Abstract
Background
Bitter taste perception and sweetness preference have been associated with dental caries. Propylthiouracil (PROP) has been used to determine the genetic sensitivity to bitter taste in early childhood caries. However, the role of the bitter threshold in dental biofilm cariogenicity has not been reported. The purpose of this study was to investigate the role of individual taste sensitivity using PROP in dental biofilm cariogenicity in orthodontic patients.
Methods
Forty orthodontic patients (12–42 years old) undergoing fixed appliance orthodontic treatment participated in this cross-sectional study. Their demographic, oral hygiene practice, and dietary habits data were obtained using a questionnaire. The patients’ bitter taste threshold was measured using a PROP assay. The patients were subsequently classified as super-tasters (STs), medium-tasters (MTs), and non-tasters (NTs). Dental biofilm cariogenicity was determined using a 3-tone disclosing gel that becomes pink (new dental biofilm), purple (mature dental biofilm), and light blue (cariogenic dental biofilm) based on dental biofilm maturity.
Results
The NT, MT, and ST groups comprised 10%, 27.5%, and 62.5% of the patients, respectively. Most of the STs (56%) and MTs (63.6%) were female, whereas no females were NTs. The dental biofilm cariogenicity was significantly different between the PROP bitterness groups (P < .05). The highest percentage of mature biofilm, followed by cariogenic and new biofilm, was found in the MT and ST groups. However, the cariogenic biofilm percentage was significantly higher compared with mature biofilm (P < .05) in the NT group. A low frequency (<1 time/d) of sugary and acidic food intake between meals was observed in the ST, MT, and NT groups with no significant difference amongst the groups (P > .05).
Conclusions
Cariogenic dental biofilm was highly present in orthodontic patients with the NT phenotype.
Key words: Taste perception, PROP, Three-tone plaque disclosing agent, Dental biofilm, Orthodontic patients
Introduction
Dental caries is the most common deleterious effect related to fixed orthodontic treatment. This risk is attributed to the presence of brackets, arch wires, ligatures, and other orthodontic appliances that complicate oral hygiene measures and leads to increased dental biofilm accumulation at the brackets’ base.1,2 Orthodontic appliances create an ecological environment favourable to qualitative and quantitative changes in dental biofilm microorganisms.3 Fixed appliances, including brackets, springs, and arch wires, impede access to the tooth surface, making it difficult to remove dental biofilm by mechanical cleaning. The ecological plaque hypothesis proposed that any major changes in local environmental conditions, for example, high sucrose consumption, will alter the competitiveness of specific bacteria within the dental biofilm and result in pathogenic dental biofilm formation.4 Acid production, acid tolerance, and intracellular and extracellular substances affect dental biofilm cariogenicity.5 The number of cariogenic bacteria, including Streptococcus mutans and lactobacilli, increase in the dental biofilm on teeth with fixed orthodontic appliances.6
The composition and properties of dental biofilm reflect the oral environment. The 3‑tone plaque disclosing gel (GC Tri Plaque ID Gel™) was developed to identify caries risk in individuals.7 This gel contains red pigment (red Bengal), blue pigment (brilliant blue FCF), and sucrose. The structure of new dental biofilm is sparse, and the blue pigment easily washes off, giving the new dental biofilm a pink/red colour. The mature dental biofilm (>48 hours) structure is dense; thus, the blue and red pigments are trapped, resulting in a blue/purple colour. When contacting the high acid-producing mature dental biofilm, the sucrose in the disclosing gel is metabolised by acidogenic bacteria in the dental biofilm, increasing its acidic content. When the dental biofilm pH drops to pH <4.5, the dental biofilm becomes light blue. The light blue–stained dental biofilm has high levels of S mutans.8 Therefore, regarding cariogenic potential, the light blue dental biofilm is the most virulent, followed by the blue/purple and pink dental biofilm.
Several studies have demonstrated the relationship of high sucrose consumption and dental caries.9 Orthodontists commonly advise patients to avoid sugary and acidic foods to prevent enamel demineralisation.10,11 However, few studies have investigated sugar intake amongst patients undergoing orthodontic treatment. A previous study demonstrated the relationship between white spot lesions (WSLs) and the frequency of consuming sugary foods/carbonated soft drinks in orthodontic patients.12 However, a cross-sectional study found no association between wearing an orthodontic appliance and daily sugar intake.13
There are multiple links amongst taste perception, taste preferences, food preferences, and food choices and the amount of food consumed. Genetic sensitivity to taste may be associated with the preference for or rejection of some foods.14 There are 3 phenotypic responses to the taste of 6-n-propylthiouracil (PROP): those perceiving minimal bitterness (non-tasters, NTs); moderate bitterness (medium tasters, MTs); or extreme bitterness (super-tasters, STs).15 These differences in perceived bitterness are due to differences in peripheral nerve sensitivity partly attributable to differences in the gene encoding for a specific bitter taste receptor (TAS2R38 gene). TAS2R38 variants (rs713598, rs1726866, and rs10246939) and bitter and sweet taste preference are significantly associated.16 Sweet preference has been linked to bitter taste sensitivity to PROP.15,17 A meta-analysis demonstrated that the NT group exhibited a significantly higher decayed, missing, and filled teeth score than the MT and ST groups.18
Most orthodontic trials have used the Silness and Loe plaque index. However, some plaque indices are inappropriate for orthodontic patients. Thus, there is a need to further assess the practicability of the advocated methods.19 Furthermore a review on the effect of sugar consumption on caries highlighted the lack of consistency and precision of dietary assessment methods in dental studies.20 Therefore, the present study used the 3-tone disclosing agent and the PROP sensitivity test to investigate dental biofilm cariogenicity and bitter taste perception, respectively. The aim of this study was to investigate the cariogenicity of dental biofilm and dietary habits amongst orthodontic patients with different genetic sensitivities to the bitter taste of PROP.
Methods
Study design and participants
This cross-sectional study received ethical approval from the Institutional Review Board, Walailak University, Thailand (Project ID: WUEC-20-224-01/2) on August 10, 2020. The principles of the Declaration of Helsinki were followed in this study.21 Patients in this study included Thai patients (≥12 years old) who were undergoing orthodontic treatment at the Advanced Oral Health Center, Walailak University. The exclusion criteria were having a systemic disease or on a long term/recent/current regimen of medications affecting taste perception or salivary flow, antibiotic therapy within the previous 6 months, and severe periodontal problems. Written informed consent was obtained from all participants on the first test day. The participants were recruited using a purposive sampling method based on the inclusion and exclusion criteria, which resulted in 40 participants.
PROP disk preparation
PROP preparation and quantification were performed as described.17 Briefly, 50 mg propylthiouracil (PTU) tablets were purchased from T.O. Chemical manufacturer. No. 1 Whatman filters (3 cm diameter, Sigma-Aldrich) were cut in half and saturated with a 50-mmol/L PROP solution. PROP was eluted from randomly selected saturated disks by incubating each disk in 20 mL methanol overnight at room temperature. The amount of PROP in the impregnated filter paper disks was quantified using spectrophotometry. Pure PROP (6-n-propylthiouracil, Sigma-Aldrich) was dissolved in methanol and used as a standard. The PROP standard was prepared at 6 concentrations (0–0.01 mg/mL). The absorbance of PROP in the methanol solution was measured at a 275-nm wavelength. A calibration curve was constructed and the extracted PROP concentration was determined. We ascertained that the mean amount of PROP per disk was 0.412 ± 0.017 mg, and the percentage coefficient of variation across the disks (n = 3) was 4.2%.
PROP sensitivity test
The PROP sensitivity test was performed as described.22 The method included a sodium chloride (NaCl) rating in the PROP screening procedure. NaCl was included because the taste intensity of NaCl does not vary based on PROP taster status. NTs give a lower intensity rating to PROP than to NaCl, whilst MTs rate the intensity of PROP and NaCl as equally intense. STs give much higher intensity ratings to PROP than to NaCl. Therefore, it is recommended to use NaCl rating as a reference standard for clarifying the classification of participants in case of a borderline PROP rating.23 Briefly, the participants placed the disks impregnated with 50 mmol/L PROP or 1.0 mol/L NaCl on the anterior two-thirds of their tongue for 30 seconds. The sensation's intensity was rated by marking the Labeled Magnitude Scale (LMS). The participants who gave lower intensity ratings to the PROP solutions than to those of NaCl or who rated the PROP disk lower than 13 mm on the LMS were classified as NTs; those who gave higher ratings to PROP solutions than to the NaCl solutions or who rated the PROP disk higher than 67 mm on the LMS were classified as STs; and those who gave similar ratings to the 2 stimuli or who rated the PROP disk with intermediate ratings (13–67 mm) were classified as MTs. Facial expressions were observed during the testing to support the verbal response and to identify any ambiguous or conflicting response.24
Questionnaire and diet analysis
Self-reported questionnaires were used to obtain the demographic characteristics (age and sex), the fixed orthodontic therapy duration, and toothbrushing frequency. Dietary analysis was performed as described.25 The patients were required to record all food and drink consumed for 5 days, which comprised 3 weekdays and a weekend. The patients were educated and motivated to enter everything that they consumed in the chart from morning until bedtime. The average number of exposures to sugar and acid between meals over the 5 days was calculated for each patient.
Dental biofilm cariogenicity assessment using the 3-tone plaque disclosing gel
The 3-tone disclosing gel (GC Tri Plaque ID Gel™) was used as described.26 The most mature dental biofilm was recorded for each tooth. Based on the colour changes on the tooth surfaces, the plaque maturing staining (PMS) was obtained using the formula:
% PMS = (number of teeth with each coloured plaque/total number of teeth examined) x 100
Statistical analysis
Data analysis was performed using the Statistical Package of Social Sciences (SPSS) version 25 (IBM Corp.). The normal distribution of the data was assessed with the Shapiro–Wilk and Kolmogorov–Smirnov tests.27 Descriptive statistics are presented as mean ± standard deviation (SD), median, range, and percentage.28 The unpaired Student t test and one-way analysis of variance (ANOVA) with Bonferroni post hoc test were used for parametric data. Otherwise, the nonparametric tests, Mann–Whitney U test,29 and Kruskal–Wallis with Dunn–Bonferroni post hoc test were used.30 The categorical data were analysed by chi-square test and Fisher exact test.31 The significance level was set at P ≤ .05.
Results
The Shapiro–Wilk and Kolmogorov–Smirnov tests demonstrated that the data were not normally distributed. Therefore, nonparametric statistics were used in the analysis.
The study population
The demographic variables, treatment duration, oral hygiene practice, and diet habits are shown in the Table. The study comprised 40 patients, aged 12 to 42 years (median = 28 years). There were 4 (10%) NT patients, 11 (27.5%) MT patients, and 25 (62.5%) ST patients. Most ST (14/25, 56%) and MT (7/11, 63.6%) patients were female; however, there were no females who were NTs.
Table.
Descriptive analysis of the demographic, fixed orthodontic therapy duration, toothbrushing frequency, and between-meal sugar/acid food intake data based on bitter taste perception.
| Variable | ST, n = 25 (62.5%) | MT, n = 11 (27.5%) | NT, n = 4 (10%) | P value |
|---|---|---|---|---|
| Age (years) | ||||
| Median (min–max) | 28 (12−41) | 28 (20–42) | 23 (20–30) | .45a |
| Sex | ||||
| Male, n (%) | 11 (44) | 4 (36.40) | 4 (100) | .08a |
| Female, n (%) | 14 (56) | 7 (63.60) | 0 (0) | |
| Fixed orthodontic therapy duration (mo) | ||||
| Median (min–max) | 9 (2−16) | 8 (2−14) | 7.50 (6−14) | .76a |
| Toothbrushing frequency, n (%) | ||||
| <2 times/d | 1 (4) | 0 (0) | 2 (50) | .02b |
| ≥2 times/d | 24 (96) | 11 (100) | 2 (50) | |
| Between-meal sugary foods (times/d) | ||||
| Median (min–max) | 0.7 (0−2.4) | 0.8 (0−2.4) | 0.1 (0−1.4) | .39a |
| Between-meal acidic foods (times/d) | ||||
| Median (min–max) | 0 (0−0.8) | 0 (0−0.2) | 0 (0−0) | .53a |
Kruskal–Wallis test.
ST and MT groups were combined as the taster group. Fisher exact test was used instead of the chi-square test because of assumption violation (P = .02; taster vs NT).
MT, medium-taster; NT, non-taster; ST, super-taster.
The orthodontic treatment duration (median and range) in the ST, MT, and NT groups were 9 (2–16), 8 (2–14), and 7.5 (6–14) months, respectively (P > .05). The brushing frequency was significantly different between the taster (ST and MT) and NT groups (P < .05). Most patients’ sugar and acid food intake between meals was <1 time/d. There was no significant difference in sugary/acidic food consumption between meals between the taster groups (P > .05).
Dental biofilm cariogenicity
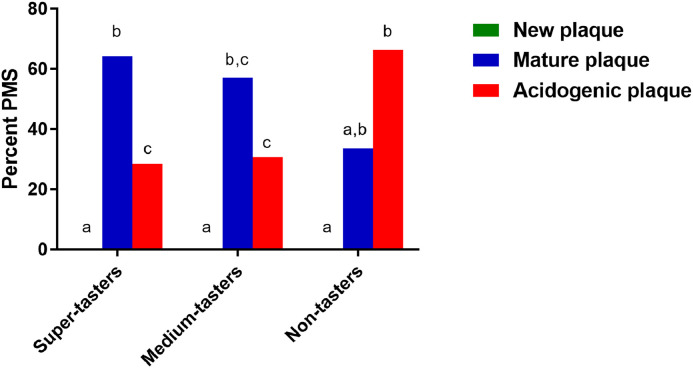
Figure 1 presents the figures for dental biofilm staining in the ST, MT, and NT groups. The medians of the percentage PMS in the ST, MT, and NT groups are illustrated in Figure 2. The highest percentage (median = 64.29, min-max = 28.57−100) of purple dental biofilm, followed by light blue dental biofilm (median = 28.57, min−max = 0−74.43) and pink dental biofilm (median = 0, min−max = 0−45.83), was found in the ST group. All pairwise comparisons of dental biofilm maturity in the ST group were significantly different (P < .05). In the MT group, the highest percentage of purple dental biofilm (median = 57.41, min−max = 12.5−75) was found, and 30.77% (min−max = 12.5−87.5) and 0% (min−max=0−66.67) light blue and pink dental biofilm, respectively, were observed. A significant difference was observed between the pink and light blue dental biofilm and pink and purple dental biofilm (P < .05). However, the highest percentage of light blue dental biofilm (median = 66.36%, min−max=41.67%−75%) was observed in the NT group, whilst 33.64% (min−max = 25−54.17) and 0% (min−max = 0−0) were found for the purple and pink dental biofilm, respectively. A pairwise difference was found between pink and light blue dental biofilm (P < .05).
Fig. 1.
Dental biofilm staining using the 3-tone plaque-disclosing gel. Representative dental biofilm colours in the (A) super-taster, (B) medium-taster, and (C) non-taster groups are shown.
Fig. 2.
Percentage plaque maturing staining by sweetness preference. Different superscript letters indicate significant differences (P < .05) in plaque maturity between each taster group (Kruskal–Wallis and Dunn–Bonferroni post hoc test).
Discussion
Our patients ranged from 12 to 42 years-old, which was similar to the range to previous studies on the association of the PROP sensitivity test and dental caries.32, 33, 34, 35 Most patients were recruited into the study after wearing fixed appliances for 2 to 9 months. This duration is long enough to observe changes related to dental caries development. Increased salivary flow rates and components were found after 1 month of orthodontic treatment.36 Furthermore, clinical WSL formation around an orthodontic attachment can occur 4 weeks after beginning treatment.37,38
In the last decade, research has focused on the relationship between genetic taste sensitivity, as detected by PROP, and dental caries.18 However, few studies have investigated the influence of taste perception and dental caries in orthodontic patients. Most of the orthodontic patients participating in this study were STs (62.5%), followed by MTs (27.5%) and NTs (10%). The prevalence of tasters in this study was relatively high. This prevalence pattern was similar to a previous study33; however, it was not in line with other studies.22,39
The prevalence of WSLs was significantly higher in adolescent orthodontic patients who were PROP NTs compared with PROP tasters.39 This study suggested that PROP taste perception could be a potential risk factor for WSL formation during fixed orthodontic treatment. However, no study has focused on bitterness perception, sweetness preference, and dental biofilm cariogenicity. In this study, similar dental biofilm maturity distribution patterns were observed in the MT and ST groups. Matured dental biofilm was markedly observed in both groups compared with new and cariogenic dental biofilm. However, although this study had a small sample size, the highest percentage of cariogenic plaque was found in the NT participants. Therefore, our findings provide additional evidence on the influence of sweetness preference on caries.8,40 Although dental caries is a multifactorial dynamic disease, the presence of dental biofilm and its interaction between host factors and sugar is very important in caries development.41 This interaction influences the oral environment as explained by the ecological plaque hypothesis, and acidogenic bacteria play an important role in dental biofilm acidogenicity.5,41 Using 3-tone plaque disclosing gels, Lee et al42 found a relationship between acidogenic bacterial counts and dental biofilm maturity. A high number of acidogenic bacteria was found in light blue dental biofilm, followed by purple and pink. Taken together, our findings suggest that dental biofilm maturity and the PROP sensitivity test might be adjunct indicators to assess caries risk status in orthodontic patients.
We found no significant difference in sugar intake amongst the NT, MT, and ST groups. Our findings did not agree with a previous report.43 However, a systematic review revealed that only a small proportion of available studies reported significant associations between taste sensitivity and dietary intake.44 Another possible explanation for the lack of a significant association between sugar consumption and bitterness preference in our study is that taste preference is not a unique determinant of the type of food consumed or the establishment of eating habits.14 Additionally, the underlying impact of socioenvironmental factors on individuals’ food choices cannot be ignored.45 Furthermore, in this study, the effects of bitterness sensitivity on dental biofilm maturity might be outweighed by the impact of oral hygiene instruction. The results of his study should be interpreted with caution, because the level of acidogenic bacteria, particularly S mutans, in dental biofilm and/or saliva was not investigated in this study. Moreover, our participants were orthodontic patients; thus, whether these results may generalise to other groups is unclear.
Conclusions
This study demonstrated a difference in dental biofilm cariogenicity amongst orthodontic patients with different genetic sensitivities to the bitter taste of PROP.
Conflict of interest
None disclosed.
Acknowledgments
Acknowledgements
We thank Christian Estacio, a native English speaker and Academic Counselor for International Affairs, Walailak University, International College of Dentistry for his English editing.
Funding
This work was supported by Walailak University, International College of Dentistry (Grant Number: WUICD-B2020).
REFERENCES
- 1.Brown MD, Campbell PM, Schneiderman ED, Buschang PH. A practice-based evaluation of the prevalence and predisposing etiology of white spot lesions. Angle Orthod. 2016;86:181–186. doi: 10.2319/041515-249.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadler-Olsen S, Sandvik K, El-Agroudi MA, Ogaard B. The incidence of caries and white spot lesions in orthodontically treated adolescents with a comprehensive caries prophylactic regimen–a prospective study. Eur J Orthod. 2012;34:633–639. doi: 10.1093/ejo/cjr068. [DOI] [PubMed] [Google Scholar]
- 3.Freitas AO, Marquezan M, Nojima Mda C, Alviano DS, Maia LC. The influence of orthodontic fixed appliances on the oral microbiota: a systematic review. Dental Press J Orthod. 2014;19:46–55. doi: 10.1590/2176-9451.19.2.046-055.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsh PD, Head DA, Devine DA. Dental plaque as a biofilm and a microbial community—implications for treatment. J Oral Biosci. 2015;57:185–191. [Google Scholar]
- 5.Marsh PD. Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawhney R, Sharma R, Sharma K. Microbial colonization on elastomeric ligatures during orthodontic therapeutics: an overview. Turk J Orthod. 2018;31:21–25. doi: 10.5152/TurkJOrthod.2018.17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh LJ, Healey DL. Prevention and caries risk management in teenage and orthodontic patients. Aust Dent J. 2019;64(Suppl 1):S37–S45. doi: 10.1111/adj.12671. [DOI] [PubMed] [Google Scholar]
- 8.Jayanthi M, Shilpapriya M, Reddy VN, Elangovan A, Sakthivel R, Vijayakumar P. Efficacy of three-tone disclosing agent as an adjunct in caries risk assessment. Contemp Clin Dent. 2015;6:358–363. doi: 10.4103/0976-237X.161887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Loveren C. Sugar restriction for caries prevention: amount and frequency. Which is more important? Caries Res. 2019;53:168–175. doi: 10.1159/000489571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HS, Walsh LJ, Freer TJ. Enamel demineralization during orthodontic treatment. Aetiology and prenvention. Aus Dent J. 1997;42:322–327. doi: 10.1111/j.1834-7819.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 11.Yip HHY, Wong R, Hagg U. Complication of orthodontic treatment: are soft drinks a risk factor? World J Orthod. 2009;10:33–40. [PubMed] [Google Scholar]
- 12.Khalaf K. Factors affecting the formation, severity and location of white spot lesions during orthodontic treatment with fixed appliances. J Oral Maxillofac Res. 2014;5:e4. doi: 10.5037/jomr.2014.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albaqami G, Abreu LG, Bernabe E. Is wearing orthodontic appliances associated with eating difficulties and sugar intake among British adolescents? A cross-sectional study. Eur J Orthod. 2021;43:193–199. doi: 10.1093/ejo/cjaa071. [DOI] [PubMed] [Google Scholar]
- 14.Drewnowski A. Taste preferences and food intake. Annu Rev Nutr. 1997;17:237–253. doi: 10.1146/annurev.nutr.17.1.237. [DOI] [PubMed] [Google Scholar]
- 15.Tepper BJ, Christensen CM, Cao J. Development of brief methods to classify individuals by PROP taster status. Physiol Behav. 2001;73:571–577. doi: 10.1016/s0031-9384(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 16.Dioszegi J, Llanaj E, Adany R. Genetic background of taste perception, taste preferences, and its nutritional implications: a systematic review. Front Genet. 2019;10:1272. doi: 10.3389/fgene.2019.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Kirkmeyer SV, Tepper BJ. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 2003;78:625–633. doi: 10.1016/s0031-9384(03)00057-x. [DOI] [PubMed] [Google Scholar]
- 18.Alkuhl H, Morgan R, Koletsi D, Kavvadia K. Genetic taste sensitivity and dental caries in children and adolescents: a systematic review and meta-analysis. Int J Paediatr Dent. 2021;32:204–222. doi: 10.1111/ipd.12845. [DOI] [PubMed] [Google Scholar]
- 19.Al-Anezi SA, Harradine NW. Quantifying plaque during orthodontic treatment. Angle Orthod. 2012;82:748–753. doi: 10.2319/050111-312.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moynihan PJ, Kelly SA. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. 2014;93:8–18. doi: 10.1177/0022034513508954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrestha B, Dunn L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J Nepal Health Res Counc. 2020;17:548–552. doi: 10.33314/jnhrc.v17i4.1042. [DOI] [PubMed] [Google Scholar]
- 22.Melis M, Tomassini Barbarossa I. Taste perception of sweet, sour, salty, bitter, and umami and changes due to l-arginine supplementation, as a function of genetic ability to taste 6-n-propylthiouracil. Nutrients. 2017;9:6. doi: 10.3390/nu9060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein GL, Daun H, Tepper BJ. Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol Behav. 2007;90:809–817. doi: 10.1016/j.physbeh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen AW, Resurreccion AVA, Paguio LP. Age appropriate hedonic scales to measure food preferences of young children. J Sens Stud. 1996;11:141–163. [Google Scholar]
- 25.Ngo HC, Wolff MS, Hume WR. In: Dental caries: activity and risk assessments as a logical and effective path to both prevention and cure. Mount GJ, Hume WR, Ngo HC, Woff MS, editors. John Wiley & Sons; Chichester: 2016. Preservation and restoration of tooth structure; pp. 33–49. [Google Scholar]
- 26.Widhianingsih D, Koontongkaew S. Enhancement of cariogenic virulence properties of dental plaque in asthmatics. J Asthma. 2021;58:1051–1057. doi: 10.1080/02770903.2020.1753211. [DOI] [PubMed] [Google Scholar]
- 27.Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCluskey A, Lalkhen AG. Statistics II: central tendency and spread of data. Cont Educ Anaesth Crit Care Pain. 2007;7:127–130. [Google Scholar]
- 29.Kim HY. Statistical notes for clinical researchers: Nonparametric statistical methods: 1. Nonparametric methods for comparing two groups. Restor Dent Endod. 2014;39:235–239. doi: 10.5395/rde.2014.39.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HY. Statistical notes for clinical researchers: Nonparametric statistical methods: 2. Nonparametric methods for comparing three or more groups and repeated measures. Restor Dent Endod. 2014;39:329–332. doi: 10.5395/rde.2014.39.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HY. Statistical notes for clinical researchers: chi-squared test and Fisher's exact test. Restor Dent Endod. 2017;42:152–155. doi: 10.5395/rde.2017.42.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drewnowski A, Kristal A, Cohen J. Genetic taste responses to 6-n-propylthiouracil among adults: a screening tool forepidemiological studies. Chem Senses. 2001;26:483–489. doi: 10.1093/chemse/26.5.483. [DOI] [PubMed] [Google Scholar]
- 33.Rupesh S, Nayak UA. Genetic sensitivity to the bitter taste of 6-n propylthiouracil: a new risk determinant for dental caries in children. J Indian Soc Pedod Prev Dent. 2006;24:63–68. doi: 10.4103/0970-4388.26018. [DOI] [PubMed] [Google Scholar]
- 34.Vandal VB, Noorani H, Shivaprakash PK, Walikar B. Genetic specificity to 6-n-propylthiouracil and its association to dental caries: a comparative study. J Indian Soc Pedod Prev Dent. 2017;35:83–85. doi: 10.4103/0970-4388.199233. [DOI] [PubMed] [Google Scholar]
- 35.Verma P, Shetty V, Hegde AM. Propylthiouracil (PROP)–a tool to determine taster status in relation to caries experience, Streptococcus mutans levels and dietary preferences in children. J Clin Pediatr Dent. 2006;31:113–117. doi: 10.17796/jcpd.31.2.34302r2857511268. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Hu B, Liu Y, Ding G, Zhang C, Wang S. The effects of fixed orthodontic appliances on saliva flow rate and saliva electrolyte concentrations. J Oral Rehabil. 2009;36:781–785. doi: 10.1111/j.1365-2842.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava K, Tikku T, Khanna R, Sachan K. Risk factors and management of white spot lesions in orthodontics. J Orthod Sci. 2013;2:43–49. doi: 10.4103/2278-0203.115081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KC Subbiksha i, MS Kannan. White spot lesions in orthodontics. J Crit Rev. 2020;7:3730–3735. [Google Scholar]
- 39.Alanzi A, Velissariou M, Al-Melh MA, Ferguson D, Kavvadia K. Role of taste perception in white spot lesion formation during orthodontic treatment. Angle Orthod. 2019;89:624–629. doi: 10.2319/091918-680.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brostek AM, Walsh LJ. Minimal intervention dentistry in general practice. Oral Health Dent Manag. 2014;13:285–294. [PubMed] [Google Scholar]
- 41.Pitts NB, Zero DT, Marsh PD, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Park H, Lee J, Seo H, Lee S. Study of bacteria associated with dental caries using a 3 tone disclosing agent. J Korean Acad Pediatr Dent. 2018;45:32–40. [Google Scholar]
- 43.Jamel HA, Sheiham A, Watt RG, Cowell CR. Sweet preference, consumption of sweet tea and dental caries; studies in urban and rural Iraqi populations. Int Dent J. 1997;47:213–217. doi: 10.1111/j.1875-595x.1997.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 44.Tan SY, Tucker RM. Sweet taste as a predictor of dietary intake: a systematic review. Nutrients. 2019:11. doi: 10.3390/nu11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt RG. Stages of change for sugar and fat reduction in an adolescent sample. Community Dent Health. 1997;14:102–107. [PubMed] [Google Scholar]