Abstract
Background
Peripheral artery disease (PAD) increases the risk of cardiovascular events and limb events including amputations. PAD is twice as prevalent in Black compared with non‐Hispanic White individuals, especially among men. Screening for PAD using the ankle–brachial index in community settings, such as the barbershop, could lead to earlier diagnosis and treatment.
Methods and Results
A pilot study was conducted at 2 barbershops in Cleveland, OH from June to December 2020 to assess the feasibility of screening for PAD in the barbershop setting and the effect of an educational intervention on PAD awareness. After screening with both automated and Doppler ankle–brachial index, PAD was identified in 5/31 (16.1%) of participants. Baseline systolic blood pressure, low‐density lipoprotein cholesterol, and random blood glucose were higher in participants who screened positive for PAD (P<0.001). PAD awareness was low overall. There was a significant improvement in PAD awareness assessment scores obtained at the initial and exit visits (9.93±4.23 to 12.50±4.41, P=0.004). An association was found between PAD awareness at baseline and highest education level achieved: compared with those with some college/associate's degree or higher, non–high school graduates scored lower on PAD awareness (P=0.022), as did those who only had a high school diploma or tests of General Educational Development (P=0.049).
Conclusions
In a pilot study, barbershop‐based screening for PAD among Black men revealed a higher than expected PAD prevalence and low PAD awareness. An educational video was effective at increasing PAD awareness. Ankle–brachial index screening and educational outreach in the barbershop may be a feasible and effective tool to diagnose PAD and reduce PAD disparities among Black men at highest risk.
Keywords: ankle brachial index, health disparities, peripheral artery disease, vascular disease
Subject Categories: Peripheral Vascular Disease, Vascular Disease
Nonstandard Abbreviations and Acronyms
- RAND SF‐36
RAND short form survey 36
- WIQ
walking impairment questionnaire
Clinical Perspective.
What Is New?
In this pilot study conducted at 2 barbershops in Cleveland, OH, Black men between the ages of 40 and 89 years were screened for peripheral artery disease (PAD) using the ankle–brachial index and provided with an educational intervention to increase PAD awareness.
The prevalence of PAD was higher than expected in this small sample and general PAD awareness was low.
PAD awareness improved over the course of the study.
What Are the Clinical Implications?
Screening for PAD with the ankle–brachial index among Black men in the barbershop setting is feasible given the relatively high prevalence in this population and a willingness to participate in this community‐based setting.
A simple, educational intervention was successful at increasing PAD awareness among Black men, who are at highest risk for the disease and its morbid consequences.
The insights from this study should be used to guide future barbershop‐based or other community‐based interventions targeting PAD awareness and detection in Black men or other at‐risk populations.
Peripheral artery disease (PAD) is a debilitating, progressive vascular disease caused by atherosclerotic obstruction of the lower extremity arteries. Although >200 million people worldwide and ≈8.5 million in the United States are affected by PAD, the burden of disease continues to rest disproportionately on Black Americans. 1 , 2 PAD has significant morbidity associated with walking impairment, claudication, and limb loss; it has also been shown to increase the risk of myocardial infarction, ischemic stroke, and death because of cardiovascular cause. 3 , 4 , 5 , 6 , 7
The estimated prevalence of PAD (as defined by an ankle–brachial index [ABI] of ≤0.90) among individuals aged 40 years and older in the United States ranges from 4.3% to 5.8%. 2 Black men, in particular, have rates of PAD that are twice as high as other races and ethnicities for any given age >40 years old. 8 Among the large cohort of MESA (Multi‐Ethnic Study of Atherosclerosis), the rate of PAD in Black men between the ages of 45 and 84 years was 6.1% compared with 3.5% in non‐Hispanic White men. Rates for Hispanic, Asian American, and Native American men are similar to rates for non‐Hispanic White men. 8 , 9 In addition to a higher prevalence of the disease, Black Americans are 2 to 4 times more likely to undergo lower extremity amputation for the most severe manifestation of PAD, known as critical limb ischemia, as compared with their non‐Hispanic White counterparts. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
Despite the significant prevalence and high rate of associated morbidity and mortality, PAD has received considerably less attention than the other forms of atherosclerotic cardiovascular disease (eg, coronary artery disease and stroke). General population awareness of PAD is estimated at 25%, which is the lowest awareness of all the cardiovascular diseases. 19 Disease awareness is almost certainly lower among Black Americans. 20 Low awareness about the signs and symptoms as well as the morbid consequences of PAD can contribute to worsening health care disparities. Black Americans may be less likely to know what conditions put them at risk for PAD and less likely to recognize or take seriously the manifestations of PAD, such as claudication, walking impairment, rest pain, and nonhealing wounds.
Community outreach in the barbershop setting may serve as a potential bridge to provide Black men with access to health care in a safe space. Previous studies have demonstrated that barbershop‐based screening for hypertension and subsequent treatment and follow‐up were effective at lowering blood pressure (BP) and promoting healthy behaviors in Black men. 21 , 22 , 23 While Black women have high rates of PAD as well (though not as high as their male counterparts), Black men have significantly less physician interaction than Black women, which can result in delayed diagnoses and treatment. 24 , 25
The ABI is a simple, reliable, and well‐validated tool that has been shown to accurately diagnose PAD when compared with diagnostic imaging studies. 26 , 27 Screening for PAD using the ABI in community settings involves an additional practical hurdle because this test requires space and time to perform correctly. Our primary hypothesis is that screening for PAD using the ABI in the barbershop setting and an educational intervention promoting awareness of the disease itself may be a feasible approach for improving early diagnosis and recognition of PAD in Black men.
METHODS
Deidentified data from this study and the PAD Awareness Assessment are available from the authors upon reasonable request.
This pilot study consisted of a prospective screening for PAD and hypertension and an educational intervention that took place in 2 barbershops in Cleveland, OH from June to December 2020. The institutional review board at the University Hospitals in Cleveland, OH approved the trial.
Study Participants
Subjects presenting to the barbershop who met specific inclusion and exclusion criteria (Table 1) were invited to participate and, if interested, provided written informed consent. Participants with known PAD and/or hypertension were eligible to participate if they met all other inclusion and exclusion criteria. A convenience sample of barbershop patrons was approached (n=98) by a study team member for eligibility screening.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
BP indicates blood pressure; and PAD, peripheral artery disease.
Study Visits
Study participants completed 3 visits in the barbershop including an initial screening visit in which they underwent PAD screening using an automated ABI device, a second visit 4 to 6 weeks later where ABI was assessed using the criterion standard of manual continuous wave Doppler to confirm the diagnosis of PAD, and a third and final visit that took place 4 to 6 weeks after the second visit, during which an exit interview and assessments were conducted. The study also involved viewing of an educational video about PAD after the second study visit. Participants were compensated up to $100 over the duration of the study ($20 each for the first 2 visits, $40 for the exit visit, and a $20 restaurant gift card for watching the educational video). Compensation for these visits was comparable to that provided in another barbershop‐based research study. 22
All procedures were administered by a trained member of the study team. Electronic surveys, BP measurements, and ABI measurements were completed before, during, or after the participant's haircut. Study team members administered in‐person questionnaires on an iPad tablet. Questionnaires included information on demographics, past medical history, family history, current medications, current lifestyle, as well as a PAD awareness assessment (Data S1). The Walking Impairment Questionnaire (WIQ) and a health‐related quality of life assessment with RAND Short Form Survey 36 (RAND SF‐36) were completed at the second and third study visits. 28 , 29 The WIQ has 4 summary scores corresponding to the domains of walking impairment, walking distance, walking speed, and stair climbing. The scores are expressed on a scale of 0% to 100%, with 0% meaning the patient was unable to perform because of claudication and 100% meaning no impairment. 28 The RAND SF‐36 has 8 scoring scales also from 0 to 100 corresponding to the domains of general health, physical functioning, role functioning/physical, role functioning/emotional, energy/fatigue, emotional well‐being, social functioning, and pain. 29
Fingerstick cholesterol and glucose readings were obtained at the second visit (Cholestech LDX analyzer, Abbott). After the second visit, participants were invited to watch an educational video about PAD either at home with a hyperlink provided or in the barbershop on a provided iPad.
For all enrolled study participants, there was a 3‐month follow‐up/exit visit. This occurred anywhere between 2 and 3 months from the initial visit. At the exit visit, outcome assessments were made using an electronic survey for cardiac and vascular events or vascular procedures and for smoking status. Electronic surveys were repeated, including the current medication list, health‐related quality of life assessment (RAND SF‐36), PAD knowledge self‐assessment, and WIQ. Automated BP measurements were recorded as done on prior visits as described above. ABI was not repeated because it would not be expected to change within a 3‐month period absent an intervention. A brief exit interview was conducted to assess the participants' subjective experience in the study including with the procedural and educational intervention components. It also queried individual concerns specific to being in the barbershop setting including concerns about privacy.
All state‐mandated precautions for COVID‐19 were adhered to during the study. 30 Participants were required to mask and adhere to social distancing during all study encounters in the barbershop. All study staff wore masks and used gloves for all study encounters.
BP and PAD Assessments
BP measurements were obtained with the use of a validated, oscillometric BP monitor (HEM‐907XL, OMRON) 31 administered by a member of the study team at all visits. The BP was obtained in the participant after at least 5 minutes seated in a chair at rest. The measurement was repeated in that arm and then repeated twice in the opposite arm (provided there were no contraindications to that arm being used). If there was a contraindication to an arm being used (eg, dialysis fistula site), then all 4 measurements were obtained from the available arm. Of the 4 consecutive BP readings taken, the last 2 readings from each arm were averaged (systolic and diastolic) to calculate a BP value. The calculated BP obtained from the screening visit using the above method was used as the baseline BP value.
The resting ABI was obtained in a separate area of the barbershop that had been cordoned off for privacy with a standing curtain (Figure 1). Initial ABI screening at the first visit was performed using a validated, automated ABI plethysmographic device (Dopplex Ability, Huntleigh) in accordance with the manufacturers' instructions. 32 The participant lay supine on a cot while ABI value was obtained once in both lower extremities. The automated ABI measurement was repeated once for reproducibility and both values were recorded. The average of the 2 ankle pressures over the highest average arm pressures (right or left) was used to calculate the automated ABI measurement. For the purposes of analysis, ABI ≤0.9 in either lower extremity was considered positive for the diagnosis of PAD by the automated device.
Figure 1. Photographs of the screening location in one of the barbershops.
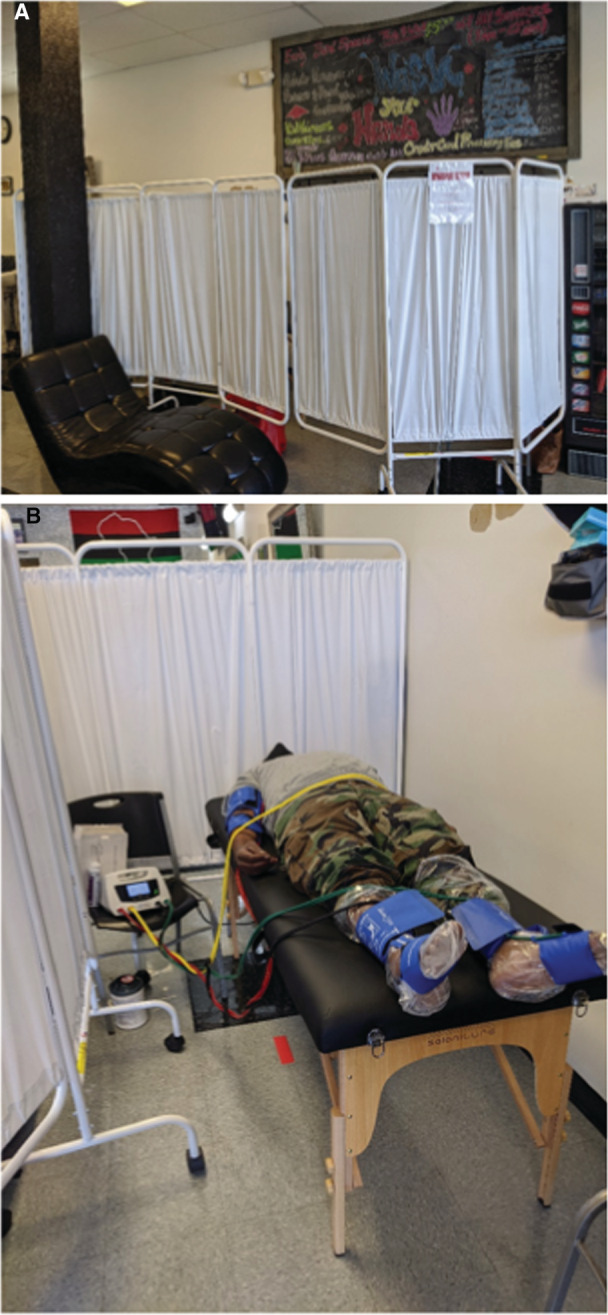
A, Sequestered area where ABI screening took place. B, Method of ABI measurement using automated oscillometric device. ABI was subsequently measured using the criterion standard continuous wave Doppler method. ABI indicates ankle–brachial index.
During the second visit, the confirmatory ABI assessment was performed manually using the Huntleigh Dopplex D900 hand‐held continuous wave Doppler device and the Welch Allyn DS66 Trigger aneroid sphygmomanometer after at least 5 minutes of rest in the supine position on a cot. The Doppler ABI was performed using standardized methods for measurement and calculation. 33 A Doppler ABI of ≤0.9 in either lower extremity was considered positive for the diagnosis of PAD.
PAD Educational Intervention
After the second visit, participants were strongly encouraged to view a prerecorded 30‐minute educational video posted online (https://www.uhhospitals.org/Health‐Talks/articles/June/what‐everyone‐should‐know‐about‐peripheral‐artery‐disease‐pad) that included information regarding PAD symptom recognition and risk factor reduction including concepts tested on the PAD awareness assessment. After viewing the video, participants completed an electronic attestation survey. The video was a recording of a live patient education webinar on PAD developed by study investigators in partnership with the Vascular Center at University Hospitals Harrington Heart & Vascular Institute.
Outcomes
The goal of this pilot study was to assess the feasibility of screening for PAD in the barbershop and the effect of an educational intervention on PAD awareness. Feasibility was assessed by multiple parameters: percentage of eligible patrons willing to participate (recruitment rate), percentage of participants who completed all 3 study visits (retention rate), and willingness to participate in this study again based on exit interviews. We also sought to sample the prevalence of PAD in the barbershop setting.
Based on results of prior community‐based research that enrolled a similar population, 34 , 35 we set the following prespecified criteria for feasibility: recruitment rate of ≥35% and retention rate of ≥70%. We hypothesized that the prevalence of PAD in our sample would be greater than or equal to the ≈6% PAD prevalence rate in similarly aged Black men in the United States. 7
Scores on the PAD awareness assessment, including subjective and objective measures of PAD awareness and knowledge, were measured at the first and third/exit visits to give a measure of the impact of the educational intervention on the participants' PAD awareness. Good PAD awareness was defined as having achieved ≥9 points out of a total of 21 on the PAD awareness assessment. Participants with <9 points were considered to have poor PAD awareness. This cut point was chosen post hoc as reflecting the median score in the participant sample.
Statistical Analysis
Continuous data were reported as means and SDs (if normally distributed), and categorical data were reported as frequencies and percentages. Differences between groups were assessed using paired t tests for continuous data, and χ2 or Fisher exact test for categorical data. ABI measurements for each limb were treated as independent for this analysis (ie, right side and left side). All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC) using SAS procedures (PROC FREQ, PROC MEANS, PROC GLM, PROC MIXED). Differences between groups were considered statistically significant at P<0.05.
RESULTS
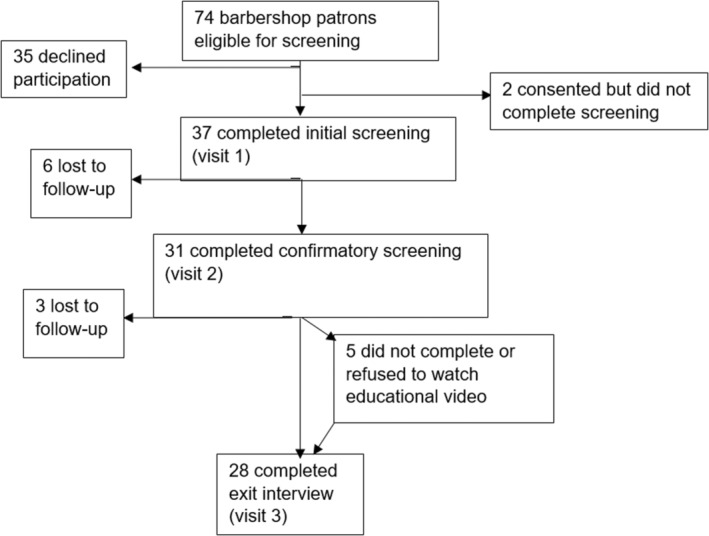
Based on the inclusion and exclusion criteria above, 74/98 (76%) of a convenience sample of barbershop patrons were screened and deemed eligible to participate in the study. Among eligible patrons, 37/74 (50%) agreed to enroll and completed the initial screening. Two additional participants signed informed consent to participate but did not complete any study procedures. The screening, enrollment, and follow‐up are detailed in Figure 2. Patrons who declined to participate did so primarily because of time constraints, because they already knew their health status, or because they did not want to participate in research. Of the 37 participants who completed the initial intake visit, 31 (84%) completed the second confirmatory testing visit, 28 (76%) completed the exit visit, and 26 (70%) watched the educational video. The overall recruitment rate (those who participated in the initial screening out of eligible participants) was 37/74 or 50%. Nine out of 37 (24%) study participants were lost to follow‐up during the course of the study, leaving an overall retention rate of 76%. With regard to attrition, those 9 who dropped out were significantly younger (50.9±8.6 versus 59.3±11.2 years), P=0.029. No other demographic factors were significantly associated with study dropout. In contrast, those who did watch the educational video were younger than those who did not watch it (58.2±11.3 versus 66.3±8.2 years), but this did not achieve statistical significance (P=0.144), likely a reflection of the small sample size for this comparison.
Figure 2. Screening, enrollment, and follow‐up of barbershop patrons.
Demographics of the 37 participants who completed the initial screening visit are shown in Table 2. The mean age was 56.8±1.4 years. There were 17 participants (44%) who reported having a known diagnosis of hypertension. None of the participants reported having a known prior diagnosis of PAD, although 4 reported having been screened with ABI in the past. The majority of participants were current smokers (57%), and 73% had achieved the equivalent education of a high school diploma or less. The average of the median household income by zip code of participant was $34 305. The majority of participants had seen a primary care physician in the past year (65%). A high proportion of participants reported having no health insurance (22%) compared with the national average of 9%. 36
Table 2.
Baseline Sociodemographics and Survey Responses (N=37 Participants)
| Total participants (%) or mean (SD) | |
|---|---|
| Age, y | 56.8 (11.4) |
| Past medical history | |
| Hypertension | 17 (44) |
| Known PAD | 0 |
| Diabetes | 5 (13) |
| Myocardial infarction | 0 |
| Stroke | 2 (5) |
| High cholesterol | 6 (15) |
| Medications taken | |
| Antihypertensive | 13 (33) |
| Statin | 3 (8) |
| Aspirin | 2 (5) |
| Other antiplatelet or anticoagulant medications | 0 (0) |
| Cigarette smoking history | |
| Never smoker | 9 (23) |
| Former smoker | 7 (26) |
| Current smoker | 21 (57) |
| Duration of smoking, y | 25.56 (16.51) |
| Packs/d | 0.74 (0.57) |
| Highest education level | |
| Not a high school graduate | 14 (38) |
| High school graduate or GED | 13 (35) |
| Some college/associate's degree | 9 (24) |
| Bachelor's degree | 0 (0) |
| Graduate or professional degree | 1 (3) |
| Median zip code household income* | $34 305 (14 952) |
| Health insurance status | |
| Insured | 29 (78) |
| Uninsured | 8 (22) |
| Self‐perceived health status | |
| Excellent or very good | 8 (22) |
| Fair or poor | 11 (30) |
| Follows with a primary care physician | 24 (65) |
| Follows with a cardiologist or vascular specialist | 7 (19) |
| Has been previously screened for PAD using ABI | 5 (14) |
| Self‐reported frequency of visits to the barbershop | |
| More than twice a month | 14 (38) |
| Once or twice a month | 21 (55) |
| Less than once a month | 3 (8) |
ABI indicates ankle–brachial index; GED, tests of General Education Development; and PAD, peripheral artery disease.
Per 2010 US Census data.
WIQ and RAND SF‐36 Questionnaires
There were 31 participants who completed the WIQ and RAND SF‐36 at the confirmatory visit. Among the 31 participants who completed the confirmatory visit, the mean score and SD for the WIQ components were as follows: walking impairment was 83.33±24.02, walking distance was 75.08±29.65, walking speed was 73.03±28.38, and climbing was 81.33±24.42. RAND SF‐36 means scores and SDs in this sample were as follows: general health (55.36±22.93), physical functioning (72.92±29.25), role functioning/physical (71.43±37.71), role functioning/emotional (69.05±37.33), energy/fatigue (60.54±24.99), emotional well‐being (76.43±21.67), social functioning (79.02±27.01), and pain (67.05±26.99).
Participants found to have PAD (ABI ≤0.9) had lower WIQ and RAND SF‐36 scores than those without PAD. For the WIQ, the walking impairment, walking distance, walking speed, and stair climbing score for individuals with PAD (n=5) were 68.75±37.50, 69.64±30.05, 39.13±25.37, and 59.38±25.77, respectively, compared with those without PAD (n=26), which were 85.87±21.09, 78.47±28.88, 78.92±24.88, and 85.14±22.61. The individuals with PAD had a lower score on the energy/fatigue (38.75±10.31 versus 64.57±25.45) domain of the SF‐36, but the other 7 domains were fairly similar between the groups (within 10 points).
ABI, BP, and LDL Cholesterol/Glucose
As identified by Doppler‐based ABI ≤0.90 in either lower extremity, PAD was confirmed in 5/31 or 16.1% of participants. Four of these participants with abnormal Doppler ABI also had PAD as determined by the automated device; one additional participant had a normal automated ABI but had a subsequent abnormal Doppler ABI. All 5 participants diagnosed with PAD based upon abnormal Doppler ABI had never been screened for PAD previously and were unaware of their PAD diagnosis before enrollment in the study.
The participants found to have PAD had a significantly higher mean baseline systolic BP compared with those without PAD (141.2 mm Hg versus 132.8 mm Hg; P<0.001). Diastolic BP was higher in those without PAD. There was no significant difference between participants' BPs obtained at the initial and exit visits. Participants with PAD also had higher low‐density lipoprotein cholesterol (98.0 mg/dL versus 91.2 mg/dL; P<0.001) and higher random blood glucose (109.3 mg/dL versus 106.6 mg/dL; P<0.001) compared with those who did not have PAD.
PAD Awareness Assessment
Baseline PAD awareness was low in this population because the vast majority of participants who completed the study (24/28 or 86%) self‐reported low or no knowledge of PAD at the initial visit (according to Question 1 of the PAD Awareness Assessment). The questions that the majority of participants answered incorrectly related to the signs and symptoms of PAD and its most critical manifestation, critical limb ischemia. In addition, only 32% of the participants knew that Black men were more likely than White men to have PAD. There was a significant improvement in mean scores pre‐ and postintervention (baseline mean score 9.93±4.23, final mean score 12.50±4.41; P=0.004). A total of 20/28 participants (71%) showed improved PAD awareness scores. As shown in Figure 3, 15/28 participants (54%) had good awareness at baseline as defined by a PAD awareness assessment composite score of ≥9 (median value) compared with 23/28 participants (82%) at the final visit (P=0.044). A significant association was found between good PAD awareness at baseline and education level (Figure 4). Compared with those with some college/associate's degree or higher (11.20±5.12), those who did not graduate high school scored lower on PAD awareness (8.08±4.29; P=0.022), as did those who only had a high school diploma or tests of General Educational Development (9.63±4.421; P=0.049). Income level, the presence of PAD, health insurance status, and age did not have any significant effect on PAD awareness assessment scores.
Figure 3. PAD awareness assessment composite score at baseline and final study visits (N=28).
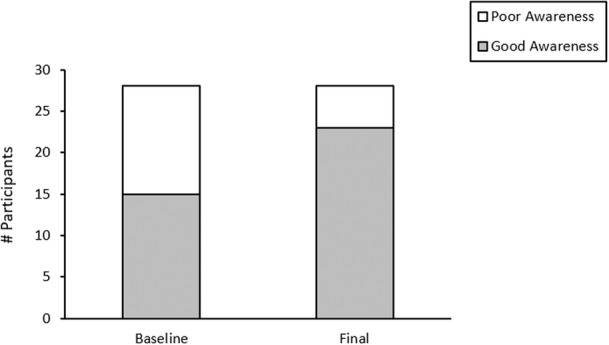
Poor awareness was defined as less than the median score of 9 on the PAD awareness assessment and good awareness was defined as a score of ≥9. Fifteen of 28 participants (54%) had good awareness at baseline compared with 23/28 (82%) at the final visit (P=0.044). A total of 20/28 (71%) participants improved their overall score with a significant difference between the baseline and final mean scores (baseline mean score 9.93±4.23, final mean score 12.50±4.41; P=0.004). PAD indicates peripheral artery disease.
Figure 4. PAD awareness assessment composite scores and highest educational level achieved.
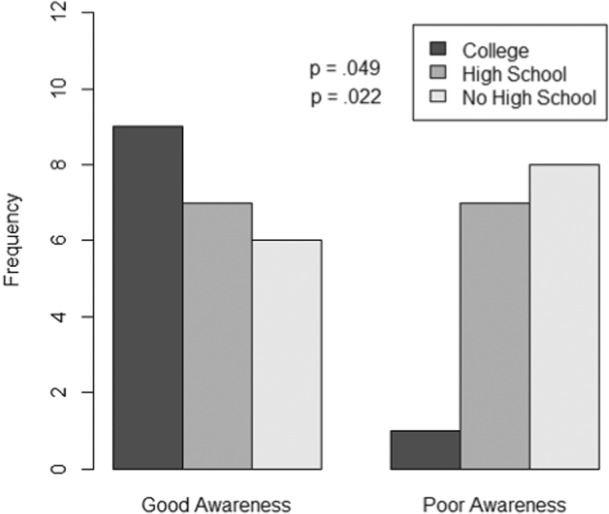
Good PAD awareness (score of ≥9) was significantly associated with higher education level and poor PAD awareness (score <9) was associated with lower educational level at baseline. Compared with those with some college/associate's degree or higher, non–high school graduates scored lower on PAD awareness (P=0.022), as did those who only had a high school diploma or GED (P=0.049). GED indicates Tests of General Educational Development; and PAD, peripheral artery disease.
Exit Interview
All 28 participants who completed the exit visit (100%) said they would participate in this study again if given the opportunity. Two participants voiced concerns about individual privacy and confidentiality in the barbershop setting, while the vast majority of participants reported no concerns with completing any of the procedures within the barbershop setting. All participants expressed satisfaction with the health screenings and most encouraged the study team to continue such initiatives for the betterment of the community.
DISCUSSION
Black men, who are at the greatest risk for developing PAD and its morbid consequences, have low PAD awareness and less physician interaction. 20 , 25 One of the barriers to the reduction of PAD‐related health disparities is disease recognition and awareness. In a study conducted in 2 barbershops in Cleveland, OH, Black men who were screened for PAD using ABI were found to have a high prevalence of previously undiagnosed PAD (16.1%). A simple educational video, which highlighted signs and symptoms as well as consequences of PAD, resulted in an improvement in PAD awareness. Together, these finding suggest that screening for PAD using the ABI in the barbershop along with an educational intervention promoting awareness of the disease improved diagnosis and recognition of PAD in this sample of Black men.
The results of the current pilot study, which uses the ABI to screen for PAD in an economically disadvantaged, urban, and Black male population, support the feasibility of the study design within the community. While previous studies have shown that the barbershop can be a successful setting for medical intervention, this is the first study to show that using the space‐consuming and time‐intensive ABI methodology can be implemented for PAD diagnosis in the community. All of the feasibility criteria in this pilot study were met, which provides support for replicating such a design on a larger scale in this underserved and difficult‐to‐reach population. The recruitment rate of 50% and retention rate of 76% were above our prespecified goals of 35% and ≥70%, respectively. The prevalence rate of 16.1% was higher than the expected 6% prevalence rate in similarly aged Black men in the United States. 8 , 9
While most participants reported being under the care of a primary care provider that they had seen at least once in the past year, the majority had never been screened for PAD in their lifetime. This is consistent with previous data that show that screening for PAD is lacking in the primary care setting. Even among patients with known PAD, less than half of their primary care physicians are aware of the diagnosis. 4 In our study, participants with PAD also had significantly higher systolic BP, low‐density lipoprotein cholesterol, and blood glucose than those without PAD, identifying opportunities for medical therapy and risk factor modification in this population. Since most patients will only have contact with a primary care physician and not a specialist, it is crucial that front‐line primary care providers be aware of the indications for PAD screening and have the ability to appropriately manage these patients or refer them to a vascular specialist when needed.
Multisocietal clinical practice guidelines for patients with PAD give a Class IA recommendation for antihypertensive therapy (for those with concomitant hypertension), statin/lipid‐lowering therapy, antiplatelet therapy with aspirin or clopidogrel (for those who are symptomatic), and smoking cessation interventions (for those who smoke). 37 Several studies have shown that Black Americans are less likely to be treated according to the clinical practice guidelines for the risk factors that predispose to PAD, including cigarette smoking, diabetes, hypertension, and hyperlipidemia. 38 , 39 , 40 , 41 , 42 Our results also support these findings.
Even after adjusting for differences in the distribution of these traditional cardiovascular risk factors among ethnic groups, PAD still remains significantly more prevalent in Black Americans, suggesting differential contributions from potential biological and social determinants of health. 43 , 44 , 45 , 46 Education status is a social determinant of health that can be a reflection of one's socioeconomic environment. In our study, participants with lower education levels had lower PAD awareness compared with those with higher levels of education. Another study by Pande and Creager found that individuals with lower education levels also had significantly higher prevalence of PAD. 47 It is therefore of the utmost importance that social policies be implemented to improve affordable access to higher education in impoverished and underserved minority communities because they are important both for economic empowerment and for health equity.
Vascular screening, which included the ABI, has previously been shown to result in a significant reduction in cardiovascular mortality, suggesting that early diagnosis of PAD can lead to interventions that may improve survival. 48 The higher rates of limb amputation in Black Americans may, in part, be because of the fact that Black Americans present at a later stage and with more severe PAD than their non‐Hispanic White counterparts. 49 The consequences of delayed diagnosis and treatment are grave. Survival rates after major amputation for critical limb ischemia are abysmally low, with only 50% of amputees surviving after 2 years. 50 , 51 Thus, there is a critical need for early detection of PAD and appropriate therapies to modify the natural history of PAD, especially for Black Americans. In addition to ABI screening, this study provided education on the signs and symptoms of PAD and critical limb ischemia, which was one key knowledge gap on the PAD awareness survey. Given that <1/3 of the participants in our study knew that Black men have PAD at higher rates than White men, this may be a ripe target for future educational interventions in this population. Black men must recognize that they are at a greater risk of developing PAD and experiencing its morbid consequences in order to become proactive about prevention, early detection, and treatment.
The major strength of this study is the design, which used a novel and trusted community‐based setting. To our knowledge, this is the first study to screen for PAD using the ABI among Black men in barbershops. The setting likely led to a higher recruitment and retention rate than would be expected in a similar hospital‐based study. A common theme in the exit interviews with participants was the sense of community that was experienced within the barbershop. The barbershop owners played a critical role as respected community members who provided an endorsement of the study. In addition, the principal investigator, a Black woman with an earned record of generating trust in this community, provided an important connection to the community and the participants. Future studies should build on the importance of barbers as advocates and include research investigators from the specific communities being studied in order to facilitate participant recruitment and retention.
There were several limitations to this pilot study. The biggest limitation is the small sample size, which precludes generalization to the population at large. Nevertheless, even in this small sample, we were able to recognize significant prevalence of PAD and trends in improved PAD awareness with a relatively minor educational intervention. Another limitation is the fact that the diagnosis of PAD was based only on ABI of ≤0.9 when in reality, patients with normal and supranormal ABI (>1.3) can also have PAD because of calcification of the lower extremity arteries. 52 However, this limitation could only result in an underestimation of the actual prevalence of PAD in this sample. Another limitation is the fact that the PAD awareness assessment tool was not standardized, which limits both the validity and generalizability of the PAD awareness results. Lastly, the study protocol may not be generalizable to all barbershops because it requires enough space to have the equipment for an ABI (including a private area and cot), highly motivated barbers, and barbershop owners willing to tolerate adjustments to their workflow, and trained study personnel to perform the Doppler ABI procedure. Future studies and applications of our findings might consider using a dedicated mobile unit parked outside of the barbershop to address both the privacy issues of performing the procedures within the shop itself as well as the feasibility of visiting more shops in a given period. One might also consider training the barbers and barbershop owners to perform the ABI and PAD educational activities because these individuals not only serve a vital role as community ambassadors, but also can promote the sustainability of the initiative.
CONCLUSIONS
Screening for PAD with the ABI among Black men in the barbershop setting is feasible. In our small community‐based sample within the barbershops, there was a high prevalence of previously undiagnosed PAD. A simple, educational intervention increased PAD awareness among Black men, which may lead to earlier recognition of potential PAD in a high‐risk population. The insights from this study should be used to guide future barbershop or other community‐based interventions targeting PAD awareness and detection in at‐risk populations.
Sources of Funding
Dr White Solaru received funding from the PRIDE‐CVD (Program to Increase Diversity Among Individuals Engaged in Health Related Research) sponsored by the National Heart, Lung, and Blood Institute (NHLBI) as well as University Hospitals Minority Faculty Award to support this research. Dr. Delozier received support from the Clinical Research Center of University Hospitals Cleveland Medical Center (UHCMC) and the Case Western Reserve University Clinical and Translational Science Collaborative (CTSC) 4UL1TR000439. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of UHCMC or NIH.
Disclosures
Dr White Solaru has received an honorarium from Abbott laboratories. Dr Ravenell is a consultant for Abbott Pharmaceuticals and HealthEfficient. Dr Wright received a consultation fee from Medtronics. Dr Gornik is named on a patent related to oscillometric measurement of the ankle–brachial index. Dr Gornik receives no royalties or compensation related to this patent and this device was not used in the current study. The remaining authors have no disclosures to report.
Supporting information
Data S1
Acknowledgments
The authors are most grateful for the generous collaboration of the barbershop owners and barbers.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026347
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence‐based facts. Atherosclerosis. 2018;275:379–381. doi: 10.1016/j.atherosclerosis.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3. Subherwal S, Patel MR, Kober L, Peterson ED, Bhatt DL, Gislason GH, Olsen AM, Jones WS, Torp‐Pedersen C, Fosbol EL. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015;22:317–325. doi: 10.1177/2047487313519344 [DOI] [PubMed] [Google Scholar]
- 4. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317 [DOI] [PubMed] [Google Scholar]
- 5. Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, Stritzky B, Tepohl G, Trampisch HJ. High prevalence of peripheral arterial disease and co‐morbidity in 6880 primary care patients: cross‐sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/S0021-9150(03)00204-1 [DOI] [PubMed] [Google Scholar]
- 6. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849 [DOI] [PubMed] [Google Scholar]
- 7. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 8. Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic‐specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 9. McDermott MM, Liu L, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle‐brachial index and subclinical cardiac and carotid disease: the multi‐ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167 [DOI] [PubMed] [Google Scholar]
- 10. Hackler EL III, Hamburg NM, White Solaru KT. Racial and ethnic disparities in peripheral artery disease. Circ Res. 2021;128:1913–1926. doi: 10.1161/CIRCRESAHA.121.318243 [DOI] [PubMed] [Google Scholar]
- 11. Arya S, Binney Z, Khakharia A, Brewster LP, Goodney P, Patzer R, Hockenberry J, Wilson PWF. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. 2018;7:e007425. doi: 10.1161/JAHA.117.007425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feinglass J, Abadin S, Thompson J, Pearce WH. A census‐based analysis of racial disparities in lower extremity amputation rates in northern Illinois, 1987‐2004. J Vasc Surg. 2008;47:1001–1007. doi: 10.1016/j.jvs.2007.11.072 [DOI] [PubMed] [Google Scholar]
- 13. Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal trends and geographic variation of lower‐extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000‐2008. J Am Coll Cardiol. 2012;60:2230–2236. doi: 10.1016/j.jacc.2012.08.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Newhall K, Splangler E, Dzebishashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: the effects of race and region. Ann Vasc Surg. 2016;30:292–298. doi: 10.1016/j.avsg.2015.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guadagnoli E, Ayanian JZ, Gibbons G, McNeil BJ, LoGerfo FW. The influence of race on the use of surgical procedures for treatment of peripheral vascular disease of the lower extremities. Arch Surg. 1995;130:381–386. doi: 10.1001/archsurg.1995.01430040043006 [DOI] [PubMed] [Google Scholar]
- 16. Huber TS, Wang JG, Wheeler KG, Cuddeback JK, Dame DA, Ozaki CK, Flynn TC, Seeger JM. Impact of race on the treatment for peripheral arterial occlusive disease. J Vasc Surg. 1999;30:417–425. doi: 10.1016/S0741-5214(99)70068-6 [DOI] [PubMed] [Google Scholar]
- 17. Hughes K, Seetahal S, Oyetuni T, Rose D, Greene W, Chang D, Cornwell E III, Obisesan T. Racial/ethnic disparities in amputation and revascularization: a nationwide inpatient sample study. Vasc Endovascular Surg. 2014;48:34–37. doi: 10.1177/1538574413510618 [DOI] [PubMed] [Google Scholar]
- 18. Hohlman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg. 2011;54:420–426. doi: 10.1016/j.jvs.2011.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roger VL, Go AS, Lloyd‐Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics 2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirsch AT, Murphy TP, Lovell MB, Twillman G, Treat‐Jacobson D, Harwood EM, Mohler ER III, Creager MA, Hobson RW II, Robertson RM, et al. Peripheral arterial disease coalition. Gaps in public knowledge of peripheral arterial disease. Circulation. 2007;116:2086–2094. doi: 10.1161/CIRCULATIONAHA.107.725101 [DOI] [PubMed] [Google Scholar]
- 21. Victor RG, Ravenell JE, Freeman A, Leonard D, Bhat DG, Shafiq M, Knowles P, Storm JS, Adhikari E, Bibbins‐Domingo K, et al. Effectiveness of a barber‐based intervention for improving hypertension control in black men. The BARBER‐1 study: a cluster randomized trial. Arch Intern Med. 2011;171:342–350. doi: 10.1001/archinternmed.2010.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx‐Drew D, et al. A cluster randomized trial of blood‐pressure reduction in black barbershops. N Engl J Med. 2018;378:1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Victor RG, Blyler CA, Li N, Lynch K, Moy NB, Rashid M, Chang LC, Handler J, Brettler J, Rader F, et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10–19. doi: 10.1161/CIRCULATIONAHA.118.038165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoenborn CA, Adams PF, Peregoy JA, Health Behaviors of Adults: United States, 2008–2010 . National Center for Health Statistics. Vital Health Stat. 2013;10:1–184. [PubMed] [Google Scholar]
- 25. Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J of Pub Health. 2007;97:1283–1289. doi: 10.2105/AJPH.2005.080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, et al. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
- 27. Casey S, Lanting S, Oldmeadow C, Chuter V. The reliability of the ankle brachial index: a systematic review. J Foot Ankle Res. 2019;12:39. doi: 10.1186/s13047-019-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Regensteiner J, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 29. Hays RD, Morales LS. The RAND‐36 measure of health‐related quality of life. Ann Med. 2011;33:350–357. [DOI] [PubMed] [Google Scholar]
- 30. Director's Updated and Revised Order for Business Guidance and Social Distancing . Ohio Department of Public Health. Signed May 29, 2020. https://coronavirus.ohio.gov/static/publicorders/revised‐business‐guidance‐sd.pdf.
- 31. Ostchega Y, Nwankwo T, Sorlie PD, Wolz M, Zipf G. Assessing the validity of the Omron HEM‐907XL oscillometric blood pressure measurement device in a National Survey environment. J Clin Hypertens. 2010;12:22–28. doi: 10.1111/j.1751-7176.2009.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis JE, Williams P, Davies JH. Non‐invasive assessment of peripheral arterial disease: automated ankle brachial index measurement and pulse volume analysis compared to duplex scan. SAGE Open Med. 2016;4:205031211665908. doi: 10.1177/2050312116659088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davies JH, Lewis JEA, Williams EM. The utility of pulse volume waveforms in the identification of lower limb arterial insufficiency. EWMA J. 2014;14:21–25. [Google Scholar]
- 34. Skolarus LE, Cowdery J, Dome M, Bailey A, Baek J, Byrd JB, Hartley SE, Valley SC, Saberi S, Wheeler NC, et al. Reach out‐churches: a community‐based participatory research pilot trial to assess the feasibility of a mobile health technology intervention to reduce blood pressure among African Americans. Health Promot Pract. 2018;19:495–505. doi: 10.1177/1524839917710893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osorio M, Ravenell JE, Sevick MA, Ararso Y, Young T, Wall S, Lee DC. Community‐based hemoglobin A1C testing in barbershops to identify black men with undiagnosed diabetes. JAMA Int Med. 2020;180:596–597. doi: 10.1001/jamainternmed.2019.6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keisler‐Starkey K, Bunch LB. Health insurance coverage in the United States: 2020. The United States Census Bureau Report Number P60‐274.
- 37. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huen KH, Chowdhury R, Shafii SM, Brewster LP, Arya S, Duwayri Y, Veeraswamy RK, Dodson TF, Rajani RR. Smoking cessation is the least successful outcome of risk factor modification in uninsured patients with symptomatic peripheral arterial disease. Ann Vasc Surg. 2015;29:42–49. doi: 10.1016/j.avsg.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 39. Collins TC, Slovut DP, Newton R Jr, Johnson WD, Larrivee S, Larrivee S, Patterson J, Johnston JA, Correa A. Ideal cardiovascular health and peripheral artery disease in African Americans: results from the Jackson heart study. Prev Med Rep. 2017;7:20–25. doi: 10.1016/j.pmedr.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goyal A, Cooper HA, Aronow WS, Nagal P, Yandrapalli S, Nabors CC, Frishman WH. Use of statins for primary prevention: selection of risk threshold and implications across race and gender. Am J Med. 2018;131:1234–1237. doi: 10.1016/j.amjmed.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 41. Lipworth L, Fazio S, Kabagambe EK, Munro HM, Nwazue VC, Tarone RE, McLaughlin JK, Blot WJ, Sampson UK. A prospective study of statin use and mortality among 67,385 blacks and whites in the southeastern United States. Clin Epidemiol. 2013;6:15–25. doi: 10.2147/CLEP.S53492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White SK. Peripheral artery disease and African Americans: review of the literature. Curr Cardiovasc Risk Rep. 2019;13:34. [Google Scholar]
- 43. Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans. The San Diego population study. J Am Coll Cardiol. 2008;51:2347–2354. [DOI] [PubMed] [Google Scholar]
- 44. Allison MA, Peralta CA, Wassel CL, Aboyans V, Arnett DK, Cushman M, Eng J, Ix J, Rich SS, Criqui MH. Genetic ancestry and lower extremity peripheral artery disease in the multi‐ethnic study of atherosclerosis. Vasc Med. 2010;15:351–359. doi: 10.1177/1358863X10375586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, et al. The effect of novel cardiovascular risk factors on the ethnic‐specific odds for peripheral arterial disease in the multi‐ethnic study of atherosclerosis (MESA). J Am Coll Cardiol. 2006;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049 [DOI] [PubMed] [Google Scholar]
- 46. Kalinowski L, Dobrucki IT, Malinski T. Race‐specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A [DOI] [PubMed] [Google Scholar]
- 47. Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. doi: 10.1161/CIRCOUTCOMES.113.000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindholt JS, Sogaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet. 2017;390:2256–2265. doi: 10.1016/S0140-6736(17)32250-X [DOI] [PubMed] [Google Scholar]
- 49. Rivero M, Nader ND, Blochle R, Harris LM, Dryjski ML, Dosluoglu HH. Poorer limb salvage in African American men with chronic limb ischemia is due to advanced clinical stage and higher anatomic complexity at presentation. J Vasc Surg. 2016;63:1318–1324. doi: 10.1016/j.jvs.2015.11.052 [DOI] [PubMed] [Google Scholar]
- 50. Cruz CP, Eidt JF, Capps C, Kirtley L, Moursi MM. Major lower extremity amputations at a veterans affairs hospital. Am J Surg. 2003;186:449–454. doi: 10.1016/j.amjsurg.2003.07.027 [DOI] [PubMed] [Google Scholar]
- 51. Pell J, Stonebridge P. Association between age and survival following major amputation. The Scottish vascular audit group. Eur J Vasc Endovasc Surg. 1999;17:166–169. doi: 10.1053/ejvs.1998.0754 [DOI] [PubMed] [Google Scholar]
- 52. Weinberg I, Giri J, Calfon MA, Hawkins BM, Weinberg MD, Margey R, Hannon K, Schainfeld RM, Jaff MR. Anatomic correlates of supra‐normal ankle brachial indices. Catheter Cardiovasc Interv. 2013;81:1025–1030. doi: 10.1002/ccd.24604 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


