Abstract
Cancer is a serious debilitating disease and one of the most common causes of death. In recent decades the high risk of various cancers enforced scientists to discover novel prevention and treatment methods to diminish the mortality of this terrifying disease. Accordingly, its prevention can be possible in near future. Based on epidemiological evidence, there is a clear link between pathogenic fungal infections and cancer development. This association is often seen in people with weakened immune systems such as the elderly and people with acquired immunodeficiency (AIDS). Carcinoma in these people is first seen chronically and then acutely. Although the different genetic and environmental risk factors are involved in carcinogenesis, one of the most important risk factors is fungal species and infections associating with cancers etiology. Now it is known that microbial infection is responsible for initiating 2.2 million new cancer cases. In this way, many recent studies have focused on investigating the role and mechanism of fungal infections in diverse cancers occurrence. This review provides a comprehensive framework of the latest clinical findings and the association of fungal infections with versatile cancers including esophageal, gastric, colorectal, lung, cervical, skin, and ovarian cancer.
Keywords: Cancer, Fungi, Infection, Carcinogenesis, Fungal species
Introduction
Diseases triggered by excessively dividing of the tissue cells that can develop into intrusive lumpy masses is called cancer, which also is mentioned to as tumors. About 200 types of cancers in human kind are currently established by the National Cancer Institute (http://www.cancer.gov/types) and among them, some cancers can spread from their origin to other body tissues in a process so-called metastasis. Cancer classification is based on the tissue and/or organ of the origin e.g. carcinomas that refers to the cancers which arise in tissues or cover body organs. Also, other classes like melanomas, sarcomas, leukemia and lymphomas, are other known kinds of cancers. 1
The cancer Tsunami is going to be more expanded. For instance, 150 000 new cases of colorectal cancers (CRC) are identified annually in the US. So, the studies have been focused on exploring the risk factors of different cancers to drop the incidence of them. Every kind of the cancers is affected by special risk factors, e.g., history of inflammatory bowel disease (IBD), type 2 diabetes and family history that are the main risk factors of CRC. 2,3 It is noticeable that there will be increased incidence, approximately 50%, and near 17 million deaths by 2030 and this fact stress the necessity of discovering the more selective treatments for cancers based on their originating factor especially for the cancers triggered by microbial infections to diminish the unintended harmful outcomes on healthy tissues. 4
Risk factors involved in cancers can vary by the area and country parallel to overall cancer incidence and mortality arises. Factors including age, smoking, diet and fat saturated regimes, body weight, alcohol consumption, exposure to pollutants and/or radioactivity, genetic background, and the infection by bacterial and fungal species have been coupled to the risk of carcinogenesis and versatile types of cancers (Figure 1). The studies revealed that infection by microbial species as a risk factor, is responsible for initiating of 2.2 million new cancer cases. 5 Nowadays, it is estimated that around 16% of the overall cancer incidences is associated to microbial infections and toxicities. On the other hand, it is proposed that the microbial infection not only can boost the cancer risk but can also assist its treatment. 6
Figure 1.
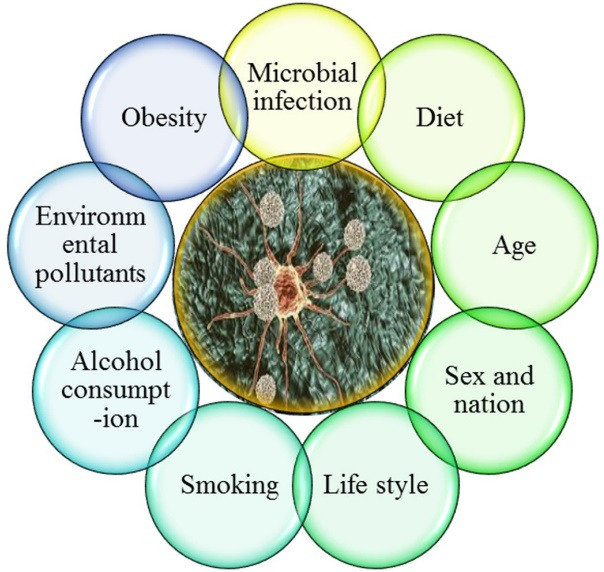
Different types of risk factors in carcinogenesis
Invasive fungal infections (IFIs) maintain to pose a challenge in cancer development and are linked with a high incidence of mortality and morbidity. By the last 20 years, despite the development of numerous diagnostics and therapeutic in the field of fungal infections, the IFI mortality has sustained to 35.7% growth. This means that the cancer growth rate is probably affected by IFI and it’s needed to be scrutinized. 7 The most common genera and species of fungi involved in cancers are Candida albicans, C. glabrata, C. tropicalis, Aspergillus flavus, A. parasiticus, Fusarium verticillioides and F. proliferatum. Nevertheless, continuous efforts to distinguish the association between the cancers and fungal infections should be nonstop since it is not expected that the combat against cancer to be won soon (Figure 2). 8
Figure 2.
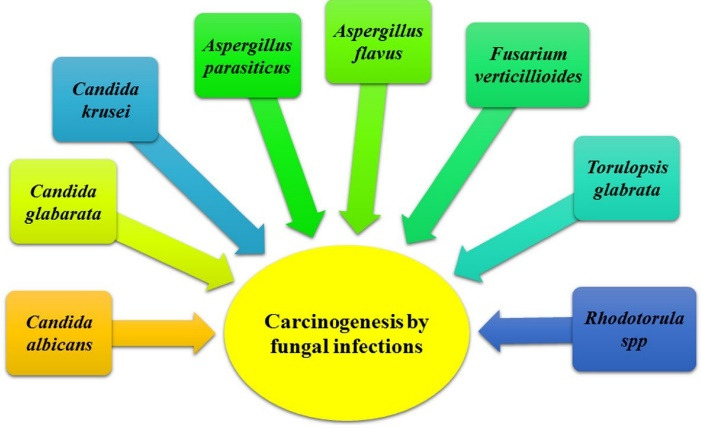
Different studied fungal genera and species in carcinogenesis.
In the present review, the role of opportunistic and pathogenic fungal infections with carcinogenesis of various organs, especially solid organs has been reviewed based on the latest clinical research studies and the most up-to-date scientific articles published in databases including PubMed and Scopus. Before this review, there was no complete report on the relationship between fungi and carcinogenesis of solid organs; however, the current study has thoroughly investigated the gap between the role of different fungi and carcinogenesis.
The role of fungal infections in esophageal cancer
Esophageal carcinoma is the sixth leading cause of cancer-related mortality and the eighth leading cause of cancer worldwide. The disease is more prevalent in men, 9 but in most countries of the world, the incidence rate per 100 000 people affects 2.5-5 men and 1.5-2.5 women. 10 Histologically, the disease is classified into two categories: esophageal adenocarcinoma, which affects the lower esophagus, and esophageal squamous cell carcinoma, which affects the middle and upper esophagus. 11 Numerous risk factors for this type of cancer have been reported, including smoking, alcohol consumption, consumption of hot beverages and foods, poor nutrition, exposure to chemicals, environmental pollutants and radiation, and exposure to pathogenic and opportunistic microorganisms. 12-14 In addition, various fungal infections can arise for esophageal carcinoma, the most common fungus is Candida spp., which is an opportunistic yeast that colonizes the mucosa of the genitourinary and gastrointestinal tract of healthy people (about 30%-50% of people) without causing infection. Candida mostly occurs during the immune system suppression which manifests by skin, nails, and mucosa (esophagus) signs. In relation to mucus, this yeast causes chronic mucocutaneous candidiasis disease (CMCD). 15 The most common genera and species of candida associated with candidiasis are the following: Candida albicans, C. glabrata, C. tropicalis, 16 C. krusei, C. parapsilosis, Torulopsis glabrata, T. tomato, Aspergillus flavus, A. parasiticus, Fusarium verticillioides, F. proliferation. 17-20 According to a study by Koo et al, in patients with primary immunodeficiency, esophageal candidiasis occurs in cancer patients as CMCD with persistent or recurrent thrush. CMCD is caused by at least five mutations that ultimately affect the IL-17 pathways; One of these is a mutation in the autosomal dominant gene gain of function of the STAT 1 signaling protein, in which a genetic defect intensifies Th1 immune responses and ultimately reduces the production of IL-17 and IL-22. 21 In another study, Domingues-Ferreira et al examined a patient with chronic mucocutaneous candidiasis who had decades of treatment-resistance esophageal candidiasis and eventually developed epidermoid esophageal cancer. In this patient, modulation of immune function induced by mitogen suppression and antigen-induced lymphoproliferation disrupted NK cytotoxic activity and IL-2 secretion, resulting in increased IL-4 secretion and lymphocyte apoptosis. 22
The role of fungal infections in gastric cancer
Gastric cancer (GC) is the fourth leading cause of malignancy and the second leading cause of mortality in all malignancies worldwide. 23,24 The five-year survival rate is 90% in Japan and 10%-30% in European countries. Due to the incidence of GC, more than 50% of new cases occur in developing countries. 25,26 GC is generally classified into four categories: Sporadic gastric cancer (SGCs), 27 Early onset gastric cancer (EOGC), 27 gastric stump cancer (GSC), 28 and hereditary diffuse gastric cancer (HDGC). 29 Risk factors for this cancer include smoking, 30 alcohol consumption, 31 obesity, 32 pernicious anemia and blood type A, 33 mycotoxins 34 and Helicobacter pylori infection. 35
Preliminary studies based on culture-dependent methods have shown that fungi make up about 70% of the digestive system of adults and their number is in the range of 102. 36 The presence of some fungi can contribute to development of GC, including C. albicans, 37 Phialemonium spp., 38 Aspergillus spp., Penicillium spp., and Fusarium spp. 39 But in general, number of commensal fungal species are predominant in the gastrointestinal tract, including S. cerevisiae, Malassezia spp., and some species are classified as transient microbiota, e.g., Aspergillus spp., Mucor spp., Cryptococcus spp., Rhodotorula spp., Trichosporon spp., Histoplasma spp., Coccidioides spp., Paracoccidioides spp. and Blastomyces spp. 40,41 Among these fungi, there is ample evidence that C. albicans is able to initiate and develop cancerous events through several mechanisms: (1) Production of carcinogens such as acetaldehyde, which contribute to the progression of cancer (C. albicans, C. tropicalis, C. parapsilosis produce more acetaldehyde than other species of Candida). 41 (2) Induction of inflammatory processes that contributes to tumor metastasis (using cytokines such as CXCL1, CXCL2, CXCL3, TNF-α, and IL-18 facilitates tumorigenesis, angiogenesis, and metastasis). 34,42-45 (3) Processes related to molecular mimicry (molecular mimic of the protein associated with complement receptor 3 (CR3-CP) C. albicans that supports cancer progression). 46 (4) The Th17 response (Th17 is a subset of CD4 + T cells that are active in response against C. albicans, and another cytokine from the Th17 family, IL-23, increases angiogenesis and tumor growth, and antagonizes IL-12 and IFN. 47,48 Another study showed that 54.2% of patients with gastric ulcer and 10.3% of patients with chronic gastritis had candidiasis, which was evident in 20% of patients with gastric cancer. 49,50
The role of fungal infections in colorectal cancer
CRC, the most common type of cancer worldwide, is the third leading cause of cancer and the fourth leading cause of cancer deaths, with a mortality rate of about 700 000. Overall, CRC accounts for 11% of all diagnosed cancers. 51 By 2018, about 1.8 million new cases of CRC have been reported. 2 The incidence of CRC in men is about 19.7 and in women 23.6 per 100 000 people and it is more common in developed countries. 52 The mortality rate until 2018 was about 881 000. 2 In general, mutations in oncogenes, tumor suppressor genes, and genes involved in DNA repair are associated with the development of CRC, and according to the primary origin of the mutation, CRCs are classified into three categories: sporadic (Most point mutations cause this type of CRC and occur in the genes APC [adenomatous polyposis coli], kRAS, Tp53, and DCC), 53 hereditary (Most point mutations occur in another allele of the mutated gene, and this inherited form is divided into two groups: FAP (familial adenomatous polyposis) and HNPCC (Hereditary non-polyposis CRC due to mutations in DNA repair proteins such as MSH2, MLH1, MLH6, PMS1, and PMS2 occur), 54,55 and familial. Environmental and hereditary risk factors play an important role in the development of CRC. CRC risk factors are classified into two categories: non-modifiable risk factors such as race, 56 sex and age, 2 IBD, 57 cystic fibrosis, 58 and cholecystectomy 59 as well as modifiable risk factors such as obesity, 60 diet, 61 smoking and alcohol, 62 and diabetes and resistance to insulin. 63
The role of fungal agents in CRC has not been well understood due to low frequency and lack of reference genome, but using high-throughput sequencing methods, they have been able to identify several fungal species, 64 and recently their role in IBD has been proven. 65 Among the fungi that are associated with CRC, the following can be mentioned: Candida genus such as C. tropicalis and C. albicans, Phoma, 66 Malassezia, 67 Trichosporon, 68 Cladosporium 69 and so on. In addition to the above, fungi such as Paracoccidioides, Histoplasma, Cryptococcus, Aspergillus, Penicillin, Zygomycetes, Pneumocystis, Scedosporiosiscause colonic involvement and may eventually be involved in CRC formation. 70 In this regard, a number of studies have revealed the relationship between fungal agents and CRC. According to a study by Luan et al, they examined fungal microbiota of biopsy specimens from intestinal adenomas and adjacent tissues using the deep sequencing technique and found that 45% of fungal microbiota contained the opportunistic pathogen Candida and Phoma and approximately 1% Cladosporium, Trichosporon, Rhodotorula, Thanatephorus, and Plectosphaerella. 66 In another study by Gao in 2017, it was found that the ratio of ascomycota to basidiomycota was different in the three groups of control, polyp, and CRC, so that ascomycota has a frequency of 37-54% and basidiomycota 4-5%. 71 In 2019, Coker et alalso examined colonic fungal microbiota, which are used as diagnostic markers for CRC, such as A. flavus, Kwoniella mangrovensis, Pseudogymnoascus sp. VKMF-4518, Debaryomyces fabryi, A. sydowii, Moniliophthora perniciosa, K. heavenensis, A. ochraceoroseus, Talaromyces islandicus, Malassezia globosa, Pseudogymnoascus sp. VKM F-4520, A. rambellii, Pneumocystis murinaand Nosema apis and also found fungi such as Malassezia, Moniliophthora, Rhodotorula, Acremonium, Thielaviopsis and Pisolithus is associated with CRC. 72
The role of fungal infections in lung cancer
Lung cancer (LC) is one of the most common causes of cancer death in the world. LC incidence is 1.35 million new cases (representing 12.4% of all new cancer cases) and mortality is 1.18 million deaths (accounting for 17.6% of the global total). 26 The five-year survival rate in newly diagnosed LC is 15%. 73 Men are more likely to develop LC than women, and it affects more African American men. 74 One of the major causes of LC is disruption of the molecular pathways of the lung cell, which alters normal cell growth, differentiation, and apoptosis. 75 In general, LC is pathologically present in two forms: NSCLC (non-small cell lung carcinoma) (85% of cases) and SCLC (small-cell lung carcinoma) (15% of cases). 76 Many internal and external factors are considered as risk factors of LC, including smoking, arsenic in drinking water, radioactive radon gas, asbestos, tuberculosis, COPD, HIV infection, 77 and mutations in genes EGFR, 78 BRAF, 79 KRAS, 80 and so on. As mentioned, one of the risk factors for LC is people with immunocompromised systems who become susceptible to lung disease and eventually LC by exposure to opportunistic fungi such as Aspergillus sp., Cryptococcus sp., Pneumocystis sp. and endemic fungi. 81 For example, in opportunistic IPA (invasive pulmonary aspergillosis), people with immunocompromised systems are susceptible to Aspergillus and Cryptococcus and pose a serious threat to people with cancer. 82 Thus, the differential diagnosis of LC solid tumors has revealed types of fungi such as Trichosporon, Fusarium, Rhizopus, Histoplasma capsulatum, H. immitis, and Cryptococcus neoformans. It has been shown that in patients with LC who receive corticosteroids, they open the way for the entry of opportunistic pathogens such as Aspergillus and P. jiroveci and exacerbate the disease. 83 Another study reported that the dimorphic and opportunistic fungal pathogen T. marneffei, the causative agent of talaromycosis, causes lung infections in people with immunocompromised systems. Following a study by Ching-López and Rodríguez Pavón, a 56-year-old HIV-free woman was reported to have talaromycosis and co-infected with stage IV NSCLC. The patient received liposomal amphotericin B but she died due to a decrease in blood oxygen levels as well as a decrease in the level of blood cells. 84 Another study by Watanabe et al was conducted in 2019 on a 69-year-old man who underwent chemoradiotherapy due to LC but later developed SIPA (subacute invasive pulmonary aspergillosis), a disease caused by A. fumigatus, due to immunocompromised system. For the treatment of fungal infections, he received amphotericin B in combination with voriconazole. 85
The role of fungal infections in cervical cancer
Cervical cancer (CC) is the third most common cancer of the female reproductive system in the world. 86 According to a 2012 report, the standard rate for cervical cancer is about 14 cases per 100 000 people. 9 In general, by 2018, about 530 000 women were diagnosed with CC, of which 257 000 died. 87 The incidence of CC is higher in African and South Asian countries, and its incidence and mortality vary in most parts of the world. As a result, developed countries account for 86% of all CC cases and 88% of all CC deaths. 24,87 The incidence rate of CC in Iran is about 2.61 per 100 000 people. 88 The five-year survival rate in developed countries is about 66%. 89 There are a number of risk factors for CC, including: reproductive factors, obesity, sexual behavior, diet, multiparity, prolonged use of hormonal contraceptives, poor socioeconomic status, smoking, Multiple pregnancies, and finally the presence of bacterial, viral and fungal infectious agents. 90 A 2018 study identified several fungi from the cervicovaginal region, including Candida, Malassezia, Sporidiobolaeae, Saccharomyces, Nakaseomyces, Gjaerumia, and Pleosporales. 91 According to a study by Moradi et al in Iran, one of the important fungi that is the link between cervicovaginal infections and cervical cancer was C. albicans, which was reported in women with high socioeconomic status in Bandar Abbas. 92 In 2015, Neves et al studied a 42-year-old woman who had received immunosuppressive therapy for cervical neoplasia and developed fungemia due to Cryptococcus laurentii with a weakened immune system and was eventually treated with fluconazole. 93
The role of fungal infections in skin cancer
Skin cancer (SC) is one of the most common types of cancer among white populations and is generally classified into two categories: melanoma and non-melanoma (NMSC). 94 According to the latest statistics from 2020, the number of new cases of skin cancer is estimated at 108 420 people and the approximate number of deaths is about 11 480 people. 95 Remember that the increase in melanoma incidence is not parallel to the increase in mortality rates. NMSC, which includes Bowens disease, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC), is more prevalent among Caucasian populations. 96 Increased incidence of SC is associated with several factors including UV exposure, light skin (albinism), old age, male gender, chemicals (such as arsenic), radiation exposure, XP, weakened immune system, smoking, and viral (HPV) and fungal infections. 97 Fungal infections have been shown to occur more frequently in people with weakened immune systems. Initially, most fungal infections were due to the presence of Candida species, but recently it has been shown that the frequency of Aspergillus sp. is increasing. 98 In general, two types of fungal infections are involved in skin cancer: infections caused by pathogenic fungi such as C. neoformans, H. capsulatum, C. immitis, Trichosporonspp. etc., as well as opportunistic fungi such as Candida sp. (e.g., C. albicans, C. tropicalis, C. glabrata, C. parapsilosis), Aspergillus, Mucorales, Fusarium, Rhizopus. 99 As mentioned, opportunistic fungal infections cause disease in people with weakened immune systems. In this regard, Pulido et al. in 2018 studied solid organ transplant recipients (SOTR) in such a way that by immunosuppression, graft survival is increased and this factor causes skin cancer and a variety of infections, followed by Those opportunistic infections are caused by black filamentous fungi such as A. alternata, A. infectoria, C. cladosporioides, M. arundinis, and E. oligosperma. 100 Another study was conducted in 2016 by Brothers and Daveluy on a 61-year-old man whose immune system was suppressed due to receiving chemotherapy drugs for multiple myeloma. Following the injury, his finger became sore with a rose thorn and despite the weak immune system, the wound developed and squamous cell carcinoma developed and C. parapsilosis was isolated from the patient. 101
The role of fungal infections in ovarian cancer
Ovarian cancer (OC) is the seventh most common type of cancer in women in the world and after breast cancer is the second most common type of cancer in women over 40 (in a developed country). 102,103 The highest incidence rates are observed in Eastern and Southern Europe and the lowest rates are observed in China. 104 The five-year survival rate in the early stages of OC is about 29%, but when the tumor progresses it reaches about 92%. 105 According to the latest statistics by 2020, the number of new OC cases is estimated at 21,750 and the number of deaths estimated at 13 940. 95 Pathologically, OC is divided into three categories: epithelial OC (90% of cases), stromal OC (5-6% of cases), and genital OC (2-3% of cases). 106 OC-related risk factors include: Reproductive risk factors (such as continuous ovulation) 107 and hormonal factors (such as gonadotropins), 108 family history and genetic predisposition (such as mutations in genes BRCA1, BRCA2 and MMR), 109 ethnicity and race (such as Jews, Dutch, and French Canadians), diet (fiber and vitamin low D intake), 110 lactation, 111 obesity, 112 smoking 113 and alcohol consumption. 114 Although fungal infections are not a risk factor for OC, a research team in 2017 studied the association between OC and fungal infections. According to the study, the following fungi were found in cancer samples: Pneumocystis, Acremonium, Cladophialophora, Malassezia, Microsporidia Pleistophora, Ajellomyces, Aspergillus, Candida, Cladosporium, Coccidioides, Cryptococcus, Cunninghamella, Issatchenkia, Nosema, Paracoccidioides, Penicillium, Pleistophora, Rhizomucor, Rhizopus, Rhodotorula, Trichophyton. 115
Results and Discussion
From the literature, it appears that fungal infections may play a significant role in the risk for precancerous lesions. The most common fungal species involved in cancers development are Aspergillus flavus, A. parasiticus, Fusarium proliferatum, F. verticillioides, Candida albicans, C. tropicalis,and C. glabarata. 115 Furthermore, the ability of fungal species in carcinogens production such as acetaldehyde, nitrosamine, mycotoxins, and the generation of proinflammatory cytokines, can be risk factors in the promotion of various cancers. 26
Moreover, the potential role of fungal infections in oncogenic processes is the subject of debate in recent surveys of this topic. There are suggestions that fungal infection is a cause of cancers development possibly when the infection is chronic and connected to risk factors such as alcohol, tobacco, and etc.; however, each class of cancer is influenced by different risk factors, such as family history, type 2 diabetes, and obesity, which are the main risk factors of cancer. 50,52
Along with the increased incidence of cancer associated with fungal infections more thorough surveys of the epidemiology of cancer patients with fungal infections can help to determine which patients are most likely to be infected. Therefore, superior monitoring and diagnostic efforts can expand the accuracy of diagnosis of fungal infection and will increase patient outcomes by permitting intervention at an earlier stage of invasive cancers. 2,3 The value of controlled clinical studies of patients with fungal infections will greatly increase our knowledge of the most operative methods of treating disseminated cancer disease in immune-compromised cancer patients.
Conclusion
Several specific fungi are associated with carcinogenesis (Table 1). Recent studies indicated that the pathogenic fungal populations were increased in cancerous patients. It has been shown that the development of carcinogenesis is closely related to a fungal profile. The most common genera of fungi that contribute to the development of versatile cancers are Candida sp., Aspergillus sp., and Fusarium sp. causing an inflammation and consequently contributing to the progression of various cancers. Although, the link between infectious fungi and carcinogenesis is not undeniable, we cannot restrict to the study of fungal microbiota. In fact, we need to explore the intimate connections that could exist with fungi and their possible role on carcinogenesis through their effect on the varied and complex immune system. It seems that the manipulation of fungal profile can be a helpful approach in the rapid improvement of patients after anti-cancer treatment. Therefore, the focus on the potential effect of infectious fungi on the initiation of cancers and consequently the treatment of fungal infectious disease as a global approach is indispensable to predicate the possible subsequences of our current public health strategies.
Table 1. The latest clinical finding and the association of fungal infections with versatile cancers.
| Cancer type | Fungal agents | References | |
| Esophageal cancer | Candida sp. | C. albicans, C. glabrata, C. tropicalis | 16 |
| C. krusei, C. parapsilosis | 116 | ||
| Torulopsissp. | T. glabrata, T. tomata | 17,117,118 | |
| Aspergillus sp. | A. flavus, A. parasiticus | 18-20,119 | |
| Fusarium sp. | F. verticillioides, F. proliferation | ||
| Gastric cancer | C. albicans, Phialemonium spp., Aspergillus spp., Penicillium spp., Fusarium spp. | 37,38,39 | |
| S. cerevisiae, Malassezia spp., Mucor spp., Cryptococcus spp., Rhodotorula spp., Trichosporon spp., Histoplasma spp., Coccidioides spp., Paracoccidioides spp. and Blastomyces spp. | 40,41 | ||
| Gallbladder cancer | Aspergillus sp. | A. flavus, A. parasiticus |
39
120,121 122 51 |
| Penicillium | |||
| Candida sp. | C. albicans, C. glabrata, C. tropicalis | ||
| Blastomyces dermatitidis, Cryptococcus neoformans | |||
| Colorectal cancer | C. tropicalis, C. albicans, Phoma, Malassezia, Trichosporon, Cladosporium |
66-69
70 66 72 |
|
| Paracoccidioides, Histoplasma, Cryptococcus, Aspergillus, Penicillin, Zygomycetes, Pneumocystis, Scedosporiosis | |||
| Rhodotorula, Thanatephorus, Plectosphaerella | |||
| A. flavus, Kwoniella mangrovensis, Pseudogymnoascus sp., Debaryomyces fabryi, A. sydowii, Moniliophthora perniciosa, K. heavenensis, A. ochraceoroseus, Talaromyces islandicus, Malassezia globosa, | |||
| Colorectal cancer | Pseudogymnoascus sp., A. rambellii, Pneumocystis murina, Nosema apis, Malassezia, Moniliophthora, Rhodotorula, Acremonium, Thielaviopsis, Pisolithus | 72 | |
| Lung cancer | Aspergillus sp., Cryptococcus sp., Pneumocystis sp. | 81 | |
| Trichosporon, Fusarium, Rhizopus, Histoplasma capsulatum, H. immitis, P. jiroveci | 83 | ||
| T. marneffei | 84 | ||
| A. fumigatus | 85 | ||
| Prostate cancer | Candida sp., Aspergillus sp., C. neoformans, C. immitis, H. capsulatum, B. dermatitidis | 123 | |
| Cervical cancer | Candida, Malassezia, Sporidiobolaeae, Saccharomyces, Nakaseomyces, Gjaerumia, Pleosporales | 91 | |
| Cryptococcus laurentii | 93 | ||
| Skin cancer | C. neoformans, H. capsulatum, C. immitis, Trichosporonspp., Mucor, Fusarium, Rhizopus | 99 | |
| Candida sp., C. albicans, C. tropicalis, C. glabrata, C. parapsilosis | 99 | ||
| A. alternata, A. infectoria, C. cladosporioides, M. arundinis, E. oligosperma | 100 | ||
| Breast cancer | Aspergillus, Candida, Coccidioides, Cunninghamella, Geotrichum, Pleistophora, Rhodotorula, Filobasidiella, Mucor, Trichophyton, Epidermophyton, Fonsecaea, Pseudallescheria, Penicillium, Ajellomyces, Alternaria, Rhizomucor, Piedraia, Malassezia | 124 | |
| Ovarian cancer | Pneumocystis, Acremonium, Cladophialophora, Malassezia, Microsporidia Pleistophora, Ajellomyces, Aspergillus, Candida, Cladosporium, Coccidioides, Cryptococcus, Cunninghamella, Issatchenkia, Nosema, Paracoccidioides, Penicillium, Pleistophora, Rhizomucor, Rhizopus, Rhodotorula, Trichophyton | 115 | |
Acknowledgments
The authors acknowledge Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran and Université de Paris, Faculté des Sciences, Paris, France.
This work was supported and funded scheme by Tabriz University of Medical Sciences (PharmD. Thesis).
Funding
This work was supported by Tabriz University of Medical Sciences (Number: 52/6456), Tabriz, Iran.
Ethical Issues
Not applicable.
Conflict of Interest
All authors indicated that they have no conflicts of interest to disclose and are aware of its submission.
References
- 1.Ashu EE, Xu J, Yuan ZC. Bacteria in cancer therapeutics: a framework for effective therapeutic bacterial screening and identification. J Cancer. 2019;10(8):1781–93. doi: 10.7150/jca.31699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Amersi F, Agustin M, Ko CY. Colorectal cancer: epidemiology, risk factors, and health services. Clin Colon Rectal Surg. 2005;18(3):133–40. doi: 10.1055/s-2005-916274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167–81. doi: 10.1002/cam4.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–16. doi: 10.1016/s2214-109x(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 6.Eyvazi S, Asghari Vostakolaei, Dilmaghani A, Borumandi O, Hejazi MS, Kahroba H, et al. The oncogenic roles of bacterial infections in development of cancer. Microb Pathog. 2020;141:104019. doi: 10.1016/j.micpath.2020.104019. [DOI] [PubMed] [Google Scholar]
- 7.Suganthini Krishnan N. Emerging fungal infections in cancer patients-a brief overview. Med Mycol. 2016;2(3):16. doi: 10.21767/2471-8521.100016. [DOI] [Google Scholar]
- 8.Chapeland-Leclerc F, Dilmaghani A, Ez-Zaki L, Boisnard S, Da Silva B, Gaslonde T, et al. Systematic gene deletion and functional characterization of histidine kinase phosphorelay receptors (HKRs) in the human pathogenic fungus Aspergillus fumigatus. Fungal Genet Biol. 2015;84:1–11. doi: 10.1016/j.fgb.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 10.Chang F, Syrjänen S, Wang L, Syrjänen K. Infectious agents in the etiology of esophageal cancer. Gastroenterology. 1992;103(4):1336–48. doi: 10.1016/0016-5085(92)91526-a. [DOI] [PubMed] [Google Scholar]
- 11.Bollschweiler E, Hölscher AH, Metzger R. Histologic tumor type and the rate of complete response after neoadjuvant therapy for esophageal cancer. Future Oncol. 2010;6(1):25–35. doi: 10.2217/fon.09.133. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai N, Wakai T, Akazawa K, Ling Y, Wang S, Shan B, et al. Heavy alcohol intake is a risk factor for esophageal squamous cell carcinoma among middle-aged men: a case-control and simulation study. Mol Clin Oncol. 2013;1(5):811–6. doi: 10.3892/mco.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrici J, Eslick GD. Hot food and beverage consumption and the risk of esophageal cancer: a meta-analysis. Am J Prev Med. 2015;49(6):952–60. doi: 10.1016/j.amepre.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Tong Y, Yang C, Gan Y, Sun H, Bi H, et al. Consumption of hot beverages and foods and the risk of esophageal cancer: a meta-analysis of observational studies. BMC Cancer. 2015;15:449. doi: 10.1186/s12885-015-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maródi L. Mucocutaneous candidiasis. InStiehm’s Immune deficiencies. Academic Press. 2014: 775-802.
- 16.Takahashi Y, Nagata N, Shimbo T, Nishijima T, Watanabe K, Aoki T, et al. Long-term trends in esophageal candidiasis prevalence and associated risk factors with or without HIV infection: lessons from an endoscopic study of 80,219 patients. PLoS One. 2015;10(7):e0133589. doi: 10.1371/journal.pone.0133589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MX, Cheng SJ. Carcinogenesis of esophageal cancer in Linxian, China. Chin Med J (Engl) 1984;97(5):311–6. [PubMed] [Google Scholar]
- 18.Chauhan NM, Washe AP, Minota T. Fungal infection and aflatoxin contamination in maize collected from Gedeo zone, Ethiopia. Springerplus. 2016;5(1):753. doi: 10.1186/s40064-016-2485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin S, Ramos AJ, Cano-Sancho G, Sanchis V. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol. 2013;60:218–37. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 20.Yazar S, Omurtag GZ. Fumonisins, trichothecenes and zearalenone in cereals. Int J Mol Sci. 2008;9(11):2062–90. doi: 10.3390/ijms9112062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo S, Kejariwal D, Al-Shehri T, Dhar A, Lilic D. Oesophageal candidiasis and squamous cell cancer in patients with gain-of-function STAT1 gene mutation. United European Gastroenterol J. 2017;5(5):625–31. doi: 10.1177/2050640616684404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domingues-Ferreira M, Grumach AS, Duarte AJ, De Moraes-Vasconcelos D. Esophageal cancer associated with chronic mucocutaneous candidiasis. Could chronic candidiasis lead to esophageal cancer? Med Mycol. 2009;47(2):201–5. doi: 10.1080/13693780802342545. [DOI] [PubMed] [Google Scholar]
- 23.Wright NA, Poulsom R, Stamp G, Van Noorden S, Sarraf C, Elia G, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 25.Stock M, Otto F. Gene deregulation in gastric cancer. Gene. 2005;360(1):1–19. doi: 10.1016/j.gene.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 27.Skierucha M, Milne AN, Offerhaus GJ, Polkowski WP, Maciejewski R, Sitarz R. Molecular alterations in gastric cancer with special reference to the early-onset subtype. World J Gastroenterol. 2016;22(8):2460–74. doi: 10.3748/wjg.v22.i8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorban S, Böttcher K, Etter M, Roder JD, Busch R, Siewert JR. Prognostic factors in gastric stump carcinoma. Ann Surg. 2000;231(2):188–94. doi: 10.1097/00000658-200002000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70(1):50–5. doi: 10.1002/1097-0142(19920701)70:1<50::aidcncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19(7):689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 31.Jedrychowski W, Wahrendorf J, Popiela T, Rachtan J. A case-control study of dietary factors and stomach cancer risk in Poland. Int J Cancer. 1986;37(6):837–42. doi: 10.1002/ijc.2910370607. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4(2):85–92. [PubMed] [Google Scholar]
- 33.Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, Ekbom A, et al. Pernicious anemia and subsequent cancer A population-based cohort study. Cancer. 1993;71(3):745–50. doi: 10.1002/1097-0142(19930201)71:3<745::aidcncr2820710316>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Cuesta J, Hernando FL, Mendoza L, Gallot N, de Cerio AA, Martínez-de-Tejada G, et al. Candida albicans enhances experimental hepatic melanoma metastasis. Clin Exp Metastasis. 2010;27(1):35–42. doi: 10.1007/s10585-009-9300-9. [DOI] [PubMed] [Google Scholar]
- 35.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 36.Schulze J, Sonnenborn U. Yeasts in the gut: from commensals to infectious agents. Dtsch Arztebl Int. 2009;106(51-52):837–42. doi: 10.3238/arztebl.2009.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karczewska E, Wojtas I, Sito E, Trojanowska D, Budak A, Zwolinska-Wcislo M, et al. Assessment of co-existence of Helicobacter pylori and Candida fungi in diseases of the upper gastrointestinal tract. J Physiol Pharmacol. 2009;60 Suppl 6:33–9. [PubMed] [Google Scholar]
- 38.von Rosenvinge EC, Song Y, White JR, Maddox C, Blanchard T, Fricke WF. Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J. 2013;7(7):1354–66. doi: 10.1038/ismej.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16(3):497–516. doi: 10.1128/cmr.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miranda LN, van der Heijden IM, Costa SF, Sousa AP, Sienra RA, Gobara S, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72(1):9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33(12):1959–67. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- 42.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68(1):1–8. doi: 10.1189/jlb.68.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, et al. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000;67(1):53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16(6):593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez-Garcia A, Arteta B, Abad-Diaz-de-Cerio A, Pellon A, Antoran A, Marquez J, et al. Candida albicans increases tumor cell adhesion to endothelial cells in vitro: intraspecific differences and importance of the mannose receptor. PLoS One. 2013;8(1):e53584. doi: 10.1371/journal.pone.0053584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hostetter MK. An integrin-like protein in Candida albicans: implications for pathogenesis. Trends Microbiol. 1996;4(6):242–6. doi: 10.1016/0966-842x(96)10036-6. [DOI] [PubMed] [Google Scholar]
- 47.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 48.Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28(5):207–12. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Scott BB, Jenkins D. Gastro-oesophageal candidiasis. Gut. 1982;23(2):137–9. doi: 10.1136/gut.23.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwolińska-Wcisło M, Budak A, Bogdał J, Trojanowska D, Stachura J. Fungal colonization of gastric mucosa and its clinical relevance. Med Sci Monit. 2001;7(5):982–88. [PubMed] [Google Scholar]
- 51. Stewart B, Wild CP. World Cancer Report 2014. Geneva, Switzerland: World Health Organization; 2014.
- 52. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2018.
- 53.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 54.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 55.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. SEER S. Explorer: An interactive website for SEER cancer statistics [Internet] Beta Version. Surveillance Research Program, National Cancer Institute; 2017. Available from: https://seer.cancer.gov/explorer/application.html.
- 57.Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19(4):789–99. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 58.Yamada A, Komaki Y, Komaki F, Micic D, Zullow S, Sakuraba A. Risk of gastrointestinal cancers in patients with cystic fibrosis: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):758–67. doi: 10.1016/s1470-2045(18)30188-8. [DOI] [PubMed] [Google Scholar]
- 59.Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001;121(3):542–7. doi: 10.1053/gast.2001.27083. [DOI] [PubMed] [Google Scholar]
- 60.Karahalios A, Simpson JA, Baglietto L, MacInnis RJ, Hodge AM, Giles GG, et al. Change in weight and waist circumference and risk of colorectal cancer: results from the Melbourne Collaborative Cohort Study. BMC Cancer. 2016;16:157. doi: 10.1186/s12885-016-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 63.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 64.Nakatsu G, Zhou H, Wu WKK, Wong SH, Coker OO, Dai Z, et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology. 2018;155(2):529–41. doi: 10.1053/j.gastro.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 65.Botschuijver S, Roeselers G, Levin E, Jonkers DM, Welting O, Heinsbroek SEM, et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology. 2017;153(4):1026–39. doi: 10.1053/j.gastro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Luan C, Xie L, Yang X, Miao H, Lv N, Zhang R, et al. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci Rep. 2015;5:7980. doi: 10.1038/srep07980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574(7777):264–7. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montoya AM, González GM, Martinez-Castilla AM, Aguilar SA, Franco-Molina MA, Coronado-Cerda E, et al. Cytokines profile in immunocompetent mice during Trichosporon asahii infection. Med Mycol. 2018;56(1):103–9. doi: 10.1093/mmy/myx018. [DOI] [PubMed] [Google Scholar]
- 69.Wu M, Li J, An Y, Li P, Xiong W, Li J, et al. Chitooligosaccharides prevents the development of colitis-associated colorectal cancer by modulating the intestinal microbiota and mycobiota. Front Microbiol. 2019;10:2101. doi: 10.3389/fmicb.2019.02101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Praneenararat S. Fungal infection of the colon. Clin Exp Gastroenterol. 2014;7:415–26. doi: 10.2147/ceg.s67776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao R, Kong C, Li H, Huang L, Qu X, Qin N, et al. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(12):2457–68. doi: 10.1007/s10096-017-3085-6. [DOI] [PubMed] [Google Scholar]
- 72.Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019;68(4):654–62. doi: 10.1136/gutjnl-2018-317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ginsburg GS, Willard HF, David S. Genomic and Precision Medicine: Primary Care. Academic Press; 2017.
- 74.Dean AG, Kreis R. Understanding lung cancer: presentation, screening, and treatment advances. J Nurse Pract. 2018;14(4):316–22. doi: 10.1016/j.nurpra.2017.12.014. [DOI] [Google Scholar]
- 75.Ross JA, Rosen GD. The molecular biology of lung cancer. Curr Opin Pulm Med. 2002;8(4):265–9. doi: 10.1097/00063198-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. doi: 10.1097/jto.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 77.Latimer KM, Mott TF. Lung cancer: diagnosis, treatment principles, and screening. Am Fam Physician. 2015;91(4):250–6. [PubMed] [Google Scholar]
- 78.Bell DW, Gore I, Okimoto RA, Godin-Heymann N, Sordella R, Mulloy R, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37(12):1315–6. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 79.Kinno T, Tsuta K, Shiraishi K, Mizukami T, Suzuki M, Yoshida A, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol. 2014;25(1):138–42. doi: 10.1093/annonc/mdt495. [DOI] [PubMed] [Google Scholar]
- 80.O’Byrne KJ, Gatzemeier U, Bondarenko I, Barrios C, Eschbach C, Martens UM, et al. Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. Lancet Oncol. 2011;12(8):795–805. doi: 10.1016/s1470-2045(11)70189-9. [DOI] [PubMed] [Google Scholar]
- 81.Li Z, Lu G, Meng G. Pathogenic fungal infection in the lung. Front Immunol. 2019;10:1524. doi: 10.3389/fimmu.2019.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denning DW. Epidemiology and pathogenesis of systemic fungal infections in the immunocompromised host. J Antimicrob Chemother. 1991;28 Suppl B:1–16. doi: 10.1093/jac/28.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- 83.Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2013;17(1):8–18. [PubMed] [Google Scholar]
- 84.Ching-López R, Rodríguez Pavón S. Talaromycosis in a lung cancer patient: a rare case. Cureus. 2020;12(9):e10615. doi: 10.7759/cureus.10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe H, Shirai T, Saigusa M, Asada K, Arai K. Subacute invasive pulmonary aspergillosis after chemoradiotherapy for lung cancer. Respirol Case Rep. 2020;8(2):e00523. doi: 10.1002/rcr2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/s2214-109x(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22(12):2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 88.Kasakura Y, Phan A, Ajani J. Adjuvant therapy for resected gastric carcinoma. Surg Oncol Clin N Am. 2002;11(2):431–44. doi: 10.1016/s1055-3207(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 89.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83(1):18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aidijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 90.Delam H, Izanloo S, Bazrafshan MR, Eidi A. Risk factors for cervical cancer: an epidemiological review. J Health Sci Surveill Syst. 2020;8(3):105–9. doi: 10.30476/jhsss.2020.86539.1092. [DOI] [Google Scholar]
- 91.Godoy-Vitorino F, Romaguera J, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F, et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a Hispanic population. Front Microbiol. 2018;9:2533. doi: 10.3389/fmicb.2018.02533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moradi S, Tadris Hasani M, Darvish L, Roozbeh N. Evaluating cervicovaginal infections and cervical cancer in women with low socioeconomic levels. Iran J Public Health. 2017;46(6):867–8. [PMC free article] [PubMed] [Google Scholar]
- 93.Neves RP, de Lima Neto RG, Leite MC, da Silva VK, dos Santos FA, Macêdo DP. Cryptococcus laurentii fungaemia in a cervical cancer patient. Braz J Infect Dis. 2015;19(6):660–3. doi: 10.1016/j.bjid.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katalinic A, Kunze U, Schäfer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) Br J Dermatol. 2003;149(6):1200–6. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 95.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 96.Apalla Z, Nashan D, Weller RB, Castellsagué X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther (Heidelb) 2017;7(Suppl 1):5–19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. DeVita VT, Lawrence TS, Rosenberg SA. Cancer of the Skin: Cancer: Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2015.
- 98.Bodey GP. Fungal infections complicating acute leukemia. J Chronic Dis. 1966;19(6):667–87. doi: 10.1016/0021-9681(66)90066-x. [DOI] [PubMed] [Google Scholar]
- 99. Bast RC Jr, Croce CM, Hait WN, Hong WK, Kufe DW, Piccart-Gebart M, et al. Holland-Frei Cancer Medicine. John Wiley & Sons; 2017.
- 100.Ferrándiz-Pulido C, Martin-Gomez MT, Repiso T, Juárez-Dobjanschi C, Ferrer B, López-Lerma I, et al. Cutaneous infections by dematiaceous opportunistic fungi: diagnosis and management in 11 solid organ transplant recipients. Mycoses. 2019;62(2):121–7. doi: 10.1111/myc.12853. [DOI] [PubMed] [Google Scholar]
- 101.Brothers RP, Daveluy SD. Squamous cell carcinoma mimicking fungal infection. IDCases. 2016;6:72–3. doi: 10.1016/j.idcr.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–21. [PubMed] [Google Scholar]
- 103.Vargas AN. Natural history of ovarian cancer. Ecancermedicalscience. 2014;8:465. doi: 10.3332/ecancer.2014.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 105. Howlader N. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute; 2011.
- 106.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20(2):207–25. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 107.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. “Incessant ovulation” and ovarian cancer. Lancet. 1979;2(8135):170–3. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 108.Cramer DW, Welch WR. Determinants of ovarian cancer risk II Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71(4):717–21. [PubMed] [Google Scholar]
- 109.Slatnik CL, Duff E. Ovarian cancer: ensuring early diagnosis. Nurse Pract. 2015;40(9):47–54. doi: 10.1097/01.NPR.0000450742.00077.a2. [DOI] [PubMed] [Google Scholar]
- 110.Huang X, Wang X, Shang J, Lin Y, Yang Y, Song Y, et al. Association between dietary fiber intake and risk of ovarian cancer: a meta-analysis of observational studies. J Int Med Res. 2018;46(10):3995–4005. doi: 10.1177/0300060518792801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schottenfeld D, Winawer SJ. Cancers of the large intestine. In: Schottenfeld D, Fraumeni JF J, eds. Cancer Epidemiology and Prevention. Oxford University Press; 1996. 10.1093/acprof:oso/9780195149616.001.0001. [DOI]
- 112.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/s0140-6736(08)60269-x. [DOI] [PubMed] [Google Scholar]
- 113.Wang T, Townsend MK, Simmons V, Terry KL, Matulonis UA, Tworoger SS. Prediagnosis and postdiagnosis smoking and survival following diagnosis with ovarian cancer. Int J Cancer. 2020;147(3):736–746. doi: 10.1002/ijc.32773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, Peto R. Ovarian cancer and smoking: individual participant meta-analysis including 28,114 women with ovarian cancer from 51 epidemiological studies. Lancet Oncol. 2012;13(9):946–56. doi: 10.1016/s1470-2045(12)70322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Alwine JC, et al. The ovarian cancer oncobiome. Oncotarget. 2017;8(22):36225–45. doi: 10.18632/oncotarget.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40(8 Pt 1):2633–44. [PubMed] [Google Scholar]
- 117.Ribeiro U Jr, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83(9):1174–85. doi: 10.1046/j.1365-2168.1996.02421.x. [DOI] [PubMed] [Google Scholar]
- 118.Li M, Lu S, Ji C, Wang M, Cheng S, Jin C. Formation of carcinogenic N-nitroso compounds in corn-bread inoculated with fungi. Sci Sin. 1979;22(4):471–7. [PubMed] [Google Scholar]
- 119.Meredith FI. Isolation and characterization of fumonisins. Methods Enzymol. 2000;311:361–73. doi: 10.1016/s0076-6879(00)11096-1. [DOI] [PubMed] [Google Scholar]
- 120.Ikoma T, Tsuchiya Y, Asai T, Okano K, Ito N, Endoh K, et al. Ochratoxin A contamination of red chili peppers from Chile, Bolivia and Peru, countries with a high incidence of gallbladder cancer. Asian Pac J Cancer Prev. 2015;16(14):5987–91. doi: 10.7314/apjcp.2015.16.14.5987. [DOI] [PubMed] [Google Scholar]
- 121.Kothavade RJ, Kura MM, Valand AG, Panthaki MH. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol. 2010;59(Pt 8):873–80. doi: 10.1099/jmm.0.013227-0. [DOI] [PubMed] [Google Scholar]
- 122.Gupta NM, Chaudhary A, Talwar P. Candidial obstruction of the common bile duct. Br J Surg. 1985;72(1):13. doi: 10.1002/bjs.1800720106. [DOI] [PubMed] [Google Scholar]
- 123.Wise GJ, Shteynshlyuger A. How to diagnose and treat fungal infections in chronic prostatitis. Curr Urol Rep. 2006;7(4):320–8. doi: 10.1007/s11934-996-0012-2. [DOI] [PubMed] [Google Scholar]
- 124.Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, et al. Distinct microbial signatures associated with different breast cancer types. Front Microbiol. 2018;9:951. doi: 10.3389/fmicb.2018.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]


