Abstract
The lytA-encoded autolysin (N-acetylmuramoyl-l-alanine amidase) of Streptococcus pneumoniae is believed to play an important role in the pathogenesis of pneumococcal infection and has been identified as a putative vaccine target. Allelic diversity of lytA in an extensive collection of clinical isolates was assessed by restriction fragment length polymorphism and confirmatory sequencing studies. Genetic diversity within lytA is limited, especially compared to the high levels of diversity seen in other pneumococcal virulence factor genes, although small blocks generating mosaic structure were identified. Sequence comparisons with genes encoding cell wall lytic enzymes of pneumococcal bacteriophage suggest that localized recombination events have occurred between host lytA and these bacteriophage genes. These results confirm earlier suggestions that recombination between DNA encoding bacteriophage autolytic enzymes and chromosomally encoded lytA might be important in the evolution of lytA. The implications of these findings for understanding the evolution of lytA and the potential utility of LytA as a vaccine target are discussed.
The lytA-encoded major autolysin (N-acetylmuramoyl-l-alanine amidase) of Streptococcus pneumoniae is a member of a widely distributed group of cell wall-degrading enzymes located in the cell envelope and postulated to play roles in a variety of physiological functions associated with cell wall growth, wall turnover, and cell separation in microorganisms (27). The pneumococcal autolysin has a modular organization; the catalytic function is located in the N-terminal domain, and the C-terminal domain, composed of six repeat units and a short tail, acts as a binding arm attaching the enzyme to the choline residues of pneumococcal cell walls (5). Many bacteriophage infecting pneumococci also possess cell wall lytic enzymes which can show high similarity to either or both domains of the host lytA. In recent years it has become clear that these cell wall lytic enzymes provide one of the clearest examples among prokaryotic proteins of a two-domain structure whereby similarity between bacteriophage and bacterial DNA allows shuffling of domains by recombination restructuring both viral and bacterial genomes (9, 17).
Although there remains some controversy regarding the importance of autolysin in pathogenesis, both direct and indirect roles in the pathogenic process have been postulated. Autolysin may play a direct role in virulence by mediating the release of cell wall components shown to be highly inflammatory in animal models (29, 30). In addition, it has been suggested that autolysin plays an indirect role in pathogenesis by mediating cell lysis and the subsequent release of virulence factors, such as pneumolysin, not actively exported from the cell (12, 20). In support of a role for lytA in virulence, isogenic lytA mutants have been found to be significantly less virulent than the parent strain in some animal models (1, 2), and when inoculated into the mouse lung in a model of pneumonia, lytA mutants are cleared rapidly and do not invade the bloodstream (3). However, there are contradictory reports claiming no role for autolysin in virulence (28). Findings that mice immunized with autolysin survived significantly longer than control mice following intranasal challenge identified autolysin as a possible vaccine candidate (1, 15). However, the degree of protection was similar to that seen in those immunized with pneumolysin, with no increased protection apparent in animals immunized with both pneumolysin and autolysin. In association with data showing that survival time was not increased in animals challenged with a pneumolysin-negative strain, these findings indicate that at least in the mouse model, antibodies against autolysin appear to mediate their effects primarily by preventing the release of pneumolysin. In contrast, in a chinchilla otitis media model, autolysin induced release of cell wall components plays a key role in middle ear inflammation whereas pneumolysin appeared to have a limited role (26). A recent study using a signature-tagged mutagenesis approach to facilitate a large-scale identification of virulence-associated genes appeared to demonstrate an important role for autolysin in establishing pneumonia, while intraperitoneal inoculation of the same mutant demonstrated no role for autolysin in septicemia (23). Thus, there remains some controversy about the relevance of autolysin in pathogenesis, with the relative contribution of particular virulence factors appearing to vary between both different disease states and different animal models (22).
As part of a systematic study investigating the allelic variation of virulence determinants of S. pneumoniae (8) examining both the molecular evolution and the potential utility of these proteins as vaccine targets, we have performed a detailed analysis of the genetic diversity of lytA. Little is known about the allelic diversity of lytA in pneumococci, although the gene from an atypical clinical isolate (101/87) shows only 81% identity with lytA (6). However, recent studies in our laboratory, involving extensive sequencing of housekeeping genes, have shown that strain 101/87 is genetically distant from clinical isolates of typical pneumococci (31). A recent study, based on single-strand conformational polymorphism (SSCP) analysis of a small number of clinical isolates, suggested that lytA is a heterogeneous gene subject to continual variation (11). This was in contrast to preliminary data obtained by us which showed only five closely related alleles of lytA in a limited collection of strains (32). Here we confirm and extend our findings and report on both restriction fragment length polymorphism (RFLP) and nucleotide sequencing studies which demonstrate that in contrast to many other genes encoding virulence factors of S. pneumoniae, lytA is a rather highly conserved gene.
MATERIALS AND METHODS
Purification of chromosomal DNA.
Chromosomal DNA was purified as described previously (33) from 62 strains of S. pneumoniae selected to represent a diverse range of isolates in terms of serotype, clinical association, and time and place of isolation (Table 1).
TABLE 1.
Allelic profiles of bacterial isolates used in this study as determined by RFLP of lytA
| Strain | City and/or country of isolation | Source | Date of isolation | Serotype | Allelic profile with indicated restriction enzyme:
|
Overall lytA profile | |||
|---|---|---|---|---|---|---|---|---|---|
| RsaI | BsrI | AciI | Hsp92II | ||||||
| Selected for lytA sequencing | |||||||||
| 494 | Liverpool, UKa | NKa | 1995 | 3 | 1 | 1 | 1 | 1 | 1 |
| 7751 | Spain | NK | NK | 6 | 1 | 1 | 1 | 1 | 1 |
| 670 | Spain | NK | 1988 | 6B | 1 | 1 | 1 | 1 | 1 |
| PN8 | Oldham, UK | NK | 1987 | 23 | 1 | 1 | 1 | 1 | 1 |
| Pn107 | Oxford, UK | NK | 1995 | 1 | 1 | 1 | 1 | 2 | 2 |
| CL2 | Spain | Vagina | 1987 | 1 | 1 | 1 | 1 | 2 | 2 |
| Pn58 | Spain | NK | 1993 | 19A | 2 | 1 | 1 | 2 | 3 |
| VA1 | USa | NK | 1983 | 19 | 1 | 2 | 1 | 3 | 4 |
| 472 | Leicester, UK | NK | NK | 3 | 1 | 1 | 1 | 4 | 5 |
| 29044 | Czechslovakia | NK | 1987 | 14 | 1 | 1 | 1 | 4 | 5 |
| 860 | NK | NK | 1994 | NK | 1 | 1 | 1 | 4 | 5 |
| CL18 | Kenya | Blood | 1991 | 10 | 3 | 1 | 1 | 2 | 6 |
| 1012 | Manchester, UK | Throat | 1993 | 35 | 1 | 1 | 2 | 1 | 7 |
| PN15 | Papua New Guinea | NK | 1969 | 12 | 1 | 1 | 1 | 5 | 8 |
| 233 | Poland | Throat | 1995 | 23F | 1 | 1 | 3 | 4 | 9 |
| 234 | Poland | Throat | 1995 | 23F | 1 | 1 | 3 | 4 | 9 |
| Others | |||||||||
| PN109 | Middlesbrough, UK | Ear | 1995 | 1 | 1 | 1 | 1 | 1 | 1 |
| 969 | Manchester, UK | Throat | 1993 | 3 | 1 | 1 | 1 | 1 | 1 |
| 940 | Oxford, UK | Throat | 1995 | 3 | 1 | 1 | 1 | 1 | 1 |
| 886 | Kenya | Throat | 1991 | 3 | 1 | 1 | 1 | 1 | 1 |
| PN112 | Cambridge, UK | Ear | 1995 | 3 | 1 | 1 | 1 | 1 | 1 |
| 1002 | Manchester, UK | Throat | 1994 | 3 | 1 | 1 | 1 | 1 | 1 |
| PN111 | Ashford, UK | Sputum | 1995 | 3 | 1 | 1 | 1 | 1 | 1 |
| CL4 | NK | NK | NK | 4 | 1 | 1 | 1 | 1 | 1 |
| CL5 | Spain | Blood | 1988 | 4 | 1 | 1 | 1 | 1 | 1 |
| PN12 | Papua New Guinea | NK | 1992 | 6 | 1 | 1 | 1 | 1 | 1 |
| CL31 | Spain | Peritoneal | 1993 | 15 | 1 | 1 | 1 | 1 | 1 |
| CL42 | Spain | Sputum | 1988 | 19 | 1 | 1 | 1 | 1 | 1 |
| CL45 | Spain | Blood | 1988 | 20 | 1 | 1 | 1 | 1 | 1 |
| SP1 | Spain | Blood | 1988 | 23 | 1 | 1 | 1 | 1 | 1 |
| Pn24 | Spain | NK | NK | 23 | 1 | 1 | 1 | 1 | 1 |
| Pn25 | Spain | NK | NK | 23 | 1 | 1 | 1 | 1 | 1 |
| 967 | Oxford, UK | Throat | 1994 | 35F | 1 | 1 | 1 | 1 | 1 |
| Pn16 | Papua New Guinea | NK | 1970 | 42 | 1 | 1 | 1 | 1 | 1 |
| Pn11 | UK | NK | 1988 | NK | 1 | 1 | 1 | 1 | 1 |
| PN108 | Oxford, UK | NK | 1995 | 1 | 1 | 1 | 1 | 2 | 2 |
| 900 | Oxford, UK | Throat | 1994 | 1 | 1 | 1 | 1 | 2 | 2 |
| 951 | Oxford, UK | Throat | 1994 | 6A | 1 | 1 | 1 | 2 | 2 |
| 990 | Manchester, UK | Throat | 1994 | 10 | 1 | 1 | 1 | 2 | 2 |
| CL21 | Kenya | Sputum | 1991 | 11 | 1 | 1 | 1 | 2 | 2 |
| CL25 | Kenya | NK | 1991 | 12 | 1 | 1 | 1 | 2 | 2 |
| CL26 | Spain | Blood | 1988 | 13 | 1 | 1 | 1 | 2 | 2 |
| PN13 | Papua New Guinea | NK | 1973 | 14 | 1 | 1 | 1 | 2 | 2 |
| 912 | Oxford, UK | Throat | 1995 | 15B | 1 | 1 | 1 | 2 | 2 |
| PN60 | Spain | NK | NK | 19A | 1 | 1 | 1 | 2 | 2 |
| 1011 | Manchester, UK | Throat | 1994 | 23 | 1 | 1 | 1 | 2 | 2 |
| PN27 | NK | NK | NK | 35 | 1 | 1 | 1 | 2 | 2 |
| 9858 | Brighton, UK | NK | 1988 | NK | 1 | 1 | 1 | 2 | 2 |
| R6 | US | NK | ca. 1930 | NTa | 1 | 1 | 1 | 2 | 2 |
| PN5 | Brighton, UK | NK | 1988 | NK | 1 | 1 | 1 | 2 | 2 |
| PN110 | Norwich, UK | Sputum | 1995 | 3 | 1 | 1 | 1 | 4 | 5 |
| CL44 | Kenya | NK | 1991 | 7 | 1 | 1 | 1 | 4 | 5 |
| CL13 | Spain | Blood | 1988 | 8 | 1 | 1 | 1 | 4 | 5 |
| 873 | Kenya | Throat | 1990 | 8 | 1 | 1 | 1 | 4 | 5 |
| 998 | Manchester, UK | Throat | 1994 | 9 | 1 | 1 | 1 | 4 | 5 |
| 880 | Kenya | Throat | 1990 | 13 | 1 | 1 | 1 | 4 | 5 |
| CL35 | Spain | Vagina | 1987 | 16 | 1 | 1 | 1 | 4 | 5 |
| CL43 | Kenya | Sputum | 1991 | 19 | 1 | 1 | 1 | 4 | 5 |
| CL41 | Kenya | Throat | 1991 | 19 | 1 | 1 | 1 | 4 | 5 |
| PN6 | Brighton, UK | NK | 1988 | NK | 1 | 1 | 1 | 4 | 5 |
| CL20 | Kenya | Blood | 1991 | 10 | 3 | 1 | 1 | 2 | 6 |
| 878 | Kenya | Throat | 1990 | 10F | 1 | 1 | 2 | 1 | 7 |
UK, United Kingdom; US, United States; NK, not known; NT, nontypeable (acapsular).
PCR analysis.
A lytA PCR product was amplified by using primers lytAup (5′ GGAGTAGAATATGGAAATTGATGTGAGTAA 3′ and lytAdn 5′ TTTATTTTACTGTAATCAAGCCATCTGGCTC 3′), corresponding to the extreme 5′ and 3′ regions of the lytA coding sequence. PCR conditions used were 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, repeated for 30 cycles.
RFLP analysis.
Approximately 5 μl of PCR product was digested with restriction enzymes according to the manufacturer’s instructions in a total volume of 25 μl. Digests were then separated on 4 and 8% polyacrylamide gels and visualized under UV illumination following staining for 15 min in ethidium bromide (0.3 μg ml−1).
Direct sequencing of PCR products.
A fraction of the PCR products used in RFLP analysis were purified by passage through QiaQuick PCR product purification columns and sequenced directly, using both primers lytAup and lytAdn and a series of internal primers. Sequencing was performed with an ABI 373 system.
Sequence analysis.
Preliminary sequence analysis was performed with the DNAStar package. Comparisons of polymorphic sites and calculations of the ratio of synonymous to nonsynonymous change were constructed by using the MEGA package (14).
Nucleotide sequence accession numbers.
The sequences of the lytA alleles have been submitted to GenBank and assigned accession no. AJ243399 to AJ43414.
RESULTS AND DISCUSSION
Assessment of allelic diversity of lytA by RFLP.
Chromosomal DNA was purified from 62 strains of S. pneumoniae selected to represent a diverse range of isolates in terms of serotype, clinical association, and time and place of isolation (Table 1). A PCR product representing the entire lytA gene was successfully amplified from each chromosomal DNA preparation. Allelic diversity of lytA was then assessed by digesting each PCR product independently with four frequently cutting restriction enzymes, RsaI, BsrI, AciI, and Hsp92II, resulting in coverage of at least 10% of the lytA sequence. The numbers of distinct alleles detected with each of these restriction enzymes were three, two, three, and five, respectively resulting in nine distinct overall allelic profiles of lytA (Table 1). The vast majority of isolates (85.5%) possessed one of the three most common alleles, lytA1, lytA2, or lytA5, with all other alleles present in no more than two isolates (Table 2). By applying the equation of Nei and Li (21) to the RFLP data, it is possible to obtain an estimate of the genetic diversity between alleles, which ranged from a minimum of 0.13% (between lytA5 and lytA8) to a maximum of 2.31% (between lytA4 and lytA6), indicating that genetic diversity in lytA is rather limited.
TABLE 2.
Distribution of lytA allelic variants as determined by RFLP
| Allele | No. (%) of isolates |
|---|---|
| lytA1 | 23 (37.1) |
| lytA2 | 17 (27.4) |
| lytA3 | 1 (1.6) |
| lytA4 | 1 (1.6) |
| lytA5 | 13 (21.0) |
| lytA6 | 2 (3.2) |
| lytA7 | 2 (3.2) |
| lytA8 | 1 (1.6) |
| lytA9 | 2 (3.2) |
Sequencing of lytA RFLP allelic variants confirms limited genetic diversity.
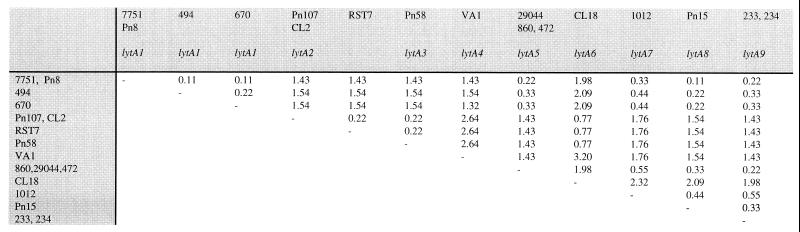
To confirm our understanding of the extent and nature of lytA genetic diversity, a PCR product representative of each of the nine RFLP allelic variants was sequenced directly. In addition, multiple representatives of the most common alleles (lytA1, lytA2, and lytA5) and lytA9 were sequenced to examine diversity within an allele as defined by RFLP analysis (Table 1). As illustrated in Fig. 1, sequencing directly confirmed the limited sequence diversity suggested by RFLP analysis. Sequence diversity ranges from a minimum of 0.11% (equivalent to one base difference) to a maximum of 3.20% between lytA4 and lytA6. The previously published lytA sequence from strain Rst7 (10) is also included in Fig. 1 for comparison; this sequence represents a distinct allele on the basis of one polymorphism not seen in any other sequence. Isolates which are largely geographically, temporally, and clinically distinct but found to possess the same RFLP allele are also closely related in terms of sequence. The lytA sequences of the three lytA5-, two lytA2-, and two lytA9-containing strains are identical, while a maximum of two base substitutions are seen between the four lytA1-containing strains sequenced. The extent of genetic diversity is broadly similar to that estimated by RFLP, and sequences are entirely consistent with the profiles obtained for each enzyme by RFLP analysis.
FIG. 1.
Percent nucleotide divergence of lytA sequences as determined by direct sequencing. The previously published sequence from strain Rst7 (10) is included for comparison. Where more than one strain is shown in a column, the lytA sequences of these strains were found to be identical. The allele designations refer to alleles identified by RFLP.
Mosaic structure of lytA resulting from localized recombination with pneumococcal bacteriophage.
Comparison of only sites polymorphic between the sequences, shown in Fig. 2, reveals that genetic variation in lytA is not randomly distributed. Three apparent blocks of diversity can be seen at bases 441 to 465 in lytA2, lytA3, lytA6, and the previously published Rst7 sequence, bases 681 to 699 in lytA4, and bases 747 to 758 in lytA6. The remaining 19 (<50%) polymorphic sites are scattered throughout the remaining ca. 95% of the lytA sequence. The predicted amino acid sequences of each allelic variant, also shown in Fig. 2, illustrate that the vast majority of the nucleotide variation seen in lytA is synonymous. The proportion of synonymous changes per synonymous site (0.0347 ± 0.008) to nonsynonymous changes per nonsynonymous site (0.0036 ± 0.0012) calculated by using the Jukes-Cantor correction in the MEGA suite of programs (14) approaches 10:1, implying that purifying selection is preferentially eliminating amino acid changes.
FIG. 2.
Illustration of the diversity of lytA alleles showing only the polymorphic sites in both nucleotide and predicted amino acid sequence alignments. Numbering begins at the first residue of the ATG start codon, although residues 1 to 20 and 922 to 951 of lytA were not sequenced in this study since they correspond to the sequences of the PCR primers. Residues identical to those in strain 7751 are indicated by dots. Numbers below the nucleotide sequence represent codon positions of the changes. Allele designations refer to alleles identified by RFLP.
The mosaic distribution of polymorphic sites suggests that very localized recombination events may have occurred, resulting in the “pock-marked” structure of the lytA genes in some pneumococci. In light of previous reports (5, 7, 9, 25), likely donors of DNA in such recombination events are genes of pneumococcal bacteriophage encoding cell wall lytic enzymes which share considerable sequence similarity with lytA. We therefore compared the available sequences of these bacteriophage genes with the mosaic lytA genes. Figure 3 shows an alignment of the polymorphic sites, comparing the lytA sequences of strains CL18 and VA1 containing putative blocks with the amidase genes hbl and ejl of the bacteriophage HB-3 and EJ-1, respectively (7, 25). The sequences are compared with that of the lytA gene of 7751 as a background strain which does not appear to have a mosaic structure. In general, the bacteriophage genes show divergence from the pneumococcal lytA sequences throughout their entire length. However the polymorphic sites which define the blocks described above for both CL18 and VA1 correspond almost perfectly with the sequence of the equivalent region of one or other of the bacteriophage genes. Thus, the CL18 block from bp 441 to 462 is identical to the equivalent region in ejl, the CL18 block from bp 753 to 758 is identical to sequence of hbl, and the VA1 block from bp 681 to 699 is also identical with the equivalent sequence of hbl. Although these blocks of identity are small, they contrast strongly with the variation seen between the bacteriophage and bacterial sequences over the rest of the alignment and strongly suggest that localized recombination events with bacteriophage DNA have been involved in the evolution of the pneumococcal lytA gene in nature. The presence of these blocks was confirmed by using the maximum chi-squared procedure with sites polymorphic over the potential recombinant strain and both potential parent strains (e.g., CL18/EJ-1/7751) to test the statistical significance of the observed mosaic structure (18). Both the larger blocks (CL18 block at bp 441 to 462 and VA1 block at bp 681 to 699) reach statistical significance (P < 0.0001).
FIG. 3.
Alignment of polymorphic sites in lytA alleles with mosaic structure represented by strains CL18 and VA1 in comparison with an allele containing no blocks represented by 7751 and the amidase genes of pneumococcal bacteriophage HB-3 (hbl) and EJ-1 (ejl). Numbering begins at the first residue of the ATG start codon, although residues 1 to 20 and 922 to 951 of lytA were not sequenced in this study since they correspond to the sequences of the PCR primers.
Molecular evolution of lytA.
In summary, our data show that lytA is essentially a rather conserved gene displaying limited genetic variation (0.11 to 3.2%). If the putative recombinant blocks are discounted from sequence comparisons, lytA genes from all strains except VA1 differ from that seen in strain 7751 by only one to three base changes. Even the maximal seven base changes seen in VA1 compared to 7751 (excluding recombinant blocks) is equivalent to variation of only 0.8%, which is similar to that reported for pneumococcal housekeeping genes (34). However, there is substantial evidence that localized recombination events with bacteriophage have occurred in the evolution of pneumococcal lytA. These findings confirm earlier reports that recombination with resident bacteriophage autolytic genes may play a role in driving the evolution of lytA. Shuffling of distinct C- and N-terminal domains is thought to have played a role in the evolution of this gene family (5, 9), and the potential for recombination to occur between bacteriophage DNA and lytA was demonstrated in the laboratory by the repair of a number of distinct point mutations following transformation with a plasmid containing the hbl gene (25). However, evidence for the occurrence of such small localized recombination events seen in clinical isolates of pneumococci not subject to laboratory manipulations has not been reported previously. It is also of interest that one of the mosaic blocks (identified in strain CL18) displays most similarity with the equivalent region of ejl from bacteriophage EJ-1. This bacteriophage was isolated from the atypical pneumococcal isolate (101/87) and reported to be unable to infect any of the typical pneumococcal strains tested (7).
Even with the occurrence of recombination, the variation seen in lytA is substantially lower than that reported for other putative pneumococcal virulence factor genes such as those encoding PspA (4, 19), immunoglobulin A protease (16, 24), or NanA (8, 13) which have been shown to be highly heterogeneous, with up to 30% diversity at the nucleotide level. The data presented here contrast with previous assertions (11), based on a small SSCP study, that lytA is a heterogeneous gene “subject to continual variation.” We suspect that the existence of these small recombinant blocks, illustrated in Fig. 3, resulted in SSCP providing a misleading picture of the evolution and overall genetic diversity of lytA. Gillespie et al. (11) sequenced only one small fragment deemed variable by SSCP. However, in support of our hypothesis, many of the changes reported in their study were also seen in this study (using a much more extensive strain collection) and found to correspond to one of the potential recombinant blocks identified in CL18. In spite of the small number of coding changes identified in this study, it remains possible that some of these could significantly alter autolysin functioning; further studies assessing the activity and affinity of purified allelic variants are required to address this issue.
This work has been driven by limited knowledge of allelic diversity of pneumococcal virulence determinants and the desire to identify conserved vaccine targets (8). Autolysin appears to represent such a conserved target. The relative infrequency of nonsynonymous change suggests that there is not a strong positive selection pressure for diversity on autolysin. This implies either that the function of the protein is unable to accommodate substantial change or that there may be little role for antibody driven evolution of autolysin in natural infections. Despite this, immunization with LytA can produce a protective response in mice and can induce antibodies capable of inhibiting spontaneous autolysis even in encapsulated organisms (1). As a protein found in all strains, associated with virulence, and apparently highly conserved, autolysin might appear to be a suitable target for inclusion in a potential vaccine. However, the results presented here suggest that any future selective pressure imposed by the use of such a vaccine could drive the selection of novel lytA alleles resulting from recombination with bacteriophage DNA. Although antisera prepared against some of the lytic enzymes of pneumococci and their bacteriophage may cross-react with one another, such events could theoretically lead to the rapid generation of immune escape mutants to a LytA-containing vaccine. Further laboratory studies are required to address this possibility.
ACKNOWLEDGMENTS
This work was supported by grants 039907/2/93/Z and 045171/Z/95/2 from The Wellcome Trust. A.M.W. is supported by a Wellcome Research Fellowship in Biodiversity.
REFERENCES
- 1.Berry A M, Lock R A, Hansman D, Paton J C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989;57:2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry A M, Paton J C, Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992;12:87–93. doi: 10.1016/0882-4010(92)90111-z. [DOI] [PubMed] [Google Scholar]
- 3.Canvin J R, Marvin A P, Sivakumaran M, Paton J C, Boulnois G, Andrew P W, Mitchell T J. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995;172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 4.Crain M J, Waltman W D, Turner J S, Yother J, Talkington D F, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Díaz E, López R, García J L. Chimeric phage-bacterial enzymes, a clue in the molecular evolution of genes. Proc Natl Acad Sci USA. 1990;87:8125–8129. doi: 10.1073/pnas.87.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díaz E, López R, García J L. Role of the major pneumococcal autolysin in the atypical response of a clinical isolate of Streptococcus pneumoniae. J Bacteriol. 1992;174:5508–5515. doi: 10.1128/jb.174.17.5508-5515.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz E, López R, García J L. EJ-1, a temperate bacteriophage of Streptococcus pneumoniae with a Myoviridae morphotype. J Bacteriol. 1992;174:5516–5525. doi: 10.1128/jb.174.17.5516-5525.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowson C G, Barcus V A, King S, Pickerill P, Whatmore A, Yeo M. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. J Appl Microbiol. 1997;83:42S–51S. doi: 10.1046/j.1365-2672.83.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 9.García E, García J L, García P, Arrarás A, Sánchez-Puelles J M, López R. Molecular evolution of lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Proc Natl Acad Sci USA. 1988;85:914–918. doi: 10.1073/pnas.85.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García P, García J L, García E, López R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43:265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie S H, McHugh T D, Ayres H, Dickens A, Efstratiou A, Whiting G C. Allelic variation in Streptococcus pneumoniae autolysin (N-acetyl muramoyl-l-alanine amidase) Infect Immun. 1997;65:3936–3938. doi: 10.1128/iai.65.9.3936-3938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson M K. Cellular location of pneumolysin. FEMS Microbiol Lett. 1977;2:243–245. [Google Scholar]
- 13.King, S. J., and C. G. Dowson. Unpublished data.
- 14.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis, version 1.01. University Park, Pa: The Pennsylvania State University; 1993. [Google Scholar]
- 15.Lock R A, Hansman D, Paton J C. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb Pathog. 1992;12:137–143. doi: 10.1016/0882-4010(92)90116-6. [DOI] [PubMed] [Google Scholar]
- 16.Lomholt H. Evidence of recombination and an antigenically diverse immunoglobulin A1 protease among strains of Streptococcus pneumoniae. Infect Immun. 1995;63:4238–4243. doi: 10.1128/iai.63.11.4238-4243.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López R, García J L, García E, Ronda C, García P. Structural analysis and biological significance of the cell wall lytic enzymes of Streptococcus pneumoniae and its bacteriophage. FEMS Microbiol Lett. 1992;100:439–448. doi: 10.1111/j.1574-6968.1992.tb14074.x. [DOI] [PubMed] [Google Scholar]
- 18.Maynard Smith J. Analyzing the mosaic structure of genes. J Mol Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel L S, McDaniel D O, Hollingshead S K, Briles D E. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified sequence from strain Rx1 and the ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998;66:4748–4754. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell T J, Alexander J E, Morgan P J, Andrew P W. Molecular analysis of virulence factors of Streptococcus pneumoniae. J Appl Microbiol. 1997;83:62S–71S. doi: 10.1046/j.1365-2672.83.s1.7.x. [DOI] [PubMed] [Google Scholar]
- 21.Nei M, Li W-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton J C, Berry A M, Lock R A. Molecular analysis of putative pneumococcal virulence proteins. Microb Drug Resist. 1997;3:1–10. doi: 10.1089/mdr.1997.3.1. [DOI] [PubMed] [Google Scholar]
- 23.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrari L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulson K, Reinholdt J, Jespersgaard C, Boye K, Brown T A, Hauge M, Kilian M. A comprehensive genetic study of streptococcal immunoglobulin A1 proteases: evidence for recombination within and between species. Infect Immun. 1998;66:181–190. doi: 10.1128/iai.66.1.181-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero A, López R, García P. Sequence of the Streptococcus pneumoniae bacteriophage HB-3 amidase reveals high homology with the major host autolysin. J Bacteriol. 1990;172:5064–5070. doi: 10.1128/jb.172.9.5064-5070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Quartey M K, Liebeler C L, Le C T, Giebink G S. Roles of autolysin and pneumolysin in middle ear inflammation caused by a type 3 Streptococcus pneumoniae strain in the chinchilla otitis media model. Infect Immun. 1996;64:1140–1145. doi: 10.1128/iai.64.4.1140-1145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomasz A. Building and breaking of bonds in the cell wall of bacteria—the role for autolysin. In: Nombela C, editor. Microbial cell wall synthesis and autolysis. Amsterdam, The Netherlands: Elsevier; 1984. pp. 3–12. [Google Scholar]
- 28.Tomasz A, Moreillon P, Pozzi G. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J Bacteriol. 1988;170:5931–5934. doi: 10.1128/jb.170.12.5931-5934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 30.Tuomanen E, Tomasz A, Hengstler B, Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985;151:535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]
- 31.Whatmore, A. M., and C. G. Dowson. Unpublished data.
- 32.Whatmore A M, Pickerill A P, Woodard G E, Dowson C G. Abstracts of the XIIIIth Lancefield International Symposium on Streptococci and Streptococcal Diseases. 1996. Allelic variation of the lytA gene of Streptococcus pneumoniae and related species, abstr. P146. Paris, France. [Google Scholar]
- 33.Whatmore A M, Barcus V A, Dowson C G. Genetic diversity of the streptococcal competence (com) locus. J Bacteriol. 1999;181:3144–3154. doi: 10.1128/jb.181.10.3144-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whatmore A M, King S J, Doherty N C, Sturgeon D, Chanter N, Dowson C G. Molecular characterization of equine isolates of Streptococcus pneumoniae: natural disruption of genes encoding the virulence factors pneumolysin and autolysin. Infect Immun. 1999;67:2776–2782. doi: 10.1128/iai.67.6.2776-2782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]