | 0.0141 | 0.00460 | 0.0000100 | 0.0000100 | 0.0000100 |
| Nausea | 8.67 | 0.0501 | 0.0390 | 0.0248 | 0.00395 | 0.149 | 0.0137 | 0.00430 |
| Vomiting | 2.67 | 0.0129 | 0.0409 | 0.0305 | 0.00510 | 0.111 | 0.0000100 | 0.136 |
| Dizziness | 5.85 | 0.0359 | 0.0351 | 0.0183 | 0.00390 | 0.0515 | 0.00400 | 0.0000100 |
| Headache | 7.59 | 0.0787 | −0.000100 | 0.00380 | 0.000100 | 0.00570 | 0.0333 | 3.01 |
| Somnolence | 3.76 | 0.0846 | 0.0405 | 0.0190 | 0.00431 | 0.0000100 | 0.0000100 | 0.126 |
| Xerostomia | 2.47 | 0.117 | 0.0229 | 0.0164 | 0.0036 | 0.0000135 | 0.0000100 | 0.0000100 |
| Pruritus | 2.23 | 0.0248 | 0.0475 | 0.0259 | 0.00397 | 0.0000100 | 0.0000100 | 0.0000100 |
| Fatigue | 2.61 | 0.00220 | 0.0260 | 0.00960 | 0.00320 | 0.0788 | 0.0000100 | 0.0000100 |
Table 4.
Summary of predicted AEs at compound recommended dose range and ED50.
| AEs | Oxycodone | Oxymorphone | Tramadol | |||
|---|---|---|---|---|---|---|
| 20–160 mg/day | ED50(47.0) | 10–80 mg/day | ED50(83.9) | 100–300 mg/day | ED50(247) | |
| Constipation | 12.6 – 99.1% | 33.9 % | 6.08 – 14.8 % | 15.5 % | 8.21 – 18.5% | 15.0 % |
| Nausea | 19.0 – 99.3% | 44.3 % | 10.9 – 41.5 % | 43.9 % | 12.4 – 23.8 % | 20.2 % |
| Vomiting | 6.40 – 97.6 % | 19.0 % | 3.59 – 23.9 % | 26.2 % | 4.69 – 13.7 % | 10.4 % |
| Dizziness | 11.5 – 65.8 % | 26.1 % | 6.95 – 21.3 % | 22.5 % | 8.40 – 16.7 % | 14.0 % |
| Somnolence | 8.07 – 96.2 % | 20.8 % | 4.51 – 15.2 % | 16.1 % | 5.99 – 14.5 % | 11.6 % |
| Headache | 7.57 – 7.44 % | 7.55 % | 7.87 – 10.1 % | 10.3 % | 8.47 – 10.5 % | 9.94 % |
| Pruritus | 5.56 – 97.9 % | 17.5 % | 2.87 – 15.3 % | 16.7 % | 3.28 – 7.00 % | 5.73 % |
| Xerostomia | 3.85 – 49.7 % | 6.92 % | 2.90 – 8.60 % | 9.12 % | 3.52 – 7.04 % | 5.88 % |
| Fatigue | 4.50 – 70.8 % | 9.15 % | 2.87 – 5.46 % | 5.66 % | 3.55 – 6.46 % | 5.52 % |
Fig. 3. Safety analysis of opioid-associated AEs.
Circles represent the observed data whilst the best fitting analysis is represented by the solid line (curve). The percentage of experiencing AEs at daily dose = 0 represents the placebo arm in each study.
| (3) |
| (4) |
3.4. Tolerability Analysis
Although data filtration steps for tolerability and safety were almost similar, final dataset was slightly different in terms of the number of trials and their corresponding data. This is because some safety trials assessed the AEs but did not report the dropouts, and vice versa. Therefore, the final dataset consisted of 23 trials, 43 arms, and approximately 5838 patients 9, 20-21, 24-33, 35-44. Similar to safety analysis, three hierarchical models were tested and OF values were reported and compared (Table S6, Supplementary Material). It was found that model #2 had a significant decline in the OF values compared to model #1. In contrast, model #3 displayed no to a slight drop in the OF values, albeit not significant (i.e., ΔOF<−3.84). Therefore, model #2 was chosen, and the structure of the selected model is described in Equations 5 and 6. Model parameters for DOs data analysis are given in the Table S7 (Supplementary Material). Also, the model predicted DOs at the recommended dose range was estimated (Table S8, Supplementary Material). It was found that there was an association between the dose and DOs due to AEs. Also, it was observed that oxycodone had the highest dropout rate due to AEs. In contrast, there was an inverse relationship between the dose and DOs due to LoE. The fit of the selected model to the combined DOs data of all trials was shown in the Figure S6 (Supplementary Material).
| (5) |
| (6) |
4. DISCUSSION
MBMA framework has been increasingly applied in drug development due to its ability to quantify the clinical trials in terms of efficacy and safety outcomes. In fact, it helps in promoting the benefits and reducing the possible risks through dose optimization. Also, it helps in making a precise decision for positioning new drugs in developing clinical trials 45. Herein, we are reporting on the MBMA to compare efficacy and safety of opioids that have been used for the management of OA pain. MBMA was successfully performed and the model that provided the best description of data was selected. It was observed that the model must possess enough complexity to capture the regularity and trends in data and, in turn, provide an adequate fit. In terms of the efficacy analysis, individual parameter estimates revealed that nearly twofold elevated Emax of the active treatment compared to the PLC response. In addition, it was noticed that Ɛ for both primary efficacy endpoint and efficacy-time course analyses was more suitable to be represented proportionally. Also, it was found that adding the total number of patients to the model did not result in any improvement as demonstrated by increases, rather than decreases, in OF values in both primary efficacy endpoint and efficacy-time course analyses. It is of note that the selected final model for primary efficacy endpoint analysis adequately described the data, and the fitted line nicely captured the pattern in the curved data. In addition to the drop in the OF values, visual goodness-of-fit plots displayed that the final model appropriately described the natural trends in the observed data.
The primary efficacy endpoint analysis also illustrated that there is a relationship between the administered dose of the opioids and the predicted response where the pain relief due to the administration of opioids (represented by the increased change in WOMAC) was gradually enhanced with increasing the dose. As expected, there was a threshold dose below which there was no effect on the WOMAC. For example, 6 mg and 11 mg daily doses of oxycodone and oxymorphone, respectively, were required to initiate the response and start observing the change in WOMAC. However, 32 mg of tramadol was necessary to give a similar response. Generally, comparing the change in WOMAC values of opioids for all doses showed a reduction in pain effects that was in the following rank order: oxycodone > oxymorphone > tramadol. Additionally, it is worth mentioning that the FDA recommended doses of opioid compounds for the management of chronic pain including OA pain were consistent with our model predicted ED50. Also, MBMA estimates at the recommended dose range suggested that there were no remarkable differences between oxycodone, oxymorphone, and tramadol in term of the model-predicted responses (i.e., change in WOMAC pain subscale). At the FDA recommended dose range, the rank order of model-predicted treatment effect indicated that oxycodone had the largest estimated drug effect with pain reduction equal to −20.2 to −25.8 points relative to BL. This was followed by tramadol and oxymorphone which can produce pain relief effect in the range of −20.1 to −23.1 and −17.9 to −22.4, respectively.
To establish a clear picture of the dose to efficacy onset of opioids over the time course of the clinical trials, efficacy-time course analysis was also investigated. The efficacy-time course was best fitted by model #3 where the efficacy analysis over the time course revealed that there were time-dependent pain relief effects at given doses. Also, the results reported herein showed that opioids have a relatively rapid time to efficacy onset in patients with OA. This was followed by a gradual amelioration in pain reduction over time. Furthermore, it was observed that the model parameters, Emax and PLC responses, obtained from efficacy-time course analysis were consistent with those obtained from the primary efficacy endpoint analysis. Moreover, it was found that the time required to produce the 50% of Emax was approximately 2–3 weeks. Also, the results revealed that PLC50 was 1.47 week indicating that the time required to reach the 50% of the maximum PLC response was less than two weeks which also suggests a rapid reduction in the pain intensity. This could be a critical factor in designing new clinical trials (i.e., the length of new developing clinical trials should be at least two weeks).
Since the drug dose is a major determinant of its safety, establishing a safe dose of drugs is one of the primary objectives during clinical pharmacokinetic studies of drug development. Herein, MBMA performed on AEs data illustrated that the selected model adequately captured the trends. Also, η of both intercept and slope seemed to play a role in data fitting model as demonstrated by a significant drop in the OF values. This may indicate the presence of variability among the trials for both untreated patients (placebo) and patients received opioids. Results on the safety demonstrated that there was a dose-dependent relationship between the total daily dose of opioids and the incidence of AEs. Particularly, it was observed that experiencing constipation, nausea, vomiting, dizziness, somnolence, and pruritus were most highly associated AEs with opioids as the slopes of the fitted lines appeared to be large compared to other AEs. The incidence of these AEs was shifted dramatically with the increase in dose (i.e., dose-dependent AEs). For example, experiencing nausea at the recommended dose range for oxycodone, oxymorphone, and tramadol was 19.0–99.3%, 10.9–41.5%, and 12.4–23.8%, respectively. In contrast, experiencing other AEs such as headache, xerostomia, and fatigue was generally none to slightly dose-dependent at the administered daily doses of the opioids as demonstrated by negligible slope values of our predicted lines. For instance, experiencing the headache at the recommended dose range for oxycodone, oxymorphone, and tramadol was 7.57–7.44%, 7.87–10.1%, and 8.47–10.5%, respectively. Surprisingly, at the high dose of oxycodone FDA recommended dose range, it was noticed that the incidence of some AEs was extremely high which may contribute to less frequent administrations of these high doses. Usually, a high dose of oxycodone treatment is reserved for opioid-tolerant patients who suffer from intractable pain, and it was reported that a total daily dose of >80 mg is used only in opioid-tolerant patients 46. Importantly, majority of AEs associated with opioids are manageable and can be alleviated by co-administering other compounds. For example, it is recommended to co-prescribe stool softener along with opioids to minimize the incidence of constipation 47. The results from safety analysis showed that tramadol appeared to be associated with less incidence rate of AEs at the recommended dose range compared to oxycodone and oxymorphone. Our observations in opioids tolerability assessment were also consistent with results obtained from safety analysis. It was noticed that oxycodone had the highest dropout incidence rates due to AEs. Also, tolerability analysis revealed that tramadol and to less extent oxymorphone are more tolerable than oxycodone, as their dropout incidence rates due to AEs were small at the recommended FDA dose range. Generally, there was an association between the dose, incidence of AEs, and dropout rates. It was observed that as the dose increases, the incidence of AEs also rises and, in turn, boosts the dropout rates. In contrast, the dropout rates due to lack of efficacy were inversely related to the dose of opioid compounds (i.e., as the dose increased, the dropout rates decreased). This demonstrates that opioids are potent compounds and can produce efficient analgesic effects and relieve intractable pain.
5. CONCLUSIONS
This study provided clinically meaningful insight into the efficacy endpoint, efficacy-time course, safety, and tolerability of oxycodone, oxymorphone, and tramadol. Selected models provided a decent fit to the data. MBMA to compare efficacy, safety, and tolerability of opioid compounds suggested that oxycodone, oxymorphone and tramadol possessed potent analgesic benefits, but they are associated with some dose-related adverse effects. The presented framework analysis has a clinical impact on drug development where it can help in optimizing the dose of opioids to manage OA pain, making precise key decisions for positioning of new drugs, and designing more efficient trials.
Supplementary Material
LIST OF ABBREVIATIONS:
- AEs
adverse events
- BL
baseline
- COX-2
cyclooxygenase-2
- DOs
dropouts
- ED50
dose of the drug that achieves 50% of maximum drug effect
- Emax
maximum drug effect
- Epsilon (Ɛ)
random residual due to within-arm variability
- Eta (η)
random residual due to between-arm variability
- FDA
Food and Drug Administration
- LL
log-likelihoods
- LoE
lack of efficacy
- MBMA
model-based meta-analysis
- NONMEM
nonlinear mixed-effects modeling
- NSAIDs
non-steroidal anti-inflammatory drugs
- OA
osteoarthritis
- OF
objective function
- P
probability
- PLC
placebo
- PLCmax
maximum response in the placebo arm over time
- PLC50
time for PLC response to drop to 50% of maximum placebo response
- TDOs
total dropouts
- WOMAC
Western Ontario and McMaster Universities OA index
Footnotes
CONFLICT OF INTREST:
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Xing D; Wang B; Liu Q; Ke Y; Xu Y; Li Z; Lin J, Intra-articular Hyaluronic Acid in Treating Knee Osteoarthritis: a PRISMA-Compliant Systematic Review of Overlapping Meta-analysis. Sci Rep 2016, 6, 32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinusas K, Osteoarthritis: diagnosis and treatment. Am Fam Physician 2012, 85 (1), 49–56. [PubMed] [Google Scholar]
- 3.Zhang W; Moskowitz RW; Nuki G; Abramson S; Altman RD; Arden N; Bierma-Zeinstra S; Brandt KD; Croft P; Doherty M; Dougados M; Hochberg M; Hunter DJ; Kwoh K; Lohmander LS; Tugwell P, OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008, 16 (2), 137–62. [DOI] [PubMed] [Google Scholar]
- 4.Neogi T, The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013, 21 (9), 1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma VY; Chan L; Carruthers KJ, Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 2014, 95 (5), 986–995 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conn PM; Benjamin SA; Finch CE; Guerin JC; Nelson JF; Olshansky SJ; Roth G; Smith RG, Handbook of Models for Human Aging. Elsevier Science: 2011. [Google Scholar]
- 7.Ungprasert P; Kittanamongkolchai W; Price C; Ratanapo S; Leeaphorn N; Chongnarungsin D; Cheungpasitporn W, What Is The “Safest” Non-Steroidal Anti-Inflammatory Drugs? 2012; Vol. 3, p 115–123. [Google Scholar]
- 8.Pirmohamed M; James S; Meakin S; Green C; Scott AK; Walley TJ; Farrar K; Park BK; Breckenridge AM, Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004, 329 (7456), 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto AK; Babul N; Ahdieh H, Oxymorphone extended-release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double-blind, placebo- and active-controlled phase III trial. Pain Med 2005, 6 (5), 357–66. [DOI] [PubMed] [Google Scholar]
- 10.American Pain Society. Guideline for the management of pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. 2002. [Google Scholar]
- 11.Osteoarthritis Treatment. https://www.arthritis.org/about-arthritis/types/osteoarthritis/treatment.php. [Google Scholar]
- 12.Dowell D; Haegerich TM; Chou R, CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 2016, 65 (1), 1–49. [DOI] [PubMed] [Google Scholar]
- 13.Manchikanti L; Helm S 2nd; Fellows B; Janata JW; Pampati V; Grider JS; Boswell MV, Opioid epidemic in the United States. Pain Physician 2012, 15 (3 Suppl), ES9–38. [PubMed] [Google Scholar]
- 14.In Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence, Geneva, 2009. [PubMed] [Google Scholar]
- 15.Mercier F; Claret L; Prins K; Bruno R, A Model-Based Meta-analysis to Compare Efficacy and Tolerability of Tramadol and Tapentadol for the Treatment of Chronic Non-Malignant Pain. Pain Ther 2014, 3 (1), 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haidich AB, Meta-analysis in medical research. Hippokratia 2010, 14 (Suppl 1), 29–37. [PMC free article] [PubMed] [Google Scholar]
- 17.Mould DR, Model-based meta-analysis: an important tool for making quantitative decisions during drug development. Clin Pharmacol Ther 2012, 92 (3), 283–6. [DOI] [PubMed] [Google Scholar]
- 18.McGrory BJ; Harris WH, Can the western Ontario and McMaster Universities (WOMAC) osteoarthritis index be used to evaluate different hip joints in the same patient? J Arthroplasty 1996, 11 (7), 841–4. [DOI] [PubMed] [Google Scholar]
- 19.Ting N, Dose Finding in Drug Development. Springer; New York: 2006. [Google Scholar]
- 20.Markenson JA; Croft J; Zhang PG; Richards P, Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain 2005, 21 (6), 524–35. [DOI] [PubMed] [Google Scholar]
- 21.Hale M; Tudor IC; Khanna S; Thipphawong J, Efficacy and tolerability of once-daily OROS hydromorphone and twice-daily extended-release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: results of a 6-week, randomized, open-label, noninferiority analysis. Clin Ther 2007, 29 (5), 874–888. [DOI] [PubMed] [Google Scholar]
- 22.Afilalo M; Etropolski MS; Kuperwasser B; Kelly K; Okamoto A; Van Hove I; Steup A; Lange B; Rauschkolb C; Haeussler J, Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig 2010, 30 (8), 489–505. [DOI] [PubMed] [Google Scholar]
- 23.Spierings EL; Fidelholtz J; Wolfram G; Smith MD; Brown MT; West CR, A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain 2013, 154 (9), 1603–12. [DOI] [PubMed] [Google Scholar]
- 24.Kivitz A; Ma C; Ahdieh H; Galer BS, A 2-week, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee. Clin Ther 2006, 28 (3), 352–64. [DOI] [PubMed] [Google Scholar]
- 25.Silverfield JC; Kamin M; Wu SC; Rosenthal N; Group C-S, Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther 2002, 24 (2), 282–97. [DOI] [PubMed] [Google Scholar]
- 26.Emkey R; Rosenthal N; Wu SC; Jordan D; Kamin M; Group C-S, Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double-blind, placebo-controlled trial. J Rheumatol 2004, 31 (1), 150–6. [PubMed] [Google Scholar]
- 27.Babul N; Noveck R; Chipman H; Roth SH; Gana T; Albert K, Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage 2004, 28 (1), 59–71. [DOI] [PubMed] [Google Scholar]
- 28.Burch F; Fishman R; Messina N; Corser B; Radulescu F; Sarbu A; Craciun-Nicodin MM; Chiriac R; Beaulieu A; Rodrigues J; Beignot-Devalmont P; Duplan A; Robertson S; Fortier L; Bouchard S, A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage 2007, 34 (3), 328–38. [DOI] [PubMed] [Google Scholar]
- 29.Fishman RL; Kistler CJ; Ellerbusch MT; Aparicio RT; Swami SS; Shirley ME; Jain AK; Fortier L; Robertson S; Bouchard S, Efficacy and safety of 12 weeks of osteoarthritic pain therapy with once-daily tramadol (Tramadol Contramid OAD). J Opioid Manag 2007, 3 (5), 273–80. [DOI] [PubMed] [Google Scholar]
- 30.Thorne C; Beaulieu AD; Callaghan DJ; O'Mahony WF; Bartlett JM; Knight R; Kraag GR; Akhras R; Piraino PS; Eisenhoffer J; Harsanyi Z; Darke AC, A randomized, double-blind, crossover comparison of the efficacy and safety of oral controlled-release tramadol and placebo in patients with painful osteoarthritis. Pain Res Manag 2008, 13 (2), 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beaulieu AD; Peloso PM; Haraoui B; Bensen W; Thomson G; Wade J; Quigley P; Eisenhoffer J; Harsanyi Z; Darke AC, Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag 2008, 13 (2), 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLemos BP; Xiang J; Benson C; Gana TJ; Pascual ML; Rosanna R; Fleming B, Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther 2011, 18 (3), 216–26. [DOI] [PubMed] [Google Scholar]
- 33.Gana TJ; Pascual ML; Fleming RR; Schein JR; Janagap CC; Xiang J; Vorsanger GJ; Study G, Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin 2006, 22 (7), 1391–401. [DOI] [PubMed] [Google Scholar]
- 34.Blue Cross Blue Shield Association. Opioids, Extended Release (ER). https://www.bcbsks.com/customerservice/Providers/MedicalPolicies/policies/policies/OpioidsExtendedRelease_2017-07-14.pdf. [Google Scholar]
- 35.Caldwell JR; Hale ME; Boyd RE; Hague JM; Iwan T; Shi M; Lacouture PG, Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial. J Rheumatol 1999, 26 (4), 862–9. [PubMed] [Google Scholar]
- 36.Roth SH; Fleischmann RM; Burch FX; Dietz F; Bockow B; Rapoport RJ; Rutstein J; Lacouture PG, Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluation. Arch Intern Med 2000, 160 (6), 853–60. [DOI] [PubMed] [Google Scholar]
- 37.Chindalore VL; Craven RA; Yu KP; Butera PG; Burns LH; Friedmann N, Adding ultralow-dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of Oxytrex. J Pain 2005, 6 (6), 392–9. [DOI] [PubMed] [Google Scholar]
- 38.Hale M; Upmalis D; Okamoto A; Lange C; Rauschkolb C, Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr Med Res Opin 2009, 25 (5), 1095–104. [DOI] [PubMed] [Google Scholar]
- 39.Hartrick C; Van Hove I; Stegmann JU; Oh C; Upmalis D, Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled study. Clin Ther 2009, 31 (2), 260–71. [DOI] [PubMed] [Google Scholar]
- 40.McIlwain H; Ahdieh H, Safety, tolerability, and effectiveness of oxymorphone extended release for moderate to severe osteoarthritis pain: a one-year study. Am J Ther 2005, 12 (2), 106–12. [DOI] [PubMed] [Google Scholar]
- 41.Malonne H; Coffiner M; Sonet B; Sereno A; Vanderbist F, Efficacy and tolerability of sustained-release tramadol in the treatment of symptomatic osteoarthritis of the hip or knee: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther 2004, 26 (11), 1774–82. [DOI] [PubMed] [Google Scholar]
- 42.Choi CB; Song JS; Kang YM; Suh CH; Lee J; Choe JY; Lee CK; Shim SC; Chung WT; Song GG; Kim HA; Ji JD; Nam EJ; Park SH; Hong YH; Sheen DH; Lim MK; Seo YI; Sung YK; Kim TH; Lee JT; Bae SC, A 2-week, multicenter, randomized, double-blind, double-dummy, add-on study of the effects of titration on tolerability of tramadol/acetaminophen combination tablet in Korean adults with knee osteoarthritis pain. Clin Ther 2007, 29 (7), 1381–9. [DOI] [PubMed] [Google Scholar]
- 43.Corsinovi L; Martinelli E; Fonte G; Astengo M; Sona A; Gatti A; Massaia M; Bo M; Zanocchi M; Michelis G; Isaia G; Molaschi M, Efficacy of oxycodone/acetaminophen and codeine/acetaminophen vs. conventional therapy in elderly women with persistent, moderate to severe osteoarthritis-related pain. Arch Gerontol Geriatr 2009, 49 (3), 378–82. [DOI] [PubMed] [Google Scholar]
- 44.Zautra AJ; Smith BW, Impact of controlled-release oxycodone on efficacy beliefs and coping efforts among osteoarthritis patients with moderate to severe pain. Clin J Pain 2005, 21 (6), 471–7. [DOI] [PubMed] [Google Scholar]
- 45.Teng Z; Gupta N; Hua Z; Liu G; Samnotra V; Venkatakrishnan K; Labotka R, Model-Based Meta-Analysis for Multiple Myeloma: A Quantitative Drug-Independent Framework for Efficient Decisions in Oncology Drug Development. Clin Transl Sci 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purdue Pharma. Oxycontin tablets: Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022272lbl.pdf. [Google Scholar]
- 47.Bell TJ; Panchal SJ; Miaskowski C; Bolge SC; Milanova T; Williamson R, The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009, 10 (1), 35–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.