Abstract
Introduction
One-fifth of emergency department presentations by ambulance are due to acute-on-chronic breathlessness. We explored the feasibility of an evaluation-phase, cluster randomised controlled trial (cRCT) of the effectiveness and cost-effectiveness of a paramedic-administered, non-pharmacological breathlessness intervention for people with acute-on-chronic breathlessness at ambulance call-out (BREATHE) regarding breathlessness intensity and conveyance to hospital.
Methods
This mixed-methods, feasibility cRCT (ISRCTN80330546) randomised paramedics to usual care or intervention plus usual care. Retrospective patient consent to use call-out data (primary end-point) and prospective patient/carer consent for follow-up was sought. Potential primary outcomes included breathlessness intensity (numerical rating scale) and conveyance. Follow-up included: interviews with patients/carers and questionnaires at 14 days, 1 and 6 months; paramedic focus groups and surveys.
Results
Recruitment was during COVID-19, with high demands on paramedics and fewer call-outs by eligible patients. We enrolled 29 paramedics; nine withdrew. Randomisation/trial procedures were acceptable. Paramedics recruited 13 patients, not meeting recruitment target (n=36); eight patients and three carers were followed-up. Data quality was good but insufficient for future sample size estimation. The intervention did not extend call-out time, was delivered with fidelity and was acceptable to patients, carers and paramedics. There were no repeat call-outs within 48 h. All trained paramedics strongly recommended BREATHE as a highly relevant, simple intervention.
Conclusion
Patient recruitment to target was not feasible during the pandemic. Training and intervention were acceptable and delivered with fidelity. Results include valuable information on recruitment, consent, attrition and data collection that will inform the design and delivery of a definitive trial.
Short abstract
A paramedic-delivered BREATHE intervention for acute-on-chronic breathlessness and study procedures were acceptable but recruitment was low (pandemic related); further work is necessary to answer remaining questions on sample size and best primary outcome https://bit.ly/3AsNQZ5
Introduction
Chronic (persistent) breathlessness – disabling despite treatment of underlying causes [1] – is prevalent in cardiorespiratory disease(s), and acute exacerbations are frightening for patients and carers. It is more common in older adults [2] with widespread impacts for patients, family carers and health systems [1–3]. Acute worsening of chronic breathlessness (acute-on-chronic breathlessness [4]) is mostly triggered by physical and/or emotional exertion but can relate to worsening of the underlying cause(s) [5]. Non-pharmacological interventions can be effective [6] and include breathing retraining, anxiety management, activity pacing [7] and cool facial airflow [8].
Severe episodes of acute-on-chronic breathlessness may be caused by a worsening of the underlying disease and/or when distress aggravates the symptom [4]. Acute-on-chronic breathlessness often triggers emergency use of health services [9]. However, one-third of these emergency department (ED) attendees do not need hospital admission and some might be avoidable with adequate community support [9]. Estimates of breathlessness as a primary reason for adult ED presentations range between 2.7% and 9.0% [10–13]. In one UK study, acute-on-chronic breathlessness was a reason for 20% of attendances conveyed by ambulance [9]. The presence and intensity of breathlessness on ED arrival predicts hospital admission [14] and subsequent presentations [15].
For many, the ED is necessary for best care. For others, the ED is less likely to be the optimal place if community-based care is working effectively [16]. Anxiety can play a significant role in people with recurrent acute-on-chronic breathlessness, and for whom targeted, community-based management plans may reduce the need for ED attendances [17].
The American Thoracic Society (ATS) consensus, whilst recognising the evidence gap in the acute setting, recommends a dual approach to acute breathlessness management [18]. Initial management should be given by first responders, using evidence-based, non-pharmacological breathlessness interventions alongside management of any underlying condition. Patients and carers should receive education and training in self-management techniques [18]. For some, an acute worsening of breathlessness can become a “teachable moment” [19] and carers may also learn techniques by observing paramedics [20]. More people with acute-on-chronic breathlessness might thereby be managed safely in the community or, if hospital admission is needed, have their breathlessness reduced more quickly.
We aimed to explore the feasibility and acceptability of conducting a definitive, cluster randomised, controlled trial (cRCT) for people with acute-on-chronic breathlessness due to medical conditions to evaluate the effectiveness and cost-effectiveness of a paramedic-administered, non-pharmacological breathlessness intervention Breathlessness RElief AT HomE (BREATHE).
Material and methods
Details of the planned methods for this mixed-methods feasibility trial are documented elsewhere [21] and summarised here with protocol amendments due to COVID-19.
Study participants
Paramedic-participants willing to undergo training in study measures, processes and the BREATHE intervention (if allocated) were recruited from Yorkshire ambulance stations. Following consent, randomisation and training, paramedics then delivered usual care or BREATHE intervention plus usual care at appropriate call-outs. Usual care was defined by the Joint Royal College Ambulance Liaison Committee (JRCALC) guidelines [22].
Eligible patients were in their usual home environment receiving an emergency response from participating paramedics because of acute-on-chronic breathlessness. They had self-reported cardiorespiratory disease, chronic breathlessness (breathless most days for ≥3 months) and gave retrospective consent for call-out data use at the end of the call-out. Patients needing immediate life-saving intervention in the paramedic's judgement were ineligible. Eligible carers were adults present at call-out to a patient-participant consenting to follow-up.
Study design
We explored the feasibility of a cRCT to evaluate the effectiveness and cost-effectiveness of a paramedic-administered non-pharmacological breathlessness intervention for people with acute-on-chronic breathlessness who have called an ambulance.
Objectives
We addressed the following uncertainties for a definitive trial:
Paramedic-participants’ and patient-participants’ recruitment and attrition rates
Randomisation and consent process: acceptability, possibility within clinical priority time constraints
Intervention: acceptability, adherence and fidelity, implementation issues (trial procedures and clinical practice), safety, contamination
Feasibility of data collection and best primary outcome
Sample size estimation using variability values for candidate primary outcomes
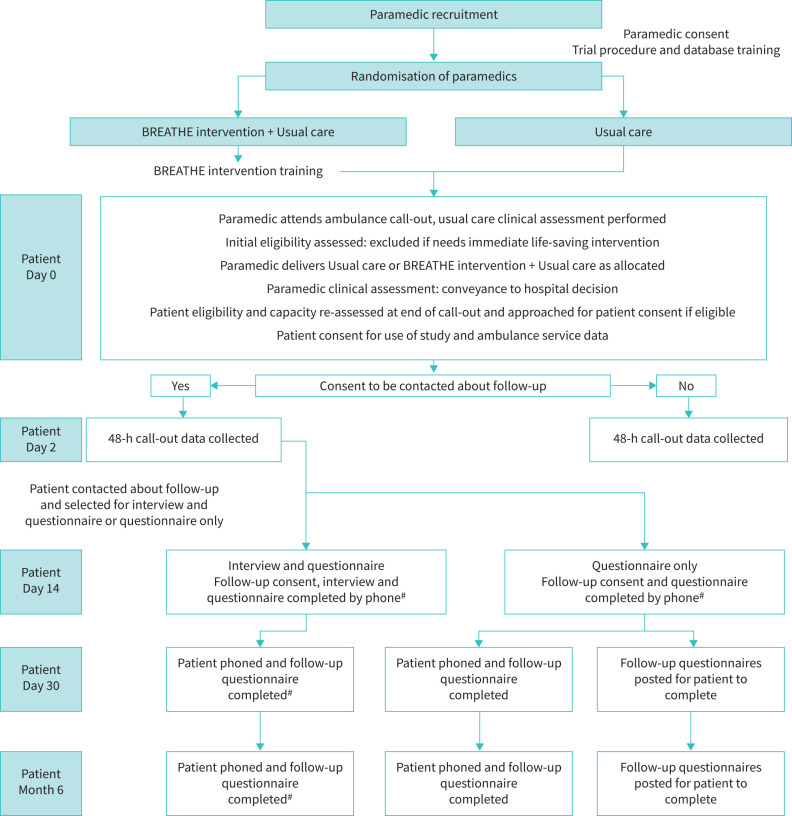
The trial procedures for patient-participants are outlined in figure 1 and table 1.
FIGURE 1.
BREATHE Study flowchart. #: indicates amendments to published protocol, trial procedures adapted due to delivery during COVID-19 pandemic.
TABLE 1.
Schedule of events for patient-participants and care-participants
| Visit | Call-out (Baseline) | 48 h | Day 14 | Day 30 | Month 6 |
| Day | 0 | 2 (±0 days) | 14 (±7 days) | 30 (±7 days) | 183 (±7 days) |
| Procedure/assessment | |||||
| Inclusion/exclusion criteria assessment | x | ||||
| Call-out informed consent | x | ||||
|
NRS 0–10 breathlessness every 2 min (patient)
0 not breathless to 10 worst breathlessness |
x | ||||
| Routinely collected paramedic data (pulse, respiratory rate, blood pressure, SpO2 with air, SpO2 with oxygen and working impression) | x | ||||
| Demographic measures (patient and carer) | x | ||||
| Index Ambulance Call-out outcome | x | ||||
| Further call-outs in 48 h after index call-out | x | ||||
| Follow-up informed consent | x | ||||
| Interview (patient and carer) | x | ||||
| Health service utilisation questionnaire (patient) | x | x | x | ||
| SF-36 (patient) | x | x | x | ||
| CRQ-dyspnoea questionnaire (patient) | x | x | X |
NRS: Numerical Rating Scale; SpO2: oxygen saturation measured by pulse oximetry; SF-36: 36-item Short-Form Health Survey; CRQ: Chronic Respiratory Questionnaire.
Sample size
As a feasibility study, a formal sample size calculation was not required. We aimed to recruit 60 patient-participants over 6 months, 30 per group, to provide sufficient data to answer our research questions [23].
Recruitment, randomisation and consent
Paramedic-participants were recruited, consented and randomised as previously described [21], with an amendment allowing electronic consent. Paramedics were randomly allocated (paramedic being the unit of randomisation) at an intervention:control ratio of 1:1 by the Hull Health Trials Unit (HHTU) using a purpose built, web-based data capture system with integrated randomisation (REDCap cloud). An independent statistician prepared the randomisation schedule with random permuted blocks of size 2–4. All researchers involved in the analysis of the quantitative data were blinded to allocation.
Patient-participants were recruited and consented at call-out, with an amendment due to pandemic restrictions allowing those who consented at call-out for further contact to be phoned to discuss follow-up, gain verbal consent and arrange Day 14 data collection.
Training
All paramedic-participants received 1-h study training on consent and study procedures, with 30-min intervention training if randomised to BREATHE. The first group was trained in-person, with an amendment due to pandemic restrictions allowing online training, and refreshers provided on request.
Data collection
Paramedics accessed REDCap cloud during call-outs via a Toughbook, their standard-issue tablet, and by researchers to input follow-up questionnaire data. A NoMAD (Normalisation Measure Development) survey was completed in REDCap by intervention paramedics. Qualitative data (online interviews and focus groups) were conducted by a researcher (AHu) using a semi-structured topic guide developed by the research team (see supplementary material), recorded and transcribed verbatim. All study-active paramedics were invited to take part in the focus groups. Patients and carers consenting to further contact were invited to take part in an interview. No participants were asked their reason for declining to take part in focus groups or interviews.
Intervention
BREATHE is described in table 2 (for evidence-based references, see protocol paper) [21] and reported in accordance with the template for intervention, description and replication (TIDieR) checklist [24] (supplementary material). Modifications to the intervention were in response to pandemic-related infection control procedures.
TABLE 2.
BREATHE intervention and usual care
| Intervention | Examples of techniques |
| BREATHE | |
| Be reassured | Reassure patient and carer; a reassuring and expert presence is sometimes sufficient to start “unwinding” escalating breathlessness |
| Resting position | Check posture; find the most comfortable and efficient position to maximise ventilation |
| Exercises (breathing) | Use to slow breathing rate and encourage breathing out to prevent air trapping (e.g. pursed lip or “breathing rectangle”). Pursed lip breathing also provides increased end-expiratory pressure |
| Airflow | Airflow across lower face/nasal passages can reduce breathlessness and recovery time The fan was not used at call-out, but recommended for future use# Use of damp cloth to cool the face# Windows opened# |
| Time | “Take it easy, nice and slow” |
| Help with fears and worries | Simple techniques to manage panic and fear |
| Education of patient/carer | Information booklet and laminated single-page leaflet about BREATHE intervention |
| Usual care | |
| Immediate clinical assessment | History, baseline vital signs and targeted examination (e.g. 12-lead ECG) |
| Reassurance | Reassurance is a mainstay of high-quality patient care |
| Oxygen | Time critical feature: oxygen saturations of 94% or less for those patients without chronic lung diseases Target range oxygen saturation in patients with chronic lung diseases: 88–92%. If SpO2 >92%, oxygen would not be administered |
| Nebuliser | Depending on the initial assessment, the paramedic may ask the patient to use their own inhalers, or proceed to nebulisation |
SpO2: oxygen saturation measured by pulse oximetry. #: indicates changes from the original protocol due to COVID-19.
Outcomes and assessments
Candidate primary outcomes were conveyance to hospital (transport of patient from their home to the hospital by ambulance) or change in breathlessness intensity measured at call-out (numerical rating score (NRS) every 2 min). Follow-up data included the 36-item Short-Form Health Survey (SF-36) and the Chronic Respiratory Questionnaire (CRQ). Participants recruited later in a funded extension (due to COVID-19) were only followed-up to 3 months. An additional paramedics’ focus group and a free text survey were conducted to gain further insight about trial experiences.
Analysis
Quantitative data were described using STATA 17 [25]. Intervention fidelity was assessed by component completion rates. Framework analysis was performed for interview, focus group and survey data informed by Normalisation Process Theory [26], managed with NVivo 12 software. Preliminary qualitative findings were discussed, then refined following open discussion with co-authors. This trial is reported consistent with relevant Consolidated Standards of Reporting Trials (CONSORT) statements [27].
Safety
At call-out paramedics were instructed to record any adverse events. A research paramedic accessed clinical records to check for repeat call-outs within 48 h of the index visit.
Ethics approval
The trial was approved by the Yorkshire and Humber-Sheffield Research Ethics Committee (Reference: 19/YH/0314) and institutional ethics committee and registered (ISRCTN80330546) prior to recruitment.
Results
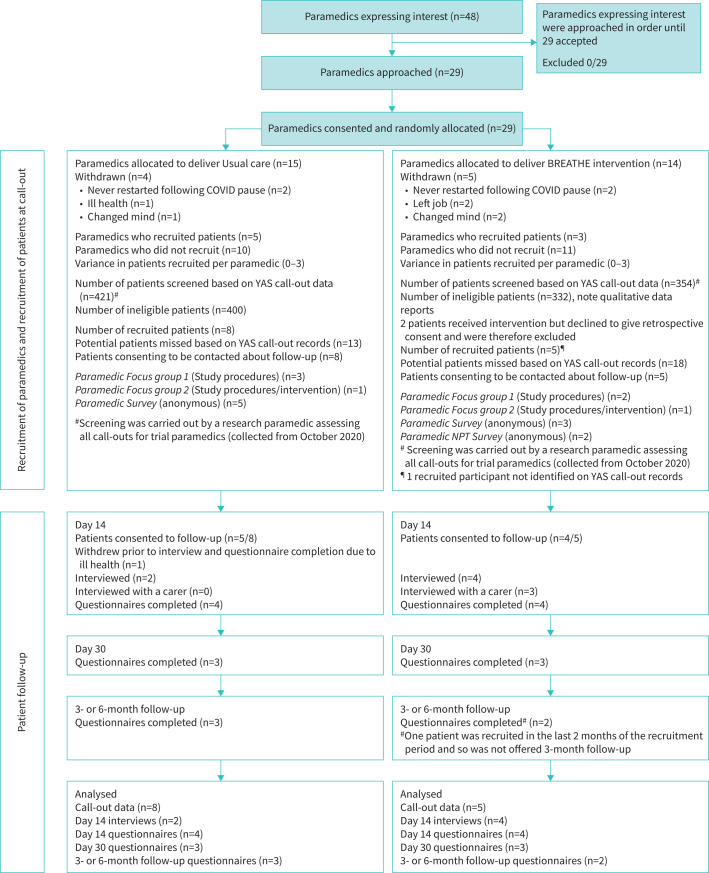
Paramedic recruitment was open between December 2019 and December 2021. Patient–participant recruitment was open for 12 months between February 2020 and June 2021 (includes 5-month COVID-19 pause); follow-up ceased in July 2021. Figure 2 details the recruitment, consent and data collection for paramedics, patient-participants and carer-participants. Quantitative data were collected at call-out for 13 patient-participants (primary end-point) and at follow-up for eight out of 13. Two paramedics completed the NoMAD survey [27]. Qualitative data were collected by interview for six patient-participants and two carer-participants and by two paramedic focus groups (n=7; n=8), and a free text survey.
FIGURE 2.
CONSORT diagram showing participant recruitment and retention throughout the trial. YAS: Yorkshire Ambulance Service.
Recruitment and retention
Paramedic (cluster) recruitment
29 paramedics were recruited; nine (31%) withdrew. Recruitment per cluster varied between zero and three.
Paramedic characteristics
Paramedic characteristics were as follows: male (52%), white (100%) and mean number of years’ experience 5 (range 1–26) years.
Patient and carer recruitment
13 patient-participants were recruited: all agreed to be contacted by a researcher about follow-up and nine (69%) consented to follow-up (one withdrew before data collection). Three carer-participants were recruited for interview. Paramedics stated they saw far fewer of our target group during the pandemic (table 3). The stop-go criterion for recruitment (≤60% target) was not met. The original recruitment period of 6 months was extended to 12 months with a funded extension of the study. Given the ongoing pandemic challenges at the end of the funded extension, the study oversight committees then agreed it was not feasible to pursue any further extension and the study was closed.
TABLE 3.
Quotes from participants
| Patient recruitment | |
| Q1 |
I agree that we normally see so many more COPD patients than we have, like when you came and gave us four packs, I was like this in't gonna be enough, I'll see four COPD patients in a week, and then COVID-19 hit. I think because we've seen so much less of them, clearly these people are managing in the community in some way, shape or form, and I don't know how that is. (ParamedicFG6) |
| Q2 | These last 18 months, it's been really difficult; I've not seen those chronic patients that I would normally see. (ParamedicFG7) |
| Intervention: patients’ and carers’ views | |
| Q3 | But some patients are just so unreceptive to help from us, like some people just think they need to be in hospital. I did them all (parts of the intervention) and he was not cooperative with any of them, but also he didn't enter the study anyway. I think a lot of COPD patients are like, ‘I don't want to go to hospital’, cos they spend their whole lives there, and so they are really receptive, but some people are just not.# (ParamedicFG6) |
| Q4 | And then they had a good talk to me and I was really distressed. All me family came, they was worried and I weren't quite all there, if you know what I mean. The paramedic, he was absolutely brilliant; he talked me through, pulled me round a bit and then after about an hour maybe I was a lot better. (Patient12) |
| Q5 | They did the right thing when they came, they sat me down and calmed the fears I had over it and gave us some good advice, yeah, it was good, thanks. (Patient11) |
| Q6 | They walked in, they were so friendly, they were very reassuring to me mum. They weren't patronising in any way, they just dealt with her but also included me, which I thought were really nice, they had time for me as well, and just chatted as well, which just reassured me mum a lot. (CarerofPatient4) |
| Q7 | Well I think it was good because they gave an immediate solution; they talked him right through what was needed to be done, but also with the written information for him to read when he felt better. Cos obviously being given lots of instructions and talking through alternative treatments, it's OK, but when you're unwell you don't absorb all the information, and to be left with really clear concise information was really good. (CarerofPatient12) |
| Q8 | I haven't used the Ventolin for two days because every time I feel like it I use that little fan and do what it says in the book, breathe in and hold it, you know, like a rectangle it is, and then breathe out. (Patient12) |
| Intervention: paramedics’ views | |
| Q9 | Opening the window and using a window to visualise the breathing is easy and effective. It calms people down and once the patient is calmer the relatives seem to take over with the coaching. (ParamedicSurvey6) |
| Q10 | I really loved the idea behind this research. (ParamedicSurvey8) |
| Q11 | If we're gonna start this intervention, we're gonna give you an alternative, instead of relying on somebody else (another clinician) that doesn't do it and we still see that patient next week, then I'm all for it. (ParamedicFG7) |
| Q12 | There was the little laminated sheet, I think that was good and I think that that's really helpful for relatives to be able to use to coach people through, because I would notice that after sorta five minutes of me coaching them they would start to step in, because it's not hard, and so I think that was effective. (ParamedicFG6) |
| Q13 | I think that coaching it and the ease of access for it, for patients to be able to do it, it might take a few minutes, but it's very doable if the patient is receptive to it. (ParamedicFG6) |
| Q14 | We usually go to a lot of breathless patients and we are making a move as a profession to try and treat more people within the community so, yeah, I definitely think it's something that's worth incorporating. (ParamedicFG7) |
| Q15 | I feel like it's definitely a worthwhile thing, because it's gonna be better for the long-term care of patients and healthcare needs to change, it needed to change cos it's not managing. So, this is something that can help with that change and help people manage on their own and that can only be a good thing. (ParamedicFG6) |
| Q16 | I think it (the intervention) is necessary for people to know, I don't think it's a specialist thing because you don't need a specialist skill set to be able to do any of it, anybody could do it. (ParamedicFG6) |
| Implementation issues | |
| Q17 | She was probably the one that was like, yes, I can take this woman, I can do it with this woman, but that was the one time where I then ended up, couldna get any access to the database at all.¶ (ParamedicFG6) |
#: did not provide retrospective consent. ¶: did not provide retrospective consent due to access issues but was prepared to join the study.
Participant characteristics
All patients recruited met the eligibility criteria. Patient-participant characteristics were as follows: male (61.5%), mean±sd age 76.4±10.7 years, from the four most deprived deciles of the Index of Multiple Deprivation (100%), lived alone (61.5%) and white (100%). The most common diagnoses were COPD (n=10 out of 13) and heart disease (n=seven out of 13). Characteristics were similar between arms. Carer-participants were adult female family members.
Acceptability of randomisation and training to paramedics
All 29 paramedics approached consented. Withdrawal was balanced across the trial arms (reasons given in figure 2). Randomisation and training (trial processes; intervention) were acceptable to all responding (qualitative data).
Feasibility and acceptability of consent processes
Qualitative data found in-person and electronic consent processes feasible, quick and acceptable to paramedics, and the two-stage consent process was acceptable and feasible to patients.
Intervention: fidelity and adherence
The intervention was delivered with fidelity and no contamination. During the pandemic, the handheld fan was discussed but not demonstrated, substituted by a damp tissue to face and/or opening a window (table 4).
TABLE 4.
Intervention fidelity and adherence
| Intervention used | n=5 |
Reasons for not doing intervention
n (reason) |
| Positions to ease breathlessness | 5 | |
| Breathing exercises | 5 | |
| The fan | 1 | 4 (COVID-19 restrictions) |
| Addressing fears and worries | 5 | |
| Go through the leaflet/action plan | 3 | 2 (not enough time for paramedic) |
| Introduce the information booklet | 2 | 1 (paperwork damaged) 2 (not enough time for paramedic) |
| Damp tissue on face | 0 | 2 (discussed) 1 (not enough time for paramedic) 1 (paramedic forgot) 1 (missing data) |
| Opening a window | 2 | 1 (not enough time for paramedic) 1 (patient already using) 1 (missing data) |
Intervention: acceptability
Qualitative and NoMAD survey data show acceptability to patients, carers and paramedics.
Intervention: patients’ and carers’ views
Patient-participants found the intervention provided them with useful techniques and resources. However, the intervention may not be acceptable to all patients; one paramedic stated she had seen a patient who engaged poorly with the intervention wishing for immediate hospital transfer (this patient was excluded because they did not give retrospective consent for data use) (see table 3). The intervention was well received by patient-participants.
Two patients did not read the information booklet or leaflet, one patient and carer read and derived benefit from both and another patient and carer read the leaflet and then dealt with two further episodes without calling an ambulance.
Intervention: paramedics’ views
NoMAD responses indicated paramedics saw potential in BREATHE. Qualitative data indicated that BREATHE was useful and easily incorporated into practice. Paramedics valued the intervention, especially improving airflow, resting positions, breathing exercises and distraction to help with anxiety. Components combined easily and helped engagement with patients (table 3). The leaflet was a useful guide for them and for carers and patients for later use. Carers got involved with breathing exercises and reassurance.
All four intervention-arm paramedics would recommend training paramedics in BREATHE to improve their skills since they see many breathless patients and it is simple to learn and to do. They felt that BREATHE would enhance the part they play in community patient care.
Some had incorporated BREATHE into practice and noted that parts of the intervention were helpful with anxious patients in general. One suggested that a GP referral post call-out would be useful for help with breathlessness management long-term.
Safety
There were no adverse events at call-out and no repeat call-outs within 48 h.
Data quality
Data collection at call-out was complete for all items of routinely collected data, except for the second temperature and pulse measurements.
Call-out
Candidate primary outcome measures
Data completion of the potential primary outcome of conveyance was 100% and for the NRS breathlessness intensity score data completion was above 75% for the control group at 6-min intervals, whereas for the intervention arm it was between 20% and 60% complete. NRS intensity scores decreased in both arms (table 5).
TABLE 5.
Numerical rating scores summary statistics (numerical rating scale of breathlessness intensity 0–10)
| Minutes from baseline | ||||||||||||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | |
| Control | ||||||||||||||||
| Mean | 6.3 | 5.0 | 5.7 | 4.9 | 4.0 | 4.3 | 3.8 | 4.3 | 3.5 | 3.3 | 3.5 | 3.5 | 3.0 | 3.0 | 3.0 | 2.7 |
| sd | 2.5 | 1.4 | 2.5 | 2.4 | 2.8 | 2.1 | 1.9 | 2.1 | 2.1 | 1.7 | 2.1 | 2.1 | 1.8 | 1.4 | 1.4 | 1.6 |
| Median | 7 | 5 | 6 | 6 | 4 | 5 | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 |
| 25th percentile | 5 | 4 | 3 | 3 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 75th percentile | 8 | 6 | 8 | 6 | 6 | 6 | 5 | 6 | 5 | 4 | 5 | 5 | 4 | 4 | 4 | 4 |
| n | 8 | 2 | 3 | 8 | 2 | 3 | 8 | 3 | 2 | 7 | 2 | 2 | 6 | 2 | 2 | 6 |
| Intervention | ||||||||||||||||
| Mean | 3.0 | 7.3 | # | 2.7 | 7.0 | 2.0 | 2.0 | 2.7 | 4.0 | 2.0 | 2.0 | 3.0 | 5.0 | # | 2.0 | 3.0 |
| sd | 0.0 | 2.1 | # | 0.6 | 1.4 | 1.4 | 1.2 | 2.0 | 2.8 | # | 1.4 | |||||
| Median | 3 | 8 | # | 3 | 7 | 2 | 2 | 2 | 4 | 2 | 2 | 3 | 5 | # | 2 | 3 |
| 25th percentile | 3 | 5 | # | 2 | 6 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 5 | # | 2 | 2 |
| 75th percentile | 3 | 9 | # | 3 | 8 | 3 | 2 | 4 | 6 | 2 | 2 | 5 | 5 | # | 2 | 4 |
| n | 2 | 3 | 0 | 3 | 2 | 2 | 1 | 3 | 3 | 1 | 1 | 2 | 1 | 0 | 1 | 2 |
#: missing data.
NRS score measurement every 6 min, but not 2 min, was acceptable to patient-participants and paramedic-participants, and they found clinical conveyance decisions acceptable. Paramedic-participants were confident in their conveyance clinical decisions. Patients interviewed found it acceptable to remain at home, where this occurred, preferring this to hospital conveyance unless necessary. This may not be the case for all in routine practice: the two (excluded because of lack of retrospective consent) reported via paramedic qualitative data insisted on conveyance.
A similar proportion in each arm were conveyed to hospital (one out of five intervention, two out of eight usual care). Though the sample size was small, our findings suggest that on-scene time for the intervention arm took no longer than controls (intervention mean: 87 min, control mean: 90 min), the intervention being incorporated into the paramedics’ routine.
Follow-up
Data collection was 100% at 14 days, 75% at 30 days and above 50% at 3 or 6 months (table 6). All health service utilisation questionnaires were fully completed. Of the SF-36 questionnaires, 14 out of 19 (74%) had data to calculate the short-form six-dimension (SF-6D) score. CRQ mastery scores could be calculated from all 19 CRQs. All data were collected by phone, taking 30–40 min. The researchers found patients had difficulty with answering the CRQ questions, which were time-consuming.
TABLE 6.
Follow-up data completion
| Intervention# | Control # | |
| Day 14 | ||
| Interview | 4 (2 with carer) | 2 |
| Health service utilisation | 4 | 4 |
| CRQ | 4 | 4 |
| SF-36 | 4 | 4 |
| Day 30 | ||
| Health service utilisation | 3 | 3 |
| CRQ | 3¶ | 3 |
| SF-36 | 3 | 3 |
| 3 months or 6 months | ||
| Health service utilisation | 2+ | 3 |
| CRQ | 2+ | 3 |
| SF-36 | 2+ | 3 |
CRQ: Chronic Respiratory Questionnaire; SF-36: 36-item Short-Form Health Survey. #: n=4; ¶: one patient partially completed the CRQ, but this still allows calculation of score; +: one patient was recruited in the last 2 months of the recruitment period and was not offered 3-month follow-up.
Implementation issues
From qualitative data, paramedics valued participation and found the intervention (as relevant) useful and acceptable. They were satisfied with intervention and trial procedure training and support. Suggestions for improvement included providing: a scenario to practice applying eligibility criteria; face-to-face and video with online training material; and regular videocalls with participating paramedics for peer support and updates on trial progression. Accessing the study database by Toughbook was problematic for some: inability to log in, poor internet access and time constraints. This led to at least one patient not being recruited into the study (table 3). Suggestions included paper case report forms (CRFs) at call-out with input to the database later, streamlining the online data entry process and database access via smart phone.
Stop-go criteria for recruitment and adherence
Recruitment stop-go criteria were not met. However, the intervention was delivered with fidelity and no contamination, and met the adherence criterion.
Sample size calculation and proposed primary outcome for a definitive trial
Although data completion was good, only 13 patients were recruited. A sample size calculation for a full trial or clarification of the best primary outcome was not possible.
Discussion
A definitive cRCT to evaluate the effectiveness and cost-effectiveness of a paramedic-administered, non-pharmacological breathlessness intervention for people with acute-on-chronic breathlessness due to medical conditions is feasible in terms of data quality, adherence, fidelity and acceptability of the intervention and acceptability of trial processes, but recruitment was not feasible to target during the pandemic. We have valuable information to inform a definitive trial, but we have insufficient data to determine a sample size, nor to identify the most appropriate primary outcome.
Most recruitment occurred under very difficult conditions (for both patients and for ambulance services) at the height of the various waves of the pandemic, with fewer call-outs to Yorkshire Ambulance Service by this particular patient population and increased demand on the service and individual paramedics.
Informing a definitive trial
It would not be possible to recruit to a definitive cRCT if patient call-out for acute-on-chronic breathlessness continued at COVID-19 pandemic rates. However, we demonstrated the acceptability of many study processes including: study and intervention training; randomisation and consent; intervention acceptability, adherence and safety; and patient-reported data collection, which informs our proposed study design adaptations.
The BREATHE intervention was simple to learn and use and acceptable to recruited participants. A future study should note how the intervention is received by patients excluded from analysis due to lack of consent; those not consenting may be those less likely to find BREATHE acceptable. The intervention needs no modification at call-out, but further primary care contact post call-out to promote sustained breathlessness management may be helpful. There were no non-conveyance safety issues and paramedics were confident in their clinical decisions.
Implications for further research
The research question remains important with ongoing distress for patients with acute-on-chronic breathlessness, and pressure on ambulance services and emergency departments; further research is needed to address this problem. Uncertainties remain about the feasibility of a future study.
We propose the following:
Include an embedded pilot to address remaining uncertainties.
Recruit from multiple NHS ambulance services.
Deliver intervention as currently described at call-out but consider triggered follow-up in primary care.
Allow both face-to-face and remote solutions for intervention training delivery.
Reduce the number of patient-reported outcomes, do not include CRQ.
Refine methods of recording data and consent at call-out.
Capture the experience of all otherwise eligible patients, e.g., Confidentiality Advisory Group approval to use call-out data without consent or use a quality improvement paradigm [28].
Given the small clusters (number of participants/paramedic), a cRCT sample size may be prohibitive. Other study designs will be considered, e.g., quasi-experimental and/or RCT using the paramedic-participant first dyad as the unit of randomisation for effectiveness.
The ADePT process [29] will be used to inform a large-scale trial design.
Strengths and limitations
The study was delivered in the NHS by usual care practitioners and in the intended setting. The use of retrospective consent ensured immediate necessary treatment. Another strength was our use of mixed-methods. Qualitative findings helped identify problems and solutions to inform a future trial.
The study had some limitations. The patient/carer sample comprised white British English speakers, and was unrepresentative of the general population. We did not recruit enough paramedics initially, adding to pandemic recruitment challenges and did not meet patient recruitment targets, nor collect sufficient data to meet all objectives. Due to COVID-19 we were unable to use the fan during call-out, which has more supportive evidence than the cold facial wipe. We kept no record of reasons for declining to take part in any aspect of the study, which may give an incomplete picture of the intervention (as well as study participation) acceptability; Suggested future study designs above would help address this.
Conclusion
Patient recruitment to target was not feasible during the COVID-19 pandemic. Training and intervention were acceptable and delivered with fidelity. Results include valuable information on recruitment, consent, attrition and data collection that will inform adaptations for the design and delivery of a definitive trial.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TIDieR checklist to describe the BREATHE intervention 00257-2022.SUPPLEMENT (83.1KB, pdf)
Semi-structured interview (patient and carer if present) 00257-2022.BREATHEInterviewTopicGuideFollow-upPatientandCarer (172KB, pdf)
Semi-structured interview/focus group with paramedics 00257-2022.BREATHEInterviewTopicGuideParamedic (91.7KB, pdf)
Acknowledgements
We wish to acknowledge the contributions of Jane Shewan, Fiona Bell, Richard Pilbery, Elisha Miller and the participating paramedics from Yorkshire Ambulance Service and Pat Hatfield (PPI representative) for her input as a co-applicant and member of the TMG. Finally, we thank Anne English for her assistance in delivery of the paramedic training sessions. We would like to thank all of the members of the trial steering committee and the stakeholder group and we are grateful for the support of Asthma and Lung UK throughout this study.
Provenance: Submitted article, peer reviewed.
This study is registered at https://www.isrctn.com with identifier number ISRCTN80330546. All of the individual participant data collected during the trial will be shared after deidentification. The study protocol paper has been published. A fully anonymised dataset will be made available to authorised researchers upon request to the corresponding author, when a data sharing agreement is in place.
Author contributions: A. Hutchinson, V. Allgar, S. Hart, A. Hodge, M.J. Johnson, S. Mason, J. Reeve, S. Griffin and F. Swan were co-applicants on the grant application. M. Northgraves, J. Cohen, V. Allgar, D.C. Currow, S. Hart, K. Hird, A. Hodge, M.J. Johnson, S. Mason, F. Swan, J. Reeve, S. Griffin and A. Hutchinson assisted in development of the protocol and implementation of the study. A. Hutchinson, M. Northgraves and M.J. Johnson drafted the manuscript. All authors read and approved the final manuscript.
Conflict of interest: A. Hutchinson reports grants from NIHR during the conduct of the study.
Conflict of interest: V. Allgar reports grants from NIHR during the conduct of the study.
Conflict of interest: J. Cohen reports grants from NIHR during the conduct of the study.
Conflict of interest: D.C. Currow reports the following. Helsinn Pharmaceuticals: advisory board member; Mayne Pharma: paid consultant and received payment for intellectual property.
Conflict of interest: S. Griffin reports grants from NIHR during the conduct of the study.
Conflict of interest: S. Hart reports grants from NIHR during the conduct of the study; and personal fees from Trevi Therapeutics, and personal fees and nonfinancial support from Chiesi and Boehringer Ingelheim, outside the submitted work. He is an associate editor of this journal.
Conflict of interest: K. Hird reports grants from NIHR, during the conduct of the study.
Conflict of interest: A. Hodge reports grants from NIHR during the conduct of the study.
Conflict of interest: S. Mason reports grants from NIHR during the conduct of the study.
Conflict of interest: M. Northgraves reports grants from NIHR during the conduct of the study.
Conflict of interest: J. Reeve reports grants from NIHR outside the submitted work.
Conflict of interest: F. Swan reports grants from NIHR during the conduct of the study.
Conflict of interest: M.J. Johnson reports grants from NIHR during the conduct of the study.
Support statement: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit Programme (Grant Reference Number PB-PG-0817-20009). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. E ur Respir J 2017; 49: 1602277. doi: 10.1183/13993003.02277-2016 [DOI] [PubMed] [Google Scholar]
- 2.Johnson MJ, Currow DC, Booth S. Prevalence and assessment of breathlessness in the clinical setting. Expert Rev Respir Med 2014; 8: 151–161. doi: 10.1586/17476348.2014.879530 [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson A, Barclay-Klingle N, Galvin K, et al. Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J 2018; 51: 1701477. 10.1183/13993003.01477-2017. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson A, Johnson MJ, Currow D. Acute-on-chronic breathlessness: recognition and response. J Pain Symptom Manage 2019; 57: e4–e5. doi: 10.1016/j.jpainsymman.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Simon ST, Higginson IJ, Benalia H, et al. Episodes of breathlessness: types and patterns – a qualitative study exploring experiences of patients with advanced diseases. Palliat Med 2013; 27: 524–532. doi: 10.1177/0269216313480255 [DOI] [PubMed] [Google Scholar]
- 6.Brighton LJ, Miller S, Farquhar M, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019; 74: 270–281. doi: 10.1136/thoraxjnl-2018-211589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spathis A, Booth S, Moffat C, et al. The Breathing, Thinking, Functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med 2017; 27: 27. doi: 10.1038/s41533-017-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luckett T, Phillips J, Johnson MJ, et al. Contributions of a hand-held fan to self-management of chronic breathlessness. Eur Respir J 2017; 50: 1700262. doi: 10.1183/13993003.00262-2017 [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson A, Pickering A, Williams P, et al. Breathlessness and presentation to the emergency department: a survey and clinical record review. BMC Pulm Med 2017; 17: 53. doi: 10.1186/s12890-017-0396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report 2010; 26: 1–31. [PubMed] [Google Scholar]
- 11.Fedullo AJ, Swinburne AJ, McGuire-Dunn C. Complaints of breathlessness in the emergency department. The experience at a community hospital. N Y State J Med 1986; 86: 4–6. [PubMed] [Google Scholar]
- 12.Langlo NM, Orvik AB, Dale J, et al. The acute sick and injured patients: an overview of the emergency department patient population at a Norwegian University Hospital Emergency Department. Eur J Emerg Med 2014; 21: 175–180. doi: 10.1097/MEJ.0b013e3283629c18 [DOI] [PubMed] [Google Scholar]
- 13.Kelly AM, Keijzers G, Klim S, et al. An Observational Study of Dyspnea in Emergency Departments: The Asia, Australia, and New Zealand Dyspnea in Emergency Departments Study (AANZDEM). Acad Emerg Med 2017; 24: 328–336. doi: 10.1111/acem.13118 [DOI] [PubMed] [Google Scholar]
- 14.Saracino A, Weiland TJ, Jolly B, et al. Verbal dyspnoea score predicts emergency department departure status in patients with shortness of breath. Emerg Med Australas 2010; 22: 21–29. [DOI] [PubMed] [Google Scholar]
- 15.Nunez S, Hexdall A, Aguirre-Jaime A. Unscheduled returns to the emergency department: an outcome of medical errors? Qual Saf Health Care 2006; 15: 102–108. doi: 10.1136/qshc.2005.016618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelinek GA, Boughey M, Marck CH, et al. “Better pathways of care”: suggested improvements to the emergency department management of people with advanced cancer. J Palliat Care 2014; 30: 83–89. doi: 10.1177/082585971403000203 [DOI] [PubMed] [Google Scholar]
- 17.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med 2014; 174: 1095–1107. doi: 10.1001/jamainternmed.2014.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mularski RA, Reinke LF, Carrieri-Kohlman V, et al. An official American Thoracic Society workshop report: assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc 2013; 10: S98–S106. doi: 10.1513/AnnalsATS.201306-169ST [DOI] [PubMed] [Google Scholar]
- 19.Lawson PJ, Flocke SA. Teachable moments for health behavior change: a concept analysis. Patient Educ Couns 2009; 76: 25–30. doi: 10.1016/j.pec.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson A. What influences the presentation of patients with chronic breathlessness to the Emergency Department? A mixed methods study. University of Hull and University of York, 2016. [Google Scholar]
- 21.Northgraves M, Cohen J, Allgar V, et al. A feasibility cluster randomised controlled trial of a paramedic-administered breathlessness management intervention for acute-on-chronic breathlessness (BREATHE). ERJ Open Res 2021; 7: 00955-2020. doi: 10.1183/23120541.00955-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joint Royal Colleges Ambulance Liaison Committee (JRCALC) and Association of Ambulance Chief Executives. 2016 UK Ambulance Services Clinical Practice Guidelines. Bridgwater, Class Professional Publishing, 2016. [Google Scholar]
- 23.Teare MD, Dimairo M, Shephard N, et al. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014; 15: 264. doi: 10.1186/1745-6215-15-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 25.StataCorp . Stata Statistical Software: Release 17. College Station, TX, StataCorp LLC, 2021. [Google Scholar]
- 26.McEvoy R, Ballini L, Maltoni S, et al. A qualitative systematic review of studies using the normalization process theory to research implementation processes. Implement Sci 2014; 9: 2. doi: 10.1186/1748-5908-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016; 2: 64. doi: 10.1186/s40814-016-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021; 374: n2061. doi: 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bugge C, Williams B, Hagen S, et al. A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse. Trials 2013; 14: 353. doi: 10.1186/1745-6215-14-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TIDieR checklist to describe the BREATHE intervention 00257-2022.SUPPLEMENT (83.1KB, pdf)
Semi-structured interview (patient and carer if present) 00257-2022.BREATHEInterviewTopicGuideFollow-upPatientandCarer (172KB, pdf)
Semi-structured interview/focus group with paramedics 00257-2022.BREATHEInterviewTopicGuideParamedic (91.7KB, pdf)