Abstract
In this paper, we studied fusogenic peptides of class I-III fusion proteins, which are relevant to membrane fusion for certain enveloped viruses, in contact with model lipid membranes. We resolved the vertical structure and examined the adsorption or penetration behavior of the fusogenic peptides at phospholipid Langmuir monolayers with different initial surface pressures with x-ray reflectometry. We show that the fusion loops of tick-borne encephalitis virus (TBEV) glycoprotein E and vesicular stomatitis virus (VSV) G-protein are not able to insert deeply into model lipid membranes, as they adsorbed mainly underneath the headgroups with only limited penetration depths into the lipid films. In contrast, we observed that the hemagglutinin 2 fusion peptide (HA2-FP) and the VSV-transmembrane domain (VSV-TMD) can penetrate deeply into the membranes. However, in the case of VSV-TMD, the penetration was suppressed already at low surface pressures, whereas HA2-FP was able to insert even into highly compressed films. Membrane fusion is accompanied by drastic changes of the membrane curvature. To investigate how the peptides affect the curvature of model lipid membranes, we examined the effect of the fusogenic peptides on the equilibration of cubic monoolein structures after a phase transition from a lamellar state induced by an abrupt hydrostatic pressure reduction. We monitored this process in presence and absence of the peptides with small-angle x-ray scattering and found that HA2-FP and VSV-TMD drastically accelerate the equilibration, while the fusion loops of TBEV and VSV stabilize the swollen state of the lipid structures. In this work, we show that the class I fusion peptide of HA2 penetrates deeply into the hydrophobic region of membranes and is able to promote and accelerate the formation of negative curvature. In contrast, we found that the class II and III fusion loops of TBEV and VSV tend to counteract negative membrane curvature.
Significance
The cell entry of enveloped viruses proceeds via protein-mediated membrane fusion. We studied fusogenic peptides of class I-III fusion proteins in contact with Langmuir monolayers with x-ray reflectivity and grazing-incidence diffraction to resolve their adsorption and penetration behavior at lipid membranes in an early state of a fusion process. In addition, we examined their interaction with cubic lipid structures to reveal their influence on the curvature of target membranes. Understanding the mechanism of viral membrane fusion is important for inhibitor development and the general understanding of fusion processes, which are fundamental to a variety of biological functions.
Introduction
Most enveloped viruses enter host cells via membrane fusion (1, 2, 3, 4). This process occurs either at the plasma membrane or at an endosomal membrane and is mediated by fusion proteins that are located on the viral surface (1). The fusion proteins connect the cell membrane and the viral envelope and pull them toward each other by a refolding mechanism (5, 6, 7). Viral membrane fusion is typically preceded by a priming event that converts the inactivated fusion protein into a fusion-competent state by proteolytic cleavage (1,2,5,8). After that, a trigger, e.g., decreasing pH in an endosome, can initiate the fusion process. The trigger causes the release of a hydrophobic segment that is originally buried in the structure of the fusion protein (2,9,10). This segment, which depends on its position in the fusion protein sequence by a fusion peptide (FP) or a fusion loop (FL) (1,11), attaches to the target membrane and causes destabilization (9,10,12). The peptide remains in direct interaction with the target membrane during the entire refolding cascade of the fusion protein (1,2). As the leaflets of cell membrane and viral envelope fuse, the FP or FL come in close proximity to a segment called the transmembrane domain (TMD), which anchors the fusion protein in the viral envelope (13, 14, 15, 16, 17). There is evidence that a structural compatibility between FP or FL and TMD is necessary for the opening of a fusion pore and that the TMD facilitates the full enlargement of the pore (18). However, the specific role of FP, FL, and TMD on the molecular mechanism of viral membrane fusion is not fully understood yet, while being highly relevant in the search for inhibitors of this fusion process.
Fusion proteins of enveloped viruses are divided into three different classes based on their structural features. Class I includes trimeric proteins that are mainly built of α-helices and exhibit a central coiled-coil structure (5,19). For class II fusion proteins, an extended β-sheet structure is characteristic. They rearrange from a homo- or heterodimeric prefusion into a homotrimeric postfusion state (6). Fusion proteins that have structural features of class I and II are assigned to a third class. A characteristic property of class III fusion proteins is that they do not require a priming event (7,20,21).
In this study, we examined the interaction of fusogenic peptides of viral fusion proteins representing all three classes with model lipid membranes. The corresponding amino acid sequences were specified by Weise and Reed (22) and are based on the host-guest system established by Han and Tamm (23). The peptides each consist of a fusogenic sequence of a viral fusion protein and an anchor unit, which ensures solubility, prevents aggregation (it was shown that all investigated peptides exist as monomers in solution (22)), and promotes interaction with anionic lipid membranes. This is achieved by four positively charged lysines. These are connected to the fusogenic peptide via a flexible linker unit, consisting of glycine-cysteine-glycine, which prevents the structure of the fusogenic peptides being affected by the anchor unit. Although the anchor unit can trigger the interaction of peptides and membranes via electrostatic attraction, it is unable to penetrate lipid layers and has no affinity to reside in the hydrophobic region of membranes (23,24). We then investigated the behavior of the FP of class I influenza virus hemagglutinin 2 (HA2) (5,19,20,25, 26, 27), the FL of class II tick-borne encephalitis virus (TBEV) glycoprotein E (6,8,28, 29, 30, 31), and the FL and TMD of class III vesicular stomatitis virus (VSV) G-protein (7,18,21,32, 33, 34, 35, 36, 37).
For all three related viruses, the membrane fusion takes place in the acidic milieu of an endosome. The HA2-FP has a high structural flexibility between α-helix and random coil and turn structure at the corresponding conditions (22,38,39). It inserts into membranes forming two α-helices that arrange in a V-shape with the open side facing toward the hydrophobic core of the membrane (25,40, 41, 42). In this conformation, hydrophilic and hydrophobic residues point in opposite directions, enabling a deep penetration into the tail group region (43). By exerting lateral pressure on the surrounding lipids, the insertion of HA2-FP was found to modify membrane structure and curvature (9,38,44, 45, 46, 47, 48, 49, 50). TBEV-FL exhibits a random coil and turn structure and shows no flexibility at fusogenic conditions (22). It is assumed that it interacts mostly with the surface of membranes and inserts only via aromatic anchors into bilayers with a penetration depth that is limited by surrounding polar residues (51,52). As the addition of TBEV-FL strongly alters the phase behavior of lipid/water mixtures, it is evident that the peptide nevertheless has a pronounced effect on the structure of membranes (46). The VSV G-protein has a bipartite FL that interacts with membranes mostly via the aromatic residues at the tip of the two involved segments (7,34, 35, 36). The sequence examined in this study involves only one part of the structure. This highly conserved segment (53, 54, 55) shows a high flexibility between α-helix and random coil and turn structure similar to class I FPs. However, there is no evidence for an α-helical insertion into membranes, as was found for HA2-FP (22). TMDs of viral fusion proteins typically form α-helices that align parallel to the membrane normal (13,14,56, 57, 58). However, VSV-TMD shows a flexibility between α-helix and β-sheet at fusogenic conditions, which is thought to be crucial for its role in membrane fusion (22,59,60).
To explore the role of FPs, FLs, and TMDs in the molecular mechanisms of viral membrane fusion, we have taken two different approaches. As a first step, we examined the adsorption or penetration behavior of the peptides at a phospholipid Langmuir monolayer. This is a suitable model to study the initial interaction of FLs and FPs with membranes, since it is known that they interact primarily with the outer monolayer of the cellular membrane (35,43,52). We conducted x-ray reflectometry (XRR) measurements to resolve the vertical structure of the lipid films before and after the addition of the fusogenic peptides. Our results confirm that the FLs of TBEV and VSV are not able to insert deep into lipid membranes, as they adsorbed mainly underneath the headgroups and exhibited only limited penetration depths into the lipid films. In contrast, we observed that HA2-FP and VSV-TMD can penetrate deeply into the membranes. However, in the case of VSV-TMD, the penetration was suppressed already at low surface pressures, whereas HA2-FP was able to insert even into highly compressed films.
Proceeding from this, we investigated how the peptides affect the curvature of lipid membranes, since the appearance of bent membranes is crucial in the initial process in membrane fusion. The lipid layers are bent strongly toward the headgroups. This negative curvature is also characteristic for inverse bicontinuous cubic lipid phases, and the architecture of the hemifusion intermediate of membrane fusion resembles the structure of these phases (46,47,49,61, 62, 63, 64, 65). Therefore, we examined the interaction of the fusogenic peptides with cubic phases formed by monoolein (66). At room temperature and ambient pressure, monoolein spontaneously forms the Pn3m phase in excess water (67). With an increase of the hydrostatic pressure, a transition into a lamellar crystalline phase can be triggered (44). An abrupt reduction of pressure in the lamellar regime causes the formation of highly swollen Pn3m crystallites that subsequently release excess water for several hours accompanied by a reduction of the lattice constant and an increase of the negative curvature of the lipid leaflets (68). We monitored this process in the presence and absence of the peptides with small-angle x-ray scattering (SAXS) and found that peptides that are able to insert deeply into lipid membranes, like HA2-FP and VSV-TMD, drastically accelerate the equilibration, while the FLs that interact mainly with the surface of membranes stabilize the swollen state of the lipid structures.
Experimental setup
XRR
For the preparation of Langmuir films, the lipids 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (Sigma Aldrich, Germany) and 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA) (Sigma Aldrich, Germany) were dissolved in chloroform in concentrations of 1 mg/mL. To improve the solubility of DPPA, 1 mL methanol was added per 10 mL solution. The lipid solution was spread dropwise on the surface of aqueous solution in a Langmuir trough until a surface pressure Π of 1–3 mN/m was reached. The surface pressure was monitored with a Wilhelmy paper plate microbalance. After an evaporation time of several minutes, the surface area was reduced with a movable barrier compressing the film to a target surface pressure between 5 and 35 mN/m. The subphase was Sørensen’s phosphate buffer solution at pH 5 consisting of 0.113 g/L potassium dihydrogen phosphate and 8.992 g/L disodium hydrogen phosphate dihydrate solved in ultrapure water (specific electrical resistivity >18 MΩcm). The fusogenic peptides (GeneCust, >90% purity) were dissolved in 1 mL buffer solution that was injected underneath the monolayer. The resulting peptide concentration in the Langmuir trough was 2 μM. The amino acid sequences of the examined peptides are stated in Fig. 1. The sample temperature was 20°C.
Figure 1.
Amino acid sequences of the investigated fusogenic peptides from Weise and Reed (22). The numbers refer to the positions of the residues in the corresponding fusion proteins. The text color indicates acidic (pink), basic (blue), polar (brown), aromatic (green), and aliphatic (gray) residues. To see this figure in color, go online.
The vertical structure of the Langmuir films was determined with XRR measurements. The reflected intensity of a monochromatic x-ray beam with a wavelength λ is recorded as a function of the angle of incidence αi. In this scattering geometry, the wave vector transfer q has only a vertical component qz = 4π sin(αi)/λ. From XRR curves, the laterally averaged electron density profiles ρe(z) perpendicular to the sample surface can be extracted (69).
XRR experiments were conducted at beamline ID10 (70) of ESRF with a photon energy of 22 keV (λ = 0.564 Å) and a beam size of 150 μm (horizontal) × 11 μm (vertical), and with a Bruker D8 diffractometer using copper Kα radiation with a photon energy of 8.048 keV (λ = 1.541 Å) and a beam size of 10 mm (horizontal) × 0.1 mm (vertical). At ID10, the scattered intensity was recorded with a horizontally aligned Mythen 1K 1D detector (71). To suppress air-scattering and prevent oxidative radiation damage, the Langmuir trough was placed in a helium atmosphere. Furthermore, the beam was shaded during motor movements and the sample was laterally shifted between scans to reduce the radiation exposure of the examined sections of the monolayers. Including motor movements, an XRR scan took approximately 5 min. The D8 diffractometer is equipped with a sodium iodide scintillation detector and XRR scans took approximately 1 h.
XRR measurements were conducted before and after peptides were injected underneath the lipid surface. The interaction of the fusogenic peptides with the Langmuir films was examined either at constant surface pressure or at constant trough surface area AT. A constant surface pressure was achieved by an automatic readjustment of the surface area with the movable barrier. We applied the software package LSFit (72) to fit the XRR curves using the Parratt algorithm (73) in combination with the effective density model (74) to determine thickness and electron density of all sublayers and the roughness of the interfaces between them. In this procedure, the Langmuir monolayers were represented by two layers for head- and tailgroup. After the addition of peptides, an additional layer was used to model an adsorption underneath the surface. Each layer is described by the parameters electron density, roughness and layer thickness. The errors of the parameters were estimated by their variation during the fitting process and amount to ±0.1 Å for the roughness, ±0.4 Å for the layer thickness, and ±0.005 e/Å3 for the electron density.
Grazing incidence diffraction
At ID10, grazing incidence diffraction (GID) measurements were also conducted, providing insight into the lateral structure of the sample. The GID data are shown in the supporting material (Fig. S1–S5). Compressed DPPC and DPPA Langmuir films align in two-dimensional crystal structures that can be examined based on the position and shape of diffraction maxima. To observe the diffraction maxima, a high surface sensitivity is necessary, which is achieved by choosing an incident angle of a monochromatic x-ray beam which is below the critical angle for external total reflection of the water-air interface (αi = 0.8 αc). In this geometry, the penetration depth of the radiation is approximately 5 nm. The intensity distribution was recorded as a function of the lateral and vertical scattering angle by a Mythen 2K 1D detector. The GID data provide information on the lattice constants of the lipid lattice and the tilt angle of the lipids. The width of the Bragg reflections is connected to the mean size of the crystallites (75).
SAXS
The pressure-jump SAXS experiments were performed in a high hydrostatic pressure cell operating at pressures up to 5 kbar (76). The cell is sealed with Poulter-type diamond windows (77) along the beam path. The sample liquid is separated from the pressure transmitting liquid by an inner sample cell with kapton windows. The sample temperature was set to 25°C with a circulating water flow through the outer shell of the high pressure cell.
Since the studied fusogenic peptides typically act in acidic milieu, 10 mM BisTris buffer at pH 5 was used as sample liquid. Monoolein and buffer solution were poured into the inner sample cell and were mixed mechanically. Subsequently, the samples were allowed to equilibrate for at least 12 h. The peptides were dissolved in the buffer solution before it was mixed with monoolein. The peptide concentration of the samples was 2 wt %.
SAXS experiments were conducted at the beamlines BL2 (78) and BL9 (79) of DELTA (Dortmund, Germany) using MAR345 image plate detectors calibrated with silver behenate (80). The photon energy was 12 keV (λ = 1.033 Å) and 13 keV (λ = 0.954 Å), and the beam size was 0.5 × 0.5 mm2 and 1 × 1 mm2, respectively. The two-dimensional detector images were integrated azimuthally to obtain the scattering intensity as a function of the wave vector transfer q and an interpolated background was subtracted. The background measurements were conducted using an empty sample cell. Phase behavior and lattice constant a (or d for lamellar phases) of the samples were then analyzed based on Bragg reflections that occur at characteristic ratios of the reciprocal lattice constant 2π/a (66). The positions of the Bragg reflections were extracted by fitting Gaussian functions.
Pressure jumps were conducted from 1 kbar to 50 bar. For every sample, reference measurements were taken at 50 bar with exposure times of 150 s before the pressure was increased. As the cubic phase can remain metastable at 1 kbar for many hours, the pressure was increased to 3.5 kbar. This triggers an immediate phase transition into a metastable lamellar phase with a spacing of approximately 43 Å. Subsequently, the pressure was reduced to 1 kbar, causing a transition into a lamellar crystalline Lc phase with a spacing of approximately 50 Å, which is the equilibrium state at high pressures. The phase behavior at 1 and 3.5 kbar was monitored by taking SAXS patterns with exposure times of 30 s. Then, the pressure jump to 50 bar was performed. The pressure reduction took approximately 10 s. After the pressure jump, SAXS scans with exposure times of 150 s were conducted at 4-min intervals for several hours.
Peptide interaction with linear membranes: DPPC and DPPA Langmuir films
Peptide adsorption at the water-air interface
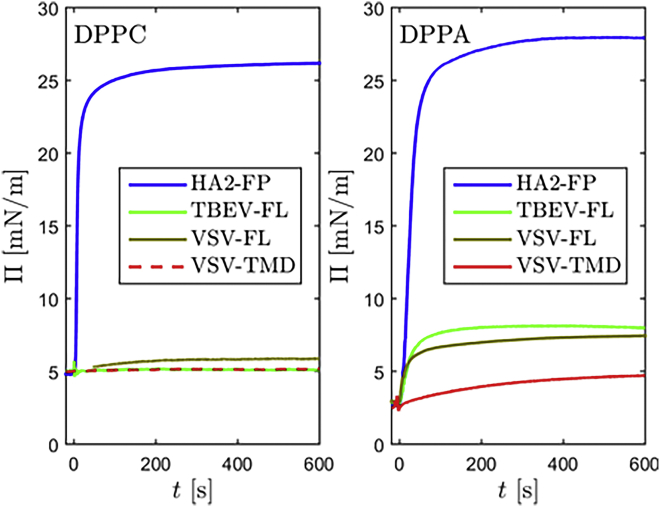
As a first step, we examined the behavior of the fusogenic peptides at an empty buffer surface in a Langmuir trough. The measurements provide information on the affinity of the peptides for hydrophobic interfaces and help to evaluate how adsorption at defect regions of Langmuir films might contribute to observed electron density profiles. Fig. 2 shows reflectivity curves taken at the bare water surface before and after peptides were added and the extracted electron density profiles. While TBEV-FL and VSV-TMD did not show any effect, HA2-FP and VSV-FL formed approximately 25 Å thick adsorbates. In the case of HA2-FP, this process was accompanied by an increase of the surface pressure to approximately 27 mN/m (see Fig. 3 [right] in gray [0 mN/m] in comparison of the pressure change at high and low lipid coverage). After the addition of VSV-FL, the surface pressure reached approximately 4 mN/m (data not shown). The TMD of VSV consists exclusively of residues with pronounced hydrophobic properties. However, the net charge of +4 provided by the anchor unit might prevent VSV-TMD from accumulating at the air-water interface.
Figure 2.
XRR data with fits (inset) and electron density profiles of the aqueous solution-air interface before and after the injection of peptides (left). To see this figure in color, go online.
Figure 3.
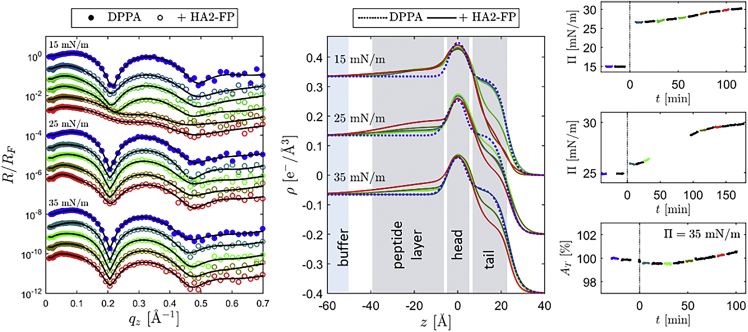
XRR data (left) and electron density profiles (center) of DPPC monolayers before (gray) and 40 min after (colored) the addition of HA2-FP. Fits to the XRR data are shown as black solid lines. Time evolution of the surface pressures of DPPC monolayers with different initial lipid coverage and of the bare water surface (gray) after the addition of HA2-FP at t = 0 (right). To see this figure in color, go online.
Likewise, TBEV-FL carries a net charge of +6. In case of this peptide, also the fusogenic sequence is interspersed with polar residues, so that its low affinity for the hydrophobic interface observed in this experiment meets the expectation. For HA2-FP and VSV-FL, negatively charged amino acids reduce the net charge of the host-guest sequence to +1 and +3, respectively. HA2-FP exhibits a large segment that consists exclusively of hydrophobic amino acids, while VSV-FL includes more polar residues. This could explain why the increase of the surface pressure is much more pronounced for HA2-FP.
HA2-FP at DPPC and DPPA monolayers
Next, we injected HA2-FP underneath phospholipid films at a low level of compression and monitored how their addition affected the surface pressure. Table S1 of the supporting material provides an overview of the XRR results for the effect of fusogenic peptides at phospholipid membranes. For DPPC, we set the initial surface pressure to 5 mN/m. At this value, crystallites of the liquid-condensed phase can already occur, but the film is mostly in the liquid-expanded phase. This means that the lipids are conformationally disordered and do not form a closed layer. For DPPA, we used an initial surface pressure of 3 mN/m. DPPA has a much smaller headgroup than DPPC. Therefore, van der Waals interactions between the lipid tail groups are more pronounced, so that the liquid condensed phase forms already at very low surface pressures. Consequently, the area per DPPC molecule at 5 mN/m is still two times larger than the area per DPPA molecule at 3 mN/m. However, also for DPPA, large disordered domains exist between the crystallites at this level of compression.
Fig. 3 (right and center) shows how the injection of HA2-FP underneath a DPPC monolayer affects surface pressure and vertical film structure at constant surface area.
While the surface pressure remains constant at 35 mN/m, an immediate response of the surface pressure is visible for lower levels of compression. For initial surface pressures of 25 mN/m and less, as well as for an empty surface, a saturation value between 25 and 30 mN/m was reached. GID experiments have shown that the structure of DPPC films that are compressed by a reduction of the surface area with a moving barrier barely differs from those in which HA2-FP has induced a comparable pressure increase (supporting material, Fig. S3). Therefore, it is assumed that HA2-FP does not insert directly into condensed domains but causes compression by penetrating disordered defect regions between the lipid crystallites. A similar behavior was found for the class I FP of HIV (12).
The structural changes that HA2-FP induced in DPPA monolayers are remarkably different. We monitored the interaction of all four peptides at DPPA films for a few hours and conducted several XRR scans at different times. During the experiments, we kept the surface pressure of the lipid film at a constant value by readjusting the barrier. Only in case of HA2-FP did we deviate from this procedure, since the strong increase in surface pressure induced by HA2-FP could not be compensated by the barrier at initial film pressures of 15 and 25 mN/m. Therefore, instead, we conducted the measurements at constant surface area for these starting values.
Fig. 4 (right) shows how the surface pressure changed after HA2-FP was injected underneath DPPA films at surface pressures of 15 and 25 mN/m. As was observed for DPPC, a pronounced increase of the surface pressure was already visible a few minutes after the injection of the peptides. However, while the surface pressure of the DPPC films remained on a constant level, a second increase occurred in the case of DPPA, which reached its maximum slope after more than 1 h. At 35 mN/m, no adaptation of the trough area was necessary to maintain a constant surface pressure immediately after the addition of the peptides. However, on a similar timescale as the second increase in surface pressure occurred at the lower initial pressures, an enlargement of the surface area was necessary to counteract an incipient increase in pressure.
Figure 4.
XRR data (left) and electron density profiles (center) of DPPA monolayers before (blue) and after the addition of HA2-FP. Fits are shown in black. Surface pressure as a function of time (right) for initial pressures of 15 and 25 mN/m and trough area as a function of time at a surface pressure of 35 mN/m. Peptides were injected at t = 0. The colored data points were taken during the XRR scans, which are shown in the same color on the left. To see this figure in color, go online.
The XRR data (Fig. 4, left and center) show that pronounced changes of the vertical film structure occur for all three initial surface pressures. In all three cases, peptides accumulated underneath the headgroups so that it was necessary to introduce an additional layer to the model. The adsorbate reached a thickness of approximately 30 Å. In contrast to DPPC, the peptides did not increase the electron density in the hydrophobic region. Instead, a reduction of the tailgroup electron density occurred. Again, the peptides spread laterally and inhomogeneously over the lipid film and the decrease of the electron density varied depending on the examined sample position. Nevertheless, the data indicate that the restructuring at the interface increased throughout the observation period. This is in good agreement with the finding that the surface pressure reaches no saturation value on the corresponding time scale. GID measurements showed no changes in the lateral film structure, apart from compression as a result of increasing surface pressure (supporting material, Fig. S4). Thus, also in the case of DPPA monolayers, the peptides seem to primarily enter the disordered domains.
The reduction of the tailgroup electron density could indicate the removal of lipids from the interface by the formation of soluble peptide-lipid complexes. However, due to the lateral inhomogeneity of the surface, the reduction can also be explained by lateral displacement of lipids by HA2-FP-rich domains with a vertical structure that is similar to the structure that HA2-FP forms at a bare water surface (Fig. 2). This structure consists of two sublayers that exhibit thicknesses comparable with the dimensions of head and tailgroups of DPPA. While the electron density of the layer facing the water is similar to the electron density of DPPA headgroups, the electron density of the layer facing the air is much lower than the electron density of DPPA tailgroups. Therefore, a displacement of DPPA domains by clusters of HA2-FP that align at the interface, as they would do at a bare water surface, could result in a laterally averaged profile with a reduced tailgroup density. Based on this assumption, the maximum observed area ratio covered by peptide clusters in the illuminated area would be slightly more than 50%. In contrast, the increased electron density in the tailgroup region of DPPC indicates that it is favorable for DPPC molecules and peptides to mix. We also assume that the large headgroups of DPPC are able to wrap around the peptides shielding hydrophobic domains from the aqueous phase and pushing them deep into the hydrophobic region. The small cross-sectional area of the DPPA headgroups does not allow such interaction and the pronounced van der Waals attraction between the hydrocarbon chains might promote the formation of segregated clusters.
TBEV-FL, VSV-FL, and VSV-TMD at DPPC and DPPA monolayers
In contrast to HA2-FP, the peptides TBEV-FL, VSV-FL, and VSV-TMD barely affect the surface pressure of DPPC monolayers (see Fig. 5, left). From XRR and GID measurements, these peptides neither change the vertical nor the lateral structure of the DPPC monolayers in the limits of the experimental resolution (see supporting material, Fig. S1). In the case of the DPPA Langmuir films, the surface pressure increased 10 min after the addition of TBEV-FL and VSV-FL by approximately 5 mN/m and after the addition of VSV-TMD by 2 mN/m. This observation suggests that the electrostatic attraction between the anionic film and the positively charged anchor unit of the peptides is necessary to trigger the interaction. Ben-Tal et al. showed that model peptides consisting of five lysine residues accumulate underneath an anionic monolayer but do not penetrate them and do not affect their surface pressure (24). Thus, it can be inferred that an insertion of the peptides into a lipid film and corresponding structural changes predominantly in the hydrophobic area can be attributed to the actual fusogenic segments of the peptides. As TBEV-FL, VSV-FL, and VSV-TMD only weakly interact with DPPC Langmuir films, we shed light on the interaction of those peptides with DPPA monolayers in the following.
Figure 5.
Surface pressure after the injection of fusogenic peptides underneath DPPC and DPPA films as a function of time (right). The peptides were injected at t = 0. To see this figure in color, go online.
The interaction of the FLs of TBEV and VSV with DPPA monolayers fundamentally differs from HA2-FP. Fig. 6 shows that the injection of TBEV-FL underneath DPPA films barely affected the surface pressure so that only minor adjustments of the barrier position were recorded. Only at a surface pressure of 15 mN/m are the changes more pronounced than the fluctuations expected for a DPPA surface. Throughout the entire observation period, the slope of the trough surface area as a function of time increased. Between 1 and 3 h after the injection of the peptides, the trough area enlarged by approximately 1%. The electron density profiles show that TBEV-FL formed a compact layer underneath the DPPA headgroups. The structure of the lipid layer itself changed much less than when HA2-FP was added. Nevertheless, there is also a reduction in electron density at the end of the hydrocarbon chains, which indicates a slight displacement of lipids from the measurement area. Close to the headgroups, the electron density increased instead. This can be observed most clearly in the case of the 15 mN/m measurement. The increase in electron density indicates that the peptides are able to penetrate the headgroup region and reach into the hydrophobic area. The penetration depth of the peptides can be estimated from the point of intersection between the profiles taken before and after the injection of the peptides. For this purpose, the profiles were superimposed so that their inflection points at the transition between the tailgroup and the air were at the same position. At 15, 25, and 35 mN/m, this procedure yields maximum penetration depths into the hydrophobic region of approximately 7, 6, and 4 Å. This is in good agreement with the penetration depths expected for interactions via aromatic anchors (52). Our data show that the insertion of the aromatic residues causes a displacement of lipid molecules and that their penetration depth decreases with increasing surface pressure.
Figure 6.
XRR data (left) and electron density profiles (center) of DPPA monolayers before (blue) and after the addition of TBEV-FL. Fits are shown in black. Trough area as a function of time (right). Peptides were injected at t = 0. The colored data points were taken during the XRR scans which are shown in the same color on the left. To see this figure in color, go online.
At 25 mN/m, the interaction mode of VSV-FL with DPPA is very similar to TBEV-FL (see Fig. 7). The peptides accumulated underneath the headgroups, forming a layer approximately twice as thick as in case of TBEV-FL. Again, the hydrophobic region is divided into a small section close to the headgroups where the electron density increased and a section where the electron density was reduced, indicating a penetration of the peptides with limited depth and a displacement of lipids. The point of intersection between profiles taken before and after the addition of the peptides is 5 Å deep in the hydrophobic area. Also in this case, a penetration via aromatic residues is assumed. At 15 mN/m, the changes of the structure of the lipid layer are much more pronounced. The distance between the maximum in the headgroup area and the inflection point at the transition between tailgroups and air reduces and the electron density at the end of the tailgroups decreases strongly. It was necessary to divide the hydrophobic region into two sublayers to obtain an adequate fit. Underneath the headgroups, an adsorbate formed with a lower density compared with the 25 mN/m profiles. As VSV-FL forms layers at an empty water surface, it is likely that VSV-FL is able to fully insert into disordered regions of the DPPA monolayer, as was also observed for HA2-FP. Thus, a displacement of lipids by VSV-FL clusters at the water surface could explain the pronounced decrease of the tailgroup electron density. This assumption is supported by the fact that the trough area had to be increased considerably to keep the surface pressure constant. Presumably, the peptides also attached to condensed domains of the DPPA layer inserting aromatic residues, so that the observed profile results from a superposition of the different effects. At 35 mN/m, the injection of the peptides did not affect the hydrophobic area. The accumulation layer underneath the headgroups is very similar to the adsorbate observed at 25 mN/m, but no indication of a partial insertion of the peptides is found. In contrast to TBEV-FL, the penetration depth of VSV-FL is not only reduced at 35 mN/m, but the insertion of aromatic anchors into the tailgroup region is fully prevented.
Figure 7.
XRR data (left) and electron density profiles (center) of DPPA monolayers before (blue) and after the addition of VSV-FL. Fits are shown in black. Trough area as a function of time (right). Peptides were injected at t = 0. The colored data points were taken during the XRR scans, which are shown in the same color on the left. To see this figure in color, go online.
Fig. 8 shows that the behavior of VSV-TMD at a DPPA monolayer is also strongly affected by the surface pressure. While no readjustment of the barrier position was necessary to maintain a surface pressure of 35 mN/m after the injection of the peptides, the surface area had to be increased almost linearly during the entire observation period of 2.5 h to hold a constant pressure of 15 mN/m. At 35 mN/m, no structural changes of the lipid layer are found with XRR. Despite the positive net charge of VSV-TMD, the peptides did not adsorb underneath the anionic headgroups. This is also not observed at 15 mN/m. Instead, the VSV-TMD inserted deeply into the monolayer indicated by an increase of the electron density in the tailgroup region. As the headgroup electron density reduced at the same time, it is assumed that the peptides are mainly located in the hydrophobic area. The reduction can be attributed to a displacement of lipids. However, a formation of VSV-TMD clusters at empty spots between condensed lipid crystallites at the surface, as it was assumed for HA2-FP, is unlikely, as the peptides did not accumulate at a bare water surface (see Fig. 2). It is likely that some residues of the peptides that are incorporated in the monolayer protrude from the headgroups causing the minor increase in electron density underneath the headgroups. Assuming an α-helical conformation, the 15 amino acids of the TMD would have a length of approximately 22.5 Å (81), which is about 1 Å longer than a fully stretched DPPA hydrocarbon chain. In combination with the anchor unit, VSV-TMD can span the whole monolayer and reach into the aqueous phase.
Figure 8.
XRR data (left) and electron density profiles (center) of DPPA monolayers before (blue) and after the addition of VSV-TMD. Fits are shown in black. Trough area as a function of time (right). Peptides were injected at t = 0. The colored data points were taken during the XRR scans, which are shown in the same color on the left. To see this figure in color, go online.
Monolayer section: Summary
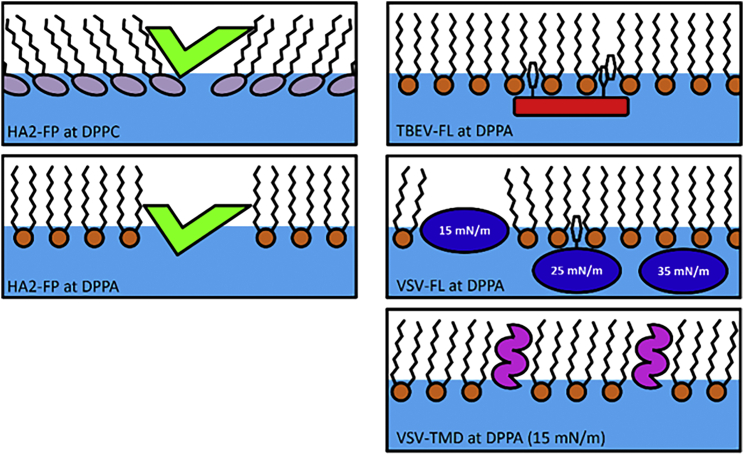
To summarize this section, the investigation of the fusogenic peptides in contact with a DPPA layer revealed different interaction mechanisms. Fig. 9 shows a sketch of the behavior of fusogenic peptides on lipid monolayers. While HA2-FP and VSV-TMD penetrated the monolayer, the FLs of TBEV and VSV mainly attached to the surface and reached into the hydrophobic region only with a limited depth, which can be attributed to the insertion of aromatic anchors. Although it is likely that electrostatic interaction of the positively charged anchor unit of the model peptides with the anionic lipid layer contributed to the adsorption, the behavior of VSV-TMD proves that the electrostatic attraction is not necessarily sufficient to cause the formation of an adsorbate underneath the headgroups. HA2-FP showed the most pronounced affinity for the hydrophobic interface and was able to penetrate even highly compressed DPPA films. In contrast, VSV-TMD did not accumulate at an empty water surface and was only incorporated into DPPA films at low surface pressures.
Figure 9.
Schematic illustration of the influence of HA2-FP on DPPC and DPPA monolayer (left). HA2-FP increases the tailgroup electron density of DPPC monolayer and decreases the tailgroup electron density of DPPA monolayers, indicating different penetration depths. Insertion behavior of TBEV-FL, VSV-FL, and VSV-TMD at DPPA monolayers at different surface pressures (right). TBEV-FL modulates the electron density profile only in proximity of the headgroup region, indicating limited penetration depths, which can be explained by the insertion of aromatic residues. For VSV-FL, the observed penetration depth strongly depends on the surface pressure of the monolayer. VSV-TMD interacts with the monolayer only at low surface pressures. Its insertion alters the vertical structure of head- and tailgroup. To see this figure in color, go online.
Peptide interaction with curved membranes
As a next step, we considered how the peptides affect the behavior of curved membrane systems, as the generation of curvature is essential for fusion mechanisms. For this purpose, we investigated the influence of peptides on the dynamics of swollen cubic monoolein structures after pressure jumps. The procedure of the pressure-jump experiments is illustrated in Fig. 10. Table S1 in the supporting material summarizes the main effects of fusogenic peptides on cubic monoolein structures.
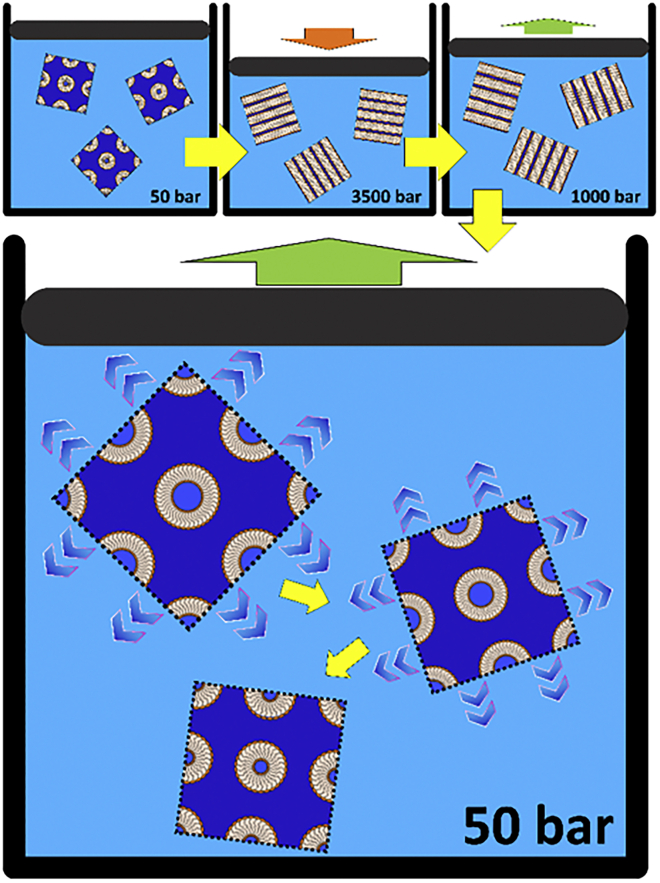
Figure 10.
Schematic illustration of the pressure-jump experiments. The yellow arrows indicate the time sequence. At the beginning of the experiment, the monoolein structures are in equilibrium at 50 bar. Then, by increasing the pressure to 3500 bar, a phase transition is induced to a metastable lamellar phase. This is followed by a pressure release to 1000 bar, causing the system to enter the stable Lc phase. After the pressure jump to 50 bar, monoolein forms a swollen cubic phase, which slowly releases excess water over time accompanied by a reduction of the lattice constant. To see this figure in color, go online.
The inset in Fig. 11 shows how the lattice constant of monoolein in excess water changes at different hydrostatic pressures when the peptides are added. Besides the four fusogenic peptides, the highly hydrophobic artificial peptide L16 (see Fig. 1) was also investigated here as a reference to distinguish between effects originating from unique structural features of the fusogenic peptides and general properties of hydrophobic peptides. It can be seen that all five peptides increase the lattice constant a of monoolein in Pn3m structure at 50 bar. Since cubic monoolein phases, especially, exhibit a high sensitivity to external conditions and equilibrate very slowly, the observed lattice constants vary between the individual samples in ranges of a few angstroms. The error of the SAXS measurements is below the size of the symbols. The SAXS data and the fits are shown in the supporting material in Fig. S6.
Figure 11.
Decrease of the Pn3m lattice constant after a pressure-induced lamellar-to-cubic transition in presence of fusogenic peptides as a function of time t (colored symbols). The dashed gray lines connect data points belonging to the same sample for clarity. The inset shows the Pn3m lattice constant at 50 bar before pressure increase into the lamellar phase regime and the lamellar spacing d at 3500 and 1000 bar before the pressure jumps. The circles represent the obtained values of different samples to illustrate the variance. The crosses mark the mean values. The reference data for pure monoolein were already shown in Surmeier et al. (68). To see this figure in color, go online.
In the metastable lamellar phase at 3500 bar, the mean values of the spacing d recorded in the presence of the peptides were all within the range of variation of the data pure monoolein. Also, in the Lc phase at 1000 bar, the influence of the peptides on the spacing is small.
In contrast, the peptides had a strong influence on the equilibration process after pressure jumps down to 50 bar. Fig. 11 shows that the Pn3m phase formed with an increased lattice constant compared with the values recorded before pressurization in all cases. In the absence of peptides, the equilibration took approximately 3–5 h. When HA2-FP, VSV-TMD, or L16 were added, the process accelerated considerably, and the lattice constant was typically close to equilibrium already after 15 min. TBEV-FL and VSV-FL had the opposite effect, causing a much slower reduction of the lattice constant. An increase of the Pn3m lattice constant can be due to an increase of the water content of the cubic phase, an increase of the surface area of the headgroups by which a decrease of the negative membrane curvature results. Therefore, there exist different mechanisms by which the additives can affect the structure of cubic phases. If solutes preferentially reside either inside of the cubic channel system or in the excess water regime, they can change the water content of the Pn3m phase, as they induce an osmotic pressure between the two reservoirs (82,83). If substances preferentially occupy the interior of the cubic structures, this can typically be attributed to favorable interactions with the lipid interface, which is an entropic driving force for an increase of the headgroup surface (84). This mechanism implies that an increased equilibrium lattice constant is connected to a slower release of excess water after a pressure jump and vice versa. If additives penetrate the lipid layers, they can directly affect the membrane structure and changes of the lattice constant can be understood as the result of an altered spontaneous membrane curvature (85).
Due to their hydrophobic properties, it is assumed that HA2-FP, VSV-TMD, and L16 are able to penetrate the monoolein layers. This assumption is confirmed by the fact that all three peptides increased the equilibrium lattice constant of the cubic phase but accelerated the water release after a pressure jump, so that their influence cannot be attributed solely to the generation of an osmotic pressure between Pn3m water channels and excess water. The increase of the equilibrium lattice constant indicates that the ratio of the cross-sectional areas that the peptides occupy in tail- and headgroup regions is lower than that of the monoolein molecules. As the lamellar phases are barely affected by the peptides, it is likely that the peptides are mostly displaced from the lipid structures. Therefore, a reinsertion of the peptides into the monoolein layers after the pressure reduction might be the reason for the accelerated equilibration process of the cubic phase. Previous studies showed that an HA2-FP molecule can expand the area of a lipid film by about 200 Å2 (86,87). The incorporation of peptides into the monoolein layers increases the ratio of the area of the Pn3m surface to the volume of the water channels and thus forces a reduction of the dimensions of the unit cell. This mechanism would bypass the slow diffusive release of water from the cubic phase by lowering the lattice parameter at constant water content of the Pn3m structure.
The increase of the equilibrium lattice constant and the stabilization of the swollen cubic phase after a pressure jump induced by the FLs of TBEV and VSV can be explained by their affinity to reside at the surface of lipid layer. However, a partial insertion of the peptides also might contribute to the observed behavior. A penetration via aromatic anchors with limited depth counteracts negative membrane curvature since it pushes the headgroups apart but does not exert lateral pressure in the center of the hydrophobic region.
With HA2-FP and TBEV-FL, we also conducted pressure jumps in the opposite direction. For this purpose, we abruptly increased the pressure from 50 to 1500 bar. At 1500 bar, monoolein forms the Lc phase at equilibrium, but the Pn3m phase can persist in a metastable state for many hours. Fig. 12 (top) shows that the lattice constant of monoolein slowly increases after the pressure jump. For two of the samples, no phase transition occurred during the observation periods of 19 and 76 h. The third sample entered the lamellar phase between 11 and 17 h.
Figure 12.
Increase of the Pn3m lattice constant after a pressure jump from 50 to 1500 bar at t = 0 in the presence of fusogenic peptides as a function of time (top). The symbols indicate different samples. For the TBEV-FL samples and one pure monoolein sample, a transition into the Lc phase was observed. The inset shows the continuation of the measurements on the monoolein sample represented by the black circles. Sum of the integrated intensity of the {110} and {111} reflections of the Pn3m phase (light red) and integrated intensity of the first order reflection of the Lc phase (dark red) of monoolein in presence of TBEV-FL as a function of time (bottom). To see this figure in color, go online.
When TBEV-FL was added, the phase transition occurred much faster. Fig. 12 (bottom) shows the intensity of the first reflection of the Pn3m and the Lc phase after the pressure jump as a function of time. The lamellar phase formed already after approximately 15 min and the intensity of the corresponding reflections increased linearly for approximately 20 min. At the same time, the intensity of the Pn3m reflections decreased and the transition was completed 40 min after the pressure jump at the latest. In contrast, no phase transition was observed when HA2-FP was added during observation periods of 13 and 16.5 h. After the pressure jump, the spacing of the Pn3m phase increased abruptly to approximately 140 Å and then slowly rose to more than 150 Å.
While TBEV-FL again promotes monoolein structures with low negative curvature, presumably due to its interactions with the surface of membranes, it is more complex to explain the behavior of HA2-FP.
Tenchov et al. (44) observed that HA2-FP reduces the lattice parameter of cubic phospholipid phases and supposed that the rigid V-shape of HA2-FP increases the negative Gaussian curvature of lipid layers, as it promotes positive curvature along its contour while, perpendicular to it, lipids might be able to wrap around the peptide causing negative curvature. Such a mechanism could have different consequences depending on the lipid composition. Possibly, HA2-FP can favorably be incorporated in monoolein structures with a negative Gaussian curvature that corresponds to a Pn3m lattice constant, which is higher than the equilibrium lattice constant of pure monoolein. This could explain the higher equilibrium lattice constant observed after addition of HA2-FP as well as the stability of the highly swollen Pn3m phase against a phase transition to the lamellar phase after the pressure jump.
Conclusion
The two approaches applied in this work to examine the interaction of viral fusogenic peptides with lipid membranes provide a consistent picture of peptide-membrane interaction. The surface sensitive experiments showed that HA2-FP has a high affinity to reside at the air-water interface and is able to penetrate lipid monolayers even at high surface pressures. HA2-FP was found to mix with DPPC membranes accumulating in the hydrophobic region and to displace DPPA lipids, indicating the formation of peptide-rich clusters. The TMD of VSV inserted into slightly compressed DPPA membranes at 15 mN/m, especially increasing the electron density in the hydrophobic region, while it did not affect the monolayer at high compression of 35 mN/m. In contrast, the FLs of TBEV and VSV mainly interacted with the surface of Langmuir films and exhibited a limited penetration depth, confirming an insertion via aromatic anchors.
Based on the pressure-jump study, the peptides can be divided into two groups as they either accelerated or decelerated the equilibration dynamics of the Pn3m phase. TBEV-FL and VSV-FL slow down the decrease of the lattice constant and thus counteract the water release of the lipid structure and the formation of negative curvature. With regard to their behavior at Langmuir films, this can be attributed to their affinity for the surface of lipid membranes. HA2-FP, VSV-TMD, and L16 have pronounced hydrophobic properties and are able to penetrate deep into lipid structures. Therefore, the increase of the equilibrium lattice constant that they cause is probably due to their direct effect on the internal structure of the monoolein membranes. As the peptides have little effect on the spacing of the lamellar phases, it is assumed that they are widely displaced from the lipid structure at high pressures. Therefore, an abrupt increase of the Pn3m surface area by reinsertion of peptides into the monoolein layers after a pressure jump might be the main driving force for the acceleration of the equilibration process of the cubic phase.
In this work, we show that class I FP of HA2 penetrates deeply into the hydrophobic region of membranes and is able to promote and accelerate the formation of negative curvature. In contrast, we found that class II and III FLs of TBEV and VSV tend to counteract negative membrane curvature. Therefore, it is likely that the peptides play very different roles in the viral fusion mechanisms. While the insertion of HA2-FP into the target membrane might directly contribute to the formation of the hemifusion intermediate, our results indicate that the FLs of TBEV and VSV primarily attach the fusion protein to the cell membrane.
Author contributions
G.S., J.N., and M.P. designed the research. G.S., C.A., J.L., C.S., E.S., S.D.-S., and M.P. performed the research. G.S. analyzed the data. G.S. and S.D.-S. wrote the paper with contributions from the co-authors.
Acknowledgments
The authors thank DELTA for providing synchrotron radiation at BL2 and BL9. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities and we would like to thank Oleg Konovalov for assistance in using beamline ID10. G.S. and J.N. acknowledge Deutsche Forschungsgemeinschaft (FOR 1979) for financial support. This work was supported by RESOLV, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germanyś Excellence Strategy – EXC 2033 – 390677874 – RESOLV.
Declaration of interests
The authors declare no competing interests.
Editor: Tommy Nylander.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.09.011.
Supporting material
References
- 1.White J.M., Whittaker G.R. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison S.C. Viral membrane fusion. Virology. 2015;479-480:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahn R., Lang T., Südhof T.C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 5.Boonstra S., Blijleven J.S., et al. van Oijen A.M. Hemagglutinin-mediated membrane fusion: a biophysical perspective. Annu. Rev. Biophys. 2018;47:153–173. doi: 10.1146/annurev-biophys-070317-033018. [DOI] [PubMed] [Google Scholar]
- 6.Modis Y. Relating structure to evolution in class II viral membrane fusion proteins. Curr. Opin. Virol. 2014;5:34–41. doi: 10.1016/j.coviro.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baquero E., Albertini A.A.V., Gaudin Y. Recent mechanistic and structural insights on class III viral fusion glycoproteins. Curr. Opin. Struct. Biol. 2015;33:52–60. doi: 10.1016/j.sbi.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Pulkkinen L.I.A., Butcher S.J., Anastasina M. Tick-borne encephalitis virus: a structural view. Viruses. 2018;10:350. doi: 10.3390/v10070350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieva J.L., Agirre A. Are fusion peptides a good model to study viral cell fusion? Biochim. Biophys. Acta. 2003;1614:104–115. doi: 10.1016/s0005-2736(03)00168-8. [DOI] [PubMed] [Google Scholar]
- 10.Epand R.M. Fusion peptides and the mechanism of viral fusion. Biochim. Biophys. Acta Biomembr. 2003;1614:116–121. doi: 10.1016/s0005-2736(03)00169-x. [DOI] [PubMed] [Google Scholar]
- 11.Kielian M. Class II virus membrane fusion proteins. Virology. 2006;344:38–47. doi: 10.1016/j.virol.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Ivankin A., Kuzmenko I., Gidalevitz D. Cholesterol mediates membrane curvature during fusion events. Phys. Rev. Lett. 2012;108 doi: 10.1103/PhysRevLett.108.238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang L., Yue L., Hunter E. Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell-cell fusion and virus infection. J. Virol. 2008;82:5417–5428. doi: 10.1128/JVI.02666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong R.T., Kushnir A.S., White J.M. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J. Cell Biol. 2000;151:425–437. doi: 10.1083/jcb.151.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroth-Diez B., Ludwig K., et al. Herrmann A. The role of the transmembrane and of the intraviral domain of glycoproteins in membrane fusion of enveloped viruses. Biosci. Rep. 2000;20:571–595. doi: 10.1023/a:1010415122234. [DOI] [PubMed] [Google Scholar]
- 16.Lee J., Nyenhuis D.A., et al. Tamm L.K. Structure of the Ebola virus envelope protein MPER/TM domain and its interaction with the fusion loop explains their fusion activity. Proc. Natl. Acad. Sci. USA. 2017;114:E7987–E7996. doi: 10.1073/pnas.1708052114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang D.-K., Cheng S.-F., et al. Liu Y.-T. Membrane interaction and structure of the transmembrane domain of influenza hemagglutinin and its fusion peptide complex. BMC Biol. 2008;6:2. doi: 10.1186/1741-7007-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ci Y., Yang Y., et al. Shi L. Vesicular stomatitis virus G protein transmembrane region is crucial for the hemi-fusion to full fusion transition. Sci. Rep. 2018;8:10669–10711. doi: 10.1038/s41598-018-28868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddy N.R., Onuchic J.N. Rotation-activated and cooperative zipping characterize class I viral fusion protein dynamics. Biophys. J. 2018;114:1878–1888. doi: 10.1016/j.bpj.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blijleven J.S., Boonstra S., et al. van Oijen A.M. Vol. 60. Elsevier; 2016. Seminars in Cell & Developmental Biology; pp. 78–88. [DOI] [PubMed] [Google Scholar]
- 21.Roche S., Rey F.A., et al. Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science. 2007;315:843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 22.Weise K., Reed J. Fusion peptides and transmembrane domains of fusion proteins are characterized by different but specific structural properties. Chembiochem. 2008;9:934–943. doi: 10.1002/cbic.200700386. [DOI] [PubMed] [Google Scholar]
- 23.Han X., Tamm L.K. A host–guest system to study structure–function relationships of membrane fusion peptides. Proc. Natl. Acad. Sci. USA. 2000;97:13097–13102. doi: 10.1073/pnas.230212097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Tal N., Honig B., et al. McLaughlin S. Binding of small basic peptides to membranes containing acidic lipids: theoretical models and experimental results. Biophys. J. 1996;71:561–575. doi: 10.1016/S0006-3495(96)79280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross K.J., Langley W.A., et al. Steinhauer D.A. Composition and functions of the influenza fusion peptide. Protein Pept. Lett. 2009;16:766–778. doi: 10.2174/092986609788681715. [DOI] [PubMed] [Google Scholar]
- 26.Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 27.Wilson I.A., Skehel J.J., Wiley D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 \AA resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 28.Stiasny K., Allison S.L., et al. Heinz F.X. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J. Virol. 2002;76:3784–3790. doi: 10.1128/JVI.76.8.3784-3790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J., Lai C.B., et al. Straus S.K. Synthetic fusion peptides of tick-borne encephalitis virus as models for membrane fusion. Biochemistry. 2010;49:287–296. doi: 10.1021/bi9017895. [DOI] [PubMed] [Google Scholar]
- 30.Lescar J., Roussel A., et al. Rey F.A. The fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell. 2001;105:137–148. doi: 10.1016/s0092-8674(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 31.Bressanelli S., Stiasny K., et al. Rey F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim I.S., Jenni S., et al. Harrison S.C. Mechanism of membrane fusion induced by vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA. 2017;114:E28–E36. doi: 10.1073/pnas.1618883114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallbracht M., Brun D., et al. Backovic M. Structure-function dissection of pseudorabies virus glycoprotein B fusion loops. J. Virol. 2018;92:e01203–e01217. doi: 10.1128/JVI.01203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X., Belouzard S., Whittaker G.R. Molecular architecture of the bipartite fusion loops of vesicular stomatitis virus glycoprotein G, a class III viral fusion protein. J. Biol. Chem. 2008;283:6418–6427. doi: 10.1074/jbc.M708955200. [DOI] [PubMed] [Google Scholar]
- 35.Roche S., Bressanelli S., et al. Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313:187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 36.Backovic M., Jardetzky T.S. Class III viral membrane fusion proteins. Curr. Opin. Struct. Biol. 2009;19:189–196. doi: 10.1016/j.sbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Poian A.T., Carneiro F.A., Stauffer F. Viral membrane fusion: is glycoprotein G of rhabdoviruses a representative of a new class of viral fusion proteins? Braz. J. Med. Biol. Res. 2005;38:813–823. doi: 10.1590/s0100-879x2005000600002. [DOI] [PubMed] [Google Scholar]
- 38.Vaccaro L., Cross K.J., et al. Fraternali F. Plasticity of influenza haemagglutinin fusion peptides and their interaction with lipid bilayers. Biophys. J. 2005;88:25–36. doi: 10.1529/biophysj.104.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang D.-K., Cheng S.-F., et al. Yang S.-H. The amino-terminal region of the fusion peptide of influenza virus hemagglutinin HA2 inserts into sodium dodecyl sulfate micelle with residues 16–18 at the aqueous boundary at acidic pH - oligomerization and the conformational flexibility. J. Biol. Chem. 2000;275:19150–19158. doi: 10.1074/jbc.M907148199. [DOI] [PubMed] [Google Scholar]
- 40.Lorieau J.L., Louis J.M., Bax A. The complete influenza hemagglutinin fusion domain adopts a tight helical hairpin arrangement at the lipid: water interface. Proc. Natl. Acad. Sci. USA. 2010;107:11341–11346. doi: 10.1073/pnas.1006142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai A.L., Park H., et al. Tamm L.K. Fusion peptide of influenza hemagglutinin requires a fixed angle boomerang structure for activity. J. Biol. Chem. 2006;281:5760–5770. doi: 10.1074/jbc.M512280200. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi S. Conformation of membrane fusion-active 20-residue peptides with or without lipid bilayers. Implication of alpha-helix formation for membrane fusion. Biochemistry. 1990;29:6257–6264. doi: 10.1021/bi00478a021. [DOI] [PubMed] [Google Scholar]
- 43.Han X., Bushweller J.H., et al. Tamm L.K. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- 44.Tenchov B.G., MacDonald R.C., Lentz B.R. Fusion peptides promote formation of bilayer cubic phases in lipid dispersions. An x-ray diffraction study. Biophys. J. 2013;104:1029–1037. doi: 10.1016/j.bpj.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volynsky P.E., Polyansky A.A., et al. Efremov R.G. Effect of lipid composition on the “membrane response” induced by a fusion peptide. Biochemistry. 2005;44:14626–14637. doi: 10.1021/bi0514562. [DOI] [PubMed] [Google Scholar]
- 46.Levin A., Jeworrek C., et al. Czeslik C. Lipid phase control and secondary structure of viral fusion peptides anchored in monoolein membranes. J. Phys. Chem. B. 2017;121:8492–8502. doi: 10.1021/acs.jpcb.7b06400. [DOI] [PubMed] [Google Scholar]
- 47.Colotto A., Epand R.M. Structural study of the relationship between the rate of membrane fusion and the ability of the fusion peptide of influenza virus to perturb bilayers. Biochemistry. 1997;36:7644–7651. doi: 10.1021/bi970382u. [DOI] [PubMed] [Google Scholar]
- 48.Ge M., Freed J.H. Fusion peptide from influenza hemagglutinin increases membrane surface order: an electron-spin resonance study. Biophys. J. 2009;96:4925–4934. doi: 10.1016/j.bpj.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegel D.P., Epand R.M. Effect of influenza hemagglutinin fusion peptide on lamellar/inverted phase transitions in dipalmitoleoylphosphatidylethanolamine: implications for membrane fusion mechanisms. Biochim. Biophys. Acta. 2000;1468:87–98. doi: 10.1016/s0005-2736(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 50.Lagüe P., Roux B., Pastor R.W. Molecular dynamics simulations of the influenza hemagglutinin fusion peptide in micelles and bilayers: conformational analysis of peptide and lipids. J. Mol. Biol. 2005;354:1129–1141. doi: 10.1016/j.jmb.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 51.Mendes Y.S., Alves N.S., et al. Oliveira A.C. The structural dynamics of the flavivirus fusion peptide–membrane interaction. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modis Y., Ogata S., et al. Harrison S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 53.Fredericksen B.L., Whitt M.A. Vesicular stomatitis virus glycoprotein mutations that affect membrane fusion activity and abolish virus infectivity. J. Virol. 1995;69:1435–1443. doi: 10.1128/jvi.69.3.1435-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delos S.E., Gilbert J.M., White J.M. The central proline of an internal viral fusion peptide serves two important roles. J. Virol. 2000;74:1686–1693. doi: 10.1128/jvi.74.4.1686-1693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L., Ghosh H.P. Characterization of the putative fusogenic domain in vesicular stomatitis virus glycoprotein G. J. Virol. 1994;68:2186–2193. doi: 10.1128/jvi.68.4.2186-2193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langosch D., Brosig B., Pipkorn R. Peptide mimics of the vesicular stomatitis virus G-protein transmembrane segment drive membrane fusion in vitro. J. Biol. Chem. 2001;276:32016–32021. doi: 10.1074/jbc.M102579200. [DOI] [PubMed] [Google Scholar]
- 57.Tatulian S.A., Tamm L.K. Secondary structure, orientation, oligomerization, and lipid interactions of the transmembrane domain of influenza hemagglutinin. Biochemistry. 2000;39:496–507. doi: 10.1021/bi991594p. [DOI] [PubMed] [Google Scholar]
- 58.Liu L.-P., Deber C.M. Uncoupling hydrophobicity and helicity in transmembrane segments - α-helical propensities of the amino acids in non-polar environments. J. Biol. Chem. 1998;273:23645–23648. doi: 10.1074/jbc.273.37.23645. [DOI] [PubMed] [Google Scholar]
- 59.Langosch D., Crane J.M., et al. Reed J. Peptide mimics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J. Mol. Biol. 2001;311:709–721. doi: 10.1006/jmbi.2001.4889. [DOI] [PubMed] [Google Scholar]
- 60.Cleverley D.Z., Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegel D.P. Fourth-order curvature energy model for the stability of bicontinuous inverted cubic phases in Amphiphile- water systems. Langmuir. 2010;26:8673–8683. doi: 10.1021/la904838z. [DOI] [PubMed] [Google Scholar]
- 62.Siegel D.P. The Gaussian curvature elastic energy of intermediates in membrane fusion. Biophys. J. 2008;95:5200–5215. doi: 10.1529/biophysj.108.140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegel D.P., Kozlov M. The Gaussian curvature elastic modulus of N-monomethylated dioleoylphosphatidylethanolamine: relevance to membrane fusion and lipid phase behavior. Biophys. J. 2004;87:366–374. doi: 10.1529/biophysj.104.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tristram-Nagle S., Nagle J.F. HIV-1 fusion peptide decreases bending energy and promotes curved fusion intermediates. Biophys. J. 2007;93:2048–2055. doi: 10.1529/biophysj.107.109181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chavarha M., Khoojinian H., et al. Hall S.B. Hydrophobic surfactant proteins induce a phosphatidylethanolamine to form cubic phases. Biophys. J. 2010;98:1549–1557. doi: 10.1016/j.bpj.2009.12.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kulkarni C.V., Wachter W., et al. Ahualli S. Monoolein: a magic lipid? Phys. Chem. Chem. Phys. 2011;13:3004–3021. doi: 10.1039/c0cp01539c. [DOI] [PubMed] [Google Scholar]
- 67.Qiu H., Caffrey M. The phase diagram of the monoolein/water system: metastability and equilibrium aspects. Biomaterials. 2000;21:223–234. doi: 10.1016/s0142-9612(99)00126-x. [DOI] [PubMed] [Google Scholar]
- 68.Surmeier G., Paulus M., et al. Nase J. A pressure-jump study on the interaction of osmolytes and crowders with cubic monoolein structures. Soft Matter. 2022;18:990–998. doi: 10.1039/d1sm01425k. [DOI] [PubMed] [Google Scholar]
- 69.Pietsch U., Holy V., Baumbach T. Springer Science & Business Media; 2004. High-resolution X-Ray Scattering: From Thin Films to Lateral Nanostructures. [Google Scholar]
- 70.Smilgies D.-M., Boudet N., et al. Konovalov O. Troika II: a versatile beamline for the study of liquid and solid interfaces. J. Synchrotron Radiat. 2005;12:329–339. doi: 10.1107/S0909049505000361. [DOI] [PubMed] [Google Scholar]
- 71.Jung D.S., Suominen L., et al. Hörmann C. Advanced materials research. Adv. Mat. Res. 2014;996:203–208. [Google Scholar]
- 72.Seeck O.H., Kaendler I.D., et al. Kolb R. Analysis of x-ray reflectivity data from low-contrast polymer bilayer systems using a Fourier method. Appl. Phys. Lett. 2000;76:2713–2715. [Google Scholar]
- 73.Parratt L.G. Surface studies of solids by total reflection of X-rays. Phys. Rev. 1954;95:359–369. [Google Scholar]
- 74.Tolan M. Springer; 1999. X-Ray Scattering from Soft-Matter Thin Films: Materials Science and Basic Research. [Google Scholar]
- 75.Als-Nielsen J., Jacquemain D., et al. Leiserowitz L. Principles and applications of grazing incidence x-ray and neutron scattering from ordered molecular monolayers at the air-water interface. Phys. Rep. 1994;246:251–313. [Google Scholar]
- 76.Wirkert F.J., Paulus M., et al. Tolan M. X-ray reflectivity measurements of liquid/solid interfaces under high hydrostatic pressure conditions. J. Synchrotron Radiat. 2014;21:76–81. doi: 10.1107/S1600577513021516. [DOI] [PubMed] [Google Scholar]
- 77.Poulter T.C. Apparatus for optical studies at high pressure. Phys. Rev. 1932;40:860–871. [Google Scholar]
- 78.Schneider E., Paulus M., et al. Tolan M. DELTA Annual Report 2019. 2019. The new wide and small angle scattering setup at beamline BL2 of DELTA; pp. 21–22. [Google Scholar]
- 79.Krywka C., Sternemann C., et al. Tolan M. The small-angle and wide-angle X-ray scattering set-up at beamline BL9 of DELTA. J. Synchrotron Radiat. 2007;14:244–251. doi: 10.1107/S0909049507009727. [DOI] [PubMed] [Google Scholar]
- 80.Huang T.C., Toraya H., et al. Wu Y. X-ray powder diffraction analysis of silver behenate, a possible low-angle diffraction standard. J. Appl. Crystallogr. 1993;26:180–184. [Google Scholar]
- 81.Pauling L., Corey R.B., Branson H.R. The structure of proteins: two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA. 1951;37:205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sukenik S., Dunsky S., et al. Harries D. TMAO mediates effective attraction between lipid membranes by partitioning unevenly between bulk and lipid domains. Phys. Chem. Chem. Phys. 2017;19:29862–29871. doi: 10.1039/c7cp04603k. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi H., Matsuo A., Hatta I. Effects of chaotropic and kosmotropic solutes on the structure of lipid cubic phase: monoolein-water systems. Mol. Cryst. Liq. Cryst. Sci. Technol. 2000;347:231–238. [Google Scholar]
- 84.Cherezov V., Clogston J., et al. Caffrey M. Room to move: crystallizing membrane proteins in swollen lipidic mesophases. J. Mol. Biol. 2006;357:1605–1618. doi: 10.1016/j.jmb.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 85.Campelo F., McMahon H.T., Kozlov M.M. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhelev D.V., Stoicheva N., et al. Needham D. Interaction of synthetic HA2 influenza fusion peptide analog with model membranes. Biophys. J. 2001;81:285–304. doi: 10.1016/S0006-3495(01)75699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Longo M.L., Waring A.J., et al. Hammer D.A. Area expansion and permeation of phospholipid membrane bilayers by influenza fusion peptides and melittin. Langmuir. 1998;14:2385–2395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.