Abstract
Introduction
Many people living with chronic kidney disease (CKD) are expected to self-manage their condition. Patient activation is the term given to describe the knowledge, skills and confidence a person has in managing their own health and is closely related to the engagement in preventive health behaviours. Self-management interventions have the potential to improve remote disease management and health outcomes. We are testing an evidence-based and theory-based digital self-management structured 10-week programme developed for peoples with CKD called ‘My Kidneys & Me’. The primary aim of the study (Self-Management Intervention through Lifestyle Education for Kidney health (SMILE-K)) is to assess the effect on patient activation levels.
Methods and analysis
A single-blind randomised controlled trial (RCT) with a nested pilot study will assess the feasibility of the intervention and study design before continuation to a full RCT. Individuals aged 18 years or older, with established CKD stage 3–4 (eGFR of 15–59 mL/min/1.73 m2) will be recruited through both primary and secondary care pathways. Participants will be randomised into two groups: intervention group (receive My Kidneys & Me in addition to usual care) and control group (usual care). The primary outcome of the nested pilot study is feasibility and the primary outcome of the full RCT is the Patient Activation Measu (PAM-13). The full RCT will assess the effect of the programme on online self-reported outcomes which will be assessed at baseline, after 10 weeks, and then after 20 weeks in both groups. A total sample size of N=432 participants are required based on a 2:1 randomisation. A substudy will measure physiological changes (eg, muscle mass, physical function) and patient experience (qualitative semi-structured interviews).
Ethics and dissemination
This study was fully approved by the Research Ethics Committee-Leicester South on the 19 November 2020 (reference: 17/EM/0357). All participants are required to provide informed consent obtained online. The results are expected to be published in scientific journals and presented at clinical research conferences. This is protocol version 1.0 dated 27 January 2021.
Trial registration number
Keywords: chronic renal failure, protocols & guidelines, clinical trials
Strengths and limitations of this study.
This study will be conducted as a large randomised controlled trial (RCT) and will assess the effect of an online self-management and lifestyle education programme (‘My Kidneys & Me’) on patient activation levels and subsequent self-management behaviours in people with kidney disease.
The secondary outcomes of the study will assess the feasibility of using such an intervention in this population and explore patient experience of the programme itself.
The nested pilot study will assess the feasibility of the intervention and study design before continuation to a full RCT; progression criteria will be developed with specified targets for progression based on feasibility outcomes.
The use of patient and public involvement in the intervention development and study design will ensure that they are acceptable, suitable and relevant to the target population.
The primary limitation of this study is the potential lack of acceptability of randomisation and assessment.
Introduction
Chronic kidney disease (CKD) is a long-term condition associated with high morbidity and premature mortality,1 and has an estimated UK prevalence of ~5%–7%.2 In UK, 70% of National Health Service (NHS) expenditure is spent on patients with long-term conditions such as CKD.3 With <1% of time spent in contact with healthcare professionals, many patients are expected to self-manage their condition.3 In CKD, the majority of people are managed in primary care rather than by kidney specialists.4 Long-term CKD management requires a high level of patient engagement, both in decision-making and in the implementation of care.5 For those with CKD, this encompasses a spectrum of behaviours ranging from adherence to medication and diet recommendations, maintaining physical activity, recognition and monitoring of risk factors (eg, blood glucose and blood pressure) and self-adjustment of home-care routines.6 Self-management interventions aim to facilitate an individual’s ability to make appropriate lifestyle changes7 and have shown beneficial impacts on various modifiable risk factors relevant to the progression of CKD (eg, proteinuria, blood pressure, exercise capacity).7 8 The COVID-19 global pandemic has presented unique challenges for people living with CKD and has further highlighted the need for and importance of self‐management.
In order to implement self-management behaviours and participate in healthcare decisions, patients must have knowledge of their condition, and patient education is a crucial pathway to ensuring that individuals can be taught to engage in self-management tasks.6 Empirical studies have shown that patient education, including an understanding of CKD, is associated with better outcomes.6 Patient activation is the term given to describe the knowledge, skills and confidence a person has in managing their own health9 and is closely related to the engagement of preventive health behaviours.5 Studies have indicated that activated patients are more likely to attend screenings, check-ups and immunisations, as well as engage in healthy behaviours such as eating a balanced diet.10 11 Increased patient activation is associated with improved health outcomes in many long-term conditions.12 13 In CKD, lower patient activation is associated with worse cardiovascular disease risk profiles14 and promoting patient activation in kidney disease care is increasingly being prioritised and has recently emerged as central to legislative policy in the USA15 and UK.16 In the UK, National Institute for Health and Care Excellence (NICE) clinical guidance recommends that informational and educational programmes are offered to those with CKD, including information regarding what people can do to manage and influence their own condition. Interventions to increase patient activation are likely to improve self-management behaviour, and consequently this may be a suitable outcome for self-management-based interventions.
A range of barriers have prevented widespread implementation of comprehensive education for people with CKD and a recent systematic review of self-management interventions in CKD found many interventions lack patient engagement in their design, and the majority of interventions do not apply behavioural change theory to inform their development.8 As such there remains a need for better and innovative self-management interventions for those with CKD.4 8 Digital self-management interventions have the potential to improve remote disease management and health outcomes17 and are increasingly becoming integrated into self-management to improve behaviour. The COVID-19 pandemic has also presented those with long-term conditions and their healthcare teams an opportunity to innovate and move towards an increasingly digitalised care, with particular emphasis on supporting patients from their own homes.
Developed in the UK, the structured DESMOND (Diabetes Education and Self-Management for Ongoing and Newly Diagnosed) diabetes self-management group education programme has recently been shown to improve patient activation in individuals living with diabetes.18 Based on the same principles, MyDESMOND is a global digital programme to provide ongoing support and guidance.19 20 Using the MyDESMOND platform, we developed an evidence-based and theory-based digital self-management programme for patients with CKD called ‘My Kidneys & Me’. The programme was developed in conjunction with patients and their families, key stakeholders and a wide range of healthcare professionals including nephrologists, psychologists, physiotherapists, dieticians, exercise scientists and pharmacists. The programme was developed using ‘Intervention Mapping’, a six-step framework used to guide behaviour change interventions and health education development.21 A full and detailed description of the development of ‘My Kidneys & Me’ can be found in Lightfoot.22
Objectives and hypotheses
The primary aim of the proposed ‘SMILE-K’ research study is to assess the effect of a structured online self-management programme—‘My Kidneys & Me’—on patient activation levels and subsequent self-management behaviour. Further objectives include assessing the feasibility of using such an intervention in this population and exploring patient experience of the programme itself. We hypothesise that access to ‘My Kidneys & Me’ will increase patient activation, compared with usual care, in people living with CKD.
Methods and analysis
This protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) reporting recommendations23 (online supplemental material S1).
bmjopen-2022-064916supp001.pdf (96.2KB, pdf)
Study design overview
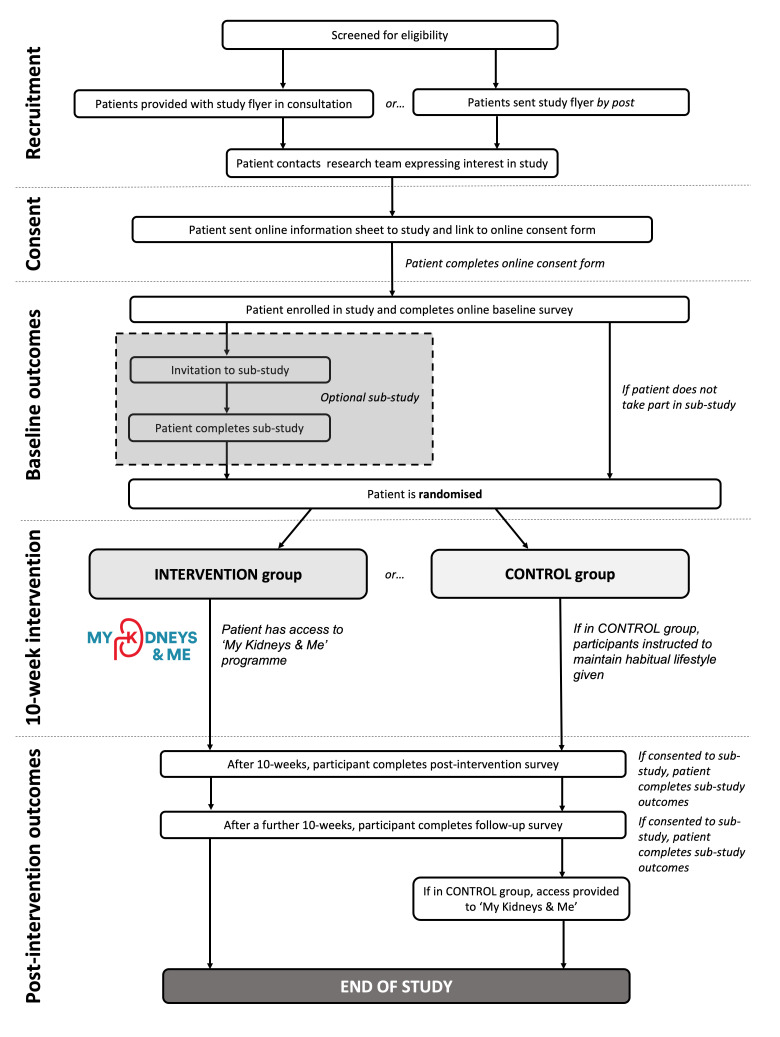
A single-blind randomised controlled trial (RCT) with a nested pilot study will be used to assess the effect of the ‘My Kidneys & Me’ programme. An initial nested pilot study will assess the feasibility of the intervention and study design before continuation to a full RCT is considered. Continuation to the full RCT will be based on a predefined ‘stop/go’ criteria assessed after n=60 participants have been recruited. The study intervention period will last 20 weeks with outcome measures assessed at baseline (preintervention), week 10 (postintervention) and week 20 (follow-up). A flow diagram showing the participant flow through the study can be found in figure 1.
Figure 1.
Study flow diagram.
An optional substudy will occur in parallel with the main study. This will consist of additional objective assessments of body composition, physical activity and physical function to the main study outcome protocol, as well as qualitative interviews with participants to discuss their expectations and experiences of the intervention and study protocol.
Eligibility criteria
Individuals aged 18 years or older, with established CKD stage 3–4 (eGFR of 15–59 mL/min/1.73 m2) according to the NICE guidelines will be included. Those requiring any form of kidney replacement therapy (ie, any modality of dialysis or transplantation) or with insufficient command of English or any other precluding factors that prevent ability to give informed consent or comply with protocol will be excluded. For the substudy, in addition, participants will be excluded if they are pregnant and/or if any element of protocol considered by own clinician or general practitioner (GP) is contraindicated and/or the individual is deemed unfit due to physical impairment, significant comorbidity or other reason (eg, unstable hypertension, arrhythmia, myocardial infarction <6 months, unstable angina, uncontrolled diabetes mellitus, advanced cerebral or vascular disease).
Recruitment
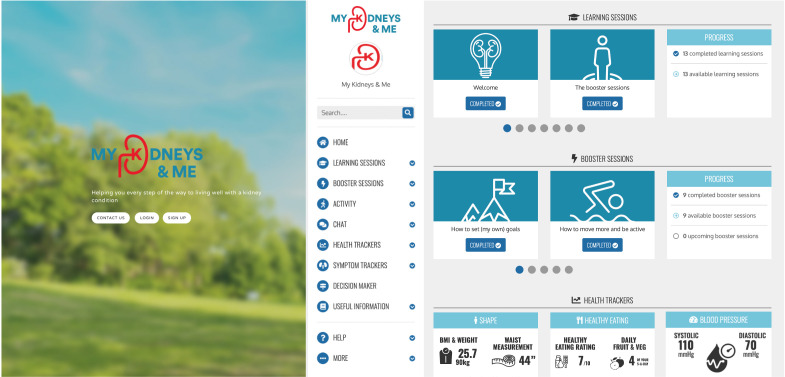
Participants will be recruited through both primary and secondary care pathways across multiple sites in England. Primary care practices will identify eligible patients and provide them with an introduction flyer and study invitation letter, either during a consultation or via the post. If recruited from secondary care, the flyer will be provided during the patient’s routine outpatient clinic visit by the nephrologist. Alternatively, patients may be recruited by postal means. Interested participants are requested to contact the research team by email who will then respond with further detailed information and an online consent form. When participants have consented, they are sent a link to complete the online outcome measures. In eligible patients, further information regarding the substudy is provided. Once the baseline outcome measures have been completed, participants are randomised and provided access to the programme if appropriate. Access will be a secure link that ask participants to register and create a unique username and password. Once created, patients can then access ‘My Kidneys & Me’ (dashboard shown in figure 2).
Figure 2.
‘My Kidneys & Me’ dashboard page.
Randomisation
Participants will be randomised into two groups: intervention group and control group. Randomisation will be performed by the research team in a single-blind fashion. Study investigators, clinicians and research staff from external recruiting sites are blinded to the group allocation of their participants. Participants will be stratified based on age (≤63, >63 years) to ensure comparatively equal representative age characteristics in both groups. These values are based on the median age attained from preliminary unpublished data from two ongoing observational studies in non-dialysis patients with CKD by our group. The control group will not be provided with the intervention during the study and will be asked to maintain their habitual lifestyle activities. The control group will be provided with the intervention on study completion. The intervention group will be provided access to the online ‘My Kidneys & Me’ intervention.
Intervention
‘My Kidneys & Me’
The ‘My Kidneys & Me’ programme forms part of the award-winning and quality-assured MyDESMOND e-learning platform.22 24 The educational sessions provide information about the kidneys, CKD, its treatment and the different ways to self-manage and is written to accommodate for those with low patient activation levels. A summary of the education sessions can be in the My Kidneys & Me development paper.22 24 ‘How to’ booster sessions are interactive educational sessions, which provide instructions on how to perform the self-management behaviours and are released weekly. A summary of these sessions is found in table 1.
Table 1.
Summary of the ‘How to’ booster sessions
| ‘How to’ session | Summary of session | Duration of session (minutes) |
| 1. Get started | This is an introduction about the ‘How to’ sessions and explains what can be expected from them. This will be available from the start. | 5 |
| 2. Set my own goals | The first ‘How to’ session introduces patients to what goals are, why they are important, how to create their own personalised goals and how to use an action plan. It is recommended that patients first read the ‘Kidney disease and general health’ and ‘Reducing my health risks’ educational sessions so that they can identify which areas they may want to set their own goals in. | 25–30 |
| 3. Move more and be active | This session includes how to set an activity goal, how to break up sedentary time, how to do aerobic activity and how to maintain activity levels. It is recommended that patients first read the ‘Moving more and being active’ educational session. | 15–20 |
| 4. How to keep my muscles healthy through strength training | This ‘How to’ session provides instruction on how to safely take part in strength training exercises and how this could help them. It is recommended that patients first read the ‘Keeping my muscles healthy’ educational session. | 15–20 |
| 5. Take control of my health | This session looks at taking medications and managing other conditions such as diabetes and hypertension. It is recommended that patients first read ‘Kidney disease and general health’ and ‘Reducing my health risks’ educational sessions. | 15–20 |
| 6. Get the most from my healthcare | This session covers information about why attending healthcare appointments is important, how to make the most out of appointments and how to decide which healthcare professional they may want to talk to for different areas so that they can share treatment decisions. It is recommended that patients first read ‘Treatment options available’ which includes information about their healthcare team and what appointments are for. | 15–20 |
| 7. Eat well | This session covers how to make healthier food choices and adapt recipes. It is recommended that patients first read the ‘Eating a healthy balanced diet’ educational sessions. | 20–25 |
| 8. Manage my symptoms | This session includes how to recognise and keep track of symptoms so that they can modify their lifestyle accordingly and speak to their healthcare professional if needed. It is recommended that patients first read ‘Reducing my health risks and ‘Managing my symptoms’ educational sessions as this explains the common symptoms of CKD. | 10–15 |
| 9. Improve my sleep quality | The penultimate session covers how to set sleep goals, improve sleep quality and monitor sleep. It is recommended that patients first read ‘Improving my sleep quality’ educational session. | 10–15 |
| 10.Look after my well-being | The final session focuses on how to balance daily life with CKD and how to manage illness-related stress. This also includes the importance of social support and how to talk to others. It is recommended that patients first read the ‘Looking after my well-being’ educational sessions. | 15–20 |
CKD, chronic kidney disease.
The health trackers feature allows patients to self-monitor different aspects of their health that are involved in the self-management of the condition: body weight and measurements, fruit and vegetable intake, symptoms, smoking, cholesterol and blood pressure. These allow patients to manually update their trackers and see their progress over time through graphs and charts. Similarly, the ‘Activity’ feature allows patients to track their physical activity, although this is broken down into different forms of activity. Patients can track how many steps they have walked and for how many minutes and can challenge others or invite up to five of their own friends and family to join these challenges. The patients can track this by either synchronising the programme with their activity tracker (eg, Fitbit) or they can enter their steps manually. They can also use a bespoke tracker created for ‘My Kidneys & Me’ to record their strength training progress and resources (eg, instructional videos) will be available online. The ‘Decision maker’ feature is a tool to help patients create and monitor their own health-related goals, as this works through a series of questions to help patients identify which goals are most important to them and how they can achieve these by overcoming identified barriers.
Outcome measures
The primary outcome of the nested pilot study is feasibility, and the primary outcome of the full RCT is the Patient Activation Measure (PAM-13).
Nested feasibility outcomes
Before progression to the full RCT, we will assess feasibility outcomes on the first n=60 participants recruited. Based on these outcomes, a decision will be made to continue with the trial based on prespecified progression criteria. These progression criteria will be developed with input from stakeholders in the study, researchers and patients. The following feasibility outcomes will be assessed:
Recruitment rate: the number of eligible patients and number consented will be recorded. Monthly recruitment rate and the time taken to recruit 25%, 50%, 75%, and 100% patients will be recorded.
Acceptability of randomisation and assessment procedure: acceptability of randomisation (and stratification variables) and procedures will be determined by comparison of randomised group characteristics, and by measuring loss to follow-up and by exploring patient’ views about their participation in the research.
Programme usage and adherence to intervention: adherence will be assessed by the completion of sessions, and the use of the goal setting and health tracking features. We will also assess patterns and frequency of programme usage.
Attrition rate: the number of dropouts (attrition rate) will be recorded.
Missing data: quantity of missing data (eg, questionnaire return rate, outcome measures not completed)
Patient experience: this will be attained through qualitative interviews in a subset of patients.
Primary study outcome
The primary outcome of the full RCT is the PAM-13, the most widely used instrument for measuring patient activation9 which was piloted in the NHS through the UK Renal Registry, and has been validated25 and recommend for use in those with kidney disease.15 25 The PAM-13 is a validated tool of 13 questions which assesses a patient’s knowledge, skills and confidence in managing their own health. The PAM-13 has demonstrated good internal consistency as well as adequate reliability and validity,9 26 including in those with kidney disease.25 Answers are weighted and combined to provide a score on a scale from 0 to 100. The PAM allows respondents to be categorised into one of four levels with lower levels indicating low activation and higher levels indicating high activation. The PAM-13 will be assessed among a battery survey of other questionnaires that will be delivered online using Jisc Online Surveys (University of Leicester). As per SPIRIT recommendations, the timepoints for each outcome can be found in table 2.
Table 2.
Outcome measure timepoints
| Item | Scale/test | Baseline | Week 10 | Week 20 |
| Main study* | ||||
| Demographics | – | X | – | – |
| Patient activation | PAM-13 | X | X | X |
| Knowledge | CKD-SMKT | X | X | X |
| Health status | SF-12 | X | X | X |
| Symptoms | KSQ | X | X | X |
| Sarcopenia | SARC-F | X | X | X |
| Illness perception | IPQ-R | X | X | X |
| Physical activity | GPPAQ | X | X | X |
| Diet | UKDDQ | X | X | X |
| Medication adherence | MARS-5 | X | X | X |
| Healthcare use | EPQ | X | X | X |
| Physical function | STS-60 | X | X | X |
| Clinical data | Full blood count, U&Es | X | X | X |
| Substudy† | ||||
| Anthropometry | Height, weight, BMI, waist and hip circumference | X | X | X |
| Muscle phenotyping | BIA, ultrasonography | X | X | X |
| Physical function | Gait speed, HGS, STS-60, TUAG | X | X | X |
| Physical activity | Accelerometery (7 days) | X | X | X |
| Qualitative component | Semistructured interview | X | X | X |
*Main study outcomes delivered online to all patients.
†Substudy outcomes assessed in a subset of patients. Qualitative interviews may be performed at any of the timepoints depending on participants’ voluntary participation and/or their status in the study.
BIA, bioelectrical impendence analysis; BMI, body mass index; CKD-SMKT, Chronic Kidney Disease Self-Management Knowledge Tool; EPQ, Modified Economic Patient Questionnaire; GPPAQ, General Practice Physical Activity Questionnaire; HGS, handgrip strength; IPQ-R, Illness Representations Questionnaire (Brief); KSQ, Kidney Symptom Questionnaire; MARS-5, Medication Adherence Report Scale; PAM-13, Patient Activation Measure; SF-12, 12 Item Short Form Health Survey; STS-60, Sit-to-stand-60; TUAG, Timed-up-and-go’ test; U&Es, Urea and electrolytes; UKDDQ, UK Diabetes and Diet Questionnaire.
Secondary outcomes
Alongside a bespoke self-reported demographic questionnaire, other validated questionnaires in the online survey, which capture different aspects of self-management and lifestyle behaviours that are addressed in ‘My Kidneys & Me’, include:
Chronic Kidney Disease Self-Management Knowledge Tool (CKD-SMKT): The CKD-SMKT is a validated 11-item questionnaire, which assesses kidney disease patients’ knowledge of various key self-management behaviours and kidney health.27
12-Item Short Form Health Survey (SF-12): the SF-12 is a multipurpose short form survey with 12 questions. The SF-12 assesses patients mental and physical functioning and overall health-related quality of life (QoL).28
Kidney Symptom Questionnaire (KSQ): the KSQ assesses the frequency, intrusiveness and total impact of a range of 13 common kidney disease-related symptoms. This questionnaire has been validated by our group29 and used widely in the literature.30
SARC-F questionnaire: the SARC‐F questionnaire includes five components—strength, assistance walking, rise from a chair, climb stairs and falls. SARC‐F scores range from 0 to 10 and a score≥4 is predictive of sarcopenia.31
Illness Representations Questionnaire (Brief) (IPQ-R): the IPQ-R is a widely accepted measure of illness representations. These components are identity, cause, timeline, consequence and controllability/cure. The questionnaire consists of three parts: an identity scale, a structure scale, and a causal scale.32
General Practice Physical Activity Questionnaire (GPPAQ): the GPPAQ was developed by the WHO and Department of Health detailing a 4-level Physical Activity Index reflecting an individual’s current physical activity. The GPPAQ is a validated measure of physical activity behaviour in CKD.33
UK Diabetes and Diet Questionnaire (UKDDQ): the UKDDQ is a 25-item questionnaire designed to assess diet and dietary behaviours. Respondents are asked to identify how often they consumed certain foods (vegetables, fruits, sugary drinks, processed meat) over that last month.34 Responses will be scored as per previous research.35 This questionnaire is sensitive to changes following an intervention.36
Medication Adherence Report Scale (MARS-5): the MARS-5 is a non-disease specific questionnaire used to measure adherence to medications and has been previously used in CKD. This comprises of five questions regarding changing medication dosage, forgetting to take medication, consciously stopping taking medication, skipping medication, and using less than prescribed.37
Modified Economic Patient Questionnaire (EPQ): we will use a modified version of the ‘EPQ’ to assess participants’ use of inpatient and outpatient services and data on non-hospital-based health and social care use at all assessment points.38
When the participant consents to the study, the researcher will access their clinical records and extract information such as blood and urine results to gather information of kidney function, proteinuria, iron status and lipid profiles. We will record prescribed medication. Self-reported comorbidities will be checked against those recorded in the medical notes.
Substudy
In an optional substudy, the following additional physical assessments will be performed during a visit to a hospital site:
Anthropometry: height, weight, body mass index and waist and hip circumference will be measured in line with established procedures. Resting heart rate and blood pressure will be assessed using a standard sphygmomanometer device.
Muscle phenotyping: muscle and fat mass/size/thickness, and body fat per cent will be measured using a free-standing bioelectrical impendence analysis (BIA) monitor and B-mode ultrasonography of the rectus femoris muscle. These are painless, non-invasive methods for measuring body composition. Our group has recently validated our BIA device against dual-energy X-ray absorptiometry,39 while ultrasonography can be used to assess sarcopenia in CKD.40 41
Gait speed: the participant is asked to walk a 4 m course at their ‘usual’ walking speed, with a walking aid if normally used.42
Handgrip strength (HGS): HGS will be assessed using a handheld dynamometer (Jamar Plus+Digital). Participants will be asked to hold the dynamometer with their shoulder at 0° flexion and elbow at 90° flexion. Participants will be asked to squeeze the dynamometer handle maximally for 3 s. The peak force output (kg) from the three attempts from both the dominant and non-dominant hand will be recorded.43
Sit-to-stand-60 test: sit-to-stand (STS) tests are a good measure of functional ability and have been used extensively in patients with CKD. Our group has extensive use and knowledge of this test, and published reliability and validation data for it.44 The patient starts from a seated position on a hard, upright chair, with the feet flat on the floor and the knees bent at 90°. For the test, the patient simply stands up fully and then sits down again to the starting position, without using the hands (one repetition). The STS-60 test involves completing as many STS cycles as possible in 60 s. Participants will also be asked to record a STS-60 score as part of the online survey. Minimal guidance via an instruction sheet will be provided for participants, but no other instructions (ie, monitoring via a video call) will be utilised.
Timed-up-and-go’ test (TUAG): the TUAG assesses mobility and requires dynamic balance. The participant will be asked to rise from a chair, walk 3 m, turn around a cone, and sit back down.42
Accelerometery: to accurately measure objective physical activity, patients will wear a wrist accelerometer (GENEActiv) for a 7-day period before the intervention and post-intervention.33 45
Familiarisation of objective physical performance measures will be performed before baseline assessments. In order to assess any potential differences in administering the questionnaires online, participants in the sub-study will also be asked to complete the same questionnaires via paper format.
In the substudy, semistructured interviews will be held with a researcher trained in qualitative methodology. Individual interviews will last between ~30 and 60 min. Interviews will take place in private area. For those unable to attend a face-to-face interview, interviews may also be performed via telephone using a secure recording device as used currently in other studies by our group. Patients recruited may be interviewed on at least one occasion (eg, before and/or after the intervention). Up to three interviews will be conducted with participants depending on their voluntary participation and/or their status in the study: (1) preintervention and/or (2) postintervention (at 10 weeks) and/or (3) after the follow-up phase (at 20 weeks). Topics in this interview will include current self-management knowledge, skills, confidence and behaviours and attitudes towards lifestyle self-management. Topics of the subsequent interviews will include experiences of the intervention, the quality of the content, the quality of the delivery method, reasons for non-adherence with the intervention, and healthcare usage.
Patient and public involvement
A full description of how patients and their families were involved in the development of the programme can be found in Lightfoot.22 24 In summary, a study patient steering group was formed consisting of 10 individuals living with kidney disease and two family members. An initial priority setting workshop determined key topics of interest to this group, including lack of educational support from healthcare professionals. Following this, we developed an educational booklet which was codesigned using our patient steering group. This booklet was disseminated to local primary care practices where individuals provided feedback on the content. The information in this booklet was then adapted for digital use and was termed ‘My Kidneys & Me’ by the patient group. We also performed semistructured interviews with patients around self-management and the use of educational resources. Key examples of feedback from patients included the following: (1) to provide a symptom tracker to enable self-monitoring of symptoms; (2) include a separate session on sleep; (3) include a separate session on well-being, emphasising the importance of looking after mental health; (4) use of myth and fact quizzes as a way to test knowledge; and (5) state how long sessions should take in the introduction. Once ‘My Kidneys & Me’ was developed, members of the steering group were provided with access to the programme to provide initial comment. The patient steering group assisted with the selection of questionnaires for the study, and reviewed the final questionnaire survey to ensure that it was acceptable. Throughout the study, the steering group will be used to develop a suitable and relevant topic guide to explore participants attitudes towards self-management and the impact these have on their lives. The group will also be used to interpret initial and final findings. In addition, the patient steering group will support the development of the lay summary outputs to be disseminated to patients and the public.
Data analysis
Sample size calculation
With PAM-13 as the primary outcome in the full RCT, a total sample size of N=432 participants are required based on a 2:1 randomisation (n=288 in the intervention group and n=144 in the control group). This was based on previously published PAM-13 data by our group14 and on the required power (β=0.8; α=0.05) to detect a minimal clinically significant difference of 4 points in the PAM-13,46–48 and an expect attrition rate of 25%. For the initial nested pilot study, a pragmatic sample size of n=60 participants (n=40 in the intervention group and n=20 in the control group, based on 2:1 randomisation) will be used. We aim to recruit at least 10 patients into the optional sub-study.
Statistical analysis
Participant demographics and clinical characteristics will be analysed using descriptive statistics. The primary outcome is the PAM-13, where answers are weighted provide a score on a scale from 0 to 100. As recommended by Twisk et al,49 estimates of treatment effect will be assessed by longitudinal analysis of covariance. In this method, the outcome variable measured at the different follow-up measurements (postintervention at week 10, follow-up at week 20) is adjusted for the baseline value of the outcome. Analysis will be performed using ‘intention-to-treat’ (ITT) analysis. In an ITT approach, patients are analysed by how they were randomised regardless of their actual compliance with treatment or intervention. For patient-reported outcomes missing data items will be handled according to established protocols for the validated surveys. Additional post-hoc analysis will be performed to determine if differences exist in PAM score changes between those with low and high PAM scores.
In the substudy, interview recordings will be professionally transcribed verbatim. The precise analytical methodology used may change based on the nature of the data collected, however thematic analysis will be used as an initial foundation for data analysis as it provides a systematic model for managing and mapping the data. Analysis will follow recognised steps (eg, familiarisation, initial and confirmation of coding (using NVivo), defining themes). In an integrative strategy, any available quantitative data will be used to inform the qualitative analysis.
Considerations
This study aims to evaluate the effect of an evidence-based and theory-based digital self-management structured 10-week programme developed for patients with CKD called ‘My Kidneys & Me’ on patient activation (PAM-13). The use of a nested pilot study to assess the feasibility of the intervention and study design before continuation to a full RCT is a strength of the study. This will enable us to make necessary adjustment and changes if the feasibility data suggest that participants do not engage with the intervention or that outcome measures are not being completed. Despite including the patient steering group in the selection of questionnaires and review of the final questionnaire survey, there is potential that the questionnaire survey may risk overburdening the participants and affect missing data and evaluation accuracy. Should the nested pilot study indicate poor questionnaire completion rates, we will revise the breadth of the outcomes collected and simplify the questionnaire survey content to improve participant acceptability.
To minimise the risk of bias (selection, performance and detection), the study is a single-blind RCT. While a higher level of blinding is often preferred to further reduce the risk of bias,50 due to the nature of the intervention being tested in this study, it is not possible to blind participants to the intervention. However, one method that can be used to reduce selection bias in an RCT is adequate allocation concealment51 to prevent intentional and unintentional assignment of participants to either of the study groups.50 Group allocation will be random and stratified by age.
An improvement of ‘4-points’ in PAM-13 score is considered the minimal clinical important difference (MCID),46–48 thus we used the required power to detect this alongside previous published data by our group on PAM-13. The MCID of PAM-13 is generic and not specific to CKD, however, we aim to identify the MCID of PAM-13 in CKD using data from the full RCT if feasible. While there is evidence to suggest that patient activation has important associations with health outcomes, including in CKD52–54 and, interventions aiming to improve patient activation are meagre.5 Interventions aimed at increasing patient activation are often designed for people other long-term conditions such as diabetes and hypertension, and to our knowledge, there are no published interventions designed to specifically target patient activation, particularly assessed using the PAM-13, in those living with CKD.
Ethics and dissemination
To carry out this study, we will consider the Good Clinical Practice guidelines of the local governance organisations (University of Leicester and University Hospitals of Leicester NHS Trust) thus guaranteeing the protection of the rights, the safety and the well-being of the participants of the trial in compliance with the principles of the Declaration of Helsinki, as well as the credibility of the data obtained in the clinical trial. All participant data entered into the ‘My Kidneys & Me’ will be managed through the MyDESMOND platform. Data entered as part of the online outcomes are managed by Jisc Online Surveys under a University of Leicester license. A full informative sheet explaining the study in detail, the voluntary nature of the research and the procedure for the protection of their personal data will be provided along with the online consent form. Patients will be given the opportunity to contact the researchers with any questions prior to the informed consent. The informed consent obtained from study participants will be online.
This study was fully approved by the Research Ethics Committee (REC)-Leicester South and Health Research Authority (HRA) on the 19 November 2020 (reference: 17/EM/0357). The results are expected to be published in scientific journals and presented at clinical research conferences in 2024. Findings will be disseminated to patients and the wider kidney and healthcare community via social media platforms, interest groups, recruiting sites and institutions associated with the research team. Any subsequent changes to the study protocol will reviewed by the REC and HRA through appropriate amendments. Any significant protocol changes will be stated on the ISRCTN Registry. The study was prospectively registered as ISRCTN18314195.
Summary
‘My Kidneys & Me’, an online self-management and lifestyle education programme, aims to increase patient activation and promote self-management behaviours. If this is achieved, it is anticipated that the intervention will achieve its distal aims of improving patient QoL, physical function, and symptom burden while experiencing fewer hospital admissions and saving costs to the NHS.
Supplementary Material
Acknowledgments
The authors would like to thank participating members of our patient and public steering group in their support in the development of the study. The authors thank members of the professional stakeholder group who have contributed to the design and development of ‘My Kidneys & Me’: Jonathan Barratt, Mike Bonar, Christopher Brough, James Burton, John Feehally, Charlie Franklin, Matthew Graham-Brown, Michelle Hadjiconstantinou, Jenny Hainsworth, Vicki Johnson, Maria Martinez, Andrew Nixon, Vicky Pursey, Sally Schreder, Hannah Young, Noemi Vadazsy, Fiona Willingham and Lucina Wilde.
Footnotes
Contributors: All authors (CJL, TW, TY, MJD, AS) contributed to the design of the SMILE-K trial protocol. CJL, TW and AS are responsible for data collection. Analyses will be conducted by CJL, TW and AS. CJL and TW drafted the manuscript contributing equally. All authors read and approved the final manuscript.
Funding: This study is funded by the Stoneygate Trust and the Leicester NIHR Biomedical Research Centre. All PPIE activities were supported by two grants from the Leicester Kidney Care Appeal awarded to TJW and ACS. Funders had no input into study protocol.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. The Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 2.Kim LG, Cleary F, Wheeler DC, et al. How do primary care doctors in England and Wales code and manage people with chronic kidney disease? results from the National chronic kidney disease audit. Nephrol Dial Transplant 2018;33:1373–9. 10.1093/ndt/gfx280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker I, Steventon A, Williamson R, et al. Self-Management capability in patients with long-term conditions is associated with reduced healthcare utilisation across a whole health economy: cross-sectional analysis of electronic health records. BMJ Qual Saf 2018;27:989–99. 10.1136/bmjqs-2017-007635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull SA, Nitsch D, Caplin B, et al. The National CKD audit: a primary care condition that deserves more attention. Br J Gen Pract 2018;68:356–7. 10.3399/bjgp18X697997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lightfoot CJ, Nair D, Bennett PN, et al. Patient activation: the cornerstone of effective self-management in chronic kidney disease? Kidney Dial 2022;2:91–105. 10.3390/kidneydial2010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narva AS, Norton JM, Boulware LE. Educating patients about CKD: the path to self-management and patient-centered care. Clin J Am Soc Nephrol 2016;11:694–703. 10.2215/CJN.07680715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng S, Shen F, Wen A, et al. Detecting lifestyle risk factors for chronic kidney disease with comorbidities: association rule mining analysis of web-based survey data. J Med Internet Res 2019;21:e14204. 10.2196/14204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donald M, Kahlon BK, Beanlands H, et al. Self-Management interventions for adults with chronic kidney disease: a scoping review. BMJ Open 2018;8:e019814. 10.1136/bmjopen-2017-019814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39:1005–26. 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene J, Hibbard JH. Why does patient activation matter? an examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 2012;27:520–6. 10.1007/s11606-011-1931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res 2007;42:1443–63. 10.1111/j.1475-6773.2006.00669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney RL, Lemon SC, Person SD, et al. The association between patient activation and medication adherence, hospitalization, and emergency room utilization in patients with chronic illnesses: a systematic review. Patient Educ Couns 2015;98:545–52. 10.1016/j.pec.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Newland P, Lorenz R, Oliver BJ. Patient activation in adults with chronic conditions: a systematic review. J Health Psychol 2021;26:103–14. 10.1177/1359105320947790 [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson M L, et al. Patient activation and CVD risk in CKD. Health Expectations 2021. 10.1111/hex.13225 [DOI] [Google Scholar]
- 15.Nair D, Cavanaugh KL. Measuring patient activation as part of kidney disease policy: are we there yet? J Am Soc Nephrol 2020;31:1435–43. 10.1681/ASN.2019121331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK Kidney Association . UKRR CKD patient measures dataset: PAM and PROM data, 2022. Available: https://ukkidney.org/audit-research/data-permissions/data/ukrr-ckd-patient-measures-dataset/pam-and-prom-data
- 17.Shen H, van der Kleij RMJJ, van der Boog PJM, et al. Electronic health self-management interventions for patients with chronic kidney disease: systematic review of quantitative and qualitative evidence. J Med Internet Res 2019;21:e12384. 10.2196/12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller VM, Davies MJ, Etherton-Beer C, et al. Increasing patient activation through diabetes self-management education: outcomes of DESMOND in regional Western Australia. Patient Educ Couns 2020;103:848–53. 10.1016/j.pec.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 19.Hadjiconstantinou M, Schreder S, Brough C, et al. Using intervention mapping to develop a digital self-management program for people with type 2 diabetes: tutorial on MyDESMOND. J Med Internet Res 2020;22:e17316. 10.2196/17316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn LM, Davies MJ, Northern A, et al. Use of MyDesmond digital education programme to support self-management in people with type 2 diabetes during the COVID-19 pandemic. Diabet Med 2021;38:e14469. 10.1111/dme.14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Educ Behav 1998;25:545–63. 10.1177/109019819802500502 [DOI] [PubMed] [Google Scholar]
- 22.Lightfoot CJ, Wilkinson TJ, Hadjiconstantinou M, et al. The Codevelopment of “My Kidneys & Me”: A Digital Self-management Program for People With Chronic Kidney Disease. Journal of Medical Internet Research 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lightfoot CJ, Wilkinson TJ, Hadjiconstantinou M, et al. The co-development of ‘My Kidneys & Me’: a digital self-management programme for people with chronic kidney disease (Preprint). J Med Internet Res 2022. 10.2196/39657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lightfoot CJ, Wilkinson TJ, Memory KE, et al. Reliability and validity of the patient activation measure in kidney disease: results of Rasch analysis. Clin J Am Soc Nephrol 2021;16:880–8. 10.2215/CJN.19611220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918–30. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devraj R, Wallace LS. Application of the content expert process to develop a clinically useful low-literacy chronic kidney disease self-management knowledge tool (CKD-SMKT). Res Social Adm Pharm 2013;9:633–9. 10.1016/j.sapharm.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health 1997;19:179–86. 10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 29.Brown SA, Tyrer F, Clarke AL. Kidney symptom questionnaire: development, content validation and relationship with quality of life. J Ren Care 2018:173. 10.1111/jorc.12247 [DOI] [PubMed] [Google Scholar]
- 30.Nixon AC, Wilkinson TJ, Young HML, et al. Symptom-burden in people living with frailty and chronic kidney disease. BMC Nephrol 2020;21:1–11. 10.1186/s12882-020-02063-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–2. 10.1016/j.jamda.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 32.Moss-Morris R, Weinman J, Petrie K, et al. The revised illness perception questionnaire (IPQ-R). Psychol Health 2002;17:1–16. 10.1080/08870440290001494 [DOI] [Google Scholar]
- 33.Wilkinson TJ, Palmer J, Gore EF, et al. The validity of the ‘General Practice Physical Activity Questionnaire’ against accelerometery in patients with chronic kidney disease. Physiother Theory Pract 2022;38:1528–37. 10.1080/09593985.2020.1855684 [DOI] [PubMed] [Google Scholar]
- 34.England CY, Thompson JL, Jago R, et al. Development of a brief, reliable and valid diet assessment tool for impaired glucose tolerance and diabetes: the UK diabetes and diet questionnaire. Public Health Nutr 2017;20:191–9. 10.1017/S1368980016002275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emadian A, England CY, Thompson JL. Dietary intake and factors influencing eating behaviours in overweight and obese South Asian men living in the UK: mixed method study. BMJ Open 2017;7:e016919. 10.1136/bmjopen-2017-016919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.England C, Leary S, Thompson C, et al. Sensitivity to change of the UK diabetes and diet questionnaire in a specialist weight management service. Proc Nutr Soc 2020;79. 10.1017/S0029665120006163 [DOI] [Google Scholar]
- 37.Chan AHY, Horne R, Hankins M, et al. The medication adherence report scale: a measurement tool for eliciting patients' reports of nonadherence. Br J Clin Pharmacol 2020;86:1281–8. 10.1111/bcp.14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells A, McNicol K, Reeves D, et al. Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group-based metacognitive therapy (pathway group MCT): study protocol for a randomised controlled trial. Trials 2018;19:1–12. 10.1186/s13063-018-2593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson TJ, Richler-Potts D, Nixon DGD, et al. Anthropometry-based equations to estimate body composition: a suitable alternative in renal transplant recipients and patients with Nondialysis dependent kidney disease? J Ren Nutr 2019;29:16–23. 10.1053/j.jrn.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 40.Gould DW, Watson EL, Wilkinson TJ, et al. Ultrasound assessment of muscle mass in response to exercise training in chronic kidney disease: a comparison with MRI. J Cachexia Sarcopenia Muscle 2019;10:748–55. 10.1002/jcsm.12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson TJ, Gore EF, Vadaszy N. Utility of ultrasound as a valid and accurate diagnostic tool for sarcopenia: Sex‐Specific cutoff values in chronic kidney disease. J Ultrasound Med 2020. 10.1002/jum.15421 [DOI] [PubMed] [Google Scholar]
- 42.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013;24:822–30. 10.1681/ASN.2012070702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkinson TJ, Gabrys I, Lightfoot CJ, et al. A systematic review of handgrip strength measurement in clinical and epidemiological studies of kidney disease: toward a standardized approach. J Ren Nutr 2022;32:371–81. 10.1053/j.jrn.2021.06.005 [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson TJ, Xenophontos S, Gould DW. Test–retest reliability, validation, and “minimal detectable change” scores for frequently reported tests of objective physical function in patients with non-dialysis chronic kidney disease. Physiotherapy theory and practice 2018:1–12. 10.1152/ajprenal.00012.2018 [DOI] [PubMed] [Google Scholar]
- 45.Dillon CB, Fitzgerald AP, Kearney PM, et al. Number of days required to estimate habitual activity using wrist-worn GENEActiv accelerometer: a cross-sectional study. PLoS One 2016;11:e0109913. 10.1371/journal.pone.0109913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hibbard JH, Tusler M. Assessing activation stage and employing a "next steps" approach to supporting patient self-management. J Ambul Care Manage 2007;30:2–8. 10.1097/00004479-200701000-00002 [DOI] [PubMed] [Google Scholar]
- 47.Hibbard JH. Using systematic measurement to target consumer activation strategies. Med Care Res Rev 2009;66:9S–27. 10.1177/1077558708326969 [DOI] [PubMed] [Google Scholar]
- 48.Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient's level of activation. Am J Manag Care 2009;15:353–60. [PubMed] [Google Scholar]
- 49.J T, L B, T H, et al. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun 2018;10:80–5. 10.1016/j.conctc.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agabegi SS, Stern PJ. Bias in research. Am J Orthop 2008;37:242–8. [PubMed] [Google Scholar]
- 51.Schulz KF. Subverting randomization in controlled trials. JAMA 1995;274:1456–8. 10.1001/jama.1995.03530180050029 [DOI] [PubMed] [Google Scholar]
- 52.Magadi W, Lightfoot CJ, Memory KE, et al. Patient activation and its association with symptom burden and quality of life across the spectrum of chronic kidney disease stages in England. BMC Nephrol 2022;23:45. 10.1186/s12882-022-02679-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gair RM, Stannard C, Wong E. Transforming participation in chronic kidney disease: programme report: renal association, 2019. [Google Scholar]
- 54.Wilkinson TJ, Memory K, Lightfoot CJ, et al. Determinants of patient activation and its association with cardiovascular disease risk in chronic kidney disease: a cross-sectional study. Health Expect 2021;24:843–52. 10.1111/hex.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064916supp001.pdf (96.2KB, pdf)