Key Points
Question
What is the proportion of use of noncontrast computed tomography of the head (CTH) in patients with acute-onset atraumatic altered mental status (AMS)?
Findings
This systematic review and meta-analysis of 25 studies with 79 201 patients found that the use of CTH in patients with AMS was very high, but the positive yield on those CTH studies was low.
Meaning
In this study, the use of CTH in patients with AMS was exceedingly high without a substantial yield.
This systematic review and meta-analysis assesses the use of noncontrast computed tomography of the head in patients with atraumatic acute-onset altered mental status.
Abstract
Importance
The usefulness of computed tomography of the head (CTH) in patients with acute-onset atraumatic altered mental status (AMS) is poorly understood, but use in these patients remains high.
Objective
To evaluate the use of CTH (event rate) in patients with AMS and the positive outcome event rate of the performed CTH studies.
Data Sources
The PubMed/MEDLINE, PubMed Central, Embase, and CINAHL databases were searched using predefined Boolean parameters. All studies that met inclusion criteria until January 31, 2022, were included.
Study Selection
Randomized clinical trials and observational, cohort, and case-control studies were included. Conference abstracts, reviews, letters, case reports, case series, systematic literature, and meta-analyses were excluded.
Data Extraction and Synthesis
The systematic literature review was performed per Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. Data were independently extracted by 2 authors. Data were pooled using a random-effects method.
Main Outcomes and Measures
Event rate of CTH use in patients with acute atraumatic AMS. The CTH event rates and positive CTH event rates were calculated with 95% CIs.
Results
Of 9338 studies identified, 26 qualified for the systematic review and 25 for the meta-analysis. The 25 studies in the meta-analysis included a total of 79 201 patients. The CTH event rate was 94% (proportion, 0.94; 95% CI, 0.76-1.00), and the positive CTH event rate was 11% (proportion, 0.11; 95% CI, 0.07-0.15). There was significant heterogeneity among the studies included (I2 > 50%, P < .001), for which a random-effects model was used. There was significant publication bias, as evident by an asymmetric funnel plot. There was no fluctuation of the results during the sensitivity analysis, which reassured the reliability of the data.
Conclusions and Relevance
In this meta-analysis, CTH use among patients with acute-onset atraumatic AMS was very high with a low yield. Large-scale studies are needed to guide clinical decision-making in such a situation.
Introduction
Since the development of computed tomography (CT) in the 1970s, the use of CT studies has been increasing exponentially. In the mid-2000s, it was estimated that 60 million CT studies were performed annually in the US, which increased to an estimated 80 million CT studies in the late 2000s.1,2 A single health system study1 performed to see the trends of CT study use during a decade revealed that the proportion of CTs ordered in the emergency department (ED) increased by 81% (from 41.4% in 2000 to 74.4% in 2010). In contrast, the total ED patient volume remained stable. The reasons for the increasing trend are multifactorial, but the easy availability of the study and the prompt result are among the top reasons.2,3
In clinical practice, when a patient with acute altered mental status (AMS) is encountered, a CT of the head (CTH) study is usually performed as a part of the workup. The American College of Radiology 2019 appropriateness criteria for AMS do not explicitly favor CTH in patients with AMS without trauma, the risk for intracranial bleeding (such as anticoagulation use), hypertensive emergency, known intracranial process (mass, recent hemorrhage, recent infarct, central nervous system infection), new-onset delirium, or psychosis. The decision to perform CTH is left to the discretion of the physician.4 Because of ease and speed of execution, CTH is the preferred modality of radiologic investigation. In a previous study, CTH was ordered for almost half of the patients who presented with AMS and confusion in the ED, with a yield of only approximately 9%.5 Similarly, in another study, an estimated one-third of the CT studies were performed on the head, and 75% were performed in the hospital setting.2 Some studies suggest that the use of CTH has increased markedly in the US during the past 2 decades.1,2 However, CTH has a low yield in the evaluation of patients with AMS and adds to the cost of care along with radiation exposure.5 It is estimated that individuals with 3 to 4 lifetime CT studies have similar cancer risks to that of nuclear bombing survivors in Japan,2 but health care professionals remain unaware of this risk.6 Thus, we performed this systematic review and meta-analysis to fully appraise the available data. We assessed the proportion of noncontrast CTH use in patients with atraumatic acute-onset AMS. Furthermore, we explored the findings of positive CTH studies and their association with change in clinical management.
Methods
Search Strategy
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.7 We searched the PubMed/MEDLINE, PubMed Central, Embase, and CINAHL databases. The Boolean parameters that were used to search AMS were [altered mental status OR confusion OR disorientation OR unconscious OR AMS]. Similarly, for CTH, the parameters were [Tomography, X-ray Computed OR CT Head OR CTH] (eTable 1 in the Supplement). We included studies published before January 31, 2022. The study's protocol was registered in PROSPERO (CRD42022324211).
Eligibility Criteria and Outcomes
Randomized clinical trials and observational, cohort, and case-control studies were included. Conference abstracts, reviews, letters, case reports, case series, systematic literature, and meta-analyses were excluded. Patients having acute AMS, confusion, loss of consciousness, or disorientation without evidence of head trauma or focal neurologic deficits in the ED or while being admitted to the inpatient unit or intensive care unit (ICU) were eligible for the study. Studies with mixed age groups were included but were excluded if the study was performed with pediatric age groups only (<18 years of age). Outcomes included acute ischemic stroke, acute intracranial hemorrhage, intracranial mass, cerebral edema, and new identifiable lesions in the CTH result.
Data Extraction
Studies were identified and screened for eligibility by 2 authors (R.A. and S.K.) independently based on inclusion criteria. EndNote software was used to maintain the records of identified and screened studies and to remove duplicated studies. Discrepancies were resolved by mutual consent obtained from another author (D.B.S.). A Microsoft Excel sheet (Microsoft Corp) was used to extract the study characteristics, such as type of study, year of publication, country, number of CTH studies, number of patients with AMS, number of CTHs in patients with AMS, CTH study outcome, cost of CTH study, and change in management.
Outcome Measures
The primary outcome was the proportion (event rate) of CTH use in patients with acute atraumatic AMS. Secondary outcomes were (1) the proportion (event rate) of positive CTH results; (2) outcomes of CTH; (3) use of CTH in the ED, ICU, or inpatient unit; (4) change in management per CTH result; and (5) cost of CTH study. We also performed a pooled proportion meta-analysis comparing the US and European studies of CTH event rates.
Evidence of Study Quality
The Joanna Briggs Institute’s critical appraisal checklist for cohort, case-control, and quasiexperimental studies was used to evaluate the quality of the studies.8 The checklist included 11 to 13 items; if the answer to an item was yes, the study was scored 1; otherwise it was scored zero. Total quality scores of 4 or less, 5 to 7, and 8 or greater were considered low, moderate, and high quality, respectively (eTables 2-4 in the Supplement).9
Statistical Analysis
The aggregate proportion of CTH prevalence (event rate) in patients with AMS and positive CTH findings was pooled using Stata software, version 17.0 (StataCorp LLC). Proportions were used to estimate the outcomes with a 95% CI. We assumed significant heterogeneity among the studies; hence, the random-effects model was used for meta-analysis. The between-study heterogeneities were assessed, with P < .10 or I2 > 50% indicating significant heterogeneity. In addition, sensitivity analyses were conducted to test the stability of the pooled proportions by excluding studies one by one. Finally, publication bias was assessed with a funnel plot.
Results
Literature Search
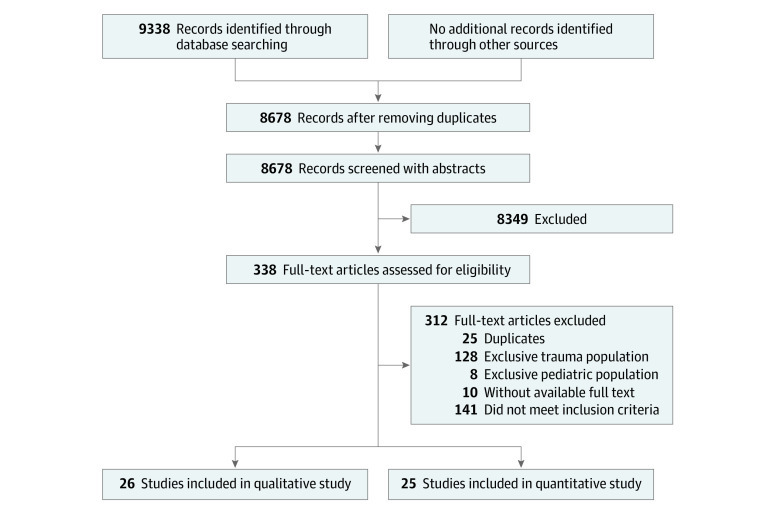
A total of 9338 studies were identified, and no additional records were obtained from other sources. Six hundred fifty-one studies were duplicated and omitted. A total of 8687 studies were screened with title and abstract, of which 338 qualified for full-text review. After applying inclusion and exclusion criteria, 26 were qualified for systematic review, and 25 were eligible for meta-analysis, with a combined total of 79 201 patients (Figure 1).
Figure 1. PRISMA Diagram of Eligible Studies.
Study Characteristics
Of 26 studies, 11 were performed in the ED,5,10,11,12,13,14,15,16,17,18,19 3 in the ICU,20,21,22 4 in an inpatient unit,23,24,25,26 and 8 in mixed settings.27,28,29,30,31,32,33,34 Fifteen studies were performed in the US,5,10,11,18,20,22,24,25,26,27,28,29,30,33,34 and 4 in Europe13,16,21,31 (Table). The studies were appraised using the Joanna Briggs Institute quality assessment. We rated 11 studies as high quality12,16,19,22,23,27,28,31,32,33,34 and 15 studies as medium quality5,10,11,13,14,15,17,18,20,21,24,25,26,29,30; none of the studies were rated as low quality (eTables 2-4 in the Supplement).
Table. Descriptive Analysis of Studiesa.
| Source | Type of study | Place of study | Total No. of patients/No. of patients with AMS | No. of CTH events in patients with AMS | No. of positive CTH events | Setting | Outcome of CTH (defined in study) | Outcome of positive CTH findings (defined in patients with AMS) | Time to CTH | Change in management |
|---|---|---|---|---|---|---|---|---|---|---|
| Bent et al,10 2015 | Retrospective, cohort | US | 500/65 | 65 | 7 | ED | Acute/subacute stroke, ICH, mass, edema, or mass effect | NA | NA | NA |
| Callen et al,11 2020 | Retrospective, case-control | US | 9593/1816 | 1816 | 130 | ED | Hemorrhage, hydrocephalus, mass effect, or worsening of prior finding | NA | NA | NA |
| Chen et al,12 2020 | Retrospective, case-control | Taiwan | 66/66 | 31 | 0 | ED | Intracerebral hemorrhage, new ischemic infarction, or space-occupying lesions | None | 12 h | None |
| Chokshi et al,20 2016 | Retrospective, cohort | US | 2846/2846 | 2846 | 566 | ICU | Hemorrhage, infarction, mass effect, or hydrocephalus | NA | 90 min | NA |
| Covino et al,13 2019 | Observational, quasi-experimental | Italy | 1664/574 | 574 | NA (not reported for AMS) | ED | Ischemia, bleeding, or hydrocephalus | NA | NA | NA |
| Detweiler et al,27 2020 | Retrospective, case-control | US | 200/100 | 79 | NA | Mixed | White matter lesion | NA | NA | NA |
| Detweiler et al,28 2017b | Retrospective, case-control | US | 200/100 | 79 | NA | Mixed | White matter lesion, cerebral atrophy, or intracerebral calcifications | NA | NA | NA |
| Donovan et al,29 2015 | Retrospective, cohort | US | 462/ 302 | 302 | 1 | Mixed | ICH | Yes (ICH) | NA | Yes, intubated |
| Finkelmeier et al,21 2019 | Retrospective, case-control | Germany | 690/162 | 162 | 16 | ICU | ICH/SAH or stroke | NA | NA | Yes, but not clear |
| Hanna et al,30 2021 | Retrospective, cohort | US | 520/408 | 408 | 3 | Mixed | Acute ischemic stroke, hemorrhagic stroke, or SDH | NA | NA | NA |
| Hufschmidt et al,31 2008 | Retrospective, cohort | Germany | 294/294 | 178 | 25 | Mixed | Diagnostic to the acute state (no clear mention of diagnosis) | NA | NA | NA |
| Khan et al,22 2014 | Retrospective, cohort | US | 901/635 | 635 | 47 | ICU | Acute and subacute or chronic changes (infarction, hemorrhage, mass, or hydrocephalus) | NA | NA | NA |
| Lai et al,23 2010 | Retrospective, case-control | Australia | 200/25 | 25 | 6 | Inpatient | Acute ischemic stroke, hemorrhagic stroke, or SDH | NA | NA | NA |
| Lim et al,32 2009 | Retrospective, cohort | Singapore | 578/578 | 327 | 128 | Mixed | Acute infarct or ICH | NA | NA | NA |
| Nesselroth et al,14 2021 | Retrospective, cohort | Israel | 1536/116 | 116 | 23 | ED | Mass effect, herniation, ischemic stroke, or ICH | NA | NA | NA |
| Patel et al,33 2002 | Retrospective, case-control | US | 152/151 | 42 | 0 | Mixed | Acute, chronic, and none | NA | 12 h | None |
| Patel et al,15 2019 | Retrospective, cohort | India | 308/55 | 55 | 17 | ED | Infarct, ICH, or mass | Yes (SCH, 9/17) | NA | NA |
| Rahimi et al,34 2016 | Retrospective, case-control | US | 349/349 | 223 | 25 | Mixed | CVA, ICH, or mass | Yes (CVA, 13/25; ICH, 9/25) | NA | NA |
| Segard et al,16 2013 | Retrospective, case-control | France | 291/139 | 139 | 63 | ED | Stroke, TIA, tumors, and ICH | NA | NA | NA |
| Shuaib et al,18 2014 | Retrospective, cohort | US | 379/49 | 49 | 12 | ED | Mass, ischemia, ICH, or calcifications | NA | NA | NA |
| Sinclair et al,17 1993 | Retrospective, cohort | Canada | 416/25 | 25 | 8 | ED | Pathology not previously demonstrated (infarction, bleed, or SAH) | NA (not clear if outcome mentioned for AMS) | NA | Yes (surgery) |
| Thacker et al,24 2021 | Retrospective, cohort | US | 83/58 | 58 | 1 | Inpatient | SDH | Yes (SDH) | NA | Yes (surgery) |
| Theisen-Toupal et al,25 2014 | Retrospective, cohort | US | 220/220 | 220 | 6 | Inpatient | Stroke/ICH | NA | NA | Yes |
| Tu et al,5 2022 | Retrospective, cohort | US | 52 799/28 332 | 6146 | 588 | ED | Critical results (ischemic infarct, ICH, or mass lesion) | NA | NA | NA |
| Wang and You,19 2013 | Retrospective, cohort | Canada | 3967/1120 | 1120 | NA | ED | Ischemia, hemorrhage, or mass needing follow-up | NA | NA | NA |
| Wong et al,26 2014 | Retrospective, cohort | US | 187/139 | 126 | 0 | Inpatient | Ischemia, hemorrhage, or acute organic disorder | None | NA | NA |
Abbreviations: AMS, altered mental status; CTH, computed tomography of head; CVA, cerebrovascular accident; ED, emergency department; ICH, intracranial hemorrhage; ICU, intensive care unit; NA, not available; SAH, subarachnoid hemorrhage; SDH, subdural hematoma; TIA, transient ischemic attack.
If there was no explicit mention of contrast-enhanced CT used in the study, noncontrast CTH was assumed. If there was no explicit mention of the study setting, mixed setting (ED, inpatient unit, or ICU) was assumed. If there was no explicit mention of focal neurologic deficit in the study, it was assumed focal neurologic deficit was not associated with AMS. Therefore, the studies with AMS and focal neurologic deficit mentioned that did not allow the authors to segregate the patients with AMS were excluded.
Study not included in the meta-analysis because the same patient sample was used in another study by the authors.
Outcome Analyses
Primary Outcome
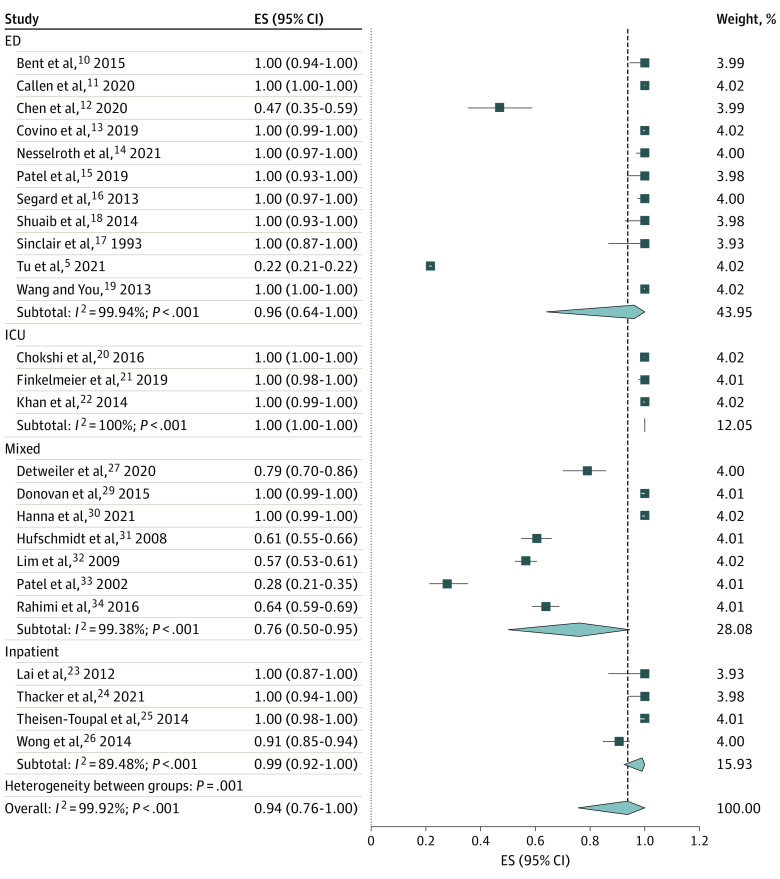
The CTH event rate among patients with AMS was 94% (proportion, 0.94; 95% CI, 0.76-1.00). Additional subgrouping for CTH event based on the settings showed 100% in the ICU (proportion, 1.00; 95% CI, 1.00-1.00), followed by 99% in the inpatient unit (proportion, 0.99; 95% CI, 0.92-1.00), 96% in the ED (proportion, 0.96; 95% CI, 0.64-1.00), and 76% among mixed settings (proportion, 0.76; 95% CI, 0.50-0.95) (Figure 2). In the event rate, total CTH performed on patients with AMS was calculated. For example, if a study had a patient who had 2 CTH studies performed for AMS and reported, we counted the CT performed as 2 CTH studies.
Figure 2. Proportion of Computed Tomography of Head in Patients With Altered Mental Status Among Studies.
Effect size (ES) represents the proportion of computed tomography of the head in patients with altered mental states. The model used is the random-effects model. ED indicates emergency department; ICU, intensive care unit.
Secondary Outcomes
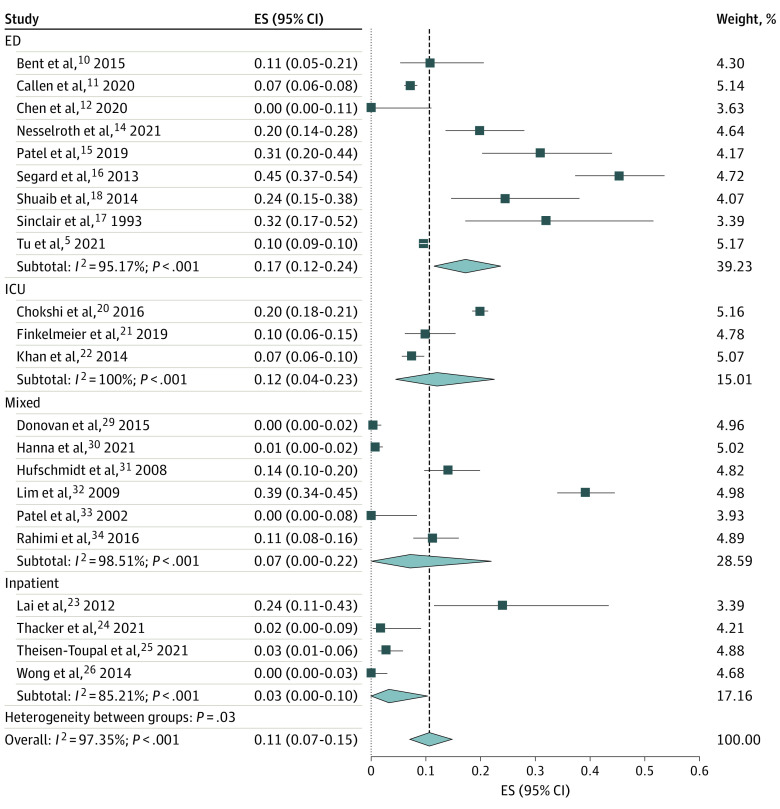
The overall positive CTH event rate within those CTH studies was 11% (proportion, 0.11; 95% CI, 0.07-0.15), with a maximum of 17% (proportion, 0.17; 95% CI, 0.12-0.24) in the ED, followed by 12% in the ICU (proportion, 0.12; 95% CI, 0.04-0.23), 7% in mixed settings (proportion, 0.07; 95% CI, 0.00-0.22), and 3% in the inpatient setting (proportion, 0.03; 95% CI, 0.00-0.10) (Figure 3). In both models, significant heterogeneity was found among the analyzed studies (I2 > 50%) (Figure 2 and Figure 3). Only 2 studies discussed the time taken to perform a CTH study, with 12 hours12,33 and 90 minutes20 being reported. Furthermore, only 1 article18 discussed the cost related to CTH study. Although all studies included declared the definition of positive CTH findings in the study, only 4 discussed the details of CTH results pertinent to patients with AMS.15,24,29,34 Four studies mentioned a change in management based on CTH results.17,21,24,29 The CTH event rates were 92% (proportion, 0.92; 95% CI, 0.61-1.00) in the US and 96% (proportion, 0.96; 95% CI, 0.74-1.00) in Europe (eFigure 1 in the Supplement).
Figure 3. Proportion of Positive Computed Tomography of Head Findings in Patients With Altered Mental Status Among Studies.
Effect size (ES) represents the proportion of positive findings on computed tomography of the head in patients with altered mental states. The model used is the random-effects model. ED indicates emergency department; ICU, intensive care unit.
Publication Bias
Publication bias was assessed using funnel plots. For both the primary and secondary outcomes evaluated, funnel plots showed a significantly asymmetric distribution of studies, suggesting significant publication bias across studies (eFigures 2-3 in the Supplement).
Sensitivity Analysis
After removing each study individually and calculating the proportions, the results did not fluctuate. The CTH event rates ranged from 85% to 88% (P < .001), and positive CTH event rates ranged from 11% to 14% (P < .001) (eFigures 4-5 in the Supplement). This finding demonstrates that our results are reliable.
Discussion
In this meta-analysis, we report the proportion of noncontrast CTH use among patients presenting with atraumatic acute AMS. The use of CTH was 94%, and a positive result was found among 11% of the CTH studies performed. We included 26 studies in the systematic review and excluded 1 study in the meta-analysis because the authors used the same set of participants in another study.
Of the studies that met the inclusion and exclusion criteria and were included in this study, 17 were retrospective cohort studies and 9 were retrospective case-control studies. The majority of the studies were on CTH use in different clinical settings, and patients with AMS were a subset. We performed a pooled analysis of the event rate in terms of CTH use in the acute AMS setting and the event rate of positive CTH findings among those CTH studies performed. Because of the inclusion of such variable studies, we had high heterogeneity in the pooled analysis (CTH event rate: I2 = 99.92%, P < .001; positive CTH event rate: I2 = 97.35%, P < .001). However, because this study aimed to calculate the event rate, we expected significant heterogeneity among the studies.
The main reasons for performing CTH studies in clinical practice on patients with AMS are the lack of clear guidelines,4 diagnostic dilemmas, and defensive medicine.2,3 However, in the subgroup analysis of the studies in the US and Europe, the use of CTH in Europe was not different from that in the US despite socialized medicine and presumed fewer medical-legal concerns. Tu et al5 reported that AMS accounted for the highest frequency of CTH ordered in the ED in a single health system study; 46% of CTH studies were ordered for AMS alone, with a 10% yield. Computed tomography of the head comprised 38% of all CT studies ordered in the ED and 8% of total ED visits.5 Our subgroup analysis found that the CTH event rate was 96% with a positive CTH event rate of 17% in the ED. A few studies suggested that the use of imaging in the ED setting was associated with increased length of stay, higher bed occupancy, and increased cost of health care.3,35,36 There is wide variation in price transparency and cost of medical imaging, and the reported cost of a CTH study can range from $211 to $2200.36 However, in our systematic review, we found only 1 study that discussed the cost of CTH studies.18
Computed tomographic studies are associated with significant radiation exposure and cancer risk. A single CT study’s mean radiation dose is typically 15 mSv in adults and 30 mSv in neonates. A significant overall risk of cancer was found in nuclear bomb survivors who had radiation exposure ranging from 5 to 150 mSv (mean, 40 mSv). This radiation dose is equivalent to the relevant organ dose received with 2 or 3 scans in an adult from a typical CT study.2 For CTH, even with newer CT machines, radiation ranges from 1 to 10 mSv per CTH in an adult,4 which means a person who received 3 to 4 CT studies in a lifetime has a similar risk of cancer as those who survived nuclear bombs in Japan. Although the cancer risk estimates calculated from a single CT study appear to be highest at the age when the CT was performed and decrease after that,2 so far, no clinical study has compared the lifetime vs short-period risks of cancer pertaining to CT studies. The linear no-threshold (LNT) concept advocates that even a small dose of radiation increases genetic alterations, which increases cancer risks.37 Some animal, cellular, and molecular studies demonstrated that the responses at the cellular level after repeated low-dose exposures were less pronounced than the total dose delivered at a high dose.38 Recently, there has been debate around the LNT concept because some epidemiologic studies showed that radiation doses less than 100 mSv may not be enough to induce carcinogenesis.38,39 The LNT model is criticized by some for not being biologically plausible, overestimating radiation risks, and preventing necessary imaging, causing more harm than benefit.37,38,39 Some even advocate for low-dose radiation for its presumed benefit, known as the radiation hormesis concept.37 Regardless of the criticism, the LNT model has been adopted by national and international advisory bodies and has guided radiation protection policies for decades.37 There is no argument regarding the wise use of imaging studies, such as CT, based on risk-benefit ratio assumptions.39
Health care professionals’ awareness regarding radiation hazards associated with CT studies is alarmingly low. In a survey study, 53% of radiologists and 91% of ED physicians did not believe that CT studies can increase the lifetime risk of cancer.6 Moreover, another study suggested that approximately one-third of the CT studies could be replaced by alternative approaches or no study at all.40 Given the risk of radiation hazards and the added cost of care, clinicians should use CTH judiciously. Prospective studies involving a larger cohort of patients with acute change in mental status are required to develop clinical risk stratification tools to facilitate rational and judicious use of CTH. Our results align with the recommendations of the Choosing Wisely and Image Wisely campaigns that advocate against the overuse of CTH.41 In addition, professional and societal bodies should use awareness campaigns considering the limited awareness among health care professionals regarding radiation hazards related to CT studies.
Limitations
Our study had some limitations. The first is heterogeneity. The studies included had a wide variation in sample size and settings, which introduced significant heterogeneity in the study. However, this variation is a common occurrence with meta-analyses for incidence or event rate. Second, in the outcome analysis, we could not report the usefulness of positive CTH results. Only 4 studies discussed the diagnoses of positive CTH findings, and none of the studies explicitly discussed the change of management from the CTH results. Third, although the quality of the studies was moderate to high, the studies had low levels of evidence. Fourth, we had a significantly asymmetric funnel plot that explains the study’s publication bias, but we suspect this asymmetry is partly attributable to heterogeneity.42 Furthermore, the sensitivity analysis supported the reliability of the results. Regardless of these limitations, this is the first systematic review and meta-analysis, to our knowledge, that investigated the use of noncontrast CTH among patients with acute atraumatic AMS.
Conclusions
This meta-analysis found that the use of noncontrast CTH in patients with acute-onset atraumatic AMS is high, with a low yield for a positive result. Computed tomographic studies are associated with significant radiation exposure. Future large-scale studies are required to provide more reliable estimates for the diagnostic yield of CTH among patients with AMS. Clinicians should exercise caution and use their clinical judgment to minimize the indiscriminate use of CTH in the evaluation of patients with AMS.
eTable 1. Database Search Strategy
eTable 2. The Joanna Briggs Institute’s (JBI) Critical Appraisal Checklist for Case-Control Study
eTable 3. The Joanna Briggs Institute’s (JBI) Critical Appraisal Checklist for Cohort Study
eTable 4. The Joanna Briggs Institute’s (JBI) Critical Appraisal Checklist for Quasi-Experimental Study
eFigure 1. The Proportion of Computed Tomography of the Head (CTH) in Patients With Altered Mental Status (AMS) Among Studies
eFigure 2. Funnel Plot Showing the Asymmetric Distribution of Studies Suggesting Significant Publication Bias for Computed Tomography of the Head (CTH) Events
eFigure 3. Funnel Plot Showing the Asymmetric Distribution of Studies Suggesting Significant Publication Bias for Positive Computed Tomography of the Head (CTH) Events
eFigure 4. Sensitivity Analysis of Studies for Computed Tomography of the Head (CTH) Events
eFigure 5. Sensitivity Analysis of Studies for Positive Computed Tomography of the Head (CTH) Events
References
- 1.Hess EP, Haas LR, Shah ND, Stroebel RJ, Denham CR, Swensen SJ. Trends in computed tomography utilization rates: a longitudinal practice-based study. J Patient Saf. 2014;10(1):52-58. doi: 10.1097/PTS.0b013e3182948b1a [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 3.Hendee WR, Becker GJ, Borgstede JP, et al. Addressing overutilization in medical imaging. Radiology. 2010;257(1):240-245. doi: 10.1148/radiol.10100063 [DOI] [PubMed] [Google Scholar]
- 4.Luttrull MD, Boulter DJ, Kirsch CFE, et al. ; Expert Panel on Neurological Imaging . ACR Appropriateness Criteria® Acute Mental Status Change, Delirium, and New Onset Psychosis. J Am Coll Radiol. 2019;16(5S):S26-S37. doi: 10.1016/j.jacr.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 5.Tu LH, Venkatesh AK, Malhotra A, et al. Scenarios to improve CT head utilization in the emergency department delineated by critical results reporting. Emerg Radiol. 2022;29(1):81-88. doi: 10.1007/s10140-021-01947-w [DOI] [PubMed] [Google Scholar]
- 6.Lee CI, Haims AH, Monico EP, Brink JA, Forman HP. Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology. 2004;231(2):393-398. doi: 10.1148/radiol.2312030767 [DOI] [PubMed] [Google Scholar]
- 7.PRISMA . PRISMA Checklist. Accessed March 6, 2022. http://www.prisma-statement.org/PRISMAStatement/Checklist.aspx
- 8.Joanna Briggs Institute. Critical Appraisal Tools. Accessed March 6, 2022. https://jbi.global/critical-appraisal-tools
- 9.Zhang Q, Mu MC, He Y, Cai ZL, Li ZC. Burnout in emergency medicine physicians: a meta-analysis and systematic review. Medicine (Baltimore). 2020;99(32):e21462. doi: 10.1097/MD.0000000000021462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bent C, Lee PS, Shen PY, Bang H, Bobinski M. Clinical scoring system may improve yield of head CT of non-trauma emergency department patients. Emerg Radiol. 2015;22(5):511-516. doi: 10.1007/s10140-015-1305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callen AL, Chow DS, Chen YA, et al. Predictive value of noncontrast head CT with negative findings in the emergency department setting. AJNR Am J Neuroradiol. 2020;41(2):213-218. doi: 10.3174/ajnr.A6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HY, Chaou CH, Chen CK, et al. Brain computed tomography in stimulant poisoning with altered consciousness. J Emerg Med. 2020;59(1):46-52. doi: 10.1016/j.jemermed.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 13.Covino M, Gilardi E, Manno A, et al. A new clinical score for cranial CT in ED non-trauma patients: definition and first validation. Am J Emerg Med. 2019;37(7):1279-1284. doi: 10.1016/j.ajem.2018.09.032 [DOI] [PubMed] [Google Scholar]
- 14.Nesselroth D, Klang E, Soffer S, et al. Yield of head CT for acute findings in patients presenting to the emergency department. Clin Imaging. 2021;73:1-5. doi: 10.1016/j.clinimag.2020.11.025 [DOI] [PubMed] [Google Scholar]
- 15.Patel RK, Choubey AK, Soni BK, Sivasankar R, Chauhan V. Pattern of emergent head computed tomography findings in a tertiary care hospital during off working hours: retrospective analysis. J Neurosci Rural Pract. 2019;10(2):207-211. doi: 10.4103/jnrp.jnrp_362_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segard J, Montassier E, Trewick D, Le Conte P, Guillon B, Berrut G. Urgent computed tomography brain scan for elderly patients: can we improve its diagnostic yield? Eur J Emerg Med. 2013;20(1):51-53. doi: 10.1097/MEJ.0b013e32834f9d51 [DOI] [PubMed] [Google Scholar]
- 17.Sinclair DE, Kovacs G, Hillis M. Cranial CT scans–emergency department utilization. J Emerg Med. 1993;11(5):643-646. doi: 10.1016/0736-4679(93)90325-2 [DOI] [PubMed] [Google Scholar]
- 18.Shuaib W, Tiwana MH, Chokshi FH, Johnson JO, Bedi H, Khosa F. Utility of CT head in the acute setting: value of contrast and non-contrast studies. Ir J Med Sci. 2015;184(3):631-635. doi: 10.1007/s11845-014-1191-3 [DOI] [PubMed] [Google Scholar]
- 19.Wang X, You JJ. Head CT for nontrauma patients in the emergency department: clinical predictors of abnormal findings. Radiology. 2013;266(3):783-790. doi: 10.1148/radiol.12120732 [DOI] [PubMed] [Google Scholar]
- 20.Chokshi FH, Sadigh G, Carpenter W, Kang J, Duszak R Jr, Khosa F. Altered mental status in ICU patients: diagnostic yield of noncontrast head CT for abnormal and communicable findings. Crit Care Med. 2016;44(12):e1180-e1185. doi: 10.1097/CCM.0000000000002005 [DOI] [PubMed] [Google Scholar]
- 21.Finkelmeier F, Walter S, Peiffer KH, et al. Diagnostic yield and outcomes of computed tomography of the head in critically ill nontrauma patients. J Intensive Care Med. 2019;34(11-12):955-966. doi: 10.1177/0885066617720901 [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Guerra C, Khandji A, Bauer RM, Claassen J, Wunsch H. Frequency of acute changes found on head computed tomographies in critically ill patients: a retrospective cohort study. J Crit Care. 2014;29(5):884.e7-884.e12. doi: 10.1016/j.jcrc.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai MMY, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x [DOI] [PubMed] [Google Scholar]
- 24.Thacker PJ, Sethi M, Sternlieb J, Schneider D, Naglak M, Patel RR. Rapid response: to scan or not to scan? the utility of noncontrast CT head for altered mental status. J Patient Saf. 2021;17(8):e1125-e1129. doi: 10.1097/PTS.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 25.Theisen-Toupal J, Breu AC, Mattison MLP, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198 [DOI] [PubMed] [Google Scholar]
- 26.Wong JC, Goyal N, McBride WC, Austin MS, Deirmengian GK. Head computed tomography is not useful for evaluating patients change in mental status following total joint arthroplasty. J Arthroplasty. 2014;29(6):1114-1118. doi: 10.1016/j.arth.2013.12.030 [DOI] [PubMed] [Google Scholar]
- 27.Detweiler MB, Lutgens BW, Choudhury D, et al. Association of renal clearance with cerebral white matter vascular disease in hospitalized veterans with and without delirium. South Med J. 2020;113(8):401-406. doi: 10.14423/SMJ.0000000000001132 [DOI] [PubMed] [Google Scholar]
- 28.Detweiler MB, Sherigar RM, Bader G, et al. Association of white matter lesions, cerebral atrophy, intracranial extravascular calcifications, and ventricular-communicating hydrocephalus with delirium among veterans. South Med J. 2017;110(6):432-439. doi: 10.14423/SMJ.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 29.Donovan LM, Kress WL, Strnad LC, et al. Low likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165-169. doi: 10.1016/j.cgh.2014.05.022 [DOI] [PubMed] [Google Scholar]
- 30.Hanna A, Gill I, Imam Z, Halalau A, Jamil LH. Low yield of head CT in cirrhotic patients presenting with hepatic encephalopathy. BMJ Open Gastroenterol. 2021;8(1):e000609. doi: 10.1136/bmjgast-2021-000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hufschmidt A, Shabarin V. Diagnostic yield of cerebral imaging in patients with acute confusion. Acta Neurol Scand. 2008;118(4):245-250. doi: 10.1111/j.1600-0404.2008.01006.x [DOI] [PubMed] [Google Scholar]
- 32.Lim BL, Lim GH, Heng WJ, Seow E. Clinical predictors of abnormal computed tomography findings in patients with altered mental status. Singapore Med J. 2009;50(9):885-888. [PubMed] [Google Scholar]
- 33.Patel MM, Tsutaoka BT, Banerji S, Blanc PD, Olson KR. ED utilization of computed tomography in a poisoned population. Am J Emerg Med. 2002;20(3):212-217. doi: 10.1053/ajem.2002.32632 [DOI] [PubMed] [Google Scholar]
- 34.Rahimi RS, Rockey DC. Overuse of head computed tomography in cirrhosis with altered mental status. Am J Med Sci. 2016;351(5):459-466. doi: 10.1016/j.amjms.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 35.Kocher KE, Meurer WJ, Desmond JS, Nallamothu BK. Effect of testing and treatment on emergency department length of stay using a national database. Acad Emerg Med. 2012;19(5):525-534. doi: 10.1111/j.1553-2712.2012.01353.x [DOI] [PubMed] [Google Scholar]
- 36.Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 37.Doss M. Are we approaching the end of the linear no-threshold era? J Nucl Med. 2018;59(12):1786-1793. doi: 10.2967/jnumed.118.217182 [DOI] [PubMed] [Google Scholar]
- 38.Health Physics Society . Radiation Risk in Perspective: Position Statement of the Health Physics Society. Health Physics Society; February 2019. Accessed October 12, 2022. http://hps.org/documents/radiationrisk.pdf
- 39.Siegel JA, Welsh JS. Does imaging technology cause cancer? debunking the linear no-threshold model of radiation carcinogenesis. Technol Cancer Res Treat. 2016;15(2):249-256. doi: 10.1177/1533034615578011 [DOI] [PubMed] [Google Scholar]
- 40.Panel discussion. Pediatr Radiol. 2002;32(4):242-244. doi: 10.1007/s00247-002-0674-y [DOI] [Google Scholar]
- 41.Choosing Wisely. Avoid head CT for asymptomatic adults with syncope. Published October 27, 2014. Accessed March 30, 2022. https://www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/
- 42.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Database Search Strategy
eTable 2. The Joanna Briggs Institute’s (JBI) Critical Appraisal Checklist for Case-Control Study
eTable 3. The Joanna Briggs Institute’s (JBI) Critical Appraisal Checklist for Cohort Study
eTable 4. The Joanna Briggs Institute’s (JBI) Critical Appraisal Checklist for Quasi-Experimental Study
eFigure 1. The Proportion of Computed Tomography of the Head (CTH) in Patients With Altered Mental Status (AMS) Among Studies
eFigure 2. Funnel Plot Showing the Asymmetric Distribution of Studies Suggesting Significant Publication Bias for Computed Tomography of the Head (CTH) Events
eFigure 3. Funnel Plot Showing the Asymmetric Distribution of Studies Suggesting Significant Publication Bias for Positive Computed Tomography of the Head (CTH) Events
eFigure 4. Sensitivity Analysis of Studies for Computed Tomography of the Head (CTH) Events
eFigure 5. Sensitivity Analysis of Studies for Positive Computed Tomography of the Head (CTH) Events