Summary
Background
Antibiotic resistance leads to longer hospital stays, higher medical costs, and increased mortality. However, research into the relationship between climate change and antibiotic resistance remains inconclusive. This study aims to address the gap in the literature by exploring the association of antibiotic resistance with regional ambient temperature and its changes over time.
Methods
Data were obtained from the China Antimicrobial Surveillance Network (CHINET), monitoring the prevalence of carbapenem-resistant Acinetobacter baumannii (CRAB), Klebsiella pneumoniae (CRKP) and Pseudomonas aeruginosa (CRPA) in 28 provinces/regions over the period from 2005 to 2019. Log-linear regression models were established to determine the association between ambient temperature and antibiotic resistance after adjustment for variations in socioeconomic, health service, and environmental factors.
Findings
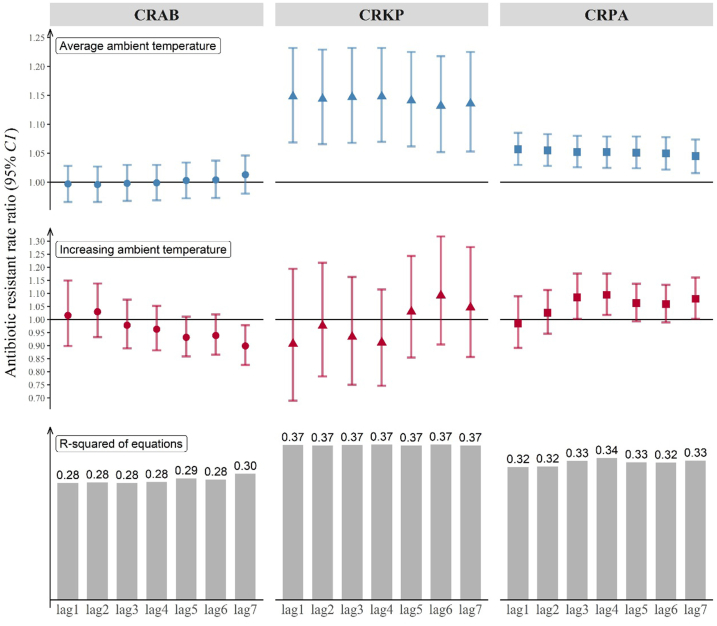
A 1 °C increase in average ambient temperature was associated with 1.14-fold increase (95%-CI [1.07–1.23]) in CRKP prevalence and 1.06-fold increase (95%-CI [1.03–1.08]) in CRPA prevalence. There was an accumulative effect of year-by-year changes in ambient temperature, with the four-year sum showing the greatest effect on antibiotic resistance. Higher prevalence of antibiotic resistance was also associated with higher antibiotic consumption, lower density of health facilities, higher density of hospital beds and higher level of corruption.
Interpretation
Higher prevalence of antibiotic resistance is associated with increased regional ambient temperature. The development of antibiotic resistance under rising ambient temperature differs across various strains of bacteria.
Funding
The National Key R&D Program of China (grant number: 2018YFA0606200), National Natural Science Foundation of China (grant number: 72074234), Fundamental Scientific Research Funds for Central Universities, P.R. China (grant number: 22qntd4201), China Medical Board (grant number: CMB-OC-19-337).
Keywords: Antibiotic resistance, Ambient temperature, Climate change, China
Research in context.
Evidence before this study
Antibiotic resistance and climate change have become the two biggest threats to global health. We searched in the PubMed between April 1, 2012 and April 1, 2022 with the terms “climate change” or “climate warming” or “temperature”, “antibiotic” or “antimicrobial” and“resistance”. Two reviews explored the potential impact of climate warming on antibiotic resistance, while three articles investigated the link between antibiotic resistance and temperature in the United States and Europe. Most available researches discussed common drug-resistant bacteria, accounting for several recognized resistance drivers like antibiotic consumption and population density. Few studies have looked at the long-term effects of increasing temperature on antibiotic resistance.
Added value of this study
We looked at the impact of a number of socioeconomic and environmental factors on three multidrug-resistant strains that require urgent research and intervention development. We discovered the prevalence of antibiotic resistance increases with higher regional ambient temperature, and highlighted year-by-year changes in ambient temperature have an accumulative effect on antibiotic resistance, with the four-year sum showing the greatest effect. These findings have added to the growing body of information from China regarding the link between antibiotic resistance and global warming.
Implications of all the available evidence
Our study revealed that fighting against antibiotic resistance is a long-term battle, as potential reduction in antibiotic resistance from reduced antibiotic use may be far slower than we expected due to the effect of climate change. More collaborative cooperation and cooperative action from the government and all sectors of the society need to be encouraged.
Introduction
Antibiotic resistance has become one of the biggest threats to global health, due to rapid spread of antibiotic resistance and slow discovery of new antibiotics. Antibiotic resistance occurs when bacteria evolve over time and cease to respond to previously effective antibiotics. Antibiotic resistance leads to longer hospital stays, higher medical costs and increased mortality.1,2 It was estimated that antibiotic resistance may have caused 1.27 million deaths in 2019, with 929,000 deaths attributable to the six leading pathogens: Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa.3 Uncontrolled antibiotic resistance also has significant implications on food and feed production, which can result in increased population poverty and inequality.4
Antibiotic resistance can be developed intrinsically or acquired by absorbing certain genetic codes from others or through self-mutation.5 Antibiotic use is the primary selective pressure promoting the genetic evolution of antibiotic resistance. Excessive and inappropriate use of antibiotics is believed to have hastened the emergence of resistant bacteria.6, 7, 8
Many socioeconomic and environmental factors have been found to be associated with the development of antibiotic resistance. Overcrowded living conditions and low income are linked to high prevalence of drug-resistant bacterial infections, such as methicillin-resistant S. aureus (MRSA).9,10 In China, the rapid socioeconomic development has witnessed increasing prevalence of antibiotic resistance. Gross domestic product (GDP) per capita, out of pocket (OOP) health expenditure, and physician density were found to be positively correlated with the prevalence of MRSA and E. coli (3GCREC) and K. pneumoniae (3GCRKP) that are resistant to the third-generation cephalosporin.11 Non-compliance with common laws and corruption are often blamed for the lack of control over antibiotic resistance.12,13
In recent years, researchers started to pay attention to the link between climate change and antibiotic resistance. Kaba and colleagues12 noticed a significant association between the warm-season change in temperature and the prevalence of carbapenem-resistant P. aeruginosa. Theoretically, bacterial activities are inextricably tied to temperature. Temperature has long been recognized to influence bacterial growth and its transfer of genomic material encoding antibiotic resistance.14,15 Global warming is usually accompanied by increased weather events such as rainstorm and flood. Heavy rainfall helps bacterial mutagenesis and expression of antibiotic resistance genes.16 MacFadden and colleagues17 revealed that the rise of local temperature in the US is linked to population level increase in antibiotic resistance from E. coli, K. pneumoniae, and S. aureus. Similar studies in Europe indicate that ambient temperature may be a key modulator of changes in antibiotic resistance rate.18 However, research into the relationship between climate change and antibiotic resistance remains inconclusive. Antibiotic resistance is likely to be developed over a certain period of time after repeated exposure to warmer ambient temperature. Therefore, this study aims to address the gap in the literature by exploring the link between local ambient temperature and its population prevalence of antibiotic resistance in China, and testing the potential accumulative effect of increased temperature.
Methods
Sample sources
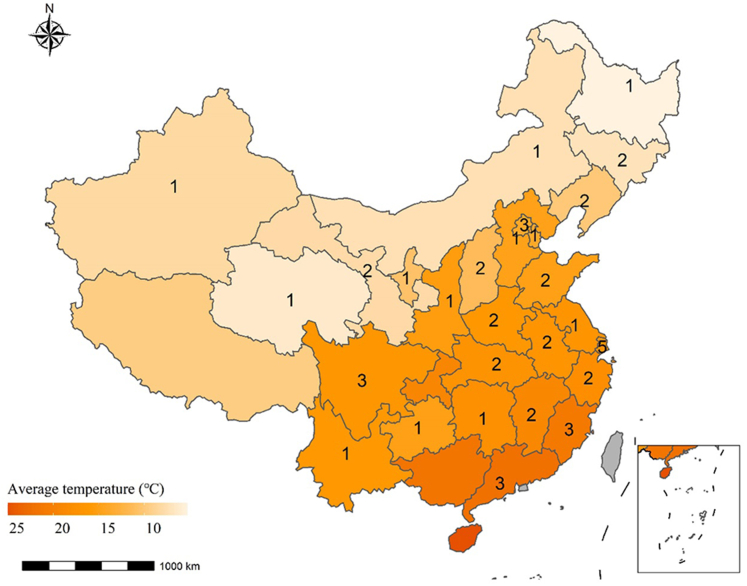
Data were obtained from the China Antimicrobial Surveillance Network (CHINET, http://www.chinets.com/Chinet), which was established in 2005. The antibiotic susceptibility testing followed the guidelines and quality control requirements specified in the Clinical and Laboratory Standards Institute (CLSI) document. CHINET totally covered 28 provinces and autonomous regions during the study period of 2005–2019. In 2019, the CHINET had covered 49 hospitals (36 tertiary and 13 secondary) across 27 provinces and autonomous regions in China (Fig. 1), and reported 0.14 million tests for the isolates of A. baumannii, 0.18 million for K. pneumoniae, and 14 million for P. aeruginosa, respectively (Table 1). The 15-year (2005–2019) panel dataset used in this study contained 603 observation records of annual prevalence of antibiotic resistance of the three isolates in the 27 provinces/regions of China.
Fig. 1.
Map with average ambient temperature. The numbers indicate the number of participating hospitals in 2019 in the China Antimicrobial Surveillance Network (CHINET) from each province or autonomous region.
Table 1.
Numbers of participating hospitals and tested isolates in the China Antimicrobial Surveillance Network (CHINET) from 2005 to 2019.
| Year | No. of hospitals | Tested isolates |
|||
|---|---|---|---|---|---|
| Total | A. baumannii | K. pneumoniae | P. aeruginosa | ||
| 2005 | 8 | 22,774 | 2095 | 2234 | 2323 |
| 2006 | 9 | 33,945 | 2968 | 3452 | 4752 |
| 2007 | 12 | 36,001 | 3157 | 3037 | 3988 |
| 2008 | 12 | 36,216 | 3625 | 3435 | 4130 |
| 2009 | 14 | 43,670 | 4796 | 4556 | 4912 |
| 2010 | 14 | 47,850 | 5523 | 5529 | 5080 |
| 2011 | 15 | 59,287 | 6723 | 6981 | 6012 |
| 2012 | 15 | 72,397 | 8739 | 9621 | 7271 |
| 2013 | 16 | 84,572 | 10,120 | 12,121 | 8257 |
| 2014 | 17 | 78,955 | 8769 | 11,308 | 7471 |
| 2015 | 20 | 88,778 | 8875 | 11,532 | 7700 |
| 2016 | 30 | 153,059 | 14,930 | 19,611 | 13,254 |
| 2017 | 34 | 190,610 | 17,602 | 26,268 | 16,562 |
| 2018 | 44 | 244,843 | 21,813 | 21,805 | 23,431 |
| 2019 | 49 | 270,497 | 23,534 | 39,379 | 23,607 |
Dependent variables
Prevalence of antibiotic resistance was measured by the detection rates of carbapenem-resistant A. baumannii (CRAB), K. pneumoniae (CRKP), and P. aeruginosa (CRPA) in each year by province/region. The three carbapenem-resistant Gram-negative bacteria (GNB) were chosen as our target species based on the following reasons: Firstly, they are the prominent causes of hospital-acquired infections (HAIs) leading to increased mortality. The World Health Organization (WHO) has labeled them as critical pathogens in need of urgent drug research and development.19,20 Secondly, carbapenems are the most reliable last-resort treatment for bacterial infections due to their broad spectrum of antibacterial activity against Gram-positive and Gram-negative bacteria and remarkable stability against most beta-lactamases.21 Thirdly, the CHINET has monitored CRAB, CRKP and CRPA over the past 15 years and made the dataset openly accessible.22, 23, 24, 25, 26, 27, 28
Independent and control variables
The independent variable, ambient temperature, was measured using two indicators: annual average ambient temperature (EQ1) and year-by-year change in average ambient temperature (EQ2). We also calculated the sum of year-by-year ambient temperature change of the current year and preceding years (Table 2).
Table 2.
Calculation of average ambient temperature and changes in average ambient temperature.
| No. | Equation |
|---|---|
| EQ1a | aver_temp = [(January … December)]2005–2019 summer_temp = [(June, July, August)]2005–2019 winter_temp = [(January, February, December)]2005–2019 |
| EQ2b | net_warming_lag1 = [(January … December)]2005–2019 – [(January … December)]2004–2018 net_warming_lag2 = net_warming_lag1 + [(January … December)]2004–2018 – [(January … December)]2003–2017 |
| net_warming_lag3 = net_warming_lag2 + [(January … December)]2003–2017 – [(January … December)]2002–2016 | |
| net_warming_lag4 = net_warming_lag3 + [(January … December)]2002–2016 – [(January … December)]2001–2015 | |
| net_warming_lag5 = net_warming_lag4 + [(January … December)]2001–2015 – [(January … December)]2000–2014 | |
| net_warming_lag6 = net_warming_lag5 + [(January … December)]2000–2014 – [(January … December)]1999–2013 | |
| net_warming_lag7 = net_warming_lag6 + [(January … December)]1999∼2013 – [(January … December)]1998–2012 |
EQ1: annual average ambient temperature over the period from 2005 to 2019.
EQ2: lag1 refers to change in average ambient temperature of the current year in contrast with the previous year; lag2 to lag7 refers to the sum of year-by-year changes in average ambient temperature of the current year and preceding years.
Historical data of monthly average ambient temperature (°C) for each province in which the participating hospitals were located, were extracted from the China Statistical Yearbook (http://www.stats.gov.cn/tjsj/ndsj/), which was calculated based on the average of major cities within each province.
Control variables
Previous studies showed that sociodemographic profiles,11 economic status,1 medical services (in particular antibiotic consumptions),1,12 environmental factors6,14,16, 17, 18 and corruption12 are associated with the development of antibiotic resistance.
China has a vast territory characterized by diversities in environmental, economic, and sociocultural contexts, as well as regional disparities in health resources and health services. In this study, population density, gross domestic production (GDP) per capita, number of health facilities, physicians and hospital beds per 10,000 population, annual average rainfall (mm), and annual average humidity (%) of the provinces in which the participating hospitals were located were extracted from the China Statistical Yearbooks and served as control variables. Annual antibiotic consumption measured by defined daily doses (DDDs) nationwide as reported by the National Health Commission was also included as a control variable. Corruption was measured by year-by-year Corruption Perceptions Index (CPI) reported by the Transparency International. CPI describes the national level of corruption of the public sector perceived by experts and businesspeople using a scale ranging from 0 to 100, with a lower score indicating higher corruption (Table 3).
Table 3.
Control variables used for statistical analysis.
| Variables | Description |
|---|---|
| DDDs | National total antibiotic consumption measured in defined daily doses per day per 100 population by year |
| CPI | National Corruption Perceptions Index by year |
| Population density | Provincial population per square kilometer by year |
| GDP | Provincial GDP (in current Chinese currency value) per capita, log-scale transformed, by year |
| Health facility | Provincial health facility density per 10,000 population by year |
| Physician | Provincial physician density per 10,000 population by year |
| Hospital bed | Provincial hospital bed density per 10,000 population by year |
| Humidity | Annual average humidity of major cities in each province (%) |
| Rainfall | Annual average rainfall of major cities in each province (mm) |
Statistical modelling
Statistical modelling on antibiotic resistance was established for the three tested isolates, respectively. The prevalence of CRAB, CRKP and CRPA (dependent variables) was transformed using the natural logarithm “LN(ABR)” function for log-linear regression modelling, because classical linear regression models could not fit proportion values between zero and one. Two types of models were established: one tested the effect of average ambient temperature adjusting for variations in the control variables; and the other tested the long-term accumulative effect of climate change on the prevalence of antibiotic resistance by adding changes in average ambient temperature. The addition of changes in average ambient temperature could help control the effect of years unrelated to ambient temperature. The sum of up to seven years of year-by-year changes in ambient temperature was considered in the modelling tests. Multicollinearity of the independent and control variables was defined as a VIF greater than 10 and a two-sided p value less than 0.05.
We used the panel data to address potential spatial–temporal heterogeneity, but found poorer model fitting results than pooled ones as indicated by the lower adjusted R2 (Supplementary Tables S1, S2, and S3). Thus, we decided to present the pooled modelling results in the manuscript.
Model validation
We predicted the 2019 prevalence (and 95% confidence interval) of antibiotic resistance in the non-participating regions (Tibet, Guangxi, Hainan and Chongqing) of the CHINET, using the established regression models, and compared the predicting values with the published data extracted from the China Antimicrobial Resistance Surveillance System (CARSS).
We performed sub-group modelling by restricting the sample to the 22 provinces (Supplementary Fig. S1), dividing the provinces/regions into northern (colder) and southern (warmer) using Qinling Mountains and Huaihe River as borderline (Supplementary Fig. S2), and testing summer (June, July, August) and winter (December, January, February) data separately.
Results
Antibiotic resistance over time
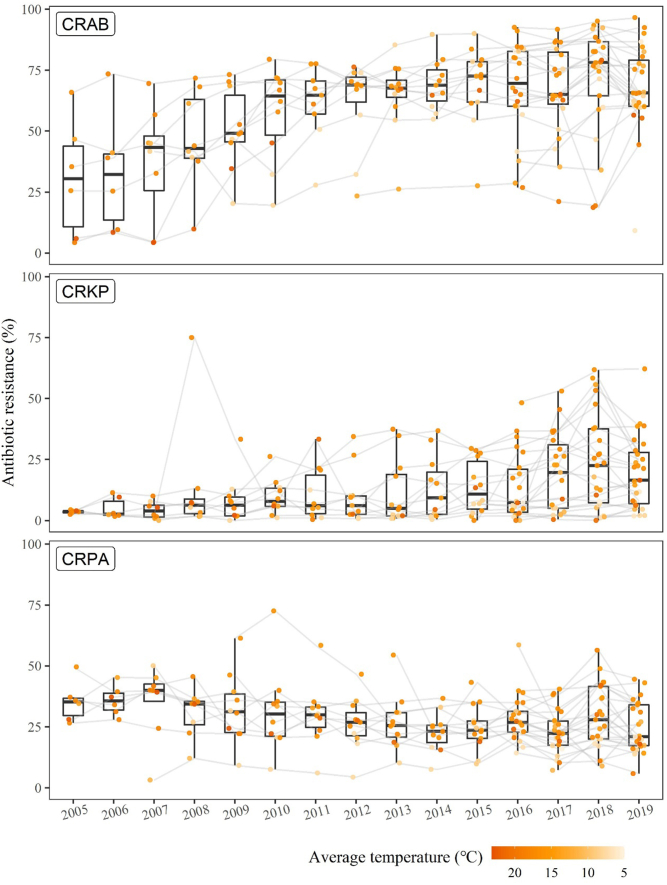
The prevalence of CRAB and CRKP showed a clear upward trend over time; whereas, the prevalence of CRPA showed a slightly downward trend. Higher prevalence of antibiotic resistance appeared in the provinces with a higher average ambient temperature, particularly for CRKP and CRPA (Fig. 2).
Fig. 2.
Prevalence of antibiotic resistance by province over the period from 2005 to 2019. Each point represents a province colored by its annual average ambient temperature. A lighter color indicates a lower average ambient temperature. The grey lines link the same province over time.
Correlation between antibiotic resistance and average ambient temperature
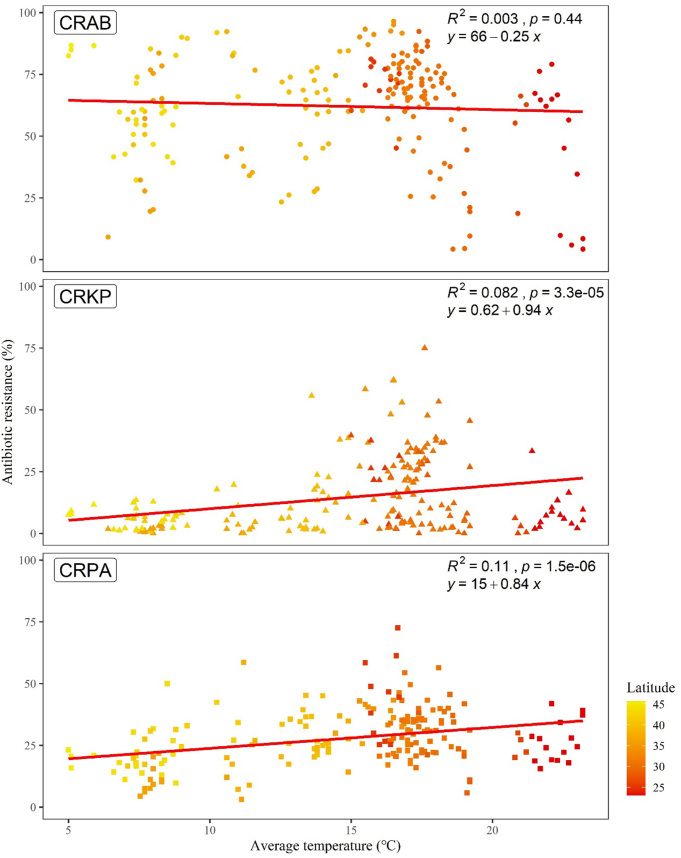
The prevalence of CRKP and CRPA increased with the rise of average ambient temperature (p < 0.001), although no such significant (p = 0.44) correlation was found for CRAB (Fig. 3).
Fig. 3.
Unadjusted linear trend line estimating the linear relationship between annual average ambient temperature and antibiotic resistance. Each point represents a province colored by latitude. The three pathogens were represented as geometric shapes.
Factors associated with antibiotic resistance
The log-linear regression models showed that average ambient temperature was a significant predictor of the prevalence of CRKP and CRPA, but not for CRAB (Table 4). An increase of 1 °C in average ambient temperature was associated with a rise of 1.14-fold (p < 0.001, 95%-CI [1.07–1.23]) of CRKP prevalence and 1.06-fold (p < 0.001, 95 %-CI [1.03–1.08]) of CRPA prevalence, respectively, after adjustment for variations of the control variables. Higher antibiotic consumption was also associated with higher CRPA prevalence, despite a lack of significant association with CRAB prevalence and CRKP prevalence. Lower health facility density and higher hospital bed density (larger numbers of beds in fewer facilities) were associated with higher prevalence of antibiotic resistance across all of the three target species. Higher perceived corruption (i.e. lower CPI) was associated with higher prevalence of CRPA (Table 4).
Table 4.
Rate ratios (RR)a of predictors on prevalence of antibiotic resistance.
| Predictor | CRAB |
CRKP |
CRPA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| RRa | p | VIF | RR | p | VIF | RR | p | VIF | |
| Antibiotic consumption (DDDs) | 1.00 | 0.275 | 3.00 | 1.01 | 0.326 | 3.01 | 1.01 | 0.012 | 2.94 |
| Corruption Perceptions Index (CPI) | 1.02 | 0.341 | 2.72 | 1.08 | 0.160 | 2.71 | 0.96 | 0.037 | 2.71 |
| Population density (person/km2) | 1.00 | 0.270 | 2.52 | 1.00 | 0.237 | 2.53 | 1.00 | 0.515 | 2.52 |
| GDP per capita (Yuan) | 0.95 | 0.679 | 4.66 | 0.71 | 0.258 | 4.53 | 1.09 | 0.434 | 4.62 |
| Health facilities per 10,000 population | 0.95 | 0.026 | 2.70 | 0.75 | <0.001 | 2.61 | 0.96 | 0.021 | 2.59 |
| Physicians per 10,000 population | 1.00 | 0.247 | 4.46 | 1.00 | 0.961 | 4.45 | 1.00 | 0.636 | 4.50 |
| Hospital beds per 10,000 population | 1.01 | 0.025 | 5.00 | 1.07 | <0.001 | 5.06 | 1.02 | 0.002 | 4.92 |
| Annual average humidity (%) | 1.01 | 0.226 | 5.14 | 0.97 | 0.105 | 4.94 | 1.00 | 0.545 | 4.81 |
| Annual average rainfall (mm) | 1.00 | 0.367 | 4.71 | 1.00 | 0.528 | 4.61 | 1.00 | 0.064 | 4.60 |
| Average ambient temperature (oC) | 1.00 | 0.862 | 4.18 | 1.14 | <0.001 | 4.13 | 1.06 | <0.001 | 4.13 |
| R2 | 0.277 | 0.366 | 0.315 | ||||||
| adj.-R2 | 0.239 | 0.332 | 0.278 | ||||||
Rate ratio was obtained from the estimated coefficient of each predictor through exponential conversion.
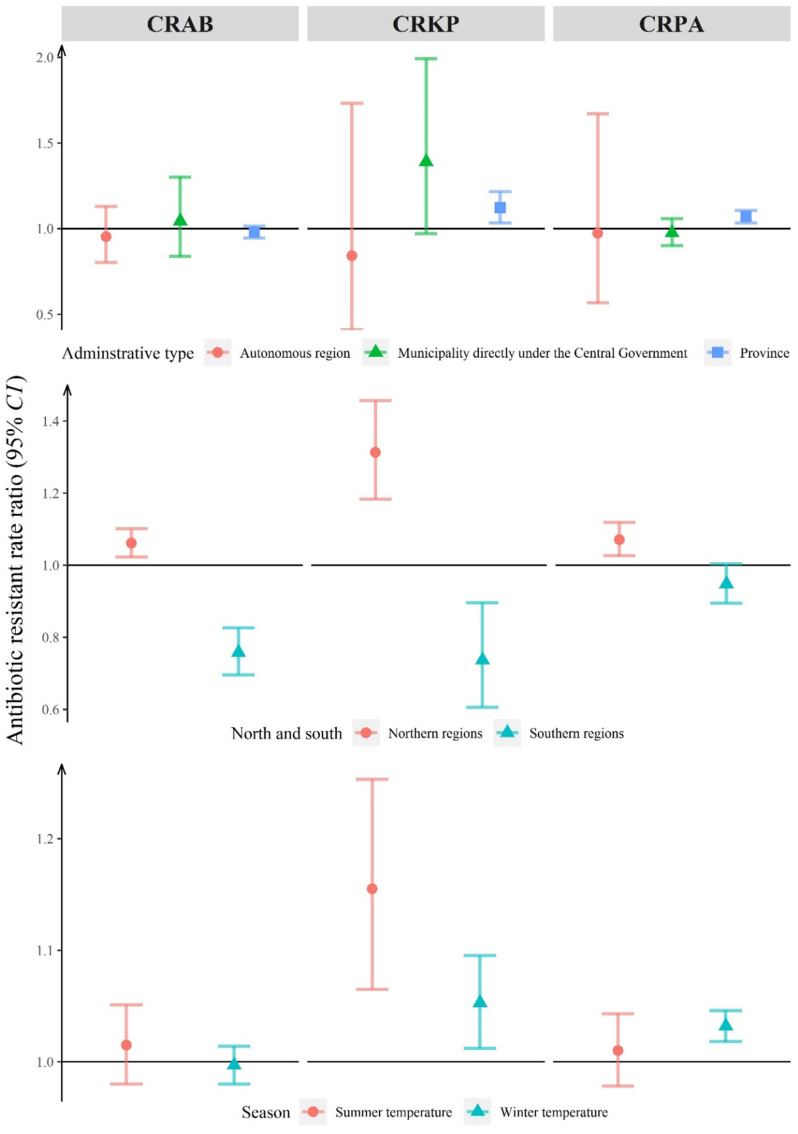
Positive associations between ambient temperature and the prevalence of antibiotic resistance remained significant for CRAB, CRKP and CRPA in northern China, for CRKP and CRPA in the 22 provinces, for CRKP and CRPA in winter, and for CRKP in summer. Negative associations between ambient temperature and the prevalence of CRAB and CRKP were found in southern China (Fig. 4).
Fig. 4.
Rate ratios of antibiotic resistance associated with ambient temperature: results of sub-group modelling.
CRAB was highly prevalent in Tibet, Guangxi, Hainan and Chongqing, compared with very low prevalence of CRKP and CRPA. The unstandardized estimations of modelling produced a higher prediction accuracy for CRAB as indicated by the Index of Estimation Deviation in comparison with CRKP and CRPA (Supplementary Table S4). The vast majority of predicting values fell within the 95% of the prediction interval even though the interval exceeded 100%, indicating a high level of uncertainty of prediction (Supplementary Table S5).
Accumulative effects of ambient temperature
Changes in average ambient temperature showed an accumulative effect, with the four-year sum in year-by-year changes in ambient temperature having the greatest effect: RR = 1.09 (p = 0.016, 95%-CI [1.02–1.18]) for CRPA. Average ambient temperature remained a significant stable predictor of antibiotic resistance for CRKP prevalence and CRPA prevalence regardless what indicators of changes in average ambient temperature were introduced in the models (Fig. 5).
Fig. 5.
Rate ratios of antibiotic resistance for the three target species (CRAB, CRKP, CRPA) when average ambient temperature and changes in average ambient temperature jumped by 1 °C. The bars represent the R-squared of the equations.
Discussion
Our study shows that population level prevalence of antibiotic resistance increases with rising regional ambient temperature and year-by-year accumulative warming. These results are consistent with the findings of studies conducted in the US and Europe.12,17,18 We found that a 1 °C increase in regional-level average ambient temperature is associated with 1.14-fold increase in CRKP prevalence and 1.06-fold increase in CRPA prevalence in China. In comparison, a 0.5 °C increase in year-wise temperature change was found to be associated with 1.02-fold increase in CRPA prevalence in Europe.12 It is important to note that some target species (such as CRPA and CRKP) are more sensitive to changes in ambient temperature than others (such as CRAB) according to the findings of our study. Using K. pneumoniae as a target strain, a 10 °C increase in ambient temperature was found to be associated with 2.2% increase in antibiotic resistance in the US17 and 1.2% increases in European countries.18
From a microcosmic perspective, the link between temperature and antibiotic resistance can be explained by their physiological similarities in triggering cell responses. Both aminoglycosides (a class of antibiotics) and heat stress enhance misfolded proteins in cells.29,30 As a result, heat exposure can stimulate bacteria to develop antibiotic resistance through a process known as cross-tolerance.31, 32, 33
We found a negative association between ambient temperature and the prevalence of antibiotic resistance in southern China. Southern China has warm weathers all year around. The growth of bacteria and their development of antibiotic resistance may be hampered by high temperature34,35 due to varied production of biofilm.36 Ambient temperature in southern China also fluctuates within a relatively small range, with most of the annual average ranging from 15 to 20 °C, which can jeopardize the reliability of modelling. Further studies are needed to explore the potential nonlinear association between ambient temperature and antibiotic resistance.
There is an accumulative effect of increasing ambient temperature on the development of CRPA, according to the findings of our study. The widespread distribution of P. aeruginosa in nature37 and its fast response to selective pressure imposed by antibiotic use provides a potential explanation.38 Another possible explanation may be related to the temperature-regulated acyltransferase (PA3242) and temperature-regulated 2-OH-lauroytransferase (PA0011). Both play a key part in changes in the composition of outer membrane of bacteria, strengthening their ability to adapt to environmental stimuli.39,40 The most likely scenario is that bacteria are exposed to antibiotics and stressful temperature simultaneously or in sequential, resulting in gradual changes over the course of their lifetime. It may take a few generations to develop antibiotic resistance. More time is needed to allow the antibiotic-resistant bacteria to become a dominant strain, displacing the susceptible ones.6 Heat shock mechanism generated by high ambient temperature can help with the process through enhancing gene expression, inducing phenotypic heterogeneity, and producing persistent antibiotic-resistant bacteria.41,42 In the long run, ambient temperature also plays a role in gene mutation and maintenance of antibiotic resistance.6,43 Higher temperature can reduce the fitness cost of mutation while preserving its resistance to antibiotics after removal of antibiotics.44, 45, 46, 47
It is undeniable that over- and inappropriate use of antibiotics remains to be an important challenge in addressing the problem of antibiotic resistance. We found that CRPA prevalence increases with antibiotic consumption even after adjustment for variations in other factors, including ambient temperature. Serious abuse of antibiotic usage is evident in China,48 which has been proved to be associated with antibiotic resistance from bacteria like E. coli and S. aureus.49 Over the past few decades, China has taken great efforts to reduce antibiotic use. In this study, we found that lower health facility density and higher hospital bed density are associated with higher prevalence of antibiotic resistance across all three target species. This is concerning as medical resources have been increasingly concentrated in large hospitals in metropolitan areas in China, which reduces the competition effect that may improve antibiotic prescribing practices.50 Indeed, hospital size in China is usually large, and inappropriate prescription of antibiotics in these hospitals is highly prevalent.51,52 Our findings about the association of corruption with antibiotic resistance is consistent with that in the European area.12 Corruption could be considered as a cultural determinant of non-compliance to common regulations, and several antibiotic–species pairs resistance was found to be associated with the corruption.13 Furthermore, association of corruption with antibiotic use was confirmed somehow, indicating that corruption might confound the influence of antimicrobial use on antibiotic resistance.53
There are several limitations in this study. Data used in this study came from the hospital monitoring system. Antibiotic products have been widely used in agriculture and animal husbandry, which are also associated with antibiotic resistance.54 Primary care facilities were not included in this study either, which served about half of outpatient visits. However, access to antibiotics in primary care is restricted to those included in the essential medicines list only. Our analysis did not consider time trends in antibiotic resistance; instead, we focused on the effect of year-by-year changes in ambient temperature. The models established in our study also have low R2 values. Further studies are needed to establish the causal relationship between climate change and antibiotic resistance and its underlying mechanisms.
Conclusion
Population level prevalence of antibiotic resistance increases with regional higher ambient temperature in China. Year-by-year changes in ambient temperature have an accumulative effect on antibiotic resistance, with the four-year sum showing the greatest effect. Variations exist among bacteria in developing antibiotic resistance under rising ambient temperature.
In light of the global warming and climate change, we have to prepare for a long-term fight against antibiotic resistance. Potential reduction in antibiotic resistance resulting from lower use of antibiotics may be far slower than we expected due to the effect of climate change.
Contributors
WBL, LS, YCZ, XYY, QXH, YP, CJL, HCH, CRH and LPY have substantial contributions to conception and design, acquisition of funding, data and interpretation of data; WBL and LPY analyzed the data, WBL and LPY drafted the article, CJL, LPY, HCH and CRH revised it critically for important intellectual content, WBL and LPY was responsible for the decision to submit the manuscript, and all authors contributed to final approval of the paper.
Data sharing statement
The datasets analyzed during the current study are publicly available at the CHINET (https://www.chinets.com/). YP is an employee of one of the CHINET member hospitals.
CHINET is not responsible for the correctness of the data and for data management, data merging and data collation. The accuracy of the authors' statistical analysis and the findings they report are not the responsibility of CHINET.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This study was funded by grants from the National Key R&D Program of China (grant number: 2018YFA0606200), National Natural Science Foundation of China (grant number: 72074234), Fundamental Scientific Research Funds for Central Universities, P.R. China (grant number: 22qntd4201), China Medical Board (grant number: CMB-OC-19-337).
We gratefully acknowledge the contribution of the members of CHINET in this study, including: Fupin Hu and Demei Zhu from Huashan Hospital, Fudan University; Yingchun Xu and Xiaojiang Zhang from Peking Union Medical College Hospital; Zhaoxia Zhang and Ping Ji from the First Affiliated Hospital of Xinjiang Medical University; Mei Kang and Chao He from West China Hospital, Sichuan University; Chuanqing Wang and Leiyan He from Children's Hospital of Fudan University; Yuanhong Xu and Ying Huang from the First Affiliated Hospital of Anhui Medical University; Zhongju Chen and Ziyong Sun from Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology; Yuxing Ni and Jingyong Sun from Ruijin Hospital, Shanghai Jiaotong University School of Medicine; Yunzhuo Chu and Sufei Tian from the First Affiliated Hospital of China Medical University; Zhidong Hu and Jin Li from Tianjin Medical University General Hospital; Yunsong Yu and Jie Lin from Sir Run Run Shaw Hospital, Zhejiang University School of Medicine; Bin Shan and Yan Du from the First Affiliated Hospital of Kunming Medical University; Sufang Guo and Yanyan Wang from the First Affiliated Hospital of Inner Mongolia Medical University; Lianhua Wei and Xin Wang from Gansu Provincial Hospital; Hong Zhang and Chun Wang from Children's Hospital of Shanghai; Yunjian Hu and Xiaoman Ai from Beijing Hospital; Chao Zhuo and Danhong Su from the First Affiliated Hospital of Guangzhou Medical University; Ruizhong Wang and Hua Fang from Pudong New Area People's Hospital; Bixia Yu from Zhejiang Ningbo Zhenhai Longsai Hospital; Ping Gong and Miao Song from the People's Hospital of Zigui, Hubei Province; Dawen Guo and Jinying Zhao from the First Affiliated Hospital of Harbin Medical University; Wen'en Liu and Yanming Li from Xiangya Hospital, Central South University; Yan Jin and Yueling Wang from Shandong Provincial Hospital; Kaizhen Weng and Yirong Zhang from Jinjiang Municipal Hospital; Xuesong Xu and Chao Yan from China–Japan Union Hospital, Jilin University; Xiangning Huang and Hua Yu from Sichuan Provincial People's Hospital; Yi Li and Shanmei Wang from Henan Provincial People's Hospital; Lixia Zhang and Juan Ma from Shaanxi Provincial People's Hospital; Shuping Zhou and Jiangwei Ke from Jiangxi Provincial Children's Hospital; Lei Zhu and Jinhua Meng from Children's Hospital of Shanxi; Han Shen and Wanqing Zhou from Nanjing Drum Tower Hospital, Affiliated Hospital of Nanjing; Gang Li and Wei Jia from General Hospital of Ningxia Medical University; Jinsong Wu and Yuemei Lu from Shenzhen People's Hospital; Jihong Li from the Second Hospital of Hebei Medical University; Jiangshan Liu from Jinchang Hospital of integrated traditional Chinese and Western Medicine; Longfeng Liao from the People's Hospital of Ganxian; Hongqin Gu from Guangrao County People's Hospital; Lin Jiang from the People's Hospital of Huixian, Henan Province; Wen He from Central Hospital of Yingkou Development Zone, Liaoning Province; Shunhong Xue from Huzhu County People's Hospital, Qinghai Province; Jiao Feng from the People's Hospital of Linshui, Sichuan Province; Rui Dou from Lixin County People's Hospital; and Chunlei Yue from Jiutai People's Hospital. Ruyi Guo and Yan Jin from Quanzhou First Hospital. Fujian; Xiaobo Ma and Yanping Zheng from The First Affiliated Hospital of Xiamen University; Fangfang Hu from Guizhou Provincial People's Hospital; Yunsheng Chen and Qing Meng from Shenzhen Children's Hospital.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2022.100628.
Appendix A. Supplementary data
References
- 1.Zhen X., Lundborg C.S., Sun X., Hu X., Dong H. The clinical and economic impact of antibiotic resistance in China: a systematic review and meta-analysis. Antibiotics. 2019;8(3) doi: 10.3390/antibiotics8030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhen X., Stålsby Lundborg C., Sun X., Zhu N., Gu S., Dong H. Economic burden of antibiotic resistance in China: a national level estimate for inpatients. Antimicrob Resist Infect Control. 2021;10(1):5. doi: 10.1186/s13756-020-00872-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutradhar I., Ching C., Desai D., et al. Computational model to quantify the growth of antibiotic-resistant bacteria in wastewater. mSystems. 2021;6(3) doi: 10.1128/mSystems.00360-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abushaheen M.A., Muzaheed, Fatani A.J., et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66(6) doi: 10.1016/j.disamonth.2020.100971. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Verdugo A., Lozano-Huntelman N., Cruz-Loya M., Savage V., Yeh P. Compounding effects of climate warming and antibiotic resistance. iScience. 2020;23(4) doi: 10.1016/j.isci.2020.101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Boeckel T.P., Brower C., Gilbert M., et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Boeckel T.P., Glennon E.E., Chen D., et al. Reducing antimicrobial use in food animals. Science. 2017;357(6358):1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Immergluck L.C., Leong T., Malhotra K., et al. Geographic surveillance of community associated MRSA infections in children using electronic health record data. BMC Infect Dis. 2019;19(1):170. doi: 10.1186/s12879-019-3682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieira M.T., Marlow M.A., Aguiar-Alves F., et al. Living conditions as a driving factor in persistent methicillin-resistant Staphylococcus aureus colonization among HIV-infected youth. Pediatr Infect Dis J. 2016;35(10):1126–1131. doi: 10.1097/INF.0000000000001246. [DOI] [PubMed] [Google Scholar]
- 11.Zhen X., Chen J., Sun X., Sun Q., Guo S., Stalsby Lundborg C. Socioeconomic factors contributing to antibiotic resistance in China: a panel data analysis. Antibiotics. 2021;10(8) doi: 10.3390/antibiotics10080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaba H.E.J., Kuhlmann E., Scheithauer S. Thinking outside the box: association of antimicrobial resistance with climate warming in Europe - a 30 country observational study. Int J Hyg Environ Health. 2020;223(1):151–158. doi: 10.1016/j.ijheh.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Collignon P., Athukorala P.C., Senanayake S., Khan F. Antimicrobial resistance: the major contribution of poor governance and corruption to this growing problem. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0116746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratkowsky D.A., Olley J., McMeekin T.A., Ball A. Relationship between temperature and growth rate of bacterial cultures. J Bacteriol. 1982;149(1):1–5. doi: 10.1128/jb.149.1.1-5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah H., Gharbia S. The impact of the environment on human infections. Microb Ecol Health Dis. 2009;11:248–254. [Google Scholar]
- 16.Burnham J.P. Climate change and antibiotic resistance: a deadly combination. Ther Adv Infect Dis. 2021;8 doi: 10.1177/2049936121991374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacFadden D.R., McGough S.F., Fisman D., Santillana M., Brownstein J.S. Antibiotic resistance increases with local temperature. Nat Clim Change. 2018;8(6):510–514. doi: 10.1038/s41558-018-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGough S.F., MacFadden D.R., Hattab M.W., Mølbak K., Santillana M. Rates of increase of antibiotic resistance and ambient temperature in Europe: a cross-national analysis of 28 countries between 2000 and 2016. Euro Surveill. 2020;25(45) doi: 10.2807/1560-7917.ES.2020.25.45.1900414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health O. World Health Organization; Geneva: 2017. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 20.Brink A.J. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32(6):609–616. doi: 10.1097/QCO.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 21.Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fupin H., Demei Z., Fu W., et al. Report of CHINET antimicrobial resistance surveillance Program in 2015. Chin J Infect Chemother. 2016;16(6):685–694. [Google Scholar]
- 23.Hu F.-P., Guo Y., Zhu D.-M., et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Chin J Infect Chemother. 2017;17(1):93–99. doi: 10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Fupin H., Yan G., Demei Z., et al. CHINET surveillance of bacterial resistance across China: report of the results in 2016. Chin J Infect Chemother. 2017;17(5):481–491. [Google Scholar]
- 25.Fupin H., Yan G., Demei Z., et al. Antimicrobial resistance profile of clinical isolates in hospitals across China: report from the CHINET Surveillance Program, 2017. Chin J Infect Chemother. 2018;18(3):241–251. [Google Scholar]
- 26.Fupin H., Yan G., Demei Z., et al. CHINET surveillance of bacterial resistance in China: 2018 report. Chin J Infect Chemother. 2020;20(1):1–10. [Google Scholar]
- 27.Fupin H., Yan G., Demei Z., et al. CHINET surveillance of bacterial resistance across tertiary hospitals in 2019. Chin J Infect Chemother. 2020;20(3):233–243. [Google Scholar]
- 28.Yonggui Z., Fupin H., Demei Z., et al. CHINET surveillance of bacterial resistance in secondary care hospitals across China: report of results in 2019. Chin J Infect Chemother. 2020;20(6):585–593. [Google Scholar]
- 29.Cardoso K., Gandra R.F., Wisniewski E.S., et al. DnaK and GroEL are induced in response to antibiotic and heat shock in Acinetobacter baumannii. J Med Microbiol. 2010;59(Pt 9):1061–1068. doi: 10.1099/jmm.0.020339-0. [DOI] [PubMed] [Google Scholar]
- 30.Cruz-Loya M., Kang T.M., Lozano N.A., et al. Stressor interaction networks suggest antibiotic resistance co-opted from stress responses to temperature. ISME J. 2019;13(1):12–23. doi: 10.1038/s41396-018-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade-Linares D.R., Lehmann A., Rillig M.C. Microbial stress priming: a meta-analysis. Environ Microbiol. 2016;18(4):1277–1288. doi: 10.1111/1462-2920.13223. [DOI] [PubMed] [Google Scholar]
- 32.Hilker M., Schwachtje J., Baier M., et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol Rev Camb Phil Soc. 2016;91(4):1118–1133. doi: 10.1111/brv.12215. [DOI] [PubMed] [Google Scholar]
- 33.Rangel D.E. Stress induced cross-protection against environmental challenges on prokaryotic and eukaryotic microbes. World J Microbiol Biotechnol. 2011;27(6):1281–1296. doi: 10.1007/s11274-010-0584-3. [DOI] [PubMed] [Google Scholar]
- 34.Guyot S., Pottier L., Ferret E., Gal L., Gervais P. Physiological responses of Escherichia coli exposed to different heat-stress kinetics. Arch Microbiol. 2010;192(8):651–661. doi: 10.1007/s00203-010-0597-1. [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Loya M., Tekin E., Kang T.M., et al. Antibiotics shift the temperature response curve of Escherichia coli growth. mSystems. 2021;6(4) doi: 10.1128/mSystems.00228-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grilo M.L., Pereira A., Sousa-Santos C., Robalo J.I., Oliveira M. Climatic alterations influence bacterial growth, biofilm production and antimicrobial resistance profiles in aeromonas spp. Antibiotics (Basel) 2021;10(8) doi: 10.3390/antibiotics10081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elshafiee E.A., Nader S.M., Dorgham S.M., Hamza D.A. Carbapenem-resistant Pseudomonas aeruginosa originating from farm animals and People in Egypt. J Vet Res. 2019;63(3):333–337. doi: 10.2478/jvetres-2019-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lister P.D., Wolter D.J., Hanson N.D. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B., Li B., Liang Y., et al. Pleiotropic effects of temperature-regulated 2-OH-lauroytransferase (PA0011) on Pseudomonas aeruginosa antibiotic resistance, virulence and type III secretion system. Microb Pathog. 2016;91:5–17. doi: 10.1016/j.micpath.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Liang Y., Guo Z., Gao L., et al. The role of the temperature-regulated acyltransferase (PA3242) on growth, antibiotic resistance and virulence in Pseudomonas aeruginosa. Microb Pathog. 2016;101:126–135. doi: 10.1016/j.micpath.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Balaban N.Q., Helaine S., Lewis K., et al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol. 2019;17(7):441–448. doi: 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruhn-Olszewska B., Szczepaniak P., Matuszewska E., et al. Physiologically distinct subpopulations formed in Escherichia coli cultures in response to heat shock. Microbiol Res. 2018;209:33–42. doi: 10.1016/j.micres.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz M.G., Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58(3):563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson D.I., Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 2010;8(4):260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 45.Trindade S., Sousa A., Gordo I. Antibiotic resistance and stress in the light of Fisher's model. Evolution. 2012;66(12):3815–3824. doi: 10.1111/j.1558-5646.2012.01722.x. [DOI] [PubMed] [Google Scholar]
- 46.Knies J.L., Cai F., Weinreich D.M. Enzyme efficiency but not thermostability drives cefotaxime resistance evolution in TEM-1 β-lactamase. Mol Biol Evol. 2017;34(5):1040–1054. doi: 10.1093/molbev/msx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez-Verdugo A., Gaut B.S., Tenaillon O. Evolution of Escherichia coli rifampicin resistance in an antibiotic-free environment during thermal stress. BMC Evol Biol. 2013;13:50. doi: 10.1186/1471-2148-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Q., Song P., Li J., Kong F., Sun L., Xu L. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci Trends. 2016;10(1):1–6. doi: 10.5582/bst.2016.01034. [DOI] [PubMed] [Google Scholar]
- 49.Tian L., Zhang Z., Sun Z. Antimicrobial resistance trends in bloodstream infections at a large teaching hospital in China: a 20-year surveillance study (1998-2017) Antimicrob Resist Infect Control. 2019;8:86. doi: 10.1186/s13756-019-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin X., Jian W., Yip W., Pan J. Perceived competition and process of care in rural China. Risk Manag Healthc Pol. 2020;13:1161–1173. doi: 10.2147/RMHP.S258812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C. Antibiotic stewardship challenges in an evolving health-care market in China. Lancet Infect Dis. 2021;21(6):753–754. doi: 10.1016/S1473-3099(20)30685-X. [DOI] [PubMed] [Google Scholar]
- 52.Li H., Yan S., Li D., Gong Y., Lu Z., Yin X. Trends and patterns of outpatient and inpatient antibiotic use in China's hospitals: data from the Center for Antibacterial Surveillance, 2012-16. J Antimicrob Chemother. 2019;74(6):1731–1740. doi: 10.1093/jac/dkz062. [DOI] [PubMed] [Google Scholar]
- 53.Ronnerstrand B., Lapuente V. Corruption and use of antibiotics in regions of Europe. Health Pol. 2017;121(3):250–256. doi: 10.1016/j.healthpol.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., Lin Z., Lu J. [One Health strategy to prevent and control antibiotic resistance] Sheng Wu Gong Cheng Xue Bao. 2018;34(8):1361–1367. doi: 10.13345/j.cjb.180249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.