Abstract
In the last decade, research on acute respiratory distress syndrome (ARDS) has made considerable progress. However, ARDS remains a leading cause of mortality in the intensive care unit. ARDS presents distinct subphenotypes with different clinical and biological features. The pathophysiologic mechanisms of ARDS may contribute to the biological variability and partially explain why some pharmacologic therapies for ARDS have failed to improve patient outcomes. Therefore, identifying ARDS variability and heterogeneity might be a key strategy for finding effective treatments. Research involving studies on biomarkers and genomic, metabolomic, and proteomic technologies is increasing. These new approaches, which are dedicated to the identification and quantitative analysis of components from biological matrixes, may help differentiate between different types of damage and predict clinical outcome and risk. Omics technologies offer a new opportunity for the development of diagnostic tools and personalized therapy in ARDS. This narrative review assesses recent evidence regarding genomics, proteomics, and metabolomics in ARDS research.
Keywords: Omics, Proteomics, Genomics, Transcriptomics, Metabolomics, ARDS, Genotypes
Background
In the latest decade, research on acute respiratory distress syndrome (ARDS) has made considerable progress in understanding the pathophysiology of the disease, diagnostic criteria, biomarkers, and rescue therapies, but it remains a leading cause of mortality in the intensive care unit (ICU) [1]. Current therapies for ARDS are mainly supportive. The failure of pharmacologic therapies for ARDS has been explained by the clinical, pathophysiologic, and biological heterogeneity of this syndrome [2]. Research on the study of biomarkers and genomic, metabolomic, and proteomic technologies is increasing. These novel approaches, which are dedicated to the identification and quantitative analysis of components from biological matrixes, may help to differentiate between different types of damage and predict clinical outcome and risk [3, 4]. Omics technologies offer a new opportunity for the development of diagnostic tools and personalized therapy in ARDS [5]. Thus, this narrative review compiles the most recent findings regarding genomics, proteomics, transcriptomics, and metabolomics approaches in ARDS research.
Current therapies for ARDS
Increasing effort has been made to elucidate which treatments or supportive interventions can be used [6, 7]. Existing treatments for ARDS are mainly supportive [8]. Using the general definition of ARDS based on the Berlin criteria, randomized controlled trials (RCTs) have found some supportive treatment strategies that can be generalized for all patients with ARDS. In contrast, pharmacologic treatments and some possible supportive therapies may benefit from personalization; specific physiologic thresholds, clinical characteristics, biological or omics subphenotypes have been targeted to find treatable traits. Supportive treatments for ARDS include protective mechanical ventilation using a low tidal volume (4–6 mL/kg of predicted body weight), plateau pressure (< 28–30 cmH2O) [9, 10], low driving pressure (< 13–15 cmH2O), and individualized levels of positive end-expiratory pressure (PEEP) [7]. In the case of refractory hypoxemia, neuromuscular blocking agents, prone positioning, recruitment maneuvers, extracorporeal membrane oxygenation should be considered [7]. Several drugs that have been tested over the years failed to demonstrate potential efficacy [8]. Current therapies include neuromuscular blocking agents, sedatives, and analgesics. RCTs that have investigated pharmacologic interventions in ARDS have not shown consistent beneficial treatments with high potential for failed drug discovery [11]. Failure of clinical trials in ARDS can be attributed to the fact that the heterogeneity of this syndrome may have affected the results. Pharmacotherapies usually do not target a specific subpopulation of patients with ARDS. Trials design should account for proper selection of patients based on their biological and clinical characteristics. In this context, omics approaches may help to identify the correct subphenotypes of patients with ARDS who can benefit from a specific pharmacotherapy [8].
ARDS classification and phenotyping
ARDS is a syndrome that can be caused by various diseases. Over the years, experimental and clinical research has focused on identifying the causative factors of ARDS heterogeneity [2]. The increased interest in addressing ARDS heterogeneity led to several clinical studies that tried to identify subphenotypes of patients with ARDS according to clinical features (i.e., dead space fraction, PEEP, ventilatory ratio, driving pressure) or biological features (i.e., specific inflammatory and coagulative biomarkers) [12–16], the causes of ARDS (i.e., pulmonary vs. extrapulmonary, acute kidney injury vs. not, trauma vs. non-trauma) and time of ARDS diagnosis (before vs. 48 h after ICU admission) [17–19], as well as stratification by omics into genotypes, i.e., the genetic material that contributes to phenotypes [20]. According to ARDS subphenotypes, we define an endotype as a subtype of a disease condition that is characterized by a distinct pathophysiologic mechanism. ARDS subphenotypes may be associated with outcome and stratify patients at the bedside, thus selecting patients according to different therapeutic strategies. However, several concerns have been identified when ARDS was classified according to the different subphenotypes: (1) broad variation in the recruited population, (2) distinct and variable timing for the assessment of biomarkers, and (3) poor association between physiologic changes and validation of biomarkers [21].
Clinical classification of ARDS
Risk stratification of patients with ARDS started in 1967 when ARDS was described as a form of hypoxemic respiratory failure due to non-cardiogenic pulmonary edema with increased work of breathing and reduced compliance of the lungs [22]. In 1992, the American-European Consensus Conference developed the first consensus to define ARDS [23]. In 2012, another consensus conference in Berlin defined ARDS as a syndrome with an acute onset within 7 days of insult, and risk stratification was suggested by categorizing patients as mild, moderate, or severe according to the ratio of arterial oxygen tension (PaO2) to fraction of inspired oxygen (FiO2) (with PEEP of 5 cmH2O or more) at ARDS onset [24]. On the clinical side, ARDS can be classified as pulmonary or extrapulmonary, depending on the pathogenic pathway [25]. When a direct insult to the alveolar epithelium causes a local alveolar inflammatory response, ARDS is defined as pulmonary; an indirect insult that affects the vascular endothelium through the bloodstream causing inflammation is defined as extrapulmonary ARDS [26].
Histopathologic classification of ARDS
Diffuse alveolar damage (DAD) is considered to be the typical histologic pattern of ARDS, but only half of patients exhibit this morphologic hallmark [27]. Interstitial and alveolar edema, hyaline membrane, alveolar hemorrhage, neutrophil infiltration, fibrin deposition, and atelectasis are features of DAD; the latter may evolve into a fibroproliferative stage and fibrotic disease [22].
Radiologic classification of ARDS
Radiologic studies revealed different lung patterns (i.e., focal [lung areas of attenuation predominating in the lower lobes or gravitationally dependent parenchyma] or diffuse [lung areas of attenuation distributed diffusely across the lungs]) among patients with ARDS. A diffuse radiologic pattern is associated with worse outcome [28–30]. According to radiologic subphenotypes, the CESAR trial adopted a Murray Lung Score > 3 points in patients with ARDS under extracorporeal membrane oxygenation (ECMO) [31]. Similarly, the RALE score, which includes a radiologic evaluation of patients with ARDS, was associated with 28-day mortality [32]. A recent study (LIVE trial) compared a personalized mechanical ventilation strategy, selected according to radiologic subphenotypes, with a standard lung protective ventilation strategy and found better outcomes with the personalized strategy [33]. No difference in 90-day mortality was found between the personalized and control groups. When patients were reallocated according to the focal or non-focal nature of ARDS, a significant difference in mortality was found between the groups [33].
Biological phenotypes of ARDS
ARDS presents substantial heterogeneity with regard to biological biomarkers. Using stepwise modeling of latent class analysis to find phenotypes based on clinical data and plasma biomarkers, Famous et al. [34] confirmed the existence of 2 subphenotypes: one characterized by hyperinflammation and hypotension, and the other characterized by a hypoinflammatory status. These 2 subphenotypes demonstrated accuracy to identify which subpopulation of patients with ARDS can benefit from a conservative or liberal fluid strategy. This confirmed the existence of different subphenotypes among patients with ARDS, as reported in previous re-analysis of RCTs by Calfee et al. [35] and Sinha et al. [36]. Calfee et al. [35], using a latent class analysis with 8 plasma biomarkers [37], distinguished between a “hyperinflammatory” and a “hypoinflammatory” phenotype in patients with ARDS, whereas Bos et al. [15] identified an “uninflamed” and a “reactive” phenotype. However, inflammatory biomarkers are usually unspecific and may not be characteristic in ARDS [38]. In this context, biological biomarkers associated with endothelial damage (i.e., angiopoietin-2, intracellular adhesion molecule-1), epithelial cell damage (i.e., soluble receptor for advanced glycation and products, surfactant protein-D), inflammation (i.e., interleukin [IL], tumor necrosis factor-α [TNF-α]), and coagulation (i.e., protein C, plasminogen activator inhibitor-1, fibrinogen, D-dimer) have been described [39, 40] and associated with different subphenotypes of ARDS, which may partially explain why some pharmacologic therapies for ARDS have failed to improve patient outcomes [41]. Numerous genomic, proteomic, transcriptomic, and metabolomic markers have been studied to find subphenotypes of patients with ARDS who share important biological features with an impact on clinical outcome [39]. Several studies have confirmed the association between ARDS subphenotypes and different treatment responses or outcomes [34, 42]. Understanding the importance of ARDS subphenotypes and their impact on patient outcome is important to plan and conduct new research projects evaluating specific therapies.
Omics in ARDS research
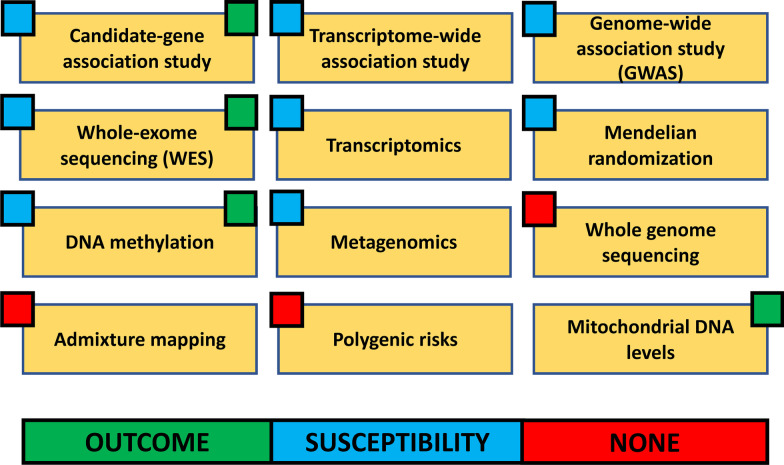
The identification of new disease-specific biomarkers is a leading approach to current research design and goals for ARDS. With the lack of effective pharmacologic therapy and high disability and mortality, advances in ARDS research have been focusing on promising technologies such as genomics, transcriptomics, proteomics, and metabolomics. Genomics refers to the ensemble of genes; transcriptomics refers to the study of ribonucleic acid molecules within a sample, providing a link between genomics and proteomics; proteomics refers to the proteins translated in an organism; and metabolomics refers to the small molecules (metabolites) identified within a biological sample [43]. Therefore, we conducted a systematic search on 4 databases (PubMed, EMBASE, Scopus, and Cochrane) up to 1 September 2022 to identify studies regarding omics approaches in ARDS research and clinical implications to present make this narrative review of the literature as comprehensive as possible. The main omics approaches applicable to ARDS and the phenotypes assessed (outcome, susceptibility, none) are reported in Fig. 1.
Fig. 1.
Main omics approaches applicable to ARDS (outcome, susceptibility, none)
Genomics
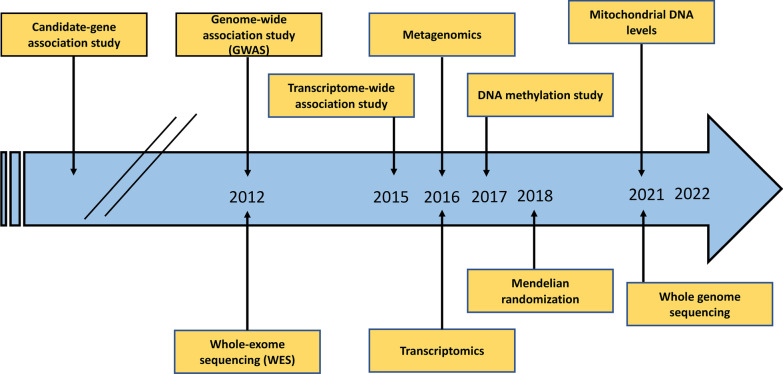
ARDS is a complex disease that activates various biological patterns that can be detected using biomarkers of lung injury [44]. Genomics is the study of genes and genetic variants of a condition, including interactions of genes with each other and with the environment [45]. Genomics has led to advances in knowledge of human disease, identifying novel pathways and genetic variants associated with human pathologic conditions. The objective of genomic technologies is to identify ARDS hyperinflammatory subphenotypes with higher risk for death or susceptibility. Many genes have limited value for risk prediction, although their aggregated impact on lung injury phenotypes in ARDS pathology is interesting and promising [44]. Since 2000, the genes that are associated with ARDS have been identified through different approaches, including a candidate gene approach [46–49], micro-array analysis [47, 50–52], whole-genome genotyping [53], and whole-exome sequencing [54, 55]. The first candidate gene study on ARDS dates to 1992 (on angiotensin-converting enzyme [ACE] polymorphism) [56], the first candidate gene study validation was developed in 2000 and 2002 [57, 58], the first gene and genome-wide association study (GWAS) was developed in 2012 [53], and the first next-generation sequencing dates to 2014 [54]. Since then, genomic research has made progress, targeting the cellular and molecular mechanisms of ARDS. Particularly, genomic research in ARDS has focused on the identification of genes that might be modulated for prevention and treatment of ARDS, targeting alveolar-capillary barrier dysfunction, alveolar fluid clearance dysfunction, and systemic inflammation [59]. Shortt et al. [54] identified a novel single nucleotide polymorphism (i.e., the presence of genetic variation within a population) associated with ARDS by exome sequencing as a potential novel biomarker in ARDS research. GWAS is the current genetic approach used in ARDS research. The evolution of genomic research over time is presented in Fig. 2.
Fig. 2.
Update on genomic research in ARDS up to 2022.
Modified from Hernandez-Beeftnik T, Guillen-Guio B, Villar J, Flores C. Genomics and the acute respiratory distress syndrome: current and future directions. Mol Sci. 2019;20(16):4004
The first GWAS study was developed with the aim of identifying risk variants for ARDS. This study identified the gene PTPRF interacting protein alpha 1 [PPFIA1] as a potential functional candidate for future research on ARDS in major trauma [53]. GWAS technology may help to predict ARDS risk and susceptibility. GWAS genetic variants were tested preferentially in white people, and only one study reported GWAS on African Americans [60]. A large GWAS was conducted on both Europeans and African Americans and reported that a novel locus within the gene BLOC-1 related complex subunit-5 [BORCS5] was a predictor of ARDS susceptibility in Europeans [61]. One of the genes more strongly associated with mortality in ARDS was the FER gene called rs4957796, which was strongly associated with 28-day survival in patients with sepsis and pneumonia [62]. Associations between FER genetic variants and mortality in ARDS have been confirmed by further studies [62, 63]. The main genes identified for the prediction of susceptibility and outcome in ARDS according to the pathophysiologic mechanism of ARDS are presented in Table 1 [45, 57, 58, 60–101].
Table 1.
The main genes identified for the prediction of susceptibility and outcome in ARDS according to the pathophysiologic mechanism
| Mechanism of ARDS | Gene |
|---|---|
| Vascular permeability [60, 61, 64–74, 99–101] | MAP3K1, FLT1, ANGPT2, AGT, EPAS1, HSPG2, KLK2, MAP3K1, MAP3K6, MYLK, NAMPT, SELPLG, S1PR3, VEGFA |
| Immune response [57, 75–88]] | IL17, DEFB1, FER, AGER, sRAGE, CHIT1, FKB1, IL1B, IL1RN, IL4, IL6, IL10, IL13, IL17, IL18, IL32, IRAK3, LTA, MBL2, MIF, NFKBIA, PDE48, PI3, SELPLG, SFTPA1, SFTPA2, SFTPD, STAT1, TIRAP, TLR1, TNF, TNFRSF11A, TRAF6 |
| Oxidative stress [89–93] | EGLN1, FTL, HIF, HIF2a, HMOX1, HMOX2, NFE2L2, NQO1, SOD3 |
| Epithelial injury [58] | SP-A, SP-B |
| Apoptosis | FAS |
| Chemotaxis | CXCL2, CXCR2, DARC, ISG15 |
| Fibrosis [94] | HAS1, MUC5B, SERPINE1 |
| Cell growth [95–97] | AQP5, ADGRV1, BCL11A, EGF, FZD2, GHR, POPDC3, TGFB2 |
| Coagulation [98] | F5, GP5, LRRC16A, PLAU, VWF |
| Metabolism [45] | ADA, ADIPOQ, AHR, APOA1, CBS, CYP1A1, DIO2, FAAH, PPARGC1A, PRKAG2, UGT2B7, VLDLR |
| Other mechanisms [45] | ABCC1, ADRBK2, CLASRP, GADD45A, GRM3, HTR2A |
ACE angiotensin-converting enzyme, ADMR2 adrenomeddulin-2, AGER advanced glycosylation end-product specific receptor, ANGPT2 angiopoietin-2, AQP1 acquaporin-1, ARL3 ADP ribosylation factor like GTPase, ARSD arylsulfatase-D, BTG1 antiproliferation factor-1, CCL2 chemokines ligand-2, CD cluster differentiation, CEBPA CCAAT enhancer binding protein alpha, CLEC4E C-type lectin like domain 4e, COX cyclooxygenase, CREBPZ pancreatic beta cell-specific gene, CXCR chemokine receptor, DEFB1 defensin beta-1, DIO1 iodothyronine deiodinase-1, EGF epidermal growth factor, EGLN prolyl hydroxylase encoding gene, F3 tissue factor precursor, FAAH fatty acid amide hydrolase, FAS Fas cell surface death receptor, FER FER tyrosine kinase, FGA fibrinogen A, FTH ferritin heavy chain, FTL ferritin light chain, GADD45A growth arrest and DNA damage-inducible-45, GJA gap junction alpha, HCAR constitutive androstane receptor, HMOX heme oxygenase-1, IL interleukin, IRAK interleukin-1 receptor associated kinases, ITGB1 integrin beta-1, KXR X-linked Kx, LCN2 lipocain-2, LRRC leucine-rich repeat containing, LTA lymphotoxin alpha, MAP mitogen-activated protein kinase-3, MBL mannose-binding lectin, MIF macrophage migration inhibitory factor, MME membrane metalloendopeptidase, MUC5B mucin5B, MYLK myosin light chain kinase, NAMPT nicotinamide phosphoribosyl transferase, NFKB nuclear factor kappa-B, NPEPL aminopeptidase-like, NQO1 NADPH dehydrogenase (quinone), NRF2 nuclear factor erythroid-2 related factor 2, OLFM olfactomedin-1, PI3 peptidase inhibitor-3, PLAUR urokinase plasminogen activator receptor, PNPLA patatin-like phospholipase domain-containing protein-3, POPDC3 Popeye domain-containing protein-3, PPFIA protein tyrosine phosphatase, RBP7 retinol binding protein-7, SERPINE serine protease inhibitor-1, SFTPB surfactant protein-B, SOC suppressor of cytokine signaling, SOD3 superoxide dismutase, TEK tyrosine kinase receptor, TFF trefoil factor family, THBS1 thrombospondin-1, TIRAP TIR domain-containing adaptor protein, TLR toll-like receptor, TNF tumor necrosis factor, VEGF vascular endothelial growth factor
Although modulation of genes could alleviate certain symptoms of ARDS, a single gene or combination of genes responsible for ARDS has not been identified yet in experimental animal research or in human studies [59]. Genomic research has great potential to elucidate ARDS pathways by identifying genetic associations and biomarkers, but ARDS is a challenging condition that may limit genomic research for various reasons: (1) ARDS is a syndrome that is a consequence of other pathologic conditions such as sepsis, trauma, or pneumonia, (2) ARDS is a syndrome that is a consequence of other pathologic conditions such as sepsis, trauma, or pneumonia, (3) ARDS lacks specific diagnostic tests and is often underrecognized, (4) blood samples for genomic research in ARDS may not reflect the expression pattern of lung endothelium or epithelium because gene expression is tissue specific, and only 10% of patients with ARDS undergo lung biopsy [102]; and (5) the epigenetic influence on disease susceptibility and outcome. This latter point is of particular interest because epigenetic changes in ARDS, probably induced by environmental interactions such as mechanical ventilation or infection, may contribute to modifications in gene expression, function, or activity without changing deoxyribonucleic sequences [103]. Genomics approaches need to be implemented in daily clinical practice to allow better understanding of ARDS and therapies and to design new clinical trials, offering a possible re-assessment of certain drugs that failed to provide benefits when administered indiscriminately to all patients with ARDS [104]. The COVID-19 pandemic has been a unique challenge and the global effort during the pandemic has led to and reinforced collaborations. This incredible effort to find effective therapeutics, also including genomics solutions, should be reconsidered within the context of ARDS research [105].
Transcriptomics
The cellular process of transcription produces ribonucleic acids (RNAs) that are based on the genomic template. The human genome is composed of approximately 21,000 protein-coding genes and several noncoding RNA genes [106]. The proteins are assembled through the process of transcription whereby RNAs are processed and spliced into mature forms. The messenger RNA (mRNA) transcripts and codes regions that promote the translation of proteins. Further, transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), short interfering RNA (siRNAs), micro-RNA (miRNAs), long noncoding RNA, and pseudogenes are involved in several cellular activities. Different from DNA-based analysis, transcriptomics allows the study of the passage of information through cell lineages, using RNA both as carrier and catalytic [107]. RNAs have tissue-specific patterns of expression that are specific and time dependent. With post-transcriptional regulation and degradation, RNA and protein expression are not always correlated. Therefore, the transcriptome includes RNAs, protein-coding, non-protein-coding, alternatively spliced, polyadenylate, initiated, sense, antisense, and RNA-edited transcripts.
The advantage of transcriptomics is that the genome-wide investigation offers a global picture instead of giving excessive importance to a single candidate gene [107]. We provide a brief overview of the current advances in transcriptomics for ARDS, specifically focusing on the most investigated techniques (miRNA and mRNA).
miRNA
Much of the evidence regarding miRNA identification in ARDS comes from preclinical studies. MiRNAs are involved in several pathophysiologic processes, including regulation of cell proliferation, differentiation, apoptosis, metabolism, and the immune response [108]. Some transcriptomics analyses of miRNAs in preclinical models revealed that inflammation during ARDS promotes macrophage proliferation and inflammation, also of the lung [109–112]. With regard to clinical evidence in humans, some miRNAs have been proposed as biomarkers of the pathophysiology, risk, and mortality in ARDS [113, 114]. Blood leukocytes of patients with ARDS showed increased expression of miRNA, and steroid therapy had no effect on the miRNA identified in patients with ARDS [115]. The clinical significance of miRNA up-/downregulation [116–127] in humans is presented in Table 2.
Table 2.
Clinical significance of miRNA up-/downregulation in humans with ARDS
| References | Micro-RNA | Model | Effect | Clinical significance |
|---|---|---|---|---|
| Lee et al. [123] | miR-106a-5p, miR-17-5p, miR-29a-3 | All-cause ARDS | Upregulated | Prediction of mortality |
| miR-126-3p, miR-191-5p, miR-223-3p | Downregulated | |||
| Wang et al. [116] | miR-103, miR-107 | Sepsis-associated ARDS | Downregulated | Increased risk of ARDS and 28-day mortality |
| Xu et al. [117] | miR-92 | Sepsis-associated ARDS | Upregulated | Risk of ARDS |
| Wang et al. [120] | miR155 | Sepsis-associated ARDS | Upregulated | Association with inflammatory lung injury |
| Wu et al. [121] | miR-27a, miR-126, miR-146a, miR-155 | Pneumonia-associated ALI | Upregulated | Risk of ARDS |
| Rahmel et al. [124] | miR-122 | All-cause ARDS | Upregulated | Association with acute liver injury and ARDS |
| Li et al. [125] | miR-140 | ALI except sepsis | Downregulated | Association with ALI and inflammation |
| Zhu et al. [126] | miR-181a, miR-92a | All-cause ARDS | Upregulated | Risk of ARDS |
| miR-424 | Downregulated | |||
| Goodwin et al. [122] | miR-887-3p | Sepsis-associated ARDS | Upregulated | Risk of ARDS |
| Lu et al. [127] | miR-22-3p, 1260b, 762, 23b, 23a | AP-associated ARDS | Upregulation | Risk of pancreatitis and ARDS |
| has-miR-550a, 324-5p, 484, 331-3p, 22-3p, 140-3p, 342-3p | Downregulated | |||
| Shi et al. [118] | miR-127 | AP-associated ALI | Upregulated | Association with acute pancreatitis and ARDS |
| miR-199a | Sepsis-associated ARDS | Downregulated | Risk of ARDS | |
| Zhu et al. [119] | miR-628-3p, miR-766, miR-922, miR-7, miR-194 | All-cause ARDS | Upregulated | Increased 28-day mortality |
miR micro-RNA, ARDS acute respiratory distress syndrome, AP acute pancreatitis
Therapeutic strategies for ARDS by modulating the transcription of genes have been proposed in the preclinical setting. Through regulation of the miR-423-5p/FOXA1 axis, silencing long noncoding RNA H19 alleviates pulmonary injury, inflammation, and fibrosis in a rat model of ARDS [128]. In rats, metformin relieved ARDS by reducing miR-138 expression [129], and mesenchymal stromal cells modulated the response in an experimental sepsis-ARDS model in rats by regulating the expression of miR-27a-5p [130]. However, the application of these therapies in the clinical setting is still limited. A limited number of studies on miRNAs have been published to date, although they found a clear association with the occurrence of inflammation in ARDS by affecting macrophages and other inflammatory pathways [131].
mRNA
Preclinical studies showed interesting results with mRNA technology in ARDS models. A study in mice identified that increased mRNA and protein expression of ELAVL-1/HuR and GSK3β was associated with difficult resolution of ARDS [132]. In another experimental murine model, heme oxygenase-1 mRNA and protein expression were increased in mice that developed malaria-associated ARDS [133]. In another mouse model of ARDS, several mRNAs were hypo-expressed, including METTL16, FTO, METTL3, KIAA1429, RBM15, ALKBH5, YTHDF2, YTHDF3, YTHDC2, and IGFBP2, and were associated with m6A methylation caused by administration of lipopolysaccharide, which was involved in the regulation of inflammation and the development of lung injury [134]. From these preclinical models, it seems clear that mRNA might be modulated to reduce inflammation in ARDS. Some therapeutic agents have also been investigated for modulating mRNA in experimental models of ARDS. Photobiomodulation of human mesenchymal stromal cells, and suppression of incRNA HOTAIR can interfere with the inflammatory response [135–137]. Andrographolide sulfonate treatment improves alveolar hypercoagulation and fibrinolytic inhibition and attenuates lung inflammation in an ARDS model in mice by inactivating the nuclear factor-κB pathway [138].
However, clinical evidence on mRNA technology in ARDS is still limited, although the results are interesting. The mRNA profile of patients with ARDS has been investigated by Ning et al. [139] who found 242 and 102 differently expressed genes in GSE32707 and GSE66890, respectively. Inflammasome-related mRNA transcripts (CASP1, IL1B, and IL18) were increased in peripheral blood of patients with sepsis- or trauma-induced ARDS [140]. The mRNA encoding proinflammatory cytokines MyD88 and IRAK1 in mononuclear cells of peripheral blood have been investigated in patients with ARDS and healthy controls; no proinflammatory alterations were found in patients with ARDS [141]. Comparing mRNA in 300 patients with ARDS and 300 controls, TNF-α rs1800629, IL-6 rs1800796, and MyD88 rs7744 SNPs were identified as markers of increased risk for ARDS and a poor prognosis [142]. N-Methyl-adenosine modification of Trim59 RNA was protective against the risk of developing ARDS during sepsis [143]. The mRNA levels of p300, CREB binding protein, tyrosine-protein kinase transmembrane receptor γt, and plasma concentration of IL-17, IL-6 were higher in patients with acute ARDS compared with controls, whereas p300/CBP expression was a risk factor for 28-day mortality [144]. All these studies, although at a preliminary stage of transcriptomics research in ARDS, demonstrate that investigations are advancing rapidly and could be a valuable support to identify biological phenotypes, thus helping to better understand the pathophysiology of ARDS and provide potential new targets for the development of effective RCTs on a specific subphenotype of patients with ARDS.
Proteomics
Proteomics is the analysis of the proteins translated in cells, tissues, and organisms at a specific time to investigate physiologic and disease conditions, molecular mechanisms, and diagnostic and prognostic biomarkers [145]. Proteomics can also identify and quantify post-translational protein modifications, localizations, activities and functions, and protein–protein interactions [146, 147]. Over the years, several proteomic technologies, together with advances in instrumentation, have been developed (e.g., shotgun two-dimensional high-performance liquid chromatography tandem mass spectrometry [148], matrix-assisted laser desorption/ionization time of flight mass spectrometry [149], and others) and have helped to quantify many proteins that were not detected with traditional methods. Proteomic research can be either targeted or untargeted, depending on multiple or single analysis of known proteins with potential for investigating disease progression or identifying biomarkers. Untargeted proteomics, also known as discovery proteomics, can be adopted to identify several proteins associated with a disease, detect several features in a single analysis, identify potential related biomarkers, and may allow large-scale studies. Despite these advantages, discovery proteomics has limit ability to quantify proteins compared with targeted proteomics, which provides higher sensitivity and accuracy for quantification of a predefined set of targeted proteins usually selected from previous discovery proteomics [150]. The advantages of targeted proteomics include the ability to select candidates to investigate their abundance in subtypes of a disease, higher analytical precision, although with a possible risk of limiting the response of interest, and limited knowledge of the protein of interest, resources, and sample size [146]. Proteome analysis in patients with ARDS can be run on various tissues and cell types, including plasma, lung tissue, lung cells, and bronchoalveolar lavage fluid (BALF) [43]. A list of proteomic studies in ARDS according to the sampling process is provided in Table 3.
Table 3.
Update on proteomic studies in ARDS
| Type of sample | References | Study aims | Number of patients | Proteomics analysis | Conclusions |
|---|---|---|---|---|---|
| Plasma | Chen et al. 2013 [151] | To examine the changes in serum protein expression in patients with ARDS | 11 patients and 15 controls | iTRAQ, MALDI-TOF, LC–MS/MS | Of the 132 proteins identified, 16 were expressed in patients with ARDS, of which 11 overlapped between the direct and indirect lung injury groups; 5 were specific to the indirect lung injury group |
| Liu et al. 2017 [152] | To examine the changes in serum protein expression in patients with ARDS undergoing ECMO | 51 patients | FC, ELISA, MS | The levels of IL10 were correlated with survival and delayed recovery | |
| Li et al. 2019 [153] | To examine the changes in serum protein expression in patients with ARDS | 5 patients and 5 healthy controls | LC–MS/MS | Of the 162 proteins identified, 128 were upregulated and 34 were downregulated in patients with ARDS | |
| Dong et al. 2021 [154] | To examine the changes in serum protein expression in patients with ARDS and association with 28-day mortality | 300 patients | SomaScan assay | IGFBP7 moderately increased ARDS 28-day mortality. The association between IGFBP7 and ARDS 28-day mortality seems to be mediated by the platelet count | |
| BALF | Bowler et al. 2004 [155] | To examine the protein profile of patients with mild ARDS and healthy volunteers | 16 patients and 12 healthy volunteers | 2D-PAGE, MALDI-TOF/MS | Of the 158 proteins identified, transferrin, IgG, clusterin, serum amyloid protein, hemopexin, IgG heavy chain, complement component 3, α2 or β‑hemoglobin, α2 or β2‑glycoprotein1, and α2‑Heremans‑Schmid‑glycoprotein were upregulated in patients with ARDS; SP-A and α1 antitrypsin were downregulated |
| de Torre et al. 2006[156] | To investigate the temporal changes of proteome in patients with ARDS and healthy volunteers | 11 patients and 33 healthy volunteers | 2D-PAGE, MALDI-TOF, SELDI-TOF | Apolipoprotein A1, S100 calcium-binding proteinsA8 and A9, AT3, transthyretin, hemoglobin-A chain-b were upregulated in ARDS | |
| Chang et al. 2008 [149] | To examine the changes in protein expression in patients with ARDS over time | 20 patients and 9 healthy volunteers | 2D-PAGE, MALDI-TOF/MS | 991 proteins were detected, of which those implicated in the immune response (complement C3, S100 calgranulin A9, fibrinogen alpha chain, α1 antitrypsin, apolipoprotein A1, and hemopexin) were overexpressed | |
| Schnapp et al. 2013[148] | To examine the changes in protein expression in patients with ARDS | 3 patients | 2D-HPLC, LC–MS/MS, Shotgun | 870 proteins were identified; albumin, ceruplasmin, fibrinogen α chain, α1 chymotrypsin, α2-HS-glycoprotein, antitrypsin inhibitor, IGFBP-3 were upregulated | |
| Nguyen et al. 2013[157] | To examine changes in proteins in patients with ARDS with and without VAP | 30 patients | 2D-HPLC, ESI–MS/MS | In patients with ARDS, 119 proteins were identified, of which 47 were downregulated; S100A8, lactotransferrin, and actinin 1 were upregulated in VAP-positive patients | |
| Bhargava et al. 2014[158] | To examine the changes in protein expression in early-phase survivors vs non-survivors and late phase survivors with ARDS | 24 patients | iTRAQ, 2D-HPLC, LC–MS/MS | Patients with ARDS showed predominance in coagulation and fibrinolysis proteins, immune responsive proteins, and proteins maintaining cation and iron homeostasis. On the other hand, early-phase non-survivors had more proteins of the carbohydrate catabolism | |
| Ren et al. 2016 [159] | To examine changes in proteins in patients with ARDS and pneumonia | 14 patients | iTRAQ, LC–MS/MS, 2D-HPLC, LC–MS/MS | DMBT1 can potentially serve as a biomarker for early ARDS diagnosis and disease severity assessment | |
| Bhargava et al. 2017 [160] | To examine changes in proteins in patients with ARDS | 36 patients | iTRAQ, 2D-HPLC, LC–MS/MS | 1115 high confidence proteins in BALF were identified, of which 142 were differentially expressed between survivors and non-survivors | |
| Lung cell | Dong et al. 2013 [161] | To examine changes in proteins in alveolar macrophages of patients with ARDS with and without VAP | 14 patients | 2D-PAGE, MALDI-TOF/MS | Between alveolar macrophages, 135 proteins were differentially expressed, of which 17 were characteristic of the recovery phase, and 10 of the exudative phase |
ARDS acute respiratory distress syndrome, AT antithrombin, BALF bronchoalveolar lavage fluid, DMBT1 deleted in malignant brain tumors 1 protein, ECMO extracorporeal membrane oxygenation, ESI electrospray ionization, IGFBP plasma insulin-like growth factor binding protein, IL interleukin, VAP ventilator-associated pneumonia, iTRAQ isobaric tags for relative and absolute quantitation, HPLC high-performance liquid chromatography, MS mass spectrometry, LC liquid chromatography, MALDI-TOF matrix-assisted laser desorption ionization time of flight, FC flow cytometry, ELISA enzyme-linked immunosorbent assay, SomaScan assay Slow Off-Rate Modified Aptamers Scan assay, SELDI-TOF surface-enhanced laser desorption/ionization time of flight
Plasma proteome
Human plasma has great potential for the identification of proteins that may have diagnostic and prognostic value in ARDS. The advantage of using human plasma is easier accessibility of sampling compared with lung tissue, which is difficult to obtain. The first untargeted proteomics assessment of patients with ARDS was performed in 2004 by Bowler et al. [155] on plasma, edema fluid, and BALF samples collected from patients with ARDS and healthy controls to identify the protein profile. In the acute phase of the disease, several proteins were identified, including albumin, serum amyloid protein, hemopexin, immunoglobulin (Ig)-G heavy chain, complement component 3, α2 or β‑hemoglobin, α2 or β2‑glycoprotein1, and α2‑Heremans‑Schmid‑glycoprotein [155]. Novel biomarkers for ARDS diagnosis/pathophysiology and treatment have been investigated by Chen et al. [151] by dividing the sample into 3 groups: direct lung injury, indirect lung injury, and control. Sixteen proteins were identified; the lung injury groups shared 11 proteins, and 5 proteins were specific to the indirect group. By finding different inflammatory pathways, this study was able to confirm a promising ability of proteomic strategies to provide the pattern of ARDS subphenotypes. In a recent analysis of plasma samples in patients with SARS-CoV-2-induced ARDS, 75% of the 368 proteins measured were significantly upregulated in moderate-severe COVID-19. Of interest, 6 proteins (IL-6, CKAP4, Gal-9, IL-1ra, LILRB4, and PD-L1) were associated with the severity of COVID-19 [162]. Li et al. [153] found 128 upregulated proteins and 34 downregulated proteins in patients with ARDS compared with healthy volunteers, allowing the possible identification of new biomarkers. The association between proteomic analysis and outcome has recently been investigated by Dong et al. [154] who observed that plasma insulin-like growth factor binding protein 7 (IGFBP7) increased ARDS 28-day mortality (odds ratio [OR], 1.11; 95% confidence interval [CI], 1.04–1.19; p = 0.002) and that the association between IGFBP7 and ARDS 28-day mortality seems to be mediated by the platelet count (OR, 1.03; 95% CI, 1.02–1.04; p = 0.01). Liu et al. [152] suggested that IL-10 can provide prognostic information on outcome in patients with ARDS undergoing ECMO. Other proteomic studies on blood samples in ARDS are reported in Table 3.
Bronchoalveolar lavage fluid proteome
The epithelial lining fluid and its proteins cover the airways and alveoli, whereas BALF represents the proteome of airways. Proteomic analysis of BALF revealed that several proteins are modified after lung injury [155]. Regarding the ARDS subphenotypes, a proteomic study investigating BALF in early (< 7 days) and late (> 8 days) survivors and non-survivors after ARDS concluded that a dynamic change of proteins occurred between the early and late timepoints and protein expression differed between survivors and non-survivors [158]. Bowler et al. [155] indicated that albumin, transferrin, IgG, clusterin, serum amyloid protein, α2 and β‐hemoglobin, α2 and β2‐glycoprotein1, α1‐antitrypsin, and α2‐Heremans‐Schmid‐glycoprotein were increased in the BALF of patients with ARDS, whereas SP-A was decreased. Similar protein expression was found by Schnapp et al. [148], including albumin, ceruloplasmin, fibrinogen α, α1 chymotrypsin, α2‐Heremans‐Schmid‐glycoprotein, insulin-like growth factor binding protein-3, and other proteins. Torre et al. [156] confirmed that patients with ARDS express several inflammatory biomarkers in BALF, including apolipoprotein A1 and S100. Chang et al. [149], for the first time, demonstrated a time-dependent modification of proteins during different inflammatory phases of ARDS. Bhargava et al. [158] found that ARDS survivors show a predominance of coagulation and fibrinolysis proteins, immune responsive proteins, and proteins maintaining cation and iron homeostasis. On the other hand, early-phase non-survivors had more proteins of carbohydrate catabolism. Nguyen et al. [157] investigated the BALF proteome of patients with ARDS and ventilator-associated pneumonia (VAP) compared with controls and found that S100A8, lactotransferrin, and actinin 1 are expressed in patients with VAP and ARDS but not in controls and patients without VAP. Yuan et al. [163] found that NADH-ubiquinone oxidoreductase chain 1 (ND-1) was overexpressed in patients with ARDS in comparison with healthy volunteers. Bhargava et al. [160] identified 142 proteins in patients with ARDS, including proteins implicated in injury, repair, and fibrosis such as coagulation/thrombosis, acute phase response, and complement activation, which differed between survivors and non-survivors. Ren et al. [159] found that the protein deleted in malignant brain tumors 1 (DMBT1), a protein implicated in cancer research, can potentially serve as a biomarker for an early ARDS diagnosis and assessment of disease severity. Factor VII activating protease (FSAP) was found to be increased in alveolar macrophages and bronchial epithelial and endothelial cells of lungs of patients with ARDS [164]. In addition, platelet-activating factor is a proinflammatory phospholipid that was found to be increased in the BALF of patients with ARDS, suggesting an alternative route to regulate inflammation [165].
Lung tissue proteome
Lung tissue samples are more difficult to obtain than serum and plasma samples, limiting their diagnostic and prognostic value [146]. Proteomics of the lung tissue are still based on in vivo experiments. In a rat ARDS model, overexpression of PRDX1 increased the release of IL-6, IL-8, and TNF-α [166]. Yen et al. [167] showed that, in a rat model, tidal volume was associated with the expression of complement/coagulation cascade proteins, and low end-expiratory lung volumes were associated with expression of mitochondrial respiratory chain protein. They concluded that that tidal stretch and lung collapse can activate different pathways. In a recent large animal study, proteomics of lung tissue revealed differences in inflammation and alveolar‑capillary barrier response between atelectasis and aerated regions. Atelectasis regions showed a predominance of negative enrichment related to the extracellular matrix, immune response, tissue development, stress, and metabolism [168]. In a mice model, Yue et al. [169] observed that the proteomic profile differs between direct lipopolysaccharide-induced lung injury and indirect lung injury. CXCL15 was upregulated in the indirect lung injury group, and liver X receptor/retinoid X receptor activation, nitric oxide expression, and reactive oxygen species in macrophages were activated by the direct injury group. Xu et al. [170] suggested 5152 proteins in lung tissues from oleic acid-treated and saline-treated mice, of which 545 were upregulated and 304 downregulated. Particularly, antithrombin III, 12-lipoxygenase, dedicator of cytokinesis 2, polycystin-2, and plasminogen are new potential biomarkers for ARDS induced by oleic acid. With the advent of the global COVID-19 pandemic, the use of proteomics approaches has made advances, providing further knowledge about the effects of infection. A study on biopsy samples from patients with ARDS induced by COVID-19 revealed that the lung underwent a huge alteration in proteins related to lung inflammation and coagulative dysregulation. In this study, other organs were investigated and showed significant protein alterations [171]. Similarly, Nie et al. [172] found 11,394 proteins in autopsy samples from patients with COVID-19, resulting in overexpression of cathepsin L1 in the lung tissue probably due to hyperinflammation, dysregulation of angiogenesis, coagulation, and fibrosis.
Lung cell proteome
As alternative to lung tissue proteome, lung cell proteome (i.e., alveolar macrophages, which represent the main defense of the airway) was collected and analyzed [173]. The role of alveolar macrophages in ARDS has been widely investigated and confirmed, showing that alveolar macrophages probably act as phagocytes for removing the infectious or toxic trigger from the airways [174]. Dong et al. identified 135 proteins, of which 27 were upregulated on alveolar macrophages in the exudative (17 proteins) and recovery (10 proteins) phases of ARDS, potentially serving as biomarkers [161]. No studies investigating alveolar macrophages during each phase of ARDS are currently available.
Metabolomics
Metabolomics refers to an emerging field targeting the study of a large set of metabolites within a single biological sample in a specific condition. Metabolomics allows detection of physiologic and pathologic changes in the concentration of metabolites using nuclear magnetic resonance spectroscopy, gas chromatography-mass spectrometry, or liquid chromatography-mass spectrometry (LC–MS) or incorporating more than one of these techniques. In contrast to other omics technologies such as proteomics and genomics, fewer metabolites are identified in humans compared with genes or proteins, and thus they are easier to access [175]. In addition, an advantage of metabolomics is that the molecules reflect the upregulation of a specific phase of a biological cascade, allowing eventual pathologic mechanisms to be detected in real time [176]. Metabolomics can be developed for a broad variety of biological samples, including BALF, exhaled breath condensate (EBC), and plasma/serum [21]. Plasma/serum sampling seems to be more suitable for the detection of pathologic metabolites both in pulmonary and extrapulmonary ARDS, whereas BALF can be more specific for identifying the changes in patients with pulmonary ARDS [21]. Problems with metabolomics technology include: (1) high dimensionality, which means that the metabolites are larger than the number of samples, (2) multicollinearity, meaning that metabolites from the same biological sample may be interconnected, (3) variability due to the analytical deviations of the technology that has been used, e.g., LC–MS [177]. Similar to proteomics, metabolomics can be targeted or untargeted. Targeted metabolomics refers to specific metabolites that belong to pathways of interest, and untargeted metabolomics refers to a concomitant measure of several metabolites from biological samples without a specific research question.
Exhaled breath condensate
Metabolomics research in ARDS started in 1998 with a study of 19 patients with ARDS and 18 ventilated controls analyzing the EBC to identify that isoprene is an ARDS-associated metabolite [178]. Many years later, Bos et al. [179] identified 3-methylheptane, octane, and acetaldehyde in the EBC of patients with ARDS. Singh et al. [180] found associations with N-acetyl glycoproteins, acetoacetate, lactate, creatinine, histidine, formate, and branched-chain amino acids, and Stringer et al. [181] confirmed the association with ARDS and phosphatidyl serine, total lipids, and total choline. Since the advent of the COVID-19 pandemic, a comparison of metabolomic signatures between patients with H1N1 and patients with COVID-19 with ARDS was performed. It was found that COVID-19 causes a significant deficit in energy supply that activates supplementary energy pathways. On the contrary, patients with H1N1 showed significantly marked inflammatory and oxidative stress responses [182]. A comparison of exhaled breath samples from patients with COVID-19 and non-COVID-19 ARDS revealed that those with COVID-19 present a specific metabolic profile, including volatile compounds methylpent-2-enal, 2,4-octadiene 1-chloroheptane, and nonanal [183].
Plasma metabolites
In 2011, Stringer et al. [181] examined the plasma of 13 patients with sepsis-induced lung injury and 6 healthy controls, finding that total glutathione, adenosine, phosphatidylserine, and sphingomyelin are metabolites associated with ARDS induced by sepsis. In 2019, the metabolomic profile of patients with H1N1 influenza was detected, revealing a strict association with the Sequential Organ Failure Assessment (SOFA) and the arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) of patients with ARDS [184]. The same research group also tested the metabolomic profile in patients with ARDS from other causes [185]. Lin et al. [186] identified 222 metabolites, of which 128 were altered in patients with ARDS in comparison with heathy controls. Phenylalanine, aspartic acid, and carbamic acid levels were significantly different between mild and severe ARDS groups, and ornithine, caprylic acid, azetidine, and iminodiacetic acid may potentially predict the severity of ARDS. Viswan et al. [187] identified biological endotypes of ARDS in 464 patients and controls, and found isoleucine, leucine, valine, lysine/arginine, tyrosine, threonine in BALF, and proline, glutamate, phenylalanine, valine in serum. The association of these biological endotypes with SOFA and APACHE II score produced a robust predictor of mortality for patients with ARDS. Xu et al. [188] investigated the metabolomic profile of 42 patients with ARDS and 28 healthy controls and found an increase in phenylalanine, D-phenylalanine, and phenylacetylglutamine in non-survivors compared with survivors of ARDS.
Bronchoalveolar lavage fluid
Rai et al. [189] compared the metabolome of the BALF of 21 patients with ARDS with 9 ventilated patients admitted to the ICU, finding an association with ARDS for BCA, arginine, glycine, aspartic acid, succinate, lactate, glutamate, ethanol, acetate, and proline. Again, on BALF, Evans et al. [190] suggested that guanosine, xanthine, hypoxanthine, lactate, and phosphatidylcholines are associated with ARDS in a comparison with healthy controls. In 2017, Rogers et al. [191] indicated 760 metabolites, of which 235 were significantly higher in patients with ARDS in comparison with those with hydrostatic pulmonary edema. Viswan et al. [192] proposed 6 biomarkers as signatures of ARDS, including proline, lysine/arginine, taurine, and threonine as signs of moderate/severe ARDS, and glutamate as a sign of mild ARDS. In addition, lung metabolism was found to be altered in patients with ARDS with acute kidney injury, suggesting a potential role of peripheral diseases in ARDS metabolic response [193].
Omics approaches in COVID-19 ARDS research
A multi-omics approach has been speeded up during the COVID-19 pandemic to find alternative treatments. In patients hospitalized with COVID-19, Ang-2, IL6, and MPO were associated with mortality, but without conclusive evidence of specificity for COVID-19. In addition, 207 differentially expressed miRNAs were found between survivors and non-survivors in the severe COVID-19 group, including miRNA pathways for platelet activation, extracellular matrix-receptor interactions, Ras, and ErbB2 [194]. Differently from non-COVID-19 ARDS, patients with COVID-19 showed better outcomes using corticosteroids. This can be explained by the fact that COVID-19 is a highly heterogenous disease with a known cause that may develop into ARDS, thus not so different from classic ARDS [195]. It seems that the combination of biomarkers can characterize the pathophysiologic responses in patients with COVID-19 or individualize management according to the biological phenotypes. Gustafson et al. [194] published a study, providing a clear example of how the incorporation of clinical data with omics should be identifying COVID-19 phenotypes and providing prognostic information. The authors confirmed that corticosteroids are useful in COVID-19 under inflammatory conditions, reinforcing the need for appropriate timing of administration and settings when designing clinical trials. However, as per omics studies, the sample size is limited, and there were several missing data. This highlights even more the need for collaborative networks and biobanks [105].
Future developments
Omics research seems promising in both preclinical and clinical settings. However, experimental models of ARDS cannot be easily translated into the clinical scenario and should be interpreted with caution. A genetic susceptibility to ARDS and its outcomes has been identified as a potential factor that can interact with the environment, affecting response to treatments, outcomes, and susceptibility to ARDS [196]. In addition, omics approaches are currently unavailable in most laboratories, treatment consequences are poorly known, and the costs are high. On the other hand, omics and biological markers may help better understand the disease, without the need to revise the definition of ARDS. Therefore, identifying subsets with similar biological features and integrating biological traits into ARDS classification may help in finding potential novel therapies [196]. Nevertheless, ARDS research based on omics approaches is still in its infancy. Several factors should be taken into account in implementing and including omics in clinical practice [197]. (1) The role of a collaborative biobank is pivotal. Biobanks of plasma and alveolar samples from patients with ARDS can allow researchers to obtain appropriate samples. To reach this milestone, collaborative networks are urgently needed. (2) Biological samples can be used to test the in vitro efficacy of certain therapies for reverse translational studies. (3) Biological samples should be collected as standard practice in RCTs on patients with ARDS to test and investigate treatments in subphenotypes of patients with ARDS [196]. (4) Biological factors that enrich the population should be measured in interventional studies. (5) The timing of assessment and ARDS evolution should always be recorded when managing biological samples. (6) Post-hoc subphenotypes analysis of RCTs should be implemented to identify biological markers of interest to translate into novel RCTs [196].
Conclusions
The heterogeneity of ARDS is the main obstacle to finding effective pharmacologic treatments. The identification of ARDS subphenotypes using omic technology offers a new opportunity for the development of diagnostic tools and personalized medicine in ARDS.
Acknowledgements
The authors acknowledge Mrs. Moira Elizabeth Schottler and Ms. Lorna O’Brien (authorserv.com) for their assistance in editing the manuscript.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- BALF
Bronchoalveolar lavage fluid
- BORCS5
BLOC-1 related complex subunit-5
- CI
Confidence interval
- DAD
Diffuse alveolar damage
- EBC
Exhaled breath condensate
- ECMO
Extracorporeal membrane oxygenation
- FiO2
Fraction of inspired oxygen
- GWAS
Genome-wide association study
- ICU
Intensive care unit
- Ig
Immunoglobulin
- IL
Interleukin
- LC–MS
Liquid chromatography-mass spectrometry
- miRNA
Micro-RNA
- mRNA
Messenger RNA
- OR
Odds ratio
- PaO2
Arterial oxygen tension
- PEEP
Positive end-expiratory pressure
- RCT
Randomized controlled trial
- rRNA
Ribosomal RNA
- siRNA
Short interfering RNA
- SOFA
Sequential Organ Failure Assessment
- TNF
Tumor necrosis factor
- tRNA
Transfer RNA
- VAP
Ventilator-associated pneumonia
Author contributions
DB and PR wrote the main manuscript text, prepared Figs. 1, 2, as well as Tables 1, 2, 3. LA, AGN, AFL, KF, MM, and PP wrote the main manuscript text. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Department of Science and Technology (DECIT)/Brazilian Ministry of Health, the Coordination for the Improvement of Higher Education Personnel (CAPES), and National Institute of Science and Technology for Regenerative Medicine.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JG, Calfee CS. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:102. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drohan CM, Nouraie SM, Bain W, Shah FA, Evankovich J, Zhang Y, et al. Biomarker-based classification of patients with acute respiratory failure into inflammatory subphenotypes: a single-center exploratory study. Crit Care Explor. 2021;3:e0518. doi: 10.1097/CCE.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battaglini D, Lopes-Pacheco M, Castro-Faria-Neto HC, Pelosi P, Rocco PRM. Laboratory biomarkers for diagnosis and prognosis in COVID-19. Front Immunol. 2022;13:857573. doi: 10.3389/fimmu.2022.857573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25:250. doi: 10.1186/s13054-021-03686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglini D, Sottano M, Ball L, Robba C, Rocco PRM, Pelosi P. Ten golden rules for individualized mechanical ventilation in acute respiratory distress syndrome. J Intensive Med. 2021;1:42–51. doi: 10.1016/j.jointm.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglini D, Rocco PRM, Pelosi P. New insights in mechanical ventilation and adjunctive therapies in ARDS. Signa Vitae. 2022;1–11.
- 8.Battaglini D, Robba C, Pelosi P, Rocco PRM. Treatment for acute respiratory distress syndrome in adults: a narrative review of phase 2 and 3 trials. Expert Opin Emerg Drugs. 2022;27:187–209. doi: 10.1080/14728214.2022.2105833. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6:e000420. doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tasaka S, Ohshimo S, Takeuchi M, Yasuda H, Ichikado K, Tsushima K, et al. ARDS clinical practice guideline 2021. J Intensive Care. 2022;10:32. doi: 10.1186/s40560-022-00615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villar J, Ferrando C, Tusman G, Berra L, Rodríguez-Suárez P, Suárez-Sipmann F. Unsuccessful and successful clinical trials in acute respiratory distress syndrome: addressing physiology-based gaps. Front Physiol. 2021;12:774025. doi: 10.3389/fphys.2021.774025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet J-F, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 13.Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199:333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 15.Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72:876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 19.Liao K-M, Chen C-W, Hsiue T-R, Lin W-C. Timing of acute respiratory distress syndrome onset is related to patient outcome. J Formos Med Assoc. 2009;108:694–703. doi: 10.1016/S0929-6646(09)60392-2. [DOI] [PubMed] [Google Scholar]
- 20.Gong MN. Genetic epidemiology of acute respiratory distress syndrome: implications for future prevention and treatment. Clin Chest Med. 2006;27:705–724. doi: 10.1016/j.ccm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metwaly S, Cote A, Donnelly SJ, Banoei MM, Mourad AI, Winston BW. Evolution of ARDS biomarkers: will metabolomics be the answer? Am J Physiol Cell Mol Physiol. 2018;315:L526–L534. doi: 10.1152/ajplung.00074.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashbaugh D, Boyd Bigelow D, Petty T, Levine B. Acute respiratory distress in adults. Lancet. 1967;290:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 23.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 24.Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi P, D’Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J. 2003;22(Suppl 42):48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 26.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Am J Respir Crit Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 27.Lorente JA, Cardinal-Fernández P, Muñoz D, Frutos-Vivar F, Thille AW, Jaramillo C, et al. Acute respiratory distress syndrome in patients with and without diffuse alveolar damage: an autopsy study. Intensive Care Med. 2015;41:1921–1930. doi: 10.1007/s00134-015-4046-0. [DOI] [PubMed] [Google Scholar]
- 28.Mrozek S, Jabaudon M, Jaber S, Paugam-Burtz C, Lefrant J-Y, Rouby J-J, et al. Elevated plasma levels of sRAGE are associated with nonfocal CT-based lung imaging in patients with ARDS. Chest. 2016;150:998–1007. doi: 10.1016/j.chest.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby J-J. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. Intensive Care Med. 2000;26:857–869. doi: 10.1007/s001340051274. [DOI] [PubMed] [Google Scholar]
- 30.Puybasset L, Gusman P, Muller J-C, Cluzel P, Coriat P, Rouby J-J, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. Intensive Care Med. 2000;26:1215–1227. doi: 10.1007/s001340051340. [DOI] [PubMed] [Google Scholar]
- 31.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 32.Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73:840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantin J-M, Jabaudon M, Lefrant J-Y, Jaber S, Quenot J-P, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7:870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 34.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinha P, Calfee CS, Cherian S, Brealey D, Cutler S, King C, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med. 2020;8:1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori M, Krumholz HM, Allore HG. Using latent class analysis to identify hidden clinical phenotypes. JAMA. 2020;324:700. doi: 10.1001/jama.2020.2278. [DOI] [PubMed] [Google Scholar]
- 38.Zador Z, Landry A, Balas M, Marshall JC, Cusimano MD. Data driven analysis reveals shared transcriptome response, immune cell composition, and distinct mortality rates across differing etiologies of critical illness. Crit Care Med. 2020;48:338–343. doi: 10.1097/CCM.0000000000004128. [DOI] [PubMed] [Google Scholar]
- 39.Walter JM, Wilson J, Ware LB. Biomarkers in acute respiratory distress syndrome: from pathobiology to improving patient care. Expert Rev Respir Med. 2014;8:573–586. doi: 10.1586/17476348.2014.924073. [DOI] [PubMed] [Google Scholar]
- 40.Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding heterogeneity in biologic phenotypes of acute respiratory distress syndrome by leukocyte expression profiles. Am J Respir Crit Care Med. 2019;200:42–50. doi: 10.1164/rccm.201809-1808OC. [DOI] [PubMed] [Google Scholar]
- 41.Santacruz CA, Pereira AJ, Celis E, Vincent J-L. Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit Care Med. 2019;47:1680–1691. doi: 10.1097/CCM.0000000000004000. [DOI] [PubMed] [Google Scholar]
- 42.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X-F, Dai H-P, Li Y-M, Xiao F, Wang C. Mass spectrometry-based proteomics in acute respiratory distress syndrome. Chin Med J (Engl) 2016;129:2357–2364. doi: 10.4103/0366-6999.190669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer NJ. Beyond single-nucleotide polymorphisms. Clin Chest Med. 2014;35:673–684. doi: 10.1016/j.ccm.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynn H, Sun X, Casanova N, Gonzales-Garay M, Bime C, Garcia JGN. Genomic and genetic approaches to deciphering acute respiratory distress syndrome risk and mortality. Antioxid Redox Signal. 2019;31:1027–1052. doi: 10.1089/ars.2018.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Cell Mol Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovach MA, Stringer KA, Bunting R, Wu X, San Mateo L, Newstead MW, et al. Microarray analysis identifies IL-1 receptor type 2 as a novel candidate biomarker in patients with acute respiratory distress syndrome. Respir Res. 2015;16:29. doi: 10.1186/s12931-015-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer N, Christie J. Genetic heterogeneity and risk of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2013;34:459–474. doi: 10.1055/s-0033-1351121. [DOI] [PubMed] [Google Scholar]
- 49.Brown SM, Grissom CK, Rondina MT, Hoidal JR, Scholand MB, Wolff RK, et al. Polymorphisms in key pulmonary inflammatory pathways and the development of acute respiratory distress syndrome. Exp Lung Res. 2015;41:155–162. doi: 10.3109/01902148.2014.983281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon BA, Easley RB, Grigoryev DN, Ma S-F, Ye SQ, Lavoie T, et al. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Cell Mol Physiol. 2006;291:L851–L861. doi: 10.1152/ajplung.00463.2005. [DOI] [PubMed] [Google Scholar]
- 51.Howrylak JA, Dolinay T, Lucht L, Wang Z, Christiani DC, Sethi JM, et al. Discovery of the gene signature for acute lung injury in patients with sepsis. Physiol Genomics. 2009;37:133–139. doi: 10.1152/physiolgenomics.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Beach D, Su L, Zhai R, Christiani DC. A genome-wide expression analysis in blood identifies pre-elafin as a biomarker in ARDS. Am J Respir Cell Mol Biol. 2008;38:724–732. doi: 10.1165/rcmb.2007-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christie JD, Wurfel MM, Feng R, O’Keefe GE, Bradfield J, Ware LB, et al. Genome wide association identifies PPFIA1 as a candidate gene for acute lung injury risk following major trauma. PLoS ONE. 2012;7:e28268. doi: 10.1371/journal.pone.0028268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shortt K, Chaudhary S, Grigoryev D, Heruth DP, Venkitachalam L, Zhang LQ, et al. Identification of novel single nucleotide polymorphisms associated with acute respiratory distress syndrome by exome-seq. PLoS ONE. 2014;9:e111953. doi: 10.1371/journal.pone.0111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wan Q-Q, Wu D, Ye Q-F. Candidate genes as biomarkers in lipopolysaccharide-induced acute respiratory distress syndrome based on mRNA expression profile by next-generation RNA-seq analysis. Biomed Res Int. 2018;2018:1–9. doi: 10.1155/2018/4384797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51:197–205. [PMC free article] [PubMed] [Google Scholar]
- 57.Marshall RP, Webb S, Hill MR, Humphries SE, Laurent GJ. Genetic polymorphisms associated with susceptibility and outcome in ARDS. Chest. 2002;121:68S–69S. doi: 10.1378/chest.121.3_suppl.68s. [DOI] [PubMed] [Google Scholar]
- 58.Lin Z, Pearson C, Chinchilli V, Pietschmann S, Luo J, Pison U, et al. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet. 2000;58:181–191. doi: 10.1034/j.1399-0004.2000.580305.x. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Dean DA. Gene therapy for acute respiratory distress syndrome. Front Physiol. 2022;12:Ahead of print. [DOI] [PMC free article] [PubMed]
- 60.Bime C, Pouladi N, Sammani S, Batai K, Casanova N, Zhou T, et al. Genome-wide association study in African Americans with acute respiratory distress syndrome identifies the selectin P ligand gene as a risk factor. Am J Respir Crit Care Med. 2018;197:1421–1432. doi: 10.1164/rccm.201705-0961OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du M, Garcia JGN, Christie JD, Xin J, Cai G, Meyer NJ, et al. Integrative omics provide biological and clinical insights into acute respiratory distress syndrome. Intensive Care Med. 2021;47:761–771. doi: 10.1007/s00134-021-06410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rautanen A, Mills TC, Gordon AC, Hutton P, Steffens M, Nuamah R, et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med. 2015;3:53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinz J, Büttner B, Kriesel F, Steinau M, Frederik Popov A, Ghadimi M, et al. The FER rs4957796 TT genotype is associated with unfavorable 90-day survival in Caucasian patients with severe ARDS due to pneumonia. Sci Rep. 2017;7:9887. doi: 10.1038/s41598-017-08540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, et al. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis. Crit Care Med. 2012;40:3034–3041. doi: 10.1097/CCM.0b013e31825fdc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.David S, Park J-K, van Meurs M, Zijlstra JG, Koenecke C, Schrimpf C, et al. Acute administration of recombinant Angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. Cytokine. 2011;55:251–259. doi: 10.1016/j.cyto.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Alfieri A, Watson JJ, Kammerer RA, Tasab M, Progias P, Reeves K, et al. Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis. Crit Care. 2012;16:R182. doi: 10.1186/cc11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medford ARL, Godinho SIH, Keen LJ, Bidwell JL, Millar AB. Relationship between vascular endothelial growth factor + 936 genotype and plasma/epithelial lining fluid vascular endothelial growth factor protein levels in patients with and at risk for ARDS. Chest. 2009;136:457–464. doi: 10.1378/chest.09-0383. [DOI] [PubMed] [Google Scholar]
- 68.Medford ARL. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medford ARL. Vascular endothelial growth factor gene polymorphism and acute respiratory distress syndrome. Thorax. 2005;60:244–248. doi: 10.1136/thx.2004.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, et al. ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med. 2011;183:1344–1353. doi: 10.1164/rccm.201005-0701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrell ED, O’Mahony DS, Glavan BJ, Harju-Baker S, Nguyen C, Gunderson S, et al. Genetic variation in MAP3K1 associates with ventilator-free days in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2018;58:117–125. doi: 10.1165/rcmb.2017-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szczepankiewicz A, Sobkowiak P, Rachel M, Bręborowicz A, Schoneich N, Bruce K, et al. Multilocus analysis of candidate genes involved in neurogenic inflammation in pediatric asthma and related phenotypes: a case–control study. J Asthma. 2012;49:329–335. doi: 10.3109/02770903.2012.669442. [DOI] [PubMed] [Google Scholar]
- 73.Nelson DS, Halteren A, Quispel WT, Bos C, Bovée JVMG, Patel B, et al. MAP2K1 and MAP3K1 mutations in langerhans cell histiocytosis. Genes Chromosomes Cancer. 2015;54:361–368. doi: 10.1002/gcc.22247. [DOI] [PubMed] [Google Scholar]
- 74.Glavan BJ, Holden TD, Goss CH, Black RA, Neff MJ, Nathens AB, et al. Genetic variation in the FAS gene and associations with acute lung injury. Am J Respir Crit Care Med. 2011;183:356–363. doi: 10.1164/rccm.201003-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer NJ, Daye ZJ, Rushefski M, Aplenc R, Lanken PN, Shashaty MG, et al. SNP-set analysis replicates acute lung injury genetic risk factors. BMC Med Genet. 2012;13:52. doi: 10.1186/1471-2350-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Mahony DS, Glavan BJ, Holden TD, Fong C, Black RA, Rona G, et al. Inflammation and immune-related candidate gene associations with acute lung injury susceptibility and severity: a validation study. PLoS ONE. 2012;7:e51104. doi: 10.1371/journal.pone.0051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flores C, Ma S-F, Maresso K, Wade MS, Villar J, Garcia JGN. IL6 gene-wide haplotype is associated with susceptibility to acute lung injury. Transl Res. 2008;152:11–17. doi: 10.1016/j.trsl.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Gong MN. Interleukin-10 polymorphism in position -1082 and acute respiratory distress syndrome. Eur Respir J. 2006;27:674–681. doi: 10.1183/09031936.06.00046405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones TK, Feng R, Kerchberger VE, Reilly JP, Anderson BJ, Shashaty MGS, et al. Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:47–56. doi: 10.1164/rccm.201810-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jabaudon M, Blondonnet R, Roszyk L, Bouvier D, Audard J, Clairefond G, et al. Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2015;192:191–199. doi: 10.1164/rccm.201501-0020OC. [DOI] [PubMed] [Google Scholar]
- 81.Jabaudon M, Berthelin P, Pranal T, Roszyk L, Godet T, Faure J-S, et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci Rep. 2018;8:2603. doi: 10.1038/s41598-018-20994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wurfel MM, Gordon AC, Holden TD, Radella F, Strout J, Kajikawa O, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med. 2008;178:710–720. doi: 10.1164/rccm.200803-462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pino-Yanes M, Ma S-F, Sun X, Tejera P, Corrales A, Blanco J, et al. Interleukin-1 receptor–associated kinase 3 gene associates with susceptibility to acute lung injury. Am J Respir Cell Mol Biol. 2011;45:740–745. doi: 10.1165/rcmb.2010-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pino-Yanes M, Corrales A, Casula M, Blanco J, Muriel A, Espinosa E, et al. Common variants of TLR1 associate with organ dysfunction and sustained pro-inflammatory responses during sepsis. PLoS ONE. 2010;5:e13759. doi: 10.1371/journal.pone.0013759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tejera P, O’Mahony DS, Owen CA, Wei Y, Wang Z, Gupta K, et al. Functional characterization of polymorphisms in the PI3 (elafin) gene and validation of their contribution to risk of ARDS. Am J Respir Cell Mol Biol. 2014;51:262–272. doi: 10.1165/rcmb.2013-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qi W. Absence of Fer protein tyrosine kinase exacerbates endotoxin induced intestinal epithelial barrier dysfunction in vivo. Gut. 2005;54:1091–1097. doi: 10.1136/gut.2004.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nickerson DA, et al. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am J Hum Genet. 2012;91:224–237. doi: 10.1016/j.ajhg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Battaglini D, Robba C, Fedele A, Trancǎ S, Sukkar SG, Di Pilato V, et al. The role of dysbiosis in critically ill patients with COVID-19 and acute respiratory distress syndrome. Front Med. 2021;8:671714. doi: 10.3389/fmed.2021.671714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirani N, Antonicelli F, Strieter RM, Wiesener MS, Ratcliffe PJ, Haslett C, et al. The regulation of interleukin-8 by hypoxia in human macrophages–a potential role in the pathogenesis of the acute respiratory distress syndrome (ARDS) Mol Med. 2001;7:685–697. [PMC free article] [PubMed] [Google Scholar]
- 90.Fan Q, Mao H, Xie L, Pi X. Prolyl hydroxylase domain-2 protein regulates lipopolysaccharide-induced vascular inflammation. Am J Pathol. 2019;189:200–213. doi: 10.1016/j.ajpath.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labrousse-Arias D, Castillo-González R, Rogers NM, Torres-Capelli M, Barreira B, Aragonés J, et al. HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc Res. 2016;109:115–130. doi: 10.1093/cvr/cvv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, et al. Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation. J Biol Chem. 2013;288:17134–17144. doi: 10.1074/jbc.M112.444059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dötsch A, Eisele L, Rabeling M, Rump K, Walstein K, Bick A, et al. Hypoxia inducible factor-2 alpha and prolinhydroxylase 2 polymorphisms in patients with acute respiratory distress syndrome (ARDS) Int J Mol Sci. 2017;18:1266. doi: 10.3390/ijms18061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rogers AJ, Solus JF, Hunninghake GM, Baron RM, Meyer NJ, Janz DR, et al. MUC5B promoter polymorphism and development of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;198:1342–1345. doi: 10.1164/rccm.201801-0140LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rump K, Unterberg M, Bergmann L, Bankfalvi A, Menon A, Schäfer S, et al. AQP5-1364A/C polymorphism and the AQP5 expression influence sepsis survival and immune cell migration: a prospective laboratory and patient study. J Transl Med. 2016;14:321. doi: 10.1186/s12967-016-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meli R, Pirozzi C, Pelagalli A. New perspectives on the potential role of aquaporins (AQPs) in the physiology of inflammation. Front Physiol. 2018;9:101. doi: 10.3389/fphys.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rahmel T, Rump K, Peters J, Adamzik M. Aquaporin 5–1364A/C promoter polymorphism is associated with pulmonary inflammation and survival in acute respiratory distress syndrome. Anesthesiology. 2019;130:404–413. doi: 10.1097/ALN.0000000000002560. [DOI] [PubMed] [Google Scholar]
- 98.Wei Y, Tejera P, Wang Z, Zhang R, Chen F, Su L, et al. A missense genetic variant in LRRC16A / CARMIL1 improves acute respiratory distress syndrome survival by attenuating platelet count decline. Am J Respir Crit Care Med. 2017;195:1353–1361. doi: 10.1164/rccm.201605-0946OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamashita M, Niisato M, Kawasaki Y, Karaman S, Robciuc MR, Shibata Y, et al. VEGF-C/VEGFR-3 signalling in macrophages ameliorates acute lung injury. Eur Respir J. 2022;59:2100880. doi: 10.1183/13993003.00880-2021. [DOI] [PubMed] [Google Scholar]
- 100.Medford ALR, Ibrahim NBN, Millar AB. Vascular endothelial growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. J Crit Care. 2009;24:236–242. doi: 10.1016/j.jcrc.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JY, Hildebrandt MAT, Pu X, Ye Y, Correa AM, Vaporciyan AA, et al. Variations in the vascular endothelial growth factor pathway predict pulmonary complications. Ann Thorac Surg. 2012;94:1079–1085. doi: 10.1016/j.athoracsur.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel SR, Karmpaliotis D, Ayas NT, Mark EJ, Wain J, Thompson BT, et al. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202. doi: 10.1378/chest.125.1.197. [DOI] [PubMed] [Google Scholar]
- 103.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 104.Verdonk F, Feyaerts D, Badenes R, Bastarache JA, Bouglé A, Ely W, et al. Upcoming and urgent challenges in critical care research based on COVID-19 pandemic experience. Anaesth Crit Care Pain Med. 2022;41:101121. doi: 10.1016/j.accpm.2022.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim W-Y. Multi-omic approach to identify risk markers specific to COVID-19. EBioMedicine. 2022;79:104009. doi: 10.1016/j.ebiom.2022.104009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 107.Liang K-H. Transcriptomics. Bioinforma Biomed Sci Clin Appl. Elsevier; 2013. p. 49–82.
- 108.Zheng F, Pan Y, Yang Y, Zeng C, Fang X, Shu Q, et al. Novel biomarkers for acute respiratory distress syndrome: genetics, epigenetics and transcriptomics. Biomark Med. 2022;16:217–231. doi: 10.2217/bmm-2021-0749. [DOI] [PubMed] [Google Scholar]
- 109.Jiang K, Yang J, Guo S, Zhao G, Wu H, Deng G. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol Ther. 2019;27:1758–1771. doi: 10.1016/j.ymthe.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang J, Do-Umehara HC, Zhang Q, Wang H, Hou C, Dong H, et al. miR-221-5p-mediated downregulation of JNK2 aggravates acute lung injury. Front Immunol. 2021;12:700933. doi: 10.3389/fimmu.2021.700933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xi X, Yao Y, Liu N, Li P. MiR-297 alleviates LPS-induced A549 cell and mice lung injury via targeting cyclin dependent kinase 8. Int Immunopharmacol. 2020;80:106197. doi: 10.1016/j.intimp.2020.106197. [DOI] [PubMed] [Google Scholar]
- 112.Huang C, Xiao X, Chintagari NR, Breshears M, Wang Y, Liu L. MicroRNA and mRNA expression profiling in rat acute respiratory distress syndrome. BMC Med Genomics. 2014;7:46. doi: 10.1186/1755-8794-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang Y, Huang L, Zhu G, Pei Z, Zhang W. Downregulated microRNA-27b attenuates lipopolysaccharide-induced acute lung injury via activation of NF-E2-related factor 2 and inhibition of nuclear factor κB signaling pathway. J Cell Physiol. 2019;234:6023–6032. doi: 10.1002/jcp.27187. [DOI] [PubMed] [Google Scholar]
- 114.Jiang W, Zhao K, Yuan W, Zhou F, Song H, Liu G, et al. MicroRNA-31-5p exacerbates lipopolysaccharide-induced acute lung injury via inactivating Cab39/AMPK α pathway. Oxid Med Cell Longev. 2020;2020:1–14. doi: 10.1155/2020/8822361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Narute P, Seam N, Tropea M, Logun C, Cai R, Sun J, et al. Temporal changes in microRNA expression in blood leukocytes from patients with the acute respiratory distress syndrome. Shock. 2017;47:688–695. doi: 10.1097/SHK.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Q, Feng Q, Zhang Y, Zhou S, Chen H. Decreased microRNA 103 and microRNA 107 predict increased risks of acute respiratory distress syndrome and 28-day mortality in sepsis patients. Medicine (Baltimore) 2020;99:e20729. doi: 10.1097/MD.0000000000020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu F, Yuan J, Tian S, Chen Y, Zhou F. MicroRNA-92a serves as a risk factor in sepsis-induced ARDS and regulates apoptosis and cell migration in lipopolysaccharide-induced HPMEC and A549 cell injury. Life Sci. 2020;256:117957. doi: 10.1016/j.lfs.2020.117957. [DOI] [PubMed] [Google Scholar]
- 118.Shi N, Deng L, Chen W, Zhang X, Luo R, Jin T, et al. Is microRNA-127 a novel biomarker for acute pancreatitis with lung injury? Dis Markers. 2017;2017:1–10. doi: 10.1155/2017/1204295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Z, Zhang R, Liang L, Su L, Lu Q, Baccarelli AA, et al. Whole blood microRNAs as a prognostic classifier for acute respiratory distress syndrome 28-day mortality. Intensive Care Med. 2016;42:1824–1825. doi: 10.1007/s00134-016-4462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Z-F, Yang Y-M, Fan H. Diagnostic value of miR-155 for acute lung injury/acute respiratory distress syndrome in patients with sepsis. J Int Med Res. 2020;48:030006052094307. doi: 10.1177/0300060520943070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu X, Wu C, Gu W, Ji H, Zhu L. Serum exosomal microRNAs predict acute respiratory distress syndrome events in patients with severe community-acquired pneumonia. Biomed Res Int. 2019;2019:1–11. doi: 10.1155/2019/3612020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Goodwin AJ, Li P, Halushka PV, Cook JA, Sumal AS, Fan H. Circulating miRNA 887 is differentially expressed in ARDS and modulates endothelial function. Am J Physiol Cell Mol Physiol. 2020;318:L1261–L1269. doi: 10.1152/ajplung.00494.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee LK, Eghbali M, Sapru A. A novel miRNA biomarker panel associated with mortality in pediatric patients with ARDS. Respir Res. 2021;22:169. doi: 10.1186/s12931-021-01761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rahmel T, Rump K, Adamzik M, Peters J, Frey UH. Increased circulating microRNA-122 is associated with mortality and acute liver injury in the acute respiratory distress syndrome. BMC Anesthesiol. 2018;18:75. doi: 10.1186/s12871-018-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li X, Wang J, Wu H, Guo P, Wang C, Wang Y, et al. Reduced peripheral blood miR-140 may be a biomarker for acute lung injury by targeting Toll-like receptor4 (TLR4) Exp Ther Med. 2018;16:3632–3638. doi: 10.3892/etm.2018.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu Z, Liang L, Zhang R, Wei Y, Su L, Tejera P, et al. Whole blood microRNA markers are associated with acute respiratory distress syndrome. Intensive Care Med Exp. 2017;5:38. doi: 10.1186/s40635-017-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu X-G, Kang X, Zhan L-B, Kang L-M, Fan Z-W, Bai L-Z. Circulating miRNAs as biomarkers for severe acute pancreatitis associated with acute lung injury. World J Gastroenterol. 2017;23:7440–7449. doi: 10.3748/wjg.v23.i41.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mu X, Wang H, Li H. Silencing of long noncoding RNA H19 alleviates pulmonary injury, inflammation, and fibrosis of acute respiratory distress syndrome through regulating the microRNA-423-5p/FOXA1 axis. Exp Lung Res. 2021;47:183–197. doi: 10.1080/01902148.2021.1887967. [DOI] [PubMed] [Google Scholar]
- 129.Yu LL, Zhu M, Zhao YM, Wen JJ, Yang XJ, Wu P. Metformin relieves acute respiratory distress syndrome by reducing miR-138 expression. Eur Rev Med Pharmacol Sci. 2018;22:5355–5366. doi: 10.26355/eurrev_201808_15737. [DOI] [PubMed] [Google Scholar]
- 130.Younes N, Zhou L, Amatullah H, Mei SHJ, Herrero R, Lorente JA, et al. Mesenchymal stromal/stem cells modulate response to experimental sepsis-induced lung injury via regulation of miR-27a-5p in recipient mice. Thorax. 2020;75:556–567. doi: 10.1136/thoraxjnl-2019-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang S, Hong Y, Liu H, Wang Q, Xu J, Zhang Y, et al. miR-584 and miR-146 are candidate biomarkers for acute respiratory distress syndrome. Exp Ther Med. 2021;21:445. doi: 10.3892/etm.2021.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoffman O, Burns N, Vadász I, Eltzschig HK, Edwards MG, Vohwinkel CU. Detrimental ELAVL-1/HuR-dependent GSK3β mRNA stabilization impairs resolution in acute respiratory distress syndrome. PLoS ONE. 2017;12:e0172116. doi: 10.1371/journal.pone.0172116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pereira MLM, Ortolan LS, Sercundes MK, Debone D, Murillo O, Lima FA, et al. Association of heme oxygenase 1 with lung protection in malaria-associated ALI/ARDS. Mediators Inflamm. 2016;2016:1–12. doi: 10.1155/2016/4158698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fei L, Sun G, Sun J, Wu D. The effect of N6-methyladenosine (m6A) factors on the development of acute respiratory distress syndrome in the mouse model. Bioengineered. 2022;13:7622–7634. doi: 10.1080/21655979.2022.2049473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.da Silva Sergio LP, Thomé AMC, da Silva Neto Trajano LA, Mencalha AL, de Souza da Fonseca A, de Paoli F. Photobiomodulation prevents DNA fragmentation of alveolar epithelial cells and alters the mRNA levels of caspase 3 and Bcl-2 genes in acute lung injury. Photochem Photobiol Sci. 2018;17:975–983. doi: 10.1039/c8pp00109j. [DOI] [PubMed] [Google Scholar]
- 136.Abreu SC, Rolandsson Enes S, Dearborn J, Goodwin M, Coffey A, Borg ZD, et al. Lung inflammatory environments differentially alter mesenchymal stromal cell behavior. Am J Physiol Cell Mol Physiol. 2019;317:L823–L831. doi: 10.1152/ajplung.00263.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang H, Song S, Mu X. Long non-coding RNA HOTAIR knockdown alleviates lipopolysaccharide-induced acute respiratory distress syndrome and the associated inflammatory response by modulating the microRNA-30a-5p/PDE7A axis. Exp Ther Med. 2021;22:1160. doi: 10.3892/etm.2021.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qian H, Yang H, Li X, Yang G, Zheng X, He T, et al. Andrographolide sulfonate attenuates alveolar hypercoagulation and fibrinolytic inhibition partly via NF-κB pathway in LPS-induced acute respiratory distress syndrome in mice. Biomed Pharmacother. 2021;143:112209. doi: 10.1016/j.biopha.2021.112209. [DOI] [PubMed] [Google Scholar]
- 139.Xu N, Guo H, Li X, Zhao Q, Li J. A five-genes based diagnostic signature for sepsis-induced ARDS. Pathol Oncol Res. 2021;27:580801. doi: 10.3389/pore.2021.580801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blumhagen RZ, Hedin BR, Malcolm KC, Burnham EL, Moss M, Abraham E, et al. Alternative pre-mRNA splicing of Toll-like receptor signaling components in peripheral blood mononuclear cells from patients with ARDS. Am J Physiol Cell Mol Physiol. 2017;313:L930–L939. doi: 10.1152/ajplung.00247.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ding Y, Feng Q, Chen J, Song J. TLR4/NF-κB signaling pathway gene single nucleotide polymorphisms alter gene expression levels and affect ARDS occurrence and prognosis outcomes. Medicine (Baltimore) 2019;98:e16029. doi: 10.1097/MD.0000000000016029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Y, Wu Y, Zhu L, Chen C, Xu S, Tang D, et al. METTL3-mediated N6-methyladenosine modification of Trim59 mRNA protects against sepsis-induced acute respiratory distress syndrome. Front Immunol. 2022;13:897487. doi: 10.3389/fimmu.2022.897487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y, Huang B, Zhao Y, Qi D, Wang D. Increased p300/CBP expression in acute respiratory distress syndrome is associated with interleukin-17 and prognosis. Clin Respir J. 2020;14:791–799. doi: 10.1111/crj.13197. [DOI] [PubMed] [Google Scholar]
- 145.Wilkins MR, Pasquali C, Appel RD, Ou K, Golaz O, Sanchez J-C, et al. From proteins to proteomes: large scale protein identification by two-dimensional electrophoresis and amino acid analysis. Nat Biotechnol. 1996;14:61–65. doi: 10.1038/nbt0196-61. [DOI] [PubMed] [Google Scholar]
- 146.Wen X-P, Zhang Y-Z, Wan Q-Q. Non-targeted proteomics of acute respiratory distress syndrome: clinical and research applications. Proteome Sci. 2021;19:5. doi: 10.1186/s12953-021-00174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fields S. The interplay of biology and technology. Proc Natl Acad Sci. 2001;98:10051–10054. doi: 10.1073/pnas.191380098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schnapp LM, Donohoe S, Chen J, Sunde DA, Kelly PM, Ruzinski J, et al. Mining the acute respiratory distress syndrome proteome: identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am J Pathol. 2006;169:86–95. doi: 10.2353/ajpath.2006.050612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang DW, Hayashi S, Gharib SA, Vaisar T, King ST, Tsuchiya M, et al. Proteomic and computational analysis of bronchoalveolar proteins during the course of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:701–709. doi: 10.1164/rccm.200712-1895OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Bentum M, Selbach M. An introduction to advanced targeted acquisition methods. Mol Cell Proteomics. 2021;20:100165. doi: 10.1016/j.mcpro.2021.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen X, Shan Q, Jiang L, Zhu B, Xi X. Quantitative proteomic analysis by iTRAQ for identification of candidate biomarkers in plasma from acute respiratory distress syndrome patients. Biochem Biophys Res Commun. 2013;441:1–6. doi: 10.1016/j.bbrc.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 152.Liu C-H, Kuo S-W, Ko W-J, Tsai P-R, Wu S-W, Lai C-H, et al. Early measurement of IL-10 predicts the outcomes of patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Sci Rep. 2017;7:1021. doi: 10.1038/s41598-017-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Q, Luo L, Lu X, Ji X, Ji D, Feng H, et al. Proteomic analysis of serum proteins at the onset of ARDS in patients. Chest. 2019;155:127A. [Google Scholar]
- 154.Dong X, Zhu Z, Wei Y, Ngo D, Zhang R, Du M, et al. Plasma insulin-like growth factor binding protein 7 contributes causally to ARDS 28-day mortality. Chest. 2021;159:1007–1018. doi: 10.1016/j.chest.2020.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bowler RP, Duda B, Chan ED, Enghild JJ, Ware LB, Matthay MA, et al. Proteomic analysis of pulmonary edema fluid and plasma in patients with acute lung injury. Am J Physiol Cell Mol Physiol. 2004;286:L1095–L1104. doi: 10.1152/ajplung.00304.2003. [DOI] [PubMed] [Google Scholar]
- 156.de Torre C, Ying S-X, Munson PJ, Meduri GU, Suffredini AF. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics. 2006;6:3949–3957. doi: 10.1002/pmic.200500693. [DOI] [PubMed] [Google Scholar]
- 157.Nguyen EV, Gharib SA, Palazzo SJ, Chow Y, Goodlett DR, Schnapp LM. Proteomic profiling of bronchoalveolar lavage fluid in critically ill patients with ventilator-associated pneumonia. PLoS ONE. 2013;8:e58782. doi: 10.1371/journal.pone.0058782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhargava M, Becker TL, Viken KJ, Jagtap PD, Dey S, Steinbach MS, et al. Proteomic profiles in acute respiratory distress syndrome differentiates survivors from non-survivors. PLoS ONE. 2014;9:e109713. doi: 10.1371/journal.pone.0109713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ren S, Chen X, Jiang L, Zhu B, Jiang Q, Xi X. Deleted in malignant brain tumors 1 protein is a potential biomarker of acute respiratory distress syndrome induced by pneumonia. Biochem Biophys Res Commun. 2016;478:1344–1349. doi: 10.1016/j.bbrc.2016.08.125. [DOI] [PubMed] [Google Scholar]
- 160.Bhargava M, Viken K, Wang Q, Jagtap P, Bitterman P, Ingbar D, et al. Bronchoalveolar lavage fluid protein expression in acute respiratory distress syndrome provides insights into pathways activated in subjects with different outcomes. Sci Rep. 2017;7:7464. doi: 10.1038/s41598-017-07791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dong H, Li J, Lv Y, Zhou Y, Wang G, Hu S, et al. Comparative analysis of the alveolar macrophage proteome in ALI/ARDS patients between the exudative phase and recovery phase. BMC Immunol. 2013;14:25. doi: 10.1186/1471-2172-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patel H, Ashton NJ, Dobson RJB, Andersson L-M, Yilmaz A, Blennow K, et al. Proteomic blood profiling in mild, severe and critical COVID-19 patients. Sci Rep. 2021;11:6357. doi: 10.1038/s41598-021-85877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yuan Z, Wang T, Wen F. iTRAQ-based proteomic analysis reveals mitochondrial ‘damage’-associated molecular patterns are involved in pulmonary inflammation in lypopolysaccharide-induced acute lung injury. Eur Respir J. 2018;52:PA4289. [Google Scholar]
- 164.Wygrecka M, Markart P, Fink L, Guenther A, Preissner KT. Raised protein levels and altered cellular expression of factor VII activating protease (FSAP) in the lungs of patients with acute respiratory distress syndrome (ARDS) Thorax. 2007;62:880–888. doi: 10.1136/thx.2006.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grissom CK, Orme JF, Richer LD, McIntyre TM, Zimmerman GA, Elstad MR. Platelet-activating factor acetylhydrolase is increased in lung lavage fluid from patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:770–775. doi: 10.1097/01.CCM.0000053647.82608.29. [DOI] [PubMed] [Google Scholar]
- 166.Liu D, Mao P, Huang Y, Liu Y, Liu X, Pang X, et al. Proteomic analysis of lung tissue in a rat acute lung injury model: identification of PRDX1 as a promoter of inflammation. Mediators Inflamm. 2014;2014:1–14. doi: 10.1155/2014/469358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yen S, Song Y, Preissner M, Bennett E, Wilson R, Pavez M, et al. The proteomic response is linked to regional lung volumes in ventilator-induced lung injury. J Appl Physiol. 2020;129:837–845. doi: 10.1152/japplphysiol.00097.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rashid A, Zeng C, Motta-Ribeiro G, Dillon ST, Libermann TA, Lessa MA, et al. Proteomics of lung tissue reveals differences in inflammation and alveolar-capillary barrier response between atelectasis and aerated regions. Sci Rep. 2022;12:7065. doi: 10.1038/s41598-022-11045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yue X, Guidry JJ. Differential protein expression profiles of bronchoalveolar lavage fluid following lipopolysaccharide-induced direct and indirect lung injury in mice. Int J Mol Sci. 2019;20:3401. doi: 10.3390/ijms20143401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu X, Zhu Q, Zhang R, Wang Y, Niu F, Wang W, et al. ITRAQ-based proteomics analysis of acute lung injury induced by oleic acid in mice. Cell Physiol Biochem. 2017;44:1949–1964. doi: 10.1159/000485885. [DOI] [PubMed] [Google Scholar]
- 171.Qiu Y, Wu D, Ning W, Xu J, Shu T, Huang M, et al. Post-mortem tissue proteomics reveals the pathogenesis of multi-organ injuries of COVID-19. Natl Sci Rev. 2021;8:nwab143. doi: 10.1093/nsr/nwab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775–791.e14. doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu HM, Jin M, Marsh CB. Toward functional proteomics of alveolar macrophages. Am J Physiol Cell Mol Physiol. 2005;288:L585–L595. doi: 10.1152/ajplung.00305.2004. [DOI] [PubMed] [Google Scholar]
- 174.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 175.Rogers AJ, Matthay MA. Applying metabolomics to uncover novel biology in ARDS. Am J Physiol Cell Mol Physiol. 2014;306:L957–L961. doi: 10.1152/ajplung.00376.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lacy P. Metabolomics of sepsis-induced acute lung injury: a new approach for biomarkers. Am J Physiol Cell Mol Physiol. 2011;300:L1–3. doi: 10.1152/ajplung.00375.2010. [DOI] [PubMed] [Google Scholar]
- 177.Patti GJ, Yanes O, Siuzdak G. Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schubert JK, Müller WPE, Benzing A, Geiger K. Application of a new method for analysis of exhaled gas in critically ill patients. Intensive Care Med. 1998;24:415–421. doi: 10.1007/s001340050589. [DOI] [PubMed] [Google Scholar]
- 179.Bos LDJ, Weda H, Wang Y, Knobel HH, Nijsen TME, Vink TJ, et al. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur Respir J. 2014;44:188–197. doi: 10.1183/09031936.00005614. [DOI] [PubMed] [Google Scholar]
- 180.Singh Y, Saklani S, Tantra T, Thareja S. Amino acid derived prodrugs: an approach to improve the bioavailability of clinically approved drugs. Curr Top Med Chem. 2021;21:2170–2183. doi: 10.2174/1568026621666210602154438. [DOI] [PubMed] [Google Scholar]
- 181.Stringer KA, Serkova NJ, Karnovsky A, Guire K, Paine R, Standiford TJ. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma 1 H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am J Physiol Cell Mol Physiol. 2011;300:L4–11. doi: 10.1152/ajplung.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lorente JA, Nin N, Villa P, Vasco D, Miguel-Coello AB, Rodriguez I, et al. Metabolomic diferences between COVID-19 and H1N1 influenza induced ARDS. Crit Care. 2021;25:390. doi: 10.1186/s13054-021-03810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grassin-Delyle S, Roquencourt C, Moine P, Saffroy G, Carn S, Heming N, et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine. 2021;63:103154. doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Izquierdo-Garcia JL, Nin N, Jimenez-Clemente J, Horcajada JP, del Arenas-Miras M, Gea J, et al. Metabolomic profile of ARDS by nuclear magnetic resonance spectroscopy in patients with H1N1 influenza virus pneumonia. Shock. 2018;50:504–510. doi: 10.1097/SHK.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 185.Izquierdo-García JL, Nin N, Cardinal-Fernandez P, Ruiz-Cabello J, Lorente JÁ. Metabolomic profile of acute respiratory distress syndrome of different etiologies. Intensive Care Med. 2019;45:1318–1320. doi: 10.1007/s00134-019-05634-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lin S, Yue X, Wu H, Han T, Zhu J, Wang C, et al. Explore potential plasma biomarkers of acute respiratory distress syndrome (ARDS) using GC–MS metabolomics analysis. Clin Biochem. 2019;66:49–56. doi: 10.1016/j.clinbiochem.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 187.Viswan A, Ghosh P, Gupta D, Azim A, Sinha N. Distinct metabolic endotype mirroring acute respiratory distress syndrome (ards) subphenotype and its heterogeneous biology. Sci Rep. 2019;9:2108. doi: 10.1038/s41598-019-39017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu J, Pan T, Qi X, Tan R, Wang X, Liu Z, et al. Increased mortality of acute respiratory distress syndrome was associated with high levels of plasma phenylalanine. Respir Res. 2020;21:99. doi: 10.1186/s12931-020-01364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rai RK, Azim A, Sinha N, Sahoo JN, Singh C, Ahmed A, et al. Metabolic profiling in human lung injuries by high-resolution nuclear magnetic resonance spectroscopy of bronchoalveolar lavage fluid (BALF) Metabolomics. 2013;9:667–676. [Google Scholar]
- 190.Evans CR, Karnovsky A, Kovach MA, Standiford TJ, Burant CF, Stringer KA. Untargeted LC–MS metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J Proteome Res. 2014;13:640–649. doi: 10.1021/pr4007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rogers AJ, Contrepois K, Wu M, Zheng M, Peltz G, Ware LB, et al. Profiling of ARDS pulmonary edema fluid identifies a metabolically distinct subset. Am J Physiol Cell Mol Physiol. 2017;312:L703–L709. doi: 10.1152/ajplung.00438.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Viswan A, Singh C, Rai RK, Azim A, Sinha N, Baronia AK. Metabolomics based predictive biomarker model of ARDS: a systemic measure of clinical hypoxemia. PLoS ONE. 2017;12:e0187545. doi: 10.1371/journal.pone.0187545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hepokoski M, Wang J, Li K, Li Y, Gupta P, Mai T, et al. Altered lung metabolism and mitochondrial DAMPs in lung injury due to acute kidney injury. Am J Physiol Cell Mol Physiol. 2021;320:L821–L831. doi: 10.1152/ajplung.00578.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gustafson D, Ngai M, Wu R, Hou H, Schoffel AC, Erice C, et al. Cardiovascular signatures of COVID-19 predict mortality and identify barrier stabilizing therapies. EBioMedicine. 2022;78:103982. doi: 10.1016/j.ebiom.2022.103982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ware LB. Physiological and biological heterogeneity in COVID-19-associated acute respiratory distress syndrome. Lancet Respir Med. 2020;8:1163–1165. doi: 10.1016/S2213-2600(20)30369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Beitler JR, Thompson BT, Baron RM, Bastarache JA, Denlinger LC, Esserman L, et al. Advancing precision medicine for acute respiratory distress syndrome. Lancet Respir Med. 2022;10:107–120. doi: 10.1016/S2213-2600(21)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bos LDJ, Artigas A, Constantin J-M, Hagens LA, Heijnen N, Laffey JG, et al. Precision medicine in acute respiratory distress syndrome: workshop report and recommendations for future research. Eur Respir Rev. 2021;30:200317. doi: 10.1183/16000617.0317-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.