Abstract
Biotechnologically useful yeast strains have been receiving important attention worldwide for the demand of a wide range of industries. Rhodotorula mucilaginosa is recognized as a biotechnologically important yeast that has gained great interest as a promising platform strain, owing to the diverse substrate appetites, robust stress resistance, and other gratifying features. Due to its attractive properties, R. mucilaginosa has been regarded as an excellent candidate for the biorefinery of carotenoids, lipids, enzymes, and other functional bioproducts by utilizing low-cost agricultural waste materials as substrates. These compounds have aroused great interest as the potential alternative sources of health-promoting food products, substrates for so-called third-generation biodiesel, and dyes or functional ingredients for cosmetics. Furthermore, the use of R. mucilaginosa has rapidly increased as a result of advancements in fermentation for enhanced production of these valuable bioactive compounds. This review focuses on R. mucilaginosa in these advancements and summarizes its potential prospects as alternative sources of natural bioproducts.
Keywords: Rhodotorula mucilaginosa, Biotechnological yeast, Industrial usage, Carotenoids, Lipids, Enzymes
Rhodotorula mucilaginosa; Biotechnological yeast; Industrial usage; Carotenoids; Lipids; Enzymes.
1. Introduction
The term "red yeast" is used for those species which can significantly produce substantial amounts of carotenoid pigments and lipid intracellularly, which impart an orange, pinkish or red color to yeast colonies (Mannazzu et al., 2015). Red yeast has excellent industrial potential and has piqued the interest of the food, pharmaceutical, cosmetics, and feed industries (Xu and Liu, 2017). Rhodotorula mucilaginosa, one of the red yeasts, is a promising yeast for industrial application in recent years.
Generally, R. mucilaginosa is considered as a natural producer of carotenoids and exhibits several advantages over bacteria, microalgae, and plants in carotenoid production due to its unicellular form, fast duplication periods, capacity to grow on a diverse range of substrates, and convenience of cultivation in large fermenters (Gualberto et al., 2022). The main carotenoid components synthesized by R. mucilaginosa generally include β-carotene, torulene, and torularhodin (Sharma and Ghoshal, 2021). And the potential for producing high-value certain carotenoids from waste raw substrates has been widely exploited (Gohain et al., 2020). These carotenoids in humans play many important health-promoting roles, such as antioxidants, precursors of vitamin A, inhibitors of cancers, and enhancers of immunity (Maoka, 2020). Furthermore, R. mucilaginosa is classified as a microbial lipid-producing microorganism for the reason that this species can synthesize more than 40% of its dry cell biomass as lipids, while the lipid accumulation of non-oil yeast is less than 10% of its cell dry biomass, such as Saccharomyces cerevisiae and Candida utilis (Ratledge, 2002; Daskalaki et al., 2019). The lipids synthesized by R. mucilaginosa mainly include triacylglycerols (TAG) (Czabany et al., 2007), which are similar to the other typically oleaginous yeasts, such as Yarrowia lipolytica, Trichosporon cutaneum, and Lipomyces starkeyi (Kolouchová et al., 2016; Dourou et al., 2018; Ganesan et al., 2019). These microbial lipids are excellent biomass fuels and good alternatives to fossil fuels (Lin et al., 2013). Moreover, R. mucilaginosa also could be developed as an industrial enzyme producer, especially in lipase and endo-β-1, 4-glucanases (Britto et al., 2013, Chennupati et al., 2009). Contingent on the cultivated environments, it has the capability to produce varied kinds of enzymes whose emergence may be industrially useful (Zhang and Cui, 2012; Wu et al., 2013; Lario et al., 2015). Furthermore, the biosynthetic pathways of carotenoid, lipid, and enzyme production in R. mucilaginosa and the influences of specific fermentation factors on their productivity have been documented previously (Kot et al., 2016; da Silva et al., 2020).
Microbial production possesses several preponderances in seasonal and geographical variability, and for that reason it takes economic advantages in cost of industrial production. A significant number of research articles have been reported on the bioprocesses of R. mucilaginosa, suggesting that it would be of benefit in the near future. Here, in line with the renewed interest towards R. mucilaginosa biotechnology, we synopsize the research progress on R. mucilaginosa and offer discussions on the prospects and perspectives for exploring it as alternative sources for advanced biotechnological applications.
2. History, taxonomy, morphology, and physiology of R. mucilaginosa
R. mucilaginosa was first isolated and named by Francis Charles Harrison in literature record, based on his research on yeast found in local cheese in the 1930s. The genus name of this species in Latin means "the yeast-colored red", while the rest means a viscous state in liquid. R. mucilaginosa is assigned to the family of Sporidiobolaceae, order of Sporidiobolales, class of Pucciniomycotina, and phylum of Basidiomycota in the Fungi kingdom as recorded by the International Mycological Association (Mycobank, 2022). The historically heterotypic synonymy of R. mucilaginosa consists of Torula mucilaginosa, Rhodotorula rubra (Demme), Rhodotorula pilimanae J. and Rhodotorula bubra (Species Fungorum, 2020).
Morphologically, the colony of R. mucilaginosa is orange-red, wetter, transparent, has a smooth surface, and picks up easily. They can form intracellular lipid droplets full of carotenoids, which makes them range in color from pink to orange in culture media. This colony morphology is similar to that of typical red yeasts assigned to the genera of Rhodosporidium, Rhodotorula, Sporobolomyces, and Sporidiobolus (Mannazzu et al., 2015). Several previous studies suggested that these carotenoids produced by R. mucilaginosa could be involved in protecting the cells from reactive oxygen species (ROS) induced by excessive irradiation of the UV, visible light spectrum, or other abiotic stresses (Frengova and Beshkova, 2009; Libkind et al., 2010; Park et al., 2018). As a reaction to certain nutritional deficiencies or abiotic stresses, red yeast cells will cline to pigmentation, tending the color to be deeper (Li et al., 2017).
R. mucilaginosa is a saprophytic eukaryote that reproduces in a manner that forms ascus and ascospores, has the characteristics of most yeasts, and exists in a unicellular and non-hyphal state. R. mucilaginosa is ubiquitous in ecological environment with wide distribution (Zhao et al., 2019), even could be separated from contaminated or polluted areas, or as endophytes exist in the rhizosphere soil of plants (Saha and Seal, 2015). Most representatives of this species are safe and show no pathogenicity. However, its low pathogenicity has been found clinically, causing dermatomycosis in patients who are critically ill and immune compromised (Sabate et al., 2002; Mohd et al., 2015; Wang et al., 2019). Therefore, R. mucilaginosa has been considered as an opportunistic pathogen, yet most of these species applied in industry pose no threat to safety and human health.
3. Characteristics of carotenoid biosynthesis by R. mucilaginosa
Carotenoids are a group of 40-carbon isoprenoids with high lipid-solubility, which widely exist in fruits, vegetables, and other dietary sources (Khan et al., 2021). Carotenoids have historically been known as food supplements and nutraceuticals (Rapoport et al., 2021). For example, lutein and zeaxanthin are reviewed synthetically to be effective antioxidants and blue light and ultraviolet filters, thus helping to prevent chronic eye diseases (Johra et al., 2020; Zafar et al., 2021). Moreover, carotenoids with unsaturated structure features have strong antioxidant activity, which endows them with strong immune activity that prevents the oxidation of low-density lipoprotein and protects cells from ROS. Humans cannot synthesize carotenoids themselves, but they can get them through the diet, which accounts for 7%–8% of the carotenoids that have been recorded in nature so far (Chacón Ordóñez et al., 2019; Honda et al., 2020). According to statistics and market data forecast, the global carotenoids market size was estimated to be USD 1.57 billion in 2022, and it is likely to reach an evaluation of USD 2.09 billion by the end of 2027, rising at a compounded average growth rate of 4.5% during the forecast period (Market Data Forecast: Carotenoids, 2022).
3.1. β-carotene
β-carotene is a well-studied pigment for its authorized dual functions as a nutrient supplement and a colorant in countries and regions around the world. β-carotene (C40H56, β, β -carotene Figure 1 A) is a fatty-soluble carotenoid with two retinyl groups and eleven conjugated double bonds. The molecular structure endows β-carotene relatively strong provitamin A and antioxidant activities (Watkins and Pogson, 2020; Burton et al., 2021). Given these excellent properties, β-carotene has been widely applied in many industries, such as medicine (Novikov et al., 2022), nourishments (Burton et al., 2021; Hambly et al., 2021), food additives (Barbosa et al., 2021), cosmetics (Borja-Martínez et al., 2020), and feed additives (Mudronova et al., 2018). Moreover, β-carotene has also been used to prevent cancer, tumors (Lee et al., 2022), and cardiovascular disease in medical and health-care proposals (Yang et al., 2022).
Figure 1.
Chemical structures of major carotenoids biosynthesis by Rhodotorula species. Groups of different carotenoids are boxed in color (A) β-carotene; (B) Torulene; (C) Torularhodin.
3.2. Torulene and torularhodin
Torulene (C40H54, 3′,4′-didehydro-β, ψ-carotene, Figure 1 B) and torularhodin (C40H52O2, 3′,4′-didehydro-β, ψ-carotene-16′-oic acid, Figure 1 C) are two principal carotenoids typically found in red yeasts of Rhodotorula genera (Tang et al., 2019; Liu et al., 2021). Torulene has one β-ionone ring linked to a polyene chain, while torularhodin bears the higher oxidation state as compared to torulene owing to the hydroxylation and oxidation from torulene. Owing to the existence of thirteen double bonds, torularhodin could be endowed with a stronger anti-oxidative capability than β-carotene and torulene in Rhodotorula species (Du C et al., 2017; Liu et al., 2019a). As two versatile fungal carotenoids acclaimed in the last decade, the biosynthetic pathways of torulene and torularhodin have been proposed, which are mainly derived from the mevalonate pathway in yeast cells (Kot et al., 2018; CU. Mussagy et al., 2022). What’s more, reports confirming their properties and potential producers are on the rise, and the present study proposes that the most promising producers of these two arresting compounds principally include the yeasts of the genera Rhodotorula, Sporidiobolus, and Sporobolomyces (Kot et al., 2018). Previous studies have proved that torulene and torularhodin are safe food additives and bait in feedstock for livestock, cosmetics, and ingredients in medicines (Li et al., 2019). Still, torulene and torularhodin have been elucidated clinically owing to their antioxidant and health-promoting properties. So far, difficulties still stand in the way of torulene and torularhodin production on a large scale (C.U. Mussagy et al., 2022). The productivity of torulene and torularhodin does rely on the yeast species or strains simply, but also on the different optimization strategies customized for their bioproduction.
3.3. Comparison with different microbial carotenoid producer
Rhodotorula species are of talent producer in producing carotenoids. A number of Rhodotorula isolates have been applied for carotenoids production since they share the same features and profiles of carotenoids as R. mucilaginosa, such as R. glutinis and R. toruloides (Kot et al., 2016; Zhao et al., 2022), that is the two red yeast which have been well-studied for β-carotene, torulene, and torularhodin production. In addition, it is worth mentioning that the carotenoids (major in lycopene and β-carotene) for food grade are mainly produced industrially by Blakeslea trispora at present (Shi et al., 2012; He et al., 2017). This mold is fast-growing, with high β-carotene yield in single unit organism,.as one of the earliest microorganisms for natural carotenoid production. Commonly, B. trispora is used for β-carotene production by means of mixing mating types species in specific ratios. During the mixed fermentation of two mating types, trisporic acid was numerously generated, and played a key role in β-carotene synthesis (Papadaki and Mantzouridou, 2021; Wang et al., 2021).
Another major carotenoid producer on industrial scale is the photosynthetic microalgae (major in β-carotene and xanthophyll), such as Chlorella, Dunaliella, Arthrospira, and Haematococcus (Cezare-Gomes et al., 2019). Compared with the carotenoid bioprocess in yeasts or molds, microalgae typically use the open pond system or closed photo-bioreactors in cultivation for carotenoid production. But problems in production and cultivation often occur in microalgae during the process of production and cultivation, including severe concerns about water loss, carbon source deficiency, other needed nutrients, and worry about weather conditions (Gong and Bassi, 2016). Furthermore, the production capacity in microalgae was still hindered and blocked by the naturally time-consuming growth, relatively low cell yielding, danger of contamination by protozoa or bacteria, scale-up for cultivation, and highly susceptibility to unfavorable weather conditions, which has become one of the bottlenecks for scaling up in light-driven carotenoids production (Liu et al., 2021). The comparison of carotenoids productivity among several R. mucilaginosa, other different red yeasts, B. trispora, and microalgae strains was summarized in Table 1.
Table 1.
The comparison between different microorganism in carotenoids production.
| Microorganism | Substrates | yield | Reference |
|---|---|---|---|
| R. glutinis TISTR 5159 | Crude glycerol | 135.2 mg/L | Saenge et al. (2011) |
| R. acheniorum | Whey ultrafiltrate | 263 mg/L (10.76 mg/g) | Nasrabadi and Razavi (2012) |
| R. mucilaginosa CCY 20-7-31 | Potato medium with 5% salt | 55.9 mg/L | Marova et al. (2012) |
| Sporobolomyces roseus CCY 19-4-8 | Whey medium | 29.4 mg/L | |
| R. glutinis CCY 20-2-26 | Whey/Whey medium | 51.22 mg/L | |
| R. glutinis 2.27 | YPD Medium | 42.32 mg/L | Li et al. (2015) |
| R. mucilaginosa CCT 7688 | Corn steep liquor, sugar cane molasses and raw glycerol | 3.73 mg/L | Dias Rodrigues et al. (2019) |
| B. trispora ATCC 14271 (+), B. trispora ATCC 14272 (-) | Lye and washing water | 64.1 mg/L | Papadaki and Mantzouridou (2021) |
| B. trispora CGMCC 3.7855 (+), B. trispora CGMCC 3.7819 (-) | Synthetic medium | 523.8 mg/L | Ding et al. (2020) |
| B. trispora ATCC 14271 (+), B. trispora ATCC 14272 (-) | Synthetic medium | 1870.0 mg/L | He et al. (2017) |
| B. trispora ATCC 14271 (+), B. trispora ATCC 14272 (-) | Synthetic medium | 578 mg/L | Shi et al. (2012) |
| Phormidium autumnale | Slaughterhouse wastewater | 183.03 μg/g | Rodrigues et al. (2014) |
| Nannochloropsis oculata NANN OCUL-1 | Low-cost medium | 311.33 μg/L | Faé Neto et al. (2018) |
| Selenastraceae sp. B10 | Kuhl medium with glucose | 7.49 mg/g | Gong and Huang (2020) |
| Coelastrum cf. pseudomicroporum | Urban wastewater | 91.2 pg/Cell | Úbeda et al. (2017) |
Although the unit yield of carotenoids by R. mucilaginosa is relatively low as compared to that of B. trispora, it is of great practical value to produce carotenoids due to their numerous advantages. For example, the specific growth rate of R. mucilaginosa is high, and a substantial amount of cell biomass is relatively effortless to acquire at lab and pilot plant scale levels (Dias Rodrigues et al., 2019; Banerjee et al., 2020). R. mucilaginosa can be cultivated in ordinary bioreactors and biomass can be utilized as feed or an additive in pharmaceutical products (Costa et al., 2020; Hamidi et al., 2020). Besides, R. mucilaginosa can suit a variety of environmental conditions, and the biomass can grow under a broad range of carbon and nitrogen sources (da Silva et al., 2020; Rodrigues et al., 2022). These advantages are both directly favorable conditions for carotenoids production in R. mucilaginosa as compared with B. trispora and microalgae.
3.4. Functions of carotenoids in ultraviolet radiation
Photo-radiation is vital for sustaining living beings. However, intense ultraviolet radiation (UVR) is extremely hazardous and causes multiple types of damage to living organisms, such as causing damage to organic molecules and leading to the accumulation of ROS, inhibiting the growth of microorganisms (Krinsky, 1989). This damage could be caused by direct damage or indirect damage. When UVR is absorbed by cellular macromolecules, such as DNA, proteins, and lipids, it causes direct harm. And the ROS wreaks indirect damage when the UVR is absorbed by an intermediate molecule. ROS is subsequently generated and leads to the impairment of biological function (Apel and Hirt, 2004).
Living organisms have different strategies to coexist with the damage caused by UVR. The most significant and widespread strategy for microorganisms may be the avoidance of harm by the synthesis of UV-cleaning chemicals, such as carotenoids. The antioxidant capacity of carotenoids has been observed in bacteria, algae, fungi, and plants. In fungi, carotenoids serve a variety of activities. They are responsible for the protection against UVR and ROS, and are associated with membrane lipids. The presence of carotenoids thus endows the cells with increased cellular resistance to radiation, heat, and oxidation (Johnson and Schroeder, 1996). Generally, radio-tolerant yeasts that survive in environments with high UVR synthesize photoprotective compounds such as pigments. More and more evidence of carotenoids' putative photoprotective effect in yeast has been discovered over the years (Moliné et al., 2010). Yeast carotenoids have long been related with antioxidant activity. Furthermore, some studies have revealed that pigments can compensate for a lack of specific antioxidant enzymes like superoxide dismutase or peroxidase (Johnson and Schroeder, 1996).
4. Mechanism of carotenoid biosynthesis by R. mucilaginosa
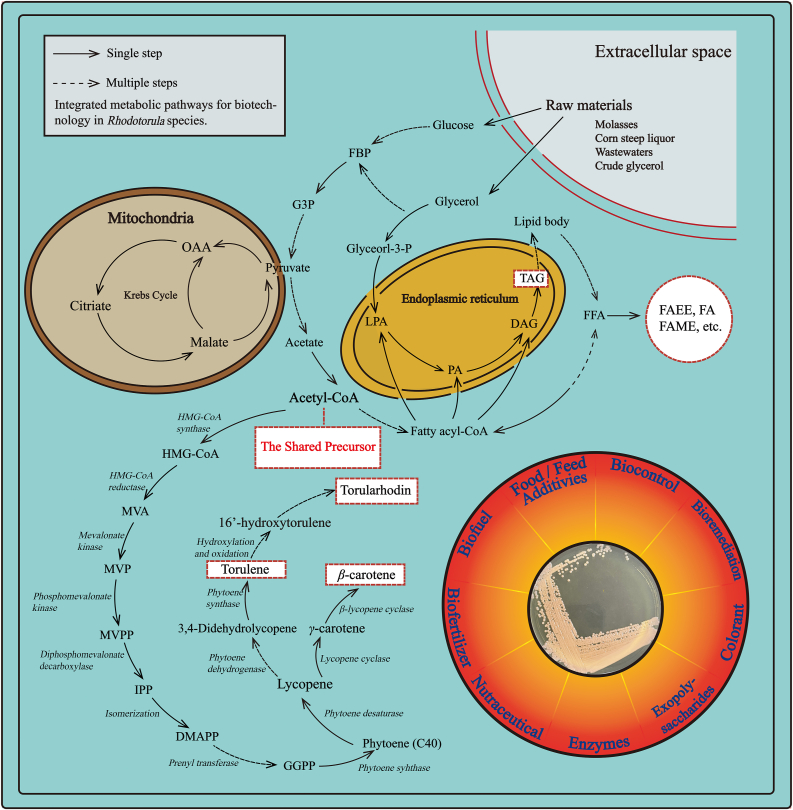
Indeed, Rhodotorula species have a similar biosynthetic pathway for carotenoids. As shown in Figure 2, the carotenoids biosynthetic pathway begins with the conversion of acetyl-CoA to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) catalyzed by the catalysis of HMG-CA synthetase. Subsequently, the HMG-CoA is reduced to mevalonic acid (MVA). Later, the MVA successively undergoes phosphorylation, and is decarboxylated to form isopentenyl pyrophosphate (IPP). Then, the IPP is isomerized to form dimethylallyl diphosphate (DMAPP), which is further transformed into geranylgeranyl pyrophosphate (GGPP) through multiple steps with three IPP molecules. Then, two molecules of GGPP are condensed, resulting in phytoene formation (the first 40-carbon compounds of carotenoid biosynthesis). Phytoene is then converted to lycopene and 3,4-didehydrolycopene, catalyzed by phytoene desaturase through four- and five-step dehydrogenation, respectively. Lycopene is transformed into γ-carotene and β-carotene catalyzed by bifunctional lycopene cyclase/phytoene synthase through one- and two-step cyclization, respectively. Moreover, 3, 4-didehydrolycopene could be converted into torulene catalyzed by bifunctional lycopene cyclase/phytoene synthase through one-step cyclization. According to the latest research and proposal, this step is currently the most possible way of torulene transforming in carotenoid biosynthetic pathway. Furthermore, torulene can be converted to torularhodin via the intermediate 16’-hydroxytorulene through a series of hydroxylation and oxidation. Remarkably, previous studies have proved that the γ-carotene could not transform into torulene, suggesting that γ-carotene is not the precursor of torulene (Hausmann and Sandmann, 2000; Sandmann et al., 2006; Herz et al., 2007). It is worth mentioning that the biosynthetic pathway of torulene and torularhodin in Rhodotorula species has been controversial for a decade. However, the hypothesis of the metabolic pathway “lycopene → 3,4-didehydrolycopene → torulene → 16’-hydroxytorulene → torularhodin” is more plausible given the available findings.
Figure 2.
Integrated metabolic pathways for biotechnology in Rhodotorula species. Metabolic pathways are depicted, along with the metabolites and enzymes. Abbreviations are used for the generally used names of metabolites and enzymes. Endoplasmic reticulum and mitochondria are also mentioned as cellular compartments. Metabolites with red-dotted lines represent prospective products to target in future methodologies.
5. Potential applications of carotenoids produced by R. mucilaginosa
5.1. As antioxidant
Reactive oxygen species (ROS) are produced in pathological processes and aerobic metabolism in living organisms, and are extremely harmful at high concentrations. When the level of ROS is out of the balance between production and elimination, cells will suffer excessive oxidative stress, and therefore trigger lipid peroxidation, protein oxidation, nucleic acid damage, and enzyme inactivation, resulting in programmed cell death (Kreiner et al., 2003; Stahl and Sies, 2005). The presence of conjugated double bonds in the carotenoid structure enables these compounds to accept electrons from oxidative stress, and neutralize free radicals as a consequence (Rutz et al., 2016; Shi et al., 2021). The beneficial effects of carotenoids on living cells are mainly attributed to their antioxidant properties as the major scavengers of ROS. β-carotene, one of the most well-studied carotenoids, possesses a relatively strong antioxidant capability in neutralizing excess ROS, and further preventing destruction of biomacromolecules. Meanwhile, the β-carotene has been served as the most-applied carotene to prevent UV irradiation from human skin cells (Borello et al., 2021; Favas et al., 2022). Previous studies have conducted that β-carotene is of great importance in human health promotion.
Previous study conducted that β-carotene treatment in workers exposed to lead (Pb) (Kasperczyk et al., 2014). The results indicated that β-carotene plays the role in antioxidant, which significantly reduced oxidative stress parameters and reduced the release of antioxidant enzymes intracellular. Another study suggested that β-carotene plays an effective antioxidant role in reducing H2O2-depent oxidative stress. The results were observed that β-carotene induces increased cell proliferation and cytokine release in the pathogenesis of Graves’ orbitopathy (GO) (Rotondo Dottore et al., 2018).
Meanwhile, studies have proved that torulene has better antioxidant activity than β-carotene. The report suggested that torulene possesses a better radical scavenging capability. A lower EC50 indicates torulene (EC50 = 2.03) as an effective scavenger of 2,20-Azino-bis diammonium salt (ABTS) than β-carotene (EC50 = 3.02) (Han et al., 2016). Further, it has been reported that torulene shows the most noticeable antioxidant effect, which protects SK-HEP-1 cells from the damage caused by hydrogen peroxide (Liu et al., 2019b). Previous study also conducted on the relative antioxidant efficiency among torulene, lycopene and β-carotene. The report observed that torulene is found to be the most easily oxidized carotenoid in polar and nonpolar environments, followed by lycopene and β-carotene (Galano, 2007).
What’s more, studies have proved that torularhodin possesses the capability to ameliorate oxidative damage and against oxidation in liver injury-induced mice (Liu et al., 2019a). The antioxidative activity of torularhodin against H2O2- induced liver damage has been reported. It was elucidated that the protective effects mechanism against oxidative damage involved cell integrity maintaining and enhancement of antioxidant enzymes activities in torularhodin treated Buffalo rat liver cells (Li J et al., 2019).
In view of the chemical structures of β-carotene, torulene, and torularhodin, it is reasonable to presume that the most potent antioxidant activity is carried by torularhodin, followed by torulene, and β-carotene. For example, Sakaki et al. confirmed that torularhodin possesses a much stronger antioxidative effect and shows a highly potent inhibitory effect on substrate degradation by singlet oxygen than that of β-carotene (Sakaki et al., 2001). Moreover, it has been observed that the addition of singlet oxygen, superoxide anion radicals, and peroxy radicals does not affect the biosynthesis of β-carotene while having significant enhancement on torulene and torularhodin in Rhodotorula glutinis. This result suggests that torulene and torularhodin possess a more effective scavenging capability of active oxygen species than β-carotene (Sakaki et al., 2002).
5.2. As provitamin A
Vitamin A (retinol) is structurally one-half of the molecule of β-carotene. Therefore, carotenoids containing at least one unsubstituted β-ionone ring with a C11 polyene chain (e.g., β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin) are classified as provitamin A carotenoids (Blomme et al., 2020; Watkins and Pogson, 2020). Each of these carotenoids has one unsubstituted β-ionone ring and could be converted into retinol by humans and animals (Tang, 2010; Bai et al., 2011). From γ-carotene, one molecule of vitamin A while from β-carotene two molecules of vitamin A could be synthesized (Grune et al., 2010). Studies related to the provitamin A property in torulene and torularhodin have not yet been reported at present. Humans cannot synthesize carotenoids naturally, and are thus dependent on diets as sources. Indeed, vitamin A is closely associated with human health, being required in bioprocesses of vision, immunity, reproduction, proliferation, cell differentiation, and growth and development of biological processes (Mayo-Wilson et al., 2011). In general, the common clinical symptoms of vitamin A deficiency may cause xerophthalmia, fetal development, an increased risk of severe infection, and other adverse effects on growth (Wirth et al., 2017; Wiseman et al., 2017; Surman et al., 2020). Increasing the provitamin A content in stable from agriculture is one way to settle vitamin A deficiency (Borel et al., 2021). However, in obtaining vitamin A from animal diets, difficulties remain in breeding area and productiveness, so it is strongly recommended that increase the consumption of microbial sources rich in vitamin A precursor carotenoids to reduce vitamin A deficiency.
5.3. As anti-cancer
Previous studies have shown that β-carotene, torulene, torularhodin exhibit anti-cancer and anti-tumor activities (Park et al., 2015; Du et al., 2017; Dulińska-Litewka et al., 2021). β-carotene could cause DNA damage on human hepatocellular carcinoma cells HepG2, and thus leading to enhanced apoptosis and necrosis of cells. Dietary β-carotene intakes may cause cytotoxic and genotoxic damage to HepG2 cells, as well as enhanced oxidative damage, in contradiction to their protective activity on healthy cells (Yurtcu et al., 2011). Still, β-carotene exhibits anti-tumorigenic properties in pituitary adenoma AtT-20 cells. The results suggested that β-carotene inhibits the growth of AtT-20 cells. The relevant mechanisms may induce cell apoptosis and cycle arrest (Haddad et al., 2013). β-carotene decreases Ku 70/80 levels and Ku-DNA-binding activity in gastric cancer cells via raising intracellular ROS levels and enhancing caspase-3 activation, ultimately causing apoptosis (Park et al., 2015). Previous studies also identified that β-carotene has an antiproliferative effect in esophageal squamous carcinoma cells. The level of anti-apoptosis protein phosphorylation is dramatically inhibited by β-carotene, which leads to an increase in the apoptosis protein Bax/Bcl-2 ratio and the activity of caspase-3 (Zhu et al., 2016). Similar results observed that β-carotene inhibits the growth and self-renewal capacity of colon cancer stem cells by regulating epigenetic modifications and regulates miRNAs with increased the expression of miRNA-mediated histone acetylation (Kim et al., 2019). What’s more, further research explained the β-carotene predominant anti-cancer activity at a concentration of 1 μM, which successfully promotes apoptosis by regulating anti-apoptotic protein expression and hindering cell survival signals (Sowmya Shree et al., 2017). β-carotene combined with other bioactive agent exhibits higher anticancer activity, compared to liposomes containing single bioactive types (Bai et al., 2019). Similar results reported that the combination of a low-dose doxorubicin treatment with β-carotene has a cell growth inhibitory and a proapoptotic impact on breast cancer cells (Vijay et al., 2018).
Recent progress in discovering the role of torulene and torularhodin in prostatic physiology has proved their excellent anti-cancer ability focused on prostate diseases (Dulińska-Litewka et al., 2021). Previous study had proved that torularhodin possesses the capability to against prostate cancer LNCaP cells. The results showed that torularhodin significantly down-regulates the expression levels of androgen receptor and prostate-specific antigen expression. The potential anti-prostate cancer mechanism of torularhodin may correspond with the loss of mitochondrial transmembrane potential, the increased intracellular calcium concentration, and the regulation of Bcl-2 proteins (Du et al., 2015). It has been observed that torulene and torularhodin treatment significantly enhanced degradation and apoptosis in tumor tissues (Du et al., 2016). The supplementation of torulene and torularhodin significantly inhibited the growth of prostate cancer in PC-3-treated mice. Additionally, torularhodin possesses the capability to maintain cell integrity, regulate the cell cycle and the activity of intracellular enzymes. The results of transcriptome analysis also revealed that the genes related to the cell cycle process regulation, response to oxygenated compounds were significantly differentially expressed as treated with torularhodin. The KEGG pathway enrichment analysis showed these genes mainly function in antioxidation, metabolic pathways, cell cycle processes, cancer, and senility (Li et al., 2019a). Moreover, the presence of torularhodin was a positively protective agent against alcoholic liver disease through transcriptome analysis (Li et al., 2019b). Previous study noted that the influence of torularhodin toward memory dysfunction and neuroinflammation induced by Alzheimer’s disease (AD). It was determined that torularhodin may be a potential therapeutic agent in treating AD via the activation of relevant anti-aging and antioxidative transcription factors (Zhang et al., 2020).
These findings highlight the importance of β-carotene, torulene and torularhodin in human anticancer. Such carotenoids may therefore be exploited for functional medicine because of their potential health benefits with various bioactivity. In other words, scientific achievements have brightened the prospects for the applications of carotenoid produced by R. mucilaginosa, yet there is still a need to evaluate the safety of carotenoids. Thus, establishing recommendations for appropriate dosages to be used in the prevention and treatment of different carcinosis in humans is necessary.
6. Influence of selected factors in carotenoid biosynthesis
Many studies have paid the efforts to augment carotenoid production in R. mucilaginosa by optimizing carotenoid fermentation conditions, tended to produce as much as possible in targeted carotenoid. However, the yield and efficiency of carotenoid biosynthesis by R. mucilaginosa depend on a good deal of factors (El-Banna et al., 2012; Mannazzu et al., 2015). Light/irradiation is a crucial factor which can affect the growth of Rhodotorula species and pigmentation (Zhang et al., 2014). It has been observed that the carotenoid productivity of R. mucilaginosa K-1 is significantly different among irradiation treatments. The results indicated that appropriate irradiation can promote the growth and productivity of carotenoids while strong illumination shows a negative effect on the growth and inhibits glucose metabolism in K-1 cells (Kong et al., 2019). Previous study found UV serves as the exogenous factor which mostly effects the production of carotenoids. Exposure to UV has a positive effect on carotenoid production in R. mucilaginosa AJB01 with 350 μg/g of carotenoids generated (Garcia-Cortes et al., 2021).
Moreover, carotenoids synthesis is highly influenced by the temperature of incubation in R. mucilaginosa. The possible mechanism may bear on the bioactivity of the enzymes that participate in the biosynthesis process. The conducted study showed the effect of temperature on R. mucilaginosa CCT 3892 carotenoid generation. The results were observed that the highest volumetric production of carotenoids (1.13 g/L) is cultured at 22 °C, while the lowest carotenoid (0.34 g/L) at 34 °C (da Silva et al., 2020). Previous study also indicated the increased temperature (up to 28 °C) accompanied with the rising carotenoid formation rates (2.43 mg/L), while sharply reduces above 35 °C (0.60 mg/L) in all cultivated R. mucilaginosa isolates (Allahkarami et al., 2021).
Carotenogenesis is an aerobic bioprocess in Rhodotorula species. The combined action of aeration and agitation can meet the demand for oxygen during fermentation. This is a necessary element for yeast growth rate, cell mass, and carotenoid production, as well as for substrate assimilation (Saenge et al., 2011). Aeration results in better mixing of the fermentation broth, which helps the maintenance of a concentration gradient between the inner and exterior environments of the yeast cell. The influence of aeration on the fermentation of carotenoids has been studied previously. It was observed that the maximum amount of carotenoids obtained by R. mucilaginosa MTCC-1403 in aerobic fermentation conditions was 819.23 mg/g, while 717.35 mg/g for the control (Sharma and Ghoshal, 2020). Aeration results in a concentration gradient, which may play a key role in fermentation provess. The improved diffusion process facilitates the intake of sources in medium by yeast cells and the removal of gases and other byproducts in the cultivation process.
Some divalent cations have been identified as the stimulants of carotenoids production in Rhodotorula species (Kot et al., 2021a). It has been reported that the carotenoid profile in R. graminis was selectively affected by certain trace elements. The results indicated that the presences of Al3+ and Zn2+ have a boosting influence on β-carotene and γ-carotene synthesis, whereas Zn2+ and Mn2+ had inhibitory effects on the biosynthesis of torulene and torularhodin (Buzzini et al., 2005). Specific carotenogenic enzymes may be activated or inhibited as a result of the action of the trace elements indicated above (Bhosale and Gadre, 2001).
The supplementation of solvents and chemical or natural agents has also been studied on carotenoid biosynthesis (Wang et al., 2001). It has been conducted that the effect of cotton seed oil for R. mucilaginosa NRRL-2502 carotenoid production. The results showed an increased carotenoids production at 57.6 mg/L in supplemented with cotton seed oil, while the control was at 39.5 mg/L (Aksu and Eren, 2005). Still, it has also been conducted that niacin has a positive effect on carotenoid genesis. According to the finding of Kot et al., R. mucilaginosa MK1 produces more torularhodin when niacin is added to the medium, increasing from 23.31% to 33.79% (Kot et al., 2021b).
The profile of carotenoids produced by Rhodotorula yeasts is greatly influenced by stress conditions. Previous study has synthetically summarized the effect of exogenous stress factors (osmotic stress, white light irradiation, low temperature, and oxidative stress) towards on the biosynthesis of carotenoids (Kot et al., 2019). The results revealed that the Rhodotorula yeasts (R. mucilaginosa, R. glutinis, and R. gracilis) are capable of growing and biosynthesizing carotenoid in the presence of applied stress stimuli. Induction of osmotic stress significantly stimulates the generation of β-carotene in R. mucilaginosa (more than 50%), and low temperature at 20 °C shows the same effect (up to 73.9% of the β-carotene in total carotenoid content). Likewise, the above results also suggested that yeast synthesizes torulene more efficiently in oxidative stress conditions (up to 82.2%) than that of the other conditions. It has been discovered that oxidative stress induced by 5 mM hydrogen peroxide promotes torulene production in R. mucilaginosa RCL-11. The proportion of torulene in the overall pool of carotenoids increased from 21.3 to 45.6%, with an increase from 1.8 m/L to 6.2 mg/L (Irazusta et al., 2013).
Yet, the presence of inhibitors will modify the profile of carotenoids. Study has been conducted on the influence of diphenylamine (DPA) and nicotine on the constituents of carotenoids in Rhodotorula yeasts. The results suggested that a prominent reduction is caused in the torularhodin/torulene ratio with the supplementation of 5 mmol DPA, from 1.5 to 0.5 for R. rubra, and 0.8 to 0.5 for R. glutinis. The β-carotene accumulation is increased from 13 to 47% in R. mucilaginosa, and 36–43% in R. glutinis. The addition of 20 mmol nicotine causes an obvious increase in lycopene by 77% of the total carotenoids, accompanied by a decrease in torulene and torularhodin (Squina and Mercadante, 2005). Researchers cultivated R. mucilaginosa RCL-11 with the addition of 50 μM DPA and 0.5 mM of CuSO4. The results indicated that 50 μM DPA causes an inhibitory effect on carotenoid synthesis, which decreases the pigment concentration to 0.26 mg/L than that of the control in 1.7 mg/L without CuSO4. And the cultivation of cells in both presences produce carotenoids below 1.0 mg/L (Irazusta et al., 2013).
The great potential of carotenoid biological features in Rhodotorula species has stimulated the advancement in industrialization culture and genetic modification technologies. Efforts have been made to improve the production of carotenoids by regulating the endogenous enzymes of these yeasts. Through homologous recombination or non-homologous end joining mechanisms, the bacterial-type II CRISPR/Cas9 system could be a prospective genome-editing application for Rhodotorula yeasts. Jiao et al. conducted studies on CARI (encoding phytoene dehydrogenase), CAR2 (encoding bifunctional protein catalyzing phytoene synthase and carotene cyclase). The results showed that an increased rate of gene deletion is performed (Jiao et al., 2019). Similarly, researchers conducted the study on carotenoid biosynthetic gene CARI with the use of agrobacterium-mediated transformation in R. toruloides NP11. It was indicated that the CRTI locus functions in RHTO_04602 (phytoene desaturase gene) in carotenoids biosynthesis (Sun et al., 2017). These findings offer valuable information for the metabolic engineering of this burgeoning yeast species.
7. Lipogenesis characteristics of R. mucilaginosa
One of the primary factors driving the expansion of the global bioenergy market is the rising concern for the environment and energy security. For this reason, some countries are exploring alternate energy sources to replace nonrenewable fossil fuels (Mohr et al., 2015). Due to the non-renewability of fossil fuels and the ever-decreasing supply, researchers are focusing on investigating clean and renewable alternatives (Abas et al., 2015). Bioenergy is quickly becoming one of the most important alternatives to traditional energy sources, helping to diversify the energy mix and lessen reliance on global oil markets, and is expected to replace conventional petroleum or diesel fuels (Sheehan et al., 2020). The global bioenergy market is rapidly expanding. It is projected that the bioenergy size will reach to 640 billion by 2027, exhibiting a compound annual growth rate of 8.0% during the forecast period (Market Data Forecast: Bioenergy, 2022).
Bioenergy is a type of renewable energy that is obtained from biological sources. Biodiesel is considered as a branch of bioenergy. Biodiesel is defined as a fuel composed of monoalkyl long-chain fatty acid esters, of which the most common are methyl esters (Knothe et al., 2006). Fuel produced from biomass feedstock by microorganisms is a useful precursor for conversion to biodiesel, with renewable and biodegradable feasibility (Kolesárová et al., 2011; Lin et al., 2013). Microbial lipids are promising substrates which can be used as new biodiesel, and are called single cell oil (SCO) in literatures (Beopoulos et al., 2011). According to the types of substrates and raw materials used for production, there are three types of biofuels: (1) the biodiesel traditionally extracted from plants (Durrett et al., 2008); (2) the biodiesel produced from oleaginous non-food raw materials (Ramos et al., 2019); (3) the biodiesel produced and synthesized from microorganisms (Papanikolaou and Aggelis, 2011).
Rhodotorula strains have a lipid storage capacity of around 40% of their dry weight, with TAG accounting for 65%–90% of the content. Lipids synthesized by R. mucilaginosa contain mainly myristic acid (0.29%), stearic (5.41%), palmitic (C16:0, 8.64%), oleic (C18:1, 35.38%), linoleic (C18:2, 28.97%), and linolenic acids (C18:3, 21.31%) (Gupta et al., 2012; Yang et al., 2014). Differences may exist in certain strains accompanied by changes in proportions of fatty acids owing to various environmental conditions. Microbial lipids produced by R. mucilaginosa could be in the service of food additives, dietary supplements, and substitutes for precious fats, and possess the capability to be used to replace high-value oils in commerce, as well as feedstocks and raw materials for biodiesel production (Béligon et al., 2016; Shields-Menard et al., 2018; Ghazani and Marangoni, 2022). The lipids produced by R. mucilaginosa have several advantages over traditional extraction methods from plants, like growing fast, covering less space, adapting better to demands, and having a broad spectrum of substrates (Jones et al., 2019). And the method of producing lipids by R. mucilaginosa is not limited by climate, season, or geographical conditions. Interestingly, another convenience of microbial lipids is that the yeast strains could be designed or engineered to synthesize high-value lipids, which are usually not generated or produced at poor yields. This engineering project usually involves the insertion or overexpression of enzymes that are related to lipid biosynthesis. Remarkably, the oleaginous yeast has attracted great attention because of its interesting potential for biotechnological applications (Kot et al., 2016; Spagnuolo et al., 2017; Park et al., 2018). More importantly, the application of biodiesel is eco-friendly to the ecosystem, mainly for the reason of reducing the emissions of greenhouse gases (Ray et al., 2021). Thus, biodiesel exhibits a sharp increase in interest owing to the ecological and economic aspects that people consider most. However, the so-called third-generation biodiesel industrialization in large-scale was hindered by the high cost of synthesis by microorganisms at present (Rajnish et al., 2021). But it is noted that costly chemical steps could be avoided because the use of these fatty acids in commerce directly is available (Szczepańska et al., 2022). Therefore, further research should focus on the economization of operating costs. This can be accomplished by using genetically modified techniques to improve microorganism biosynthesis efficiency or by utilizing waste as medium components (Sheehan et al., 2020).
8. Mechanism of lipid biosynthesis by R. mucilaginosa
Lipid biosynthesis occurs mostly in the cytoplasm of yeast cells, via a sequence of enzyme processes that convert the biosynthetic substrates saccharides, glycerol, or acetyl-CoA to long chain fatty acids. This bioprocess keeps a close relationship with glycolysis, the Krebs cycle, and the citrate/malate shuttle, as shown in Figure 2 (Beopoulos et al., 2011; Dourou et al., 2018). Lipids may be accumulated in yeast cells by de novo pathway that metabolizes saccharides or glycerol as the substrates for lipid production (Fakas, 2017). The biosynthetic precursor of lipids in this process is glycerol-3-phosphate (G3P), which is derived from the glycolytic pathway. Then, lipid synthesis starts with the acylation of G3P by the G3P-acyltransferases (encoded by SCT and GPT2 genes) to yield lysophosphatidic acid (LA), which is acylated to phosphatidic acid (PA) by the lysophospholipid acyltransferases (encoded by SLC and ALE genes). Then that is diacylglycerol (DAG) which is formed by the dephosphorylation of PA catalyzed by PA phosphatase (encoded by PAH gene). With the acylation by diacylglycerol acyltransferase (encoded by DGAT gene), DAG is to form TAG in the final step of triglyceride synthesis (Beopoulos et al., 2009; Papanikolaou et al., 2011; Beopoulos and Nicaud, 2012).
It is worth noting that after the depletion of nitrogen supplies from the culture environment, the overproduction of intracellular lipids occurs, which is associated to the activation of myoadenylate (AMP) deaminase (AMP deaminase) in de novo synthesis. AMP deaminase catalyzes the breakdown of AMP into inosine monophosphate (IMP) and ammonium, which serve as a supplementary nitrogen source. The drop in adenosine monophosphate levels impedes the Krebs cycle process for that it activates isocitrate dehydrogenase, which catalyzes the conversion of isocitrate to α-ketoglutarate. With the participation of aconitase, the mitochondrion accumulates isocitrate to balance the concentration of citrate. When citric acid reaches a critical concentration, it is divided by ATP-citrate lyase into acetyl-CoA and oxaloacetate, which are then transported from the mitochondrion to the cytoplasm. The carboxylation of acetyl-CoA produces malonyl CoA, which is the initial step in the production of fatty acids. The complex of fatty acid synthase then catalyzes a series of enzymatic processes (FAS). The fatty acids produced could be used in the triacylglycerol production pathway. The main pathways and major metabolites in lipogenesis by Rhodotorula species, with initial substrates of glucose or glycerol, is shown in Figure 2.
9. Influence of selected factors in lipid biosynthesis
Nowadays, the lipid biosynthesis mechanism in microorganisms has not been fully clarified. The yield and efficiency of lipid biosynthesis by R. mucilaginosa are determined by many factors. The high C/N molar ratio is the crucial factor for yeast accumulating lipids. Researchers have conducted lipid production at different C/N ratios by cultivating R. mucilaginosa LP-2. The results were observed that R. mucilaginosa LP-2 yields the highest lipid at the C/N ratio of 65 (6.0 g/L, 46.7%), compared with the ratio of 25 at 5.7 g/L (35.7%), 45 at 5.7 g/L (38.7%), and 85 at 4.4 g/L (44.6%), respectively. Most of the fatty acids are oleic acid (C18: 1), palmitic acid (C16: 1), and stearic acid (C18: 0) (Liang et al., 2021). It has been reported that R. mucilaginosa R2 exhibits the maximum lipid content at a C/N ratio of 40, which leads to a lipid concentration of 0.58 g/L (22.21%) after 96 h of cultivation (Bardhan et al., 2020).
Furthermore, the bioprocess of lipid production by R. mucilaginosa is also temperature dependent. The effect of incubation temperature on lipid accumulation and the profile of fatty acid unsaturation in R. mucilaginosa has been investigated. Previous study proposed that intracellular lipid content varied at 7, 15, and 26 °C. The reports observed that the profile of fact acids content of R. mucilaginosa varied widely, yet performed no significant effect on the yield of lipids. The highest unsaturated fatty acid (63.4%) is obtained at 15 °C with a monounsaturated fatty acid of 31.38% and a polyunsaturated fatty acid of 32.02%, compared with unsaturated fatty acid of 57.4% and 43.49% cultivated at 7 °C and 26 °C, respectively (Adel et al., 2021).
The effect of medial pH value or acidity also plays a significant role in lipid biosynthesis. It has been conducted that the experiments were performed at pH 4 to 7 for the purpose of investigating the influence of pH value. The results suggested that R. mucilaginosa shows its maximum lipid content as 69.5% (dry cell weight) and methyl ester as 92.3% at pH 5, whereas at pH 4, 6, and 7, its lipid content amounts to 50.8%, 51.7%, and 64.5%, respectively (Karatay and Dönmez, 2010). Moreover, the carbon composition of various starchy wastes also has a considerable effect on yeast lipid generation. Previous study used different types of wastes as medium to support R. mucilaginosa Y-1 growth and lipid production. The fermentation performances showed the highest lipid productivity (8.3 g/g starch) was obtained from barley residue with 14.91% lipid content (dry yeast biomass), followed by potato peel of 6.0 g/g with 13.42%, banana peel of 6.0 g/g with 6.04%, and corn residue of 5.9 g/g with 14.09%, respectively (Chaturvedi et al., 2019).
Studies on culture parameters in bio-progress are of great importance which greatly affect the productivity and profile of targeted products. These findings raise the possibility of cultivating R. mucilaginosa for lipid production, provide available options to researchers who purposed in perfecting lipid production. Regarding R. mucilaginosa applications, it appears evident that more advances will occur in production through metabolic engineering, which has not yet been completely utilized in lipid genesis.
10. Valorization of low-cost substrate
What is even more interesting is that R. mucilaginosa can thrive on a variety of carbon sources and is resistant to inhibitory chemicals common in unrefined substrates, which could be applied to product carotenoids and lipids in favored form (Kot et al., 2019; Costa et al., 2020). The industrialization of microbial production tremendously depends on the raw material cost for the purpose of raising productivity. Usually, in most biotechnological processes, the cost of the medium components is the deciding issue. Carbon and nitrogen sources can cost more than a third of the entire cost of the bioprocess (Bautista et al., 2012). Nonetheless, some articles have looked into alternate and less expensive carbon sources like glycerol, sugarcane, wheat straw hydrolysate, and other waste products from agricultural activities to replace the use of traditional carbon sources such as glucose. Several studies have examined the viability of employing raw materials as carbon sources and found that these substrates are economically viable (Kot et al., 2019, 2021). A strategy for carotenoids and lipids by cultivating with low-cost substrates can satisfy the needs of growth in yeast cells and industrial desired products.
Sugarcane bagasse and molasses are economical carbon sources in waste substrates with cost-saving interest. R. mucilaginosa CCT-7688 yields carotenoid and lipid content of 1794.2 μg/L (121.4 μg/g) and 43.2% (w/w), respectively, with the cultivation medium composed of sugarcane molasses and corn steep liquor (Rodrigues et al., 2022). Researchers cultivated R. mucilaginosa CCT3892 on medium containing residual sugarcane molasses. It was determined that the yeast produces total lipids and carotenoids at 16.50% and 53.0 μg/g, respectively, and the lipid generates the most interesting profile by the content of oleic acid at 74.05% (Costa et al., 2020). Previous study was conducted with the use of sugarcane bagasse for R. mucilaginosa IIPL32 growth and lipogenesis. The results obtained 97.23 mg of lipid as FAME per gram of dry biomass, and showed the major presence of monounsaturated fatty esters at 35–55%, which is suitable for the properties of biodiesel (18:1, 16:1) (Khot and Ghosh, 2017).
It has also been conducted that banana peel extract can serve as the substrate for R. mucilaginosa UANL-001L. Yields are highest in 317 μg/g carotenoids and 0.55 mg/g fatty acids in dry biomass, compared with the results in 298 μg/g and 0.46 mg/g while obtained in the yeast malt broth (Torres-Alvarez et al., 2022). It has also been found that onion peel powder and mung bean husks serve as the appropriate composition for R. mucilaginosa MTCC-1403 carotenoids genesis. The results suggested that the highest concentration of carotenoids obtained is 819.23 μg/g in aerobic conditions (Sharma and Ghoshal, 2021). R. mucilaginosa KKUSY14 was conducted on the medium composited by durian peel hydrolysate to produce biodiesel. It was observed that the fatty acid methyl ester (FAME) production is 81.57% in wet yeast cells (Leesing et al., 2021). It has been found that R. mucilaginosa ATCC 66034 exhibits high lipid production potential in waste glycerol with a yield of 3.24 g/L (Gientka et al., 2017). The utilization of wheat bran also composed the fermentation medium for R. mucilaginosa Y-MG1 lipid production. The results showed that yields are highest at 11 g/L dry biomass and fatty acid of 38.7%, respectively, while relative fatty acid composition shows the presence of increased levels of monounsaturated and saturated fatty acids in lipids at 66.8% and 23.4%, respectively (Ayadi et al., 2019). Cheng and Yang conducted carotenoids production with the use of reused molasses and waste ketchup as the cultivating medium for R. mucilaginosa F-1. It was observed that total carotenoids are produced at 268.6 μg/g, and 376.3 μg/g, respectively, with the composition of carotenoids in β-carotene, torulene, and torularhodin, at 23.8%, 67.5%, and 8.7%, respectively (Cheng and Yang, 2016). Previous study also conducted the use of bagasse hydrolyzate for R. mucilaginosa CCT 3892 production. It was observed that the yeast yielded the highest carotenoid at 1.13 mg/L, and lipids at 0.54 g/L, rich in oleic and linoleic (Allahkarami et al., 2021). Still, study has tested the olive mill waste for carotenoid production. The report observed that total carotenoid produced by R. mucilaginosa sp. is achieved at a maximum of 7.3 mg/L, mainly in torulene and torularhodin (Ghilardi et al., 2020). All these findings demonstrate the feasibility of using low-cost mediums as cheap substrates to produce targeted bioproducts. Here, we summarized the information mentioned above in Table 2.
Table 2.
Targeted products produced using agro-industrial waste by different strains of R. mucilaginosa.
| Strains | Agro-industrial waste | Targeted product | Cultivation method | Cultivation conditions | Biosynthesis efficiency | References |
|---|---|---|---|---|---|---|
| CCT-7688 |
Sugarcane molasses and corn steep liquor | Lipids Carotenoids |
Batch Fed-Batch Batch Fed-Batch |
25 °C, pH 6.0 180 rpm, 144 h |
43.20 % 34.70 % 121.3 μg/g 121.1 μg/g |
Rodrigues et al. (2022) |
| UANL-001L | Banana peel extract | Carotenoids, Lipids |
- | - | 317 μg/g 550 μg/g |
Torres-Alvarez et al. (2022) |
| KKUSY14 | Durian peel hydrolysate | Lipids | - | - | 15.83 % | Leesing et al. (2021) |
| CCT 3892 | Sisal bagasse hydrolyzate | Carotenoids Lipids |
- | 28 °C, pH 6.0 150 rpm, 120 h |
1.13 mg/L 0.54 g/L |
Allahkarami et al. (2021) |
| MTCC-1403 | Onion peel powder and mung bean husks | Carotenoids | - | 25.8 °C, pH 6.1 119.6 rpm, 84 h |
819.2 μg/g | Sharma and Ghoshal (2020) |
| - | Olive mill waste | Carotenoids | - | 30 °C, pH 5.15–5.39 150 rpm, 6 d |
7.30 mg/L | Ghilardi et al. (2020) |
| CCT 3892 | Sugarcane molasses | Carotenoids Lipids |
- | 30 °C, pH 6.49 200 rpm, 120 h |
53.0 μg/g 16.50% |
Costa et al. (2020) |
| CCT-7688 | Sugarcane molasses, corn steep liquor and raw glycerol | Carotenoids | Batch Fed-Batch |
25 °C 180 rpm 216 h |
152.5 μg/g 118.8 μg/g |
Amore et al. (2019) |
| Y-MG1 | Wheat bran and sugarcane bagasse | Lipids | Fed-Batch | - | 32.76 % | Ayadi et al. (2019) |
| IIPL32 | Sugarcane bagasse | Lipids | - | - | 97.23 mg/gd.w. | Khot and Ghosh (2017) |
| F-1 | Molasses and waste ketchup | Carotenoids | - | 25 °C, pH 5.0, 120 rpm, 216 h |
268.6 μg/g | Cheng and Yang (2016) |
Note: “-”: Name of strain was not mentioned in the article.
11. Enzymes produced by R. mucilaginosa
Enzymes are extremely efficient catalysts, their application in industries can greatly increase efficiency and productivity (Hammes-Schiffer, 2013). Compared with traditional physical and chemical extraction or degradation, enzymatic extraction shows the mildest demand for biological activation (Nandy, 2016). From this, a large amount of energy could be saved for cost expenditure. Still, reactions catalyzed by biological enzymes are time-saving (Frey, 2007). However, an abundant and stable enzyme source is a prerequisite for biocatalysts in commercial applications. R. mucilaginosa is of great interest as it is capable to synthesize enzymes naturally, which can be applied in various industries, especially in the production of phenylalanine ammonia lyase, endo-1,4-glucanases, and lipase. All are relevant to the agricultural and chemical industries.
11.1. L-phenylalanine ammonia-lyase
L-phenylalanine ammonia-lyase (PAL, E.C. 4.3.1.24) catalyzes the non-oxidative deamination of L-phenylalanine to trans-cinnamic acid and ammonia. PAL is widely distributed in higher plants and yeasts, and thus plays a key role in phenylpropanoid metabolism (MacDonald and D'Cunha, 2007; Barros and Dixon, 2020). PAL functions as a catabolic enzyme in microorganisms, allowing yeasts, fungi, and Streptomyces to use L-phenylalanine as the only carbon and nitrogen source (Orndorff et al., 1988; Hyun et al., 2011). It has multiplex potential and functions in industrial and medical applications (Kawatra et al., 2020). Clinically, it could be used as a reliable method for the treatment of phenylketonuria, a metabolic disorder of amino acid metabolism for the deficiency of PAL by the nature property of decomposing L-phenylalanine (Lee et al., 2016; Levy et al., 2018). It should be noted that the reverse process has been implemented to produce L-phenylalanine under controlled conditions. L-phenylalanine is one of the necessary amino acids that humans cannot produce or synthesize at a rate that is insufficient to meet the needs of the organism (Mahalakshmi et al., 2006). L-phenylalanine could be used to produce the noncaloric sweetener, aspartame, in further industrial production (MacDonald and D'Cunha, 2007).
Previous studies have shown that Rhodotorula species are capable of producing PAL. It has been shown that the presence of the PAL in R. glutinis is dependent on the addition of L-phenylalanine. Yet, the opinion was soon to be overturned by the study showing that the PAL was also induced significantly during carbon starvation, indicating that the appearance of the PAL enzyme in R. glutinis was not dependent (Wick et al., 1982; Kane and Fiske, 1985). Based on its industrial importance, PAL with viability and stability are determinants in production. Researchers are devoted to making PAL keep stable activity due to the rapid loss of enzyme activity and stability in vitro. Subunit dissociation, autolysis, or proteolysis by proteases could be avoided by using the immobilization methods, which would allow the PAL to maintain a relatively high activity for a certain time (Ateş and Dogan, 2010). The report showed R. glutinis cells retain nearly 85% of the original PAL activity for at least 12 weeks, with the addition of 0.01% Mn2+ in the enzyme buffer, compared to 73% in the case of isolated PAL (Wall et al., 2008). Yet the expected characteristics of PAL from Rhodotorula species show low expectations in repeat-does studies. Researchers applied a technique called cross-linked enzyme aggregates for PAL (PAL-CLEAs) which encapsulated the PAL of R. glutinis in polyallylamine mediated biomimetic silica. The encapsulated PAL retained 46% of its original enzymatic activity after storage for about 3 weeks, whereas the free enzyme retained only 10% (Cui et al., 2015). What’s more, novel CLEAs got 70% of the original PAL activity remained after 13 cycles (Cui et al., 2017). There have also been investigators focused on PAL heterologous expression, making it not restricted to the Rhodotorula species. The full-length gene coding for the R. glutinis PAL (RgPAL) was sequenced, length of 2121 bp, encoding 706 amino acids, was first reported and isolated by Zhu et al. and expressed in E. coli successfully (Zhu et al., 2013). Immediately afterward, site-directed mutagenesis of PAL was performed by the above researchers to construct RgPAL with higher catalytic efficiency, resulting in 29% conversion to D-PAL and 93% full conversion efficiency at pH 7 in R. glutinis JN-1. More than that, some scientists are making efforts to access the new PTAL (EC 4.3.1.26), the enzyme that shows dual specificity toward L-Phe and L-Tyr. When present in R. glutinis, it catalyzes the conversion of L-tyrosine methyl ester (L-TM) to the methyl ester of para-hydroxycinnamic acid (p-HCAM). Derivatives of p-HCAM can be widely used in health, pharmaceutical, as well as food applications and can act as antibacterial agents with significant antibacterial activity against three common pathogenic bacteria (E. coli, S. aureus, and Klebsiella pneumoniae). Compared with the standard antibiotic, Ampicillin, with a 9 mm zone of inhibition, the diameters of the inhibition zones were 5 mm, 6 mm, and 8 mm, respectively (MacDonald et al., 2016). However, currently available reports on PAL production of R. mucilaginosa are still very limited at present. Zhang and Cui reported that R. mucilaginosa CICC 32917 produced PAL in optimized fermentation. The reports indicated that PAL production was enhanced by 8.0 folds of 41 U/g dry cell weight and found that L-phenylalanine could induce the biosynthesis of PAL by 18.5% more than that of control (Zhang and Cui, 2012). Yet reports of R. mucilaginosa related to PAL production are still scarce in the literature.
11.2. Endo-β-1,4-glucanases
Cellulose is the most abundant carbohydrate produced by plants. As a renewable bio-resource on earth, this substance is considered as a potential alternative energy source (Kulkarni et al., 2011). The hydrolysis of plant polysaccharides could be completed by microbial cellulases, which catalyze the hydrolytic cleavage of cellulose into cello-oligosaccharides or glucose, allowing the organisms to utilize cellulose as carbon sources (Oikawa et al., 1998). These enzymes include endo-β-1,4-glucanases (EC 3.2.1.4, EGase), cellobiohydrolases, cellodextrinases, and β-glucosidases (Béguin and Aubert, 1994; Boyce and Walsh, 2007). Converting cellobiose by EGase is an important factor for reducing cellobiose piling up and enhancing the efficiency of cellulolytic enzymes for bio-energy production. This enzyme could be found in many species, of which the most commercialized method for EGase is extracted from Bacillus species (Chaari et al., 2012; Cezare-Gomes et al., 2019). And likewise, the Rhodotorula species possess the ability to produce EGase. Previous study found that R. glutinis KUJ 2731 is capable of producing EGase extracellularly. The reports indicated that this enzyme is active at a wide range of temperatures (0–70 °C), catalyzes hydrolysis of cellulose, glucose, cellobiose, and cellotriose (Oikawa et al., 1998). Pi et al. pioneered the engineering of R. glutinis for simultaneous β-carotene and cellulase production. Transformed the β-carotene biogenesis genes (crtI, crtE, crtYB, and HMG1) and cellulase genes (CBHⅠ, CBHⅡ, EgⅠ, EgⅢ, EglA and BGS, coding for cellobiohydrolases and endo-β-1,4-glucanases) into the R. glutini genome. The transformant produces β-carotene 27.13 mg/g than the wild type and exhibits cellulase activity (Pi et al., 2018). Researchers have also proved that supplementation with organics has a promoting effect on the catalytic activity of EGase in R. glutinis (Oikawa et al., 2001).
11.3. Lipase
Lipase (triacylglycerol acyl hydrolase, EC 3.1.1.3) belongs to the class of hydrolases, which are ubiquitous enzymes with considerable industrial potential, which are among the most important classes of industrial enzymes (Singh and Mukhopadhyay, 2012). Lipases catalyze the hydrolysis and the synthesis of esters formed from glycerol and long-chain fatty acids, which are extremely versatile because they also catalyze numerous other different reactions. Lipases are frequently implemented by various applications such as dairy and food manufacturing, leather and detergent manufacturing, pharmaceutical and cosmetics production, and organic synthesis processes, particularly those that operate in both aquatic and non-aquatic environments, which distinguishes them from esterase (Orlando Beys Silva et al., 2005; Goswami et al., 2013; Chandra et al., 2020). And cold active lipase has a wide-range of application in various sectors and industries (Marx et al., 2007; Joseph et al., 2008; Duarte et al., 2018). Numerous microorganisms have been reported for their capability to produce lipase (Sharma et al., 2001; Chennupati et al., 2009). Rhodotorula species are considered as microbial-derived lipases producer of that lipase also seems to be the expected bio-platform for the biorefinery strategy (Papaparaskevas et al., 1992; Szotkowski et al., 2019). Previous studies have proved R. mucilaginosa can produce lipase.
It has been reported that R. mucilaginosa sp. generates lipase which exhibits maximum activity at pH 4.0 and 5.0. The maximum lipase production by the isolate is obtained by the utilization of olive oil and maltose, and the highest lipase activity is shown with the utilization of peptone (Hammamchı and Cihangir, 2017). Previous study demonstrated that R. mucilaginosa P11I89 is a producer of whole cell lipase at its maximal lipase production of 272.72 U/L. It was observed that R. mucilaginosa P11I89 achieves high activity of transesterification between palm-oil and methanol, which increased FAME yield from 84.0 to 92.89% than that of the initial medium (Nuylert and Hongpattarakere, 2013). The lipase produced by R. mucilaginosa MTCC 8737 was also reported, which suggested that the activity of lipase could reach up to 72 U/mL in fermentation with 1% molasses concentration for only medium with 2 vvm aeration and 200 rpm agitation (Potumarthi et al., 2008). The yeast of R. mucilaginosa with lipase produce-property is also found in contaminated environments. The isolates of R. mucilaginosa D17 and D42 show lipase activity in the medium consisted of tributyrin, yet the certain characteristic of lipase in these two isolates was not been fully demonstrated (Yalçın et al., 2014).
12. Beneficial effect of R. mucilaginosa on plants and environment
Present studies have demonstrated that R. mucilaginosa is a good natural generator of carotenoids and lipids, both of which are of great relevance in industry. Moreover, the beneficial effect of R. mucilaginosa yeast on plants and the environment emerges as a potentially new direction has been reported in recent years. These new directions include but are not limited to microbial fertilizers, biocontrol against pathogenic microorganisms, and environmental bioremediation agents. The rising innovative studies of R. mucilaginosa have enriched its applications in various sectors.
12.1. Effect of plant growth promoting
Atmospheric nitrogen is extremely abundant. However, the extremely low nitrogen use efficiency in cultivated land is a common cosmopolitan problem. With the rapid development of the chemical fertilizer industry, the amount of nitrogen fertilizer application is being exponentially expanded, but the nitrogen recovery is often less than 40% (Good and Beatty, 2011; Kant, 2018; Anas et al., 2020), which causes a serious waste of resources. Therefore, raising nitrogen use efficiency is thus considered to be a key research topic.
Plant nitrogen metabolism is close to growth and development, yet plants cannot use nitrogen directly and need to reduce atmospheric N2 into absorbable nitrogen, which can be absorbed and utilized by plants (Poulton et al., 2012). This process is known as biological nitrogen fixation, which can biochemically turn the nitrogen into ammonia nitrogen that can be utilized by plants through microbial nitrogenase (de Bruijn, 2015). Biological nitrogen fixation plays an important role in agricultural production practice, which can provide nitrogen nutrition for plant use effectively, especially in crop cultivation. Aiming at perfect nitrogen supply for plants, microorganisms play a key role in raising crop yield, reducing chemical fertilizer consumption, and cutting production costs (Geisseler and Scow, 2014; Li et al., 2019).
The strain of R. mucilaginosa JGTA-S1, an endophytic yeast of plants living in heavy metal-contaminated areas in India (Saha and Seal, 2015). It is able to act as a microbial fertilizer and probiotic, raising the plant nutrient supply, and providing it with ammonium nitrogen due to its biological nitrogen fixation capability by some prokaryotic genes. As JGTA-S1 possesses the nitrogen reductase gene of Pseudomonas aeruginosa, a wonderful synergistic relationship (plant-yeast-bacteria) provides the plant with rich nitrogen nutrition (Sen et al., 2019). Treated with R. mucilaginosa JGTA-S1 to rice (Oryza sativa), the research found that the growth and yield of rice were remarkably promoted by its plant growth-promoting (PGP) effect. The relative nitrogen usage efficiency of JGTA-S1 treated biomass rose 2.62-fold greater than that of control (Paul et al., 2020).
The large existed aluminum (Al) was reported to be serve as the phytotoxic element which inhibits the growth of plant in acidic arable lands. Al could be converted into a soluble state at high acidity in soil (pH < 5) and consequently release toxic Al3+. The negative effect of uptake Al3+ is mainly relevant to the damage of plant roots, which interrupts essential element absorption and transmembrane transport, resulting in poor growth in plants (Kochian, 1995; Kochian et al., 2004). It has been reported that R. mucilaginosa CAM4 is capable to reduce the accumulation of Al in Lactuca sativa, which confers Al tolerance to the plants. Research has been conducted that R. mucilaginosa CAM4 could be applied as a biofertilizer for crop production that is healthy and safe. The results proposed that the PGP effect was achieved by several mechanisms, such as the tolerance to abiotic stress by inoculation of R. mucilaginosa CAM4, the enhanced morphological and biochemical characteristics of L. sativa, which enables the plants to grow under abiotic stress conditions, and the increased accumulation of antioxidant enzymes that scavenge ROS generated by the overexposure of Al (Silambarasan et al., 2019). Yet the application of R. mucilaginosa in agriculture is still relatively rare.
12.2. Plant pathogen antagonist and biocontrol agent
With the development of human civilization, the food supply has restricted the fate of human survival. The great famine in Ireland was caused by Phytophthora infestans, the pathogenic fungi of potato (Solanum tuberosum L.) late blight disease, resulting in significant economic and social upheaval (Huang et al., 2021). Human beings have never stopped phytopathology since they realized the severity of plant pathogens to crops. Ensuring the quality and productivity of stable crops is of great importance in agricultural production, which has a significant impact on the economy. One of the effective methods against plant pathogens is the application of biological control by using antagonistic organisms (Cook, 1993; Haggag and Mohamed, 2007). This organism could cooperate with the immune response of the plant or coordinate with substances such as reactive oxygen species to eliminate the extrinsic infections caused by pathogens. R. mucilaginosa is used as a biocontrol agent against Phytophthora capsica in vivo, the cause of basal stem rot disease in black pepper plants. Safitri et al. found two isolates of R. mucilaginosa to be effective in controlling basal stem rot (BSR) disease caused by Phytophthora capsica in black pepper plants. The results indicated that R. mucilaginosa (isolate END6) exhibits anti-pathogen activities against P. capsica. The biocontrol effects of END6 were achieved by several mechanisms, such as the hyperparasitism process, which damaged the hyphae cell walls of P. capsica (Safitri et al., 2021). It has been suggested that the EPS produced by R. mucilaginosa UANL-001L was capable of inhibiting the growth of three bacterial pathogens: E. coli, Pseudomonas aeruginosa, and S. aureus (Vazquez-Rodriguez et al., 2018). Previous study conducted the effect of R. mucilaginosa on its antagonistic activity against two postharvest fungi diseases of strawberries, Rhizopus stolonifer and Botrytis cinerea, which cause Rhizopus decay and gray mold separately. The findings revealed that the biocontrol activity of yeast is significantly upgraded by cultured in media containing chitosan. This substance enhances the ability of nutrient competitiveness and the generation of defense enzymes against the fungal pathogen (Zhang et al., 2014). Previous researches have shown that R. glutinis is capable of producing spores and a crystalliferous protein per yeast cell, and produces a salmon/red pigment in the logarithmic growth phase. This pigment is consistent with melanin both physically and chemically. Previous study indicated that the R. glutinis suspensions possessed insecticidal activity against Aedes aegypti and Trichoplusia ni. Still, the salmon/red pigment genes are successfully expressed in E. coli. The results showed that transformants contain melanin pigments and insecticidal toxins. Therefore, it is reasonable to presume that melanin pigments and insecticidal toxins are co-expressed and may co-evolve in Rhodotorula species (Oloke and Glick, 2006).
12.3. Environmental bioremediate agent
Countless heavy metal pollutants are produced through frequent industrial activities. Polluted water without effective treatment will continue to harm the ecological environment. In contrast to physical or chemical treatment, researchers have found the benefits of applying biological systems to remediate contaminants as a natural alternative. For waste-water treatment, bioremediation has gained attention for its natural process of treating waste by biotransformation (Singh and Prasad, 2014; Gavrilescu et al., 2015). It is a feasible choice to apply microorganisms with the ability to degrade heavy metals as the bioremediate agent for ecological restoration (Sun et al., 2020). The treatment of environmental pollution can be actively promoted by microorganisms whose growth environment is demanding modestly and possess the ability to transform toxic contaminants into toxic-free, and stable substances, which can achieve the purpose of pollutant degradation, and meet the needs of contaminant decomposition (Zafar et al., 2007; Gavrilescu et al., 2015).
The application of R. mucilaginosa for heavy metal detoxification comes as a cost-effective and environment-friendly option for the treatment of polluted ecosystems. Cudowski and Pietryczuk determined the influence of iron (III) ions on the growth and metabolism of the yeast R. mucilaginosa commonly occurring in water. The results indicated that iron (III) ions have an adverse influence on the yeast cells since the activity of antioxidant enzymes was increased at higher iron (III) ion concentrations. The bioremediation efficiency of R. mucilaginosa was observed that iron (III) ions in yeast cells was improved 9-fold in comparison to the control under the influence of 1 mg/L iron (III) ions and 11-fold under the influence of 100 mg/L and showed no decrease in the R. mucilaginosa biomass at high concentrations of iron (III) ions in the medium (Cudowski and Pietryczuk, 2019). Aibeche et al. isolated R. mucilaginosa RO7 with high lead (Pb) resistance. The isolate exhibits lead removal ability in living yeast cells. The experiments indicated that a lead concentration of 31.25 ppm resulted in strong purification activity in the presence of 1 g of biomass, with a high percentage of purification at 95.32%. The removal efficiency of lead by R. mucilaginosa RO7 reached its maximum level at 15.55 mg/g with the smallest amount of fresh yeast biomass (0.6 g) and the highest amount of lead (125 ppm) (Aibeche et al., 2022). Zhang et al. conducted the study on the response mechanisms to cadmium (Cd) exposure and provided the potential bioremediation capability of R. mucilaginosa AN5. The results indicated that the composition and contents of saccharides, amino acids, and organic acids are significantly changed. This change is to form a metabolic network to against cadmium stress (Zhang et al., 2022). Different strains of R. mucilaginosa exhibit various capabilities, involving protease (Lario et al., 2015, 2020; Chaud et al., 2016), laccase (Zhang et al., 2020) and cutinase (Zhang et al., 2015). Table 3 summarizes different R. mucilaginosa strains with various functions.
Table 3.
Other bio-properties of R. mucilaginosa in different strains.
| Strains | Property | Target | Reference |
|---|---|---|---|
| JGTA-S1 | Plant Growth Promoting | Nitrogen Fertilizer in Rice | Paul et al. (2020) |
| CAM4 | Plant Growth Promoting | Biofertilizer for Al Detoxification, Salt and Drought Stress | Silambarasan et al. (2019) |
| - | Antimycotics | Phytopthora capsici in Black Pepper Plants | Safitri et al. (2021) |
| T1 | Antimycotics | Destructive Molds in Apple Fruit | Sukmawati et al. (2020) |
| UANL-001L | Anti-bacterium | E. coli, P.aeruginosa and S.aureus | Vazquez-Rodriguez et al. (2018) |
| - | Antimycotics | R. stolonifer and B. cinerea in Strawberries | Zhang et al. (2014) |
| AN5 | Bioremediation | Cadmium (Cd) Adaptability | Zhang et al. (2022) |
| RO7 | Bioremediation | Lead (Pb) Removal | Aibeche et al. (2022) |
| CGMCC-16597 | Biosorption | Lead (Pb) Removal | Wang et al. (2021) |
| - | Biosorption | Iron (III) (Fe) Removal | Cudowski (2019) |
| R1 | Bioremediation | Hydrargyrum (Hg) Removal | Liu et al. (2017) |
| CCMB 604 | Enzyme producing | Inulinase | da Silva et al. (2020) |
| LSL | Enzyme producing | Aldo-keto reductase | Anello et al. (2020) |
| CBMAI 1528 | Enzyme producing | Protease | Lario et al. (2020) |
| JM401 | Enzyme producing | Laccase | Duarte et al., 2018 |
| L7 | Enzyme producing | Protease | Chaud et al. (2016) |
| L7 | Enzyme producing | Acid Protease | Lario et al. (2015) |
| - | Enzyme producing | Glycosidase | Hu et al. (2016) |
| - | Enzyme producing | Cutinase | Zhang et al. (2015) |
| - | Enzyme producing | Urethanase | Wu et al. (2013) |
| MTCC 8737 | Enzyme producing | Lipase | Potumarthi et al. (2008) |
Note: ‘-’ means the name of strain was not shown in the article.
13. Other bio-properties of R. mucilaginosa
13.1. Exopolysaccharides
As a natural source, yeast EPS is increasingly being developed and used. Exopolysaccharides or extracellular polysaccharides are known as extracellular polymeric substances which have shown the properties in antioxidant, immunomodulatory, anti-radiation, anti-inflammation, anti-tumor, and anti-fatigue, in previous studies (Castro et al., 2014; Huang et al., 2017; Li et al., 2020). These functional biopolymers are mostly monosaccharides with some non-carbohydrate substituents such as proteins, phospholipids, glycoproteins, and other acids. EPS secreted by microorganisms during the metabolic process and then released into the surrounding environment may be effective in protecting microorganisms from extreme environments or abiotic stress, and with physiological abilities in nourishment storage, and tolerance to toxic heavy metals (Ma et al., 2018; Li et al., 2019; Rahbar et al., 2021). R. mucilaginosa is one of the outstanding microorganisms in producing EPS used in industrial applications. EPS produced by R. mucilaginosa YL-1 was characterized in immunomodulatory activity. The results revealed that these EPS are made up of four monosaccharides: glucose, mannose, galactose, and fucose, with an average molecular weight of 1200 kDa. The trial also showed that it has the function of stimulating the expression of more cytokines (IL-6, 18, TNF-α, and IL-1β) with great immunomodulatory activity on macrophage cell lines RAW264.7 (Li et al., 2020). EPS produced by R. mucilaginosa sp. GUMS16 was reported to have an anti-tumor effect on chronic myeloid leukemia K562 cell line. The results suggested that apoptosis is induced in EPS-treated cells by an antiproliferative and growth-inhibitory effect (Kheyrandish et al., 2022). EPS from Rhodotorula sp. RY 1801 was investigated. It has been reported that these EPS are capable of scavenging both superoxide anion and hydroxyl radicals in vitro. The max scavenging rates for them, are 29% and 84%, respectively. Results also showed that these EPS may be used as a carbon and energy source, which could be applied to promote the growth of two prebiotic strains, Lactobacillus acidophilus and L. plantarum (Wang et al., 2021).
Microbial EPS are also considered as critical extracellular zones to shield the cells against stress (Andrew and Jayaraman, 2020). Some R. mucilaginosa with EPS secreted properties were explored to bioaccumulate and detoxify heavy metal ions owing to the oversupply of heavy metals in human activities (Duda-Chodak et al., 2012; Gientka et al., 2015). It was conducted that R. mucilaginosa R1 exhibited the talent of Cu2+ sorption and chelation. The results were observed that physiological activity of R. mucilaginosa R1 is kept by the EPS when the Cu2+ is less than 100 mg/L, but might have limited ability to high Cu2+ stress (more than 200 mg/L) (Wang et al., 2020). It was purposed that R. mucilaginosa R1 also exhibits strong lead (Pb) adsorbing ability owing to the presence of EPS assembled with glutathione, which is responsible for the nature to resist the Pb toxicity (Li et al., 2019). EPS are important substances in yeast bioproducts with significance from industrial perspectives. However, relevant mechanisms of yeast EPS for their nature still need to be elucidated for the purposes of pharmaceutical and food industries in the future.
13.2. Antifungal activity
Interestingly, R. mucilaginosa also exhibits the biosynthesis capability of an antifungal agent. Previous study has conducted that the antifungal agent of silver nanoparticles (AgNPs) produced by R. mucilaginosa possesses antagonistic activity against Candida parapsilosis. The results were observed that the minimum inhibition concentration (MIC) of AgNPs against C. parapsilosis is in the range of 0.80–0.025 μg/mL, while fluconazole ranges from 2-8 μg/mL as the control, which proved C. parapsilosis is extremely susceptible to the AgNPs. Still, the activity of fluconazole conjugated with produced AgNPs against C. parapsilosis was promoted by 29.7%. It was also reported that the AgNPs cell toxicity effect was only observed in high concentrations at 10.0 μg/mL (Cunha et al., 2018).
14. Conclusion and future directions
Apparently, R. mucilaginosa has attracted major interest in academia and industry since the unique physiological features and a wide range of valuable potentials which have been applied in various industrial sectors. R. mucilaginosa is capable to conveniently produce carotenoids fulfill the needs of growing consumers who are aware of the detrimental impact of synthetic colorants on health. These carotenoids have been proved clinically safe which used in medicine and pharmacy to against oxidative stress, anti-cancer, and health promoting. Still, R. mucilaginosa belongs to the group of oleaginous microorganisms and is capable of producing and accumulating more than 40% of lipids in dry cell weight. These lipids meet the needs and standards of the newly third biodiesel, which shows minimal stress on the ecological environment. The generation of yeast biodiesel is the result of a diminution in the reliance on nonrenewable fossil fuels. Enzymes are substances with bioactivity that have great practical value for industrial production due to their energy saving, mild condition to activate, and catalytic efficiency. These features would bring industries a competitive advantage.
Producing high-value products can easily increase the economic feasibility of the R. mucilaginosa bioprocess, and the development of low-cost biotechnologies for R. mucilaginosa biotechnological manufacturing is energizing scientists and businesses. Bioprocessing could be operated by utilizing low-cost substrates from agro-industrial waste by means of metabolic engineering strategies, bioreactor design, and customized product control strategies, which will help to decompose and reuse pollutants in the ecosystem. Generally, the waste contains a large amount of carbon, nitrogen sources, and some other useful salt components for fermentation bioprocesses, which may improve economic feasibility and mitigate environmental impacts. Furthermore, using low-cost substrates as an alternative natural resource will help to lower industrial expenditures. To meet the demand of industries for microbial products with biotechnology properties, direct study into the utilization of agro-industrial waste is necessary.
Existing molecular biology technologies are then available and may be applied to R. mucilaginosa to speed up biotechnological progress. The following future directions are written below here. Studies available on the genetic engineering and gene editing of R. mucilaginosa need to be upgraded and synchronized at present. Fruitful efforts have been established and yielded in two traditional red yeasts, R. toruloides and R. glutinis for industrial application (Kot et al., 2016; Park et al., 2018; Wen et al., 2020). Relevant researchers may get inspired by the results and achievements that have been achieved in other Rhodotorula species, and get motivated to perform corresponding molecular biology or phenotypic editing on R. mucilaginosa. Based on the fact that acetyl-CoA is a common precursor for fatty acids and carotenoid biosynthesis in Rhodotorula species, it is achievable to genetically engineer R. mucilaginosa to boost or impair carbon flow from one of the pathways, allowing more flow of acetyl-CoA to another biosynthesis production. Tkáčová et al. conducted a relevant study on R. mucilaginosa C2.5t1. With T-DNA insertion, R. mucilaginosa C2.5t1 generated a mutation in the CAR1 gene (coding for phytoene desaturase), which resulted in an increase in CAR2 gene (coding for phytoene synthase/lycopene cyclase) expression. The mutation of the CAR1 gene causes significant increases in the production of phytoene, CoQ10, and sterols, all of which are high-value metabolites in industrials (Tkáčová et al., 2022). This was a successful experiment that confirmed the hypothesis that the impairment of carotenoid biosynthesis would redirect carbon flux towards to the bioprocessing of lipids, which can be conducted to advance the biotechnological exploitation of R. mucilaginosa for the upgraded production of secondary metabolites with practical production.
However, the main weakness of the study is the neglect to consider how to balance the production and growth of yeast. As a producer of carotenoids, lipids, enzymes, and some other bio-functional secondary metabolites, researchers should pay attention to the balance between the production and growth of R. mucilaginosa for that there is a high possibility of adverse impact corresponding with the growth and development process in yeast cells. For Rhodotorula species, the overproduction of carotenoids or lipids results from a signal that the yeast cells sense the deficiency in nitrogen elements since this is the cell stress response to nutrition in natural circumstances. In conclusion, R. mucilaginosa holds the promise of accessing more secondary metabolites of great academic and economic importance. Well-established engineered strains for application in biotechnology and genetically engineered approaches will probably be a practicable choice.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr. Chunji Li was supported by the National Natural Science Foundation of China [32101930].
Dr. Guohui Yu was supported by the Guangdong Province Basic and Applied Basic Research Fund [2020A1515011015] and the Research Project fund at the Innovative Institute for Plant Health [KA20131H102].
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Chunji Li, Email: lichunji0804@126.com.
Ping Cheng, Email: nkpcheng@163.com.
Guohui Yu, Email: ygh76411@zhku.edu.cn.
References
- Abas N., Kalair A., Khan N. Review of fossil fuels and future energy technologies. Futures. 2015;69:31–49. [Google Scholar]
- Adel A., El-Baz A., Shetaia Y., Sorour N.M. Biosynthesis of polyunsaturated fatty acids by two newly cold-adapted Egyptian marine yeast. 3 Biotech. 2021;11(11):461. doi: 10.1007/s13205-021-03010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aibeche C., Selami N., Zitouni-Haouar F.E., Oeunzar K., Addou A., Kaid-Harche M., Djabeur A. Bioremediation potential and lead removal capacity of heavy metal-tolerant yeasts isolated from Dayet Oum Ghellaz Lake water (northwest of Algeria) Int. Microbiol. 2022;25(1):61–73. doi: 10.1007/s10123-021-00191-z. [DOI] [PubMed] [Google Scholar]
- Aksu Z., Eren A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005;40(9):2985–2991. [Google Scholar]
- Allahkarami S., Sepahi A.A., Hosseini H., Razavi M.R. Isolation and identification of carotenoid-producing Rhodotorula sp. from Pinaceae forest ecosystems and optimization of in vitro carotenoid production. Biotechnol. Rep. 2021;32 doi: 10.1016/j.btre.2021.e00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anas M., Liao F., Verma K.K., Sarwar M.A., Mahmood A., Chen Z., Li Q., Zeng X., Liu Y., Li Y. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020;53(1):47. doi: 10.1186/s40659-020-00312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew M., Jayaraman G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020;487 doi: 10.1016/j.carres.2019.107881. [DOI] [PubMed] [Google Scholar]
- Anello A.L., Aguilera L., Kurina-Sanz M., Juri Ayub M., Mascotti M.L. Broadening the repertoire of microbial aldo-keto reductases: Cloning and characterization of AKR3B4 from Rhodotorula mucilaginosa LSL strain. Enzyme Microb. Technol. 2020;132 doi: 10.1016/j.enzmictec.2019.109415. [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Ateş S., Dogan N. Properties of immobilized phenylalanine ammonia lyase and investigation of its use for the prediagnosis of phenylketonuria. Turk. J. Biochem. 2010;35:58–62. [Google Scholar]
- Ayadi I., Belghith H., Gargouri A., Guerfali M. Utilization of wheat bran acid hydrolysate by Rhodotorula mucilaginosa Y-MG1 for microbial lipid production as feedstock for biodiesel synthesis. BioMed Res. Int. 2019;29:1–11. doi: 10.1155/2019/3213521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C., Twyman R.M., Farré G., Sanahuja G., Christou P., Capell T., Zhu C. A golden era—Pro-vitamin A enhancement in diverse crops. In Vitro Cell. Dev. Plant. 2011;47(2):205–221. [Google Scholar]
- Bai C., Zheng J., Zhao L., Chen L., Xiong H., McClements D.J. Development of oral delivery systems with enhanced antioxidant and anticancer activity: Coix seed oil and β-carotene coloaded liposomes. J. Agric. Food Chem. 2019;67(1):406–414. doi: 10.1021/acs.jafc.8b04879. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Sharma T., Nautiyal A.K., Dasgupta D., Hazra S., Bhaskar T., Ghosh D. Scale-up strategy for yeast single cell oil production for Rhodotorula mucilagenosa IIPL32 from corn cob derived pentosan. Bioresour. Technol. 2020;309 doi: 10.1016/j.biortech.2020.123329. [DOI] [PubMed] [Google Scholar]
- Barbosa C.H., Andrade M.A., Séndon R., Silva A.S., Ramos F., Vilarinho F., Khwaldia K., Barbosa-Pereira L. Industrial fruits by-products and their antioxidant profile: Can they be exploited for industrial food applications? Foods. 2021;10(2):272. doi: 10.3390/foods10020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan P., Gupta K., Kishor S., Chattopadhyay P., Chaliha C., Kalita E., Goud V.V., Mandal M. Oleaginous yeasts isolated from traditional fermented foods and beverages of Manipur and Mizoram, India, as a potent source of microbial lipids for biodiesel production. Ann. Microbiol. 2020;70(1):27. [Google Scholar]
- Barros J., Dixon R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020;25(1):66–79. doi: 10.1016/j.tplants.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Bautista L.F., Vicente G., Garre V. In: Advances in Biodiesel Production. Luque R., Melero J.A., editors. Woodhead Publishing; 2012. 8 - biodiesel from microbial oil; pp. 179–203. [Google Scholar]
- Béguin P., Aubert J.P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994;13(1):25–58. doi: 10.1111/j.1574-6976.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Béligon V., Christophe G., Fontanille P., Larroche C. Microbial lipids as potential source to food supplements. Curr. Opin. Food Sci. 2016;7:35–42. [Google Scholar]
- Beopoulos A., Nicaud J.M. Yeast: A new oil producer? Corps gras, Lipides. 2012;19(1):22–28. [Google Scholar]
- Beopoulos A., Cescut J., Haddouche R., Uribelarrea J.L., Molina-Jouve C., Nicaud J.M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009;48(6):375–387. doi: 10.1016/j.plipres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Beopoulos A., Nicaud J., Gaillardin C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011;90(4):1193–1206. doi: 10.1007/s00253-011-3212-8. [DOI] [PubMed] [Google Scholar]
- Bhosale P.B., Gadre R.V. Production of beta-carotene by a mutant of Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2001;55(4):423–427. doi: 10.1007/s002530000570. [DOI] [PubMed] [Google Scholar]
- Blomme G., Ocimati W., Nabuuma D., Sivirihauma C., Davey M., Buah S., Van den Bergh I., Vutseme L., Bahati L., Ekesa B. Pro-vitamin A carotenoid content of 48 plantain (Musa AAB genome) cultivars sourced from eastern Democratic Republic of Congo. J. Sci. Food Agric. 2020;100(2):634–647. doi: 10.1002/jsfa.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel P., Hammaz F., Morand-Laffargue L., Creton B., Halimi C., Sabatier D., Desmarchelier C. Using black soldier fly larvae reared on fruits and vegetables waste as a sustainable dietary source of provitamin A carotenoids. Food Chem. 2021;359 doi: 10.1016/j.foodchem.2021.129911. [DOI] [PubMed] [Google Scholar]
- Borello E., Roncucci D., Domenici V. Study of the evolution of pigments from freshly pressed to ‘On-the-Shelf’ extra-virgin olive oils by means of near-UV visible spectroscopy. Foods. 2021;10(8):1891. doi: 10.3390/foods10081891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borja-Martínez M., Lozano-Sánchez J., Borrás-Linares I., Pedreño M.A., Sabater-Jara A.B. Revalorization of broccoli by-products for cosmetic uses using supercritical fluid extraction. Antioxidants. 2020;9(12):1195. doi: 10.3390/antiox9121195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce A., Walsh G. Production, purification and application-relevant characterisation of an endo-1,3(4)-beta-glucanase from Rhizomucor miehei. Appl. Microbiol. Biotechnol. 2007;76(4):835–841. doi: 10.1007/s00253-007-1058-x. [DOI] [PubMed] [Google Scholar]
- Britto D.S., Pirovani C.P., Andrade B.S., Dos Santos T.P., Pungartnik C., Cascardo J.C., Micheli F., Gesteira A.S. Recombinant beta-1,3-1,4-glucanase from Theobroma cacao impairs Moniliophthora perniciosa mycelial growth. Mol. Biol. Rep. 2013;40(9):5417–5427. doi: 10.1007/s11033-013-2640-1. [DOI] [PubMed] [Google Scholar]
- Burton G.W., Mogg T.J., Riley W.W., Nickerson J.G. β-Carotene oxidation products - Function and safety. Food Chem. Toxicol. 2021;152 doi: 10.1016/j.fct.2021.112207. [DOI] [PubMed] [Google Scholar]
- Buzzini P., Martini A., Gaetani M., Turchetti B., Pagnoni UM., Davoli P. Optimization of carotenoid production by Rhodotorula graminis DBVPG 7021 as a function of trace element concentration by means of response surface analysis. Enzyme Microb. Tech. 2005;36(5):687–692. [Google Scholar]
- Castro A.J.G., Castro L.S.E.P.W., Santos M.S.N., Faustino M.G.C., Pinheiro T.S., Dore C.M.P.G., Baseia I.G., Leite E.L. Anti-inflamatory, anti-angiogenenic and antioxidant activities of polysaccharide-rich extract from fungi Caripia montagnei. Biomed. Prev. Nut. 2014;4(2):121–129. [Google Scholar]
- Cezare-Gomes E.A., Mejia-da-Silva L.D.C., Pérez-Mora L.S., Matsudo M.C., Ferreira-Camargo L.S., Singh A.K., de Carvalho J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019;188(3):602–634. doi: 10.1007/s12010-018-02945-4. [DOI] [PubMed] [Google Scholar]
- Chaari F., Blibech M., Bhiri F., Maktouf S., Ellouz-Ghorbel R., Ellouz-Chaabouni S. Production of mixed-linkage beta-oligosaccharides from lichenan using immobilized Bacillus licheniformis UEB CF lichenase. Appl. Biochem. Biotechnol. 2012;168(3):616–628. doi: 10.1007/s12010-012-9804-7. [DOI] [PubMed] [Google Scholar]
- Chacón Ordóñez T., Carle R., Schweiggert R. Bioaccessibility of carotenoids from plant and animal foods. J. Sci. Food Agric. 2019;99(7):3220–3239. doi: 10.1002/jsfa.9525. [DOI] [PubMed] [Google Scholar]
- Chandra P., Enespa, Singh R., Arora P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories. 2020;19(1):169. doi: 10.1186/s12934-020-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S., Bhattacharya A., Nain L., Prasanna R., Khare S.K. Valorization of agro-starchy wastes as substrates for oleaginous microbes. Biomass Bioenerg. 2019;127 [Google Scholar]
- Chaud L.C.S., Lario L.D., Bonugli-Santos R.C., Sette L.D., Pessoa Junior A., Felipe M.D.G.D. Improvement in extracellular protease production by the marine antarctic yeast Rhodotorula mucilaginosa L7. N. Biotech. 2016;33(6):807–814. doi: 10.1016/j.nbt.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Yang C. Using strain Rhodotorula mucilaginosa to produce carotenoids using food wastes. J. Taiwan Inst. Chem. Eng. 2016;61:270–275. [Google Scholar]
- Chennupati S., Potumarthi R., Gopal Rao M., Manga P.L., Sridevi M., Jetty A. Multiple responses optimization and modeling of lipase production by Rhodotorula mucilaginosa MTCC-8737 using response surface methodology. Appl. Biochem. Biotechnol. 2009;159(2):317–329. doi: 10.1007/s12010-009-8547-6. [DOI] [PubMed] [Google Scholar]
- Cook R.J. Making greater use of introduced microorganisms for biological control of plant pathogens. Annu. Rev. Phytopathol. 1993;31(1):53–80. doi: 10.1146/annurev.py.31.090193.000413. [DOI] [PubMed] [Google Scholar]
- Costa W.A.D., Padilha C.E.D.A., Júnior S.D.D.O., Silva F.L.H.D., Silva J., Ancântara M.A., Ferrari M., Santos E.S.D. Oil-lipids, carotenoids and fatty acids simultaneous production by Rhodotorula mucilaginosa CCT3892 using sugarcane molasses as carbon source. Braz. J. Food Technol. 2020;23(1-2) [Google Scholar]
- Cudowski A., Pietryczuk A. Biochemical response of Rhodotorula mucilaginosa and Cladosporium herbarum isolated from aquatic environment on iron (III) ions. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-56088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Liang L., Han C., Lin Liu R. Stabilization of phenylalanine ammonia lyase from Rhodotorula glutinis by encapsulation in polyethyleneimine-mediated biomimetic silica. Appl. Biochem. Biotechnol. 2015;176(4):999–1011. doi: 10.1007/s12010-015-1624-0. [DOI] [PubMed] [Google Scholar]
- Cui J., Zhao Y., Feng Y., Lin T., Zhong C., Tan Z., Jia S. Encapsulation of spherical cross-linked phenylalanine ammonia lyase aggregates in mesoporous biosilica. J. Agric. Food Chem. 2017;65(3):618–625. doi: 10.1021/acs.jafc.6b05003. [DOI] [PubMed] [Google Scholar]
- Cunha F.A., Cunha M., da Frota S.M., Mallmann E.J.J., Freire T.M., Costa L.S., Paula A.J., Menezes E.A., Fechine P.B.A. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: Antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 2018;4(9):127. doi: 10.1007/s11274-018-2514-8. [DOI] [PubMed] [Google Scholar]
- Czabany T., Athenstaedt K., Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta. 2007;1771(3):299–309. doi: 10.1016/j.bbalip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- da Silva J., Honorato da Silva F.L., Santos Ribeiro J.E., Nóbrega de Melo D.J., Santos F.A., Lucena de Medeiros L. Effect of supplementation, temperature and pH on carotenoids and lipids production by Rhodotorula mucilaginosa on sisal bagasse hydrolyzate. Biocatal. Agric. Biotechnol. 2020;30 [Google Scholar]
- Daskalaki A., Perdikouli N., Aggeli D., Aggelis G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019;103(20):8585–8596. doi: 10.1007/s00253-019-10088-7. [DOI] [PubMed] [Google Scholar]
- de Bruijn F.J. In: Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture. Lugtenberg B., editor. Springer International Publishing; Cham: 2015. Biological nitrogen fixation; pp. 215–224. [Google Scholar]
- Dias Rodrigues T.V., Amore T.D., Teixeira E.C., de Medeiros Burkert J.F. Carotenoid production by Rhodotorula mucilaginosa in batch and fed-batch fermentation using agroindustrial byproducts. Food Technol. Biotechnol. 2019;57(3):388–398. doi: 10.17113/ftb.57.03.19.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C.H., Yin D.H., Meng Y., Li H.L. Study on the optimization of β-carotene production by fermentation of Blakeslea trispora. Food Res. Dev. 2020;41(61):88–194. [Google Scholar]
- Dourou M., Aggeli D., Papanikolaou S., Aggelis G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018;102(6):2509–2523. doi: 10.1007/s00253-018-8813-z. [DOI] [PubMed] [Google Scholar]
- Du C., Li Y., Guo Y., Han M., Zhang W., Qian H. Torularhodin, isolated from Sporidiobolus pararoseus, inhibits human prostate cancer LNCaP and PC-3 cell growth through Bcl-2/Bax mediated apoptosis and AR down-regulation. RSC Adv. 2015;5(129):106387–106395. [Google Scholar]
- Du C., Li Y., Guo Y., Han M., Zhang W., Qian H. The suppression of torulene and torularhodin treatment on the growth of PC-3 xenograft prostate tumors. Biochem. Bioph. Res. Co. 2016;469(4):1146–1152. doi: 10.1016/j.bbrc.2015.12.112. [DOI] [PubMed] [Google Scholar]
- Du C., Guo Y., Cheng Y., Han M., Zhang W., Qian H. Torulene and torularhodin, protects human prostate stromal cells from hydrogen peroxide-induced oxidative stress damage through the regulation of Bcl-2/Bax mediated apoptosis. Free Radic. Res. 2017;51(2):113–123. doi: 10.1080/10715762.2017.1285024. [DOI] [PubMed] [Google Scholar]
- Duarte A.W.F., Dos Santos J.A., Vianna M.V., Vieira J.M.F., Mallagutti V.H., Inforsato F.J., Wentzel L.C.P., Lario L.D., Rodrigues A., Pagnocca F.C., Pessoa Junior A., Durães Sette L. Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit. Rev. Biotechnol. 2018;38(4):600–619. doi: 10.1080/07388551.2017.1379468. [DOI] [PubMed] [Google Scholar]
- Duda-Chodak A., Tarko T., Milotta KJJoE. Applicability of different kinds of yeast biomass to lead removal from water. J. Elementol. 2012;17(1):7–18. [Google Scholar]
- Dulińska-Litewka J., Sharoni Y., Hałubiec P., Łazarczyk A., Szafrański O., McCubrey J.A., Gąsiorkiewicz B., Laidler P., Bohn T. Recent progress in discovering the role of carotenoids and their metabolites in prostatic physiology and pathology with a focus on prostate cancer—A review—Part I: molecular mechanisms of carotenoid action. Antioxidants. 2021;10(4):585. doi: 10.3390/antiox10040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrett T.P., Benning C., Ohlrogge J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008;54(4):593–607. doi: 10.1111/j.1365-313X.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- El-Banna A.A., El-Razek A.M.A., El-Mahdy A.R. Some factors affecting the production of carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr. Sci. 2012;3(1):64–71. [Google Scholar]
- Faé Neto W.A., Borges Mendes C.R., Abreu P.C. Carotenoid production by the marine microalgae Nannochloropsis oculata in different low-cost culture media. Aquacult. Res. 2018;49(7):2527–2535. [Google Scholar]
- Fakas S. Lipid biosynthesis in yeasts: a comparison of the lipid biosynthetic pathway between the model nonoleaginous yeast Saccharomyces cerevisiae and the model oleaginous yeast Yarrowia lipolytica. Eng. Life Sci. 2017;17(3):292–302. doi: 10.1002/elsc.201600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favas R., Morone J., Martins R., Vasconcelos V., Lopes G. Cyanobacteria secondary metabolites as biotechnological ingredients in natural anti-aging cosmetics: Potential to overcome hyperpigmentation, loss of skin density and UV radiation-deleterious effects. Mar. Drugs. 2022;20(3):183. doi: 10.3390/md20030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frengova G.I., Beshkova D.M. Carotenoids from Rhodotorula and Phaffia: Yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 2009;36(2):163–180. doi: 10.1007/s10295-008-0492-9. [DOI] [PubMed] [Google Scholar]
- Frey P.A., Hegeman A.D. Oxford University Press; 2007. Enzymatic Reaction Mechanisms. [Google Scholar]
- Galano A. Relative antioxidant efficiency of a large series of carotenoids in terms of one electron transfer reactions. J. Phys. Chem. B. 2007;11(44):12898–12908. doi: 10.1021/jp074358u. [DOI] [PubMed] [Google Scholar]
- Ganesan V., Spagnuolo M., Agrawal A., Smith S., Gao D., Blenner M. Advances and opportunities in gene editing and gene regulation technology for Yarrowia lipolytica. Microb. Cell Factories. 2019;18(1):208. doi: 10.1186/s12934-019-1259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cortes A., Garcia-Vásquez J.A., Aranguren Y., Ramirez-Castrillon M. Pigment production improvement in Rhodotorula mucilaginosa AJB01 using design of experiments. Microorganisms. 2021;9(2):387. doi: 10.3390/microorganisms9020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilescu M., Demnerová K., Aamand J., Agathos S., Fava F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotech. 2015;32(1):147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Geisseler D., Scow K.M. Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biol. Biochem. 2014;75:54–63. [Google Scholar]
- Ghazani S.M., Marangoni A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2022;119:593–607. [Google Scholar]
- Ghilardi C., Sanmartin N.P., Carelli A.A., Borroni V. Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020;7(1):1–11. [Google Scholar]
- Gientka I., Błażejak S., Stasiak-Różańska L., Chlebowska-Śmigiel A. Exopolysaccharides from yeast: Insight into optimal conditions for biosynthesis, chemical composition and functional properties - Review. Acta Sci. Polon. Techn. 2015;14(4):283–292. doi: 10.17306/J.AFS.2015.4.29. [DOI] [PubMed] [Google Scholar]
- Gientka I., Gadaszewska M., Błażejak S., Kieliszek M., Bzducha-Wróbel A., Stasiak-Różańska L., Kot A.M. Evaluation of lipid biosynthesis ability by Rhodotorula and Sporobolomyces strains in medium with glycerol. Eur. Food Res. Technol. 2017;243(2):275–286. [Google Scholar]
- Gohain M., Bardhan P., Laskar K., Sarmah S., Mandal M., Bora U., Chandra K.M., Goud V.V., Deka D. Rhodotorula mucilaginosa: A source of heterogeneous catalyst for biodiesel production from yeast single cell oil and waste cooking oil. Renew. Energy. 2020;160:220–230. [Google Scholar]
- Gong M., Bassi A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016;34(8):1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Gong Y., Huang J. Characterization of four untapped microalgae for the production of lipids and carotenoids. Algal Res. 2020;49 [Google Scholar]
- Good A.G., Beatty P.H. Fertilizing nature: A tragedy of excess in the commons. PLoS Biol. 2011;9(8) doi: 10.1371/journal.pbio.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D., Basu J.K., De S. Lipase applications in oil hydrolysis with a case study on castor oil: A review. Crit. Rev. Biotechnol. 2013;33(1):81–96. doi: 10.3109/07388551.2012.672319. [DOI] [PubMed] [Google Scholar]
- Grune T., Lietz G., Palou A., Ross A.C., Stahl W., Tang G., Thurnham D., Yin S.A., Biesalski H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010;140(12):2268s–2285s. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto N.C., Nogueira J.P., Silva AdSd., Barbosa P.F., Santana Matos C.M., Rajan M., Santos Leite Neta M.T., Narain N. Optimization of the biotechnological process using Rhodotorula mucilaginosa and acerola (Malpighia emarginata L.) seeds for the production of bioactive compounds. LWT. 2022;160 [Google Scholar]
- Gupta A., Vongsvivut J., Barrow C.J., Puri M. Molecular identification of marine yeast and its spectroscopic analysis establishes unsaturated fatty acid accumulation. J. Biosci. Bioeng. 2012;114(4):411–417. doi: 10.1016/j.jbiosc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Haddad N.F., Teodoro A.J., Leite de Oliveira F., Soares N., de Mattos R.M., Hecht F., Dezonne R.S., Vairo L., Goldenberg R.C., Gomes F.C., de Carvalho D.P., Gadelha M.R., Nasciutti L.E., Miranda-Alves L. Lycopene and beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggag W.M., Mohamed H.A.A. Biotechnological aspects of microorganisms used in plant biological control. Am-Eurasian J. Sustain. Agric. 2007;1(1):7–12. [Google Scholar]
- Hambly A.J., van Duijneveldt J.S., Gates P.J. Identification of β-carotene oxidation products produced by bleaching clay using UPLC-ESI-MS/MS. Food Chem. 2021;353(1) doi: 10.1016/j.foodchem.2021.129455. [DOI] [PubMed] [Google Scholar]
- Hamidi M., Gholipour A.R., Delattre C., Sesdighi F., Mirzaei Seveiri R., Pasdaran A., Kheirandish S., Pierre G., Safarzadeh Kozani P., Safarzadeh Kozani P., Karimitabar F. Production, characterization and biological activities of exopolysaccharides from a new cold-adapted yeast: Rhodotorula mucilaginosa sp. GUMS16. Int. J. Biol. Macromol. 2020;151:268–277. doi: 10.1016/j.ijbiomac.2020.02.206. [DOI] [PubMed] [Google Scholar]
- Hammamchı H., Cihangir N. Production of lipase by newly isolated Rhodotorula mucilaginosa by using molasses, important environmental pollutant. CEST. 2017;2017:1. [Google Scholar]
- Hammes-Schiffer S. Catalytic efficiency of enzymes: A theoretical analysis. Biochemistry. 2013;52(12):2012–2020. doi: 10.1021/bi301515j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Xu Z., Du C., Qian H., Zhang W.G. Effects of nitrogen on the lipid and carotenoid accumulation of oleaginous yeast Sporidiobolus pararoseus. Bioproc. Biosyst. Eng. 2016;39(9):1425–1433. doi: 10.1007/s00449-016-1620-y. [DOI] [PubMed] [Google Scholar]
- Hausmann A., Sandmann G. A single five-step desaturase is involved in the carotenoid biosynthesis pathway to β-carotene and torulene in Neurospora crassa. Fungal Genet. Biol. 2000;30(2):147–153. doi: 10.1006/fgbi.2000.1212. [DOI] [PubMed] [Google Scholar]
- He Z., Wang S., Yang Y., Hu J., Wang C., Li H., Ma B., Yuan Q.J.P.B. β-carotene production promoted by ethylene in Blakeslea trispora and the mechanism involved in metabolic responses. Process Biochem. 2017;57:57–63. [Google Scholar]
- Herz S., Weber R.W.S., Anke H., Mucci A., Davoli P. Intermediates in the oxidative pathway from torulene to torularhodin in the red yeasts Cystofilobasidium infirmominiatum and C. capitatum (Heterobasidiomycetes, Fungi) Phytochemistry. 2007;68(20):2503–2511. doi: 10.1016/j.phytochem.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Honda M., Kageyama H., Hibino T., Ichihashi K., Takada W., Goto M. Isomerization of commercially important carotenoids (Lycopene, β-carotene, and astaxanthin) by natural catalysts: isothiocyanates and polysulfides. J. Agric. Food Chem. 2020;68(10):3228–3237. doi: 10.1021/acs.jafc.0c00316. [DOI] [PubMed] [Google Scholar]
- Hu K., Zhu X.L., Mu H., Ma Y., Ullah N., Tao Y.S. A novel extracellular glycosidase activity from Rhodotorula mucilaginosa: Its application potential in wine aroma enhancement. Lett. Appl. Microbiol. 2016;62(2):169–176. doi: 10.1111/lam.12527. [DOI] [PubMed] [Google Scholar]
- Huang G., Mei X., Hu J. The antioxidant activities of natural polysaccharides. Curr. Drug Targets. 2017;18(11):1296–1300. doi: 10.2174/1389450118666170123145357. [DOI] [PubMed] [Google Scholar]
- Huang X.Q., You Z.Y., Luo Y., Yang C.J., Ren J., Wei G.J., Dong P., Ren M.Z. Antifungal activity of chitosan against Phytophthora infestans, the pathogen of potato late bright. Int. J. Biol. Macromol. 2021;166(102):1365–1376. doi: 10.1016/j.ijbiomac.2020.11.016. [DOI] [PubMed] [Google Scholar]
- Hyun M.W., Yun Y.H., Kim J.Y., Kim S.H. Fungal and plant phenylalanine ammonia-lyase. Mycobiology. 2011;39(4):257–265. doi: 10.5941/MYCO.2011.39.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazusta V., Nieto-Peñalver C.G., Cabral M.E., Amoroso M.J., de Figueroa L.I.C. Relationship among carotenoid production, copper bioremediation and oxidative stress in Rhodotorula mucilaginosa RCL-11. Process Biochem. 2013;48(5):803–809. [Google Scholar]
- Jiao X., Zhang Y., Liu X., Zhang Q., Zhang S., Zhao Z.K. Developing a CRISPR/Cas9 system for genome editing in the basidiomycetous yeast Rhodosporidium toruloides. Biotechnol. J. 2019;14(7) doi: 10.1002/biot.201900036. [DOI] [PubMed] [Google Scholar]
- Johnson E.A., Schroeder W.A. Microbial carotenoids. Adv. Biochem. Eng. Biot. 1996;53:119–178. doi: 10.1007/BFb0102327. e00657. [DOI] [PubMed] [Google Scholar]
- Johra F.T., Bepari A.K., Bristy A.T., Reza H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants. 2020;9(11):1046. doi: 10.3390/antiox9111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.D., Boundy-Mills K.L., Barla G.F., Kumar S., Ubanwa B., Balan V. In: Microbial Lipid Production: Methods and Protocols. Balan V., editor. Springer New York; New York, NY: 2019. Microbial lipid alternatives to plant lipids; pp. 1–32. [DOI] [PubMed] [Google Scholar]
- Joseph B., Ramteke P.W., Thomas G. Cold active microbial lipases: Some hot issues and recent developments. Biotechnol. Adv. 2008;26(5):457–470. doi: 10.1016/j.biotechadv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kane J.F., Fiske M.J. Regulation of phenylalanine ammonia lyase in Rhodotorula glutinis. J. Bacteriol. 1985;161(3):963–966. doi: 10.1128/jb.161.3.963-966.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Semin. Cell Dev. Biol. 2018;74:89–96. doi: 10.1016/j.semcdb.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Karatay S.E., Dönmez G. Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour. Technol. 2010;101(20):7988–7990. doi: 10.1016/j.biortech.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Kasperczyk S., Dobrakowski M., Kasperczyk J., Ostałowska A., Zalejska-Fiolka J., Birkner E. Beta-carotene reduces oxidative stress, improves glutathione metabolism and modifies antioxidant defense systems in lead-exposed workers. Toxicol. Appl. Pharmacol. 2014;280(1):36–41. doi: 10.1016/j.taap.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Kawatra A., Dhankhar R., Mohanty A., Gulati P. Biomedical applications of microbial phenylalanine ammonia lyase: Current status and future prospects. Biochimie. 2020;177:142–152. doi: 10.1016/j.biochi.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Khan U.M., Sevindik M., Zarrabi A., Nami M., Ozdemir B., Kaplan D.N., Selamoglu Z., Hasan M., Kumar M., Alshehri M.M., Sharifi-Rad J. Lycopene: Food sources, biological activities, and human health benefits. Oxid. Med. Cell Longev. 2021 doi: 10.1155/2021/2713511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheyrandish S., Rastgar A., Hamidi M., Sajjadi S.M., Sarab G.A. Evaluation of anti-tumor effect of the exopolysaccharide from new cold-adapted yeast, Rhodotorula mucilaginosa sp. GUMS16 on chronic myeloid leukemia K562 cell line. Int. J. Biol. Macromol. 2022;206:21–28. doi: 10.1016/j.ijbiomac.2022.02.113. [DOI] [PubMed] [Google Scholar]
- Khot M., Ghosh D. Lipids of Rhodotorula mucilaginosa IIPL32 with biodiesel potential: Oil yield, fatty acid profile, fuel properties. J. Basic Microbiol. 2017;57(4):345–352. doi: 10.1002/jobm.201600618. [DOI] [PubMed] [Google Scholar]
- Kim D., Kim Y., Kim Y. Effects of β-carotene on expression of selected microRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J. Cancer. Prev. 2019;24(4):224–232. doi: 10.15430/JCP.2019.24.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe G., Sharp C.A., Ryan T.W. Exhaust emissions of biodiesel, petrodiesel, neat methyl esters, and alkanes in a new technology engine. Energy Fuel. 2006;20(1):403–408. [Google Scholar]
- Kochian L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46(1):237–260. [Google Scholar]
- Kochian L.V., Hoekenga O.A., Pineros M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004;55(1):459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Kolesárová N., Hutňan M., Bodík I., Špalková V. Utilization of biodiesel by-products for biogas production. J. Biomed. Biotechnol. 2011;2011(11) doi: 10.1155/2011/126798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolouchová I., Maťátková O., Sigler K., Masák J., Řezanka T. Lipid accumulation by oleaginous and non-oleaginous yeast strains in nitrogen and phosphate limitation. Folia Microbiol. 2016;61(5):431–438. doi: 10.1007/s12223-016-0454-y. [DOI] [PubMed] [Google Scholar]
- Kong W., Yang S., Agboyibor C., Chen D., Zhang A., Niu S. Light irradiation can regulate the growth characteristics and metabolites compositions of Rhodotorula mucilaginosa. J. Food Sci. Technol. 2019;56(12):5509–5517. doi: 10.1007/s13197-019-04023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot A.M., Blazejak S., Kurcz A., Gientka I., Kieliszek M. Rhodotorula glutinis – Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016;100(14):6103–6117. doi: 10.1007/s00253-016-7611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot A.M., Błażejak S., Brzezińska R., Sęk W., Kieliszek M. Effect of selected cations and B vitamins on the biosynthesis of carotenoids by Rhodotorula mucilaginosa yeast in the media with agro-industrial wastes implications. Appl. Sci. 2021;11(24) [Google Scholar]
- Kot A.M., Blazejak S., Gientka I., Kieliszek M., Brys J. Torulene and torularhodin: “New” fungal carotenoids for industry? Microb. Cell Fact. 2018;17(1):49. doi: 10.1186/s12934-018-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot A.M., Blazejak S., Kieliszek M., Gientka I., Brys J. Simultaneous production of lipids and carotenoids by the red yeast Rhodotorula from waste glycerol fraction and potato wastewater. Appl. Biochem. Biotechnol. 2019;189(2):589–607. doi: 10.1007/s12010-019-03023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot A.M., Kieliszek M., Piwowarek K., Błażejak S., Mussagy C.U. Sporobolomyces and Sporidiobolus – Non-conventional yeasts for use in industries. Fungal Biol. Rev. 2021;37:41–58. [Google Scholar]
- Kreiner M., Harvey L.M., McNeil B. Morphological and enzymatic responses of a recombinant Aspergillus niger to oxidative stressors in chemostat cultures. J. Biotechnol. 2003;100(3):251–260. doi: 10.1016/s0168-1656(02)00245-6. [DOI] [PubMed] [Google Scholar]
- Krinsky N.I. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 1989;7(6):617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Kulkarni P., Dixit M., Raavi P.K., Krishna L.N.V. Sources of cellulose and their applications- A review. Int. J. Drug For. Res. 2011;2:19–38. [Google Scholar]
- Lario L.D., Chaud L., Almeida M.D.G., Converti A., Durães S.L., Pessoa A. Production, purification, and characterization of an extracellular acid protease from the marine antarctic yeast Rhodotorula mucilaginosa L7. Fungal Biol. 2015;119(11):1129–1136. doi: 10.1016/j.funbio.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Lario L.D., Pillaca-Pullo O.S., Durães S.L., Converti A., Casati P., Spampinato C., Pessoa A. Optimization of protease production and sequence analysis of the purified enzyme from the cold adapted yeast Rhodotorula mucilaginosa CBMAI 1528. Biotechnol. Rep. (Amst). 2020;28 doi: 10.1016/j.btre.2020.e00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J.L., Chen L., Cao B., Chen W.N. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture. Appl. Microbiol. Biotechnol. 2016;100(2):869–877. doi: 10.1007/s00253-015-7102-3. [DOI] [PubMed] [Google Scholar]
- Lee K.E., Kwon M., Kim Y.S., Kim Y., Chung M.G., Heo S.C., Kim Y. β-carotene regulates cancer stemness in colon cancer in vivo and in vitro. Nutr. Res. Pract. 2022;16(2):161–172. doi: 10.4162/nrp.2022.16.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesing R., Siwina S., Fiala K. Yeast-based biodiesel production using sulfonated carbon-based solid acid catalyst by an integrated biorefinery of durian peel waste. Renew. Energy. 2021;171:647–657. [Google Scholar]
- Levy H.L., Sarkissian C.N., Scriver C.R. Phenylalanine ammonia lyase (PAL): From discovery to enzyme substitution therapy for phenylketonuria. Mol. Genet. Metabol. 2018;124(4):223–229. doi: 10.1016/j.ymgme.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Li N., Wu X., Wu Z.J.B.B. A effect of liquid alkane oxygen-vectors on Rhodotorula fermentations and lycopene production. Biol. Bull. 2015;31(2):196. [Google Scholar]
- Li C., Zhang N., Li B., Xu Q., Song J., Wei N., Wang W., Zou H. Increased torulene accumulation in red yeast Sporidiobolus pararoseus NGR as stress response to high salt conditions. Food Chem. 2017;237:1041–1047. doi: 10.1016/j.foodchem.2017.06.033. [DOI] [PubMed] [Google Scholar]
- Li J., Guo Y., Cheng Y., Pi F., Yao W., Xie Y., Qian H. Determination of the molecular mechanism of torularhodin against hepatic oxidative damage by transcriptome analysis. Oxid. Med. Cell. Longev. 2019;(1):1–11. doi: 10.1155/2019/7417263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Jiang Z., Chen S., Wang T., Jiang L., Wang M., Wang S., Li Z. Biochemical changes of polysaccharides and proteins within EPS under Pb (II) stress in Rhodotorula mucilaginosa. Ecotoxicol. Environ. Saf. 2019;174:484–490. doi: 10.1016/j.ecoenv.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Li J., Liu C., Guo Y., Pi F., Yao W., Xie Y., Cheng Y., Qian H. Determination of the effects of torularhodin against alcoholic liver diseases by transcriptome analysis. Free Radic. Biol. Med. 2019;143(54):47–54. doi: 10.1016/j.freeradbiomed.2019.07.033. [DOI] [PubMed] [Google Scholar]
- Li Y., Pan F., Yao H. Response of symbiotic and asymbiotic nitrogen-fixing microorganisms to nitrogen fertilizer application. J. Soils Sediments. 2019;19(4):1948–1958. [Google Scholar]
- Li H., Huang L., Zhang Y., Yan Y. Production, characterization and immunomodulatory activity of an extracellular polysaccharide from Rhodotorula mucilaginosa YL-1 isolated from sea salt field. Mar. Drugs. 2020;18(12) doi: 10.3390/md18120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Yang C., Du J. Lipid production and waste reutilization combination using yeast isolate Rhodotorula mucilaginosa LP-2. Bioenerg. Res. 2021;14(4):1184–1195. [Google Scholar]
- Libkind D., Moline M., Broock M.V. Production of the UVB-absorbing compound mycosporine-glutaminol-glucoside by Xanthophyllomyces dendrorhous (Phaffia rhodozyma) FEMS Yeast Res. 2010;11(1):52–59. doi: 10.1111/j.1567-1364.2010.00688.x. [DOI] [PubMed] [Google Scholar]
- Liu C., Cui Y., Pi F., Guo Y., Cheng Y., Qian H. Torularhodin ameliorates oxidative activity in vitro and galactose-induced liver injury via the Nrf2/HO-1 signaling pathway in vivo. J. Agric. Food Chem. 2019;67(36):10059–10068. doi: 10.1021/acs.jafc.9b03847. [DOI] [PubMed] [Google Scholar]
- Lin H., Wang Q., Shen Q., Zhan J., Zhao Y. Genetic engineering of microorganisms for biodiesel production. Bioengineered. 2013;4(5):292–304. doi: 10.4161/bioe.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Cheng Y., Du C., Lv T., Guo Y., Han M., Pi F., Zhang W., Qian H. Study on the wall-breaking method of carotenoids producing yeast Sporidiobolus pararoseus and the antioxidant effect of four carotenoids on SK-HEP-1 cells. Prep. Biochem. Biotechnol. 2019;49(8):767–774. doi: 10.1080/10826068.2019.1608448. [DOI] [PubMed] [Google Scholar]
- Liu C., Hu B., Cheng Y., Guo Y., Yao W., Qian H.J.B.T. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021;337 doi: 10.1016/j.biortech.2021.125398. [DOI] [PubMed] [Google Scholar]
- Liu B., Wang C., Liu D., He N., Deng X. Hg tolerance and biouptake of an isolated pigmentation yeast Rhodotorula mucilaginosa. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0172984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Chen X., Wang B., Lou W., Chen X., Hua J., Sun Y.J., Zhao Y., Peng T. Characterization, antioxidativity, and anti-carcinoma activity of exopolysaccharide extract from Rhodotorula mucilaginosa CICC 33013. Carbohyd. polym. 2018;181:768–777. doi: 10.1016/j.carbpol.2017.11.080. [DOI] [PubMed] [Google Scholar]
- MacDonald M.J., D'Cunha G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell. Biol. 2007;85(3):273–282. doi: 10.1139/o07-018. [DOI] [PubMed] [Google Scholar]
- MacDonald M.C., Arivalagan P., Barre D.E., MacInnis J.A., D'Cunha G.B. Rhodotorula glutinis phenylalanine/tyrosine ammonia lyase enzyme catalyzed synthesis of the methyl ester of para-hydroxycinnamic acid and its potential antibacterial activity. Front. Microbiol. 2016;7:281. doi: 10.3389/fmicb.2016.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalakshmi R., Jesuraja S.X., Das S.J. Growth and characterization of L-phenylalanine. Cryst. Res. Technol. 2006;41(8):780–783. [Google Scholar]
- Mannazzu I., Landolfo S., Da Silva T.L., Buzzini P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015;31(11):1665–1673. doi: 10.1007/s11274-015-1927-x. [DOI] [PubMed] [Google Scholar]
- Maoka T. Carotenoids as natural functional pigments. J. Nat. Med. 2020;74(1):1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Market Data Forecast Report Page: Bioenergy 2022. https://www.marketdataforecast.com/market-reports/bio-energy-market
- Market Data Forecast Report Page: Carotenoids 2022. https://www.marketdataforecast.com/market-reports/global-carotenoids-market
- Marova I., Carnecka M., Halienova A., Certik M., Dvorakova T., Haronikova A.J. Use of several waste substrates for carotenoid-rich yeast biomass production. J. Environ. Manag. 2012;95:S338–S342. doi: 10.1016/j.jenvman.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Marx J.C., Collins T., D'Amico S., Feller G., Gerday C. Cold-adapted enzymes from marine Antarctic microorganisms. Mar. Biotechnol. 2007;9(3):293–304. doi: 10.1007/s10126-006-6103-8. [DOI] [PubMed] [Google Scholar]
- Mayo-Wilson E., Imdad A., Herzer K., Yakoob M.Y., Bhutta Z.A. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta-analysis. BMJ. 2011;343 doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd N.F., Tan L.H., Na S.L., Ng K.P. Meningitis caused by Rhodotorula mucilaginosa in HIV-infected patient: A case report and review of the literature. Mycopathologia. 2015;180(1–2):95–98. doi: 10.1007/s11046-015-9879-0. [DOI] [PubMed] [Google Scholar]
- Mohr S.H., Wang J., Ellem G., Ward J., Giurco D. Projection of world fossil fuels by country. Fuel. 2015;141:120–135. [Google Scholar]
- Moliné M., Flores M.R., Libkind D., Del Carmen Diéguez M., Farías M.E., van Broock M. Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: The role of torularhodin. Photochem. Photobiol. Sci. 2010;9(8):1145–1151. doi: 10.1039/c0pp00009d. [DOI] [PubMed] [Google Scholar]
- Mudronova D., Karaffova V., Koscova J., Bartkovsky M., Marcincakova D., Popelka P., Klempova T., Certik M., Macanga J., Marcincak S. Effect of fungal gamma-linolenic acid and beta-carotene containing prefermented feed on immunity and gut of broiler chicken. Poultry Sci. 2018;97(12):4211–4218. doi: 10.3382/ps/pey306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussagy C.U., Gonzalez-Miquel M., Santos-Ebinuma V.C., Pereira J.F.B. Microbial torularhodin - A comprehensive review. Crit. Rev. Biotechnol. 2022 doi: 10.1080/07388551.2022.2041540. [DOI] [PubMed] [Google Scholar]
- Mycobank . 2022. Database Report Page: Rhodotorula mucilaginosa.https://www.mycobank.org/MB/271749 [Google Scholar]
- Nandy S. Bioprocess technology governs enzyme use and production in industrial biotechnology: An overview. Enzyme Eng. 2016;5 [Google Scholar]
- Nasrabadi M.R.N., Razavi S.H. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci. Biotechnol. 2011;20(2):445–454. [Google Scholar]
- Novikov V.S., Kuzmin V.V., Darvin M.E., Lademann J., Sagitova E.A., Prokhorov K.A., Ustynyuk L.Y., Nikolaeva G.Y. Relations between the Raman spectra and molecular structure of selected carotenoids: DFT study of α-carotene, β-carotene, γ-carotene and lycopene. Spectrochim. Acta Mol. Biomol. Spectrosc. 2022;270(9) doi: 10.1016/j.saa.2021.120755. [DOI] [PubMed] [Google Scholar]
- Nuylert A., Hongpattarakere T. Improvement of cell-bound lipase from Rhodotorula mucilaginosa P11I89 for use as a methanol-tolerant, whole-cell biocatalyst for production of palm-oil biodiesel. Ann. Microbiol. 2013;63(3):929–939. [Google Scholar]
- Oikawa T., Tsukagawa Y., Soda K. Endo-beta-glucanase secreted by a psychrotrophic yeast: Purification and characterization. Biosc. Biotech. Biochem. 1998;62(9):1751–1756. doi: 10.1271/bbb.62.1751. [DOI] [PubMed] [Google Scholar]
- Oikawa T., Tsukagawa Y., Chino M., Soda K. Increased transglycosylation activity of Rhodotorula glutinis endo-beta-glucanase in media containing organic solvent. Biosc. Biotech. Biochem. 2001;65(8):1889–1892. doi: 10.1271/bbb.65.1889. [DOI] [PubMed] [Google Scholar]
- Oloke J.K., Glick B.R. Expression of melanin and insecticidal protein from Rhodotorula glutinis in Escherichia coli. Afr. J. Biotechnol. 2006;5(4):327–332. [Google Scholar]
- Orlando Beys Silva W., Mitidieri S., Schrank A., Vainstein M.H. Production and extraction of an extracellular lipase from the entomopathogenic fungus Metarhizium anisopliae. Process Biochem. 2005;40(1):321–326. [Google Scholar]
- Orndorff S.A., Costantino N., Stewart D., Durham D.R. Strain improvement of Rhodotorula graminis for production of a novel L-phenylalanine ammonia-lyase. Appl. Environ. Microbiol. 1998;54(4):996–1002. doi: 10.1128/aem.54.4.996-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadaki E., Mantzouridou F.T. Natural β-carotene production by Blakeslea trispora cultivated in Spanish-style green olive processing wastewaters. Foods. 2021;10(2) doi: 10.3390/foods10020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou S., Aggelis G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011;113(8):1031–1051. [Google Scholar]
- Papaparaskevas D., Christakopoulos P., Kekos D., Macris B.J. Optimizing production of extracellular lipase from Rhodotorula glutinis. Biotechnol. Lett. 1992;14(5):397–402. [Google Scholar]
- Park Y., Choi J., Lim J.W., Kim H. β-Carotene-induced apoptosis is mediated with loss of Ku proteins in gastric cancer AGS cells. Genes Nutr. 2015;10(4):17. doi: 10.1007/s12263-015-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Nicaud J., Ledesma-Amaro R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol. 2018;36(3):304–317. doi: 10.1016/j.tibtech.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Paul K., Saha C., Nag M., Mandal D., Naiya H., Sen D., Mitra S., Kumar M., Bose D., Mukherjee G., Naskar N., Lahiri S., Ghosh U.D., Tripathi S., Sarkar M.P., Banerjee M., Kleniert A., Valentine A.J., Tripathy S., Sinharoy S., Seal A. A tripartite interaction among the basidiomycete Rhodotorula mucilaginosa, N2-fixing endobacteria, and rice improves plant nitrogen nutrition. Plant Cell. 2020;32(2):486–507. doi: 10.1105/tpc.19.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H.W., Anandharaj M., Kao Y.Y., Lin Y.J., Chang J.J., Li W.H. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-29194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potumarthi R., Subhakar C., Vanajakshi J., Jetty A. Effect of aeration and agitation regimes on lipase production by newly isolated Rhodotorula mucilaginosa-MTCC 8737 in stirred tank reactor using molasses as sole production medium. Appl. Biochem. Biotechnol. 2008;151(2-3):700–710. doi: 10.1007/s12010-008-8293-1. [DOI] [PubMed] [Google Scholar]
- Poulton J.E., Romeo J.T., Conn E.E. Springer Science & Business Media; 2012. Plant Nitrogen Metabolism. [Google Scholar]
- Rahbar S.Y., Yari K.A., Pourghassem G.B. Yeast exopolysaccharides and their physiological functions. Folia Microbiol. 2021;66(2):171–182. doi: 10.1007/s12223-021-00856-2. [DOI] [PubMed] [Google Scholar]
- Rajnish K.N., Samuel M.S., John J.A., Datta S., Chandrasekar N., Balaji R., Jose S., Selvarajan E. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-A narrative review. Int. J. Biol. Macromol. 2021;182(6):1793–1802. doi: 10.1016/j.ijbiomac.2021.05.176. [DOI] [PubMed] [Google Scholar]
- Ramos M., Dias A.P.S., Puna J.F., Gomes J., Bordado J.C. Biodiesel production processes and sustainable raw materials. Energies. 2019;12(23):4408. [Google Scholar]
- Rapoport A., Guzhova I., Bernetti L., Buzzini P., Kieliszek M., Kot A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites. 2021;11(2) doi: 10.3390/metabo11020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 2002;30(6):1047–1050. doi: 10.1042/bst0301047. [DOI] [PubMed] [Google Scholar]
- Ray A., Banerjee S., Das D. Microalgal bio-flocculation: Present scenario and prospects for commercialization. Environ. Sci. Pollut. Res. Int. 2021;28(21):26294–26312. doi: 10.1007/s11356-021-13437-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues D.B., Flores É.M.M., Barin J.S., Mercadante A.Z., Jacob-Lopes E., Zepka L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014;65:144–148. [Google Scholar]
- Rodrigues T.V.D., Teixeira E.C., Macedo L.P., Santos G.M.D., Burkert C.A.V., de Medeiros Burkert J.F. Agroindustrial byproduct-based media in the production of microbial oil rich in oleic acid and carotenoids. Bioproc. Biosyst. Eng. 2022;45(4):721–732. doi: 10.1007/s00449-022-02692-1. [DOI] [PubMed] [Google Scholar]
- Rotondo Dottore G., Ionni I., Menconi F., Casini G., Sellari-Franceschini S., Nardi M., Vitti P., Marcocci C., Marinò M. Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves’ orbitopathy (GO) J. Endocrinol. Invest. 2018;41(7):815–820. doi: 10.1007/s40618-017-0809-5. [DOI] [PubMed] [Google Scholar]
- Rutz J.K., Borges C.D., Zambiazi R.C., Da Rosa C.G., Da Silva M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016;202:324–333. doi: 10.1016/j.foodchem.2016.01.140. [DOI] [PubMed] [Google Scholar]
- Sabate J., Cano J., Esteve-Zarzoso B., Guillamón J.M. Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol. Res. 2002;157(4):267–274. doi: 10.1078/0944-5013-00163. [DOI] [PubMed] [Google Scholar]
- Saenge C., Cheirsilp B., Suksaroge T.T., Bourtoom T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011;46(1):210–218. [Google Scholar]
- Safitri D., Wiyono S., Soekarno B.P.W., Achmad Mode of action of the endophytic yeast Rhodotorula mucilaginosa in controlling basal stem rot caused by Phytopthora capsici. IOP Conf. Ser. Earth Environ. Sci. 2021;667(1) [Google Scholar]
- Saha C., Seal A. Early changes in shoot transcriptome of rice in response to Rhodotorula mucilaginosa JGTA-S1. Genom. Data. 2015;6:237–240. doi: 10.1016/j.gdata.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki H., Nakanishi T., Komemushi S., Namikawa K., Miki W. Torularhodin as a potent scavenger against peroxyl radicals isolated from a soil yeast, Rhodotorula glutinis. J. Clin. Biochem. Nutr. 2001;30:1–10. [Google Scholar]
- Sakaki H., Nochide H., Komemushi S., Miki W. Effect of active oxygen species on the productivity of torularhodin by Rhodotorula glutinis No. 21. J. Biosci. Bioeng. 2002;93(3):338–340. doi: 10.1263/jbb.93.338. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Zhu C., Krubasik P., Fraser P.D. The biotechnological potential of the al-2 gene from Neurospra crassa for the production of monocyclic keto hydroxy carotenoids. Biochim. Biophys. Acta. 2006;1761(9):1085–1092. doi: 10.1016/j.bbalip.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sen D., Paul K., Saha C., Mukherjee G., Nag M., Ghosh S., Das A., Seal A., Tripathy S. A unique life-strategy of an endophytic yeast Rhodotorula mucilaginosa JGTA-S1-A comparative genomics viewpoint. DNA Res. 2019;26(2):131–146. doi: 10.1093/dnares/dsy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Ghoshal G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. (Amst). 2020;25 doi: 10.1016/j.btre.2019.e00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Ghoshal G. Characterization and cytotoxic activity of pigment extracted from Rhodotorula mucilaginosa to assess its potential as bio-functional additive in confectionary products. J. Food Sci. Technol. 2021;58(7):2688–2698. doi: 10.1007/s13197-020-04775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Chisti Y., Banerjee U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001;19(8):627–662. doi: 10.1016/s0734-9750(01)00086-6. [DOI] [PubMed] [Google Scholar]
- Sheehan N.P., Ng A., Murray K., Martinez E., Quell K., Ouellette C., Flagg T., Boyle J. Bioenergy from biofuel residues and waste. Water Environ. Res. 2020;92(10):1433–1439. doi: 10.1002/wer.1381. [DOI] [PubMed] [Google Scholar]
- Shi Y., Xin X., Yuan Q. Improved lycopene production by Blakeslea trispora with isopentenyl compounds and metabolic precursors. Biotechnol. Lett. 2012;34(5):849–852. doi: 10.1007/s10529-011-0839-6. [DOI] [PubMed] [Google Scholar]
- Shi G., Gu L., Jung H., Chung W., Koo S. Apocarotenals of phenolic carotenoids for superior antioxidant activities. ACS Omega. 2021;6(38):25096–25108. doi: 10.1021/acsomega.1c04432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields-Menard S.A., Amirsadeghi M., French W.T., Boopathy R. A review on microbial lipids as a potential biofuel. Bioresour. Technol. 2018;259:451–460. doi: 10.1016/j.biortech.2018.03.080. [DOI] [PubMed] [Google Scholar]
- Silambarasan S., Logeswari P., Cornejo P., Abraham J., Valentine A. Simultaneous mitigation of aluminum, salinity and drought stress in Lactuca sativa growth via formulated plant growth promoting Rhodotorula mucilaginosa CAM4. Ecotoxicol. Environ. Saf. 2019;180(1):63–72. doi: 10.1016/j.ecoenv.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Mukhopadhyay M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012;166(2):486–520. doi: 10.1007/s12010-011-9444-3. [DOI] [PubMed] [Google Scholar]
- Singh A., Prasad S.M. Remediation of heavy metal contaminated ecosystem: An overview on technology advancement. Int. J. Environ. Sci. Te. 2014;12(1):353–366. [Google Scholar]
- Sowmya Shree G., Yogendra Prasad K., Arpitha H.S., Deepika U.R., Nawneet Kumar K., Mondal P., Ganesan P. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol. Cell. Biochem. 2017;436(1):1–12. doi: 10.1007/s11010-017-3071-4. [DOI] [PubMed] [Google Scholar]
- Spagnuolo M., Yaguchi A., Blenner M. Oleaginous yeast for biofuel and oleochemical production. Curr. Opin. Biotechnol. 2017;57:73–81. doi: 10.1016/j.copbio.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Species Fungorum . http://www.speciesfungorum.org/GSD/GSDspecies.asp?RecordID=271749; 2020. Report Page: Rhodotorula mucilaginosa. [Google Scholar]
- Squina F.M., Mercadante A.Z. Influence of nicotine and diphenylamine of the carotenoid composition of Rhodotorula strains. J. Food Biochem. 2005;29(6):638–652. [Google Scholar]
- Stahl W., Sies H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta. 2005;1740(2):101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Sun W., Yang X., Wang X., Lin X., Wang Y., Zhang S., Luan Y., Zhao Z.K. Homologous gene targeting of a carotenoid biosynthetic gene in Rhodosporidium toruloides by agrobacterium-mediated transformation. Biotechnol. Lett. 2017;39(7):1001–1007. doi: 10.1007/s10529-017-2324-3. [DOI] [PubMed] [Google Scholar]
- Sukmawati D., Shabrina A., Indrayanti R., Kurniati T.H., Nurjayadi M., Hidayat I., Al Husna S.N., Ratnaningtyas N.I., El Enshasy H., Dailin D.J., Hesham A.E. Antifungal mechanism of Rhodotorula mucilaginosa and Aureobasidium sp. nov. isolated from Cerbera manghas L. against the growth of destructive molds in post harvested apples. Recent Pat. Food Nutr. Agric. 2020;11(3):219–228. doi: 10.2174/2212798411666200423101159. [DOI] [PubMed] [Google Scholar]
- Sun G.L., Reynolds E.E., Belcher A.M. Using yeast to sustainably remediate and extract heavy metals from waste waters. Nat. Sustain. 2020;3(4):1–9. [Google Scholar]
- Surman S.L., Penkert R.R., Sealy R.E., Jones B.G., Marion T.N., Vogel P., Hurwitz J.L. Consequences of vitamin A deficiency: Immunoglobulin dysregulation, squamous cell metaplasia, infectious disease, and death. Int. J. Mol. Sci. 2020;21(15):5570. doi: 10.3390/ijms21155570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepańska P., Hapeta P., Lazar Z. Advances in production of high-value lipids by oleaginous yeasts. Crit. Rev. Biotechnol. 2022;42(1):1–22. doi: 10.1080/07388551.2021.1922353. [DOI] [PubMed] [Google Scholar]
- Szotkowski M., Byrtusova D., Haronikova A., Vysoka M., Rapta M., Shapaval V., Marova I. Study of metabolic adaptation of red yeasts to waste animal fat substrate. Microorganisms. 2019;7(11) doi: 10.3390/microorganisms7110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010;91(5):1468s–1473s. doi: 10.3945/ajcn.2010.28674G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Wang Y., Zhang J., Cai Y., He Z. Biosynthetic pathway of carotenoids in Rhodotorula and strategies for enhanced their production. J. Microbiol. Biotechnol. 2019;29(4):507–517. doi: 10.4014/jmb.1801.01022. [DOI] [PubMed] [Google Scholar]
- Tkáčová J., Zara G., Ianiri G., Castoria R., Čertík M., Mannazzu I. Impairment of carotenoid biosynthesis through CAR1 gene mutation results in CoQ10, sterols, and phytoene accumulation in Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2022;106(1):317–327. doi: 10.1007/s00253-021-11673-5. [DOI] [PubMed] [Google Scholar]
- Torres-Alvarez D., León-Buitimea A., Albalate-Ramírez A., Rivas-García P., Hernández-Núñez E., Morones-Ramírez J.R. Conversion of banana peel into diverse valuable metabolites using an autochthonous Rhodotorula mucilaginosa strain. Microb. Cell Fact. 2022;21(1) doi: 10.1186/s12934-022-01834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Úbeda B., Gálvez J.Á., Michel M., Bartual A. Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 2017;228:210–217. doi: 10.1016/j.biortech.2016.12.095. [DOI] [PubMed] [Google Scholar]
- Vazquez-Rodriguez A., Vasto-Anzaldo X.G., Barboza Perez D., Vázquez-Garza E., Chapoy-Villanueva H., García-Rivas G., Garza-Cervantes J.A., Gómez-Lugo J.J., Gomez-Loredo A.E., Garza Gonzalez M.T., Zarate X., Morones-Ramirez J.R. Microbial competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of non-toxic exopolysaccharide with applications as a wide-spectrum antimicrobial. Sci. Rep. 2018;8(1):798. doi: 10.1038/s41598-017-17908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay K., Sowmya P.R.-R., Arathi B.P., Shilpa S., Shwetha H.J., Raju M., Baskaran V., Lakshminarayana R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018;118:675–690. doi: 10.1016/j.fct.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Wall M.J., Quinn A.J., D'Cunha G.B. Manganese (Mn2+)-dependent storage stabilization of Rhodotorula glutinis phenylalanine ammonia-lyase activity. J. Agric. Food Chem. 2008;56(3):894–902. doi: 10.1021/jf072614u. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang X., Zhang H., Lin K.J.F.S.T. Effects of some additives on the growth and carotenoids content of Rhodotorula. Food. Sci. Tech-Brazil. 2001;2:20–21. [Google Scholar]
- Wang C., Hsueh P., Chen F., Lee W. Breakthrough fungemia caused by Rhodotorula mucilaginosa during anidulafungin therapy. J. Microbiol. Immunol. Infect. 2019;52(4):674–675. doi: 10.1016/j.jmii.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Wang M., Ma J., Wang X., Wang Z., Tang L., Chen H., Li Z. Detoxification of Cu (II) by the red yeast Rhodotorula mucilaginosa: from extracellular to intracellular. Appl. Microbiol. Biotechnol. 2020;104(23):10181–10190. doi: 10.1007/s00253-020-10952-x. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhao Y., Jiang Y., Chu W. Prebiotic, antioxidant, and immunomodulatory properties of acidic exopolysaccharide from marine Rhodotorula RY1801. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.710668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liu Z., Jiang H., Mao X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021;111:322–332. [Google Scholar]
- Watkins J.L., Pogson B.J. Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci. 2020;25(5):501–512. doi: 10.1016/j.tplants.2019.12.021. [DOI] [PubMed] [Google Scholar]
- Wen Z., Zhang S., Odoh C.K., Jin M., Zhao Z.K. Rhodosporidium toruloides - A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020;20(5):foaa038. doi: 10.1093/femsyr/foaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick J.F., Willis J.E. Phenylalanine-dependent de novo synthesis of phenylalanine ammonia-lyase from Rhodotorula glutinis. Arch. Biochem. Biophys. 1982;216(2):385–391. doi: 10.1016/0003-9861(82)90226-0. [DOI] [PubMed] [Google Scholar]
- Wirth J., Petry N., Tanumihardjo S., Rogers L., McLean E., Greig A., Garrett G., Klemm R., Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients. 2017;9(3):190. doi: 10.3390/nu9030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman E.M., Bar-El Dadon S., Reifen R. The vicious cycle of vitamin a deficiency: A review. Crit. Rev. Food Sci. Nutr. 2017;57(17):3703–3714. doi: 10.1080/10408398.2016.1160362. [DOI] [PubMed] [Google Scholar]
- Wu Q., Zhao Y., Wang D., Xu Y. Immobilized Rhodotorula mucilaginosa: A novel urethanase-producing strain for degrading ethyl carbamate. Appl. Biochem. Biotechnol. 2013;171(8):2220–2232. doi: 10.1007/s12010-013-0493-7. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu D. Exploitation of genus Rhodosporidium for microbial lipid production. World J. Microbiol. Biotechnol. 2017;33(3):54. doi: 10.1007/s11274-017-2225-6. [DOI] [PubMed] [Google Scholar]
- Yalçın H.T., Çorbacı C., Uçar F.B. Molecular characterization and lipase profiling of the yeasts isolated from environments contaminated with petroleum. J. Basic Microbiol. 2014;54(S1):S85–S92. doi: 10.1002/jobm.201300029. [DOI] [PubMed] [Google Scholar]
- Yang K., Yang Y.Y., Jia L.Z., Lin H.Z., Wei Y., Wang J., Xia D.M., Wen X.C. Analysis on nutrition components in Rhodotorula mucilaginosa. Guangdong Agric. Sci. 2014;5:40. [Google Scholar]
- Yang J., Zhang Y., Na X., Zhao A. β-carotene supplementation and risk of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Nutrients. 2022;14(6):1284. doi: 10.3390/nu14061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurtcu E., Iseri O.D., Sahin F.I. Effects of ascorbic acid and β-carotene on HepG2 human hepatocellular carcinoma cell line. Mol. Biol. Rep. 2011;38(7):4265–4272. doi: 10.1007/s11033-010-0549-5. [DOI] [PubMed] [Google Scholar]
- Zafar S., Aqil F., Ahmad I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007;98(13):2557–2561. doi: 10.1016/j.biortech.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Zafar J., Aqeel A., Shah F.I., Ehsan N., Gohar U.F., Moga M.A., Festila D., Ciurea C., Irimie M., Chicea R. Biochemical and immunological implications of lutein and zeaxanthin. Int. J. Mol. Sci. 2021;22(20) doi: 10.3390/ijms222010910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cui J.-D. Enhancement of phenylalanine ammonia lyase production from Rhodotorula mucilaginosa by optimization of culture conditions in batch and fed-batch. Biotechnol. Biotechnol. Equip. 2012;26(6):3418–3423. [Google Scholar]
- Zhang H., Ge L., Chen K., Zhao L., Zhang X. Enhanced biocontrol activity of Rhodotorula mucilaginosa cultured in media containing chitosan against postharvest diseases in strawberries: Possible mechanisms underlying the effect. J. Agric. Food Chem. 2014;62(18):4214–4224. doi: 10.1021/jf500065n. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhang X., Tan T. Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour. Technol. 2014;157:149–153. doi: 10.1016/j.biortech.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Zhang W., Hua H., Guo Y., Cheng Y., Pi F., Yao W., Xie Y., Qian H. Torularhodin from Sporidiobolus pararoseus attenuates d-galactose/AlCl3-induced cognitive impairment, oxidative stress, and neuroinflammation via the Nrf2/NF-kappaB pathway. J. Agric. Food Chem. 2020;68(24):6604–6614. doi: 10.1021/acs.jafc.0c01892. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ran Q., Zhang X. Screening and identification of a cutinase-producing Rhodotorula mucilaginosa and properties of the cutinase. Appl. Biochem. Biotechnol. 2015;175(2):1221–1233. doi: 10.1007/s12010-014-1291-6. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhao L., Li Y., Wang F., Li S., Shi G., Ding Z. Comparative transcriptomics and transcriptional regulation analysis of enhanced laccase production induced by co-culture of Pleurotus eryngii var. ferulae with Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2020;104(1):241–255. doi: 10.1007/s00253-019-10228-z. [DOI] [PubMed] [Google Scholar]
- Zhang C., Shi C., Zhang H., Yu K., Wang Y., Jiang J., Kan G. Metabolomics reveals the mechanism of antarctic yeast Rhodotorula mucliaginosa AN5 to cope with cadmium stress. Biometals. 2022;35(1):53–65. doi: 10.1007/s10534-021-00350-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Guo L., Xia Y., Zhuang X., Chu W. Isolation, identification of carotenoid-producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Mar. Drugs. 2019;17(3):161. doi: 10.3390/md17030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Song B., Li J., Zhang J. Rhodotorula toruloides: An ideal microbial cell factory to produce oleochemicals, carotenoids, and other products. World J. Microbiol. Biotechnol. 2022;38(1):1–19. doi: 10.1007/s11274-021-03201-4. [DOI] [PubMed] [Google Scholar]
- Zhu L., Cui W., Fang Y., Liu Y., Gao X., Zhou Z. Cloning, expression and characterization of phenylalanine ammonia-lyase from Rhodotorula glutinis. Biotechnol. Lett. 2013;35(5):751–756. doi: 10.1007/s10529-013-1140-7. [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhang Y., Li Q., Yang L., Zhang N., Ma S., Zhang K., Song J., Guan F. β-carotene induces apoptosis in human esophageal squamous cell carcinoma cell lines via the Cav-1/AKT/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2016;30(3):148–157. doi: 10.1002/jbt.21773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.