Abstract
While the majority of Legionnaire’s disease has been attributed to Legionella pneumophila, Legionella micdadei can cause a similar infection in immunocompromised people. Consistent with its epidemiological profile, the growth of L. micdadei in cultured macrophages is less robust than that of L. pneumophila. To identify those features of the Legionella spp. which are correlated to efficient growth in macrophages, two approaches were taken. First, a phenotypic analysis compared four clinical isolates of L. micdadei to one well-characterized strain of L. pneumophila. Seven traits previously correlated with the virulence of L. pneumophila were evaluated: infection and replication in cultured macrophages, evasion of phagosome-lysosome fusion, contact-dependent cytotoxicity, sodium sensitivity, osmotic resistance, and conjugal DNA transfer. By nearly every measure, L. micdadei appeared less virulent than L. pneumophila. The surprising exception was L. micdadei 31B, which evaded lysosomes and replicated in macrophages as efficiently as L. pneumophila, despite lacking both contact-dependent cytopathicity and regulated sodium sensitivity. Second, in an attempt to identify virulence factors genetically, an L. pneumophila genomic library was screened for clones which conferred robust intracellular growth on L. micdadei. No such loci were isolated, consistent with the multiple phenotypic differences observed for the two species. Apparently, L. pneumophila and L. micdadei use distinct strategies to colonize alveolar macrophages, causing Legionnaire’s disease.
At the 1976 American Legion convention in Philadelphia, an outbreak of pneumonia, termed Legionnaire’s disease, led to the identification of the genus Legionella (31, 52). Although more than 40 species of Legionella are now known (7), surveillance studies have attributed 80 to 90% of Legionnaire’s disease cases to Legionella pneumophila (14, 50), and it has been studied in the greatest detail. L. pneumophila is an opportunistic pathogen: disease is typically restricted to people with underlying health conditions, including the elderly, cigarette smokers, individuals receiving immunosuppressive therapy, or organ transplant recipients (14, 50). In contrast, healthy individuals associated with outbreaks, recognized by their low titers of Legionella-specific antibodies, are often asymptomatic (34).
In nature, legionellae are found in fresh water and soil as parasites of amoebae (11, 28, 65). L. pneumophila may adapt to its distinct intracellular and aquatic environments by alternating between a “replicative” and a “virulent” form in response to growth conditions (13). When amino acids and other conditions are favorable, L. pneumophila replicates within a host cell vacuole. When amino acids are limiting, the bacteria become cytotoxic, resistant to osmotic stress, motile, sensitive to sodium, and competent to evade phagosome-lysosome fusion (13), traits which likely enable the progeny to survive and to disperse in the environment, then reestablish a protected intracellular replication niche. Many of the traits required by L. pneumophila to parasitize amoebae also contribute to its survival and growth in macrophages (18, 33, 72).
Although the molecular mechanisms of L. pneumophila pathogenesis remain largely undefined, a number of traits which are correlated with virulence have been described. When aerosols containing L. pneumophila are inhaled, alveolar macrophages ingest the bacterium by coiling or conventional phagocytosis (39, 62). Phagosomal bacteria can evade phagosome-lysosome fusion, associate sequentially with mitochondria and the rough endoplasmic reticulum (ER), then replicate to high numbers (37, 38, 77). To establish this replication niche, L. pneumophila may secrete virulence factors by a specialized transport apparatus. A number of dot and icm genes are required by L. pneumophila for both intracellular growth in macrophages and conjugal DNA transfer (70, 80), and some of the predicted Dot and Icm proteins resemble components of the Agrobacterium tumafaciens vir complex (17), the Bordetella pertussis ptl system (84), and the Helicobacter pylori cag complex (16), three secretion systems important for virulence. In addition, it has been shown that a type II secretion system contributes to the virulence of L. pneumophila (49).
The second most common etiologic agent of Legionnaire’s disease is Legionella micdadei (27, 50, 63), which infects immunocompromised hosts primarily (27, 47, 55, 56, 64, 68). Consistent with its epidemiology, L. micdadei is less virulent than L. pneumophila in guinea pig and tissue culture models of infection (29, 83). The genetic basis for the differential virulence of L. micdadei and L. pneumophila has not been determined. Indeed, very few studies have directly compared the pathogenesis of L. pneumophila with that of L. micdadei, and the variety of L. micdadei clinical isolates examined further complicates interpretation of this literature.
L. micdadei and L. pneumophila do share some features associated with virulence but not others. For example, like L. pneumophila, L. micdadei encodes flagella (6, 35), acid phosphatase (26), Mip (19, 57), and common antigen, or GroEL (5, 61), and both species inhibit superoxide anion generation by macrophages (25, 26). However, L. micdadei lacks phospholipase C and zinc metalloprotease, two factors thought to play a role in L. pneumophila virulence (26). And unlike L. pneumophila, macrophage internalization of L. micdadei via coiling phagocytosis (62) and association with the host ER (83) has not been observed. Indeed, whether L. micdadei belongs in the genus Legionella is controversial. As judged by the 16S rRNA similarity (30, 32) and DNA relatedness profiles (10), it can be argued that L. micdadei and L. pneumophila are distinct taxonomically. In spite of their genetic heterogeneity, some contend that the legionellae form a natural and practical phenotypic genus (7, 10).
A number of quantitative assays have been developed to evaluate particular traits associated with L. pneumophila virulence (13, 15, 41, 46, 60, 69, 79, 80, 82). To determine whether robust intracellular growth correlates with expression of one, or several, of these traits, four L. micdadei clinical isolates were compared to one well-characterized L. pneumophila strain. In addition, using a genetic gain-of-function strategy, we investigated whether any L. pneumophila loci could stimulate L. micdadei growth in cultured macrophages. Results obtained from both the approaches suggest that L. pneumophila and L. micdadei employ different strategies to replicate within amoebae and macrophages.
MATERIALS AND METHODS
Cell culture.
Bone marrow-derived macrophages were prepared from female A/J mice (Jackson Laboratory) as described previously (77). After a 7-day culture period in L-cell conditioned medium, macrophages were collected by centrifugation, suspended in RPMI 1640 containing 10% fetal bovine serum (RPMI-FBS; Gibco BRL), and plated as described below for intracellular infection and replication, phagosome-lysosome fusion, and cytotoxicity assays. Cells of the human monocyte cell line U937 (American Type Culture Collection) were cultured in RPMI-FBS containing 25 mM HEPES buffer, pH 7.0, and differentiated by treatment with phorbol 12-myristate 13-acetate (Sigma) as described previously (60).
Bacterial strains and media.
L. pneumophila Lp02, a virulent thymine auxotroph derived from the serogroup 1 Philadelphia-1 strain, was chosen as the prototype because it has been studied extensively (1, 8, 9, 12, 13, 21, 22, 36–38, 40, 46, 66, 67, 69, 70, 73, 74, 77–79). Four clinical isolates of L. micdadei were examined: 31B (University of Pittsburgh Hospital), Rivera (Stanford University Medical Center), and Camilleri (Stanford University Medical Center), kindly provided by Nicholas P. Cianciotto, and D-2676 (National Center for Infectious Diseases), kindly provided by Barry S. Fields. Bacterial strains were maintained at −70°C as glycerol stocks. Prior to experiments, strains were colony-purified either on N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered charcoal-yeast extract agar (CYE) or on CYE supplemented with 100 μg of thymidine/ml (CYET) and then cultured in ACES-buffered yeast extract broth (AYE) or in AYE supplemented with 100 μg of thymidine/ml (AYET).
Broth cultures of L. pneumophila were shown previously to express the following virulence traits exclusively in the stationary phase: sodium sensitivity, osmotic resistance, contact-dependent cytotoxicity, efficient infection initiation, and evasion of phagosome-lysosome fusion (13). Therefore, to learn when L. micdadei virulence was maximal, the relationship between growth phase, optical density at 600 nm (OD600), and virulence was determined for each of the L. micdadei strains. Entry and survival in macrophages by L. micdadei was similar for exponential- and post-exponential-phase cultures (data not shown). However, based upon sodium sensitivity (Nas) (see Fig. 5) and osmotic sensitivity (see Fig. 7) assays, L. micdadei was maximally virulent in the post-exponential phase of growth, which typically began at OD600s of 1.0 for 31B, 1.2 for Camilleri, 1.3 for D-2676, and 1.5 for Rivera (data not shown). Hence, unless stated otherwise, post-exponential-phase cultures were analyzed.
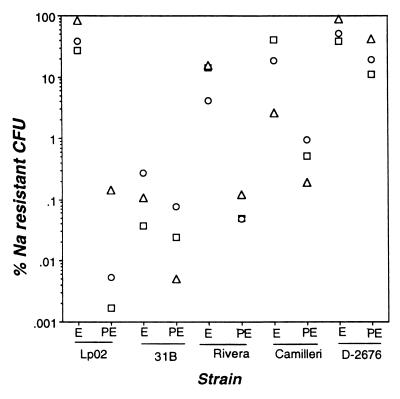
FIG. 5.
Sodium sensitivities of Legionella strains. Bacteria cultured to the exponential (E) and post-exponential (PE) phase were plated in duplicate onto CYE or CYET to quantify total CFU and onto CYE or CYET containing 100 mM NaCl to enumerate sodium-resistant CFU. Shown is the percent plating efficiency on sodium-containing medium determined in three experiments, each represented by a unique symbol (open circles, open squares, or solid triangles).
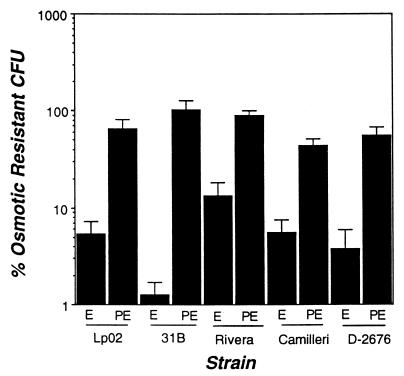
FIG. 7.
Osmotic sensitivity of Legionella. Exponential- and post-exponential-phase cultures of Legionella were incubated for 1 h in broth with or without 0.3 M KCl, diluted into distilled water, then plated in duplicate onto CYE or CYET to quantify CFU. Shown are the means and standard errors of the means obtained in six experiments.
Intracellular bacterial growth.
The abilities of Legionella strains to enter and survive in macrophages and to replicate within macrophages were measured as described previously (13, 77). Macrophages and U937 cells plated at a density of 2 × 105 to 3 × 105 cells per well in 24-well tissue culture dishes were infected for 2 h; next, extracellular bacteria were removed by washing the monolayers with RPMI-FBS, then incubating with 10 μg of gentamicin/ml in RPMI-FBS for 30 min. Infection initiation efficiency was calculated for triplicate samples by dividing the number of CFU associated with the monolayer at 2 h by the number of CFU added initially to the monolayer, then multiplying by 100. Bacterial replication in mouse macrophages and U937 cells was measured in triplicate over the subsequent 2- or 3-day period. Macrophages were infected with 31B and Lp02 at an approximate multiplicity of infection (MOI) of 1. For Camilleri, Rivera, and D-2676 infections, the MOI was increased to 5 to 10 to ensure that the number of CFU at 2 h was within the range of detection; neither the efficiency of infection initiation nor the growth rate of the strains was affected by the MOI (data not shown).
Microscopic assay for intracellular growth.
The ability of individual intracellular bacteria to replicate was analyzed by fluorescence microscopy essentially as described previously (79). Macrophages cultured on 12-mm glass coverslips were infected at approximates MOI of 1 for Lp02 and 31B, 10 for Rivera, 50 for Camilleri, and 25 for D-2676, conditions which ensured that only very rarely were macrophages infected by more than one bacterium. After 2 h, extracellular bacteria were killed by incubating the cultures with gentamicin (10 μg/ml) for 30 min. At this time, one set of coverslips was fixed with periodate-lysine-paraformaldehyde (53) containing 4.5% sucrose and prewarmed to 37°C, washed three times with phosphate-buffered saline (PBS), then stored at 4°C until further use. The remaining coverslips were incubated for an additional 16 h at 37°C to allow Lp02 to replicate within, but not escape from, the primary host cell. The 18-h samples were fixed as described above; then all of the samples were methanol extracted and washed three times with PBS before the DNA was stained fluorescently with 0.1 μg of 4′,6-diamidino-2-phenylindole (DAPI) per ml of PBS. For each time point, at least 50 infected macrophages were located, and the number of intact bacteria per macrophage was counted.
Phagosome-lysosome fusion assay.
The ability of each Legionella strain to evade phagosome-lysosome fusion was analyzed 2 h after infection by using fluorescence microscopy and the soluble endocytic probe Texas red-ovalbumin (TRov) as described previously (77). Prior to methanol extraction of the preparations, extracellular bacteria were stained with rabbit serum specific for L. micdadei (Monoclonal Technologies, Inc., Norcross, Ga.) or L. pneumophila (77) (a kind gift from Ralph R. Isberg) followed by Cascade Blue-conjugated anti-rabbit immunoglobulin G (IgG) antibody (Molecular Probes, Eugene, Oreg.). After methanol extraction of the preparations, both intracellular and extracellular bacteria were stained with L. micdadei- or L. pneumophila-specific antiserum followed by Oregon Green-conjugated anti-rabbit IgG (Molecular Probes). The efficiency of phagosome-lysosome fusion was calculated by dividing the number of intracellular bacteria that colocalized with TRov by the total number of intracellular bacteria, then multiplying by 100.
Strain 31B stained poorly with the L. micdadei-specific antiserum used in this study, making intracellular bacteria difficult to locate. Therefore, an alternate technique was used to label 31B cells fluorescently prior to infection as described before (76). 31B cells were incubated with 5(6)-carboxyfluorescein-N-hydrosuccinimide ester (FLUOS; Boehringer Mannheim Biochemica) for 30 min on ice and were washed twice with PBS and once with RPMI-FBS prior to use. FLUOS-labeled bacteria replicated within macrophages as efficiently as untreated cells, as judged by quantifying the yield of CFU from macrophage cultures 24 h after infection (data not shown). Extracellular bacteria were labeled preferentially by incubating the fixed preparations with DAPI, which does not stain phagosomal bacteria brightly when preparations are fixed but not methanol extracted (data not shown).
Cytotoxicity assay.
To quantify contact-dependent cytotoxicity, macrophages plated at a density of 3 × 104 to 5 × 104 cells per well of a 96-well tissue culture dish were incubated for 1 h with dilutions of each Legionella strain. Next, the infection medium was aspirated, the monolayers were incubated for 4 h with 0.1 ml of 10% (vol/vol) Alamar Blue (Accumed, Inc., Chicago, Ill.) in RPMI-FBS, and the redox-specific absorbance of the dye was measured as the OD570 and OD600 with a SpectraMax 250 spectrophotometer (Molecular Devices). The percentage of macrophages that were viable was calculated for triplicate samples from the slope of a plot of the A570/A600 determined for triplicate samples of six known densities of uninfected macrophages in the range of 103 to 5 × 104 cells per well. To determine the actual MOI, duplicate samples of the infection medium were plated onto CYE or CYET.
Sodium sensitivity and osmotic sensitivity.
The Nas of exponential- and post-exponential-phase broth cultures was determined as described previously (13) and calculated as (CFU on CYE or CYET agar containing 100 mM NaCl)/(CFU on CYE or CYET) × 100. The osmotic sensitivity of exponential- and post-exponential-phase broth cultures was determined as described previously (13) by using AYE or AYET broth which did or did not contain 0.3 M KCl and was calculated as (CFU of KCl-treated samples)/(CFU of untreated samples) × 100.
Conjugation efficiency assay.
How efficiently each Legionella strain donated plasmid DNA by conjugation was quantified by the method of Vogel et al. (80). First, each of the Legionella strains was transformed with pMS8 (1), a derivative of the mobilizable IncQ RSF1010 plasmid, which confers kanamycin resistance. Approximately 109 CFU of exponential-phase donor cells was mixed with 108 to 109 CFU of the recipient strain, Escherichia coli DH5α. The bacteria were collected by centrifugation, spread onto CYE or CYET, then incubated for 4 h at 37°C. To select for transconjugants, the mating mixture was collected onto a sterile swab, diluted into water, and plated onto Luria broth (LB)-kanamycin, which does not permit Legionella growth. Conjugation efficiency was calculated as (CFU of transconjugants)/(CFU of donor) × 100.
Genetic screen for genes that confer increased growth and survival in macrophages on L. micdadei.
To identify L. pneumophila genetic loci which enhance the intracellular growth of L. micdadei, we chose strain D-2676 as a cloning vehicle to minimize the background of false-positives. An L. pneumophila genomic library (1) consisting of 5- to 10-kb Sau3A Lp02 genomic fragments cloned into vector pMS8, which confers kanamycin resistance, was transferred by conjugation to D-2676. Transconjugants were collected as three separate pools, each containing approximately 104 members and representing more than 15 genome equivalents. To enrich for L. micdadei strains that replicated efficiently intracellularly, each pool of transconjugants was added at an approximate MOI of 1 to cultures of 2 × 106 macrophages in a six-well tissue culture dish. After a 4.5-h incubation, extracellular bacteria were removed by washing the monolayers with RPMI-FBS, then incubating the infected cultures with 10 μg of gentamicin/ml for 4 h. After 3 days, monolayers were lysed in PBS, and the surviving bacteria were recovered on CYE-kanamycin. Each pool of surviving bacteria was then subjected to a second enrichment cycle.
Next, individual colonies from each enrichment pool were tested for the ability to kill macrophages. For this purpose, clones were cultured to the post-exponential phase in 96-well microtiter dishes containing AYE-kanamycin. An aliquot of each culture was then transferred to a second set of microtiter dishes containing 105 macrophages per well, yielding an MOI of 5 to 10. After 3 days, the incubation medium was replaced with 0.1 ml of 10% Alamar Blue in RPMI (vol/vol); then macrophage viability was quantified as described above for the cytotoxicity assay.
To test whether the enhanced intracellular growth phenotype was linked to the L. pneumophila genomic clone or to the L. micdadei chromosome, plasmid DNA was isolated from each L. micdadei candidate by a standard nonalkaline procedure, followed by sequential precipitation with hexadecyltrimethylammonium bromide and ethanol (24). Plasmids that contained genomic fragments, as judged by restriction endonuclease mapping, were reintroduced into D-2676 by conjugation, then retested for the ability to confer increased growth in macrophages.
RESULTS
Intracellular replication of Legionella.
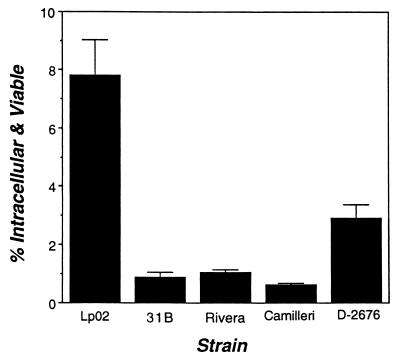
A hallmark of Legionella pathogenesis is the capacity to survive and to replicate within macrophages (48, 49, 57). Therefore, entry and survival in primary bone marrow macrophages by L. micdadei 31B, Rivera, Camilleri, and 31B and L. pneumophila Lp02 were compared. Macrophages were infected for 2 h; then extracellular bacteria were killed with gentamicin and cell-associated CFU were enumerated. As observed previously (13), approximately 8% of the inoculum of post-exponential-phase L. pneumophila cells initiated an infection of macrophages (Fig. 1). In comparison, only 1 to 3% of the L. micdadei strains did so, indicating less-efficient binding, entry, and/or survival in macrophages.
FIG. 1.
Efficiency of initiation of infection. Macrophages were incubated with each Legionella strain for 2 h; then the numbers of viable and cell-associated bacteria were determined by a standard gentamicin resistance assay (see Materials and Methods). Shown are the means and standard errors of the means calculated in one experiment performed in triplicate; similar results were obtained in six additional experiments.
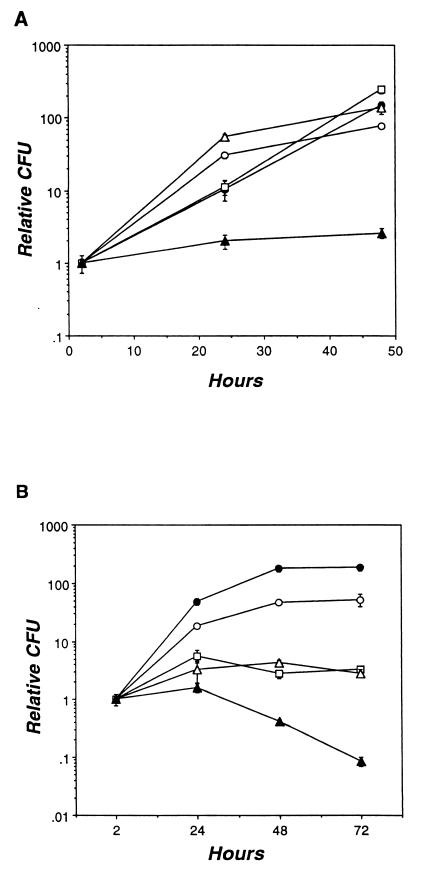
Next, the ability of intracellular bacteria to replicate in mouse macrophages and in the human monocyte cell line U937 was assessed. Consistent with previous reports (57, 60), L. pneumophila and L. micdadei 31B, Rivera, and Camilleri all showed robust growth in U937 cells, with a 100-fold increase in the yield of CFU over a 2-day period (Fig. 2A). Strain D-2676 was markedly less virulent: its CFU increased only slightly. Primary mouse macrophages were generally more restrictive for Legionella growth (Fig. 2B). The yield of CFU for L. pneumophila and L. micdadei 31B increased approximately 100-fold during a 72-h infection. In contrast, the numbers of Rivera and Camilleri CFU increased less than 10-fold during the first 24 h, then declined slightly. Again, the least virulent strain was D-2676: 24 h after infection, its yield of CFU declined markedly. Thus, as judged by growth in primary macrophages, L. micdadei 31B appeared as virulent as L. pneumophila, D-2676 appeared attenuated, and Rivera and Camilleri had an intermediate phenotype.
FIG. 2.
Intracellular growth of Legionella strains. (A) U937 cells were infected with the Legionella strains shown; then, at the times indicated, the numbers of viable bacteria were determined. The relative number of CFU was calculated by dividing the CFU at each time point by the CFU at the first time point; the actual initial CFU was 3.5 × 104 for Lp02 (open circles), 1.4 × 104 for 31B (solid circles), 2.7 × 104 for Rivera (open squares), 1.0 × 104 for Camilleri (open triangles), and 1.1 × 104 for D-2676 (solid triangles). The mean CFU was determined from triplicate samples; the standard error of the mean is indicated by error bars. Similar results were obtained in two other experiments. (B) Macrophages were infected with the Legionella strains shown; then intracellular growth was determined as described above. The actual initial CFU was 2.9 × 104 for Lp02 (open circles), 8.6 × 103 for 31B (solid circles), 2.1 × 104 for Rivera (open squares), 8.5 × 103 for Camilleri (open triangles), and 2.4 × 104 for D-2676 (solid triangles). Similar results were obtained in six other experiments.
Intracellular growth by individual bacteria.
The lower yield of CFU in primary macrophages observed for L. micdadei Rivera, Camilleri, and D-2676 could indicate that the majority of cells in the inoculum may replicate at a lower rate than L. pneumophila. Alternatively, a subset of cells in the inoculum may replicate efficiently while the majority do not multiply at all or are killed. To differentiate between these two possibilities, intracellular replication by individual cells was analyzed microscopically under conditions which ensured that only one bacterium entered each macrophage. Late in the primary infection period, approximately one-third of the macrophages infected with L. pneumophila contained more bacteria than could be counted, an indication of robust intracellular growth (Table 1). As expected, strain 31B showed a similar profile, confirming its efficient replication in macrophages (Table 1; Fig. 2). In contrast, more than 85% of the macrophages infected with Rivera, Camilleri, or D-2676 contained no more than 10 bacteria, indicating that most intracellular bacteria had not replicated efficiently. Interestingly, when infected with Rivera, Camilleri, or D-2676, a small minority of macrophages (<10%) contained bacteria too numerous to count. That a subpopulation of each of these strains established a primary infection in macrophages may reflect heterogeneity in the virulence of the bacterial population; alternatively, macrophages may not respond uniformly to infection.
TABLE 1.
Microscopic assay of intracellular growtha
| Strain | % Infected macrophages containing the following no. of bacteria/cell:
|
||
|---|---|---|---|
| 1–10 | 11–20 | >20 | |
| Lp02 | 50 ± 6.4 | 14 ± 2.0 | 36 ± 7.4 |
| 31B | 46 ± 1.3 | 20 ± 3.9 | 34 ± 4.9 |
| Rivera | 87 ± 2.9 | 6.3 ± 2.2 | 6.7 ± 1.8 |
| Camilleri | 88 ± 2.0 | 4.3 ± 0.3 | 7.7 ± 1.9 |
| D-2676 | 89 ± 4.1 | 4.0 ± 1.8 | 7.0 ± 2.3 |
Macrophage monolayers were infected with Legionella strains, incubated for 18 h, fixed, and stained with the DNA dye DAPI; then the number of bacteria per macrophage was counted. Each value represents the mean fraction of at least 50 infected macrophages which contained the indicated number of bacteria. Shown are the means and the standard errors of the means determined in three experiments.
Three of the L. micdadei strains grew poorly in primary macrophages, whereas strain 31B appeared as virulent as L. pneumophila Lp02. To investigate whether the relative intracellular growth levels of the four L. micdadei strains correlated to expression levels of particular virulence traits, a detailed phenotypic comparison was performed.
Evasion of phagosome-lysosome fusion.
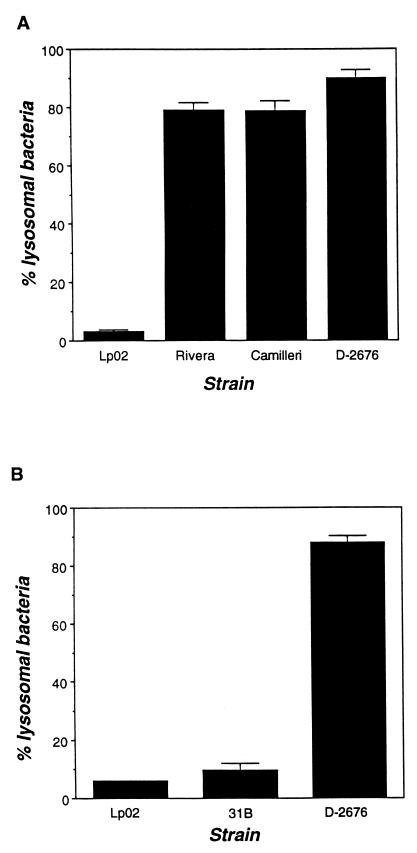
To determine whether the reduced initiation of infection (Fig. 1) reflected decreased survival of intracellular L. micdadei, the interaction between macrophage lysosomes and phagosomes harboring each of the strains was examined. First, macrophage lysosomes were labeled by endocytosis of the soluble fluorescent marker TRov; 2 h after infection with post-exponential-phase Legionella, colocalization of bacteria and TRov was evaluated by fluorescence microscopy (77). As expected, fewer than 4% of phagosomes harboring virulent L. pneumophila contained the lysosomal marker (Fig. 3A). In contrast, approximately 80% of Rivera, Camilleri, and D-2676 phagosomes had fused with lysosomes (Fig. 3A), a result consistent with the lower numbers of viable intracellular bacteria 2 h after infection (Fig. 1).
FIG. 3.
Evasion of phagosome-lysosome fusion by Legionella strains. (A) Macrophages prelabeled with the endocytic probe TRov were infected with the Legionella strain indicated for 2 h; then the percentage of intracellular bacteria that colocalized with TRov was determined by fluorescence microscopy. Intracellular and extracellular bacteria were stained differentially by using Legionella-specific antisera (Materials and Methods). (B) Evasion of phagosome-lysosome fusion was determined essentially as described above, except that macrophages were infected with Lp02, 31B, and D-2676 which had been prelabled with FLUOS. At least 50 intracellular bacteria were scored in each experiment; shown are the means and the standard errors of the means calculated from three experiments.
Strain 31B stained poorly with the L. micdadei-specific antiserum, making intracellular bacteria difficult to locate by this method. Instead, strain 31B was labeled directly with the fluorescent probe FLUOS prior to infection of macrophages. As expected, only 6% of phagosomes containing FLUOS-L. pneumophila fused with lysosomes, but nearly 90% of FLUOS–D-2676 phagosomes colocalized with TRov (compare Fig. 3A and B). Consistent with its efficient growth in macrophages (Fig. 2A and Table 1), phagosomes harboring 31B rarely fused with lysosomes (Fig. 3B). Thus, unlike the three other L. micdadei strains examined, the majority of intracellular 31B bacteria escaped lysosomal killing and established a replication niche in mouse macrophages.
Cytotoxicity for macrophages.
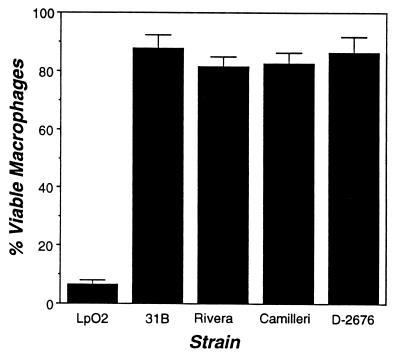
L. pneumophila can kill macrophages by a replication-independent but contact-dependent mechanism (41). Cytotoxicity has been correlated with L. pneumophila virulence, although its role in pathogenesis is not known (46). To measure L. micdadei cytotoxicity, macrophages and bacteria were cocultured for 1 h; then macrophage viability was measured by using the colorimetric dye Alamar Blue (51). As expected, at an MOI greater than 5, L. pneumophila killed approximately 95% of the macrophage monolayer (Fig. 4). In contrast, none of the L. micdadei strains were cytotoxic (Fig. 4). Even at the high MOI of 50, neither exponential- nor post-exponential-phase cultures of L. micdadei affected macrophage viability (data not shown). Surprisingly, even strain 31B, which replicates efficiently in macrophages, lacked contact-dependent cytotoxicity. Therefore, if the cytotoxicity of L. pneumophila is critical for establishment of its unique replication niche (45) and/or escape from amino acid-depleted host cells (13), L. micdadei must use an alternative strategy.
FIG. 4.
Cytotoxicity of Legionella strains for macrophages. Macrophages were incubated for 1 h with the bacterial strain indicated; then macrophage viability was assessed by using the redox-sensitive dye Alamar Blue (see Materials and Methods). Shown are the percentages (means and standard errors) of viable macrophages infected at an MOI of 5 with Camilleri, Rivera, and 31B (n = 6), D-2676 (n = 4), and Lp02 (n = 2).
Sodium sensitivity.
The ability of L. pneumophila to survive and to replicate in macrophages has been correlated with Nas (15, 69, 82), a trait expressed in the post-exponential phase (13). Yet two broad surveys of Legionella indicate that this correlation is not complete (2, 58). To assess the association between the virulence and Nas of L. micdadei, bacteria collected from exponential- and post-exponential-phase cultures were plated onto CYE which did or did not contain 100 mM NaCl; then colony formation was quantified. Consistent with the differences observed between virulent and avirulent L. pneumophila, the severely attenuated L. micdadei strain D-2676 was Nar, while the most virulent strain, 31B, was relatively Nas in both the exponential and the post-exponential phase (Fig. 5). Rivera and Camilleri, which appear to be partially attenuated, were slightly Nas during the exponential phase and more so in the post-exponential period, consistent with a previous report (58). In general, two rules held: relative Nas correlated with virulence, and the Nas of each of the strains increased in the post-exponential phase.
Conjugal transfer of DNA.
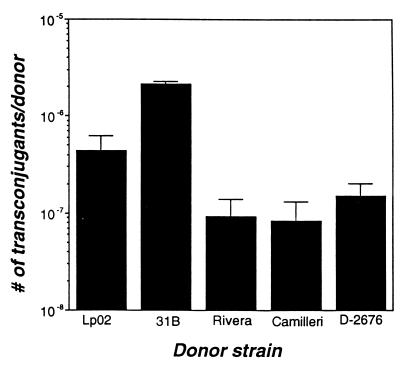
It has been proposed that L. pneumophila secretes virulence factors via the putative Dot-Icm complex, which also mediates the conjugal transfer of plasmid DNA (73, 80). To determine whether conjugation activity correlates with the virulence of L. micdadei, we compared the conjugation efficiencies of the four strains of L. micdadei with that of L. pneumophila. As predicted by the Dot-Icm secretion model (73, 80), the virulent L. micdadei strain 31B transferred DNA to the recipient more efficiently than L. pneumophila, whereas the three attenuated L. micdadei strains donated DNA at a rate approximately 10-fold lower than that of L. pneumophila (Fig. 6). In contrast, L. pneumophila dotB mutants donate plasmid DNA 100- to 1,000-fold less efficiently than the wild type (80) (data not shown). Whether transfer of plasmid DNA by L. micdadei is mediated by a Dot-Icm complex remains to be tested.
FIG. 6.
Conjugal DNA transfer. Legionella organisms cultured to exponential phase were mixed with an approximately equal number of CFU of the recipient strain DH5α, then incubated for 4 h. Appropriate dilutions of the mating mix were plated in duplicate onto LB-kanamycin plates to quantify conjugation efficiency. Shown is the number (mean and standard error) of conjugants obtained per donor in one experiment; similar results were obtained in at least one other experiment. The difference in conjugal efficiency between L. pneumophila and the L. micdadei strains was significant (P < 0.001 by the χ2 test of independence).
Osmotic resistance.
Like E. coli (43), L. pneumophila becomes osmotically resistant as it exits the exponential phase (13). A similar phenotypic switch was observed for all four strains of L. micdadei. As cultures of each of the strains entered the post-exponential phase, their osmotic resistance increased approximately 10-fold (Fig. 7). Thus, osmotic resistance correlated with the growth phase, but not the virulence, of L. micdadei.
Gain-of-function genetic screen for L. pneumophila virulence factors.
Two observations indicated that the L. micdadei strains are equipped for intracellular growth: all of the strains replicated in the U937 cell line (Fig. 2A), and a subpopulation of each exhibited robust growth in primary macrophages (Table 1). Yet, in nearly every phenotypic test, L. micdadei appeared less virulent than L. pneumophila. In an attempt to identify the genetic basis for this difference, we designed a gain-of-function, interspecies complementation strategy applied previously for a variety of pathogenesis model systems (4, 20, 23, 42, 54, 59, 75, 85). In particular, we screened an L. pneumophila genomic library for loci which conferred a robust intracellular growth phenotype on L. micdadei. To maximize the selective pressure exerted by the macrophages, we chose as the parent strain D-2676, a clinical isolate which is killed in macrophage lysosomes (Fig. 2B and 3).
To enrich for genomic clones which conferred increased intracellular survival and/or replication on L. micdadei, pools of D-2676 transconjugants were passaged twice in macrophage cultures. On the basis of previous infection and intracellular growth assays (Fig. 1 and 2A), we estimated that approximately 0.5% of the parental strain would survive each enrichment cycle. Judging from the percent recovery of each enrichment, none of the transconjugant pools appeared to contain strains with increased virulence (Table 2).
TABLE 2.
Genetic screen for L. pneumophila loci which stimulate L. micdadei intracellular growth
| Pool (no. of members)a | Enrichment cycle 1
|
Enrichment cycle 2
|
||||
|---|---|---|---|---|---|---|
| CFU at 0 h | CFU at 72 h | % Re-coveryb | CFU at 0 h | CFU at 72 h | % Re-coveryb | |
| A (1.5 × 104) | 5.1 × 106 | 6.0 × 103 | 0.12 | 6.4 × 106 | 2.9 × 103 | 0.05 |
| B (1.8 × 104) | 2.2 × 106 | 6.0 × 103 | 0.29 | 3.7 × 106 | 5.0 × 103 | 0.14 |
| C (1.6 × 104) | 3.3 × 106 | 7.5 × 103 | 0.23 | 11 × 106 | 5.2 × 103 | 0.05 |
Based on an average insert size of 5 to 10 kb and an estimated L. pneumophila genome size of 4 × 106, each pool represents the equivalent of 16 to 19 complete genomes.
To examine the pools obtained after enrichment in more detail, a total of 405 strains collected from each of the enrichment pools were screened individually for their ability to kill macrophages. Eighteen candidates exhibited a modest increase in macrophage killing, as measured by the Alamar Blue viability technique (see Materials and Methods). To determine whether the increased cytotoxicity was likely conferred by an L. pneumophila or an L. micdadei locus, the plasmid-borne L. pneumophila genomic clones were analyzed by restriction endonuclease mapping. Surprisingly, 13 of 18 candidate plasmids had sustained an extensive deletion and/or lacked an L. pneumophila insert. Moreover, upon retransformation of the parent, D-2676, none of the remaining five plasmids, which contained genomic fragments of varying sizes, conferred increased killing of macrophages. Therefore, passage in macrophage culture appeared to enrich for two classes of mutations: L. micdadei chromosomal mutations which enhanced virulence and plasmid mutations which relieved a growth inhibition, perhaps encoded by the original L. pneumophila genomic fragment or exerted by mobilization sequences of the IncQ RSF1010 plasmid (73). Neither class of mutants was analyzed further.
DISCUSSION
We took a comparative approach toward understanding the pathogenesis of the two causative agents of Legionnaire’s disease, L. pneumophila and L. micdadei. Using a series of quantitative phenotypic assays, we sought to identify those traits most likely to be required by Legionella for robust growth in macrophages. In nearly every test, L. micdadei strains appeared less virulent than L. pneumophila (Table 3). One hypothesis consistent with these results is that L. pneumophila and L. micdadei encode the same battery of virulence factors, but L. micdadei lacks a factor critical for high-level expression of one or more traits. Attempts to augment the intracellular growth of L. micdadei by providing L. pneumophila genomic fragments in trans were not successful (Table 2). Therefore, simplistic models in which a single virulence determinant or locus differentiates these pathogenic Legionella spp. were not supported. Instead, results of this study and others (62, 83) suggest that L. pneumophila and L. micdadei, though similar in some respects, use different strategies to parasitize host cells.
TABLE 3.
Summary of L. pneumophila and L. micdadei phenotypes
| Strain | Growth in U937 cellsa | Growth in macrophagesb | Entry and survivalc | Evasion of lysosomesd | Cyto-toxicitye | Sodium sensitivityf | Conjugationg | Osmotic resistanceh |
|---|---|---|---|---|---|---|---|---|
| L. pneumophila Lp02 | +++ | ++ | ++ | +++ | + | + | ++ | + |
| L. micdadei | ||||||||
| 31B | +++ | +++ | + | +++ | − | + | +++ | + |
| Rivera | +++ | + | + | + | − | + | + | + |
| Camilleri | +++ | + | + | + | − | + | + | + |
| D-2676 | + | − | + | + | − | − | + | + |
L. pneumophila expresses a contact-dependent cytotoxicity (41) concomitant with exit from the exponential phase of growth (13). Recently, a pore-forming activity that correlated with L. pneumophila virulence was characterized. When added to either erythrocytes or macrophages at a high MOI, L. pneumophila organisms insert a pore which leads to osmotic lysis and rapid cell death (46). Under normal infection conditions, L. pneumophila may insert pores into its nascent phagosomal membrane to block, by some undefined mechanism, subsequent fusion with the lysosomal network (45). Alternatively, or additionally, the cytotoxin may facilitate the lysis of host membranes by starved bacteria, which must escape to locate a new supply of nutrients (13). Surprisingly, none of the L. micdadei strains were cytotoxic for macrophages (Fig. 4), yet three of the strains replicated efficiently in U937 cells, and strain 31B also exhibited robust growth in the more-stringent environment of primary mouse macrophage cultures (Fig. 2). Moreover, strain 31B efficiently evaded phagosome-lysosome fusion in macrophages (Fig. 3B). Therefore, L. micdadei and L. pneumophila may establish and/or escape from replication vacuoles by different mechanisms. Alternatively, cytotoxicity may be dispensable for L. pneumophila growth in macrophages, despite a genetic or regulatory linkage to virulence (45, 46).
The L. pneumophila dot and icm genes are required for intracellular growth and for efficient conjugal transfer of plasmid DNA (71, 81). Thus, like A. tumefaciens (17) and B. pertussis (84), L. pneumophila may use a type IV secretion system to export virulence factors. A functional Dot-Icm apparatus has been correlated genetically with two other L. pneumophila virulence traits: Nas and contact-dependent cytotoxicity (69, 81, 82). By one model, export of a cytotoxin or other virulence factors by the Dot-Icm transport apparatus permits the accumulation of inhibitory levels of NaCl in the cytosol; mutational inactivation of this machinery confers salt resistance, loss of cytotoxicity, and avirulence (82). In our studies of L. micdadei, a strict correlation between conjugation efficiency, Nas, and cytotoxicity was not observed. For example, although the Rivera, Camilleri, and D-2676 strains of L. micdadei appeared similarly competent to transfer plasmid DNA by conjugation (Fig. 6), their degrees of Nas differed (Fig. 5), and none were cytotoxic (Fig. 4). Apparently, conjugal transport systems of Legionella do not necessarily confer Nas.
To begin to address whether L. micdadei encodes a Dot-Icm transport system, we tested whether L. micdadei contained genomic sequences homologous to dotA, dotB, dotE, dotF, or dotG of L. pneumophila. Under low-stringency Southern hybridization conditions which allowed a 30% base pair mismatch (Tm − 47°C) (3), full-length probes of dotA, dotB, dotE, and dotFG genes each hybridized with genomic fragments from each of the four L. micdadei strains (44). When the stringency was increased to allow a 20% base pair mismatch (Tm − 30°C) (3), only the dotA probe hybridized with L. micdadei genomic DNA. These data indicate that L. micdadei harbors sequences homologous to both dot-icm region I and region II (71). Whether L. micdadei encodes functional copies of the 23 dot-icm genes or assembles a functional Dot-Icm complex important for conjugation or intracellular growth remains to be established.
In macrophages and amoebae, L. pneumophila replicates in a compartment bounded by the ER (33, 37, 77). Based on morphological and kinetic studies of replication vacuole formation, Swanson and Isberg had proposed that L. pneumophila acquires a rich supply of nutrients by stimulating the eukaryotic autophagy pathway (77). In contrast, none of the L. micdadei strains examined to date appear to associate with the ER (83); instead, L. micdadei occupies a dilated phagosome (62). According to the autophagy exploitation model, the inability of L. micdadei to associate with the ER to obtain nutrients could account for its poor intracellular growth. However, L. micdadei 31B, which replicated in macrophages as efficiently as did L. pneumophila, occupied a dilated phagosome which was not associated with the ER, as judged by immunofluorescence microscopic localization of the ER luminal protein Bip (44, 77). Therefore, even though ER association has been correlated with the intracellular growth of L. pneumophila (37, 77, 79), it does not appear to play a role in L. micdadei pathogenesis. Thus, the intriguing question of how intracellular Legionella organisms obtain the amino acids needed for replication remains.
Designation of 31B as L. micdadei was suspect because its intracellular growth characteristics differed from those of the three other L. micdadei strains studied and because it stained poorly with the L. micdadei-specific antiserum. In particular, immunofluorescent staining of broth-grown 31B required fourfold-higher antiserum concentrations to achieve the intensity seen for Rivera, Camilleri, and D-2676. Interestingly, genetic divergence of 31B was also suggested by Southern hybridization analysis, which revealed restriction fragment polymorphisms in the chromosomal loci homologous to L. pneumophila dotA, dotB, dotE, and dotFG for 31B compared to the other three L. micdadei strains. On the other hand, 31B did not react strongly with L. pneumophila-specific antiserum (data not shown). Furthermore, previous molecular studies of the Mip gene and protein of strain 31B established its relatedness to several other L. micdadei clinical isolates (57). Additional molecular genetic studies are required to explain the potent virulence of 31B relative to other clinical isolates of L. micdadei.
Taken together, our data indicate that the differential virulence of L. pneumophila and L. micdadei is not attributable to a single phenotypic or genetic trait. It remains possible that L. micdadei lacks a particular genetic determinant which is critical for robust intracellular growth but which either was not represented in the L. pneumophila library, was poorly expressed by L. micdadei, or was not sufficient to bypass the more extensive genetic differences of D-2676. However, we favor the alternative view that Legionella is a diverse genus whose species appear to use quite different strategies to parasitize host cells. Perhaps the most intriguing questions raised by this comparative study are whether cytotoxicity, sodium sensitivity, the conjugation apparatus, and the host ER contribute to L. pneumophila pathogenesis, and if so, how.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R29AI40694-01 BM), the Rackham Faculty Research Fund, and the Department of Microbiology and Immunology at the University of Michigan.
REFERENCES
- 1.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa M, Hashimoto Y, Ezaki T, Yabuuchi E. Virulence of Legionella species does not correlate with their sodium chloride resistance. Med Microbiol Lett. 1992;1:30–37. [Google Scholar]
- 3.Ausubel F M, Brent R B, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 4.Banerjee A, Dubnau E, Quermard A, Balasubramanian V, Um K S, Wilson T, Collins D, de Lisle G, Jacobs W R. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 5.Bangsborg J M, Collins M T, Hoiby N, Hindersson P. Cloning and expression of the Legionella micdadei “common antigen” in Escherichia coli. APMIS. 1989;97:14–22. [PubMed] [Google Scholar]
- 6.Bangsborg J M, Hindersson P, Shand G, Hoiby N. The Legionella micdadei flagellin gene: expression in Escherichia coli K12 and DNA sequence of the gene. APMIS. 1995;103:869–877. doi: 10.1111/j.1699-0463.1995.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 7.Benson R F, Fields B S. Classification of the genus Legionella. Semin Respir Infect. 1998;13:90–99. [PubMed] [Google Scholar]
- 8.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 9.Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 10.Brenner D. Classification of legionellae. Semin Respir Infect. 1987;2:190–205. [PubMed] [Google Scholar]
- 11.Brown M R W. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 1999;7:46–50. doi: 10.1016/s0966-842x(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 12.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Investig. 1988;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carratala J, Gudiol F, Pallares R, Dorca J, Verdaguer R, Ariza J, Manresa F. Risk factors for nosocomial Legionella pneumophila pneumonia. Am J Respir Crit Care Med. 1994;149:625–629. doi: 10.1164/ajrccm.149.3.8118629. [DOI] [PubMed] [Google Scholar]
- 15.Catrenich C E, Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989;57:1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type-I specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in Eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cianciotto N P, Long R, Eisenstein B I, Engleberg N C. A Legionella pneumophila gene encoding a species-specific surface protein potentiates the initiation of intracellular infection. Infect Immun. 1989;57:1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark-Curtiss J E. Identification of virulence determinants in pathogenic mycobacteria. Curr Top Microbiol Immunol. 1998;225:57–79. doi: 10.1007/978-3-642-80451-9_4. [DOI] [PubMed] [Google Scholar]
- 21.Clemens D L, Horwitz M A. Hypoexpression of major histocompatibility complex molecules on Legionella pneumophila phagosomes and phagolysosomes. Infect Immun. 1993;61:2803–2812. doi: 10.1128/iai.61.7.2803-2812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens D L, Horwitz M A. Membrane sorting during phagocytosis: selective exclusion of major histocompatibility complex molecules but not complement receptor CR3 during conventional and coiling phagocytosis. J Exp Med. 1992;175:1317–1326. doi: 10.1084/jem.175.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins D M, Kawakami R P, de Lisle G W, Pascopella L, Bloom B R, Jacobs W R. Mutation of the principal ς factor causes loss of virulence in a strain of the Mycobacterium tuberculosis complex. Proc Natl Acad Sci USA. 1995;92:8036–8040. doi: 10.1073/pnas.92.17.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Sal G, Manfioletti G, Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages, or plasmids suitable for sequencing. BioTechniques. 1989;7:514–519. [PubMed] [Google Scholar]
- 25.Donowitz G R, Reardon I, Dowling J, Rubin L, Focht D. Ingestion of Legionella micdadei inhibits human neutrophil function. Infect Immun. 1990;58:3307–3311. doi: 10.1128/iai.58.10.3307-3311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowling J N, Saha A K, Glew R H. Virulence factors of the family Legionellaceae. Microbiol Rev. 1992;56:32–60. doi: 10.1128/mr.56.1.32-60.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang G, Yu V L, Vickers R M. Disease due to the Legionellaceae (other than Legionella pneumophila) Medicine. 1989;68:116–132. doi: 10.1097/00005792-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 29.Fields B S, Barbaree J M, Shotts E B, Jr, Feeley J C, Morrill W E, Sanden G N, Dykstra M J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986;53:553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox K F, Brown A. Properties of the genus Tatlockia. Differentiation of Tatlockia (Legionella) maceachernii and micdadei from each other and from other legionellae. Can J Microbiol. 1993;39:486–491. doi: 10.1139/m93-069. [DOI] [PubMed] [Google Scholar]
- 31.Fraser D W, Tsai T R, Orenstin W, Parken W E, Beechan H J, Sharrar R G, Harris J, Mallison G F, Martin S M, McDade J E, Shepard C C, Brachman P S. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 32.Fry N K, Warwick S, Saunders N A, Embley M T. The use of 16S ribosomal RNA analysis to investigate the phylogeny of the family Legionellaceae. J Gen Microbiol. 1991;137:1215–1222. doi: 10.1099/00221287-137-5-1215. [DOI] [PubMed] [Google Scholar]
- 33.Gao L Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haley C E, Cohen M L, Halter J, Meyer R D. Nosocomial Legionnaire’s disease: a continuing common-source epidemic at the Wadsworth Medical Center. Ann Intern Med. 1979;90:583–586. doi: 10.7326/0003-4819-90-4-583. [DOI] [PubMed] [Google Scholar]
- 35.Heuner K, Bender-Beck L, Brand B C, Luck P C, Mann K-H, Marre R, Ott M, Hacker J. Cloning and genetic characterization of the flagellum subunit gene (flaA) of Legionella pneumophila serogroup 1. Infect Immun. 1995;63:2499–2507. doi: 10.1128/iai.63.7.2499-2507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz M A, Silverstein S C. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwitz M A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isberg R R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins D E, Chaisson S A, Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990;172:2779–2781. doi: 10.1128/jb.172.5.2779-2781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi, A. D., and M. S. Swanson. Unpublished data.
- 45.Kirby J E, Isberg R R. Legionnaire’s disease: the pore macrophage and the legion of terror within. Trends Microbiol. 1998;6:256–258. doi: 10.1016/s0966-842x(98)01310-9. [DOI] [PubMed] [Google Scholar]
- 46.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 47.Korvick J A, Yu V L, Fang G-D. Legionella species as hospital-acquired respiratory pathogens. Semin Respir Infect. 1987;2:34–47. [PubMed] [Google Scholar]
- 48.Levi M H, Pasculle A W, Dowling J N. Role of alveolar macrophage in host defense and immunity to Legionella micdadei pneumonia in the guinea pig. Microb Pathog. 1987;2:269–282. doi: 10.1016/0882-4010(87)90125-2. [DOI] [PubMed] [Google Scholar]
- 49.Liles M R, Edelstein P H, Cianciotto N P. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol. 1999;31:959–970. doi: 10.1046/j.1365-2958.1999.01239.x. [DOI] [PubMed] [Google Scholar]
- 50.Marston B J, Lipman H B, Breiman R F. Surveillance for Legionnaires’ disease. Arch Intern Med. 1994;154:2417–2422. [PubMed] [Google Scholar]
- 51.McClain M S, Hurley M C, Brieland J K, Engleberg N C. The Legionella pneumophila hel locus encodes intracellularly induced homologs of heavy-metal ion transporters of Alcaligenes spp. Infect Immun. 1996;64:1532–1540. doi: 10.1128/iai.64.5.1532-1540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory diseases. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 53.McLean I W, Nakane P K. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 54.Miller V L, Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988;56:1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muder R R, Yu V L, Zuravleff J J. Pneumonia due to the Pittsburgh pneumonia agent: new clinical perspective with a review of the literature. Medicine. 1983;62:120–128. doi: 10.1097/00005792-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Myerowitz R L, Pasculle A W, Dowling J N, Pazin G J, Puerzer M, Yee R B, Rinaldo C R, Hakala T R. Opportunistic lung infection due to “Pittsburgh pneumonia agent”. N Engl J Med. 1979;301:953–958. doi: 10.1056/NEJM197911013011801. [DOI] [PubMed] [Google Scholar]
- 57.O’Connell W, Bangsborg J M, Cianciotto N P. Characterization of a Legionella micdadei mip mutant. Infect Immun. 1995;63:2840–2845. doi: 10.1128/iai.63.8.2840-2845.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Connell W A, Dhand L, Cianciotto N P. Infection of macrophage-like cells by Legionella species that have not been associated with disease. Infect Immun. 1996;64:4381–4384. doi: 10.1128/iai.64.10.4381-4384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pascopella L, Collins F M, Martin J M, Lee M H, Hatfull G F, Stover C K, Bloom B R, Jacobs W R. Use of in vivo complementation in Mycobacterium tuberculosis to identify a genomic fragment associated with virulence. Infect Immun. 1994;62:1313–1319. doi: 10.1128/iai.62.4.1313-1319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearlman E, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 61.Plikaytis B B, Carlone G M, Pau C-P, Wilkinson H W. Purified 60-kilodalton Legionella protein antigen with Legionella-specific and nonspecific epitopes. J Clin Microbiol. 1987;25:2080–2084. doi: 10.1128/jcm.25.11.2080-2084.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rechnitzer C, Blom J. Engulfment of the Philadelphia strain of Legionella pneumophila within the pseudopod coils in human phagocytes. Comparison with other Legionella strains and species. APMIS. 1989;97:105–114. [PubMed] [Google Scholar]
- 63.Reingold A L, Thomason B M, Brake B J, Thacker L, Wilkinson H W, Kuritsky J N. Legionella pneumophila in the United States: the distribution of serogroups and species causing human illness. J Infect Dis. 1984;149:819. doi: 10.1093/infdis/149.5.819. [DOI] [PubMed] [Google Scholar]
- 64.Rogers B H, Donowitz G R, Walker G K, Harding S A, Sande M A. Opportunistic pneumonia. A clinicopathological study of five cases by an unidentified acid-fast bacterium. N Engl J Med. 1979;301:959–961. doi: 10.1056/NEJM197911013011802. [DOI] [PubMed] [Google Scholar]
- 65.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 67.Roy C R, Isberg R R. Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect Immun. 1997;65:571–578. doi: 10.1128/iai.65.2.571-578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudin J E, Wing E J. A comparative study of Legionella micdadei and other nosocomial acquired pneumonia. Chest. 1984;86:675–680. doi: 10.1378/chest.86.5.675. [DOI] [PubMed] [Google Scholar]
- 69.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Segal G, Shuman H A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–255. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 72.Segal G, Shuman H A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segal G, Shuman H A. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol Microbiol. 1998;30:197–208. doi: 10.1046/j.1365-2958.1998.01054.x. [DOI] [PubMed] [Google Scholar]
- 74.Skerrett S J, Martin T R. Roles for tumor necrosis factor alpha and nitric oxide in resistance of rat alveolar macrophages to Legionella pneumophila. Infect Immun. 1996;64:3236–3243. doi: 10.1128/iai.64.8.3236-3243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snapper S B, Melton R E, Kieser M T, Jacobs W R. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 76.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 77.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:bp3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swanson M S, Isberg R R. Analysis of the intracellular fate of Legionella pneumophila mutants by fluorescence microscopy. Ann N Y Acad Sci. 1996;797:8–18. doi: 10.1111/j.1749-6632.1996.tb52944.x. [DOI] [PubMed] [Google Scholar]
- 79.Swanson M S, Isberg R R. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:bp873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 81.Vogel J P, Isberg R R. Cell biology of Legionella pneumophila. Curr Opin Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- 82.Vogel J P, Roy C, Isberg R R. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann N Y Acad Sci. 1996;797:271–272. doi: 10.1111/j.1749-6632.1996.tb52975.x. [DOI] [PubMed] [Google Scholar]
- 83.Weinbaum D L, Benner R R, Dowling J N, Alpern A, Pasculle A W, Donowitz G R. Interaction of Legionella micdadei with human monocytes. Infect Immun. 1984;46:68–73. doi: 10.1128/iai.46.1.68-73.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson T M, de Lisle G W, Collins D M. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium avium. Mol Microbiol. 1995;15:1009–1015. doi: 10.1111/j.1365-2958.1995.tb02276.x. [DOI] [PubMed] [Google Scholar]