Background
We sought to determine the role of obesity in adolescent men on development of atrial fibrillation (AF) and subsequent associated clinical outcomes in subjects diagnosed with AF.
Methods and Results
We conducted a nationwide, register‐based, cohort study of 1 704 467 men (mean age, 18.3±0.75 years) enrolled in compulsory military service in Sweden from 1969 through 2005. Height and weight, blood pressure, fitness, muscle strength, intelligence quotient, and medical disorders were recorded at baseline. Records obtained from the National Inpatient Registry and the Cause of Death Register were used to determine incidence and clinical outcomes of AF. During a median follow‐up of 32 years (interquartile range, 24–41 years), 36 693 cases (mean age at diagnosis, 52.4±10.6 years) of AF were recorded. The multivariable‐adjusted hazard ratio (HR) for AF increased from 1.06 (95% CI, 1.03–1.10) in individuals with body mass index (BMI) of 20.0 to <22.5 kg/m2 to 3.72 (95% CI, 2.44–5.66) among men with BMI of 40.0 to 50.0 kg/m2, compared with those with BMI of 18.5 to <20.0 kg/m2. During a median follow‐up of ≈6 years in patients diagnosed with AF, we identified 3767 deaths, 3251 cases of incident heart failure, and 921 cases of ischemic stroke. The multivariable‐adjusted HRs for all‐cause mortality, incident heart failure, and ischemic stroke in AF‐diagnosed men with baseline BMI >30 kg/m2 compared with those with BMI <20 kg/m2 were 2.86 (95% CI, 2.30–3.56), 3.42 (95% CI, 2.50–4.68), and 2.34 (95% CI, 1.52–3.61), respectively.
Conclusions
Increasing BMI in adolescent men is strongly associated with early AF, and with subsequent worse clinical outcomes in those diagnosed with AF with respect to all‐cause mortality, incident heart failure, and ischemic stroke.
Keywords: adolescence and ischemic stroke, atrial fibrillation, body mass index, heart failure, mortality
Subject Categories: Atrial Fibrillation, Obesity, Ischemic Stroke, Heart Failure, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- IS
ischemic stroke
- W max
maximum work capacity
Clinical Perspective.
What Is New?
In adolescent men, increased risk of future atrial fibrillation (AF) started already at low‐normal body mass index and continued to increase with increasing body mass index.
Adolescent men with severe or morbid obesity, corresponding to body mass index levels >35 kg/m2, had a 3‐ to 4‐fold increased risk of AF in early adulthood (mean age at diagnosis, 43.4 years).
The risk for all‐cause mortality, heart failure, and ischemic stroke among men diagnosed with AF in adulthood was strongly associated with body mass index in adolescence.
What Are the Clinical Implications?
Assessment of long‐standing and early‐onset obesity among men diagnosed with AF could help to identify individuals with a particularly high risk of clinical outcomes, such as all‐cause mortality, heart failure, and ischemic stroke.
A more vigilant monitoring of obese individuals may be warranted in patients with AF, and these issues should be addressed in future randomized studies.
Obesity is a complex metabolic condition defined as an excessive accumulation of body fat and is associated with a range of disorders, including cardiovascular disease. 1 , 2 , 3 , 4 Individuals with a body mass index (BMI) of >30 kg/m2 are classified as obese, those with a BMI of 35 to >40 kg/m2 are classified as severely obese, and those with a BMI of >40 kg/m2 are classified as morbidly obese.
In 2016, >650 million individuals, equivalent to 12% of the world's population, were estimated to be obese. 5 The prevalence of obesity has approximately tripled during the past 50 years, and it is anticipated that by 2025, 33 of 53 European countries will reach an obesity prevalence exceeding 20%, with increasing rates in children and adolescents. 5 , 6 , 7 In Swedish adolescent men, the prevalence of severe and morbid obesity increased from 0.1% in 1969 to 1974 to 1.1% in 1996 to 2005. 8 Accordingly, with unprecedented numbers of young people overweight and obese, there is an urgent need to address the long‐term consequences.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults worldwide, with 43.6 million diagnosed in 2016, equivalent to a prevalence of 2% to 4%, and is more common in elderly individuals and in men. 9 Individuals with AF are at a 1.5‐ to 3.5‐fold risk of death, with about 21% of those deaths attributed to heart failure (HF) and ischemic stroke (IS). 9 , 10 , 11 , 12 Studies have shown lower rates of all‐cause mortality in obese subjects with AF, a phenomenon known as the “obesity paradox.” 13 , 14 The observed obesity paradox that emerged from these studies may potentially be related to an older and sicker study population. Recent data from a prospective cohort of middle‐aged patients with AF did not show obesity to be associated with higher risk of poor clinical outcomes. 15 Other studies have suggested both overweight and obesity as a risk factor for death and a composite end point of IS, thromboembolism, and death, but not for HF or IS alone, in patients with AF. 16 Accordingly, there is a need for further studies in this area, in particular among those diagnosed already when young, where a double burden of early obesity and AF may theoretically be more harmful than AF alone.
In this long‐term cohort study, we aimed to establish the relationship of BMI in adolescence with later diagnosis of AF using a nationwide register of men enrolled in compulsory military service in Sweden. A further goal was to determine the influence of adolescent overweight and obesity on clinical outcomes in individuals later diagnosed with AF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Data of conscripts (n=1 918 466) into military service from 1969 through 2005 were drawn from the Swedish Military Service Conscription Register (Figure S1). Military service was mandatory in Sweden until 2005, except for prison inmates, those with severe medical conditions, or those with functional disability (≈2%–3% annually). The personal identity number was reused for some enlistees; these were excluded (n=1154). Women (n=10 228), men aged <15 and >40 years (n=14 204), and those with missing BMI data (n=188 274) were excluded from the analysis. We also excluded a limited number of subjects with BMI >50 kg/m2 (n=139) because there might have been errors in registering their weight or height.
All military conscripts underwent a 2‐day assessment at 1 of 6 centers in Sweden. 2 The assessment protocols were performed according to standard procedures and included measures of weight, height, and blood pressure and evaluation of cognition, cardiorespiratory fitness, and muscle strength. A global intelligence score (intelligence quotient [IQ]) was obtained from the cognitive evaluations and used as a proxy for general cognitive ability. The test results were standardized and transformed to Standard Nine (stanine) scores 1 to 9.
A bicycle ergometric test was performed to evaluate cardiorespiratory fitness. Briefly, 5 minutes of submaximal exercise was performed at work rate of 75 to 175 W, depending on the body weight. 17 The work rate was continuously increased by 25 W/min until limited by exhaustion. The final work rate (maximum work capacity [W max]) was recorded and divided by kilograms (W max/kg). This measure was used because W max/kg yielded a better correlation with maximum oxygen consumption (correlation coefficient of ≈0.9) than the predicted maximum oxygen consumption (correlation coefficient of 0.6–0.7). 18 The resulting value (W max/kg) served as a measure of cardiorespiratory fitness. W max/kg was recorded as raw data for the conscription in years 1972 through 2005. No raw data were recorded for the conscripts performing at the lowest 3 fitness levels (=stanines) in years 2000 through 2005. For the rest of the years, these conscripts were categorized into the lowest fitness group. 2 From 1969 through 1971, raw data were not recorded, but the values of W max/kg were converted to 9 levels that served as a measure of fitness. Isometric muscle strength was measured by hand grip, elbow flexion, and knee extension. Weighted values were integrated into an overall estimate in kiloponds until April 1, 1979, and in Newtons after that date. 19 Test results were standardized against data from previous years, resulting in scores from 1 to 9 (low to high).
The Longitudinal Integration Database for Health Insurance and Labour Market was used to obtain data about the highest completed level of parental education, available for 80% of the study population and stratified into ≤9, 10 to 12, and >12 years.
Outcomes
Data were obtained from the Swedish National Inpatient Registry, recording hospital inpatient and outpatient visits or discharge. The coverage of inpatient discharge increased during the period from 1970 through 1986 and is complete since 1987, with outpatient visits recorded from 2001. AF cases and clinical outcomes (IS and HF) after a recorded case of AF were identified according to the International Classification of Diseases, Eight Revision (ICD‐8), International Classification of Diseases, Ninth Revision (ICD‐9), and International Classification of Diseases, Tenth Revision (ICD‐10), and linked to the Hospital Register and Cause of Death Register through the Swedish 12‐digit personal identity number with diagnoses coded as in both main and secondary diagnosis (Table S1). We were unable to differentiate between various subtypes of AF, such as paroxysmal, persistent, or permanent AF as well as atrial flutter.
Statistical Analysis
Enlistee records were reviewed from date of conscription until an initial AF diagnosis, death, emigration, or December 31, 2019, whichever occurred first (14–50 years). Men with a registered AF diagnosis during follow‐up formed a subcohort that was followed up with respect to clinical outcomes, including all‐cause mortality, HF, and IS.
Unadjusted incidence rates and 95% Poisson CIs (exact) were calculated. Cox regression was used to estimate the association of BMI in adolescence with risk of future AF. To not underestimate the true risk associated with high adolescent BMI, we did not adjust for interim diagnoses of comorbidities, such as hypertension and diabetes, conditions that are strongly associated with elevated BMI and involved in the pathophysiological mechanism of AF. BMI was classified as <18.5, 18.5 to <20.0, 20.0 to <22.5, 22.5 to <25.0, 25.0 to <27.5, 27.5 to <30.0, 30.0 to <35.0, 35 to <40.0, and 40.0 to 50.0 kg/m2. For Cox regressions of BMI and subsequent clinical outcomes, BMI “normal” reference was set at <20.0 kg/m2, contrasting to the more traditional definition of normal BMI of 18.5 to 20 kg/m2, 5 because of the near‐continuous increase in risk of AF from low BMI values observed in prior analyses from this cohort. 20
Subjects who developed AF were followed up until a clinical cardiovascular outcome of death, HF, or IS. Incidence rates and corresponding 95% CIs for clinical events were calculated with Poisson regression. Cox regression was used to estimate the association of adolescent BMI with future hospitalization for clinical outcomes in patients with AF. This analysis included fewer subject groups with BMI <20.0 (reference), 20.0 to <25.0, 25.0 to <30.0, and >30.0 kg/m2, because relatively few clinical outcomes developed in those exhibiting the most extreme obesity.
Systolic blood pressure was stratified into 100 to 119, 120 to 125, 126 to 130, 131 to 138, and 139 to 180 mm Hg, and diastolic blood pressure was stratified as 40 to 59, 60 to 65, 66 to 70, 71 to 76, and 77 to 100 mm Hg. Cardiorespiratory fitness was categorized as low, 1 , 2 , 3 , 4 moderate, 5 , 6 , 7 or high, 8 , 9 and muscle strength was categorized as low, 1 , 2 , 3 moderate, 4 , 5 , 6 or high. 7 , 8 , 9 Parental education was classified as ≤9, 10 to 12, or >12 years.
Differences among conscription centers, conscription years, and age at conscription were adjusted for as potential confounders. Spline plots were produced with BMI as a restricted cubic spline with knots placed at 5% (18.1 kg/m2), 35% (19.9 kg/m2), 65% (23.2 kg/m2), and 95% (27.2 kg/m2). Year of conscription was included in the spline with knots placed at 5% (1972), 35% (1979), 65% (1994), and 95% (2002). Effects of interactions of BMI with other variables on the incidence of AF were tested with BMI as a continuous variable. Systolic and diastolic blood pressure, cardiorespiratory fitness, muscle strength, parent education, baseline diabetes, hypertension, and congenital heart disease were analyzed as factors. The unadjusted models are presented in Figures S2 through S5.
Random forest, a robust and nonparametric machine learning statistical method that optimizes predictive accuracy by fitting an ensemble of trees, was used to study the interactions among baseline risk factors on clinical outcomes in patients with AF. 21 Finally, to aid interpretation of the random forest results, partial dependence plots were produced to show the interaction effects among the most important cardiovascular risk factors.
Data were prepared with SAS version 9.4 (SAS Institute, Cary, NC), and statistical analyses were conducted using R software version 3.4.2 (dplyr, moonBook, ggplot, caret, rms, plotmo, and survival packages; R Foundation for Statistical Computing, Vienna, Austria).
Ethical Approval
The regional ethical review board of Gothenburg approved the study (registration number 567‐15), which conformed to the principles outlined in the Declaration of Helsinki. Informed consent was not required because data were not collected for a research purpose.
Results
Study Population and Follow‐Up
Table 1 shows the characteristics of the 1 704 467 men in the study (mean age, 18.3±0.75 years) after exclusions (Figure S1); 8.1% were underweight (BMI <18.5 kg/m2), 79.5% were normal weight (BMI 18.5–<25 kg/m2), 10.0% were overweight (BMI 25 to <30 kg/m2), 1.8% were obese, 0.4% were severely obese, and 0.1% were morbidly obese (Table 1). There were no major differences in age or height among the BMI categories of the subjects. Systolic and diastolic blood pressure increased slightly with BMI. The proportion of subjects with low cardiorespiratory fitness, muscle strength, and IQ was higher in obese, compared with normal weight, men. Few comorbidities were present at baseline, but in the 2 most obese categories, 1% to 2% were diagnosed with hypertension already at conscription.
Table 1.
Baseline Characteristics of Study Population Relative to BMI
| Characteristic | All | BMI <18.5 kg/m2 | BMI 18.5–<20.0 kg/m2 | BMI 20.0–<22.5 kg/m2 | BMI 22.5–<25.0 kg/m2 | BMI 25.0–<27.5 kg/m2 | BMI 27.5–<30.0 kg/m2 | BMI 30.0–<35.0 kg/m2 | BMI 35.0‐<40.0 kg/m2 | BMI 40.0–50.0 kg/m2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Men, n (%) | 1 704 467 (100) | 138 645 (8.1) | 307 135 (18.0) | 687 070 (40.3) | 362 485 (21.3) | 126 193 (7.4) | 44 592 (2.6) | 30 800 (1.8) | 6244 (0.4) | 1302 (0.1) |
| Age, mean (SD), y | 18.3 (0.8) | 18.3 (0.7) | 18.3 (0.7) | 18.3 (0.7) | 18.4 (0.9) | 18.4 (1.0) | 18.4 (0.9) | 18.4 (0.8) | 18.4 (0.8) | 18.4 (0.9) |
| Height, mean (SD), cm | 179 (6.6) | 179 (7.0) | 179 (6.6) | 179 (6.5) | 179 (6.6) | 179 (6.6) | 179 (6.8) | 179 (6.8) | 180 (6.8) | 179 (7.0) |
| Weight, mean (SD), kg | 70.4 (11.1) | 57.0 (5.3) | 62.2 (4.8) | 68.3 (5.4) | 75.6 (6.0) | 83.5 (6.6) | 91.8 (7.4) | 102.6 (9.1) | 118.8 (10.0) | 135.9 (11.9) |
| BMI, mean (SD), kg/m2 | 21.9 (3.1) | 17.7 (1.0) | 19.3 (0.4) | 21.2 (0.7) | 23.6 (0.7) | 26.1 (0.7) | 28.6 (0.7) | 31.9 (1.4) | 36.8 (1.4) | 42.4 (2.1) |
| Systolic BP, mean (SD), mm Hg | 128.5 (11.1) | 125.4 (11.2) | 126.6 (10.9) | 128.2 (10.9) | 129.9 (10.9) | 131.3 (10.9) | 132.5 (11.3) | 133.9 (11.1) | 136.2 (11.7) | 137.9 (13.7) |
| Diastolic BP, mean (SD), mm Hg | 67.6 (9.9) | 67.4 (9.8) | 67.3 (9.7) | 67.4 (9.8) | 67.7 (9.9) | 68.5 (10.1) | 69.3 (10.4) | 70.5 (10.6) | 72.6 (11.0) | 74.3 (12.2) |
| W max, mean (SD) | 251.7 (96.5) | 196.6 (94.8) | 225.3 (95.3) | 254.8 (94.8) | 276.6 (91.8) | 278.8 (88.9) | 275.8 (84.9) | 272.8 (82.9) | 267.7 (83.8) | 256.5 (83.9) |
| W max/kg, mean (SD) | 3.2 (1.8) | 3.0 (1.9) | 3.6 (1.5) | 3.7 (1.4) | 3.7 (1.2) | 3.4 (1.1) | 3.0 (0.9) | 2.7 (0.82) | 2.3 (0.7) | 1.9 (0.6) |
| Cardiorespiratory fitness, n (%) | ||||||||||
| Low (1–4) | 258 951 (15.8) | 42 847 (32.5) | 53 355 (17.9) | 69 133 (10.4) | 36 212 (10.4) | 23 620 (19.6) | 14 797 (35.0) | 15 040 (53.9) | 3332 (72.4) | 615 (82.8) |
| Moderate (5–6) | 799 867 (48.7) | 73 921 (56.1) | 170 421 (57.2) | 320 911 (48.2) | 150 854 (43.1) | 55 917 (46.3) | 18 186 (43.0) | 8757 (31.4) | 823 (17.9) | 77 (10.4) |
| High (7–9) | 583 072 (35.5) | 15 094 (11.5) | 74 262 (24.9) | 276 043 (41.4) | 162 622 (46.5) | 41 167 (34.1) | 9291 (22.0) | 4094 (14.7) | 448 (9.7) | 51 (6.9) |
| Muscle strength, n (%) | ||||||||||
| Low (1–3) | 235 891 (14.1) | 58 779 (43.6) | 67 097 (22.2) | 73 146 (10.8) | 23 255 (6.5) | 7992 (6.5) | 3003 (6.9) | 2086 (7.1) | 419 (8.1) | 114 (12.4) |
| Moderate (4–6) | 946 216 (56.5) | 72 116 (53.5) | 203 875 (67.4) | 416 908 (61.6) | 170 486 (47.8) | 52 006 (42.0) | 17 485 (40.1) | 11 129 (37.8) | 1879 (36.2) | 332 (36.1) |
| High (7–9) | 491 679 (29.4) | 3867 (2.9) | 31 753 (10.5) | 186 638 (27.6) | 162 753 (45.7) | 63 898 (51.57) | 23 166 (53.1) | 16 232 (55.1) | 2897 (55.8) | 475 (51.6) |
| IQ, n (%) | ||||||||||
| Low (1–3) | 340 882 (20.4) | 30 658 (22.6) | 60 595 (20.0) | 124 952 (18.5) | 70 930 (19.9) | 29 928 (24.2) | 12 359 (28.3) | 9107 (30.7) | 1931 (34.6) | 422 (39.0) |
| Moderate (4–6) | 915 535 (54.7) | 70 704 (52.1) | 161 485 (53.4) | 370 363 (54.8) | 199 738 (56.1) | 69 491 (56.2) | 24 004 (55.0) | 16 258 (54.8) | 2946 (52.8) | 546 (50.5) |
| High (7–9) | 417 656 (25.0) | 34 319 (25.3) | 80 445 (26.6) | 180 851 (26.8) | 85 372 (24.0) | 24 279 (19.6) | 7250 (16.6) | 4318 (14.6) | 708 (12.7) | 114 (10.5) |
| Parental education, n (%) | ||||||||||
| ≤9 y | 226 062 (16.2) | 20 849 (19.1) | 43 040 (17.4) | 89 673 (15.9) | 45 515 (15.2) | 16 277 (15.6) | 5902 (15.9) | 3903 (15.2) | 731 (13.9) | 172 (15.4) |
| 10–12 y | 865 245 (62.1) | 66 138 (60.7) | 149 334 (60.5) | 343 571 (61.0) | 189 310 (63.0) | 68 948 (65.9) | 25 177 (68.0) | 18 081 (70.4) | 3883 (73.5) | 803 (71.9) |
| >12 y | 301 594 (21.7) | 21 946 (20.2) | 54 409 (22.1) | 129 807 (23.1) | 65 610 (21.8) | 19 377 (18.5) | 5955 (16.1) | 3683 (14.4) | 665 (12.6) | 142 (12.7) |
| Diagnosis at baseline, n (%) | ||||||||||
| ACH | 2469 (0.2) | 235 (0.2) | 423 (0.1) | 937 (0.1) | 543 (0.2) | 187 (0.2) | 75 (0.2) | 58 (0.2) | 10 (0.2) | 1 (0.1) |
| Diabetes | 1331 (0.1) | 89 (0.1) | 189 (0.1) | 532 (0.1) | 341 (0.1) | 116 (0.1) | 29 (0.1) | 24 (0.1) | 11 (0.2) | 0 (0.0) |
| Hypertension | 2825 (0.2) | 115 (0.1) | 268 (0.1) | 860 (0.1) | 654 (0.2) | 376 (0.3) | 229 (0.5) | 223 (0.7) | 77 (1.3) | 23 (1.8) |
ACH indicates adult congenital heart disease; BMI, body mass index; BP, blood pressure; IQ, intelligence quotient; and W max, maximum work capacity.
During a median follow‐up of 32 years, 36 693 men were registered with a diagnosis of AF (Table S2). The number of AF cases in the total cohort was 68 per 100 000 observed person‐years, increasing from 61 per 100 000 person‐years in those with BMI of 18.5 to <20 kg/m2 to 131 per 100 000 person‐years in the BMI group of 40 to 50 kg/m2 (Table S2). The median age of AF diagnosis in the total cohort was 52.4 years, decreasing from a median of 54 years at AF diagnosis for those with BMI of 18.5 kg/m2 to 43.4 years in the BMI group of 40 to 50 kg/m2.
Risk of AF
The cumulative incidence rate of AF after a maximum 50‐year follow‐up until December 31, 2019, was 20% among men fulfilling criteria for obesity at baseline, 12.4% in overweight men, and 8.3% in those with BMI 20 to <25 kg/m2, compared with 6.5% in men with BMI <20 kg/m2 (Figure 1A).
Figure 1. A, Cumulative incidence of atrial fibrillation with respect to body mass index (BMI) group.
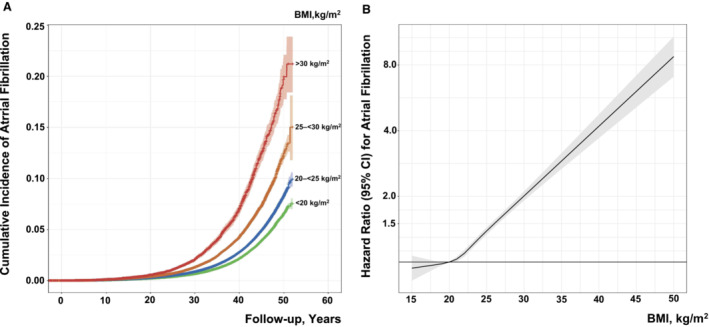
B, Association of BMI at conscription into military service with risk of early‐onset atrial fibrillation. The model was adjusted for age, conscription year as a spline with knots at 5%, 25%, 75%, and 95% (1972, 1979, 1994, and 2002), test center, systolic blood pressure, diastolic blood pressure, cardiorespiratory fitness, muscle strength, parental education, and baseline comorbidities (diabetes, hypertension, and adult congenital heart disease) (n=1 287 197). BMI of 15 to 50 kg/m2 was modeled as a restricted cubic spline with knots at 5%, 25%, 75%, and 95% (18.1, 19.9, 23.2, and 27.2 kg/m2) with BMI of 20 kg/m2 as reference.
Compared with the BMI reference of 18.5 to <20.0 kg/m2, the adjusted risk for AF at low‐normal BMI levels of 20.0 to <22.5 kg/m2 was hazard ratio (HR) of 1.06 (95% CI, 1.03–1.10) and this increased gradually to 3.72 (95% CI, 2.44–5.66) at BMI of 40 to 50 kg/m2 (Figure 1B and Table 2). The multivariable‐adjusted HR showed a 6.4% higher risk of AF (HR, 1.064 [95% CI, 1.059–1.068]) for each kg/m2 increase in BMI.
Table 2.
HRs (95% CIs) for AF Relative to BMI Group
| Variable | Model 1* | Model 2† | Model 3‡ | Model 4§ |
|---|---|---|---|---|
| Events/population | 36 693/1 704 466 | 36 275/1 614 868 | 36 126/1 587 607 | 24 897/1 297 909 |
| BMI <18.5 kg/m2 | 0.94 (0.90–0.98) | 0.94 (0.90–0.98) | 0.99 (0.95–1.04) | 1.00 (0.95–1.06) |
| BMI 18.5–<20.0 kg/m2 | 1.00 | 1.00 | 1.00 | 1.00 |
| BMI 20.0–<22.5 kg/m2 | 1.15 (1.12–1.19) | 1.14 (1.11–1.18) | 1.09 (1.05–1.12) | 1.06 (1.03–1.10) |
| BMI 22.5–<25.0 kg/m2 | 1.43 (1.39–1.48) | 1.41 (1.36–1.46) | 1.29 (1.24–1.33) | 1.23 (1.18–1.28) |
| BMI 25.0–<27.5 kg/m2 | 1.74 (1.66–1.81) | 1.69 (1.62–1.77) | 1.55 (1.48–1.62) | 1.45 (1.37–1.54) |
| BMI 27.5–<30.0 kg/m2 | 2.28 (2.14–2.42) | 2.19 (2.06–2.33) | 2.03 (1.91–2.16) | 1.87 (1.73–2.02) |
| BMI 30.0–<35.0 kg/m2 | 2.91 (2.71–3.11) | 2.76 (2.58–2.96) | 2.56 (2.39–2.75) | 2.39 (2.19–2.61) |
| BMI 35.0–<40.0 kg/m2 | 3.75 (3.24–4.33) | 3.64 (3.14–4.22) | 3.41 (2.93–3.97) | 2.80 (2.30–3.40) |
| BMI 40.0–50.0 kg/m2 | 4.70 (3.44–6.41) | 4.39 (3.13–6.15) | 4.30 (3.05–6.06) | 3.72 (2.44–5.66) |
| Per unit BMI | 1.083 (1.080–1.087) | 1.080 (1.077–1.084) | 1.071 (1.068–1.075) | 1.064 (1.059–1.068) |
The HRs were derived from Cox regressions (Methods) with different models of adjustments as follows. AF indicates atrial fibrillation; BMI, body mass index; and HR, hazard ratio.
Model 1 adjusted for age at conscription, conscription year, and test center.
Model 2 as model 1, additionally adjusted for systolic and diastolic blood pressure at baseline, and baseline comorbidities diabetes, hypertension, and adult congenital heart disease.
Model 3 as model 2, additionally adjusted for cardiorespiratory fitness and muscle strength.
Model 4 as model 3, additionally adjusted for parental education and intelligence quotient.
In the multivariable‐adjusted models, age, year of conscription, systolic blood pressure, cardiorespiratory fitness, IQ, and baseline comorbidities were independent predictors of AF (Table S3). There were significant negative interactions between BMI and IQ (ie, the effect of high BMI on risk of AF was attenuated in subjects with high compared with low IQ). In contrast, we found positive interactions between BMI and muscle strength and adult congenital heart disease (namely, the effect of high BMI on the risk of AF was increased in subjects with higher muscle strength or in those with adult congenital heart disease) (Table S3).
Event Rates and Risk of Clinical Outcomes in Subjects With AF
Among men diagnosed with AF (n=36 693), there were 3767 deaths, representing 13.2 cases per 1000 person‐years (Table S4), increasing from 13.0 per 1000 person‐years in subjects with BMI <20 kg/m2 to 22.6 per 1000 person‐years for BMI >30 kg/m2. Similarly, 3251 men developed HF, and 921 developed IS (Table S4). The number of cases per 1000 person‐years was 11.7 for HF and 3.4 for IS in those with AF. The figures per 1000 person‐years in subjects with a BMI <20 kg/m2 were 11.8 for HF and 3.6 for IS compared with 19.2 and 5.4, respectively, for BMI >30 kg/m2.
All‐cause mortality after a maximum 10‐year follow‐up after AF index diagnosis was 19.1% in obese subjects, 16.8% in overweight subjects, and 11.4% in those with BMI 20 to <25 kg/m2, compared with 11.7% in men with BMI <20 kg/m2. The corresponding cumulative incidence of HF was 12.2% in obese subjects, 8.7% in overweight subjects, and 5.1% in those with BMI 20 to <25 kg/m2, compared with 5.2% in men with BMI <20 kg/m2; and for IS, the values were 4.8%, 3.8%, 3.0%, and 3.2%, respectively.
The risk for all‐cause mortality, incident HF, and IS gradually increased with increasing BMI levels in subjects diagnosed with AF (Figure 2). The multivariable‐adjusted HR for all‐cause mortality was 2.86 (95% CI, 2.30–3.56) in individuals with BMI >30 kg/m2 in adolescence compared with those having BMI levels <20 kg/m2 (Table 3), with an 8.8% higher risk of all‐cause mortality per BMI unit. Obese and overweight subjects showed a significantly higher multivariable‐adjusted HR for incident HF (HR, 3.42 [95% CI, 2.50–4.68] and HR, 2.17 [95% CI, 1.73–2.72], respectively) compared with subjects with BMI levels <20 kg/m2. In similarly adjusted models, those subjects with AF with BMI >30 kg/m2 at baseline exhibited a higher risk of developing IS (HR, 2.34 [95% CI, 1.52–3.61]) compared with those with BMI levels <20 kg/m2.
Figure 2. Association of adolescent body mass index (BMI) diagnosed with atrial fibrillation with mortality and clinical outcome in early adulthood.
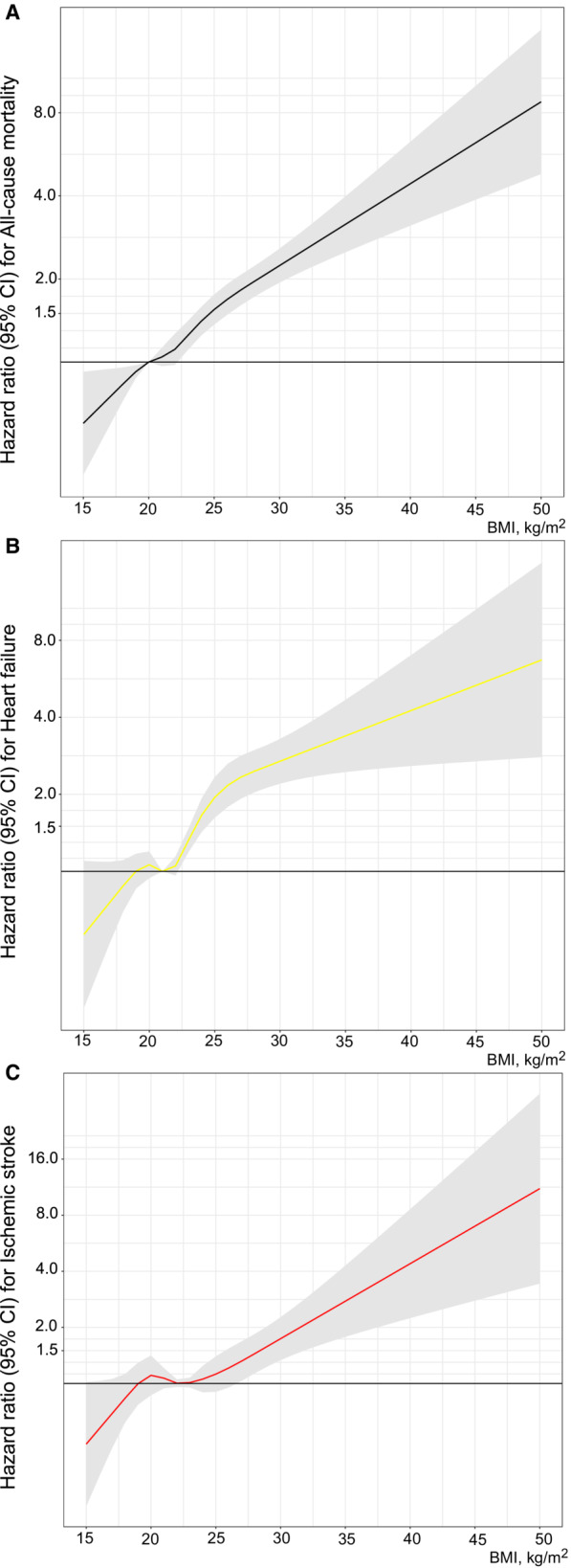
Risk of all‐cause mortality (A), heart failure (B), and ischemic stroke (C) in patients with atrial fibrillation relative to BMI. The models were adjusted for age, conscription year as a spline with knots at 5%, 25%, 75%, and 95% (years 1970, 1972, 1982, and 1992), test center, systolic blood pressure, diastolic blood pressure, cardiorespiratory fitness, muscle strength, parental education, and baseline comorbidities (diabetes, hypertension, and congenital heart disease). BMI of 15 to 50 kg/m2 was modeled as a restricted cubic spline with knots at 5%, 25%, 75%, and 95% (18.1, 20.0, 23.5, and 28.0 kg/m2) with BMI of 20 kg/m2 as reference for all‐cause mortality, 21 kg/m2 for heart failure, and 22.5 kg/m2 for ischemic stroke.
Table 3.
HRs (95% CIs) for Clinical Outcomes in Patients With AF Relative to BMI Group
| Variable | Model 1* | Model 2† | Model 3‡ | Model 4§ |
|---|---|---|---|---|
| All‐cause mortality | ||||
| Events/population, n | 3767/36 459 | 3742 /36 041 | 3735/35 892 | 2145/24 757 |
| BMI <20.0 kg/m2 | 1.00 | 1.00 | 1.00 | 1.00 |
| BMI 20–<25.0 kg/m2 | 1.01 (0.93–1.09) | 1.01 (0.93–1.09) | 1.25 (1.15–1.35) | 1.26 (1.13–1.41) |
| BMI 25–<30.0 kg/m2 | 1.62 (1.46–1.80) | 1.60 (1.43–1.78) | 2.02 (1.80–2.26) | 2.11 (1.81–2.45) |
| BMI ≥30 kg/m2 | 2.12 (1.82–2.49) | 2.10 (1.79–2.47) | 2.50 (2.12–2.97) | 2.86 (2.30–3.56) |
| Per unit BMI | 1.063 (1.053–1.072) | 1.063 (1.053–1.072) | 1.081 (1.071–1.091) | 1.088 (1.074–1.010) |
| Heart failure | ||||
| Events/population, n | 1648/34 534 | 1629/34 131 | 1626/33 983 | 956/23 568 |
| BMI <20.0 kg/m2 | 1.00 | 1.00 | 1.00 | 1.00 |
| BMI 20–<25.0 kg/m2 | 1.08 (0.96–1.22) | 1.08 (0.95–1.22) | 1.28 (1.12–1.46) | 1.22 (1.03–1.45) |
| BMI 25–<30.0 kg/m2 | 1.87 (1.59–2.19) | 1.86 (1.58–2.19) | 2.28 (1.92–2.71) | 2.17 (1.73–2.72) |
| BMI ≥30 kg/m2 | 3.04 (2.44–3.79) | 2.88 (2.29–3.63) | 3.40 (2.68–4.32) | 3.42 (2.50–4.68) |
| Per unit BMI | 1.085 (1.071–1.099) | 1.083 (1.068–1.098) | 1.099 (1.084–1.114) | 1.104 (1.084–1.125) |
| Ischemic stroke | ||||
| Events/population, n | 921/35 025 | 917/34 616 | 916/34 468 | 550/ 23 846 |
| BMI <20.0 kg/m2 | 1.00 | 1.00 | 1.00 | 1.00 |
| BMI 20–<25.0 kg/m2 | 0.95 (0.82–1.11) | 0.93 (0.80–1.08) | 1.08 (0.92–1.27) | 1.09 (0.88–1.34) |
| BMI 25–<30.0 kg/m2 | 1.21 (0.97–1.52) | 1.15 (0.91–1.44) | 1.37 (1.07–1.74) | 1.29 (0.93–1.78) |
| BMI ≥30 kg/m2 | 1.88 (1.36–2.60) | 1.74 (1.25–2.43) | 1.99 (1.40–2.81) | 2.34 (1.52–3.61) |
| Per unit BMI | 1.040 (1.021–1.061) | 1.034 (1.014–1.055) | 1.052 (1.031–1.074) | 1.058 (1.030–1.086) |
The HRs were derived from Cox regressions (Methods) with different models of adjustments as follows. AF indicates atrial fibrillation; BMI, body mass index; and HR, hazard ratio.
Model 1 adjusted for age at conscription, conscription year, and test center.
Model 2 as model 1, additionally adjusted for systolic and diastolic blood pressure at baseline, and baseline comorbidities diabetes, hypertension, adult congenital heart disease, and heart failure.
Model 3 as model 2, additionally adjusted for cardiorespiratory fitness and muscle strength.
Model 4 as model 3, additionally adjusted for parental education and intelligence quotient.
To visualize the complex interaction effects of BMI with the most important risk factors, we constructed 3‐dimensional partial dependence plots. We found the interaction effects between BMI and IQ and systolic and diastolic blood pressure on all‐cause mortality and incident HF (Figure 3). The probability of all‐cause mortality in subjects with AF gradually increased with lower IQ, and higher systolic and diastolic blood pressure (Figure 3A). Similar trends were observed for incident HF (Figure 3B); however, for subjects with high BMI levels, the probability for incident HF was high, independent of IQ and blood pressure. There were no significant interactions among BMI and risk factors on IS (data not shown).
Figure 3. Three‐dimensional partial dependence plots generated from the random forest models.
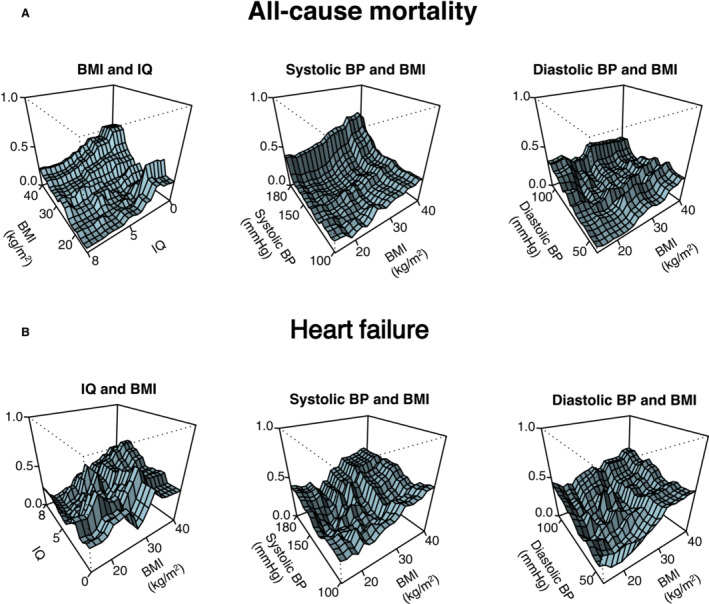
Graphical visualization of the most important interactions between body mass index (BMI) and risk factors (x 1 and x 2 axis) on all‐cause mortality (A) and incident heart failure (B) (y axis). The y axis denotes probability of all‐cause mortality or incident heart failure. A, Intelligence quotient (IQ) is in the reverse direction from high to low. BP indicates blood pressure.
Discussion
In this large nationwide long‐term study, elevated BMI in adolescence was associated with increased risk of AF in adulthood in comparatively young men. An increased risk was observed already at mildly elevated BMI within the normal range, with a 3‐ to 4‐fold increase in risk among men with severe or morbid obesity, corresponding to BMI levels >35 kg/m2, suggesting a causal role of adiposity on pathophysiological processes leading to AF. In addition, our data also suggest that adolescent obesity is prognostic of poorer outcomes in subjects later diagnosed with AF with respect to all‐cause mortality, HF, and IS. Investigating the true association of obesity with AF is challenging because of the complex interactions of obesity with other factors associated with AF, such as poor diet, low physical activity, hypertension, diabetes, smoking, elevated blood lipids, and sleep apnea.9 However, young obese individuals may not have accumulated these risk factors to the same extent as older cohorts, and the present study cohort accordingly provides a valuable opportunity to investigate the impact of obesity per se on AF. We found that age, year of conscription, systolic blood pressure, cardiorespiratory fitness, IQ, and baseline comorbidities, such as hypertension and adult congenital heart disease, in adolescence were additional factors associated with development of AF in adulthood, but the relationship with BMI in adolescence persisted independent of these factors and comorbidities.
An increased risk of AF in obese subjects has been a consistent finding of several register‐ and population‐based prospective cohort studies. 16 , 20 , 22 , 23 In the prospective Danish Diet, Cancer, and Health Study of 47 589 randomly selected participants aged 50 to 64 years followed up for 5.7 years, 553 subjects developed AF or flutter. 16 The adjusted HR for AF in obese, compared with normal weight, men was 2.35 (95% CI, 1.70–3.25), with each SD increment of BMI significantly related to incident AF (HR, 1.08). A prospective study from Sweden of 4021 subjects aged 60 years, with a mean follow‐up of 13.6 years and 285 cases of incident AF, revealed obesity to be associated with an ≈2‐fold risk of AF, regardless of whether metabolic syndrome was present or not. 22
A previous study of the present cohort, followed up for a median of 26 years with 9777 cases of incident AF, showed an ≈2‐fold risk of hospitalizations for AF among men who were obese in adolescence compared with those with BMI 20 kg/m2. 20 However, with a longer follow‐up and addition of outpatient cases, and analysis of important post‐AF outcomes, we were able to follow the severely obese category, relevant to the current obesity pandemic with severe and morbid obesity increasing worldwide.
Accordingly, there is a need to address how the issue should be addressed. To the best of our knowledge, the present study of ≈1.7 million subjects with a median follow‐up of 32 years and 36 693 cases of incident AF is the largest, with the longest follow‐up and the highest number of identified AF outcomes. The cumulative incidence of 20% among obese subjects was high, also in absolute terms, and approaching that of lifetime risk (about 25%), whereas the men of the present study were much younger. 24 We found an increased risk of AF starting at normal BMI levels in adolescence and ≈3‐ and 4‐fold risks of incident AF in the severely and morbidly obese subjects, respectively, compared with normal weight subjects. The European Society of Cardiology 2020 guidelines pertaining to AF recommend screening for AF in individuals aged ≥65 years and in patients with hypertension, and it should be considered in those with obstructive sleep apnea, but not in obese subjects. 9 Our data generate questions to be further addressed in randomized trials: whether screening for AF in severely and morbidly obese subjects may increase survival or prevent HF or stroke in this group. In a previous study from the MESA (Multi‐Ethnic Study of Atherosclerosis), the relationship between BMI and development of AF was J shaped. 25 The nearly linear association in the present study may be explained by the fact that we measured weight and height in adolescence, and not in adulthood; hence, the association may differ, with a stronger relationship among younger people, to that seen in adulthood.
The mechanisms by which obesity may lead to the development of AF are complex and not completely understood. Obese individuals may be susceptible to AF via alterations in hemodynamics and cardiac structures, such as increased blood volume and increased stroke volume, eventually causing elevation in left atrial pressure, increase in left ventricular wall stress, and left ventricular remodeling and diastolic dysfunction that may trigger AF. 26 An excessive accumulation of adipose tissue may result in release of proinflammatory cytokines and oxidative stress and excess deposition of epicardial fat, contributing to left atrial enlargement and left ventricular diastolic dysfunction and remodeling, including collagen deposition and fibrosis. 26
Individuals with AF are at 1.5‐ to 3.5‐fold risk of death compared with those with sinus rhythm. 9 , 10 , 12 A prospective study of Japanese men (n=4045) showed that common causes of death among patients with AF were malignancy (23.1%), infection/sepsis (17.3%), or HF (14.5%), with stroke‐related mortality at 6.5%. 11 However, the impact of obesity on death in relation to other comorbidities in patients with AF has not been extensively studied. Further on, in subgroup analyses of the AFFIRM (Atrial Fibrillation Follow‐Up Investigation of Rhythm Management) study, 304 deaths occurred in 2492 subjects (mean age, 66.4 years at baseline; 35.7% obese) who were followed up for a mean of 3 years. Obese subjects with AF showed higher rates of all‐cause mortality, but not of stroke; however, the adjusted HR for all‐cause mortality was not significantly associated with obesity. 27 In a second subgroup analysis, a Cox regression analysis adjusted only for those baseline variables that differed significantly revealed a significant inverse relationship between obesity and all‐cause mortality. 13 Findings of the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) randomized trial subgroup analysis of 17 913 subjects (mean age, 69.4 years; 40.0% obese) were similar, with a median follow‐up of 1.8 years, 1229 deaths, and 465 strokes or systemic emboli. In multivariable adjusted models, obesity in those with AF was associated with lower risk of all‐cause mortality, but not of stroke or systemic embolism. 14 Findings from these 2 studies were compatible with a potential obesity paradox, indicating that obese subjects with AF are “protected” from cardiovascular outcomes, but where this effect is mainly attributable to older and sicker individuals being included. Investigating the association between AF and HF might be complex, as the 2 conditions often overlap. 28
Findings of observational prospective studies have differed from subgroup analyses of randomized trials that suggest an obesity paradox. In a recent prospective study of 1956 patients (mean age, 73.8 years; 37.2% obese) with AF receiving anticoagulants, and a median follow‐up of 3 years, observing 255 deaths and 45 strokes, obesity was not associated with all‐cause mortality or stroke. This contrasts to the findings of the prospective observational Danish Diet, Cancer and Health study (aged 50–64 years at baseline; n=57 053), where the subcohort with AF (n=3135; followed up for a median 4.9 years) had a significant association for obesity with a composite end point of IS, thromboembolism, and death, even after adjustment for CHA2DS2‐VASc (HR, 1.36 [95% CI, 1.11–1.65]). However, obesity was not significantly associated with IS or HF alone.
Our data gained from a median follow‐up of ≈6 years of 37 000 subjects with AF suggest that obesity in adolescence is associated with an ≈3‐fold risk of all‐cause mortality, 3.5‐fold risk of incident HF, and 2.5‐fold risk of incident IS. In addition, the probability for all‐cause mortality gradually increased with lower IQ and higher blood pressure levels, whereas the probability for incident HF was high in subjects with high BMI levels independent of blood pressure. We believe these findings are novel and suggest that the question of whether more rigorous monitoring of obese subjects with AF may improve prognosis and survival should be addressed in future trials. For example, otherwise healthy men aged <65 years with AF would normally not be considered for anticoagulant therapy; our findings might indicate a potential beneficial effect that might be explored in a clinical trial.
Strengths and Limitations
Strengths of the present study include a large sample size with a high number of AF cases, long‐term follow‐up, and the population‐based study design comprising a cohort largely representative of the male adolescent population of Sweden. In addition, we investigated the association of BMI in early adolescence with clinical outcomes in patients diagnosed with AF.
There are limitations to our study that should be addressed. First, we used BMI as a proxy for overweight and obesity. Mildly elevated BMI may reflect high muscle mass, not adiposity, but this is unlikely to be the case when BMI is extremely high. Second, our data and statistical analysis did not account for variation of BMI over time, which might have introduced a time‐related bias. This has been demonstrated in a previous study in which a decrease in BMI over time was associated with lower rates of AF. 29 However, it should also be addressed that obesity in adolescence may cause irreversible changes in cardiac structures strongly associated with AF, such as left atrial enlargement. This has been shown in a previous study investigating changes in cardiac geometric structures before and after bariatric surgery in 38 adolescents. 30 Indeed, there were no changes in the dimension of left atrium or the left ventricle, which might suggest that obesity in adolescence is a crucial determinant of AF irrespective of changes in weight during adulthood. 31
Third, our homogeneous study population with respect to sex and ethnicity precludes the generalizability of results to women and to populations of other ethnicities. Age‐adjusted incidence, prevalence, and life‐time risk of AF are lower in women compared with men and varies with race and/ethnicity. Fourth, another inherent limitation is the inability to account or adjust for the development of comorbid medical diagnoses. One might hypothesize that the compendium of risk factors that closely track with obesity and morbid obesity explain the development of AF, as opposed to a direct causal role of elevated adolescent BMI levels. This might suggest BMI is a mere marker of downstream risk factors (eg, type 2 diabetes, obstructive sleep apnea, and essential hypertension), which predispose to AF. Still, given the young age of the cohort at baseline, it is more likely that high BMI exerts an effect on future AF through these intermediary factors, which would likely not have developed in the absence of an elevated BMI, or at least not to the same extent. In addition, we were unable to include potential lifestyle risk factors, such as smoking, diet, alcohol consumption, and physical activity occurring during follow‐up as potential mediating factors, nor did we have information on adult BMI. Fifth, we were unable to differentiate between AF and atrial flutter and various subtypes of AF, such as paroxysmal, persistent, or permanent AF. Finally, lack of data on oral anticoagulant use is a limitation as we cannot exclude that body weight might impact on the use of anticoagulants and subsequent AF‐related strokes.
Conclusions
Severe and morbid obesity in adolescence in men is strongly associated to development of AF in early adulthood. Documented obesity in adolescence in men later diagnosed with AF is associated with higher risk of all‐cause mortality, incident HF, and IS. Increasing obesity prevalence in the young is likely to lead to increasing rates of early AF, with markedly elevated risk of poor outcomes.
Sources of Funding
This work was supported by grants from: the Swedish state under an agreement concerning research and education of physicians (ALFGBG‐717211); the Swedish Heart and Lung Foundation (2018‐0366); and the Swedish Research Council (2018‐02527 and VRREG 2019‐00193).
Disclosures
None.
Supporting information
Table S1–S4
Figure S1–S5
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025984
For Sources of Funding and Disclosures, see page 11.
References
- 1. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi‐Sunyer FX, Eckel RH, American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism . Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 2. Robertson J, Schaufelberger M, Lindgren M, Adiels M, Schiöler L, Torén K, McMurray J, Sattar N, Åberg M, Rosengren A. Higher body mass index in adolescence predicts cardiomyopathy risk in midlife. Circulation. 2019;140:117–125. doi: 10.1161/CIRCULATIONAHA.118.039132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim MS, Kim WJ, Khera AV, Kim JY, Yon DK, Lee SW, Shin JI, Won HH. Association between adiposity and cardiovascular outcomes: an umbrella review and meta‐analysis of observational and Mendelian randomization studies. Eur Heart J. 2021;42:3388–3403. doi: 10.1093/eurheartj/ehab454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK biobank: a Mendelian randomization study. Eur Heart J. 2020;41:221–226. doi: 10.1093/eurheartj/ehz388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO . Obesity and overweight. 2020. https://www.who.int/en/news‐room/fact‐sheets/detail/obesity‐and‐overweight.
- 6. Pineda E, Sanchez‐Romero LM, Brown M, Jaccard A, Jewell J, Galea G, Webber L, Breda J. Forecasting future trends in obesity across Europe: the value of improving surveillance. Obes Facts. 2018;11:360–371. doi: 10.1159/000492115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lissner L, Mehlig K, Rosengren A, Toren K, Åberg M. A growing social divide in body mass index, strength, and fitness of Swedish male conscripts. J Adolesc Health. 2019;65:232–238. doi: 10.1016/j.jadohealth.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 9. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 10. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all‐cause mortality and heart failure: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2017;24:1555–1566. doi: 10.1177/2047487317715769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. An Y, Ogawa H, Yamashita Y, Ishii M, Iguchi M, Masunaga N, Esato M, Tsuji H, Wada H, Hasegawa K, et al. Causes of death in Japanese patients with atrial fibrillation: the Fushimi atrial fibrillation registry. Eur Heart J Qual Care Clin Outcomes. 2019;5:35–42. doi: 10.1093/ehjqcco/qcy033 [DOI] [PubMed] [Google Scholar]
- 12. Andersson T, Magnuson A, Bryngelsson IL, Frøbert O, Henriksson KM, Edvardsson N, Poçi D. All‐cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995‐2008: a Swedish nationwide long‐term case‐control study. Eur Heart J. 2013;34:1061–1067. doi: 10.1093/eurheartj/ehs469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, Jacob S. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123:646–651. doi: 10.1016/j.amjmed.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 14. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, et al. The 'obesity paradox' in atrial fibrillation: observations from the ARISTOTLE (Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. 2016;37:2869–2878. doi: 10.1093/eurheartj/ehw124 [DOI] [PubMed] [Google Scholar]
- 15. Bertomeu‐Gonzalez V, Moreno‐Arribas J, Esteve‐Pastor MA, Roldán‐Rabadán I, Muñiz J, Raña‐Míguez P, Ruiz‐Ortiz M, Cequier Á, Bertomeu‐Martínez V, Badimón L, et al. Association of body mass index with clinical outcomes in patients with atrial fibrillation: a report from the FANTASIIA registry. J Am Heart Assoc. 2020;9:e013789. doi: 10.1161/JAHA.119.013789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish diet, cancer, and health study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 17. Lindgren M, Åberg M, Schaufelberger M, Åberg D, Schiöler L, Torén K, Rosengren A. Cardiorespiratory fitness and muscle strength in late adolescence and long‐term risk of early heart failure in Swedish men. Eur J Prev Cardiol. 2017;24:876–884. doi: 10.1177/2047487317689974 [DOI] [PubMed] [Google Scholar]
- 18. Glassford RG, Baycroft GH, Sedgwick AW, Macnab RB. Comparison of maximal oxygen uptake values determined by predicted and actual methods. J Appl Physiol. 1965;20:509–513. doi: 10.1152/jappl.1965.20.3.509 [DOI] [PubMed] [Google Scholar]
- 19. Åberg ND, Kuhn HG, Nyberg J, Waern M, Friberg P, Svensson J, Torén K, Rosengren A, Åberg MA, Nilsson M. Influence of cardiovascular fitness and muscle strength in early adulthood on long‐term risk of stroke in Swedish men. Stroke. 2015;46:1769–1776. doi: 10.1161/STROKEAHA.115.009008 [DOI] [PubMed] [Google Scholar]
- 20. Andersen K, Rasmussen F, Neovius M, Tynelius P, Sundström J. Body size and risk of atrial fibrillation: a cohort study of 1.1 million young men. J Intern Med. 2018;283:346–355. doi: 10.1111/joim.12717 [DOI] [PubMed] [Google Scholar]
- 21. Breiman L. Random forests. 2001.
- 22. Nyström PK, Carlsson AC, Leander K, de Faire U, Hellenius ML, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS One. 2015;10:e0127111. doi: 10.1371/journal.pone.0127111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Persson CE, Adiels M, Björck L, Rosengren A. Young women, body size and risk of atrial fibrillation. Eur J Prev Cardiol. 2018;25:173–180. doi: 10.1177/2047487317740644 [DOI] [PubMed] [Google Scholar]
- 24. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, et al. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42 [DOI] [PubMed] [Google Scholar]
- 25. Singleton MJ, German CA, Carnethon M, Soliman EZ, Bertoni AG, Yeboah J. Race, body mass index, and the risk of atrial fibrillation: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2021;10:e018592. doi: 10.1161/JAHA.120.018592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 27. Ardestani A, Hoffman HJ, Cooper HA. Obesity and outcomes among patients with established atrial fibrillation. Am J Cardiol. 2010;106:369–373. doi: 10.1016/j.amjcard.2010.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conner SC, Lodi S, Lunetta KL, Casas JP, Lubitz SA, Ellinor PT, Anderson CD, Huang Q, Coleman J, White WB, et al. Refining the association between body mass index and atrial fibrillation: G‐formula and restricted mean survival times. J Am Heart Assoc. 2019;8:e013011. doi: 10.1161/JAHA.119.013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, Glascock BJ, Garcia VF, Kimball TR. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol. 2008;51:1342–1348. doi: 10.1016/j.jacc.2007.12.029 [DOI] [PubMed] [Google Scholar]
- 31. Wang TJ, Parise H, Levy D, D'Agostino RB, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Figure S1–S5


