Abstract
Background
Pain is the most frequent symptom experienced by cancer patients, its intensity dependent on the site of the tumour. Tumours that compromise bone or nervous structures due to the bone destruction process are the most painful. There are several treatments to deal with pain (and other symptoms) caused by bone metastases. The hormone calcitonin has the potential to relieve pain and also retain bone density, thus reducing the risk of fractures. This review is an update of a previously published review in the Cochrane Library (2003, Issue 3) which was also updated in 2006 (Issue 3) and in 2011 (Issue 9).
Objectives
The main objective of the review is to determine the effectiveness of calcitonin to reduce metastatic bone pain in patients with painful bone metastases. Secondary objectives are to assess the benefits of calcitonin in reducing the incidence of bone complications (hypercalcemia, fractures and nervous compression) and improving patient survival, and to report any adverse effects of the treatment.
Search methods
We updated the electronic searches in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE up to February 2015. We also searched registers of clinical trials in progress up to February 2015.
Selection criteria
We included studies if they were randomised, double‐blind clinical trials of patients with metastatic bone pain, treated with calcitonin, where the major outcome measure was pain, assessed at four weeks or longer.
Data collection and analysis
Two independent review authors performed study selection and data extraction. Only two studies (90 participants) were eligible for inclusion in the review and therefore meta‐analysis of the data was not possible. We performed intention‐to‐treat (ITT) analysis by imputing all missing values as adverse effects.
Main results
The overall quality of the evidence was very low. In this update no new studies were identified for inclusion; one additional study was excluded. Of the two small included studies, one study showed a non‐significant effect of calcitonin on the number of patients with complete pain relief (risk ratio (RR) 2.50; 95% confidence interval (CI) 0.55 to 11.41). The second study provided no evidence that calcitonin reduced analgesia consumption (RR 1.05; 95% CI 0.90 to 1.21) in patients with painful bone metastases. There was no evidence that calcitonin was effective in controlling complications due to bone metastases, for improving quality of life or for patients' survival. Although not statistically significant, a greater number of adverse effects were observed in the groups given calcitonin in the two included studies (RR 3.50; 95% CI 0.77 to 15.88).
Authors' conclusions
Current available research evidence is of very low quality and there is a lack of evidence to support the use of calcitonin for managing bone pain from bone metastases. Since the last version of this review, we did not identify any additional studies.
Plain language summary
Calcitonin used to treat metastatic bone pain
People who have cancer which has spread to their bones and the nerves adjacent to the bones often suffer severe pain. There are several treatments to help relieve this pain: radiotherapy, analgesic drugs (pain killers) such as opioids, and bone‐modulating drugs such as bisphosphonates and calcitonin. Calcitonin has the potential to relieve pain and maintain bone strength, thus reducing the risk of broken bones. This review looked at the effectiveness of calcitonin for controlling pain from bone metastases. However, only two studies were found with very low quality evidence to support the use of calcitonin for patients suffering from bone pain. We updated the review in 2015 and did not find any more studies. There were slightly more side effects for the patients given calcitonin. Unless new studies provide additional relevant information about this treatment, other therapeutic approaches should be considered.
Summary of findings
Summary of findings 1. Summary of findings.
| Calcitonin compared with placebo for metastatic bone pain | |||||
|
Patient or population: Patients withmetastatic bone pain Settings: Hospital Intervention: Calcitonin Comparison: Placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Calcitonin | ||||
|
Complete pain relief Follow‐up: mean 1 month |
100 per 1000 | 250 per 1000 (55 to 1000) | RR 2.5 (0.55 to 11.41) | 40 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
|
Less or equal analgesia consumption Follow‐up: mean 12 months |
917 per 1000 | 962 per 1000 (825 to 1000) | RR 1.05 (0.9 to 1.21) | 48 (1 study) | ⊕⊝⊝⊝ very low1,2,4 |
|
Adverse effects Follow‐up: mean 1‐12 months |
45 per 1000 | 159 per 1000 (35 to 722) | RR 3.5 (0.77 to 15.88) | 88 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The randomisation was unclear 2 The confidence interval overlaps no effect, small sample size. 3 Wide confidence interval 4 The blinding was unclear
Background
This review is an update of a previously published review originally published in the Cochrane Library in Issue 3, 2003 and updated in Issue 3, 2006 and Issue 9, 2011, on 'Calcitonin for metastatic bone pain'. Pain is the most frequent symptom of cancer patients, its intensity dependent on the site of the tumour (Portenoy 1989). Tumours that compromise bone or nervous structures are the most painful.
Description of the condition
The incidence of malignant bone tumours is relatively low but the development of bone metastases in primary breast, lung, prostate and kidney tumours is common. A high proportion of patients diagnosed with malignant breast, lung and prostate tumours (80% to 85%) develop a bone metastasis (Nelson 1991). Pain and functional disability occur in 45% to 75% of cases, whereas major complications will be observed in up to one‐third of patients whose first relapse occurs in bone. Hypercalcaemia occurs in 10% to 15% of these patients and fractures in 10% to 20% (Body 2000).
Pain arises as a result of bone destruction and, as more destruction ensues, the patient experiences more pain. Pain is also caused by periosteal narrowing due to tumour growth and cortical bone deformation. Radiculopathies, plexopathies and the shrinkage of spinal nerves due to tumour growth and fractures are frequent events in these patients. This situation makes both diagnosis and treatment more complicated, limiting the patient's mobility, confining them to bed rest and thereby weakening the patient and increasing the risk of thromboembolic disease, hypercalcaemia, atelectasis (failure of the normal expansion of part or all of the lung) and pneumonia (as a result of rib metastases).
It is too simplistic to explain bone pain on purely mechanistic grounds, as bone metastases are manifested through pain which can arise even before the injury is radiologically detected. The pain also occurs as a result of the release of substance P (neuropeptide), bradykinins, prostaglandins and other cytokines by the neoplastic cells, which lead to stimulation of C‐type fibres within the bone (Paterson 2000).
Description of the intervention
Several treatments are available to manage the pain caused by bone metastasis. Some are directed at the tumour itself and thus also treat the pain. Others specifically address the pain because pain‐induced impairment can become more debilitating than the symptoms of the illness itself. Despite the availability of therapies, however, cancer‐related pain continues to be under treated.
The therapeutic strategies for painful bone metastases are based on different mechanisms, for example:
base treatments or treatments directed towards oncological illness;
conventional analgesics, e.g. paracetamol and non‐steroidal anti‐inflammatory drugs (NSAIDs) with or without opioid;
radiotherapy for localised bone metastases;
specific drugs for bone pain that work on the bone tumour‐induced alteration, e.g. bisphosphonates which inhibit osteoclastic activity and reduce bone resorption (Eisenberg 1994), radioactive agents such as estronium‐89 (Robinson 1995) or calcitonin.
How the intervention might work
Calcitonin is a hormone produced in the thyroid glands of some animals. It has a hypocalcaemic action that is due primarily to the inhibition of osteoclastic bone resorption, and secondarily by action on the kidneys that results in increased urinary excretion of calcium and phosphorus. Naturally occurring porcine calcitonin, synthetic salmon calcitonin (salcatonin) and synthetic human calcitonin are in clinical use. Salcatonin is the most potent and is used to control bone pain due to malignant neoplasms (Parfitt 1999). Salcatonin can cause nausea, vomiting and flushing, as well as unpleasant taste sensations, tingling in the hands and pain at the injection site. Some patients also experience an allergic reaction. In addition, salcatonin is immunogenic and antibodies may form; this may result in resistance to its pharmacological effects during long‐term therapy (Grahame‐Smith 2002). Periodic monitoring of calcium and phosphorus is advisable during treatment (Lussier 2004).
Why it is important to do this review
Currently, the use of calcitonin for the relief of metastatic bone pain is not very common and little information is available about its use in this context. The most frequent routes of administration are subcutaneous and intranasal. The current analgesic dose by the intranasal route is 200 IU/day and the optimal dose by the subcutaneous route is not well defined (Lussier 2004).
There is some evidence regarding the use of calcitonin to prevent bone fractures in post menopausal women with osteoporosis (Chesnut 2000; MacLean 2008; Palmer 2007), in kidney transplant recipients (Palmer 2007), to prevent corticosteroid‐induced osteoporosis (Craney 2000), and to prevent hypercalcaemia in neoplastic processes (Matsumoto 2002; Zojer 1999), as a second‐line treatment. However, there is unclear evidence on the effectiveness of calcitonin in treating phantom limb pain (Alviar 2011) or complex regional pain syndrome (O'Connell 2013). It was therefore important that a systematic review of the data was undertaken to assess whether this treatment is effective to control bone pain and metastatic complications in patients with bone metastases. This review is an update of a previously published review in the Cochrane Library on this topic (Martínez‐Zapata 2006).
Objectives
The main objective of the review is to determine the effectiveness of calcitonin to reduce metastatic bone pain in patients with painful bone metastases. Secondary objectives are to assess the benefits of calcitonin in reducing the incidence of bone complications (hypercalcemia, fractures and nervous compression) and improving patient survival, and to report any adverse effects of the treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind clinical trials.
Types of participants
Participants were adults (18 years old or more), men or women, with bone metastatic pain caused by any primary tumour, diagnosed by computed tomography, bone gammagraphy, nuclear magnetic resonance or other radiographic process.
Types of interventions
Inclusion criteria
Calcitonin plus a rescue medication versus placebo plus a rescue medication.
Any comparison between different models and intervention doses of interest.
The use of steroids or analgesic radiotherapy were accepted if administration was equal in each treatment arm.
Exclusion criteria
We excluded studies where bisphosphonates were administered before participants were randomised to different treatment groups from the study.
We excluded studies in which the efficacy of calcitonin in treating pain from bone metastases was measured over a short period (less than four weeks), considering only studies with a minimum follow‐up.
Types of outcome measures
We collected data on the following outcome measures.
Primary outcomes
Complete pain relief: percentage of patients with complete relief at ≥ 4 weeks follow‐up.
Less or equal analgesia consumption.
Percentage of patients with less or equal analgesia consumption at ≥ 4 weeks follow‐up.
Secondary outcomes
Pain: intensity of pain measured by a standardised scale (e.g. analogical visual scale) at baseline, 4 weeks, 6 months, 9 months and 12 months.
Partial pain relief:percentage of patients' partial reduction of pain, defined as a reduction greater or equal to 50% from baseline.
Rescue medication at baseline and post intervention: percentage of patients with rescue medication.
Length of time of improvement.
Bone metastases complications: percentage of patients with bone metastases complications (hypercalcaemia, bone fracture, nerve root and bone marrow compression).
Quality of life measured by a standardised questionnaire.
Hospitalisation: percentage of patients with hospitalisation due to adverse effects.
Adverse effects: percentage of patients with adverse effects.
We did not collect subjective evaluations of pain rated by physicians, nurses or carers, only those rated by the patient.
Search methods for identification of studies
Electronic searches
This review is an update of a review previously published in the Cochrane Library in 2003 (Martinez‐Zapata 2003), 2006 (Martínez‐Zapata 2006) and 2011. The last update was conducted in 2011, with no changes in the review or its citation.
In this update we searched for randomised controlled clinical trials (RCTs) related to this review electronically using:
The Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 1);
MEDLINE (1966 to week 4 February 2015);
EMBASE (1974 to week 4 February 2015).
We combined the general strategies to identify RCTs with specific search commands to identify trials of calcitonin and metastatic bone pain. The MEDLINE search strategy is detailed in Appendix 1; the details of the other search strategies are included in Appendix 2.
We searched the clinical trials database held by the Cochrane Cancer Network (where the results of handsearching relevant journals and other literature worldwide are collated), as well as the Cochrane Pain, Palliative and Supportive Care (PaPaS) Group Specialised Register. For the current update we did not search the PaPaS Specialised Register as it is no longer updated.
Searching other resources
We sought ongoing trials from the following databases searching for "calcitonin" AND "all recruitment status" (from 1/1/2006 until 16/2/15):
WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/)
We reviewed identified systematic reviews, meta‐analyses and reference lists of identified RCTs in order to identify further RCTs. We undertook no additional handsearching of journals. There was no language restriction in the search strategy and selection of papers.
Data collection and analysis
Selection of studies
The authors critically appraised the studies identified through the search strategy for inclusion in the systematic review. Two independent review authors (MM and YR) assessed each study in an open fashion: we undertook no blinding of authors, institutions or source of publication of the study. There were no major discrepancies between the review authors in the selection of studies.
Data extraction and management
We designed a data collection form to record:
the characteristics of the study participants;
details of the intervention and the comparison groups;
the results from each participant subgroup;
risk of bias.
With regard to studies with a cross‐over design, we only collected and analysed data corresponding to the first period of treatment.
Two independent review authors sorted the data and completed a standard spreadsheet form designed for that purpose. If the information provided in the studies was incomplete, we contacted the study authors to provide the required data.
Assessment of risk of bias in included studies
In this update two independent review authors (EC and YR) assessed the risk of bias. Where there was disagreement, MM participated in the final decision.
We based our 'Risk of bias' assessment on the proposals described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We examined the adequacy of the method used to generate the allocation sequence, the allocation concealment and the level of blinding (clinician, participant or outcome assessor). We further examined the presence of incomplete outcome data and selective reporting.
We classified each study to be at high, unclear or low risk of bias. We described the reason for each judgement from details provided in the studies or from data sought from the original authors. We considered a study to be at low risk of bias when it concealed allocation and blinded participants and outcome assessors, if it reported complete outcome data and there was no selective reporting or other biases. If one or more of these key domains were not met, we considered the study to be at a high risk of bias. If one or more of these key domains were unclear, we considered the study as 'unclear' with respect to risk of bias (see table 8.7a of the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011).
We also evaluated the quality of the evidence with the GRADE (Grades of recommendation, Assessment, Development and Evaluation) system and developed a 'Summary of findings' table (Guyatt 2008). The quality (certainty) of the evidence is rated as high, moderate, low or very low and takes into account several components (risk of bias, consistency, directness, precision and publication bias).
Measures of treatment effect
We estimated the effect of bone pain treatment by calculating the risk ratio (RR) and standardised mean difference (SMD), with their corresponding confidence intervals (CIs). If the results had been statistically significant, we would have computed clinical effect measures such as the number needed to treat to benefit one patient (NNTB) or the number needed to treat to harm one patient (NNTH).
We calculated the global estimate of the effect of each variable by conducting a meta‐analysis of single effect measures of the study. We used the Mantel‐Haezsel (M‐H) method (Mantel 1959) for dichotomised variables and the inverse of variance for continuous variables.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
We performed an intention‐to‐treat (ITT) analysis, analysing each participant in their corresponding randomised treatment group, independent of either completion or withdrawal from the study. For dichotomous variables, we imputed missing values due to drop‐outs or withdrawals by categorising the participants as non‐respondents. In the analysis of adverse effects, we considered all participants who withdrew from the study as having suffered an adverse effect, or not to have experienced a positive treatment effect. For continuous variables, we analysed data as provided by the study authors, either per protocol or per ITT.
Assessment of heterogeneity
We quantified the impact of statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). If percentage of heterogeneity (I2) was less than 50% we applied a fixed‐effect model, and where it was between 50% and 75% we applied a random‐effects model (DerSimonian 1986). Where heterogeneity was significant (> 75%) we investigated possible causes of the heterogeneity by exploring the impact of participants' characteristics and the risk of bias of the studies.
Assessment of reporting biases
We checked if the results of all variables specified in the methods of the included studies were reported.
Data synthesis
When we considered the included studies to be comparable enough and to provide sufficiently similar methods to measure the outcomes, we summarised their findings by pooling the results using a fixed‐effect model using Review Manager software (RevMan 2014). If there were doubts about the similarity of participant characteristics or the methods used to measure the outcomes we pooled the data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
It was not possible to perform subgroup analyses by primary tumour location, stage and route of treatment due to the low number of studies included in the review.
Sensitivity analysis
Only two studies met the criteria for inclusion in this review, therefore it was not possible to perform a sensitivity analysis to assess how robust the estimate of the global effect was regarding, per protocol analysis, published studies, risk of bias of studies and imputation strategies.
Whereas in the main analyses we assumed all participants withdrawing from studies to present an absence of a positive effect or an adverse effect, for the sensitivity analyses we repeated imputation based on the assumption that the participants who withdrew presented adverse effects in the same proportion as the participants observed in the control group.
Results
Description of studies
Results of the search
For the previous version of this review bibliographic searches retrieved a total of 11 studies likely to be included in the review. Following assessment, we excluded nine studies. We included two randomised, double‐blind clinical trials with a total of 90 women with breast cancer and pain from bone metastasis. There were no ongoing clinical trials. For this update the search identified seven records that did not fulfil the inclusion criteria of this review (Figure 1).
1.
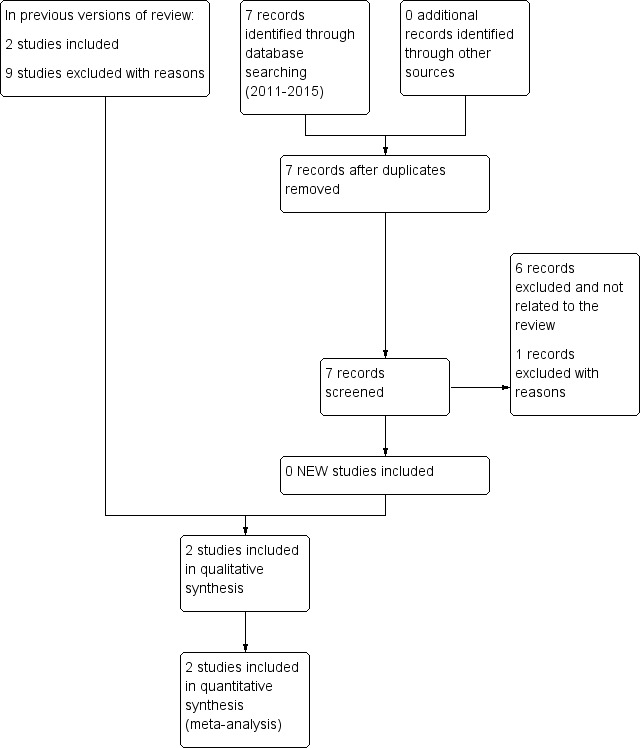
Study flow diagram.
Included studies
The two studies that met the inclusion criteria were, as for the last update, Blomqvist 1988 and Roth 1986. We found no new studies which impacted on the original outcome of this systematic review.
Roth 1986 compared calcitonin (100 IU administered subcutaneously each day for 28 days) versus placebo administered to a group of 40 women with breast cancer and pain from bone metastasis. The analgesic effect of calcitonin was assessed through analgesic consumption, functional capacity, pain duration and patient pain self assessment. Participants were followed for one month. There were two drop‐outs in the intervention group.
Blomqvist 1988 compared calcitonin (100 IU administered subcutaneously each day for three months) versus placebo in 50 women with breast cancer and painful bone metastases. Pain control was assessed over one year. There was one drop‐out per treatment group. Participants were followed for up to 24 months. Although this study assessed both pain and use of analgesics, the study did not provide quantifiable data for pain reduction and allowed quantitative analysis of use of analgesics only.
A detailed description of the included studies can be found in the Characteristics of included studies table.
Excluded studies
In the present update the search identified seven studies. One study assessed denosumab for refractory hypercalcaemia of malignancy.Three studies assessed calcitonin for hypercalcemia, and one study for Paget's disease. Two studies assessed calcitonin for cancer pain, but one was not a clinical trial, and the other was a randomised clinical trial that compared pamidronate versus calcitonin without a placebo group (El Wasseef 2012). We excluded nine studies from the previous version of the review (Allan 1983; Beaufort 1984; Berrenssen 1985; Gennari 1989; Hindley 1982; Kleibel 1984; Lepidini 1992; Serdengeçti 1986; Tsavaris 2006) and one new study from the 2015 update (El Wasseef 2012). The reasons for exclusion were as follows:
assessment of pain was performed less than four weeks after the treatment (Allan 1983; Beaufort 1984; Berrenssen 1985; Gennari 1989; Hindley 1982; Kleibel 1984);
single‐blind design (Serdengeçti 1986);
open study with no control group (Lepidini 1992; Tsavaris 2006);
Kleibel 1984 was identified in the search strategy of the old version of this review and was ignored due to different inclusion criteria. In the present update the review authors have re‐evaluated and excluded it because outcome assessment was less than one month after treatment. Berrenssen 1985 has also been identified as an excluded study in the update due to some changes in the design of the search strategy, in comparison with the previous review (outcome assessment less than one month after treatment);
there was no placebo group used in the study (Beaufort 1984; El Wasseef 2012; Gennari 1989).
A detailed description of the excluded studies can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
The global risk of bias of the included studies was unclear (Figure 2; Figure 3), because some items did not specifically mention the method of randomisation and allocation concealment. One study (Roth 1986) presented some imprecision defining the blinding of treatments.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method to generate the sequence of randomisation and allocation concealment was not described in the two included studies (Blomqvist 1988; Roth 1986).
Blinding
In the Roth 1986 study, the placebo was a saline solution, but it is not specified if it was identical in appearance to calcitonin. Therefore, there is doubt around the blinding of participants and investigators.
In the Blomqvist 1988 study, the concealment of treatment to the participant and investigators was well described.
Incomplete outcome data
Only two participants were lost in both included studies (Blomqvist 1988; Roth 1986), therefore we excluded attrition bias.
Selective reporting
The results of all outcomes specified in the methods are presented in both Blomqvist 1988 and Roth 1986 , therefore we excluded outcome reporting bias in these studies.
Effects of interventions
See: Table 1
Only the two studies included in the original review (Blomqvist 1988; Roth 1986) were included in this update; we included no new studies. The overall quality of the evidence was very low due to risk of bias and imprecision (Table 1).
The study by Roth 1986 provided data for the number of participants with complete pain relief. This was assessed one month after the start of treatment. More participants with complete pain relief were found in the group given calcitonin, but the difference did not reach a level of statistical significance (risk ratio (RR) 2.50; 95% confidence interval (CI) 0.55 to 11.41). Therefore, there was insufficient evidence that calcitonin is effective for complete pain relief.
Blomqvist 1988 was the only study that provided information on analgesic consumption during the trial period. Results showed that almost all the participants assigned to both calcitonin and placebo groups had equal analgesic consumption at baseline, or otherwise the use was similarly decreased (RR 1.05; 95% CI 0.90 to 1.21). These results should be interpreted along with the results for pain relief.
Unfortunately, there were no studies providing information on both variables. The two included studies did not provide sufficient evidence for the efficacy of calcitonin either for the treatment of pain or for complications from bone metastases to be quantitatively assessed. In addition, evidence for the effect of calcitonin on controlling complications due to bone metastases, improving quality of life and on patients' survival was not found.
Although not statistically significant, a greater number of participants with any adverse effect were observed in the groups given calcitonin in both included studies (RR 3.50; 95% CI 0.77 to 15.88) (Analysis 1.1). The observed adverse effects were related to facial blushing in Blomqvist 1988 and subcutaneous pain in Roth 1986. In a sensitivity analysis, the results were minimally altered when we performed a per protocol analysis (Analysis 2.1). An ITT analysis of the participants who withdrew presented adverse effects in the same proportion in both groups (Analysis 3.1).
1.1. Analysis.

Comparison 1: Calcitonin vs placebo (ITT: worst‐case scenario analysis), Outcome 1: Adverse effects
2.1. Analysis.

Comparison 2: Calcitonin vs placebo (per protocol analysis), Outcome 1: Adverse effects
3.1. Analysis.

Comparison 3: Calcitonin vs placebo (ITT: same as control scenario), Outcome 1: Adverse effects
The included studies did not report quantitative information about other outcomes like:
pain: intensity;
partial pain relief;
rescue medication;
length of time of improvement;
bone metastases complications;
quality of life;
hospitalisation due to adverse effects.
Sensitivity analysis
Due to the small number of studies included in this review, and the features common to both, sensitivity analyses (by publication status or risk of bias) were unnecessary. In addition, because a low number of participants were lost to follow‐up (two in each study) there was no difference when the data were analysed per protocol or by imputing missing data.
Discussion
This review is an update of a previously published review in the Cochrane Library (2011, Issue 9) on calcitonin for metastatic bone pain.
Summary of main results
This systematic review was conducted to assess the efficacy of calcitonin in the control of pain due to bone metastases and bone complications. This updated review does not provide additional information on this treatment as no new relevant studies were included. All seven new studies identified in the search were excluded. Six of them were not related to the review and one of them was excluded with reasons.
This review, as in the previous reviews, includes two clinical trials involving a total of 90 participants who were treated for painful bone metastases with either a dose of 100 IU/day subcutaneous calcitonin or placebo. The follow‐up and patient assessments were undertaken only in the short term.
Due to its antireabsorptive effects, by inhibiting osteoclasts and analgesic activity, it has been hypothesised that calcitonin has a likely benefit for bone pain. Only one study provided data about complete pain relief (Roth 1986). However, more participants with complete pain relief were found in the group given calcitonin; the difference with respect to the placebo group was not significant. The other study provided information on analgesic consumption during the trial period, but the results also showed no differences between groups (Blomqvist 1988).
There was no evidence for the treatment of bone complications. Although not significant, the studies showed that calcitonin produced a greater number of adverse effects when compared with placebo. This result did not significantly change in the sensitivity analysis.
Overall completeness and applicability of evidence
We have included all relevant studies that assessed calcitonin to treat metastatic bone pain. The small number of participants in the included studies assessing the efficacy of calcitonin may explain the non‐conclusive results. The conclusions cannot be considered reliable and are susceptible to change in the future after publication of new reports that may provide further information. Moreover, the low number of participants that were included in the review led to a lack of power to detect small but significant changes of the intervention.
Only two studies in women with breast cancer and pain from bone metastasis were included. Therefore, there is a lack of evidence in men.
Quality of the evidence
In general, the quality of evidence was very low due to risk of bias and the imprecision of the included studies. Although the two included studies were defined as randomised and double‐blinded, there was a lack of description of randomisation generation and allocation concealment. One study did not describe the blinding of participants in enough depth. It was possible that there was reporting bias due to the publication years of the studies, because the requirements for conducting a study may have been less rigorous than they are today.
Potential biases in the review process
We identified a low number of studies despite an exhaustive bibliographic search and after establishing contact with authors of identified studies to provide information on further studies. Similarly, we applied no restrictions on status or language of publication.
In order to reduce the impact of attrition bias, we determined the number of losses to follow‐up and their distribution per treatment group in the two included studies. This allowed an intention‐to‐treat (ITT) analysis and the exploration of the impact that losses to follow‐up have on effect estimates. Due to the characteristics of the two included studies and the short follow‐up period, losses were kept to a minimum and therefore no modifications to the analyses were necessary.
Agreements and disagreements with other studies or reviews
Pain relief must be the first objective in the treatment of bone metastases to improve the quality of life of patients. There are different treatment levels for achieving relief from pain, which normally start with an analgesic (paracetamol (acetaminophen)) or a non‐steroidal anti‐inflammatory drug (NSAID), and continue with low‐potency opioids and then with another more powerful opioid (e.g. morphine). Frequently this drug sequence fails for a variety of reasons and often because the drugs are not prescribed at the highest therapeutic doses. When pain is not controlled with these options there are alternative treatments such as radiotherapy, radionucleotides, bisphosphonates or calcitonin.
Radiotherapy is commonly used to provide pain relief for localised painful bone metastases (Hoskin 1995; Sze 2002). About 75% of patients achieve pain relief and half of those stay free from pain (Jacox 1994). However, when there are multiple bone metastases, systemic treatments such as bisphosphonates, radionucleotides and calcitonin with different evidence levels for efficacy may be necessary.
This review constitutes a contribution to the available scientific evidence on the use of calcitonin in this field, because we have not identified any systematic review that assesses calcitonin for metastatic bone pain. Nevertheless, due to the lack of primary studies, strong conclusions on the efficacy of calcitonin cannot be drawn.
Some systematic reviews of bisphosphonates (Wong 2002; Wong 2012; Yuen 2006) have shown some evidence to indicate the efficacy of bisphosphonates for this indication, and also radionucleotides (Roqué 2011). Based on the limited results of this review on the efficacy of calcitonin in relieving cancer‐related bone pain, consideration should be given to different therapeutic approaches when making decisions on the management of pain from bone metastases.
Authors' conclusions
Implications for practice.
There is very low quality evidence from two small studies on the effect of calcitonin on pain control and its associated adverse effects. There is a lack of evidence to support clinicians' use of calcitonin to control patients' pain from bone metastases. No additional studies were included in the present update. Until new studies provide additional information on this treatment, other therapeutic approaches should be considered.
Implications for research.
Calcitonin has been assessed in studies with small sample sizes, risk of bias and short‐term evaluations. More double‐blind, parallel clinical trials using long‐term evaluations are needed. Future clinical trials should use realistic sample size calculations to evaluate the efficacy of calcitonin better, including adequate estimations of the number of participants likely to be lost to follow‐up. This is the only way to guarantee a minimum level of quality, significance and power to demonstrate the actual efficacy of interventions.
Alternatively, the variables studied should be confined to reduction/absence of pain, adverse effects and quality of life. Possible complications of bone metastases, such as hypercalcemia, bone fractures or radicular compression, should also be quantified.
What's new
| Date | Event | Description |
|---|---|---|
| 23 June 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 7 July 2015 | Review declared as stable | This review will be assessed for further updating in 2020. |
| 25 February 2015 | New citation required but conclusions have not changed | No new studies were identified for inclusion; one additional study was excluded. The conclusions remain unchanged. |
| 25 February 2015 | New search has been performed | Searches updated and Summary of findings table added. |
| 27 June 2012 | Amended | Contact details updated. |
| 29 November 2011 | New search has been performed | The update of this systematic review did not change the conclusions or add any new included studies to the previously published review. The bibliographic searches retrieved a total of 11 studies likely to be included in the review. Following assessment, we excluded nine studies as they did not meet the inclusion criteria. Three of the nine excluded studies were newly identified studies that we then excluded because they were not controlled (Tsavaris 2006) or had no placebo (Beaufort 1984; Gennari 1989). |
| 12 August 2009 | Amended | Contact details updated. |
| 24 October 2008 | Amended | Converted to new review format. |
| 23 May 2006 | New search has been performed | This is an update of the review originally published in Issue 3, 2003 of The Cochrane Library. Two new excluded studies were identified but no new studies were included and therefore there was no change to the original conclusion. |
Notes
Assessed for updating in 2020
At June 2020, we found one potentially relevant study but the authors and editors judged that the new information is unlikely to change the review findings [Jain 2020]. We have now stabilised this review following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Jain PN, Chatterjee A. A Randomized Placebo‐Controlled Trial Evaluating the Analgesic Effect of Salmon Calcitonin in Refractory Bone Metastasis Pain. Indian J Palliat Care. 2020;26(1):4‐8. doi:10.4103/IJPC.IJPC_167_19
Acknowledgements
To Dr Suheyla Serdengeçti for replying to our requests for clarification, to Ms Miren Fernández for performing the bibliographic searches and to Dr Garcia Jl and Dr Ferrandiz for providing their clinical experience in the first version. To Sylvia Bickley for performing the searches for the previous update version. To Jane Hayes (Trials Search Co‐ordinator of the previous version) and Joanne Abbott (Trials Search Co‐ordinator of the present version of this review) for performing the bibliographic searches.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy (2015 update)
via OVID
1. exp Bone Neoplasms/sc
2. exp Neoplasm Metastasis/
3. exp "Bone and Bones"/
4. 2 and 3
5. (bone$ adj10 metasta$).mp.
6. 1 or 4 or 5
7. Pain, Intractable/ or Pain/
8. Palliative Care/
9. complicat$.ti,ab.
10. complications.hw.
11. Hypercalcemia/
12. hypercalc$.ti,ab.
13. exp Fractures, Bone/
14. fractur$.ti,ab.
15. Spinal Cord Compression/
16. spin$ compression.ti,ab.
17. Nerve Compression Syndromes/
18. radicular compres$.ti,ab.
19. or/7‐18
20. Calcitonin/
21. calcitonin.ti,ab.
22. 20 or 21
23. 6 and 19 and 22
24. randomized controlled trial.pt.
25. controlled clinical trial.pt.
26. randomized.ab.
27. placebo.ab.
28. drug therapy.fs.
29. randomly.ab.
30. trial.ab.
31. or/24‐30
32. exp animals/ not humans.sh.
33. 31 not 32
34. 23 and 33
35. (201109* or 201110* or 201111* or 201112* or 2012* or 2013* or 2014*).ed.
36. 34 and 35
Appendix 2. Other search strategies (2015 update)
CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Bone Neoplasms] explode all trees
#2 MeSH descriptor: [Neoplasm Metastasis] explode all trees
#3 MeSH descriptor: [Bone and Bones] explode all trees
#4 (#2 and #3)
#5 (osseous metasta*) or (bone* near metasta*):ti,ab,kw (Word variations have been searched)
#6 (#1 or #4 or #5)
#7 MeSH descriptor: [Palliative Care] this term only
#8 MeSH descriptor: [Pain] explode all trees
#9 complicat*:ti,ab,kw (Word variations have been searched)
#10 MeSH descriptor: [Hypercalcemia] this term only
#11 hypercalcemia or hypercalcaemia:ti,ab,kw (Word variations have been searched)
#12 MeSH descriptor: [Fractures, Bone] explode all trees
#13 fractur*:ti,ab,kw (Word variations have been searched)
#14 MeSH descriptor: [Spinal Cord Compression] this term only
#15 (spin* cord compress*):ti,ab,kw (Word variations have been searched)
#16 MeSH descriptor: [Nerve Compression Syndromes] explode all trees
#17 (nerve or radicular) near compress*:ti,ab,kw (Word variations have been searched)
#18 pain*:ti,ab,kw (Word variations have been searched)
#19 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17)
#20 MeSH descriptor: [Calcitonin] this term only
#21 calcitonin:ti,ab,kw (Word variations have been searched)
#22 (#20 or #21)
#23 (#6 and #19 and #22) from 2011 to 2014
EMBASE (OVID)
1. Bone Metastasis/
2. osseous metasta$.mp.
3. (bone$ adj6 metasta$).ti,ab.
4. or/1‐3
5. exp Palliative Therapy/
6. complication.sh.
7. complicat$.ti,ab.
8. Hypercalcemia/
9. (hypercalcemia or hypercalcaemia).mp.
10. exp Fracture/
11. fractur$.ti,ab.
12. Spinal Cord Compression/
13. spin$ cord compress$.ti,ab.
14. "Nerve Root Compression"/
15. radicular compress$.mp. or nerve compress$.ti,ab. or nerve‐root‐compress$.mp.
16. exp Pain/
17. pain$.ti,ab.
18. or/5‐17
19. Calcitonin/
20. calcitonin.mp.
21. 19 or 20
22. 4 and 18 and 21
23. random$.tw.
24. factorial$.tw.
25. crossover$.tw.
26. cross over$.tw.
27. cross‐over$.tw.
28. placebo$.tw.
29. (doubl$ adj blind$).tw.
30. (singl$ adj blind$).tw.
31. assign$.tw.
32. allocat$.tw.
33. volunteer$.tw.
34. Crossover Procedure/
35. double‐blind procedure.tw.
36. Randomized Controlled Trial/
37. Single Blind Procedure/
38. or/23‐37
39. (animal/ or nonhuman/) not human/
40. 38 not 39
41. 22 and 40
42. (201109* or 201110* or 201111* or 201112* or 2012* or 2013* or 2014*).dd.
43. 41 and 42
Data and analyses
Comparison 1. Calcitonin vs placebo (ITT: worst‐case scenario analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adverse effects | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.77, 15.88] |
Comparison 2. Calcitonin vs placebo (per protocol analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adverse effects | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.54, 19.24] |
Comparison 3. Calcitonin vs placebo (ITT: same as control scenario).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Adverse effects | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.50, 18.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blomqvist 1988.
| Study characteristics | ||
| Methods | Randomised, double‐blind trial | |
| Participants | N = 50 Women with breast cancer and metastatic bone pain | |
| Interventions | 100 IU sc/24 hours every 21 out of 31 days, for 3 months Follow‐up: 1 year |
|
| Outcomes | Pain assessment: use of analgesic drugs, VAS (20 points) and 5‐point pain score General performance: 5‐point pain scale Biochemical parameters Mortality |
|
| Notes | 2 participants (1 in each group) lost to follow‐up due to progression of illness
Adverse effects: 1 facial flush in the calcitonin group Funding: Sandoz Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors do not specify how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants | Unclear risk | The placebo was a saline solution, but it is not specified if it was identical in appearance to calcitonin. Therefore, there is doubt around the blinding of participants |
| Blinding of investigators that implemented the intervention | Unclear risk | The placebo was a saline solution, but it is not specified if it was identical in appearance to calcitonin. Therefore, there is doubt around the blinding of investigators |
| Incomplete outcome data | Low risk | There are 2 participants lost from the data |
| Selective reporting | Low risk | The results of all outcomes specified in the methods are presented |
Roth 1986.
| Study characteristics | ||
| Methods | Randomised, double‐blind trial | |
| Participants | N = 40 Women with breast cancer and metastatic bone pain | |
| Interventions | 100 IU sc/24 hours for 28 days | |
| Outcomes | Daily analgesic consumption by the participant Lasting pain Participant's own assessment of pain (VAS) Assessment of the efficacy of treatment by the investigator |
|
| Notes | 2 participants lost to follow‐up
Adverse effects: 3 calcitonin participants and 1 placebo participant felt pain at the site of subcutaneous administration Founding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants | Low risk | The placebo is a physiological solution in identical ampoules to calcitonin |
| Blinding of investigators that implemented the intervention | Low risk | The placebo is a physiological solution in identical ampoules to calcitonin |
| Incomplete outcome data | Low risk | There were only 2 losses |
| Selective reporting | Low risk | The results of all outcomes specified in the methods are presented |
IU: international units sc: subcutaneous VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allan 1983 | Outcome assessment before 1 month |
| Beaufort 1984 | Assessment of pain was performed less than 4 weeks after the treatment There was no placebo |
| Berrenssen 1985 | Outcome assessment before 1 month |
| El Wasseef 2012 | There was no placebo |
| Gennari 1989 | There was no placebo |
| Hindley 1982 | Outcome assessment before 1 month |
| Kleibel 1984 | Outcome assessment before 1 month |
| Lepidini 1992 | Open study with no control group |
| Serdengeçti 1986 | The study design was single‐blind |
| Tsavaris 2006 | Non‐randomised study. Open study with no control group. The doses of salmon calcitonin were 300 IU intravenously daily for 5 consecutive days and repeated every 2 weeks until no response was noticeable |
IU: international units
Differences between protocol and review
In this 2015 updated review we have assessed the quality of evidence and added a 'Summary of findings' table using the GRADE approach. We have specified primary and secondary outcomes.
Contributions of authors
All authors contributed to the interpretation of data, and commented on the final text and gave their final approval.
Dr. Martinez and Dr. Roqué were responsible for the conception and design of the study.
Dr. Martinez, Dr. Roqué and Dr. Alonso‐Coello contributed to the analysis and interpretation of data.
Dr. Martinez was responsible for the data extraction process and the day‐to‐day work of the review.
Dr. Roman performed duplicate data extraction. Dr. Alonso‐Coello revised the manuscript and collaborated on methodological aspects. Dr. Roqué revised the statistical and methodological aspects of the review and revised the manuscript. Dr. Català performed duplicate data extraction and provided clinical experience and insight on the protocol and review reports.
Dr. Martinez was responsible for co‐ordinating the review update.
Sources of support
Internal sources
No sources of support supplied
External sources
Agencia Evaluación Tecnologías Sanitarias, Fondo de Investigaciones Sanitarias FIS. Instituto de Salud Carlos III. Subdirección General de Investigación Sanitaria. (01/A060), Spain
Instituto de Salud Carlos III. Subdirección General de Investigación Sanitaria. Ministerio de Salud y Consumo. (01/F070), Spain
-
Iberoamerican Cochrane Center. IIB Sant Pau, Spain
The first version was funded by the institutions above mentioned. The updates of the review were funded by the Iberoamerican Cochrane Center, IIB Sant Pau
Pablo Alonso‐Coello is supported by a Miguel Servet investigator contract from the Instituto de Salud Carlos III (CP09/00137), Spain
Declarations of interest
Dr. Martinez has no relevant conflicts of interest to declare.
Dr. Roqué has no relevant conflicts of interest to declare.
Dr. Roman has no relevant conflicts of interest to declare.
Dr. Català has no relevant conflicts of interest to declare.
Dr. Pablo Alonso‐Coello is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP09/00137).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Blomqvist 1988 {published data only}
- Blomqvist C, Elomaa I, Porkka L, Karonen SL, Lamberg-Allardt C. Evaluation of salmon calcitonin treatment in bone metastases from breast cancer - a controlled trial. Bone 1988;9(1):45-51. [DOI] [PubMed] [Google Scholar]
Roth 1986 {published data only}
- Roth A, Kolaric K. Analgesic activity of calcitonin in patients with painful osteolytic metastases of breast cancer. Results of a controlled randomized study. Oncology 1986;43(5):283-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allan 1983 {published data only}
- Allan E. Calcitonin in the treatment of intractable pain from advanced malignancy. Pharmatherapeutica 1983;3(7):482-6. [PubMed] [Google Scholar]
Beaufort 1984 {published data only}
- Beaufort F, Gölles M. Calcitonin in bone-metastasizing breast carcinoma. Results of a pilot study [Calcitonin beim skelettmetastasierten Mammakarzinom. Ergebnisse einer Pilot-Studie]. Onkologie 1984;7(3):137-40. [PUBMED 6205336] [DOI] [PubMed] [Google Scholar]
Berrenssen 1985 {published data only}
- Berressem P, Dambacher MA, Schmidt G. Controlled trials of salmon calcitonin in patients with bone metastases [Kontrollierte wirkungsprufungen von lackhs-calcitonin bei patienten mit knochenmetatasen therapiewoche]. Therapiewoche 1985;35(9):992-5. [Google Scholar]
El Wasseef 2012 {published data only}
- El Wasseef M, Ibrahim SE, Osman AM, El Kafrawy S. Pamidronate disodium versus calcitonin: a comparative study in the management of bone metastases in patients with breast cancer. In: Regional Anesthesia and Pain Medicine. Vol. Conference abstract. 2012.
Gennari 1989 {published data only}
- Gennari C, Francini G, Chierichetti SM, Nami R, Gonelli S, Piolini M. Salmon calcitonin treatment in bone metastases. Current Therapeutic Research, Clinical and Experimental 1989;45(5):804-12. [EMBASE: 1989138640] [Google Scholar]
Hindley 1982 {published data only}
- Hindley AC, Hill EB, Leyland MJ, Wiles AE. A double-blind controlled trial of salmon calcitonin in pain due to malignancy. Cancer Chemotherapy and Pharmacology 1982;9(2):71-4. [DOI] [PubMed] [Google Scholar]
Kleibel 1984 {published data only}
- Kleibel F, Schmidt G. Salmon calcitonin in metastatic bone pain. Demonstration of acute analgesia in tumor patients. Deutsche Medizinische Wochenschrift 1984;109(24):944-7. [DOI] [PubMed] [Google Scholar]
Lepidini 1992 {published data only}
- Lepidini G. Analgesic therapy in bone metastases: effect of association of calcitonin-HD and 6-methyl-prednisolone [Terapia antalgica in corso di metastasi ossee: effetto dell´associazione calcitonina-HD e 6-metil prednisolone]. Giornale Italiano di Ricerche Cliniche e Terapeutiche 1992;13:123-7. [Google Scholar]
Serdengeçti 1986 {published and unpublished data}
- Serdengecti S, Serdengecti K, Derman U, Berkarda B. Salmon calcitonin in the treatment of bone metastases. International Journal of Clinical Pharmacology Research 1986;6(2):151-5. [PubMed] [Google Scholar]
Tsavaris 2006 {published data only}
- Tsavaris N, Kopterides P, Kosmas C, Vadiaka M, Dimitrakopoulos A, Scopelitis H, et al. Analgesic activity of high-dose intravenous calcitonin in cancer patients with bone metastases. Oncology Reports 2006;16(4):871-5. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Alviar 2011
- Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD006380. [DOI: 10.1002/14651858.CD006380.pub2] [DOI] [PubMed] [Google Scholar]
Body 2000
- Body JJ. Bisphosphonates as chemotherapeutic agents. Current Opinion in Oncologic, Endocrine and Metabolic Investigational Drugs 2000;2(2):155-61. [Google Scholar]
Chesnut 2000
- Chesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. American Journal of Medicine 2000;109(4):267-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Craney 2000
- Cranney A, Welch V, Adachi JD, Homik J, Shea B, Suarez-Almazor ME, et al. Calcitonin for the treatment and prevention of corticosteroid-induced osteoporosis. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD001983. [DOI: 10.1002/14651858.CD001983] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Eisenberg 1994
- Eisenberg E, Berkey CS, Carr DB, Mosteller F, Chalmers TC. Efficacy and safety of nonsteroidal antiinflammatory drugs for cancer pain: a meta-analysis. Journal of Clinical Oncology 1994;12:2756-65. [DOI] [PubMed] [Google Scholar]
Grahame‐Smith 2002
- Grahame-Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug Therapy. 3rd edition. Oxford: Oxford University Press, 2002. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks, JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org..
Hoskin 1995
- Hoskin P. Radiotherapy for bone pain. Pain 1995;63:137-9. [DOI] [PubMed] [Google Scholar]
Jacox 1994
- Jacox A, Carr DB, Payne R. Management of cancer pain. In: Clinical Practice Guideline No 9. Agency for Health Care Policy and Research, US Department of Health and Human Services, 1994:41-74. [AHCPR Publication No. 94-0592] [Google Scholar]
Lussier 2004
- Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004;9(5):571-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
MacLean 2008
- MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Annals of Internal Medicine 2008;148(3):197-213. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from the retrospective analysis of disease. Journal of the National Cancer Institute 1959;22(4):719–48. [PubMed] [Google Scholar]
Matsumoto 2002
- Matsumoto T, Nagata N, Horikoshi N, Adachi I, Ohashi Y, Ogata E. Comparative study of incadronate and elcatonin in patients with malignancy-associated hypercalcaemia. Journal of International Medical Research 2002;30(3):230-43. [PMID: 12166339.] [DOI] [PubMed] [Google Scholar]
Nelson 1991
- Nelson OS, Munro AJ, Tannock IF. Bone metastatic: pathophysiology and management policy. Journal of Clinical Oncology 1991;9:509-24. [DOI] [PubMed] [Google Scholar]
O'Connell 2013
- O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL. Interventions for treating pain and disability in adults with complex regional pain syndrome- an overview of systematic reviews. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD009416. [DOI: 10.1002/14651858.CD009416.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2007
- Palmer SC, McGregor DO, Strippoli GFM. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD005015. [DOI: 10.1002/14651858.CD005015.pub3] [DOI] [PubMed] [Google Scholar]
Parfitt 1999
- Parfitt K (Ed). Martindale: The Complete Drug Reference. Vol. 32. London: Pharmaceutical Press, 1999. [Google Scholar]
Paterson 2000
- Paterson AHG. The potential role of bisphosphonates as adjuvant therapy in the prevention of bone metastases. Cancer 2000;88(Suppl 12):3038-46. [PMID: 10898349] [DOI] [PubMed] [Google Scholar]
Portenoy 1989
- Portenoy RK. Cancer pain. Epidemiology and pain syndromes. Cancer 1989;6:2298-307. [PMID: 2655867] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre; The Cochrane Collaboration Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre; The Cochrane Collaboration, 2014.
Robinson 1995
- Robinson RG, Preston DF, Schiefelbein M, Baxter KG. Strontium 89 therapy for the palliation of pain due to osseous metastases. JAMA 1995;274:420-4. [PubMed] [Google Scholar]
Roqué 2011
- Roqué M, Martinez MJ, Alonso P, Català E, Garcia JL, Ferrandiz M. Radioisotopes for metastatic bone pain. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD003347. [DOI: 10.1002/14651858.CD003347.pub2] [DOI] [PubMed] [Google Scholar]
Sze 2002
- Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No.: CD004721. Art. No: CD004721. [DOI: 10.1002/14651858.CD004721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2002
- Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD002068. [DOI: 10.1002/14651858.CD002068] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong MHF, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD003474. [DOI: 10.1002/14651858.CD003474.pub3] [DOI] [PubMed] [Google Scholar]
Yuen 2006
- Yuen KK, Shelley M, Sze WM, Wilt T, Mason MD. Bisphosphonates for advanced prostate cancer. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.: CD006250. Art. No: CD006250. [DOI: 10.1002/14651858.CD006250] [DOI] [PubMed] [Google Scholar]
Zojer 1999
- Zojer N, Keck AV, Pecherstorfer M. Comparative tolerability of drug therapies for hypercalcaemia of malignancy. Drug Safety 1999;21(5):389-406. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martinez‐Zapata 2003
- Martinez MJ, Roqué i Figuls M, Alonso Coello P, Català E, Garcia JL, Ferrandiz M. Calcitonin for metastatic bone pain. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD003223. [DOI: 10.1002/14651858.CD003223.pub2] [DOI] [PubMed] [Google Scholar]
Martínez‐Zapata 2006
- Martinez-Zapata MJ, Roqué M, Alonso-Coello P, Català E. Calcitonin for metastatic bone pain. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD003223. [DOI: 10.1002/14651858.CD003223.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


