Abstract
Neonatal late-onset sepsis (LOS) continues to threaten morbidity and mortality within the neonatal intensive care unit (NICU) and poses ongoing diagnostic and therapeutic challenges. Early recognition of clinical signs, rapid evaluation, and prompt initiation of treatment are critical to prevent life-threatening deterioration. Preterm infants – born at ever-decreasing gestational ages – are at particularly high risk for life-long morbidities and death. This changing NICU population necessitates continual reassessments of diagnostic and preventive measures and evidence-based treatment for LOS. The clinical presentation of LOS is varied and nonspecific. Despite ongoing research, identification of reliable, specific laboratory biomarkers facilitating early diagnosis is lacking. These limitations drive an ongoing practice of liberal initiation of empiric antibiotics among infants with suspected LOS. Subsequent promotion of multidrug-resistant microorganisms threatens the future of antimicrobial therapy and puts preterm and chronically ill infants at even higher risk of nosocomial infection. Efforts to identify adjunctive therapies counteracting sepsis-driven hyper-inflammation and sepsis-related functional immunosuppression are ongoing. However, most approaches have either failed to improve LOS prognosis or are not yet ready for clinical application. This article provides an overview of epidemiology, risk factors, diagnostic tools, and treatment options of LOS in the context of increasing numbers of extremely preterm infants. It addresses the question of whether LOS could be identified earlier and more precisely to allow for earlier and more targeted therapy and discusses rational approaches to antibiotic therapy to avoid over-use. Finally, this review elucidates the necessity of long-term follow-up of infants with a history of LOS.
Evidence / Article Summary
Neonatal LOS is a life-threatening condition and a major cause of neonatal morbidity and mortality worldwide.
Preterm infants and chronically ill neonates are at highest risk, and VLBW infants account for up to 50% of LOS cases in high-income countries.
Early recognition and prompt initiation of therapy are key to prevent life-threatening deterioration.
Antibiotic therapy includes initial empiric and organism-specific therapy. The choice of empiric antibiotic agents should be based on the likely organism and patterns of antibiotic susceptibility and resistance in the individual NICU setting. Duration should be as short as possible and selection of antibiotic regimes as narrow as possible.
Extended hygiene measures based on routine colonization screening of NICU patients and CLABSI prevention efforts have been associated with reduced rates of LOS.
Promotion of resistant microorganisms threatens the future of antimicrobial therapy. High exposure rates to 3rd/4th generation cephalosporins, vancomycin, and meropenem likely reflect increasingly complex critically ill neonates and resistant organisms but should also prompt antimicrobial stewardship efforts where feasible.
INTRODUCTION
Despite advances in overall neonatal care and implementation of quality improvement measures, neonatal late-onset sepsis (LOS) remains a persistent threat in neonatal intensive care units (NICUs). LOS disproportionately affects the most premature infants and is associated with mortality and considerable morbidity among survivors.1–7 LOS recognition and diagnosis remain challenging; LOS often presents with varied, nonspecific clinical signs,8 and common laboratory biomarkers perform inconsistently in discriminating infected from uninfected infants.9 10 This review focuses on current approaches to LOS risk assessment, diagnostic testing, antimicrobial management, and infection prevention. Finally, we summarize survival outcomes and long-term morbidities associated with neonatal LOS and highlight the need for neurodevelopmental follow-up of LOS survivors.
DEFINING LATE-ONSET SEPSIS
Sepsis is a life-threatening condition caused by systemic infections that prompt a cascade of often fatal inflammatory immune responses. Neonatal sepsis is defined as sepsis presenting in the first 28 days after birth.11 In view of distinct pathogenesis and pathogen epidemiology, neonatologists discriminate early-onset sepsis (EOS) and LOS according to the timing of infection onset.2 11 EOS is mostly defined as manifesting in the first 48–72 hours after birth.1 11 12 In the preterm NICU population, sepsis may occur much later; thus, in research contexts, LOS encompasses sepsis presenting ≥72 hours after birth and through NICU hospitalization.
Although there is general research consensus defining the timing of neonatal sepsis subtypes, there is substantial heterogeneity in neonatal LOS definitions among observational studies and major neonatal organizations, and there is no standard approach to diagnosing LOS across NICUs.11–13 In variable combinations, the definition is based upon assessment of microbiological cultures, clinical signs of infection, and adjunctive laboratory data.11 12 Most definitions include a positive blood culture as the essential criterion, although culture collection requirements and procedures may vary widely. Clinical signs of sepsis are the second major criterion. However, there is no consensus on key indicator signs among a multitude of symptoms (Figure).11 In summary, “culture-proven sepsis” may be defined by positive blood culture results, while the diagnosis of “culture-negative sepsis” or “clinical sepsis” relies on variable clinical signs consistent with infection.11 “Septic shock” is discriminated from sepsis when criteria for neonatal sepsis are met and blood pressure is below the 5th percentile for age requiring hemodynamic stabilization with fluids or inotropic agents.11
Figure 1:
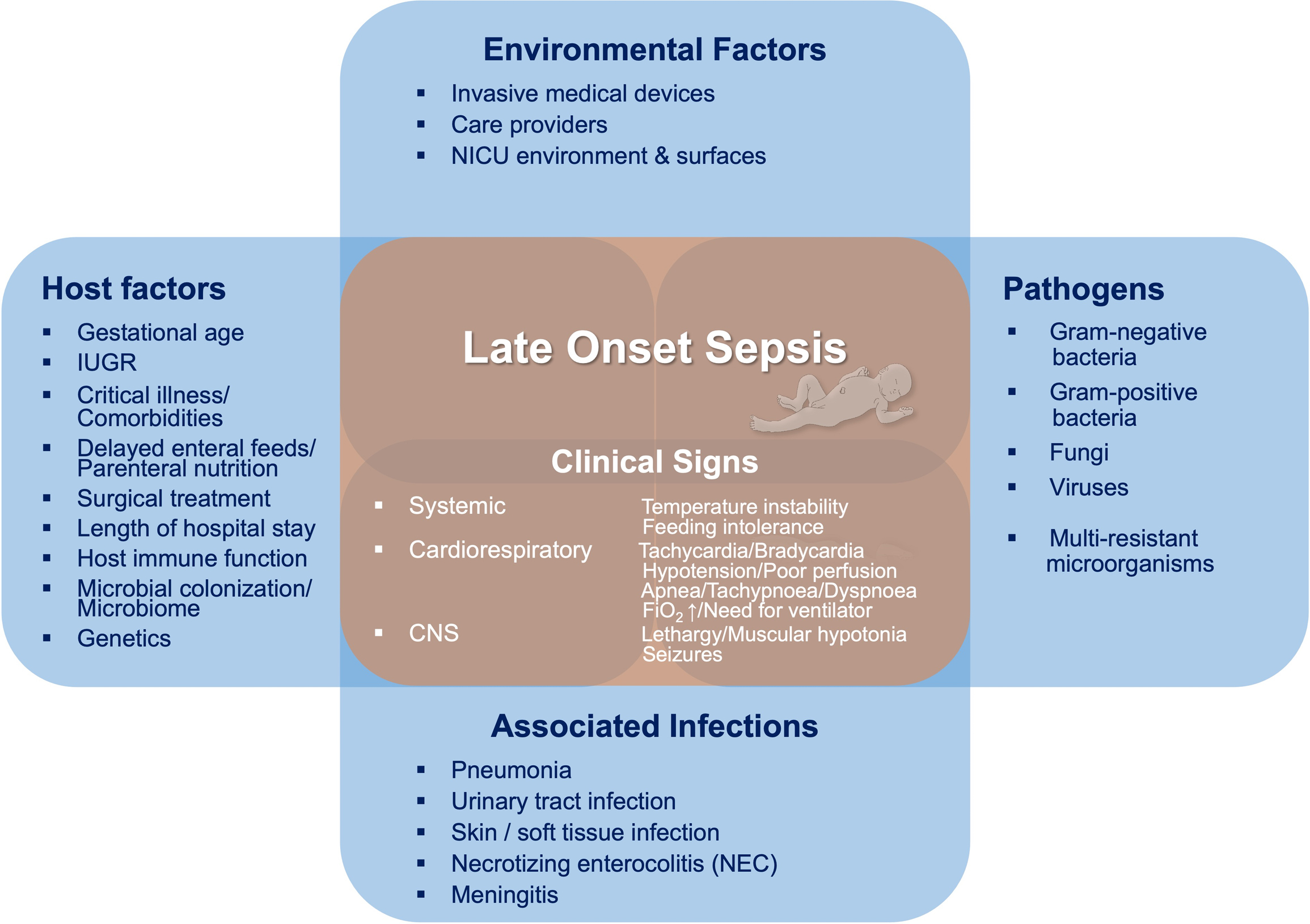
Etiology and Clinical Signs and Symptoms of Neonatal Late Onset Sepsis
Heterogeneity in defining LOS hampers interpretation and comparability of clinical trials and development of evidence-based guidelines for LOS diagnosis and management.13 There are ongoing attempts to establish a consensus definition of neonatal LOS, aiming to identify neonatal-specific objective physiologic and laboratory characteristics that may allow more rapid recognition and initiation of therapy. Furthermore, this may facilitate more standardized, comparable data collection worldwide that could contribute to diagnostic and therapeutic innovations in LOS.11 12
EPIDEMIOLOGY, RISK FACTORS AND CAUSATIVE PATHOGENS
LOS is primarily attributed to nosocomial or horizontal pathogen acquisition, and exposures to hospital or community environments. Pathogen exposure may occur due to contamination or colonization of indwelling invasive medical devices, contact with care providers, and/or other environmental sources and surfaces. Preterm birth and critical illness are major risk factors for LOS given their associated needs for central catheters, mechanical ventilation, prolonged parenteral nutrition, and surgical interventions.3 14 15 Predisposing factors further include maternal and perinatal risk factors, such as preeclampsia, chorioamnionitis, and intrauterine growth restriction, as well as length of hospital stay and comorbidities (Figure).1 3 4 15 16 The most immature infants experience the highest infectious burden; LOS rates are reported at 1.6% in term neonates, compared to 12–50% among very preterm and/or very low birth weight (VLBW) infants.1 2 4 6 LOS-associated mortality varies by gestation and by organism, and may be as high as 35% in the most vulnerable, lowest-gestation infants.6 14
Host response factors shape the inflammatory response to sepsis and contribute to the severity of clinical presentation. Gestational age (GA)-specific patterns of immune function place preterm infants at increased risk of infection, adverse or sustained inflammation, and organ dysfunction.17–19 Moreover, microbial colonization and aberrations in microbiome development are implicated in increased susceptibility to LOS.3 20 Specifically, prolonged empiric antibiotic therapy >4 days at birth is associated with 1.25 to 2.5-fold higher adjusted odds of later LOS and combined LOS/death.21 The role of genetics remains unclear, and LOS rates are not significantly different among infants born from singleton versus multiple gestation pregnancies.14 Sex differences in immune function, infection development, and potentially increased susceptibility to sepsis in male infants are poorly understood.22
Ultimately, infection risk and clinical manifestation are driven by host factors (e.g., baseline organ dysfunction or immaturity), the pathogen type, and potential antimicrobial resistance patterns. Causative pathogens vary widely across geographical regions and NICUs, and infectious epidemiology may change over time within the same unit.4 5 Gram-positive bacteria constitute the majority of pathogens isolated in high-income countries,1 6 14 23 while gram-negative organisms are predominant in some low- and middle-income countries.5 Notably, >50% of gram-positive bacteremia among preterm infants is due to coagulase-negative Staphylococci (CoNS),1 6 14 23 an organism considered to be a skin commensal in term neonates. However, in preterm infants, CoNS may represent true pathogens causing clinically significant infections.17 23 Ultimately, isolation of CoNS from blood cultures necessitates discrimination between potential culture contamination and true bacteremia in the individual patient and NICU setting.
Other important gram-positive bacteria implicated in LOS include S. aureus (isolated in 4–18%), Enterococcus species (spp.) (3–16%) and Group B Streptococcus (1.8–8%), with large variation among nationwide data reported by the US National Institute of Child Health and Human Development (NICHD), the NeonIN surveillance network in England, and the German Neonatal Network (GNN).1 2 4 6 24 Against the background of rising numbers of multidrug-resistant organisms, methicillin-resistant S. aureus (MRSA) represents an increasingly prevalent pathogen in gram-positive LOS, responsible for 11% of S. aureus infections in the NeonIN surveillance cohort and 23% of S. aureus infections reported to the Center for Disease Control’s National Nosocomial Infections Surveillance system.24 25 The GNN and data from nationwide Australian and New Zealand cohorts still report a MRSA prevalence of <1% of all pathogens isolated in VLBW infants with LOS (6% of S. aureus infections in GNN infants).6 26 Other observational studies do not differentiate MRSA from methicillin-sensitive S. aureus.1 2 4
LOS caused by gram-negative pathogens is associated with higher illness severity, significantly higher mortality, and higher likelihood of short- and long-term neonatal morbidities.2 3 15 In nationwide cohorts in the United States, England, and Germany, Escherichia (E.) coli (proportions range from 3 to 13%), Klebsiella spp. (4–5%), Pseudomonas spp. (2–5%), Enterobacter spp. (2.5–21%), Serratia (0.8–2%), and Acinetobacter (0.1–2%) account for the majority of cases of gram-negative LOS.1 2 4 6 24 In recent decades, a growing number of infections due to multidrug-resistant gram-negative organisms (e.g., extended-spectrum beta-lactamase [ESBL]-producing bacteria) have challenged antimicrobial therapy selection in LOS in high-risk NICU patients.5
Fungal organisms are isolated in about 3–10% of cases of neonatal LOS, with Candida (C.) spp. (mainly C. albicans and C. parapsilosis) most frequently detected.2 4 6 Yeast infections have been associated with high mortality, and should be particularly considered in LOS evaluation and empiric therapy in ill preterm and term neonates who demonstrate clinical features possibly consistent with invasive fungal infections (e.g., rash, neutropenia/thrombocytopenia, hyperglycemia).4 Finally, viral pathogens (e.g., parainfluenza, echo-, entero-, coxsackie-, adeno-, rhino- and coronavirus) have been increasingly acknowledged as causative agents of sepsis-like syndromes in preterm and term infants.27 28
CLINICAL PRESENTATION
The clinical presentation of LOS is nonspecific and varied, with respiratory signs, lethargy, tachycardia, feeding intolerance, and temperature instability (fever or hypothermia) commonly reported (Figure). The spectrum of illness severity ranges from moderate signs of infection to critical illness with severe organ dysfunction and potential multiorgan failure.8 Secondary sites of infection that are most frequently associated with late-onset bacteremia include pneumonia, urinary tract infections (UTIs), and skin and soft tissue infections, as well as necrotizing enterocolitis. Translocation of pathogens colonizing the neonatal gut is a major cause of neonatal sepsis, especially in very immature preterm infants and infants with compromised intestinal integrity. In infants with clinically apparent necrotizing enterocolitis, concurrent bloodstream infections (mostly of gram-negative origin) were detected in 40–60% of cases.29 LOS is complicated by meningitis in approximately 5% of cases (in which a lumbar puncture (LP) was performed).30 Clinically, sepsis cannot be distinguished from meningitis, since presentation is again nonspecific and includes apnea, lethargy, and temperature instability, among other signs.
DIAGNOSIS
The ideal LOS biomarker would facilitate early diagnosis of culture-confirmed infections with high positive and/or negative predictive value, generalizability across gestational and postnatal age strata, and rapid turnaround time. However, this biomarker has not yet been identified (Table 1). The complete blood count with differential has limitations in both preterm and term LOS diagnosis. Complete blood count indices may be normal in infected infants, and individual indices including white blood count, absolute neutrophil count, immature-to-total (I:T) neutrophil ratio, and platelet count do not have sufficient sensitivity or specificity alone for reliable LOS diagnosis.31 C-reactive protein (CRP) is an acute-phase reactant primarily produced in the liver with peak expression 36–48 hours post-stimulation. CRP test characteristics in identification of culture-proven LOS in VLBW infants are modest at best, with median sensitivity 62% and specificity 74%.32 Single CRP measurements have limited diagnostic efficiency and cannot reliably identify nor exclude infection in a symptomatic infant at the time of sepsis evaluation.32 However, three serial CRP measurements obtained over days improve sensitivity to 98% and negative predictive value to 99%.33 Thus, assessing CRP at the time of presentation is unlikely to assist in clinical decision making, while repeated negative measurements may serve as a useful adjunct in the decision to discontinue antibiotics.
Table 1:
Diagnostic Characteristics of the Most-Studied Laboratory Adjuncts in LOS
| Biomarker | Mechanism/Physiology | Ref. | Test Characteristics | Notes |
|---|---|---|---|---|
|
| ||||
| Complete Blood Count (CBC) | Cellular-based defense meachnisms | (31) | WBC <5000/mm3: Sensitivity 7%, specificity 96% WBC >20,000/mm3: Sensitivity 23%, specificity 80% ANC <1000/mm3: Sensitivity 2%, specificity 98% I/T ratio >0.2: Sensitivity 54%, specificity 62% Platelet <50,000/mm3: Sensitivity 8%, specificity 98% |
CBC indices often normal in infants with culture-confirmed LOS |
|
| ||||
| C-reactive protein (CRP) | Acute phase reactant | (34) | Sensitivity (median, range): 85% (12 – 100%) | Peaks at 24–36 hours |
| Produced in liver after stimulation by IL-6, IL-1β, TNF-α | Specificity (median, range): 86% (41– 100%) | Sensitivity and negative predictive value both improve with serial measurements No consensus cut-off value studied - range >1 – 111 mg/L | ||
| (32) | Pooled sensitivity 62% (95% CI 50 – 72%), reported at median specificity 74% | |||
|
| ||||
| Procalcitonin (PCT) | Acute phase reactant | (34) | Sensitivity (median, range): 92% (69 – 100%) | Peaks at 12–24 hours |
| Largely produced in liver, similar cytokine stimulation as CRP. Down-regulated by interferon-γ. | Specificity (median, range); 80% (36 – 92%) | May have better diagnostic utility for bacterial infections (vs viral infections) compared to CRP. | ||
| Response appears to be less affected by postoperative inflammation, compared to CRP No consensus cut-off value studied: range >0.5 – 6.1 ng/mL | ||||
|
| ||||
| Interleukin 6 (IL-6) | Proinflammatory cytokine | (35, 40) | Sensitivity range 78 – 94%, specificity range 92 – 99% | Short measurement window: peaks at 2–3 hours, returns back to baseline by 6–8 hours May have improved diagnostic accuracy in combination with CRP or PCT |
|
| ||||
| Interleukin 8 (IL-8) | Proinflammatory cytokine | (37, 39) | Studies are widely variable: Sensitivity range 44 – 95%, specificity range 89 – 100% | Similar to IL-6 |
|
| ||||
| CD64 | Neutrophil surface marker; upregulated in setting of bacterial infection | (38) | Sensitivity (95% CI): 79% (75 – 82%), Specificity (95% CI): 71% (64 – 74%) | May have better diagnostic accuracy in term infants compared to preterm infants |
| Wide range of cutoff values (CD-64 index 2.19 – 46) | ||||
|
| ||||
| CD11b | Leukocyte β2-integrin surface protein, expression↑ in inflammation | (36) | Sensitivity range 72 – 100%, Specificity range 56 – 70% | CD11b↑ not limited to infection-mediated inflammation |
Procalcitonin (PCT), like CRP, is an acute-phase reactant that is mostly synthesized in the liver in response to interleukin-6 (IL-6) and tumor necrosis factor (TNF)-alpha but appears to have faster kinetics with peak levels being detected 12–24 hours post-stimulation. Test characteristics for PCT are variably reported, with median sensitivity 92% and median specificity 80%.34 In summary, single CRP and/or PCT values obtained at the time of LOS evaluation have limited diagnostic utility, as they do not reliably “rule-in” or “rule-out” culture-confirmed infections. However, serial values trended over time may assist as adjuncts in decision-making surrounding antibiotic discontinuation in the context of clinical assessments and culture data.
Multiple other biomarkers have been evaluated in LOS diagnosis. Proinflammatory cytokines (e.g., IL-6, IL-8, TNF-alpha) and cell surface markers (e.g., CD64, CD11b, soluble CD14, HLA-DR) have moderate diagnostic efficiency,35–40 which increases with serial measurements.10 However, these markers may not have available assays in hospital laboratories, and they are not routinely used in most centers. In the future, machine learning techniques may aid in constructing combined panels of biomarkers offering better diagnostic efficiency than single biomarkers.
Positive blood cultures remain the “gold standard” for neonatal LOS diagnosis, given that sensitivity for bacteremia detection may be as high as >98%.41 Improvements in laboratory technology, including automated blood culture detection systems, have contributed to faster time to organism detection and speciation. However, adequate blood culture volume is still key for pathogen detection (Infobox).41 Most LOS evaluations, especially in preterm infants, yield negative blood culture results;42 in a cohort of 99,796 VLBW infants with episodes of suspected LOS, only 8.9% of 164,744 blood cultures obtained were positive.14 This count reflects the uncertainty facing clinicians: on one hand, LOS evaluations are initiated in the face of clinical instability (which in the great majority of cases results from a non-infectious etiology), though truly infected infants may also have “falsely negative” blood cultures if there is low-level circulating bacteremia, insufficient blood culture volume, or antibiotic administration prior to culture collection. Emerging molecular diagnostic adjuncts (e.g., reverse transcription - polymerase chain reaction [RT-PCR] of bacterial 16s ribosomal RNA) amplify small amounts of genetic material of pathogens, with sensitivity and specificity reported as high as 90% and 96%.9 However, these techniques are expensive, do not differentiate between live and dead bacteria, and may lead to amplification of pathogenic material unrelated to the clinical phenotype.
Infobox. Best Practices for Blood Culture Collection & Volume Requirements.
-
Disinfection (May vary based on gestational age)
Topical disinfectants mainly include chlorhexidine gluconate and octenidine dihydrochloride (povidone iodine is no longer recommended in neonates for reason of potential systemic absorption and risk of hypothyroidism).97
1–2% chlorhexidine gluconate aqueous formulation with good efficacy; alcohol component likely accounts for lower contamination than with 10% povidone iodine.98
Skin irritation due to chlorhexidine gluconate reported (ascribed to alcohol component).
Longer skin disinfection duration (>30 sec) has been reported more efficacious than shorter durations (10 sec) in removing skin flora;97 allow to dry.
Do not re-palpate phlebotomy site after disinfected
-
Sites
Cultures should be obtained from a peripheral site (arterial or venous puncture).
If a central catheter is present, consider concurrent central catheter sourced blood culture.
Culture growth & differential time to positivity between peripheral and central catheter culture may aid in CLABSI diagnosis.
However, potential risks of central catheter contamination exist
-
Blood volume 41
At least 1 mL recommended for blood culture
1 mL blood culture volume ≈ 63% probability of pathogen detection at 1 CFU/mL,
0.5 mL blood culture volume ≈ 39% probability at 1 CFU/mL
3 mL blood culture volume ≈ 95% probability at 1 CFU/mL,
CFU: colony-forming units, CLABSI: central line-associated bloodstream infections
Although urine cultures should be routinely obtained during LOS evaluations, inclusion is variable and occurs in as few as 7% and up to 50% of LOS evaluations.43 44 UTIs were reported in 8–11% of LOS evaluations in which a urine culture was sent, and tend to occur more often in preterm infants with lower birth weight and higher postnatal age (reported mean UTI diagnosis at 42 postnatal days).44 45 Variation in urine culture practices may stem from lack of clinical suspicion for UTI, technical challenges in obtaining sterile cultures (particularly in very immature infants or those with anatomic differences), and/or perceived lack of patient stability to obtain a urine sample. Moreover, there is no consistent definition of UTI applicable to NICU patients, cutoffs for urinalysis indices vary considerably, and UTI may frequently occur in the absence of positive blood cultures. Urine specimens should ideally be collected in a sterile fashion (via urethral catheterization or suprapubic tap), as opposed to external bag collection which carries higher risks of contaminant pathogen growth. Antibiotic therapy prior to urine culture collection reduces the diagnostic efficiency for UTIs, emphasizing the importance of urine sample collection at the time of sepsis evaluation and prior to antibiotic exposure. 43 44
Inclusion of cerebrospinal fluid (CSF) diagnostics in LOS evaluation should be considered, particularly among preterm infants and febrile neonates <28 days of age – since clinical signs of meningitis are nonspecific and may overlap with other infectious processes.46 47 CSF cultures obtained prior to antibiotic administration are the “gold standard”. However, it is challenging to accurately estimate meningitis rates complicating LOS. LPs are often not performed as part of LOS evaluations, and those performed after antibiotic initiation may reveal false-negative culture growth in CSF samples. Variations in rates of LP performance could be attributable to several factors, including clinician awareness of the low estimated prevalence of meningitis associated with LOS (~2–5%) and reluctance to perform LPs (particularly in very small or clinically unstable infants).15 45 48 In a cohort of 2,989 VLBW infants, only 24% of LOS evaluations included CSF cultures, with significant practice variation (7–49% across 8 centers). Of those patients for whom CSF cultures were obtained, only 2% were deemed to have meningitis.45 Of note, consistent evidence suggests that a significant proportion of meningitis cases (30–70%) occur in the absence of bacteremia.45–47 Diagnostic adjuncts, particularly in infants with pre-treated cultures or uninterpretable CSF indices, may include pathogen RT-PCR or cytokine profiling.49
RISK ASSESSMENT
Improvements in LOS risk assessment strategies are needed for better discrimination among heterogeneous NICU populations and earlier identification of evolving LOS. Due to a lack of sensitivity and specificity, sepsis definitions based upon systemic inflammatory response syndrome (SIRS) criteria have largely been abandoned in adult patients, with a shift to metrics focused on infection-associated organ dysfunction that also have utility in predicting sepsis-attributable morbidity and mortality.50 51 Revisions to pediatric sepsis definitions are in progress and also appear to be shifting towards metrics focusing on organ dysfunction.52 The nSOFA (neonatal Sequential Organ Failure Assessment) is one proposed tool for quantifying neonatal multi-organ dysfunction and associated mortality risk.53 It quantifies respiratory, cardiovascular, and hematologic dysfunction using readily available clinical data in the electronic medical record, and observational studies have demonstrated that changes in the nSOFA over time correlate with LOS-attributable and all-cause mortality in preterm infants.53 54
Computer-based algorithms have attracted considerable interest as “early-warning systems” for LOS diagnosis. Vital sign-based approaches include heart rate characteristic algorithms to identify inflammation-induced periods of minimal heart rate variability, decelerations, and/or tachycardia. Monitoring has been associated with reduced all-cause and sepsis-related mortality in randomized controlled trials.55 Moreover, there is an evolution of predictive bioinformatic approaches, such as machine learning modeling and artificial intelligence methods. These aim at patient-level risk assessment based upon clinical data, including vital signs, laboratory results, and clinical parameters (e.g., mechanical ventilation or vasopressor support).56 Approaches are promising, though further evaluation of performance in sepsis recognition and assessment of outcomes are needed prior to clinical implementation. “Omics”-based strategies for sepsis diagnosis (metabolomic, proteomics and genomic approaches) are also being evaluated, but are not yet ready for clinical application.57
MANAGEMENT
Prompt initiation of antibiotic therapy is crucial. Additionally, hemodynamic stabilization via volume resuscitation and/or vasopressor support may be required to counteract vasodilatation and capillary leakage and subsequent hypoperfusion and hypovolemia. Supportive care may also include supplemental oxygen and/or mechanical ventilation, management of acid/base and electrolyte disturbances, and transfusion of blood products. Aggressive supportive interventions are particularly required in infants with fulminant sepsis and development of septic shock.
Antibiotic Treatment
Antibiotic therapy should be administered as quickly as possible once concern for LOS is identified, and ideally after cultures have been obtained. Delayed antibiotic administration for suspected LOS in a level IV NICU was independently associated with increased 14-day mortality (47% increased risk of death for each additional 30-minute delay).58 While prompt empiric antibiotic therapy is critical, the use of antibiotics for suspected LOS must be balanced against potential risks (including drug toxicity, negative interference with healthy skin and gut microbiota, and antibiotic selection pressures).
There is no consensus upon the ideal empiric antibiotic regimen for LOS, and considerable practice variation exists in antibiotic selection and treatment durations.26 59–61 Broad-spectrum empiric antibiotics for LOS generally include two agents with complementary spectra of activity. Beta-lactam agents (e.g., ampicillin, oxacillin, nafcillin) are commonly utilized to provide gram-positive bacterial coverage.60 However, given high rates of LOS due to CoNS (often resistant to beta-lactam antibiotics), vancomycin may be preferred for initial gram-positive coverage. Additional patient factors that may influence decision-making surrounding empiric vancomycin use include presence of indwelling central catheters and known colonization with MRSA, as well as local resistance patterns. Vancomycin use in the NICU is widespread: it was the sixth-most frequently prescribed medication among NICU patients in a large US-based cohort – and after ampicillin and gentamicin, it was the third-most common antibiotic.62 However, due to associated risks of acute kidney injury, vancomycin use requires renal function and drug level monitoring to surveil for toxicity. Agents targeting gram-negative bacteria include aminoglycosides (e.g., gentamicin), 3rd/4th generation cephalosporins (e.g., cefotaxime, cefepime) and carbapenems (e.g., meropenem).63 64 Given its broad aerobic and anaerobic coverage, piperacillin-tazobactam, a β-lactam antibiotic with β-lactamase inhibitor, is frequently used in the context of presumed intra-abdominal sources of infection. Due to its poor central nervous system (CNS) penetration, piperacillin-tazobactam is not recommended in cases of clinical concern for meningitis. Instead, a 3rd/4th generation cephalosporin, or carbapenem, is preferred for gram-negative CNS coverage.
Carbapenems are powerful β-lactam antibiotics with the broadest range of in-vitro activity against gram-positive and gram-negative bacteria (including ESBL-producing Enterobacteriaceae) and are essential reserve antibiotics.64 Linezolid, fosfomycin, and daptomycin are additional reserve antibiotics that may be used in multi-resistant gram-positive infections. Ciprofloxacin and colistin have been used – despite concerns for adverse effects – in neonatal infections with multi-resistant gram-negative bacteria.56 Use of these antibiotics should be limited to definitive and antibiogram-guided therapy of infections with a multidrug-resistant pathogen. They are not recommended as routine empiric therapy. We suggest that clinicians consider consultation with an infectious disease specialist in cases where the use of a “reserve” antibiotic might be indicated.
Major concerns exist about increasing numbers of multidrug-resistant bacteria among NICU cohorts worldwide,60 65 driven by widespread use of vancomycin, 3rd/4th generation cephalosporins and carbapenems.63 64 Empiric antibiotics are often inappropriately used, in terms of unnecessarily broad spectra and prolonged durations of treatment in the setting of negative cultures. In an evaluation of NICU antibiotic utilization as defined by the Centers for Disease Control’s 12-Step Campaign to Prevent Antimicrobial Resistance, up to 25% of all antibiotic courses were considered inappropriate (39% were antibiotic regimens inappropriately continued >72 hours’ duration, others related to inappropriate pathogen targeting).65 Ultimately, local organism epidemiology, resistance patterns, and antibiotic stewardship should guide individual regimens. Judicious and rational use is mandatory, and antibiotic regimens should be narrowed as soon as organism speciation and antibiotic sensitivity data are available. Of note, quality improvement initiatives in settings with low MRSA prevalence have demonstrated a reduction in vancomycin utilization (in favor of anti-staphylococcal penicillins) and reduction in vancomycin-associated acute kidney injury, without impacts on mortality.66 67 The duration of antibiotics for culture-proven infections varies by organism and site. While bacteremia is usually treated with 10–14 days of antibiotics (depending on organism), meningitis requires longer courses of 14–21 days, especially in gram-negative meningitis.68 Empiric antibiotic durations to “rule-out” LOS commonly range 48–72 hours, with discontinuation upon receipt of negative cultures. Given that most blood culture growth occurs within 36 hours (with late culture growth largely driven by CoNS), shorter empiric antibiotic durations may be adequate.69
Adjunctive Therapeutic Interventions
There is an ongoing search for effective adjunctive therapies for neonatal sepsis – mainly aiming at a beneficial modulation of both sepsis-driven hyper-inflammation and sepsis-related functional immunosuppression.17–19 70 Sepsis is characterized by excessive induction of pro-inflammatory and anti-inflammatory pathways, activation of the coagulation cascade and complement system, sepsis-induced neutropenia and thrombocytopenia, and biochemical imbalances resulting in an oxidant state associated with reduced plasma and tissue levels of antioxidants (such as glutathione).70 Clinical and experimental data indicate exaggerated and sustained pro-inflammatory but impaired counter-regulatory responses in preterm infant sepsis, and impaired resolution of inflammation. Numerous therapeutic interventions have been studied for potential utility in counteracting these mechanisms. However, many of these approaches have either failed to affect the prognosis of LOS or are not yet ready for clinical application (Table 2).60 70–89
Table 2:
Adjunctive and Preventive Immunomodulatory Therapies Evaluated in LOS
| Intervention | Mechanism | Meta-analyses/RCTs | Overall Results | Recommendation |
|---|---|---|---|---|
| Adjunctive Therapies | ||||
| Pentoxifylline | Non-specific phosphodiesterase inhibitor with immunomodulatory and anti-inflammatory properties. | (79) | Pentoxifylline used as adjunct to antibiotics decreased mortality without adverse effects - however, overall quality low. | No recommendation for routine use. Future studies needed. |
| Immunoglobulins (IVIG) | Low levels of immunoglobulins (Ig) in preterm infants and reduced Ig levels in severe sepsis. | (74, 78) | No effect on in-hospital mortality and death/major disability at 2y age in preterm infants with suspected/proven LOS | Routine administration not recommended. |
| Granulocyte Transfusion | Quantitative and qualitative deficits in neonatal granulocyte function have been described. | (75) | No significant difference in all-cause mortality. Pulmonary complications. | Not recommended due to inconclusive evidence of safety and efficacy. |
| Granulocyte / Granulocyte-Macrophage Colony Stimulating Factors (G-CSF / GM-CSF) | Reversion of sepsis-induced neutropenia to promote phagocytosis and granulocyte-driven mechanisms of resolution of inflammation. | (71) | No survival advantage at 14 days of G-CSF / GM-CSF treatment. One study: prophylactic GM-CSF may protect against infection in infants with neutropenia/high risk of neutropenia. |
Insufficient evidence both for prophylactic administration and treatment. |
| Antioxidants (Selenium, Vitamin A, Melatonin) | Reduced blood levels of antioxidants in preterm infants and increased risk of oxidative stress. Melatonin has additional anti-inflammatory and anti-apoptotic properties. |
(72, 77, 80, 86) | Routine selenium supplementation reduced number of sepsis episodes. No effect on overall mortality or major neonatal morbidities. Adjunctive Melatonin improved condition. No effect of routine Vitamin A. |
Not yet recommended. |
| Recombinant Activated Human Protein C (rhAPC) | Role of rhAPC in modulating coagulation and inflammation. | (76) | Increased risk of bleeding and higher mortality in trials in adults and children. Withdrawn from the market. |
Neonates should NOT be treated with rhAPC. |
| Antibiotics with Anti-inflammatory Activity (Azithromycin) | Anti-inflammatory and immunomodulatory properties of macrolide antibiotics by inhibition of IL-1 p response. | (88) | Promising results in adult sepsis. No existing trial in neonatal sepsis. Ongoing study on the effect of early IV azithromycin as anti-inflammatory therapy on survival without BPD. |
Research ongoing. |
| Preventive Strategies | ||||
| Human Milk | Contains antimicrobial proteins and peptides and other beneficial components (lyzozymes, secretory IgA, lactoferrin, growth factors, antioxidants, microbiota) protecting against infection and contibuting to healthy microbiome. | (73, 84) | Many benefits of human milk. Formula feeding might be associated with NEC|. However, existing studies provide inconclusive evidence that human milk fe eding p revents infection and mortality. |
Human milk feeding, esp. breast feeding, highly recommended for many reasons. |
| Probiotics & Prebiotics | Dysbiosis of skin and gut microbiome has been associated with increased risk of infection. | (83) | Beneficial effect of probiotic supplementation on LOS risk. However, optimal composition remains to be determined. No evidence of efficacy of prebiotics. |
AAP does not recommend routine and universal use in preterm infants (conflicting data on safety, efficacy and potential harm) |
| Lactoferrin | Iron-binding protein, present in high concentrations in human milk. Wide range of antimicrobial/ immuno- modulatory/anti-inflammatory properties. | (85, 87) | Low quality/no evidence that routine routine enteral lactoferrin reduces the incidence of infection. No effect on mortality or morbidity in preterm infants. | Not recommended. Future studies needed. |
| Glutamine | Insufficiently synthesized in conditions of metabolic sress. | (82) | No effect of preventive supplementation on mortality or major neonatal morbidities. | No evid ence for supplementation apart from clinical trials. |
| Zinc | Vital trace element for growth, cell differentiation and immune function (oxidative stress↓ and pro-inflammatory↓) Low zinc stores in preterm infants. | (89) | Enteral zinc moderately decreased mortality, while no effect on LOS incidence and common morbidities in preterm infants. | No recommendation for use. Future studies needed. |
| Future Therapies? | ||||
| Inflammasome Inhibitors (e.g., Anakinra, MCC950) | Specific blocking of pro-inflammatory IL-1 cytokine cascades initiated by multiprotein complexes of the innate immune system acknowledged as the “inflammasome”. | (70) | Evidence from animal models. Promising results in adult inflammatory diseases. | Research is ongoing. |
| Antimicrobial Proteins & Peptides (α-/β defensins, cathelicidins, bactericidal/permeability-i increasing protein (BPI)) | Antimcrobial peptides and proteins released by innate immune cells and mucosal surfaces contribute to mucosal immunity. Low levels in early life. |
(81) | Evidence of potential benefits in pediatric sepsis. | Research is ongoing. |
PREVENTION
Strategies focused on infection prevention are key to reduce LOS burden.90–92 Preventive measures include hand hygiene, adherence to infection control protocols, implementation of antimicrobial stewardship programs (ASPs), and care practices including early initiation of enteral feeds and use of breast milk.91 93 Preventive immunomodulatory strategies aim at a beneficial modulation of skin and gut microbiome, inflammatory immune responses, and oxidative stress (Table 2).70
Hand Hygiene, Antiseptic Measures, and Colonization Screening
Hand hygiene remains one of the most effective measures to reduce infections associated with care providers.94 Staff use of nonsterile gloves for patient contact may confer additional protection – additive to hand hygiene. A randomized controlled trial demonstrated reductions in late-onset infections among extremely preterm infants whose caregivers used nonsterile gloves following hand hygiene, compared to hand hygiene alone.95 Strict adherence to aseptic protocols prior to line and catheter insertion, in particular, is a key preventive measure.96 Antiseptics, such as chlorhexidine gluconate provided in aqueous and alcoholic forms (0.05% to 2%) and octenidine dihydrochloride, effectively reduce skin colonization with pathogens in preterm and term neonates.97 However, national surveys reveal substantial variation in disinfection practices. In fact, there is no robust evidence in favor of any specific skin disinfectant, and there is no consensus on whether alcoholic or aqueous formulations should be preferred. In very immature preterm infants, there are potential safety and toxicity issues to be taken into account, such as risk of thyroid dysfunction associated with povidone-iodine use.97 Notably, 1% chlorhexidine gluconate was found to be even more effective than 1% povidone-iodine in reducing blood culture contamination rates in moderate preterm and term neonates.98 However, adverse skin reactions to chlorhexidine have been reported in very immature preterm infants.97 Apart from topical and systemic side effects, antiseptic regimens have the potential to promote bacterial resistance. An observational study in two NICUs in the UK and Germany, using chlorhexidine gluconate versus octenidine, demonstrated that long-term use of chlorhexidine for skin antisepsis may select for chlorhexidine and octenidine tolerance among CoNS isolates.99 Finally, there is no convincing evidence of any beneficial effect of full-body washing with antiseptics, such as chlorhexidine bathing, in preterm infants.100 Instead, there are concerns about disturbance of skin pH and skin microbiome as well as disruption of innate antimicrobial and immunological skin properties.100
In addition to antiseptic placement techniques, central line-associated bloodstream infection (CLABSI) prevention quality improvement efforts stipulate a bundle of care measures, including dressing change practices, reduction of daily central catheter accesses, and timely central catheter removal, with the goal of preventing colonization of indwelling central vascular catheters and systemic dissemination of pathogens. In a US-based initiative, a 19% reduction in CLABSI rates was documented among a collaborative of tertiary and quaternary NICUs following implementation of standardized CLABSI prevention bundles.101 Implementation of weekly colonization screening of VLBW infants for high-risk or multidrug-resistant bacteria and subsequent individual extension of hygiene measures has been associated with reduced sepsis rates.102 Routine use of prophylactic systemic antibiotics for CLABSI prevention and routine vancomycin catheter locks, on the contrary, are not recommended – for substantial risk of selection of resistant organisms and rather high numbers needed to treat.103
Antimicrobial Stewardship
ASPs are collaborations between prescribing clinicians, infectious disease specialists, and pharmacists, aiming at critical evaluation and reduction of antibiotic exposures. Examples of ASP-guided interventions include guidance of empiric antibiotic selection and restrictions on broad-spectrum antibiotic use as well as standardization of durations of antibiotic treatment. Single-center reports of ASP implementation in NICUs have demonstrated reductions in antibiotic initiation, improved narrow-spectrum antibiotic selection, and improved rates of timely antibiotic discontinuation. However, programs have had variable impact on overall antibiotic utilization.93 104
OUTCOMES
LOS contributes significantly to neonatal mortality and morbidity.1 3 5 7 Outcomes are affected by etiology and causative pathogen, GA, underlying comorbidities, presence of organ dysfunction, and the cumulative number of infectious insults. Lower GA, higher illness severity, and intra-abdominal, pulmonary, and CNS sites of primary infection are associated with increased mortality in neonatal LOS.15 53
Mortality
Estimates of LOS-associated mortality vary based on the neonatal subpopulation of interest. In a large NICHD Neonatal Research Network cohort study including >10,000 ELBW infants, those with LOS experienced significantly higher all-cause mortality compared to uninfected infants (24% vs. 18%).4 Among VLBW infants, all-cause mortality estimates range from 4.2% of infants with LOS in the GNN,6 to 15% of infants in a large US cohort from the Pediatrix database.14 LOS-related mortality further varies by organism class; specifically, fungal and gram-negative sepsis is associated with higher mortality compared to gram-positive sepsis. Among a large US cohort of ELBW infants, sepsis-attributable mortality occurred in 15% cases of gram-positive LOS, 20% of gram-negative LOS, and 31% of fungal LOS.4 In another large cohort study of >108,000 VLBW infants, organism-specific mortality was highest in Pseudomonas LOS (occurring in 35% of all Pseudomonas infections), followed by H. influenzae (33%), Candida (29%), and S. aureus (21%).14 In 4,094 VLBW infants with culture-proven sepsis in the German Neo-KISS surveillance system, infection with Klebsiella spp. (HR 3.17; 95% CI 1.69–5.95), Enterobacter spp. (hazard ratio (HR) 3.42; 95% CI 1.86–6.27), Escherichia coli (HR 3.32; 95% CI 1.84–6.00) and Serratia spp. (HR 3.30; 95% CI 1.44–7.57) were associated with significantly higher mortality risk compared to S. aureus.105 Of note, available epidemiologic data report CoNS-related mortality in VLBW infants ranging from 1.6% to as high as 11.5%.23 106
Morbidity
Neurodevelopmental impairment (NDI) is a major sequela of LOS.107–109 Central nervous system (CNS) injury results from direct bacterial cytotoxicity, adverse systemic inflammation (even without pathogen invasion into the CNS) and altered brain perfusion in the setting of hemodynamic instability.108 110 Bacterial meningitis has potentially devastating outcomes, and affected infants are at highest risk of poor neurocognitive development – as high as 10-fold increase in risk of moderate or severe neurologic disability at the age of 5 years (up to 15% of meningitis survivors).111 Moreover, white matter is particularly vulnerable to oligodendrocyte injury and aberrant maturation in the face of inflammatory cascades, especially in preterm infants.109 110 In a US cohort of >6,000 ELBW infants, adverse neurodevelopmental outcomes at 18–22 months corrected age were identified in almost 50% of infants with a history of culture-confirmed sepsis.112 Compared to uninfected neonates, those with culture-proven sepsis had significantly higher odds of NDI.112 Emerging literature is investigating the relationship between NDI and “culture-negative” LOS syndromes. A Swiss cohort study of 541 infants born at 24–28 weeks’ GA identified that culture-proven sepsis, but not culture-negative “suspected sepsis”, was associated with increased risks of NDI and CP, compared to uninfected infants.113 A recent US study of >3,900 ELBW infants born at 22–26 weeks’ GA found infants with culture-negative sepsis at increased risk of NDI.7 Increased risks of LOS-associated NDI seem to persist into childhood. A French cohort study identified LOS as a significant risk factor for CP at the age of 5 years (adjusted OR 1.7, 95% CI 1.1–2.6).114 Among children born <28 weeks’ GA, those with a history of LOS were at higher risk of NDI at the age of 10 years compared to uninfected infants. NDI appeared to be largely manifested as intellectual impairment, assessed as low IQ.107
Ultimately, adverse and/or sustained inflammatory immune responses in LOS are a major contributor in the multifactorial pathogenesis of diseases of prematurity, and drive organ injury and life-long morbidity, such as bronchopulmonary dysplasia.18 108 110 115 Finally, LOS has been associated with postnatal growth failure, potentially attributable to inflammation and/or nutritional deficiencies in the setting of critical illness.112 116 In a matched cohort study of ~700 VLBW infants born <32 weeks with sepsis (the majority of which was LOS) growth failure manifested at least 3 weeks after LOS and persisted until NICU discharge.116
CONCLUSIONS AND OUTLOOK
LOS management and prevention pose ongoing challenges in current neonatal care, particularly in the context of increasing populations of very immature preterm infants and rising rates of multi-drug resistant organisms. Early recognition of infants with suspected sepsis is critical to improve timeliness of therapy and optimize outcomes. Future advancements in LOS care may focus on improving diagnostic accuracy via biomarker discovery, incorporation of technology and/or computer-based algorithms for use in LOS recognition, and quality improvement-based implementation of preventive measures. LOS-driven adverse or sustained inflammation has been associated with increased neonatal morbidity, including poor neurodevelopmental outcomes, particularly in preterm neonates. Ongoing efforts to better elucidate the unique features of early life immunity and adverse inflammation may facilitate the development of targeted immunomodulatory therapies. Finally, follow-up of neurocognitive and motor development into childhood and adolescence is necessary to characterize enduring sequelae of infection. Ultimately, antimicrobial stewardship is vital, and clinicians should critically evaluate prescribing practices to target the narrowest effective antimicrobial regimens based on local antibiograms and sensitivity patterns. Moreover, further research is needed to better define ideal empiric antibiotic regimens, acknowledging center-specific variation in patient populations, care practices, and infection epidemiology.
Content Specifications:
Understand the impact of neonatal late onset sepsis on neonatal morbidity and mortality worldwide, particularly among extremely preterm neonates and chronically ill infants (ABP Content 10.A.1)
Review the infectious epidemiology of neonatal late-onset sepsis and approaches to antimicrobial therapy (ABP Content 10.A.1, 10.B.e, f)
Recognize the need for early sepsis recognition, rapid initiation of therapy, and the vital importance of LOS prevention – largely relying upon hand hygiene and adherence to infection control protocols (ABP Content 10.C.1,4,5).
Practice Gap(s) or Education Gap(s):
Neonatal late-onset sepsis (LOS) remains a significant cause of morbidity and mortality in the NICU, particularly among extremely preterm and/or chronically ill infants.
Early recognition and diagnosis of LOS is challenging due to often non-specific presenting features and limited diagnostic efficiency of commonly used biomarkers.
Advances in LOS prevention are needed and may primarily lie in quality-improvement efforts in infection control.
Antimicrobial stewardship practices are vital to reduce antibiotic overuse and emergence of antimicrobial resistance.
Learning Objectives:
After completing this article, readers should be able to
Recognize preventable and non-preventable risk factors for LOS in preterm infants and chronically ill neonates.
Identify the need for continuous evaluation and critical judgment of indwelling central lines, ongoing mechanical ventilation, and prolonged parenteral nutrition, especially in high-risk infants.
Recognize existing limitations in early sepsis recognition and the vital role of rapid initiation of empiric antibiotic therapy and supportive care whenever LOS is suspected.
Understand the importance of judicious empiric antibiotic prescribing and duration of therapy – to avoid adverse effects for individual patients and to reduce selection pressure promoting multi-drug resistant organisms across NICUs and communities.
Funding Source:
SAC is supported by NIH training grant T32HL007891.
Abbreviations
- ASP
antimicrobial stewardship program
- CFU
colony-forming units
- CI
confidence interval
- CLABSI
central line-associated bloodstream infections
- CNS
central nervous system
- CoNS
coagulase negative Staphylococci
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- ELBW
extremely low birth weight
- EOS
early-onset sepsis
- ESBL
extended-spectrum beta lactamase
- GA
gestational age
- HR
hazard ratio
- IL-6
interleukin 6
- LP
lumbar puncture
- LOS
late-onset sepsis
- MRSA
Methicillin-resistant Staphylococcus aureus
- NICU
neonatal intensive care unit
- NDI
neurodevelopmental impairment
- OR
odds ratio
- nSOFA
neonatal-specific sequential organ failure assessment
- PCT
procalcitonin
- SIRS
systemic inflammatory response syndrome
- spp
species
- TNF-alpha
tumor necrosis factor alpha
- UTI
urinary tract infection
- VLBW
very low birth weight
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291 [DOI] [PubMed] [Google Scholar]
- 2.Boghossian NS, Page GP, Bell EF, et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr. 2013;162(6):1120–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah J, Jefferies AL, Yoon EW, et al. Risk Factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks’ gestation. Am J Perinatol 2015;32(7):675–682 [DOI] [PubMed] [Google Scholar]
- 4.Greenberg RG, Kandefer S, Do BT, et al. Late-onset sepsis in extremely premature infants: 2000–2011. Pediatr Infect Dis J. 2017;36(8):774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Glaser K, Speer CP. Late-onset sepsis caused by Gram-negative bacteria in very low birth weight infants: a systematic review. Expert Rev Aanti-infective Therapy. 2019;17(3):177–188 [DOI] [PubMed] [Google Scholar]
- 6.Kostlin-Gille N, Hartel C, Haug C, et al. Epidemiology of early and late onset neonatal sepsis in very low birthweight infants: data from the German Neonatal Network. Pediatr Infect Dis J. 2021;40(3):255–259 [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay S, Puopolo KM, Hansen NI, et al. Neurodevelopmental outcomes following neonatal late-onset sepsis and blood culture-negative conditions. Arch Dis Child Fetal Neonatal Ed. 2021;106(5):467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan BA, Nagraj VP, Berry KL, et al. Clinical and vital sign changes associated with late-onset sepsis in very low birth weight infants at 3 NICUs. J Neonatal Perinatal Med. 2021;14(4):553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan L, Harris MC. New technologies for the rapid diagnosis of neonatal sepsis. Current Opinion Pediatr. 2012;24(2):165–171 [DOI] [PubMed] [Google Scholar]
- 10.Celik IH, Hanna M, Canpolat FE, et al. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. 2022;91(2):337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes R, Hartnett J, Semova G, et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr Res. 2021. doi: 10.1038/s41390-021-01749-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGovern M, Giannoni E, Kuester H, et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res. 2020;88(1):14–26 [DOI] [PubMed] [Google Scholar]
- 13.Wynn JL, Wong HR, Shanley TP, et al. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. 2014;15(6):523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88 Suppl 2:S69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai M-H, Hsu J-F, Chu S-M, et al. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J. 2014;33(1):e7–e13 [DOI] [PubMed] [Google Scholar]
- 16.Villamor-Martinez E, Lubach GA, Rahim OM, et al. Association of histological and clinical chorioamnionitis with neonatal sepsis among preterm infants: a systematic review, meta-analysis, and meta-regression. Front Immunol 2020;11:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strunk T, Prosser A, Levy O, et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Ped Res. 2012;72(1):10–18 [DOI] [PubMed] [Google Scholar]
- 18.Glaser K, Speer CP. Toll-like receptor signaling in neonatal sepsis and inflammation: a matter of orchestration and conditioning. Expert review Clin Immunol. 2013;9(12):1239–1252 [DOI] [PubMed] [Google Scholar]
- 19.Collins A, Weitkamp J-H, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F391–F394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mai V, Torrazza RM, Ukhanova M, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One. 2013;8(1):e52876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuppala VS, Meinzen-Derr J, Morrow AL, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Driscoll DN, McGovern M, Greene CM, et al. Gender disparities in preterm neonatal outcomes. Acta Paediatrica. 2018. doi: 10.1111/apa.14390 [DOI] [PubMed] [Google Scholar]
- 23.Dong Y, Speer CP, Glaser K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence. 2018;9(1):621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergnano S, Menson E, Kennea N, et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F9–F14 [DOI] [PubMed] [Google Scholar]
- 25.Lessa FC, Edwards JR, Fridkin SK, et al. Trends in incidence of late-onset methicillin-resistant Staphylococcus aureus infection in neonatal intensive care units. Pediatr Infect Dis J. 2009;28(7):577–581 [DOI] [PubMed] [Google Scholar]
- 26.Carr JP, Burgner DP, Hardikar RS, et al. Empiric antibiotic regimens for neonatal sepsis in Australian and New Zealand neonatal intensive care units. J Paediatr Child Health. 2017;53(7):680–684 [DOI] [PubMed] [Google Scholar]
- 27.Civardi E, Tzialla C, Baldanti F, et al. Viral outbreaks in neonatal intensive care units: what we do not know. Am J Inect Control. 2013;41(10):854–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan M, Khalil A, Magboul S, et al. Neonates and young infants with COVID-19 presented with sepsis-like syndrome: a retrospective case controlled study. Front Pediatr. 2021;9:634844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinat. 2013;40(1):27–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoll BJ, Hansen N, Fanaroff AA, et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics. 2004;113(5):1181–1186 doi: 10.1542/peds.113.5.1181 [DOI] [PubMed] [Google Scholar]
- 31.Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in late-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31(8):803–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JVE, Meader N, Wright K, et al. Assessment of C-Reactive protein diagnostic test accuracy for late-onset infection in newborn infants: a systematic review and meta-analysis. JAMA Pediatr. 2020;174(3):260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benitz WE, Han MY, Madan A, et al. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics. 1998;102(4):E41. [DOI] [PubMed] [Google Scholar]
- 34.Eschborn S, Weitkamp J-H. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinat. 2019;39(7):893–903 [DOI] [PubMed] [Google Scholar]
- 35.Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turunen R, Andersson S, Nupponen I, et al. Increased CD11b-density on circulating phagocytes as an early sign of late-onset sepsis in extremely low-birth-weight infants. Pediatr Res. 2005;57(2):270–275 [DOI] [PubMed] [Google Scholar]
- 37.Boskabadi H, Maamouri G, Afshari JT, et al. Serum interleukin 8 level as a diagnostic marker in late neonatal sepsis. Iranian J Pediatr. 2010;20(1):41–47 [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J, Tang J, Chen D. Meta-analysis of diagnostic accuracy of neutrophil CD64 for neonatal sepsis. Italian J Pediatr. 2016;42(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillenseger L, Langlet C, Iacobelli S, et al. Early inflammatory markers for the diagnosis of late-onset sepsis in neonates: the Nosodiag study. Front Pediatr. 2018;6:346–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berka I, Korček P, Straňák Z. C-reactive protein, interleukin-6, and procalcitonin in diagnosis of late-onset bloodstream infection in very preterm infants. J Pediatr Infect Dis Soc. 2021. doi: 10.1093/jpids/piab071 [DOI] [PubMed] [Google Scholar]
- 41.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129(2):275–278 [DOI] [PubMed] [Google Scholar]
- 42.Modi N, Doré CJ, Saraswatula A, et al. A case definition for national and international neonatal bloodstream infection surveillance. Arch Disease Child Fetal Neonatal Ed. 2009;94(1):F8–12 [DOI] [PubMed] [Google Scholar]
- 43.Foglia EE, Lorch SA. Clinical predictors of urinary tract infection in the neonatal intensive care unit. J Neonatal-Perinatal Med.2012;5(4):327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downey LC, Benjamin DK, Jr., Clark RH, et al. Urinary tract infection concordance with positive blood and cerebrospinal fluid cultures in the neonatal intensive care unit. J Perinatol. 2013;33(4):302–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weitkamp JH, Aschner JL, Carlo WA, et al. Meningitis, urinary tract, and bloodstream infections in very low birth weight infants enrolled in a heart rate characteristics monitoring trial. Pediatr Res. 2020;87(7):1226–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith PB, Garges HP, Cotton CM, et al. Meningitis in preterm neonates: importance of cerebrospinal fluid parameters. Am J Perinatol. 2008;25(7):421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Naggar W, Afifi J, McMillan D, et al. Epidemiology of meningitis in Canadian neonatal intensive care units. Pediatr Infect Dis J. 2019;38(5):476–480 [DOI] [PubMed] [Google Scholar]
- 48.Jiang S, Yang C, Yang C, et al. Epidemiology and microbiology of late-onset sepsis among preterm infants in China, 2015–2018: A cohort study. Int J Infect Dis. 2020;96:1–9 [DOI] [PubMed] [Google Scholar]
- 49.Chiba N, Murayama SY, Morozumi M, et al. Rapid detection of eight causative pathogens for the diagnosis of bacterial meningitis by real-time PCR. J Infect Chemotherapy. 2009;15(2):92–98 [DOI] [PubMed] [Google Scholar]
- 50.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menon K, Schlapbach LJ, Akech S, et al. Criteria for pediatric sepsis-a systematic review and meta-analysis by the Pediatric Sepsis Definition Taskforce. Critical Care Med.2022;50(1):21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleiss N, Coggins SA, Lewis AN, et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Network Open. 2021;4(2):e2036518–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wynn JL, Mayampurath A, Carey K, et al. Multicenter validation of the neonatal sequential organ failure assessment score for prognosis in the neonatal intensive care unit. J Pediatr. 2021;236:297–300.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan BA, Fairchild KD. Vital signs as physiomarkers of neonatal sepsis. Pediatric Res. 2022;91(2):273–282 [DOI] [PubMed] [Google Scholar]
- 56.Masino AJ, Harris MC, Forsyth D, et al. Machine learning models for early sepsis recognition in the neonatal intensive care unit using readily available electronic health record data. PloS One 2019;14(2):e0212665–e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itenov TS, Murray DD, Jensen J. US. sepsis: personalized medicine utilizing ‘Omic’ Technologies-a paradigm shift? Healthcare. 2018;6(3) doi: 10.3390/healthcare6030111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmatz M, Srinivasan L, Grundmeier RW, et al. Surviving sepsis in a referral neonatal intensive care unit: association between time to antibiotic administration and in-hospital outcomes.J Pediatr. 2020;217:59–65.e1 [DOI] [PubMed] [Google Scholar]
- 59.Litz JE, Goedicke-Fritz S, Härtel C, et al. Management of early- and late-onset sepsis: results from a survey in 80 German NICUs. Infection. 2019;47(4):557–564 [DOI] [PubMed] [Google Scholar]
- 60.Mukhopadhyay S, Wade KC, Puopolo KM. Drugs for the prevention and treatment of sepsis in the newborn. Clin Perinatal. 2019;46(2):327–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korang SK, Safi S, Nava C, et al. Antibiotic regimens for late-onset neonatal sepsis. Cochrane Database Syst Rev. 2021;5:CD013836–CD36. doi: 10.1002/14651858.CD013836.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stark A, Smith PB, Hornik CP, et al. Medication use in the neonatal intensive care unit and changes from 2010 to 2018. J Pediatr. 2022;240:66–71.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dancer SJ. The problem with cephalosporins. J Antimicrobial Chemo. 2001;48(4):463–478 [DOI] [PubMed] [Google Scholar]
- 64.Elshamy AA, Aboshanab KM. A review on bacterial resistance to carbapenems: epidemiology, detection and treatment options. Future Sci OA. 2020;6(3):FSO438–FSO438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel SJ, Oshodi A, Prasad P, et al. Antibiotic use in neonatal intensive care units and adherence with Centers for Disease Control and Prevention 12 Step Campaign to Prevent Antimicrobial Resistance. Pediatr Infect Dis J. 2009;28(12):1047–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holzmann-Pazgal G, Khan AM, Northrup TF, et al. Decreasing vancomycin utilization in a neonatal intensive care unit. Am J Infect Control. 2015;43(11):1255–1257 [DOI] [PubMed] [Google Scholar]
- 67.Hamdy RF, Bhattarai S, Basu SK, et al. Reducing vancomycin use in a Level IV NICU. Pediatrics. 2020;146(2) doi: 10.1542/peds.2019-2963 [DOI] [PubMed] [Google Scholar]
- 68.Sivanandan S, Soraisham AS, Swarnam K. Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Internat J Pediatr. 2011:712150–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukhopadhyay S, Briker SM, Flannery DD, et al. Time to positivity of blood cultures in neonatal late-onset bacteraemia. Arch Dis Childhood Fetal Neonatal Ed. 2022. doi: 10.1136/archdischild-2021-323416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schüller SS, Kramer BW, Villamor E, et al. Immunomodulation to Prevent or Treat Neonatal Sepsis: Past, Present, and Future. Frontiers Pediatr. 2018;6:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carr R, Modi N, Doré C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. 2003(3):CD003066–CD66. doi: 10.1002/14651858.CD003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003(4):CD003312–CD12. doi: 10.1002/14651858.CD003312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Silva A, Jones PW, Spencer SA. Does human milk reduce infection rates in preterm infants? A systematic review. Arch Dis Childhood Fetal Neonatal Ed. 2004;89(6):F509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Group IC, Brocklehurst P, Farrell B, et al. Treatment of neonatal sepsis with intravenous immune globulin. New Eng J Med. 2011;365(13):1201–1211 [DOI] [PubMed] [Google Scholar]
- 75.Pammi M, Brocklehurst P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropenia. Cochrane Database Syst Rev. 2011(10):CD003956–CD56. doi: 10.1002/14651858.CD003956.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kylat RI, Ohlsson A. Recombinant human activated protein C for severe sepsis in neonates. Cochrane Database Syst Rev. 2012(4):CD005385–CD85. doi: 10.1002/14651858.CD005385.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uberos J, Miras-Baldo M, Jerez-Calero A, et al. Effectiveness of Vitamin A in the prevention of complications of prematurity. Pediatr Neonatol. 2014;55(5):358–362 [DOI] [PubMed] [Google Scholar]
- 78.Ohlsson A, Lacy JB. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst Rev. 2015. doi: 10.1002/14651858.CD001239.pub5 [DOI] [PubMed] [Google Scholar]
- 79.Pammi M, Haque KN. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2015(3):CD004205–CD05. doi: 10.1002/14651858.CD004205.pub3 [DOI] [PubMed] [Google Scholar]
- 80.Aggarwal R, Gathwala G, Yadav S, et al. Selenium supplementation for prevention of late-onset sepsis in very low birth weight preterm neonates. J Tropical Pediatr. 2016;62(3):185–193 [DOI] [PubMed] [Google Scholar]
- 81.Battersby AJ, Khara J, Wright VJ, et al. Antimicrobial proteins and peptides in early life: ontogeny and translational opportunities. Front Immunol. 2016;7:309–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moe-Byrne T, Brown JVE, McGuire W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2016(1):CD001457–CD57. doi: 10.1002/14651858.CD001457.pub5 [DOI] [PubMed] [Google Scholar]
- 83.Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics. 2016;137(3):e20153684–e84 [DOI] [PubMed] [Google Scholar]
- 84.Cacho NT, Parker LA, Neu J. Necrotizing enterocolitis and human milk feeding: a systematic review. Clin Perinatol. 2017;44(1):49–67 [DOI] [PubMed] [Google Scholar]
- 85.Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2017;6:CD007137–CD37. doi: 10.1002/14651858.CD007137.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Gendy FM, El-Hawy MA, Hassan MG. Beneficial effect of melatonin in the treatment of neonatal sepsis. J Mat-Fetal Neonatal Med. 2018;31(17):2299–2303 [DOI] [PubMed] [Google Scholar]
- 87.Griffiths J, Jenkins P, Vargova M, et al. Enteral lactoferrin to prevent infection for very preterm infants: the ELFIN RCT. Health Tech Assessment. 2018;22(74):1–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lowe J, Gillespie D, Hubbard M, et al. Study protocol: azithromycin therapy for chronic lung disease of prematurity (AZTEC) - a randomised, placebo-controlled trial of azithromycin for the prevention of chronic lung disease of prematurity in preterm infants. BMJ Open. 2020;10(10):e041528–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Staub E, Evers K, Askie LM. Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates. Cochrane Database Syst Rev. 2021;3:CD012797–CD97. doi: 10.1002/14651858.CD012797.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horbar JD, Carpenter JH, Buzas J, et al. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ (Clinical Res Ed). 2004;329(7473):1004–04. doi: 10.1136/bmj.329.7473.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schulman J, Stricof R, Stevens TP, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–44. doi: 10.1542/peds.2010-2873 [published Online First: 20110221] [DOI] [PubMed] [Google Scholar]
- 92.Wirtschafter DD, Powers RJ, Pettit JS, et al. Nosocomial infection reduction in VLBW infants with a statewide quality-improvement model. Pediatrics. 2011;127(3):419–26. doi: 10.1542/peds.2010-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Araujo da Silva AR, Marques A, Di Biase C, et al. Effectiveness of antimicrobial stewardship programmes in neonatology: a systematic review. Arch Dis Child. 2020;105(6):563–568 [DOI] [PubMed] [Google Scholar]
- 94.Polin RA, Denson S, Brady MT, et al. Strategies for prevention of health care-associated infections in the NICU. Pediatrics. 2012;129(4):e1085–93 [DOI] [PubMed] [Google Scholar]
- 95.Kaufman DA, Blackman A, Conaway MR, et al. Nonsterile glove use in addition to hand hygiene to prevent late-onset infection in preterm infants: randomized clinical trial. JAMA Pediatr. 2014;168(10):909–916 [DOI] [PubMed] [Google Scholar]
- 96.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ponnusamy V, Venkatesh V, Clarke P. Skin antisepsis in the neonate: what should we use? Current Opin Infect Dis. 2014;27(3):244–250 [DOI] [PubMed] [Google Scholar]
- 98.Nuntnarumit P, Sangsuksawang N. A randomized controlled trial of 1% aqueous chlorhexidine gluconate compared with 10% povidone-iodine for topical antiseptic in neonates: effects on blood culture contamination rates. Infect Control Hosp Epidemiol. 2013;34(4):430–432 [DOI] [PubMed] [Google Scholar]
- 99.Sethi DK, Felgate H, Diaz M, et al. Chlorhexidine gluconate usage is associated with antiseptic tolerance in staphylococci from the neonatal intensive care unit. JAC-Antimicrobial Resistance. 2021;3(4):dlab173–dlab73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sankar MJ, Paul VK. Efficacy and safety of whole body skin cleansing with chlorhexidine in neonates--a systemic review. Pediatr Infect Dis J. 2013;32(6):e227–234 [DOI] [PubMed] [Google Scholar]
- 101.Piazza AJ, Brozanski B, Provost L, et al. SLUG Bug: Quality Improvement With Orchestrated Testing Leads to NICU CLABSI Reduction. Pediatrics. 2016;137(1) doi: 10.1542/peds.2014-3642 [DOI] [PubMed] [Google Scholar]
- 102.Härtel C, Faust K, Fortmann I, et al. Sepsis related mortality of extremely low gestational age newborns after the introduction of colonization screening for multi-drug resistant organisms. Antimicrobial Resist Infect Control. 2020;9(1):144–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jardine LA, Inglis GDT, Davies MW. Prophylactic systemic antibiotics to reduce morbidity and mortality in neonates with central venous catheters. Cochrane Database Syst Rev. 2008(1):CD006179–CD79. doi: 10.1002/14651858.CD006179.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ting JY, Paquette V, Ng K, et al. Reduction of inappropriate antimicrobial prescriptions in a tertiary neonatal intensive care unit after antimicrobial stewardship care bundle implementation. Pediatr Infect Dis J. 2019;38(1):54–59 [DOI] [PubMed] [Google Scholar]
- 105.Piening BC, Geffers C, Gastmeier P, et al. Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection. PloS One 2017;12(6):e0180134–e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cantey JB, Anderson KR, Kalagiri RR, et al. Morbidity and mortality of coagulase-negative staphylococcal sepsis in very-low-birth-weight infants. World J Pediatr. 2018;14(3):269–273 [DOI] [PubMed] [Google Scholar]
- 107.Bright HR, Babata K, Allred EN, et al. Neurocognitive outcomes at 10 years of age in extremely preterm newborns with late-onset bacteremia. J Pediatr. 2017;187:43–49.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leviton A, Dammann O, Allred EN, et al. Neonatal systemic inflammation and the risk of low scores on measures of reading and mathematics achievement at age 10 years among children born extremely preterm. Internat J Dev Neurosci. 2018;66:45–53. doi: 10.1016/j.ijdevneu.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sewell E, Roberts J, Mukhopadhyay S. Association of infection in neonates and long-term neurodevelopmental outcome. Clin Perinatol. 2021;48(2):251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75(3):376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bedford H, de Louvois J, Halket S, et al. Meningitis in infancy in England and Wales: follow up at age 5 years. BMJ (Clinical Res Ed). 2001;323(7312):533–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 [DOI] [PubMed] [Google Scholar]
- 113.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128(2):e348–357 [DOI] [PubMed] [Google Scholar]
- 114.Mitha A, Foix-L’Hélias L, Arnaud C, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2):e372–380 [DOI] [PubMed] [Google Scholar]
- 115.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123(5):1314–1319 [DOI] [PubMed] [Google Scholar]
- 116.Flannery DD, Jensen EA, Tomlinson LA, et al. Poor postnatal weight growth is a late finding after sepsis in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2021;106(3):298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]


