ABSTRACT
Background
Fusobacterium nucleatum (F. nucleatum) is an anaerobic oral commensal and the major coaggregation bridge organism linking early and late colonisers. In recent years, a large number of studies suggest that F. nucleatum is closely related to the development of various systemic diseases, such as cardiovascular diseases, adverse pregnancy outcomes, inflammatory bowel diseases, cancer, Alzheimer's disease, respiratory infection, rheumatoid arthritis, etc.
Objective
To review the effect of F. nucleatum on systemic diseases and its possible pathogenesis and to open new avenues for prevention and treatment of F. nucleatum-associated systemic diseases.
Design
The research included every article published up to July 2022 featuring the keywords 'Systemic diseases' OR 'Atherosclerotic cardiovascular diseases' OR 'Atherosclerosis' OR 'Adverse pregnancy outcomes' OR 'Inflammatory bowel disease' OR 'Ulcerative colitis' OR 'Crohn’s disease' OR 'Cancers' OR 'Oral squamous cell carcinomas' OR 'Gastrointestinal cancers' OR 'Colorectal cancer' OR 'Breast cancer' OR 'Genitourinary cancers' OR 'Alzheimer’s disease ' OR 'Rheumatoid arthritis' OR 'Respiratory diseases' AND 'Fusobacterium nucleatum' OR 'Periodontal pathogen' OR 'Oral microbiota' OR 'Porphyromonas gingivalis' and was conducted in the major medical databases.
Results
F. nucleatum can induce immune response and inflammation in the body through direct or indirect pathways, and thus affect the occurrence and development of systemic diseases. Only by continuing to investigate the pathogenic lifestyles of F. nucleatum will we discover the divergent pathways that may be leveraged for diagnostic, preventive and therapeutic purposes.
KEYWORDS: Fusobacterium nucleatum, systemic diseases, periodontal disease, FadA, microbiome
Introduction
Periodontal disease (PD), one of the most common inflammatory diseases in adults, comprises a wide range of inflammatory conditions that jeopardize the supporting structures of the teeth (the gingiva, bone and periodontal ligament). PD is initiated by bacterial biofilm, which interacts with the host immune defense system, further aggravating the inflammatory response [1]. Recently, mounting evidence has supported PD as a potential risk factor for multiple systemic diseases [2–6]. These diseases include cardiovascular diseases, adverse pregnancy outcomes, gastrointestinal and colorectal cancer, Alzheimer’s disease, respiratory infection, rheumatoid arthritis, etc. In addition, studies show that periodontal pathogens can spread to different parts of the body through direct dissemination, blood transmission, immunization and other ways, causing systemic or local infection, thus exerting influence on the occurrence and development of systemic diseases [7–9]. F. nucleatum, a Gram-negative anaerobe, is one of the most abundant species in the oral cavity for both diseased and healthy individuals [6]. It is a coaggregation bridge organism, which links primary and late colonisers by coaggregation-mediated mechanisms and by promoting growth of other anaerobes [10,11]. Its virulence mechanism involves colonization, invasion, as well as induction of aberrant inflammation and tumorigenesis [12]. In the past decade, F. nucleatum has become a hot research topic because of its increasingly revealed associations with extraoral diseases. The surface adhesin FadA expressed by F. nucleatum could increase permeability and promote F. nucleatum penetration of endothelial cells by binding to vascular endothelial cadherin (VE-cadherin) [13–15]. This phenomenon also supported the opinion that hematogenous transmission might be one route used by bacteria to spread from the oral cavity to deeper organs with crossing of the endothelial barrier as a key step in the process [16]. In this review, we delve into recent discoveries and prospects of F. nucleatum-related researches, including our evolving understanding of its mechanistic role in promoting systemic diseases and the challenges of developing diagnostic and therapeutic methods for F. nucleatum-associated systemic diseases.
Association between F. nucleatum and systemic diseases
Atherosclerotic cardiovascular diseases (ACVDs)
ACVDs, including coronary artery disease and stroke, are one of the most common causes of death in the elderly [17]. Atherosclerosis (AS) is the pathological basis of ACVDs, and the widely recognized risk factors of AS include hyperlipidemia, hypertension, smoking, diabetes, obesity, immune damage and genetic factors. However, studies have shown that up to half of AS patients may not be under those risks [18]. In recent years, researchers have found that oral bacteria play a role in the occurrence and development of AS. Oral bacterial DNA was initially detected in human atherosclerotic plaques by Haraszthy et al. [19], as well as in coronary artery biopsies from patients with coronary artery disease and endarterectomy specimens from patients undergoing surgical treatment for atherosclerosis by Ford et al. [20,21]. Some of these bacteria are well-known periodontal pathogens, such as Aggregatibacter actinomycetecomitans (A. actinomycetecomitans), Porphyromonas gingivalis (P. gingivalis), Tannerella forsythia (T. forsythia), Prevotella intermedia (P. intermedia), F. nucleatum, Campylobacter rectus, and Treponema denticola (T. denticola) [19–21], suggesting that PD may be associated with AS. Recent meta-analyses also highlighted the close correlation between PD and AS [22,23]. Moreover, the treatment of PD was also proved to have a certain inhibitory effect on progression of ACVDs independent of traditional ACVDs risk factor management [24].
F. nucleatum is one of the oral bacteria detected in the atherosclerotic plaques [21,25,26] and the frequency of detected F. nucleatum in atherosclerotic plaques and blood vessels is directly related to the severity of PD [27]. In 31 carotid arterectomy specimens, the detection rate of F. nucleatum was 34% [21]. Subsequent animal experiments proved that infection of F. nucleatum alone did not promote AS [28], but in the multi-bacterial infection model, F. nucleatum would act synergistically with other organisms in the development of AS [29,30]. There are several etiological hypotheses for AS, all of which can be attributed to the ‘injury response’ [31]. During the formation of AS, serum lipoprotein concentration, endothelial permeability, and binding of lipoprotein to intima are considered to be three important pathogenic factors [32]. Permeability of endothelial cells is a key factor in the pathogenesis of ACVDs, because the formation of AS requires not only monocytes and lipoprotein to penetrate the endothelium, but also lipoprotein to accumulate in intima [33].
Firstly, F. nucleatum can promote the progression of AS by affecting endothelial cell permeability through various mechanisms. F. nucleatum possesses a best-characterized surface adhesion called FadA [34]. FadA has two forms: one is an undamaged pre-FadA consisting of 129 amino acids and the other is a secreted mature FadA (mFadA) consisting of 111 aa residues [14]. Pre-FadA and mFadA form a high molecular weight complex, FadAc, which can bind to VE-cadherin on endothelial cells, causing the latter to migrate from cell–cell junctions to intracellular compartments [15]. This results in an endothelium so permeable that even passage of bacteria is allowed, a likely reason why F. nucleatum is often found in mixed infections at extra-oral sites. In addition, F. nucleatum challenge markedly impaired cell proliferation and apoptosis in endothelial cells, destroying the original equilibrium state and leading to endothelial damage [35]. F. nucleatum infection was also observed to affect the expression of endothelial cell surface markers and modulate receptors for VEGF on endothelial cells, resulting in impaired tissue vascularization during inflammation [36]. On the whole, after entering the bloodstream, F. nucleatum further impairs vascular endothelial integrity by inhibiting endothelial cell proliferation, destroying endothelial cell structure and function, and inhibiting the vascularization of damaged tissues during inflammation, thus paving the way for the invasion of other bacteria [34–36].
Secondly, many studies have reported that human heat shock proteins (hHSPs) are closely related to AS. Antibodies directed against bacterial GroEL cross-react with hHSP60 on endothelial cells may result in endothelial dysfunction and the subsequent development of AS [37]. Lee et al. proved that the heat-shock protein GroEL of F. nucleatum stimulated atherosclerotic risk factors through several mechanisms [38]. F. nucleatum GroEL upregulated the expression of chemokines, such as interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1), and cell adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin. This is an important step in the pathogenesis of endothelial dysfunction. In addition, GroEL could increase procoagulant activity by upregulating tissue factor (TF) and downregulating TF pathway inhibitor (TFPI) in endothelial cells. This prothrombotic response may be associated with plaque progression and instability. GroEL also induced monocyte adhesion to and transmigration through endothelial cells in concert with increased uptake of lipids in atherosclerotic lesions, and promoted monocyte differentiation into pro-inflammatory macrophages and, eventually, foam cells.
Thirdly, chronic oral infection with F. nucleatum as monoinfection alone does not promote the induction of AS, but may conversely inhibit plaque formation [28]. In this research, 24-week F. nucleatum-infected mice developed minimal aortic plaque, which was significantly smaller than sham-infected mice, and fewer F4/80+ macrophages were detected in the intimal layer of 24-week-infected mice than 12-week-infected mice. Additionally, there was no increase in macrophage infiltration or T-cell infiltration into the inner and outer membrane layers in infected mice at 24 weeks, suggesting that chronic F. nucleatum infection reduces inflammation in the aorta, which may in part explain the minimal development of atherosclerotic plaques. Although all lipid fractions were statistically elevated in F. nucleatum-infected mice, serum NO was not altered. Consistent with the minimal plaque observed in 24-week-infected mice, the result demonstrated no vascular endothelial dysfunction in animal model.
Some scholars used the multi-bacterial infection models to show that the inclusion of F. nucleatum promoted the endothelial dysfunction and AS plaque progression by upregulating expression of different aortic Toll-like receptors (TLRs) and inflammasome signal transduction [29,30]. Subsequent studies further demonstrated that F. nucleatum up-regulated pro-inflammatory factors such as IL-1α, IL-6 and TNF-α through activation of TLR-MyD88-NF -κB in endothelial cells, resulting in the upregulation of ICAM, VCAM and MCP-1 [35].
Due to the interdependence (physical, metabolic, and nutritional) of periodontal bacteria, no single periodontal bacterial species is effective in inducing aortic disease pathology, for which a multimicrobial consortium is responsible. In summary, more researches are needed to confirm these arguments that F. nucleatum may not be involved in AS alone, but rather act synergistically with other organisms, in particular by disrupting cell–cell junctions, breaking down endothelial integrity and leading to invasion [28–31].
Furthermore, we found that both P. gingivalis and F. nucleatum in vitro can induce the expression of fatty acid-binding protein 4 (FABP4) mRNA and protein depending on the JNK/AP-1 pathway, causing an increase of lipid uptake and foam cell transformation in macrophages [39]. Human studies showed that serum levels of antibodies against P. gingivalis correlated with serum levels of FABP4 in humans, whereas no association occurred between F. nucleatum antibody titers and FABP4 levels, which might indicate that P. gingivalis is superior to F. nucleatum in inducing FABP4 in humans. Further study is needed to define the reason underlying the difference in cell-based models and human studies. The mechanisms between F. nucleatum and AS are summarized in Figure 1.
Figure 1.
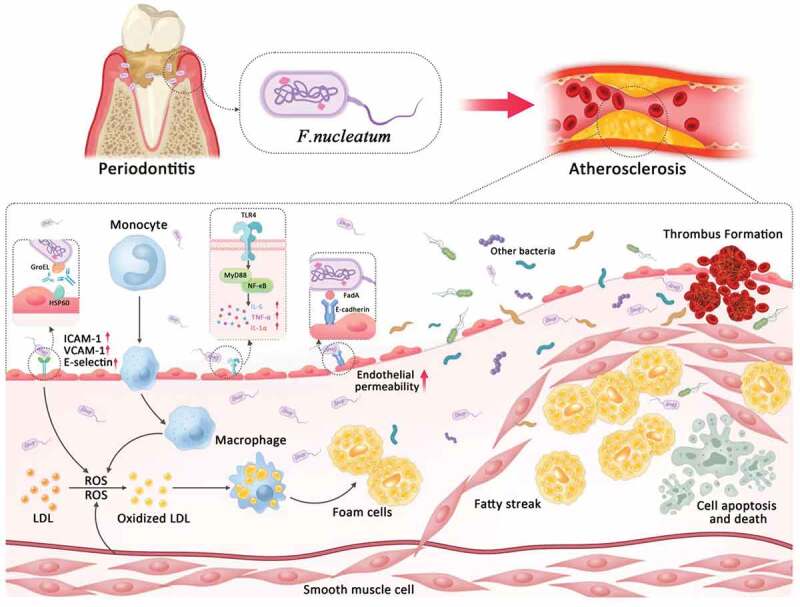
The possible mechanisms by which F. nucleatum contributes to atherosclerosis. HSP60, heat shock protein-60; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; LDL, low-density lipoprotein; ROS, reactive oxygen species; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Adverse pregnancy outcomes (APOs)
APO is a broad term including preterm labor, chorioamnionitis, preterm premature rupture of membranes, preeclampsia, miscarriage, intrauterine growth retardation, low birth weight, stillbirth, neonatal sepsis, etc.
F. nucleatum is one of the most prevalent species and by far the most prevalent oral species implicated in APOs and has been found to be significantly enriched in a wide variety of placental and fetal tissues including amniotic fluid, fetal membranes, cord blood, neonatal gastric aspirates, fetal lung and stomach, associated with preterm birth, preterm premature rupture of membranes, chorioamnionitis, early-onset neonatal sepsis, stillbirth and preeclampsia, either as the sole infectious agent or in mixed infections [40].
The strains of F. nucleatum identified in amniotic fluid and placenta appear to match those from the maternal or the partner subgingival sites, providing the human evidences that the bacteria originated from the mother’s subgingival plaque and translocated to the placenta and fetus, causing acute inflammation that eventually leads to APOs [41]. Animal studies also show that injecting saliva or subgingival plaque samples into mice leads to infection of the murine placenta with oral commensal species, including F. nucleatum, demonstrating that the oral bacteria are capable of translocation to the fetal-placental unit [16]. Concurrent detection of F. nucleatum in amniotic fluid and cord blood associated with preterm birth indicates its ability to spread to different placental and fetal compartments [16].
It has been assumed that F. nucleatum translocates from the maternal oral cavity to the intrauterine cavity via hematogenous transmission [42–44]. This hypothesis is supported by results from animal studies [45,46]. In a pregnant mouse model, F. nucleatum was injected into the tail vein of pregnant mice to mimic dental bacteremia, which led to bacterial colonization in the decidua of the mouse placenta, followed by spread to the fetal membranes, mimicking chorioamnionitis in humans [45]. A recent case-cohort design based on placental specimens from 320 subjects showed that the human placenta harbors a low abundant microbiome closely mimicking the human oral microbiome, further supporting the hypothesis of blood-borne transmission [47].
Periodontal pathogens can cause adverse pregnancy outcomes through two major mechanisms identified in the consensus report from the joint European Federation of Periodontology/American Academy of Periodontology workshop on periodontitis and systematic diseases: one is that periodontal pathogens directly enter the blood circulation system, or an ascending route via the genitourinary tract, thus invading the fetal-placenta unit; the other is that pro-inflammatory cytokines, such as IL-6, IL-8, and TNF-α, which are locally produced in periodontal tissues, enter the circulatory system, directly exerting negative influence on the fetal-placenta unit, or circulate to the liver and increase the systemic inflammatory state through the acute phase of protein reactions, which in turn affects the fetal placental unit [48,49]. In both ways, periodontal pathogens may cause inflammation in the placental tissues of pregnant women, increase levels of prostaglandin E2 and TNF-α in the amniotic fluid, and contribute to the onset of APOs [50].
Several adhesin molecules, including Fap2 and FadA, have been identified for colonization of the F. nucleatum in the placenta of mice [13,51–53]. Among them, FadA is the best characterized and plays a critical role in the murine model of infection [15]. Binding of F. nucleatum FadA to VE-cadherin not only increases the endothelial permeability, but also allows F. nucleatum and other oral bacteria to disseminate into and from the circulation. This may explain why F. nucleatum is frequently detected concurrently with other oral species in intrauterine infections in humans [6]. In vivo, an F. nucleatum mutant without FadA is significantly defective in placental colonization, while its complement clone restores the colonization [45,54].
More studies reported that colonization of F. nucleatum in the mouse placenta led to preterm and/or term fetal death, occurring 2–3 days following bacterial injection, accompanied by placental neutrophil infiltration, similar to that observed in humans [45]. Although F. nucleatum activates both TLR2 and TLR4 in vitro and in murine placentas, it induces inflammatory responses via TLR4, accompanied by neutrophil infiltration into the decidua [46]. In mice lacking TLR4, or in wild-type mice treated with a TLR4 antagonist, F. nucleatum colonizes the placenta to a similar extent as in untreated wild-type mice without eliciting inflammatory responses, resulting in a reduced fetal death rate, which suggests that inflammation rather than the bacteria per se is the cause of fetal demise. In contrast, the effect of TLR2 is insignificant because there is no change in fetal loss or inflammatory response in TLR2-knockout mice compared to wild-type mice.
Scholars have confirmed that inflammatory biomarkers in the maternal serum may include IL-6,C-X-C motif chemokine 8 (CXCL8) and CC chemokine ligand 2 (CCL2) [55–59]. Receptor-interacting protein kinase 2 (Ripk2) may contribute to F. nucleatum-induced production of IL-6 by activating NF-κB signaling in murine macrophages and human decidual stromal cells (hDSCs) [60]. Ripk2 also contributed to inducible nitric oxide synthase (iNOS) gene expression and NO production in macrophages and promoted the production of CXCL8 and CCL2, which was reduced by Ripk2 inhibitors. These results suggested that F. nucleatum infection results in APOs by inducing aberrant production of cytokines and chemokines through NOD1/NOD2-Ripk2-mediated signaling. In conclusion, although it would be beneficial to regulate Ripk2 signaling to prevent APOs caused by bacterial infections, further studies using animal models are needed to elucidate whether Ripk2 is involved in F. nucleatum-induced APOs and Ripk2 inhibitors are beneficial to prevent the occurrence of APOs.
Inflammatory bowel disease (IBD)
IBD, a heterogeneous set of inflammatory disorders of the gastrointestinal (GI) tract and a global public health issue of increasing importance, presents as two major clinical phenotypes: ulcerative colitis (UC) and Crohn’s disease (CD). UC is a continuous inflammation of the colonic mucosa and submucosa, usually involving the rectum initially, then gradually spreading to the whole colon, whereas CD is usually transmural and can affect any area of the gastrointestinal tract, which is a discontinuous full-layer inflammation, most commonly involving the terminal ileum, colon, and perianal [61]. The etiology of IBD is still not completely understood. Yet, several studies have supported the hypothesis that its onset is due to a convergence of host genetic factors and environmental triggers resulting in changes of the host immune response to intestinal microbes [62,63]. Therefore, the role of intestinal microbiota alteration is repeatedly discussed in literatures [64].
Back in 2011, Strauss et al. have reported that F. nucleatum isolated from inflamed biopsy tissue from IBD patients is significantly more common and invasive than from healthy controls [65]. It has been reported that F. nucleatum is obviously enriched in feces of IBD patients and its abundance has a positive correlation with patients’ disease activity, and administration of F. nucleatum markedly exacerbates colitis in DSS mice model [66]. This confirms the findings of previous clinical studies [67–69]. In addition, scholars found that patients with IBD have a significantly increased risk of PD, and exhibit more severe periodontal symptoms when oral hygiene conditions are similar [70–72]. Recent studies also have suggested a bidirectional association between IBD and PD based on statistics of the prevalence and clinical manifestations of both diseases, whereas the microbial etiological correlation and common risk factors of the two diseases remained unclear [72–74].
Kitamoto et al. discussed that the mode of the relocation of oral bacteria from the oral cavity to the gut mucosa may include hematogenous route, enteral route and other possible factors [75]. First, oral mechanical injuries and dental procedures enable oral bacteria to spread into the systemic circulation hematogenously, and inflammatory conditions of the oral cavity, namely periodontitis, may facilitate bacteremia [76,77]. In addition, oral bacteria are known to invade and survive inside immune cells, such as dendritic cells and macrophages, indicating that oral bacteria may hijack host immune cells to serve as Trojan horses for dissemination from oral mucosa to gut mucosa [50]. Second, enteral spreading is a plausible route worth paying attention to. The colonization resistance by the gut resident microbiota is considered to be the major barrier that prevents the gut colonization of swallowed oral bacteria [75,78]. Meanwhile, the majority of oral resident bacteria are so sensitive to the gastric acid that ingested oral bacteria will be sharply reduced when they are passing the stomach [75,79,80]. Therefore, when dysfunction of gastric barrier or/and disruption of gut colonization resistance happens, there will be a significant increase in the ectopic gut colonization by oral bacteria. Other possible factors include immune depression and poor oral health [81,82]. Yet further studies are needed to clarify the transmission methods to the gut mucosa by oral bacteria.
In this review, we only discuss the possible pathogenesis of F. nucleatum in IBD. Firstly, F. nucleatum could damage the epithelial barrier integrity and increase permeability by downregulating the expression of the tight junction proteins zonula occludens-1 (ZO-1) and occludin, which are markers of intestinal mucosal barrier function [66]. Additionally, F. nucleatum-mediated mucosal barrier damage could be promoted by targeting caspase activation and recruitment domain 3 (CARD3) which in term activated the endoplasmic reticulum stress (ERS) pathway [83]. Secondly, F. nucleatum could regulate M1 macrophage skewing to induce colitis [84]. Liu et al. also reported that F. nucleatum could exacerbate intestinal inflammation through upregulating cytokine secretion, such as IL-1β, IL-6, and IL-17, activating STAT3 signaling pathway, enhancing proliferation of CD4+ T cell and differentiation to Th1 and Th17 [66]. Li et al. found that the presence of F. nucleatum and FadA gene increased in UC patients, especially in patients with severe colitis and pancolitis, suggesting that FadA may play a significant role in the pathogenesis of UC [85]. Nevertheless, the exact mechanism of this association is not fully understood, more studies are required to better elucidate the role of F. nucleatum and FadA gene in UC.
Using ApoE−/− mice model, Yan et al. reported that non-surgical periodontal treatment triggered modulation of gut microbiota, facilitating recovery to a healthy microbiome situation [86]. It also strengthened the intestinal mucosal barrier which was impaired by periodontitis, resulting in a stronger nonspecific immune function. Based on this paper and the above relationship between F. nucleatum and IBD, we reasonably assume that F. nucleatum abundance maybe indicative of development of IBD. Moreover, targeting F. nucleatum may be effective to shorten disease course and prevent the development of IBD. Since there is no explicit cure for IBD, understanding the intrinsic mechanism of F. nucleatum may provide a new insight in optimizing current therapeutic strategies.
In addition, IBD has been recognized as a risk factor for colorectal cancer (CRC), the infection of F. nucleatum in intestinal tract may be the common pathogenesis of these two diseases [87]. The connection between F. nucleatum and CRC will be discussed in detail later.
Cancers
Oral squamous cell carcinomas (OSCC)
OSCC is a malignant tumor occurring in the oral epithelium, which is the main type of head and neck squamous cell carcinoma (HNSCC). Al-Hebshi,et al. firstly showed that F. nucleatum is associated with OSCC from an epidemiological perspective [88]. Subsequently, scholars used 16S rRNA to analyze the microbiome within healthy normal and tumorous (primary and metastatic) human tissues from the oral cavity, larynx-pharynx, and lymph nodes [89]. The microbiota associated with tumors supported altered abundances in the phyla Fusobacteria, Firmicutes, Actinobacteria and Proteobacteria. Most notably, a significant reduction in the abundance of Streptococcus species and an increase in the abundance of Fusobacterium species were observed in both primary and metastatic samples. Resphera Insight applied to saliva samples from HNSCC patients and healthy controls led to the first discovery that F. nucleatum enriched in a subset of saliva samples from HNSCC patients, when compared with controls [90]. Additionally, many species of anaerobic bacteria have been proposed to be involved in carcinogenesis [91]. Nagy et al. detected significantly larger quantities of Porphyromonas and Fusobacterium species in OSCC tissue samples compared to samples from healthy mucosa [92]. F. nucleatum is closely associated with the development of oral cancer by several mechanisms.
Firstly, when F. nucleatum invades gingival epithelial cells, NF-κB and NOD-like receptor 3 (NLRP3) are simultaneously activated [93]. NF-κB would subsequently translocate to the nucleus where it stimulates expression of pro-IL-1β gene. NLRP3 inflammasome would induce autocatalytic activation of caspase 1, resulting in release of IL-1β, one of the most important proinflammatory cytokines associated with cancer pathogenesis [94]. Furthermore, once caspase 1 is activated, other danger-associated molecular patterns (DAMPs) (also known as danger signals) will be released, such as highmobility group box 1 protein (HMGB1) and apoptosis-associated speck-like protein (ASC), which further amplify immune response [93]. The inflammasome/IL-1β pathway has been reported to be involved in HNSCC progression [94,95]. Aral et al. further verified that F. nucleatum could promote IL-1β by increasing AIM2 and downregulating POP1 in HNSCC in vitro [96]. P. gingivalis and adenosine triphosphate (ATP) with or without F. nucleatum upregulated NLRP3, IL-1β by downregulating POP1. Moreover, P. gingivalis and F. nucleatum can initiate the overexpressed NLRP3, activate upstream signal molecules of ataxia-telangiectasia and Rad3 related (ATR)-checkpoint kinase 1 (CHK1), promoting the growth and proliferation of oral cancers [97].
Secondly, F. nucleatum induces activation of protein kinase p38 in infected cells, promoting the secretion of matrix metalloproteinase-13 (MMP-13) and MMP-9, which contributes to tumor invasiveness [98].
Thirdly, DNA damage plays a significant role in the development and progression of oral cancer. It has been reported that the Ku70/p53 signaling pathway may be involved in the excessive proliferation of F. nucleatum-infected OSCC cells due to DNA damage [99]. If Ku70 protein levels are too low to repair severely damaged DNA, OSCC cells will proliferate abnormally [100,101]. Nevertheless, the interplay between F. nucleatum and Ku70 is yet to be explained.
In addition, the levels of tumor suppressor protein p27, a member of the cyclin-dependent kinase inhibitor (CDK) family, would be downregulated in F. nucleatum-infected cells, which leads to cell-cycle arrest in the S phase and to increased cell proliferation [100]. This process is correlated with adverse cancer prognosis.
Moreover, infection of oral epithelial cells with F. nucleatum was found to contribute to the induction of epithelial–mesenchymal transition (EMT) through lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 pathway [102]. EMT is an important biological process through which epithelial-derived malignant tumor cells acquire the ability to migrate and invade [103].
Gastrointestinal (GI) cancers
GI cancers are in the forefront of all malignant tumor in morbidity and mortality, including esophageal, gastric, colorectal, liver, and pancreatic cancer. A case-control study examined the salivary microbiota in patients with GI cancers and evaluated their differential distribution based on the cancer sites, suggesting the bacterial diversity and composition of saliva are related to GI cancers [104].
It was reported that F. nucleatum can not only promote the progression of GI tumor, but also contribute to the chemo-resistance of GI cancer [105]. We have summarized recent progress in the pathogenesis of F. nucleatum-related GI cancers.
Colorectal cancer (CRC)
CRC is one of the most common malignant tumors. Its morbidity and mortality rank among the top three cancers in the world, and are increasing in recent years [106]. Recent studies have revealed an enrichment of Fusobacterium species in human CRCs and adenomas compared with adjacent normal tissue [87,107–110], and the most abundant species is F. nucleatum, suggesting that F. nucleatum is involved in the development of CRC. Castellarin et al. verified overabundance of F. nucleatum in colorectal tumor specimens versus matched normal control tissue by quantitative PCR analysis and observed that F. nucleatum was positively associated with lymph node metastasis [87]. Subsequent studies implied that F. nucleatum can be used as a risk biomarker of CRC [111–114]. Increased levels of F. nucleatum correlated with the genetic and epigenetic aberrancies in CRC, such as the CpG island methylator phenotype (CIMP), microsatellite instability (MSI), and MLH1 methylation [108,115, 116–118].
F. nucleatum can alter the composition of the residual microbiota, with a consortium of inflammatory responses, virulence factors and impaired epithelial signaling in the context of a polymicrobial oral biofilm with synergistic properties, resulting in intestinal dysbiosis, contributing to the initiation and progression of CRC [118].
In respect to association between F. nucleatum and CRC oncogenesis, two pathways must be mentioned. One of the pathways is the virulence factor FadA of F. nucleatum which would activate the β-catenin signaling pathway when present on the VE-cadherin, initiating inflammatory responses, thus boosting the genes of the transcription factors NF-κB, pro-inflammatory cytokines such as IL-6, IL-8, and IL-18, Wnt7a, Wnt7b, Wnt9a, transcription factors lymphoid enhancer factor (LEF) /T cell factor (TCF), Myc, and Cyclin D1, and the parts of the Wnt pathway [119]. F. nucleatum also can activate β-catenin signaling through TLR4/P-PAK1 cascade [120]. In pathway two, the Fap2 of F. nucleatum mediated its enrichment in CRC by binding to tumor-overexpressed Gal-GalNAc, further intensifying the inflammatory response [121]. In pathway three, F. nucleatum lipopolysaccharide (LPS) could activate TLR4/MyD88/NF-κB, inducing high expression of miR21; the elevated miR21 could reduce levels of RASA1, while the RASA1 could active MAPK pathway [122. Meanwhile, F. nucleatum was found to inhibit the expression of miR-18* and miR-4802 through TLR4/MyD88 immune signals [123]. The loss of these miRs avoided chemotherapy-induced apoptosis by activating autophagy in CRC cells. In addition, when F. nucleatum invades into CRC cells, reactive oxygen species (ROS) could be induced, which subsequently lead to DNA damage [124,125].
The above findings bring us to the next question: how F. nucleatum modulates tumor immune microenvironment in CRC? On the one hand, F. nucleatum could selectively recruit tumor-infiltrating myeloid cells and promotes tumor progression [126]. On the other hand, Fap2 of F. nucleatum could interact with TIGIT, an immunoregulatory signaling receptor on NK and T cells, and this Fap2-TIGIT interaction could reduce killing of tumor cells by NK and tumor-infiltrating lymphocytes and inducing lymphocyte apoptosis [127–129].
Due to the biological characteristics of F. nucleatum and its relationship with CRC, studies on F. nucleatum are increasing gradually, but our understanding of F. nucleatum is still insufficient at present. Further researches are needed on how F. nucleatum can be a therapeutic target to reduce the risk of CRC. The researchers found that detection of fecal abundance of F. nucleatum can be used as a rapid and non-invasive diagnostic technique for CRC screening [112]. Combined with fecal occult blood test, the positive rate and accuracy of CRC diagnosis can be improved. In addition, serum IgA or IgG antibodies against F. nucleatum also have potential diagnostic value [130]. Scholars found that a ‘robust’ diet rich in whole grains and fiber could effectively reduce the individual’s risk of developing F. nucleatum-associated CRC, but the risk of CRC did not seem to change in patients lacking F. nucleatum [131]. Moreover, metronidazole treatment can significantly reduce tumor volume in a xenograft model of CRC enriched with F. nucleatum [132]. However, metronidazole broadly targets anaerobic bacteria. Therefore, it is important to search for narrow-spectrum antibiotics that are specific to F. nucleatum and only targeted at tumor tissue.
Other gastrointestinal (GI) cancers
Yamamura et al. reported that F. nucleatum enrichment was associated with a worse prognosis in esophageal cancer by activating chemokines, such as CCL20 [133]. In a study by Mitsuhashi et al., the detection rate of Fusobacterium spp. in pancreatic cancer tissue specimens was 8.8% [134], while F. nucleatum was not detected in pancreatic cancer tissue in another study by Yamamura et al. [135]. Castan˜o-Rodrı´guez et al. found that several bacterial groups, including Lactococcus, Veilonella, Fusobacterium, and Leptotrichia species were enriched in gastric cancer [136]. Heish et al. implied that Clostridium colicanis and F. nucleatum could be used as diagnosis biomarkers of gastric cancer [137]. Abed et al. found that Gal-GalNAC antigen was highly expressed in gastric cancer and esophageal cancer tissues, suggesting that gastric cancer and esophageal cancer could enrich F. nucleatum chemotaxis and affect disease progression [138].
Breast cancer (BC)
BC is the leading cause of cancer mortality in women and a type of cancer with different presentations among women [139]. F. nucleatum has recently been detected in human BC tissues and was shown to promote BC progression in a murine model [140,141]. Parhi et al. reported that F. nucleatum colonizes not only CRC, but also BC through recognition of Gal-GalNAc by Fap2 [140]. Also, metronidazole treatment can inhibit F. nucleatum-induced tumor exacerbation. Van der Merwe et al. have suggested that F. nucleatum may promote CRC and BC progression by activating the TLR4/ MyD88 pathway, and F. nucleatum also exhibits immunomodulatory effects [142]. These observations provide valuable therapeutic insights into CRC and BC: Gal/GalNAc antagonists or Fap2 antibodies could be used to inhibit the binding of F. nucleatum to tumours [121,140]; autophagy inhibitors, including chloroquine and hydroxychloroquine, have the potential to weaken the drug resistance of F. nucleatum [123,143]; PD-L1 and CD-47 antagonists would be beneficial for boosting the immune system in cancer patients [143–146].
Genitourinary (GU) cancers
Shuai Yuan et al. have summarized epidemiological studies exploring the association between PD and GU cancers, and indicated that the presence of an oral-genitourinary axis and oral microbiota may be involved in the pathogenesis of GU cancers [147,148]. Bučević Popović et al. are the first to report that there exists possible association of Fusobacterium spp. with urothelial carcinomas, at least in some bladder cancer patients [48]. They showed that F. nucleatum is indeed present in approximately one quarter of the tested samples by PCR-based analysis, and indicated the bacteria may play a key role in the exacerbation of the bladder cancer based on the 16S rDNA gene sequence approach. Nevertheless, more epidemiological studies and animal experiments are needed to elucidate the exact role of F. nucleatum in bladder cancer formation and progression. Alluri et al. have identified the periodontal pathogen F. nucleatum in prostate glands diagnosed with adenocarcinoma, but they have no evidence of whether the F. nucleatum found in the prostatic tissue of the individual originated from the oral cavity [149]. Meanwhile, the role of F. nucleatum in pathological prostate changes is unclear yet. Huang et al. found that there was a distinct observation of higher levels of F. nucleatum in cervical cancer, especially for recurrent tissues [150]. Patients with high burdens of F. nucleatum intratumoral infiltration exhibited correspondingly poor rates of both overall survival and progression-free survival, suggesting that F. nucleatum might be one potential cervical cancer diagnostic and prognostic biomarker.
Alzheimer’s disease (AD)
AD is the most common form of dementia in older adults and refers to a central nervous system disease characterized by progressive cognitive dysfunction and memory loss [151]. In the past decade, studies have identified a relationship between periodontitis and AD, suggesting that the pathogens of periodontitis have significant implications on the development of AD [152–155].
Several putative mechanisms that could explain how periodontitis affects the central nervous system (CNS) homeostasis have been described [156], including: (i) Bacterial diffusion into the bloodstream, and once in the cerebral vessels, the inflammatory response associated with cerebrovascular atherosclerosis may induce rupture of the blood–brain barrier (BBB); (ii) Oral bacteria would migrate through the peripheral terminations of the trigeminal nerve to the trigeminal ganglion and then to the brain; (iii) Bacteria could migrate by the lymphatic circulation. While these three theories explain the possible route by which oral bacteria migrate to the brain, it has not yet been demonstrated which contributed most to the onset of AD.
With the increase of age, the permeability of the BBB increases in elderly patients. Therefore, pathogenic microorganisms could easily cross the BBB and enter the brain tissue, directly act on neurons, activate the inflammatory cascade reaction, and cause direct damage to the CNS. The bacteria entering brain tissue and their secreted virulence factors could activate a classic immune response similar in some aspects to that observed in AD, through TLR2 and TLR4 pathway, and turn inactive microglia into active ones [157,158] When activated, they could produce several inflammatory mediators such as TNF-α, IL-1β, IL-6, iNOS, and reactive oxygen species (ROS) that trigger necrosis and apoptosis of dopaminergic neurons in the CNS [159]. Additionally, oral microbial populations indirectly affect AD by secreting bacterial toxins, outer membrane vesicles (OMVs) and proinflammatory factors that flow into the brain with blood [160–163]
Earlier studies have reported that antibodies to F. nucleatum can be detected in the serum of patients with AD or cognitive impairment [164]. Scholars also found that the oral microbial load of F. nucleatum were significantly more abundant in the AD group than controls [165,166]. Recently, in vitro and animal experiments were conducted to preliminarily explore the pathogenesis of AD exacerbated by F. nucleatum [167]. Scholars reported that F. nucleatum LPS promoted the proliferation of microglia, which promoted the enhancement of inflammatory immune function, leading to changes in cell morphology and increased expression of inflammatory genes. F. nucleatum LPS could increase permeability of the BBB, and then affect the onset and development of AD. Previous studies have shown that local inflammation in the central nervous system could lead to cognitive impairment [168]. This report showed the elevations of TNF-α and IL-1β message in the brain tissue of 5XFAD mice after infection with F. nucleatum. In addition, in the presence of F. nucleatum infection, the expression of P38 protein in mouse brain tissue was significantly upregulated, as was phosphorylated P38 protein. Likewise, MyD88 protein was upregulated. F. nucleatum also stimulated the JNK pathway; the expression levels of phosphorylated JNK and JNK proteins were raised. Moreover, quantitative proteomics analyses were performed to detect the proteins in the brain tissues of 5XFAD mice with or without F. nucleatum infection [168]. The result showed that 31 proteins were obviously differentially expressed by the two groups of mice [168]. Further studies are needed to validate and explore the results of quantitative proteomics in order to find key proteins that play a significant role in signaling pathways. Furthermore, how other virulence factors of F. nucleatum affect the progression of AD remains unkonwn. On the whole, more efforts are required to elucidate the mechanism of F. nucleatum actions in AD in future studies.
Other organ inflammation and abscesses
Han et al. have suggested that F. nucleatum is associated with brain, lung, liver, and splenic abscesses [34]. Recent studies also indicated that F. nucleatum was involved in rheumatoid arthritis (RA) and acute appendicitis [164,166,169–171]. Ebbers et al. firstly reported that a triple oral inoculation of pathobionts (P. gingivalis, F. nucleatum, and A. actinomycetemcomintans) combined with collagen could induce arthritis in the mouse, and oral inoculation with either F. nucleatum or A. actinomycetemcomintans alone could accelerate subsequent arthritis onset and progression [171]. Several scholars have found that P. gingivalis, F. nucleatum and A. actinomycetecomitans inhaled into respiratory tract could promote the invasion of Pseudomonas aeruginosa into respiratory epithelial cells and induce cytokine production and cell apoptosis [172]. F. nucleatum is one of the most common causative agents of Lemierre’s syndrome, a rare form of upper airways infection with a life-threatening secondary septic thrombophlebitis of internal or external jugular veins [173].
Conclusions
F. nucleatum has been considered as an opportunistic pathogen that interacts with other microorganisms and plays a crucial role in many infectious diseases. With the development of omics technology, the occurrence and development of many diseases are closely related to the infection of F. nucleatum. The latest trend has been to focus on exploring possible direct and indirect links between F. nucleatum and certain systemic diseases, especially ACVDs and CRC, and an increasing amount of evidence has been accumulated. F. nucleatum not only promotes inflammation, but also binds to or invades multiple cell types, including oral, colon and placental epithelial cells, T cells, keratinocytes, and macrophages [174]. The answer to the question why F. nucleatum can invade the human body and influence general health lies in several key virulence mechanisms which can be broadly classified into three groups (Figure 2): (i) Colonization and invasion: F. nucleatum can enter the circulation and cause transient bacteremia following daily life activities, such as toothbrushing, chewing, and flossing, and after dental treatment procedures. Frequent circulation enables bacteria to access organs around the body and take part in local pathogenesis [175]. In addition, F. nucleatum can colonize intestinal mucosa through digestive tract [75]. (ii) Induction of host responses: F. nucleatum is a potent stimulator of inflammatory cytokines. Persistent local infection caused by F. nucleatum induces the upregulation of inflammatory cascades [38,48–50]. Chronic inflammation is believed to be the root cause of systemic disorders and is one of the leading causes of long-term health problems. Furthermore, F. nucleatum promotes the progression of various systemic diseases by upregulating expression of different TLRs [29,121,141], promoting macrophage M1 polarization [84], and enhancing proliferation of CD4+ T cell and differentiation to Th1 and Th17 [66]. (iii) Specific toxins of F. nucleatum, especially FadA, Fap2 and LPS, play a significant role in the induction of local diseases [15,84,114,140].
Figure 2.
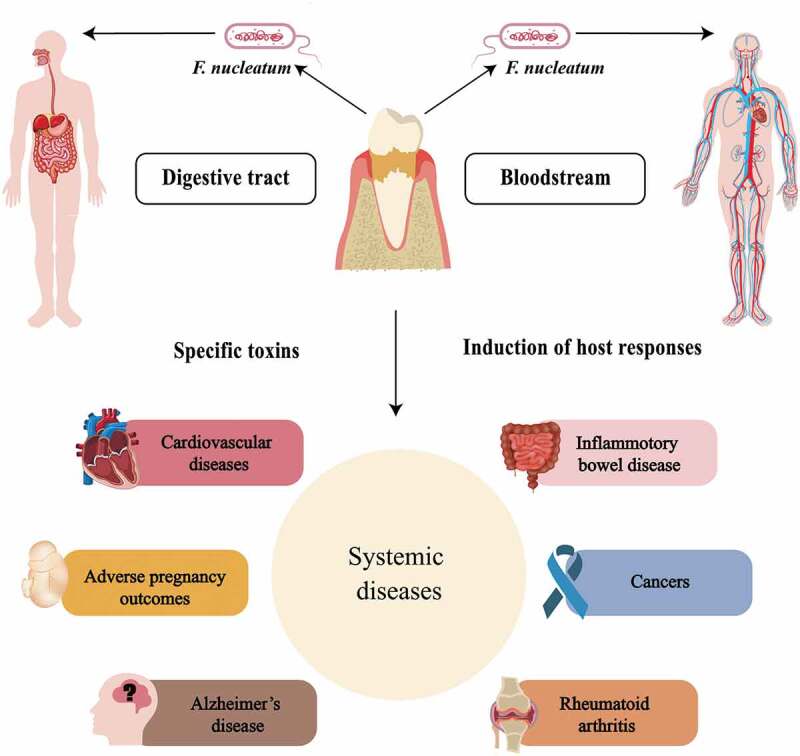
Patterns by which F. nucleatum invades the whole body, along with simple a schematic representation of F. nucleatum-associated systemic diseases.
In this article, we not only summarize the adverse effects of F. nucleatum on multiple systemic diseases, but also discuss the impact of targeting F. nucleatum on the treatment of related systemic diseases. However, many of the epidemiological studies only show the correlation but not necessarily causation between F. nucleatum and specific diseases. Precise pathogenicity of F. nucleatum-related systemic diseases has yet to be uncovered, and a few conclusions are controversial. Only by continuing to investigate mechanisms involved in transformation of this oral commensal organism into systemic pathogens will we discover possible strategies for diagnostic, preventive and therapeutic purposes. Based on current research, some recommendations can be given. Firstly, it must be emphasized that oral health should be maintained as an indispensable part of a healthy lifestyle to reduce risk of bacteremia, especially for immune-compromised patients. Secondly, by investigating into the epidemiological background of F. nucleatum infection, high-risk groups for various related diseases can be effectively revealed, facilitating early detection and prevention among the population. Thirdly, it is of great significance for F. nucleatum-associated cancer patients with poor prognosis or chemotherapy-resistance to apply multi-drug combination therapy against F. nucleatum and employ drug administration methods similar to Helicobacter pylori triple therapy.
Funding Statement
This work was supported by the National Natural Science Foundation of China [82201072], the project of cadre health of Jiangsu Commission of health [BJ19033] and the Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD, 2018-87].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Kinane DF, Stathopoulou PG, Papapanou PN.. Periodontal diseases. Nat Rev Dis Primers. 2017;3(1):17038. [DOI] [PubMed] [Google Scholar]
- [2].Graves DT, Corrêa JD, Silva TA. The oral microbiota is modified by systemic diseases. J Dent Res. 2019;98(2):148–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Debelian GJ, Olsen I, Tronstad L. Systemic diseases caused by oral microorganisms. Endodontics & Dental Traumatology. 1994;10(2):57–65. [DOI] [PubMed] [Google Scholar]
- [4].Bourgeois D, Inquimbert C, Ottolenghi L, et al. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-is there cause for consideration? Microorganisms. 2019;7(10):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bui FQ, Almeida-da-Silva C, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92(6):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Salhi L, Rompen E, Sakalihasan N, et al. Can periodontitis influence the progression of abdominal aortic aneurysm? A systematic review. Angiology. 2019;70(6):479–491. [DOI] [PubMed] [Google Scholar]
- [8].Yeo BK, Lim LP, Paquette DW, et al. Periodontal disease – the emergence of a risk for systemic conditions: pre-term low birth weight. Ann Acad Med Singapore. 2005;34(1):111–116. [PubMed] [Google Scholar]
- [9].Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sharma A, Inagaki S, Sigurdson W, et al. Synergy between tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol Immunol. 2005;20(1):39–42. [DOI] [PubMed] [Google Scholar]
- [11].Bradshaw DJ, Marsh PD, Watson GK, et al. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun. 1998;66(10):4729–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han YW, Ikegami A, Rajanna C, et al. Identification and characterization of a novel adhesin unique to oral fusobacteria. J Bacteriol. 2005;187(15):5330–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu M, Yamada M, Li M, et al. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem. 2007;282(34):25000–25009. [DOI] [PubMed] [Google Scholar]
- [15].Fardini Y, Wang X, Témoin S, et al. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol Microbiol. 2011;82(6):1468–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fardini Y, Chung P, Dumm R, et al. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun. 2010;78(4):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. [DOI] [PubMed] [Google Scholar]
- [18].Seymour GJ, Ford PJ, Cullinan MP, et al. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(4):3–10. [DOI] [PubMed] [Google Scholar]
- [19].Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71(10):1554–1560. [DOI] [PubMed] [Google Scholar]
- [20].Ford PJ, Gemmell E, Hamlet SM, et al. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol Immunol. 2005;20(5):296–302. [DOI] [PubMed] [Google Scholar]
- [21].Ford PJ, Gemmell E, Chan A, et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. 2006;21(4):206–211. [DOI] [PubMed] [Google Scholar]
- [22].Khader YS, Albashaireh ZSM, Alomari MA. Periodontal diseases and the risk of coronary heart and cerebrovascular diseases: a meta-analysis. J Periodontol. 2004;75(8):1046–1053. [DOI] [PubMed] [Google Scholar]
- [23].Humphrey LL, Fu R, Buckley DI, et al. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23(12):2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grau AJ, Buggle F, Ziegler C, et al. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. 1997;28(9):1724–1729. [DOI] [PubMed] [Google Scholar]
- [25].Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82(10):1469–1477. [DOI] [PubMed] [Google Scholar]
- [26].Pyysalo MJ, Pyysalo LM, Pessi T, et al. The connection between ruptured cerebral aneurysms and odontogenic bacteria. J Neurol Neurosurg Psychiatry. 2013;84(11):1214–1218. [DOI] [PubMed] [Google Scholar]
- [27].Elkaïm R, Dahan M, Kocgozlu L, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodontal Res. 2008;43(2):224–231. [DOI] [PubMed] [Google Scholar]
- [28].Velsko IM, Chukkapalli SS, Rivera-Kweh MF, et al. Fusobacterium nucleatum alters atherosclerosis risk factors and enhances inflammatory markers with an atheroprotective immune response in ApoE(null) mice. PloS one. 2015;10(6):e0129795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Velsko IM, Chukkapalli SS, Rivera-Kweh MF, et al. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in αvβ6 integrin-deficient mice. Infect Immun. 2015;83(12):4582–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chukkapalli SS, Velsko IM, Rivera-Kweh MF, et al. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoE null mice. PloS one. 2015;10(11):e0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. [DOI] [PubMed] [Google Scholar]
- [32].Wilkins JT, Li RC, Sniderman A, et al. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, et al. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. 2021;22(8):3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nithianantham S, Xu M, Wu N, et al. Crystallization and preliminary X-ray data of the FadA adhesin from Fusobacterium nucleatum. Acta crystallographica, Section F, Structural Biology and Crystallization Communications. 2006;62(Pt 12):1215–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Q, Zhao L, Xu C, et al. Fusobacterium nucleatum stimulates monocyte adhesion to and transmigration through endothelial cells. Arch Oral Biol. 2019;100:86–92. [DOI] [PubMed] [Google Scholar]
- [36].Mendes RT, Nguyen D, Stephens D, et al. Endothelial cell response to Fusobacterium nucleatum. Infect Immun. 2016;84(7):2141–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wick G, Perschinka H, Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001;22(12):665–669. [DOI] [PubMed] [Google Scholar]
- [38].Lee H-R, Jun H-K, Kim H-D, et al. Fusobacterium nucleatum GroEL induces risk factors of atherosclerosis in human microvascular endothelial cells and ApoE−/− mice. Mol Oral Microbiol. 2012;27(2):109–123. [DOI] [PubMed] [Google Scholar]
- [39].Kim DJ, Rho JH, Woo BH, et al. Periodontal pathogens modulate lipid flux via fatty acid binding protein 4. J Dent Res. 2019;98(13):1511–1520. [DOI] [PubMed] [Google Scholar]
- [40].Vander Haar EL, So J, Gyamfi-Bannerman C, et al. Fusobacterium nucleatum and adverse pregnancy outcomes: epidemiological and mechanistic evidence. Anaerobe. 2018;50:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hill GB. Preterm birth: associations with genital and possibly oral microflora. Ann Periodontol. 1998;3(1):222–232. [DOI] [PubMed] [Google Scholar]
- [42].Han YW. Oral health and adverse pregnancy outcomes - what’s next? J Dent Res. 2011;90(3):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Han YW. Can oral bacteria cause pregnancy complications? Women’s health (London. England). 2011;7(4):401–404. [DOI] [PubMed] [Google Scholar]
- [44].Hill GB. Investigating the source of amniotic fluid isolates of fusobacteria. Clin Infect Dis. 1993;16(4):S423–S424. [DOI] [PubMed] [Google Scholar]
- [45].Han YW, Redline RW, Li M, et al. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72(4):2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol (Baltimore, Md: 1950). 2007;179(4):2501–2508. [DOI] [PubMed] [Google Scholar]
- [47].Aagaard K, Ma J, Antony KM, et al. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sanz M, Kornman K, Working group 3 of joint EFP/AAP workshop . Periodontitis and adverse pregnancy outcomes: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40(14):S164–S169. [DOI] [PubMed] [Google Scholar]
- [49].Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. J Periodontol. 2013;84(4 Suppl):S170–S180. [DOI] [PubMed] [Google Scholar]
- [50].Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaplan A, Kaplan CW, He X, et al. Characterization of aid1, a novel gene involved in Fusobacterium nucleatum interspecies interactions. Microb Ecol. 2014;68(2):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kaplan CW, Lux R, Haake SK, et al. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol. 2009;71(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Coppenhagen-Glazer S, Sol A, Abed J, et al. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect Immun. 2015;83(3):1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ikegami A, Chung P, Han YW. Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect Immun. 2009;77(7):3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stallmach T, Hebisch G, Joller-Jemelka HI, et al. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab Invest. 1995;73(3):384–392. [PubMed] [Google Scholar]
- [56].Sakai M, Sasaki Y, Yoneda S, et al. Elevated interleukin-8 in cervical mucus as an indicator for treatment to prevent premature birth and preterm, pre-labor rupture of membranes: a prospective study. American Journal of Reproductive Immunology (New York, N.Y.: 1989). 2004;51(3):220–225. [DOI] [PubMed] [Google Scholar]
- [57].Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26(8–9):661–671. [DOI] [PubMed] [Google Scholar]
- [58].Pearce BD, Garvin SE, Grove J, et al. Serum macrophage migration inhibitory factor in the prediction of preterm delivery. Am J Obstet Gynecol. 2008;199(1):46.e1–46.e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Szarka A, Rigó J Jr, Lázár L, et al. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Park JY, Lee TS, Noh EJ, et al. Receptor-interacting protein kinase 2 contributes to host innate immune responses against Fusobacterium nucleatum in macrophages and decidual stromal cells. American Journal of Reproductive Immunology (New York, N.Y.: 1989). 2021;86(1):e13403. [DOI] [PubMed] [Google Scholar]
- [61].Veauthier B, Hornecker JR. Crohn’s Disease: diagnosis and Management. Am Fam Physician. 2018;98(11):661–669. [PubMed] [Google Scholar]
- [62].Di Stasi LC. Coumarin derivatives in inflammatory bowel disease. Molecules. 2021;26(2):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dixon LJ, Kabi A, Nickerson KP, et al. Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2015;21(4):912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Allen-Vercoe E, Jobin C. Fusobacterium and enterobacteriaceae: important players for CRC? Immunol Lett. 2014;162(2 Pt A):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Strauss J, Kaplan GG, Beck PL, et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17(9):1971–1978. [DOI] [PubMed] [Google Scholar]
- [66].Liu H, Hong XL, Sun TT, et al. Fusobacterium nucleatum exacerbates colitis by damaging epithelial barriers and inducing aberrant inflammation. J Dig Dis. 2020;21(7):385–398. [DOI] [PubMed] [Google Scholar]
- [67].Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tahara T, Shibata T, Kawamura T, et al. Fusobacterium detected in colonic biopsy and clinicopathological features of ulcerative colitis in Japan. Dig Dis Sci. 2015;60(1):205–210. [DOI] [PubMed] [Google Scholar]
- [69].Ozmeric N, Bissada N, da Silva A. The association between inflammatory bowel disease and periodontal conditions: is there a common bacterial etiology? Journal of the International Academy of Periodontology. 2018;20(2):40–51. [PubMed] [Google Scholar]
- [70].Zhang L, Gao X, Zhou J, et al. Increased risks of dental caries and periodontal disease in Chinese patients with inflammatory bowel disease. Int Dent J. 2020;70(3):227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kang EA, Chun J, Kim JH, et al. Periodontitis combined with smoking increases risk of the ulcerative colitis: a national cohort study. World J Gastroenterol. 2020;26(37):5661–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Agossa K, Dendooven A, Dubuquoy L, et al. Periodontal manifestations of inflammatory bowel disease: emerging epidemiologic and biologic evidence. J Periodontal Res. 2017;52(3):313–324. [DOI] [PubMed] [Google Scholar]
- [73].Baima G, Massano A, Squillace E, et al. Shared microbiological and immunological patterns in periodontitis and IBD: a scoping review. Oral Dis. 2022;28(4):1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schmidt J, Weigert M, Leuschner C, et al. Active matrix metalloproteinase-8 and periodontal bacteria-interlink between periodontitis and inflammatory bowel disease? J Periodontol. 2018;89(6):699–707. [DOI] [PubMed] [Google Scholar]
- [75].Kitamoto S, Nagao-Kitamoto H, Hein R, et al. The bacterial connection between the oral cavity and the gut diseases. J Dent Res. 2020;99(9):1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Parahitiyawa NB, Jin LJ, Leung WK, et al. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. 2009;22(1):46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358(6361):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Paganelli FL, Luyer M, Hazelbag CM, et al. Roux-Y gastric bypass and sleeve gastrectomy directly change gut microbiota composition independent of surgery type. Sci Rep. 2019;9(1):10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Walker MY, Pratap S, Southerland JH, et al. Role of oral and gut microbiome in nitric oxide-mediated colon motility. Nitric Oxide. 2018;73:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Crakes KR, Jiang G. Gut microbiome alterations during HIV/SIV infection: implications for HIV cure. Front Microbiol. 2019;10:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Momen-Heravi F, Babic A, Tworoger SS, et al. Periodontal disease, tooth loss and colorectal cancer risk: results from the nurses. Health Study. International Journal of Cancer. 2017;140(3):646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cao P, Chen Y, Guo X, et al. Fusobacterium nucleatum activates endoplasmic reticulum stress to promote crohn’s disease development via the upregulation of CARD3 expression. Front Pharmacol. 2020;11(106). DOI: 10.3389/fphar.2020.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Liu L, Liang L, Liang H, et al. Fusobacterium nucleatum aggravates the progression of colitis by regulating M1 macrophage polarization via AKT2 pathway. Front Immunol. 2019;10:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Li DH, Li ZP, Zhang Y, et al. Fecal Fusobacterium nucleatum harbored virulence gene fadA are associated with ulcerative colitis and clinical outcomes. Microb Pathog. 2021;157:104964. [DOI] [PubMed] [Google Scholar]
- [86].Huang Y, Liao Y, Luo B, et al. Non-surgical periodontal treatment restored the gut microbiota and intestinal barrier in apolipoprotein E-/- mice with periodontitis. Front Cell Infect Microbiol. 2020;10:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Al-Hebshi NN, Nasher AT, Maryoud MY, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7(1):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Shin JM, Luo T, Kamarajan P, et al. Microbial communities associated with primary and metastatic head and neck squamous cell carcinoma - a high fusobacterial and low streptococcal signature. Sci Rep. 2017;7(1):9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Guerrero-Preston R, White JR, Godoy-Vitorino F, et al. High-resolution microbiome profiling uncovers Fusobacterium nucleatum, Lactobacillus gasseri/johnsonii, and Lactobacillus vaginalis associated to oral and oropharyngeal cancer in saliva from HPV positive and HPV negative patients treated with surgery and chemo-radiation. Oncotarget. 2017;8(67):110931–110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sun J, Tang Q, Yu S, et al. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020;9(17):6306–6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nagy KN, Sonkodi I, Szöke I, et al. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34(4):304–308. [PubMed] [Google Scholar]
- [93].Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lee CH, Chang JS, Syu SH, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230(4):875–884. [DOI] [PubMed] [Google Scholar]
- [95].Wang H, Luo Q, Feng X, et al. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer. 2018;18(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Aral K, Milward MR, Gupta D, et al. Effects of porphyromonas gingivalis and Fusobacterium nucleatum on inflammasomes and their regulators in H400 cells. Mol Oral Microbiol. 2020;35(4):158–167. [DOI] [PubMed] [Google Scholar]
- [97].Yao Y, Shen X, Zhou M, et al. Periodontal pathogens promote oral squamous cell carcinoma by regulating ATR and NLRP3 Inflammasome. Front Oncol. 2021;11:722797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Uitto VJ, Baillie D, Wu Q, et al. Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect Immun. 2005;73(2):1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66(1):311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Geng F, Zhang Y, Lu Z, et al. Fusobacterium nucleatum caused DNA damage and promoted cell proliferation by the Ku70/p53 pathway in oral cancer cells. DNA Cell Biol. 2020;39(1):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fujiwara N, Kitamura N, Yoshida K, et al. Involvement of Fusobacterium species in oral cancer progression: a literature review including other types of cancer. Int J Mol Sci. 2020;21(17):6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhang S, Li C, Liu J, et al. Fusobacterium nucleatum promotes epithelial-mesenchymal transition through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020;287(18):4032–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nisticò P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2):a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kageyama S, Takeshita T, Takeuchi K, et al. Characteristics of the salivary microbiota in patients with various digestive tract cancers. Front Microbiol. 2019;10:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu Y, Baba Y, Ishimoto T, et al. Progress in characterizing the linkage between Fusobacterium nucleatum and gastrointestinal cancer. J Gastroenterol. 2019;54(1):33–41. [DOI] [PubMed] [Google Scholar]
- [106].Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- [107].Proença MA, Biselli JM, Succi M, et al. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World J Gastroenterol. 2018;24(47):5351–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015;137(6):1258–1268. [DOI] [PubMed] [Google Scholar]
- [109].Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Eklöf V, Löfgren-Burström A, Zingmark C, et al. Cancer-associated fecal microbial markers in colorectal cancer detection. Int J Cancer. 2017;141(12):2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wong SH, Kwong T, Chow TC, et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut. 2017;66(8):1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Liang Q, Chiu J, Chen Y, et al. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res off J Am Assoc Cancer Res. 2017;23(8):2061–2070. [DOI] [PubMed] [Google Scholar]
- [114].Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2018;53(4):517–524. [DOI] [PubMed] [Google Scholar]
- [115].Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22(2):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74(5):1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lee DW, Han SW, Kang JK, et al. Association between Fusobacterium nucleatum, pathway mutation, and patient prognosis in colorectal cancer. Ann Surg Oncol. 2018;25(11):3389–3395. [DOI] [PubMed] [Google Scholar]
- [118].Koliarakis I, Messaritakis I, Nikolouzakis TK, et al. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int J Mol Sci. 2019;20(17):4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen Y, Peng Y, Yu J, et al. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget. 2017;8(19):31802–31814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Abed J, Emgård JE, Zamir G, et al. Fap2 mediates fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20(2):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851–866.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kumar A, Thotakura PL, Tiwary BK, et al. Target identification in Fusobacterium nucleatum by subtractive genomics approach and enrichment analysis of host-pathogen protein-protein interactions. BMC Microbiol. 2016;16(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tang B, Wang K, Jia YP, et al. Fusobacterium nucleatum-induced impairment of autophagic flux enhances the expression of proinflammatory cytokines via ROS in Caco-2 cells. PloS one. 2016;11(11):e0165701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kaplan CW, Lux R, Huynh T, et al. Fusobacterium nucleatum apoptosis-inducing outer membrane protein. J Dent Res. 2005;84(8):700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. [DOI] [PubMed] [Google Scholar]
- [130].Wang HF, Li LF, Guo SH, et al. Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer. Sci Rep. 2016;6(1):33440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3(7):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Dejea CM, Fathi P, Craig JM, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(6375):592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yamamura K, Baba Y, Nakagawa S, et al. Human microbiome fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res off J Am Assoc Cancer Res. 2016;22(22):5574–5581. [DOI] [PubMed] [Google Scholar]
- [134].Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6(9):7209–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Yamamura K, Baba Y, Miyake K, et al. Fusobacterium nucleatum in gastroenterological cancer: evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol Lett. 2017;14(6):6373–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Castaño-Rodríguez N, Goh KL, Fock KM, et al. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7(1):15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hsieh YY, Tung SY, Pan HY, et al. Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep. 2018;8(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Abed J, Maalouf N, Parhi L, et al. Tumor targeting by fusobacterium nucleatum: a pilot study and future perspectives. Front Cell Infect Microbiol. 2017;7:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Barzaman K, Karami J, Zarei Z, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535. [DOI] [PubMed] [Google Scholar]
- [140].Parhi L, Alon-Maimon T, Sol A, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020;11(1):3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Van der Merwe M, Van Niekerk G, Botha A, et al. The onco-immunological implications of Fusobacterium nucleatum in breast cancer. Immunol Lett. 2021;232:60–66. [DOI] [PubMed] [Google Scholar]
- [143].Liu J, Yue W, Chen H. The correlation between autophagy and tamoxifen resistance in breast cancer. Int J Clin Exp Pathol. 2019;12(6):2066–2074. [PMC free article] [PubMed] [Google Scholar]
- [144].Noman MZ, Janji B, Abdou A, et al. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6(1):e1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Yuan S, Fang C, Leng WD, et al. Oral microbiota in the oral-genitourinary axis: identifying periodontitis as a potential risk of genitourinary cancers. Mil Med Res. 2021;8(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bučević Popović V, Šitum M, Chow CT, et al. The urinary microbiome associated with bladder cancer. Sci Rep. 2018;8(1):12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Alluri L, Paes Batista da Silva A, Verma S, et al. Presence of specific periodontal pathogens in prostate gland diagnosed with chronic inflammation and adenocarcinoma. Cureus. 2021;13(9):e17742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Huang ST, Chen J, Lian LY, et al. Intratumoral levels and prognostic significance of Fusobacterium nucleatum in cervical carcinoma. Aging (Albany NY). 2020;12(22):23337–23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Atri A. The Alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019;103(2):263–293. [DOI] [PubMed] [Google Scholar]
- [152].Kantarci A, Tognoni CM, Yaghmoor W, et al. Microglial response to experimental periodontitis in a murine model of Alzheimer’s disease. Sci Rep. 2020;10(1):18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Chen CK, Wu YT, Chang YC. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimer’s Res Ther. 2017;9(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Liccardo D, Marzano F, Carraturo F, et al. Potential bidirectional relationship between periodontitis and Alzheimer’s disease. Front Physiol. 2020;11:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hsu CC, Hsu YC, Chen HJ, et al. Association of periodontitis and subsequent depression: a nationwide population-based study. Medicine (Baltimore). 2015;94(51):e2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sansores-España D, Carrillo-Avila A, Melgar-Rodriguez S, et al. Periodontitis and alzheimer´s disease. Med Oral Patol Oral Cir Bucal. 2021;26(1):e43–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kaur T, Uppoor A, Naik D. Parkinson’s disease and periodontitis–the missing link? A review. Gerodontology. 2016;33(4):434–438. [DOI] [PubMed] [Google Scholar]
- [158].Abbayya K, Puthanakar NY, Naduwinmani S, et al. Association between periodontitis and Alzheimer’s disease. N Am J Med Sci. 2015;7(6):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–3924. [DOI] [PubMed] [Google Scholar]
- [160].Lee DC, Rizer J, Selenica ML, et al. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J. Neuroinflamm. 2010;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Poole S, Singhrao SK, Kesavalu L, et al. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers dis. 2013;36(4):665–677. [DOI] [PubMed] [Google Scholar]
- [162].Al-Obaidi M, Desa M. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol. 2018;38(7):1349–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Han EC, Choi SY, Lee Y, et al. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 2019;33(12):13412–13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Sparks Stein P, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’s Dementia. 2012;8(3):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Taati Moghadam M, Amirmozafari N, Mojtahedi A, et al. Association of perturbation of oral bacterial with incident of Alzheimer’s disease: a pilot study. J Clin Lab Anal. 2022;36(7):e24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Panzarella V, Mauceri R, Baschi R, et al. Oral health status in subjects with amnestic mild cognitive impairment and Alzheimer’s disease: data from the zabút aging project. J Alzheimers dis. 2022;87(1):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wu H, Qiu W, Zhu X, et al. The periodontal pathogen Fusobacterium nucleatum exacerbates Alzheimer’s pathogenesis via specific pathways. Front Aging Neurosci. 2022;14(14):912709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Teixeira AL, Reis HJ, Coelho FM, et al. All-or-nothing type biphasic cytokine production of human lymphocytes after exposure to Alzheimer’s beta-amyloid peptide. Biol Psychiatry. 2008;64(10):891–895. [DOI] [PubMed] [Google Scholar]
- [169].Témoin S, Chakaki A, Askari A, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012;18(3):117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Schmickler J, Rupprecht A, Patschan S, et al. Cross-sectional evaluation of periodontal status and microbiologic and rheumatoid parameters in a large cohort of patients with rheumatoid arthritis. J Periodontol. 2017;88(4):368–379. [DOI] [PubMed] [Google Scholar]
- [171].Ebbers M, Lübcke PM, Volzke J, et al. Interplay between P. gingivalis,F. nucleatum and A. actinomycetemcomitans in murine alveolar bone loss, arthritis onset and progression. Sci Rep. 2018;8(1):15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Pan Y, Teng D, Burke AC, et al. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb Pathog. 2009;46(2):73–79. [DOI] [PubMed] [Google Scholar]
- [173].Williams MD, Kerber CA, Tergin HF. Unusual presentation of Lemierre’s syndrome due to Fusobacterium nucleatum. J Clin Microbiol. 2003;41(7):3445–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kinder Haake S, Lindemann RA. Fusobacterium nucleatum T18 aggregates human mononuclear cells and inhibits their PHA-stimulated proliferation. J Periodontol. 1997;68(1):39–44. [DOI] [PubMed] [Google Scholar]
- [175].Forner L, Larsen T, Kilian M, et al. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–407. [DOI] [PubMed] [Google Scholar]


