Abstract
Background:
Language characteristics of logopenic variant primary progressive aphasia (lvPPA) are well-defined; however, there is a paucity of research investigating behavioral characteristics specific to this subtype.
Objective:
To determine the relationship between language abilities and behavior across the three variants of primary progressive aphasia (PPA). We hypothesized that, associations between language and behavior (measured by the Frontal Behavioral Inventory; FBI) would vary across subtypes due to distinct neuropathologies. More specifically, we hypothesized that the severity of language and behavioral problems would be independent in semantic (svPPA) and nonfluent (nfaPPA) PPA subtypes and related in lvPPA.
Methods:
We used Spearman’s rank correlation coefficients to investigate correlations between language and behavioral scores. Total scores on the FBI-mod significantly correlated with scores on the Pyramids and Palm Trees Test (r = −0.589, p = 0.001), a Sentence Repetition test (r = −0.559, p = 0.003), and the Hopkins Assessment of Naming Actions (r = −0.550, p = 0.003) in lvPPA.
Conclusions:
Results were interpreted as evidence that individuals with lvPPA show a correlation between language scores and negative behavior scores because they do not develop negative behaviors until language deficits are severe, consistent with the common underlying cause of lvPPA—Alzheimer’s disease. In contrast, behavioral symptoms are early symptoms in semantic variant PPA and nonfluent agrammatic PPA. Such findings are crucial to clinical care because, after receiving a PPA diagnosis, patients, families and caregivers often seek prognoses specific to language and behavior, especially when considering the progressive nature of this disease.
Keywords: primary progressive aphasia, logopenic variant primary progressive aphasia, semantic variant primary progressive aphasia, nonfluent agrammatic primary progressive aphasia, language symptoms, behavior
Introduction
Primary progressive aphasia (PPA) is a language disorder defined by gradual onset, relative sparing of non-language cognitive domains, and disproportionate atrophy of the left hemisphere. This progressive deterioration of language abilities manifests across domains of word finding, word usage, comprehension, and/or sentence construction (Mesulam, 2001; 2013), and sometimes grammatical production. There are three main phenotypic clinical presentations: logopenic variant PPA (lvPPA), nonfluent agrammatic PPA (nfaPPA), and semantic variant PPA (svPPA) (Gorno-Tempini et al., 2011; Josephs et al., 2008). Although impaired naming is common across all three PPA variants (Grossman et al., 2004; Hurley et al., 2009), each PPA subtype is classified based on the distinct nature and severity of language deficits. In addition, the PPA variants differ in underlying neuropathology and patterns of atrophy.
Logopenic variant PPA (lvPPA) is defined by hesitant speech, impaired word retrieval, and repetition deficits at the phrase and sentence level. With disease progression, a generalized cognitive decline specific to the domains of language, memory, and visuospatial skills is typical, while grammar and single word comprehension are relatively preserved (Rohrer et al., 2013). Auditory-verbal short term memory impairments are considered inclusionary criteria at the early stages of disease onset, and manifest in impaired sentence repetition and phonological errors in naming (Gorno-Tempini et al., 2008; Grossman, 2010). LvPPA is commonly associated with the underlying pathology of Alzheimer’s disease (AD) (Josephs et al., 2008; Modirrousta, Price & Dickerson, 2013). With disease progression, patients with a clinical diagnosis of lvPPA may present with episodic memory impairments consistent with the underlying AD neuropathology (Grossman, 2010, Josephs et al., 2008). Imaging abnormalities in the left temporoparietal junction, left posterior perisylvian and parietal regions are typically observed (Mesulam, 2001).
Nonfluent agrammatic PPA (nfaPPA) is classified by features of effortful speech and/or agrammatic language production (Gorno-Tempini et al., 2011, Gorno-Tempini et al., 2004a; Mesulam, Wieneke, Thompson, Rogalski & Weintraub, 2012; Rogalski et al., 2011). Effortful speech presents as a disruption in rate of speech and is characterized by slow, labored production. This typically results from an articulation planning deficit, the most common of which is apraxia of speech (AOS). These patients usually present with inconsistent speech sound errors and prosodic disruption (Gorno-Tempini et al., 2011). Agrammatic speech is characterized by short, simple phrases and omissions of grammatical morphemes (Gorno-Tempini, et al., 2011). Patients with nfaPPA may become mute early in disease progression (Gorno-Tempini et al., 2006) and develop clinical features consistent with parkinsonism and related syndromes, such as corticobasal syndrome, progressive supranuclear palsy (PSP), or behavioral variant frontotemporal dementia (due to frontotemporal lobar degeneration-tau; FTLD-t) (Gorno-Tempini, Murray, Rankin, Weiner & Miller, 2004b). Tau has been identified as an underlying pathology in 70% of those with nfaPPA (Irwin, Trojanowski, & Grossman, 2013). Though nfaPPA is typically associated with a tau-positive pathology (e.g. corticobasal degeneration, PSP, or FTLD-t), non-tau pathologies that have been reported in nfaPPA include AD pathology (Alladi et al., 2007; Grossman et al., 2008; Kertesz, McMonagle, Blair, Davidson, & Munoz, 2005), FTLD-U (Knopman et al., 2005; Mesulam et al., 2008), or more specifically, frontotemporal lobar degeneration trans-activator regulatory DNA binding protein 43 (FTLD-TDP-43) (Josephs, Stroh, Dugger, & Dickson, 2009; Mackenzie et al., 2006; Snowden, Neary & Mann, 2007). Neuroimaging reveals abnormalities of the left posterior frontal region (Gorno-Tempini et al., 2004a; Josephs et al., 2006; Wilson et al., 2011) as well as atrophy in the insula, premotor, and supplementary motor areas (Gorno-Tempini et al., 2011; Josephs et al., 2008; Wilson et al., 2011).
Semantic variant PPA (svPPA) is a fluent subtype of PPA and distinctive from nfaPPA and lvPPA due to a deterioration of semantic knowledge across modalities, particularly for low frequency and less familiar items (Gorno-Tempini et al., 2011). Both impaired confrontational naming and impaired comprehension at the single word level must be present for a classification of svPPA (Gorno-Tempini et al., 2011). Other speech and language characteristics such as single word repetition, speech fluency, syntax, and motor speech remain spared (Gorno-Tempini et al., 2004a). The most common underlying disease pathology in svPPA is frontotemporal lobar degeneration-ubiquitin (FTLD-U) (Grossman et al., 2008; Kertesz et al., 2005; Knopman et al., 2005), generally the variant, FTLD-TDP-43 (Snowden et al., 2007). Less commonly, svPPA is associated with AD pathology (Alladi et al., 2007) and Pick bodies (Davies et al., 2005). This variant is associated with atrophy in ventrolateral anterior temporal lobes bilaterally, usually left greater than right (Gorno-Tempini et al., 2004a; Wilson et al., 2011).
While the language characteristics, patterns of atrophy, and underlying neuropathologies defining the three phenotypes of PPA have been described extensively, the behavioral characteristics unique to each of these conditions, particularly lvPPA, have been investigated to a lesser extent, and relationships between language and behavioral characteristics have not been explored. In general, neuropsychiatric symptoms in patients diagnosed with lvPPA are infrequent early on, but increase with disease progression to include apathy, anxiety, irritability, and agitation with a maintenance of self-awareness (Rohrer, 2010; Rosen et al., 2006; Van Langenhove, Leyton, Piguet, & Hodges, 2016). Similarly, neuropsychiatric symptoms are reported to be infrequent in the early stages of nfaPPA, (Modirrousta et al., 2013; Neary, Snowden, Gustafson, 1998). Although, behaviors such as apathy, agitation, loss of empathy and depression, in addition to limited self-awareness of comportment changes, become apparent with disease progression (Rohrer & Warren, 2010; Eslinger et al., 2005). Unlike lvPPA and nfaPPA subtypes, behavioral symptoms in svPPA manifest relatively early in disease progression (Modirrousta et al., 2013) and include emotional distance, irritability, and disruption of physiologic drives (Seeley et al., 2005). Loss of empathy, change in appetite, presence of compulsive behaviors and disinhibition have also been noted in svPPA with disease advancement (Modirrousta et al., 2013; Seeley et al., 2005).
Few studies have compared behavioral manifestations of the individual variants of PPA. Banks and Weintraub (2009) compared patient and caregiver reports of behavior on the Frontal Behavioral Inventory (FBI) in 16 individuals with PPA, 10 individuals with behavioral variant frontotemporal lobar degeneration (bvFTLD), and 23 individuals with AD. The FBI is a caregiver questionnaire originally designed to capture behavioral impairments in individuals with bvFTLD (Blair et al., 2007, Kertesz, Davidson, & Fox, 1997) and changes in such behaviors over time (Kertesz, Blair, McMonagle, & Munoz, 2007; Marczinski, Davidson, & Kertesz, 2004). The FBI interrogates deficit/negative behaviors (i.e. apathy, aspontaneity, indifference, inflexibility) and disinhibition/positive behaviors (i.e. perseveration/obsession, irritability, excessive jocularity, poor judgment) on a 4 point Likert scale (0=no change in behavior/no symptom, 3=important change/severe symptoms). The original version requires affirmative and negative responses to indicate presence of a symptom (e.g. Apathy: Has s/he lost interest in friends or activities, or is s/he interested in seeing people and doing things). Banks and Weintraub (2009) found that patients with bvFTLD had overall reduced insight compared to PPA patients. PPA patients, however, demonstrated worse awareness specific to behavioral symptoms while the bvFTLD groups had intact insight into some language symptoms.
In another study, the FBI was used to investigate behavioral disturbances in 30 individuals with AD and 87 with FTLD, including 19 with nfaPPA and svPPA (Konstantinopoulous, Aretouli, Ioannidis, Karacostas, & Kosmidis, 2013). On most items of the FBI, individuals with PPA had higher (more pathological) ratings than those with AD, but lower scores than those with bvFTLD. PPA patients had the highest ratings on language related items, such as logopenia, verbal apraxia and comprehension, compared to those with AD and bvFTLD. Across non-language items (e.g. aspontaneity, inflexibility, disorganization, attention and hoarding), PPA and bvFTLD patients scored similarly.
In 2007, Heidler-Gary and colleagues modified the FBI so only one affirmative question is asked about each symptom to achieve a more efficient interview and used this adaptation to investigate whether the FBI-mod could distinguish between AD and FTLD subtypes. In a study of 30 individuals with AD and 50 with FTLD (including 25 with bvFTD, 13 with nfaPPA and 12 with svPPA), results revealed significant between group differences on the FBI-mod negative behavior scores, disinhibition scores and total score. Individuals with a diagnosis of bvFTD yielded the most pathological FBI-mod scores. NfaPPA and svPPA scores were relatively normal on the FBI-mod as compared to other groups, although there were deficits across other domains of testing.
A recent study by our group employed the FBI-mod to discriminate between distinct subtypes of PPA and individuals with a diagnosis of mild cognitive impairment (MCI) (Tippett et al., 2017). Results from this study differentiated PPA from MCI. Both PPA and MCI groups of similar age and duration post symptom onset had higher scores for negative behaviors (i.e. apathy, aspontaneity, indifference, inflexibility) than for disinhibition (i.e. perseveration, irritability, excessive jocularity, poor judgment). More severe personal neglect was demonstrated in svPPA and nfaPPA groups than lvPPA. More severe judgment impairments distinguished nfaPPA from the lvPPA group. While Tippett et al. (2017) found differential behavioral results for the PPA subtypes, language performance was not considered, nor were relationships between language assessment scores and behavioral measures compared.
In the current study, our objective was to investigate correlations between language abilities and behavioral presentations across the three variants of PPA. This is an important consideration because while aphasia is the primary cause of impaired function in these patients, neuropsychiatric symptoms, including distress, sadness, and apathy with secondary changes in aberrant motor behavior, agitation, disinhibition, irritability, and fluctuations in appetite routine are significantly disabling (Fatemi et al., 2011; Mesulam, 1982; Modirrousta, et al., 2013). Investigation of potential relationships between language abilities and behavioral manifestations may yield information that can enable health care providers to provide more informed prognoses and education to patients with PPA and their families and caregivers.
In this study, we hypothesized that variants of PPA would differ with regard to relationships between scores on language tests and changes in behavior and comportment measured with the FBI-mod because of the distinct neuropathologies associated with each variant. Specifically, we hypothesized that language and behavior would be correlated in lvPPA with disease progression, and that language and behavior in nfaPPA and svPPA would not be correlated because behavior changes are manifested early in disease course when language abilities are still relatively spared.
Methods
Participants
Prior to initiation of the study, the data collection, review and analysis were approved by the Johns Hopkins Medicine Institutional Review Board. All participants or their spouses provided written informed consent and agreed to participate. Candidates for inclusion were individuals with PPA. Fifty-eight individuals with PPA (mean age = 69.19 ± 8.02 years; 29 female; mean education =15.68 ± 2.65 years; average disease duration = 39.88 ± 30.18 months) were enrolled, including 24 with lvPPA (mean age = 69.13 ± 8.53; mean education = 15.58 ± 2.43; average disease duration = 42.43 ± 39.20), 19 with nfaPPA (mean age = 71.42 ± 6.58; mean education = 15.84 ± 3.02; average disease duration = 38.79 ± 25.68), and 15 with svPPA (mean age = 66.47 ± 8.48; mean education = 15.64 ± 2.64; average symptom duration=37.13 ± 17.84). Nine of the 19 nfaPPA patients presented with a clinical diagnosis of apraxia of speech (AOS). These individuals were evaluated in one author’s (AEH) outpatient cognitive neurology clinic. Participants were diagnosed with PPA based on a presentation of predominant and progressive deterioration in language abilities in the absence of major change in personality, behavior, or cognition other than praxis (Mesulam, 1982). PPA subtype was diagnosed by an experienced behavioral neurologist (AEH) based on medical history, comprehensive neurological examination, imaging, and a battery of cognitive and language tests. Patients were classified using consensus criteria for each variant (Gorno-Tempini et al., 2011).
Language and Behavioral Assessment
We focused on language components that are inclusionary criteria and/or commonly impaired for one or more of the three variants of PPA (Gorno Tempini, et al., 2011): auditory comprehension (word level), semantic knowledge, repetition (sentence level), and naming (nouns and verbs). We measured performance on five language tests: Semantic Word Picture Matching (an auditory comprehension task), Pyramids and Palm Trees Test, short form (PPTT; Breining et al., 2015b), Sentence Repetition, Hopkins Assessment of Naming Actions (HANA; Breining et al., 2015a); and Boston Naming Test, short form (BNT; Mack et al, 1992). The Semantic Word Picture Matching and Sentence Repetition, are from the Frontotemporal Lobar Degeneration-National Alzheimer’s Coordinating Center neuropsychological battery (Weintraub, S. et al., 2009; Version 2.0, November 2011).
The Semantic Word Picture Matching subtest evaluates spoken word comprehension at the single word level. Participants were asked to point to a picture matching the orally presented stimulus. Visual stimuli consist of five displays of pictures containing a set of four pictures of semantically related objects. For example, a set of four pictures: witch, ghost, pumpkin, and bat, were presented and the participant was asked to point to the picture matching the verbal stimulus “ghost.” The five displays were each presented four times (once with each picture as the target) for a total of 20 trials. The location of the target picture was counterbalanced across all of the trials and the order of presentation was pseudo-randomized across all trials. The total score was the sum of correctly identified pictures (0–20).
On the short form of the PPTT, participants were asked to identify a line drawing of an object semantically related to target pictured objects on the PPTT (score range 0 – 14). Fourteen target pictures were presented along with two additional pictures. Participants were asked to point to the picture related to the target. For example, the target picture of eye glasses (spectacles) was presented along with pictures depicting an eye and an ear. Participants decided which picture best relates in meaning to the target picture (eye glasses).
In the Sentence Repetition subtest, patients were asked to repeat a sentence exactly as the clinician said it. One repetition by the clinician was permitted if the patient did not hear the sentence, and explicitly asked for a repetition. Patient responses were scored based on total number of completely accurate sentences (0–5).
On the HANA, participants were instructed to name 35 line drawings of actions (e.g., run, spill, whisper), which are matched in frequency to the items on the BNT. If participants named an object in the test picture, they were re-instructed to name actions. Total score was based on a total number of correct responses (0–35).
On the short form of the BNT, participants were asked to verbally name 30 line drawings of objects (score range 0 – 30). Objects ranged from high familiarity items, such as “bed,” to low frequency items, such as “sphinx.” If a participant experienced difficulty naming a pictured object, a phonemic cue was provided; however, these responses were not included in the total correct.
Family members and/or caregivers of these individuals with PPA completed the FBI-mod (Heidler-Gary et al., 2007). Informants were instructed to rate the extent of behavioral change for each symptom across two scored domains: negative behaviors and disinhibition. Responses were rated on a Likert scale as “0” or “none,” “1” or “mild,” “2” or “moderate,” or “3” or “severe.” Caregivers were asked to base their responses on the extent of behavioral change for each item since the onset of symptoms. Negative behaviors included apathy, aspontaneity, indifference/emotional flatness, inflexibility, personal neglect, disorganization, inattention, loss of insight, and logopenia. Disinhibition included perseveration/obsessions, irritability, excessive jocularity, poor judgment, hoarding, inappropriateness, impulsivity, restlessness, aggression, hyperorality, hypersexuality, utilization behavior, and incontinence. A question accompanied each symptom to elucidate that behavior (e.g., Apathy: Has s/he lost interest in friends or daily activities?). Total scores, negative behavior scores, and disinhibition scores were calculated. Percent scores were calculated for each group by dividing the total scores by the total possible score for negative behavior (i.e. 27) and disinhibition (i.e. 39).
Data Analysis
Spearman’s rank correlation coefficients (rs) were calculated for language test scores and total FBI-Mod scores for all groups. We applied Bonferroni corrections for multiple comparisons; therefore an alpha level of p= 0.003 was considered statistically significant.
Results
Table 1 shows the age, gender, education, and disease duration for the groups. Disease duration was defined as number of months between participants and/or caregivers first noticing symptoms and the language/behavioral assessment. The groups were not significantly different across these characteristics. Table 2 summarizes language test scores. Table 3 and Figure 1 summarize FBI-mod scores.
Table 1:
Age, Gender, Education, and Disease Duration for PPA subtypes and for Participants Overall
| Variant | Age (yrs) (mean, SD) |
Gender (F) N (%) |
Education (yrs) (mean, SD) |
Disease Duration (mos) (mean, SD) |
|---|---|---|---|---|
| lvPPA (n=24) |
69.13 (8.53) | 15 (63) | 15.58 (2.43) | 42.43 (39.20) |
| nfaPPA (n=19) |
71.42 (6.58) | 7 (37) | 15.84 (3.02) | 38.79 (25.68) |
| svPPA (n=15) |
66.47 (8.48) | 7 (47) | 15.64 (2.64) | 37.13 (17.84) |
|
Overall
(n=58) |
69.19 (8.02) | 29 (50) | 15.68 (2.65) | 39.88 (30.18) |
| P values* | 0.203 | 0.237 | 0.951 | 0.852 |
F, female; SD, standard deviation; yrs, years; mos, months; lvPPA, logopenic primary progressive aphasia; nfaPPA, nonfluent agrammatic primary progressive aphasia; svPPA semantic variant primary progressive aphasia;
p values were calculated using one-way ANOVA for age, education, and disease duration and using chi square for gender.
Table 2:
Language Test Scores for PPA subtypes and for Participants Overall
| Variant | Semantic Word Picture Matching (mean, SD) |
PPT (mean, SD) |
Sentence Repetition (mean, SD) |
HANA (mean, SD) |
BNT (mean, SD) |
|---|---|---|---|---|---|
| lvPPA (n=24) |
19.25 (2.38) | 13.17 (1.61) | 2.50 (1.77) | 14.33 (9.21) | 14.42 (8.02) |
| nfaPPA (n=19) |
19.47 (1.50) | 13.84 (0.37) | 3.47 (1.95) | 21.00 (8.67) | 20.11 (8.92) |
| svPPA (n=15) |
17.93 (2.91) | 10.73 (3.35) | 3.13 (1.36) | 9.13 (7.94) | 7.00 (6.24) |
| Overall (n=58) |
18.98 (2.34) | 12.76 (2.32) | 2.98 (2.34) | 15.17 (9.73) | 14.36 (9.27) |
BNT, Boston Naming Test, short form, normal = 27.5 ± 2.4; Mack et al., 2002; HANA, Hopkins Assessment of Naming Actions, short form, normal = 23.92 ± 3.46; Breining et al., in preparation; PPT, Pyramids and Palm Trees Test, short form, normal = 13.9 ± 0.24, Breining et al., 2015b; SD, standard deviation; lvPPA, logopenic primary progressive aphasia; nfaPPA, nonfluent agrammatic primary progressive aphasia; svPPA semantic variant primary progressive aphasia
Table 3:
Frontal Behavioral Inventory Scores for Negative Behaviors, Disinhibition, and Total Scores for PPA subtypes and for Participants Overall
| Variant | FBI-mod Negative Behavior Score: mean (SD), [percent] |
FBI-mod Disinhibition Score: mean (SD), [percent] |
Total FBI-mod Score: mean (SD), [percent] |
|---|---|---|---|
| lvPPA (n=24) |
10.50 (5.21) [39] | 4.58 (5.21) [12] | 15.17 (7.67) [23] |
| nfaPPA (=19) |
8.21 (5.86) [30] | 4.11 (4.29) [11] | 12.32 (9.06) [19] |
| svPPA (n=15) |
14.13 (6.67) [52] | 10.80 (8.09) [28] | 24.93 (13.61) [38] |
| Overall (n=58) |
10.66 (6.16) [39] | 6.03 (5.98) [15] | 16.76 (10.99) [25] |
FBI-mod, Modified Frontal Behavioral Inventory; SD, standard deviation; lvPPA, logopenic primary progressive aphasia; nfaPPA, nonfluent agrammatic primary progressive aphasia; svPPA semantic variant primary progressive aphasia
Figure 1:
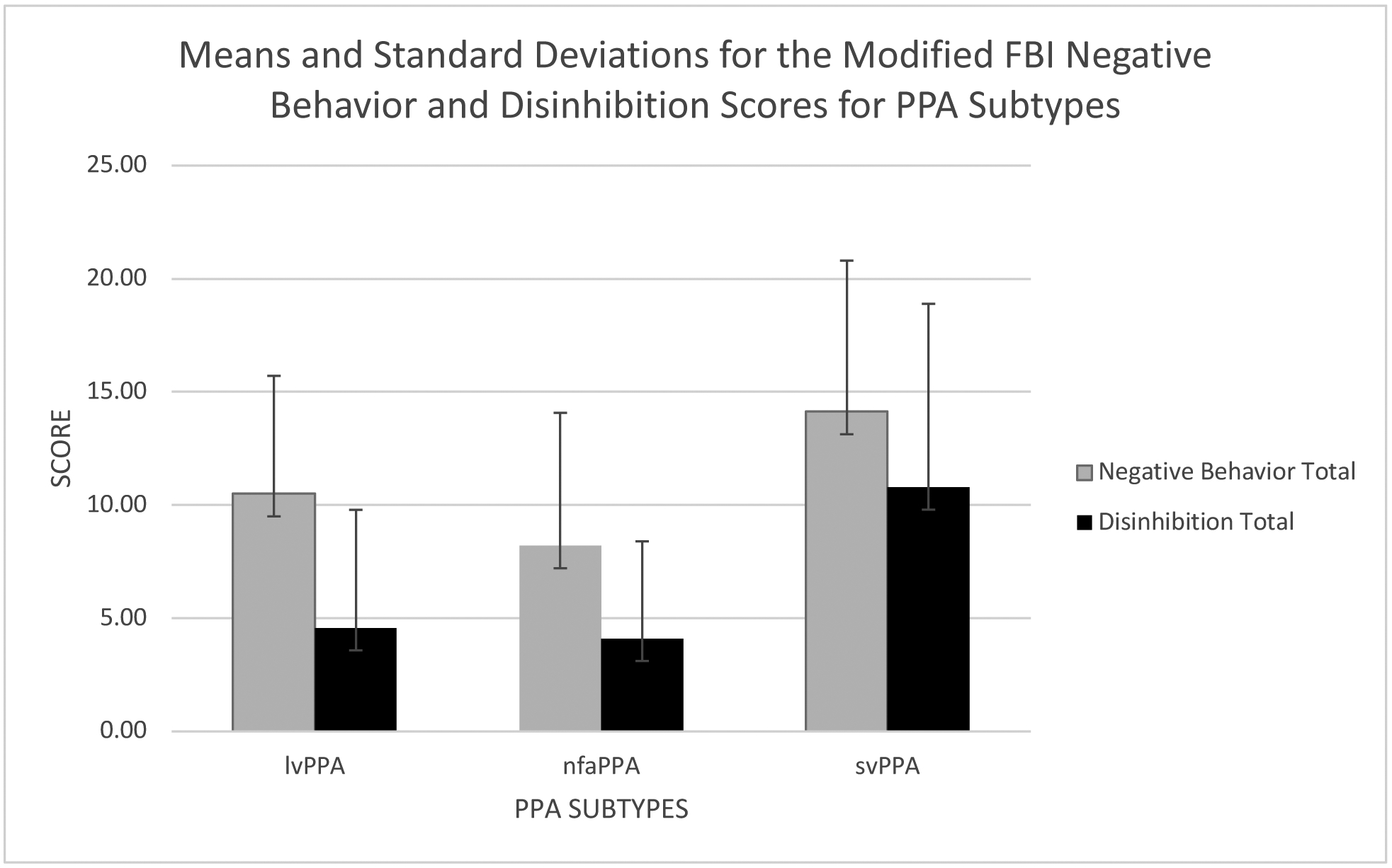
Means and standard deviations for modified FBI negative behavior and disinhibition scores for PPA subtypes
lvPPA, logopenic primary progressive aphasia; nfaPPA, nonfluent agrammatic primary progressive aphasia; svPPA semantic variant primary progressive aphasia
For the lvPPA group, total FBI-mod scores significantly negatively correlated with PPTT scores (r = −0.589, p = 0.001), Sentence Repetition scores (r = −0559, p = 0.003), and HANA scores (r = −0.550, p = 0.003). Table 4 summarizes correlations of language test scores and FBI-mod scores for each PPA variant. Figure 2 displays significant correlations for the lvPPA group with language test scores converted to z scores for comparison purposes. There were no significant correlations between language scores and total FBI-mod scores for the nfaPPA and svPPA groups.
Table 4:
Spearman Correlation Coefficients (rs) between Language Tests and Total Frontal Behavioral Inventory Scores for the PPA Subtypes
| lvPPA | nfaPPA | svPPA | ||||
|---|---|---|---|---|---|---|
| rs | P values* | rs | P values* | rs | P values* | |
| Semantic Word Picture Matching | −0.358 | 0.043 | 0.116 | 0.319 | −0.360 | 0.094 |
| Pyramids and Palm Trees Test | −0.589 | 0.001 | −0.370 | 0.060 | −0.657 | 0.004 |
| Sentence Repetition | −0.559 | 0.003 | −0.098 | 0.343 | 0.055 | 0.422 |
| Hopkins Assessment of Naming Actions | −0.550 | 0.003 | −0.356 | 0.067 | −0.538 | 0.019 |
| Boston Naming Tests | −0.352 | 0.046 | −0.366 | 0.062 | −0.143 | 0.306 |
lvPPA, logopenic primary progressive aphasia; nfaPPA, nonfluent agrammatic primary progressive aphasia; svPPA semantic variant primary progressive aphasia;
p values were calculated using Spearman rank-order correlation; Bonferroni corrected/adjusted p value = 0.003
Figure 2:
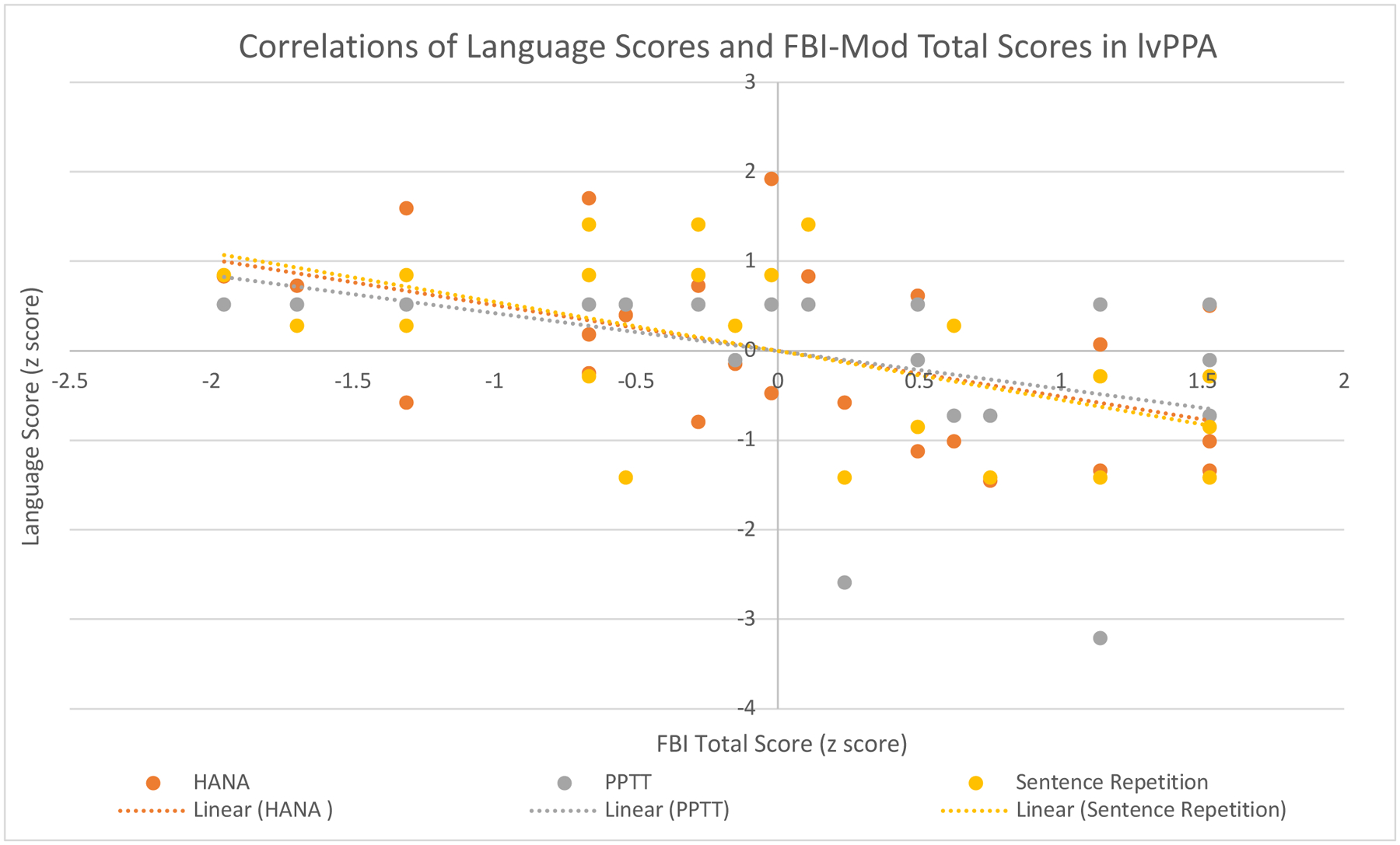
Correlations of Language Scores and FBI Total Scores in lvPPA
lvPPA, logopenic primary progressive aphasia; HANA, Hopkins Action Naming Assessment; PPTT, Pyramids and Palm Trees Test
Discussion
This study unveiled a novel finding that language and behavioral disturbances are correlated in lvPPA, but not other PPA subtypes. Specifically, we identified significant negative correlations between total FBI-mod and each of three domains (language repetition, semantic knowledge, and action naming). Results can be accounted for by proposing that individuals with lvPPA show significant correlations between language scores and total FBI-mod scores, because they do not develop negative behaviors until their language deficits are severe. Negative correlations suggest that higher language scores indicate lower (less severe behavior) scores on the FBI-mod. This is an important finding because it suggests that in the early stages of lvPPA, language is still largely intact and behavior disturbances are mild or nonexistent. With disease progression, language performance deteriorates in a manner consistent with the clinical characteristics of lvPPA and behavioral changes become evident (indicated by higher FBI-mod scores). The indications from our results and the known onset of behavioral disturbance in later and more severe stages of lvPPA are consistent with the underlying cause of lvPPA— AD (Josephs et al., 2008; Modirrousta et al., 2013). In contrast, nfaPPA and svPPA develop negative behaviors and disinhibition behaviors reported by caregivers, even when their language deficits are mild, which is consistent with the underlying cause of these variants, FTLD-TDP43, FTLD-t or related tau-opathies (Grossman et al., 2008; Kertesz et al., 2005; Knopman et al., 2005; Mesulam et al., 2008; Snowden et al., 2007). In AD, memory problems, rather than behavioral disturbances, are predominant manifestations (Levy, Miller, Cummings, Fairbanks, & Craig, 1996; Lindau et al., 2000), whereas in bvFTLD, which shares the same underlying pathologies as nfaPPA and svPPA, behavioral and neuropsychiatric symptoms are commonly present early in disease progression (Levy et al., 1996; Snowden et al., 2001). Thus, the underlying pathophysiology of the PPA variants influences these correlations.
Our findings are consistent with previous studies showing that behavioral changes appear to be common in patients who exhibit core features of svPPA and nfvPPA, even before language is severely impaired (e.g. Harris et al., 2016). In this study, we do not find correlations between language and behavior in svPPA and nfaPPA, yet FBI-mod scores (indicating the presence and severity of comportment changes) for each variant are consistent with the literature. In a study of svPPA, comportment abnormalities were present early and increased with disease progression (Rosen et al., 2006). Banks and Weintraub (2008) used the Neuropsychiatric Inventory to compare caregiver reported behavioral symptoms in bvFLTD and PPA. Their results demonstrated that patients with bvFTD had more symptoms than the PPA group, but patients with PPA with long duration had similar symptoms to the bvFTD group. Another study implemented the Neuropsychiatric Inventory and found that individuals with svPPA demonstrated early onset of increased socioemotional behavioral dysfunction (i.e. increased disinhibition, deviant motor behavior and eating disorders) as compared to lvPPA, nfaPPA, and AD (Modirrousta et al., 2013). Behavioral profiles of lvPPA and nfaPPA did not differ from each other or from AD in type or severity of dysfunction. A longitudinal study specific to behavior in PPA revealed significantly more behavioral disturbances in svPPA as compared to other PPA variants based on the Cambridge Behavioral Inventory-Revised (Van Langenhove et al., 2016). Behavioral changes in svPPA were similar to those observed in bvFTD. No differences in anxiety, irritability, apathy, perseveration, hyperorality, or abnormal motor behaviors were noted between svPPA and nfaPPA (Gómez-Tortosa et al., 2016). Patients with svPPA have been found to have increased damage to the uncinate fasciculus. Damage to this pathway is associated with a wide range of behavioral disturbances: apathy, impulsivity, and irresponsibility (D’Anna et al., 2016).
Macoir and colleagues (2017) identified the presence of impaired executive function and associated neuropsychiatric symptoms across all variants of PPA in a longitudinal report of a patient with svPPA. The case study revealed an emergence of behavioral changes in conjunction with frontal atrophy and is consistent with findings of D’Anna et al. (2016). These studies acknowledge that while executive function and behavioral changes are not inclusion criteria for any variant of PPA, it is important to identify behavioral and non-language cognitive changes with disease progression to aid in clinical care and management (Macoir et al., 2017).
While most studies of behavioral changes in PPA have focused on those observed in svPPA (and less in nfaPPA), the behavioral manifestations of lvPPA have been less well described, especially in relation to language abilities. Funayama et al. (2013) reported the clinical course of three individuals with lvPPA who eventually developed apraxia (typically associated with nfaPPA) and semantic memory deficits (typically associated with svPPA) as they progressed to the advanced stages of their disease. We also previously found that semantic knowledge remained intact until late in disease course of lvPPA, with less than one point decline per month on the PPTT (Sebastian et al., 2018). None of these previous studies have identified the correlations between behavioral decline and language decline in lvPPA.
Identifying correlations (and the absence of correlations) between language abilities and behavioral manifestations in each PPA variant is crucial to clinical care, patient support, and caregiver education. After receiving a PPA diagnosis, families want to know what they can expect, especially when considering the progressive course of PPA. It is important for clinicians to be able to describe the behavioral and language based changes to come and explain that some behavioral changes appear earlier in some variants compared to others. Understanding typical language and behavioral trajectories, and their temporal relationships, in each PPA variant enables clinicians to counsel patients, families, and caregivers about anticipated difficulties. Early counseling regarding behavioral management is essential in nfaPPA and svPPA, whereas this may be a topic that is deferred during initial counseling in lvPPA, particularly if language deficits are mild. Quantifiable behavioral inventories such as the FBI and Hypersensory and Social/Emotional Scale (HSS) questionnaire (Midorikawa et al., 2016) can supplement caregiver communication with healthcare providers and assist in delineating a plan for future care. Preparation for behavioral manifestations may facilitate caregiver coping and compensations to promote engagement in home and community life.
Limitations of this study include the cross-sectional design, self-reported symptom onset, and use of a single caregiver-reporting assessment tool (FBI-mod). Our scope of behavioral data is limited to five language tests as we did not investigate correlations between other language tests and the FBI-mod. Further study across other behavioral inventories and populations is needed. Another limitation is that our sample sizes are fairly small. We attempted to limit spurious associations by applying a Bonferroni correction. However, replication of this investigation with larger sample sizes is indicated. In addition to determining the reliability of this linguistic-behavioral correlation, there is a need for continued research specific to the lvPPA which is the least consistently defined across studies.
Despite its limitations, our study provides novel evidence that in lvPPA (but not other PPA variants), behavioral and comportment changes increase as language scores decline, indicating that behavioral changes increase with disease severity. In nfaPPA and svPPA, behavioral and comportment changes appear to be independent of language decline, and may arise even before language substantially declines.
Funding
This study was supported by research grants from the National Institutes of Health / National Institute on Deafness and Other Communication Disorders (NIDCD): R01DC005375, P50 DC011739, and by a research grant from the National Institutes of Health/National Institute of Deafness and Communication Disorders (NCDCD) and the National Institute on Aging: R01DC011317.
Footnotes
Disclosures
Argye E. Hillis, MD, MA receives compensation from AHA for editorial activities for Stroke and Elsevier for editorial activities for Practice Update Neurology.
References
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, & Hodges JR (2007). Focal cortical presentations of Alzheimer’s disease. Brain, 130, 2636–2645. [DOI] [PubMed] [Google Scholar]
- Banks S, & Weintraub S (2008). Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and primary progressive aphasia. J Geriatr Psychiatry Neurol. 2008; 21(2): 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, & Weintraub S (2009). Generalized and symptom-specific insight in behavioral variant frontotemporal dementia and primary progressive aphasia. Journal of Neuropsychiatry and Clinical Neuroscience, 21, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair M, Kertesz A, Davis-Faroque N, Hsiung GYR, Black SE, Bouchard RW, … .& Feldman H (2007). Behavioral measures in frontotemporal lobar dementia and other dementias: The utility of the Frontal Behavioral Inventory and the Neuropsychiatric Inventory in a national cohort study. Dementia and Geriatric Cognitive Disorders, 23, 406–415. [DOI] [PubMed] [Google Scholar]
- Breining BL, Tippett DC, Davis C, Posner J, Sebastian R, Oishi K, … .& Hillis AE (2015a, May). Assessing dissociations of object and action naming in acute stroke. Paper presented at the Clinical Aphasiology Conference, Monterey, CA. [Google Scholar]
- Breining BL, Lala T, Martínez Cuitiño M, Manes F, Peristeri E, Tsapkini K, … .& Hillis AE (2015b). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29(4), 488–505. [Google Scholar]
- D’Anna L, Mesulam MM, Thiebaut de Schotten M, Dell’Acqua F, Murphy D, Wieneke C, … .& Catani M (2016). Frontotemporal networks and behavioral symptoms in primary progressive aphasia. Neurology, 86(15), 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, & Xuereb J,H (2005). The pathological basis of semantic dementia. Brain, 128, 1984–1995. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Anatani S, Hauck R, Grossman M Metacognitive deficits in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2005;76(12): 1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi Y, Boeve BF, Duffy J, Petersen RC, Knopman DS, Cejka V, … .& Geda YE (2011). Neuropsychiatric aspects of primary progressive aphasia. Journal of Neuropsychiatry and Clinical Neurosciences, 23, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama M, Nakagawa Y, Yamaya Y, Yoshino F, Mimura M, & Kato M (2013). Progression of logopenic variant primary progressive aphasia to apraxia and semantic memory deficit. BMC Neurology, 13, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Tortosa E, Rigual R, Prieto-Jurczynska C, Mahillo-Fernández I, Guerrero-López R, Pérez-Pérez J, & Sainz MJ (2016). Behavioral evolution of progressive semantic aphasia in comparison with nonfluent aphasia. Dementia and Geriatric Cognitive Disorders, 41(1–2), 1–8. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, … .& Miller BL (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ , … .& Miller BL (2004a). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … .& Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, & Miller BL (2004b). Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: A case report. Neurocase, 10, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Ogar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, … . & Miller BL (2006). Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology, 67, 1849–1851. [DOI] [PubMed] [Google Scholar]
- Grossman M Primary progressive aphasia: Clinicopathological correlations. (2010). Nature Reviews Neurology, 6(2), 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, & Gee J (2004). What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain, 127, 628–649. [DOI] [PubMed] [Google Scholar]
- Grossman M, Xie SX, Libon DJ, Wang X, Massimo L, Moore P, … .& Trojanowski JQ (2008). Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology, 70, 2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Jones M, Gall C, Richardson AMT, Neary D du Plessis D, … .& Mann DM (2016). Co-occurrence of language and behavioural change in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders, 6, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler-Gary J, Gottesman R, Newhart M, Chang S, Ken L, & Hillis AE (2007). Utility of behavioral versus cognitive measures in differentiating between subtypes of frontotemporal lobar degeneration and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 23, 184–193. [DOI] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Wieneke CA, Weintraub S, Thompson CK, Federmeier KD, & Mesulam M-M (2009). Electrophysiology of object naming in primary progressive aphasia. Journal of Neuroscience, 29, 15762–15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Trojanowski JQ, & Grossman M (2013). Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer’s disease. Frontiers in Aging Neuroscience, 5, 6. 10.3389/fnagi.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, … .& Petersen RC (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Stroh A, Dugger B, & Dickson DW (2009). Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathologica, 118, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, … .& Petersen RC (2008). Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology, 70, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Blair M, McMonagle P, & Munoz DG (2007). The diagnosis and course of frontotemporal dementia. Alzheimer Disease and Associated Disorders, 21, 155–163. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, & Fox H (1997). Frontal Behavioral Inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences, 24, 29–36. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, & Munoz DG (2005). The evolution and pathology of frontotemporal dementia. Brain, 128, 1996–2005. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boeve BF, Parisi JE, Dickson DW, Smith GE, Ivnik RJ, … .& Petersen RC (2005). Antemortem diagnosis of frontotemporal lobar degeneration. Annals of Neurology, 57, 480–488. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulou E, Aretouli E, Ioannidis P, Karacostas D, & Kosmidis MH (2013). Behavioral disturbances differentiate frontotemporal lobar degeneration subtypes and Alzheimer’s disease: Evidence from the Frontal Behavioral Inventory. International Journal of Geriatric Psychiatry, 28, 939–946. [DOI] [PubMed] [Google Scholar]
- Levy ML, Miller BL, Cummings JL, Fairbanks LA, & Craig A (1996). Alzheimer disease and frontotemporal dementias: Behavioral distinctions. Archives of Neurology, 53, 687–690. [DOI] [PubMed] [Google Scholar]
- Lindau M, Almkvist O, Kushi J, Boone K, Johansson SE, Wahlund LO, …& Miller BL (2000). First symptoms: Frontotemporal dementia versus Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 11, 286–293. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, & Henderson VW (1992). Boston naming test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology, 47, 154–158. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, … .& Mann DM (2006). Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathologica, 112, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoir J, Lavoie M, Laforce R, Brambati S, Wilson M (2017) Dysexcutive symptoms in primary progressive aphasia: beyond diagnostic criteria. Journal of Geriatric Psychiatry, 30(3), 151–161. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Davidson W, & Kertesz A (2004). A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cognitive and Behavioral Neurology, 17, 185–190. [PubMed] [Google Scholar]
- Mesulam M-M (1982). slowly progressive aphasia without generalized dementia. Annals of Neurology, 11, 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M (2001). Primary progressive aphasia. Annals of Neurology, 49, 425–432. [PubMed] [Google Scholar]
- Mesulam M-M (2013). Primary progressive aphasia and the language network: The 2013 H. Houston Merritt Lecture. Neurology, 81, 456–462. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, … .& Bigio EH (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology, 63(6):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke C, Thompson C, Rogalski E, & Weintraub S (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135, 1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa A, Leyton C, Foxe D, Landin-Romero R, Hodges J, & Piguet O (2016). All is not lost: positive behaviors in Alzheimer’s disease and behavioral-variant frontotemporal dementia with disease severity. Journal of Alzheimers Disease, 54: 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Price BH, & Dickerson BC (2013). Neuropsychiatric symptoms in primary progressive aphasia: phenomenology, pathophysiology, and approach to assessment and treatment. Neurodegenerative Disease Management, 3, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS., Gustafson L, Passant U, Stuss D, Black S, . … &Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998; 51(6): 1546–1554. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, & Mesulam M-M (2011). Anatomy in language impairments in primary progressive aphasia. Journal of Neuroscience, 31, 3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, … .& Gorno-Tempini ML (2013). Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and Language, 127, 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD., Warren JD. Phenomenology and anatomy of abnormal beahaviours in primary progressive aphasia. J Neurol Sci. 2010; 293(1–2): 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, … .& Gorno-Tempini ML (2006). Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology, 67, 1752–1756. [DOI] [PubMed] [Google Scholar]
- Sebastian R, Thompson CB, Wang N-Y, Wright A, Meyer A, Friedman RB, … Tippett DC (2018). Patterns of decline in naming and semantic knowledge in primary progressive aphasia. Aphasiology. 32, 1010–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, & Rosen HJ (2005). The natural history of temporal variant frontotemporal dementia. Neurology, 64, 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, & Neary D (2001). Journal of Neurology, Neurosurgery, and Psychiatry, 70, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J, Neary D, & Mann D (2007). Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathologica, 114, 31–38. [DOI] [PubMed] [Google Scholar]
- Tippett DC, Thompson CB, Demsky C, Sebastian R, Wright A, & Hillis AE (2017). Differentiating between subtypes of primary progressive aphasia and mild cognitive impairment on a modified version of the Frontal Behavioral Inventory. PLoS ONE, 12(8), e0183212. 10.1371/journal.pone.0183212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove T, Leyton CE, Piguet O, & Hodges JR (2016). Comparing longitudinal behavior changes in the primary progressive aphasias. Journal of Alzheimers Disease, 53(3), 1033–42. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford N, Chui H, Cummings J, DeCarli, Foster N, Galasko D, Peskind E, Dietrich W, Beekly D, Kukull W, Morris J The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The Neuropsychological Test Battery. Alzheimer Dis Assoc Disorder. 2009; 23(2): 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, … .& Gorno-Tempini ML (2011). Syntactic processing depends on dorsal language tracts. Neuron, 72, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]


